Abstract
The divalent metal transporter 1 (DMT1) is a major iron transporter required for iron absorption and erythropoiesis. Loss of DMT1 function results in microcytic anemia. While iron plays an important role in neural function, the behavioral consequences of DMT1 deficiency are largely unexplored. The goal of this study was to define the neurobehavioral and neurochemical phenotypes of homozygous Belgrade (b/b) rats that carry DMT1 mutation and explore potential mechanisms of these phenotypes. The b/b rats (11–12 wk old) and their healthy littermate heterozygous (+/b) Belgrade rats used as controls, were subject to elevated plus maze tasks. The b/b rats spent more time in open arms, entered open arms more frequently and traveled more distance in the maze than +/b controls, suggesting increased impulsivity. Impaired emotional behavior was associated with down-regulation of GABA in the hippocampus in b/b rats. Also, b/b rats showed increased GABAA receptor α1 and GABA transporter, indicating altered GABAergic function. Furthermore, metal analysis revealed that b/b rats have decreased total iron, but normal non-heme iron, in the brain. Interestingly, b/b rats exhibited unusually high copper levels in most brain regions, including striatum and hippocampus. Quantitative PCR analysis showed that both copper importer Ctr1 and exporter Atp7a were up-regulated in the hippocampus from b/b rats. Finally, b/b rats exhibited increased 8-isoprostane levels and decreased GSH/GSSG ratio in the hippocampus, reflecting elevated oxidative stress. Combined, our results suggest that copper loading in DMT1 deficiency could induce oxidative stress and impair GABA metabolism, which promote impulsivity-like behavior.
Keywords: Belgrade rats, elevated plus maze, emotion, GABA, oxidative stress, Wilson’s disease
JNC-2015-1053.R2_InThisIssue
Iron-copper model: Mutations in the divalent metal transporter 1 (DMT1) decrease body iron status and up-regulate copper absorption, which leads to copper loading in the brain and consequently increases metal-induced oxidative stress. This event disrupts GABAergic neurotransmission and promotes impulsivity-like behavior. Our model provides better understanding of physiological risks associated with imbalanced metal metabolism in mental function and, more specifically, the interactions with GABA and redox control in the treatment of emotional disorders.

INTRODUCTION
Iron plays an established role in proper brain function and emotional behavior (Beard et al. 1993, Delinard et al. 1981, Han & Kim 2015, Menon et al. 2016). For example, iron deficiency in children causes increased anxiety and depression along with social and attention problems (Lozoff et al. 2000). Animal studies demonstrated that dietary iron-deficient rats display increased anxiety with hypoactivity and impaired memory function (Youdim et al. 1989, Pinero et al. 2001, Li et al. 2011). These behavioral problems are closely associated with imbalanced monoaminergic function and alterations in γ-amino butyric acid (GABA)-mediated neurotransmission (Beard et al. 2003). In particular, GABA levels are elevated in both iron-deficient anemia and hypoxia (Batra & Seth 2002, Madl & Royer 2000). Conversely, iron overload has been implicated in neuropsychiatric disorders (Maaroufi et al. 2009, Sobotka et al. 1996) as well as age-related neurodegenerative diseases (Zecca et al. 2004, Barnham et al. 2004). Experimental evidence indicates that excess iron increases oxidative stress that impairs dopaminergic neurons and alters GABA homeostasis (Sobotka et al. 1996). Together, these findings suggest that abnormal iron metabolism, including both iron-deficient anemia and iron overload, promotes emotional dysfunction.
Like iron, copper (Cu) is also essential for brain development, required for cellular respiration and neurotransmitter synthesis (Tainer et al. 1983, Prohaska 1990, Schlief & Gitlin 2006). Copper deficiency is associated with Menkes disease and neurobehavioral deficits in cognitive (Pajonk et al. 2005) and motor function (Penland & Prohaska 2004). In contrast, excessive copper results in neurotoxicity, including emotional liability, hyperactivity and cognitive impairment (Madsen & Gitlin 2007). Elevated Cu levels are associated with Wilson’s disease (WD), which is caused by mutations in the copper transporter gene ATP7B (Bull et al. 1993, Tanzi et al. 1993). WD is characterized by several neurological dysfunction and psychiatric disturbance (Das & Ray 2006), including memory deficit and impulsivity (Dening & Berrios 1989). It is generally accepted that Cu’s neurotoxic effects arise due to increased oxidative stress promoted by excess brain Cu (Jomova & Valko 2011). Moreover, the neurobehavioral deficits in Cu overload are accompanied by inappropriate metabolism of monoamines (Pfeiffer & Mailloux 1987) and impaired GABAergic function through blockade of GABA-GABA receptor binding (Sharonova et al. 1998).
The intimate relationship between iron and copper in human nutrition has long been acknowledged (Fox 2003), but the mechanism of interaction between these two metals still remains elusive. For example, copper is accumulated in the body during iron deficiency in several mammalian species (Fox 2003, Ravia et al. 2005), likely due to altered expression of copper transporters, including copper transporter 1 (Ctr1) and copper-transporting ATPase 1 and 2 (Atp7a and Atp7b). Conversely, the assimilation of iron and synthesis of heme are impaired in swine with copper deficiency (Williams et al. 1976). In addition, ceruloplasmin (Cp), the major copper carrier in blood, has been considered a direct link between copper and iron (Hellman & Gitlin 2002) since it mediates oxidation of ferrous iron.
The divalent metal transporter 1 (DMT1) is a major iron transporter required for intestinal iron absorption and erythropoiesis (Gunshin et al. 2005). The homozygous Belgrade (b/b) rat is an animal model of DMT1 deficiency since it carries a mutation in DMT1 protein due to a glycine-to-arginine substitution at amino acid 185 (G185R) (Fleming et al. 1998). As a result, b/b rats display hypochromic, microcytic anemia (Sladic-Simic et al. 1969). However, b/b rats also exhibit high serum iron and hepatic iron loading due to increased circulating iron which results from ineffective erythropoiesis (Thompson et al. 2006). This unique feature of iron loading anemia resembles several types of transfusional iron overload, such as thalassemia, sideroblastic anemia and myelodysplastic syndrome (Mehta et al. 1989). While DMT1 plays an essential role in the absorption of iron, it also transports several other divalent metals, including manganese (Mn), zinc (Zn), lead and cadmium (Garrick et al. 2006, Gunshin et al. 1997). However, conflicting results exist about copper transport by DMT1; It has been suggested that copper transport could be mediated by DMT1 (Arredondo et al. 2014, Jiang et al. 2013), while others (Illing et al. 2012, Shawki et al. 2012) claimed that DMT1 does not contribute to copper uptake. These results imply that altered iron status due to DMT1 deficiency could affect Cu homeostasis.
While both iron and copper contribute to proper CNS function and emotional behavior, the influence of DMT1 on brain copper metabolism and behavioral outcome has been largely unexplored. Hence, the goal of this study was to define the neurobehavioral and neurochemical phenotypes of homozygous Belgrade (b/b) rats that carry DMT1 mutation and explore the potential molecular mechanisms of these phenotypes. We demonstrated that b/b rats display increased impulsivity-like behavior with normal non-heme iron levels in the brain. Moreover, b/b rats exhibited copper loading and decreased GABA in the brain along with increased oxidative stress markers. Combined, our results suggest that elevated copper in the brain resulting from impaired iron homeostasis could disrupt GABA metabolism and alter emotional behavior.
METHODS
Animals and diets
Animal protocols were approved by the Division of Laboratory Animal Medicine and the Northeastern University-Institutional Animal Care and Use Committee. Breeders of heterozygous (+/b) and homozygous (b/b) Belgrade rats (Fischer F344 background) were kindly provided by Dr. Michael Garrick (SUNY Buffalo). The rats were maintained on a 12:12-h light/dark cycle and given water ad libitum. Weanling male rats (3–4 weeks old) were given facility chow for 3 weeks, followed by an iron-supplemented diet containing 500 mg iron/kg (TD.02385, Harlan Teklad, Madison, WI) to support anemic condition of b/b rats for 5 weeks. Since iron metabolism is affected by estrogen (Hou et al. 2012), only male rats were used in this study.
Elevated plus maze test
Emotional behavior is commonly tested by the elevated plus maze task in rodents (Walf & Frye 2007). The elevated plus maze (Harvard Apparatus, Holliston, MA) consists of two open arms and two closed arms. The test was conducted as previously described (Li et al. 2011). Briefly, each rat was placed on the center of the maze facing one of the open arms and allowed to explore the maze for 5 min. The test area was enclosed by curtains with dim light. Time spent in the open and closed arms, entries into the open arms and total distance traveled were recorded by a CMOS camera and analyzed by ANY-Maze software (Stoelting Co., Wood Dale, IL). The apparatus was cleaned with Quatricide TB.
Tissue collection
After the behavior tests, rats were euthanized by isoflurane overdose, followed by exsanguination and collection of tissues, including blood, liver, urine and brain. The brain was further microdissected to harvest olfactory bulb, cortex, striatum, hippocampus and cerebellum. Serum was harvested from blood. All tissues were flash-frozen in liquid nitrogen and stored at −80°C until analysis.
Metal analysis
Wet tissues were weighed and digested in nitric acid as previously described (Chang et al. 2014) in the presence of yttrium as internal standard. The levels of Fe, Mn, Cu and Zn were quantified by inductively coupled plasma mass spectrometry (ICP-MS) and analyzed by a calibration method using ICP-MS standard solutions (ICP-MS calibration standard 3-A; High-Purity Standards, Charleston, SC).
Non-heme iron analysis
Following tissue digestion in an acid solution (10% trichloroacetic acid, 3 M HCl) at 65°C for 20 h, non-heme iron concentrations were quantified by a colorimetric assay, as previously described (Torrance & Bothwell 1968).
GABA analysis
The hippocampus samples from Belgrade rats were homogenized in 10 volumes (w/v) of 0.4 M perchloric acid containing 50 μM EDTA. L-norvaline was added as an internal standard. After neutralization by sodium borate buffer (10 mM, 10-volume) and centrifugation at 15,000 g for 15 min at 4°C, the supernatant was derivatized with o-phthalaldehyde (16.4 mM, 20:1, v/v) (Rowley et al. 1995) and injected (100 μL) into an HPLC system (Shimadzu). Mobile phase consisted of aqueous phase (0.1 M monosodium phosphate, 0.5 mM EDTA, pH 4.5) and methanol at a 3:1 ratio (v/v). The GABA peak was detected at 344 nm by an UV detector (Shimazu), which was normalized to norvaline.
Real-time qPCR
RNA was isolated from snap-frozen tissues of Belgrade rats using TRI reagent (Sigma-Aldrich) as per the manufacturer’s instructions. RNA (1 μg) was reversely transcribed into cDNA, which was used for real-time polymerase chain reaction assays. The iScript™ reverse transcription supermix and iTaq™ universal SYBR® green supermix were obtained from Bio-Rad (Hercules, California). Primers were obtained from Eurofins, MWG Operon (Huntsville, AL). These primers are copper-related genes, including Atp7a, Atp7b, Ctr1 (Bauerly et al. 2005), Cp (Lestaevel et al. 2009) and metallothionein 1 (Mt1) and 2 (Mt2) (Pankhurst et al. 2012), and GABA-related genes, including GABAA receptor α1 (GABRA1), GABAA receptor α2 (GABRA2) (Fujimura et al. 2005), glutamate decarboxylase 65 (GAD65), glutamate decarboxylase 67 (GAD67) and GABA transporter (GAT) (Takano et al. 2014). The expression level of each gene was normalized to that of cyclophilin and analyzed by the comparative Ct method (2−ΔCt) (Schmittgen & Livak 2008).
Analysis of isoprostanes and glutathione
The hippocampus and liver samples were homogenized using Tris buffer (100 mM, pH 7.4; 10-time dilution) containing 0.005% butylated hydroxytoluene. The lysate was centrifuged at 8,000 g for 10 min for 8-isoprostane analysis. Another aliquot of tissue samples was homogenized using Tris buffer (100 mM, pH 7.4; 10-time dilution). The lysate was centrifuged at 10,000 g for 15 min and deproteinized for glutathione (GSH) assay. The levels of 8-isoprostane, GSH and glutathione disulfide (GSSG) in the hippocampus and liver were determined using assay kits (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions.
Statistical analysis
Values reported were expressed as means ± SEM. Comparisons between b/b and control +/b rats were performed by the Student’s t-test. Differences were considered significant at p < 0.05.
RESULTS
Belgrade rats display iron loading anemia
The homozygous Belgrade (b/b) rats displayed significantly decreased body weight compared with +/b rats (Table 1; 14% decrease, p < 0.001). Hematocrit was decreased (16% decrease, p < 0.001), but liver non-heme iron levels were significantly higher in b/b rats (320% increase, p = 0.015), indicating the condition of iron loading anemia (Kim et al. 2013).
Table 1. Physiological and hematological parameters in Belgrade rats.
Homozygous Belgrade (b/b) rats and control heterozygous (+/b) rats (6–7 weeks old) were fed an iron-supplemented diet (500 mg iron/kg diet) for 5 weeks and euthanized to collect tissues. Data were presented as means ± SEM and analyzed using the two-sample t-test.
| Parameter (unit) | +/b | b/b | n | p value |
|---|---|---|---|---|
| Body weight (g) | 256 ± 1 | 220 ± 4 | 10–12 | < 0.001 |
| Hematocrit (%) | 48.7 ± 0.5 | 40.9 ± 0.7 | 10–12 | < 0.001 |
| Liver non-heme iron level (μg/g) | 103 ± 11 | 434 ± 107 | 4 | 0.015 |
| Brain non-heme iron level (μg/g) | 5.6 ± 0.1 | 5.0 ± 0.6 | 4 | 0.428 |
Belgrade rats exhibit iron and copper loading in the liver
In b/b rats, total iron concentrations were decreased in blood (Figure 1 and Supplementary Material), likely due to impaired hemoglobin synthesis (Bowen & Morgan 1987). Interestingly, the Mn level was decreased, whereas Cu and Zn levels were increased in the blood of b/b rats (Figure 1A). In the liver of b/b rats, levels of Fe and Cu were increased, while those of Mn and Zn were not altered (Figure 1B). There was a trend of elevated urinary Fe, Mn, Cu and Zn in b/b rats (Table 2).
Figure 1. Metal levels in blood and liver of Belgrade rats.
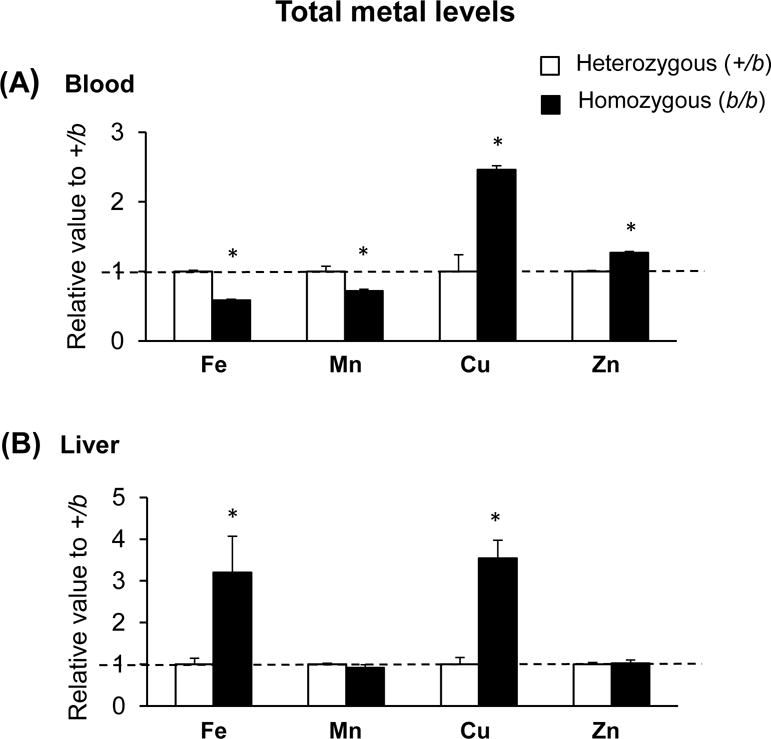
The concentrations of essential metals including iron (Fe), manganese (Mn), copper (Cu) and zinc (Zn) were determined in blood (A) and liver (B) using inductively-coupled plasma mass spectrometry and analyzed using the two-sample t-test. Open and closed bars represent control +/b and b/b rats, respectively. Data were shown as ratios of b/b to +/b rats (means ± SEM, n = 4 per group). * p < 0.05 vs. +/b rats.
Table 2. Metal levels in urine of Belgrade rats.
Urine samples from 4 b/b rats and 4 +/b rats were pooled and analyzed for metals levels by ICP-MS. Data were presented as ppm.
| Metal (unit) | +/b | b/b |
|---|---|---|
| Iron (ppm) | 0.31 | 1.45 |
| Manganese (ppm) | 0.14 | 2.5 |
| Copper (ppm) | 0.64 | 2.3 |
| Zinc (ppm) | 0.35 | 0.81 |
Belgrade rats display copper loading in the brain
In b/b rats, total iron levels were significantly decreased in the olfactory bulb, cortex, striatum, hippocampus and cerebellum (Figure 2). However, non-heme iron in the brain of b/b rats was not different from that of +/b rats (Table 1). Mn levels in the brain were not different between the two genotypes. Interestingly, Cu levels were elevated in the cortex, striatum and hippocampus of b/b rats compared with +/b rats, suggesting that Cu loading in the brain upon DMT1 mutation. Zn levels were higher in the striatum, but not in other brain regions, of b/b rats.
Figure 2. Metal status in the brain of Belgrade rats.

Brain tissues were microdissected to obtain olfactory bulb, cortex, striatum, hippocampus and cerebellum. Metal concentrations were determined using ICP-MS and analyzed using the two-sample t-test. Open and closed bars represent control +/b and b/b rats, respectively. Data were shown as ratios of b/b to +/b rats (means ± SEM, n = 4 per group). * p < 0.05 vs. +/b rats.
Copper transporters are abnormally expressed in Belgrade rats
To examine if increased copper levels in b/b rats result from elevated expression of copper transporters, we characterized copper transporters by qPCR (Figure 3). In the duodenum, there was no significant difference between +/b and b/b rats in the expression of either copper importer (Ctr1) or exporters (Atp7a and Atp7b). In contrast, both Ctr1 and Atp7b were down-regulated in the liver of b/b rats, while Cp, Mt1 and Mt2 were not altered. In the brain of b/b rats, both Ctr1 and Atp7a were up-regulated by 31% (p = 0.023) and 39% (p = 0.043), respectively. These data indicate that copper loading in the liver and brain is not likely caused by increased absorption of copper from the intestine, but is associated with altered expression of copper transporters and storage proteins at tissue levels.
Figure 3. Copper transporters in Belgrade rats.

The qRT-PCR was used to quantify the mRNA levels of copper transporter 1 (Ctr1), copper-transporting ATPase 1 (Atp7a) and 2 (Atp7b), ceruloplasmin (Cp) and metallothionein 1 (Mt1) and 2 (Mt2) in the duodenum, liver and hippocampus. Open and closed bars represent control +/b and b/b rats, respectively. The expression level of each gene was normalized to that of cyclophilin according to the comparative Ct method (2−ΔCt) and analyzed using the two-sample t-test. Data were presented as ratios of b/b to +/b rats (means ± SEM, n = 6–8 per group). * p < 0.05 vs. +/b rats. N/D, not detected.
Emotional behavior is impaired in Belgrade rats
In order to characterize emotional behavior in Belgrade rats, the elevated plus maze task was employed (Figure 4). The b/b rats spent more time in the open arms (196% increase; p < 0.001) and less time in the closed arms (49% decrease; p < 0.001) compared with +/b rats. In addition, b/b rats entered open arms more frequently (93% increase; p < 0.001) and traveled more distance in the maze (41% increase; p = 0.017) than +/b controls. These results suggest increased impulsivity and hyperactivity in b/b rats.
Figure 4. Emotional behavior in Belgrade rats.
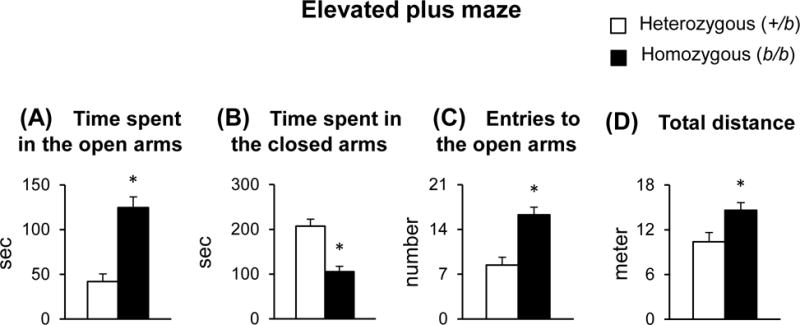
Rats were placed in the central area of the elevated plus maze, facing an open arm, and were allowed to explore the maze for 5 min. Time spent in the open arms (A), time spent in the closed arms (B), entries to the open arms (C) and total distance traveled (D) were analyzed by ANY-Maze behavioral tracking software. Open and closed bars represent control +/b and b/b rats, respectively. Data were presented as means ± SEM (n = 12–14 per group) and analyzed using the two-sample t-test. * p < 0.05 vs. +/b rats.
GABA and GABA-related protein transcript levels are altered in Belgrade rats
Since impaired emotional behavior is associated with abnormal GABA metabolism, molecules related to GABAergic neurotransmission were examined in the brain of b/b rats (Figure 5). The b/b rats demonstrated decreased levels of GABA in the hippocampus (19% decrease; p = 0.017). Moreover, GABRA1 (78% increase; p = 0.015) and GAT (78% increase; p = 0.018) were significantly up-regulated in b/b rats. However, there was no change in GABRA2 expression between b/b and +/b rats. The mRNA expression of GAD65 and GAD67, the enzymes that catalyze the production of GABA from glutamate at different locations in the cells, did not differ between the two genotypes.
Figure 5. GABA and related protein mRNAs in the brain of Belgrade rats.

HPLC was used to measure the levels of GABA in the hippocampus. The mRNA expression of GABAA receptor α1 (GABRA1), GABAA receptor α2 (GABRA2), GABA transporter (GAT), glutamate decarboxylase 65 (GAD65) and glutamate decarboxylase 67 (GAD67) were determined by qRT-PCR. The expression level of each gene was normalized to that of cyclophilin according to the comparative Ct method (2−ΔCt) and analyzed using the two-sample t-test. Data were presented as ratios of b/b to +/b rats; means ± SEM (n = 4–8 per group). * p < 0.05 vs. +/b rats.
Oxidative stress is elevated in Belgrade rats
Since oxidative stress plays an important role in behavioral deficits and neurochemical alterations, we quantified the levels of 8-isoprostane and GSH/GSSG in the liver and hippocampus of Belgrade rats (Figure 6). In b/b rats, hepatic isoprostane levels were significantly elevated compared with +/b rats (248% increase; p < 0.001). Moreover, isoprostane was up-regulated in the hippocampus of b/b rats (42% increase; p = 0.013). Concordantly, the ratio of GSH/GSSG was decreased in the liver (78% decrease; p < 0.001) and brain (21% decrease; p = 0.042) of b/b rats. Combined, these results indicate increased oxidative stress and decreased antioxidant reserves in the brain of b/b rats.
Figure 6. Oxidative stress in Belgrade rats.
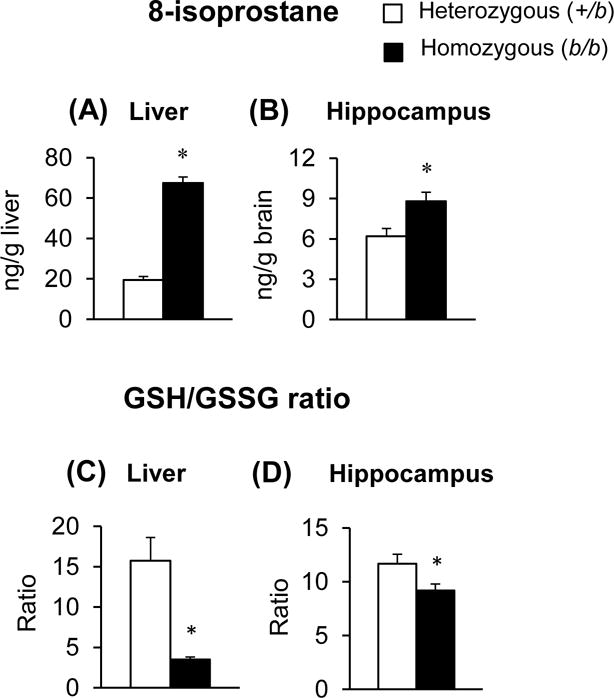
The liver (A and C) and hippocampus (B and D) were analyzed for 8-isoprostane levels and the ratio of glutathione/glutathione disulfide (GSH/GSSG). Open and closed bars represent control +/b and b/b rats, respectively. Data were presented as means ± SEM (n = 6–8 per group) and analyzed using the two-sample t-test. * p < 0.05 vs. +/b rats.
DISCUSSION
While there is a significant association between impaired iron metabolism and emotional behavior, our study demonstrates that b/b rats display risk-taking behavior and thereby increased impulsivity. These results are distinctively different from the case of iron-deficient anemia and/or hypoxia which typically demonstrate increased anxiety with hypoactivity (Li et al. 2011, Dratcu 2000). In addition, increased GABA in the brain, including hippocampus, is found in both iron-deficient anemia (Batra & Seth 2002, Rao et al. 2003, Mittal et al. 2003) and hypoxia (Madl & Royer 2000), but hippocampal GABA levels in b/b rats were decreased. These findings suggest that impaired emotional behavior and GABA homeostasis in b/b rats are unlikely influenced by their anemic status. With respect to brain iron, our b/b rats displayed decreased total iron in the brain, consistent with findings by Carlson et al. (Carlson et al. 2009) who demonstrated decreased brain iron in hippocampal DMT1-knockout mice. Notably, these mice exhibit impaired spatial memory and prepulse inhibition (Carlson et al. 2009, Pisansky et al. 2013). These results suggest that brain iron deficiency in b/b rats could contribute to abnormal emotional behavior. However, we found that iron deficiency alone does not fully explain behavioral and neurochemical changes in b/b rats; For example, non-heme iron in the brain of b/b rats was within a normal range, while the information of hippocampal non-heme iron in DMT1-knockout mice is unavailable (Carlson et al. 2009). It has been reported that non-heme iron plays an important role in a variety of physiological processes in the brain, including myelination (Connor & Menzies 1996) and enzymatic and biosynthetic activity (Magaki et al. 2007), as well as neurobehavioral function (Black 2003, Halterman et al. 2001, Blanton et al. 2013). In addition, while iron deficiency impairs recognition memory (Pinero et al. 2001), b/b rats showed no evidence of altered memory (Supplementary Material), which was consistent with unchanged non-heme iron, but poorly associated with decreased total iron in the brain. For these reasons, we hypothesized that, in addition to iron deficiency, there could be other mechanism(s) involved in the development of behavioral deficits in b/b rats, which we investigated in the current study.
Metal levels in tissues of Belgrade rats were in good agreement with reported values from other rat studies (Kucukatay et al. 2006, Mercadante et al. 2016, Tarohda et al. 2004, Abdel-Mageed & Oehme 1991, Molina et al. 2011). It has been known that abnormal levels of several trace metals are associated with psychiatric disorders. For examples, Islam et al. demonstrated that generalized anxiety disorder patients display elevated Cu, Mn and Fe, but decreased Zn, in serum (Islam et al. 2013). In contrast, Yanik et al. found higher Cu and lower Fe and Mn in serum from schizophrenic patients (Yanik et al. 2004). Moreover, patients with WD have very high levels of Cu in the liver and brain, but serum Cu is deficient (Dening & Berrios 1989). These studies indicate that serum metal levels do not necessarily represent brain metal status and therefore may not be directly correlated with emotional behavior.
Several lines of evidence have indicated that copper overload, including WD, results in increased impulsivity (Russo 2010, Stock et al. 2015). Since Cu was consistently elevated across the brain regions with brain non-heme iron unchanged in b/b rats, we speculate that increased brain copper plays a significant role in the progression of abnormal emotional behavior. Notably, Atp7b-deficient mice, an animal model of WD, display age-associated copper accumulation in the brain; brain Cu level does not differ at the age of 2-month-old between Atp7b-deficient and wild-type mice, but increases 50–130% in Atp7b-deficient mice when they become 11–24 months old (Boaru et al. 2014). Our b/b rats exhibit increased brain Cu (up to 50%) at the age of 4 months old, providing a relevant rat model of WD, potentially with early onset. However, brain Cu loading in b/b rats is less severe than in WD patients who exhibit 5–10 times higher Cu concentrations in the brain than normal levels (Scheinberg & Sternlieb 1975). It remains to be explored if older b/b rats show a similar extent of Cu deposition in the brain as observed in WD patients.
With respect to the iron-copper relationship, our observation of Cu loading in blood and liver of b/b rats is consistent with increased Cu during anemia (Fox 2003, Ravia et al. 2005). In contrast, Jiang et al. reported that serum Cu level is reduced, but unchanged in the liver in b/b rats (Jiang et al. 2011). This difference could be due to different ages/sex tested and/or different iron and copper content in chow. For example, both female and male rats at ages ranging from 3.5 to 22 month were fed semipurified AIN93G-based diet with 200 ppm iron in chow for +/b rats and 300 ppm iron for b/b rats (Jiang et al. 2011), whereas we used 11–12 weeks old male rats with 500 ppm iron in chow for both +/b and b/b rats (Supplementary Material). Since iron and copper levels change with age (Yunice et al. 1974, Mohri et al. 2007), our study using age- and diet-matched Belgrade rats provides an important insight into neurobehavioral and neurochemical phenotypes under impaired iron and copper metabolism.
Recent investigations have suggested that the hippocampus is closely involved in emotional behavior (Barkus et al. 2010, Xiang et al. 2011). For example, the spontaneously hypertensive rats, a model of ADHD, demonstrated decreased GABA levels in the hippocampus (Sterley et al. 2013), consistent with our b/b rats that display increased impulsivity with decreased hippocampal GABA concentrations. We also found that GAT mRNA levels were increased in b/b rats, which suggests a decrease in synaptic GABA levels. A future study is necessary to directly determine extracellular GABA concentrations using microdialysis. While several studies have indicated that the GABRA1 gene is linked to mood disorders (Brambilla et al. 2003, Horiuchi et al. 2004), mRNA expression of GABRA1 is up-regulated in schizophrenic subjects with no significant changes in GABRA2 (Mudge et al. 2008). Heckers et al. demonstrated that GABRAs are elevated in the brain regions, including the hippocampus, along with reduced GABA input in schizophrenia (Heckers & Konradi 2002). Combined, these findings suggest that abnormal emotional behavior in b/b rats could be associated with a compensatory up-regulation of GABRA1 expression in response to decreased brain GABA. Furthermore, GABA receptor activity is inhibited by several divalent metals, such as nickel, cadmium, zinc and copper (Fisher & Macdonald 1998, Kim & Macdonald 2003, Draguhn et al. 1990, Ma & Narahashi 1993, Narahashi et al. 1994). In particular, Cu inhibits extrasynaptic GABRA in the striatum and cerebellum (McGee et al. 2013). The effect of Cu on GABRA inhibition is concentration-dependent (Kardos et al. 1989), but this effect is attenuated by increased GABA levels (Sharonova et al. 1998). Interestingly, GABA agonists (e.g. clonazepam) are used for treating individuals with WD (Das & Ray 2006). These results indicate that Cu-induced neurotoxicity could in part be mediated by chronic GABRA blockade (Sharonova et al. 1998) and that activation of the GABA system could reverse the neurotoxic effect of Cu.
Copper accumulation in b/b rats prompted us to further examine if the expression of copper transporters was altered. In the duodenum, there was a trend of increase, although insignificant, in the expression of these transporters in b/b rats. Our results are different from those reported by Jiang et al. who showed that Atp7a is significantly up-regulated in the duodenum of b/b rats (Jiang et al. 2011). Again, these differences could result from different ages and metal contents in chow. We indeed note that duodenal copper transport using everted gut sacs is unchanged in b/b rats when the identical diet was given to both +/b and b/b rats (Jiang et al. 2013). By contrast, iron-deficient rats by diet display increased expression of both Ctr1 and Atp7a in the duodenum (Collins et al. 2009), consistent with greater intestinal uptake of Cu (Jiang et al. 2013). These findings suggest that a distinct mechanism may exist in Cu transport between genetically anemic b/b rats and postnatally iron-depleted rats. Whether or not DMT1 mediates copper transport is controversial. In our study, copper levels increased upon DMT1 mutation, favoring the claim that DMT1 does not directly transport copper. It is possible, however, that intestinal copper uptake could be increased due to potential up-regulation in other copper-transporting proteins, such as other members of Ctr family or zinc-regulated-transporter/iron-regulated-transporter-like protein (ZIP) family (Ohrvik et al. 2013, Grotz et al. 1998), in response to DMT1 deficiency.
We also examined copper transporters in the liver since copper is mainly excreted by bile secretion (Roberts & Sarkar 2008). It has been known that patients with WD display impaired copper excretion (Roberts & Sarkar 2008) and increased urinary copper (Das & Ray 2006). Similarly, we observed decreased hepatic Atp7b expression along with high urinary copper content in b/b rats. Therefore, copper excretion by the biliary route is likely reduced in b/b rats. Combined, both increased uptake and decreased excretion of copper in b/b rats could result in systemic copper overload, which could facilitate copper transport into the brain. Future study is needed to directly measure copper levels in the bile juice and also to determine the pharmacokinetics of copper in Belgrade rats. Our results also revealed increased levels of Ctr1 and Atp7a in the brain of b/b rats. Interestingly, hypoxia is a stimulus for enhanced intracellular copper transport in murine macrophages (White et al. 2009). Thus, it is possible that up-regulation of Ctr1 and Atp7a could be induced by hypoxia resulting from anemic effect in b/b rats, and this effect could be more obvious in the brain (hypoxia-sensitive tissue) than in duodenum or liver. The molecular mechanism of brain copper transport under altered iron metabolism should be explored in the future study.
Increased Cu is potentially toxic due to the ability to generate free radicals via the Fenton reaction (Jomova & Valko 2011). Cu is typically bound in tissues by metallothioneins as a nontoxic form. However, increased Cu beyond the detoxification capacity can elevate oxidative stress and lead to tissue damage. In support of this, elevated isoprostanes are found in copper overload (Viquez et al. 2008). Importantly, oxidative stress reduces GABA levels (Rego et al. 1996) and alters GABA uptake (Braughler 1985). Therefore, it is plausible that increased brain copper in b/b rats promotes metal-induced oxidative stress that consequently impairs GABA function and associated behavior. Together, our study provides a molecular and neurochemical basis for the development and progression of abnormal emotional behavior in loss of DMT1 function. It remains to be tested whether a reversal of oxidative stress (i.e. supplementation of antioxidants) and/or a removal of excess copper (e.g. copper chelators; D-penicillamine and trientine) could correct abnormal GABAergic function and emotional dysfunction in b/b rats. These approaches will contribute to the better understanding of physiological risks associated with imbalanced metal metabolism in mental function and, more specifically, the interactions with GABA and redox control in the treatment of emotional disorders.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH K99/R00 ES017781 (J.K.). Authors are grateful to Ms. Qi Ye and Mr. Helal Alsulimani for help during animal experiments.
Abbreviations
- +/b rats
heterozygous Belgrade rats
- Atp7a
copper-transporting ATPase 1
- Atp7b
copper-transporting ATPase 2
- b/b rats
homozygous Belgrade rats
- Cp
ceruloplasmin
- Ctr1
copper transporter 1
- DMT1
divalent metal transporter 1
- Fe
iron
- GABRA1
GABAA receptor α1
- GABRA2
GABAA receptor α2
- GAD
glutamate decarboxylase
- GAT
GABA transporter
- GSH
glutathione
- GSSG
glutathione disulfide
- Mt
metallothionein
- Mn
manganese
- ROS
reactive oxygen species
- Zn
zinc
Footnotes
ARRIVE guidelines have been followed: Yes => if No, skip complete sentence => if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.” Conflicts of interest: none => if ‘none’, insert “The authors have no conflict of interest to declare. ” => otherwise insert info unless it is already included
Authors have no conflicts of interest.
References
- Abdel-Mageed AB, Oehme FW. The effect of various dietary zinc concentrations on the biological interactions of zinc, copper, and iron in rats. Biol Trace Elem Res. 1991;29:239–256. doi: 10.1007/BF03032681. [DOI] [PubMed] [Google Scholar]
- Arredondo M, Mendiburo MJ, Flores S, Singleton ST, Garrick MD. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2014;27:115–123. doi: 10.1007/s10534-013-9691-6. [DOI] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nature reviews. Drug discovery. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Batra J, Seth PK. Effect of iron deficiency on developing rat brain. Indian journal of clinical biochemistry : IJCB. 2002;17:108–114. doi: 10.1007/BF02867982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerly KA, Kelleher SL, Lonnerdal B. Effects of copper supplementation on copper absorption, tissue distribution, and copper transporter expression in an infant rat model. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1007–1014. doi: 10.1152/ajpgi.00210.2004. [DOI] [PubMed] [Google Scholar]
- Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–1179. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr. 2003;133:3927S–3931S. doi: 10.1093/jn/133.11.3927S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton CA, Green MW, Kretsch MJ. Body iron is associated with cognitive executive planning function in college women. Br J Nutr. 2013;109:906–913. doi: 10.1017/S0007114512002620. [DOI] [PubMed] [Google Scholar]
- Boaru SG, Merle U, Uerlings R, Zimmermann A, Weiskirchen S, Matusch A, Stremmel W, Weiskirchen R. Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson’s disease by laser ablation inductively coupled plasma mass spectrometry. BMC Neurosci. 2014;15:98. doi: 10.1186/1471-2202-15-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen BJ, Morgan EH. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood. 1987;70:38–44. [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Molecular psychiatry. 2003;8:721–737, 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Braughler JM. Lipid peroxidation-induced inhibition of gamma-aminobutyric acid uptake in rat brain synaptosomes: protection by glucocorticoids. J Neurochem. 1985;44:1282–1288. doi: 10.1111/j.1471-4159.1985.tb08755.x. [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Carlson ES, Tkac I, Magid R, O’Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Kueon C, Kim J. Influence of lead on repetitive behavior and dopamine metabolism in a mouse model of iron overload. Toxicological research. 2014;30:267–276. doi: 10.5487/TR.2014.30.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JF, Hua P, Lu Y, Ranganathan PN. Alternative splicing of the Menkes copper Atpase (Atp7a) transcript in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;297:G695–707. doi: 10.1152/ajpgi.00203.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- Delinard A, Gilbert A, Dodds M, Egeland B. Iron deficiency and behavioral deficits. Pediatrics. 1981;68:828–833. [PubMed] [Google Scholar]
- Dening TR, Berrios GE. Wilson’s disease. Psychiatric symptoms in 195 cases. Archives of general psychiatry. 1989;46:1126–1134. doi: 10.1001/archpsyc.1989.01810120068011. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Dratcu L. Panic, hyperventilation and perpetuation of anxiety. Progress in neuro-psychopharmacology & biological psychiatry. 2000;24:1069–1089. doi: 10.1016/s0278-5846(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. The role of an alpha subtype M2-M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2003;16:9–40. doi: 10.1023/a:1020799512190. [DOI] [PubMed] [Google Scholar]
- Fujimura J, Nagano M, Suzuki H. Differential expression of GABA(A) receptor subunits in the distinct nuclei of the rat amygdala. Brain research. Molecular brain research. 2005;138:17–23. doi: 10.1016/j.molbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, et al. DMT1: which metals does it transport? Biol Res. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–1386. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- Han M, Kim J. Effect of dietary iron loading on recognition memory in growing rats. PLoS One. 2015;10:e0120609. doi: 10.1371/journal.pone.0120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of neural transmission. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Horiuchi Y, Nakayama J, Ishiguro H, et al. Possible association between a haplotype of the GABA-A receptor alpha 1 subunit gene (GABRA1) and mood disorders. Biological psychiatry. 2004;55:40–45. doi: 10.1016/s0006-3223(03)00689-9. [DOI] [PubMed] [Google Scholar]
- Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012;287:30485–30496. doi: 10.1074/jbc.M112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Ahmed MU, Mitu SA, Islam MS, Rahman GK, Qusar MM, Hasnat A. Comparative analysis of serum zinc, copper, manganese, iron, calcium, and magnesium level and complexity of interelement relations in generalized anxiety disorder patients. Biol Trace Elem Res. 2013;154:21–27. doi: 10.1007/s12011-013-9723-7. [DOI] [PubMed] [Google Scholar]
- Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF. Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr. 2013;143:1927–1933. doi: 10.3945/jn.113.181867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Ranganathan P, Lu Y, Kim C, Collins JF. Exploration of the copper-related compensatory response in the Belgrade rat model of genetic iron deficiency. Am J Physiol Gastrointest Liver Physiol. 2011;301:G877–886. doi: 10.1152/ajpgi.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kardos J, Kovacs I, Hajos F, Kalman M, Simonyi M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- Kim H, Macdonald RL. An N-terminal histidine is the primary determinant of alpha subunit-dependent Cu2+ sensitivity of alphabeta3gamma2L GABA(A) receptors. Mol Pharmacol. 2003;64:1145–1152. doi: 10.1124/mol.64.5.1145. [DOI] [PubMed] [Google Scholar]
- Kim J, Jia X, Buckett PD, Liu S, Lee CH, Wessling-Resnick M. Iron loading impairs lipoprotein lipase activity and promotes hypertriglyceridemia. FASEB J. 2013;27:1657–1663. doi: 10.1096/fj.12-224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukatay V, Turgut S, Kocamaz E, Emmungil G, Bor-Kucukatay M, Turgut G, Akca H, Bagci H. Effect of sulfite exposure on zinc, iron, and copper levels in rat liver and kidney tissues. Biol Trace Elem Res. 2006;114:185–195. doi: 10.1385/BTER:114:1:185. [DOI] [PubMed] [Google Scholar]
- Lestaevel P, Romero E, Dhieux B, Ben Soussan H, Berradi H, Dublineau I, Voisin P, Gourmelon P. Different pattern of brain pro-/anti-oxidant activity between depleted and enriched uranium in chronically exposed rats. Toxicology. 2009;258:1–9. doi: 10.1016/j.tox.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Li Y, Kim J, Buckett PD, Bohlke M, Maher TJ, Wessling-Resnick M. Severe postnatal iron deficiency alters emotional behavior and dopamine levels in the prefrontal cortex of young male rats. The Journal of nutrition. 2011;141:2133–2138. doi: 10.3945/jn.111.145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Ma JY, Narahashi T. Differential modulation of GABAA receptor-channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Res. 1993;607:222–232. doi: 10.1016/0006-8993(93)91510-y. [DOI] [PubMed] [Google Scholar]
- Maaroufi K, Ammari M, Jeljeli M, Roy V, Sakly M, Abdelmelek H. Impairment of emotional behavior and spatial learning in adult Wistar rats by ferrous sulfate. Physiol Behav. 2009;96:343–349. doi: 10.1016/j.physbeh.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Madl JE, Royer SM. Glutamate dependence of GABA levels in neurons of hypoxic and hypoglycemic rat hippocampal slices. Neuroscience. 2000;96:657–664. doi: 10.1016/s0306-4522(99)00548-5. [DOI] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annual review of neuroscience. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- Magaki S, Mueller C, Yellon SM, Fox J, Kim J, Snissarenko E, Chin V, Ghosh MC, Kirsch WM. Regional dissection and determination of loosely bound and non-heme iron in the developing mouse brain. Brain Res. 2007;1158:144–150. doi: 10.1016/j.brainres.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TP, Houston CM, Brickley SG. Copper block of extrasynaptic GABAA receptors in the mature cerebellum and striatum. J Neurosci. 2013;33:13431–13435. doi: 10.1523/JNEUROSCI.1908-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta BC, Pandya BG, Mehta JB, Bisariya BN. Iron loading anaemias. The Journal of the Association of Physicians of India. 1989;37:754–756. [PubMed] [Google Scholar]
- Menon AV, Chang J, Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology. 2016;339:58–72. doi: 10.1016/j.tox.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante CJ, Herrera C, Pettiglio MA, Foster ML, Johnson LC, Dorman DC, Bartnikas TB. The effect of high dose oral manganese exposure on copper, iron and zinc levels in rats. Biometals. 2016 doi: 10.1007/s10534-016-9924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal RD, Pandey A, Mittal B, Agarwal KN. Effect of latent iron deficiency on GABA and glutamate neuroreceptors in rat brain. Indian journal of clinical biochemistry : IJCB. 2003;18:111–116. doi: 10.1007/BF02867677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri M, Sharifi K, Eidi S. Hematology and serum biochemistry of Holstein dairy calves: age related changes and comparison with blood composition in adults. Research in veterinary science. 2007;83:30–39. doi: 10.1016/j.rvsc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Molina RM, Phattanarudee S, Kim J, Thompson K, Wessling-Resnick M, Maher TJ, Brain JD. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology. 2011;32:413–422. doi: 10.1016/j.neuro.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge J, Miller NA, Khrebtukova I, et al. Genomic convergence analysis of schizophrenia: mRNA sequencing reveals altered synaptic vesicular transport in post-mortem cerebellum. PloS one. 2008;3:e3625. doi: 10.1371/journal.pone.0003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. GABA receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cell Mol Neurobiol. 1994;14:599–621. doi: 10.1007/BF02088671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrvik H, Nose Y, Wood LK, Kim BE, Gleber SC, Ralle M, Thiele DJ. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4279–4288. doi: 10.1073/pnas.1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk FG, Kessler H, Supprian T, et al. Cognitive decline correlates with low plasma concentrations of copper in patients with mild to moderate Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2005;8:23–27. doi: 10.3233/jad-2005-8103. [DOI] [PubMed] [Google Scholar]
- Pankhurst MW, Gell DA, Butler CW, Kirkcaldie MT, West AK, Chung RS. Metallothionein (MT) -I and MT-II expression are induced and cause zinc sequestration in the liver after brain injury. PloS one. 2012;7:e31185. doi: 10.1371/journal.pone.0031185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. The Journal of nutrition. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CC, Mailloux R. Excess Copper as a Factor in Human-Diseases. J Orthomol Med. 1987;2:171–182. [Google Scholar]
- Pinero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131:311–318. doi: 10.1093/jn/131.2.311. [DOI] [PubMed] [Google Scholar]
- Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan LL, Sun M, Gewirtz JC, Georgieff MK. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus. 2013;23:952–962. doi: 10.1002/hipo.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR. Biochemical changes in copper deficiency. J Nutr Biochem. 1990;1:452–461. doi: 10.1016/0955-2863(90)90080-5. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes Copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. The Journal of biological chemistry. 2005;280:36221–36227. doi: 10.1074/jbc.M506727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego AC, Santos MS, Oliveira CR. Oxidative stress, hypoxia, and ischemia-like conditions increase the release of endogenous amino acids by distinct mechanisms in cultured retinal cells. Journal of neurochemistry. 1996;66:2506–2516. doi: 10.1046/j.1471-4159.1996.66062506.x. [DOI] [PubMed] [Google Scholar]
- Roberts EA, Sarkar B. Liver as a key organ in the supply, storage, and excretion of copper. The American journal of clinical nutrition. 2008;88:851S–854S. doi: 10.1093/ajcn/88.3.851S. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. Journal of neuroscience methods. 1995;57:93–99. doi: 10.1016/0165-0270(94)00132-z. [DOI] [PubMed] [Google Scholar]
- Russo AJ. Decreased Serum Cu/Zn SOD Associated with High Copper in Children with Attention Deficit Hyperactivity Disorder (ADHD) Journal of central nervous system disease. 2010;2:9–14. doi: 10.4137/jcnsd.s4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg IH, Sternlieb I. Chapter 5: Wilson’s Disease: Biology of Brain Dysfunction. Plenum Press; New York and London: 1975. [Google Scholar]
- Schlief ML, Gitlin JD. Copper homeostasis in the CNS: a novel link between the NMDA receptor and copper homeostasis in the hippocampus. Molecular neurobiology. 2006;33:81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharonova IN, Vorobjev VS, Haas HL. High-affinity copper block of GABA(A) receptor-mediated currents in acutely isolated cerebellar Purkinje cells of the rat. The European journal of neuroscience. 1998;10:522–528. doi: 10.1046/j.1460-9568.1998.00057.x. [DOI] [PubMed] [Google Scholar]
- Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Current topics in membranes. 2012;70:169–214. doi: 10.1016/B978-0-12-394316-3.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladic-Simic D, Martinovitch PN, Zivkovic N, Pavic D, Martinovic J, Kahn M, Ranney HM. A thalassemia-like disorder in Belgrade laboratory rats. Ann N Y Acad Sci. 1969;165:93–99. doi: 10.1111/j.1749-6632.1969.tb27779.x. [DOI] [PubMed] [Google Scholar]
- Sobotka TJ, Whittaker P, Sobotka JM, Brodie RE, Quander DY, Robl M, Bryant M, Barton CN. Neurobehavioral dysfunctions associated with dietary iron overload. Physiol Behav. 1996;59:213–219. doi: 10.1016/0031-9384(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Sterley TL, Howells FM, Russell VA. Evidence for reduced tonic levels of GABA in the hippocampus of an animal model of ADHD, the spontaneously hypertensive rat. Brain research. 2013;1541:52–60. doi: 10.1016/j.brainres.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Stock AK, Reuner U, Gohil K, Beste C. Effects of copper toxicity on response inhibition processes: a study in Wilson’s disease. Archives of toxicology. 2015 doi: 10.1007/s00204-015-1609-3. [DOI] [PubMed] [Google Scholar]
- Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983;306:284–287. doi: 10.1038/306284a0. [DOI] [PubMed] [Google Scholar]
- Takano K, Yatabe MS, Abe A, Suzuki Y, Sanada H, Watanabe T, Kimura J, Yatabe J. Characteristic expressions of GABA receptors and GABA producing/transporting molecules in rat kidney. PloS one. 2014;9:e105835. doi: 10.1371/journal.pone.0105835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Tarohda T, Yamamoto M, Amamo R. Regional distribution of manganese, iron, copper, and zinc in the rat brain during development. Anal Bioanal Chem. 2004;380:240–246. doi: 10.1007/s00216-004-2697-8. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade rats display liver iron loading. J Nutr. 2006;136:3010–3014. doi: 10.1093/jn/136.12.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance JD, Bothwell TH. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci. 1968;33:9–11. [PubMed] [Google Scholar]
- Viquez OM, Valentine HL, Amarnath K, Milatovic D, Valentine WM. Copper accumulation and lipid oxidation precede inflammation and myelin lesions in N,N-diethyldithiocarbamate peripheral myelinopathy. Toxicol Appl Pharmacol. 2008;229:77–85. doi: 10.1016/j.taap.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Kambe T, Fulcher YG, Sachdev SW, Bush AI, Fritsche K, Lee J, Quinn TP, Petris MJ. Copper transport into the secretory pathway is regulated by oxygen in macrophages. Journal of cell science. 2009;122:1315–1321. doi: 10.1242/jcs.043216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Loukopoulos D, Lee GR, Cartwright GE. Role of copper in mitochondrial iron metabolism. Blood. 1976;48:77–85. [PubMed] [Google Scholar]
- Xiang X, Huang W, Haile CN, Kosten TA. Hippocampal GluR1 associates with behavior in the elevated plus maze and shows sex differences. Behavioural brain research. 2011;222:326–331. doi: 10.1016/j.bbr.2011.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res. 2004;98:109–117. doi: 10.1385/BTER:98:2:109. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Ben-Shachar D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am J Clin Nutr. 1989;50:607–615. doi: 10.1093/ajcn/50.3.607. discussion 615–617. [DOI] [PubMed] [Google Scholar]
- Yunice AA, Lindeman RD, Czerwinski AW, Clark M. Influence of age and sex on serum copper and ceruloplasmin levels. Journal of gerontology. 1974;29:277–281. doi: 10.1093/geronj/29.3.277. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


