Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) has an etiologic role in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. These diseases are most common in immunocompromised individuals, especially those with AIDS. Similar to all herpesviruses, KSHV infection is lifelong. KSHV infection in tumor cells is primarily latent, with only a small subset of cells undergoing lytic infection. During latency, the KSHV genome persists as a multiple copy, extrachromosomal episome in the nucleus. In order to persist in proliferating tumor cells, the viral genome replicates once per cell cycle and then segregates to daughter cell nuclei. KSHV only expresses several genes during latent infection. Prominent among these genes, is the latency-associated nuclear antigen (LANA). LANA is responsible for KSHV genome persistence and also exerts transcriptional regulatory effects. LANA mediates KSHV DNA replication and in addition, is responsible for segregation of replicated genomes to daughter nuclei. LANA serves as a molecular tether, bridging the viral genome to mitotic chromosomes to ensure that KSHV DNA reaches progeny nuclei. N-terminal LANA attaches to mitotic chromosomes by binding histones H2A/H2B at the surface of the nucleosome. C-terminal LANA binds specific KSHV DNA sequence and also has a role in chromosome attachment. In addition to the essential roles of N- and C-terminal LANA in genome persistence, internal LANA sequence is also critical for efficient episome maintenance. LANA’s role as an essential mediator of virus persistence makes it an attractive target for inhibition in order to prevent or treat KSHV infection and disease.
Keywords: KSHV, latency-associated nuclear antigen, chromosome, DNA binding, viral persistence
Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV) (or human herpesvirus 8) is a large, enveloped, double-stranded DNA virus. KSHV is the only human gamma-2 herpesvirus, belonging to the Rhadinovirus genus. The KSHV genome is ~165 kb, and encodes nearly 100 genes. Similar to all herpesviruses, after infection, KSHV persists for the lifetime of its host. KSHV persists by latently infecting cells. During latent infection, the viral genome exists as a multi copy, extrachromosomal, circular episome (plasmid). During latent infection, only a small subset of viral genes is expressed. These genes include the latency-associated nuclear antigen (LANA), v-FLIP, and v-Cyclin (Chang et al., 1996; Kedes et al., 1997; Kellam et al., 1997; Rainbow et al., 1997; Dittmer et al., 1998). Low levels of K1 transcript are also detected in latent infection, and v-IL6 (K2) is expressed at low levels during latency in certain cell types (Chandriani and Ganem, 2010). vIRF3 (LANA2) is expressed during latent infection, but only in B cells (Rivas et al. 2001). The KSHV K12 locus encodes viral miRNAs which are expressed in latency (Samols et al., 2005), and this locus also encodes kaposins A, B, and C, of which the kaposin B protein is the predominant product and most easily detected during lytic infection (Sadler et al., 1999).
LANA Mediates KSHV Genome Persistence
Latency-associated nuclear antigen is an 1162 amino acid protein encoded by KSHV open reading frame (ORF) 73 (Figure 1) and is the viral protein responsible for virus persistence. LANA acts on KSHV terminal repeat (TR) sequence to mediate episome persistence. The viral TRs are 801 bp repeated sequences that are highly GC-rich (~80%) and comprise ~ 20% of the genome (Figure 2). Upon initial infection, the linear KSHV genome circularizes by fusing at the TR elements, located at each end of the genome. LANA is necessary and sufficient to mediate episome persistence of TR associated DNA in the absence of other virus genes (Ballestas et al., 1999; Ballestas and Kaye, 2001; Garber et al., 2001; Fejer et al., 2003; Grundhoff and Ganem, 2003, 2004; Skalsky et al., 2007). Knockdown of LANA using siRNA leads to loss of episomes, while deletion of LANA from a bacterial artificial chromosome (BAC) containing the viral genome results in loss of the ability of virus to persist in an episomal state (Zhang et al., 2007; Li et al., 2008). Similarly, knockout or mutating MHV-68 ORF73, the murine LANA homolog, from murine γ-herpesvirus 68 abolishes the ability of virus to efficiently establish latent infection (Fowler et al., 2003; Moorman et al., 2003; Forrest et al., 2007; Paden et al., 2010; Habison et al., 2012; Correia et al., 2013).
FIGURE 1.
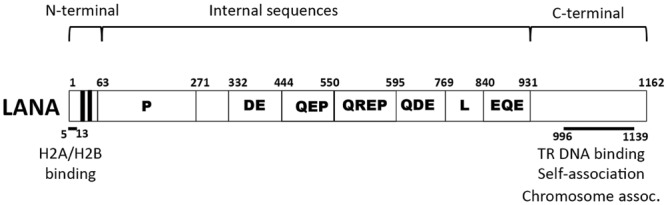
Schematic diagram of Kaposi’s sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen (LANA). Indicated are the proline-rich region (P), and the repetitive regions (DE, QEP, QREP, QDE, L, EQE). The black areas indicate the bipartite N-terminal nuclear localization signal (NLS) within amino acids 24 to 30 and 41 to 47. Amino acids 5 to 13 mediate chromosome association through interaction with histones H2A/H2B. Amino acids 996 to 1139 contain TR DNA binding, self-association, and chromosome association functions.
FIGURE 2.
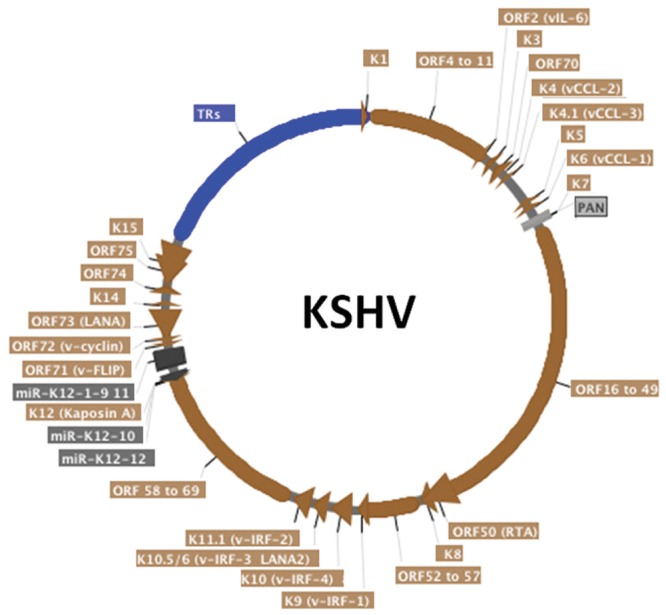
Schematic representation of the circularized KSHV genome. The terminal repeats (TRs), in blue, comprise around 20% of the genome. KSHV open reading frames (ORF) are in brown, miRNAs are in dark gray and non-coding long RNA PAN is in light gray. Alternative protein names are written in parenthesis. Arrows indicate transcription direction. Although KSHV persistence is the focus of this article, most KSHV genes are shown. This map was generated from the HHV-8 complete genome sequence (Genbank accession AF148805) strain GK18 using SerialCloner software.
There are two key components to episome persistence. First, episomal DNA must replicate with each cell division. Second, episomes must segregate to progeny nuclei following mitosis to avoid destruction in the cytoplasm. LANA is responsible for both of these functions. LANA mediates episomal DNA replication. LANA also serves to segregate KSHV episomes to daughter nuclei. LANA accomplishes this segregation by acting as a molecular tether for viral episomes, bridging virus DNA to host cell mitotic chromosomes.
This review focuses on current understanding of LANA’s mediation of genome persistence through tethering KSHV DNA to mitotic chromosomes to effect distribution of episomes to daughter nuclei. LANA’s role in KSHV DNA replication is addressed separately in another chapter.
LANA Binds Chromosomes to Mediate Viral Genome Persistence
LANA Tethers the KSHV Genome to Host Cell Chromosomes
Using simultaneous immune fluorescence to detect LANA and fluorescent in situ hybridization to detect KSHV DNA, LANA was shown to colocalize with KSHV episomes along metaphase chromosomes in KSHV latently infected cells (Ballestas et al., 1999; Cotter and Robertson, 1999). This finding suggested LANA had a role in KSHV episome persistence analogous to EBNA1 of Epstein-Barr virus (EBV) (Reedman and Klein, 1973; Grogan et al., 1983; Yates et al., 1984; Harris et al., 1985), and in fact, LANA expressing cells were shown to allow persistence of plasmids containing KSHV TR DNA (Ballestas et al., 1999; Ballestas and Kaye, 2001). This work led to a model in which LANA bridges KSHV DNA to chromosomes during mitosis through concomitantly binding to the cis-acting TRs sequence in the KSHV genome and to mitotic chromosomes. Therefore, LANA tethers KSHV episomes to mitotic chromosomes during cell division to ensure segregation of the viral genome to the nuclei of daughter cells.
N- and C-terminal LANA Independently Bind Mitotic Chromosomes
Both N- and C-terminal regions of LANA are involved in mitotic chromosome binding. N-terminal LANA residues 5–22 were originally reported by Piolot et al. (2001) to target chromatin during interphase and mitosis. N-terminal LANA also encodes a nuclear localization signal, which is bipartite, located within amino acids 24 to 30 and 41 to 47. Cherezova et al. (2011) consistent with its role in tethering KSHV DNA to mitotic chromosomes, the LANA N-terminal chromosome association region was later shown to be essential for LANA mediated episome persistence. Of interest, the N-terminal region was also found to be critical for efficient LANA mediated DNA replication, despite having no role in binding KSHV DNA (Barbera et al., 2004; Lim et al., 2004). In addition, the N-terminal LANA chromosome binding region also has effects on LANA transcriptional regulation (Wong et al., 2004). Other than microscopy, biochemical evidence also supports N- and C-LANA interactions with chromatin (Viejo-Borbolla et al., 2003; Wong et al., 2004).
N-terminal LANA binds directly to the surface of the nucleosome to attach to chromosomes. X-ray crystal structure of N-terminal LANA complexed with a nucleosome core particle showed that the first 23 amino acids of LANA forms a hairpin that binds exclusively to the acidic patch at the interface of histones H2A and H2B (Barbera et al., 2006a) (Figure 3A). The nucleosome binding property of N-terminal LANA determines its broad distribution across mitotic chromosomes in the absence of episomal DNA (Piolot et al., 2001; Barbera et al., 2004, 2006a; Wong et al., 2004; Chodaparambil et al., 2007). Whether or not histone epigenetic modifications exert a modulatory role on N-terminal LANA binding to the nucleosome surface is not currently known.
FIGURE 3.
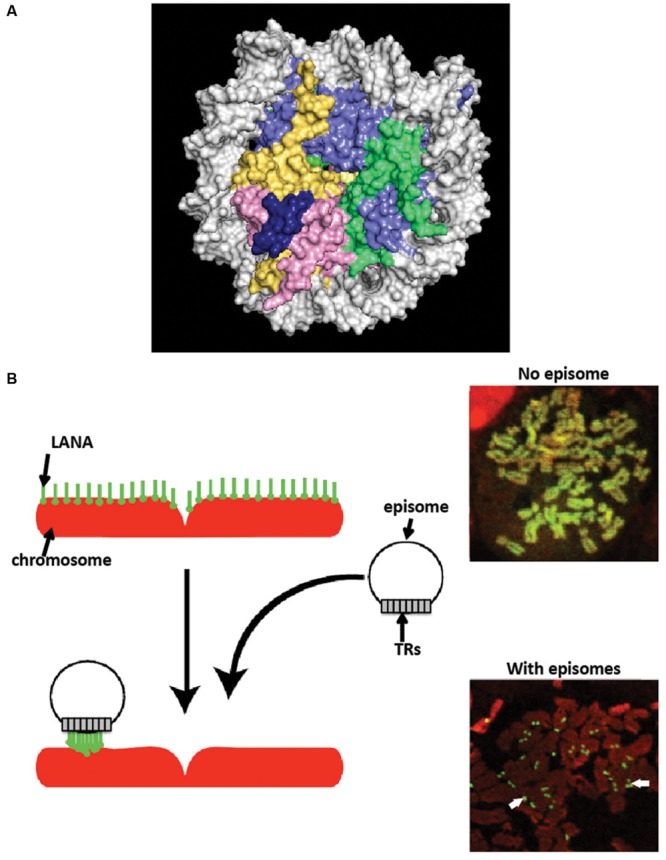
Latency-associated nuclear antigen associates with chromosomes. (A) X-ray crystal structure of N-terminal LANA complexed with the nucleosome. A space-filling representation is shown. (histone H2A, yellow; H2B, red; H3 light blue; H4, green; LANA, dark blue; DNA, silver). From PDB 1zla (Barbera et al., 2006a). (B) LANA associates with cellular chromosomes in a broad distribution in the absence of episomes, and concentrates to dots on chromosomes at sites of episomes. Cells expressing LANA were arrested in metaphase with colcemid. LANA (green) was detected with antibody directed against LANA. DNA was counterstained with propidium iodide (red) (overlay of red and green generates yellow.) White arrows indicate LANA dots at sites of KSHV episomes.
C-terminal LANA contains an independent chromosome binding domain, which consists of amino acids 996–1139 (Krithivas et al., 2002; Kelley-Clarke et al., 2007a, 2009). The LANA C-terminal domain is conserved among the LANA orthologs from different gamma-2 herpesviruses, including ORF73s from Retroperitoneal fibromatosis herpesvirus (RFHV) (Burnside et al., 2006), Rhesus rhadinovirus (RRV), Herpesvirus saimiri (HVS) (Calderwood et al., 2004), Herpesvirus ateles (HVA), and murine gammaherpesivrus 68 (MHV68) (Grundhoff and Ganem, 2003; Paden et al., 2012).
C-terminal LANA binds chromatin in a unique pattern in the absence of episomal DNA, concentrating to punctate foci at pericentromeric and peri-telomeric regions of a subset of mitotic chromosomes (Krithivas et al., 2002; Kelley-Clarke et al., 2007a). This pattern contrasts sharply with the diffuse chromosome distribution of N-terminal LANA. Self-association of LANA C-terminal region is required for LANA C-terminal chromosome binding (Komatsu et al., 2004; Kelley-Clarke et al., 2009). Of other episome maintenance proteins, EBV EBNA1 does not localize to pericentromeric regions of sister chromatids during mitosis (Szekely et al., 1999; Kanda et al., 2007; Nanbo et al., 2007). The binding pattern of human papillomavirus (HPV) E2 episome maintenance protein to chromosomes can be pericentromeric or not, depending on the virus type (Oliveira et al., 2006).
LANA Broadly Associates with Chromatin in the Absence of Episomes, but Concentrates to Dots on Chromosomes at Sites of Episomal DNA
In the absence of KSHV episomes, full length LANA broadly distributes on mitotic chromosomes (Ballestas et al., 1999; Piolot et al., 2001). However, LANA’s distribution is uneven, and not diffuse, when expressed at physiological levels (Kelley-Clarke et al., 2009). Since N-terminal LANA diffusely paints mitotic chromosomes through binding to histones H2A/H2B, and since C-terminal LANA concentrates to pericentromeric and peri-telomeric regions of mitotic chromosomes (as described above), the result of combining these two localization targeting mechanisms in full length LANA effects broad distribution over mitotic chromosomes, but with more intense staining at peri-telomeric and pericentromeric regions.
In stark contrast to its broad chromosomal distribution in the absence of episomes, LANA concentrates to dots in the presence of KSHV episomal DNA (Figure 3B), both in interphase and along mitotic chromosomes (Ballestas et al., 1999; Cotter and Robertson, 1999; Sakakibara et al., 2004). The concentration to dots occurs at the sites of KSHV episomal genomes, as shown by simultaneous immune fluorescent detection of LANA and fluorescent in situ hybridization with KSHV DNA (Ballestas et al., 1999; Cotter and Robertson, 1999). The strong concentration of LANA to dots at sites of episomal DNA is likely a result of the higher affinity that C-terminal LANA has for its DNA binding site in TR DNA [Kd of binding to LANA adjacent binding sites 1 and 2 ~13.7 nM (Garber et al., 2002; Ponnusamy et al., 2015)] compared to a lower affinity for N-terminal LANA binding to the nucleosome [Kd ~184 nM (Beauchemin et al., 2014)]. Further, each KSHV genome contains ~40 TR copies, and each TR contains three adjacent LANA binding sites (Garber et al., 2002; Hellert et al., 2015). Therefore, each KSHV genome contains ~120 LANA binding sites within its TR elements, to which a LANA dimer binds at each site, resulting in ~240 LANA molecules binding to TR DNA per KSHV genome. LANA bound at TR DNA and simultaneously binding to nucleosomes within mitotic chromosomes results in tethering of the viral genome to mitotic chromosomes. What is less clear, however, is whether or not a subset of LANA molecules bound at TR DNA bind nucleosomes within the KSHV episome; such binding would not result in tethering to mitotic chromosomes but instead would result in LANA doubly bound to the episome: through direct TR DNA binding and also through nucleosomal attachment. If such binding occurs, it would potentially compete with binding to chromosomes, and perhaps could serve as a regulatory mechanism.
N-terminal LANA Is the Dominant Chromosome Attachment Region
Although both N- and C-terminal LANA contain independent chromosome binding regions, N-terminal LANA appears to be the primary effector. Alanine substitution of key chromosome attachment residues in N-terminal LANA abolished LANA’s chromosome association and its ability to mediate episome persistence (Barbera et al., 2004). In contrast, alanine substitutions that dramatically impair C-terminal LANA’s ability to bind mitotic chromosomes did not reduce full length LANA’s association with chromosomes or its ability to mediate episome persistence (Kelley-Clarke et al., 2009). It is important to note, however, that these experiments could not use LANA that was completely abolished for C-terminal chromosome binding. Such null chromosome binding mutations also impaired other critical C-terminal LANA functions, such as DNA binding. Therefore, it remains possible that N-terminal LANA may have “rescued” the impaired (but not abolished) C-terminal LANA chromosome binding, possibly through a cooperative effect. In fact, when N-terminal LANA was mutated so as to reduce (but not abolish) N-terminal LANA chromosome association, the C-terminal chromosome binding mutations resulted both in a reduction of full length LANA binding to mitotic chromosomes and also in a reduction of LANA’s ability to mediate episome persistence (Kelley-Clarke et al., 2009).
Altogether, these data suggest that N-terminal LANA is the dominant effector for chromosome binding, and that C-terminal LANA exerts an auxiliary role. It is tempting to consider that C-terminal LANA was the original chromosome tether, and that N-terminal LANA evolved subsequently as a more efficient alternative. Consistent with this possibility, C-terminal LANA is the most highly conserved region among LANA orthologs.
LANA, Including N-terminal LANA, Undergoes Post Translational Modifications
Latency-associated nuclear antigen has been described to undergo several types of post translational modifications. LANA is a phosphoprotein and is the target of several host cell kinases. Pim-1 and Pim-3 phosphorylate LANA serine residues 205 and 206 to allow lytic reactivation (Bajaj et al., 2006; Cheng et al., 2009). RSK1 and ERK1/2 interact with LANA along with GSK-3 and phosphorylate LANA residues located within 246–258 (Fujimuro et al., 2005; Liu et al., 2007). Notably, RSK3 phosphorylates LANA serine 13 and threonine 14, within the N-terminal chromosome binding region. RSK small molecule inhibition decreased LANA binding to histone H2B as well as LANA protein levels. Treatment of KSHV infected PEL cells with the RSK inhibitor resulted in growth cessation and a decrease in viable cell numbers. In contrast, treatment of uninfected BJAB B cell lymphoma cells resulted in growth cessation, but there was not a decrease in the number of viable cells. These findings show that inhibition of RSKs can impact KSHV persistence (Woodard et al., 2012). DNA-PK was also shown to phosphorylate LANA in at least two sites, one located between amino acids 31–52, and a second between amino acids 91–340, although the specific residues were not identified. The phosphorylation between residues 31–52 may have reduced LANA mediated DNA replication (Cha et al., 2010). C-terminal LANA is also phosphorylated at serine and threonine residues between amino acids 951–1107, and this is mediated by a kinase recruited by RING3 (Brd2) (Platt et al., 1999).
In addition to phosphorylation, N-terminal LANA arginine residue 20, located near the chromosome attachment region, was shown to be methylated by protein arginine methyltransferase 1 and to have a role in antagonizing viral lytic reactivation (Campbell et al., 2012). LANA is also acetylated, and sodium butyrate (a histone deacetylase inhibitor) treatment, reduced LANA’s ability to co immunoprecipitate with histones H2A/H2B (Lu et al., 2006). LANA is also a target of poly (ADP-ribose) polymerase 1 (PARP-1) and is poly (ADP-ribosyl) ated (Ohsaki et al., 2004). The sites of acetylation or poly (ADP-ribosyl) ation within LANA are not currently known. In addition, C-terminal LANA is SUMOylated at lysine 1140 (Cai et al., 2013).
Cellular Proteins Associated with LANA at the Chromosome
In addition to histones, LANA interacts with other chromosome associated proteins. Although N-terminal LANA binding to histones H2A/H2B is likely the primary chromosome attachment mechanism, other cell proteins may modulate this interaction. LANA N- and C-terminal regions both interact with the methyl CpG binding protein 2 (MeCP2), which binds methylated CpG dinucleotides (Krithivas et al., 2002; Matsumura et al., 2010). MeCP2 was initially proposed to mediate N-terminal LANA chromosome association based on the inability of LANA to associate with murine chromosomes in the absence of human MeCP2, although other work demonstrated LANA associates with native murine chromosomes (Krithivas et al., 2002; Barbera et al., 2006b; Kelley-Clarke et al., 2007a). The interaction between LANA and MeCP2 may confer LANA with the potential to regulate cellular gene expression in latently infected cells (Matsumura et al., 2010). Of note, MeCP2 was shown to be important for herpesvirus saimiri LANA episome persistence (Griffiths and Whitehouse, 2007). LANA also interacts with the DEK protein, which associates with chromatin. The DEK-interacting region was mapped to LANA amino acids 986 to 1043 and DEK was proposed to mediate C-terminal LANA attachment to chromosomes (Krithivas et al., 2002), although unlike C-terminal LANA’s concentration to pericentromeres and peri-telomeres, DEK diffusely paints mitotic chromosomes (Krithivas et al., 2002; Kelley-Clarke et al., 2007a). Full length LANA was shown to interact with histone H1 (Cotter and Robertson, 1999), and a chimeric LANA with the N-terminal chromosome association region replaced by histone H1 protein was functional for episomal maintenance (Shinohara et al., 2002). Results differ as to whether histone H1 can interact with N-terminal LANA (Barbera et al., 2006a; Verma et al., 2013).
KSHV LANA also interacts with BET (Bromodomain and Extra Terminal domain) proteins (Platt et al., 1999; Ottinger et al., 2006). The BET protein BRD4 binding sites on LANA have been mapped to LANA amino acids 475 to 777 and C-terminal amino acids 982 to 1162 (Viejo-Borbolla et al., 2005; You et al., 2006). BET proteins such as BRD2 and BRD4 have two bromodomains and an extraterminal (ET) domain. BET proteins interact with C-terminal LANA through the BET protein ET domain and also through a conserved serine rich region downstream of the ET domain (Hellert et al., 2013). BET protein bromodomains bind acetylated tails of histones H3 and H4 and may have a role in LANA’s transcriptional properties (Viejo-Borbolla et al., 2005; Ottinger et al., 2006; You et al., 2006). Notably, bovine papillomavirus E2, which is an episomal maintenance protein, attaches to chromosomes through the bromodomain of BRD4 (You et al., 2004).
Nuclear Mitotic Apparatus (NuMA), a protein interacting with microtubule dynein/dynactin during mitosis, binds C-terminal LANA and associates with LANA during interphase and at the end of telophase. Repressing NuMA function led to a reduction in episomal maintenance (Si et al., 2008). LANA also associates with the kinetochore proteins CENPF and BUB1. Both N- and C-terminal LANA are involved in CENPF and BUB1 association and the interaction between CENPF and LANA leads to colocalization of LANA with these kinetochore proteins in a subset of ~50% of mitotic chromosomes in KSHV infected PEL cells (Xiao et al., 2010). Bub1, but not CENPF depletion with RNA interference led to a reduction in KSHV episome copy number in PEL cells (Xiao et al., 2010). LANA also targets BUB1 for degradation which can lead to chromosomal instability (Sun Z. et al., 2014). PARP-1 binds KSHV TR DNA. PARP-1 activity may control viral copy number in infected cells. Treatment of BC3 PEL cells with hydroxyurea, a compound that elevates PARP activity, decreased KSHV copy number and treatment with niacinamide and 3-aminobenzamide, which decrease PARP activity, increased KSHV copy numbers (Ohsaki et al., 2004). The DNA-PK/Ku complex associates with N-terminal LANA, and phosphorylates LANA. Overexpression of the catalytic subunit Ku70, but not Ku86, impaired LANA replication of a TR-containing plasmid in 293T cells. This suggests that DNA-PK/Ku may negatively regulate KSHV latent replication (Cha et al., 2010). LANA localizes to heterochromatin and interacts with heterochromatin protein 1 (HP1), which is a chromodomain containing protein, and SUV39H1, a histone methyltransferase, at chromosomes (Szekely et al., 1999; Lim et al., 2003; Sakakibara et al., 2004). Histone H2AX also interacts with LANA and its phosphorylated form, gamma H2AX colocalized with a subset of LANA dots. Knockdown of H2AX decreased KSHV episome copy number (Jha et al., 2013). HMGA1 or HMGB1 also interact with LANA and chromosomes (Shamay et al., 2012).
LANA Binds Viral TR DNA through Its C-terminal Domain to Mediate Viral Genome Persistence
LANA Binds Specific Sequence in the KSHV TR Element
Latency-associated nuclear antigen binds TR DNA to mediate KSHV DNA replication and to tether episomes to mitotic chromosomes. The finding that LANA acted on KSHV TR associated DNA to mediate episome persistence in the absence of other virus genes led to the hypothesis that LANA can directly interact with TR DNA. Subsequently, a 20 bp sequence in the TR was identified as a LANA binding site (LBS1) and was bound by the LANA C-terminal domain, also referred to as the DNA binding domain (DBD) (Ballestas and Kaye, 2001; Cotter et al., 2001; Garber et al., 2001). Mutagenesis of the LANA binding site revealed that the 6CCC8 nucleotides of LBS1 are critical for LANA interaction. Substitutions of these nucleotides severely impacted LANA binding. In contrast, mutations within other nucleotides had only modest effects on binding (Srinivasan et al., 2004). A second LBS (LBS2) was identified downstream of LBS1 through electrophoretic mobility shift assay (EMSA) and DNase I footprinting experiments (Garber et al., 2002). Compared to LBS1, LBS2 is a low affinity LANA binding site. However, since LBS2 is adjacent to LBS1, LBS1 is capable of facilitating cooperative binding of LANA to LBS2 (Garber et al., 2002). Recently, a third LBS (LBS3) was identified, also adjacent to LBS1, but with inverse orientation compared to LBS1 and LBS2 (Hellert et al., 2015) (Figure 4A). Similar to LBS2, LBS3 is a low affinity binding site with about a 100-fold lower LANA binding affinity compared to LBS1. Interestingly, LANA DBD binding to LBS1 and LBS3, or to LBS2–LBS1–LBS3 proceeds in a biphasic manner, with LANA binding to LBS1 (or cooperatively to LBS2–LBS1) in the first phase, and then binding LBS3 in the second phase (Ponnusamy et al., 2015). The biphasic binding to LBS3 could be due to the slightly greater distance between LBS1 and LBS3 as compared to LBS1 and LBS2 (Ponnusamy et al., 2015). LBS3 was previously recognized as a 32 bp sequence essential for LANA mediated replication of TR DNA, and had been termed the replication element (Hu and Renne, 2005). Although all three LBSs share homologous sequence, both LBS2 and LBS3 harbor a cytosine to guanine transversion at position 8 (Hellert et al., 2015). Considering the critical role of this cytosine for LBS1 binding affinity (Srinivasan et al., 2004), it is very likely that the low binding affinities for LANA of LBS2 and LBS3 arises from this cytosine to guanine substitution at position 8. The spacing of 22 bp between each LBS results in location of the LBS’s on the same face of DNA, thereby allowing adjacent LANA dimers to bind cooperatively (Garber et al., 2002; Hu and Renne, 2005; Hellert et al., 2015). In addition to binding viral DNA, LANA is capable of binding host genomic DNA. Chip-seq data shows that host LANA-binding sites are generally found within transcriptionally active promoters with sequence homology to the characterized LBS’s within TR DNA (Lu et al., 2012; Hu et al., 2014; Mercier et al., 2014). A novel LANA binding motif (TCCAT)3, which is only present in host LANA-binding sites, and to which LANA binds with low affinity, similar to LBS2, was also confirmed by gel shift analysis (Hu et al., 2014).
FIGURE 4.
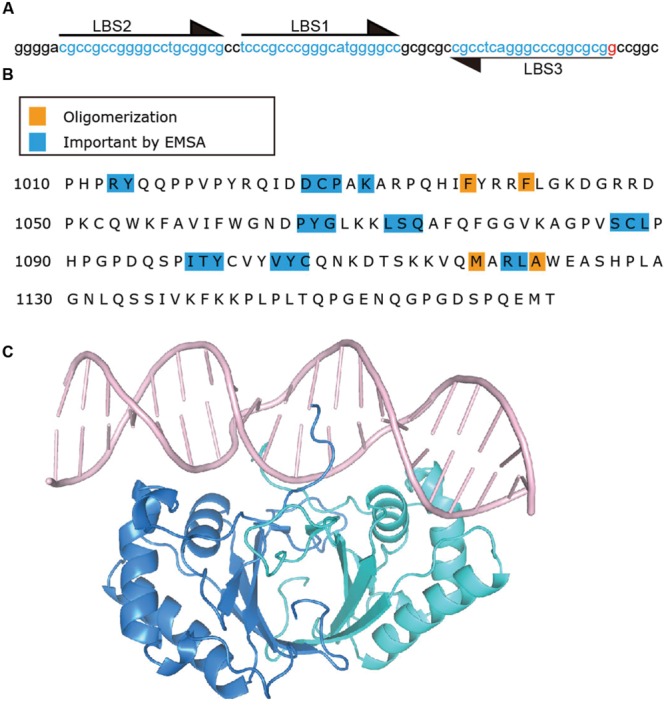
Latency-associated nuclear antigen binds specific sequence in KSHV TR DNA. (A) TR sequence with LBS sequences indicated by arrows and highlighting. (B) C-terminal LANA residues. Residues which impact DNA binding by EMSA (Kelley-Clarke et al., 2007b; Han et al., 2010) are highlighted in blue and those involved in oligomerization (Correia et al., 2013; Domsic et al., 2013; Hellert et al., 2013, 2015; Ponnusamy et al., 2015) of LANA dimers are highlighted in orange. (C) Crystal structure of C-terminal LANA complexed with LBS1 (Hellert et al., 2015) PDB:4UZB. One LANA monomer is shown in blue, and the other in green.
Mutagenesis has also defined residues within the LANA DBD as important for DNA binding (Figure 4B). LANA mutants which are deficient for, or lack DNA binding ability, are also deficient or abolished for the ability to mediate KSHV DNA replication or episome persistence (Komatsu et al., 2004; Han et al., 2010). The inability of LANA abolished for DNA binding to mediate episome persistence is due both to an inability to replicate KSHV DNA as well as loss of the ability to tether KSHV episomes to mitotic chromosomes to segregate episomes to daughter nuclei. C-terminal LANA also mediates LANA’s self-association, and self-association is necessary for DNA binding; deletions in C-terminal LANA that result in the loss of LANA self-association also result in loss of DNA binding and episome persistence (Schwam et al., 2000; Komatsu et al., 2004). As noted above, C-terminal LANA concentrates to percicentromeres and peri-telomeres of a subset of mitotic chromosomes in addition to binding KSHV TR DNA. Alanine scanning mutagenesis of C-terminal LANA revealed that different subsets of residues are important for binding to TR DNA versus mitotic chromosomes (Kelley-Clarke et al., 2007b; Han et al., 2010). Therefore, C-terminal LANA does not appear to attach to mitotic chromosomes through recognition of its TR DNA binding sequence.
Structure of the LANA DNA Binding Domain
The x-ray crystal structures of the KSHV and MHV68 LANA DNA binding domains were recently solved (Correia et al., 2013; Domsic et al., 2013; Hellert et al., 2013, 2015; Ponnusamy et al., 2015). These revealed an alpha-beta fold that assembles as a dimer, and that is structurally similar to the EBV EBNA1 and papillomavirus E2 DBDs. The hydrophobic interface of LANA DBD dimerization interface is mediated by an eight-strand anti-parallel intermolecular β-barrel to which each monomer contributes four β-sheets (Domsic et al., 2013; Hellert et al., 2013, 2015). However, in contrast to EBNA1 and E2, a unique feature of LANA is a positive electrostatic patch on the surface opposite of the DNA binding surface. This positive patch serves to interact with BET proteins, although BET proteins also interact with a second region (residues 1125–1129) in C-terminal LANA (Hellert et al., 2013). Mutation of the positive patch reduced LANA’s ability to mediate DNA replication and episome persistence, and mutation of the peripheral region of the positive patch generated the most severe effects, compared to mutation of other portions of the patch (Correia et al., 2013; Domsic et al., 2013; Hellert et al., 2013; Li et al., 2015). These deficiencies in replication and maintenance were unrelated to BET protein binding (Correia et al., 2013; Li et al., 2015). Mutation of the analogous positive patch in MHV68 LANA impacted the ability of MHV68 to establish latent infection in mice (Correia et al., 2013; Hellert et al., 2013).
Another notable finding of the LANA structure was an oligomerization interface between LANA dimers. This ability of adjacent LANA dimers to interact underlies LANA’s cooperative binding to adjacent DNA binding sites. Disruption of this interface results in loss of LANA cooperative binding to LANA binding sites such as LBS1 and LBS2, deficient DNA replication, and deficient episome persistence (Domsic et al., 2013; Hellert et al., 2013). Importantly, the hydrophobic interface that mediates the dimer–dimer interaction results in a bend and also is a flexible pivot point that allows rotation and bending between two adjacent dimers (Ponnusamy et al., 2015). Variation in the degree of bend between adjacent dimers can result in LANA DBD dimers oligomerizing to form ring structures consisting of different numbers of dimers (e.g., four or five dimers) (Figure 5A). In these LANA structures, the DNA binding surface of each dimer is on the exterior face (Domsic et al., 2013; Hellert et al., 2013, 2015). In addition, LANA dimers can form non-ring structures (Figure 5B) as well as a spiral (Hellert et al., 2015; Ponnusamy et al., 2015). MHV68 LANA also forms oligomers through interactions between adjacent dimers, which mediates cooperative binding to adjacent binding sites in MHV68 TR DNA. However, in contrast to KSHV LANA, MHV68 LANA forms a rigid, linear conformation compared to KSHV LANA’s bent, flexible configuration (Hellert et al., 2013; Ponnusamy et al., 2015).
FIGURE 5.

Higher-order crystal structures of the KSHV LANA DNA binding domain. (A) Ring form structure of the KSHV LANA DNA binding domain composed of 5 dimers (from PDB:4K2J.) (Domsic et al., 2013). (B) Non-ring form tetramer structure of KSHV LANA DNA binding domain (from PDB:5A76.) (Ponnusamy et al., 2015).
LANA Recognition of DNA
A recent x-ray crystal structure of the KSHV LANA DBD bound to the high affinity LBS1 recognition site was solved, revealing insight into LANA’s recognition of DNA (Hellert et al., 2015) (Figure 4C). To obtain the structure, point mutations were introduced in the DBD in order to obtain well-behaved complexes with DNA. Notably, and in contrast to EBNA1 or E2’s recognition of DNA (Hegde et al., 1992; Bochkarev et al., 1996), the LANA DNA dimer binds LBS1 with remarkable asymmetry, with the primary sequence recognition occurring within G5 to G10 of the 20 bp LBS1. These findings are consistent with mutagenesis studies of LBS1 as discussed above, which revealed that alteration of LBS1 6CCC8 nucleotides resulted in severe deficiency of LANA binding as compared to other mutations (Srinivasan et al., 2004). The N-terminal arm residues of one monomer, and helices 1 and 2 of the second monomer make sequence specific contacts with base pairs G5 to G10. The corresponding residues of the other monomer within the dimer make contact only with the DNA backbone. Deletion (LANAΔ1007–1021) or point mutations (R1013, Y1014) within the N-terminal arm of the LANA DBD ablates TR DNA binding, replication and episome maintenance (Komatsu et al., 2004; Domsic et al., 2013). A bend of 25° is introduced in DNA by the bound LBS1 DNA, which is less than the previously reported bend of 57°, but could be due to crystal packing constraints (Wong and Wilson, 2005).
In addition to sequence specific binding, the LANA DBD was also proposed to bind DNA in a sequence independent manner (Hellert et al., 2015). In both the ring forms (Figure 5A) and spiral structures (as described above) formed by oligomerizing LANA dimers, the sequence specific binding surface is located on the outside of the rings or spiral, and the positive electrostatic patch is located on the inside of the rings or circle, to form a continuous, positively charged inner surface. The inner diameter of the rings or spiral is large enough to accommodate double stranded DNA. In fact, negative staining electron microscopy demonstrated that LANA DBDs mutated for sequence specific binding, were able to form complexes on an arbitrary dsDNA fragment. Mutations of the oligomerization surface or of the positive electrostatic patch abolished the LANA DBD’s ability to complex with the DNA fragment (Hellert et al., 2015). It is possible that related, higher order structures of LANA may account for the LANA dots observed when LANA concentrates at episomes in cells.
Role of Internal LANA Sequences in Viral Persistence
Although both the N-terminal LANA chromosome attachment region and the C-terminal DBD are essential for episome maintenance, they are insufficient for efficient KSHV episome persistence. Accordingly, fusion of N-terminal (aa 1–32) and C-terminal (aa 929–1162) LANA is highly deficient for episome maintenance, despite its ability to bind mitotic chromosomes and TR DNA at WT levels (De Leon Vazquez and Kaye, 2011). The internal LANA sequence can be divided into two major components: unique sequence, located between amino acids 33 and 331, which includes a proline-rich region from amino acids 63 to 271, and large region of imperfect repeat elements between residues 332 and 931 (Figure 1). Both these regions contribute to viral episome maintenance.
The unique internal sequence exerts an important role for episome maintenance. LANAΔ33–273, which contains a deletion of amino acids 33 to 273, was highly impaired for episome maintenance of a plasmid containing eight TR elements. LANAΔ33–273 was also deficient for the ability to replicate TR DNA in transient assays. A number of different host cell proteins involved in DNA replication have been described to interact physically or functionally with LANA including origin recognition complex (ORC) proteins 1-6, mini-chromosome maintenance complex (MCM), HBO1, a histone acetyltransferase important for DNA replication licensing, topoisomerase IIbeta (TopoIIbeta), ubiquitin specific protease USP7, structure-specific recognition protein 1 (SSRP1), replication proteins A1 and A2, replication factor C (RFC), and the proliferating cell nuclear antigen (PCNA) (Lim et al., 2002; Stedman et al., 2004; Verma et al., 2006; Jager et al., 2012; Purushothaman et al., 2012; Shamay et al., 2012; Sun Q. et al., 2014, Sun et al., 2015). However, none of these proteins have yet been mapped to this region of LANA, although deletion of residues 262–320, which partially overlaps residues 33–273, abolished RFC interaction. It is possible that one of these, or other host cell proteins may interact with this region to exert effects on LANA’s DNA replication.
In addition to replication deficiency, the unique internal sequence 33–331 was found to be important for segregation of TR DNA to progeny nuclei. Segregation ability was assessed with a GFP reporter plasmid containing two TR elements that are incompetent for LANA mediated DNA replication due to deletion of LBS3 (formerly termed the replication element) (Hu and Renne, 2005; Skalsky et al., 2007). LANA still maintains the ability to bind this plasmid since LBS1 and LBS2 remain present. Despite preservation of DNA binding and chromosome binding, which allow tethering of TR DNA to mitotic chromosomes, LANA deleted for residues within 33–331 was deficient for the ability to segregate DNA to daughter nuclei (as assessed by retention of GFP expression), while deletion mutants containing residues 33–331 were WT for segregation (De Leon Vazquez et al., 2013). It is possible that this sequence may interact with a host cell protein important for a process such as ensuring that histones bound with LANA are deposited onto newly formed nucleosomes during DNA replication. Alternatively, this region could be responsible for ensuring that LANA molecules with bound episomes are distributed onto sister chromatids. It is possible that facilitating chromatin transcription (FACT), which disrupts and reassembles nucleosomes through removal and deposition of histones H2A/H2B, could have such a role since LANA interacts with the FACT component, SSRP1 (Reinberg and Sims, 2006; Tan et al., 2006; Hu et al., 2009). It is also possible that HMGA1 or HMGB1, which interact with LANA and remodel chromatin (Shamay et al., 2012), could have a role in LANA segregation. The interactions of HMGA1, HMGB1, and SSRP1 within LANA have not been mapped. The LANA interacting kinetochore proteins, CENPF or Bub1, are other intriguing possibilities, and these interact with LANA 1–340 and amino acids 842–1162, which therefore includes the unique internal region (Xiao et al., 2010).
Latency-associated nuclear antigen also has several motifs within the unique internal sequence that could have roles in replication or segregation. A bipartite LANA SUMO-interacting motif (SIM), which interacts with SUMO-2 modified proteins, such as the transcription factor KAP1, is located at residues 244–250 and 264–270, and has been shown to be important for LANA mediated episome persistence (Campbell and Izumiya, 2012; Cai et al., 2013). LANA contains at least one phosphorylation site within amino acids 31–52, and is also phosphorylated at additional sites within 91–340 as discussed above (Fujimuro et al., 2005; Liu et al., 2007; Cha et al., 2010). In addition, there is a suppressors of cytokine signaling (SOCS) box-like motif which contains an Elongin B and C box located at LANA residues 212–222. LANA also has another component of the SOCS box motif, a Cullin box, which is spatially separated from the elongin B/C motif, and is located at residues 1085–1100 within the C-terminal domain (Cai et al., 2006). It is possible that any of these sites within the unique internal sequence may contribute to LANA mediated segregation or replication.
The LANA internal repeat region consists of glutamine rich and acidic imperfect repeat elements spanning from amino acids 332–931 (Russo et al., 1996). The length of each region and the number of repeats varies depending on the virus isolate (Rainbow et al., 1997). Deletion of the entire repeat region between residues 332 to 929 diminishes LANA’s ability to mediate episome persistence of a small plasmid containing eight TRs to a modest degree compared to deletion of most of the unique internal sequence (residues 33–273) (~10-fold vs. ~275-fold, respectively) (De Leon Vazquez et al., 2013). However, deletion of the repeat elements had a potent effect on persistence of the entire KSHV genome. A BAC containing the KSHV genome with LANA deleted for amino acids 329–931 lost the ability to persist. After transfection of cells with this BAC, no colonies formed under drug selection. This loss was not due to a major replication defect, as the LANA mutant retained the ability to replicate a plasmid containing TR DNA in a transient assay (Alkharsah and Schulz, 2012). Therefore, both the unique internal sequence and the internal repeat elements contribute to LANA episome persistence function.
KSHV Episome Persistence Is a Multi Step Process and May Require Epigenetic Changes for Longterm Stability
As discussed above, there are two steps necessary for viral episome maintenance in proliferating cells (Figure 6). First, the viral episomes replicate in concert with cell DNA once per cell cycle using a semiconservative mode of replication (Verma et al., 2007). Second, replicated episomes must be efficiently dispatched to daughter cells. This critical process involves tethering the viral genomes to cellular mitotic chromosomes to allow migration of episomes to the nuclei of daughter cells, thereby avoiding viral DNA degradation in the cytoplasm. Although the terms “segregation” or “partitioning” are sometimes used to describe different components of this process, such terms tend to be arbitrary as the process is a continuous one that accomplishes the global goal of ensuring that replicated episomes are distributed to daughter nuclei.
FIGURE 6.
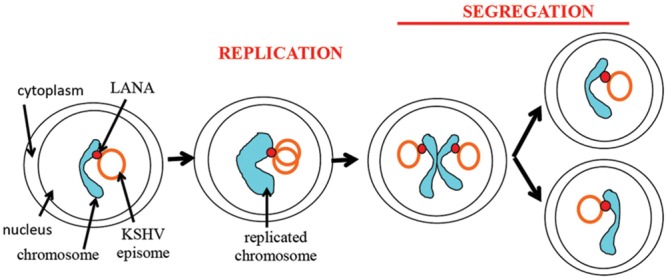
Schematic representation of episome persistence through the cell cycle. LANA tethers the episome to the cellular chromosome. During S phase, the viral episome replicates once in concert with cell DNA (Verma et al., 2007). A potential model is shown of two replicated episomes attaching to adjacent sister chromatids and segregating to daughter nuclei through tethering to chromatids.
The attachment of LANA bound KSHV episomes to nucleosomes is likely a multi-step process. For instance, histones are removed from DNA prior to its replication and re-deposited following replication. It is possible that LANA may remain bound to histones throughout this process or that LANA may be removed from histones, and then reattached to nucleosomes following DNA replication. Further, if LANA deposition on sister chromatids is not random, a mechanism must exist to ensure equal episome distribution to each sister chromatid.
A faithful segregation system of newly replicated episomes to daughter cells would seemingly be the most efficient method of distribution. Notably, it has been observed that a similar KSHV genome copy number appears to be maintained in infected cells within a population (Ueda et al., 2006), consistent with a faithful partitioning mechanism. In addition, equivalent amounts of chromosome associated GFP LANA appeared to partition in dividing, uninfected cells, also suggesting faithful partitioning (Tetsuka et al., 2004). However, work using a KSHV BAC marked with tandem lactose operator sequences to allow binding of a lactose repressor protein fused to fluorescent protein suggested KSHV may partition randomly (Norby et al., 2012). For EBV, episome segregation has been shown to be a non-random process (Nanbo et al., 2007).
Despite the persistence of stable copy numbers of KSHV episomes in infected cell lines, such as primary effusion lymphoma (PEL) cells, the establishment of stable KSHV persistence is a relatively inefficient process in vitro (Grundhoff and Ganem, 2004; Ueda et al., 2006). In fact, the use of a selection marker is necessary for efficient selection of cells with persistent maintenance of episomes (Tetsuka et al., 2004; Ueda et al., 2006). This situation is similar to that of EBV, where the establishment of stable EBNA1 mediated episome persistence of oriP plasmids is also inefficient (Leight and Sugden, 2001). It is likely that cis acting, epigenetic changes in the viral episome are necessary for stable persistence, and that such changes occur at a relatively low frequency. However, once they have occurred, episome maintenance remains stable (Grundhoff and Ganem, 2004; Skalsky et al., 2007). It has been hypothesized that lytic infection may be important for KS tumorigenesis due to the inefficiency of KSHV persistence following infection. Lytic infection could potentially recruit new infected cells to replace those that lose KSHV infection (Grundhoff and Ganem, 2004). However, an alternative explanation is that the local microenvironment in vivo may provide critical conditions that are necessary for efficient persistence of KSHV following infection.
Conclusion
Kaposi’s sarcoma-associated herpesvirus genome persistence is mediated by the viral LANA protein. LANA mediates viral DNA replication and tethers KSHV DNA (Figure 7) to mitotic chromosomes to segregate episomes to progeny nuclei (Figure 6). To tether episomes to chromosomes, N-terminal LANA directly interacts with the folded portion of the nucleosome at the conserved acidic patch at the interface of histones H2A and H2B. C-terminal LANA also interacts with chromosomes, likely by binding a chromosomal protein. In addition, C-terminal LANA mediates the interaction with viral DNA by binding sequence within the TR elements. The structure of the LANA DBD was recently solved and suggests that cooperative binding of LANA to its adjacent binding sites within each TR is mediated by interactions between LANA dimers. LANA dimers can assemble into higher-order structures, mediated by dimer-dimer interactions. LANA interacts with a number of cellular proteins, including at the chromosome. Through these interactions, LANA exerts its functions, including KSHV DNA replication and segregation. Although N and C-terminal LANA are necessary, they are not sufficient for efficient episome maintenance. LANA internal regions also exert important roles necessary for maintenance of episomes. The essential role of LANA in episome persistence makes it an ideal target for inhibition for the treatment and prevention of KSHV malignancies. Inhibition of any critical component of LANA’s episome persistence function, including binding to TR DNA or to mitotic chromosomes, is expected to abolish KSHV persistence, and eliminate virus infection from proliferating cells.
FIGURE 7.
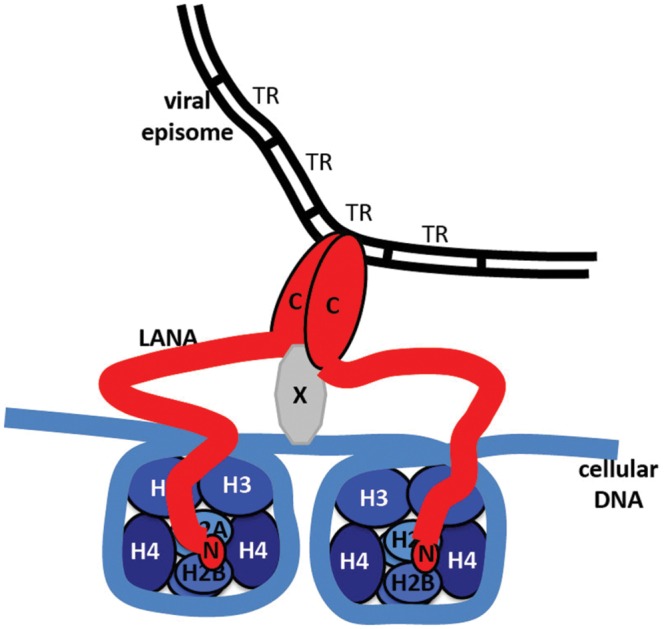
Model of LANA tethering the KSHV genome to a chromosome. N-terminal LANA (N) binds to core histones H2A/H2B. C-terminal LANA (C) self-associates, binds to KSHV TR DNA in the KSHV genome, and binds to a putative protein (X) that associates with the chromosome.
Author Contributions
All authors have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Chantal Beauchemin provided the photomicrograph of LANA in the absence of episomes in Figure 3B.
Footnotes
Funding. This work was supported in part by National Institutes of Health grants CA082036 (NCI), DE025208, and DE024971 (both NIDCR), to KMK.
References
- Alkharsah K. R., Schulz T. F. (2012). A role for the internal repeat of the Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen in the persistence of an episomal viral genome. J. Virol. 86 1883–1887. 10.1128/JVI.06029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj B. G., Verma S. C., Lan K., Cotter M. A., Woodman Z. L., Robertson E. S. (2006). KSHV encoded LANA upregulates Pi-1 and is a substrate for its kinase activity. Virology 351 18–28. 10.1016/j.virol.2006.03.037 [DOI] [PubMed] [Google Scholar]
- Ballestas M. E., Chatis P. A., Kaye K. M. (1999). Efficient persistence of extrachromosomal KSHV DNA mediated by latency- associated nuclear antigen. Science 284 641–644. 10.1126/science.284.5414.641 [DOI] [PubMed] [Google Scholar]
- Ballestas M. E., Kaye K. M. (2001). Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75 3250–3258. 10.1128/JVI.75.7.3250-3258.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera A. J., Ballestas M. E., Kaye K. M. (2004). The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78 294–301. 10.1128/JVI.78.1.294-301.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera A. J., Chodaparambil J. V., Kelley-Clarke B., Joukov V., Walter J. C., Luger K., et al. (2006a). The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science 311 856–861. 10.1126/science.1120541 [DOI] [PubMed] [Google Scholar]
- Barbera A. J., Chodaparambil J. V., Kelley-Clarke B., Luger K., Kaye K. M. (2006b). Kaposi’s sarcoma-associated herpesvirus LANA hitches a ride on the chromosome. Cell Cycle 5 1048–1052. 10.4161/cc.5.10.2768 [DOI] [PubMed] [Google Scholar]
- Beauchemin C., Moerke N. J., Faloon P., Kaye K. M. (2014). Assay development and high-throughput screening for inhibitors of Kaposi’s sarcoma-associated herpesvirus N-terminal latency-associated nuclear antigen binding to nucleosomes. J. Biomol. Screen. 19 947–958. 10.1177/1087057114520973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkarev A., Barwell J. A., Pfuetzner R. A., Bochkareva E., Frappier L., Edwards A. M. (1996). Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein, EBNA1, bound to DNA. Cell 84 791–800. 10.1016/S0092-8674(00)81056-9 [DOI] [PubMed] [Google Scholar]
- Burnside K. L., Ryan J. T., Bielefeldt-Ohmann H., Gregory Bruce A., Thouless M. E., Tsai C. C., et al. (2006). RFHVMn ORF73 is structurally related to the KSHV ORF73 latency-associated nuclear antigen (LANA) and is expressed in retroperitoneal fibromatosis (RF) tumor cells. Virology 354 103–115. 10.1016/j.virol.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Cai Q., Cai S., Zhu C., Verma S. C., Choi J. Y., Robertson E. S. (2013). A unique SUMO-2-interacting motif within LANA is essential for KSHV latency. PLoS Pathog. 9:e1003750 10.1371/journal.ppat.1003750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q. L., Knight J. S., Verma S. C., Zald P., Robertson E. S. (2006). EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2:e116 10.1371/journal.ppat.0020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood M. A., Hall K. T., Matthews D. A., Whitehouse A. (2004). The herpesvirus saimiri ORF73 gene product interacts with host-cell mitotic chromosomes and self-associates via its C terminus. J. Gen. Virol. 85 147–153. 10.1099/vir.0.19437-0 [DOI] [PubMed] [Google Scholar]
- Campbell M., Chang P. C., Huerta S., Izumiya C., Davis R., Tepper C. G., et al. (2012). Protein arginine methyltransferase 1-directed methylation of Kaposi Sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Biol. Chem. 287 5806–5818. 10.1074/jbc.M111.289496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., Izumiya Y. (2012). Post-translational modifications of Kaposi’s sarcoma-associated herpesvirus regulatory proteins – SUMO and KSHV. Front. Microbiol. 3:31 10.3389/fmicb.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S., Lim C., Lee J. Y., Song Y. J., Park J., Choe J., et al. (2010). DNA-PK/Ku complex binds to latency-associated nuclear antigen and negatively regulates Kaposi’s sarcoma-associated herpesvirus latent replication. Biochem. Biophys. Res. Commun. 394 934–939. 10.1016/j.bbrc.2010.03.086 [DOI] [PubMed] [Google Scholar]
- Chandriani S., Ganem D. (2010). Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi’s sarcoma-associated herpesvirus. J. Virol. 84 5565–5573. 10.1128/JVI.02723-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Moore P. S., Talbot S. J., Boshoff C. H., Zarkowska T., Godden K., et al. (1996). Cyclin encoded by KS herpesvirus. Nature 382 410 10.1038/382410a0 [DOI] [PubMed] [Google Scholar]
- Cheng F., Weidner-Glunde M., Varjosalo M., Rainio E. M., Lehtonen A., Schulz T. F., et al. (2009). KSHV reactivation from latency requires Pim-1 and Pim-3 kinases to inactivate the latency-associated nuclear antigen LANA. PLoS Pathog. 5:e1000324 10.1371/journal.ppat.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezova L., Burnside K. L., Rose T. M. (2011). Conservation of complex nuclear localization signals utilizing classical and non-classical nuclear import pathways in LANA homologs of KSHV and RFHV. PLoS ONE 6:e18920 10.1371/journal.pone.0018920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodaparambil J. V., Barbera A. J., Lu X., Kaye K. M., Hansen J. C., Luger K. (2007). A charged and contoured surface on the nucleosome regulates chromatin compaction. Nat. Struct. Mol. Biol. 14 1105–1107. 10.1038/nsmb1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia B., Cerqueira S. A., Beauchemin C., Pires de Miranda M., Li S., Ponnusamy R., et al. (2013). Crystal structure of the gamma-2 herpesvirus LANA DNA binding domain identifies charged surface residues which impact viral latency. PLoS Pathog. 9:e1003673 10.1371/journal.ppat.1003673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter M. A., II, Robertson E. S. (1999). The latency-associated nuclear antigen tethers the Kaposi’s sarcoma- associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264 254–264. 10.1006/viro.1999.9999 [DOI] [PubMed] [Google Scholar]
- Cotter M. A., II, Subramanian C., Robertson E. S. (2001). The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291 241–259. 10.1006/viro.2001.1202 [DOI] [PubMed] [Google Scholar]
- De Leon Vazquez E., Carey V. J., Kaye K. M. (2013). Identification of Kshv Lana regions important for episome segregation, replication and persistence. J. Virol. 87 12270–12283. 10.1128/JVI.01243-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon Vazquez E., Kaye K. M. (2011). The internal Kaposi’s sarcoma-associated herpesvirus LANA regions exert a critical role on episome persistence. J. Virol. 85 7622–7633. 10.1128/JVI.00304-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D., Lagunoff M., Renne R., Staskus K., Haase A., Ganem D. (1998). A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J. Virol. 72 8309–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsic J. F., Chen H. S., Lu F., Marmorstein R., Lieberman P. M. (2013). Molecular basis for oligomeric-DNA binding and episome maintenance by KSHV LANA. PLoS Pathog. 9:e1003672 10.1371/journal.ppat.1003672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejer G., Medveczky M. M., Horvath E., Lane B., Chang Y., Medveczky P. G. (2003). The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84 1451–1462. 10.1099/vir.0.18940-0 [DOI] [PubMed] [Google Scholar]
- Forrest J. C., Paden C. R., Allen R. D., III, Collins J., Speck S. H. (2007). ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J. Virol. 81 11957–11971. 10.1128/JVI.00111-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler P., Marques S., Simas J. P., Efstathiou S. (2003). ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J. Gen. Virol. 84 3405–3416. 10.1099/vir.0.19594-0 [DOI] [PubMed] [Google Scholar]
- Fujimuro M., Liu J., Zhu J., Yokosawa H., Hayward S. D. (2005). Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Virol. 79 10429–10441. 10.1128/JVI.79.16.10429-10441.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. C., Hu J., Renne R. (2002). Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277 27401–27411. 10.1074/jbc.M203489200 [DOI] [PubMed] [Google Scholar]
- Garber A. C., Shu M. A., Hu J., Renne R. (2001). DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 75 7882–7892. 10.1128/JVI.75.17.7882-7892.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R., Whitehouse A. (2007). Herpesvirus saimiri episomal persistence is maintained via interaction between open reading frame 73 and the cellular chromosome-associated protein MeCP2. J. Virol. 81 4021–4032. 10.1128/JVI.02171-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan E. A., Summers W. P., Dowling S., Shedd D., Gradoville L., Miller G. (1983). Two Epstein-Barr viral nuclear neoantigens distinguished by gene transfer, serology, and chromosome binding. Proc. Natl. Acad. Sci. U.S.A. 80 7650–7653. 10.1073/pnas.80.24.7650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A., Ganem D. (2003). The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77 2779–2783. 10.1128/JVI.77.7.4470.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A., Ganem D. (2004). Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 113 124–136. 10.1172/JCI200417803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habison A. C., Beauchemin C., Simas J. P., Usherwood E. J., Kaye K. M. (2012). Murine gammaherpesvirus 68 LANA acts on terminal repeat DNA to mediate episome persistence. J. Virol. 86 11863–11876. 10.1128/JVI.01656-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. J., Hu J., Pierce B., Weng Z., Renne R. (2010). Mutational analysis of the latency-associated nuclear antigen DNA-binding domain of Kaposi’s sarcoma-associated herpesvirus reveals structural conservation among gammaherpesvirus origin-binding proteins. J. Gen. Virol. 91 2203–2215. 10.1099/vir.0.020958-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A., Young B. D., Griffin B. E. (1985). Random association of Epstein-Barr virus genomes with host cell metaphase chromosomes in Bukitt’s lymphoma-derived cell lines. J. Virol. 56 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R. S., Grossman S. R., Laimins L. A., Sigler P. B. (1992). Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA- binding domain bound to its DNA target [see comments]. Nature 359 505–512. 10.1038/359505a0 [DOI] [PubMed] [Google Scholar]
- Hellert J., Weidner-Glunde M., Krausze J., Lunsdorf H., Ritter C., Schulz T. F., et al. (2015). The 3D structure of Kaposi sarcoma herpesvirus LANA C-terminal domain bound to DNA. Proc. Natl. Acad. Sci. U.S.A. 112 6694–6699. 10.1073/pnas.1421804112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellert J., Weidner-Glunde M., Krausze J., Richter U., Adler H., Fedorov R., et al. (2013). A structural basis for BRD2/4-mediated host chromatin interaction and oligomer assembly of Kaposi sarcoma-associated herpesvirus and murine gammaherpesvirus LANA proteins. PLoS Pathog. 9:e1003640 10.1371/journal.ppat.1003640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Liu E., Renne R. (2009). Involvement of SSRP1 in latent replication of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 83 11051–11063. 10.1128/JVI.00907-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Renne R. (2005). Characterization of the minimal replicator of Kaposi’s sarcoma-associated herpesvirus latent origin. J. Virol. 79 2637–2642. 10.1128/JVI.79.4.2637-2642.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Yang Y., Turner P. C., Jain V., Mcintyre L. M., Renne R. (2014). LANA binds to multiple active viral and cellular promoters and associates with the H3K4Methyltransferase hSET1 complex. PLoS Pathog. 10:e1004240 10.1371/journal.ppat.1004240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager W., Santag S., Weidner-Glunde M., Gellermann E., Kati S., Pietrek M., et al. (2012). The ubiquitin-specific protease USP7 modulates the replication of Kaposi’s sarcoma-associated herpesvirus latent episomal DNA. J. Virol. 86 6745–6757. 10.1128/JVI.06840-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha H. C., Upadhyay S. K., Prasad A. J. M., Lu J., Cai Q., Saha A., et al. (2013). H2AX phosphorylation is important for LANA-mediated Kaposi’s sarcoma-associated herpesvirus episome persistence. J. Virol. 87 5255–5269. 10.1128/JVI.03575-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Kamiya M., Maruo S., Iwakiri D., Takada K. (2007). Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. J. Cell Sci. 120 1529–1539. 10.1242/jcs.03434 [DOI] [PubMed] [Google Scholar]
- Kedes D. H., Lagunoff M., Renne R., Ganem D. (1997). Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J. Clin. Invest. 100 2606–2610. 10.1172/JCI119804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P., Boshoff C., Whitby D., Matthews S., Weiss R. A., Talbot S. J. (1997). Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1 19–29. [PubMed] [Google Scholar]
- Kelley-Clarke B., Ballestas M. E., Komatsu T., Kaye K. M. (2007a). Kaposi’s sarcoma herpesvirus C-terminal LANA concentrates at pericentromeric and peri-telomeric regions of a subset of mitotic chromosomes. Virology 357 149–157. 10.1016/j.virol.2006.07.052 [DOI] [PubMed] [Google Scholar]
- Kelley-Clarke B., Ballestas M. E., Srinivasan V., Barbera A. J., Komatsu T., Harris T. A., et al. (2007b). Determination of Kaposi’s sarcoma-associated herpesvirus C-terminal latency-associated nuclear antigen residues mediating chromosome association and DNA binding. J. Virol. 81 4348–4356. 10.1128/JVI.01289-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley-Clarke B., De Leon-Vazquez E., Slain K., Barbera A. J., Kaye K. M. (2009). Role of Kaposi’s sarcoma-associated herpesvirus C-terminal LANA chromosome binding in episome persistence. J. Virol. 83 4326–4337. 10.1128/JVI.02395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T., Ballestas M. E., Barbera A. J., Kelley-Clarke B., Kaye K. M. (2004). KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology 319 225–236. 10.1016/j.virol.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Krithivas A., Fujimuro M., Weidner M., Young D. B., Hayward S. D. (2002). Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76 11596–11604. 10.1128/JVI.76.22.11596-11604.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leight E. R., Sugden B. (2001). Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol. Cell. Biol. 21 4149–4161. 10.1128/MCB.21.13.4149-4161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhou F., Ye F., Gao S. J. (2008). Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology 379 234–244. 10.1016/j.virol.2008.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Tan M., Juillard F., Ponnusamy R., Correia B., Simas J. P., et al. (2015). The Kaposi’s sarcoma herpesvirus latency-associated nuclear antigen DNA binding domain dorsal positive electrostatic patch facilitates DNA replication and episome persistence. J. Biol. Chem. 290 28084–28096. 10.1074/jbc.M115.674622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Choi C., Choe J. (2004). Mitotic chromosome-binding activity of latency-associated nuclear antigen 1 is required for DNA replication from terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 78 7248–7256. 10.1128/JVI.78.13.7248-7256.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Lee D., Seo T., Choi C., Choe J. (2003). Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278 7397–7405. 10.1074/jbc.M211912200 [DOI] [PubMed] [Google Scholar]
- Lim C., Sohn H., Lee D., Gwack Y., Choe J. (2002). Functional dissection of latency-associated nuclear antigen 1 of Kaposi’s sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 76 10320–10331. 10.1128/JVI.76.20.10320-10331.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Martin H., Shamay M., Woodard C., Tang Q. Q., Hayward S. D. (2007). Kaposi’s sarcoma-associated herpesvirus LANA protein downregulates nuclear glycogen synthase kinase 3 activity and consequently blocks differentiation. J. Virol. 81 4722–4731. 10.1128/JVI.02548-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Day L., Gao S. J., Lieberman P. M. (2006). Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi’s sarcoma-associated herpesvirus lytic transcription. J. Virol. 80 5273–5282. 10.1128/JVI.02541-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Tsai K., Chen H. S., Wikramasinghe P., Davuluri R. V., Showe L., et al. (2012). Identification of host-chromosome binding sites and candidate gene targets for Kaposi’s sarcoma-associated herpesvirus LANA. J. Virol. 86 5752–5762. 10.1128/JVI.07216-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S., Persson L. M., Wong L., Wilson A. C. (2010). The latency-associated nuclear antigen interacts with MeCP2 and nucleosomes through separate domains. J. Virol. 84 2318–2330. 10.1128/JVI.01097-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A., Arias C., Madrid A. S., Holdorf M. M., Ganem D. (2014). Site-specific association with host and viral chromatin by Kaposi’s sarcoma-associated herpesvirus LANA and its reversal during lytic reactivation. J. Virol. 88 6762–6777. 10.1128/JVI.00268-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman N. J., Willer D. O., Speck S. H. (2003). The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 77 10295–10303. 10.1128/JVI.77.19.10295-10303.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbo A., Sugden A., Sugden B. (2007). The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 26 4252–4262. 10.1038/sj.emboj.7601853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby K., Chiu Y. F., Sugden B. (2012). Monitoring plasmid replication in live mammalian cells over multiple generations by fluorescence microscopy. J. Vis. Exp. 70:e4305 10.3791/4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaki E., Ueda K., Sakakibara S., Do E., Yada K., Yamanishi K. (2004). Poly(ADP-ribose) polymerase 1 binds to Kaposi’s sarcoma-associated herpesvirus (KSHV) terminal repeat sequence and modulates KSHV replication in latency. J. Virol. 78 9936–9946. 10.1128/JVI.78.18.9936-9946.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J. G., Colf L. A., Mcbride A. A. (2006). Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc. Natl. Acad. Sci. U.S.A. 103 1047–1052. 10.1073/pnas.0507624103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger M., Christalla T., Nathan K., Brinkmann M. M., Viejo-Borbolla A., Schulz T. F. (2006). Kaposi’s sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80 10772–10786. 10.1128/JVI.00804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C. R., Forrest J. C., Moorman N. J., Speck S. H. (2010). Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J. Virol. 84 7214–7224. 10.1128/JVI.00133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C. R., Forrest J. C., Tibbetts S. A., Speck S. H. (2012). Unbiased mutagenesis of MHV68 LANA reveals a DNA-binding domain required for LANA function in vitro and in vivo. PLoS Pathog. 8:e1002906 10.1371/journal.ppat.1002906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolot T., Tramier M., Coppey M., Nicolas J. C., Marechal V. (2001). Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75 3948–3959. 10.1128/JVI.75.8.3948-3959.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt G. M., Simpson G. R., Mittnacht S., Schulz T. F. (1999). Latent nuclear antigen of Kaposi’s sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73 9789–9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R., Petoukhov M. V., Correia B., Custodio T. F., Juillard F., Tan M., et al. (2015). KSHV but not MHV-68 LANA induces a strong bend upon binding to terminal repeat viral DNA. Nucleic Acids Res. 43 10039–10054. 10.1093/nar/gkv987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman P., Mcdowell M. E., Mcguinness J., Salas R., Rumjahn S. M., Verma S. C. (2012). Kaposi’s sarcoma-associated herpesvirus-encoded LANA recruits topoisomerase iibeta for latent DNA replication of the terminal repeats. J. Virol. 86 9983–9994. 10.1128/JVI.00839-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow L., Platt G. M., Simpson G. R., Sarid R., Stoiber S.-J. G., Herrington C. S., et al. (1997). The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71 5915–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. (1973). Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int. J. Cancer 11 499–520. 10.1002/ijc.2910110302 [DOI] [PubMed] [Google Scholar]
- Reinberg D., Sims R. J., III. (2006). de FACTo nucleosome dynamics. J. Biol. Chem. 281 23297–23301. 10.1074/jbc.R600007200 [DOI] [PubMed] [Google Scholar]
- Rivas C., Thlick A. E., Parravicini C., Moore P. S., Chang Y. (2001). Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75 429–438. 10.1128/JVI.75.1.429-438.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J. J., Bohenzky R. A., Chien M.-C., Chen J., Yan M., Maddalena D., et al. (1996). Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. U.S.A. 93 14862–14867. 10.1073/pnas.93.25.14862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R., Wu L., Forghani B., Renne R., Zhong W., Herndier B., et al. (1999). A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma- associated herpesvirus. J. Virol. 73 5722–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S., Ueda K., Nishimura K., Do E., Ohsaki E., Okuno T., et al. (2004). Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78 7299–7310. 10.1128/JVI.78.14.7299-7310.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols M. A., Hu J., Skalsky R. L., Renne R. (2005). Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 79 9301–9305. 10.1128/JVI.79.14.9301-9305.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwam D. R., Luciano R. L., Mahajan S. S., Wong L., Wilson A. C. (2000). Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies [In Process Citation]. J. Virol. 74 8532–8540. 10.1128/JVI.74.18.8532-8540.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay M., Liu J., Li R., Liao G., Shen L., Greenway M., et al. (2012). A protein array screen for KSHV LANA interactors links LANA to TIP60, PP2A activity and telomere shortening. J. Virol. 86 5179–5191. 10.1128/JVI.00169-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H., Fukushi M., Higuchi M., Oie M., Hoshi O., Ushiki T., et al. (2002). Chromosome binding site of latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 76 12917–12924. 10.1128/JVI.76.24.12917-12924.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H., Verma S. C., Lampson M. A., Cai Q., Robertson E. S. (2008). Kaposi’s sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation. J. Virol. 82 6734–6746. 10.1128/JVI.00342-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky R. L., Hu J., Renne R. (2007). Analysis of viral cis elements conferring Kaposi’s sarcoma-associated herpesvirus episome partitioning and maintenance. J. Virol. 81 9825–9837. 10.1128/JVI.00842-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V., Komatsu T., Ballestas M. E., Kaye K. M. (2004). Definition of sequence requirements for latency-associated nuclear antigen 1 binding to Kaposi’s sarcoma-associated herpesvirus DNA. J. Virol. 78 14033–14038. 10.1128/JVI.78.24.14033-14038.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman W., Deng Z., Lu F., Lieberman P. M. (2004). ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J. Virol. 78 12566–12575. 10.1128/JVI.78.22.12566-12575.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Tsurimoto T., Juillard F., Li L., Li S., De Leon Vazquez E., et al. (2014). Kaposi’s sarcoma-associated herpesvirus LANA recruits the DNA polymerase clamp loader to mediate efficient replication and virus persistence. Proc. Natl. Acad. Sci. U.S.A. 111 11816–11821. 10.1073/pnas.1404219111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Jha H. C., Robertson E. S. (2015). Bub1 in complex with LANA recruits PCNA to regulate Kaposi’s sarcoma-associated herpesvirus latent replication and DNA translesion synthesis. J. Virol. 89 10206–10218. 10.1128/JVI.01524-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Xiao B., Jha H. C., Lu J., Banerjee S., Robertson E. S. (2014). Kaposi’s sarcoma-associated herpesvirus-encoded LANA can induce chromosomal instability through targeted degradation of the mitotic checkpoint kinase Bub1. J. Virol. 88 7367–7378. 10.1128/JVI.00554-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely L., Kiss C., Mattsson K., Kashuba E., Pokrovskaja K., Juhasz A., et al. (1999). Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin- associated nuclear bodies. J. Gen. Virol. 80 2889–2900. 10.1099/0022-1317-80-11-2889 [DOI] [PubMed] [Google Scholar]
- Tan B. C., Chien C. T., Hirose S., Lee S. C. (2006). Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication. EMBO J. 25 3975–3985. 10.1038/sj.emboj.7601271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsuka T., Higuchi M., Fukushi M., Watanabe A., Takizawa S., Oie M., et al. (2004). Visualization of a functional KSHV episome-maintenance protein LANA in living cells. Virus Genes 29 175–182. 10.1023/B:VIRU.0000036377.48454.d8 [DOI] [PubMed] [Google Scholar]
- Ueda K., Sakakibara S., Ohsaki E., Yada K. (2006). Lack of a mechanism for faithful partition and maintenance of the KSHV genome. Virus Res. 122 85–94. 10.1016/j.virusres.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Verma S. C., Cai Q., Kreider E., Lu J., Robertson E. S. (2013). Comprehensive analysis of LANA interacting proteins essential for viral genome tethering and persistence. PLoS ONE 8:e74662 10.1371/journal.pone.0074662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. C., Choudhuri T., Kaul R., Robertson E. S. (2006). Latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 80 2243–2256. 10.1128/JVI.80.5.2243-2256.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. C., Choudhuri T., Robertson E. S. (2007). The minimal replicator element of the Kaposi’s sarcoma-associated herpesvirus terminal repeat supports replication in a semiconservative and cell-cycle-dependent manner. J. Virol. 81 3402–3413. 10.1128/JVI.01607-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Borbolla A., Kati E., Sheldon J. A., Nathan K., Mattsson K., Szekely L., et al. (2003). A Domain in the C-terminal region of latency-associated nuclear antigen 1 of Kaposi’s sarcoma-associated Herpesvirus affects transcriptional activation and binding to nuclear heterochromatin. J. Virol. 77 7093–7100. 10.1128/JVI.77.12.7093-7100.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Borbolla A., Ottinger M., Bruning E., Burger A., Konig R., Kati E., et al. (2005). Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 79 13618–13629. 10.1128/JVI.79.21.13618-13629.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. Y., Matchett G. A., Wilson A. C. (2004). Transcriptional activation by the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen is facilitated by an N-terminal chromatin-binding motif. J. Virol. 78 10074–10085. 10.1128/JVI.78.18.10074-10085.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. Y., Wilson A. C. (2005). Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces a strong bend on binding to terminal repeat DNA. J. Virol. 79 13829–13836. 10.1128/JVI.79.21.13829-13836.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard C., Shamay M., Liao G., Zhu J., Ng A. N., Li R., et al. (2012). Phosphorylation of the chromatin binding domain of KSHV LANA. PLoS Pathog. 8:e1002972 10.1371/journal.ppat.1002972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Verma S. C., Cai Q., Kaul R., Lu J., Saha A., et al. (2010). Bub1 and CENP-F can contribute to Kaposi’s sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores. J. Virol. 84 9718–9732. 10.1128/JVI.00713-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. (1984). A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. U.S.A. 81 3806–3810. 10.1073/pnas.81.12.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J., Croyle J. L., Nishimura A., Ozato K., Howley P. M. (2004). Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117 349–360. 10.1016/S0092-8674(04)00402-7 [DOI] [PubMed] [Google Scholar]
- You J., Srinivasan V., Denis G. V., Harrington W. J., Jr., Ballestas M. E., Kaye K. M., et al. (2006). Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80 8909–8919. 10.1128/JVI.00502-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. J., Wang K. Y., Stein D. A., Patel D., Watkins R., Moulton H. M., et al. (2007). Inhibition of replication and transcription activator and latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus by morpholino oligomers. Antiviral Res. 73 12–23. 10.1016/j.antiviral.2006.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]


