Abstract
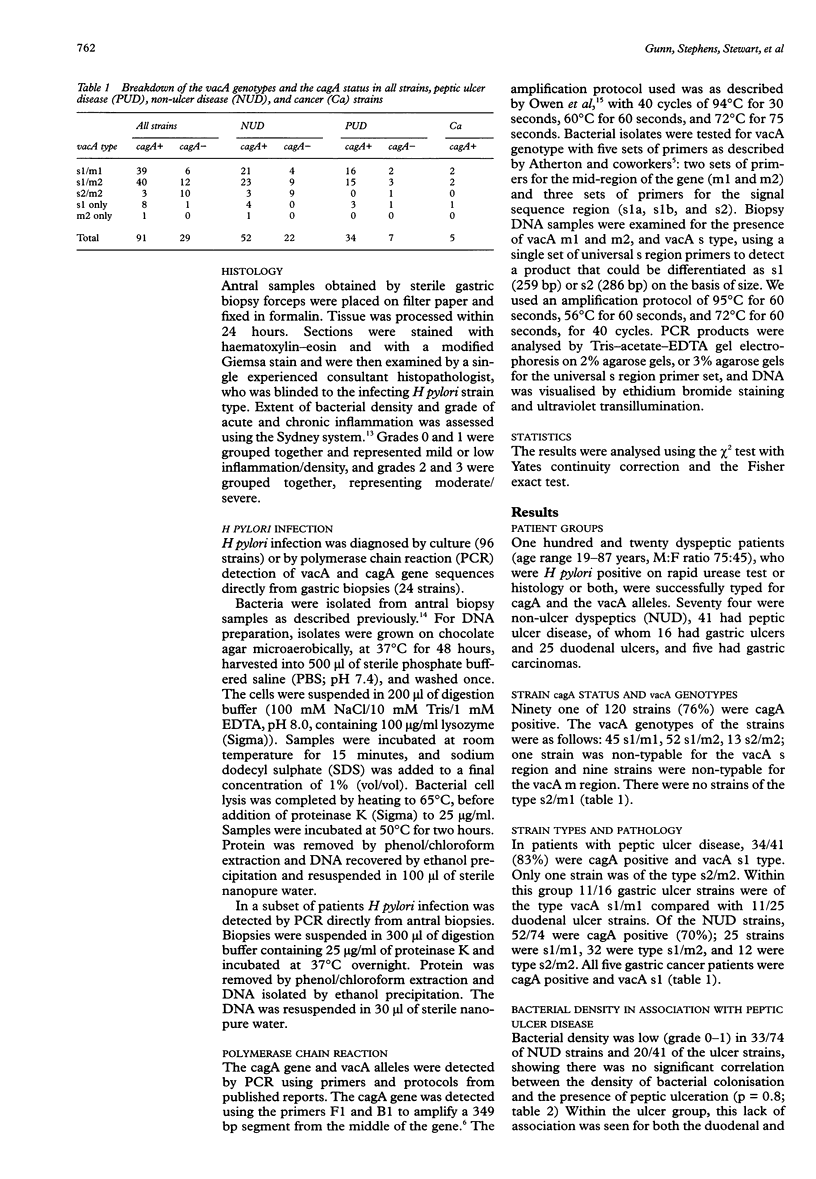
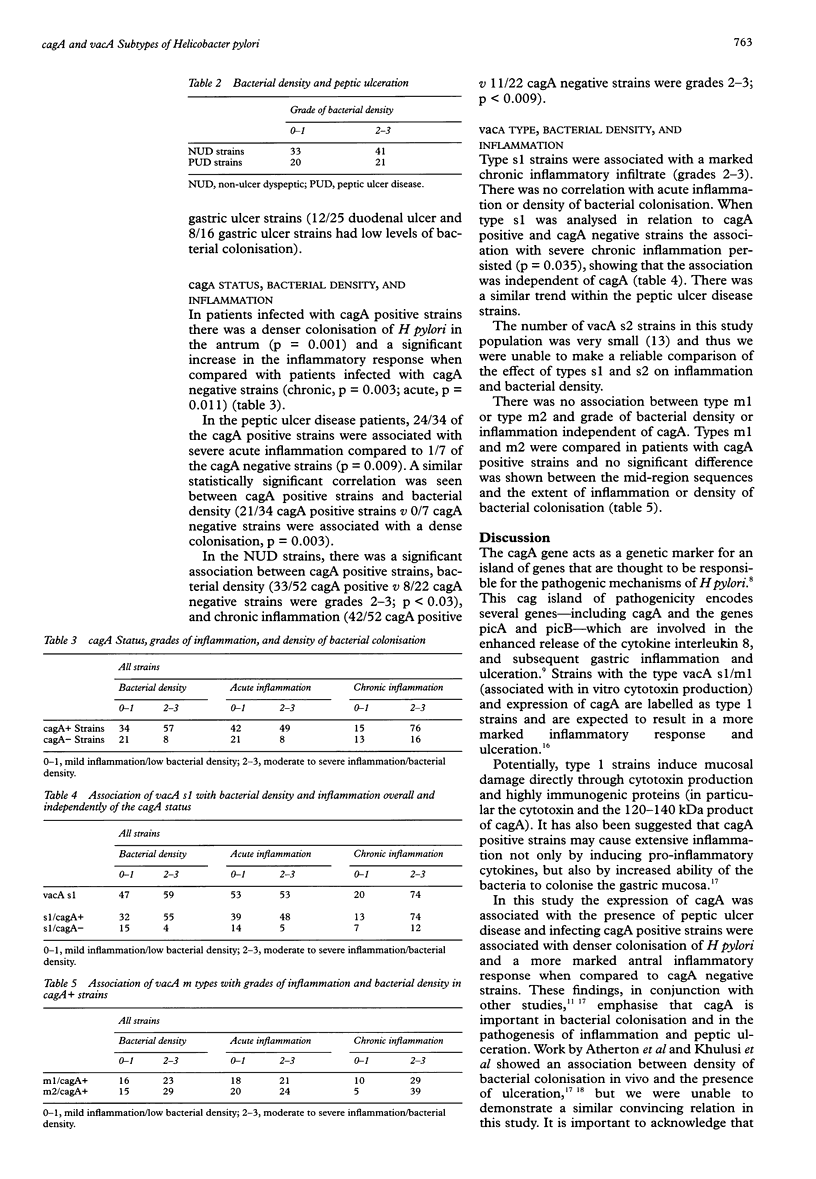
AIMS: To assess the significance of cagA and vacA subtypes of Helicobacter pylori in relation to inflammation and density of bacterial colonisation in vivo within a dyspeptic UK population. METHODS: Dyspeptic patients who were Helicobacter pylori positive had antral samples taken for histology and culture. Gastroduodenal pathology was noted. The grade of bacterial density and inflammation was assessed using the Sydney system. Bacterial DNA was extracted and the vacA alleles and the cagA/gene typed using PCR. RESULTS: 120 patients were studied. There was high rate of cagA positive strains in this population. Bacterial density did not correlate with the presence of peptic ulceration. There was a significant association between cagA positive strains and increased inflammation and bacterial density. The vacA s1 type independently correlated with extensive chronic inflammation but there was no association with bacterial density. The vacA m type did not correlate with extent of inflammation or bacterial density. CONCLUSIONS: The results suggest that cagA is important in the pathogenesis of inflammation and peptic ulceration. These findings are in keeping with the hypothesis that cagA acts as a marker for a cag pathogenicity island which encodes several genes involved in inflammation. The vacA s1 allele correlates with inflammation independently of cagA, possibly through its enhanced ability to produce the vacuolating cytotoxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995 Jul 28;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Peek R. M., Jr, Tham K. T., Cover T. L., Blaser M. J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997 Jan;112(1):92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Tham K. T., Peek R. M., Jr, Cover T. L., Blaser M. J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996 Sep;174(3):552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996 Apr;20(2):241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Tummuru M. K., Cao P., Thompson S. A., Blaser M. J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994 Apr 8;269(14):10566–10573. [PubMed] [Google Scholar]
- Crabtree J. E., Xiang Z., Lindley I. J., Tompkins D. S., Rappuoli R., Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995 Oct;48(10):967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Khulusi S., Mendall M. A., Patel P., Levy J., Badve S., Northfield T. C. Helicobacter pylori infection density and gastric inflammation in duodenal ulcer and non-ulcer subjects. Gut. 1995 Sep;37(3):319–324. doi: 10.1136/gut.37.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hurtado A., Banatvala N., Abdi Y., Davies G. R., Feldman R., Hardie J. M. Conservation of the cytotoxin-associated (cagA) gene of Helicobacter pylori and investigation of association with vacuolating-cytotoxin activity and gastroduodenal disease. FEMS Immunol Med Microbiol. 1994 Oct;9(4):307–315. doi: 10.1111/j.1574-695X.1994.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Miller G. G., Tham K. T., Perez-Perez G. I., Zhao X., Atherton J. C., Blaser M. J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995 Dec;73(6):760–770. [PubMed] [Google Scholar]
- Stephens J. C., Larkins A., James R. F., Rathbone B. J. Production of a monoclonal antibody against the 128 kDa (CagA) protein of Helicobacter pylori. J Immunol Methods. 1996 Apr 19;190(2):163–169. doi: 10.1016/0022-1759(95)00265-0. [DOI] [PubMed] [Google Scholar]
- Telford J. L., Covacci A., Rappuoli R., Chiara P. Immunobiology of Helicobacter pylori infection. Curr Opin Immunol. 1997 Aug;9(4):498–503. doi: 10.1016/s0952-7915(97)80101-x. [DOI] [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994 Jun;62(6):2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]


