Abstract
Objective
The object of this study was to determine culture conditions that create stable scaffold-free cartilage-like cell-sheets from human bone marrow–derived mesenchymal stem cells (hBMSCs) and to assess their effects after transplantation into osteochondral defects in nude rats.
Design
(Experiment 1) The hBMSCs were harvested from 3 males, the proliferative and chondrogenic capacities were assessed at passage 1, and the cells were expanded in 3 different culture conditions: (1) 5% fetal bovine serum (FBS), (2) 10% FBS, and (3) 5% FBS with fibroblast growth factor 2 (FGF-2). The cells were harvested and made chondrogenic pellet culture. The cell proliferation rate, glycosaminoglycan/DNA ratio, and safranin-O staining intensity of pellets cultured condition 3 were higher than those of conditions 1 and 2. (Experiment 2) The hBMSCs were expanded and passaged 3 times under culture condition 3, and fabricate the cell-sheets in chondrogenic medium either with or without FBS. The cell-sheets fabricated with FBS maintained their size with flat edges. (Experiment 3) The cell-sheets were transplanted into osteochondral defects in nude rats. Histological analysis was performed at 2, 4, and 12 weeks after surgery.
Results
The osteochondral repair was better after sheet transplantation than in the control group and significantly improved Wakitani score. Immunostaining with human-specific vimentin antibody showed that the transplanted cells became fewer and disappeared at 12 weeks.
Conclusions
These results indicate that culture with FGF-2 may help to quickly generate sufficient numbers of cells to create stable and reliable scaffold-free cartilage-like cell-sheets, which contribute to the regeneration of osteochondral defects.
Keywords: animal models, articular cartilage, knee, mesenchymal stem cells, rodent
Introduction
Articular cartilage defects show a poor capacity for self-repair. Large defects in articular cartilage, including full-thickness defects, often progress to osteoarthritis (OA). Current surgical treatments for defects include microfracture and mosaicplasty.1,2 The microfracture technique often results in repair with fibrocartilage,3 which has inadequate biomechanical properties compared with hyaline cartilage. Therefore, the progression to OA remains a concern.4 Mosaicplasty requires the harvesting of cylindrical osteochondral grafts from healthy, non-weightbearing areas of cartilage, which is invasive and destructive. Regarding cell therapies, autologous chondrocyte implantation (ACI) has been explored and performed more than 20,000 times.5 However, this therapy remains controversial because of problems with harvesting normal cartilage and periosteum, as well as with securing the periosteum over the defect to retain the transplanted cells, which increases the operation time and surgical stress.6 These disadvantages have led to the development of bio-absorbable material covers manufactured from porcine-derived type I/type III collagen, which have achieved similar clinical outcomes as ACI.7 The implantation of cultured chondrocytes in suspension, as is done during ACI covered with either periosteum or a collagen membrane, raises concerns about the uneven distribution of chondrocytes within the defect and the potential for cell leakage.8 To address those problems, matrix-induced autologous chondrocyte implantation (MACI) was introduced, which used a collagen bilayer seeded with chondrocytes (vergen, Leverkusen, Germany).9 However, this method does not address the problem that normal cartilage must be harvest and uses porcine-derived collagen as the scaffold.
Our group has been developing cell therapies using bone marrow–derived mesenchymal stem cells (BMSCs) for cartilage defect repair.10-12 BMSCs can be harvested easily using more minimally invasive methods than ACI.11 Furthermore, there is no need to harvest normal cartilage tissue. BMSC transplantation is likely a safe procedure because neither tumors nor infections were observed from 5 to 137 months (mean: 75 months) of follow-up in our study.12 That study reported some success repairing osteochondral defects with human BMSCs (hBMSC), but did not regenerate new hyaline cartilage with the same properties as native articular cartilage.12 To find better methods for regenerating articular cartilage defects using hBMSC,s Agung et al.13 injected a large number of MSCs into multiple tissues of an injured knee joint. They found that the cells mobilized to the injured area and contributed to tissue regeneration. They also found that a large number of the injected MSCs generated free bodies of scar tissue in the joint.13 We hypothesized that the induction of chondrogenic differentiation and seeding of high density of cells into the tissue matrix directly within the defect are important for regenerating cartilage. The transplantation of cell-sheets may enhance the repair of osteochondral defects and produce better quality hyaline cartilage, because of the high density of cells on the sheet that are seeded into the tissue matrix within the defect.
In order to provide large, stable cell-sheets, a sufficient number of cells are needed within a short period of time. However, hBMSCs lose their differentiation potential after extensive passaging.14 Fibroblast growth factor 2 (FGF-2) supplementation in hBMSCs growth media was reported to increase cell proliferation and enhance chondrogenic potential.15 Our colleagues previously reported that scaffold-free cartilage-like cell-sheets derived from hBMSCs shrink when prepared with serum-free chondrogenic medium, but not when prepared with chondrogenic medium containing serum.16 While they cultured their cell-sheets in medium supplemented with 10% fetal bovine serum (FBS).We are planning to use autologous serum for clinical applications, using a large amount of serum during cell culture require, large samples of peripheral blood. To reduce the amount of necessary serum, we expected that FGF-2 was a suitable compensation. Thus, the purpose of this study was to determine the culture conditions that provide a sufficient number of hBMSCs to create stable scaffold-free cartilage-like cell-sheets and to assess their effects after transplantation into osteochondral defects in nude rats.
Materials and Methods
Isolation of hBMSCs
hBMSCs were prepared from three male donors aged 21 (#1), 27 (#2), and 31 (#3) years and from one commercially available bone marrow (Allcells LLC, Alameda, CA, USA) from a 20-year-old male (#4). The donor cells were derived from bone marrow aspirated from the iliac crest of normal healthy human donors at the Osaka City University Hospital and Hyogo College of Medicine after obtaining informed consent from each patient before they underwent lower extremity joint surgeries. The protocols used in this study were approved by the institutional committees on human research.
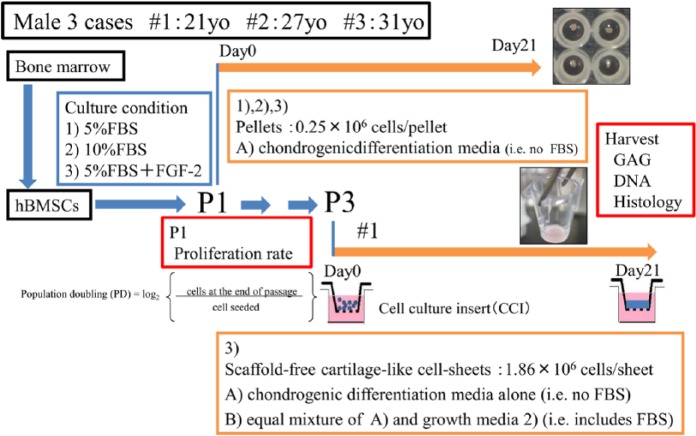
The bone marrow samples were seeded on T175 flasks (175 cm 2; Corning, NY, USA) and cultured in Dulbecco’s modified Eagle’s medium–low glucose (DMEM-LG; Nacalai Tesque, Kyoto, Japan) supplemented with 100 U/L penicillin and 0.1 mg/L streptomycin (Nacalai Tesque), as well as (1) 5% fetal bovine serum (FBS; Sigma-Aldrich, Tokyo, Japan), (2) 10% FBS, or (3) 5% FBS and 10 ng/mL of FGF-2 (Kaken Pharmaceutical, Osaka, Japan). All the cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was first changed 3 days after seeding and was then replaced twice a week. When the adherent cells reached sub confluent, they were collected using trypsin–ethylenediamine tetraacetic (trypsin-EDTA) (Sigma Chemical, Tokyo, Japan) and further subcultured by counting and reseeding at a density of 1.0 × 106 cells per 175 cm2 ( Fig. 1 ).
Figure 1.
Chondrogenic differentiation induction and culture. Human bone marrow–derived mesenchymal stem cells (hBMSCs) were derived from bone marrow aspirates from the iliac crest of 3 male donors aged 21 (#1), 27 (#2), and 31 (#3) years. The bone mallow cells were seeded on flasks in media supplemented with 3 conditions: (1) 5% fetal bovine serum (FBS), (2) 10% FBS, and (3) 5% FBS + 10 ng/mL fibroblast growth factor 2 (FGF-2). The cells were subcultured at passage 1 (P1). Cell pellets were prepared using one of the following conditions: chondrogenic medium A that was chondrogenic differentiation media (i.e., no FBS). The trypsinized cell suspensions were manually counted using a hemocytometer, and population doublings were calculated using the equation shown. Cells from donor #1 were expanded to passage 3 (P3) in media from condition 3. Scaffold-free cartilage-like cell-sheets were prepared using two different conditions: one was A only and another was B, which was equal mixture of A and growth media 2 (i.e., includes FBS). The pellets and sheets were harvested and analyzed for glycosaminoglycan (GAG) and DNA contents and tissue matrix composition.
Cell Expansion
The trypsinized cell suspensions were manually counted using a hemocytometer. Population doublings for each of the 3 treatment groups—(1) 5% FBS, (2) 10% FBS, and (3) 5% FBS + FGF-2—were calculated based on the number of population doublings in that passage divided by the duration of the passage in days, as previously described ( Fig. 1 ).14
Flow Cytometric Analysis
The surface protein expression of the hBMSCs was examined by flow cytometry. Passage 1 hBMSCs before chondrogenic differentiation were stained using antibodies against CD44-FITC, CD105-PE, isotype control PE, and isotype control FITC for 20 minutes. After staining, the cells were washed twice in phosphate buffered saline (PBS) and analyzed on a standard Becton–Dickinson FACS Aria instrument (BD, San Jose, CA, USA). The data were acquired and analyzed using a FACSCalibur (BD, Franklin Lakes, NJ, USA). All the antibodies and isotype controls were purchased from BD.
Chondrogenic Differentiation
Pellet Culture
During passage 1 (P1) the cells were subcultured in chondrogenic medium A), which consisted of high-glucose DMEM (Nacalai Tesque) supplemented with 1% ITS Premix (BD Biosciences, Franklin Lakes, NJ, USA), 100 mM ascorbate-2-phosphate (Wako, Osaka, Japan), 10−7 M dexamethasone (Sigma Chemical), and 10 ng/mL transforming growth factor (TGF)-β1 (Peprotech, Rocky Hill, NJ, USA). Aliquots of 200 µL containing 0.25×106 cells were placed in polypropylene conical, nonadherent, V-bottom 96-well plates (Evergreen Scientific, Los Angeles, CA, USA), centrifuged at 2400 rpm, and placed in a 37°C, 5% CO2 incubator. The chondrogenic medium was replaced every 2 days. On day 21, the cell aggregates (pellets) were harvested. Replicate pellets from each of the 3 groups were processed for histology and glycosaminoglycan (GAG) and DNA quantification using the methods described below ( Fig. 1 ).15
Cell-Sheet Culture
Cells from donor #1 (a 21-year-old male) were grown until passage 3 (P3) in condition 3 (5% FBS + FGF-2). Then, using the method of Takagi’s group,16,17 we prepared scaffold-free cartilage-like cell-sheets using 2 different conditions: (A) a chondrogenic differentiation media alone (i.e., no FBS) and (B) equal mixtures of A and growth media 2 (10% FBS). The cell suspensions in chondrogenic medium (0.3 mL, 1.86 × 106 cells/well) were added to empty wells of a cell culture insert (CCI; 0.3 cm2, thickness 20-25 µm, polyethylene terephthalate; BD Biosciences) in wells of a 24-well plate containing 1.0 mL of the chondrogenic medium. The cells were incubated at 37°C in 5% CO2 for 21 days, and the chondrogenic medium was replaced every 2 days ( Fig. 1 ).17
Cell-Sheet Culture for Transplantation
To create cell-sheets for transplantation, the cells from 2 donors (#1 and #4) were grown to P1 in condition 3 (5% FBS + FGF-2). Then, we prepared scaffold-free cartilage-like cell-sheets using condition B, which included FBS.
DNA and GAG Quantification
The P1 cell 3 pellets from each group were digested with papain (Sigma Chemical), stained with safranin-O (Sigma Chemical) and Hoechst33258 (Sigma Chemical), and the GAG and DNA was assayed in triplicate as previously described.15 The GAG content of the pellets was normalized to the DNA content.
Histology of Cell-Sheets and Pellet Cultures
The pellets and cell-sheets were harvested and fixed in buffered formalin containing 2.5% cetylpyridinium chloride monohydrate (Wako Pure Chemical Industries, Osaka, Japan) at 4°C and embedded in paraffin. Histological imaging using light microscopy was performed on the safranin-O stained sections of the pellets and cell-sheets at a thickness of 3µm and the images were analyzed.
Animals
Fifteen 11-week-old female immunodeficient rats (F344/Njcl-run/run) purchased from Clea Japan, Inc. (Tokyo, Japan) were maintained on a 24-hour light-dark cycle with food and water ad libitum. All animal protocols were approved by and conducted in accordance with the regulations of the Osaka City University School of Medicine Committee on Animal Research.
Osteochondral Defect Creation
The rats were anesthetized by subcutaneous injections of ketamine (50 mg/mL; Sankyo Co., Ltd., Tokyo, Japan) and xylazine (0.2 mg/mL; Bayer Co., Ltd., Tokyo, Japan) in a ratio of 10:3 at a dose of 1 mL/kg body weight. An incision through the skin was made at the midline of the knee to expose the joint, and the patella was dislocated laterally using medial parapatellar methods. Osteochondral defects (diameter 2.0 mm, depth 1.0 mm) were created in the patellar groove of the femoral bone in both knees using a hand drill. In one knee, the defect was filled with a scaffold-free cartilage-like cell-sheet and fixed with fibrin glue. In the other knee, the defect was left empty. The dislocated patella was sutured and returned to the original position, and the incision was sutured closed. The animals were allowed to recover and move freely after surgery.
Histological Evaluation of the Osteochondral Defects in Animals
At 2 weeks (n = 5), 4 weeks (n = 5), and 12 weeks (n = 5) after the osteochondral defect surgery, rats at each time point were euthanized by CO2 inhalation. The knees were harvested and fixed in 4% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan) for 24 hours at 4°C. The knees were decalcified with 0.5 M EDTA at pH 7.4 for 3 weeks, and then embedded in paraffin and sagittally sectioned to the femur at a thickness of 4 µm. The sections were stained with hematoxylin/eosin or toluidine blue. Microscopy images of the sections were evaluated and quantified using the Wakitani score.10 Sections from each animal were scored independently by 3 of the authors (M.I., H.M., and Y.T.) who were blinded to the study groups.
Immunohistochemistry
To evaluate chondrogenesis in the transplanted tissues, sections were prepared and stained for collagen types I (ColI), II (ColII), X (ColX) and human-specific vimentin (hVIM) using anti-colI (ab34710, 1:50; Abcam, Cambridge, MA, USA), anti-colII (ab34712, 1:50; Abcam), anti-colX (ab58632, 1:8000; Abcam) and anti-hVIM (ab16700, 1:100; Abcam) antibodies. All of the sections were deparaffinized in xylene, and those for ColI and ColII were digested with target retrieval solution (S1699; Dako, Glostrup, Denmark) for 20 minutes. For ColX, antigen retrieval was performed with Pronase E (Sigma) at room temperature for 10 minutes. All the sections were then blocked with goat serum for 60 minutes. The primary antibodies and normal goat IgG (negative control) were diluted in 0.01 M PBS at pH 7.4 and applied at 4°C overnight. The sections were then incubated with goat anti-rabbit IgG (Chemicon, Temecula, CA, USA) secondary antibody for 60 minutes and then treated with a Vectastain ABC kit (Funakoshi Co., Tokyo, Japan). The ColI, ColII, ColX, and hVIM were visualized by the reactions of the secondary antibody with 0.01% diaminobenzidine (Wako, Osaka, Japan) in Tris-buffered saline containing with 0.01% H2O2.
Statistical Analyses
The statistical significance of differences in the number of population doublings and the GAG/DNA ratio were determined using t tests. Comparisons of the Wakitani score7 between the 2 treatment groups were analyzed using Wilcoxon signed-rank tests. P values less than 0.05 were considered statistically significant.
Results
Surface Protein Expression
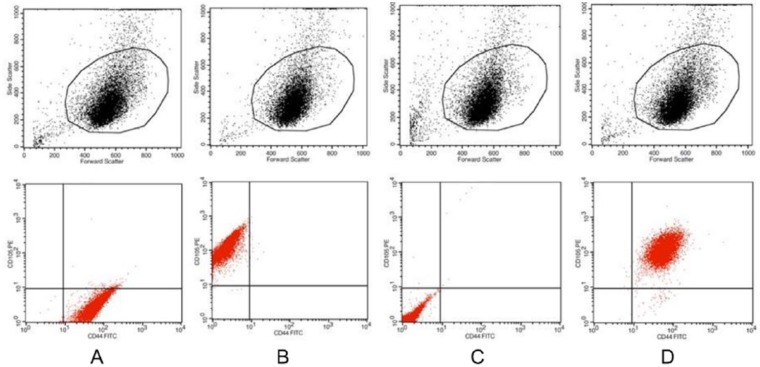
Passage 1 hBMSCs, that is, before chondrogenic differentiation, were examined for surface protein molecule expression by flow cytometry. We examined the expression of CD44, CD105, and their isotype controls. Representative surface marker expression patterns are shown in Figure 2 . More than 90% of the hBMSCs expressed both CD44 and CD105. These results indicated that most of the cultured cells were in fact hBMSCs ( Fig. 2 ).
Figure 2.
The surface marker expression was determined by flow cytometry for donor #1, who was 21 years old. (A) CD44-positive, (B) CD105-positive, (C) both negative, and (D) both positive. FACS analysis showed that more than 93.5% of the bone marrow–derived mesenchymal stem cells (BMSCs) expressed both CD44 and CD105.
Proliferation and GAG/DNA of Pellets
The proliferation rates of condition 2 (10% FBS) were twice as high as those found for condition 1 (5% FBS), while those of condition 3 (5% FBS + FGF-2) were 4.5 times higher than those of condition 1 (5% FBS) (P < 0.05; Fig. 3A ). No significant difference was found in the GAG/DNA ratio between conditions 1 (5% FBS) and 2 (10% FBS), but the ratio of condition 3 (5% FBS + FGF-2) was 3-fold higher than the other conditions (P < 0.05, n = 3, assayed in triplicate; Fig. 3B ). These results indicated that addition of FGF-2 during the expansion of hBMSCs not only increased cell proliferation over a short period of time, and enhanced their chondrogenic potential as previous reports had described,18-21 but it was also possible to lower the concentration of FBS.
Figure 3.
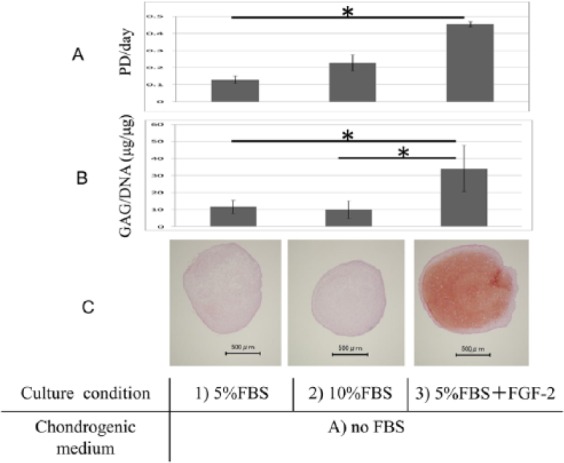
Human bone marrow–derived mesenchymal stem cells (hBMSCs) from donors #1, 21 years old; #2, 27 years old; #3, 31 years old at passage (P1) were used to assess population doubling (PD) per day described in Figure 1 , pellets glycosaminoglycan (GAG)/DNA (μg/μg), and donor #2 was histological safranin-O staining. (A) The proliferation rates of cells in condition 3, 5% fetal bovine serum (FBS) + 10 ng/mL fibroblast growth factor 2 (FGF-2) were higher than those in condition 1 5% FBS (*P < 0.05, t test). (B) No difference in GAG/DNA ratio was found between conditions 1 and 2, 10% FBS; the ratio in condition 3 was higher than the others (*P < .05, n = 3, assayed in triplicate, t test). (C) The safranin-O staining was more intense in condition 3 than in conditions 1 and 2.
Safranin-O Staining of the Pellets, Cell-Sheets, and Shape of the Cell-Sheets
The safranin-O staining in condition 3 (5% FBS + FGF-2) was more intense than that in conditions 1 (5% FBS) and 2 (10% FBS) ( Fig. 3C ). The macroscopic observations of scaffold-free cartilage-like cell-sheets, the center of group A (with no FBS) was recessed slightly, but did not shrink. The surface of group B (with FBS) was stable and flat ( Fig. 4A ). The histology showed that the margins of the sheets in A were curled. The surface of B was flat. In addition, compared with group A (with no FBS), group B (with FBS) was more intensely stained by safranin-O ( Fig. 4B ).
Figure 4.
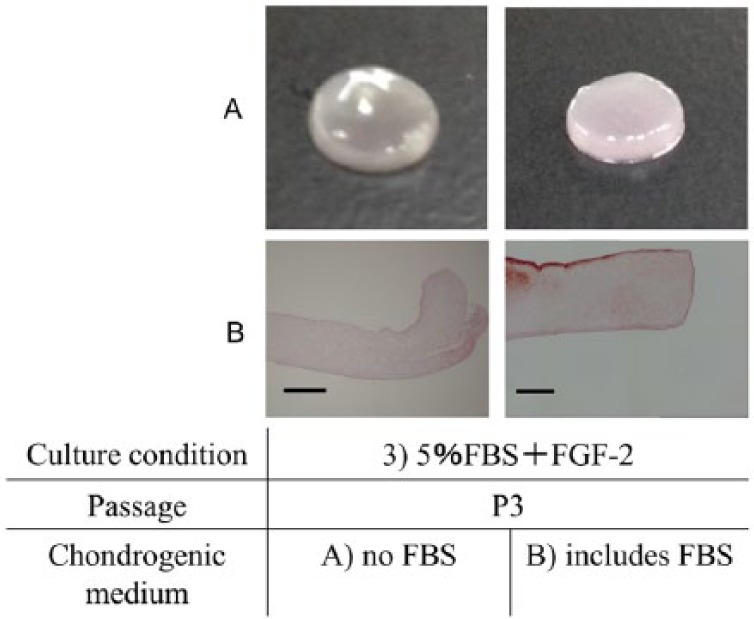
Human bone marrow–derived mesenchymal stem cells (hBMSCs) from donor #1, 21 years old, at passage 3 (P3) were prepared into scaffold-free cartilage-like cell-sheets and assessed macroscopically and by histology for safranin-O staining. (A) The center of sheets cultured in condition A, no fetal bovine serum (FBS), was recessed slightly but did not shrink. The surface of sheets cultured in condition B, includes FBS, was stable at 5 mm diameter and flat. (B) As assessed by histology, the margins of sheets in condition A had curled, while the surface of those in condition B were flat. The sheet cultured in condition A stained more intensely for safranin-O than those cultured in condition B. Bar: 500 μm.
Animal Experiments
Macroscopic Observations
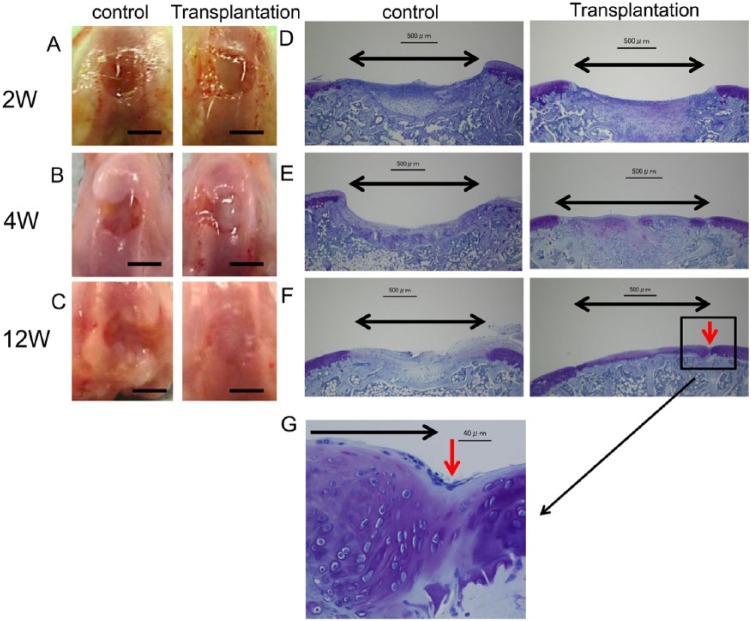
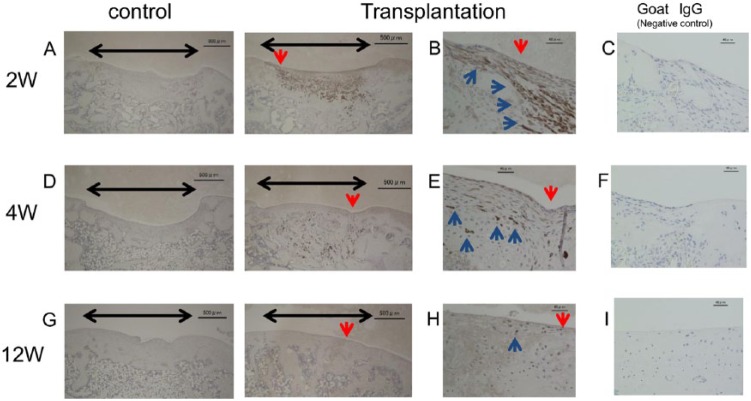
In the sheet-transplanted groups, at 2 weeks after transplant of the cartilage-like cell-sheet, the surfaces of the defects were covered with a smooth, white tissue layer that resembled articular cartilage, though the margins of the defects were recognizable ( Fig. 5A ). At 4 weeks, the margins of the defects were unclear, and by 12 weeks, the repair tissue looked similar to the surrounding articular cartilage ( Fig. 5B and C ).
Figure 5.
Macroscopic and histological observation of the animals transplanted with cartilage-like cell-sheets. (A) At 2 weeks after transplantation, the surfaces of the defects were covered with a smooth, white, tissue layer. Control group, the defect was left empty. (B) At 4 weeks, the margins of the defects were unclear compared with control. (C) At 12 weeks, the repair tissue appeared similar to the surrounding articular cartilage. Control group, the defect was still left empty. Bar: 2 mm. Toluidine blue histology of the transplanted animals. Black arrow: cartilage defect. Red arrow: boundary between the transplant section and the normal cartilage. (D) At 2 weeks, the defects transplanted with cell-sheets were covered with a matrix that stained faintly with the metachromatic stain. Bar: 500 μm. (E) At 4 weeks, the defects were filled with stained tissue compared with control. Bar: 500 μm. (F) At 12 weeks, the defects transplanted with cell-sheets were filled with repair tissue compared with control. That repair tissue resembled hyaline cartilage. The repair tissue appeared continuous with the surrounding native cartilage tissue. Panel (G) is an enlarged view of the section in (F) identified by the red arrow. Bar: 40 μm.
In the control groups, at 2 weeks the defects were covered with red tissue and the margins were discernible ( Fig. 5A ). At 4 weeks, the defects were covered with white tissue and the defect edges were still distinguishable ( Fig. 5B ). At 12 weeks, the defects showed irregular tissue and a depression at the defect site was evident ( Fig. 5C ).
Histological Observation by Toluidine Blue Staining
In the sheet-transplanted groups, at 2 weeks after the operation the defects transplanted with cell-sheets were covered with tissue that showed faint metachromatic staining ( Fig. 5D ). At 4 weeks, more metachromatic-stained tissue was found in the defect, and that tissue was thicker than the surrounding host cartilage ( Fig. 5E ). At 12 weeks, the defects transplanted with cell-sheets were filled with intensely stained metachromatic matrix, suggesting the active production of GAG, and the reparative tissue resembled hyaline cartilage. In addition, the subchondral bone was completely remodeled by new bone ( Fig. 5F ). At higher magnification, the cells appeared similar to differentiated chondrocytes, and continuity with the surrounding host cartilage was observed ( Fig. 5G ).
In the control groups, at 2 weeks the defects were not completely filled with repair tissue, and the deep part of the defect was filled with cancellous bone, while the surface of the defect was filled with fibrous tissue ( Fig. 5D ). At 4 weeks, the defects were still not yet completely filled. De novo bone was found in the proximal areas and fibrous tissue expanding to the distal edge was observed ( Fig. 5E ). At 12 weeks, the defects were filled with an irregular fibrous tissue without cartilage ( Fig. 5F ).
Histological Grading of the Repair Tissue
The Wakitani scores of the transplanted groups at 2, 4, and 12 weeks were significantly improved compared with those of the control groups (P < 0.05, n = 5, Wilcoxon signed-rank tests; Table 1 ).
Table 1.
Average Wakitani Scores at Each Week of the Experiment Scored by 3 Different Investigators.
| Group | Interval until Animals were Euthanized (Wks.) | No. of Specimens | Wakitani Score |
|||||
|---|---|---|---|---|---|---|---|---|
| Cell Morphology (Maximum 4) | Matrix-Staining (max 3) | Surface Regular (Maximum 3) | Thickness of Cartilage (Maximum 2) | Integration of Donor with Adjacent Host Cartilage (Maximum 2) | Total (Maximum 14) | |||
| Control | 2 | 5 | 3.7 | 2.6 | 2.5 | 1.7 | 1.1 | 11.7 |
| 4 | 5 | 3.5 | 2.7 | 2.3 | 1.5 | 0.9 | 10.9 | |
| 12 | 5 | 2.2 | 2.5 | 1.6 | 1.2 | 0.4 | 7.9 | |
| Transplantation | 2 | 5 | 3.1 | 2.2 | 1.4* | 1.5 | 0.9 | 9.2* |
| 4 | 5 | 2.6 | 2.2 | 1.2* | 1.0 | 0.4* | 7.4* | |
| 12 | 5 | 1.4 | 1.6* | 0.8 | 0.7 | 0.2* | 4.7* | |
P < 0.05 (n = 5, Wilcoxon signed-rank tests).
Immunohistochemistry
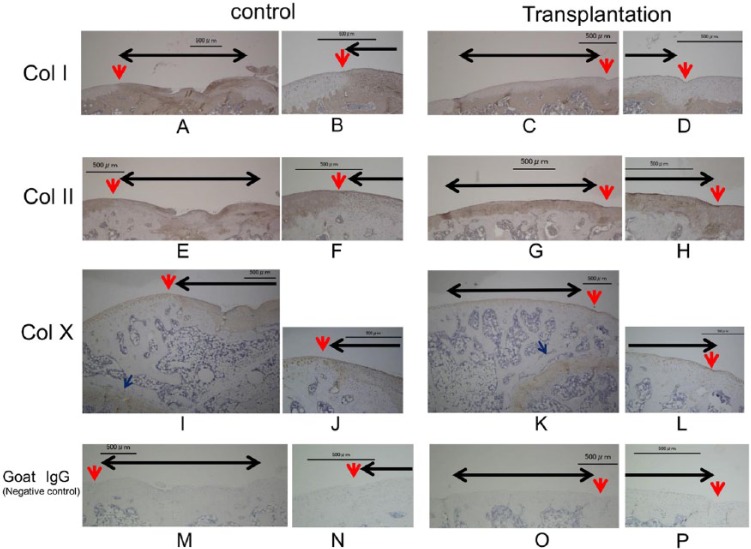
At 12 weeks after the surgery, the regenerated cartilage showed strong staining for ColII ( Fig. 6G and H ) and no staining for ColI, similar to the surrounding host cartilage ( Fig. 6C and D ). The surface of the regenerated cartilage-like repair tissue was slightly positive for ColX, but that intensity was weak compared with the growth plate and control defect, similar to the surrounding host cartilage ( Fig. 6I-L ).
Figure 6.
Immunostaining for collagen types I (A, B, C, D), II (E, F, G, H), X (I, J, K, L), and negative control goat IgG (M, N, O, P) at 12 weeks after transplantation. B, D, F, H, J, L, N, P: magnified views of the sections in A, C, E, G, I, K, M, and O labeled with red arrows. Black arrow: cartilage defect. Red arrow: boundary between the transplant section and the normal cartilage. Blue arrow: growth plate. In the transplanted defects, staining was positive for collagen type II, but not collagen type I. Type X staining was weak compared with the growth plate and control defect. Bar: 500 μm.
Immunostaining with the hVIM antibody showed a high level of human cell chimerism in the repair tissue at 2 weeks ( Fig. 7A and B ). At 4 weeks, this chimerism was decreased in the repair tissue, but increased in the subchondral bone ( Fig. 7D and E ). At 12 weeks, however, very low levels of human cell chimerism were found ( Fig. 7G and H ). No clear specific staining was found in the control sections incubated with normal goat IgG (negative control, Figs. 6M-P and 7C , F , and I )
Figure 7.
Immunostaining for human vimentin (hVIM). Black arrow: cartilage defect. Blue arrow: positively stained cells. (A) At 2 weeks after transplant of the cartilage-like cell-sheets, the hVIM antibody showed a high level of human cell chimerism. (D) At 4 weeks, the amount of chimerism was decreased in the regenerated cartilage layer, but increased in the subchondral bone. (G) At 12 weeks, very low levels of chimerism were found. (B, E, H) Magnified views of the sections indicated by the red arrows in A, D, and G. (C, F, I) Goat IgG negative control sections. Bars: 500 μm (A, D, G); 40 μm (B, C, E, F, H, I).
Discussion
The positive effect of FGF-2 on enhancing proliferation and chondrogenic potential of MSCs is well extablished.18-21 Our results indicate that the addition of FGF-2 during the expansion of hBMSCs not only increased cell proliferation over a short period of time and enhanced their chondrogenic potential, but also it was possible to lower the concentration of FBS. This culture condition may be useful to quickly yield enough cells under the lower concentration of FBS to reliably create stable scaffold-free cartilage-like cell-sheets. But FGF-2 does not prevent the subsequent formation of hypertrophic cartilage.22,23 Recently Narcisi et al.24 reported that the signaling protein Wnt3A, in combination with FGF-2, supports the long-term expansion of hBMSCs. They found that endogenous Wnt signals are the main drivers of the hypertrophic maturation that follows chondrogenic differentiation. Further inhibition of Wnt signals during differentiation prevented calcification and maintained the cartilage properties following implantation in a mouse model.24 Including Wnt signaling modulation in our culture protocol may allow us to create better quality scaffold-free cartilage-like cell-sheets.
There have been conflicting reports on whether age causes changes in the differentiation capacity of MSCs.25 Zaim et al.26 found that the adipogenic, osteogenic, and neurogenic differentiation potentials of hBMSCs declined with age, but that the chondrogenic potential did not. In contrast, Kanawa et al.27 reported that the adipogenic and osteogenic differentiation capacities of human BMSCs were unaffected by age, but the chondrogenic capacity of BMSCs declined with age. We used 3 volunteers as sources of BMSCs in the present study with an average age of 26 years. While we found no effect of age, it will be necessary to consider age for future clinical use.
Many different scaffolds for implantation have been described, including those made from synthetic polymers such as poly-l-lactic acid, polylactic acid-co-polyglycolic acid, and poly-l-lactide-co-glycolide28-30 and from biological materials such as collagen, fibrin, hyaluronan, chitosan, and alginate.31-34 However, several issues remain concerning their long-term safety. Synthetic polymers may have problems with retention and degradation in situ,35 while biological materials carry the risk of infection and immunological reactions.36,37 Therefore, new approaches that avoid the use of such scaffolds have been developed, including the use of thermos-responsive polymeric surfaces,38 acellular sheet technology,39 and electrospun sheet technology.40 Unfortunately, these technologies are not easy to manipulate.41 Compared with these other sheet technologies, our scaffold-free cartilage-like cell-sheets are easy to manipulate, harvest, and transplant.
One common general complication of BMSCs-based strategies is that they produce fibrocartilaginous repair tissue and graft calcification through the endochondral route. Type X collagen (ColX) is a well-established marker of hypertrophic chondrocyte differentiation. The repair tissue generated after scaffold-free cartilage-like cell-sheets implantation was positive for colII and negative colI. The surface of the regenerated cartilage like repair tissue was lightly for ColX compared with the growth plate and control defect. These results suggest that our cell-sheets may be able to generate a matrix composition similar to that of articular cartilage.
We transplanted scaffold-free cartilage-like cell-sheets created using hBMSCs into osteochondral defects in immunodeficient rats. At 2 weeks after transplantation, immunostaining with hVIM shows that the cell sheets were still present in the defects. By 4 weeks after transplantation, human cell chimerism had spread to the deep portions of the defect, though very low levels of human cell chimerism were found at 12 weeks. We believe this result can be explained by the following hypothesis. Over time, most of the proximal chondrocytes and the cartilage tissue itself became hypertrophic, showing vascular infiltration. The repair cartilage, up to the junction between the subchondral bone and cartilage, was eventually replaced by host-derived vascular and bone-forming cells. The articular cartilage distal from the defect site is also affected by injurious signal communication through the synovial fluid because the rats were allowed to move freely after surgery.
As the cartilage repair process proceeds during cell therapy with BMSCs, the influence of TGF-β and bone morphogenetic protein (BMP) created by the host tissue on the transplanted BMSCs should be considered.10 The TGF-β superfamily is one of the most investigated and biologically active substances within the field of cartilage tissue engineering.42 For example, TGF-β1 stimulates the synthetic activity of chondrocytes and acts against the catabolic activity of inflammatory mediator interleukin 1 in vivo.43 In addition, treatment of MSCs with TGF-β1enhanced the synthesis of sulfated GAG and induced production of cartilaginous extracellular matrix (ECM).44 BMPs are homodimeric molecules that belong to the TGF-β superfamily. Treatment with BMP-2 increases cell proliferation and cartilaginous ECM production and upregulates collagen type II gene expression.45 Based on those findings, we believe that the creation of a defect in the cartilage creates an osseous receptacle that stores and releases host bone-derived bioactive factors, such as TGF-β and BMPs. These bioactive factors promote the differentiation of the cells in the scaffold-free cartilage-like cell-sheets into chondrocytes after transplantation into defects in the distal femoral condyle of the experimental rats. Furthermore, under physiological conditions, chondrocytes experience dynamic changes in the osmolarity of their microenvironment caused by loading induced consolidation and recovery of the cartilage ECM. The downstream effects of chondrocyte swelling are multifocal and include increases in intracellular Ca2+, cytoskeletal rearrangement,46 gene activation,47 and activation of a regulatory volume decrease.48 One recent study suggested that this mechano-osmotic transduction occurs via the ion channel transient receptor potential vanilloid channel 4 (TRVP4).49 TRPV4-mediated Ca2+ influx can also activate the expression of SOX9, and TRPV4 appears to play a critical role in the transduction of mechanical loading into an intracellular signal that regulates chondrocyte matrix production.50,51 The articular cartilage distal from the defect site is also affected by biomechanical stimuli because the rats were allowed to move freely after surgery.
There are several limitations to this study. First, 2-, 4-, and 12-week outcomes are fairly short for cartilage regeneration. Assessing regeneration at longer time points will be necessary. Second, no biomechanical testing was performed. Third, we did not compare the use of BMSCs with other cell sources. However, we successfully prepared scaffold-free cartilage-like cell-sheets using the methods of Takagi et al.16,17 with the addition of FGF-2 during the cell expansion. This method does not require harvesting of normal cartilage or synovium, and can be performed without securing of the periosteum or a collagen membrane over the cell-sheets.
Conclusions
The proliferative and chondrogenic differentiation capacity of hBMSCs were enhanced by FGF-2 treatment during expansion, even with a reduced serum concentration, suggesting that these conditions may be able to reliably provide stable scaffold-free cartilage-like cell-sheets. We successfully transplanted the scaffold-free cartilage-like cell-sheets made using hBMSCs into osteochondral defects in nude rats for up to 12 weeks. The results suggested that the cell sheets are useful for the treatment of cartilage defects. We speculate that this scaffold-free cartilage-like cell-sheet fabrication technique could be used for the treatment of cartilage defects in humans. Further studies are needed to confirm this new therapeutic approach for the treatment of cartilage defects in humans.
Footnotes
Acknowledgments and Funding: The authors thank Kanako Hata from the Department of Orthopedic Surgery, Osaka City University Graduate School of Medicine, Osaka, Japan, for providing technical assistance in the animal experiments and histology. No external funding was used for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from our institutional review board.
Informed Consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-84. [DOI] [PubMed] [Google Scholar]
- 2. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;1:65-72. [PubMed] [Google Scholar]
- 3. Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781-9. [DOI] [PubMed] [Google Scholar]
- 4. Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62:79-89. [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 6. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84:571-8. [DOI] [PubMed] [Google Scholar]
- 7. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30. [DOI] [PubMed] [Google Scholar]
- 8. Sohn DH, Lottman LM, Lum LY, Kim SG, Pedowitz RA, Coutts RD, et al. Effect of gravity on localization of chondrocytes implanted in cartilage defects. Clin Orthop Relat Res. 2002;394:254-62. [DOI] [PubMed] [Google Scholar]
- 9. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 10. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full thickness defect of articular cartilage. J Bone Surg. 1994;76:579-92. [DOI] [PubMed] [Google Scholar]
- 11. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199-206. [DOI] [PubMed] [Google Scholar]
- 12. Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146-50. [DOI] [PubMed] [Google Scholar]
- 13. Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intra-articular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14:1307-14. [DOI] [PubMed] [Google Scholar]
- 14. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275-81. [DOI] [PubMed] [Google Scholar]
- 15. Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato Y, Wakitani S, Takagi M. Xeno-free and shrinkage-free preparation of scaffold-free cartilage-like disc-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2013;116:734-9. [DOI] [PubMed] [Google Scholar]
- 17. Maeda S, Fujitomo T, Okabe T, Wakitani S, Takagi M. Shrinkage-free preparation of scaffold-free cartilage-like disk-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2011;111:489-92. [DOI] [PubMed] [Google Scholar]
- 18. Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001;9:S36-40. [DOI] [PubMed] [Google Scholar]
- 19. Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413-9. [DOI] [PubMed] [Google Scholar]
- 20. Solchaga LA, Penick K, Porter JD. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398-409. [DOI] [PubMed] [Google Scholar]
- 21. Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98-105. [DOI] [PubMed] [Google Scholar]
- 22. Farrell E, van der Jagt OP, Koevoet W, Kops N, van Manen CJ, Hellingman CA, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15(2):285-95. [DOI] [PubMed] [Google Scholar]
- 23. Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254-66. [DOI] [PubMed] [Google Scholar]
- 24. Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, et al. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Rep. 2015;4:459-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014;10:289-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175-86. [DOI] [PubMed] [Google Scholar]
- 27. Kanawa M, Igarashi A, Ronald VS, Higashi Y, Kurihara H, Sugiyama M, et al. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy. 2013;15:1062-72. [DOI] [PubMed] [Google Scholar]
- 28. Fujihara Y, Asawa Y, Takato T, Hoshi K. Tissue reactions to engineered cartilage based on poly-L-lactic acid scaffolds. Tissue Eng Part A. 2009;15:1565-77. [DOI] [PubMed] [Google Scholar]
- 29. Moran JM, Pazzano D, Bonassar LJ. Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tissue Eng. 2003;9:63-70. [DOI] [PubMed] [Google Scholar]
- 30. Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. Poly(lactide-co-glycolide) microspheres as a moldable scaffold for cartilage tissue engineering. Biomaterials. 2005;26:1945-52. [DOI] [PubMed] [Google Scholar]
- 31. Scotti C, Mangiavini L, Boschetti F, Vitari F, Domeneghini C, Fraschini G, et al. Effect of in vitro culture on a chondrocyte-fibrin glue hydrogel for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18:1400-6. [DOI] [PubMed] [Google Scholar]
- 32. Solchaga LA, Temenoff JS, Gao J, Mikos AG, Caplan AI, Goldberg VM. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthritis Cartilage. 2005;13:297-309. [DOI] [PubMed] [Google Scholar]
- 33. Hao T, Wen N, Cao JK, Wang HB, Lü SH, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage. 2010;18:257-65. [DOI] [PubMed] [Google Scholar]
- 34. Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922-30. [DOI] [PubMed] [Google Scholar]
- 35. Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26:309-24. [DOI] [PubMed] [Google Scholar]
- 36. Schakenraad JM, Dijkstra PJ. Biocompatibility of poly(dl-lactic acid/glycine) copolymers. Clin Mater. 1991;7:253-69. [DOI] [PubMed] [Google Scholar]
- 37. Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228-32. [DOI] [PubMed] [Google Scholar]
- 38. Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297-303. [DOI] [PubMed] [Google Scholar]
- 39. Xue JX, Gong YY, Zhou GD, Liu W, Cao Y, Zhang WJ. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33:5832-40. [DOI] [PubMed] [Google Scholar]
- 40. Man Z, Yin L, Shao Z, Zhang X, Hu X, Zhu J, et al. The effects of co-delivery of BMSC-affinity peptide and rhTGF-β1 from coaxial electrospun scaffolds on chondrogenic differentiation. Biomaterials. 2014;35:5250-60. [DOI] [PubMed] [Google Scholar]
- 41. Ge Y, Gong YY, Xu Z, Lu Y, Fu W. The application of sheet technology in cartilage tissue engineering. Tissue Eng Part B Rev. Epub 2015. October 23. [DOI] [PubMed] [Google Scholar]
- 42. Danišovič L, Varga I, Polák S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell. 2012;44:69-73. [DOI] [PubMed] [Google Scholar]
- 43. Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-β and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597-604. [DOI] [PubMed] [Google Scholar]
- 44. Thorpe SD, Buckley CT, Vinardell T, O’Brien FJ, Campbell VA, Kelly DJ. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Ann Biomed Eng. 2010;38:2896-909. [DOI] [PubMed] [Google Scholar]
- 45. Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269-76. [DOI] [PubMed] [Google Scholar]
- 46. Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage. 2003;11:187-97. [DOI] [PubMed] [Google Scholar]
- 47. Hung CT, LeRoux MA, Palmer GD, Chao PH, Lo S, Valhmu WB. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology. 2003;40:61-72. [PubMed] [Google Scholar]
- 48. Bush PG, Hall AC. Regulatory volume decrease (RVD) by isolated and in situ bovine articular chondrocytes. J Cell Physiol. 2001;187:304-14. [DOI] [PubMed] [Google Scholar]
- 49. Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282:32158-67. [DOI] [PubMed] [Google Scholar]
- 51. O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316-21. [DOI] [PMC free article] [PubMed] [Google Scholar]