Abstract
The aims of this study were to compare cut points for weakness proposed by Foundation for the National Institutes of Health (FNIH) Sarcopenia Project with cut points estimated with our own data; to assess the prevalence of clinically relevant handgrip strength (HGS) weakness according to published criteria across distinct populations of older adults; to estimate the ability of HGS weakness to identify slowness. This is a cross-sectional analysis of International Mobility in Aging Study (IMIAS) involving 1935 community-dwelling older adults, between 65 and 74 years, who completed HGS and gait speed assessment. We used baseline data from Tirana (Albania), Natal (Brazil), Manizales (Colombia), Kingston (Ontario, Canada), and Saint-Hyacinthe (Quebec, Canada). Weakness was defined according to sex-specific HGS cut points associated with slowness proposed by FNIH Sarcopenia Project. Slowness was defined as gait speed <0.8 m/s. IMIAS cut points for clinical weakness had good agreement with those proposed by FNIH. Weakness prevalence across the research sites ranged from 1.1 % (Saint-Hyacinthe) to 19.2 % (Manizales) among men. Women from Manizales (13.5 %) and Natal (19.3 %) had higher prevalence of weakness than their counterparts. FNIH cut points had a strong association with slowness, for both sexes. The IMIAS population generated cut points which were close to those proposed by FNIH. There was large variability in prevalence of weakness across our research sites. The HGS cut points for weakness proposed by FNIH performed well in IMIAS populations, providing a useful tool for screening older adults at risk for functional problems.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9888-z) contains supplementary material, which is available to authorized users.
Keywords: Handgrip strength, Mobility limitation, Sarcopenia, Aging
Introduction
Population aging is accelerating in middle-income countries, and there is a need for research on valid clinical predictors of physical function decline (García-Peña et al. 2013). A recognized change associated with aging is a progressive decline in skeletal muscle strength (Bohannon 2008a; Walston 2012). Declines in skeletal muscle strength predicts changes in physical functioning (Lauretani et al. 2003; Vermeulen et al. 2011), disability(Cooper et al. 2011), falls (Tanimoto et al. 2014), and mortality(Legrand et al. 2014).
A measure widely used for assessment of muscle strength in geriatric patients is the handgrip strength (HGS), a simple, quick, and inexpensive method. HGS assessment is relatively easy to implement and can be assessed even with bedridden patients (Savino et al. 2013; Beseler et al. 2014). This makes it an attractive and frequently used tool for clinical purposes and epidemiologic studies. Low HGS values have been associated with limited mobility (Sillanpää et al. 2014) and hospitalization (Cawthon et al. 2009). HGS is considered to be a key component of sarcopenia (Lauretani et al. 2003; Cruz-Jentoft et al. 2010), the frailty phenotype (Fried et al. 2001), and a marker of nutritional status (Norman et al. 2011).
Recently, several HGS cut points have been proposed to identify clinically relevant weakness (Zalewski et al. 2009; Cruz-Jentoft et al. 2010; Sallinen et al. 2010; Hicks et al. 2012; Seino et al. 2014; Alley et al. 2014). The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project working group published cut points of handgrip weakness associated with slowness in walking (Alley et al. 2014). This study was based on 11 diverse cohorts from the USA and Italy with considerable functional variability going from very high function assessed in the cohort of the Framingham Offspring Study to poor function assessed in the Boston Puerto Rican cohort. However, with few exceptions (García-Peña et al. 2013; Lourenço et al. 2015), all published studies have been carried out on populations living in high-income countries. Globally validated cut points for clinically relevant weakness could provide health professionals an important tool to identify older adults at risk for functional problems (Hicks et al. 2012; Alley et al. 2014).
Using the International Mobility in Aging Study (IMIAS), a study on older adult urban populations from different societies in four countries with varying degree of human development: Canada, Brazil, Colombia, and Albania (United Nations Development Programme 2014; Zunzunegui et al. 2015), the aims of this research are the following: (a) to compare FNIH-proposed criteria with IMIAS internally defined cut points of handgrip weakness associated with slowness, (b) to assess the prevalence of clinically relevant handgrip strength weakness according to FNIH-proposed criteria, and (c) to estimate the ability of handgrip strength weakness to identify slowness in gait speed across distinct populations of older adults.
Methods
Design and participants
This is a cross-sectional study using data from the International Mobility in Aging Study (IMIAS). IMIAS is a population-based prospective cohort study that is ongoing at five sites: Tirana (Albania), Natal (Brazil), Manizales (Colombia), Kingston (Ontario, Canada), and Saint-Hyacinthe (Quebec, Canada). Data were collected at baseline in 2012. Details of the study design have been described elsewhere (Sousa et al. 2014; Pirkle et al. 2014; Zunzunegui et al. 2015).
The study population was composed of community-dwelling men and women aged 65 to 74 years. Stratification by sex aimed at recruiting 200 men and 200 women at each site. The sample size at each site was calculated to allow for comparison of baseline mobility disability prevalence of men and women assuming a prevalence ratio of 1.8, error type I of 0.05, and power of 0.80. Baseline data was collected in 2012: from January to June in Manizales, Natal, and Saint-Hyacinthe; from January to December in Kingston; and from September to December in Tirana.
The final sample size was composed of 1995 older adults. Of these, 921 men and 1014 women had complete data on height, weight, grip strength, and gait speed and were included in this study. There were no differences in age and sex between subjects with complete data and those with incomplete data; however, subjects with incomplete data had worse self-reported mobility (assessed by the Life Space Assessment scale).
Sampling strategy
Participants were recruited through neighborhood primary care center registers at Tirana, Manizales, and Natal. At these sites, a random sample of elderly people registered at the health centers was drawn and participants were approached directly by our interviewers to invite them to participate in the study. In Kingston and Saint-Hyacinthe, participants received a letter from their primary care doctors inviting them to contact our field coordinator to make an appointment for the home visits. Since Albania, Brazil, and Canada have universal health care systems; more than 90 % of the population in the 65 to 74 age range are registered at a health center or have a primary care doctor. In Tirana and Natal, two and five neighborhood health centers, respectively, were covered by our sampling scheme. These neighborhoods are located in middle and low socioeconomic areas in both cities, and most of our participants in these two sites were of low and middle socioeconomic status. In choosing the study neighborhoods, we purposefully avoided the extremes of the socioeconomic spectrum: the wealthy and the very poor areas. In Manizales, a random sample of all subjects between 65 and 74 years of age registered in the Public Health Insurance of the city was drawn. Most of the population in this age group is covered by this Public Health Insurance.
Data collection
At all research sites, study procedures were carried out at the participant’s home unless that person requested otherwise. In Manizales, physical performance was evaluated at the local hospital. Interviewers at each site were trained using the same standard training based on videotapes, protocol instructions, and data entry forms. The questionnaires, data collection documents, and procedure manuals were available in each local language.
Measures
Muscle strength was assessed by handgrip strength using a handheld dynamometer (Jamar Hydraulic Hand Dynamometer®). Participants were instructed and verbally encouraged to grip the handle as hard as possible using their dominant hand. The measurement protocol for handgrip strength followed the recommendations of The American Society of Hand Therapists. That protocol calls for participants to be seated, shoulders adducted and neutrally rotated, elbow flexed at 90, forearm in a neutral position, and the wrist between 0 and 30 of dorsiflexion (Fess 1992). Three trials were performed, and the highest value in kilograms was used in the analyses. The reliability of the HGS test measured using intra-class correlation has been excellent (ICC >0.90) (Schrama et al. 2014).
The HGS cut points to define weakness were based on the FNIH criteria. HGS values less than 26 kg for men and 16 kg for women were considered weak, values between 26 to 31.9 kg for men and 16 to 19.9 kg for women were classified in the intermediate group, and values greater than 32 kg for men and 20 kg for women were classified as normal strength. These specific cut points may reflect weakness due to low muscle mass (Alley et al. 2014).
Assessment of gait speed was done over on a 4-m course at usual walking speed from a standing position. Gait speed was assessed twice for each participant, and the average was calculated in meters per second. Slowness was defined as a speed lower than 0.8 m/s. This cut point was used based on a previous research assessing its association with risk of adverse outcomes (Cruz-Jentoft et al. 2010; Studenski et al. 2011; Alley et al. 2014).
Height (m) was measured to the nearest 0.1 cm with a stadiometer. Weight (kg) was measured with an electronic scale with participants wearing light indoor clothes and no shoes. Body mass index (BMI) was calculated from weight and height2 (kg/m2). Waist circumference (cm) was assessed using a non-elastic tape at the midpoint between the lower border of the rib cage and the iliac crest.
Statistical analysis
Sample characterization was provided using descriptive statistics. All analyses are presented separately by sex and research site because strength and body size differ significantly by sex and across cities. Distributions of categorical variables are presented as frequencies and percentages. Continuous variables are presented as means and standard deviations (SD). The differences of general characteristics between research sites and FNIH Project sites were analyzed using independent t test for continuous variables. In some analyses, the research sites were categorized as follows: Kingston and Saint Hyacinthe (Canada), Tirana (Albania), and Natal and Manizales (Latin America).
We used Classification Regression Trees (CART), as explained in Alley et al. (2014), to identify IMIAS cut points for grip strength associated with slowness. This statistical procedure can identify subgroups of a population whose members share common characteristics that influence the dependent variable of interest (De’ath and fabricius 2000; Lemon et al. 2003; Razi and Athappilly 2005). CART analysis was performed using IBM SPSS Statistics 20.0. The tree was pruned to the most parsimonious model within one standard prediction error of the tree with the smallest prediction error. CART analysis to verify the associations between muscle strength and slowness has been used in previous studies (Hicks et al. 2012; Alley et al. 2014). We then estimated weighted kappa coefficients to assess the agreement between the FNIH and the IMIAS classifications of clinically relevant weakness.
For external comparison, we added data from the two population samples participating in the FNIH Sarcopenia Project which had similar age distribution as the IMIAS populations, as published in the supplementary material of Alley et al. (2014): first, the BPRHS subsample with average age of 69.9 ± 3.5 in men and 69.0 ± 3.1 in women and, second, the Framingham Offspring with an average age of 70.8 ± 4.2 in men and 70.4 ± 4.1 in women.
We estimated, in the IMIAS samples, the prevalence of clinically relevant weakness using handgrip strength categories proposed by the FNIH Project (Alley et al. 2014; Studenski et al. 2014) and compared them with the FNIH published data on their total population aged 65 to 79 and to the Framingham Offspring cohort and the Boston Puerto Rican cohort. We then estimated the odds ratio of slowness with weakness compared with the “normal strength” group as the referent. Lastly, we conducted sensitivity analyses by examining this association between weakness and slowness by categories of height, BMI, and abdominal obesity. We also examined the associations between the ratio of handgrip strength to body size (HGS/BMI) and slow walking, because previous studies have demonstrated that mobility impairment and weakness may be differently associated across BMI categories (Sallinen et al. 2010; Alley et al. 2014).
Results
The five IMIAS populations are very different in socioeconomic indicators, as reflected by education and current levels of insufficient income to cover basic needs. Table 1 shows the socioeconomic indicators for each research site and by sex.
Table 1.
Socioeconomic indicators from each research site and by sex
| IMIAS sites | |||||
|---|---|---|---|---|---|
| Kingston, n (%) | Saint-Hyacinthe, n (%) | Tirana, n (%) | Manizales, n (%) | Natal, n (%) | |
| Men | |||||
| Education level | |||||
| Less secondary | 2 (1.1 %) | 14 (7.4 %) | 15 (8.2 %) | 123 (67.6 %) | 132 (69.8 %) |
| Secondary | 41 (23.0 %) | 72 (38.1 %) | 37 (20.2 %) | 25 (13.7 %) | 44 (23.3 %) |
| Post-secondary | 135 (75.8 %) | 103 (54.5 %) | 131 (71.6 %) | 34 (18.7 %) | 13 (6.9 %) |
| Income | |||||
| Very sufficient | 113 (63.5 %) | 99 (52.4 %) | 4 (2.2 %) | 10 (5.6 %) | 9 (4.8 %) |
| Sufficient | 59 (33.1 %) | 81(42.9 %) | 74 (40.4 %) | 44 (24.7 %) | 48 (25.4 %) |
| Insufficient | 6 (3.4 %) | 9 (4.8 %) | 105 (57.4 %) | 124 (69.7 %) | 133 (69.8 %) |
| Living arrangements | |||||
| Alone | 31 (17.5 %) | 27 (14.4 %) | 4 (2.2 %) | 22 (12.1 %) | 9 (4.8 %) |
| Only spouse | 73 (41.2 %) | 138 (72.9 %) | 94 (51.4 %) | 41 (22.5 %) | 47 (24.9 %) |
| Children, spouse, or others | 73 (41.2 %) | 24 (12.8 %) | 85 (46.4 %) | 119 (65.4 %) | 133 (70.4 %) |
| Self-rated health | |||||
| Good | 154 (87.0 %) | 159 (84.1 %) | 77 (42.1 %) | 99 (54.4 %) | 67 (35.4 %) |
| Fair | 19 (10.7 %) | 28 (14.8 %) | 86 (47.0 %) | 72 (39.6 %) | 101 (53.4 %) |
| Poor | 4 (2.3 %) | 2 (1.1 %) | 20 (10.9 %) | 11 (6.0 %) | 21 (11.1 %) |
| Women | |||||
| Education level | |||||
| Less secondary | - | 12 5.9 %) | 29 (14.5 %) | 149 (77.2 %) | 180 (84.9 %) |
| Secondary | 44 (21.3 %) | 92 (45.5 %) | 66 (33.0 %) | 34 (17.6 %) | 27 (12.7 %) |
| Post-secondary | 163 (78.7 %) | 98 (48.5 %) | 105 (52.5 %) | 10 (5.2 %) | 5 (2.4 %) |
| Income | |||||
| Very sufficient | 126 (60.9 %) | 78 (38.1 %) | 4 (2.0 %) | 7 (3.7 %) | 7 (3.3 %) |
| Sufficient | 67 (32.4 %) | 106 (52.5 %) | 61 (30.7 %) | 44 (23.5 %) | 40 (18.9 %) |
| Insufficient | 14 (6.8 %) | 19 (9.4 %) | 134 (67.3 %) | 136 (72.7 %) | 165 (77.8 %) |
| Living arrangements | |||||
| Alone | 86 (41.5 %) | 69 (34.3 %) | 32 (16.01 %) | 24 (12.4 %) | 14 (6.6 %) |
| Only spouse | 96 (46.4 %) | 116 (57.7 %) | 75 (37.7 %) | 24 (12.4 %) | 29 (13.7 %) |
| Children, spouse or others | 25 (12.1 %) | 16 (8.0 %) | 92 (46.2 %) | 145 (75.1 %) | 169 (79.7 %) |
| Self-rated health | |||||
| Good | 172 (83.5 %) | 166 (82.2 %) | 59 (29.5 %) | 86 (44.8 %) | 48 (22.7 %) |
| Fair | 27 (13.1 %) | 32 (15.8 %) | 110 (55.0 %) | 94 (49.0 %) | 120 (56.9 %) |
| Poor | 7 (3.4 %) | 4 (2.0 %) | 31 (15.5 %) | 12 (6.2 %) | 43 (20.4 %) |
Anthropometric measures and functional indicators by sex and research sites are shown in Table 2. Men residing in the Canadian cities of Kingston and Saint-Hyacinthe were on average stronger and taller and had faster gait speed and significantly higher BMI than men residing in Manizales or Natal. Mean BMI of Tirana’s men was similar to that of Canadian men. In respect to average values of HGS, Tirana’s men were in intermediate range (34.09 kg ± 8.86), between those of Canadian men (Kingston, 41.68 ± 8.55; Saint-Hyacinthe, 42.42 kg ± 7.52) and those of Latin American men (Manizales, 31.07 kg ± 6.37; Natal, 31.88 kg ± 7.28). Tirana’s men had slower gait (0.87 m/s ± 0.24) than men from the two Canadian cities (Kingston, 1.03 m/s ± 0.19; Saint-Hyacinthe, 1.07 m/s ± 0.22) and were similar in gait speed to those from the Latin American cities (Manizales, 0.88 m/s ± 0.19; Natal, 0.85 m/s ± 0.19).
Table 2.
Distribution of physical function indicators and anthropometric measures in research site and by sex (mean ± SD)
| IMIAS sites | FNIH Project | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Kingston | Saint-Hyacinthe | Tirana | Manizales | Natal | Total | BPRHS | Framingham Offspring | |
| Men | |||||||||
| N | 921 | 178 | 189 | 183 | 182 | 189 | 9897 | 31 | 325 |
| Primary variables | |||||||||
| Walking speed (m/s) | 0.94 ± 0.23 | 1.03 ± 0.19 | 1.07 ± 0.22 | 0.87 ± 0.24 | 0.88 ± 0.19 | 0.85 ± 0.19 | 1.16 ± 0.26 | 0.71 ± 0.16a | 1.13 ± 0.21b |
| Maximum grip strength (kg) | 36.22 ± 9.15 | 41.68 ± 8.55 | 42.42 ± 7.52 | 34.09 ± 8.86 | 31.07 ± 6.37 | 31.88 ± 7.28 | 40.12 ± 9.09 | 32.43 ± 9.11 | 37.37 ± 9.20b |
| Stratification variables | |||||||||
| Age (year) | 69.13 ± 2.93 | 69.06 ± 2.77 | 68.59 ± 2.74 | 69.66 ± 3.25 | 69.18 ± 2.99 | 69.19 ± 2.78 | 74.9 ± 5.90 | 69.9 ± 3.48 | 70.76 ± 4.21b |
| BMI (kg/m2) | 27.26 ± 4.33 | 27.88 ± 4.64 | 28.37 ± 4.63 | 28.18 ± 3.90 | 25.27 ± 3.77 | 26. 59 ± 3.85 | 27.22 ± 3.86 | 31.07 ± 6.01a | 28.25 ± 4.13 |
| Height (m) | 1.68 ± 0.07 | 1.74 ± 0.06 | 1.70 ± 0.05 | 1.67 ± 0.06 | 1.63 ± 0.07 | 1.64 ± 0.07 | 1.74 ± 0.07 | 1.65 ± 0.05 | 1.73 ± 0.06c |
| Women | |||||||||
| N | 1014 | 207 | 202 | 200 | 193 | 212 | 10,950 | 89 | 339 |
| Primary variables | |||||||||
| Walking speed (m/s) | 0.88 ± 0.26 | 1.08 ± 0.25 | 1.02 ± 0.21 | 0.78 ± 0.25 | 0.79 ± 0.17 | 0.72 ± 0.19 | 0.91 ± 0.23 | 0.67 ± 0.16a | 1.09 ± 0.21b |
| Maximum grip strength (kg) | 21.32 ± 5.43 | 22.85 ± 5.95 | 23.96 ± 5.04 | 20.78 ± 5.25 | 20.08 ± 4.36 | 18.94 ± 4.81 | 20.79 ± 5.76 | 19.23 ± 4.95 | 20.35 ± 6.71b |
| Stratification variables | |||||||||
| Age (year) | 69.07 ± 2.79 | 69.13 ± 260 | 68.49 ± 2.58 | 69.14 ± 3.05 | 69.32 ± 3.02 | 69.26 ± 2.65 | 78.13 ± 5.43 | 68.96 ± 3.13 | 70.43 ± 4.13b |
| BMI (kg/m2) | 28.26 ± 5.46 | 28.16 ± 6.25 | 27.84 ± 5.52 | 29.67 ± 4.65 | 26.87 ± 4.28 | 28.68 ± 5.90 | 27.05 ± 5.09 | 31.22 ± 5.88a | 27.41 ± 5.5 |
| Height (m) | 1.54 ± 0.07 | 1.60 ± 0.05 | 1.57 ± 0.05 | 1.54 ± 0.05 | 1.49 ± 0.05 | 1.50 ± 0.05 | 1.58 ± 0.06 | 1.53 ± 0.06a | 1.59 ± 0.05b |
a p < 0.05 between BPRHS with Natal and Manizales
b p < 0.05 between Framingham Offspring with Kingston and Saint-Hyacinthe
c p < 0.05 between Framingham Offspring and Saint-Hyacinthe
Women living in the Canadian cities were stronger and had a faster gait speed than those living in Manizales, Natal, or Tirana. BMI was not different across women from different cities although Canadian women were significantly taller than their Latin American counterparts and Tirana’s women were in the intermediate range of values for height (Table 2).
The Canadian IMIAS populations of men and women were closer to the Framingham Offspring in grip strength and gait speed (although they were statistically different), and they were also similar in terms of weight and height. The Boston Puerto Rican population had distributions of functional and anthropometric indicators closer to those of the Latin American cities of Manizales and Natal. As for Tirana, gait speed and grip strength were intermediate between the Framingham Offspring cohort and the Puerto Rican cohort. As for weight and height, the Tirana male participants were close to the Framingham Offspring men although Tirana women had greater BMI than Framingham women (Table 2).
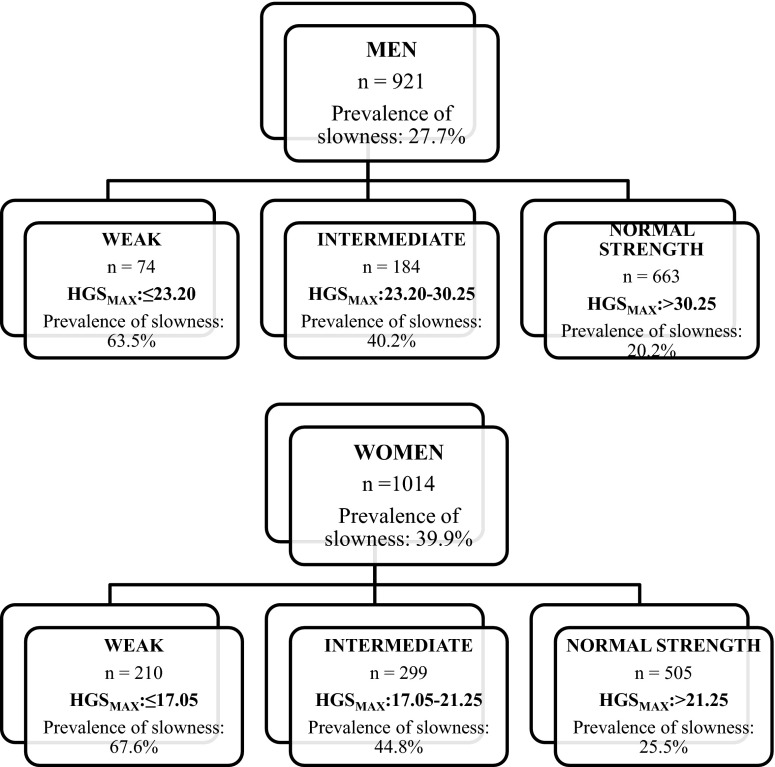
Figure 1 presents the decision tree obtained for men and women in the pooled IMIAS populations. The results are similar to those determined in the FNIH Project (<26 kg for men, <16 kg for women). To assess agreement between categories of HGS using internal IMIAS classification and the FNIH classification, we classified all IMIAS subjects by both sets of criteria and then computed a weighted kappa coefficient (wK). Agreement among men was wK = 0.89 (95 % CI, 0.86–0.92). In women, wK equals 0.64 (95 % CI, 0.60–0.67). Among men, the few cases of disagreement showed that, compared with IMIAS cut points, FNIH cut points tended to classify slightly more men as weak. The opposite was observed in women, since FNIH cut points tended to classify more women as strong compared with the IMIAS internally defined cut points. Disagreements between FNIH and IMIAS cut points were consistent at all research site (data available upon request).
Fig. 1.
Cutoff points of handgrip strength to identify slowness in IMIAS population by sex
Table 3 shows the distribution of handgrip strength categories as proposed by the FNIH Sarcopenia Project (Alley et al. 2014). Large differences were observed across study cities. For men, FNIH cohort’s observed prevalence of weakness in the 65 to 79 age groups was 3.1 %, which is consistent with men from Kingston (3.9 %) and Saint-Hyacinthe (1.1 %). Men from Tirana (17.5 %), Manizales (19.2 %), and Natal (14.8 %) had higher prevalence of weakness than the FNIH cohorts. Among women, FNIH cohorts’ weakness prevalence was 12.3 % in the age group 65–79. For women, prevalence of weakness was higher in Natal, similar in Tirana and Manizales, and lower in the Canadian sites.
Table 3.
Distribution of study population by FNIH categories of handgrip strength
| Men | |||
|---|---|---|---|
| Normal strength | Intermediate | Weak | |
| ≥32 kg | 26–32 kg | <26 kg | |
| IMIAS, n (%) | |||
| Kingston | 161 (90.4 %) | 10 (5.6 %) | 7 (3.9 %) |
| Saint-Hyacinthe | 175 (92.6 %) | 12 (6.3 %) | 2 (1.1 %) |
| Tirana | 118 (64.5 %) | 33 (18.0 %) | 32 (17.5 %) |
| Manizales | 88 (48.4 %) | 59 (32.4 %) | 35 (19.2 %) |
| Natal | 99 (52.4 %) | 62 (32.8 %) | 28 (14.8 %) |
| FNIH Project, n (%) | |||
| All population (65–79) | 6801 (89.5 %) | 566 (7.5 %) | 232 (3.1 %) |
| BPRHS | 16 (51.6 %) | 7 (22.6 %) | 8 (25.8 %) |
| Framingham Offspring | 242 (74.5 %) | 50 (15.4 %) | 33 (10.2 %) |
| Women | |||
| Normal strength | Intermediate | Weak | |
| ≥20 kg | 16–19.9 kg | <16 kg | |
| IMIAS, n (%) | |||
| Kingston | 161 (77.8 %) | 30 (14.5 %) | 16 (7.7 %) |
| Saint-Hyacinthe | 171 (84.7 %) | 21 (10.4 %) | 10 (5.0 %) |
| Tirana | 131 (65.5 %) | 41 (20.5 %) | 28 (14.0 %) |
| Manizales | 113 (58.5 %) | 54 (28.0 %) | 26 (13.5 %) |
| Natal | 112 (52.8 %) | 59 (27.8 %) | 41 (19.3 %) |
| FNIH Project, n (%) | |||
| All population (65–79) | 4523 (66.8 %) | 1417 (20.9 %) | 832 (12.3 %) |
| BPRHS | 37 (41.6 %) | 26 (29.2 % | 26 (29.2 %) |
| Framingham Offspring | 99 (41.4 %) | 77 (32.2 %) | 63 (26.4 %) |
Additional analyses (Supplementary Tables 1) examined the prevalence of weakness based on established criteria proposed by Fried et al. (2001). The Fried criteria for HGS, stratified by sex and BMI, results in higher prevalence of weakness at each site compared with the FNIH Project criteria. We also compared the prevalence of weakness by definitions from Fried et al. (2001), the FNIH Sarcopenia Project, and our internally defined cut points (Supplementary Tables 2), and it was observed that among men, FNIH and internally defined cut points produce similar categorization; among women, FNIH is closer to Fried criteria and lower than internally defined cut points.
Men from Canada and Tirana had weakness prevalence below the Framingham Offspring (10.2 %), while men in the Puerto Rican cohort (25.8 %) had higher prevalence of weakness than men in Manizales (19.2 %) or Natal (14.8 %). Women from Framingham Offspring (26.4 %) and the Puerto Rican cohorts (29.2 %) had higher prevalence of weakness than those from any IMIAS city (Table 3).
The overall prevalence of slowness and the prevalence of slowness (gait speed <0.8 m/s) according to each category of age, BMI, height, and waist circumference are shown in Table 4, for men and women in each study site. Men from Canada had low prevalence of slowness (10.6 %) in relation to their counterparts from Albania (43.2 %) and Latin America (36.9 %). The same pattern was observed in women from Canada (12.7 %), Albania (55.0 %), and Latin America (60.0 %).
Table 4.
Prevalence of slowness (gait speed <0.8 m/s) for each research site and by sex
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Canada | Albania | Latin America | Canada | Albania | Latin America | |
| All, n (%) | 39 (10.6 %) | 79 (43.2 %) | 137 (36.9 %) | 52 (12.7 %) | 110 (55.0 %) | 243 (60.0 %) |
| Age (year), n (%) | ||||||
| 65–69 | 18 (8.1 %) | 34 (40.0 %) | 69 (34.8 %) | 19 (8.2 %) | 50 (49.0 %) | 117 (55.5 %) |
| 70–74 | 20 (14.9 %) | 43 (47.3 %) | 65 (39.6 %) | 32 (18.6 %) | 55 (60.4 %) | 120 (64.5 %) |
| BMI, n (%) | ||||||
| Normal weight | 7 (7.6 %) | 17 (47.2 %) | 46 (30.7 %) | 11 (8.0 %) | 19 (63.3 %) | 54 (45.8 %) |
| Overweight | 10 (6.6 %) | 47 (46.1 % | 65 (41.1 %) | 10 (6.8 %) | 38 (51.4 %) | 106 (62.7 %) |
| Obese | 38 (18.1 %) | 15 (33.3 %) | 25 (43.9 %) | 31 (25.2 %) | 52 (55.9 %) | 81 (71.1 %) |
| Height, n (%)a | ||||||
| Tertile 1 | 16 (11.7 %) | 29 (43.9 %) | 52 (39.7 %) | 22 (14.0 %) | 48 (62.3 %) | 107 (66.9 %) |
| Tertile 2 | 15 (13.2 %) | 24 (41.4 %) | 46 (37.4 %) | 29 (11.6 %) | 33 (53.2 %) | 68(61.3 %) |
| Tertile 3 | 8 (6.9 %) | 26 (44.1 %) | 39 (33.3 %) | 1 (33.3 %) | 29 (47.5 %) | 68 (50.7 %) |
| Waist circumference, n (%) | ||||||
| Non-obese | 34 (9.7 %) | 75 (42.1 %) | 137 (37.0 %) | 8 (5.5 %) | 11 (55.0 %) | 57 (44.9 %) |
| Abdominal obesity | 5 (33.3 %) | 4 (80.0 %) | – | 44 (16.7 %) | 99 (55.0 %) | 184 (66.9 %) |
aTertiles values for men—Canada: 1.52 ≤ Tertile 1 < 1.70; 1.70 ≤ Tertile 2 < 1.75; 1.75 ≤ Tertile 3 < 1.98. Albania: 1.58 ≤ Tertile 1 < 1.68; 1.68 ≤ Tertile 2 < 1.73; 1.73 ≤ Tertile 3 < 1.84. Latin America: 1.42 ≤ Tertile 1 < 1.61; 1.61 ≤ Tertile 2 < 1.67; 1.67 ≤ Tertile 3 < 1.87. Tertiles values for women- Canada: 1.40 ≤ Tertile 1 < 1.57; 1.57 ≤ Tertile 2 < 1.61; 1.61 ≤ Tertile 3 < 1.76. Albania: 1.39 ≤ Tertile 1 < 1.52; 1.52 ≤ Tertile 2 < 1.56; 1.56 ≤ Tertile 3 < 1.77. in Latin America: 1.32 ≤ Tertile 1 < 1.48; 1.48 ≤ Tertile 2 < 1.52; 1.52 ≤ Tertile 3 < 1.75
Table 5 provides results of HGS cut points associated with slow gait speed defined according to FNIH as applied to men from Canada, Albania, and Latin America. Due to the low prevalence of weakness and slowness in our sample of Canadian men, we had to collapse handgrip strength in two groups (normal strength >32 kg; weak ≤32), instead of using the three FNIH categories. First, the cut points of HGS discriminate well those that were slow in each study site. However, confidence intervals were very wide given the small numbers of men who were slow in those Canadian cities. Second, in sensitivity analyses, we observed that the FNIH cut points were able to discriminate the slow walkers in most subgroups of BMI and height in men from Latin America. In Albanian men, weakness was significantly associated with slow gait speed in some subgroups of BMI and height.
Table 5.
Odds ratios of slowness according to handgrip weakness categories in each research site in men, by selected characteristics
| Canada | Albania | Latin America | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal strength | Weak | Normal strength | Intermediate | Weak | Normal strength | Intermediate | Weak | |
| >32 kg | ≤32 | ≥32 kg | 26–32 kg | <26 kg | ≥32 kg | 26–32 kg | <26 kg | |
| All | 1.0 (referent) | 2.77 (1.10–6.93)ǂ | 1.0 (referent) | 1.35 (0.62–2.94) | 2.37 (1.06–5.26)ǂ | 1.0 (referent) | 1.45 (0.89–2.37) | 3.74 (2.06–6.79)ǂ |
| Age (year) | ||||||||
| 65–69 | 1.0 (referent) | 5.57 (1.54–20.04)ǂ | 1.0 (referent) | 2.40 (0.65–8.82) | 2.66 (0.81–8.73) | 1.0 (referent) | 1.78 (0.91–3.49) | 4.75 (2.02–11.14)ǂ |
| 70–74 | 1.0 (referent) | 1.37 (0.35–5.32) | 1.0 (referent) | 1.02 (0.35–2.97) | 2.13 (0.68–6.66) | 1.0 (referent) | 1.29 (0.62–2.65) | 2.83 (1.19–6.74)ǂ |
| BMI | ||||||||
| Normal weight | 1.0 (referent) | 8.10 (1.18–55.40)ǂ | 1.0 (referent) | 5.00 (0.93–26.78) | 12.50 (1.19–130.61)ǂ | 1.0 (referent) | 1.34 (0.60–3.00) | 2.34 (0.92–5.93) |
| Overweight | 1.0 (referent) | 1.00 (0.11–8.55) | 1.0 (referent) | 0.92 (0.31–2.78) | 1.19 (0.42–3.37) | 1.0 (referent) | 2.47 (1.17–5.46)ǂ | 5.20 (1.68–16.14)ǂ |
| Obese | 1.0 (referent) | 3.49 (0.88–13.69) | 1.0 (referent) | 0.87 (0.14–5.27) | 4.37 (0.84–22.70) | 1.0 (referent) | 1.92 (0.54–6.78) | 8.80 (1.57–49.16)ǂ |
| Height (m)a | ||||||||
| Tertile 1 | 1.0 (referent) | 2.87 (0.68–11.96) | 1.0 (referent) | 1.60 (0.49–5.19) | 4.00 (1.09–14.60)ǂ | 1.0 (referent) | 1.50 (0.71–3.17) | 3.85 (1.48–10.01)ǂ |
| Tertile 2 | 1.0 (referent) | 4.70 (0.99–22.19) | 1.0 (referent) | 0.80 (0.16–3.79) | 0.80 (0.16–3.79) | 1.0 (referent) | 1.20 (0.52–2.79) | 5.62 (1.85–17.09)ǂ |
| Tertile 3 | 1.0 (referent) | 1.40 (0.15–12.55) | 1.0 (referent) | 2.25 (0.44–11.36) | 3.37 (0.74–15.39) | 1.0 (referent) | 1.76 (0.73–4.24) | 7.50 (0.74–75.72) |
| Waist circumference | ||||||||
| Non-obese | 1.0 (referent) | 2.88 (1.08–7.70) | 1.0 (referent) | 1.48 (0.66–3.34) | 2.10 (0.25–17.59) | 1.0 (referent) | 1.44 (0.89–2.35) | 3.71 (2.08–6.74) ǂ |
| Abdominal obesity | 1.0 (referent) | 1.00 (0.68–14.64)ǂ | 1.0 (referent) | – | – | 1.0 (referent) | – | – |
aTertiles values for men—Canada: 1.52 ≤ Tertile 1 < 1.70; 1.70 ≤ Tetile 2 < 1.75; 1.75 ≤ Tertile 3 < 1.98. Albania: 1.58 ≤ Tertile 1 < 1.68; 1.68 ≤ Tertile 2 < 1.73; 1.73 ≤ Tertile 3 < 1.84. Latin America: 1.42 ≤ Tertile 1 < 1.61; 1.61 ≤ Tertile 2 < 1.67; 1.67 ≤ Tertile 3 < 1.87
ǂ p value <0.05
Among women, similar results were obtained (Table 6). HGS weakness (<16 kg for women) remained significantly associated with slow walk, and between research sites, this association remained significant in most subgroups according to age, BMI, and height. We also observed that weakness and slowness were strongly associated in those participants with abdominal obesity in Canadian and Latin American sites.
Table 6.
Odds ratios of slowness according to handgrip weakness categories in each research site in women, by selected characteristics
| Canada | Albania | Latin America | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal strength | Intermediate | Weak | Normal strength | Intermediate | Weak | Normal strength | Intermediate | Weak | |
| ≥20 kg | 16–19.9 kg | <16 kg | ≥20 kg | 16–19.9 kg | <16 kg | ≥ 20 kg | 16–19.9 kg | <16 kg | |
| All | 1.0 (referent) | 1.49 (0.62–3.58) | 9.37 (4.00–21.95)ǂ | 1.0 (referent) | 2.39 (1.14–5.03) ǂ | 2.78 (1.14–6.76) ǂ | 1.0 (referent) | 1.81 (1.13–2.89) ǂ | 3.97 (2.05–7.68)ǂ |
| Age (year) | |||||||||
| 65–69 | 1.0 (referent) | 2.14 (0.43–10.53) | 12.88 (3.80–43.67)ǂ | 1.0 (referent) | 2.73 (0.92–8.20) | 2.18 (0.65–7.35) | 1.0 (referent) | 1.83 (0.95–3.54) | 3.82 (1.53–9.52)ǂ |
| 70–74 | 1.0 (referent) | 0.91 (0.31–2.62) | 6.11 (1.70–21.89)ǂ | 1.0 (referent) | 2.10 (0.70–6.23) | 3.30 (0.83–13.09) | 1.0 (referent) | 1.52 (0.76–3.05) | 3.69 (1.40–9.70)ǂ |
| BMI | |||||||||
| Normal weight | 1.0 (referent) | 0.94 (0.11–8.14) | 3.78 (0.67–21.31)ǂ | 1.0 (referent) | 0.87 (0.17–4.47) | - | 1.0 (referent) | 1.41 (0.62–3.28) | 2.68 (0.92–7.81) |
| Overweight | 1.0 (referent) | 2.11 (0.39–11.27) | 9.50 (1.44–62.60)ǂ | 1.0 (referent) | 1.22 (0.34–4.34) | 4.09 (1.00–16.71) ǂ | 1.0 (referent) | 2.63 (0.94–7.35) | 6.25 (1.33–29.21) ǂ |
| Obese | 1.0 (referent) | 1.44 (0.41–5.00) | 19.50 (3.87–98.10)ǂ | 1.0 (referent) | 5.86 (1.55–22.15)ǂ | 1.32 (0.36–4.77) | 1.0 (referent) | 2.13 (0.62–7.30) | 4.93 (1.54–15.72)ǂ |
| Height (m)a | |||||||||
| Tertile 1 | 1.0 (referent) | 2.00 (0.63–6.31) | 11.56 (3.03–44.13)ǂ | 1.0 (referent) | 2.34 (1.10–4.97) ǂ | 2.78 (1.13–6.83)ǂ | 1.0 (referent) | 1.50 (0.71–3.17) | 3.87 (1.48–10.01) ǂ |
| Tertile 2 | 1.0 (referent) | 0.96 (0.20–4.41) | 8.40 (2.75–25.59)ǂ | 1.0 (referent) | – | – | 1.0 (referent) | 2.28 (0.89–5.82) | 3.90 (0.78–19.38) |
| Tertile 3 | 1.0 (referent) | – | – | 1.0 (referent) | 1.41 (0.64–3.09) | 2.40 (1.04–5.51) ǂ | 1.0 (referent) | 1.58 (0.67–3.72) | 2.97 (0.87–10.18) |
| Waist circumference | |||||||||
| Non-obese | 1.0 (referent) | 2.55 (0.47–13.82) | – | 1.0 (referent) | – | – | 1.0 (referent) | 2.16 (0.94–4.94) | 4.48 (1.53–13.08) ǂ |
| Abdominal obesity | 1.0 (referent) | 1.22 (0.43–3.45) | 15.41 (5.39–44.09)ǂ | 1.0 (referent) | 2.47 (1.16–5.47) ǂ | 2.31 (0.92–5.76) | 1.0 (referent) | 1.68 (0.94–3.03) | 3.96 (1.66–9.49)ǂ |
aTertile values for women- Canada: 1.40 ≤ Tertile 1 < 1.57; 1.57 ≤ Tertile 2 < 1.61; 1.61 ≤ Tertile 3 < 1.76. Albania: 1.39 ≤ Tertile 1 < 1.52; 1.52 ≤ Tertile 2 < 1.56; 1.56 ≤ Tertile 3 < 1.77. in Latin America: 1.32 ≤ Tertile 1 < 1.48; 1.48 ≤ Tertile 2 < 1.52; 1.52 ≤ Tertile 3 < 1.75
ǂ p value <0.05
The results for the associations between HGS/BMI and slowness are reported in Supplementary Appendix and briefly summarized here. Weakness was also associated with slowness in men and in women, using the definition of weakness based on HGS/BMI. Although in men, the associations between weakness and slowness were weaker than in women, they were statistically significant, except for Albania where HGS-defined weakness was significantly associated with slowness (OR = 2.4) while HGS/BMI-defined association with slowness was not (OR = 1.3). In women, these associations were stronger in Canada (HGS weakness OR = 9.4; HGS/BMI weakness OR = 9.3) than in Latin America (HGS weakness OR = 4.0; HGS/BMI weakness = 4.7); in Albania, the corresponding values were OR = 2.4 and OR = 3.5.
Discussion
Based on the diverse populations of IMIAS, we developed internally defined IMIAS cut points and compared their agreement with the FNIH Project in the classification of participants by degree of weakness. In addition, we estimated the prevalence of weakness associated with slow gait speed using cut points proposed by the FNIH Project criteria and examined their performance in identifying gait speed across selected characteristics.
Main findings
Our results showed large variability across study sites in relation to body composition measures, muscle strength, and gait speed. Older adults from Latin America (Manizales and Natal) had poorer physical performance than their Canadian and Albanian counterparts. These findings could be due to ethnic diversity, but they could also be explained by the varying degree of social and economic adversity during the life course (Bohannon 2008b; Sousa et al. 2014; Lourenço et al. 2015). Comparing levels of physical performance obtained in our population with Puerto Rican and Framingham cohorts, we observed that Canadian men and women are characterized by very high levels, higher than those observed in the Framingham cohort. Residents of Natal (Northeast Brazil) show the lowest levels of physical function; however, they appear to have faster gait speeds than Puerto Rican men and women residing in Boston.
CART analyses of our pooled IMIAS data further confirmed the validity of the FNIH classification. The IMIAS population generated cut points which were close to those proposed by FNIH. Additionally, there was good agreement between the IMIAS internal classification and the FNIH classification. Thus, the HGS cut points of less than 16 kg for women and less than 26 kg for men proposed by the FNIH Project could be used in the populations of older adults from Canada, Albania, Colombia, and Brazil.
The FNIH Sarcopenia Project (Studenski et al. 2014) aimed to identify criteria for clinically relevant weakness associated with mobility impairment using multiple data sources in a large sample of older adults. Applying those cutoffs (<26 kg for men, <16 kg for women) for HGS in our diverse population showed large differences in weakness prevalence. The prevalence of weakness in non-Canadian men was higher than what was observed in the FNIH populations while Canadian men had even lower prevalence than observed according to FNIH. Women in the two Latin American cities had slightly higher prevalence of clinical weakness than FNIH populations. Tirana women had a prevalence of weakness close to what was observed in FNIH. However, Canadian women were stronger than the FNIH average for that age group.
Compared to similar cohorts, we found low prevalence of weakness in older adults from Canada and Tirana. Buttery et al. (2015) found a weakness prevalence of 10.8 % in men and 12.6 % in women, in a sample of German older adults, using different cut points of HGS (cut points specific by sex and BMI and <20 kg for women and <30 kg for men). In the Bus Santé study, using cut points specific by sex and BMI, older adults from a Swiss region had a prevalence of weakness of 13.8 %, and weakness was one of the most frequently frailty indicators (Guessous et al. 2014). Another study from the USA, using the same cut points for weakness proposed by FNIH Project, found a weakness prevalence of 2 % in older adults aged 60–79 years (Looker and Wang 2015), which it was closer to our Canadian sample.
Comparing our Latin American older adults to other studies, our sample had lower prevalence of weakness. Lourenco et al. (2015) found higher prevalence of weakness using established grip strength values (<20 kg for women and <30 kg for men) in three different cohorts (from countries of Latin America and Spain), ranging from 89 to 18.5 %, respectively. Two studies with Brazilian older adults found a prevalence of weakness of 18 % (Vieira et al. 2013) and 23.85 % for men and 23.82 % for women (de Oliveira Bez and Neri 2014).
A part of the large variability in prevalence of weakness across studies may be due to the difficulty in establishing internationally valid cut points for HGS weakness. The majority of research has been using distribution-based cut points to define low strength (Hairi et al. 2010) or cut points in function of body mass index quartiles and sex (Vieira et al. 2013; de Oliveira Bez and Neri 2014). However, using this approach has limitations: a distribution-based cut point may not distinguish groups at major risk for disability (Hicks et al. 2012). Previously, studies have reported that cut points for handgrip strength based on mobility outcomes may be a useful tool for to identify populations at risk for future mobility impairment (Sallinen et al. 2010; Hicks et al. 2012; McLean et al. 2014).
Weakness was associated with slowness in our populations using two definitions of weakness (HGS weakness and HGS/BMI weakness). Since HGS cut points unadjusted by BMI may reflect more directly the population with limited ability to generate strength due to low lean muscle mass (Alley et al. 2014), we consider the indicator of weakness unadjusted by BMI, a better indicator of sarcopenia.
Slowness was measured in this study by gait speed less than 0.8 m/s. Our results showed large variability in the prevalence of slowness. Generally, Canadian older adults had lower prevalence of slowness than Albanian and Latin American. This may be a consequence of the large variability in socioeconomic aspects in our population. Differences in gait speed may reflect not only the influence of anthropometric characteristics but it may also be due to ethnic differences (Blanco et al. 2012), socioeconomic conditions such as low educational level (Coppin et al. 2006; Busch et al. 2015), low employment grade (Brunner et al. 2009), and life course social and economic adversity (Sousa et al. 2014).
Our results suggest that applying the HGS cut points proposed by FNIH Project resulted in a strong association between slowness and HGS both in men and in women in the Canadian cities, in the Latin American cities, and in Albania, and this strong association between clinical weakness and slowness persists in some BMI categories, height groups, and the presence of abdominal obesity. These results suggest that the FNIH cut points for weakness are clinically relevant to detect those people who are at high risk of frailty with low muscle strength and slowness, even in very distinct populations.
However, height seems to play a significant role in the relationship between HGS and slowness in Canadian men, since HGS is not associated with slowness in any of the tertiles of height. In fact, in this population, the association between HGS and slowness is confounded by height. This lack of association between HGS and slowness is also observed among the tallest men in Latin America and Albania.
A life course perspective can be used to interpret these results. Childhood growth, a strong predictor of adult height, is strongly determined by good nutrition and living conditions in utero and during childhood (Peck and Lundberg 1995; Li et al. 2004; Barros et al. 2006; Ehounoux et al. 2009). On average, a man from the study birth cohorts (born between 1937 and 1948) who had good nutrition and living conditions in utero and during childhood should have attained higher stature than a man who had poor nutrition and living conditions during early life. Previous studies have shown that shorter people have lower HGS than taller people (Samson et al. 2000) and subjects with lower stature also have smaller length in gait (Bohannon 2008b). Thus, attained adult height could be a common determinant of HGS and gait speed among older adults. In Latin America and Albania, slowness was associated with low HGS among men in the lowest tertile of height. We propose that these discrepant findings could be explained by differential survival, since those short men are likely survivors among the population exposed to the largest childhood adversity of their birth cohort. They may have survived to old age but childhood adversity has taken a significant toll in their physical function, as demonstrated by their lower HGS and their slowness (Dodds et al. 2012; Sousa et al. 2014; Bielemann et al. 2015).
In women, associations between HGS and slowness are strong, independently of height. Other factors, such as reproductive history could be implicated.
Implications for clinical practice and research
Our results suggest that the cut points proposed by FNIH performed well in our diverse populations. Thus, a simple inexpensive HGS test with specific cut points may be a useful instrument for assessment of risk for mobility impairment and could constitute a good screening tool for sarcopenia in older adults. Although there was a strong relationship between specific cut points for HGS and poor mobility in this cross-sectional analysis, we suggest verifying the validity of these criteria in a longitudinal study. Consequently, the longitudinal study could assess the prognostic importance of this measurement in sociocultural and economically diverse contexts.
Strengths and limitations
The main strength of this study is its use of diverse populations to compare prevalence of weakness according to specific criteria and to assess the strength of the association between weakness and slowness in international samples. Information on HGS distribution is mostly available from high-income countries, and data from low-income or middle-income countries on these functional measures are unusual (Leong et al. 2015). Furthermore, we used standardized procedures for functional assessment at the five research sites, diminishing measurement error.
Despite these strengths, some limitations must be considered. First, the relatively small sample limited the addition of important variables like BMI, age, or weight in the CART analyses (Sallinen et al. 2010; Hicks et al. 2012; McLean et al. 2014). We considered mobility impairment as a gait speed less than 0.8 m/s. Using this specific cut point gave a high prevalence of slowness, mainly in our sample from Latin America (Lourenço et al. 2015). The majority of normative values of gait speed is from developed countries (Abellan van Kan et al. 2009; Bohannon and Williams Andrews 2011) and did not include Latin American countries. As the gait speed is highly sensitive to anthropometric and socioeconomic (Bohannon 2008b) characteristics, perhaps it may be the reason for the large variability of slowness in our population. However, as shown in a recent systematic review, this specific cut point has good predictive value for adverse health outcomes in older adults (Abellan van Kan et al. 2009).
Conclusion
Our analysis confirmed the validity of the cut points for weakness to identify slowness as proposed by the FNIH Project. Using these cut points, we observed large variations in prevalence of weakness across IMIAS populations. Latin American older adults had higher prevalence of weakness than corresponding Canadians or Albanian. FNIH classification was in agreement with the classification produced by internally defined IMIAS cutoff points. Further work is needed using longitudinal data to demonstrate the predictive validity of the proposed cut points for mortality, frailty, and mobility disability in such different populations.
Electronic supplementary material
(PDF 10kb)
(PDF 132kb)
(PDF 160kb)
(PDF 360kb)
Compliance with ethical standards
Ethical considerations
This study was approved by the ethics committees of each site and written informed consent was obtained at the baseline visit from all the participants.
References
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition AND Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros AJD, Victora CG, Horta BL, et al. Effects of socioeconomic change from birth to early adulthood on height and overweight. Int J Epidemiol. 2006;35:1233–1238. doi: 10.1093/ije/dyl160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler MR, Rubio C, Duarte E, et al. Clinical effectiveness of grip strength in predicting ambulation of elderly inpatients. Clin Interv Aging. 2014;9:1873–1877. doi: 10.2147/CIA.S62002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielemann RM, Gigante DP, Horta BL. Birth weight, intrauterine growth restriction and nutritional status in childhood in relation to grip strength in adults: from the 1982 Pelotas (Brazil) birth cohort. Nutrition. 2015 doi: 10.1016/j.nut.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco I, Verghese J, Lipton RB, et al. Racial differences in gait velocity in an urban elderly cohort. J Am Geriatr Soc. 2012;60:922–926. doi: 10.1111/j.1532-5415.2012.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Population representative gait speed and its determinants. J Geriatr Phys Ther. 2008;31:49–52. doi: 10.1519/00139143-200831020-00002. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Williams Andrews A. Normal walking speed: A descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Brunner E, Shipley M, Spencer V, et al. Social inequality in walking speed in early old age in the Whitehall II Study. J Gerontol Ser A Biol Sci Med Sci. 2009;64A:1082–1089. doi: 10.1093/gerona/glp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch TDA, Duarte YA, Pires Nunes D, et al. Factors associated with lower gait speed among the elderly living in a developing country: a cross-sectional population-based study. BMC Geriatr. 2015;15:35. doi: 10.1186/s12877-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery AK, Busch MA, Gaertner B, et al (2015) Prevalence and correlates of frailty among older adults: findings from the German health interview and examination survey. BMC Geriatr 15:22. doi:10.1186/s12877-015-0022-3 [DOI] [PMC free article] [PubMed]
- Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin AK, Ferrucci L, Lauretani F, et al. Low socioeconomic status and disability in old age: evidence from the InChianti study for the mediating role of physiological impairments. J Gerontol A Biol Sci Med Sci. 2006;61:86–91. doi: 10.1093/gerona/61.1.86. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Bez JP, Neri AL. Velocidade da marcha, força de preensão e saúde percebida em idosos: dados da rede FIBRA Campinas, São Paulo, Brasil. Cien Saude Colet. 2014;19:3343–3353. doi: 10.1590/1413-81232014198.09592013. [DOI] [PubMed] [Google Scholar]
- De’ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. doi: 10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2. [DOI] [Google Scholar]
- Dodds R, Denison HJ, Ntani G, et al. Birth weight and muscle strength: a systematic review and meta-analysis. J Nutr Heal Aging. 2012;16:609–615. doi: 10.1007/s12603-012-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehounoux NZ, Zunzunegui M-V, Séguin L, et al. Duration of lack of money for basic needs and growth delay in the Quebec Longitudinal Study of Child Development birth cohort. J Epidemiol Community Health. 2009;63:45–49. doi: 10.1136/jech.2007.072157. [DOI] [PubMed] [Google Scholar]
- Fess E. Grip strength. 2. Chicago: American Society of Hand Therapists; 1992. [Google Scholar]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- García-Peña C, García-Fabela LC, Gutiérrez-Robledo LM, et al. Handgrip strength predicts functional decline at discharge in hospitalized male elderly: a hospital cohort study. PLoS one. 2013;8:e69849. doi: 10.1371/journal.pone.0069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous I, Luthi J-C, Bowling CB, et al. Prevalence of frailty indicators and association with socioeconomic status in middle-aged and older adults in a Swiss region with universal health insurance coverage: a population-based cross-sectional study. J Aging Res. 2014;2014:198603. doi: 10.1155/2014/198603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the concord health and ageing in men project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Legrand D, Vaes B, Matheï C, et al. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. 2014;62:1030–1038. doi: 10.1111/jgs.12840. [DOI] [PubMed] [Google Scholar]
- Lemon SC, Roy J, Clark MA, et al. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015 doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- Li L, Manor O, Power C. Early environment and child-to-adult growth trajectories in the 1958 British birth cohort. Am J Clin Nutr. 2004;80:185–192. doi: 10.1093/ajcn/80.1.185. [DOI] [PubMed] [Google Scholar]
- Looker AC, Wang C-Y (2015) Prevalence of reduced muscle strength in older U.S. adults: United States, 2011–2012. NCHS Data Brief 1–8. [PubMed]
- Lourenço RA, Pérez-Zepeda M, Gutiérrez-Robledo L, et al. Performance of the European Working Group on Sarcopenia in Older People algorithm in screening older adults for muscle mass assessment. Age Ageing. 2015;44:334–338. doi: 10.1093/ageing/afu192. [DOI] [PubMed] [Google Scholar]
- McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman K, Stobäus N, Gonzalez MC, et al. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135–142. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Peck MN, Lundberg O. Short stature as an effect of economic and social conditions in childhood. Soc Sci Med. 1995;41:733–738. doi: 10.1016/0277-9536(94)00379-8. [DOI] [PubMed] [Google Scholar]
- Pirkle CM, de Albuquerque SACPP, Alvarado B, Zunzunegui M-V. Early maternal age at first birth is associated with chronic diseases and poor physical performance in older age: cross-sectional analysis from the International Mobility in Aging Study. BMC Public Health. 2014;14:293. doi: 10.1186/1471-2458-14-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi MA, Athappilly K. A comparative predictive analysis of neural networks (NNs), nonlinear regression and classification and regression tree (CART) models. Expert Syst Appl. 2005;29:65–74. doi: 10.1016/j.eswa.2005.01.006. [DOI] [Google Scholar]
- Sallinen J, Stenholm S, Rantanen T, et al. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson MM, Meeuwsen IB, Crowe A, et al. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–242. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- Savino E, Martini E, Lauretani F, et al. Handgrip strength predicts persistent walking recovery after hip fracture surgery. Am J Med. 2013;126:1068–1075. doi: 10.1016/j.amjmed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Schrama PPM, Stenneberg MS, Lucas C, van Trijffel E. Intraexaminer reliability of hand-held dynamometry in the upper extremity: a systematic review. Arch Phys Med Rehabil. 2014;95:2444–2469. doi: 10.1016/j.apmr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Seino S, Shinkai S, Fujiwara Y, et al. Reference values and age and sex differences in physical performance measures for community-dwelling older Japanese: a pooled analysis of six cohort studies. PLoS one. 2014;9 doi: 10.1371/journal.pone.0099487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää E, Stenroth L, Bijlsma AY, et al. Associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults. Age (Dordr) 2014;36:9667. doi: 10.1007/s11357-014-9667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa DA, Guerra RO, Tu MT, Patrı AC. Lifecourse adversity and physical performance across countries among men and women aged 65-74. PLoS one. 2014;9:1–10. doi: 10.1371/journal.pone.0102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski SA, Peters KW, Alley DE, et al. The FNIH Sarcopenia Project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto Y, Watanabe M, Sun W, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. Arch Gerontol Geriatr. 2014;59:295–299. doi: 10.1016/j.archger.2014.04.016. [DOI] [PubMed] [Google Scholar]
- United Nations Development Programme . Human development report 2014: sustaining human progress: reducing vulnerabilities and building resilience. 2014. [Google Scholar]
- Vermeulen J, Neyens J, van Rossum E, et al. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:1–11. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira RA, Guerra RO, Giacomin KC, et al. Prevalência de fragilidade e fatores associados em idosos comunitários de Belo Horizonte, Minas Gerais, Brasil: dados do estudo FIBRA. Cad Saude Publica. 2013;29:1631–1643. doi: 10.1590/S0102-311X2013001200015. [DOI] [PubMed] [Google Scholar]
- Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski KR, Smith JC, Malzahn J, et al. Measures of physical ability are unrelated to objectively measured physical activity behavior in older adults residing in continuing care retirement communities. Arch Phys Med Rehabil. 2009;90:982–986. doi: 10.1016/j.apmr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Zunzunegui MVV, Alvarado BEE, Guerra R, et al. The mobility gap between older men and women: the embodiment of gender. Arch Gerontol Geriatr. 2015;61:140–148. doi: 10.1016/j.archger.2015.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 10kb)
(PDF 132kb)
(PDF 160kb)
(PDF 360kb)