Abstract
Objective
To assess serum B12, folate and the associated homocysteine (Hcy) levels among women of childbearing age in the UK and examine their association with dietary intake in relation to the UK Recommended Nutrient Intakes (RNIs) for B12 and folate.
Design
Cross-sectional study.
Setting
Data from two publicly available National Diet and Nutrition Surveys (NDNS 2000/2001 and 2008/2012) were used. These were population-based surveys of randomly selected samples of adults which were carried out in their households.
Participants
Women of childbearing age (aged 19–39 years), representative of the UK population. Those who were pregnant or breastfeeding were excluded.
Outcome measures
The associations between micronutrient intakes and blood levels of B12, folate and Hcy were assessed by correlation and stepwise linear regression. B12 intake was divided into quintiles and plotted against blood B12 and Hcy concentrations to determine the threshold of any associations.
Results
299 women from the first NDNS cohort had complete intake and biomarker data. The prevalence of serum vitamin B12 (≤150 pmol/L) and serum folate (≤10 nmol/L) deficiency and hyperhomocysteinemia (≥12 µmol/L) was 12.4%, 6.4% and 21.2%, respectively, despite seemingly adequate B12 intakes (median 3.8 μg/day, 96% consumed more than the UK RNI of 1.5 μg/day). B12 concentrations increased across all quintiles of intake with serum levels in quintiles 4 and 5 (median intake 4.9 and 7.1 μg/day, respectively) significantly higher than quintile 1. However, Hcy concentrations levelled off between quintiles 4 and 5. Comparison of micronutrient intake between the two surveys found that folate intake has reduced in the more recent cohort.
Conclusions
The UK RNI for B12 intake should be increased for women of childbearing age with intakes of around 5–7 μg/day likely to be associated with stable biomarker levels. B12 levels should also be measured in women preconceptionally or in early pregnancy given the high rates of deficiency.
Keywords: NUTRITION & DIETETICS
Strengths and limitations of this study.
Two publicly available data sets from the British National Diet and Nutrition Surveys (NDNS) were used to investigate the association between dietary intake of B12 and folate and their blood concentrations in women of childbearing age.
The women sampled in the surveys were representative of UK adults; therefore, the findings can be generalised to the wider UK population.
Information about the participants, including demographics, dietary intakes, blood results and anthropometry, was used for detailed analysis of the outcomes of interest.
The availability of two separate NDNS data sets allowed comparison of micronutrient intakes over the last 15 years in young women.
Blood micronutrient concentrations were not analysed in the more recent survey. Therefore, the associations observed in the first survey could not be replicated.
Introduction
Vitamin B12 (B12), also known as cobalamin, is a micronutrient essential for cellular growth, differentiation and development.1 Along with folate, B12 is necessary for the synthesis of DNA, RNA, lipids and protein, and an essential step in this process is the conversion of homocysteine (Hcy) to methionine.2 Therefore, a deficiency of either B12 or folate leads to increased Hcy which can have significant clinical implications such as cardiovascular disease and atherosclerosis in adults.3 4
During pregnancy, low circulating levels of B12 or folate have been associated with complications such as neural tube defects (NTDs),5 spontaneous abortion,6 premature birth7 and possibly low birth weight.8 Mandatory folic acid fortification in North America has resulted in reduction of NTD by over 40% in the last 10 years,9 but there has been a tripling of the condition attributable to B12 deficiency during the same time frame in this population.10 Maternal hyperhomocysteinemia has been linked to early pregnancy losses,11 pre-eclampsia12 and small for gestational age babies.13 Although the exact mechanisms are not known, some of these effects may be mediated by vascular compromise to the fetus and insufficient placental development which occurs in the early embryonic period.14 As around half of the pregnancies are unplanned, even in developed countries,15 there is increasing attention on optimising the nutrient status of women in the periconceptional period.
B12 deficiency is prevalent during pregnancy as shown by a large systematic review recently conducted by our group.8 We showed that the rates were in the order of 20–30% in all three trimesters across the world, with particular high rates in studies from the Indian subcontinent, Eastern Mediterranean and South American regions.16–18 A longitudinal study has shown that cobalamin levels can decrease by around 10–20% from preconception to the first and second trimesters, respectively.19 However, there are no studies in the UK that assessed the B12 intake or circulating levels in women of reproductive age prior to conception.
The UK Department of Health (DOH) has stipulated that the Recommended Nutrient Intake (RNI) for B12 and folate in adults is 1.5 and 200 μg per day, respectively, with no different recommendations for women (pregnant or otherwise) and the elderly.20 These recommendations were published in 1991 and state that the RNI for B12 ‘represent the level of intake considered likely to be sufficient to meet the requirements of 97.5% of the group’.21 The evidence for this RNI is from reports in the 1970s, which estimated the average requirement to prevent diet-related B12 deficiency and megaloblastic anaemia.22–24 B12 deficiency in these studies was arbitrarily defined as <150 pmol/L without use of any functional markers of B12 deficiency.25 26 Thus, there are little contemporary data to support how accurately the recommended intakes correlate with serum values of B12 and functional indicators such as Hcy. The primary aim of this study is to determine the serum B12, folate and Hcy status of women of childbearing age in the UK and to assess the correlation between estimated B12 intake and blood concentrations of B12 and Hcy using the data from two British National Diet and Nutrition Survey (NDNS) in 2000/2001 and 2008/2012. The secondary aim is to compare the nutritional intake between the two NDNS cohort to provide more recent data on B12 intake.
Methods
Subjects
We used data collected from the two British NDNS between July 2000 and June 2001 and 2008–2012. The surveys provide detailed quantitative assessment of nutritional status and laboratory results of the participants. The methods used in the survey have previously been described in detail.27–29 The samples were made up of randomly selected adults aged 19–64 years living in private households, who were representative of the UK adult population. Their selection was done by a multistage, stratified, probability sampling with postal sectors as first stage units. If there were more than one adult in the same household, one was selected randomly. Women who were pregnant or breast feeding were excluded.
The selection of the participants included in our analysis is provided in online supplementary figures S1 and S2. For the first survey (2000/2001), the fieldwork was carried out over a 12-month period, with respondents being surveyed over four 3-month periods to account for seasonal variations in nutritional behaviour and content. Out of 3704 potentially eligible adults identified for the study, 299 women between 19 and 39 years of age (known as women of ‘childbearing age’ for the purpose of our analysis) with complete dietary and biomarker information were included in the final analysis. In an independent study,30 no evidence of was found of non-response bias in this NDNS data.
bmjopen-2016-011247supp_figures.pdf (351.9KB, pdf)
The second survey (2008/2012) consisted of 2424 adults of whom 395 were women of childbearing age. Unfortunately, blood samples were only obtained from 157 of these women (ie, 39.7% of all females aged 19–39 years). The main reasons for this low return of blood samples were ‘no nurse visit’, ‘participant refused’ and ‘blood sample inapplicable’. Owing to the potential bias from a sample of women not representative of the larger NDNS cohort, we only included and compared the micronutrient intakes from the two surveys to assess the secular trend.
Assessment of dietary intake
This has been described in detail elsewhere.27 Briefly, dietary assessment for each participant was done by a two-stage process: (1) two face-to-face interviews using computer-assisted personal interviewing methods, and (2) a 7-day dietary record using weighed food diaries. Each participant was provided with a set of Soehnle Quanta digital food scales and two recording diaries (for use at home and outside). Additional information was obtained about the use of medicines, vitamin and mineral supplements. Information provided in the food diaries was later used to determine nutrient intakes by linking to the Food Standards Agency nutrient database, which holds details for 56 nutrients for each of 6000 food codes.27
Laboratory methods
Venous blood samples were taken at the non-fasting state by trained phlebotomists in participants' homes. Serum folate and B12 measurements were performed on the Abbott IMx semiautomated analyser, which uses microparticle enzyme immunoassay (MEIA) technology (z). Quality control consisted of an internal pooling of serum samples with each run for use as a drift control, and an external quality assessment by the UK National External Quality Assessment Service (NEQAS). Outlying results were defined as serum B12 level >1000 pmol/L and serum folate >60 nmol/L and were excluded. Plasma Hcy measurements were performed by the Abbott IMx assay on the IMx analyser. Quality control consisted of participation in an international external quality assessment scheme based in Denmark31 and by the manufacturer's QV samples.
Statistical analysis
Statistical analysis was performed with SPSS V.22.0 (IBM SPSS Statistics for Windows, Version 22.0 (program). Armonk, NY: IBM Corp, Released 2013). For continuous variables (eg, mean B12/folate intakes and levels between RNI threshold groups), the Student’s t-test and for categorical variables (eg, B12 and folate deficiency rates), the χ2 test for independence or the Fisher's exact test was used. Stepwise multiple linear regression analysis with each of serum B12, folate and plasma Hcy as the dependent variable was done, with predictors entered or removed following the criteria: probability of F to enter ≤0.050, probability of F to remove ≥0.100. The regression models included the following covariates: age, body mass index (BMI), total cholesterol:high-density lipoprotein (TC:HDL) ratio, alcohol intake, smoking status, oral contraceptive use, vegan/vegetarian, serum folate and where appropriate daily folate intake (with supplements), daily B12 intake (with supplements) and serum B12 levels. Race was not included in the analysis as the majority of the participants (95%) were white. To determine the trend of B12 and Hcy across the spectrum of B12 intakes, we divided the cohort into quintiles of B12 intake. One-way analysis of variance (ANOVA) with Tukey's post hoc test was applied to compare the serum levels against the lowest quintile. Logistic regression was then done for incremental B12 intake thresholds of 0.25 μg to determine predictors of B12 deficiency and hyperhomocysteinemia. In order to estimate the prevalence of inadequate B12 intake in our population and observe how this was related to abnormal biomarker levels, we used the Estimated Average Requirement (EAR) cut-point method.32 The EAR was calculated as 1.25 μg/day based on a coefficient of variation of 10% below the UK RNI.25
The definitions for micronutrient deficiencies were as follows: serum B12≤150 pmol/L,33 serum folate ≤10 nmol/L34 and red cell folate <350 nmol/L.34 The upper limit of normal for plasma Hcy in non-pregnant adults aged 15–65 has been variably defined as 12 µmol/L in non-pregnant adults with folic acid fortification or supplementation35 and 15 µmol/L in those without fortification or supplementation, which applies to our population. However, Hcy levels above 10.7 µmol/L in women during preconception has been associated with pre-eclampsia, prematurity and very low birthweight infants if they become pregnant.12 In addition, other experts recommend <12 µmol/L for all adults.36 Therefore, we have presented results for 12 and 15 µmol/L and stated these clearly throughout.
Results
From the NDNS 2000/01 cohort, 299 women of childbearing age had complete dietary and serum micronutrient level results. The demographics and clinical characteristics of these women are shown in table 1. Their mean age was 31.6 years and BMI 25.3 kg/m2. To determine how the B12/folate intakes women of childbearing age compared with older women from the same NDNS survey, we analysed these parameters in women aged 40–64 years. The younger women consumed significantly less B12 and folate (median 3.83 vs 5.16 and 237 vs 279 μg/day, respectively, p<0.001 for both) and consisted of more vegetarians/vegans (8.7% vs 4.2%, p<0.05) and less B12 supplement users (10.4% vs 16.2%, p<0.05). Folic acid supplement use was the same (data not shown).
Table 1.
Demographics and B12 and folate intakes of women of childbearing age (NDNS 2000/2001 cohort)
| Female 19–39 years | All subjects (n=299) |
|---|---|
| Age (years) | 31.6±5.7 |
| BMI (kg/m2) | 25.4±5.2 |
| Obesity, n (%) | 46 (15.7) |
| Current smokers, n (%) | 117 (39.1) |
| Regular alcohol drinkers, n (%) | 267 (89.3) |
| Oral contraceptive use, n (%) | 103 (34.4) |
| Ethnicity, n (%) | |
| White | 283 (94.6) |
| Afro-Caribbean | 3 (1.0) |
| Asian | 8 (2.7) |
| Other | 5 (1.7) |
| Vegetarians, n (%) | 26 (8.7) |
| B12 supplement users, n (%) | 31 (10.4) |
| B12 intake, diet only (μg/day) | 3.82 (2.75, 5.02) |
| B12 intake, diet+supplements (μg/day) | 3.83 (2.82, 5.20) |
| Folic acid supplement users, n (%) | 32 (10.7) |
| Folate intake with supplements (μg/day) | 237 (177, 315) |
Continuous variables are mean±SD or median (IQR).
Categorical variables are n (%).
BMI, body mass index; NDNS, National Diet and Nutrition Surveys.
The blood levels of B12, folate and Hcy in women of childbearing age and according to categories of UK RNI for B12 intake (adequate/inadequate) are given in table 2. The median serum B12 concentration was 241 pmol/L. Overall, 12% of women were B12 deficient (<150 pmol/L), despite the median B12 intake of the deficient women being nearly two times the UK RNI (2.96 μg/day; data not shown). In total, 3.7% of the surveyed population had B12<150 pmol/L and Hcy>12 µmol/L, with a significantly higher proportion having the combination of abnormalities when their estimated was lower than the UK RNI (9.1% vs 3.5%, p=0.001). There is evidence that B12 levels <258 pmol/L may be indicative of B12 deficiency in certain individuals with concomitant elevation of Hcy and methylmalonic acid (MMA).37 In total, 44.0% of women had B12 levels in this borderline range of 150–258 pmol/L. In this subgroup, mean Hcy levels were significantly higher than the group with B12>258 pmol/L (10.4 vs 9.2 µmol/L, p=0.02) despite similar folate levels (21.4 vs 22.0 nmol/L, p=NS).
Table 2.
Comparison of B12, folate and plasma homocysteine concentrations in women according to the UK RNI for vitamin B12 intake
| Female 19–39 years | All subjects | UK RNI (μg/day) |
p value* | |
|---|---|---|---|---|
| <1.5 | ≥1.5 | |||
| Number (%) | 299 (100) | 11 (3.7) | 288 (96.3) | |
| B12 intake, diet only (μg/day) | 3.82 (2.75, 5.02)† | 1.29 (0.64, 1.46) | 3.86 (2.86, 5.09) | <0.001 |
| B12 intake, diet+supplements (μg/day) | 3.83 (2.82, 5.20) | 1.29 (0.98, 1.46) | 3.92 (2.88, 5.32) | <0.001 |
| Serum B12 (pmol/L) | 241 (188, 324) | 169 (153, 256) | 244 (189, 325) | 0.05 |
| B12 deficiency (<150 pmol/L), n (%) | 36 (12.0) | 2 (18.2) | 34 (11.8) | NS |
| Serum folate (nmol/L) | 19.5 (14.1, 26.7) | 14.3 (13.6, 21.3) | 19.7 (14.2, 27.0) | NS |
| Serum folate deficiency (<10 nmol/L), n (%) | 18 (6.1) | 0 (0) | 18 (6.3) | NS |
| Red cell folate (nmol/L) | 584 (473.9, 750.6) | 460 (372, 739) | 585 (478, 751) | NS |
| Red cell folate deficiency (<350 nmol/L), n (%) | 13 (4.4) | 2 (18.2) | 11 (3.8) | NS |
| Hcy (μmol/L) | 9.4 (9.1, 9.8) | 11.9 (9.6, 14.3) | 9.2 (7.8, 11.4) | <0.05 |
| High Hcy (>12 μmol/L), n (%) | 62 (21.2) | 5 (50) | 57 (20.1) | <0.05 |
| High Hcy (>15 μmol/L), n (%) | 24 (8.2) | 2 (20) | 22 (7.8) | NS |
*Comparison between lower and higher B12 intake groups. For categorical variables, Student’s t-test was used (after log transformation); for continuous variables, Fisher’s exact test was used.
†Median, 25–75th centile in parentheses (all such values).
Hcy, homocysteine; NS, not significant; UK RNI, UK Recommended Nutrient Intake.
The plasma Hcy concentrations were higher in the lower intake group (11.9 vs 9.2 µmol/L, p<0.05) although hyperhomocysteinemia (Hcy>12 µmol/L) was present in 20% of women with apparently adequate B12 intake (table 2). Serum and red cell folate deficiency rates were 6.1% and 4.4%, respectively, in the whole population. There were no differences in the folate deficiency rates between above and below the UK RNI B12 intake groups. In total, 34.4% of the women were taking the oral contraceptives and their B12 values were lower than those who were not (median 211.5 vs 267.5 pmol/L, p<0.001).
In total, 8.7% of women in the whole cohort were vegetarian or vegan and their median dietary intake of B12 was non-significantly lower than non-vegetarians (2.95 vs 3.87 μg/day), while folate consumption in the former group was higher (see online supplementary table S1). Vegetarians had lower serum B12 concentrations (median 192 vs 248 pmol/L, p<0.01) but their folate or Hcy concentrations did not vary significantly.
Biochemical indices in vegetarian and vegan women of child-bearing age compared to non-vegetarians
bmjopen-2016-011247supp_table.pdf (60.4KB, pdf)
Predictors of B12, folate and Hcy
There was a positive correlation between serum B12 values and daily B12 intake (Pearson's r=0.27, p<0.001) (figure 1). Simple linear regression analyses of the predictors of B12, folate and Hcy are shown in table 3. After adjusting for the likely confounders, daily B12 and folic acid intakes were positive predictors of serum B12 (β=0.28, p<0.001) and serum folate (β=0.33, p<0.001), respectively. Along with age, serum B12, serum folate and B12 intake were independent predictors of Hcy, though it would appear that serum folate is strongest based on the value of the β coefficient (table 3).
Figure 1.
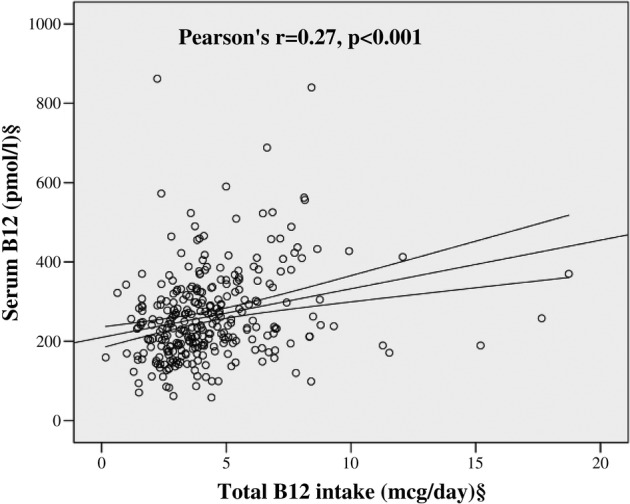
Correlation between daily B12 intake and serum B12 values. §Log transformed for statistical comparisons.
Table 3.
Multiple linear regression analysis of predictors of serum B12, folate and homocysteine
| Variables | Serum B12* |
Serum folate |
Homocysteine* |
|||
|---|---|---|---|---|---|---|
| β coefficient | p Value | β coefficient | p Value | β coefficient | p Value | |
| Age | – | NS | – | NS | 0.18 | 0.001 |
| BMI | – | NS | – | NS | – | NS |
| Smoking | –0.12 | <0.05 | – | NS | – | NS |
| Alcohol | – | NS | – | NS | – | NS |
| Oral contraceptive use | −0.29 | <0.001 | 0.11 | <0.05 | – | NS |
| TC:HDL ratio* | – | NS | – | NS | – | NS |
| Vegetarian or vegan diet | −0.18 | <0.01 | – | NS | – | NS |
| B12 supplement use | – | NS | Not included | NA | – | NS |
| Daily B12 intake* | 0.28 | <0.001 | Not included | NA | –0.16 | 0.001 |
| Folic acid supplement use | Not included | NA | 0.18 | <0.01 | – | NS |
| Daily folate intake* | Not included | NA | 0.33 | <0.001 | – | NS |
| Serum B12* | – | NS | −0.20 | <0.001 | ||
| Serum folate* | – | NS | −0.35 | <0.001 | ||
*Log transformed for statistical comparison.
–, Tested but not significant in the model;
BMI, body mass index; NA, not applicable; NS, not significant; TC:HDL, total cholesterol:high-density lipoprotein ratio.
Relationship between B12 intake and associated biomarkers
In total, 5 out of 299 (1.7%) women consumed below the EAR of 1.25 μg/day, but none of them had B12 levels <150 pmol/L. Conversely, in the 98.3% of women with ‘adequate’ EAR category for B12 consumption, 12.2% of them had serum levels below 150 pmol/L.
In order to determine the trend of blood B12 and Hcy concentrations with increasing intakes of B12, we divided the cohort in quintiles of B12 intake. The median levels in each quintile and biomarker values are represented in figures 2A, B. Women in quintiles 4 and 5 (median intake 4.9 and 7.1 μg/day, respectively) had significantly higher mean B12 and lower mean Hcy concentrations than quintile 1 (291 and 322 vs 229 pmol/L; 9.0 and 9.0 vs 11.4 µmol/L, respectively). Additionally, the Hcy levels appear to level off between quintiles 4 and 5, suggesting that increasing intakes above 5 μg/day is unlikely to provide higher B12 at the tissue level (figure 2B). To confirm this, we performed logistic regression to determine the intake threshold at which the odds of hyperhomocysteinemia would reduce significantly after correcting for confounders. Using 0.25 μg increments, a threshold of 4.75 μg/day significantly reduced the odds of Hcy>12 µmol/L (adjusted OR (AOR) 0.35, 95% CI 0.14 to 0.88). When serum B12 was added to the model, the significance was lost, suggesting that the influence of dietary B12 intake on circulating Hcy is mediated through serum B12 values in a folate-replete population (data not shown).
Figure 2.
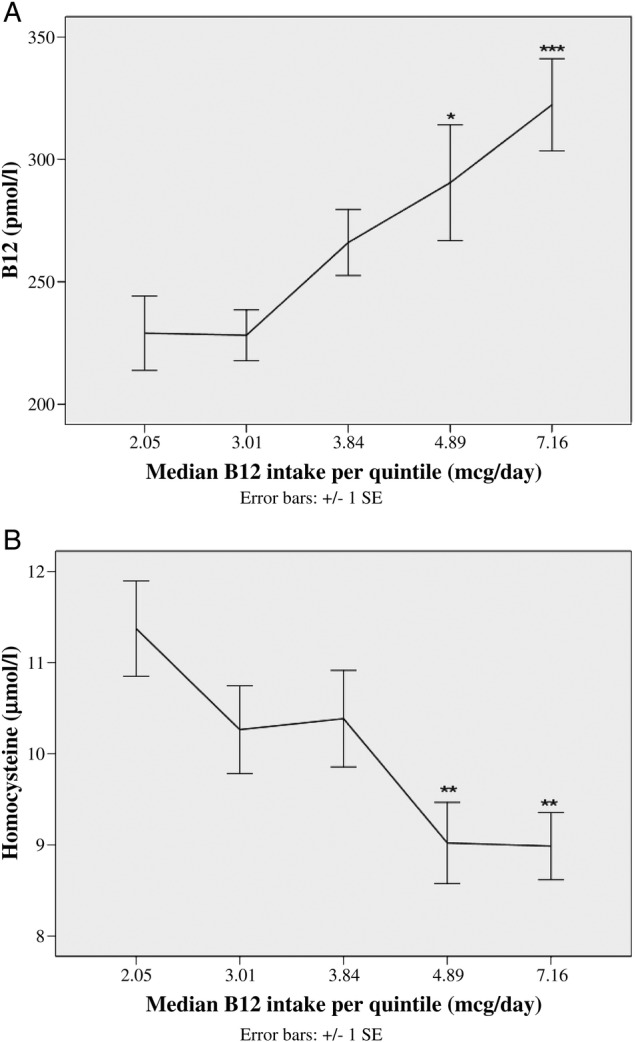
Relationship between B12 intake in quintiles and (A) mean serum B12 and (B) mean plasma homocysteine concentrations. Mean±SEM values are plotted against the median B12 intake in each quintile. One-way analysis of variance (ANOVA) test used to compare the means between the quintiles (after log transformation) and Tukey’s post hoc analysis done. Mean biomarker levels differed as compared with quintile 1 as follows: *p<0.05; **p<0.01; ***p<0.001.
Micronutrient intake: comparisons between NDNS 2000/2001 and 2008/2012 data
Comparison of the mean nutrient intake among young women between the two surveys is shown in table 4. The proportion of women consuming below the UK RNI for folate increased in the more recent survey (44% vs 33%, p<0.01) and median intake levels fell by 14% (206 vs 235 pmol/L, p<0.001). There were no significant differences in the B12 intake values.
Table 4.
Comparison between folic acid and B12 intakes between the NDNS 2000/2001 and 2008/2011 cohorts
| Female 19–39 years | NDNS 2000/2001 cohort | NDNS 2008/2011 cohort |
|---|---|---|
| Number | 299 | 395 |
| Folic acid supplement users, n (%) | 32 (10.7) | 12 (3.0)***,† |
| Folate intake, diet only (μg/day) | 234.9 (175.7, 302.7)‡ | 205.8 (162.3, 261.2)*** |
| n (%) consuming below UK RNI of folate | 99 (33.1) | 173 (43.8)** |
| B12 supplement users, n (%) | 31 (10.4) | 33 (8.4) |
| B12 intake, diet only (μg/day) | 3.82 (2.75, 5.01) | 3.78 (2.64, 4.84) |
| n (%) consuming below UK RNI of B12 | 11 (3.7) | 24 (6.1) |
Significance: **p<0.01; ***p<0.001
†p Values from independent-samples t-test for continuous variables (after log transformation) or χ2 test for categorical variables.
‡Median, 25–75th centile in parentheses (all such values).
UK RNI, UK Recommended Nutrient Intake; NDNS, National Diet and Nutrition Survey.
Discussion
Our study shows contemporary data of B12 and folate intakes and serum levels in population-based nutritional surveys involving women of childbearing age, who are representative of the UK population. The key findings are that, despite an apparent adequate daily intake of B12, a high proportion of women have B12 deficiency and hyperhomocysteinemia.
Optimum B12 intake and biomarker levels
Our data showed positive correlation between B12 intake and blood levels with a trend of increasing B12 concentration even in intakes up to 7 μg/day, although the corresponding reductions in Hcy levels level off around the intake of 5–7 μg/day. Additionally, the consumption of around 4.75 μg/day was independently associated with a decrease in the odds of hyperhomocysteinemia. Our findings are supportive of the dose–response relationship between intake and blood levels of B12 found previously, which showed doubling B12 intakes would increase B12 concentrations by around 10%.38
A daily intake of 4–10 μg has been suggested by other studies to stabilise levels of B12, Hcy and the associated MMA in adults,39 40 but more evidence is needed before extrapolating these figures to women of childbearing age due to the different requirements and implications should they become pregnant. In a randomised controlled trial where all subjects consumed 8.6 μg/day of B12, pregnant women had higher holotranscobalamin (holoTC):total B12 ratios than non-pregnant, non-lactating controls, suggesting that such high intakes were required to provide sufficient supply to the developing fetus.41 More research is required to decide on the upper limit of B12 intake in women before and during pregnancy, as higher B12 intake in the third trimester was positively associated with offspring birth weight in high-BMI women (who are already at high risk of having macrosomic babies), although circulating B12 levels were not reported here.42 There is also no consistent evidence that increased B12 intake or supplementation is associated with reduction in the prevalence of subclinical B12 deficiency in adults (particularly neurocognitive decline in the elderly).43 44 Therefore, until the availability of further studies, our data call for revision of the UK RNI (and EAR) for B12 to be at least in line with the current European recommendations (4.5 μg/day).24
The estimated B12 intake in preconceptional or women in early pregnancy and specifically its relationship with serum levels has not been widely studied. In developing countries, over 50% of women of reproductive age do not meet the RNIs for B1245 46 and separate surveys from three developing countries (Turkey, Iran and China) found B12 deficiency rates of 21–23%.47–49 An Australian study on young females found a similar rate to ours (11.4%), albeit using a lower threshold of 120 pmol/L.50 As the B12 levels fall by around 10% from preconception to early pregnancy,19 if we extrapolate our findings, the proportion of pregnant women with B12 levels below 150 pmol/L is likely to be much higher. More than a third of vegetarian/vegan women in our study had B12 deficiency, which was in keeping with studies from other UK adult population51 and elsewhere.52 Thus our findings highlight the need for specific advice for the vegetarian/vegan population about potential sources of B12, as well as recommending them to have their B12 levels checked (and corrected) if they are planning pregnancy.
Folate intake
Our study shows that consumption of dietary folate has fallen by around 14% in the 10 years between the two surveys and nearly 50% of women are now consuming below the UK RNI. The Scientific Advisory Committee on Nutrition within the UK Food Standards Agency has recommended mandatory folic acid fortification of food products to the UK DOH,53 which, if implemented, would increase folate intake and consequently serum levels in the population as it did in the USA.54 However, if mandatory fortification does occur, it is possible that improving folate levels can reduce B12 levels due to usage of the available B12.55 In addition, B12 deficiency is the strongest driver of Hcy in a folate-replete population.56 Therefore fortification of food products with B12 together with folate should be considered if there is to be a policy change in the UK or, as a minimum, B12 supplements recommended for women in the peri-conceptional period, especially if they are at high risk of deficiency.
The strengths of our study are that this is the first study of its kind from the UK to evaluate B12 and folate among women in the preconceptional stage. Extensive data on these women including anthropometry, biochemical markers and dietary information allowed comparison of intake and serum levels adjusted for possible confounders. There were three important limitations of this data set: (1) lack of sufficient biochemical data in the second survey, (2) lack of data on other biomarkers such as holoTC or MMA and (3) lack of haematological and clinical information relevant to the effects of B12. Since we were able to compare the nutrient intakes between the two cohorts, which was predictive of serum levels in the earlier survey, we believe that the availability of serum B12 in the latter cohort may not necessarily change the findings. Hcy is a readily available marker in clinical practice as opposed to holoTC and MMA. Therefore, our findings are applicable to wider clinical practice. Any future prospective studies involving preconceptional or pregnant women should include more clinical information as well as these biomarkers for a better evaluation of B12 status. In conclusion, our study supports revision of the UK RNI to at least match the European recommendations and also calls for assessing maternal B12 status in preconception or early pregnancy.
Acknowledgments
The authors acknowledge the British National Diet and Nutrition Survey for kindly providing the public database for detailed quantitative assessment of the samples, nutritional, dietary and laboratory characteristics.
Footnotes
Contributors: PS conceived the research question and study design. NS and AA contributed to data collection, statistical analysis and data interpretation and NS drafted the initial manuscript. HM and HV contributed to data collection. All authors contributed, revised and edited the manuscript. PS is the guarantor of this work and had full access to all the data presented in the study and takes full responsibility for the integrity and the accuracy of the data analysis.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. PS is partly supported by a project grant from MRC which is designed to assess the impact of B12 levels in early pregnancy on gestational diabetes.
Competing interests: None declared.
Ethics approval: Ethical approval was obtained from a multicentre Research Ethics Committee and the Oxfordshire A Research Ethics Committee for the first and second surveys, respectively, and all local research ethics committees covering areas where the fieldwork was conducted.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Yajnik CS, Deshmukh US. Fetal programming: maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord 2012;13:121–7. 10.1007/s11154-012-9214-8 [DOI] [PubMed] [Google Scholar]
- 2.Saravanan P, Yajnik CS. Role of maternal vitamin B12 on the metabolic health of the offspring: a contributor to the diabetes epidemic? Br J Diabetes Vasc Dis 2010;10:109–14. 10.1177/1474651409358015 [DOI] [Google Scholar]
- 3.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002;325:1202 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors 2009;35:120–9. 10.1002/biof.17 [DOI] [PubMed] [Google Scholar]
- 5.Ray JG, Blom HJ. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM 2003;96:289–95. 10.1093/qjmed/hcg043 [DOI] [PubMed] [Google Scholar]
- 6.George L, Mills JL, Johansson AL et al. . Plasma folate levels and risk of spontaneous abortion. JAMA 2002;288:1867–73. 10.1001/jama.288.15.1867 [DOI] [PubMed] [Google Scholar]
- 7.Ronnenberg AG, Goldman MB, Chen D et al. . Preconception homocysteine and B vitamin status and birth outcomes in Chinese women. Am J Clin Nutr 2002;76:1385–91. [DOI] [PubMed] [Google Scholar]
- 8.Sukumar N, Rafnsson SB, Kandala NB et al. . Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am J Clin Nutr 2016;103:1232–51. 10.3945/ajcn.115.123083 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Spina bifida and anencephaly before and after folic acid mandate— United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep 2004;53:362–5. [PubMed] [Google Scholar]
- 10.Ray JG, Wyatt PR, Thompson MD et al. . Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology 2007;18:362–6. 10.1097/01.ede.0000257063.77411.e9 [DOI] [PubMed] [Google Scholar]
- 11.Nelen WL, Blom HJ, Steegers EA et al. . Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril 2000;74:1196–9. 10.1016/S0015-0282(00)01595-8 [DOI] [PubMed] [Google Scholar]
- 12.Vollset SE, Refsum H, Irgens LM et al. . Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr 2000;71:962–8. [DOI] [PubMed] [Google Scholar]
- 13.Hogeveen M, Blom HJ, den Heijer M. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr 2012;95:130–6. 10.3945/ajcn.111.016212 [DOI] [PubMed] [Google Scholar]
- 14.Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review). Mol Med Rep 2012;5:883–9. 10.3892/mmr.2012.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011;84:478–85. 10.1016/j.contraception.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balcı YI, Ergin A, Karabulut A et al. . Serum vitamin B12 and folate concentrations and the effect of the Mediterranean diet on vulnerable populations. Pediatr Hematol Oncol 2014;31:62–7. 10.3109/08880018.2013.829894 [DOI] [PubMed] [Google Scholar]
- 17.Dwarkanath P, Barzilay JR, Thomas T et al. . High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am J Clin Nutr 2013;98:1450–8. 10.3945/ajcn.112.056382 [DOI] [PubMed] [Google Scholar]
- 18.García-Casal MN, Osorio C, Landaeta M et al. . High prevalence of folic acid and vitamin B12 deficiencies in infants, children, adolescents and pregnant women in Venezuela. Eur J Clin Nutr 2005;59:1064–70. 10.1038/sj.ejcn.1602212 [DOI] [PubMed] [Google Scholar]
- 19.Murphy MM, Molloy AM, Ueland PM et al. . Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J Nutr 2007;137:1863–7. [DOI] [PubMed] [Google Scholar]
- 20.[No authors listed] Dietary reference values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep Health Soc Subj (Lond) 1991;41:1–210. [PubMed] [Google Scholar]
- 21.Whitehead RG. Dietary reference values. Proc Nutr Soc 1992;51:29–34. 10.1079/PNS19920007 [DOI] [PubMed] [Google Scholar]
- 22.Baker SJ, Mathan VI. Evidence regarding the minimal daily requirement of dietary vitamin B12. Am J Clin Nutr 1981;34:2423–33. [DOI] [PubMed] [Google Scholar]
- 23.Cooper BA, Lowenstein L. Vitamin B-12-folate interrelationships in megaloblastic anaemia. Br J Haematol 1966;12:283–96. 10.1111/j.1365-2141.1966.tb05635.x [DOI] [PubMed] [Google Scholar]
- 24.EFSA NDA Panel (EFSA Panel on Dietetic Products NaA. Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J 2015;13:4150 10.2903/j.efsa.2015.4150 [DOI] [Google Scholar]
- 25.Doets EL, Cavelaars AE, Dhonukshe-Rutten RA et al. . Explaining the variability in recommended intakes of folate, vitamin B12, iron and zinc for adults and elderly people. Public Health Nutr 2012;15:906–15. 10.1017/S1368980011002643 [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington DC: National Academies Press, 1998:306–56. [PubMed] [Google Scholar]
- 27.Henderson L, Gregory J, Swan G. The national diet & nutrition survey: adults aged 19 to 64 years. Volume 1. Types and quantities of foods consumed. London: The Stationery Office, 2002. [Google Scholar]
- 28.Henderson L, Irving K, Gregory J et al. . The national diet & nutrition survey: adults aged 19 to 64 years. Volume 3. Vitamin and mineral intake and urinary analytes. London: The Stationery Office, 2003. [Google Scholar]
- 29.Bates B, Lennox A, Prentice A, et al. 2014. National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009–2011/2012) https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/310995/NDNS_Y1_to_4_UK_report.pdf.
- 30.Skinner C, Holmes D.2002. The 2000-01 National Diet and Nutrition Survey of Adults aged 19-64 years: The impact of non-response. In National Diet and Nutrition survey Adults Aged 19–64 years. Appendix E. http://wwwfoodgovuk/science/101717/ndnsdocuments/ndnsappendicies.
- 31.Møller J, Christensen L, Rasmussen K. An external quality assessment study on the analysis of methylmalonic acid and total homocysteine in plasma. Scand J Clin Lab Invest 1997;57:613–19. 10.3109/00365519709055285 [DOI] [PubMed] [Google Scholar]
- 32.Beaton GH. Approaches to analysis of dietary data: relationship between planned analyses and choice of methodology. Am J Clin Nutr 1994;59(1 Suppl):253S–61S. [DOI] [PubMed] [Google Scholar]
- 33.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94:348S–58S. 10.3945/ajcn.111.013441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.[No authors listed] Folate and vitamin B12 deficiencies. Proceedings of a WHO technical consultation held 18–21 October, 2005, in Geneva, Switzerland. Food Nutr Bull 2008;29(2 Suppl):S1–246. [PubMed] [Google Scholar]
- 35.Refsum H, Smith AD, Ueland PM et al. . Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50:3–32. 10.1373/clinchem.2003.021634 [DOI] [PubMed] [Google Scholar]
- 36.Fokkema MR, Weijer JM, Dijck-Brouwer DA et al. . Influence of vitamin-optimized plasma homocysteine cutoff values on the prevalence of hyperhomocysteinemia in healthy adults. Clin Chem 2001;47:1001–7. [PubMed] [Google Scholar]
- 37.Lindenbaum J, Rosenberg IH, Wilson PW et al. . Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60:2–11. [DOI] [PubMed] [Google Scholar]
- 38.Dullemeijer C, Souverein OW, Doets EL et al. . Systematic review with dose-response meta-analyses between vitamin B-12 intake and European Micronutrient Recommendations Aligned's prioritized biomarkers of vitamin B-12 including randomized controlled trials and observational studies in adults and elderly persons. Am J Clin Nutr 2013;97:390–402. 10.3945/ajcn.112.033951 [DOI] [PubMed] [Google Scholar]
- 39.Bor MV, von Castel-Roberts KM, Kauwell GP et al. . Daily intake of 4 to 7 microg dietary vitamin B-12 is associated with steady concentrations of vitamin B-12-related biomarkers in a healthy young population. Am J Clin Nutr 2010;91:571–7. 10.3945/ajcn.2009.28082 [DOI] [PubMed] [Google Scholar]
- 40.Vogiatzoglou A, Smith AD, Nurk E et al. . Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: the Hordaland Homocysteine Study. Am J Clin Nutr 2009;89:1078–87. 10.3945/ajcn.2008.26598 [DOI] [PubMed] [Google Scholar]
- 41.Bae S, West AA, Yan J et al. . Vitamin B-12 Status Differs among Pregnant, Lactating, and Control Women with Equivalent Nutrient Intakes. J Nutr 2015;145:1507–14. 10.3945/jn.115.210757 [DOI] [PubMed] [Google Scholar]
- 42.Horan MK, McGowan CA, Gibney ER et al. . The association between maternal dietary micronutrient intake and neonatal anthropometry—secondary analysis from the ROLO study. Nutr J 2015;14:105 10.1186/s12937-015-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AD, Refsum H. Do we need to reconsider the desirable blood level of vitamin B12? J Intern Med 2012;271:179–82. 10.1111/j.1365-2796.2011.02485.x [DOI] [PubMed] [Google Scholar]
- 44.Smith AD, Smith SM, de Jager CA et al. . Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE 2010;5:e12244 10.1371/journal.pone.0012244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arsenault JE, Yakes EA, Islam MM et al. . Very low adequacy of micronutrient intakes by young children and women in rural Bangladesh is primarily explained by low food intake and limited diversity. J Nutr 2013;143:197–203. 10.3945/jn.112.169524 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen PH, Nguyen H, Gonzalez-Casanova I et al. . Micronutrient intakes among women of reproductive age in Vietnam. PLoS One 2014;9:e89504 10.1371/journal.pone.0089504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdollahi Z, Elmadfa I, Djazayeri A et al. . Folate, vitamin B12 and homocysteine status in women of childbearing age: baseline data of folic acid wheat flour fortification in Iran. Ann Nutr Metab 2008;53:143–50. 10.1159/000170890 [DOI] [PubMed] [Google Scholar]
- 48.Karabulut A, Guler ÖT, Karahan HT et al. . Premarital screening of 466 Mediterranean women for serum ferritin, vitamin B12, and folate concentrations. Turk J Med Sci 2015;45:358–63. 10.3906/sag-1401-25 [DOI] [PubMed] [Google Scholar]
- 49.Zhu JH, Hu DJ, Hao L et al. . Iron, folate, and B(12) deficiencies and their associations with anemia among women of childbearing age in a rural area in Northern China. Int J Vitam Nutr Res 2010;80:144–54. 10.1024/0300-9831/a000014 [DOI] [PubMed] [Google Scholar]
- 50.Fayet-Moore F, Petocz P, Samman S. Micronutrient status in female university students: iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients 2014;6:5103–16. 10.3390/nu6115103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilsing AM, Crowe FL, Lloyd-Wright Z et al. . Serum concentrations of vitamin B12 and folate in British Male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr 2010;64:933–9. 10.1038/ejcn.2010.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawlak R, Parrott SJ, Raj S et al. . How prevalent is vitamin B(12) deficiency among vegetarians? Nutr Rev 2013;71:110–17. 10.1111/nure.12001 [DOI] [PubMed] [Google Scholar]
- 53.Scientific Advisory Committee on Nutrition. Folate and disease prevention. Food Standards Agency and the Department of Health, 2006. [Google Scholar]
- 54.Pfeiffer CM, Johnson CL, Jain RB et al. . Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr 2007;86:718–27. [DOI] [PubMed] [Google Scholar]
- 55.Selhub J, Morris MS, Jacques PF et al. . Folate–vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009;89:702S–6S. 10.3945/ajcn.2008.26947C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 2007;104:19995–20000. 10.1073/pnas.0709487104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011247supp_figures.pdf (351.9KB, pdf)
Biochemical indices in vegetarian and vegan women of child-bearing age compared to non-vegetarians
bmjopen-2016-011247supp_table.pdf (60.4KB, pdf)


