Abstract
AIM: To describe the pattern of distribution of thrombospondin (TSP1) and its receptors, alpha root of beta 3 integrin and CD36, in normal human thyroid tissue and to compare their expression in different benign and malignant thyroid conditions. METHODS: Immunohistochemistry was used to study TSP1 and its receptors in 40 surgical thyroidectomy specimens (normal parenchyma, 7; follicular adenoma, 4; multinodular goitre, 13; papillary carcinoma, 6; follicular carcinoma, 8; anaplastic carcinoma, 2). RESULTS: In the normal thyroid parenchyma, there was weak expression of TSP1 limited to the vessels with no staining of the extracellular matrix. In goitres, the expression of TSP1 was more pronounced in areas of fibrosis, with staining of alpha root of beta 3 on thyrocytes located in the vicinity. In thyroid adenomas, expression of TSP1 was slightly enhanced compared with normal tissue, located in the basement membrane of vessels. In papillary carcinomas, TSP1 was abundant in the desmoplastic stroma with a cytoplasmic distribution of alpha root of beta 3 integrin in thyrocytes. In follicular carcinomas, TSP1 was less abundant in the extracellular matrix, limited to the vessels of the stroma with a weaker expression of alpha root of beta 3 on thyrocytes than in papillary carcinomas. In anaplastic carcinomas, TSP1 was only present in the numerous capillaries of the stroma, with a marked positivity for alpha root of beta 3 in one case. No immunostaining of thyrocytes is observed with CD36. CONCLUSIONS: These results suggest the importance of the interaction between alpha root of beta 3 integrin and TSP1 during remodelling of the matrix in fibrous goitres with areas of early sclerosis comparable with wound healing. In papillary carcinomas, the overexpression of TSP1 restricted to the stroma suggests protective effects against tumour progression.
Full text
PDF

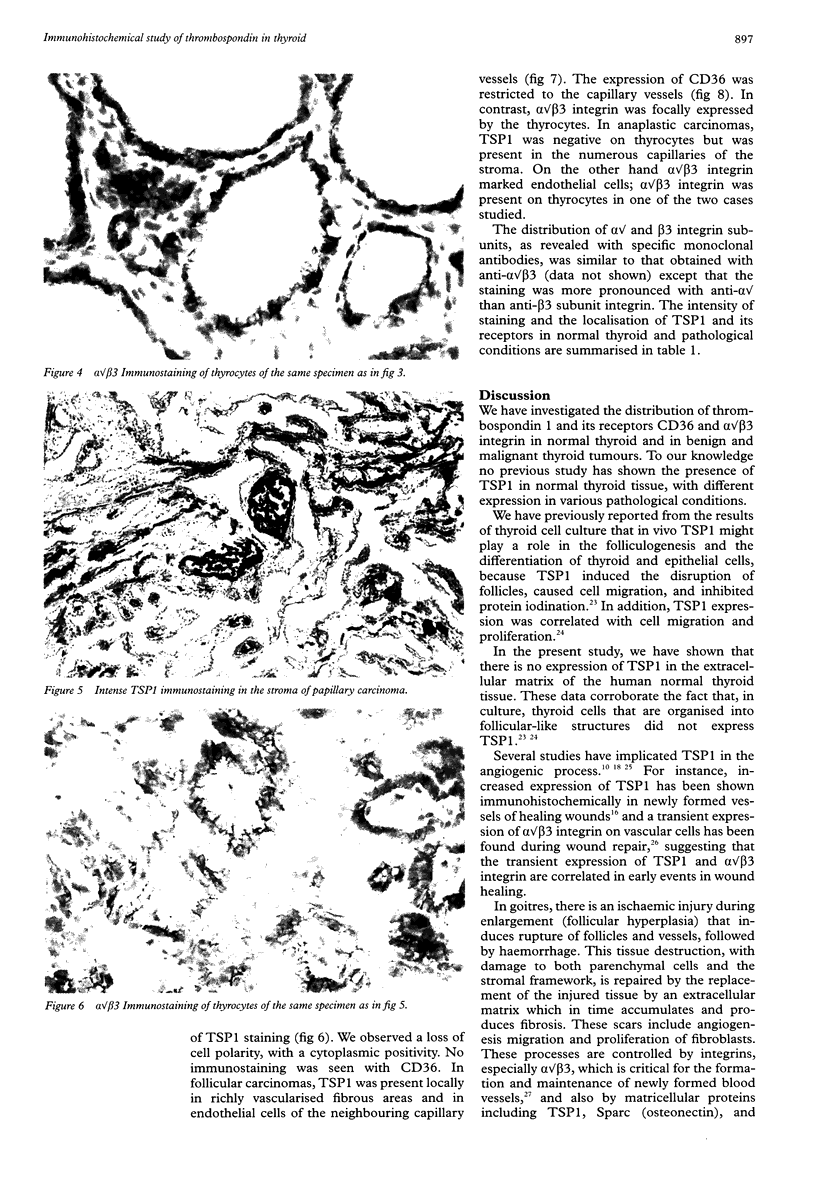
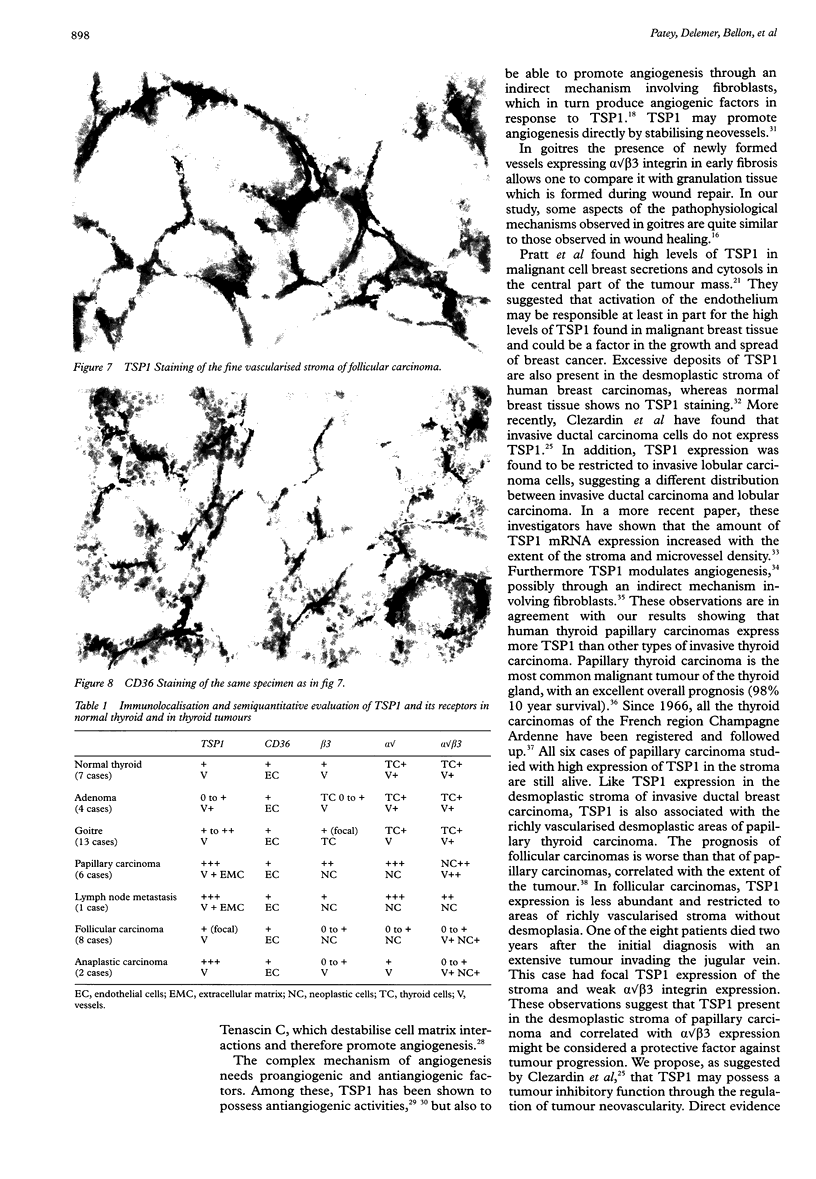


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Liu I., Briccetti F. M., Barnwell J. W., Kwakye-Berko F., Dokun A., Goldberger J., Pernambuco M. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science. 1993 Nov 26;262(5138):1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Silbiger S., Heimer E., Nachman R. L. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Bellon G., Chaqour B., Antonicelli F., Wegrowski J., Claisse D., Haye B., Borel J. P. Differential expression of thrombospondin, collagen, and thyroglobulin by thyroid-stimulating hormone and tumor-promoting phorbol ester in cultured porcine thyroid cells. J Cell Physiol. 1994 Jul;160(1):75–88. doi: 10.1002/jcp.1041600110. [DOI] [PubMed] [Google Scholar]
- Bertin N., Clezardin P., Kubiak R., Frappart L. Thrombospondin-1 and -2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res. 1997 Feb 1;57(3):396–399. [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995 Aug;130(3):503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. D., Bergstralh E. J., van Heerden J. A., McConahey W. M. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991 Jan;66(1):11–22. doi: 10.1016/s0025-6196(12)61170-7. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Castle V., Varani J., Fligiel S., Prochownik E. V., Dixit V. Antisense-mediated reduction in thrombospondin reverses the malignant phenotype of a human squamous carcinoma. J Clin Invest. 1991 Jun;87(6):1883–1888. doi: 10.1172/JCI115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claisse D., Martiny I., Chaqour B., Wegrowski Y., Petitfrere E., Schneider C., Haye B., Bellon G. Influence of transforming growth factor beta1 (TGF-beta1) on the behaviour of porcine thyroid epithelial cells in primary culture through thrombospondin-1 synthesis. J Cell Sci. 1999 May;112(Pt 9):1405–1416. doi: 10.1242/jcs.112.9.1405. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Tonnesen M. G., Gailit J., Cheresh D. A. Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol. 1996 May;148(5):1407–1421. [PMC free article] [PubMed] [Google Scholar]
- Clezardin P., Frappart L., Clerget M., Pechoux C., Delmas P. D. Expression of thrombospondin (TSP1) and its receptors (CD36 and CD51) in normal, hyperplastic, and neoplastic human breast. Cancer Res. 1993 Mar 15;53(6):1421–1430. [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- Dawes J., Clezardin P., Pratt D. A. Thrombospondin in milk, other breast secretions, and breast tissue. Semin Thromb Hemost. 1987 Jul;13(3):378–384. doi: 10.1055/s-2007-1003514. [DOI] [PubMed] [Google Scholar]
- Delisle M. J., Schvartz C., Theobald S., Maes B., Vaudrey C., Pochart J. M. Les cancers de la thyroïde. Intérêt d'un registre régional de 627 patients diagnostiqués, traités et suivis par une équipe multidisciplinaire. Ann Endocrinol (Paris) 1996;57(1):41–49. [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992 Jun;89(6):2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossfeld G. D., Ginsberg D. A., Stein J. P., Bochner B. H., Esrig D., Groshen S., Dunn M., Nichols P. W., Taylor C. R., Skinner D. G. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997 Feb 5;89(3):219–227. doi: 10.1093/jnci/89.3.219. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989 Nov 1;74(6):2022–2027. [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferri E. L., Jhiang S. M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994 Nov;97(5):418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- McPherson J., Sage H., Bornstein P. Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture. Apparent identity with platelet thrombospondin. J Biol Chem. 1981 Nov 10;256(21):11330–11336. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Nicosia R. F., Tuszynski G. P. Matrix-bound thrombospondin promotes angiogenesis in vitro. J Cell Biol. 1994 Jan;124(1-2):183–193. doi: 10.1083/jcb.124.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran D., Kim P., Kim K. R., Arvan P. Polarized secretion of thrombospondin is opposite to thyroglobulin in thyroid epithelial cells. J Biol Chem. 1993 Apr 25;268(12):9041–9048. [PubMed] [Google Scholar]
- Pratt D. A., Miller W. R., Dawes J. Thrombospondin in malignant and non-malignant breast tissue. Eur J Cancer Clin Oncol. 1989 Feb;25(2):343–350. doi: 10.1016/0277-5379(89)90028-x. [DOI] [PubMed] [Google Scholar]
- Ragno P., Montuori N., Covelli B., Hoyer-Hansen G., Rossi G. Differential expression of a truncated form of the urokinase-type plasminogen-activator receptor in normal and tumor thyroid cells. Cancer Res. 1998 Mar 15;58(6):1315–1319. [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Puolakkainen P., Lane T. F., Dickerson D., Bornstein P., Sage E. H. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 1996 Aug;10(10):1183–1191. [PubMed] [Google Scholar]
- Sandler M. A., Zhang J. N., Westerhausen D. R., Jr, Billadello J. J. A novel protein interacts with the major transforming growth factor-beta responsive element in the plasminogen activator inhibitor type-1 gene. J Biol Chem. 1994 Aug 26;269(34):21500–21504. [PubMed] [Google Scholar]
- Tolsma S. S., Volpert O. V., Good D. J., Frazier W. A., Polverini P. J., Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993 Jul;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Gasic T. B., Rothman V. L., Knudsen K. A., Gasic G. J. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987 Aug 1;47(15):4130–4133. [PubMed] [Google Scholar]
- Tuszynski G. P., Nicosia R. F. The role of thrombospondin-1 in tumor progression and angiogenesis. Bioessays. 1996 Jan;18(1):71–76. doi: 10.1002/bies.950180113. [DOI] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Riser B. L., Hughes L. A., Carey T. E., Fligiel S. E., Dixit V. M. Characterization of thrombospondin synthesis, secretion and cell surface expression by human tumor cells. Clin Exp Metastasis. 1989 May-Jun;7(3):265–276. doi: 10.1007/BF01753679. [DOI] [PubMed] [Google Scholar]
- Weinstat-Saslow D. L., Zabrenetzky V. S., VanHoutte K., Frazier W. A., Roberts D. D., Steeg P. S. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994 Dec 15;54(24):6504–6511. [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- Wong S. Y., Purdie A. T., Han P. Thrombospondin and other possible related matrix proteins in malignant and benign breast disease. An immunohistochemical study. Am J Pathol. 1992 Jun;140(6):1473–1482. [PMC free article] [PubMed] [Google Scholar]