ABSTRACT
Bacteriocin 41 (Bac41) is the plasmid-encoded bacteriocin produced by the opportunistic pathogen Enterococcus faecalis. Its genetic determinant consists of bacL1 (effector), bacL2 (regulator), bacA (effector), and bacI (immunity). The secreted effectors BacL1 and BacA coordinate to induce the lytic cell death of E. faecalis. Meanwhile, the immunity factor BacI provides self-resistance to the Bac41 producer, E. faecalis, against the action of BacL1 and BacA. In this study, we demonstrated that more than half of the 327 clinical strains of E. faecalis screened had functional Bac41 genes. Analysis of the genetic structure of the Bac41 genes in the DNA sequences of the E. faecalis strains revealed that the Bac41-like genes consist of a relatively conserved region and a variable region located downstream from bacA. Based on similarities in the variable region, the Bac41-like genes could be classified into type I, type IIa, and type IIb. Interestingly, the distinct Bac41 types had specific immunity factors for self-resistance, BacI1 or BacI2, and did not show cross-immunity to the other type of effector. We also demonstrated experimentally that the specificity of the immunity was determined by the combination of the C-terminal region of BacA and the presence of the unique BacI1 or BacI2 factor. These observations suggested that Bac41-like bacteriocin genes are extensively disseminated among E. faecalis strains in the clinical environment and can be grouped into at least three types. It was also indicated that the partial diversity results in specificity of self-resistance which may offer these strains a competitive advantage.
IMPORTANCE Bacteriocins are antibacterial effectors produced by bacteria. In general, a bacteriocin-coding gene is accompanied by a cognate immunity gene that confers self-resistance on the bacteriocin-producing bacterium itself. We demonstrated that one of the bacteriocins, Bac41, is disseminated among E. faecalis clinical strains and the Bac41 subtypes with partial diversity. The Bac41-like bacteriocins were found to be classified into type I, type IIa, and type IIb by variation of the cognate immunity factors. The antibacterial activity of the respective effectors was specifically inhibited by the immunity factor from the same type of Bac41 but not the other types. This specificity of effector-immunity pairs suggests that bacteriocin genes might have evolved to change the immunity specificity to acquire an advantage in interbacterial competition.
INTRODUCTION
Enterococcus faecalis is a Gram-positive commensal bacterium present in the intestinal tract of healthy humans or animals, but it is also a causative agent of opportunistic infectious diseases, including urinary infectious disease, bacteremia, infective endocarditis, and others (1–4). As represented by the development of drug resistance, the acquisition of new genes via mobile genetic elements (MGEs), such as plasmids, raises the concern of increased severity of these enterococcal diseases (5). Enterococcal plasmids also encode various bacteriocins, which are bactericidal peptides or proteins produced by bacteria (6). Enterococcal bacteriocins are generally divided into three classes (7, 8). Heat- and acid-stable bacteriocin peptides are called class I and class II (6). Class I bacteriocin peptides are referred to as lantibiotics, and this class contains nonproteinogenic amino acids generated by posttranslational modification. Only two class I bacteriocins, beta-hemolysin/bacteriocin (cytolysin) and enterocin W, have been identified in enterococci (1, 3, 6, 9–11). Class II bacteriocin peptides are not posttranscriptionally modified and include most enterococcal bacteriocins, such as AS-48, enterocin A, bacteriocin 21 (Bac21), Bac31, Bac32, Bac43, and Bac51, which were isolated from clinical strains of E. faecalis or Enterococcus faecium (6, 7, 12–18). In contrast, class III bacteriocins, which are also referred to as bacteriolysins, are heat-labile antimicrobial proteins showing enzymatic bactericidal activity (7, 19, 20). To date, the only enterococcal bacteriolysins to be identified are enterolysin A and bacteriocin 41 (Bac41) (21–23).
Bac41 was originally cloned from the pheromone-responsive plasmid pYI14 of the clinical strain E. faecalis YI714 (21). Like other common bacteriocins, the bactericidal activity of Bac41 has a narrow spectrum against E. faecalis but is not active against E. faecium, Enterococcus hirae, Streptococcus pyogenes, Streptococcus pneumoniae, Staphylococcus aureus, or Listeria monocytogenes (21, 24). The determinant genetic element of Bac41 consists of six open reading frames (ORFs), including the four characterized genes bacL1, bacL2, bacA, and bacI (Fig. 1) (21). The Bac41 lytic system has a classical antimicrobial effector/immunity module acting in interbacterial interaction (25). The bacL1- and bacA-encoded proteins, BacL1 and BacA, are effector proteins secreted into the environment to actually express the antimicrobial activity against E. faecalis. The lytic effector, BacL1, has the NlpC/P60-type d-isoglutamyl-l-lysine endopeptidase domain located in its amino acid (aa)-163-to-315 region to degrade E. faecalis peptidoglycan (24). The binding of BacL1 via the aa-329-to-590 region is limited to the cell division-associated area on the E. faecalis cell surface, and it specifically recognizes peptidoglycans with stem peptides cross-bridged by l-Ala–l-Ala, which is a unique structure existing in E. faecalis (46). The other effector, BacA, is required in addition for the bacteriolysis of target E. faecalis cells, although its action has not been elucidated by experimental work to date. Therefore, Bac41 is a unique two-component bacteriolysin consisting of two effector proteins, BacL1 and BacA. Again, BacI is the cognate immunity factor providing self-resistance to protect a Bac41-producing E. faecalis from Bac41 activity (21).
FIG 1.
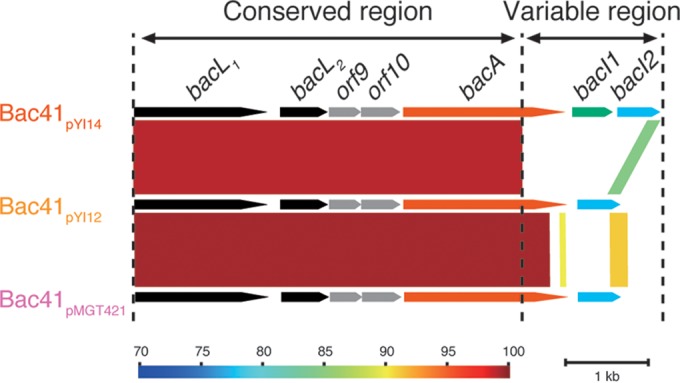
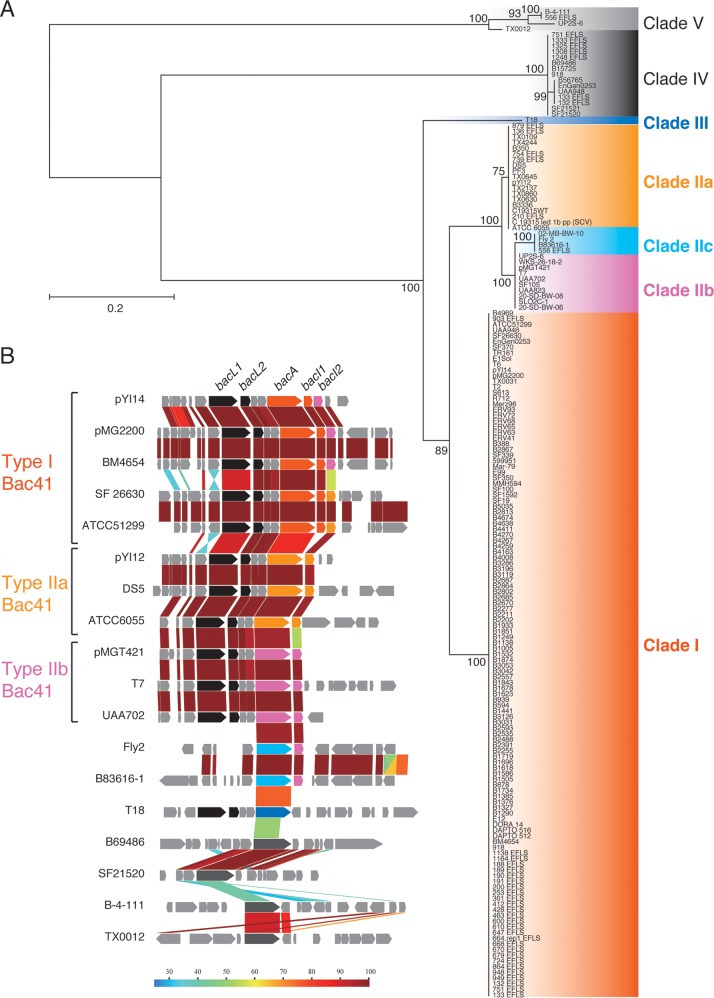
Genetic organization comparison of type I, type IIa, and type IIb Bac41 gene clusters. The genetic organization of each of type of Bac41 gene cluster sequenced in this study was aligned, and their nucleotide sequences compared by bl2seq (BLASTn) using Genome Matcher software. Bac41pYI14 (AB271686.1), Bac41pYI12 (LC114487), and Bac41pMGT421 (LC114488) represent the type I, type IIa, and type IIb Bac41 gene clusters, respectively. The color scale represents nucleotide sequence similarity (%).
In this study to understand the physiology of the Bac41 system in the clinical environment, we carried out an epidemiological study and demonstrated that functional Bac41-type bacteriocins with a degree of diversity were detected in more than half of the E. faecalis clinical isolates tested. A comparison of Bac41-like genes of the E. faecalis DNA sequences present in public databases and our collections revealed that there was diversity in bacA and bacI but not in bacL1 and bacL2. Our experimental study also revealed that these diversities could be classified into three types based on their amino acid sequences and that each type appeared to generate a specific immunity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and antimicrobial reagents.
The bacterial strains and plasmids used in this study are shown in Table 1. The oligonucleotides used in this study are shown in Table 2. The clinical isolates of E. faecalis used in this study are listed in Table S1 in the supplemental material (26). Enterococcal strains were routinely grown in Todd-Hewitt broth (THB; Difco, Detroit, MI) at 37°C (27), unless otherwise noted. Escherichia coli strains were grown in Luria-Bertani (LB; Difco) medium at 37°C. The antibiotic concentrations for the selection of E. coli were 100 mg liter−1 ampicillin, 12.5 mg liter−1 tetracycline, and 50 mg liter−1 chloramphenicol. The concentration of chloramphenicol for the routine selection of E. faecalis harboring pAM401 derivatives was 20 mg liter−1. All antibiotics were obtained from Sigma Co. (St. Louis, MO).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain(s) or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| KRY14031 to KRY140330 | 30 enterococcal strains isolated from individual hospital patients in Japan in 2014 | This study |
| ML12VRE1 to ML12VRE84 | 60 VRE strains isolated throughout Japan in 2012 | This study |
| ML12Efs1 to ML12Efs96 | 96 E. faecalis strains isolated throughout Japan in 2012 | This study |
| ML11Efs1 to ML11Efs200 | 197 E. faecalis strains isolated throughout Japan in 2011 | This study |
| MIVRE41 to MIVRE674 | 22 VRE (E. faecalis) strains isolated from University of Michigan Medical School Hospital from 1995 to 1999 | 26 |
| E. faecalis OG1S | Strr; derivative of OG1 | 27 |
| E. faecalis YI712 | Clinical strain isolate from inpatient in Gunma University Hospital; pYI12 (70 kb) Bac41 (type IIa) | 21 |
| E. faecalis GUHEfs421 | Clinical strain isolate from inpatient in Gunma University Hospital; pMGT421 Bac41 (type IIb) | This study |
| E. coli DH5α | Host for DNA cloning | Bethesda Research Laboratories |
| E. coli BL21 Rosetta | Host for protein expression | Novagen |
| Plasmids | ||
| pYI14 | Pheromone responsive, highly conjugative, encodes Bac41 bacteriocin (type I) | 21 |
| pYI12 | Pheromone responsive, highly conjugative, encodes Bac41-like bacteriocin (type IIa) | 21 |
| pMGT421 | Pheromone responsive highly conjugative, encodes Bac41-like bacteriocin (type IIb) | This study |
| pMG2200 | Pheromone responsive highly conjugative, encodes Bac41-like bacteriocin (type I); vanB | 39 |
| pAM401 | E. coli-E. faecalis shuttle plasmid; cat tet | 32 |
| pMGS100 | E. coli-E. faecalis shuttle expression plasmid; cat tet | 33 |
| pBac41pYI14 | pAM401 derivative containing whole Bac41 genes; identical to pHT1100 | 21 |
| pBac41pYI12 | pAM401 derivative containing whole Bac41 genes from pYI12; a relational clone of five BamHI fragments (F, E, H, G, D) spanning a 14.2-kb region of pYI12 | This study |
| pBac41pMGT421 | pAM401 derivative containing whole Bac41 genes from pMGT421 | This study |
| pbacI1pYI14 | pMGS100 containing bacI1 derived from pYI14 | This study |
| pbacI2pYI14 | pAM401 containing bacI2 derived from pYI14 | This study |
| pbacI2pYI12 | pAM401 containing bacI2 derived from pYI12 | This study |
| pbacI2pMGT421 | pAM401 containing bacI2 derived from pMGT421 | This study |
| pET22(+) | Expression vector for His-tagged protein in E. coli | Novagen |
| pET22ΔpelB | pET22(+) derivative with intrinsic pelB sequence removed | This study |
| pET22::bacL1pYI14 | pET22b(+) containing bacL1 gene derived from pYI14 | 24 |
| pET22::bacL1pYI12 | pET22b(+) containing bacL1 gene derived from pYI12 | This study |
| pET22::bacL1pMGT421 | pET22b(+) containing bacL1 gene derived from pMGT421 | This study |
| pET22::bacApYI14 | pET22b(+) containing bacA gene derived from pYI14 | 24 |
| pET22::bacApYI12 | pET22bΔpelB containing bacA gene derived from pYI12 | This study |
| pET22::bacApMGT421 | pET22bΔpelB containing bacA gene derived from pMGT421 | This study |
VRE, vancomycin-resistant Enterococcus.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′–3′)a | Purpose | Reference or source |
|---|---|---|---|
| F-ddl | atcaagtacagttagtct | Detection of species-specific ddl gene to identify E. faecalis | 28 |
| F-ddl | acgattcaaagctaactg | Detection of species-specific ddl gene to identify E. faecalis | 28 |
| F-bacL1 | atgaattacagtcaaaaagc | Detection of entire bacL1 gene | This study |
| R-bacL1 | ctaattaaagaatcctttgc | Detection of entire bacL1 gene | This study |
| F-bacL2 | gtggaattttgggcagaagt | Detection of entire bacL2 gene | This study |
| R-bacL2 | ttataaagattcacacagca | Detection of entire bacL2 gene | This study |
| F-Bac41_pMGT421 | ggcgaaTTCAGAAGATCAATTAGATGTTATGATGGAG | Construction of pBac41pMGT421 | This study |
| R-Bac41_pMGT421 | ccggaattcaAGCTTTATAAAAATATAGAAATACATTATGAGG | Construction of pBac41pMGT421 | This study |
| F-EagI-bacI1 | tttttcggccggcATGAATAGTATTATAGGGGA | Construction of pbacI1pYI14 | This study |
| R-NruI-bacI1 | attttttcgcgaCTAATACACAGTAGAATTAA | Construction of pbacI1pYI14 | This study |
| F-bacI2_pYI14 | ggcgggatccGCAGCAGAATTAGCAGGAGCG | Construction of pbacI2pYI14 | This study |
| R-bacI2_pYI14 | gccggatCCAATTCTAATACACAGTAG | Construction of pbacI2pYI14 | This study |
| F-bacI1del | GGGGAAATGGATTTGACGTCTGTGATATTGGCAGGT | Construction of pbacI2pYI14, 453-bp (151-aa) in-frame deletion of bacI1 | This study |
| R-bacI1del | ACCTGCCAATATCACAGACGTCAAATCCATTTCCCC | Construction of pbacI2pYI14, 453-bp (151-aa) in-frame deletion of bacI1 | This study |
| F-bacI2_pYI12 | gccggatccGCAGCTACTTTAAGCGTTGC | Construction of pbacI2pYI12 | This study |
| R-bacI2_pYI12 | gccggatcCGTTAAATAGGTATCATTTG | Construction of pbacI2pYI12 | This study |
| F-bacI2_pMGT421 | gccggatccCCTTTCAACTATGTTTTCCG | Construction of pbacI2pMGT421 | This study |
| R-bacI2_pMGT421 | gccggatccTTATTTCGTAACATGTTTCTC | Construction of pbacI2pMGT421 | This study |
| F-NdeI-bacL1 | cgccatatgAATTACAGTCAAAAAGC | Construction of pET::bacL1pYI12 and pET::bacL1pMGT421 | 24 |
| R-XhoI-bacL1 | ccgctcgagATTAAAGAATCCTTTGCCCC | Construction of pET::bacL1pYI12 and pET::bacL1pMGT421 | 24 |
| F-BamHI-bacA_pMGT421 | cgggatccATGGATGAAATGGTTTTAGG | Construction of pET::bacApYI12 and pET::bacApMGT421 | This study |
| R-XhoI-bacA_pYI12 | ccgctcgagTTTATTACCAAACATAGATG | Construction of pET::bacApYI12 | This study |
| R-XhoI-bacA_pMGT421 | ccgctcgagTTTTTCGGAAAACATAGTTG | Construction of pET::bacApMGT421 | This study |
| F-BamHI-pET | cgggatccGAATTCGAGCTCCG | Construction of pETΔpel | This study |
| R-BamHI-pET_rbs | cgggatccTATATCTCCTTCTTAAAGTT | Construction of pETΔpel | This study |
Lowercase letters indicate additional nucleotides forming tags to be digested by restriction enzymes for plasmid construction.
In vitro competition assay.
To assess the advantage of carrying a plasmid in a population in vitro, equal volumes of the overnight cultures of E. faecalis OG1S (plasmid null) and E. faecalis OG1S harboring pAM401 derivatives were diluted 100-fold with fresh THB broth, followed by cocultivation at 37°C. At set time periods, serial dilutions of coculture samples were plated on THB with or without chloramphenicol (20 mg/liter). As pAM401 derivatives carry the catB gene, the CFU count for plasmid-harboring cells was estimated by counting colonies grown on THB agar containing chloramphenicol. The total cell count, including cells with and without a plasmid, was obtained by determining the total count of CFU on THB without antibiotics.
Antimicrobial susceptibility testing.
The MICs of the antibiotics were determined by the agar dilution method. An overnight culture of each strain grown in Mueller-Hinton broth (Nissui, Tokyo, Japan) was diluted 100-fold with fresh broth. An inoculum of approximately 5 × 105 cells was spotted onto a series of Mueller-Hinton agar (Eiken, Tokyo, Japan) plates containing a range of concentrations of the test drug. After incubation at 37°C for 24 h, the number of colonies that had grown on the plates was determined. The MIC breakpoints for resistance to antibiotics were defined as suggested by Clinical and Laboratory Standards Institute (CLSI) guidelines (http://clsi.org/).
Colony-directed PCR.
To simultaneously detect bacL1, bacL2, and the E. faecalis-specific ddl gene (28), multiplex PCR was performed with KOD FX plus (Toyobo, Tokyo, Japan) using a bacterial colony from an agar plate as the template (29, 30). The PCR cycling conditions comprised 5 min at 94°C, followed by 35 cycles of 10 s at 98°C, 30 s at 55°C, and 1 min 30 s at 68°C, using the GeneAmp PCR 9700 thermal cycler (Bio-Rad, Hercules, CA).
Soft-agar assay for bacteriocin activity.
The soft-agar assay for bacteriocin activity was performed as described previously (24, 31). Briefly, the test bacterial strain or 1 μl of recombinant protein solution was inoculated into THB soft agar (0.75%) containing the indicator strain and was then incubated at 37°C for 24 h. The formation of an inhibitory zone was evaluated as a sign of bacteriocinogenic activity of the test strain.
Isolation of type II Bac41 plasmids pYI12 and pMGT421 and cloning and DNA sequence analysis of the Bac41-like genes they carry.
In this study, we examined the previously reported Bac41-producing pheromone-responsive conjugative plasmid pYI12 that had been isolated from E. faecalis clinical strain YI712 (21). To determine the restriction map and to clone the Bac41-like bacteriocin genes of pYI12, the relational cloning methodology was used as described in our previous reports (15, 16, 21). pYI12 plasmid DNA was digested with BamHI, EcoRI, SalI, or XbaI or doubly digested with a combination of two of these restriction enzymes. The molecular sizes of the eight BamHI fragments, A to H, were found to be 36.7, 13.7, 5.8, 4.5, 4.1, 3.3, 2.0, and 0.2 kb, respectively. To determine the order of the BamHI fragments of pYI12, a relational clone set was constructed. After agarose gel electrophoresis of plasmid pYI12 DNA partially digested with BamHI, fragments greater than 7 kb in size were eluted and used for cloning. The cloning vector used was pAM401 (32), and the host strain was E. coli DH5α. DNA sequence analysis was carried out as previously reported (15, 16, 21). A deletion kit (Nippon Gene, Tokyo, Japan) and a Bac41 expression plasmid (pBacpYI12, containing five BamHI fragments, F, E, H, G, and D, of pYI12) obtained from the screening were used. The resulting constructs were sequenced in both orientations with a Taq BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA), a model 377 DNA sequencer, and an ABI Prism 310 gene analyzer (Applied Biosystems). The DNA sequence data of the Bac41-expressing plasmid (pAM401 derivative containing five BamHI fragments of pYI12 spanning a 14.2-kb region) revealed that the BacA of pYI12 was classified as a type IIa Bac41 in this study (Fig. 1; see Fig. S3 in the supplemental material). The genetic analysis of the bacteriocin determinants of pBac41pYI12 by Tn5-transposon mutagenesis was conducted as previously described (15, 16, 21).
To find whether various other Bac41 types differed from the prototype Bac41 encoded on pYI14, we screened the bacteriocinogenic E. faecalis clinical isolates by PCR using the specific primer sets for the bacL1, bacA, and bacI (bacI1) genes, listed in Table 2. The bacL1-positive, bacA (N-terminal-region)-positive, and bacI1-negative strains were picked up for further analysis in this study. The representative Bac41-like bacteriocin-encoding plasmid pMGT421 from a bacteriocinogenic clinical isolate, E. faecalis GUHEfs421, was used in this study. The locations of the determinants were examined, and DNA sequence analysis was performed as previously described (15, 16, 21). To examine the variable region spanning the C-terminal end of bacA and the immunity gene, inverse PCR using the HindIII restriction enzyme was performed. The Bac41 expression plasmid pBac41pMGT421, a derivative of pAM401, was constructed by PCR methodology utilizing the DNA sequence data and specific primer set used in this study (Tables 1 and 2). The immunity genes were cloned into a shuttle vector, pAM401 or pMGS100 (33), using the corresponding primers listed in Table 2.
Construction of expression plasmids.
The amplification of the respective genes for plasmid construction was carried out by PCR using the corresponding primers, indicated in Table 2. The amplified DNA fragments were inserted into the respective expression vectors using restriction enzymes (NEB, Ipswich, MA) and a DNA ligation kit (TaKaRa, Shiga, Japan) as described elsewhere. Overlap extension PCR was used for the construction of pbacI2pYI14, containing ORF13 (named bacI2 in this study) along with the original promoter region upstream from bacI (renamed bacI1) and an in-frame deletion (453 bp/151 aa) of bacI (bacI1) encoded on pYI14. The constructs of the pET22 derivative were designed without the pelB sequence. For the construction of pET22::bacApYI12 or pET22::bacApMGT421, where the BamHI site was used, the pelB sequence in pET22(+) was eliminated by inverse PCR. The constructed plasmids were sequenced to confirm that the desired sequence had been inserted.
Purification of His-tagged recombinant proteins.
The preparation of the recombinant proteins was carried out as previously described. Briefly, the recombinant-expressing E. coli strains were inoculated into 500 ml of fresh LB and cultured at 37°C with shaking until an optical density at 600 nm of 0.5 to 0.7 was obtained. Then, isopropyl-β-d-thiogalactoside was added to a final concentration of 0.5 mM, and an additional incubation at 30°C with shaking for 3 h was carried out. The collected bacterial cells were resuspended in 10 ml of lysis buffer (25 mM Tri-HCl, 150 mM NaCl, 10 mM imidazole, 10 mg ml−1 lysozyme, pH 8.0) with EDTA-free protease inhibitor cocktail (cOmplete, mini, EDTA-free; Roche Diagnostic Corporation, Indianapolis, IN) to be enzymatically lysed at 37°C for 30 min following sonication in ice using a sonicator (ultrasonic disruptor UD-201; TOMY Digital Biology Co., Tokyo, Japan) set at power level 6, 40% duty cycle, for 20 min. The resulting clarified and soluble lysate was added to 1 ml 50% Ni-nitrilotriacetic acid (NTA) nickel chromatography resin (Ni-NTA purification system; Invitrogen, Carlsbad, CA). After washing with 40 ml wash buffer (25 mM Tris-HCl, 150 mM NaCl, 20 mM imidazole, pH 8.0), the His-tagged protein was eluted with elution buffer (25 mM Tris-HCl, 150 mM NaCl, 200 mM imidazole, pH 8.0). The resulting His-tagged protein solution was subjected to ultracentrifugation using an Amicon ultra centrifugal filter (catalog no. UFC801024; Millipore Billerica, MA) and then resuspended in phosphate-buffered saline (PBS). The protein concentration was determined by the Bradford method (protein assay kit; Bio-Rad).
Statistical analysis.
The statistical significance of the findings was evaluated by using the chi-square and Fisher exact tests. Results were considered to be statistically significant at P values of <0.05.
Bioinformatic analysis.
Genetic information for the E. faecalis strains was obtained from the draft genome database in NCBI (https://www.ncbi.nlm.nih.gov/). Amino acid sequence alignments were generated by using ClustalW version 2.0 (http://clustalw.ddbj.nig.ac.jp/) (34). The evolutionary history was inferred by using the maximum-likelihood method based on the JTT matrix-based model. Evolutionary analyses were conducted in MEGA7 (35). A graphic alignment showing the organization (36) of the genes was produced using GenomeMatcher software (http://www.ige.tohoku.ac.jp/joho/gmProject/gmhomeJP.html) (37). The transmembrane helix domain prediction was obtained by using the TMHMM server (http://www.cbs.dtu.dk/services/TMHMM/) (38).
Accession numbers.
The nucleotide sequences reported in this article are available from the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers LC114487 (pYI12-bac41) and LC114488 (pMGT421-bac41).
RESULTS
Functional Bac41 genes are extensively disseminated among clinical E. faecalis strains.
Since the Bac41 system excludes E. faecalis cells without a Bac41-coding plasmid, it is postulated that Bac41 promotes the expansion of the plasmid on which it is encoded in the E. faecalis population. Indeed, this hypothesis was confirmed by an in vitro competition assay, as follows. An E. faecalis strain harboring a shuttle vector with the catB gene and pAM401 or pHT1100 was mixed with a plasmid-null E. faecalis strain at a 1:1 ratio, and the ratio of chloramphenicol-resistant E. faecalis cells to the whole E. faecalis population was assessed (see Fig. S1 in the supplemental material). The CFU count of E. faecalis cells harboring the control vector, pAM401, was equivalent to half of the total population and equal to that counted at the initial period of incubation even after 6 or 12 h of incubation. In contrast, E. faecalis cells harboring pHT1100, which is the pAM401 vector containing the entire Bac41 operon, became dominant in the total population and excluded the plasmid-null population in 6 and 12 h of incubation. This result indicated that carrying the Bac41 system is advantageous for the competitive survival of the E. faecalis strain carrying the plasmid.ddl.
To experimentally verify this hypothesis in the strains isolated from a clinical environment, we screened for Bac41 production in our collection of 405 clinical strains of enterococci (see Table S1 in the supplemental material). The screening was performed using PCR to detect the bacL1 and bacL2 genes and by examining the strains for growth inhibition activity against E. faecalis OG1S in the soft-agar test (Table 3). The strains that were positive in both these tests were defined as Bac41-positive strains. Detection of the E. faecalis-specific ddl gene (ddlE. faecalis) was also carried out to confirm the species. Among the 405 enterococcal strains, 327 strains gave rise to the ddlE. faecalis PCR product, indicating that those strains were E. faecalis (Table 3). Of the 327 E. faecalis isolates, 195 (59.6%) strains were Bac41 positive. Sixty-one (18.7%) strains of E. faecalis still appeared to have Bac41-independent bacteriocin activity against E. faecalis. In contrast, non-E. faecalis enterococci (ddlE. faecalis negative) did not include any Bac41-positive strains, although 17 (21.8%) strains showed undefined bacteriocin activity against E. faecalis. Collectively, the Bac41-positive strains accounted for more than half of the clinical isolates of E. faecalis strains, and the Bac41 system appears to be specific to E. faecalis and not to occur in other Enterococcus species.
TABLE 3.
Proportions of bacL1 and bacL2 gene-positive strains and growth inhibition of E. faecalis OG1S among enterococcal strains
| Enterococcal speciesa | Presence of: |
No. (%) of strains with indicated profile | ||
|---|---|---|---|---|
| OG1S inhibition | bacL1 | bacL2 | ||
| E. faecalis (n = 327) | + | + | + | 195 (59.6) |
| + | + | − | 1 (0.3) | |
| + | − | + | 6 (1.8) | |
| + | − | − | 61 (18.7) | |
| − | + | + | 6 (1.8) | |
| − | + | − | 2 (0.6) | |
| − | − | + | 3 (0.9) | |
| − | − | − | 53 (16.2) | |
| E. faecium or other (n = 78) | + | − | − | 17 (21.8) |
| − | − | − | 61 (78.2) | |
The presence of the E. faecalis-specific ddl gene (ddlE. faecalis) was used to identify strains as E. faecalis.
Relationships of Bac41 systems to types of clinical specimens from which strains were isolated and drug resistance profiles.
The clinical strains used in this study were derived from a variety of sources and showed resistance to several drugs. Most of the Bac41-positive (66%) or -negative (69%) strains were derived from urine (see Table S1 in the supplemental material), and thus, no relationship between the specimen that was isolated and Bac41 possession was shown (Table 4). It is known that antibiotic resistance, such as vancomycin resistance or high-level resistance to aminoglycosides, is often horizontally transferred by plasmids in E. faecalis (39). There was a statistically significant increase in the frequency of resistance to erythromycin, gentamicin, kanamycin, or tetracycline in Bac41-positive strains (Table 5). Resistance to the other drugs showed equal rates of incidence in Bac41-positive and -negative strains.
TABLE 4.
Specimen types from which Bac41-negative or -positive E. faecalis strains were isolated
| Source | No. (%) of strains that were: |
|
|---|---|---|
| Bac41 negative (n = 132) | Bac41 positive (n = 195) | |
| Urine/urinary tract catheter | 89 (67.4) | 133 (68.2) |
| Vagina | 8 (6.1) | 16 (8.1) |
| Pus | 7 (5.3) | 2 (1.0) |
| Blood/vascular catheter | 4 (3.0) | 7 (3.6) |
| Decubitus ulcer | 3 (2.3) | 7 (3.6) |
| Sputum | 3 (2.3) | 6 (3.1) |
| Ascitic fluid | 2 (1.5) | 0 (0) |
| Bile | 1 (0.8) | 2 (1.2) |
| Feces | 0 (0) | 2 (1.2) |
| Other | 9 (6.8) | 8 (4.1) |
| Unknown | 6 (4.5) | 12 (6.2) |
TABLE 5.
Frequencies of drug resistance among Bac41-negative or -positive E. faecalis strains
| Druga | No. (%) of strains resistant to drug that were: |
Statistical significanceb | |
|---|---|---|---|
| Bac41 negative (n = 132) | Bac41 positive (n = 195) | ||
| AMP | 9 (6.8) | 17 (8.7) | NS |
| MIN | 7 (5.3) | 11 (5.6) | NS |
| VAN | 6 (4.5) | 17 (8.7) | NS |
| ERY | 59 (44.7) | 151 (77.4) | <0.0001 |
| CHL | 6 (4.5) | 6 (3.1) | NS |
| CIP | 13 (9.8) | 24 (12.3) | NS |
| GENc | 29 (22.0) | 72 (36.4) | 0.0054 |
| KANc | 45 (34.2) | 130 (66.7) | <0.0001 |
| STRc | 25 (18.9) | 44 (22.6) | NS |
| TET | 67 (51.1) | 123 (63.1) | 0.0322 |
AMP, ampicillin; MIN, minocycline; VAN, vancomycin; ERY, erythromycin; CHL, chloramphenicol; CIP, ciprofloxacin; GEN, gentamicin; KAN, kanamycin; STR, streptomycin; TET, tetracycline.
NS, not significant.
The levels of resistance to aminoglycosides were high.
The BacA homologues in E. faecalis strains are divided into five clades.
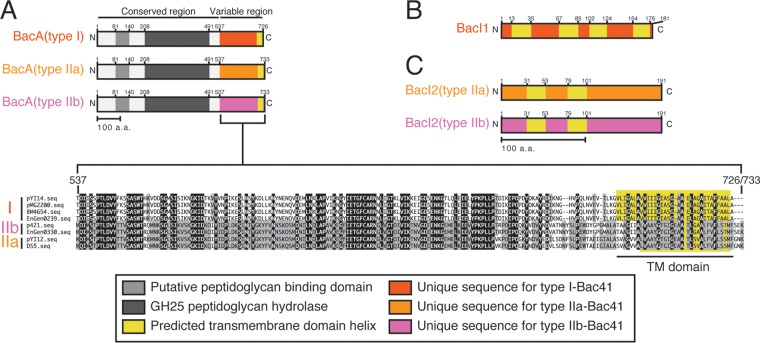
To date, 440 E. faecalis (NCBI Taxonomy accession number 1351) genome assemblies have been registered at the NCBI database (http://www.ncbi.nlm.nih.gov). Consistent with the results from our collection (Table 3), 160 Bac41 homologues were found in the public database, although partial sequences were identified in some genomes (see Table S2 in the supplemental material). In contrast with poor diversity of BacL1 and BacL2 homologues (see Fig. S2), BacA homologues consist of seven variants, comprising clades I, IIa, IIb, IIc, III, IV, and V (Fig. 2A). Clade I, which includes the BacA of pYI14, formed the majority group, containing 125 coding sequences (CDSs). The second major group, clade II, included three subclades, IIa, IIb, and IIc, containing 19, 10, and 4 CDSs, respectively. Clade III BacA was found in only one strain. Clades IV and V (15 and 4 CDSs, respectively) seemed to be phylogenetically distant from the other clades. Indeed, only clades I, IIa, IIb, and III were accompanied by BacL1 and BacL2 and suggested to be related to the Bac41 system (Fig. 2B). Thus, we newly defined the Bac41-like gene clusters as type I, type IIa, type IIb, and type III based on the clades of the bacA homologues. The amino acid sequences of BacL1 and BacL2 homologues showed high identity with each other among the distinct types of Bac41-like gene clusters. In contrast, although there is a highly conserved region from the N terminus through the middle of BacA (aa 1 to 536), diversity among the distinct clades converged within the C-terminal moiety of BacA (aa 537 to 726/723) (Fig. 3A; see also Fig. S3). Furthermore, the immunity gene bacI, which is located downstream from bacA, is represented in type I but not type IIa or IIb Bac41 gene clusters (Fig. 2B). These data suggest that, among the diverse Bac41-like genes, there is a conserved region from bacL1 to the 5′ region of bacA and a variable region from the 3′ region of bacA that includes bacI (Fig. 1).
FIG 2.
Diversity of BacA proteins in E. faecalis strains. (A) The phylogenic tree of BacA homologues of E. faecalis strains was constructed using the JTT model in MEGA7 based on the amino acid sequence alignment generated by ClustalW version 2.0. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to each branch. The initial tree(s) for the heuristic search was obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model and then selecting the topology with the superior log-likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 174 amino acid sequences. All positions containing gaps and missing data were eliminated. The names of the source strains are shown. (B) Genetic structure alignment for the flanking regions of the respective BacA homologues. Color scale represents amino acid sequence similarity (%). Conserved CDSs, including bacL1 and bacL2, are represented in black. Specific CDSs including bacA, bacI1, and bacI2 are represented as follows: type I, orange; type IIa, yellow; type IIb, pink; type IIc, cyan; type III, blue; types IV and V, gray.
FIG 3.
Diversity of Bac41-like gene clusters. (A) Molecular structures of type I, IIa, and IIb BacA proteins are represented. The region from the N terminus through the middle is conserved, and aa-81-to-140 and aa-208-to-491 regions are considered to be the putative peptidoglycan binding domain and GH25 peptidoglycan hydrolase, respectively. The C-terminal end is predicted to be a transmembrane (TM) helix domain. The aa-537-to-726/733 region is a variable region that is unique to each type. (B and C) The molecular structures of BacI1 and type I, IIa, and IIc BacI2 proteins are represented. The predicted four or two transmembrane helices are located in BacI1 and BacI2 proteins, respectively. Type IIa and type IIb BacI2 proteins show low degrees of similarity to each other throughout the whole molecule.
Identification of an alternative immunity gene, bacI2, for type II Bac41.
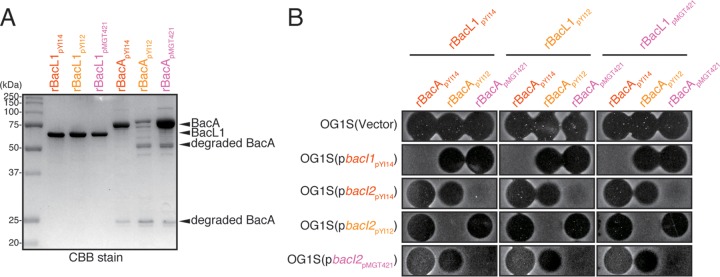
To test whether the type II Bac41s are functional, we cloned the representative type IIa or type IIb Bac41 genes from plasmid pYI12 or pMGT421, respectively (Fig. 1). The supernatant of E. faecalis OG1S harboring either pAM401::Bac41pYI12 or pAM401::Bac41pMGT421 showed bacteriocinogenic activity against E. faecalis (Fig. 4). In addition, both of the recombinant effector components, recombinant BacL1 (rBacL1) and rBacA, from either Bac41pYI12 or Bac41pMGT421 exerted bactericidal activity against E. faecalis (Fig. 5), demonstrating that both type IIa and type IIb Bac41s are functional, as well as type I Bac41. In general, a gene encoding a bacteriocin is accompanied by its cognate immunity gene, resulting in self-resistance in the bacteriocin-producing strain (7). In the type I Bac41 system, the highly conserved immunity gene bacI, which is located downstream from bacA, encodes a transmembrane protein that provides self-resistance via an undefined mechanism (Fig. 3B; see also Fig. S5A in the supplemental material) (21). Unexpectedly, the type IIa and type IIb Bac41 genetic clusters completely lacked a bacI homologue (Fig. 1 and 2B). In addition, the bacI of type I Bac41 did not function to provide immunity against type II Bac41s (Fig. 4). Therefore, it was supposed that the type II Bac41 utilizes an alternative immunity factor rather than the type I-encoded bacI. To avoid confusion, the immunity gene for type I Bac41 that has been referred to simply as bacI is hereinafter renamed bacI1. To identify the cognate immunity factor responsible for the self-resistance in type II Bac41 systems, we carried out a Tn5 transposon mutagenesis screening study and identified the gene, designated bacI2, located immediately downstream from the bacA gene in the type IIa Bac41 derived from pYI12. The bacI2 gene was conserved in type IIa, type IIb, and even type I Bac41 gene clusters (see Fig. S4). BacI2 also has two predicted transmembrane domains but does not show any sequence similarity to the type I Bac41 immunity factor BacI1 (Fig. 3C; see also Fig. S5B). The introduction of the pYI12-derived bacI2 (bacI2pYI12) clone effectively provided resistance to the type II Bac41pYI12 (Fig. 4). In addition, the pMGT421-derived bacI2 (bacI2pMGT421) also conferred self-resistance to Bac41pMGT421. These data demonstrated that the cognate immunity system in type II Bac41 was distinct from that of the type I Bac41 but that bacI2 could function as an alternative cognate immunity factor in the type II Bac41. However, it was unexpected that the type IIb bacI2pMGT421 did not show cross immunity against type IIa Bac41, derived from pYI12, and vice versa (Fig. 4), despite the similarity between the amino acid sequences of type IIa and type IIb BacI2 (56% identity). These observations indicated that, in addition to the differing immunity specificities between type I and type II Bac41s, there are also different immunity specificities between type IIa and type IIb Bac41s, and they suggested that the slight difference in amino acid sequence between type IIa and type IIb BacI2s results in the specificity of self-resistance.
FIG 4.
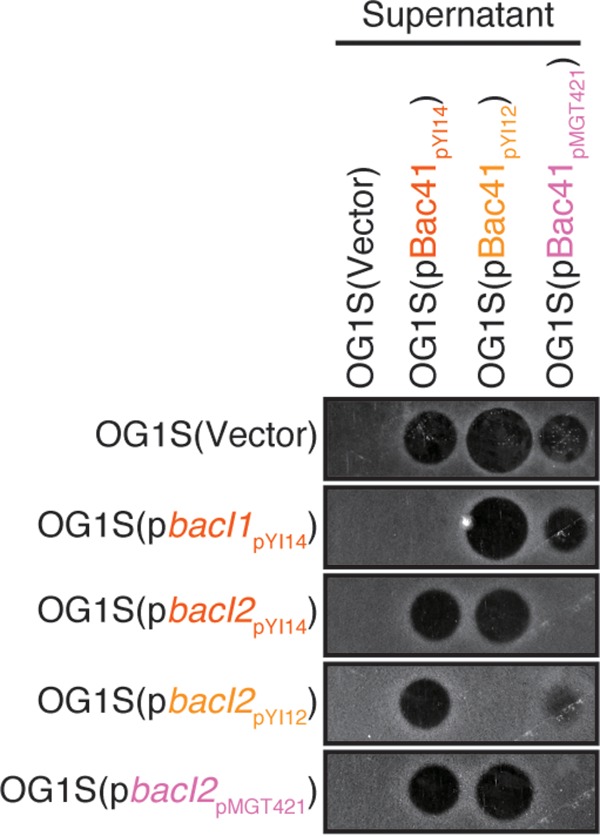
Specific protection in E. faecalis strains with introduced bacI genes that confer immunity against distinct types of Bac41s. The culture supernatants of E. faecalis OG1S harboring a plasmid vector carrying type I Bac41 (pBac41pYI14), type IIa Bac41 (pBac41pYI12), or type IIb Bac41 (pBac41pMGT421) were spotted onto THB soft-agar (0.75%) plates containing the indicator strains. OG1S strains with the introduced immunity gene type I bacI1 (pbacI1pYI14), type I bacI2 (pbacI2pYI14), type IIa bacI2 (pbacI2pYI12), or type IIb bacI2 (pbacI2pMGT421) were used as indicator strains. The plates were incubated at 37°C for 24 h, and the formation of halos was evaluated.
FIG 5.
Specific protection in E. faecalis strains with introduced bacI genes against chimeric combinations of recombinant BacL1 and BacA effectors. (A) Recombinant His-tagged BacL1 and BacA proteins derived from pYI14 (rBacL1pYI14 and rBacApYI14), pYI12 (rBacL1pYI12 and rBacApYI12), or pMGT421 (rBacL1pMGT421 and rBacApMGT421) proteins (400 ng) were separated by SDS-PAGE and stained with Coomassie brilliant blue (CBB). (B) Combinations of recombinant His-tagged BacL1 and BacA proteins derived from pYI14 (rBacL1pYI14 and rBacApYI14), pYI12 (rBacL1pYI12 and rBacApYI12), or pMGT421 (rBacL1pMGT421 and rBacApMGT421) proteins (25 ng each) were spotted onto THB soft-agar (0.75%) plates containing the indicator strains. OG1S strains with the introduced immunity gene type I bacI1 (pbacI1pYI14), type I bacI2 (pbacI2pYI14), type IIa bacI2 (pbacI2pYI12), or type IIb bacI2 (pbacI2pMGT421) were used as indicator strains. The plates was incubated at 37°C for 24 h, and the formation of halos was evaluated.
BacA but not BacL1 is key to the immunity specificity.
In Bac41, the bacteriocinogenic activity is actually exerted by the combination of two secreted effector proteins, BacL1 and BacA (21, 24). To reveal which effector component is responsible for the resistance specificity, we prepared recombinants of BacL1 and BacA derived from pYI14 (type I Bac41), pYI12 (type IIa Bac41), and pMGT421 (type IIb Bac41) (Fig. 5A). The susceptibility of E. faecalis harboring the control vector, pbacI1pYI14, pbacI2pYI14, pbacI2pYI12, or pbacI2pMGT421 to chimeric combinations of each BacL1 and BacA derived from a different type of Bac41 was examined (Fig. 5B). As expected, when the origin of the BacA component was identical to that of the introduced immunity factor, resistance was provided. In contrast, each immunity gene did not confer resistance against a different type of BacA. The origin of BacL1 did not affect the specificity of immunity. This result clearly demonstrated that the relationship between BacA and BacI1/BacI2 determined the specificity of self-resistance in different Bac41-like bacteriocins.
DISCUSSION
Epidemiological relationship of Bac41 systems in certain E. faecalis lineages.
In this report, our epidemiological study demonstrated that functional Bac41 genes were extensively propagated among E. faecalis clinical strains (Table 3). Therefore, the frequency of detection of Bac41 in clinical strains was notably high compared to the frequency of detection of the known major virulence factor beta-hemolysin/bacteriocin (3, 10, 31). As far as our investigation showed, Bac41 genes are carried on the pheromone-responsive conjugative plasmids belonging to the RepA_N-type plasmid family, whose host range is narrow and restricted to E. faecalis (40). In this aspect, the Bac41 gene element is, at least in theory, suspected to be freely disseminated among E. faecalis lineages without restriction by host background. Indeed, the epigenetic backgrounds of our collected isolates were geographically diverse, and the isolates varied as to the characteristics of the individual inpatients (gender and age) and the hospitals from which they were obtained, suggesting that they were independent and unlikely to belong to certain clonal strains (see Table S1 in the supplemental material) (41). However, the collected isolates were largely from urine-associated specimens, and fewer were obtained from blood or cases of infective endocarditis. Besides, their multilocus sequence typing (MLST)-based strain lineages (sequence types [STs]) were not yet defined. Alternatively, the sequence types in some genome-sequenced strains are available from previous reports (42, 43), and we could partially investigate the correlations of Bac41 types with ST clonal lineages (Table 6; see also Table S1). Notably, of 100 strains belonging to clonal complex 2 (CC2), which is the major hospital-adapted lineage, containing ST2 and ST6 (44, 45), 71 strains (71%) were Bac41 positive. In contrast, for 11 strains of the other major lineage, ST40, there were no Bac41-positive strains. The other lineages also include Bac41-positive strains at rates of 20 to 30%. Therefore, there must be at least some tropism by which Bac41 tends to be possessed by strains in a certain lineage, CC2. It is notable that strain FA2-2, belonging to ST8, shows lower susceptibility (tolerance) to Bac41 than the E. faecalis OG1RF strain and its derivatives belonging to ST1 (CC1) (46). The genetic lineages that show tolerance to Bac41 might be scarcely affected by the selective force of Bac41 and so might take only slight advantage from the possession of Bac41. Therefore, it could be difficult for Bac41 to spread among such a lineage in the natural environment, resulting in the formation of tropism. However, any conclusion regarding a correlation of Bac41 with specific lineages needs further extensive and detailed epidemiological studies.
TABLE 6.
Frequencies of Bac41 genes in genome-sequenced E. faecalis strainsa
| ST (CC) | No. of strains with Bac41 assemblies of type: |
Total no. of strains | No. (%) possessing Bac41 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None | I (type IIa bacI2) | I (type IIb bacI2) | IIa | IIb | IIc | III | |||
| 2 (2) | 12 | 7 | 19 | 7 (37) | |||||
| 6 (2) | 17 | 3 | 59 | 1 | 1 | 81 | 64 (79) | ||
| 4 (4) | 2 | 1 | 1 | 4 | 2 (50) | ||||
| 32 (4) | 4 | 4 | 0 (0) | ||||||
| 8 (8) | 4 | 4 | 0 (0) | ||||||
| 64 (8) | 3 | 1 | 1 | 5 | 2 (40) | ||||
| 9 (9) | 9 | 1 | 1 | 11 | 2 (18) | ||||
| 21 (21) | 6 | 6 | 0 (0) | ||||||
| 40 (40) | 11 | 11 | 0 (0) | ||||||
| 103 | 5 | 5 | 5 (100) | ||||||
| Other | 52 | 3 | 4 | 6 | 1 | 1 | 1 | 68 | 16 (24) |
| Unknown | 160 | 1 | 40 | 12 | 6 | 3 | 222 | 62 (28) | |
| Total | 280 | 8 | 117 | 20 | 10 | 4 | 1 | 440 | 160 (36) |
These 440 E. faecalis genomes were obtained from the NCBI database (http://www.ncbi.nlm.nih.gov).
Features of Bac41-unrelated BacA homologues.
Unlike the low degree of diversity in BacL1 and BacL2 proteins, the BacA homologues are divided into five clades (Fig. 2A). Clades I and II of the Bac41 subtypes were widely distributed among the E. faecalis strains; however, clade III BacA was only found in one strain. As previously demonstrated, BacA alone does not function as a bacteriolysin; it requires cooperation with BacL1 (24). Type IIc and type III Bac41 loci lacked bacL1/bacL2 and immunity genes, respectively, suggesting that they are pseudo-bacteriocin loci. In addition, the genetic structures flanking clade IV or V bacA also did not contain any other Bac41-related genes, suggesting that they were not functional homologues (Fig. 2B). As shown in our previous study, a BacA homologue with a relatively low level of similarity was found in the Bacillus subtilis chromosome (21). Although its activity is unclear, the clade IV or V bacA gene may be present at a locus distinct from the Bac41-containing genetic element on the chromosome.
Deduced mechanism of BacA-BacI1/-BacI2 interaction for specific resistance.
The bacteriolytic proteins secreted by type VI secretion systems of Gram-negative bacteria are extensively studied examples of effector-immunity interaction. For instance, the Tae effectors of Pseudomonas aeruginosa are degradation enzymes that are injected into target bacterial cells to induce bacteriolysis (25). Tae-producing bacterial cells also express the cognate immunity Tai proteins for self-resistance to the toxicity of the corresponding effectors. A structural biology study has shown that Tai directly binds to Tae and inhibits its enzymatic activity, thus providing protection from the toxic effect. Our recent studies revealed the molecular mechanism of bacteriolysis by the Bac41 effectors BacL1 and BacA (21, 24). In particular, BacL1 acts as an endopeptidase enzyme that degrades E. faecalis peptidoglycan and binds to the cell division site on the bacterial cell wall through specific recognition of the l-Ala–l-Ala cross-bridge. On the other hand, the cell wall degradation activity of BacL1 is not sufficient to kill bacterial cells. In addition to BacL1, an as-yet-undefined triggering action of BacA is required to induce the bacteriolysis of target cells. The precise molecular function of BacA still remains unclear, except for speculation based on its amino acid sequence. The C-terminal end of BacA has a predicted transmembrane domain (Fig. 3A), and it is postulated that BacA disrupts the plasma membrane to trigger bacteriolysis. This study revealed that the unique C-terminal moiety of BacA was also a critical determinant for protection by the immunity protein BacI. The TMHMM-predicted sequence suggests that BacI1 is anchored to the plasma membrane via four transmembrane domains (Fig. 3B). The predicted sequence also suggests that aa 36 to 66 and aa 125 to 153 are exposed on the exterior of the cell. The two outside domains are thought to interact with the immunity-determining domain, namely, the C terminus of type I BacA. Similarly, BacI2 is predicted to be anchored to the plasma membrane (Fig. 3C) and its aa-54-to-78 region is thought to be exposed externally to interact with the C terminus of type IIa or type IIb BacA. There is no sequence similarity among these external regions that were presumed to be BacA-interacting domains. BacIs show sequence diversity through the entire molecule in BacI1, type IIa BacI2, and type IIb BacI2. A number of effector/immunity studies have shown that immunity proteins do not show any common sequence features compared to effectors sharing functionally conserved domains. Collectively, it is possible that BacI1 or BacI2 physically interacts with the variable C-terminal region of BacA and inhibits its membrane disruption activity to protect host E. faecalis cells. It remains unresolved precisely how BacI1 or BacI2 inhibits cognate BacA activity and, also, how BacA triggers the bacteriolysis of target cells at the molecular level. Further study is required to understand the precise molecular mechanism of the Bac41 effector/immunity system.
Xenoresistance behavior of BacI2 in type I Bac41 systems.
Our data clearly showed that, unlike type I Bac41 systems, self-resistance immunity of type IIa or IIb Bac41 systems was provided by the newly identified BacI2 but not BacI1 (Fig. 4). Interestingly, BacI2 was also conserved in type I Bac41 systems, despite BacI2 not providing immunity against type I BacA (Fig. 2B). Understanding the immunity systems was further complicated as the BacI2 proteins encoded in type I Bac41 systems were further divided into two subgroups which were similar to type IIa or type IIb BacI2 proteins, respectively (see Fig. S4B in the supplemental material). Type I Bac41pYI14 contained a BacI2 that was similar to type IIb BacI2 rather than type IIa BacI2 (Table 7). Although it no longer served to provide self-resistance, BacI2pYI14 still functioned to provide immunity against type IIb BacApMGT421 but not against type IIa BacApYI12 (Fig. 4). Although the physiological role of BacI2 in type I Bac41 systems remains obscure, it may serve as a xenoresistance mechanism to provide cells with a competitive advantage against even unrelated type IIb Bac41 proteins.
TABLE 7.
Similarity score table for BacL1, BacL2, BacA, BacI1, and BacI2 homologues represented in Fig. 2B
| Plasmid or strain | % identities of Bac41 proteins (BacL1/BacL2/BacA/BacI1/BacI2) froma: |
|||||||
|---|---|---|---|---|---|---|---|---|
| pYI14 | pMG2200 | BM4653 | SF26630 | pYI12 | DS5 | pMGT421 | T7 | |
| pYI14 | 100/100/100/100/100 | |||||||
| pMG2200 | 100/100/100/99/98 | 100/100/100/100/100 | ||||||
| BM4654 | 100/100/100/99/98 | 100/100/100/100/100 | 100/100/100/100/100 | |||||
| SF26630 | 90/99/100/98/56 | 90/100/100/97/57 | 90/100/100/97/57 | 100/100/100/100/100 | ||||
| pYI12 | 100/100/86/NA/55 | 100/100/86/NA/56 | 100/100/86/NA/56 | 89/99/85/NA/97 | 100/100/100/NA/100 | |||
| DS5 | 100/100/86/NA/54 | 100/100/86/NA/54 | 100/100/86/NA/54 | 90/100/85/NA/95 | 100/100/100/NA/96 | 100/100/100/NA/100 | ||
| pMGT421 | 100/100/85/NA/84 | 100/100/85/NA/86 | 100/100/85/NA/86 | 89/99/85/NA/54 | 100/100/97/NA/56 | 100/100/97/NA/55 | 100/100/100/NA/100 | |
| T7 | 100/100/85/NA/85 | 100/100/85/NA/85 | 100/100/85/NA/85 | 90/100/85/NA/55 | 100/100/97/NA/57 | 100/100/97/NA/56 | 100/100/100/NA/99 | 100/100/100/NA/100 |
NA, not applicable; type IIa (pYI12 and DS5) and type IIb (pMGT421 and T7) Bac41 systems lack a BacI1 homologue.
Role of Bac41 in competitive advantage in a microbial ecosystem.
Since Bac41 excluded E. faecalis without a Bac41-encoding plasmid from the population, this system was interpreted as an example of selfish behavior by the plasmids themselves, so that the Bac41-coding plasmids can be efficiently propagated among populations of E. faecalis cells (see Fig. S1 in the supplemental material). This propagation force appears to be more effective under conditions where E. faecalis cells are allowed to actively proliferate, leading to fluctuation of strain populations, because Bac41 activity is only exerted against dividing cells and not static cells (26). In fact, in the competition experiment, the predominance of Bac41-harboring cells was no longer observed after 24 h of incubation when bacterial cells were postconfluent (data not shown). This self-selection module appears to be one of the reasons why Bac41 was extensively distributed among E. faecalis isolates. In general, the possession of bacteriocin genes is considered to be a competitive tool for acquiring a niche. Here, we focused on Bac41-mediated plasmid maintenance, as shown in Fig. S1. The toxin-antitoxin (TA) system is a well-known example of a plasmid maintenance system that prevents a plasmid from being lost from host cells (47). The role of Bac41 appeared to be similar to that of the TA system. On the other hand, Bac41 excludes not only daughter cells but also unassociated cells, because the Bac41 effectors BacL1 and BacA are secreted proteins that diffuse into the environment (24). Therefore, it is proposed that Bac41 is a more effective maintenance system at the population level than is the TA system. The recent studies focusing on incompatibility of a vancomycin-resistant E. faecalis strain, V583, with commensal E. faecalis demonstrated that temperate phage or mobile genetic elements (MGEs) play a role in competition in microbial ecosystems (48, 49). Here, we suggested another example of an MGE-associated player, Bac41, that could influence the social behavior of E. faecalis. It has been described elsewhere that MGEs drive bacterial genomic evolution (50). Our results presented here suggest that an MGE component itself might also be evolved to have partial-variant-generating effector-resistance specificity.
Supplementary Material
ACKNOWLEDGMENTS
We thank Takako Inoue for technical assistance and preliminary data for this study and Elizabeth Kamei for proofreading the English of the manuscript.
Funding Statement
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology [Grant-in-Aid for Young Scientists (B) 25870116, Gunma University Operation Grants] and the Japanese Ministry of Health, Labor and Welfare (the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development [AMED], H27-Shokuhin-Ippan-008).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00348-16.
REFERENCES
- 1.Clewell DB. 1981. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev 45:409–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jett BD, Huycke MM, Gilmore MS. 1994. Virulence of enterococci. Clin Microbiol Rev 7:462–478. doi: 10.1128/CMR.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ike Y, Hashimoto H, Clewell DB. 1984. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun 45:528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray BE. 1990. The life and times of the Enterococcus. Clin Microbiol Rev 3:46–65. doi: 10.1128/CMR.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nes IF, Diep DB, Ike Y. 2014. Enterococcal bacteriocins and antimicrobial proteins that contribute to niche control. In Gilmore MS, Clewell DB, Ike Y, Shanker N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 7.Jack RW, Tagg JR, Ray B. 1995. Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nes IF, Diep DB, Holo H. 2007. Bacteriocin diversity in Streptococcus and Enterococcus. J Bacteriol 189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Tyne D, Martin MJ, Gilmore MS. 2013. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel) 5:895–911. doi: 10.3390/toxins5050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jett BD, Jensen HG, Nordquist RE, Gilmore MS. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun 60:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawa N, Wilaipun P, Kinoshita S, Zendo T, Leelawatcharamas V, Nakayama J, Sonomoto K. 2012. Isolation and characterization of enterocin W, a novel two-peptide lantibiotic produced by Enterococcus faecalis NKR-4-1. Appl Environ Microbiol 78:900–903. doi: 10.1128/AEM.06497-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita H, Tomita H, Inoue T, Ike Y. 2011. Genetic organization and mode of action of a novel bacteriocin, bacteriocin 51: determinant of VanA-type vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 55:4352–4360. doi: 10.1128/AAC.01274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todokoro D, Tomita H, Inoue T, Ike Y. 2006. Genetic analysis of bacteriocin 43 of vancomycin-resistant Enterococcus faecium. Appl Environ Microbiol 72:6955–6964. doi: 10.1128/AEM.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Tomita H, Ike Y. 2006. Bac 32, a novel bacteriocin widely disseminated among clinical isolates of Enterococcus faecium. Antimicrob Agents Chemother 50:1202–1212. doi: 10.1128/AAC.50.4.1202-1212.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita H, Fujimoto S, Tanimoto K, Ike Y. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J Bacteriol 178:3585–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomita H, Fujimoto S, Tanimoto K, Ike Y. 1997. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J Bacteriol 179:7843–7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aymerich T, Holo H, Håvarstein LS, Hugas M, Garriga M, Nes IF. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol 62:1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Bueno M, Maqueda M, Galvez A, Samyn B, Van Beeumen J, Coyette J, Valdivia E. 1994. Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J Bacteriol 176:6334–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaenhammer TR. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 20.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 21.Tomita H, Kamei E, Ike Y. 2008. Cloning and genetic analyses of the bacteriocin 41 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI14: a novel bacteriocin complemented by two extracellular components (lysin and activator). J Bacteriol 190:2075–2085. doi: 10.1128/JB.01056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsen T, Nes IF, Holo H. 2003. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl Environ Microbiol 69:2975–2984. doi: 10.1128/AEM.69.5.2975-2984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey RM, Twomey DP, Ross RP, Hill C. 2003. Production of enterolysin A by a raw milk enterococcal isolate exhibiting multiple virulence factors. Microbiology 149(Pt 3):655–664. doi: 10.1099/mic.0.25949-0. [DOI] [PubMed] [Google Scholar]
- 24.Kurushima J, Hayashi I, Sugai M, Tomita H. 2013. Bacteriocin protein BacL1 of Enterococcus faecalis is a peptidoglycan d-isoglutamyl-l-lysine endopeptidase. J Biol Chem 288:36915–36925. doi: 10.1074/jbc.M113.506618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benz J, Meinhart A. 2014. Antibacterial effector/immunity systems: it's just the tip of the iceberg. Curr Opin Microbiol 17:1–10. doi: 10.1016/j.mib.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Tomita H, Pierson C, Lim SK, Clewell DB, Ike Y. 2002. Possible connection between a widely disseminated conjugative gentamicin resistance (pMG1-like) plasmid and the emergence of vancomycin resistance in Enterococcus faecium. J Clin Microbiol 40:3326–3333. doi: 10.1128/JCM.40.9.3326-3333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunny GM, Craig RAR, Carron RLR, Clewell DB. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2:454–465. doi: 10.1016/0147-619X(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 28.Dutka-Malen S, Evers S, Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 33:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozawa Y, Tanimoto K, Fujimoto S, Tomita H, Ike Y. 1997. Cloning and genetic analysis of the UV resistance determinant (uvr) encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pAD1. J Bacteriol 179:7468–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozlowicz BK, Bae T, Dunny GM. 2004. Enterococcus faecalis pheromone-responsive protein PrgX: genetic separation of positive autoregulatory functions from those involved in negative regulation of conjugative plasmid transfer. Mol Microbiol 54:520–532. doi: 10.1111/j.1365-2958.2004.04286.x. [DOI] [PubMed] [Google Scholar]
- 31.Ike Y, Clewell DB, Segarra RA, Gilmore MS. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol 172:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirth R, An FY, Clewell DB. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol 165:831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto S, Ike Y. 2001. pAM401-based shuttle vectors that enable overexpression of promoterless genes and one-step purification of tag fusion proteins directly from Enterococcus faecalis. Appl Environ Microbiol 67:1262–1267. doi: 10.1128/AEM.67.3.1262-1267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. [DOI] [PubMed] [Google Scholar]
- 37.Ohtsubo Y, Ikeda-Ohtsubo W, Nagata Y, Tsuda M. 2008. GenomeMatcher: a graphical user interface for DNA sequence comparison. BMC Bioinformatics 9:376. doi: 10.1186/1471-2105-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krogh A, Larsson B, Heijne von G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 39.Zheng B, Tomita H, Inoue T, Ike Y. 2009. Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections: first report of the isolation and identification of the pheromone-responsive plasmids pMG2200, encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac). Antimicrob Agents Chemother 53:735–747. doi: 10.1128/AAC.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver KE, Kwong SM, Firth N, Francia MV. 2009. The RepA_N replicons of Gram-positive bacteria: a family of broadly distributed but narrow host range plasmids. Plasmid 61:94–109. doi: 10.1016/j.plasmid.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tedim AP, Ruiz-Garbajosa P, Corander J, Rodríguez CM, Cantón R, Willems RJ, Baquero F, Coque TM. 2015. Population biology of intestinal Enterococcus isolates from hospitalized and nonhospitalized individuals in different age groups. Appl Environ Microbiol 81:1820–1831. doi: 10.1128/AEM.03661-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hullahalli K, Rodrigues M, Schmidt BD, Li X, Bhardwaj P, Palmer KL. 2015. Comparative analysis of the orphan CRISPR2 locus in 242 Enterococcus faecalis strains. PLoS One 10:e0138890. doi: 10.1371/journal.pone.0138890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury SA, Nallapareddy SR, Arias CA, Murray BE, Gilligan PH. 2014. The majority of a collection of U.S. endocarditis Enterococcus faecalis isolates obtained from 1974 to 2004 lack capsular genes and belong to diverse, non-hospital-associated lineages. J Clin Microbiol 52:549–556. doi: 10.1128/JCM.02763-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuch A, Willems RJL, Werner G, Coque TM, Hammerum AM, Sundsfjord A, Klare I, Ruiz-Garbajosa P, Simonsen GS, van Luit-Asbroek M, Hryniewicz W, Sadowy E. 2012. Insight into antimicrobial susceptibility and population structure of contemporary human Enterococcus faecalis isolates from Europe. J Antimicrob Chemother 67:551–558. doi: 10.1093/jac/dkr544. [DOI] [PubMed] [Google Scholar]
- 45.Raven KE, Reuter S, Gouliouris T, Reynolds R, Russell JE, Brown NM, Török ME, Parkhill J, Peacock SJ. 2016. Genome-based characterization of hospital-adapted Enterococcus faecalis lineages. Nat Microbiol 1:15033. doi: 10.1038/nmicrobiol.2015.33. [DOI] [PubMed] [Google Scholar]
- 46.Kurushima J, Nakane D, Nishizaka T, Tomita H. 2015. Bacteriocin protein BacL1 of Enterococcus faecalis targets cell division loci and specifically recognizes l-Ala2-cross-bridged peptidoglycan. J Bacteriol 197:286–295. doi: 10.1128/JB.02203-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuster CF, Bertram R. 2013. Toxin-antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol Lett 340:73–85. doi: 10.1111/1574-6968.12074. [DOI] [PubMed] [Google Scholar]
- 48.Duerkop BA, Clements CV, Rollins D, Rodrigues JLM, Hooper LV. 2012. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci U S A 109:17621–17626. doi: 10.1073/pnas.1206136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaca AO, Gilmore MS. 2016. Killing of VRE Enterococcus faecalis by commensal strains: evidence for evolution and accumulation of mobile elements in the absence of competition. Gut Microbes 7:90–96. doi: 10.1080/19490976.2015.1127482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer KL, van Schaik W, Willems RJL, Gilmore MS. 2014. Enterococcal genomics. In Gilmore MS, Clewell DB, Ike Y, Shanker N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA: Available at: http://www.ncbi.nlm.nih.gov/books/NBK190425/. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.