Abstract
BACKGROUND
Partial hepatectomy and cyst fenestration (PHCF) selectively provides clinical benefit in highly symptomatic patients with polycystic liver disease (PLD). This study aims to ascertain whether the reduction in liver volume (LV) achieved by PHCF is sustained long term.
STUDY DESIGN
Clinical data were retrieved from the electronic records of all patients with PLD who underwent PHCF between 1985 and 2014. Preoperative LVs (LV1), postoperative LVs (LV2), and late follow-up LVs (LV3) were measured from magnetic resonance or CT images.
RESULTS
Among 186 patients who underwent PHCF, 91% were Caucasian women with autosomal dominant polycystic kidney disease with a mean age of 49 years. Major perioperative complications (Clavien III/IV) occurred in 21% of the patients. Operative mortality (<90 days) was 2.7%. Eleven patients had liver failure develop, received liver transplants, or had liver-related deaths. Overall survival was 95.7%, 93.3%, 85.6%, and 77.7% at 1, 5, 10, and 15 years respectively. Imaging records for volumetry were unavailable in 32 patients. Of the remaining 154 patients, 34 had imaging for 1 LV, 64 for 2 LVs, and 55 for all 3 LVs. Median LV was 6,781 mL (interquartile range 4,903 to 8,341 mL) preoperatively and 2,502 mL (interquartile range 2,089 to 3,136 mL) after PHCF, leading to a median postoperative LV reduction of 61%. At follow-up (mean 8 years), median LV was 2,519 mL (interquartile range 2,083 to 3,752 mL). Interestingly, 33 of 62 patients with available LV2 and LV3 showed additional regression in LV at follow-up (median –14.1%), and the rest showed mild growth of 9.9%. Overall volumetric comparison of preoperative with follow-up liver imaging showed sustained LV reduction (median 61%).
CONCLUSIONS
Sustained long-term reductions in LV after PHCF can be achieved in selected patients with severe, highly symptomatic PLD. In our experience, liver-related death and subsequent liver transplantation are infrequent after PHCF.
Polycystic liver disease (PLD) is characterized by the presence of multiple cholangiocyte-derived epithelial cysts that cause progressive liver enlargement. Most commonly, PLD co-exists with autosomal dominant polycystic kidney disease (ADPKD),1 and occurs less commonly as a genetically distinct disease with few or no renal cysts in autosomal dominant polycystic liver disease.2 Polycystic liver disease is one of the most common extrarenal manifestations of ADPKD3,4 and is defined clinically by the presence of any liver cyst. Hepatic cyst prevalence and total hepatic cyst volume increase with age and in women compared with men. Hepatic cysts are evident on MRI in 94% of patients with ADPKD who are older than 35 years of age.1,5 Although most patients are asymptomatic initially, extensive PLD can lead to altered hepatic-related biochemical features and affect quality of life.5 Cystic enlargement causes liver enlargement, which can be marked and result in dyspnea, early satiety, gastroesophageal reflux, mechanical back pain, hepatic venous outflow obstruction, portal vein and inferior vena cava compression, and, rarely, jaundice from bile duct compression.6 When lifestyle is impaired substantially, surgical intervention is indicated to alleviate symptoms and restore quality of life. Partial hepatectomy and cyst fenestration (PHCF) selectively provides clinical benefit in highly symptomatic patients with massive hepatomegaly with an acceptable surgical risk when performed by an experienced liver surgeon. Our center has reported short-term and long-term effectiveness of PHCF for symptom control on limited number of patients previously.7,8 This study aims to ascertain whether the reduction in liver volume (LV) achieved by PHCF is sustained long-term and to assess operative risk and survival.
METHODS
Between July 1985 and April 2014, 186 patients with PLD underwent PHCF at Mayo Clinic Rochester, MN. Demographic characteristics and clinical data were reviewed retrospectively. Hepatic resection was offered to patients who had massive and symptomatic PLD that resulted in decreased clinical performance status, quality of life, and, in some cases, complications such as cholestasis and hepatic venous outflow obstruction. The primary indication for PHCF was the patients’ decision that their lifestyle impairment from liver enlargement precluded or severely limited physical activity, social interaction, employment, or combinations of these findings. Partial hepatectomy and cyst fenestration was undertaken if at least one hepatic section (sector) was relatively spared of PLD in comparison with those sections diffusely involved with PLD, afferent and efferent hepatic vasculature was patent, and hepatic function was maintained. Preoperative evaluation of patients for resection has been detailed previously and routinely included hepatic, renal, and cerebrovascular imaging.8 In brief, patients undergoing PHCF had type C PLD based on our earlier classification scheme.8 Patients with PLD who underwent hepatic operations for reasons other than control of volume-related symptoms with or without concurrent complications from liver cysts were specifically excluded. All patients were operated on by a single surgeon (DMN). Perioperative morbidity included complications within the hospital stay or within 30 days of operation. Early mortality was defined as death within the hospital stay or within 90 days of operation. Clinical follow-up was defined as last recorded communication or visit with the patient. Imaging follow-up was defined as the last imaging study available at long-term follow-up (>1 year). Ten patients were enrolled into clinical trials of somatostatin analogues for severe PLD, 4 several years preoperatively (the administration of the somatostatin analogue was discontinued at the time of the surgery) and 6 postoperatively (postoperative liver images included in these patients were obtained before the initiation of somatostatin analogue therapy). Survival status was obtained on all patients using vital records website (www.archives.com). Patient survival was analyzed using the Kaplan-Meier method. Cause of death was obtained from medical records or through obtaining the official death certificate. The study was approved by the Mayo Clinic Rochester IRB and research authorization was provided by all patients. Radiographic volumetric measurements of the liver were performed on axial or coronal CT or MRI studies using Analyze software (Analyze 120.0, Biomedical Image Resource, Mayo Clinic). Preoperative LVs (LV1), postoperative LVs (<6 months, LV2), and late follow-up LVs (>1 year, LV3) were measured when available. Data are reported as mean ± SD for normally distributed data or median and interquartile range (IQR) for skewed data. Comparisons between groups were done by Student's t-test (2 groups) or ANOVA (more than 2 groups) for normally distributed data and by Wilcoxon rank sum test or Kruskal-Wallis test as appropriate for skewed data.
RESULTS
Among the 186 patients who underwent PHCF for symptomatic PLD, 170 (91.4%) patients had ADPKD and 16 (8.6%) had autosomal dominant polycystic liver disease. The majority of patients were women (90.5%) and Caucasian (91.8%). Mean age at operation was 49 ± 9.6 years. Mean number of hepatic segments resected per patient was 4.42 ± 1.2. At the time of PHCF, 38% of patients had chronic kidney disease stage II, 32% had stage III, 11% had stage IV, 3% had stage V without renal replacement therapy, 4.3% had end-stage renal disease on dialysis, and 1.1% received a kidney transplant. Indications for surgery were abdominal distention, abdominal pain, early satiety, supine dyspnea, fatigue, uterine pro-lapse, hepatic venous outflow obstruction with ascites, obstruction of the inferior vena cava and dialysis hypotension, bile duct obstruction, and infected cysts. Mean duration of clinical follow-up was 8.04 ± 6.8 years (range 0 to 28.5 years). Fourteen patients had a follow-up at <1 month and 11 additional patients had follow-up at <1 year. These patients were followed locally by their referring physicians.
Morbidity and mortality
Major perioperative morbidity (Clavien III/IV) occurred in 21% of the patients. Clavien III/IV complications included bile leaks requiring stenting (n = 13 [7%]), hepatic venous outflow obstruction requiring IVC stenting (n = 9 [4.8%]), reoperation for hemorrhage (n = 6 [3.2%]), acute kidney injury (Acute Kidney Injury Network stage 1: n = 5 [2.7%]; Acute Kidney Injury Network stage 2: n = 1 [0.05%]). Overall, liver function was preserved pre- and postoperatively; however, there were significant reductions in peripheral leukocyte and platelet counts consistent with a mild degree of hyper-splenism from subclinical portal hypertension postoperatively, which is confirmed by a significant increase in splenic volume postoperatively (Table 1).
Table 1.
Biochemical and Hematologic Features Before and After Surgery
| Feature | Preoperative | Postoperative (>6 mo) | p Value |
|---|---|---|---|
| Alanine transaminase, U/L | 22 (15–34) | 24 (17.8–34.5) | 0.43 |
| Aspartate transaminase, U/L | 25 (20–31) | 23 (19–32.3) | 0.61 |
| Alkaline phosphatase, U/L | 162 (101.2–264.7) | 97.5 (70–202.5) | <0.001 |
| International normalized ratio | 1 (1–1.1) | 1.1 (1–1.2) | 0.043 |
| Hemoglobin, g/dL | 12 (10.9–13.2) | 12 (11.4–13.5) | 0.25 |
| WBC, 109/L | 6 (5.1–7) | 5.3 (4.5–6.7) | 0.031 |
| Platelets, 109/L | 218 (184–259) | 160 (120–199) | <0.001 |
| Spleen volume, mL | 261 (191–353) | 335 (238–501) | <0.001 |
Data are presented as median (interquartile range).
Screening for intracranial aneurysms was routinely performed preoperatively. Among the 186 patients, 15 had intracranial aneurysms with mean size of 3.54 ± 1.3 mm. No aneurysm that was detected by preoperative screening subsequently ruptured. One patient with a negative magnetic resonance angiography before surgery had a subarachnoid hemorrhage and died 9 months after the surgery, presumably from a ruptured de novo intracranial aneurysms. Another patient had undergone clipping of a ruptured basilar artery aneurysm 3 years before the surgery and had no evidence of recurrent aneurysm on a magnetic resonance angiography obtained preoperatively. Two years later, this patient suffered a fatal subarachnoid hemorrhage, presumably from a de novo aneurysm.
Eleven patients had liver failure develop, received liver transplants, or had liver-related deaths. Three patients had acute hepatic failure (within 8 to 28 days), 1 had subacute liver failure (4 to 12 weeks), and 7 received liver transplants or had liver-related deaths at later dates (>12 weeks). One patient with acute liver failure had complete recovery, 1 patient had liver transplantation alone, and the other required combined liver and kidney transplantation within 5 weeks of the hepatectomy, but died perioperatively. The patient with subacute liver failure underwent transplantation after 7.7 months. Among the remaining 7 patients, 1 underwent liver transplantation after 4.3 years, another underwent combined liver and kidney transplantation after 9.7 years and died perioperatively from cardiac failure. The cause of death in 6 patients was listed as end-stage liver disease or hepatic failure after a mean postoperative duration of 11.4 ± 8.1 years.
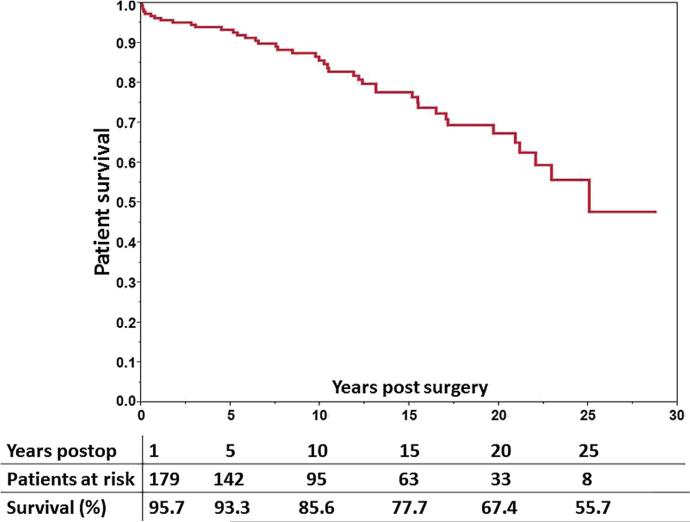
Overall survival was 95.7%, 93.3%, 85.6%, and 77.7% at 1, 5, 10, and 15 years, respectively (Fig. 1). Operative mortality ( 90 days) was 2.7%. Causes of operative mortality included sepsis (n = 2), liver failure (n = 1), cerebral infarct (n = 1), and intracerebral bleeding (n = 1). Mean age of the 38 patients who had late mortality (>3 months) was 63.8 ± 12 years and mean time elapsed after surgery was 11 ± 6.8 years. Causes of late death in addition to the 7 liver-related deaths described in the previous paragraph included malignancy (n = 7), sepsis (n = 4), subarachnoid hemorrhage (n = 2), gastrointestinal hemorrhage (n = 2), and acute ischemic stroke, acute myocardial infarction, cholestatic hepatitis, and intestinal ischemia in 1 patient each.
Figure 1.
Long-term survival of patients who underwent partial hepatectomy and cyst fenestration up to 25 years.
Liver volume reduction
The CT or magnetic resonance images for volumetry were unavailable in 32 of 186 patients. Those patients had their images and surgery before the electronic records era and images were discarded after 10 years by institution protocol at that time, or had been returned to outside facilities or referral physicians. Ten of the 32 patients with unavailable images had been included in a previous report from our institution of 31 patients who underwent PHCF between July 1985 and June 1993.7 The LV1 images of the remaining 21 patients in the previous report had also been discarded, but LV2 and/or LV3 images were available and therefore these patients are included in the current study. In the previous report, LVs were measured manually from hard copies; mean LV1 and LV2 were 9357 mL and 3567 mL, respectively.7
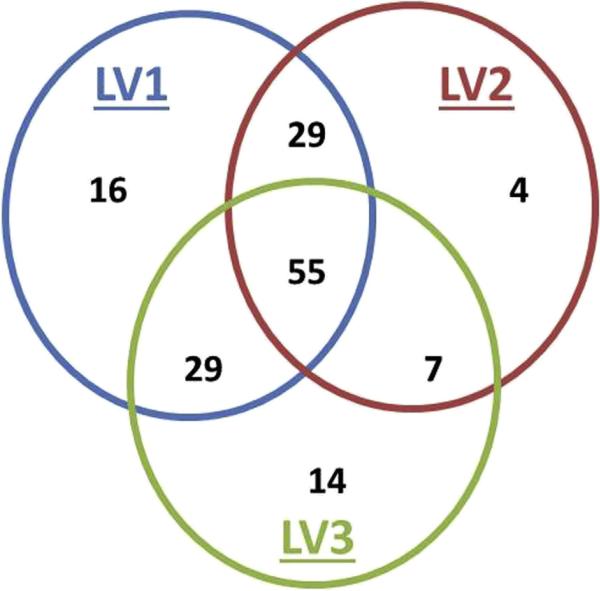
Of the remaining 154 patients, 34 had imaging for 1 LV, 65 for 2 LVs, and 55 for all 3 LVs. Figure 2 shows the overall distribution of measured LVs per patient. Single LVs per patient were: LV1 (n = 16), LV2 (n = 4),and LV3 (n = 140). Two LVs per patient were: LV1 and LV2 (n = 29), LV1 and LV3 (n = 29), and LV2 and LV3 (n = 7). All 3 LVs were measured in 55 patients. Baseline demographic characteristics, hepatorenal function, and outcomes for all groups were similar regardless of imaging availability (Table 2).
Figure 2.
Proportion of patients with preoperative (LV1), immediate postoperative (LV2), and follow-up (LV3) liver volumes.
Table 2.
Baseline Characteristics of Patients Based on Imaging Availability
| Characteristic | Missing images (n = 32) | With images (n = 154) | p Value |
|---|---|---|---|
| Female, n (%) | 26 (81.3) | 143 (92.9) | 0.038 |
| White, % | 92.8 | 91.6 | 0.62 |
| Age at surgery, y, mean ± SD | 49.6 ± 13.7 | 48.7 ± 8.5 | 0.64 |
| ADPKD, n (%) | 29 (90.6) | 141 (91.6) | 0.86 |
| ADPLD, n (%) | 3 (9.4) | 13 (8.4) | |
| No. of segments resected, mean ± SD | 4.2 ± 1.1 | 4.4 ± 1.2 | 0.34 |
| Major perioperative morbidity, n (%) | 1 (3.2) | 38 (24.7) | 0.006 |
| Operative mortality, n (%) | 2 (6.3) | 3 (2) | 0.17 |
| Later need for liver transplantation, n (%) | 0 | 4 (2.6) | 0.35 |
ADPKD, autosomal dominant polycystic kidney disease; ADPLD, autosomal dominant polycystic liver disease.
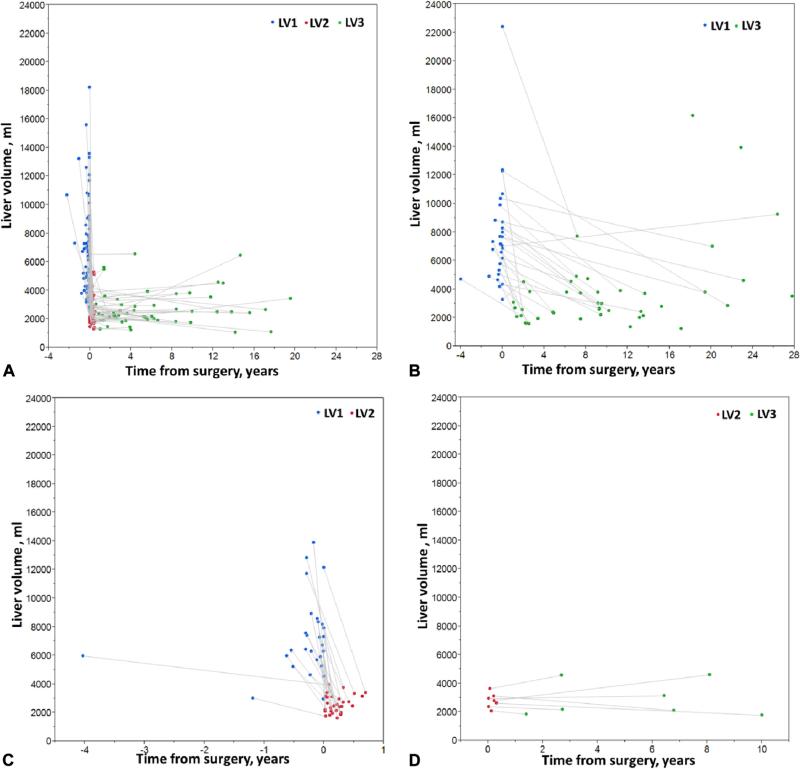
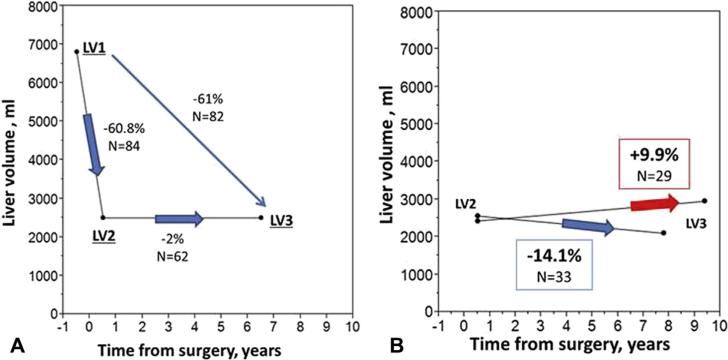
Median LV was 6,781 mL (IQR 4,903 to 8,341 mL) preoperatively and 2,502 mL (IQR 2,089 to 3,136 mL) after PHCF, with a median postoperative LV reduction of 61% (IQR 51.5% to 68.4%). At long-term imaging follow-up (mean 7.97 ± 6.5 years), median LV was 2,519 mL (IQR 2,083 to 3,752 mL). The reduction in LV postoperatively and the progressive changes in LV several years after PHCF are shown in Figures 3 and 4. Overall volumetric comparison of preoperative to follow-up liver imaging showed sustained LV reduction (median 61% [IQR 51% to 68%], Fig. 4A). Preoperative to follow-up liver imaging showed sustained LV reduction (median 61% [IQR 50% to 67%]). There was no difference in LVs or reduction rates among the patients who had 1 of the 3 images unavailable. One male and 1 female patient who underwent PHCF at age 43.6 years and 44.3 years, respectively, and did not have available LV1 and LV2 images, had marked recurrent hepatomegaly 18 and 23 years after PHCF, respectively. Interestingly, 29 of 62 patients with available LV2 and LV3 showed additional regression in LV at follow-up (median –14.1% [IQR –31.9 to –8.3]); and the rest showed mild growth of 9.9% (IQR 6.93% to 29.9%) (Fig. 4B). We analyzed the patients who had their LV3 at least 3 years apart from LV2 (n = 37) and compared baseline factors in Table 3.
Figure 3.
Liver volumes in the patients treated by partial hepatectomy and cyst fenestration according to the availability of images of pre-operative (LV1), immediate postoperative (LV2), and follow-up (LV3) liver volumes. Individual patients are shown in each of the 4 panels. (A) Patients with all 3 images available (LV1, LV2, and LV3) in addition to patients with only LV1 available. (B) Patients with both LV1 and LV3 or only LV3. (C) Patients with only LV1 and LV2 available or LV2 alone. (D) Patients with LV2 and LV3.
Figure 4.
(A) Reduction of liver volumes after surgery in early and long-term postoperative phase, comparing patients with both LV1 and LV2, LV2 and LV3, or LV1 and LV3. (B) Negative and positive postoperative liver growth in patients with both LV2 and LV3. LV1, preoperative liver volume; LV2, immediate postoperative liver volume; LV3, liver volume at follow-up.
Table 3.
Univariate Analysis of Variable in the Patients with Negative or Positive Postoperative Growth of Liver Volumes at Long-Term Follow-Up
| Variable | Positive growth (n = 19) | Negative growth (n = 18) | p Value |
|---|---|---|---|
| Females, n (%) | 17 (89.5) | 16 (88.9) | 0.95 |
| Age at operation, y, mean ± SD | 47 ± 26.2 | 51 ± 59.4 | 0.11 |
| Age of female at operation, y, mean ± SD | 46.6 ± 6.3 | 50.4 ± 9.4 | 0.18 |
| LV1, mL, median (IQR) | 6,505 (4,498–11,447) | 7,226 (3,766–8,782) | 0.79 |
| LV2, mL, median (IQR) | 2,776 (1,894–3365) | 2,590 (2,292–2,904) | 0.66 |
| Time PHCF to LV2, mo, mean ± SD | 2 ± 1.5 | 1.84 ± 1.5 | 0.75 |
| Time LV2 to LV3, y, mean ± SD | 8.4 ± 4.2 | 9.1 ± 4.8 | 0.71 |
| LV3, mL, median (IQR) | 3,447 (2,519–4,508) | 2,048 (1,768–2,447) | <0.001 |
| Annualized growth rate, % per year, mean ± SD | 3.42 ± 4.6 | −2.98 ± 1.6 | <0.001 |
IQR, interquartile range; LV1, preoperative liver volume; LV2, immediate postoperative liver volume; LV3, liver volume at follow-up; PHCF partial hepatectomy with cyst fenestration.
Liver imaging of 120 patients with PLD with preoperative, short-term, and/or long-term postoperative follow-up is shown in Appendix 1 (Available online). Supplementary Figure 1 (Available online) shows the number of patients with PLD who underwent PHCF or orthotropic liver transplantation in our center for treatment of PLD from 1985 until 2014 in increments of 5 years.
DISCUSSION
The major finding of this study is that hepatic volume reduction after PHCF can be both significant and durable for selected patients with PLD. Although such an operative approach is associated with considerable perioperative risk, resolution of symptoms with improved quality of life and survival is noteworthy. These findings extend earlier clinical outcomes after PHCF by quantitating volume reduction and confirming that postoperative cyst progression is not inexorable.8,9 Reduction of operative risk and subsequent therapy to delay cyst progression in patients who demonstrate substantial hepatic enlargement after PHCF are issues for future investigation.
Polycystic liver disease is the most common extrarenal manifestation of ADPKD, but can also occur as a distinct genetic entity without renal cysts (autosomal dominant polycystic liver disease). Polycystic liver disease is more prevalent and more severe in women. Although often asymptomatic, some patients suffer considerably from symptoms that affect the quality of life. Occasionally, symptoms in some patients are caused by a small number of large dominant cysts that can be treated by percutaneous drainage and sclerosis or by laparoscopic fenestration (these patients are not included in this report). The size, extent, and distribution of cysts in most patients preclude minimally invasive intervention. Combined PHCF has been used in such patients with massive PLD. Liver transplantation might be considered in patients who are not candidates for PHCF. We have previously described anatomic selection criteria for these major operative approaches.8 Recent clinical trials have shown that somatostatin analogues, octreotide and lanreotide, can produce a small but significant reduction in hepatic volume from decreased cyst size. Benefit is mostly seen during the first year and in women with severe PLD who are younger than age 48 years of age, because this subset of patients experiences the fastest rates of increase in LV.10 Currently, somatostatin analogues are not approved for long-term use to slow PLD progression.
Previously, we and others have reported short-term and long-term outcomes for PHCF in symptomatic patients with symptomatic hepatomegaly from PLD.7-9 Partial hepatectomy and cyst fenestration can be performed with acceptable morbidity and mortality, prompt and durable relief of symptoms, and maintenance of liver function. This study is the largest series reporting PHCF in PLD in a single tertiary referral center. Patients with massive PLD had hepatic volumes exceeding 6 times the mean volumes of noncystic, noncirrhotic livers.11,12 Most patients in this series were Caucasian women with ADPKD. The overall favorable results from PHCF in our experience might be due, in part, to the sex (91% female) and age (48 years, female) of our patients. Recent studies have shown a substantial reduction in the rate of growth or even a reduction in LV in female patients after menopause.13,14 Therefore, young premenopausal women and men with severe PLD might have a higher risk for sustained and faster enlargement of the liver after PHCF. Perioperative complications associated with PHCF were common but manageable in most cases. Postoperative death, including hepatic failure-related, was infrequent, given the extent of PHCF. The major factor contributing to mortality or early liver transplantation was hemorrhage near the hepatic veins and compromise of hepatic venous outflow in controlling hemorrhage. We believe that detailed mapping of the hepatic veins by imaging has decreased this issue with experience. Although the operative risk associated with PHCF is greater than similar resections in patients without PLD historically, perioperative risk is similar or less than that after liver transplantation.
There is a general reluctance to advise PHCF due to the complexity of and lack of expertise with PHCF and inconsistent selection criteria. Additionally, the widespread assumptions that cyst proliferation and enlargement with or without hepatic failure will progress inexorably after PHCF, that PHCF significantly increases perioperative risk of liver transplantation, and that late hepatic failure is frequent, have prompted the recommendation of preemptive liver transplantation regardless of type of PLD for massive symptomatic hepatomegaly.15 Our data conversely show that cyst progression with recurrent hepatomegaly or hepatic failure is not the predominant end point and supports the recommendation for careful selection of patients for PHCF before consideration of hepatic transplantation. Patients with relative cyst preservation of at least one hepatic sector with widely patent hepatic and portal veins to that sector with type C PLD are potential candidates for PHCF. In contrast, patients with PLD who have obstruction or compromise of all hepatic veins from diffuse PLD without parenchymal sparing are classified as type D and are only candidates for liver transplantation. Although PHCF is clearly associated with extensive perihepatic adhesions, which are technically challenging at reoperation and increase the risk of subsequent liver transplantation, preemptive liver transplantation for type C PLD cannot be justified because our data show that PHCF is durable and not associated with hepatic failure in most patients.
Although hepatic transplantation optimally normalizes hepatic volume and completely precludes cyst progression in patients with PLD, organ allocation remains problematic because hepatic function is almost always preserved. Consequently, the waiting time for hepatic transplantation for PLD is prolonged in the United States and has increased in the Model for End-Stage Liver Disease (MELD) era from 272 to 337 days, with more than half of the patients transplanted based on MELD exceptions.16 Patients with severe PLD (Mayo type C, not candidates for alternative therapy, or type D) and concurrent renal failure on dialysis can benefit from MELD exception points and are expected to receive a combined liver/kidney transplantation within 1 year.17 Therefore, dialysis status is another factor to consider when recommending PHCF vs combined liver/kidney transplantation. Interestingly, based on United Network for Organ Sharing data, patient and allograft 5-year survival rates in the MELD era (2002 to 2009) were 80% and 75%, respectively, although greater for some single institutions.15 In contrast, patient and allograft 5-year survival rates were only 40% in the pre-MELD era (1990 to 2001). In the European experience, the 5-year survival rate for patients who underwent liver transplantation for PLD was in the range of 80% to 92%.15,18,19 Our patient survival rates are equivalent to transplantation, with 5- and 15-year patient survival rates of 93.3% and 77.7%, respectively.
The major weakness of our study is the incomplete imaging data in all patients. This deficiency is due to both the referral nature of our tertiary practice and the absence of planned follow-up imaging program. Follow-up imaging was generally left to the discretion of referring physicians or local providers based on subsequent symptoms or clinical concerns and not a prescribed schedule. Acquisition of imaging was requested for all patients. Some imaging was missing due to institutional policies before electronic records that discarded imaging after 10 years. Another weakness of our study was that interval intervention of PLD after PHCF could have affected subsequent hepatic volume measurements. In fact, few patients had such intervention. No patient underwent repeat hepatic resection, and intervention was limited to aspiration/sclerosis of a dominant cyst or percutaneous drainage of an infected cyst. Finally, somatostatin analogues were used postoperatively in very few patients as part of clinical trials for severe PLD and not as compassionate use for symptomatic relief. Given that these patients’ imaging was obtained before initiation of somatostatin analogues, this therapy would not affect the LVs in this study.
Selection of the patients who can benefit from this surgery is crucial. We provide evidence of the extent and degree of PLD in our selected patients (Appendix 1; available online) by showing the actual hepatic images before and after surgery. Our selection criteria evolved throughout our experience. Currently, at least one hepatic sector spared of cysts with patent hepatic and portal venous systems to that sector is an absolute criterion. Hepatic function must also be preserved. Evidence of portal hypertension, such as splenomegaly, varices, or thrombocytopenia, is a contraindication for PHCF. Intrahepatic venous collaterals in the spared sector are indicative of hepatic venous outflow obstruction and are a relative contraindication to PHCF. Previously, we have anatomically classified patients with PLD into 4 types based on extent and distribution of cystic disease and noncompromised patency of the major hepatic veins (Supplementary Figure 2; available online).9 Type C PLD is the primary indication for PHCF. Type D PLD is the primary indication for hepatic transplantation. Demographic characteristics are not factors for selection of patients for PHCF. Impairment of clinical performance status, to the degree that normal activity is precluded and lifestyle is continually altered (ie, resignation from employment, limiting fatigue), remains a key selection factor. We have not found that PHCF has been precluded by cyst progression of the spared parenchyma during the interval between initial evaluation and development of subsequent impairment of performance status when PHCF is clinically indicated. Finally, renal failure can influence operative treatment. Most of our patients had some degree of renal insufficiency. However, in general, the recommendation for PHCF is independent of renal failure. Combined renal and hepatic transplantation in patients with renal failure requiring dialysis is favored primarily in patients with borderline type C or type D PLD. Based on our data, the frequency of late hepatic failure does not support broadening the indication for hepatic transplantation in the setting of renal failure.
CONCLUSIONS
Sustained long-term reductions in LV after PHCF can be achieved in selected patients with severe, highly symptomatic PLD. In our experience, liver-related death and subsequent liver transplantation are infrequent after PHCF.
Supplementary Material
Acknowledgments
Support: This study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Mayo Translational PKD Center (DK090728).
Abbreviations and Acronyms
- ADPKD
autosomal dominant polycystic kidney disease
- IQR
interquartile range
- LV
liver volume
- LV1
preoperative liver volume
- LV2
postoperative liver volume
- LV3
liver volume at follow-up
- MELD
Model for End-Stage Liver Disease
- PHCF
partial hepatectomy and cyst fenestration
- PLD
polycystic liver disease
Footnotes
Presented at the Western Surgical Association 123rd Scientific Session, Napa Valley, CA, November 2015.
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Chebib, Kamath, Torres, Nagorney
Acquisition of data: Chebib, Harmon, Irazabal Mira, Jung, Edwards, Hogan, Kamath, Torres, Nagorney
Analysis and interpretation of data: Chebib, Harmon, Irazabal Mira, Jung, Edwards, Hogan, Kamath, Torres, Nagorney
Drafting of manuscript: Chebib, Harmon, Irazabal Mira, Jung, Edwards, Hogan, Kamath, Torres, Nagorney
Critical revision: Chebib, Harmon, Irazabal Mira, Jung, Edwards, Hogan, Kamath, Torres, Nagorney
REFERENCES
- 1.Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 2.Van Keimpema L, De Koning DB, Van Hoek B, et al. Patients with isolated polycystic liver disease referred to liver centers: clinical characterization of 137 cases. Liver Int. 2011;31:92–98. doi: 10.1111/j.1478-3231.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 3.Everson GT. Hepatic cysts in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65:1020–1025. doi: 10.1016/s0025-6196(12)65165-9. [DOI] [PubMed] [Google Scholar]
- 4.Everson GT, Taylor MR, Doctor RB. Polycystic disease of the liver. Hepatology. 2004;40:774–782. doi: 10.1002/hep.20431. [DOI] [PubMed] [Google Scholar]
- 5.Hogan MC, Abebe K, Torres VE, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13:155–164. e6. doi: 10.1016/j.cgh.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres VE, Rastogi S, King BF, et al. Hepatic venous outflow obstruction in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1186–1192. doi: 10.1681/ASN.V551186. [DOI] [PubMed] [Google Scholar]
- 7.Que F, Nagorney OM, Gross JB, Jr, et al. Liver resection and cyst fenestration in the treatment of severe polycystic liver disease. Gastroenterology. 1995;108:487–494. doi: 10.1016/0016-5085(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 8.Schnelldorfer T, Torres VE, Zakaria S, et al. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250:112–118. doi: 10.1097/SLA.0b013e3181ad83dc. [DOI] [PubMed] [Google Scholar]
- 9.Aussilhou B, Doufle G, Hubert C, et al. Extended liver resection for polycystic liver disease can challenge liver transplantation. Ann Surg. 2010;252:735–743. doi: 10.1097/SLA.0b013e3181fb8dc4. [DOI] [PubMed] [Google Scholar]
- 10.Neijenhuis MK, Gevers TJ, Nevens F, et al. Somatostatin analogues improve health-related quality of life in polycystic liver disease: a pooled analysis of two randomized, placebo-controlled trials. Aliment Pharmacol Ther. 2015;42:591–598. doi: 10.1111/apt.13301. [DOI] [PubMed] [Google Scholar]
- 11.Chan SC, Liu CL, Lo CM, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217–2222. doi: 10.3748/wjg.v12.i4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urata K, Hashikura Y, lkegami T, et al. Standard liver volume in adults. Transplant Proc. 2000;32:2093–2094. doi: 10.1016/s0041-1345(00)01583-9. [DOI] [PubMed] [Google Scholar]
- 13.Gevers TJ, lnthout J, Caroli A, et al. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology. 2013;145:357–365. e1, e2. doi: 10.1053/j.gastro.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 14.Chebib F, Jung Y, Heyer C, et al. Effect of genotype on the severity and volume progression of polycystic liver disease in ADPKD. Nephrol Dial Transplant. doi: 10.1093/ndt/gfw008. published online ahead of print February 29, 2016 doi: 10.1093/ndt/gfw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baber JT, Hiatt JR, Busuttil RW, et al. A 20-year experience with liver transplantation for polycystic liver disease: does previous palliative surgical intervention affect outcomes? J Am Coll Surg. 2014;219:695–703. doi: 10.1016/j.jamcollsurg.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 16.Saidi RF, Jabbour N, Shah SA, et al. Improving outcomes of liver transplantation for polycystic disease in MELD era. Int J Organ Transplant Med. 2013;4:27–29. [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services [October 22, 2015];Organ Procurement and Transplantation Network. Available at: http://optn.transplant.hrsa.gov/resources/by-organ/liver-intestine/guidance-on-meld-peld-exception-review.
- 18.van Keimpema L, Nevens F, Adam R, et al. Excellent survival after liver transplantation for isolated polycystic liver disease: an European Liver Transplant Registry study. Transpl Int. 2011;24:1239–1245. doi: 10.1111/j.1432-2277.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 19.Adam R, McMaster P, O'Grady JG, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transplant. 2003;9:1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.