Abstract
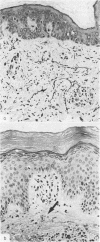
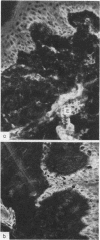
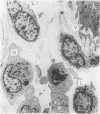
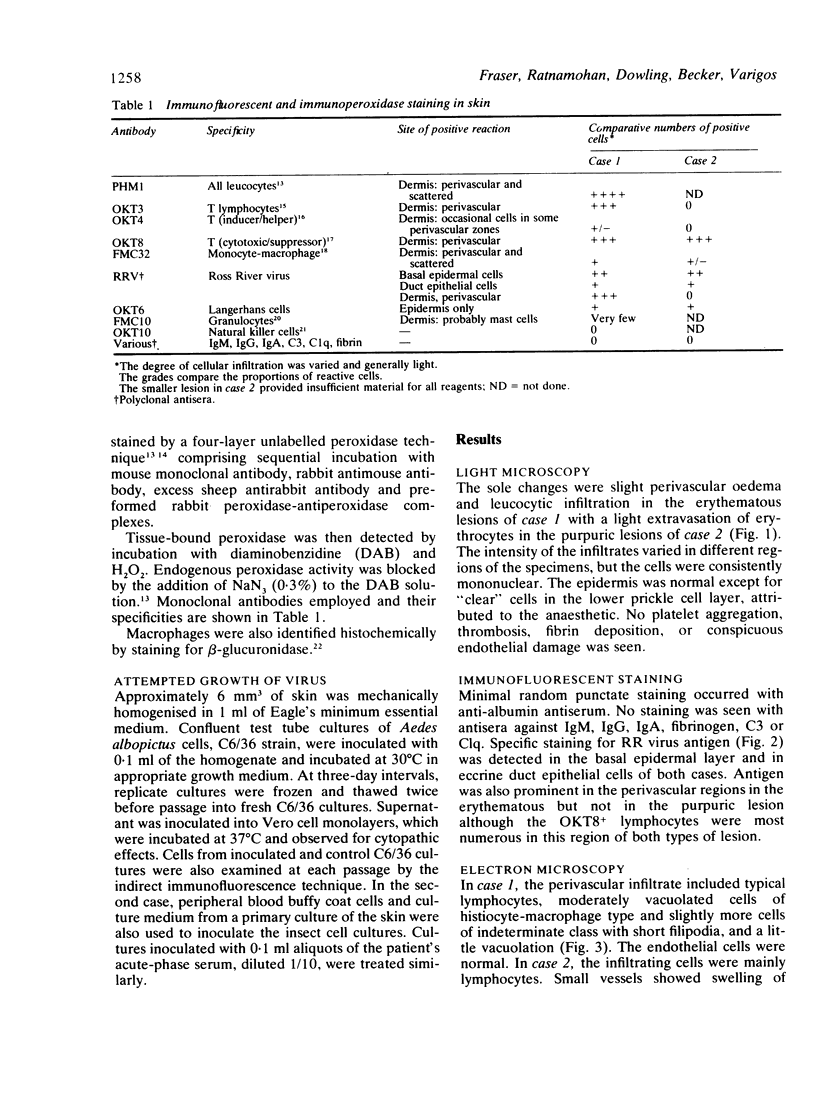
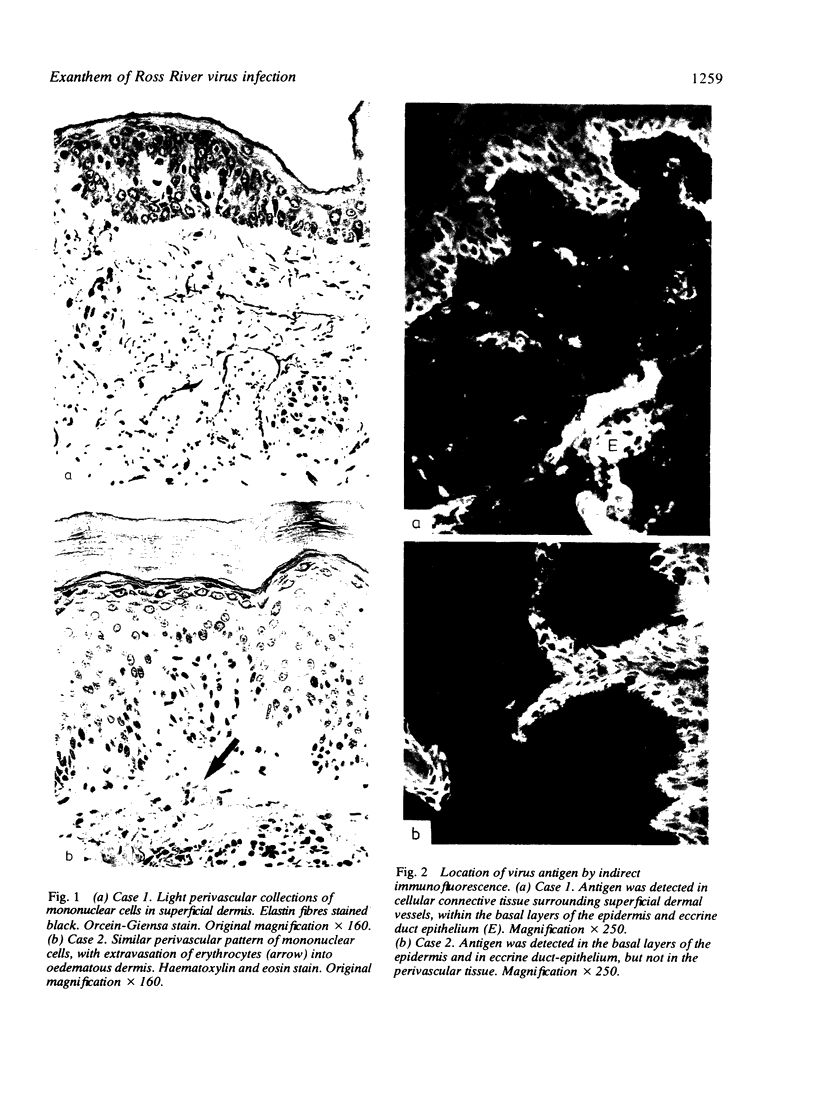
The exanthem of epidemic polyarthritis, a disease caused by Ross River (RR) virus, was examined three days after onset of the common erythematous and the rare purpuric forms of the eruption. The dermis showed a light perivascular infiltrate of mononuclear cells in both, with extravasation of erythrocytes in the latter. No immunoglobulins (IgM, IgG, IgA) or complement components (Clq, C3) were detected. Most of the infiltrating cells were T lymphocytes of the T suppressor-cytotoxic class. Their perivascular location, the scarcity of other lymphocytes or phagocytes, and rapid resolution of the rash indicated that the T lymphocytes were responsible for cytotoxic destruction of virus-infected cells. A few monocyte-macrophage cells were identified in the perivascular infiltrate. RR virus antigen was found in the basal epidermal and eccrine duct epithelial cells of both types of lesion and in the perivascular zone of the erythematous lesion, but appeared to have been eliminated from this region in the purpuric lesion. It is suggested that secondary effects of the T-cytotoxic reaction on nearby capillaries are responsible for erythema, oedema and purpura in the exanthem.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON S. G., FRENCH E. L. An epidemic exanthem associated with polyarthritis in the Murray valley, 1956. Med J Aust. 1957 Jul 27;44(4):113–117. doi: 10.5694/j.1326-5377.1957.tb57732.x. [DOI] [PubMed] [Google Scholar]
- Becker G. J., Hancock W. W., Kraft N., Lanyon H. C., Atkins R. C. Monoclonal antibodies to human macrophage and leucocyte common antigens. Pathology. 1981 Oct;13(4):669–680. doi: 10.3109/00313028109086640. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Harrist T. J., Murphy G. F., Mihm M. C., Jr T cell subsets and Langerhans cells in lichen planus: in situ characterization using monoclonal antibodies. Br J Dermatol. 1981 Dec;105(6):617–622. doi: 10.1111/j.1365-2133.1981.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Brooks D. A., Zola H., McNamara P. J., Bradley J., Bradstock K. F., Hancock W. W., Atkins R. C. Membrane antigens of human cells of the monocyte/macrophage lineage studied with monoclonal antibodies. Pathology. 1983 Jan;15(1):45–52. doi: 10.3109/00313028309061401. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Doherty R. L., Fraser J. R., French E. L., Muirden K. D. Epidemic polyarthritis: a cytological, virological and immunochemical study. Aust N Z J Med. 1975 Oct;5(5):450–457. doi: 10.1111/j.1445-5994.1975.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Brickell P., Tidman N., McConnell I., Hoffbrand A. V., Janossy G. Increased numbers of cells with suppressor T cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol. 1981 Feb;43(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. Immunoregulatory T cells in mononucleosis and toxoplasmosis. N Engl J Med. 1981 Jul 23;305(4):228–228. doi: 10.1056/NEJM198107233050422. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., Cunningham A. L., Clarris B. J., Aaskov J. G., Leach R. Cytology of synovial effusions in epidemic polyarthritis. Aust N Z J Med. 1981 Apr;11(2):168–173. doi: 10.1111/j.1445-5994.1981.tb04226.x. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., NAKAJIMA Y., FISHMAN W. H. THE CYTOLOGIC DEMONSTRATION OF BETA-GLUCURONIDASE EMPLOYING NAPHTHOL AS-BI GLUCURONIDE AND HEXAZONIUM PARAROSANILIN; A PRELIMINARY REPORT. J Histochem Cytochem. 1964 Apr;12:293–297. doi: 10.1177/12.4.293. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Becker G. J., Atkins R. C. A comparison of fixatives and immunohistochemical technics for use with monoclonal antibodies to cell surface antigens. Am J Clin Pathol. 1982 Dec;78(6):825–831. doi: 10.1093/ajcp/78.6.825. [DOI] [PubMed] [Google Scholar]
- Heggie A. D. Pathogenesis of the rubella exanthem: distribution of rubella virus in the skin during rubella with and without rash. J Infect Dis. 1978 Jan;137(1):74–77. doi: 10.1093/infdis/137.1.74. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Macfarlan R. I., Burns W. H., White D. O. Two cytotoxic cells in peritoneal cavity of virus-infected mice: antibody-dependent macrophages and nonspecific killer cells. J Immunol. 1977 Nov;119(5):1569–1574. [PubMed] [Google Scholar]
- Macfarlan R. I., White D. O. Further characterization of natural killer cells induced by Kunjin virus. Aust J Exp Biol Med Sci. 1980 Feb;58(1):77–89. doi: 10.1038/icb.1980.8. [DOI] [PubMed] [Google Scholar]
- McMillan E. M., Beeman K., Wasik R., Everett M. A. Demonstration of OKT 6-reactive cells in mycosis fungoides. J Am Acad Dermatol. 1982 May;6(5):880–887. doi: 10.1016/s0190-9622(82)70077-5. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rosen L., Gubler D. J., Bennett P. H. Epidemic polyarthritis (Ross River) virus infection in the Cook Islands. Am J Trop Med Hyg. 1981 Nov;30(6):1294–1302. doi: 10.4269/ajtmh.1981.30.1294. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Research on dengue during World War II. Am J Trop Med Hyg. 1952 Jan;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Irigoyen O., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol. 1980 Dec;125(6):2402–2408. [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Zola H., McNamara P., Thomas M., Smart I. J., Bradley J. The preparation and properties of monoclonal antibodies against human granulocyte membrane antigens. Br J Haematol. 1981 Jul;48(3):481–490. doi: 10.1111/j.1365-2141.1981.tb02740.x. [DOI] [PubMed] [Google Scholar]