This study has demonstrated for the first time that adipose-derived mesenchymal stem cell infusion can significantly suppress the increase in body weight and remarkably improve dyslipidemia in db/db obese mice and diet-induced obesity mice. Induction of white fat tissue “browning” and activation of adenosine monophosphate-activated protein kinase and its downstream hormone-sensitive lipase in adipose tissue contribute to the antiobesity and lipid-lowering effects.
Keywords: Adipose-derived mesenchymal stem cell, Anti-obesity, Lipid metabolism, Adenosine monophosphate-activated protein kinase
Abstract
Adipose-derived mesenchymal stem cells (AD-MSCs) have been shown to ameliorate hyperglycemia in diabetic animals and individuals. However, little is known about whether AD-MSCs affect lipid metabolism. Here we have demonstrated for the first time that AD-MSC infusion can significantly suppress the increase in body weight and remarkably improve dyslipidemia in db/db obese mice and diet-induced obesity mice. Induction of white fat tissue “browning” and activation of adenosine monophosphate-activated protein kinase and its downstream hormone-sensitive lipase in adipose tissue contribute to the antiobesity and lipid-lowering effects. Thus, AD-MSC infusion holds great therapeutic potential for dyslipidemia and associated cardiovascular diseases.
Significance
Mesenchymal stem cells (MSCs) are considered one of the most promising types of stem cells for translational application because of their rich tissue sources, multilineage differentiation capacity, and easy amplification in vitro and unique immunobiological properties. This study demonstrated that adipose-derived MSCs (AD-MSCs) infusion can significantly suppress the increase in body weight and remarkably improve dyslipidemia in obese mice. Induction of white fat tissue “browning” and activation of adenosine monophosphate-activated protein kinase and its downstream hormone-sensitive lipase in adipose tissue were demonstrated to contribute to the antiobesity and lipid-lowering effects. Thus, AD-MSC infusion holds great therapeutic potential for dyslipidemia.
Introduction
Overweight or obesity, defined as excessive accumulation or abnormal distribution of fat tissues, is strongly associated with chronic inflammation, insulin resistance, and dyslipidemia, which contribute to the high mortality and morbidity of type 2 diabetes mellitus and cardiovascular disease [1, 2]. The incidence of obesity has seen a dramatic increase worldwide in the last three decades, and currently, the prevalence of obesity has reached epidemic proportions. It is estimated that overweight and obesity affect approximately 2.1 billon people worldwide [3]. Clearly, research on the effective prevention and treatment of obesity and associated diseases would have great benefits.
There are two major types of adipose tissues or adipocytes, namely, white and brown adipose tissues. Brown adipose tissue (BAT) has long been viewed as a tissue that plays a key role in maintaining body temperature in response to cold, whereas white adipose tissue (WAT) is known for its role in energy storage. However, recent research into adipocytes, especially into WAT, has altered our understanding of adipose tissue. In contrast to the traditional view of WAT as a quiescent fat-storage tissue, it is now thought to be highly dynamic and to actively control metabolism by “browning” and secreting lipids, hormones, and other factors [4–7]. Recently, a distinct type of adipocyte, beige adipocyte, has been identified. This type of adipocyte is different from brown adipocyte in several aspects and accounts for the browning of WAT [8, 9].
One of the most important findings in WAT research is the isolation of white adipose stem cells (ASCs) and the identification of its niche [10–12]. These stem/progenitor cells reside perivascularly within WAT and undergo adipogenesis to support WAT expansion in response to increased energy intake. The perivascular localization of ASCs also indicates that these cells could be mobilized in the blood stream or regulated by various endocrine factors and could therefore play a pivotal role in metabolic balance or disturbance. However, the physiological functions of ASCs in energy homeostasis and systemic metabolism are poorly understood.
ASCs can be easily isolated from the stromal-vascular fraction of fat tissue and are also termed adipose-derived mesenchymal stem cells (AD-MSCs) or lipoblasts. Apart from adipogenesis, AD-MSCs could be induced to undergo abundant expansion in vitro and to differentiate into osteocytes, chondrocytes, myocytes, neurons, and other cell types [13]. From the point of view of clinical translation, both AD-MSCs and bone marrow-derived mesenchymal stem cells (BM-MSCs) are ethically acceptable. However, AD-MSCs are particularly abundant and easy to obtain without invasive surgery, which makes it is the most attractive candidate for clinical application. Many preclinical and clinical studies have been performed to test the safety and efficacy of AD-MSCs in tissue regeneration, primary wound repair, and immune regulation [14–16]. In addition, AD-MSCs have also been used to treat type 2 diabetes because of their potential to differentiate into β-like insulin-producing cells [17]. Recently, Si et al. demonstrated that systemically infused umbilical cord-derived MSCs (UC-MSCs) could significantly improve insulin resistance in adipose and muscle tissues in a streptozotocin-induced type 2 diabetic rat model [18]. These results suggest that apart from their functions in tissue repair/regeneration and immune regulation, MSCs may play an important role in the regulation of glucose metabolism and homeostasis. Although type 2 diabetes is closely associated with obesity, obesity and dyslipidemia are a direct result of lipid or fat metabolic disorder. It would be interesting to explore whether culture-expanded AD-MSCs can modulate lipid metabolism. This investigation was conducted to test our hypothesis that AD-MSCs might play a role in ameliorating disturbances in lipid metabolism, such as hyperlipidemia and obesity.
Here, using db/db mice and diet-induced obesity (DIO) mice as hyperlipidemia and obesity models, respectively, we show that AD-MSCs, but not UC-MSCs, have antiobesity properties and significantly improve serum lipid profiles. We also found that the expression and activity of hormone-sensitive lipase (HSL) and its upstream kinase adenosine monophosphate-activated protein kinase (AMPK) were markedly increased in the adipose tissues of AD-MSC-treated mice. These observations indicate that AD-MSCs play a crucial role in lipid metabolism and hold great promise in treating dyslipidemia and associated diseases.
Materials and Methods
Isolation of Human AD-MSCs and UC-MSCs
Liposuction aspirates (approximately 50 ml) from abdominal subcutaneous adipose tissue sites were obtained from three volunteers (the anthropometric and clinical characteristics of donors are supplied in supplemental online Table 1) using a simple negative pressure plunger technique, as has been described recently [19]. AD-MSCs were isolated and cultured according to a collagenase digestion protocol [20]. UC-MSCs were isolated as described before with mild modifications [21]. The brief protocols of isolation and phenotype identification are supplied in the supplemental online data. MSCs were isolated, cultured, and characterized as described above in accordance with the ethics committee of the Beijing Institute of Radiation Medicine.
Animals
All animals used in this study were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). The animals received humane care, and all the animal experimental procedures were carried out with the approval of the Animal Use and Care Committee of the Beijing Institute of Radiation Medicine. The ethics decision number is 2013-018.
Establishment of DIO Model
Male C57BL/6J mice at the age of 6 weeks were fed a high-fat diet (HFD; 60 kcal%; Biotech Co. Ltd., Beijing, China, http://biotech-hd.cn.gongchang.com) to establish the diet-induced obesity (DIO) mouse model. Normal male C57BL/6J mice at the age of 6 weeks were fed a standard diet (10 kcal%) and served as normal controls. After 6 weeks of feeding, the average weight of the obese mice was 32 g.
In Vivo Studies
Male db/db mice at the age of 6 weeks or DIO mice with an average weight of 32 g were divided randomly into three groups. Each group contained six to eight mice that were kept in two cages. Mice in the three groups were administered physiologic saline (vehicle, 0.2 ml), AD-MSCs (2 × 106 cells suspended in 0.2 ml of physiological saline), and UC-MSCs (2 × 106 cells suspended in 0.2 ml of physiological saline) once a week for 3 weeks. Meanwhile, male C57BL/6J mice age 6 weeks were used as the normal control. The four groups of mice were weighed, and fasting glucose was measured every week. Six weeks later, the mice were sacrificed, and the adipose, liver, and pancreatic tissues were isolated for further examination.
Fasting Glucose and Glucose Tolerance Tests
For the weekly fasting glucose test, the mice were starved overnight to assess glycemia. At the end of the experiment, after overnight fasting, the mice were administered glucose (1 g/kg) by oral gavage, and blood samples were collected from the tail vein for glucose determination. Glycemia was assessed using an Accu-Chek glucometer (Roche, Basel, Switzerland, http://www.roche.com), and the area under the curve was calculated.
Metabolic Measurements
After 6–8 weeks of treatment, blood was collected from the retro-orbital sinus of each nonanesthetized mouse to measure the C-peptide, glucagon, cholesterol, triglyceride, high- and low-destiny lipoprotein, free fatty acid, aspartate amino-transaminase (AST), alanine amino-transaminase (ALT), and alkaline phosphatase (ALP) levels. The measurements were carried out by the Beijing North Institute of Biological Technology.
Tissue Preparation, Histological Analysis, and Immunostaining Assays
Protocols are supplied in the supplemental online data.
Real-Time Polymerase Chain Reaction
Protocols are supplied in the supplemental online data.
Western Blot Analysis
Protocols are supplied in the supplemental online data.
Results
AD-MSCs Decreased Body Weight of Obese Mice
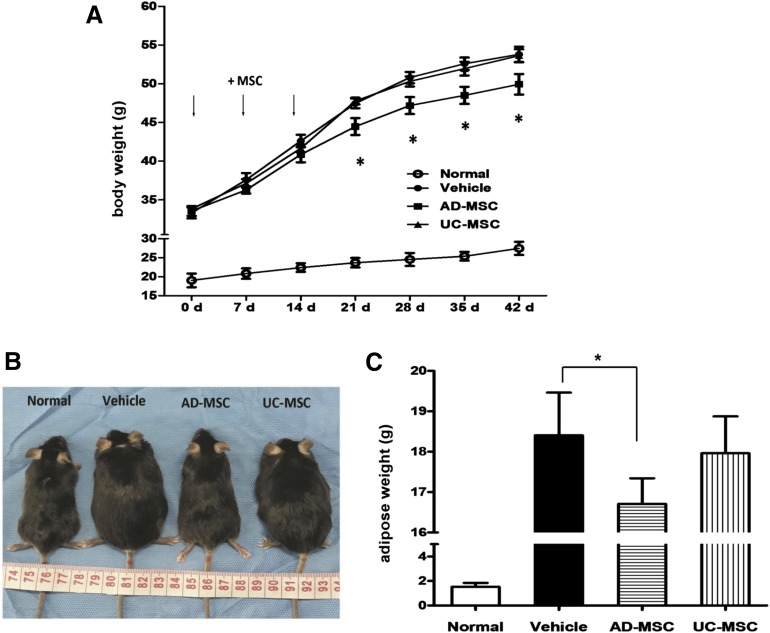
To test our hypothesis, we investigated the effect of AD-MSCs in system metabolic disturbance. We used db/db mice in which the leptin receptor was genetically deleted; this model is characterized by obesity, insulin resistance, hyperglycemia, and dyslipidemia. UC-MSCs were used as the control. AD-MSCs and UC-MSCs are identical with regard to their cell surface immunophenotype, and both types of cells are capable of differentiating into osteoblasts, adipocytes, and chondrocytes under the appropriate in vitro conditions (supplemental online Fig. 1). As was expected, 3 weeks after AD-MSC administration, the increase in body weight (BW) was suppressed, and adipose tissue weight (ATW) was decreased in the db/db mice in comparison with the no-treatment group. Intriguingly, UC-MSCs had little or no effect on the BW and ATW of the animals (Fig. 1A–1C). Our data suggest that MSCs isolated from different sources may differ in their physiological functions.
Figure 1.
Resistance against obesity in AD-MSC treated mice. (A): The time course of body weight during 6 weeks in normal mice and db/db obese mice injected i.p. with saline (vehicle), AD-MSCs, and UC-MSCs. The cells were injected three times, as indicated. (B): Gross appearance of normal mice and db/db mice injected i.p. with saline, AD-MSCs, and UC-MSCs. (C): The abdominal adipose weights in mice of the indicated groups. Results are expressed as the mean ± SEM (n = 6). ∗, p < .05 vs. the vehicle group. Abbreviations: AD-MSC, adipose-derived mesenchymal stem cell; d, days; MSC, mesenchymal stem cell; UC-MSC, umbilical cord-derived mesenchymal stem cell.
To confirm the antiobesity and lipid-lowering effects of AD-MSCs, we also established a diet-induced obesity (DIO) mice model. Male C57B/6J mice were fed a standard diet or an HFD for 6 weeks. Similar to the db/db mouse model, only AD-MSC administration significantly reduced the BW of the DIO mice (supplemental online Fig. 2A).
AD-MSC Transplantation Ameliorated Blood Glucose and Lipid Disorders in Mice
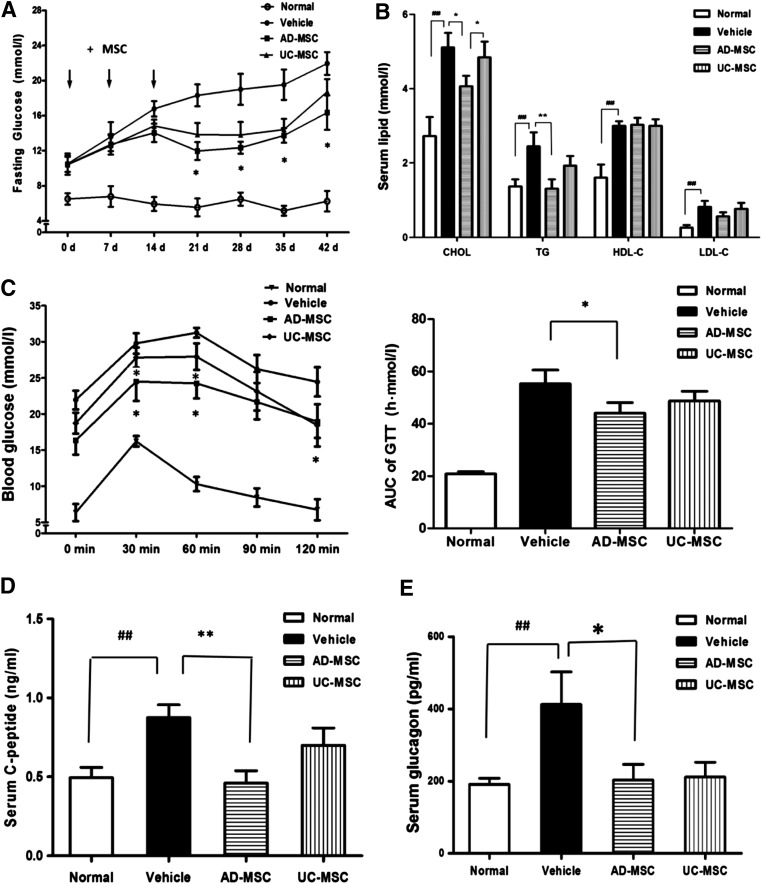
Both AD-MSCs and UC-MSCs have an antidiabetic effect. Here, the fasting serum glucose level of db/db mice was determined once a week for 6 weeks. As is shown in Figure 2A, both AD-MSC and UC-MSC administration remarkably decreased the fasting blood glucose levels of db/db mice. It was noted that the glucose-lowering effect of AD-MSCs was slightly greater than that of UC-MSCs, but the difference was not significant. The same phenomenon was observed from the results of the oral glucose tolerance test (Fig. 2C). Interestingly, and in accordance with our initial expectations, the plasma lipid profiles of AD-MSC-treated mice were significantly improved, as manifested by the markedly reduced triglyceride (TG), cholesterol (CHOL), and low-density lipoprotein cholesterol (LDL-C) levels. In contrast, UC-MSCs had little or no effect on dyslipidemia in the animals (Fig. 2B). Similar results were also observed in the DIO mice (supplemental online Fig. 2B). In addition, the plasma C-peptide and glucagon levels of the db/db mice were decreased after AD-MSC and UC-MSC treatment, but only the C-peptide level was significantly decreased after AD-MSC treatment (Fig. 2D, 2E). These observations suggest that AD-MSCs could enhance insulin sensitivity.
Figure 2.
AD-MSC induced reduction in blood glucose and improvement of the plasma lipid profiles of db/db mice. (A): Time course of the fasting blood glucose concentrations of normal mice and db/db mice injected i.p. with saline, AD-MSCs, and UC-MSCs. (B): The serum TG, CHOL, HDL-C, and LDL-C levels in the indicated groups. (C): Blood glucose concentration from oral glucose tolerance tests in normal mice and db/db mice injected i.p. with AD-MSCs and UC-MSCs. Curve of GTT (left) and AUC of GTT (right) are illustrated. Serum C-peptide (D) and glucagon levels (E) in each group. The results are expressed as the mean ± SEM (n = 6). ∗, p < .05; ∗∗, p < .01 vs. the vehicle group; ##, p < .01 vs. the normal group. Abbreviations: AD-MSC, adipose-derived mesenchymal stem cell; AUC, area under the curve; CHOL, cholesterol; d, days; GTT, glucose tolerance test; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; UC-MSC, umbilical cord-derived mesenchymal stem cell.
AD-MSCs Promoted Recovery of Adipose Tissues
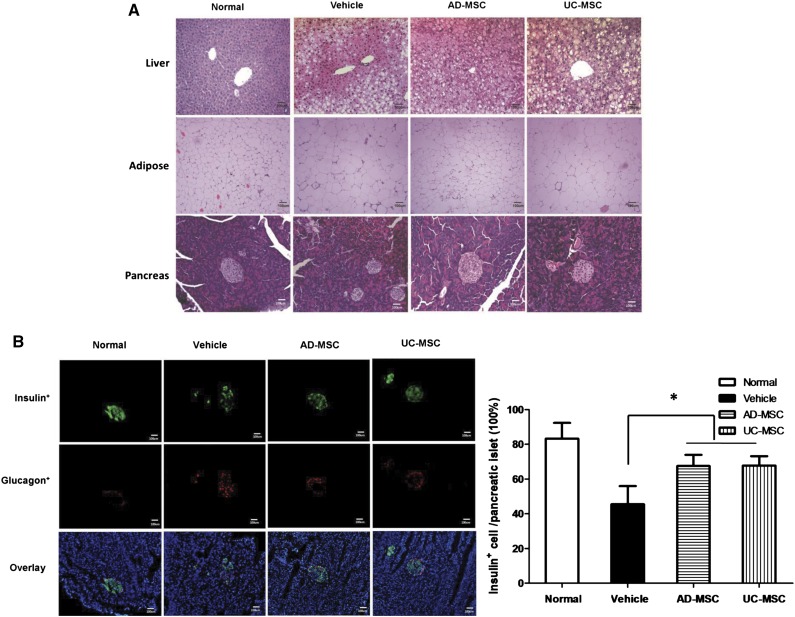
Next, we performed histopathological analysis to determine the potential target tissues or organs of AD-MSCs. Microscopic observation showed that administration of both AD-MSCs and UC-MSCs resulted in the recovery of pancreatic islets and liver structures of the db/db mice (Fig. 3A). Enzyme measurements showed that AST, ALT, and ALP levels in the AD-MSC- and UC-MSC-treated db/db mice were dramatically reduced; thus, the histopathological findings were confirmed (supplemental online Fig. 3). Likewise, the β cell percentage was noticeably increased in the pancreatic islets of both AD-MSC- and UC-MSC-treated db/db mice (Fig. 3A, 3B), as evidenced by the results of immunofluorescence staining. However, histological observation of adipose tissues showed that the size of abdominal adipocytes in the AD-MSC-treated mice had decreased substantially, but this was not observed in the UC-MSC-treated mice (Fig. 3A). This is interesting, because this response of adipose tissues to AD-MSC treatment may partially explain the antiobesity and lipid-lowering effects.
Figure 3.
Improvement in the tissue structures of the liver, adipose, and pancreas in db/db mice (scale bars = 100 µm). (A): Hematoxylin and eosin staining of representative liver, adipose, and pancreas sections obtained from mice of the indicated groups. (B): Immunofluorescence staining for insulin-positive cells (green) and glucagon-positive cells (red) in representative pancreas sections obtained from mice of the indicated groups. The results are expressed as the mean ± SEM (n = 5 sections per group). ∗, p < .05 vs. the vehicle group. Abbreviations: AD-MSC, adipose-derived mesenchymal stem cell; UC-MSCs, umbilical cord-derived mesenchymal stem cells.
AD-MSCs Influenced Expression of BAT-Specific Genes in Adipose Tissues
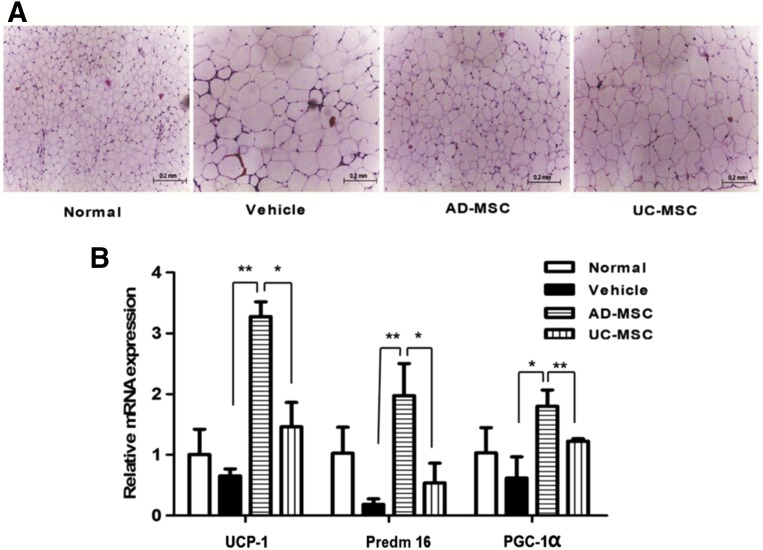
Using the DIO mouse model, we further confirmed the adipose tissue-targeted effect of AD-MSCs (Fig. 4A). WAT browning refers to the development of brown or beige adipocytes in WAT and holds great promise in counteracting metabolic disease, including obesity and type 2 diabetes. In fact, increased activity of brown and beige adipocytes has been demonstrated to have antiobesity effects in several mouse models [22–24]. As was expected, the mRNA expression of brown adipose-specific genes, uncoupling protein 1, PRD1-BF1-RIZ1 homologous domain-containing 16, and peroxisome proliferator-activated receptor γ coactivator 1-α were markedly increased in the visceral adipose tissues of AD-MSC-treated mice. This increase was not observed in the mice treated with UC-MSCs (Fig. 4B).
Figure 4.
Improvement in the structure of adipose tissues and changes in gene expression in brown/beige fat in diet-induced obesity mice. (A): Hematoxylin and eosin staining of representative adipose sections from mice of the indicated groups (scale bar = 200 µm). (B): Quantitative polymerase chain reaction for brown adipose-specific genes in abdominal adipose tissues from mice of the indicated groups (∗, p < .05; ∗∗, p < .01). Abbreviations: AD-MSC, adipose-derived mesenchymal stem cell; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-α; Predm 16, PRD1-BF1-RIZ1 homologous domain-containing 16; UC-MSCs, umbilical cord-derived mesenchymal stem cells; UCP-1, uncoupling protein 1.
AD-MSCs Regulated Activity of Lipid Metabolism Enzymes in Adipose Tissues by Activating AMPK Signaling Pathway
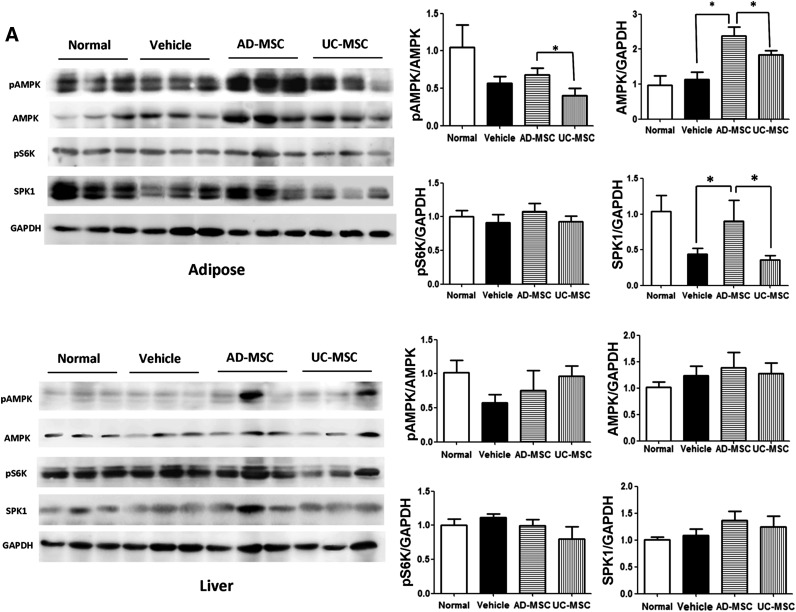
AMPK is a central regulator of cellular metabolism, and an increase in AMPK activity accelerates lipolysis [25, 26]. AMPK plays a role in the maintenance of cellular as well as whole-body energy homeostasis in adipose and liver tissues [27]. Using immunoblot analysis, we found that the expression of and phosphorylation of the AMPK-α subunit were enhanced in the adipose tissues of AD-MSC-treated DIO mice, but not in the UC-MSC-treated or nontreated mice. Interestingly, the expression of and phosphorylation of AMPK were not significantly different in the liver tissues between the groups. Ribosome protein subunit 6 kinase 1 (S6K1) and sphingosine kinase 1 (SPK1), two other crucial kinases involved in energy metabolism, have also been analyzed in adipose and liver tissues. Similar to the findings for sphingosine-1-phosphate receptor 1 (S1P1-4), enhanced SPK1 expression was observed in the adipose tissues of AD-MSC-treated mice, but not in the UC-MSC-treated mice. S6K1 activity showed no obvious change in the liver and adipose tissues of both groups (Fig. 5A).
Figure 5.
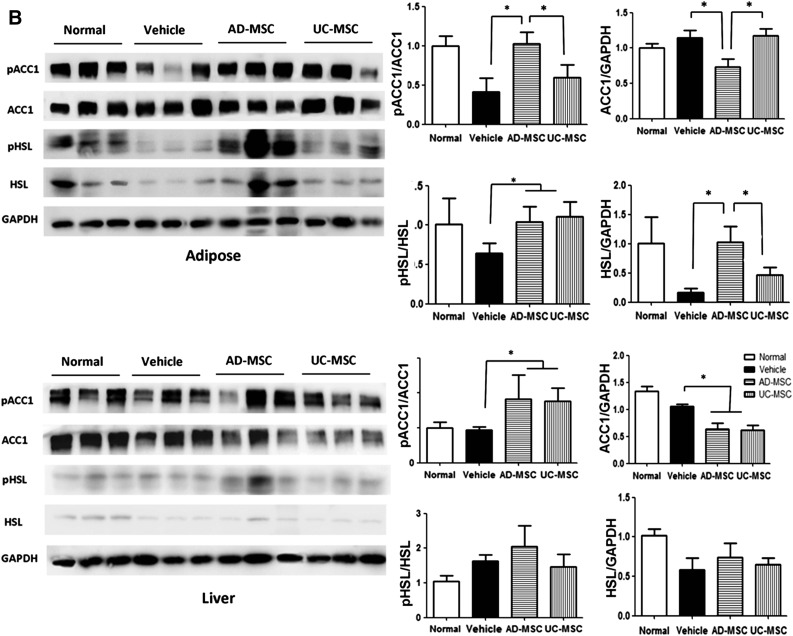
Activation of AMPK HSL/ACC1 signaling cascades in the adipose tissues of diet-induced obesity mice after AD-MSC administration. (A): Western blotting against the indicated molecules in the liver and adipose tissues from the mice of each group. (B): Western blotting analysis of pACC1 and pHSL or total ACC1 and HSL in the liver and adipose tissues of mice. The results are expressed as the mean ± SEM (n = 3 samples per group). ∗, p < .05 vs. the vehicle group. Abbreviations: AD-MSC, adipose-derived mesenchymal stem cell; AMPK, adenosine monophosphate-activated protein kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSL, hormone-sensitive lipase; pACC1, phospho-ACC1; pAMPK, phospho-AMPK; pHSL, phospho-HSL; UC-MSCs, umbilical cord-derived mesenchymal stem cells.
Acetyl-CoA carboxylase1 (ACC1), a key enzyme in the lipogenic pathway, synthesizes malonyl-CoA from acetyl-CoA and is one of the most important substrates of AMPK [28]. HSL, which is a key triglyceride lipase in adipose tissue, is also a substrate of AMPK. Lipolysis in the WAT of rodents and humans is regulated by HSL [29]. In the liver and visceral adipose tissues of mice, we found that ACC1 phosphorylation increased after AD-MSC treatment in comparison with UC-MSC treatment or no treatment, which means that the ACC1 activity was inhibited after AD-MSC treatment. However, ACC1 expression and phosphorylation showed no significant difference in the liver tissues between the groups (Fig. 5B). We also found that the expression and phosphorylation levels of HSL were considerably increased in the adipose tissues of AD-MSC-treated mice (Fig. 5B). These results indicate that the decreased adiposity in AD-MSC-treated mice is a result of enhanced lipolysis and decreased lipogenesis via activation of HSL and inhibition of ACC1 activity.
Discussion
Twenty years have elapsed since the results of the Scandinavian Simvastatin Survival Study (4S) were published in The Lancet in 1994 [30]. Currently, dyslipidemia is well established as the cause of atherosclerotic vascular disease. In a recent issue of The Lancet, a series of three articles about lipids (high-density lipoprotein cholesterol [HDL-C], LDL-C, and TG) and cardiovascular disease were published [31–33], which further underlined the significance of dyslipidemia in cardiovascular disease. Here we demonstrate for the first time that AD-MSC infusion corrected dyslipidemia in mouse models. The therapeutic effects look promising, as is evidenced by the reduced CHOL, TG, and LDL-C levels and the increased HDL-C level. In both the db/db and DIO mouse models, AD-MSC treatment also significantly suppressed BW increase in the mice. Thus, AD-MSC transplantation may be of much benefit and hold great promise for counteracting dyslipidemia and its associated diseases.
The 4S study was the first to provide evidence of the functions of statins, and statins are now the most widely prescribed class of drugs in the history of medicine. However, although this class of drug could lower LDL-C in the most effective way and prevent atherosclerosis, it has several side effects, such as liver damage, muscle ache, and diabetes. Currently, several novel drugs targeting different key molecules in lipid metabolism are under intense investigation, from the viewpoint of improving dyslipidemia and reducing cardiovascular events. These drugs include the monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) [34], the cholesteryl ester transfer protein inhibitors [35], and the antisense apolipoprotein inhibitor [36], among others. The primary results from clinical trials have demonstrated their safety and efficacy. Here we found that AD-MSC infusion dramatically improved the serum lipid profiles in mice. We believe that this is an important contribution to research on metabolic disorders and cardiovascular disease.
In the last few years, stem cell therapy has received great interest as a treatment for miscellaneous difficult-to-treat diseases, such as nervous system diseases, ischemic heart diseases, and autoimmune diseases [37–39]. Moreover, several stem cell drugs have been approved for clinical application. For example, Cartistem (umbilical cord blood-derived MSCs) was approved for cartilage regeneration, and Prochymal (BM-MSCs) was approved for treating acute graft versus host disease. Cell-based therapeutics is regarded as the third pillar of medicine, along with small-molecule drugs and engineered antibodies or proteins [40]. Among the different cell types that have been used, MSCs are considered to be one of the most promising types of stem cells for translational application because of their rich tissue sources, multilineage differentiation capacity, easy amplification in vitro, and unique immune biological properties [41, 42]. Perhaps one of the most exciting findings in MSC research is the discovery of an abundant number of MSCs within the adipose tissues of the adult body. Currently, AD-MSCs are widely used in regenerative medicine—such as wound healing, breast reconstruction, and augmentation mammoplasty—and in the treatment of diseases [43, 44]. Here we found another important potential application of AD-MSCs, namely, as antimetabolic therapy for obesity, dyslipidemia, and associated diseases. From the point of view of pharma projects, AD-MSCs may be one of the best candidates for antimetabolic drugs. Unlike statins and other drugs, AD-MSCs have little or no side effects, and they actually balance lipid metabolism and do not just lower the levels of harmful lipoproteins or TGs. We can also prevent or treat dyslipidemia or associated cardiovascular diseases through autologous AD-MSC infusion, thus turning “waste” into wealth. In addition, our results showed that the lipid-lowering effect of AD-MSCs is long lasting; this is also largely different from the effects of routine drugs. However, we still need to investigate how long this effect can be maintained.
As is shown in many studies, MSCs could home in to injured tissues. In diabetic nonobese diabetic/severe combined immunodeficiency mice, intracardiac-infused human MSCs were demonstrated to home in to pancreas and kidney tissues but not to other noninjured tissues [45]. The differences in homing tissues between intraperitoneal and intravenous injection were investigated in an article by Castelo-Branco et al. [46]. As is shown by their results, the intraperitoneally injected MSCs migrated toward the injured site (inflamed colon) but not the noninjured tissues, whereas the intravenously infused MSCs accumulated first in the lungs and then migrated to other noninjured organs including liver, spleen, kidney, and bladder through blood circulation. In our study, MSCs were injected intraperitoneally. Genomic DNA were collected from treated mouse tissues that were preserved at −80°C. By real-time polymerase chain reaction, β-globin of human was detected in liver, adipose, and pancreas in both AD-MSC- and UC-MSC-treated mice. No significant difference in homing was observed between AD-MSCs and UC-MSCs (data not shown).
Increased expression and activity of AMPK and HSL in adipose tissues appear to be crucial in mediating the antimetabolic effect of AD-MSCs. AMPK, a key kinase in energy metabolism, has been proven to be an important target of antimetabolic drugs [47]. For example, statins and metformin, two widely used antimetabolic drugs, are potent activators of AMPK [48, 49]. Additionally, two phase 2 studies showed that a novel agent that targets hepatic adenosine triphosphate citrate lyase and AMPK reduced LDL-C by 27% [50] in patients with hypercholesterolemia and by 43% in those with diabetes [51]. It is not clear why AD-MSC treatment selectively activated AMPK and HSL in adipose tissue. It is possible that AD-MSCs reach adipose tissue and activate AMPK and HSL via secretion of certain factors. This observation suggests that AMPK-mediated activation of HSL is critical for the antimetabolic effects of AD-MSCs.
Conclusion
AD-MSC infusion can significantly suppress the increase in body weight and remarkably improve dyslipidemia in db/db obese mice and diet-induced (DIO) mice. AD-MSC holds great therapeutic potential for dyslipidemia and associated cardiovascular diseases.
Supplementary Material
Acknowledgments
This study was supported by the National Science Foundation of China (Grants 81270894 and 81201760).
Author Contributions
G.-Y.L. and J.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.-L.W., Y.L., Y.S., Y.H., Y.-R.Q., F.-J.X., P.-F.L., L.-J.Z., E.-Y.G., and S.-Y.C.: collection and/or assembly of data; L.-H.G.: administrative support, provision of study material or patients; C.-T.W.: financial support; X.-W.H. and H.-F.D.: conception and design, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwald M, Perdikari A, Rülicke T, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 6.Lo JC, Ljubicic S, Leibiger B, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms M, Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traktuev DO, Prater DN, Merfeld-Clauss S, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 15.Rajashekhar G, Ramadan A, Abburi C, et al. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9:e84671. doi: 10.1371/journal.pone.0084671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nae S, Bordeianu I, Stăncioiu AT, et al. Human adipose-derived stem cells: Definition, isolation, tissue-engineering applications. Rom J Morphol Embryol. 2013;54:919–924. [PubMed] [Google Scholar]
- 17.Kono TM, Sims EK, Moss DR, et al. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. stem cells. 2014;32:1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si Y, Zhao Y, Hao H, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61:1616–1625. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amit A, Tuabin R. A simple plunger technique for negative pressure during fat harvesting. J Plast Reconstr Aesthet Surg. 2014;67:e105–e106. doi: 10.1016/j.bjps.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 21.Di G, Wang J, Liu M, et al. Development and evaluation of a trehalose-contained solution formula to preserve hUC-MSCs at 4°C. J Cell Physiol. 2012;227:879–884. doi: 10.1002/jcp.23066. [DOI] [PubMed] [Google Scholar]
- 22.Cederberg A, Grønning LM, Ahrén B, et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 23.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopecky J, Clarke G, Enerbäck S, et al. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 26.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SP, Carling D, Munday MR, et al. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203:615–623. doi: 10.1111/j.1432-1033.1992.tb16591.x. [DOI] [PubMed] [Google Scholar]
- 29.Watt MJ, Holmes AG, Pinnamaneni SK, et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 30.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 31.Ridker PM. LDL cholesterol: Controversies and future therapeutic directions. Lancet. 2014;384:607–617. doi: 10.1016/S0140-6736(14)61009-6. [DOI] [PubMed] [Google Scholar]
- 32.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 33.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 34.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 35.Rader DJ, deGoma EM. Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med. 2014;65:385–403. doi: 10.1146/annurev-med-050311-163305. [DOI] [PubMed] [Google Scholar]
- 36.Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 37.Fox IJ, Daley GQ, Goldman SA, et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernal A, Gálvez BG. The potential of stem cells in the treatment of cardiovascular diseases. Stem Cell Rev. 2013;9:814–832. doi: 10.1007/s12015-013-9461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 40.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: The next pillar of medicine. Sci Transl Med. 2013;5:179ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 42.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Sato K, Aoi N et al. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 2008;32:48–55; discussion 56–47. [DOI] [PMC free article] [PubMed]
- 44.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med. 2012;7:225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castelo-Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 48.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W, Lee TS, Zhu M, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 50.Ballantyne CM, Davidson MH, Macdougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: Results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62:1154–1162. doi: 10.1016/j.jacc.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–683. doi: 10.1161/ATVBAHA.113.302677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.