Abstract
Chemokines direct the migration of cells during various immune processes and are involved in many disease states. For example, CCL19 and CCL21, through activation of the CCR7 receptor, recruit dendritic cells and naïve T-cells to the secondary lymphoid organs aiding in balancing immune response and tolerance. However, CCL19 and CCL21 can also direct the metastasis of CCR7 expressing cancers. Chemokine binding to glycosaminoglycans, such as heparin, is as important to chemokine function as receptor activation. CCL21 is unique in that it contains an extended C-terminus not found in other chemokines like CCL19. Deletion of this extended C-terminus reduces CCL21’s affinity for heparin and transferring the CCL21 C-terminus to CCL19 enhances heparin binding mainly through non-specific, electrostatic interactions.
Keywords: Chemokines, glycosaminoglycans, CCL19, CCL21, heparin, chimeric protein
1. Introduction
Chemokines are known for their ability to direct cell migration in support of a variety of immune processes and their involvement in numerous disease states [1, 2]. The chemokines CCL19 and CCL21 recruit antigen presenting dendritic cells and naïve T-cells to the secondary lymphoid organs though activation of CCR7 thereby aiding in the balance of immune response and tolerance [3]. Metastatic breast and other forms of cancers increase expression of CCR7, the CCL19 and CCL21 chemokine receptor, which is correlated with lymph node metastases as well as poor patient prognosis [4–11]. Binding of chemokines, like CCL21, to lymphatic glycosaminoglycans (GAGs) is essential for this lymph node metastasis [12]. Additionally, CCL19 and CCL21 can contribute to HIV-1 latency, which is a major reason behind the inability of current highly active antiretrovirals to eradicate HIV-1 [13, 14].
Chemokines orchestrate cell trafficking through activating cellular chemokine receptors and through GAG binding [1, 15], an interaction that is required for in vivo recruitment of cells by chemokines [16]. GAG binding, as was shown for CCL21, allows for the formation of a stationary chemokine concentration gradient that migrating cells follow through haptotaxis [17]. Other chemokines with lower GAG binding capacity, like CCL19, are thought to form more soluble gradients that cells follow through chemotaxis [17].
CCL21 is unique among chemokines because, at 111 amino acids, it contains an extended C-terminus making it approximately 30 to 50 amino acids longer than the average chemokine. The typical chemokine domain contains about 70 amino acid residues [18]. The domain consists of a flexible N-terminus followed by an N-loop, a three-stranded antiparallel beta sheet with strands connected by the 30s and 40s loops, and an alpha helix [18]. The domain is often followed by a very short flexible C-terminus [18]. For example, CCL19 contains 77 residues with the seven most C-terminal residues making up the short unstructured C-terminus [19]. CCL21 contains a chemokine domain but residues 71–111 are unstructured with the exception of a disulfide bond linking C80 to C99 [20]. This extended C-terminus of CCL21 contributes to GAG binding as truncation dramatically reduces binding to GAGs, like heparin and chondroitin sulfate [21]; and plays a significant role in forming a GAG bound CCL21 gradient [17]. Truncated CCL21, which is observed in vivo due to proteolysis, is thought to form a more soluble gradient similar to CCL19 [17, 22].
Interestingly, the C-terminus of CCL21 also interacts with polysialic acid (PSA), a rare posttranslational modification recently observed in CCR7 that controls dendritic cell trafficking by regulating chemokine recognition [23]. Mouse dendritic cells expressing polysialylated CCR7 readily migrate in response to CCL21 and a truncated version of CCL21 lacking the extended C-terminus [23]. This truncated CCL21, called CCL21trunc, lacks residues 80–111. However, cells expressing unmodified CCR7 migrate only in response to CCL21trunc signifying that the PSA modification of CCR7 is essential for receptor activation by full-length CCL21 [23]. Protein NMR shows differences in the spectra of CCL21 and CCL21trunc indicating the two proteins do not have the same conformation [23]. Changes in spectra of full length CCL21 show binding of PSA leads to a conformational change in which full length CCL21 appears to adopt a conformation similar to CCL21trunc, the PSA independent agonist [23]. CCL19, which is similar in length to truncated CCL21, is also capable of receptor activation in the presence or absence of the PSA modification of CCR7 [23]. Convincingly, the interaction between PSA and the C-terminus of CCL21 was also shown through transferring the extended C-terminus of CCL21 to CCL19. The resulting chimera (CCL19 residues 1–77, CCL21 residues 78–111) is only able to activate PSA modified CCR7, which is similar to full length CCL21 [23].
Given that the C-terminus of CCL21 confers dependence on PSA for CCL19 receptor activation, we hypothesized that transferring the C-terminus of CCL21 to CCL19 would enhance binding to GAGs. To test this hypothesis, protein NMR and truncation studies were used to first confirm the importance of the extended C-terminus of CCL21 for heparin binding. Subsequently qualitative analysis of chemokine binding using heparin affinity chromatography indicated transferring the C-terminus of CCL21 to CCL19 enhances heparin affinity. Examination of chemokine binding using cation exchange chromatography suggests the CCL21 C-terminus contributes to heparin binding mainly through non-specific, electrostatic interactions.
2. Materials and Methods
2.1 Protein expression and purification
Human CCL21, CCL21trunc (CCL21 residues 1–79), CCL19, and the chimera (CCL19, which contains 77 residues, with residues 78–111 of CCL21) were expressed, refolded and purified as previously described [23, 24].
2.2 Protein NMR and chemical shift mapping
100 µM U-15N labeled CCL21 in 25 mM deuterated MES pH 6.0, 10% D2O, and 0.2% NaN3 was titrated with heparin disaccharide I-S (Sigma–Aldrich) and monitored using 15N-1H HSQC spectra as previously described [19, 25]. Combined amide chemical shift perturbations were computed as [(ΔδH)2+(ΔδN)2]1/2, where ΔδH and ΔδN are changes in backbone amide 1H and 15N chemical shifts in ppm, respectively. Dose dependent changes in chemical shift perturbations from titration with heparin disaccharide I-S were used to determine a dissociation constant (Kd) through fitting to an equation that takes into account ligand depletion as previously described [19]. Dose dependent chemical shift perturbations for residues V21, K45, R46, S47, L63, M64, H66, L67, D68, K69, T70, K75, Q78, C80, R81, K82, D83, K88, K91, K94, K97, C99, K100, R101, T102 and E103 were used in determining the Kd.
2.3 Heparin and SP-Sepharose binding assay
Heparin binding was assessed using heparin sepharose chromatography while electrostatic binding was assessed using cation exchange (SP-sepharose) chromatography similar to Fox et al. [26, 27] and others [28, 29]. Roughly 50 µg of chemokine was loaded onto either a 1 mL HiTrap® heparin HP or 1 mL HiTrap® SP-sepharose HP column (GE Healthcare) and eluted with a 0 to 1.3 M NaCl gradient in 20 mM sodium phosphate at pH 7 over 39 minutes using a Shimadzu LC-20 HPLC system. Protein elution was monitored at 220 nm. The elution peak for each chemokine was recorded both as a function of retention time and NaCl concentration. The difference between the NaCl concentrations at which the chemokines eluted from the heparin sepharose column (heparin [NaCl] mM) and the cation exchange column (SP-sepharose [NaCl] mM) was labeled Δ [NaCl] mM and was calculated as Δ [NaCl] mM = heparin [NaCl] mM minus SP-sepharose [NaCl] mM.
3. Results
3.1 Heparin disaccharide causes chemical shift perturbations in the CCL21 C-terminus
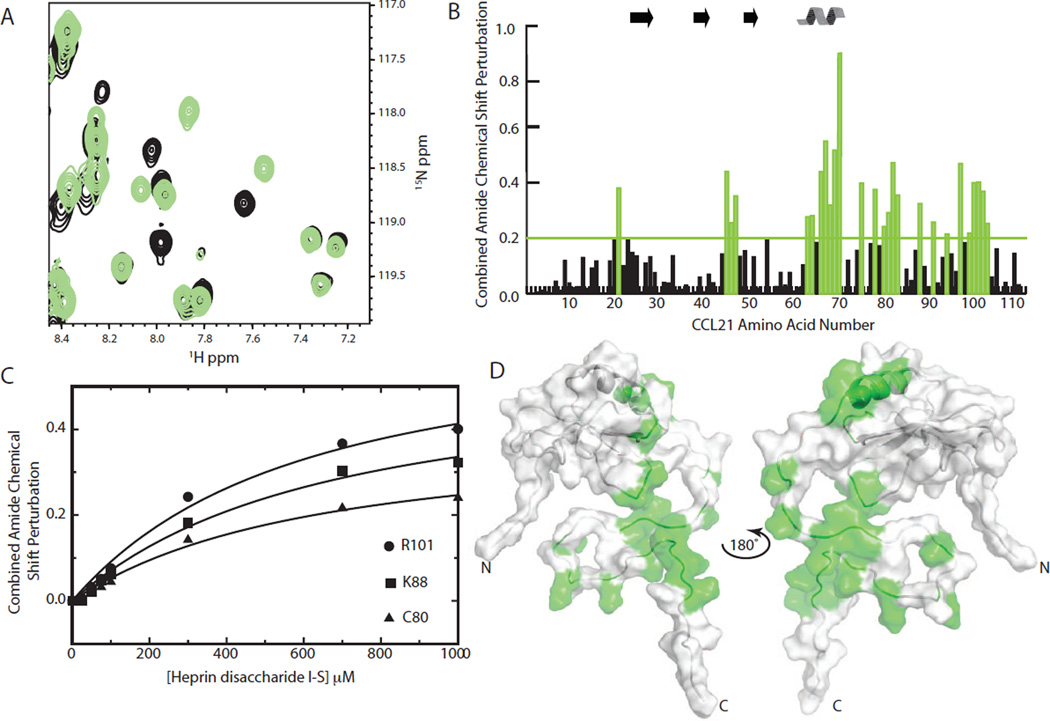
CCL21 contains 111 amino acids and a highly basic C-terminus not found in other chemokines. For example, CCL19, at 77 residues, has a length typical of most chemokines [19]. The extended, basic C-terminus of CCL21 is reported to be necessary for GAG binding with deletions of CCL21 residues 80–111 resulting in greatly reduced or eliminated affinity for GAGs as described by Hirose et al. [21] and Dyer et al. [30]. Heparin is often used when studying chemokine GAG interactions [15], but longer chain heparin and chemokine complexes are not conducive to structural studies using protein NMR [25]. Hence, heparin disaccharide I-S, representing the smallest repeating subunit of heparin, was chosen to investigate the interaction of CCL21 with heparin. As seen in Figure 1, chemical shift mapping using the heparin disaccharide I-S implicates the CCL21 C-terminus in heparin binding, which supports the results of Hirose et al. [21] and Dyer et al. [30]. For many residues, significant changes in chemical shift are observed in CCL21 in the presence and absence of heparin disaccharide I-S, an indication of binding (Fig. 1A). Plotting the combined amide chemical shift perturbation versus the CCL21 amino acid residue number shows heparin disaccharide I-S induced perturbations localize to the extended C-terminus, the alpha helix and the 40s loop that connects the β2 and β3 strands (Fig. 1B). A majority of residues with a significant perturbation (>0.2) are basic amino acids or are near basic amino acids (V21, K45, R46, S47, L63, M64, H66, L67, D68, K69, T70, K75, Q78, C80, R81, K82, D83, K88, K91, K94, K97, C99, K100, R101, T102 and E103). For example, V21 is next to R20 and E103 is next to R104. D83 is within a canonical heparin binding BBXB sequence motif (where B is a basic and X is any amino acid) [31] consisting of R81, K82, D83 and R84. While K91 and K94 are within another BBXB sequence motif (K91, K92, G93, and K94) and show perturbations. Combined amide chemical shift perturbation induced by increasing concentrations of heparin disaccharide I-S were analyzed using non-linear regression to determine a dissociation constant (Kd). The binding model used for fitting incorporates ligand depletion and yields a Kd of approximately 500 µM (Fig. 1C). While a 500 µM Kd may seem weak, surface plasmon resonance studies indicating nano-molar Kd values for CCL21 and heparin utilize much longer chain heparins [30]. Mapping heparin disaccharide I-S induced chemical shift perturbations onto the CCL21 structure reveals a binding interface that incorporates much of CCL21’s extended C-terminus (Fig. 1D). GAGs are known to promote chemokine dimer formation [16, 25, 29, 32]. However, analytical ultracentrifugation studies show that CCL21 is an obligate monomer in isolation [20]. Here we observe no large chemical shift perturbations in the N-terminus or the β1 strand, both regions that can be involved in chemokine dimer formation [33]. The lack of perturbations in these regions may suggest CCL21 remains monomeric in the presence of the heparin disaccharide I-S.
Figure 1. Identification of a heparin-binding surface on CCL21.
A) 15N-1H HSQC spectra of 100 µM 15N CCL21 in the absence (black) or presence (green) of 1000 µM heparin disaccharide I-S. B) Combined amide chemical shift perturbation plotted versus the CCL21 amino acid residue number. C) Non-linear fitting to a binding equation that accounts for ligand depletion yields a Kd of ~500 µM for CCL21 and heparin disaccharide I-S. Fits for three representative residues are shown. D) CCL21 structure with residues having heparin-induced perturbation greater than 0.2 in panel B colored green.
Clearly, the C-terminus of CCL21 is involved in heparin binding. However, with heparin disaccharide I-S induced chemical shift perturbations located in the extended C-terminus and in the 40’s loop and alpha helix of the chemokine domain, a more complete test of the C-terminus’s role may be to see if transferring it to another chemokine enhances the heparin binding of that chemokine. Additionally, most CCL21 residues with heparin prompted chemical shift perturbations are basic amino acids or are found near one. This led us to question whether or not the binding to heparin resulted from any specificity for heparin or if this interaction was more electrostatic in nature.
3.2 Transferring the CCL21 C-terminus to CCL19 enhances heparin binding mainly through non-specific electrostatic interactions
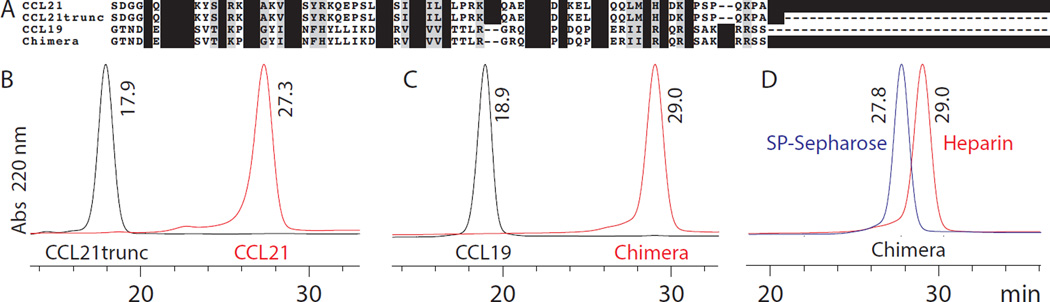
The relative affinity to heparin in relation to the specificity of binding can be determined through comparing a protein’s elution profile from heparin sepharose and cation exchange (SP-sepharose) chromatography as demonstrated most recently by Fox et al. [26, 27] and others [28, 29]. This method is utilized to elucidate whether affinity is a result of a specific binding affinity to heparin or nonspecific electrostatic interactions. As seen in the sequence alignment (Fig. 2A), transferring residues 80–111 of CCL21 to CCL19 results in the formation of a CCL19/CCL21 chimera. The chimera is useful in confirming the importance of CCL21’s C-terminus for binding to GAGs such as heparin. The sequences of CCL21, CCL21trunc and CCL19 are also shown in Figure 2A.
Figure 2. Transfer of the C-terminus of CCL21 to CCL19 enhances heparin binding mainly through electrostatic interactions.
A) Sequence alignment of CCL21, CCL21trunc, CCL19 and the chimera (CCL19 with the CCL21 C-terminus). B) Elution profile of CCL21trunc (black) and CCL21 (red) from a HiTrap® Heparin HP column. C) Elution profile of CCL19 (black) and chimera (red) from a HiTrap® Heparin HP column. D) Elution profile of the chimera from a HiTrap® SP-sepharose HP column (blue) and a HiTrap® Heparin HP column (red). The Abs at 220 nm has been normalized so maximum peak intensities are the same for all chemokines; example full chromatograms showing raw data are in the electronic supplemental materials.
Similar to results from Hirose et al. [21] and Dyer et al. [30], deletion of the CCL21 C-terminus results in a reduction in heparin binding affinity. This is seen by the lower retention time and NaCl concentration at which CCL21trunc elutes from the heparin column (Fig. 2B and Table 1). The transfer of the basic C-terminus of CCL21 to CCL19 induces greater heparin interaction as seen in Figure 2C and Table 1. The chimera eluting from the heparin column at a significantly higher retention time and NaCl concentration than CCL19 highlights this enhanced heparin binding.
Table 1.
Elution of chemokines from heparin and SP-sepharose columns.a
| Protein | Heparin [NaCl] mM | SP-sepharose [NaCl] mM | Δ [NaCl] mMb |
|---|---|---|---|
| CCL21 | 911 | 806 | 105 |
| CCL21trunc | 597 | 566 | 31 |
| CCL19 | 632 | 689 | −57 |
| Chimera | 968 | 927 | 41 |
Chemokine elution reported as a function of NaCl concentration.
Δ [NaCl] mM = Heparin [NaCl] mM minus SP-sepharose [NaCl] mM.
It is clear that the C-terminus of CCL21 has intrinsic properties that contribute to higher affinity for heparin. However, it is necessary to further interrogate the nature of CCL21’s heparin interaction. Using a SP-sepharose cation exchange column and a heparin affinity column helps distinguish between specific heparin interactions and affinity resulting from general electrostatic interactions [26–29]. Figure 2D shows that the chimera elutes at similar retention times from both the heparin and SP-sepharose columns, this is shown in Table 1 in terms of NaCl concentrations at elution. The chimera eluted from the heparin column at 968 mM NaCl and at 927 mM NaCl from the SP-sepharose column (Table 1). The small difference, 41 mM, in these NaCl concentrations provides evidence that the transfer of CCL21’s C-terminus to CCL19 enhances heparin affinity mainly through non-specific electrostatic interactions.
Small differences in the concentration at which CCL21trunc or CCL19 eluted from the heparin and cation exchange column were also observed (Table 1). For CCL21trunc this difference was 31 mM NaCl. For CCL19 this difference was −57 mM NaCl, indicating CCL19 bound slightly better to the cation exchange column than the heparin column. These results suggest any heparin affinity for CCL21trunc and CCL19 is mostly derived from non-specific electrostatic interactions (Table 1). Sixt and colleagues report that CCL21trunc and CCL19 form more soluble gradients than full length CCL21 [17, 34]. Perhaps this lack of specificity for heparin plays a role in CCL21trunc or CCL19 forming these more soluble gradients. Fittingly, full length CCL21, which forms an immobilized gradient for haptotaxing cells to follow[17, 34], shows higher affinity for heparin and some heparin specific binding. This specificity is seen in the slightly larger difference, 105 mM NaCl, between the concentration at which CCL21 elutes from the heparin and cation exchange columns (Table 1) [27].
4. Discussion
Deleting the extended CCL21 C-terminus results in reduced GAG binding as observed in other reports [17, 21, 30] and confirmed here using the GAG heparin. Heparin binding may be due to electrostatic interaction between the basic CCL21 C-terminus and negatively charged heparin, specificity for heparin, or a combination of both. Comparison of elution profiles from heparin and cation exchange sepharose columns has been used to address specific heparin binding versus non-specific electrostatic interactions [26–29]. Comparing the elution profile for CCL21 reveals a difference in NaCl concentration at elution of 105 mM from heparin and cation exchange columns (Table 1). Fox et al. categorize differences in elution concentration that are broadly near 0 mM NaCl as having little to no specificity for heparin (largely electrostatic interactions), between 100–200 mM NaCl as an intermediate specificity for heparin, and differences >200 mM as having high specificity for heparin [27]. For example, the chemokine XCL1 elutes at 920 mM NaCl from heparin and 590 mM NaCl from cation exchange columns; a difference of 330 mM NaCl that makes XCL1’s binding to heparin highly specific [27]. With a 31 mM change in NaCl elution concentration for the heparin versus the cation exchange column, CCL21trunc shows little to no specificity for heparin. Similarly, CCL19 also falls into this category with a difference of −57 mM NaCl (Table 1). Again, Sixt and colleagues report that CCL21trunc, which is produced in vivo from proteolysis of full length CCL21, and CCL19 form more soluble gradients [17, 34]. We hypothesize that the lack of specific binding to heparin for CCL21trunc and CCL19 may be related to the ability of these chemokines to form more soluble gradients for migrating or chemotaxing cells to follow in vivo.
Transferring the C-terminus of CCL21 to CCL19 creating the chimera greatly enhances heparin binding (Fig. 2C and Table 1). Yet, the chimera, with similar elution times and concentrations (Fig. 2D and Table 1) from the heparin and cation exchange columns, shows little to no specificity for heparin suggesting interactions are electrostatic in nature. Mild specificity for heparin is only observed in the context of full length CCL21 (Table 1). As such, our results indicate CCL21 has a higher affinity for heparin that is partly due to specific heparin binding, which fits with CCL21 forming GAG bound, immobilized gradients that haptotaxing cells follow[17, 34]. Sixt and colleagues propose a combination of chemotaxis in response to more soluble chemokine gradients and haptotaxis toward immobilized chemokine gradients are likely necessary for cellular recruitment to and within the lymph nodes [17, 34]. Overall our results start to provide a mechanistic explanation as to how CCL19 and CCL21trunc might form more soluble gradients while full length CCL21 with its higher affinity and mild specificity for heparin forms an immobilized gradient.
Subsequent investigations into CCL21’s limited specificity for heparin, or potentially other GAGs, must involve investigating CCL21 mutants in the context of GAGs with various sulfation and epimerization patterns [15]. This may be a difficult task as reduced sulfation inherently means a reduction in negative charge making parsing out changes in binding affinity that equate to loss of specific interactions versus generic electrostatic interactions challenging. For example, reducing GAG sulfation through enzymatic treatment, eliminating the cellular enzymes responsible for GAG sulfation, or controlling the level of sulfation in synthetic GAGs reduces CCL21 GAG binding [12, 35, 36]. The lack of specificity for heparin observed for CCL19 and CCL21trunc and their lower binding observed here and elsewhere [21, 30] surely contribute to CCL19 and CCL21trunc forming more soluble chemokine gradients as described by Sixt and colleagues [17, 22]. The chimera may be useful in future investigations into soluble or stationary chemokine gradient formation and, hence, chemotaxis versus haptotaxis.
Supplementary Material
Highlights.
Identification of a putative heparin binding interface in CCL21
The CCL21 C-terminus enhances heparin binding of a CCL19/CCL21 chimeric protein
The CCL21 C-terminus confers heparin binding through non-specific interactions
Acknowledgments
This work was supported by the NCI of the NIH through grant 1R15CA159202-01 to C.T.V. Undergraduates completed the majority of this work as a part of a biochemistry laboratory course; we thank the University of Wisconsin-Whitewater and the Department of Chemistry for supporting the use of research in teaching.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baggiolini M. Chemokines in pathology and medicine. J. Intern. Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib. Microbiol. 2006;13:191–199. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- 3.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 4.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin. Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 6.Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Forster R. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int. J. Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi K, Kozawa Y, Ohashi Y, Nakamura ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T, Saiki I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol. Rep. 2007;17:1511–1516. [PubMed] [Google Scholar]
- 8.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 9.Malietzis G, Lee GH, Bernardo D, Blakemore AI, Knight SC, Moorghen M, Al-Hassi HO, Jenkins JT. The prognostic significance and relationship with body composition of CCR7-positive cells in colorectal cancer. Journal of Surgical oncology. 2015;112:86–92. doi: 10.1002/jso.23959. [DOI] [PubMed] [Google Scholar]
- 10.Du P, Liu Y, Ren H, Zhao J, Zhang X, Patel R, Hu C, Gan J, Huang G. Expression of chemokine receptor CCR7 is a negative prognostic factor for patients with gastric cancer: a meta-analysis. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016 doi: 10.1007/s10120-016-0602-8. [DOI] [PubMed] [Google Scholar]
- 11.van den Bosch T, Koopmans AE, Vaarwater J, van den Berg M, de Klein A, Verdijk RM. Chemokine receptor CCR7 expression predicts poor outcome in uveal melanoma and relates to liver metastasis whereas expression of CXCR4 is not of clinical relevance. Invest. Ophthalmol. Vis. Sci. 2013;54:7354–7361. doi: 10.1167/iovs.13-12407. [DOI] [PubMed] [Google Scholar]
- 12.Yin X, Truty J, Lawrence R, Johns SC, Srinivasan RS, Handel TM, Fuster MM. A critical role for lymphatic endothelial heparan sulfate in lymph node metastasis. Molecular Cancer. 2010;9:316. doi: 10.1186/1476-4598-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh S, Wightman F, Ramanayake S, Alexander M, Kumar N, Khoury G, Pereira C, Purcell D, Cameron PU, Lewin SR. Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells. Retrovirology. 2011;8:80. doi: 10.1186/1742-4690-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 15.Monneau Y, Arenzana-Seisdedos F, Lortat-Jacob H. The sweet spot: how GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2015 doi: 10.1189/jlb.3MR0915-440R. [DOI] [PubMed] [Google Scholar]
- 16.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu. Rev. Pharmacol. Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 19.Veldkamp CT, Kiermaier E, Gabel-Eissens SJ, Gillitzer ML, Lippner DR, DiSilvio FA, Mueller CJ, Wantuch PL, Chaffee GR, Famiglietti MW, Zgoba DM, Bailey AA, Bah Y, Engebretson SJ, Graupner DR, Lackner ER, LaRosa VD, Medeiros T, Olson ML, Phillips AJ, Pyles H, Richard AM, Schoeller SJ, Touzeau B, Williams LG, Sixt M, Peterson FC. Solution Structure of CCL19 and Identification of Overlapping CCR7 and PSGL-1 Binding Sites. Biochemistry. 2015;54:4163–4166. doi: 10.1021/acs.biochem.5b00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love M, Sandberg JL, Ziarek JJ, Gerarden KP, Rode RR, Jensen DR, McCaslin DR, Peterson FC, Veldkamp CT. Solution structure of CCL21 and identification of a putative CCR7 binding site. Biochemistry. 2012;51:733–735. doi: 10.1021/bi201601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirose J, Kawashima H, Swope Willis M, Springer TA, Hasegawa H, Yoshie O, Miyasaka M. Chondroitin sulfate B exerts its inhibitory effect on secondary lymphoid tissue chemokine (SLC) by binding to the C-terminus of SLC. Biochim. Biophys. Acta. 2002;1571:219–224. doi: 10.1016/s0304-4165(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 22.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Kiermaier E, Moussion C, Veldkamp CT, Gerardy-Schahn R, de Vries I, Williams LG, Chaffee GR, Phillips AJ, Freiberger F, Imre R, Taleski D, Payne RJ, Braun A, Forster R, Mechtler K, Muhlenhoff M, Volkman BF, Sixt M. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science. 2016;351:186–190. doi: 10.1126/science.aad0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veldkamp CT, Koplinski CA, Jensen DR, Peterson FC, Smits KM, Smith BL, Johnson SK, Lettieri C, Buchholz WG, Solheim JC, Volkman BF. Production of Recombinant Chemokines and Validation of Refolding. Methods Enzymol. 2016;570:539–565. doi: 10.1016/bs.mie.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldkamp CT, Peterson FC, Pelzek AJ, Volkman BF. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin. Protein. Sci. 2005;14:1071–1081. doi: 10.1110/ps.041219505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox JC, Tyler RC, Guzzo C, Tuinstra RL, Peterson FC, Lusso P, Volkman BF. Engineering Metamorphic Chemokine Lymphotactin/XCL1 into the GAG-Binding, HIV-Inhibitory Dimer Conformation. ACS Chem. Biol. 2015;10:2580–2588. doi: 10.1021/acschembio.5b00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox JC, Tyler RC, Peterson FC, Dyer DP, Zhang F, Linhardt RJ, Handel TM, Volkman BF. Examination of Glycosaminoglycan Binding Sites on the XCL1 Dimer. Biochemistry. 2016;55:1214–1225. doi: 10.1021/acs.biochem.5b01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu. Rev. Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 29.Hamel DJ, Sielaff I, Proudfoot AE, Handel TM. Chapter 4. Interactions of chemokines with glycosaminoglycans. Methods Enzymol. 2009;461:71–102. doi: 10.1016/S0076-6879(09)05404-4. [DOI] [PubMed] [Google Scholar]
- 30.Dyer DP, Salanga CL, Johns SC, Valdambrini E, Fuster MM, Milner CM, Day AJ, Handel TM. The anti-inflammatory protein TSG-6 regulates chemokine function by inhibiting chemokine:glycosaminoglycan interactions. J. Biol. Chem. 2016 doi: 10.1074/jbc.M116.720953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proudfoot AEI, Fritchley S, Borlat F, Shaw JP, Vilbois F, Zwahlen C, Trkola A, Marchant D, Clapham PR, Wells TNC. The BBXB Motif of RANTES Is the Principal Site for Heparin Binding and Controls Receptor Selectivity. J. Biol. Chem. 2001;276:10620–10626. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Sharp JS, Handel TM, Prestegard JH. Chemokine oligomerization in cell signaling and migration. Prog. Mol. Biol. Transl. Sci. 2013;117:531–578. doi: 10.1016/B978-0-12-386931-9.00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salanga CL, Handel TM. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp. Cell. Res. 2011;317:590–601. doi: 10.1016/j.yexcr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sixt M, Lammermann T. In vitro analysis of chemotactic leukocyte migration in 3D environments. Methods Mol. Biol. 2011;769:149–165. doi: 10.1007/978-1-61779-207-6_11. [DOI] [PubMed] [Google Scholar]
- 35.Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Werb Z, Rosen SD. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochemistry. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Paz JL, Moseman EA, Noti C, Polito L, von Andrian UH, Seeberger PH. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem. Biol. 2007;2:735–744. doi: 10.1021/cb700159m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.