Abstract
Mammalian cells deploy autophagy to defend their cytosol against bacterial invaders. Anti‐bacterial autophagy relies on the core autophagy machinery, cargo receptors, and “eat‐me” signals such as galectin‐8 and ubiquitin that label bacteria as autophagy cargo. Anti‐bacterial autophagy also requires the kinase TBK1, whose role in autophagy has remained enigmatic. Here we show that recruitment of WIPI2, itself essential for anti‐bacterial autophagy, is dependent on the localization of catalytically active TBK1 to the vicinity of cytosolic bacteria. Experimental manipulation of TBK1 recruitment revealed that engagement of TBK1 with any of a variety of Salmonella‐associated “eat‐me” signals, including host‐derived glycans and K48‐ and K63‐linked ubiquitin chains, suffices to restrict bacterial proliferation. Promiscuity in recruiting TBK1 via independent signals may buffer TBK1 functionality from potential bacterial antagonism and thus be of evolutionary advantage to the host.
Keywords: anti‐bacterial autophagy, PI(3)P, Salmonella, TBK1, WIPI
Subject Categories: Autophagy & Cell Death; Microbiology, Virology & Host Pathogen Interaction
Introduction
Anti‐bacterial autophagy provides potent cell‐autonomous immunity against bacterial attempts to colonize the cytosol of mammalian cells (Kuballa et al, 2012; Deretic et al, 2013; Randow et al, 2013). The defense of the gut epithelium against bacteria in particular is crucially dependent on anti‐bacterial autophagy, since mice lacking the essential autophagy gene Atg5 in enterocytes suffer from tissue invasion by commensal bacteria and from increased pathology upon infection with Salmonella enterica serovar Typhimurium (S. Typhimurium), a specialized enteropathogen (Benjamin et al, 2013).
Macro‐autophagy, hereafter autophagy, is an evolutionarily conserved quality control and degradation pathway that engulfs cytosolic content into double‐membrane vesicles called autophagosomes. Autophagosome biogenesis requires the concerted activity of about 15 core AuTophaGy genes (ATGs), among them the VPS34 lipid kinase complex (Mizushima et al, 2011). VPS34 produces membrane patches rich in phosphatidylinositol 3‐phosphate (PI(3)P) that recruit PI(3)P‐binding proteins such as WIPI and DFCP1 to the site of phagophore formation (Axe et al, 2008). In contrast to the non‐selective engulfment of cytosol into starvation‐induced autophagosomes, anti‐bacterial autophagy is mediated by cargo receptors including NDP52, optineurin, and p62 (Thurston et al, 2009; Zheng et al, 2009; Wild et al, 2011). Cargo receptors bind members of the LC3/GABARAP family of ubiquitin‐like proteins on the autophagosomal membrane and specific “eat‐me” signals associated with cytosol‐invading bacteria, thereby selectively tethering bacteria to phagophore membranes (Weidberg et al, 2011; Rogov et al, 2014).
Salmonella enterica serovar Typhimurium reaches the cytosol from a vesicular compartment, the Salmonella‐containing vacuole (SCV). Damage to the limiting membrane of the SCV during bacterial escape exposes host glycans otherwise hidden inside the vacuole as ligands for a family of cytosolic lectins, the galectins (Dupont et al, 2009; Paz et al, 2010). By binding the cargo receptor NDP52, galectin‐8 provides an “eat‐me” signal for anti‐bacterial autophagy (Thurston et al, 2012). The dense layer of poly‐ubiquitylated proteins that accumulates on cytosol‐exposed S. Typhimurium serves as an alternative “eat‐me” signal, which is sensed by multiple cargo receptors, namely NDP52, optineurin, and p62 (Perrin et al, 2004; Thurston et al, 2009; Zheng et al, 2009; Wild et al, 2011). Failure of “eat‐me” signals to associate with cytosolic bacteria or interference with cargo receptor function prevents efficient anti‐bacterial autophagy and allows hyper‐proliferation of cytosolic S. Typhimurium (Boyle & Randow, 2013).
Restricting the proliferation of S. Typhimurium also requires the kinase TBK1, a member of the IKK (inhibitor of nuclear factor κB kinase) family (Radtke et al, 2007; Thurston et al, 2009). The anti‐bacterial function of TBK1 is distinct from its well‐characterized role of inducing type I interferons by phosphorylating IRF3 in virally infected cells (Randow et al, 2013; Wu & Chen, 2014). TBK1 accumulates in the vicinity of cytosol‐exposed bacteria together with its adaptor proteins Nap1, Sintbad, and their binding partner NDP52 (Fujita et al, 2003; Ryzhakov & Randow, 2007; Thurston et al, 2009; Verlhac et al, 2015). TBK1 also associates with optineurin and it has been reported to phosphorylate both optineurin and p62, thereby enhancing their affinity for LC3B and ubiquitin, respectively (Morton et al, 2008; Wild et al, 2011; Pilli et al, 2012; Heo et al, 2015; Richter et al, 2016). While these findings imply that TBK1 strengthens the tethering function of cargo receptors, TBK1 has also been suggested to promote autophagosome maturation (Pilli et al, 2012).
Here we show that in order to restrict Salmonella proliferation TBK1 activity is required in the proximity of cytosolic bacteria for the recruitment of WIPI2, a PI(3)P‐binding upstream autophagy component itself essential for anti‐bacterial autophagy. To investigate the recruitment requirements for TBK1 in restricting bacterial proliferation, we deployed a TBK1 variant unable to bind any of its known adaptors. Recruitment of TBK1 to S. Typhimurium via any of several eat‐me signals, including galectin‐8 and K48‐ or K63‐linked ubiquitin, is sufficient to provide TBK1 functionality for anti‐bacterial autophagy, suggesting that robust and promiscuous recruitment of TBK1 to cytosol‐invading bacteria may be beneficial in thwarting potential bacterial evasion attempts.
Results
The autophagic capture of Salmonella requires enzymatically active TBK1 in the bacterial vicinity
TBK1 is essential for anti‐bacterial autophagy but its precise function in the pathway, as well as its mode of activation, remain poorly understood. TBK1 comprises an N‐terminal kinase domain, a ubiquitin‐like domain, and two C‐terminal coiled‐coils. To explore the role of TBK1 in antagonizing S. Typhimurium replication inside host cells we utilized TBK1 knockout mouse embryonic fibroblasts (MEFs). We confirmed previous findings of unrestricted proliferation of S. Typhimurium in Tbk1 −/− MEFs, a phenotype complemented with wild‐type but not catalytically inactive TBK1K38M (Figs 1A and EV1A) (Pomerantz & Baltimore, 1999; Radtke et al, 2007). We have previously shown that TBK1 physically associates with those intracellular Salmonella that are positive for the TBK1 adaptor proteins Nap1 and Sintbad and the autophagy cargo receptor NDP52 (Thurston et al, 2009). To test whether the function of TBK1 in anti‐bacterial autophagy requires interactions with its adaptor proteins we truncated TBK1 at its C‐terminus (TBK1N685 hereafter referred to as TBK1ΔC), thereby generating a molecule deficient in binding to all its known adaptors, that is Nap1, Sintbad, Tank, and optineurin (Fig EV1B) (Goncalves et al, 2011), while maintaining kinase activity as indicated by the activation of an ISRE reporter (Fig EV1C). Complementation of Tbk1 −/− MEFS with TBK1ΔC failed to restrict proliferation of S. Typhimurium (Figs 1A and EV1A). The double mutant lacking catalytic activity and adaptor binding had a phenotype no more severe than either single mutant in the Salmonella assay (Figs 1A and EV1A). We therefore conclude that the catalytic activity of TBK1 and its ability to bind adaptor proteins are equally important to protect cells against S. Typhimurium, most likely because adaptor binding controls TBK1 spatially and/or temporally.
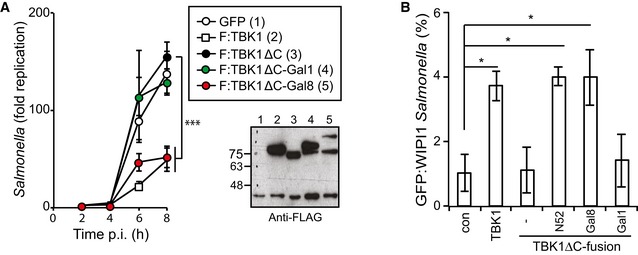
Figure 1. TBK1 kinase activity and C‐terminal domain are required for restriction of Salmonella enterica serovar Typhimurium replication.
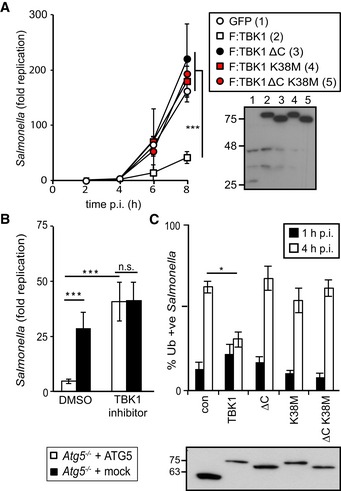
- Kinetics of S. Typhimurium replication in Tbk1 −/− MEFs stably expressing the indicated TBK1 alleles. Intracellular bacteria were enumerated by their ability to form colonies on agar plates following cell lysis at the indicated time points. Statistical analysis comparing Tbk1 −/− MEFs and cells expressing TBK1 alleles. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates.
- Replication of S. Typhimurium in Atg5 −/− MEFs complemented with ATG5 or mock and treated with the TBK1 kinase inhibitor MRT68843 (10 nM) or DMSO. Fold replication was determined by counting bacterial colonies on agar plates at 2 and 8 h post‐inoculation (p.i.) following cell lysis.
- Analysis of Tbk1 −/− MEFs stably expressing the indicated TBK1 alleles and infected with S. Typhimurium. Percentage of S. Typhimurium coated with ubiquitin (detected by FK2 antibody) at 1 or 4 h p.i. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates.
Figure EV1. Characterization of TBK1 alleles.
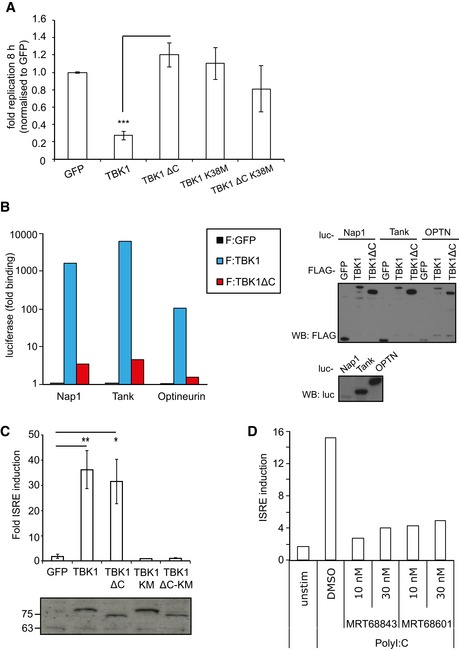
- Replication of Salmonella enterica serovar Typhimurium in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC fusion proteins. Replication normalized to cells expressing GFP. Mean and SEM. N = 3, ***P < 0.001 one‐way ANOVA with Dunnett's multiple comparisons test.
- Lumier binding assay. Binding of luciferase‐tagged Nap1, Tank, or optineurin to beads coated with Flag:GFP, Flag:TBK1, or Flag:TBK1ΔC. Western blot for luciferase‐tagged or Flag‐tagged proteins in total cell lysates.
- ISRE reporter induction upon transient expression of the indicated Flag:TBK1 alleles into 293ET cells. Western blot for Flag‐tagged proteins. Mean and SEM of four independent experiments. *P < 0.05, **P < 0.01, Student's t‐test.
- ISRE reporter induction upon stimulation of 293ET cells with polyI:C (8 h) in the presence of DMSO or TBK1 inhibitors MRT68843 or MRT68601.
Precisely how TBK1 restricts bacterial proliferation is controversial; the enhanced bacterial load in TBK1‐deficient cells has been suggested to be caused by either the cells' inability to maintain SCV integrity (Radtke et al, 2007) or the cells' inability to execute anti‐bacterial autophagy (Thurston et al, 2009). The TBK1 inhibitor MRT68843, which inhibits poly(I:C)‐induced ISRE reporter activity similar to the related TBK1 inhibitor MRT68601 (Newman et al, 2012) (Fig EV1D), was used to analyze the relationship between TBK1 activity and autophagy. As expected, Atg5 −/− cells failed to suppress proliferation of S. Typhimurium (Fig 1B). Addition of MRT68843 increased bacterial replication only 1.5‐fold in Atg5 −/− MEFs but more than eightfold in cells complemented with ATG5, consistent with TBK1 controlling anti‐bacterial autophagy due to its kinase activity. To substantiate this finding, we next investigated where in the anti‐bacterial autophagy pathway TBK1 acts. By complementing Tbk1 −/− MEFs, we confirmed that lack of TBK1 increased the percentage of ubiquitin‐coated cytosolic S. Typhimurium at 4 h post‐infection (Fig 1C) (Radtke et al, 2007; Thurston et al, 2009). TBK1K38M and TBK1ΔC, which are catalytically inactive and deficient in binding adaptor proteins, respectively, did not complement the ubiquitin phenotype, in line with the lack of these alleles to control proliferation of S. Typhimurium in the cytosol of host cells (Fig 1A and C).
The recruitment of WIPI2, itself essential for anti‐bacterial autophagy, is controlled by TBK1
The anti‐bacterial autophagy attack can be visualized by assessing the association of S. Typhimurium with LC3B, a mammalian Atg8 ortholog. However, complementation of Tbk1 −/− MEFs with wild‐type TBK1 did not significantly alter the percentage of GFP:LC3B‐positive S. Typhimurium at 1 h post‐infection, nor did complementation with TBK1ΔC or TBK1K38M (Fig 2A). Such apparently normal recruitment of LC3B to S. Typhimurium in cells failing to restrict bacterial proliferation (Fig 1A and C) may be due to conjugation of LC3 to the remnants of SCV membranes rather than anti‐bacterial phagophores, a phenotype well‐documented for MEFs with defects in upstream autophagy components such as FIP200 or ATG9 (Kageyama et al, 2011). The phenotype in Tbk1 −/− MEFs therefore points to an upstream defect in the autophagy pathway. Phagophore formation requires the PI3 kinase VPS34 to generate PI(3)P as a recruitment signal for WIPI proteins, the mammalian orthologs of yeast Atg18 (Proikas‐Cezanne et al, 2004). Since wortmannin, a potent inhibitor of VPS34, prevents colocalization of WIPI1 but not LC3 with S. Typhimurium (Kageyama et al, 2011), we tested whether TBK1 was similarly required for the recruitment of WIPI proteins to bacteria. We found that GFP‐tagged WIPI1 and WIPI2B but not WIPI3 and WIPI4 accumulated on S. Typhimurium, as did endogenous WIPI2 (Figs 2B and C, and EV2A and B). The recruitment of WIPI1 and WIPI2B was sensitive to wortmannin treatment and abrogated by mutations in their PI(3)P‐binding sites (GFP:WIPI1FTTG and GFP:WIPI2BFTTG). Importantly, accumulation of WIPI1 and WIPI2B also required expression of wild‐type TBK1 and was not supported by either catalytically inactive TBK1K38M or TBK1ΔC deficient in binding adaptor proteins (Fig 2B). In contrast, recruitment of DFCP1 did not require TBK1, although it was also sensitive to wortmannin treatment and mutational inactivation of its PI(3)P‐binding site (DFCP1FYVE*) (Fig 2D–F). WIPI proteins also bind PI(3,5)P2 (Baskaran et al, 2012). However, the accumulation of PI(3,5)P2 on S. Typhimurium was independent of TBK1, as revealed by the normal recruitment of GFP:ML1N*2, a PI(3,5)P2‐specific probe (Li et al, 2013b) (Fig 2G and H). We therefore conclude that TBK1 and VPS34 independently control the recruitment of WIPI1 and WIPI2 to S. Typhimurium and that TBK1 functionality requires catalytic activity as well as its C‐terminal adaptor‐binding coiled‐coil domain.
Figure 2. TBK1 kinase activity and C‐terminal adaptor‐binding domain are required to recruit WIPI1 and WIPI2 to Salmonella enterica serovar Typhimurium.
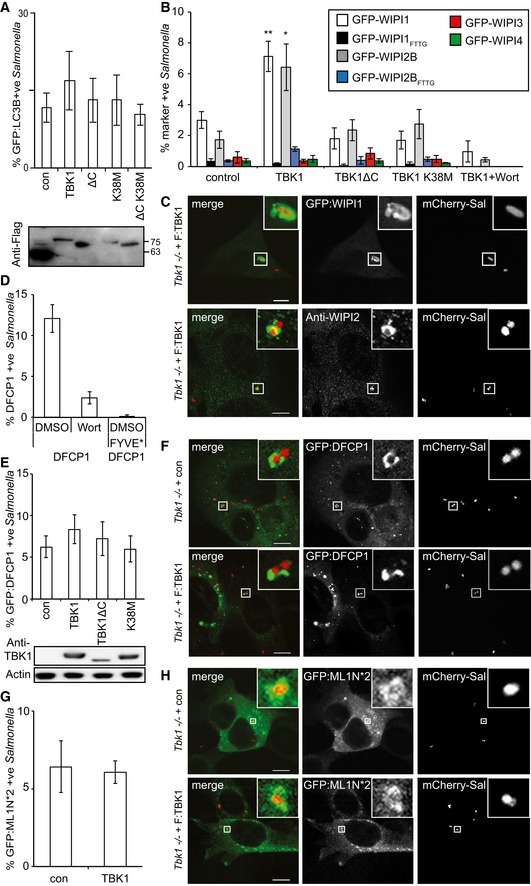
-
A–HAnalysis of Tbk1 −/− MEFs stably expressing the indicated TBK1 alleles and infected with S. Typhimurium for 1 h. Percentage of S. Typhimurium coated with GFP:LC3B (A), the indicated GFP:WIPI alleles (B), GFP:DFCP1 (FYVE* denotes a PI(3)P‐binding mutant of DFCP1) (D, E), or GFP:ML1N*2, a probe for PI(3,5)P2 (G). Where indicated, wortmannin (Wort) was added at 100 nM. Confocal micrographs of MEFs expressing the indicated GFP fusion proteins or immunolabeled for WIPI2 and infected with mCherry‐expressing S. Typhimurium (C, F and H). Mean and SEM of at least three independent experiments with duplicate coverslips. > 200 bacteria counted per coverslip. *P < 0.05, **P < 0.01 one‐way ANOVA with Dunnett's multiple comparisons test. Scale bar, 10 μm.
Figure EV2. Recruitment of WIPI1 and WIPI2B to Salmonella enterica serovar Typhimurium requires TBK1 but not optineurin.
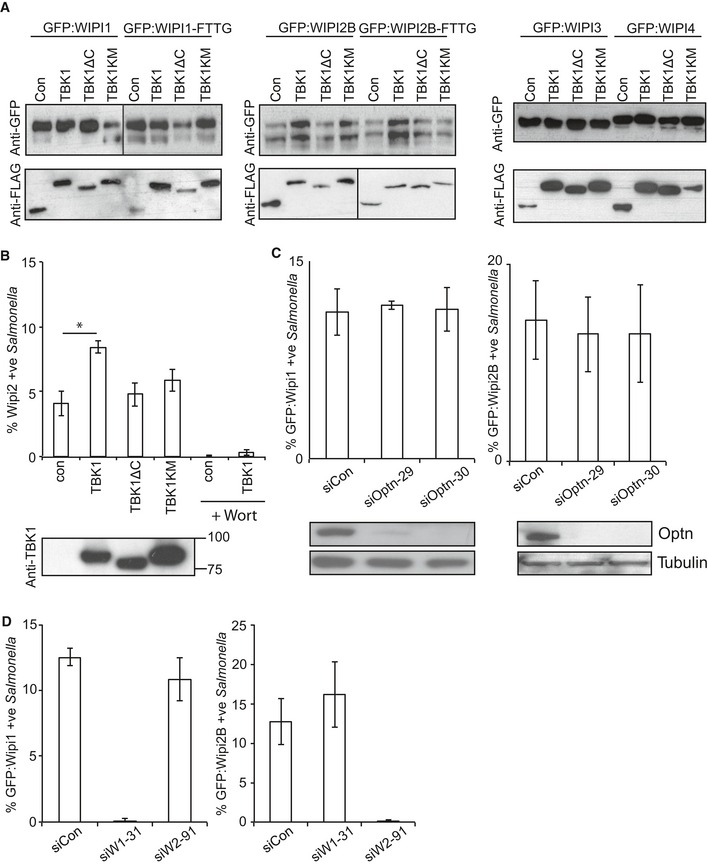
-
AWestern blots of GFP‐tagged WIPI alleles and Flag‐tagged TBK1 alleles corresponding to Fig 2B.
-
BPercentage of WIPI2‐positive S. Typhimurium at 1 h p.i., in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1 proteins. Wortmannin (Wort) was added as indicated at 100 nM. Mean and SEM. N = 3, *P < 0.05, one‐way ANOVA with Dunnett's multiple comparisons test. Western blot of TBK1 alleles in post‐nuclear lysates.
-
C, DAnalysis of MEFs, stably expressing GFP:WIPI1 or GFP:WIPI2B, treated with the indicated siRNAs and infected with S. Typhimurium for 1 h. Mean and SEM. N = 3. Western blot for optineurin and tubulin upon the indicated siRNA treatment.
To further investigate the mechanism of how TBK1 recruits WIPI1 and WIPI2 to cytosol‐invading bacteria, we depleted cells of optineurin, the only known TBK1 substrate in anti‐bacterial autophagy (Wild et al, 2011). Cells lacking optineurin recruited WIPI1 and WIPI2B normally to S. Typhimurium, suggesting that phosphorylation of a substrate other than optineurin is essential for WIPI1/2 recruitment in anti‐bacterial autophagy (Fig EV2C). We also tested the interdependence of WIPI1 and WIPI2B recruitment and found that neither protein was required for the recruitment of the other (Fig EV2D).
We next investigated whether WIPIs are essential to protect cells against bacterial proliferation. Cells lacking WIPI2 failed to restrict proliferation of S. Typhimurium, confirming a recent finding (Dooley et al, 2014), while the presence of WIPI1 was not required (Fig 3). We therefore conclude that the recruitment of WIPI2 to cytosol‐invading bacteria is likely an essential function of TBK1 in cell‐autonomous defense.
Figure 3. WIPI2 restricts Salmonella proliferation.
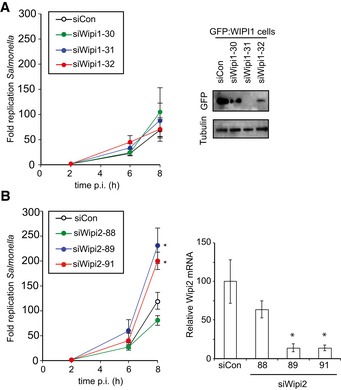
-
A, BKinetics of Salmonella enterica serovar Typhimurium replication in MEFs depleted of WIPI1 (A) or WIPI2 (B). Intracellular bacteria were enumerated by their ability to form colonies on agar plates. Western blot for GFP:WIPI1 and quantitative RT–PCR for WIPI2 upon the indicated siRNA treatments. Mean and SEM. N = 6 (A), n = 3 (B). *P < 0.05, Student's t‐test.
NDP52‐mediated recruitment of TBK1 to S. Typhimurium suffices to restrict bacterial proliferation
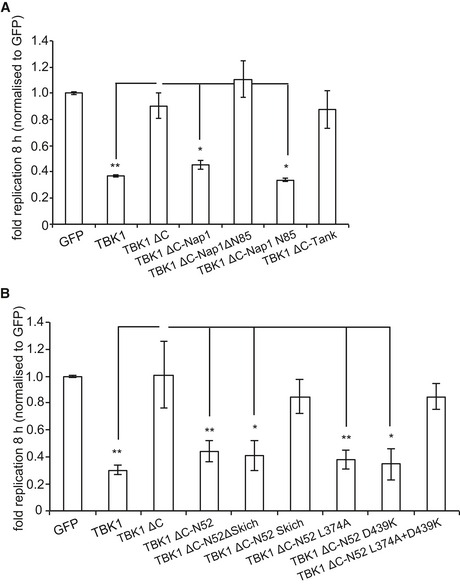
Since the C‐terminal domain of TBK1 is required to restrict bacterial proliferation and mediates adaptor binding (Fig EV1A) we thought to repair TBK1ΔC by fusing it directly to individual adaptor proteins. This strategy enables the evaluation of individual adaptors in the TBK1‐mediated restriction of S. Typhimurium and structure–function analyses without interference from potentially redundant adaptor function. As cytosol‐exposed S. Typhimurium recruit Nap1 but not Tank (Thurston et al, 2009), we compared these two adaptors by fusing them to TBK1ΔC. Consistent with their differential recruitment to cytosolic Salmonella, TBK1ΔC:Tank did not restrict Salmonella proliferation in Tbk1 −/− MEFs; in contrast, TBK1ΔC:Nap1 reduced bacterial replication as efficiently as full‐length TBK1 (Figs 4A and EV3A). Complementation of Tbk1 −/− MEFs with TBK1ΔC:Nap1 but not with TBK1ΔC:Tank also restored localization of GFP‐WIPI1 to Salmonella (Fig 4B).
Figure 4. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via NAP1 or NDP52, but not TANK, restricts bacterial proliferation and recruits WIPI1.
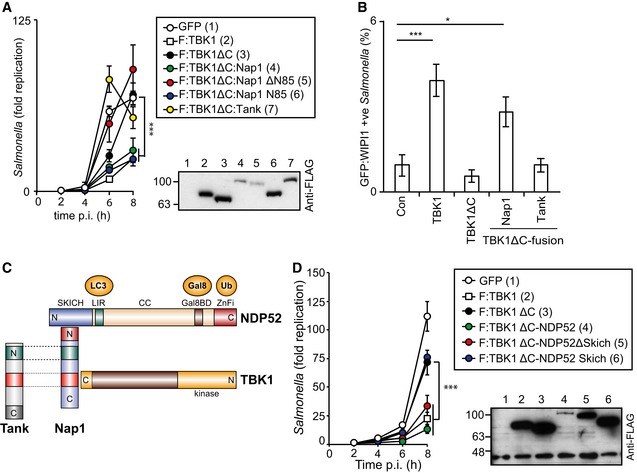
-
A–DAnalysis of Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC‐adaptor fusion proteins. S. Typhimurium replication kinetics (A, D). Infected cells were lysed at the indicated time points post‐inoculation (p.i.), and bacteria were enumerated by their ability to form colonies on agar plates. Mean and SD of triplicate MEF cultures and duplicate colony counts. Data are representative of at least two repeats. Statistical significance to Tbk1 −/− MEFs expressing TBK1ΔC is indicated. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates. (B) Percentage of GFP:WIPI1‐positive S. Typhimurium at 1 h p.i. in the indicated complemented MEFs. Mean and SEM of four independent experiments. > 200 bacteria counted per coverslip. *P < 0.05, ***P < 0.001, one‐way ANOVA with Dunnett's multiple comparisons test. (C) TBK1–Nap1–NDP52 complex. N‐ and C‐termini, domains and binding partners are indicated.
Figure EV3. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via NAP1 or NDP52, but not TANK, restricts bacterial proliferation.
-
A, BReplication of S. Typhimurium in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC alleles. Replication normalized to cells expressing GFP. Mean and SEM. N = 3, *P < 0.05, **P < 0.01, one‐way ANOVA with Dunnett's multiple comparisons test.
How does Nap1 contribute to TBK1 function? Considering the overall structural similarity between Nap1 and Tank (Fig 4C) (Ryzhakov & Randow, 2007), efficient complementation with TBK1ΔC:Nap1 but not TBK1ΔC:Tank suggested a role for the N‐terminal coiled‐coil region of Nap1. Indeed, TBK1ΔC:Nap1ΔN85, in contrast to TBK1ΔC:Nap1, did not prevent hyper‐proliferation of S. Typhimurium in Tbk1 −/− MEFs (Figs 4A and EV3A). Complementation with TBK1ΔC:Nap1N85 also restricted bacterial proliferation; the N‐terminal 85 residues of Nap1 are therefore required and sufficient to provide functionality to TBK1ΔC.
Nap1N85 forms a coiled‐coil that contributes to the dimerization of Nap1 and binds the autophagy cargo receptor NDP52 (Thurston et al, 2009). We therefore speculated that recruitment of TBK1 to Salmonella via NDP52 antagonizes bacterial replication. To test this hypothesis, we fused TBK1ΔC directly to NDP52 and found that it restricted bacterial proliferation in Tbk1 −/− MEFs as efficiently as full‐length TBK1 (Figs 4D and EV3B). However, although endogenous Nap1 cannot bind TBK1ΔC directly (Fig EV1B), Nap1 may be recruited to TBK1ΔC:NDP52 via its binding site in the NDP52 SKICH domain. To test whether such indirect recruitment of Nap1 was required for the complementation of Tbk1 −/− MEFs with TBK1ΔC:NDP52, we examined TBK1ΔC:NDP52SKICH, which was inactive, and TBK1ΔC:NDP52ΔSKICH, which retained activity (Figs 4D and EV3B). We therefore conclude that in anti‐bacterial autophagy, the interaction of TBK1 with adaptor proteins can be replaced entirely by fusing TBK1 directly to NDP52.
Recruitment of TBK1 to S. Typhimurium via either galectin‐8 or ubiquitin suffices to restrict bacterial proliferation
We speculated that the function of NDP52 in TBK1ΔC:NDP52 might be provided by its ability to sense cytosol‐invading bacteria via autophagy‐inducing “eat‐me” signals, that is the bacterial ubiquitin coat and/or galectin‐8 on damaged bacteria‐containing vacuoles. Binding of NDP52 to galectin‐8 is understood in structural detail; it is mediated by a hook‐like structure formed by residues 371–381 and is abrogated in NDP52L374A (Kim et al, 2013; Li et al, 2013a). NDP52 binds ubiquitin via its C‐terminal zinc finger and structural information on the interaction has been recently published (Xie et al, 2015). We also determined the solution structure of the C‐terminal ubiquitin‐binding zinc finger of NDP52 by NMR spectroscopy, which confirmed the existence of an UBZ‐like fold consisting of an α‐helix and a two‐stranded β‐sheet (Fig 5A) (Xie et al, 2015). The zinc ion is coordinated by residues His440 and His444 as well as residues Cys422 and Cys425, which are located in the helix and in the loop connecting the two β‐strands, respectively. This fold is found in several ubiquitin‐binding proteins with the NDP52 structure most similar to the C‐terminal ubiquitin‐binding Zn fingers of Nemo and optineurin (Fig 5B). Addition of mono‐ubiquitin to the NDP52 zinc finger produced changes in the chemical shift primarily of residues in the helix (Fig 5C and Appendix Fig S1). Analysis of the chemical shift changes as a function of ubiquitin concentration revealed a dissociation constant of 60 μM (Appendix Fig S2). Perturbed ubiquitin residues upon binding to NDP52 are centered on the so‐called Ile44 patch in ubiquitin, a common binding site for UBDs (Fig 5C and Appendix Fig S1). These data suggest that the NDP52 zinc finger interacts with ubiquitin similar to other UBZ domains, that is via binding of the exposed face of the helix to the hydrophobic Ile44 patch on ubiquitin. Consistent with such a binding mode and a crystallographic analysis of the NDP52 Zn finger bound to ubiquitin (Xie et al, 2015), residues on the exposed face of the helix are highly conserved among NDP52 orthologs (Fig 5D). To test our model, we mutated Asp439, a conserved residue with a large ubiquitin‐induced chemical shift change located on the exposed helix surface (Appendix Fig S3). NDP52D439K failed to bind ubiquitin (GST‐4xUB)similar to NDP52D439R (Xie et al, 2015), while the interaction with GST‐galectin‐8 was maintained (Fig 5E). In contrast, NDP52L374A bound GST‐4xUb but not GST‐galectin‐8. As expected, the double‐mutant NDP52L374A+D439K did not bind to either ligand. We used the NDP52 mutants specifically deficient in binding to galectin‐8 or ubiquitin to test (i) whether the recruitment of TBK1 to cytosol‐invading Salmonella via bacteria‐associated danger signals is essential to restrict bacterial growth and (ii) whether recruitment via both galectin‐8 and ubiquitin is required. Bacterial proliferation in Tbk1 −/− MEFs was potently restricted by both TBK1ΔC:NDP52D439K and TBK1ΔC:NDP52L374A, which selectively bind galectin‐8 or ubiquitin, respectively (Figs 5F and EV3B). In contrast, TBK1ΔC:NDP52L374A+D439K, which binds neither galectin‐8 nor ubiquitin, was inactive in this assay. We therefore conclude that the recruitment of TBK1 to cytosol‐invading Salmonella via galectin‐8 or the bacterial ubiquitin coat is essential to restrict bacterial proliferation and that either signal, that is galectin‐8 or the ubiquitin coat, suffices in recruiting TBK1. To directly test this prediction for galectin‐8, we complemented Tbk1 −/− MEFs with TBK1ΔC fused to galectin‐8 (TBK1ΔC:Gal8). Indeed, TBK1ΔC:Gal8, similar to wild‐type TBK1 but unlike TBK1ΔC, prevented hyper‐proliferation of S. Typhimurium and enabled the recruitment of GFP:WIPI1 to bacteria (Figs 6 and EV4A).
Figure 5. Structure of the NDP52 zinc finger domain.
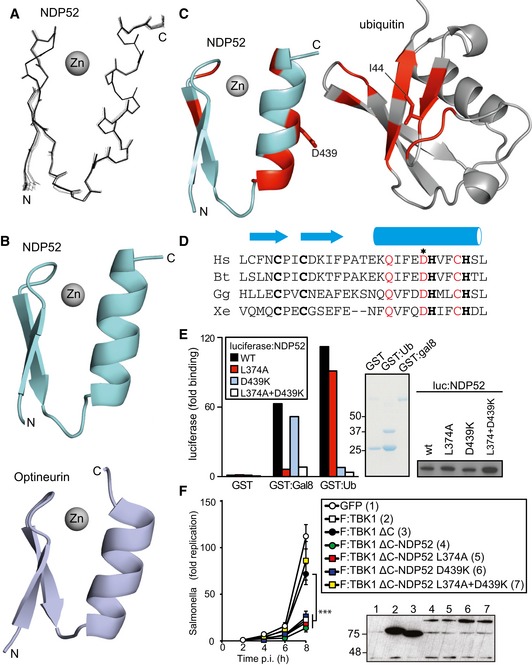
- Superposition of the 20 lowest energy solution structures of the NDP52 domain
- Cartoon representation of the zinc finger domains of NDP52 and optineurin (pdb id: 5AAZ)
- Cartoon representation of the NDP52 zinc finger and ubiquitin. In red are those residues whose signals alter upon binding.
- Phylogenetic alignment of the NDP52 zinc finger. Zinc‐coordinating residues in bold, conserved residues in the helix in red. The asterisk indicates D439, the residue mutated in subsequent experiments. Hs: Homo sapiens, Bt: Bos taurus, Gg: Gallus gallus, and Xe: Xenopus laevis.
- Lumier binding assay. Binding of the indicated luciferase‐tagged NDP52 variants to beads coated with GST, GST:galectin‐8, or GST:ubiquitin. Coomassie stain of purified GST proteins. Western blot for luciferase‐tagged NDP52 alleles in total cell lysates.
- Kinetics of Salmonella enterica serovar Typhimurium replication in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC‐NDP52 fusion proteins. Infected cells were lysed at the indicated times post‐inoculation (p.i.) and bacteria were enumerated by their ability to form colonies on agar plates. Mean and SD of triplicate MEF cultures and duplicate colony counts. Data are representative of at least two repeats. Statistical comparison with Tbk1 −/− MEFs expressing TBK1ΔC is indicated. ***P < 0.001, one‐way ANOVA with Dunnett's multiple comparisons test. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates.
Figure 6. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via galectin‐8 restricts bacterial proliferation and recruits WIPI1.
-
A, BAnalysis of Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC‐galectin fusion proteins. S. Typhimurium replication kinetics (A). Infected cells were lysed at the indicated times post‐inoculation (p.i.), and bacteria were enumerated by their ability to form colonies on agar plates. Mean and SD of triplicate MEF cultures and duplicate colony counts. Data are representative of at least two repeats. Statistical differences to Tbk1 −/− MEFs expressing TBK1ΔC are shown. ***P < 0.001, one‐way ANOVA with Dunnett's multiple comparisons test. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates. (B) Percentage of GFP:WIPI1‐positive S. Typhimurium at 1 h p.i. in the indicated complemented MEFs. Mean and SEM of three independent experiments. > 200 bacteria counted per coverslip. *P < 0.05, one‐way ANOVA with Dunnett's multiple comparisons test.
Figure EV4. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via galectin restricts bacterial proliferation.
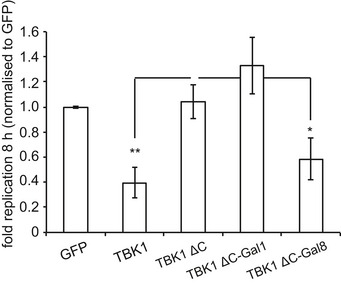
Replication of S. Typhimurium in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC fusion proteins. Replication normalized to cells expressing GFP. Mean and SEM. N = 4, *P < 0.05, **P < 0.01, one‐way ANOVA with Dunnett's multiple comparisons test.
Recruitment of TBK1 to S. Typhimurium via K48‐ or K63‐linked ubiquitin chains suffices to restrict bacterial proliferation
To test whether the bacterial ubiquitin coat also provides recruitment signals for TBK1 sufficient to protect cells against hyper‐proliferation of Salmonella, we fused ubiquitin‐binding domains to TBK1ΔC. The ubiquitin coat of S. Typhimurium contains different linkage types; at minimum, M1‐ and K63‐linked chains are present but their functional contribution to anti‐bacterial autophagy remains unknown (van Wijk et al, 2012). NDP52 can bind M1‐, K48‐, and K63‐linked ubiquitin chains (Wild et al, 2011) with similar affinities for di‐ubiquitin molecules and tetra‐ubiquitin chains that are M1‐linked (Xie et al, 2015). To investigate whether NDP52 binds longer chains in a linkage‐specific manner, we exposed NDP52 to an equimolar mixture of M1‐, K48‐, and K63‐linked ubiquitin tetramers (Fig 7A). Control proteins of known linkage specificity (Trempe et al, 2005; Kulathu et al, 2009; Rahighi et al, 2009b) precipitated their ligands selectively; the UBAN domain of Nemo bound M1‐linked chains, the NZF domain of TAB2 K63‐linked chains, and the UBA domain of Mud1 K48‐linked chains. In contrast, NDP52 bound equally well to all three ubiquitin chains, suggesting lack of linkage specificity for NDP52, at least among the tested linkages types.
Figure 7. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via K48‐ or K63‐ubiquitin chains restricts bacterial proliferation.
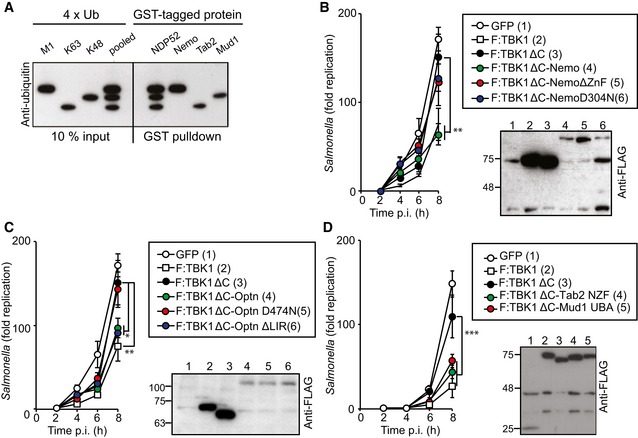
-
ABinding of NDP52 to ubiquitin tetramers of different linkage types. Beads coated with the indicated GST‐tagged proteins were incubated with pooled M1‐, K63‐, and K48‐linked tetra‐ubiquitin chains. Anti‐ubiquitin Western blot of input and proteins bound to beads.
-
B–DKinetics of S. Typhimurium replication in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC fusion proteins. Infected cells were lysed at the indicated time points post‐inoculation (p.i.) and bacteria were enumerated by their ability to form colonies on agar plates. Mean and SD of triplicate MEF cultures and duplicate colony counts. Data are representative of at least two repeats. Statistical differences to Tbk1 −/− MEFs expressing TBK1ΔC are shown. *P < 0.5, **P < 0.01, ***P < 0.001, one‐way ANOVA with Dunnett's multiple comparisons test. Western blot for Flag‐tagged TBK1 variants in post‐nuclear cell lysates.
To directly test the importance of individual ubiquitin‐linkage types in recruiting TBK1 to the ubiquitin coat of cytosolic S. Typhimurium, we fused TBK1ΔC to ubiquitin‐binding proteins of defined specificity. Complementing Tbk1 −/− MEFs with TBK1ΔC:Nemo restricted the proliferation of S. Typhimurium (Figs 7B and EV5A). The UBAN domain of Nemo selectively binds M1‐linked ubiquitin (Rahighi et al, 2009a), while full‐length Nemo engages additional chain types due to combined contributions from its UBAN domain and C‐terminal Zn finger (Hadian et al, 2011). Consistent with a role for ubiquitin, and possibly linear chains, in recruiting TBK1 to cytosol‐invading bacteria, TBK1ΔC:NemoD304N carrying a point mutation in the UBAN domain as well as TBK1ΔC:NemoΔZnF failed to antagonize bacterial growth. Because of its homology to Nemo and its ability to bind TBK1 directly (Morton et al, 2008), we complemented Tbk1 −/− MEFs with TBK1ΔC:optineurin, which prevented bacterial hyper‐proliferation (Figs 7C and EV5B). Similar to TBK1ΔC:NemoD304N, TBK1ΔC:optineurinD474N was inactive due to loss of ubiquitin binding via its UBAN domain, while TBK1ΔC:optineurinΔLIR remained active. Recruitment of TBK1 via LC3/GABARAP, in contrast to ubiquitin‐mediated recruitment is therefore insufficient to productively enroll TBK1 against cytosol‐invading S. Typhimurium, likely because TBK1 function is required for an essential step in autophagy upstream of LC3/GABARAP conjugation to the phagophore, such as the recruitment of WIPI2. To assess whether K48‐ or K63‐linked ubiquitin chains would be sufficient in recruiting TBK1 to cytosol‐exposed S. Typhimurium, we deployed the ubiquitin‐binding domains of Mud1 or TAB2, respectively. Complementation of Tbk1 −/− MEFs with either TBK1ΔC:Mud1UBA or TBK1ΔC:TAB2NZF prevented hyper‐proliferation of S. Typhimurium (Figs 7D and EV5C). Taken together, recruitment of TBK1 to cytosol‐invading S. Typhimurium via K48‐ or K63‐linked ubiquitin chains is sufficient to control TBK1 activity in anti‐bacterial autophagy while conscription via LC3/GABARAP is not.
Figure EV5. Recruitment of TBK1 to Salmonella enterica serovar Typhimurium via ubiquitin restricts bacterial proliferation.
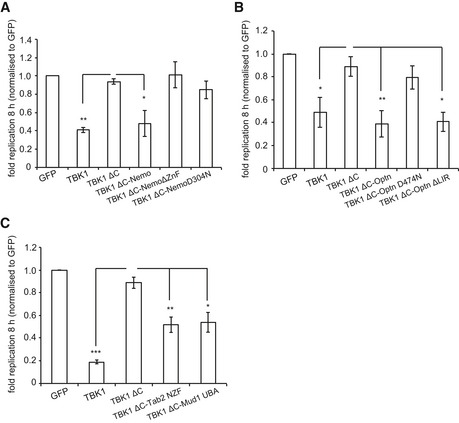
-
A–CReplication of S. Typhimurium in Tbk1 −/− MEFs complemented with the indicated Flag‐tagged TBK1ΔC fusion proteins. Replication normalized to cells expressing GFP. Mean and SEM. N = 4, *P < 0.05, **P < 0.01, ***P < 0.001, one‐way ANOVA with Dunnett's multiple comparisons test.
Discussion
Our data establish that the recruitment of TBK1 to S. Typhimurium is essential to restrict bacterial proliferation by autophagy and that a variety of bacteria‐associated “eat‐me” signals, including galectin‐8 and K48‐ and K63‐linked ubiquitin chains, can mediate TBK1 recruitment. Promiscuity in recruiting TBK1 may be of evolutionary advantage to the host by providing a backup mechanism against potential bacterial interference. We find that the localization of TBK1 activity to S. Typhimurium is required to control an upstream step in anti‐bacterial autophagy, namely the recruitment of the ATG18 orthologue WIPI2 to cytosol‐invading bacteria.
TBK1 is essential for cell‐autonomous immunity against both viral and bacterial infections. In anti‐viral immunity, TBK1 acts downstream of several important receptors—Toll‐like receptors, RIG‐like receptors, and cGAS—to phosphorylate IRF3 (Wu & Chen, 2014). IRF3 controls expression of type I interferons and other anti‐viral genes that establish the so‐called anti‐viral state of increased resistance against a broad spectrum of viruses. In cell‐autonomous anti‐bacterial immunity, TBK1 specifically protects the host cytosol independently of IRF3 (Radtke et al, 2007; Thurston et al, 2009). While originally thought to control vesicular integrity and thus access of bacteria to the cytosol (Radtke et al, 2007), the unchanged recruitment of galectins to S. Typhimurium in cells lacking TBK1 placed TBK1 firmly downstream of bacterial entry (Thurston et al, 2012), while the epistatic relationship between ATG5 and TBK1 revealed in this study demonstrates that TBK1 protects cells against cytosol‐invading S. Typhimurium by controlling autophagy. Consistent with TBK1 controlling anti‐bacterial autophagy is the enzyme's ability to phosphorylate functionally important sites in cargo receptors, namely the LC3‐interacting region (LIR) of optineurin (Wild et al, 2011) and the ubiquitin‐binding site of p62 (Pilli et al, 2012), thereby enhancing their affinity for LC3 and ubiquitin, respectively. While the spatial and temporal control of the tethering function of cargo receptors appears as an important contribution of TBK1 to anti‐bacterial autophagy, we found that in Tbk1 −/− MEFs, LC3 is still recruited to S. Typhimurium. Apparently normal LC3 recruitment while failing to control the growth of cytosolic S. Typhimurium also occurs in MEFs deficient in FIP200 and ATG9, where it is caused by conjugation of LC3 to the damaged membrane of SCVs rather than to de novo phagophores attacking bacteria (Kageyama et al, 2011). Lack of TBK1, and failure to phosphorylate the LIR motif in optineurin, could result in a similarly subtle but nevertheless functionally important mislocalization of LC3.
However, irrespective of the precise membrane localization of LC3 in Tbk1 −/− MEFs, the lack of WIPI2 recruitment, whose essential role in anti‐bacterial autophagy we confirm (Dooley et al, 2014), provides a sufficient and more parsimonious explanation for the failure of autophagy to control bacterial proliferation. WIPI2 is recruited to bacteria via PI(3)P, which initially suggested insufficient PI(3)P in the bacterial vicinity may hinder anti‐bacterial autophagy in Tbk1 −/− MEFs. During autophagy PI(3)P is produced by the VPS34 lipid kinase complex and inhibition of VPS34 with wortmannin or knockout of ATG14L, a subunit of the VPS34 complex, abrogates anti‐bacterial autophagy (Kageyama et al, 2011). However, a defect in PI(3)P production in Tbk1 −/− MEFs can be ruled out since DFCP1, a bona fide probe for the autophagic pool of PI(3)P, is recruited normally to S. Typhimurium. A defect in PI(3,5)P2 levels (Baskaran et al, 2012), an alternative recruitment signal for WIPI2, can also be excluded since recruitment of GFP:ML1N*2, a PI(3,5)P2‐specific probe, did not depend on TBK1 either. We therefore conclude that an essential function of TBK1 in anti‐bacterial autophagy is to control WIPI2 recruitment to cytosol‐invading Salmonella independently of PIP‐related signals. We propose that WIPI2 detects the coincidence of TBK1 and VPS34‐generated signals on cytosol‐invading bacteria in order to direct autophagy toward cognate cargo and away from cellular structures. The requirement for both TBK1 kinase activity and TBK1 localization to S. Typhimurium for the recruitment of WIPI2 furthermore suggests that the TBK1 substrates crucial for anti‐bacterial autophagy are also located in the vicinity of the invading bacterium. Further experiments will be required to reveal their identity.
Anti‐bacterial autophagy targets cytosol‐invading bacteria in response to several distinct “eat‐me” signals (Boyle & Randow, 2013). The earliest known “eat‐me” signal comprises galectin‐8, which detects damage to SCV membranes by binding cytosol‐exposed host glycans liberated by S. Typhimurium during its entry into the cytosol (Thurston et al, 2012). Galectin‐8 is a selective ligand for the cargo receptor NDP52, which is physically linked to TBK1 via Nap1 and Sintbad, two homologous adaptor proteins (Ryzhakov & Randow, 2007; Thurston et al, 2009, 2012). The bacterial ubiquitin coat that develops subsequent to SCV damage comprises another “eat‐me” signal (Perrin et al, 2004). Two E3 ligases, Parkin and LRSAM1, have been suggested to generate the bacterial ubiquitin coat, possibly in response to different bacterial species (Huett et al, 2012; Manzanillo et al, 2013). The ubiquitin “eat‐me” signal is detected by several cargo receptors, of which optineurin and NDP52 provide independent physical links to TBK1. To overcome the redundancy in TBK1 recruitment regarding both “eat‐me” signals and cargo receptors, we generated TBK1ΔC, a TBK1 allele unable to bind any of its known adaptors and thus unable to sense invading bacteria. TBK1ΔC failed to complement Tbk1 −/− MEFs, suggesting that adaptor binding is essential for TBK1 function in anti‐bacterial autophagy. Importantly, TBK1 functionality was reconstituted by fusing TBK1ΔC directly to NDP52 or optineurin but not to mutants deficient in binding “eat‐me” signals, thus providing strong evidence that TBK1 recruitment to “eat‐me” signals is essential for autophagy against cytosol‐invading S. Typhimurium. Fusing TBK1ΔC to NDP52 alleles selectively deficient in ubiquitin binding or directly to galectin‐8 demonstrated that glycan‐driven recruitment suffices to provide TBK1 functionality for anti‐bacterial autophagy. In contrast, NDP52C425A, a mutation that destabilizes the C‐terminal zinc finger, has been reported not to support anti‐bacterial autophagy because of a defect in phagosome maturation, an apparently TBK1‐independent function of NDP52 (Verlhac et al, 2015).
Similarly, fusing TBK1ΔC to optineurin or NDP52 deficient in galectin‐8 binding revealed that ubiquitin‐driven recruitment also suffices. Why would such apparent redundancy in recruiting TBK1 to invading bacteria be needed? Glycan‐ and ubiquitin‐encoded signals can provide functionality to TBK1 for cytosolic defense against invading bacteria at different stages of infection. Recruitment of TBK1 via galectin‐8 enables autophagy to catch invading bacteria as soon as they appear in the cytosol but would fail against bacteria that thwart the attack long enough for cytosol‐exposed glycans to become degraded by cytosolic glycosidases or against those bacteria that proliferate and thereby outgrow the vacuolar membrane remnants. The ubiquitin coat, on the other hand, requires time to develop but could be maintained even on moderately proliferating bacteria for long enough that ultimately anti‐bacterial autophagy may succeed. We therefore suggest that redundancy in TBK1 recruitment via the galectin‐8 and ubiquitin route equips anti‐bacterial autophagy with sufficient robustness to efficiently combat cytosol‐invading bacteria throughout the invasion process.
Additional sturdiness to TBK1 recruitment is provided by the ability of NDP52 to bind M1‐, K48‐, and K63‐linked ubiquitin chains similarly (Xie et al, 2015; this study), which enables anti‐bacterial autophagy to sense a variety of ubiquitin‐derived “eat‐me” signals, for example those generated by different E3 ligases on bacterial and host‐derived substrates. The ubiquitin coat on S. Typhimurium comprises, at minimum, M1‐ and K63‐linked chains (van Wijk et al, 2012). By fusing linkage‐specific ubiquitin‐binding domains to TBK1ΔC, we demonstrate that the ubiquitin coat on S. Typhimurium also contains K48‐linked chains and that recruitment of TBK1 via K48‐ and K63‐linked chains suffices for anti‐bacterial autophagy. The inability of the UBAN domain in NemoΔZnF to recruit TBK1 implies that M1‐linked ubiquitin chains may not be a sufficient signal to provide functionality to TBK1. However, we refrain from explicitly ruling out that M1‐linked chains could provide such a signal since the combined effect of zinc finger and adjacent UBAN domain on Nemo's affinity and specificity for M1 and other linkage types is insufficiently understood. Nevertheless, not all signals found on cytosol‐invading S. Typhimurium are sufficient to provide functionality to TBK1ΔC, as demonstrated by the failure of NDP52 and optineurin to recruit TBK1 functionality to bacteria‐associated LC3 via their LIR motifs.
Taken together, in this study, we demonstrated that recruitment of TBK1 to cytosol‐invading bacteria is essential for anti‐bacterial autophagy and that multiple recruitment signals provide TBK1 functionality for anti‐bacterial autophagy throughout distinct stages of bacterial invasion. We suggest that such robustness in providing TBK1 functionality to anti‐bacterial autophagy protects the pathway from potential bacterial antagonism and is of evolutionary advantage to the host.
Materials and Methods
Antibodies
Antibodies were from Enzo Life Science (ubiquitin FK2), Sigma (Flag M2), Abcam (WIPI2, ab101985; TBK1, ab40676), Dabco (HRP‐conjugated reagents), and Invitrogen (Alexa‐conjugated anti‐mouse and anti‐goat antisera).
Bacteria
Salmonella enterica serovar Typhimurium (strain 12023) was grown overnight in LB and subcultured (1:33) in fresh LB for 3.5 h prior to infection. MEF cells in 24‐well plates were infected with 5 μl of such cultures for 7 min. Following two washes with warm PBS and a 2‐h incubation with 100 μg/ml gentamycin, cells were cultured in 20 μg/ml gentamycin. Where indicated, wortmannin (Sigma; 100 nM) or DMSO vehicle control was added 15 min prior to infection and maintained during the experiment. To enumerate intracellular bacteria, cells from triplicate wells were lysed in 1 ml cold PBS containing 0.1% Triton X‐100. Serial dilutions were plated in duplicate on LB agar.
Cell culture
Tbk1 −/− MEFs (gift from Kate Fitzgerald, University of Massachusetts), Atg5 −/− MEFs (gift from Noboru Mizushima, University of Tokyo), and 293ET cells (gift from Brian Seed, Harvard University), confirmed as mycoplasma free, were grown in IMDM supplemented with 10% FCS at 37°C in 5% CO2. MEFs expressing GFP‐tagged LC3B were obtained by limiting dilution of retrovirally transduced cells. MEFs expressing GFP‐tagged WIPI proteins, DFCP1, or ML1N*2 were transduced with retrovirus and selected in blasticidin at 5 μg/ml. To complement Tbk1 −/− MEFs, cells were transduced with retrovirus and selected in puromycin at 1.5 μg/ml.
LUMIER assays
Binding assays with pairs of putative interactors, one fused to luciferase and the other fused to GST or Flag, were performed in Lumier lysis buffer (150 mM NaCl, 0.1% Triton X‐100, 20 mM Tris–Cl (pH 7.4), 5% glycerol, 5 mM EDTA and protease inhibitors). GST fusion proteins were immobilized on beads before incubation with the luciferase‐tagged binding partner for 2 h. For Flag‐based assays, both proteins were expressed in 293ET cells and immobilized using Flag‐agarose. After washing in lysis buffer, proteins were eluted with glutathione or Flag peptide in Renilla lysis buffer (Promega). Relative luciferase activity represents the ratio of activity eluted from beads and present in lysates.
Ubiquitin‐binding assay
Ubiquitin chains and assay conditions have been described previously (Komander et al, 2009).
Western blot
Post‐nuclear supernatants from 1 × 106 MEF cells or 293ET cells expressing the indicated fusion proteins were separated on a 12% denaturing Bis‐Tris gels. Proteins were transferred to PVDF membrane and incubated with relevant primary and secondary antibodies. Visualization following immunoblotting was performed using ECL detection reagents (Amersham Bioscience).
Microscopy
MEFs were grown on poly‐L‐lysine pre‐treated glass cover slips prior to infection. Following infections, cells were washed twice with warm PBS and fixed in 4% paraformaldehyde in PBS for 20 min. Cells were washed twice in PBS and then quenched with PBS pH 7.4 containing 1 M glycine and 0.1% Triton X‐100 for 30 min prior to blocking for 30 min in PBTB (PBS, 0.1% Triton X‐100, 2% BSA). Where required, cover slips were incubated with primary antibody for 2 h, followed by secondary antibodies and DAPI (4′,6‐diamidino‐2‐phenylindole) for 1 h in PBTB before being mounted (Vector Laboratories). Confocal images were taken with a 100× 1.4 objective on a Zeiss 710 microscope.
Plasmids
M5P or closely related plasmids were used to express proteins in mammalian cells. For TBK1ΔC fusions (N685), genes encoding full‐length or deletion constructs of murine Nemo, Nap1, Tab2, or human NDP52, galectin‐1, galectin‐8, optineurin or Tank were amplified by PCR and ligated BspHI to NotI, in frame with TBK1ΔC. Mutations were generated by PCR and verified by sequencing. Open reading frames for LC3B, DFCP1, WIPI1, WIPI2B, WIPI3, and WIPI4 were amplified from human cDNA. PI(3)P‐binding mutants in WIPI (FRRG‐FTTG) were introduced by mutational PCR, that is WIPI1RR226/227TT and WIPI2RR224/225TT. Plasmids encoding GFP‐DFCP1 double FYVE mutant (FYVE*) and GFP‐ML1N*2 have been described (Ridley et al, 2001; Li et al, 2013b).
| Construct name | Further construct information | References |
|---|---|---|
| TBK1 | Full‐length TBK1 | |
| TBK1ΔC | TBK1 N685, lacks the C‐terminal adaptor‐binding region | |
| TBK1 K38M | TBK1 catalytic mutant | Pomerantz and Baltimore (1999) |
| TBK1ΔC K38M | TBK1 N685 K38M | |
| TBK1ΔC:Nap1 | TBK1 N685 fused to full‐length Nap1 | |
| TBK1ΔC:Nap1 ΔN85 | TBK1 N685 fused to Nap1 ΔN85, Nap1 lacks the CC for binding NDP52 | Ryzhakov and Randow (2007) |
| TBK1ΔC:Nap1 N85 | TBK1 N685 fused to N85 of Nap1 | Ryzhakov and Randow (2007) |
| TBK1ΔC:Tank | TBK1 N685 fused to full‐length Tank | |
| TBK1ΔC:NDP52 | TBK1 N685 fused to full‐length NDP52 | |
| TBK1ΔC:NDP52ΔSkich | TBK1 N685 fused to NDP52 ΔN127, NDP52 lacks the Skich domain | Gurung (2003) |
| TBK1ΔC:NDP52 Skich | TBK1 N685 fused to N127 of NDP52 (the Skich domain) | Gurung (2003) |
| TBK1ΔC:NDP52 L374A | TBK1 N685 fused to NDP52 L374A, a galectin‐8‐binding mutant of NDP52 | Thurston et al (2012), Li et al (2013a) |
| TBK1ΔC:NDP52 D439K | TBK1 N685 fused to NDP52 D439K, a ubiquitin‐binding mutant of NDP52 | This study |
| TBK1ΔC:NDP52 L374A+D439K | TBK1 N685 fused to NDP52 L374A + D439K, a mutant of NDP52 lacking ubiquitin binding and galectin‐8 binding | This study |
| TBK1ΔC:Gal1 | TBK1 N685 fused to full‐length galectin‐1 | |
| TBK1ΔC:Gal8 | TBK1 N685 fused to full‐length galectin‐8 | |
| TBK1ΔC:Nemo | TBK1 N685 fused to full‐length Nemo | |
| TBK1ΔC:NemoΔZnF | TBK1 N685 fused to Nemo ΔC389, lacking the C‐terminal ZnF | Bloor et al (2008) |
| TBK1ΔC:NemoD304N | TBK1 N685 fused to Nemo D304N, a ubiquitin‐binding mutant of Nemo | Bloor et al (2008) |
| TBK1ΔC:Optn | TBK1 N685 fused to full‐length optineurin | |
| TBK1ΔC:Optn D474N | TBK1 N685 fused to Optn D474N, a ubiquitin‐binding mutant | Zhu et al (2007) |
| TBK1ΔC:Optn ΔLIR | TBK1 N685 fused to Optn F178S+V179S+I181S, an LC3 interacting mutant | Wild et al (2011) |
| TBK1ΔC:Tab2 NZF | TBK1 N685 fused to Tab2 AA663–693 | Kulathu et al (2009) |
| TBK1ΔC:Mud1 UBA | TBK1 N685 fused to Mud1 UBA AA293–332 | Trempe et al (2005) |
RNA interference
About 5 × 104 MEFs were reverse‐transfected with 6 pmol of siRNA (Optn siRNA, Invitrogen) or 40 pmol siRNA against WIPI1 (Oligo IDs MSS225230, MSS225231, and MSS225232, Invitrogen) or WIPI2 (Oligo IDs MSS232888, MSS232890, and MSS232891, Invitrogen), using Lipofectamine RNAiMAX (Invitrogen). Control siRNAs were purchased from Invitrogen. siRNA transfection was repeated after 2 days, and experiments were performed 4 days after the first siRNA treatment.
siOPTN #29 5′‐GAAGCUAAAUAAUCAAGCU
siOPTN #30 5′‐GCCUCGCAGUAUUCCGAUU
Quantitative PCR
RNA was extracted (Qiagen) from siRNA‐treated MEF cells and converted into cDNA (SuperScript III reverse transcriptase kit, Invitrogen) according to the manufacturer's protocol. SYBR Green qPCR kit (Applied Biosystems) was used to quantify gene expression using the following primer pairs:
muWipi2 5′‐ATGAACCTGGCGAGCCAGAGC‐3′
and 5′‐GCTGGAGAACAATCTCTCTAC‐3′
muRsp9 5′‐CTGGACGAGGGCAAGATGAAGC‐3′
and 5′‐TGACGTTGGCGGATGAGCACA‐3′
Data were normalized to Rsp9 levels in each sample after relative cDNA levels were calculated from a standard curve.
NMR
NDP52 zinc finger domain samples for NMR spectroscopy experiments were typically at 1.0 mM in 90% H2O and 10% D2O in PBS containing 10 mM 2‐mercaptoethanol. All spectra were acquired with either a Bruker Advance 700 or a DRX600 spectrometer at 20°C and referenced relative to external sodium 2,2‐dimethyl‐2‐silapentane‐5‐sulfonate (DSS) for proton and carbon signals, or liquid ammonium for nitrogen. Assignments were obtained using standard NMR methods using 13C/15N‐labeled and 15N‐labeled samples. Distance constraints were derived from 2D NOESY spectra recorded on 1.5 mM samples with a mixing time of 100 ms. The three‐dimensional structure of the domain was calculated using the standard torsion angle dynamics‐simulated annealing protocol in the program CNS 1.2. Structures were accepted where no distance violation was > 0.25 Å and no dihedral angle violations > 5°. To map the binding interface between the NDP52 zinc finger and ubiquitin, a series of 1H‐15N HSQC spectra of 15N‐labeled zinc finger domain in the presence of increasing molar ratios of unlabeled ubiquitin were recorded; a reciprocal titration was carried out with the unlabeled zinc finger domain added to 15N‐labeled ubiquitin. Dissociation constants were obtained by fitting the concentration dependence of the normalized chemical shift changes to a single site‐binding model.
Accession numbers
Coordinates have been deposited under PDB code 5AAQ and 5AAZ.
Statistical analysis
Student's t‐test or one‐way ANOVA with Dunnett's multiple comparisons test were used as indicated. *P < 0.05, **P < 0.01 and ***P < 0.001.
Author contributions
TLMT, KBB, BJR, MK, SB, AK, JN, AF, and SAM planned, performed, and analyzed the experiments; MA and MB performed and analyzed NMR experiments; DK provided reagents and advice; and TLMT and FR designed the overall research and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank Medical Research Council Technology for providing MRT68843. Work in the authors' laboratories was supported by the Medical Research Council (MC_U105170648, F.R., MC_U105192732, D.K.), the Wellcome Trust (WT104752MA, F.R.), the Leverhulme Trust (T.L.M.T.), the European Research Council (309756, D.K.), and the Lister Institute for Preventive Medicine (D.K.). We thank Drs. Haoxing Xu (University of Michigan, USA) for GFP:ML1N*2, Nicholas Ktistakis (Babraham Institute, Cambridge, UK) for GFP:DFCP1 double FYVE mutant, Kate Fitzgerald (Univsersity of Massachusetts Medical School) for Tbk1 −/− MEFs, and Noboru Mizushima (University of Tokyo, Japan) for Atg5 −/− MEFs.
The EMBO Journal (2016) 35: 1779–1792
References
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3‐phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran S, Ragusa MJ, Boura E, Hurley JH (2012) Two‐site recognition of phosphatidylinositol 3‐phosphate by PROPPINs in autophagy. Mol Cell 47: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JL, Sumpter R Jr, Levine B, Hooper LV (2013) Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 13: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor S, Ryzhakov G, Wagner S, Butler PJG, Smith DL, Krumbach R, Dikic I, Randow F (2008) Signal processing by its coil zipper domain activates IKK gamma. Proc Natl Acad Sci USA 105: 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KB, Randow F (2013) The role of “eat‐me” signals and autophagy cargo receptors in innate immunity. Curr Opin Microbiol 16: 1–10 [DOI] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13: 722–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley HC, Razi M, Polson HEJ, Girardin SE, Wilson MI, Tooze SA (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12–5‐16L1. Mol Cell 55: 238–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N, Lacas‐Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, Van Der Goot FG, Sansonetti PJ, Lafont F (2009) Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6: 137–149 [DOI] [PubMed] [Google Scholar]
- Fujita F, Taniguchi Y, Kato T, Narita Y, Furuya A, Ogawa T, Sakurai H, Joh T, Itoh M, Delhase M, Karin M, Nakanishi M (2003) Identification of NAP1, a regulatory subunit of IkappaB kinase‐related kinases that potentiates NF‐kappaB signaling. Mol Cell Biol 23: 7780–7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves A, Bürckstümmer T, Dixit E, Scheicher R, Górna MW, Karayel E, Sugar C, Stukalov A, Berg T, Kralovics R, Planyavsky M, Bennett KL, Colinge J, Superti‐Furga G (2011) Functional dissection of the TBK1 molecular network. PLoS ONE 6: e23971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung R (2003) Identification of a novel domain in two mammalian inositol‐polyphosphate 5‐phosphatases that mediates membrane ruffle localization. J Biol Chem 278: 11376–11385 [DOI] [PubMed] [Google Scholar]
- Hadian K, Griesbach RA, Dornauer S, Wanger TM, Nagel D, Metlitzky M, Beisker W, Schmidt‐Supprian M, Krappmann D (2011) NEMO interaction with linear and K63 ubiquitin chains contributes to NF‐B activation. J Biol Chem 286: 26107–26117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J‐M, Ordureau A, Paulo JA, Rinehart J, Harper JW (2015) The PINK1‐PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell 60: 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huett A, Heath RJ, Begun J, Sassi SO, Baxt LA, Vyas JM, Goldberg MB, Xavier RJ (2012) The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin‐dependent autophagy of intracellular Salmonella Typhimurium. Cell Host Microbe 12: 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Omori H, Saitoh T, Sone T, Guan J‐L, Akira S, Imamoto F, Noda T, Yoshimori T (2011) The LC3 recruitment mechanism is separate from Atg9L1‐dependent membrane formation in the autophagic response against Salmonella . Mol Biol Cell 22: 2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B‐W, Hong SB, Kim JH, Kwon DH, Song HK (2013) Structural basis for recognition of autophagic receptor NDP52 by the sugar receptor galectin‐8. Nat Commun 4: 1613 [DOI] [PubMed] [Google Scholar]
- Komander D, Reyes‐Turcu F, Licchesi JDF, Odenwaelder P, Wilkinson KD, Barford D (2009) Molecular discrimination of structurally equivalent Lys 63‐linked and linear polyubiquitin chains. EMBO Rep 10: 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuballa P, Nolte WM, Castoreno AB, Xavier RJ (2012) Autophagy and the immune system. Annu Rev Immunol 30: 611–646 [DOI] [PubMed] [Google Scholar]
- Kulathu Y, Akutsu M, Bremm A, Hofmann K, Komander D (2009) Two‐sided ubiquitin binding explains specificity of the TAB 2 NZF domain. Nat Struct Mol Biol 16: 1328–1330 [DOI] [PubMed] [Google Scholar]
- Li S, Wandel MP, Li F, Liu Z, He C, Wu J, Shi Y, Randow F (2013a) Sterical hindrance promotes selectivity of the autophagy cargo receptor NDP52 for the danger receptor galectin‐8 in antibacterial autophagy. Sci Signal 6: ra9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang X, Zhang X, Zhao M, Tsang WL, Zhang Y, Yau RGW, Weisman LS, Xu H (2013b) Genetically encoded fluorescent probe to visualize intracellular phosphatidylinositol 3,5‐bisphosphate localization and dynamics. Proc Natl Acad Sci USA 110: 21165–21170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS (2013) The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501: 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27: 107–132 [DOI] [PubMed] [Google Scholar]
- Morton S, Hesson L, Peggie M, Cohen P (2008) Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett 6: 997–1002 [DOI] [PubMed] [Google Scholar]
- Newman AC, Scholefield CL, Kemp AJ, Newman M, McIver EG, Kamal A, Wilkinson S (2012) TBK1 kinase addiction in lung cancer cells is mediated via autophagy of tax1 bp1/Ndp52 and non‐canonical NF‐κB signalling. PLoS ONE 7: e50672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost M‐C, Lafont F, Sansonetti P (2010) Galectin‐3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol 12: 530–544 [DOI] [PubMed] [Google Scholar]
- Perrin A, Jiang X, Birmingham C, So N, Brumell J (2004) Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr Biol 14: 806–811 [DOI] [PubMed] [Google Scholar]
- Pilli M, Arko‐Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun J‐A, Hansen TE, Johansen T, Deretic V (2012) TBK‐1 promotes autophagy‐mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37: 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D (1999) NF‐kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK‐related kinase. EMBO J 18: 6694–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proikas‐Cezanne T, Waddell S, Gaugel A, Frickey T, Lupas A, Nordheim A (2004) WIPI‐1alpha (WIPI49), a member of the novel 7‐bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation‐induced autophagy. Oncogene 23: 9314–9325 [DOI] [PubMed] [Google Scholar]
- Radtke AL, Delbridge LM, Balachandran S, Barber GN, O'riordan MXD (2007) TBK1 protects vacuolar integrity during intracellular bacterial infection. PLoS Pathog 3: e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D (2009a) Specific recognition of linear ubiquitin chains by NEMO Is Important for NF‐κB Activation. Cell 136: 1098–1109 [DOI] [PubMed] [Google Scholar]
- Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, Randow F, Wakatsuki S, Dikic I (2009b) Specific recognition of linear ubiquitin chains by NEMO is important for NF‐kappaB activation. Cell 136: 1098–1109 [DOI] [PubMed] [Google Scholar]
- Randow F, MacMicking JD, James LC (2013) Cellular self‐defense: how cell‐autonomous immunity protects against pathogens. Science 340: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, Zaffagnini G, Wild P, Martens S, Wagner SA, Youle RJ, Dikic I (2016) Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc Natl Acad Sci USA 113: 4039–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley SH, Ktistakis N, Davidson K, Anderson KE, Manifava M, Ellson CD, Lipp P, Bootman M, Coadwell J, Nazarian A, Erdjument‐Bromage H, Tempst P, Cooper MA, Thuring JW, Lim ZY, Holmes AB, Stephens LR, Hawkins PT (2001) FENS‐1 and DFCP1 are FYVE domain‐containing proteins with distinct functions in the endosomal and Golgi compartments. J Cell Sci 114: 3991–4000 [DOI] [PubMed] [Google Scholar]
- Rogov V, Dötsch V, Johansen T, Kirkin V (2014) Interactions between autophagy receptors and ubiquitin‐like proteins form the molecular basis for selective autophagy. Mol Cell 53: 167–178 [DOI] [PubMed] [Google Scholar]
- Ryzhakov G, Randow F (2007) SINTBAD, a novel component of innate antiviral immunity, shares a TBK1‐binding domain with NAP1 and TANK. EMBO J 26: 3180–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston TLM, Ryzhakov G, Bloor S, von Muhlinen N, Randow F (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin‐coated bacteria. Nat Immunol 10: 1215–1221 [DOI] [PubMed] [Google Scholar]
- Thurston TLM, Wandel MP, von Muhlinen N, Foeglein A, Randow F (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482: 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J‐F, Brown NR, Lowe ED, Gordon C, Campbell ID, Noble MEM, Endicott JA (2005) Mechanism of Lys48‐linked polyubiquitin chain recognition by the Mud1 UBA domain. EMBO J 24: 3178–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac P, Grégoire IP, Azocar O, Petkova DS, Baguet J, Viret C, Faure M (2015) Autophagy receptor NDP52 regulates pathogen‐containing autophagosome maturation. Cell Host Microbe 17: 1–12 [DOI] [PubMed] [Google Scholar]
- Weidberg H, Shvets E, Elazar Z (2011) Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem 80: 125–156 [DOI] [PubMed] [Google Scholar]
- van Wijk SJL, Fiskin E, Putyrski M, Pampaloni F, Hou J, Wild P, Kensche T, Grecco HE, Bastiaens P, Dikic I (2012) Fluorescence‐based sensors to monitor localization and functions of linear and K63‐linked ubiquitin chains in cells. Mol Cell 47: 797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dötsch V, Bumann D, Dikic I (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333: 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Chen ZJ (2014) Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32: 461–488 [DOI] [PubMed] [Google Scholar]
- Xie X, Li F, Wang Y, Wang Y, Lin Z, Cheng X, Liu J, Chen C, Pan L (2015) Molecular basis of ubiquitin recognition by the autophagy receptor CALCOCO2. Autophagy 11: 1775–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 183: 5909–5916 [DOI] [PubMed] [Google Scholar]
- Zhu G, Wu C‐J, Zhao Y, Ashwell JD (2007) Optineurin negatively regulates TNFalpha‐ induced NF‐kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol 17: 1438–1443 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


