Abstract
Background
The neural correlates of working memory (WM) impairment in schizophrenia remain a key puzzle in understanding the cognitive deficits and dysfunction of dorsolateral prefrontal cortex observed in the disorder. We sought to determine whether patients with schizophrenia exhibit an alteration in the inverted-U relationship between WM load and activation that we recently observed in healthy individuals, and whether this could account for WM deficits in this population.
Methods
Medicated (N=30) and unmedicated (N=21) patients with schizophrenia and healthy controls (N=45) performed the self-ordered WM task during functional Magnetic Resonance Imaging. We identified regions exhibiting an altered fit to an inverted-U relationship between WM load and activation that were also predictive of WM performance.
Results
A blunted inverted-U response was observed in left DLPFC in patients and was associated with behavioral deficits in WM capacity. In addition, suppression of medial prefrontal cortex (mPFC) during WM was reduced in patients, and was also associated with poorer WM capacity in patients. Finally, activation of visual cortex in the cuneus was elevated in patients and associated with improved WM capacity. Together, these findings explained 55% of the interindividual variance in WM capacity when combined with diagnostic and medication status, which alone accounted for only 22% of the variance in WM capacity.
Conclusions
These findings identify a novel biomarker and putative mechanism of WM deficits in patients with schizophrenia, a reduction or flattening of the inverted-U relationship between activation and WM load observed in healthy individuals in left dorsolateral prefrontal cortex.
Keywords: schizophrenia, working memory, functional magnetic resonance imaging, inverted-u, cognitive impairment, short-term memory
Introduction
For several decades researchers have attempted to characterize the neurobiological mechanisms of deficits in working memory (WM) in patients with schizophrenia (1), which have been closely linked to poorer functional outcomes (2, 3). Performance on WM tasks depends on dopamine function in dorsolateral prefrontal cortex (DLPFC; 4–7), which along with norepinephrine exerts a neuromodulatory influence on glutamatergic and GABAergic networks that are critical to WM representations (8). Given the numerous dopaminergic abnormalities present in schizophrenia (4, 9–11) and the mounting evidence for a disruption in glutamate (12, 13) and GABA (14, 15) neurotransmission, disruption of WM representations in the DLPFC of patients with schizophrenia seems clear, although the precise nature of the disruption has yet to be elucidated.
A widely used approach to assaying DLPFC function during WM performance has been non-invasive in vivo hemodynamic imaging using functional Magnetic Resonance Imaging (fMRI). Initial fMRI studies demonstrated reduced activation in DLPFC in patients during the performance of WM tasks (16–19), which was taken to be consistent with impaired dopamine function in DLPFC, given that dopamine in DLPFC had been shown to be critical for WM performance in non-human primates (20, 21). However, subsequent studies failed to confirm these findings, instead showing greater DLPFC activation by patients (22–24), and our meta-analysis revealed no difference in DLPFC activation between patients and controls across 29 studies (25).
To account for these inconsistent findings, multiple authors proposed that the normal response of DLPFC to parametric variations in WM load may be non-monotonic (i.e. an ‘inverted-U’; 26–28), such that DLPFC activation declines at higher WM loads, while patients with schizophrenia exhibit a ‘left-shift’ in this inverted-U, leading to greater DLPFC activation at lower WM loads and reduced activation at greater loads. While this notion became prevalent and received considerable discussion (see, e.g., 25, 29, 30–33), thus far there has been no direct evidence to support it. Jansma and colleagues (29) did observe a reduction in activation in DLPFC in patients from a 2-back to a 3-back load of the n-back task, but their finding has not been replicated in the very large literature using n-back tasks, was carried out with only 10 participants in each group, included error trials, and no inverted-U was observed in healthy participants. We propose that the failure thus far to demonstrate the hypothesized inverted-U may be due to the relative coarseness of the WM load manipulations allowed by commonly used WM tasks, such as the n-back and Sternberg tasks, where a limited number of steps limits the ability of the task to demonstrate an inverted-U. Consequently, we adapted the Self-Ordered WM Task (SOT), a classic neuropsychological test of DLPFC function (34), for use with fMRI. Our version of the task allows for a gradual increase in WM load from 0 to 7 items in a single trial, and SOT performance correlates with performance on a visual change detection task (35), the gold standard for estimating visual WM capacity. Critically, two independent cohorts of healthy individuals showed an inverted-U response to increasing WM load in the SOT in a network of regions including DLPFC (36).
Thus, we hypothesized that patients with schizophrenia would exhibit a similar but left-shifted variant of this inverted-U. Moreover, we hypothesized that this would relate to task performance and WM deficit in patients. Consequently, we sought to determine whether either an inverted-U pattern of activation or the magnitude of activation (first in DLPFC, but also elsewhere in the brain) was 1) altered in patients with schizophrenia relative to matched controls, and 2) predictive of performance on the task, as only regions showing evidence of both 1 and 2 can be taken as putative neurobiological substrates explaining WM deficits in schizophrenia. Furthermore, as chronic dopamine-D2 receptor antagonism by antipsychotic medications may impact DLPFC function, we included both unmedicated and medicated groups of psychiatrically stable patients in our study. Consistent with our work in healthy individuals, we restricted analysis of DLPFC activation to correct trials, to limit the impact of poor performance, which has been shown to produce reduced DLPFC activation in patients with schizophrenia (25, 37).
Methods and Materials
Participants
All procedures were approved by the New York State Psychiatric Institute (NYSPI) Institutional Review Board. Participants provided written informed consent, and patient participants were deemed to have capacity to provide consent by an independent psychiatrist. Patients were outpatients recruited from research facilities at NYSPI, and control participants were recruited via advertisements. The final sample included 21 unmedicated patients, 30 medicated patients, and 45 healthy control participants (see supplement for details).
Inclusion criteria for patients were: (1) lifetime DSM-IV diagnosis of schizophrenia, schizoaffective, or schizophreniform disorder and (2) negative urine toxicology. Unmedicated patients were medication free for at least two weeks, while medicated patients were on stable doses of risperidone, aripiprazole, lurasidone, paliperidone, or haloperidol for at least 4 weeks, with no antipsychotic polypharmacy and no psychiatric ER visit or hospitalization for at least 3 months. Inclusion criteria for healthy controls were: (1) no history of DSM-IV Axis-I disorder; (2) no family history (first-degree) of psychotic illness; and (3) negative urine toxicology. Exclusion criteria for all groups included significant medical and neurological illnesses, current misuse of substances other than nicotine, pregnancy, and nursing. Groups were matched for age, gender, and parental socioeconomic status. See Table 1 for demographic and clinical data.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Unmedicated Patients | Medicated Patients | Healthy Controls | |
|---|---|---|---|
| N | 21 | 30 | 45 |
| Age (SD) | 33.2 (10.6) | 36.4 (7.5) | 34.0 (8.9) |
| Gender | 11 M/10 F | 17 M/13 F | 21 M/24 F |
| Parental SES (SD) | 43.4 (13.7) | 41.0 (12.6) | 42.1 (14.0) |
| Handedness | 19 R/2 L | 27 R/3 L | 44 R/1 L |
| Age at diagnosisa (SD) | 18.1 (4.9) | 22.2 (7.1) | - |
| Antipsychotic medication history | 12 DF/9 DN | - | - |
| Current CPE (mg) (SD) | - | 270.6 (227.8)b | - |
| PANSS General | 29.7 (8.8) | 29.2 (7.9) | - |
| PANSS Positive | 14.4 (5.9) | 13.0 (6.6) | - |
| PANSS Negative | 15.9 (5.9) | 14.8 (6.0) | - |
| SANS | 8.8 (4.4) | 8.4 (3.4) | - |
Note.
Age at diagnosis refers only to primary psychotic disorder. Data were only available for 20 (95%) of the unmedicated sample and 13 (43%) of the medicated sample.
Three patients were also on a non-antipsychotic mood stabilizer at time of participation and 11 were on an antidepressant.
N = number of participants in each group; SD = standard deviation; M = male; F = female; SES = socioeconomic status; R = right; L = left; DF = antipsychotic drug free for at least 2 weeks (at least 6 weeks for aripiprazole); DN = antipsychotic drug naïve; PANSS = Positive and Negative Syndrome Scale; SANS = Scale for the Assessment of Negative Symptoms; CPE = chlorpromazine equivalent dose, where current antipsychotic dose compares to 100 mg oral chlorpromazine, using 2 mg haloperidol, 2 mg risperidone, 7.5 mg aripiprazole, 20 mg lurasidone, 25 mg risperidone (depot), 30 mg haloperidol (depot) (51). For depot paliperidone, we use the manufacturers’ recommended equivalent for the depot to oral conversion (234 mg paliperidone palmitate (depot) every 28 days = 12 mg oral paliperidone daily) and then converted to oral chlorpromazine equivalents.
Task Procedures
Task procedures are detailed in the supplement, and have been described previously (36). Briefly, eight line-drawings of difficult-to-verbalize objects were presented, and participants were instructed to select each object once, in any order. After each object was selected, all objects were pseudo-randomly rearranged on the screen. Participants then had to select an object not already selected, so that at each step there was one more previously-selected object to remember. A perceptual and motor control task was used following identical procedures, except that one object was marked with an asterisk and participants were instructed to simply select the marked object. Participants were paid $0.25 per correct response for both tasks. Our primary measure of performance was WM capacity, as estimated by a maximum-likelihood model (see supplement and 35).
fMRI Procedures
fMRI acquisition, preprocessing and first-level modeling
Data was acquired on a Philips 1.5 Tesla Intera scanner and preprocessed as described elsewhere (36) and in the supplement. Briefly, images were slice-timing corrected, motion realigned, normalized to a standard template, and smoothed. Time series values were transformed to percent signal change on a per-voxel basis. First-level modeling followed prior work (see supplement and 36). Regressors of interest were those reflecting correct trials in the control task or each of the first seven steps of the SOT, modeled separately. Step eight was excluded due to poor performance by patients. Incorrect trials were modeled separately and are not reported.
Two primary outcome measures were calculated for each subject. First, the fit to an empirical inverted-U shape (obtained from an independent healthy sample; study 1 in 36) was calculated at each voxel for each participant. This fit was obtained by regressing observed task activation at each step on the inverted-U shape, such that larger positive values indicate better fit. Second, a task - control contrast was calculated as the average activation across the first seven steps of the SOT minus activation to the control task. Although we did not hypothesize a between-group difference in this contrast, it is a commonly used and straightforward measure of regional brain activation to the SOT relative to the control task.
Second-level modeling
Both outcome measures (regression betas indicating fit to the inverted-U pattern, and contrast values for overall activation) were analyzed in a series of robust models (38); t-tests for group comparisons or multiple regressions for testing effects of WM capacity and group, as appropriate. These models evaluated 1) whether there were group differences in activation or inverted-U fit, and 2) whether activation or inverted-U fit related to WM capacity in each group, and whether there were group differences in this relationship (group by WM capacity interaction). See supplement for multiple comparison corrections.
Region of Interest
Second-level analyses were first carried out in an anatomically- and functionally-defined a priori region of interest (ROI) of bilateral DLPFC (see supplement), and subsequently in an exploratory whole-brain analysis.
Results
Task Performance
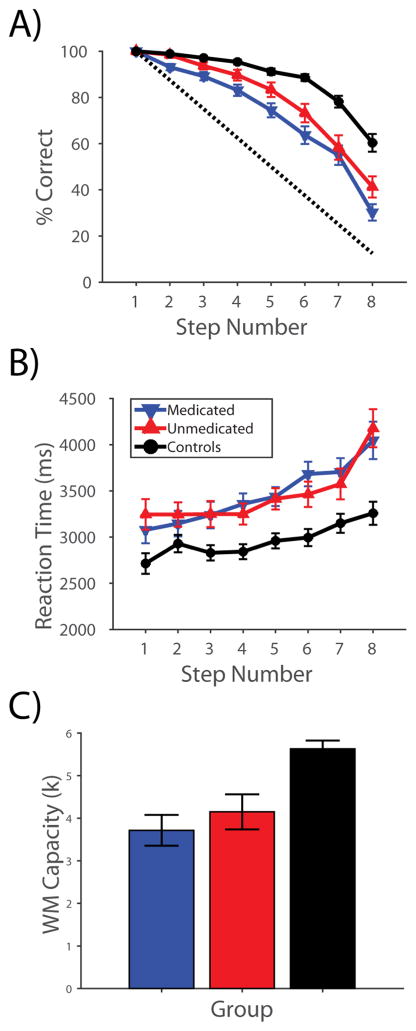
Accuracy, reaction times, and estimated WM capacity are shown in Figure 1. Healthy participants had a mean (SD) WM capacity of 5.63 (1.32), consistent with prior observations (35, 36), while patients had a WM capacity of 3.89 (1.93), which differed significantly from controls (P<0.00001). Unmedicated and medicated patients had WM capacities of 4.15 (1.88) and 3.72 (1.97) respectively (no significant difference; P=0.43).
Figure 1. Performance on the Self-ordered Working Memory Task.
A) Accuracy, B) reaction time, and C) WM capacity data for participants in all three groups over all eight steps of the task. The dotted line in A) shows the level of accuracy expected by chance at each step.
All groups performed above chance at every step (one-sample t-tests, all P<0.0001), and control participants performed better than patients at all steps (two-sample t-tests, all P<0.0001). Unmedicated patients performed better than medicated patients at step 2 (P<0.005) and showed a trend towards better performance at all other steps excluding step 7 (all P<0.1). Controls responded significantly faster than patients at all steps (all P<0.05) except step 2, at which a trend was observed (P=0.06). Patient groups did not differ in reaction time (all P>0.26).
fMRI Results
Activation to the Self-Ordered Working Memory Task
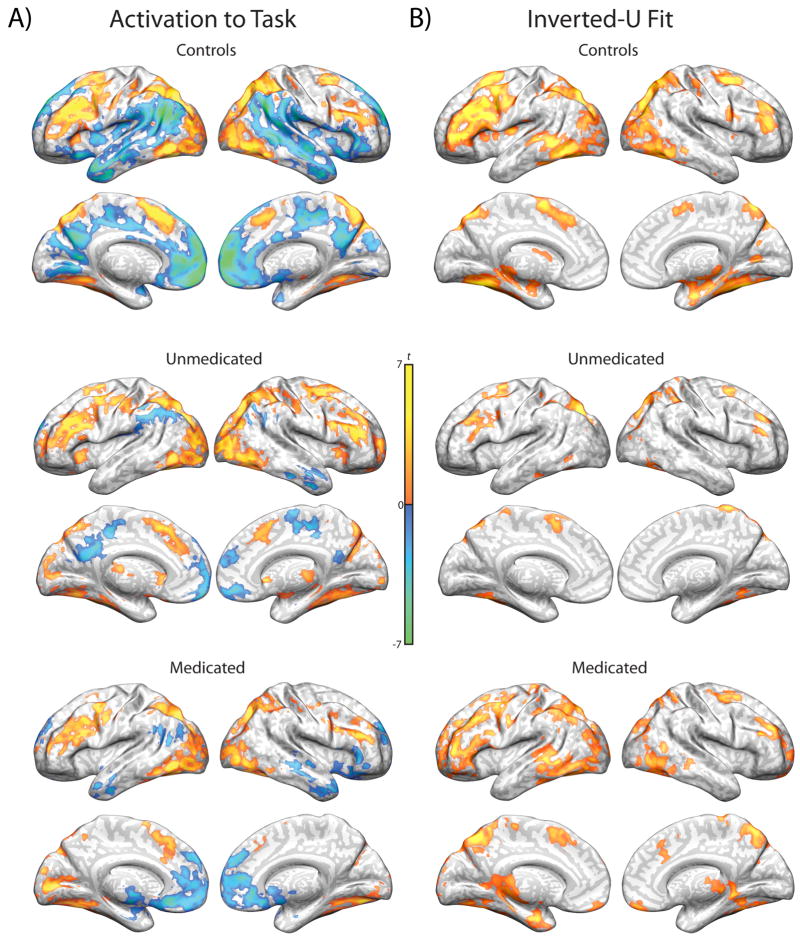
Regions showing significant differences in activation between the SOT and control task are reported in Figure 2a and supplementary Tables S1–3. Patient subgroups are displayed for illustrative purposes, and between-group analyses are reported separately (below). Consistent with our prior report (36), healthy controls showed robust activation of the classic WM network, including bilateral DLPFC, posterior parietal cortex (PPC), pre-supplementary motor area (pre-SMA), and left dorsal anterior insula, as well as premotor areas and most of the lateral occipital lobe and fusiform gyrus. They also demonstrated substantial deactivations throughout the default mode network, including medial prefrontal cortex (mPFC), posterior dorsal cingulate, precuneus, and lateral temporal lobes, as well as the temporal parietal junction. Both patient groups showed similar patterns of activation and deactivation to those observed in healthy individuals.
Figure 2. Within-group activation and inverted-U fits during working memory.
A) Regions showing significant activation (hot color spectrum) or deactivation (cool colors) to the self-ordered working memory task, as compared to the perceptual and motor control task. B) Regions in the current study cohort that show a significant positive fit to the inverted-U pattern of activation identified in our previous report in an independent sample of healthy individuals.
Regions Exhibiting Inverted-U Activation Pattern
Regions that significantly fit an inverted-U activation pattern are shown in Figure 2b and supplementary Tables S4–6. Again, these regions closely matched those in prior work (36), including bilateral DLPFC, PPC, pre-SMA, premotor areas, lateral occipital lobe, fusiform gyrus, and medial temporal lobe in healthy individuals, with a similar but less robust activation pattern observed in both patient groups.
Group Differences and Relationships to Performance in DLPFC
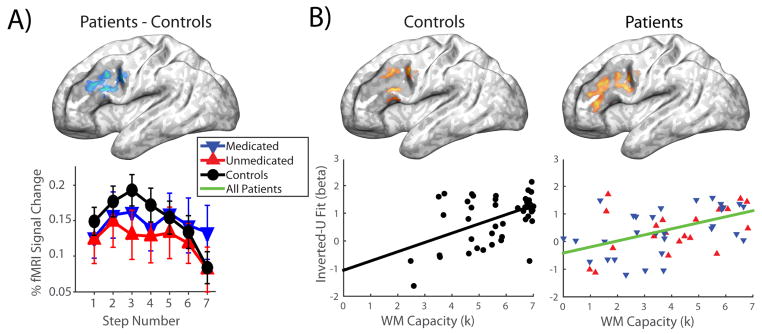
A region of left DLPFC (141 voxels; MNI coordinates −48,15,28; max t-value 3.28) showed a poorer fit to an inverted-U in patients than in healthy controls (see Figure 3a). Activation in this region did not appear to be ‘left-shifted’ in the patient group; rather, patients failed to show the clear rise and fall of activation in this region observed in healthy individuals, leading to a flatter pattern of response. Moreover, in both patient and control groups we observed a positive relationship between inverted-U fit and WM capacity in left DLPFC, which overlapped with the region showing a group difference (see Figure 3b; controls: 109 voxels; MNI coordinates −51,15,31; max t-value 3.17; patients: 71 and 54 voxels; MNI coordinates −45,33,16 and −42,9,28; max t-values 3.24 and 3.42). Thus, the reduction in inverted-U fit in this region of left DLPFC can be considered a putative neurobiological marker of WM deficit in schizophrenia, as it is both deficient in patients and associated with WM capacity. No differences were observed within the DLPFC ROI between the two patient subgroups.
Figure 3. Group differences in inverted-U activation in dorsolateral prefrontal cortex, and associations with working memory capacity.
A) Top: Region showing a significant difference between patients and controls in inverted-U fit within the dorsolateral prefrontal cortex region-of-interest. Bottom: Line plot showing activation at each step of the task in each of the three groups, within the region above. B) Regions showing a significant relationship between inverted-U fit and working memory capacity within the dorsolateral prefrontal cortex region-of-interest. Scatter plots show the average inverted-U fit in significant voxels for each participant, plotted against working memory capacity. Shaded regions on brain surfaces show the spatial extent of the dorsolateral prefrontal cortex region-of-interest.
Regions within the DLPFC ROI showing differences in overall activation to the task (relative to the control task) are shown in supplemental Figure S1. Briefly, inferior right prefrontal cortex showed greater activation in patients than controls, while a more dorsal and anterior area showed a positive relationship between activation and WM capacity in patients, but not controls.
Whole-Brain Group Differences and Relationships to Performance
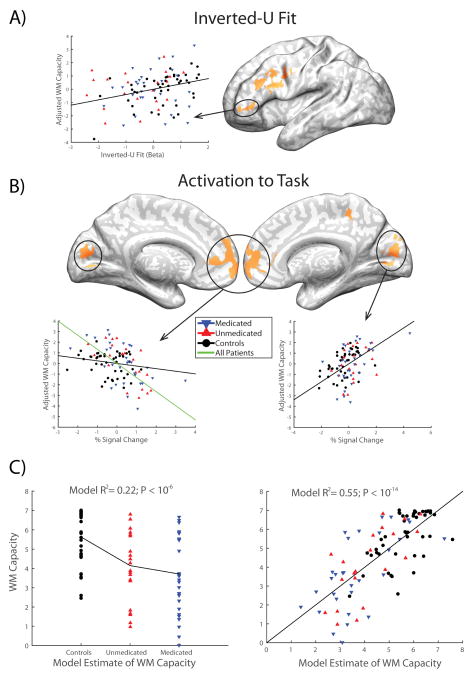
The full set of regions showing significant group differences or relationships to performance in inverted-U fit or task activation are shown in supplemental Figures S2–3 and Tables S7–8. We were primarily interested in identifying regions that exhibit both 1) a group difference and 2) a relationship to WM capacity in either inverted-U fit or task activation, precisely like the left DLPFC region identified in the ROI analysis above, and consistent with a neurobiological substrate of WM deficit. Consequently, for both inverted-U fit and task activation we produced conjunction maps of regions showing both of these effects (1 and 2; see Figure 4). This analysis recapitulated our ROI-based finding of a putative substrate of WM deficit in left-DLPFC for inverted-U fit, and further identified a region of mPFC which showed greater overall activation in patients and a negative relationship with performance in patient and control groups, demonstrating that failure by patients to adequately suppress activation in this region during task performance is associated with their deficit in WM. In addition, a region of visual cortex in the cuneus demonstrated increased activation in patients with schizophrenia, along with a positive relationship to WM capacity observed in healthy individuals (but not patients).
Figure 4. Brain regions showing group differences and an association with working memory for either inverted-U fit or task activation.
A) Regions in a whole-brain analysis showing both a significant difference between patients and controls and an association with working memory capacity in at least one group for the inverted-U fit. B) Regions in a whole-brain analysis showing both a significant difference between patients and controls and an association with working memory capacity in at least one group for activation to the self-ordered working memory task. Scatter plots in both A) and B) show the relationship between the circled region and working memory capacity, after adjusting working memory capacity for other predictors in the full model described in the main text. C) Working memory capacity regressions for a simple model including only diagnostic and medication grouping variables (left) and for a full model determined with step-forward model selection (right), including the four circled regions identified in panels A) and B).
In order to tease apart the relative, unique contributions of each of these findings to individual differences in WM capacity, we extracted mean values (either inverted-U fit betas or task - control contrast values, as appropriate) from each cluster (minimum 10 voxels) identified in the conjunction images shown in Figure 4. These were averaged within each cluster to produce a scalar value for each participant in each cluster, which was then related to WM capacity in a step-forward linear regression model selection framework (see supplement). The impact of symptoms (the three PANSS subscales) on WM capacity were also evaluated in this framework. The resulting model demonstrated independent contributions to WM capacity from a) the inverted-U fit in the smaller left prefrontal cortex region, which behaved very similarly to the larger DLPFC cluster (see below), and was positively associated with WM capacity; b) precuneus, in which activation was positively associated with WM capacity; and c) the suppression of activation in mPFC, where activation was negatively associated with WM capacity in patients (but not controls). No symptom variables were significant. In addition, diagnosis still had a significant impact on WM capacity in the model, and a trend was observed toward further WM deficit in medicated patients. Moreover, all variables retained in the regression model were also significant predictors in bivariate simple linear regression models. All told, the model accounted for 55.5% of the variance in WM capacity, while a model with diagnostic information removed accounted for 37.9% of the variance in WM capacity. Critically, inverted-U fit in the larger left DLPFC region was correlated with the smaller region that remained in the model (r=0.57, P<0.00005), and if we substituted it into the model in place of the smaller region the model remained largely unchanged, although the P value for this region was then only at trend level (P=0.071). Taken together, these observations suggest that the contributions of the two left prefrontal regions in Figure 4 were largely indistinguishable, though slightly more robust in the smaller of the two regions.
Discussion
These data identify two potential neurobiological mechanisms of WM deficits in individuals with schizophrenia, one of which has not been previously identified in the literature. First, patients with schizophrenia demonstrated a flattening of the inverted-U pattern of activation over increasing WM loads observed in the DLPFC of healthy individuals, a pattern that was associated with reduced WM capacity. This represents the first clear demonstration of an alteration in the long-hypothesized inverted-U relationship between WM load and DLPFC activation in patients with schizophrenia, although the alteration we observed does not take on the form of a ‘left-shift’, as initially described (26–28). Rather, the flattening or blunting of the inverted-U observed here was proposed later, to explain findings of a reduced load-activation slope in patients relative to controls (30), although no inverted-U was observed directly in that study. Second, patients demonstrated a relative failure to deactivate mPFC during WM task performance, which was also associated with greater deficits in WM capacity among patients with schizophrenia. This failure to deactivate portions of the so-called default-mode network by patients with schizophrenia has been widely reported (39–42), although we are aware of only one prior report linking deactivation of this region to WM task performance, in healthy individuals (but not patients; 43). Finally, activation in medial visual cortex (precuneus) was both significantly increased in schizophrenia and positively associated with WM capacity. Critically, multiple regression indicated an independent role for each of these three mechanisms in WM deficits. Moreover, these findings were obtained in an analysis using only task steps that were performed correctly, mitigating contamination of fMRI activation measures by trials on which participants were disengaged or otherwise unable to effectively utilize their WM.
Despite tremendous interest in DLPFC dysfunction as the (putative) primary neuropathological substrate of WM deficits in schizophrenia, clear evidence for a functional abnormality in this region that is associated with individual differences in WM capacity has failed to emerge until this report, and confidence in our finding is enhanced by the (relatively) large sample employed here, and by the inclusion of a substantial number of medication-free patients. While the present results cannot speak directly to the molecular underpinnings of WM deficits in schizophrenia, and would certainly benefit from replication, here we report a direct connection between DLPFC dysfunction and WM deficits in schizophrenia in a clinical sample. Critically, this dysfunction does not come in the form of simply increased or decreased activation, but rather in a more subtle alteration of an activation pattern associated with strong WM capacity across diagnostic groups, which can (presumably) only be elucidated under conditions that allow for fine-grained manipulation of WM load over a broad WM loads; namely, a failure to show a robust inverted-U relationship between WM load and activation in left DLPFC. This has important implications for future work, which will be needed to better understand how this functional alteration in inverted-U activation in DLPFC operates in clinical samples and animal models, and which could potentially identify new targets for treatment of cognitive deficits in schizophrenia. That is, the overwhelming majority of WM tasks that have been used to probe WM in both clinical and basic research employ at most two or three WM loads, which is likely insufficient to characterize the inverted-U activation pattern (or lack thereof) shown to be associated with WM capacity in the present study.
A critical unresolved question relates to the functional significance of the inverted-U observed in healthy controls, which we have discussed at some length elsewhere (36). The initial formulation of the inverted-U hypothesis (26, 27) speculated that it may occur as a result of task disengagement, which is an unlikely explanation for the present findings given that we analyzed only correctly performed trials, and that reaction times increase at higher loads even in poor performers (36). Moreover, the positive association between the inverted-U pattern and WM capacity observed also suggests that this activation pattern is adaptive. Although we are at present unable to definitively adjudicate between competing possibilities, in general terms it seems most likely that the inverted-U reflects an adaptive shift in cognitive strategy that patients (or at least many patients) fail to engage. For example, as we have argued elsewhere, participants may gradually shift from a WM-mediated to a long-term memory mediated strategy that requires less reliance on active maintenance (36). In this case, the failure by patients to exhibit this neural response could reflect inefficient strategy use, direct impairment to the WM system that limits the extent of DLPFC activation (thereby flattening the inverted-U), impairment in long-term memory that renders a strategy switch maladaptive, or impairment in whatever mechanism initiates such a switch. All of these possibilities bear careful consideration in future work specifically designed to determine the mechanism and functional significance of this inverted-U.
In addition to the finding in DLPFC discussed above, an unhypothesized potential substrate of WM deficit in schizophrenia was identified in the mPFC. This region of the so-called default mode network has been linked to self-referential thought in studies of social cognition (44–46) as well to auto-biographical memory retrieval (47–50), suggesting that its association with poor WM capacity when activation of the region is not fully suppressed by patients during the SOT may reflect a failure to fully suppress task-irrelevant, self-referential or autobiographical cognition during WM task performance in individuals with schizophrenia. We also observed a region of the precuneus which was activated more by patients with schizophrenia than healthy controls, but which also showed a positive association between activation and WM capacity. While we can only speculate as to the precise implications of this finding, it is consistent with a compensatory mechanism in at least some patients, such that in spite of deficits in WM capacity, at least some gains were possible due to (or were indexed by) increases in activation in downstream visual cortical regions representing the task stimuli.
In our view, this work highlights the critical importance of linking observed differences in neural activity (or BOLD signal) between patients and healthy individuals with measurable non-neural outcomes, such as task performance, cognitive deficit, or clinical symptoms. Without such a link (even one that is only correlational), observations such as the widely reported reduction in activation of DLPFC during WM task performance in patients with schizophrenia are difficult to interpret. If such a reduction is not clearly linked to poorer performance, which it is not in the large majority of the literature, it cannot be concluded that such a finding is related to cognitive deficits or WM impairment; indeed, it could be epiphenomenal to some other disease process that is not relevant to the process being studied. Here we observed no relationship between the overall level of activation in DLPFC and WM capacity in either healthy individuals or patients with schizophrenia, consistent with the literature, although the pattern of activation across loads within DLPFC was predictive of capacity. This is suggestive of a dynamic process that may depend more on the ability to flexibly alter the neural processes brought to bear on a behavioral goal in the course of a single trial than it does on the ability to produce a large increase in BOLD signal in a region thought to carry out executive control processes. In our view, the field should strive to move away from simply describing alterations in BOLD signal in patient samples, and attempt to rigorously characterize biomarkers of WM or other cognitive deficits, such as those described here. Once a biomarker has been established, and ideally replicated, researchers can then attempt to characterize other cognitive, neural, molecular, or genetic mechanisms associated with the biomarker and ultimately attempt to identify interventions (pharmacological or otherwise) that target the biomarker and could potentially produce improvements in WM or other cognitive deficits.
As a final note, it is important to bear in mind that our two patient samples were identified more naturalistically than experimentally. Our unmedicated group consisted of psychiatrically stable outpatients who either refuse to take medications or were off medications for reasons unrelated to this study. Thus, they represent a population that is distinct from our medicated sample in ways that extend beyond medication status, and so any differences (or similarities) cannot necessarily be attributed to the medications per se. For example, the close correspondence in overall symptomatology between the two groups (see Table 1) strongly suggests that our unmedicated sample is more mildly psychotic than our medicated sample. Similarly, there was considerable suggestive (i.e. trend-level) evidence that the medicated group may have performed more poorly on the SOT than unmedicated patients. While, if real, this could plausibly be an effect of antipsychotic medications, it could also reflect more serious impairment in the medicated group. Thus, while the lack of differences between patient groups in any of our neural outcome measures or the observed relationships between neural outcomes and WM capacity suggests that the phenomenon under consideration are not strongly impacted by antipsychotic medication, it remains possible that these medications do exert important influences that have been missed here as a result of other systematic differences between these two groups.
Supplementary Material
Acknowledgments
Funding for the study was provided by National Institute of Mental Health (NIMH) grant #1P50MH086404 and by National Institutes of Health grant 5U01MH076544. Dr. Van Snellenberg and Dr. Weinstein were supported by NIMH grant T32MH018870, and Dr. Van Snellenberg was also supported by NIMH grant 1K01MH107763. Dr. Horga was supported by National Institute of Mental Health grant 1K23MH101637. The authors would like to acknowledge the staff of the Division of Translational Imaging at the New York State Psychiatric Institute, whose hard work and expertise made this study possible, in particular Juan Sanchez, who assisted in the preparation of figures for this manuscript.
Footnotes
Financial Disclosures
Dr Girgis has received research support from Otsuka, Genentech, and Pharmanac. Dr Slifstein has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka and has provided consultation for Amgen. Dr. Lieberman serves on the advisory boards of Clintara and Intra-Cellular Therapies. He receives grant support from Alkermes, Forum, Novartis, and Sunovion. Dr Abi-Dargham has received research support from Takeda and Forest Laboratories and has served on advisory boards for Roche, Forum, and Otsuka. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of abnormal psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? The American journal of psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Green MF. Schizophrenia from a neurocognitive perspective: Probing the impenetrable darkness. Boston, MA: Allyn and Bacon; 1998. [Google Scholar]
- 4.Slifstein M, van de Geissen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extra-striatal dopamine release in schizophrenia: A Positron Emission Tomographic Functional Magnetic Resonance Imaging Study. JAMA psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am J Psychiat. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 10.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of general psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 11.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. The American journal of psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 13.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. International review of neurobiology. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia bulletin. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callicott JH, Ramsey NF, Tallen K, Bertolino A, Knable MB, Coppola R, et al. Functional magnetic resonance imaging brain mapping in psychiatry: Methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 17.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. The American journal of psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 18.Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Archives of general psychiatry. 1998;55:1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- 19.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiat. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 20.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 21.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 22.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 23.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 24.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 26.Manoach DS. Functional neuroimaging investigations of working memory deficits in schizophrenia: Reconciling discrepant findings. In: Lenzenweger MF, Hooley JM, editors. Principles of experimental psychopathology: Essays in honor of Brendan A Maher. Washington, DC: American Psychological Association; 2002. pp. 119–134. [Google Scholar]
- 27.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 28.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. The American journal of psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 29.Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophrenia research. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biological psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophrenia bulletin. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, et al. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: An fMRI multi-center study. Schizophrenia research. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 35.Van Snellenberg JX, Conway AR, Spicer J, Read C, Smith EE. Capacity estimates in working memory: Reliability and interrelationships among tasks. Cognitive, affective & behavioral neuroscience. 2014;14:106–116. doi: 10.3758/s13415-013-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Snellenberg JX, Slifstein M, Read C, Weber J, Thompson JL, Wager TD, et al. Dynamic shifts in brain network activation during supracapacity working memory task performance. Human brain mapping. 2015;36:1245–1264. doi: 10.1002/hbm.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophrenia research. 2009;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuro Image. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in cognitive sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of Working Memory and Default-Mode Networks in Schizophrenia Using Independent Component Analysis, an fBIRN and MCIC Study. Human brain mapping. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ETC, Woodward TS. Decreased Efficiency of Task-Positive and Task-Negative Networks During Working Memory in Schizophrenia. Schizophrenia bulletin. 2012;38:803–813. doi: 10.1093/schbul/sbq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological medicine. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 43.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuro Image. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cognitive Neurosci. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- 47.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 49.Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study on recent and remote memory retrieval. Neuro Image. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in cognitive sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of clinical psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.