Abstract
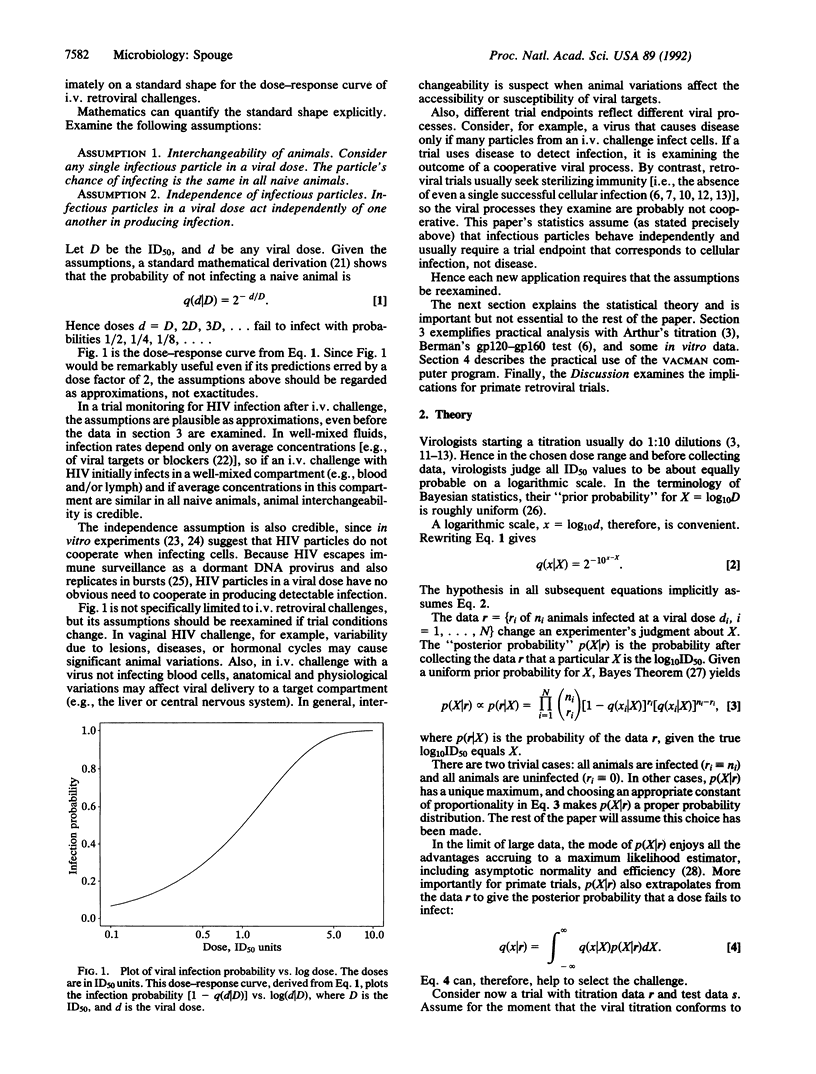
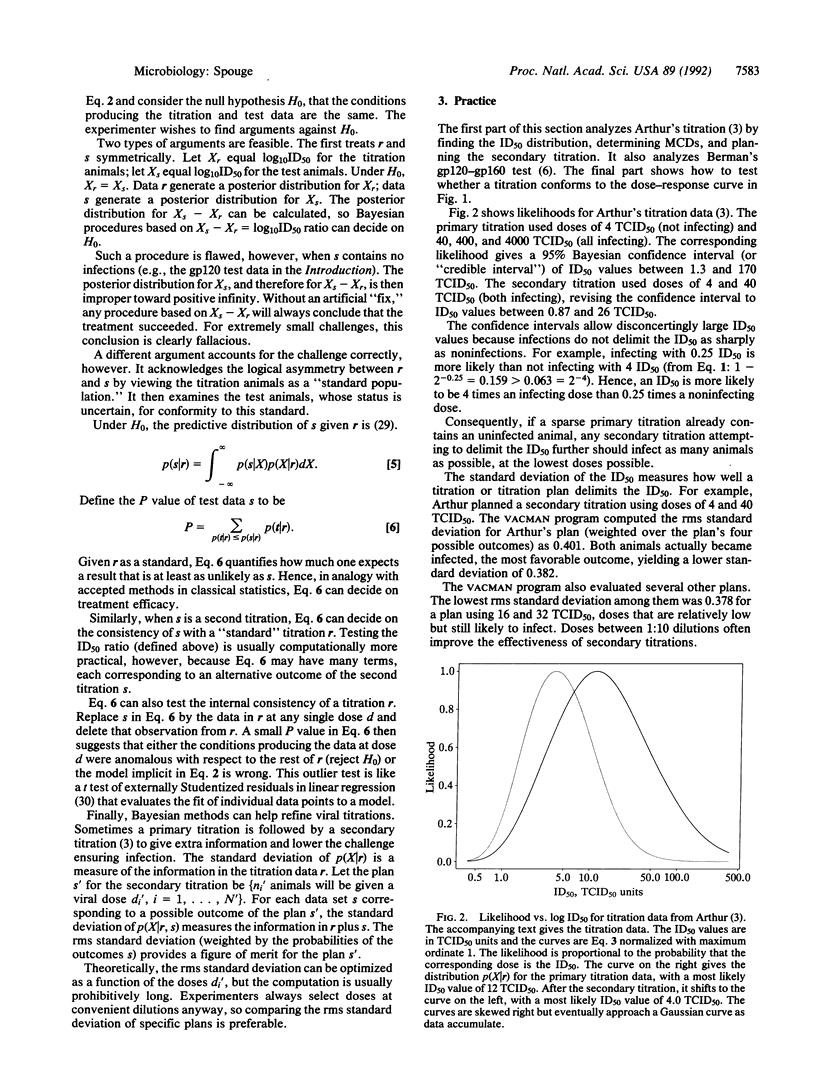
Reports on retroviral primate trials rarely publish any statistical analysis. Present statistical methodology lacks appropriate tests for these trials and effectively discourages quantitative assessment. This paper describes the theory behind VACMAN, a user-friendly computer program that calculates statistics for in vitro and in vivo infectivity data. VACMAN's analysis applies to many retroviral trials using i.v. challenges and is valid whenever the viral dose-response curve has a particular shape. Statistics from actual i.v. retroviral trials illustrate some unappreciated principles of effective animal use: dilutions other than 1:10 can improve titration accuracy; infecting titration animals at the lowest doses possible can lower challenge doses; and finally, challenging test animals in small trials with more virus than controls safeguards against false successes, "reuses" animals, and strengthens experimental conclusions. The theory presented also explains the important concept of viral saturation, a phenomenon that may cause in vitro and in vivo titrations to agree for some retroviral strains and disagree for others.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bess J. W., Jr, Waters D. J., Pyle S. W., Kelliher J. C., Nara P. L., Krohn K., Robey W. G., Langlois A. J., Gallo R. C. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989 Dec;63(12):5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur L. O., Pyle S. W., Nara P. L., Bess J. W., Jr, Gonda M. A., Kelliher J. C., Gilden R. V., Robey W. G., Bolognesi D. P., Gallo R. C. Serological responses in chimpanzees inoculated with human immunodeficiency virus glycoprotein (gp120) subunit vaccine. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8583–8587. doi: 10.1073/pnas.84.23.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. M. Obstacles to an AIDS vaccine. Science. 1988 May 6;240(4853):719–721. doi: 10.1126/science.2834823. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Wyand M. S., Kodama T., Ringler D. J., Arthur L. O., Sehgal P. K., Letvin N. L., King N. W., Daniel M. D. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Fultz P. N., McClure H. M., Eichberg J. W., Thomas E. K., Zarling J., Singhal M. C., Kosowski S. G., Swenson R. B., Anderson D. C. Effect of immunization with a vaccinia-HIV env recombinant on HIV infection of chimpanzees. Nature. 1987 Aug 20;328(6132):721–723. doi: 10.1038/328721a0. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Dembo M., Spouge J. L., Nara P. L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990 Jul 19;346(6281):277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Spouge J. L., Dembo M., Nara P. L. Blocking of human immunodeficiency virus infection depends on cell density and viral stock age. J Virol. 1991 Jun;65(6):3293–3300. doi: 10.1128/jvi.65.6.3293-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne S. P., Spouge J. L., Dembo M. Quantifying the infectivity of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4644–4648. doi: 10.1073/pnas.86.12.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A. M., Howanitz J., van Gemert M., Miller M. Clofibrate-induced antidiuresis. J Clin Invest. 1973 Mar;52(3):535–542. doi: 10.1172/JCI107213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Davison-Fairburn B., Montelaro R. C., Miller M., West M., Ohkawa S., Baskin G. B., Zhang J. Y., Putney S. D. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989 Dec 8;246(4935):1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Horowitz B., Baker L., Shulman R. W., Ralph H., Valinsky J., Cundell A., Brotman B., Boehle W., Rey F. Failure of a human immunodeficiency virus (HIV) immune globulin to protect chimpanzees against experimental challenge with HIV. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6944–6948. doi: 10.1073/pnas.85.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkonen P., Thorstensson R., Ghavamzadeh L., Albert J., Hild K., Biberfeld G., Norrby E. Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature. 1991 Aug 1;352(6334):436–438. doi: 10.1038/352436a0. [DOI] [PubMed] [Google Scholar]
- Ramsey F. L. A Bayesian approach to bioassay. Biometrics. 1972 Sep;28(3):841–858. [PubMed] [Google Scholar]
- Ward R. H., Capon D. J., Jett C. M., Murthy K. K., Mordenti J., Lucas C., Frie S. W., Prince A. M., Green J. D., Eichberg J. W. Prevention of HIV-1 IIIB infection in chimpanzees by CD4 immunoadhesin. Nature. 1991 Aug 1;352(6334):434–436. doi: 10.1038/352434a0. [DOI] [PubMed] [Google Scholar]


