Abstract
Despite the fact that appropriate social behaviors are vital to thriving in one’s environment, little is understood of the molecular mechanisms controlling social behaviors or how social experience sculpts these signaling pathways. Here, we determine if Phosphodiesterase 11A (PDE11A), an enzyme that is restricted to the ventral hippocampal formation (VHIPP) and that breaks down cAMP and cGMP, regulates social behaviors. PDE11 wild-type (WT), heterozygous (HT), and knockout (KO) mice were tested in various social approach assays and gene expression differences were measured by RNA sequencing. The effect of social isolation on PDE11A4 compartmentalization and subsequent social interactions and social memory was also assessed. Deletion of PDE11A triggered age- and sex-dependent deficits in social approach in specific social contexts but not others. Mice appear to detect altered social behaviors of PDE11A KO mice, because C57BL/6J mice prefer to spend time with a sex-matched PDE11A WT vs. its KO littermate; whereas, a PDE11A KO prefers to spend time with a novel PDE11A KO vs. its WT littermate. Not only is PDE11A required for intact social interactions, we found that 1 month of social isolation vs. group housing decreased PDE11A4 protein expression specifically within the membrane fraction of VHIPP. This isolation-induced decrease in PDE11A4 expression appears functional because social isolation impairs subsequent social approach behavior and social memory in a PDE11A genotype-dependent manner. Pathway analyses following RNA sequencing suggests PDE11A is a key regulator of the oxytocin pathway and membrane signaling, consistent with its pivotal role in regulating social behavior.
Keywords: phosphodiesterase, hippocampus, social buffering, social approach, social odor recognition, social memory
Graphical abstract
INTRODUCTION
Several neuropsychiatric disorders are associated with deficits in social behaviors (c.f., (Young, 2008)) and no medicines remedy these symptoms. Without acceptable social behaviors, our ability to attract a mate, acquire resources from society, and establish a safe/secure environment is severely compromised (Ferguson et al., 2002). When an individual lacks proper social behaviors, one is often ostracized (Reidpath et al., 2005). Social isolation worsens mental and physical health and increases mortality, particularly among adolescents, the elderly and the mentally ill (Pompili et al., 2008; Bartels and Pratt, 2009; Dickens et al., 2011; Hawton et al., 2011; Kondo et al., 2013; Slavich and Cole, 2013; Holt-Lunstad et al., 2015; Yang et al., 2016). To make matters worse, social isolation further impairs subsequent social behaviors—thus, creating a vicious cycle (Kogan et al., 2000; Reidpath et al., 2005; Derntl et al., 2011; Shahar-Gold et al., 2013). Despite the fact that appropriate social behaviors are vital to thriving in one’s environment (Young, 2008), little is understood of the molecular mechanisms that control social behaviors or how social experiences modify these signaling pathways.
Cyclic nucleotide (cAMP/cGMP) signaling appears to be a molecular mechanism of social behavior that is conserved from amobas (Gregor et al., 2010) to mammals (Bickle, 2008). For example, oxytocin, a neuropeptide that regulates social behaviors (Lukas and Neumann, 2013; Stoesz et al., 2013; Feldman et al., 2015), couples to both the cAMP and cGMP cascades (Cheng et al., 2009; Viero et al., 2010). Further, cAMP-response element binding protein (CREB) and exchange protein activated by cAMP (Epac) are required for various types of social behaviors (Bickle, 2008; Srivastava et al., 2012; Yang et al., 2012). Phosphodiesterases (PDE), the only known enzymes to break down cAMP and cGMP (Francis et al., 2011), also modulate social behaviors (Boess et al., 2004; Grauer et al., 2009; Kelly et al., 2010; Hutson et al., 2011; Liebenberg et al., 2012). Cyclic nucleotide signaling specifically within the ventral hippocampal formation (VHIPP) may be particularly important because VHIPP lesions and knockdown of CREB in the rodent VHIPP impair social behaviors (Kogan et al., 2000; Brightwell et al., 2005; Tseng et al., 2009). This is consistent with the fact that both cyclic nucleotide signaling deficits (Ebstein et al., 1976; Belmaker et al., 1978; Bowers and Study, 1979; Kafka et al., 1979, 1986; Hoshino et al., 1980; Garver et al., 1982; Gattaz et al., 1983; Kafka and van Kammen, 1983; Memo et al., 1983; Goldberg et al., 1984; Kanof et al., 1986, 1987, 1989; Lerer et al., 1987; Ofuji et al., 1989; Kaiya et al., 1990; Kang, 1990; Young et al., 1991, 1993, 1994; Kaiya, 1992; Cowburn et al., 1994; Avissar et al., 1997; Gurguis et al., 1997, 1999a,b; Rahman et al., 1997; Avissar et al., 2001a,b; Bacchelli et al., 2003; Edmunds et al., 2008; Kelley et al., 2008; Turetsky and Moberg, 2009; Woolfrey et al., 2009; Ji et al., 2011) and abnormalities in the VHIPP (Suddath et al., 1990; Shenton et al., 1992; Rajarethinam et al., 2001; Jessen et al., 2003; Pegues et al., 2003; Lee et al., 2004; Rametti et al., 2007; Rusch et al., 2008; Zhou et al., 2008; Ghose et al., 2009; Nesvaderani et al., 2009; Schobel et al., 2009a,b; Hall et al., 2010; Goldman et al., 2011; Zhang et al., 2011) (referred to as the anterior hippocampus in primates) are observed in patients with neuropsychiatric disorders in which social deficits are known to occur.
Phosphodiesterase 11A (PDE11A, specifically isoform PDE11A4) is the only PDE with expression in brain restricted to the hippocampal formation, with a 3–10-fold enrichment in the VHIPP versus dorsal HIPP (Fig. 1A) (Kelly et al., 2010, 2014; Kelly, 2014, 2015; Hegde et al., 2016; Pathak et al., in press) and little to no expression in peripheral organs (c.f., (Kelly, 2015)). PDE11A has been genetically and/or functionally associated with clinical phenotypes related to changes in social function, including major depressive disorder (Wong et al., 2006; Cabanero et al., 2009; Luo et al., 2009), suicide risk (an inactivating mutation, (Coon et al., 2013)), and lithium responsivity (Couzin, 2008; Kelsoe, 2010; Mertens et al., 2015; Pathak et al., in press). Previously, we showed that reducing PDE11A expression was sufficient to reduce social approach behavior towards a novel mouse in adult male mice, but not female mice (Kelly et al., 2010). Here, we determine if social context and age differentially affect the manifestation of social deficits in PDE11A mutant mice and if social experience feeds back to shape the brain and subsequent behavior via PDE11A4. We show that PDE11A4 is a key regulator of social behaviors and the oxytocin signaling pathway, and that social experience shapes the brain, at least in part, by altering PDE11A4 compartmentalization.
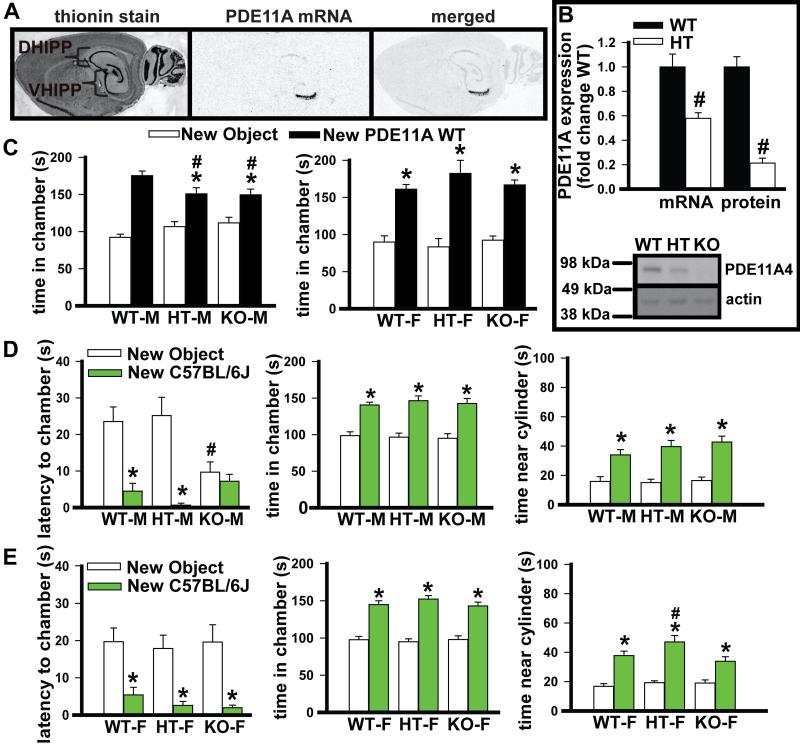
Fig. 1.
PDE11A mRNA expression is restricted to the hippocampal formation and deletion alters social approach in a manner that depends on the strain of the stimulus mouse. (A) As previously reported (Kelly et al., 2010, 2014; Kelly, 2014, 2015), PDE11A mRNA is selectively expressed in CA1 (and possibly ventral CA2), subiculum, and the adjacent amygdalohippocampal area (not shown at this level), with a 3- to 10-fold enrichment in ventral (VHIPP) versus dorsal hippocampus (DHIPP). (B) As expected, PDE11A heterozygous (HT; n = 6 (3 females)) mice show a 50% reduction in ventral hippocampal (VHIPP) PDE11A mRNA expression relative to wild-type (WT) littermates (n = 7 (4 females)), as measured by Q-PCR (t(11) = 3.47, P = 0.005). Note, no signal was detected in samples from PDE11A knockout (KO) mice, proving specificity of the primer/probe sets (data not shown). Surprisingly, however, PDE11A HT mice (n = 9 (5 females)) show an 80% reduction in VHIPP PDE11A4 protein expression relative to WT littermates (n = 10 (5 females)), as measured by Western blot (t(17) = 8.20, P < 0.001; shown in lower panel). Identical results were obtained in dorsal hippocampal samples from these mice as well as whole hippocampus samples from a second cohort of mice (data not shown). The mechanism underlying this disconnect between mRNA and protein remains to be determined, but alterations in social behavior driven by the HT deletion may further drive down PDE11A4 protein expression in the PDE11A HT mice (e.g., see Fig. 5A). (C) (Left) When given a chance to explore a novel object vs. a novel PDE11A WT mouse, adult male PDE11A mutant mice (i.e., HT, n = 8, and KO mice, n = 10) exhibited reduced social approach relative to that of male WT littermates (n = 14; F(2,26) = 4.50, P = 0.021). (Right) in contrast, female PDE11A WT (n = 7), HT (n = 3), and KO mice (n = 9) spent equivalent amounts of time with a novel mouse from the PDE11A colony (F(1,16) = 68.77, P < 0.001). This sex-specific phenotype in group-housed mice bred onsite is consistent with our previous report in single-housed mice bred offsite (Kelly et al., 2010). Note: latency to chamber and time near the cylinder were not available for this experiment. (D) Changing the stimulus mouse to a C57BL/6J altered the pattern of behavior seen in PDE11A mutant mice. (Left) male PDE11A KO mice (n = 18) failed to show a significant social preference in terms of their latency to approach the new C57BL/6J mouse vs. the new object, having approached the novel object much more quickly than their male HT (n = 14) and WT littermates (n = 20; effect replicated in 2 cohorts of mice, combined analysis: F(2,49) = 4.82, P = 0.012). (Middle) despite this difference in latency to approach, PDE11A KO males exhibited significant social preference in terms of their time in each chamber (F(1,49) = 55.22, P < 0.001) and (right) time near each cylinder (F(1,49) = 54.60, P < 0.001). (E) Unlike PDE11A KO males, (left) PDE11A KO females (n = 17) showed a significance preference for the new C57BL/6J mouse vs. the new object, as did PDE11A WT and HT females (WT, n = 27; HT, n = 22), in terms of their (left) latency to approach (F(1,63) = 31.78, P < 0.001) as well as their (middle) time in chamber (F(1,63) = 87.15, P < 0.001), and (right) time near the cylinder (F(2,57) = 4.57, P = 0.014), with PDE11A HT females showing significantly greater social approach on the last measure. Post hoc, *vs. object, P < 0.001; #vs. WT, P < 0.05–0.001.
EXPERIMENTAL PROCEDURES
Subjects
PDE11A knockout (KO) mice were originally developed by Deltagen (San Mateo, CA, USA), and are maintained on a mixed C57BL/6J-C57BL/6N-129S6 background, as previously described (Kelly et al., 2010; Hegde et al., 2016). PDE11A mice were bred onsite in heterozygous (HT) × HT matings, with same-sex wild-type (WT), HT, and knockout(KO) littermates weaned together into the same cage at P28 (3–5/cage; but see “mouse psychiatrist” assay below). C57BL/6J mice were either obtained from Jackson Laboratories or bred onsite and were also group housed (3/cage). For social isolation studies, C57BL/6J mice were single-housed for 1 month and mice from the PDE11A colony were single-housed for 1–2 months. Approximately equal numbers of male and female off-spring were tested between P28 - P42 for adolescent studies and 2–9 months of age for adult studies. In all experiments, PDE11A KO mice were compared to sex-matched littermates. Animals are housed on a 12:12-h light:dark cycle with unlimited access to food and water. See figure legends for experimental n’s. Experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Pub 85-23, revised 1996) and were fully approved by the Institutional Animal Care and Use Committee of the University of South Carolina.
In situ hybridization, quantitative PCR (Q-PCR) and Western blotting
In situ hybridization, quantitative PCR (Q-PCR) and Western blotting were performed as previously described (Kelly et al., 2010, 2014). In situ hybridization for PDE11A was performed using an S35-labelled antisense probe (5′gccacctgtctggagatctcccacggtttggtcacggc-3′). Q-PCR was performed using pre-designed gene-specific oligonucleotide primer-probe sets (Table 1; Integrated DNA Technologies (IDT), Coralville, IA, USA). For Western blotting, antibodies were used to detect PDE11A (Fabgennix PD11-112 Lot 173, 1:500) and actin as a loading control (Sigma #A2066; 1:10,000).
Table 1.
Primers used to assess PDE11A mRNA expression
| Gene | Set IDT Cat# | Sequence Name | Sequence |
|---|---|---|---|
| PDE11A exon 12–13 |
Mm.PT.58.42976146 | PrimeTime Probe | 5′-/6-FAM/CAG CTC TAC /ZEN/GGG ACC TCG GC/IBFQ/-3′ |
| PrimeTime Primer 2 | GAA CCA ACA ATG CCT TCC AAG | ||
| PrimeTime Primer 1 | CCT CAC TCT GAA GGA TCA TCA C | ||
| TBP exon 3–4 | Mm.PT.58.10867035 | PrimeTime Probe | 5′-/6-FAM/CA CTC CTG C/Zen/C ACA CCA GCT TCT /IBFQ/-3′ |
| PrimeTime Primer 2 | TTC ACC AAT GAC TCC TAT GAC C | ||
| PrimeTime Primer 1 | CAA GTT TAC AGC CAA GAT TCA CG | ||
| ACTB exon 4–5 | Mm.PT.58.33257376. gs |
PrimeTime Probe | 5′-/6-FAM/AT TCC ATA C/Zen/C CAA GAA GGA AGG CTG G/IBFQ/-3′ |
| PrimeTime Primer 2 | ATT GGC AAC GAG CGG TT | ||
| PrimeTime Primer 1 | AGG TCT TTA CGG ATG TCA ACG | ||
| POLR2A exon 22–23 |
Mm.PT.58.13811327 | PrimeTime Probe | 5′-/6-FAM/AC CAC CTC T/Zen/T CCT CCT CTT GCA TCT /IBFQ/-3′ |
| PrimeTime Primer 2 | TCG AAT CCG CAT CAT GAA CAG | ||
| PrimeTime Primer 1 | GTC AGC ATG TTG GAC TCA ATG |
PDE11A was normalized to the average of values for the housekeeping genes TBP, ACTB, and POLR2A. Primer-probe sets were used with 5′ 6-FAM (6-Carboxyfluorescein) fluorophore, 3′ IBFQ (Iowa black FQ) and internal ZEN quenchers (IDT) using iTaq™ Universal Probes Supermix (Bio-Rad, Hercules, CA).
Social approach assay
As we previously described (Kelly et al., 2010), we measured social approach behavior using Brodkin’s version of the 3-compartment chamber in which a perforated plexiglass cylinder is placed in either end of the compartment (Sankoorikal et al., 2006). After subjects habituated to the apparatus and empty cylinders for 5 min, a novel object (50 mL conical tube) and either a novel PDE11A WT, a novel C57BL/6J, or a cagemate were placed in the cylinders (position counterbalanced across subjects). Littermates were tested against the same stimulus mice, with order of genotypes tested counterbalanced across littermate groups. In the “cagemate vs. novel mouse” version of the assay, the cagemate remained and the object was first replaced with a novel PDE11A WT and then a novel C57BL/6J. Each approach trial lasted 5 min. For the “new object vs. new PDE11A WT” assay, chamber time was hand scored. For the “new object vs. new C57BL/6J” assay, latency and chamber time were collected using AnyMaze to track center of body, and cylinder data was hand scored. For the cagemate assays, latency and chamber/cylinder time were collected by tracking head position using Ethovision 7.0, with the exception of the adolescent study that was hand scored. For all studies using AnyMaze or Ethovision, locomotor activity was recorded but no effects of genotype were noted (Table 2).
Table 2.
There was no significant difference in locomotor activity between genotypes as measured by total centimeters travelled in the social approach chamber
| Social approach test | Source | WT-M | HT-M | KO-M | WT-F | HT-F | KO-F |
|---|---|---|---|---|---|---|---|
| New object vs. new PDE11A WT |
Kelly et al. (2010), PNAS |
1862 (105) | 1970 (86) | 1753 (111) | 1890 (167) |
1542 (186) | 2250 (516) |
| New object vs. new C57BL/6J | Fig. 1D,E | 1484 (54) | 1576 (68) | 1517 (50) | 1629 (62) | 1645 (79) | 1786 (94) |
| New object vs. cagemate | Fig. 2A | 3078 (787) | 4299 (1325) |
2998 (953) | 2525 (658) |
3463 (969) | 1744 (169) |
| Cagemate vs. new PDE11A WT |
Fig. 2B | 3393 (896) | 6104 (2243) |
3406 (1236) |
3231 (826) |
7241 (4062) |
2297 (379) |
| Cagemate vs. new C57BL/6J | Fig. 2C | 3574 (1113) |
6133 (1352) |
2753 (757) | 2865 (786) |
2393 (570) | 2682 (600) |
Data expressed as mean (SEM).
“Mouse psychiatrist” assay
This assay is somewhat similar to the Preference for Typical Social Interaction Test (Shah et al., 2013) but with substantial differences in methodology/design. A C57BL/6J mouse, a “social” mouse strain (Brodkin et al., 2004; Fairless et al., 2012), was first habituated to the social approach apparatus for 5 min. Next, an age- and sex-matched PDE11A WT and either a HT or KO littermate were placed in the two cylinders (i.e., 1 mouse/cylinder). The position of genotypes in the cylinders was counterbalanced across trials. Each C57BL/6J was tested against a new pair of WT-HT/KO littermates. The C57BL/6J “mouse psychiatrist” was then allowed to assess the WT and HT/KO mice for 30 min. Data were binned in 5-min epochs. This assay was also run using PDE11A KO mice as the “mouse psychiatrist”. These PDE11A KO mice were raised in HT × HT matings but were weaned exclusively with other sex-matched KO mice. Chamber data were calculated using AnyMaze, and cylinder data were hand scored.
Biochemical fraction
Animals were sacrificed by cervical dislocation, with individual brain regions immediately dissected onto dry ice and stored at −80 °C. Each biological replicate contained tissue from 2 - 3 mice to provide sufficient starting material. Tissue samples were homogenized, biochemically fractionated, and processed by Western blotting as previously described (Kelly et al., 2010, 2014; Pathak et al., in press).
Social odor recognition
As previously described (Kelly et al., 2010; Hegde et al., 2016), mice were individually placed in a clean home cage and allowed to explore 3 wooden beads—2 from their home cage and 1 from the cage of a novel mouse strain (C57BL/6J, BALB/cJ, 129S6/SvEv). During a 2-min memory test 24 h after training, mice were again presented with 3 beads: home cage, bead from training, and a bead from another strain. Time investigating each bead was scored, and “memory” was operationally defined as spending significantly more time investigating the novel versus the familiar odor.
RNA sequencing
Animals were sacrificed by cervical dislocation, with individual brain regions immediately dissected onto dry ice and stored at −80 °C. RNA and library preparation, sequencing, and post-processing of the raw data were performed by the Epigenomics Core at Weill Cornell Medicine. Tissue stored at −80 °C was dry-fractured in a tissueTUBE device using a cryoPREP TM Impactor as per manufacturer recommendations (Covaris, Woburn, MA, USA). Each pulverized sample was resuspended in 350uls of Qiagen RNeasy Plus Mini RTL lysis buffer and RNA extracted as per manufacturer recommendations (Qiagen, Valencia, CA, USA). RNA integrity was assessed using the Lab Chip GX (Perkin Elmer, Waltham, MA, USA), and samples had a quality score > 8.0. TruSeq RNA libraries were prepared using established Illumina methods (Illumina, San Diego, CA, USA, Part #RS-122-2001). Each library was made with one of the TruSeq barcode index sequences and samples were sequenced across 3 lanes. The pools were clustered at 6.5 pM on a pair end read flow cell and sequenced for 100 cycles on an Illumina HiSeq 2500. Primary processing of sequencing images was done using Illumina’s Real Time Analysis software (RTA). CASAVA 1.8.2 software was used to perform image capture, base calling and demultiplexing. Sequences were aligned to the mouse mm10 genome (http://hgdownload.soe.ucsc.edu/goldenPath/mm10/bigZips/) using STAR v2.3.1 (Dobin et al., 2013). Reads were counted using the featureCounts function of the Subreads package (Liao et al., 2013) in R (https://www.R-project.org/) using Gencode M6 GTF (http://www.gencodegenes.org/mouse_stats/archive.html) and summarized at exon, transcript, or gene level. Only reads that were mapped uniquely to the genome were used. Mapping quality (MAPQ) minimum threshold was set at 10. Differential expression analysis was performed in R using the edgeR package (Robinson et al., 2010). The average read depth for the samples was 64 million reads and only genes with at least 1 count per million average depth were considered for differential expression analysis. Raw counts were normalized using the trimmed mean of m-values (TMM) method. Dispersion estimates were then calculated using the estimateGLMRobustDisp function (Zhou et al., 2014). The normalized read counts were then fitted to a generalized linear model using the function glmFit (McCarthy et al., 2012). Genewise tests for significant differential expression were performed using the function glmLRT. The P-value was then corrected for multiple testing using Benjamini-Hochburg’s FDR (Benjamini and Hochberg, 1995). Genes that differed in expression between PDE11A WT and KO mice (FDR P < 0.05; Table 3) were examined for enrichment in KEGG and GO pathway using STRING 10 (string-db.org) (Szklarczyk et al., 2015).
Table 3.
RNA sequencing reveals 169 genes that significantly differ in expression between PDE11A KO versus WT littermates (sorted by KO vs. WT FDR P-value)
| Gene ID | KO vs. WT |
HT vs. WT |
KO vs. HT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| logFC | P-Value | FDR P-value | logFC | P-Value | FDR P-value | logFC | P-value | FDR P-value | |
| Xist | 4.6819 | 2.351E–11 | 1.736E–07 | 0.8148 | 3.052E–01 | 9.998E–01 | 1.9335 | 4.048E–07 | 2.990E–03 |
| Mylk | −0.6646 | 3.077E–08 | 1.515E–04 | −0.2890 | 1.542E–02 | 9.443E–01 | −0.1878 | 1.368E–03 | 2.617E–01 |
| Samd4 | 0.6946 | 5.943E–08 | 2.194E–04 | 0.4917 | 1.025E–04 | 4.619E–01 | 0.1015 | 1.120E–01 | 8.715E–01 |
| Fgl2 | −1.9915 | 2.400E–07 | 7.088E–04 | −0.7670 | 3.958E–02 | 9.998E–01 | −0.6122 | 1.233E–03 | 2.617E–01 |
| Hivep1 | 1.1266 | 3.264E–07 | 8.036E–04 | 0.8016 | 2.502E–04 | 4.619E–01 | 0.1625 | 1.324E–01 | 8.955E–01 |
| Syce2 | −1.3233 | 5.799E–07 | 1.224E–03 | −0.5006 | 4.899E–02 | 9.998E–01 | −0.4113 | 1.245E–03 | 2.617E–01 |
| Pitpnm3 | 0.8443 | 8.292E–07 | 1.531E–03 | 0.4902 | 3.295E–03 | 7.487E–01 | 0.1771 | 3.853E–02 | 7.123E–01 |
| Hlf | 0.7903 | 1.064E–06 | 1.746E–03 | 0.2144 | 1.765E–01 | 9.998E–01 | 0.2879 | 2.640E–04 | 1.950E–01 |
| Satb1 | 0.8303 | 1.264E–06 | 1.867E–03 | 0.5077 | 2.779E–03 | 7.309E–01 | 0.1613 | 6.132E–02 | 7.703E–01 |
| Asap1 | 0.8505 | 1.638E–06 | 2.199E–03 | 0.3569 | 3.840E–02 | 9.998E–01 | 0.2468 | 5.392E–03 | 4.525E–01 |
| Unc13c | 1.9464 | 2.114E–06 | 2.602E–03 | 1.2594 | 1.634E–03 | 7.120E–01 | 0.3435 | 8.163E–02 | 8.359E–01 |
| Cacnb4 | 0.6627 | 2.398E–06 | 2.724E–03 | 0.3364 | 1.525E–02 | 9.443E–01 | 0.1631 | 1.999E–02 | 6.357E–01 |
| Mtcl1 | 0.8188 | 2.949E–06 | 3.046E–03 | 0.2353 | 1.849E–01 | 9.998E–01 | 0.2917 | 9.939E–04 | 2.617E–01 |
| Fam26e | −0.6501 | 3.096E–06 | 3.046E–03 | −0.2762 | 4.846E–02 | 9.998E–01 | −0.1869 | 8.562E–03 | 5.038E–01 |
| Hecw1 | 0.7093 | 3.300E–06 | 3.046E–03 | 0.3463 | 2.109E–02 | 9.852E–01 | 0.1815 | 1.467E–02 | 5.640E–01 |
| St3gal1 | 1.0880 | 3.808E–06 | 3.309E–03 | 0.6929 | 2.668E–03 | 7.297E–01 | 0.1976 | 8.500E–02 | 8.392E–01 |
| Cobl | 1.3253 | 5.535E–06 | 4.542E–03 | 1.0592 | 2.278E–04 | 4.619E–01 | 0.1331 | 3.443E–01 | 9.457E–01 |
| Nbl1 | −0.7475 | 6.227E–06 | 4.812E–03 | −0.4266 | 7.894E–03 | 8.388E–01 | −0.1604 | 5.065E–02 | 7.449E–01 |
| Grm8 | 1.2196 | 6.516E–06 | 4.812E–03 | 0.8843 | 9.596E–04 | 6.450E–01 | 0.1677 | 2.027E–01 | 9.260E–01 |
| Etl4 | 0.7987 | 7.032E–06 | 4.946E–03 | 0.5497 | 1.983E–03 | 7.120E–01 | 0.1245 | 1.608E–01 | 9.132E–01 |
| Rab26 | −0.6809 | 7.532E–06 | 5.057E–03 | −0.2208 | 1.347E–01 | 9.998E–01 | −0.2301 | 2.150E–03 | 3.125E–01 |
| Rora | 0.7188 | 7.926E–06 | 5.090E–03 | 0.4800 | 2.485E–03 | 7.120E–01 | 0.1194 | 1.270E–01 | 8.897E–01 |
| Mlip | 0.6955 | 1.174E–05 | 7.223E–03 | 0.5119 | 7.089E–04 | 5.847E–01 | 0.0918 | 2.473E–01 | 9.415E–01 |
| Il1rapl2 | 1.3039 | 1.480E–05 | 8.743E–03 | 0.6506 | 3.221E–02 | 9.998E–01 | 0.3267 | 2.532E–02 | 6.812E–01 |
| Plcb4 | 0.8478 | 1.581E–05 | 8.979E–03 | 0.5576 | 3.578E–03 | 7.738E–01 | 0.1451 | 1.348E–01 | 8.971E–01 |
| RP24–490B17.1 | 2.0479 | 1.765E–05 | 9.435E–03 | 0.8881 | 6.392E–02 | 9.998E–01 | 0.5799 | 1.327E–02 | 5.401E–01 |
| Cntn4 | 0.8808 | 1.840E–05 | 9.435E–03 | 0.5997 | 3.201E–03 | 7.471E–01 | 0.1406 | 1.486E–01 | 9.053E–01 |
| Vwc2l | 1.8754 | 1.853E–05 | 9.435E–03 | 1.3651 | 1.367E–03 | 7.120E–01 | 0.2551 | 2.188E–01 | 9.322E–01 |
| Zdhhc22 | 1.3832 | 2.075E–05 | 1.021E–02 | 0.6864 | 3.144E–02 | 9.998E–01 | 0.3484 | 2.750E–02 | 6.839E–01 |
| Frmd7 | −1.1582 | 2.399E–05 | 1.074E–02 | −0.5211 | 4.737E–02 | 9.998E–01 | −0.3186 | 2.140E–02 | 6.517E–01 |
| Pamr1 | 0.9322 | 2.317E–05 | 1.074E–02 | 0.5266 | 1.588E–02 | 9.443E–01 | 0.2028 | 6.407E–02 | 7.763E–01 |
| Dlg1 | 0.4058 | 2.361E–05 | 1.074E–02 | 0.2297 | 1.534E–02 | 9.443E–01 | 0.0881 | 6.472E–02 | 7.763E–01 |
| Cpne7 | −0.9417 | 2.905E–05 | 1.226E–02 | −0.2759 | 1.905E–01 | 9.998E–01 | −0.3329 | 2.800E–03 | 3.504E–01 |
| Nr4a2 | 0.9161 | 2.828E–05 | 1.226E–02 | 0.7585 | 6.396E–04 | 5.847E–01 | 0.0788 | 4.777E–01 | 9.518E–01 |
| Fut9 | 0.6358 | 3.079E–05 | 1.263E–02 | 0.3092 | 4.054E–02 | 9.998E–01 | 0.1633 | 2.764E–02 | 6.839E–01 |
| Pknox2 | 0.5421 | 3.193E–05 | 1.275E–02 | 0.1613 | 1.994E–01 | 9.998E–01 | 0.1904 | 3.439E–03 | 3.681E–01 |
| Igfbp3 | −0.8821 | 3.446E–05 | 1.339E–02 | 0.0503 | 7.989E–01 | 9.998E–01 | −0.4662 | 9.618E–06 | 4.735E–02 |
| Deptor | 1.1437 | 3.608E–05 | 1.366E–02 | 0.7056 | 1.015E–02 | 8.766E–01 | 0.2190 | 1.002E–01 | 8.567E–01 |
| Camk2g | 0.4572 | 3.942E–05 | 1.399E–02 | 0.0855 | 4.258E–01 | 9.998E–01 | 0.1858 | 8.338E–04 | 2.512E–01 |
| Astn2 | 0.5271 | 3.796E–05 | 1.399E–02 | 0.1916 | 1.256E–01 | 9.998E–01 | 0.1677 | 8.933E–03 | 5.095E–01 |
| Lnp | 0.4311 | 3.977E–05 | 1.399E–02 | 0.3909 | 2.466E–04 | 4.619E–01 | 0.0201 | 7.065E–01 | 9.797E–01 |
| Adra1d | −0.8237 | 4.383E–05 | 1.407E–02 | −0.3748 | 5.364E–02 | 9.998E–01 | −0.2245 | 2.522E–02 | 6.812E–01 |
| Smad6 | −1.6239 | 4.283E–05 | 1.407E–02 | −0.9034 | 2.135E–02 | 9.852E–01 | −0.3603 | 6.840E–02 | 7.885E–01 |
| Chrna4 | 0.8001 | 4.221E–05 | 1.407E–02 | 0.4968 | 1.161E–02 | 9.421E–01 | 0.1516 | 1.176E–01 | 8.771E–01 |
| Sowahb | 1.4828 | 4.328E–05 | 1.407E–02 | 1.0883 | 2.371E–03 | 7.120E–01 | 0.1973 | 2.776E–01 | 9.441E–01 |
| Tshz3 | 1.3431 | 4.644E–05 | 1.459E–02 | 0.6382 | 4.970E–02 | 9.998E–01 | 0.3525 | 2.655E–02 | 6.812E–01 |
| Rnf128 | −0.5481 | 5.331E–05 | 1.637E–02 | −0.1518 | 2.552E–01 | 9.998E–01 | −0.1981 | 2.978E–03 | 3.605E–01 |
| Gm23935 | −1.2578 | 5.431E–05 | 1.637E–02 | −0.4726 | 1.597E–01 | 9.998E–01 | −0.3926 | 1.825E–02 | 6.130E–01 |
| Slc17a6 | 1.4644 | 5.748E–05 | 1.687E–v02 | 0.8239 | 1.942E–02 | 9.723E–01 | 0.3202 | 7.196E–02 | 8.035E–01 |
| Pth1r | 1.0572 | 5.825E–05 | 1.687E–02 | 0.8738 | 6.847E–04 | 5.847E–01 | 0.0917 | 4.763E–01 | 9.518E–01 |
| Epha10 | 0.5337 | 5.999E–05 | 1.698E–02 | 0.2302 | 8.915E–02 | 9.998E–01 | 0.1518 | 2.389E–02 | 6.784E–01 |
| Hspa1b | −0.9499 | 6.174E–05 | 1.698E–02 | −0.4208 | 7.725E–02 | 9.998E–01 | −0.2646 | 2.445E–02 | 6.804E–01 |
| Osbpl3 | 0.6298 | 6.207E–05 | 1.698E–02 | 0.3116 | 4.510E–02 | 9.998E–01 | 0.1591 | 4.309E–02 | 7.265E–01 |
| Tusc1 | −0.8527 | 6.390E–05 | 1.716E–02 | −0.1130 | 5.845E–01 | 9.998E–01 | −0.3699 | 5.334E–04 | 2.462E–01 |
| Ubac2 | −0.3748 | 6.566E–05 | 1.732E–02 | −0.2280 | 1.552E–02 | 9.443E–01 | −0.0734 | 1.204E–01 | 8.771E–01 |
| Nova1 | 0.6060 | 6.856E–05 | 1.777E–02 | 0.2648 | 7.041E–02 | 9.998E–01 | 0.1706 | 2.498E–02 | 6.812E–01 |
| Ptgds | −1.6596 | 7.009E–05 | 1.785E–02 | −0.4602 | 2.558E–01 | 9.998E–01 | −0.5997 | 2.959E–03 | 3.605E–01 |
| Cntnap5a | 0.7787 | 7.409E–05 | 1.797E–02 | 0.3909 | 4.037E–02 | 9.998E–01 | 0.1939 | 4.467E–02 | 7.329E–01 |
| Cit | 0.9118 | 7.371E–05 | 1.797E–02 | 0.6235 | 6.443E–03 | 8.209E–01 | 0.1441 | 2.035E–01 | 9.263E–01 |
| Slc20a1 | 0.3071 | 7.478E–05 | 1.797E–02 | 0.2245 | 4.194E–03 | 7.738E–01 | 0.0413 | 2.905E–01 | 9.443E–01 |
| Lrrtm3 | 0.8080 | 7.542E–05 | 1.797E–02 | 0.8092 | 6.359E–05 | 4.619E–01 | −0.0006 | 9.949E–01 | 9.994E–01 |
| Fam53b | 0.5043 | 7.863E–05 | 1.834E–02 | 0.0684 | 5.987E–01 | 9.998E–01 | 0.2180 | 7.599E–04 | 2.512E–01 |
| Cdon | 0.7665 | 7.947E–05 | 1.834Ex–02 | 0.3634 | 6.201E–02 | 9.998E–01 | 0.2015 | 3.872E–02 | 7.130E–01 |
| R3hdm2 | 0.3424 | 8.228E–05 | 1.870E–02 | 0.1582 | 6.914E–02 | 9.998E–01 | 0.0921 | 3.418E–02 | 7.000E–01 |
| Rprml | −0.8683 | 8.893E–05 | 1.976E–02 | −0.2619 | 2.362E–01 | 9.998E–01 | −0.3032 | 6.772E–03 | 4.713E–01 |
| Gm22405 | −2.2935 | 9.097E–05 | 1.976E–02 | −1.4109 | 1.486E–02 | 9.443E–01 | −0.4413 | 1.328E–01 | 8.955E–01 |
| Fgd2 | 1.2255 | 9.010E–05 | 1.976E–02 | 0.8777 | 4.495E–03 | 7.738E–01 | 0.1739 | 2.363E–01 | 9.398E–01 |
| Zfp365 | 0.3791 | 9.500E–05 | 1.976E–02 | 0.0822 | 3.955E–01 | 9.998E–01 | 0.1485 | 1.970E–03 | 2.984E–01 |
| Obscn | 0.9565 | 9.380E–05 | 1.976E–02 | 0.5057 | 3.871E–02 | 9.998E–01 | 0.2254 | 6.629E–02 | 7.829E–01 |
| Gm28370 | 0.6501 | 9.390E–05 | 1.976E–02 | 0.4202 | 1.352E–02 | 9.443E–01 | 0.1150 | 1.574E–01 | 9.128E–01 |
| Agps | 0.3853 | 1.036E–04 | 2.125E–02 | 0.1543 | 1.258E–01 | 9.998E–01 | 0.1155 | 2.106E–02 | 6.480E–01 |
| Snca | −0.4367 | 1.050E–04 | 2.125E–02 | −0.2042 | 6.800E–02 | 9.998E–01 | −0.1162 | 3.756E–02 | 7.077E–01 |
| Rcan2 | 0.4698 | 1.078E–04 | 2.151E–02 | 0.3143 | 9.596E–03 | 8.688E–01 | 0.0777 | 1.996E–01 | 9.255E–01 |
| Galnt13v | 0.6550 | 1.108E–04 | 2.183E–02 | 0.2291 | 1.673E–01 | 9.998E–01 | 0.2130 | 1.025E–02 | 5.271E–01 |
| Kdm5d | −4.6329 | 1.151E–04 | 2.208E–02 | −1.3754 | 6.847E–02 | 9.998E–01 | −1.6287 | 1.937E–02 | 6.282E–01 |
| B930095G15Rik | 0.6217 | 1.147E–04 | 2.208E–02 | 0.4885 | 3.026E–03 | 7.406E–01 | 0.0666 | 4.017E–01 | 9.478E–01 |
| Stard5 | −0.5076 | 1.270E–04 | 2.406E–02 | −0.3388 | 1.051E–02 | 8.974E–01 | −0.0844 | 2.051E–01 | 9.272E–01 |
| Olfm3 | 0.6911 | 1.309E–04 | 2.448E–02 | 0.6636 | 2.247E–04 | 4.619E–01 | 0.0138 | 8.778E–01 | 9.924E–01 |
| Tshz2 | 1.8146 | 1.336E–04 | 2.466E–02 | 0.8524 | 6.725E–02 | 9.998E–01 | 0.4811 | 3.457E–02 | 7.013E–01 |
| Rxfp1 | 1.7468 | 1.420E–04 | 2.527E–02 | 0.6832 | 1.310E–01 | 9.998E–01 | 0.5318 | 1.641E–02 | 5.801E–01 |
| Satb2 | 1.2285 | 1.390E–04 | 2.527E–02 | 0.6804 | 3.054E–02 | 9.998E–01 | 0.2740 | 8.687E–02 | 8.402E–01 |
| Yam1 | −1.8381 | 1.415E–04 | 2.527E–02 | −1.8508 | 1.697E–04 | 4.619E–01 | 0.0064 | 9.785E–01 | 9.989E–01 |
| Dcaf12l2 | −0.9540 | 1.438E–04 | 2.529E–02 | −0.2825 | 2.562E–01 | 9.998E–01 | −0.3358 | 7.761E–03 | 4.838E–01 |
| Kcnab3 | 0.7636 | 1.541E–04 | 2.677E–02 | 0.4629 | 2.285E–02 | 9.959E–01 | 0.1503 | 1.469E–01 | 9.053E–01 |
| Gm11914 | −0.7652 | 1.648E–04 | 2.750E–02 | −0.0130 | 9.484E–01 | 9.998E–01 | −0.3761 | 2.296E–04 | 1.785E–01 |
| Tesc | −0.6218 | 1.652E–04 | 2.750E–02 | −0.2546 | 1.118E–01 | 9.998E–01 | −0.1836 | 2.571E–02 | 6.812E–01 |
| Slc35e2 | 0.4057 | 1.625E–04 | 2.750E–02 | 0.2064 | 5.572E–02 | 9.998E–01 | 0.0996 | 6.372E–02 | 7.763E–01 |
| Hkdc1 | 0.8737 | 1.657E–04 | 2.750E–02 | 0.5539 | 1.801E–02 | 9.498E–01 | 0.1599 | 1.720E–01 | 9.210E–01 |
| Rnf217 | 0.5521 | 1.696E–04 | 2.784E–02 | 0.5010 | 5.547E–04 | 5.847E–01 | 0.0255 | 7.228E–01 | 9.797E–01 |
| Dcdc2a | −0.6627 | 1.716E–04 | 2.785E–02 | −0.2704 | 1.225E–01 | 9.998E–01 | −0.1962 | 2.578E–02 | 6.812E–01 |
| Calcrl | −0.5534 | 1.910E–04 | 2.794E–02 | −0.0688 | 6.409E–01 | 9.998E–01 | −0.2423 | 6.780E–04 | 2.512E–01 |
| Rph3a | 0.5848 | 1.841E–04 | 2.794E–02 | 0.0880 | 5.685E–01 | 9.998E–01 | 0.2484 | 1.260E–03 | 2.617E–01 |
| Tacc1 | 0.4630 | 1.885E–04 | 2.794E–02 | 0.1262 | 3.085E–01 | 9.998E–01 | 0.1684 | 6.032E–03 | 4.713E–01 |
| Fcgbp | −1.2194 | 1.743E–04 | 2.794E–02 | −0.3520 | 2.712E–01 | 9.998E–01 | −0.4337 | 7.536E–03 | 4.829E–01 |
| Grm2 | 0.9173 | 1.909E–04 | 2.794E–02 | 0.3328 | 1.632E–01 | 9.998E–01 | 0.2922 | 1.665E–02 | 5.801E–01 |
| Syt2 | 1.1424 | 1.802E–04 | 2.794E–02 | 0.4946 | 9.704E–02 | 9.998E–01 | 0.3239 | 3.012E–02 | 6.878E–01 |
| Plxdc1 | 1.2835 | 1.835E–04 | 2.794E–02 | 0.7993 | 1.605E–02 | 9.443E–01 | 0.2421 | 1.538E–01 | 9.084E–01 |
| Mcc | 0.5805 | 1.899E–04 | 2.794E–02 | 0.4434 | 4.223E–03 | 7.738E–01 | 0.0685 | 3.637E–01 | 9.457E–01 |
| Dsc3 | 2.9992 | 1.850E–04 | 2.794E–02 | 2.3767 | 2.806E–03 | 7.309E–01 | 0.3113 | 4.064E–01 | 9.479E–01 |
| Brinp3 | 0.7647 | 1.800E–04 | 2.794E–02 | 0.6185 | 2.243E–03 | 7.120E–01 | 0.0731 | 4.726E–01 | 9.510E–01 |
| Epha5 | 0.6288 | 1.943E–04 | 2.813E–02 | 0.2785 | 9.477E–02 | 9.998E–01 | 0.1751 | 3.901E–02 | 7.130E–01 |
| Grik3 | 0.9567 | 1.978E–04 | 2.837E–02 | 0.6005 | 1.851E–02 | 9.518E–01 | 0.1781 | 1.559E–01 | 9.089E–01 |
| Nxph3 | 1.2644 | 2.091E–04 | 2.970E–02 | 0.8348 | 1.607E–02 | 9.443E–01 | 0.2148 | 2.023E–01 | 9.256E–01 |
| Smarcd3 | −0.4134 | 2.275E–04 | 3.171E–02 | −0.1088 | 3.258E–01 | 9.998E–01 | −0.1523 | 6.890E–03 | 4.713E–01 |
| Scn4b | 1.3640 | 2.263E–04 | 3.171E–02 | 0.6231 | 8.663E–02 | 9.998E–01 | 0.3705 | 4.042E–02 | 7.215E–01 |
| Grb7 | 1.9920 | 2.303E–04 | 3.179E–02 | 1.4475 | 6.829E–03 | 8.209E–01 | 0.2723 | 2.977E–01 | 9.443E–01 |
| Crym | −0.5688 | 2.336E–04 | 3.195E–02 | −0.3365 | 2.362E–02 | 9.998E–01 | −0.1162 | 1.241E–01 | 8.818E–01 |
| Tbc1d30 | 0.4939 | 2.387E–04 | 3.235E–02 | 0.2748 | 3.946E–02 | 9.998E–01 | 0.1095 | 1.030E–01 | 8.576E–01 |
| Cbln2 | 0.9211 | 2.441E–04 | 3.248E–02 | 0.4791 | 5.943E–02 | 9.998E–01 | 0.2210 | 8.262E–02 | 8.359E–01 |
| Wdr83 | −0.4093 | 2.432E–04 | 3.248E–02 | −0.2675 | 1.656E–02 | 9.498E–01 | 80.0709 | 2.002E–01 | 9.255E–01 |
| Cecr2 | 0.6876 | 2.490E–04 | 3.284E–02 | 0.0912 | 6.358E–01 | 9.998E–01 | 0.2982 | 1.826E–03 | 2.900E–01 |
| Vipr1 | 0.5246 | 2.558E–04 | 3.314E–02 | 0.1278 | 3.893E–01 | 9.998E–01 | 0.1984 | 7.324E–03 | 4.822E–01 |
| Gm27177 | 4.2839 | 2.545E–04 | 3.314E–02 | 2.8819 | 1.195E–02 | 9.425E–01 | 0.7010 | 1.897E–01 | 9.218E–01 |
| Mgp | −1.1996 | 2.700E–04 | 3.468E–02 | −0.8580 | 8.336E–03 | 8.610E–01 | −0.1708 | 2.776E–01 | 9.441E–01 |
| Limk2 | 0.2955 | 2.756E–04 | 3.509E–02 | 0.1775 | 3.353E–02 | 9.998E–01 | 0.0590 | 1.560E–01 | 9.089E–01 |
| Fam217b | 0.2971 | 2.896E–04 | 3.538E–02 | 0.1064 | 1.954E–01 | 9.998E–01 | 0.0953 | 1.996E–02 | 6.357E–01 |
| Zdhhc14 | −0.4323 | 2.898E–04 | 3.538E–02 | −0.1936 | 9.898E–02 | 9.998E–01 | −0.1194 | 4.400E–02 | 7.328E–01 |
| Ttc30b | −0.3918 | 2.893E–04 | 3.538E–02 | −0.1829 | 9.113E–02 | 9.998E–01 | −0.1044 | 5.473E–02 | 7.501E–01 |
| Cacng3 | 0.4936 | 2.898E–04 | 3.538E–02 | 0.2444 | 6.276E–02 | 9.998E–01 | 0.1246 | 6.737E–02 | 7.843E–01 |
| Kcnt1 | 0.6121 | 2.888E–04 | 3.538E–02 | 0.4703 | 6.106E–03 | 8.209E–01 | 0.0709 | 4.073E–01 | 9.479E–01 |
| Cdh7 | 0.7944 | 2.973E–04 | 3.599E–02 | 0.6049 | 5.484E–03 | 8.100E–01 | 0.0948 | 3.819E–01 | 9.457E–01 |
| Sh3bgrl | −0.3908 | 3.202E–04 | 3.845E–02 | −0.2139 | 4.429E–02 | 9.998E–01 | −0.0885 | 1.032E–01 | 8.576E–01 |
| Tyro3 | 0.3389 | 3.257E–04 | 3.880E–02 | 0.0525 | 5.711E–01 | 9.998E–01 | 0.1432 | 2.374E–03 | 3.217E–01 |
| Ank1 | 0.6262 | 3.296E–04 | 3.894E–02 | 0.3612 | 4.014E–02 | 9.998E–01 | 0.1325 | 1.306E–01 | 8.922E–01 |
| Rhobtb2 | 0.4320 | 3.418E–04 | 4.006E–02 | 0.2404 | 3.852E–02 | 9.998E–01 | 0.0958 | 1.074E–01 | 8.639E–01 |
| Kif5a | 0.3307 | 3.524E–04 | 4.066E–02 | 0.1806 | 5.095E–02 | 9.998E–01 | 0.0750 | 1.044E–01 | 8.586E–01 |
| Gm5639 | −1.0324 | 3.520E–04 | 4.066E–02 | −0.7001 | 1.528E–02 | 9.443E–01 | −0.1661 | 2.621E–01 | 9.423E–01 |
| Gatsl2 | 0.4026 | 3.590E–04 | 4.110E–02 | 0.1782 | 1.159E–01 | 9.998E–01 | 0.1122 | 4.651E–02 | 7.355E–01 |
| Arhgef10l | 0.5565 | 3.676E–04 | 4.141E–02 | 0.2019 | 1.723E–01 | 9.998E–01 | 0.1773 | 2.326E–02 | 6.713E–01 |
| Trim66 | 0.8119 | 3.701E–04 | 4.141E–02 | 0.5932 | 8.757E–03 | 8.649E–01 | 0.1094 | 3.261E–01 | 9.457E–01 |
| Pcdh15 | 0.9567 | 3.665E–04 | 4.141E–02 | 0.9113 | 7.126E–04 | 5.847E–01 | 0.0227 | 8.649E–01 | 9.921E–01 |
| Lama3 | 1.1681 | 3.737E–04 | 4.150E–02 | 0.5850 | 7.442E–02 | 9.998E–01 | 0.2916 | 7.112E–02 | 8.013E–01 |
| Stk3 | −0.3788 | 3.809E–04 | 4.179E–02 | −0.0391 | 6.976E–01 | 9.998E–01 | −0.1698 | 1.468E–03 | 2.617E–01 |
| Prss23 | −0.7360 | 3.820E–04 | 4.179E–02 | −0.5581 | 6.054E–03 | 8.209E–01 | −0.0889 | 3.671E–01 | 9.457E–01 |
| G8m24447 | −1.3571 | 3.849E–04 | 4.180E–02 | −0.9883 | 1.243E–02 | 9.425E–01 | −0.1844 | 3.447E–01 | 9.457E–01 |
| Arap2 | 0.5580 | 3.929E–04 | 4.236E–02 | 0.2518 | 1.097E–01 | 9.998E–01 | 0.1531 | 5.159E–02 | 7.478E–01 |
| Pi15 | −1.0084 | 3.990E–04 | 4.270E–02 | −0.1340 | 6.215E–01 | 9.998E–01 | −0.4372 | 2.229E–03 | 3.149E–01 |
| Galk2 | −0.3509 | 4.069E–04 | 4.317E–02 | −0.0144 | 8.856E–01 | 9.998E–01 | −0.1682 | 8.177E–04 | 2.512E–01 |
| Rapgef3 | 0.4784 | 4.092E–04 | 4.317E–02 | 0.2501 | 6.167E–02 | 9.998E–01 | 0.1142 | 8.934E–02 | 8.430E–01 |
| Arhgef15 | 0.6324 | 4.133E–04 | 4.329E–02 | 0.3132 | 7.582E–02 | 9.998E–01 | 0.1596 | 6.946E–02 | 7.918E–01 |
| Sytl2 | 0.5686 | 4.203E–04 | 4.372E–02 | 0.2429 | 1.333E–01 | 9.998E–01 | 0.1629 | 4.235E–02 | 7.249E–01 |
| Zmat4 | 0.5322 | 4.252E–04 | 4.392E–02 | 0.1010 | 4.954E–01 | 9.998E–01 | 0.2156 | 3.267E–03 | 3.676E–01 |
| Pik3c2b | 0.6788 | 4.317E–04 | 4.398E–02 | 0.2323 | 2.393E–01 | 9.998E–01 | 0.2232 | 2.332E–02 | 6.713E–01 |
| Rgs6 | 0.9177 | 4.299E–04 | 4.398E–02 | 0.5679 | 2.817E–02 | 9.998E–01 | 0.1749 | 1.717E–01 | 9.210E–01 |
| Cacna2d2 | 0.4855 | 4.381E–04 | 4.432E–02 | 0.3532 | 9.762E–03 | 8.688E–01 | 0.0662 | 3.401E–01 | 9.457E–01 |
| Epb4.1 | 0.7239 | 4.545E–04 | 4.532E–02 | 0.1620 | 4.260E–01 | 9.998E–01 | 0.2810 | 5.182E–03 | 4.424E–01 |
| Dock3 | 0.7209 | 4.613E–04 | 4.532E–02 | 0.2911 | 1.533E–01 | 9.998E–01 | 0.2149 | 3.668E–02 | 7.063E–01 |
| Slc8a3 | 0.6069 | 4.511E–04 | 4.532E–02 | 0.2673 | 1.186E–01 | 9.998E–01 | 0.1698 | 4.152E–02 | 7.240E–01 |
| Ncald | 0.5018 | 4.622E–04 | 4.532E–02 | 0.2284 | 1.039E–01 | 9.998E–01 | 0.1367 | 4.919E–02 | 7.420E–01 |
| Gm5616 | −0.4293 | 4.634E–04 | 4.532E–02 | −0.2619 | 3.185E–02 | 9.998E–01 | −0.0837 | 1.756E–01 | 9.210E–01 |
| Tmem180 | −0.3773 | 4.738E–04 | 4.604E–02 | −0.1906 | 7.419E–02 | 9.998E–01 | −0.0933 | 8.470E–02 | 8.384E–01 |
| Agap1 | 0.3895 | 4.858E–04 | 4.607E–02 | 0.1665 | 1.329E–01 | 9.998E–01 | 0.1115 | 4.560E–02 | 7.348E–01 |
| Map3k9 | 0.7193 | 4.847E–04 | 4.607E–02 | 0.3765 | 6.769E–02 | 9.998E–01 | 0.1714 | 9.319E–02 | 8.465E–01 |
| Atp10b | 1.2795 | 4.897E–04 | 4.607E–02 | 0.6771 | 6.617E–02 | 9.998E–01 | 0.3012 | 1.031E–01 | 8.576E–01 |
| Arhgap32 | 0.5381 | 4.873E–04 | 4.607E–02 | 0.3709 | 1.611E–02 | 9.443E–01 | 0.0836 | 2.780E–01 | 9.442E–01 |
| Mapk6 | 0.4079 | 4.857E–04 | 4.607E–02 | 0.3133 | 6.890E–03 | 8.209E–01 | 0.0473 | 4.179E–01 | 9.479E–01 |
| Fibcd1 | −0.8530 | 4.940E–04 | 4.618E–02 | −0.4837 | 5.282E–02 | 9.998E–01 | −0.1847 | 1.484E–01 | 9.053E–01 |
| Cpne2 | −1.2501 | 5.141E–04 | 4.757E–02 | −0.1021 | 7.650E–01 | 9.998E–01 | −0.5740 | 1.408E–03 | 2.617E–01 |
| Gm6788 | −0.5516 | 5.250E–04 | 4.757E–02 | −0.0535 | 7.368E–01 | 9.998E–01 | −0.2490 | 1.715E–03 | 2.814E–01 |
| Gst8m6 | −0.5525 | 5.179E–04 | 4.757E–02 | −0.1977 | 2.082E–01 | 9.998E–01 | −0.1774 | 2.568E–02 | 6.812E–01 |
| Nrros | −0.5870 | 5.241E–04 | 4.757E–02 | −0.3482 | 4.270E–02 | 9.998E–01 | −0.1194 | 1.722E–01 | 9.210E–01 |
| Gucy1a3 | 0.5850 | 5.231E–04 | 4.757E–02 | 0.5019 | 2.507E–03 | 7.120E–01 | 0.0416 | 6.213E–01 | 9.711E–01 |
| Dock10 | 0.6370 | 5.379E–04 | 4.786E–02 | 0.0765 | 6.802E–01 | 9.998E–01 | 0.2802 | 2.561E–03 | 3.328E–01 |
| Ssbp4 | −0.4606 | 5.317E–04 | 4.786E–02 | −0.1781 | 1.727E–01 | 9.998E–01 | −0.1412 | 3.273E–02 | 6.915E–01 |
| Wwox | 0.4364 | 5.376E–04 | 4.786E–02 | 0.3074 | 1.511E–02 | 9.443E–01 | 0.0645 | 3.053E–01 | 9.455E–01 |
| Syt6 | −0.7500 | 5.528E–04 | 4.889E–02 | −0.0377 | 8.615E–01 | 9.998E–01 | −0.3561 | 1.080E–03 | 2.617E–01 |
| Nlk | 0.3939 | 5.674E–04 | 4.939E–02 | 0.1694 | 1.339E–01 | 9.998E–01 | 0.1122 | 4.566E–02 | 7.348E–01 |
| Prrg3 | 0.6091 | 5.649E–04 | 4.939E–02 | 0.4106 | 1.976E–02 | 9.762E–01 | 0.0992 | 2.529E–01 | 9.423E–01 |
| Orai3 | −0.3671 | 5.684E–04 | 4.939E–02 | −0.2533 | 1.729E–02 | 9.498E–01 | −0.0569 | 2.767E–01 | 9.441E–01 |
KO–PDE11A knockout; WT–PDE11A wild-type; HT–PDE11A heterozygous mouse.
Note: In the HT vs. WT and KO vs. HT analyses, no genes were significant at the level of the whole genome (i.e., all FDR-corrected P-values were >0.05). That said, P-values for a number of genes reached significance in both analyses when the stricter Bonferroni correction was applied but limited to the number of genes within this specific gene set (i.e., 0.05/169 = P < 0.00029; shown in bold).
Data analyses
Behavioral and biochemical data were collected in a blinded fashion and analyzed using Statistica 9.0. As previously described (Kelly et al., 2010; Kelly, 2014; Pathak et al., 2015), to mitigate non-specific effects related to transfer efficiencies, film exposures, etc. across gels, Western blot data on a given gel were normalized to a reference group (e.g., GH). Data were analyzed by an analysis of variance (ANOVA) or repeated measure ANOVA for genotype and/or housing condition and compartment. With regard to the effect of sex, male and female data in Figs. 1 and 3A were analyzed separately based on our previous sex-specific findings in an “object vs. novel mouse” version of the approach assay. For remaining datasets, sex was included along with genotype in ANOVAs where n ⩾ 6/sex/genotype. Data were graphed collapsed across sex unless a significant genotype × sex interaction was detected to best reflect the conclusions of the statistical tests. In cases of significant ANOVAs, post hoc analyses were conducted using the Student–Newman–Keuls Method. As previously described (Kelly et al., 2008, 2010; Kelly, 2014; Pathak et al., 2015), statistical outliers >2 standard deviations from the mean were removed from analyses (outliers/total data points: Fig. 1C Left, 2/62; Fig. 1E Right, 6/132;Fig. 2A Left 8/28; Fig. 2A Middle, 7/128; Fig. 2A Right, 7/128; Fig. 2B Left, 3/64; Fig. 2B Middle, 8/128; Fig. 2B Right, 10/128; Fig. 2C Left, 6/128; Fig. 2C Middle, 5/128; Fig. 2C Right 10/128; Fig. 3A Left, 2/109; Fig. 3A Right, 3/56; Fig. 3B, 4/101; Fig. 3C, 3/101;Fig. 3D, 4/101; Fig. 4A, 5/104; Fig. 4B, 4/104;Fig. 4C, 5/104; Fig. 4D, 6/88; Fig. 4E, 1/88;Fig. 4F, 4/88; Fig. 4H, 1/56; Fig. 4I, 1/56). Significance was defined as P < 0.05. Data are graphed as means ± SEM.
Fig. 3.
Adolescent PDE11A heterozygous (HT) and knockout (KO) mice show no deficits in social approach behavior relative to PDE11A wild-type (WT) littermates. (A) Unlike adult male PDE11A HT and KO mice (see Figs. 1 and 2), adolescent PDE11A HT and KO mice show no deficits in social approach relative to PDE11A WT littermates in the “object vs. novel PDE11A” version of the assay (females, F(2,47) = 162.60, P < 0.001; WT, n = 9; HT, n = 9; KO, n = 10) (males, F(2,78) = 304.12, P < 0.001; WT, n = 13; HT, n = 13; KO, n = 17). (B) In the presence of a cagemate, male and female adolescent PDE11A HT and KO mice similarly show normal or better social approach behavior relative to sex-matched PDE11A WT littermates in terms of the latency to approach (F(2,40) = 3.56, P = 0.038), (C) the time spent in the chamber (F(1,41) = 201.31, P < 0.001), and (D) the time spent near the cylinder (F(1,42) = 190.30, P < 0.001). 3B-D WT-F, n = 8; HT-F, n = 8; KO-F, n = 12; WT-M, n = 6; HT-M, n = 6; KO-M, n = 7. Post hoc, *vs. object or cagemate, P < 0.01–0.001; #vs. WT, P = 0.014.
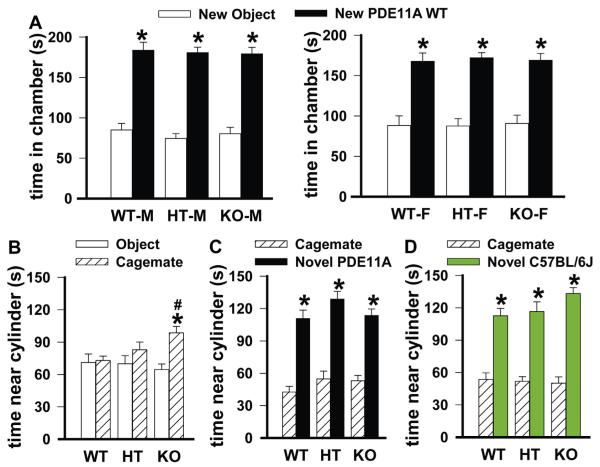
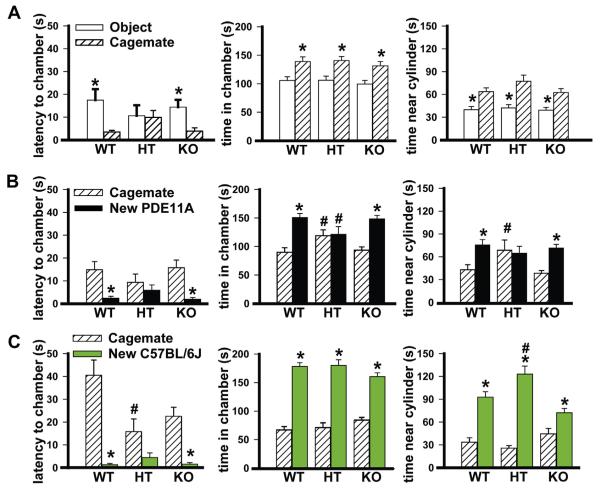
Fig. 2.
Replacing the new object with a cagemate aligned social approach behaviors of PDE11A mutant males and females, with PDE11A mutant mice showing context-dependent changes in social approach behavior. (A) Relative to PDE11A wild-type (WT) mice, PDE11A HT and KO mice generally demonstrated intact social approach towards their own cagemate. (Left) an exception exists in that HTs fail to differentiate their latency to approach a cagemate vs. a novel object (latency: F(1,60) = 5.94, P = 0.018); however, all mice demonstrate significant social preference for the cagemate in terms of (middle) chamber time (F(1,63) = 15.01, P < 0.001) and (right) cylinder time (F(1,60) = 32.79, P < 0.001). *vs. cagemate, P < 0.001. (B) When given a choice between a cagemate and a novel PDE11A WT, PDE11A KO mice exhibited significant social approach in terms of (left) their latency to approach the chamber (F(1,24) = 13.75, P = 0.001), (middle) time in chamber (F(1,25) = 28.39, P = 0.001), and (right) time near the cylinder (F(1,23) = 20.41, P < 0.001), as did PDE11A WT mice (latency: F(1,22) = 15.18, P < 0.001; chamber time: F(1,23) = 16.24, P < 0.001; cylinder time: F(1,23) = 7.46, P = 0.012). Surprisingly, PDE11A HT mice showed further impairments in social approach such that both male and female HTs failed to demonstrate any preference for the new PDE11A WT over their own cagemate on any measure. Further, PDE11A HT mice spent significantly more time in the chamber of the cagemate than did PDE11A WT and KO mice (F(2,63) = 5.12, P = 0.009) and significantly less time in the chamber of the new PDE11A WT than did PDE11A WT and KO mice (F(2,65) = 4.52, P = 0.015). (C) Similarly, PDE11A HT mice failed to show a significant preference in their latency to approach a new C57BL/6J mouse vs. their own cagemate, unlike the PDE11A WT (F(1,24) = 31.54, P < 0.001) and KO mice (F(1,25) = 28.15, P < 0.001). This is due to the fact that PDE11A HT approached their cagemate significantly sooner than did PDE11A WT mice (F(2,65) = 3.85, P = 0.026). A similar trend was observed in the PDE11A KO mice (Post hoc, P = 0.079). (Middle) despite these differences in latency to approach, PDE11A WT (F(1,23) = 85.83, P < 0.001), HT (F(1,14) = 45.43, P < 0.001), and KO mice (F(1,26) = 48.12, P < 0.001) all showed a significant preference for the new C57BL/6J mouse over their own cagemate in terms of time spent in each chamber. (Right) PDE11A WT (F(1,23) = 30.91, P < 0.001), HT (F(1,13) = 83.04, P < 0.001), and KO mice (F(1,22) = 6.84, P = 0.016) also showed a significant preference for the new C57BL/6J mouse over their own cagemate in terms of time spent near the cylinder. That said, there was a significant difference among the genotypes in terms of how much time was spent near the cylinder of the C57BL/6J mice (F(2,63) = 10.90, P < 0.001), with PDE11A HT mice spending significantly more time relative to WT mice and PDE11A KO mice showing a strong trend towards spending less time than WT mice (Post hoc, P = 0.057). (F, G) WT-F, n = 14; HT-F, n = 9; KO-F, n = 15; WT-M, n = 12; HT-M, n = 7; KO-M, n = 11. Post hoc, *vs. cagemate, P < 0.05–0.001; #vs. WT, P = 0.025–0.004.
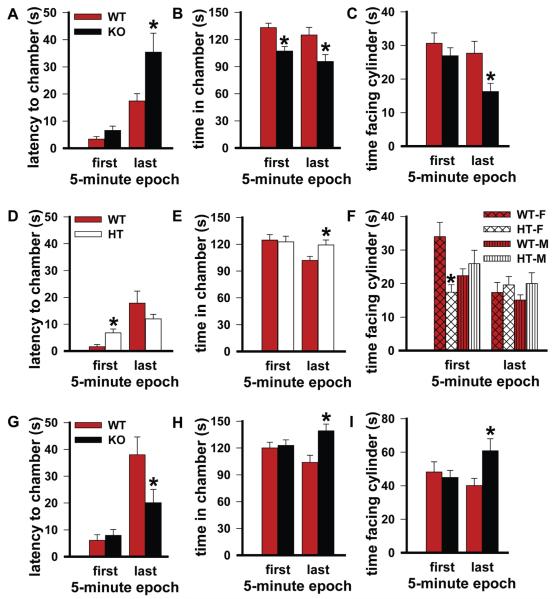
Fig. 4.
Social C57BL/6J mice spend more time with a PDE11A wild-type (WT) mouse relative to a PDE11A knockout (KO) mouse; whereas, PDE11A KO mice spend more time with a PDE11A KO mouse relative to a WT mouse. (A) During a 30-min session, C57BL/6J mice (males, n = 10; females, n = 14) were given access to a sex-matched PDE11A WT and its PDE11A KO littermate (consistent effects observed across 2 cohorts and combined analyses are shown here). During the last 5 min, C57BL/6J mice approached the chamber of the PDE11A WT significantly faster than that of the KO littermate (F(1,22) = 4.94, P = 0.018). (B) In both the first (F(1,23) = 4.37, P = 0.048) and last 5 min (F(1,23) = 4.94, P = 0.036), C57BL/6J mice spent more time in the chamber of the PDE11A WT versus KO littermate. (C) During the last 5 min, C57BL/6J mice spent more time directly interacting with the cylinder of the PDE11A WT versus KO littermate (F(1,21) = 7.6, P = 0.011). (D) The pattern of results differed when C57BL/6J mice (males, n = 12; females, n = 10) were given access to a sex-matched PDE11A WT and its heterozygous (HT) littermate (consistent effects observed across 2 cohorts and combined analyses are shown here). C57BL/6J mice approached the PDE11A WT significantly faster than the HT littermate only during the first 5 min (F(1,17) = 8.82, P = 0.009). (E) In the last 5 min, C57BL/6J mice actually spent significantly more time in the chamber of the HT vs. WT littermate (F(1,19) = 4.61, P = 0.045). (F) Consistent with these effects shown in panels D and E, female C57BL/6J mice spent significantly more time interacting with the cylinder of the female PDE11A WT vs. HT littermate during the first 5 min (F(1,17) = 9.61, P = 0.017), and yet male and female C57BL/6J mice showed a trend towards spending more time interacting with the cylinder of the HT vs. WT littermate during the last 5 min (F(1,17) = 4.05, P = 0.07). (G) Interestingly, when PDE11A KO mice (male, n = 6; female, n = 8) were given access to a novel PDE11A WT and that WT’s KO littermate, the PDE11A KO mice approached the novel PDE11A KO mouse chamber more quickly than that of the WT littermate (F(1,12) = 6.90, P = 0.022) and (H) spent significantly more time with the PDE11A KO mouse vs. the WT littermate in the chamber (F(1,11) = 6.20, P = 0.03) and (I) at the cylinder (F(1,11) = 5.31, P = 0.042). Post hoc, *vs. WT, P = 0.048–0.005.
RESULTS
Social approach behaviors of PDE11A HT and KO mice depend on the social context
In our previous laboratory (Kelly et al., 2010), we reported that within a colony that was bred offsite and single-housed, male PDE11A KO mice—but not female KO mice—exhibited significantly reduced social approach behavior relative to sex-matched WT littermates. Here, we replicated this sex-specific effect with group-housed (GH) mice bred onsite (Fig. 1C). Next we determined if social approach behavior of PDE11A mutant mice might depend on the social context. Changing the stimulus mouse from a novel PDE11A WT to a novel C57BL/6J mouse (Fig. 1D-E) and/or replacing the alternative option of the novel object with a cagemate (Fig. 2A) altered the social approach deficits exhibited by PDE11A HT and KO mice, in some cases lessening the deficits and in other cases worsening the deficits. Together, these data suggest that social approach deficits exhibited by PDE11A mutant mice differ depending on the social context.
Effects of PDE11A deletion on social approach behavior are age dependent
Previously we showed that PDE11A expression is minimal at postnatal day 7 but dramatically increases to young adulthood levels between postnatal day 21–28 (Hegde et al., 2016). Social approach behaviors become reliably measurable in the mouse around postnatal day 19 (Laviola et al., 1994; Fairless et al., 2012), suggesting PDE11A mutant mice might exhibit social approach deficits early in adolescence. That said, a number of neurodevelopmental insults, including neonatal ventral hippocampal lesions, do not yield immediate behavioral deficits but, rather, require a period of development to occur before deficits fully manifest (e.g., (Tseng et al., 2009)). As such, we determined if adolescent PDE11A HT and KO mice would exhibit similar social approach deficits relative to WT littermates as did their adult counterparts. Adolescent PDE11A HT and KO mice largely performed normally in social approach behaviors where adult HT and KO mice showed deficits (Fig. 3). In fact, adolescent PDE11A KO mice demonstrated significant social approach towards their cagemate vs. a novel object when adolescent PDE11A WT and HT littermates demonstrated no such preference (Fig. 3B). Overall, these data suggest deficits observed in the adult mutant mice are age dependent.
A social mouse prefers to spend time with a PDE11A WT versus a PDE11A KO but a PDE11A KO prefers to spend time with another KO
The above experiments suggest that adult PDE11A mutant mice exhibit altered social behaviors in specific social contexts; however, this is based on a human definition of what is an appropriate social behavior for a mouse. Therefore, we determined if a C57BL/6J “mouse psychiatrist” would be able to detect abnormal behavior in PDE11A mutant mice. C57BL/6J mice strongly preferred to spend time with PDE11A WT versus KO mice, particularly during the last 5 min of the 30-min session (Fig. 4A–C). In contrast, C57BL/6J mice preferred PDE11A WT over HT mice during the first 5 min, but then switched to prefer the HTs over the WTs during the last 5 min (Fig. 4D–F). In contrast, PDE11A KO mice preferred to spend time with another PDE11A KO vs. a WT mouse, particularly during the final 5 min of the session (Fig. 4G–I). These data suggest other mice are able to detect differences in PDE11A mutant mouse behavior.
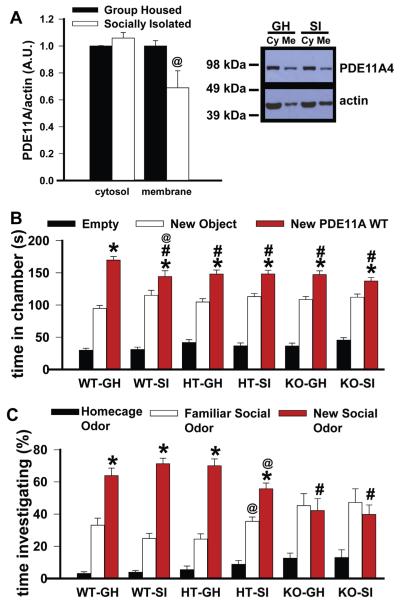
Social isolation decreases PDE11A4 protein expression in a subcellular compartment-specific manner
When humans lack proper social behaviors, they are often ostracized. We show this phenomenon extends to mice as well (Fig. 4). As such, we determined if social isolation might alter PDE11A4 protein expression. In biochemically fractionated ventral hippocampi of C57BL/6J mice, 1 month of social isolation decreased PDE11A4 expression in the membrane but not the cytosolic compartment (Fig. 5A).
Fig. 5.
Isolation-induced decreases in PDE11A4 expression are sufficient to impair subsequent social behavior. (A) Socially isolated (SI) C57BL/6J mice expressed significantly less PDE11A4 protein in the membrane compartment of the ventral hippocampal formation (VHIPP) relative to group-housed (GH) C57BL/6J mice (F(1,12) = 5.65, P = 0.035; n = 7 biological replicates (five males + two females)/group). (B) Isolation, then, induced a dose–response shift across PDE11A genotypes such that isolated PDE11A wild-type (WT) mice exhibited a behavioral profile similar to that of GH PDE11A heterozygous (HT) mice in social approach (assessed in males only, F(10,156) = 2.61, P = 0.006). WT-GH, n = 16; WT-SI, n = 12; HT-GH, n = 14; HT-SI, n = 15; KO-GH, n = 16; KO-SI, n = 11. (C) Similarly, isolated HTs shifted their behavioral profile towards that of PDE11A knockout (KO) mice in 24-h long-term memory for social odor recognition (F(2,58) = 5.43, P = 0.007). All males except where noted: WT-GH, n = 17; WT-SI, n = 9; HT-GH, n = 17 (includes three females); HT-SI, n = 14 (includes four females); KO-GH, n = 10; KO-SI, n = 5. #WT-GH, P < 0.03–0.001; *vs. novel object/familiar odor, P ⩽ 0.002; @vs. GH, P = 0.019–0.003.
Isolation impairs subsequent social approach and social odor recognition memory in a PDE11A genotype-dependent manner
Given that social isolation impairs subsequent social behaviors in humans (Kogan et al., 2000; Derntl et al., 2011; Shahar-Gold et al., 2013), we determined if isolation would impair subsequent social behaviors in a PDE11A genotype-dependent manner. If so, this would suggest that the isolation-induced decreases in PDE11A4 expression noted above are of functional consequence. To do so, we compared social behaviors in GH versus single-housed PDE11A WT, HT, and KO mice, reasoning that isolation should induce a dose–response shift across PDE11A genotypes. We tested social approach behavior in male mice in the “novel object vs. novel PDE11A” version of the assay and social odor recognition in both male and female mice. Across the 2 assays, behavior of socially isolated (SI) WTs shifted towards that of group-housed (GH) HTs and the behavior of SI HTs shifted towards that of GH KOs, with no effect of isolation on PDE11A KO behavior (Fig. 5B-C).
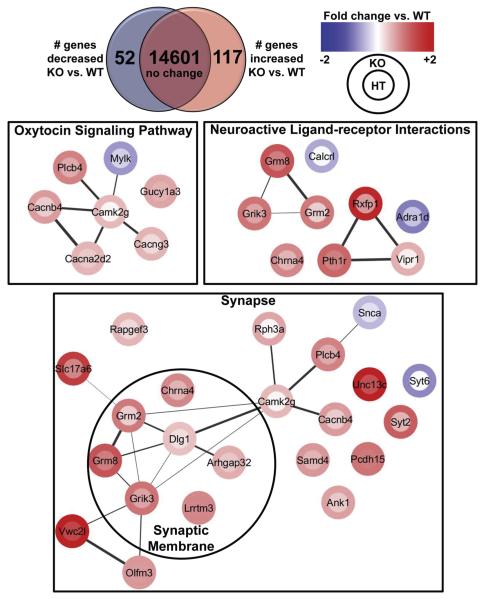
Deletion of PDE11A triggers gene expression changes in the oxytocin pathway
To determine the molecular mechanisms by which PDE11A deletion alters social behavior, we conducted RNA sequencing on VHIPP samples from adult male and female PDE11A WT, HT, and KO mice (n = 7/genotype). After FDR correction, 52 genes were down regulated and 117 genes were upregulated in PDE11A KO vs. WT mice (Table 3; Fig. 6). KEGG pathway analyses identified enrichment in only three pathways (Table 4). 1 of these 3 pathways is the oxytocin pathway, a major signaling pathway underlying social behaviors (c.f., (Viero et al., 2010; Ebstein et al., 2012; Lukas and Neumann, 2013; Stoesz et al., 2013; Feldman et al., 2015)) and the effects of social buffering (Chen et al., 2011; Hostinar et al., 2014). Consistent with a role for PDE11A in regulating behavior, the GO Biological Process and GO Cellular compartment most significantly affected by PDE11A deletion was synaptic transmission and the synapse, respectively (Table 4; Fig. 6). Given that social isolation specifically decreased PDE11A4 expression in membrane, it is interesting to note that PDE11A deletion also significantly affected several membrane-related GO pathways (e.g., synaptic membrane, Table 4).
Fig. 6.
RNA sequencing of VHIPP reveals 52 downregulated and 116 upregulated genes in PDE11A knockout (KO) mice relative to PDE11A wild-type (WT) littermates, with significant enrichment of genes in pathways related to social behavior. Pathway genes and edges generated by STRING 10 (medium confidence) for the oxytocin (FDR P = 0.014), neuroactive-ligand (FDR P = 0.014), synapse (FDR P < 0.001), and synaptic membrane pathways (FDR P = 0.038) are shown here. Nodes are color coded for the log-fold change in gene expression of PDE11A KO mice (outer rim) and PDE11A heterozygous (HT) mice (inner circle) relative to WT littermates. See Table 3 for the complete list of genes that significantly differed between PDE11A KO vs. WT mice that were included in the pathway analyses and Table 4 for the full list of KEGG and GO pathways demonstrating significant gene enrichment. Gene changes in PDE11A HT mice are also detailed in Table 3. n = 7 (three males, four females)/genotype. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Pathway analyses following RNA sequencing of VHIPP from male and female adult PDE11A wild-type versus knockout mice
| GO_id | Term | # Genes | p-value_fdr |
|---|---|---|---|
| KEGG pathways | |||
| 4261 | Adrenergic signaling in cardiomyocytes | 8 | 1.74E–03 |
| 4921 | Oxytocin signaling pathway | 7 | 1.37E–02 |
| 4080 | Neuroactive ligand-receptor interaction | 9 | 1.37E–02 |
| GO biological processes | |||
| G0:0007268 | Synaptic transmission | 12 | 2.97E–02 |
| G0:0051239 | Regulation of multicellular organismal process | 35 | 2.97E–02 |
| G0:0030001 | Metal ion transport | 13 | 2.97E–02 |
| G0:0007196 | Adenylate cyclase-inhibiting G-protein-coupled glutamate receptor signaling pathway | 3 | 3.07E–02 |
| GO:0006812 | Cation transport | 15 | 3.07E–02 |
| G0:0051056 | Regulation of small gtpase-mediated signal transduction | 12 | 3.07E–02 |
| GO:0065008 | Regulation of biological quality | 36 | 3.46E–02 |
| G0:0006816 | Calcium ion transport | 8 | 4.21E–02 |
| GO Cellular componenets | |||
| G0:0045202 | Synapse | 22 | 2.83E–06 |
| G0:0042995 | Cell projection | 34 | 2.20E–05 |
| G0:0097458 | Neuron part | 25 | 4.47E–04 |
| G0:0044456 | Synapse part | 15 | 1.05E–03 |
| G0:0005886 | Plasma membrane | 49 | 1.42E–03 |
| G0:0043005 | Neuron projection | 21 | 1.42E–03 |
| G0:0034702 | Ion channel complex | 10 | 1.98E–03 |
| G0:1902495 | Transmembrane transporter complex | 10 | 2.98E–03 |
| G0:0071944 | Cell periphery | 48 | 2.98E–03 |
| G0:1990351 | Transporter complex | 10 | 2.98E–03 |
| G0:0008328 | Ionotropic glutamate receptor complex | 5 | 4.10E–03 |
| G0:0005623 | Cell | 107 | 7.23E–03 |
| G0:0005622 | Intracellular | 96 | 8.78E–03 |
| G0:0044464 | Cell part | 106 | 9.25E–03 |
| G0:0043235 | Receptor complex | 9 | 1.50E–02 |
| G0:0030054 | Cell junction | 19 | 1.95E–02 |
| G0:0097060 | Synaptic membrane | 8 | 3.84E–02 |
| G0:0042734 | Presynaptic membrane | 4 | 4.52E–02 |
| G0:0044424 | Intracellular part | 92 | 4.57E–02 |
| GO molecular functions | |||
| G0:0043167 | Ion binding | 61 | 3.93E–03 |
| G0:0005543 | Phospholipid binding | 11 | 1.70E–02 |
| G0:0001640 | Adenylate cyclase inhibiting G-protein-coupled glutamate receptor activity | 3 | 1.70E–02 |
| G0:0008289 | Lipid binding | 14 | 1.70E–02 |
| G0:0043169 | Cation binding | 43 | 1.70E–02 |
| G0:0030276 | Clathrin binding | 5 | 1.70E–02 |
| G0:0046872 | Metal ion binding | 42 | 1.79E–02 |
| G0:0098772 | Molecular function regulator | 19 | 2.05E–02 |
| G0:0005261 | Cation channel activity | 9 | 2.73E–02 |
| G0:0005089 | Rho guanyl-nucleotide exchange factor activity | 5 | 2.74E–02 |
| G0:0005088 | Ras guanyl-nucleotide exchange factor activity | 6 | 3.99E–02 |
Pathway analyses conducted using STRING 10.0. n = 7/genotype. VHIPP–ventral hippocampal formation.
Note: 5/8 genes included in the “Adrenergic signaling in cardiomyocytes” pathway are represented in the “Oxytocin” pathway.
DISCUSSION
Here we showed that PDE11A4, an enzyme enriched in the VHIPP, is a key regulator of social behavior and a key mechanism by which social experience feeds back to shape signaling in the brain. We showed that (i) PDE11A deletion impairs social behaviors of adults much more so than adolescents (Figs. 1–3), (ii) social deficits caused by the loss of PDE11A are sensitive to the social context (Figs. 1, 2 and 4), (iii) social isolation decreases PDE11A4 expression in a subcellular compartment-specific manner (Fig. 5A), (iv) isolation impairs subsequent social behaviors in a PDE11A genotype-dependent manner, suggesting that isolation-induced decreases in PDE11A4 expression do contribute to subsequent impairments in social behaviors (Fig. 5B-C), and (v) deletion of PDE11A4 significantly alters expression of genes in a major signaling pathway underlying social behavior (Table 4,Fig. 6), namely the oxytocin pathway (c.f. (Viero et al., 2010; Ebstein et al., 2012; Lukas and Neumann, 2013; Stoesz et al., 2013)). We believe that social approach deficits measured here in PDE11A mutant mice likely reflect a fundamental deficit in social approach behavior as opposed to a simple failure to detect social novelty because PDE11A HT and KO mice retain the ability to differentiate familiar versus novel social odors when presented via wooden beads, although the consolidation of long-term social memory is impaired (Fig. 5C; (Kelly et al., 2010; Hegde et al., 2016)). Thus, phenotypes exhibited by PDE11A mutant mice are reminiscent of social deficits observed in patients with certain neurodevelopmental disorders (e.g., (Miller et al., 2002; Johnstone et al., 2005; Jahr et al., 2007; Locke et al., 2010; Giacco et al., 2012; Dean et al., 2014)), in that mutant mice initiate social interactions differently relative to WT mice and a social mouse chooses to interact less with the PDE11A KOs.
Although the effect sizes of the social approach deficits noted in the PDE11A mutant mice are arguably limited, they are reproducible and their magnitude is well in line with the sparse nature of PDE11A4 expression in the brain (i.e., limited to a subpopulation of neurons in CA1, subiculum, and the amygdalohippocampal area (Kelly et al., 2010, 2014; Kelly, 2015; Hegde et al., 2016)). The effect of PDE11A deletion on social interactions is clearly dependent on the social context as changing the stimulus mouse strain (i.e., new PDE11A vs. cagemate vs. new C57BL/6J mouse) or the nature of the alternative option (i.e., new object vs. cagemate), distinctly alters the phenotype of PDE11A HT and KO mice. Although PDE11A mutant mice clearly show context-dependent alterations in their social approach behaviors, it remains to be determined what is driving the context specificity of the effects. It may be that deletion of PDE11A affects motivational aspects of task performance. For example, the social approach deficits noted in male PDE11A mutant mice in the “new object vs. new PDE11A mouse” context may reflect a slight aversion for the new mouse or simply a stronger attraction toward the novel object. Similarly, the fact that male PDE11A KO mice exhibit reduced social approach in the “object vs. new PDE11A mouse” context, but not in the “cagemate vs. new PDE11A mouse” context, may reflect a positive effect of social buffering in the latter (i.e., the stress-reducing effect elicited by the presence of a conspecific (Ditzen and Heinrichs, 2014; Hostinar et al., 2014; Gobrogge and Wang, 2015)), since it does not appear that a PDE11A mutant mouse is simply more motivated to explore a novel object vs. a familiar mouse (Fig. 2A). Indeed, PDE11A4 is expressed in VHIPP neurons that project to the nucleus accumbens (Cembrowski et al., 2016), suggesting PDE11A4 may be important in regulating how motivated one is to engage in social interactions or how rewarding one finds those social interactions. Whatever motivations drive the altered social interactions of a PDE11A mutant mouse, those alterations appear to be recognizable by another mouse as the “mouse psychiatrist” assay shows a C57BL/6J mouse prefers to interact with a PDE11A WT mouse over a PDE11A KO mice while a PDE11A KO mouse prefers to interact with another PDE11A KO mouse over a PDE11A WT mouse. The fact that PDE11A KO mice prefer another KO vs. a WT in the “mouse psychiatrist” assay suggests that a PDE11A KO mouse might even show stronger social approach relative to a WT when given the choice between a novel object and another PDE11A KO mise. Findings in the “mouse psychiatrist” assay are consistent with reports in humans showing people tend to associate with like-minded people (Launay and Dunbar, 2015) and individuals with neurodevelopmental or psychiatric illnesses tend to socially withdraw and/or be ostracized (e.g., (Miller et al., 2002; Johnstone et al., 2005; Locke et al., 2010; Giacco et al., 2012; Dean et al., 2014)).
Interestingly, deleting PDE11A changes social behaviors in a manner that does not strictly follow a gene-dosage relationship (Figs. 1, 2 and 4). This suggests that full deletion of PDE11A may trigger a protective compensatory mechanism that partial deletion does not. Consistent with this idea, the RNAseq experiment shows that PDE11A KO mice exhibit a significant reduction relative to PDE11A WT and HT mice in insulin-like growth factor binding protein 3 (IGFBP3), elevated expression of which is associated with chronic loneliness in humans (Cole et al., 2007). Although some questions remain, data presented here strongly argue that PDE11A is a key regulator of social interactions, which is consistent with the fact that PDE11A has been genetically and/or functionally associated with clinical phenotypes related to changes in social function, including major depressive disorder (Wong et al., 2006; Couzin, 2008; Cabanero et al., 2009; Luo et al., 2009; Kelsoe, 2010; Coon et al., 2013), suicide risk (an inactivating mutation, (Coon et al., 2013)), and lithium responsivity (Couzin, 2008; Kelsoe, 2010; Mertens et al., 2015; Pathak et al., in press).
It remains to be determined whether PDE11A is more relevant to social deficits observed in adulthood disorders, such as schizophrenia, or those observed in childhood disorders, such as autism. Adolescent PDE11A mutant mice exhibit normal social approach behaviors in social contexts where adult PDE11A mutant mice show reduced social approach (Fig. 3). Such an age-dependent trajectory is thought to be relevant in modeling aspects of schizophrenia in rodents (Feleder et al., 2010), since this schizophrenia is thought to be caused by neurodevelop-mental insults that do not fully manifest until early adulthood. That said, PDE11A KO mice are impaired in other types of social behaviors. Previously we showed that both adolescent and adult PDE11A mutant mice show significant impairments in consolidating long-term memories involving social information (Kelly et al., 2010; Hegde et al., 2016). This raises the interesting possibility that adolescent PDE11A mutant mice have a fundamental inability to form long-term memories for what is and what is not a socially-acceptable behavior, which ultimately leads to alterations in social approach behavior at a later age. Indeed, adolescents with intellectual disabilities were found to have lower quality friendships relative to typically developing adolescents, which was predicted by impaired social skills earlier in development (Tipton et al., 2013). It will be of interest to future studies to determine if social interaction deficits observed in adult PDE11A mutant mice are, in fact, directly related to their inability to form social memories.
Our work argues that it may be necessary to not only stimulate PDE11A4 catalytic activity but also to restore PDE11A4 to a specific subcellular localization to treat social deficits. The importance of the compartment-specific effects noted herein in response to social isolation are underscored by the fact that patients with neuropsychiatric and neurodegenerative diseases may have cyclic nucleotide deficits in one subcellular compartment but not another (Rahman et al., 1997; Bonkale et al., 1999; Fields et al., 1999; Chang et al., 2003). It is possible to stimulate PDE11A4 catalytic activity via its GAF-A domain (Jager et al., 2012). Further, it is possible to increase insertion of PDE11A4 into the VHIPP membrane by promoting homodimerization of the protein at its GAF-B domain (Pathak et al., in press). Importantly, PDE11A4 GAF domains are less than 50% homologous to those in other PDE families (84), and PDE11A1–3 do not contain a full GAF-A domain (Yuasa et al., 2000). This suggests selectivity for PDE11A4 relative to other PDE families, and even other PDE11A isoforms, is possible. Selective targeting of PDE11A4 may reduce side effect potential given its minimal expression outside of the brain (Kelly, 2015).
Highlights.
Adult—not adolescent—PDE11A mutant mice exhibit sex-dependent social deficits in select social contexts but not others.
C57BL/6J mice prefer to interact with PDE11A WT mice while PDE11A KO mice prefer to interact with other PDE11A KO mice.
Social isolation decreases PDE11A4 protein and impairs subsequent social behaviors in a PDE11A genotype-dependent manner.
Deletion of PDE11A4 alters gene expression in the oxytocin signaling pathway, a key regulator of social behaviors.
Acknowledgements
The authors would like to thank Marcelo Wood, Ted Abel, and Igor Roninson for helpful advice on RNA sequencing as well as Alex Gasparian for project management support. Research supported by 1P20GM109091 from NIGMS (Project 1, M.S.; Project 3, M.P.K.), a Research Starter Grant in Pharmacology & Toxicology from the PhRMA Foundation (M. P.K.), an ASPIRE award from the Office of the Vice President for Research from the University of South Carolina (individual awards to M.S. and M.P.K.), a Research Development Fund Award from the University of South Carolina School of Medicine (M.P.K.), a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation (M.P.K.), and 1R01MH101130 from NIMH (M.P.K.). M.P.K. receives consulting fees from ASUBIO, Inc. and Deallus for projects unrelated to the current manuscript.
Glossary
- ANOVA
analysis of variance
- CREB
cAMP-response element binding protein
- GH
group housed
- HT
heterozygous
- KO
knockout
- PDE11A
Phosphodiesterase 11A
- Q-PCR
quantitative PCR
- SI
socially isolated
- VHIPP
ventral hippocampal formation
- WT
wild-type
Footnotes
S.H., H.J., D.O., N.P. and M.S. have no financial conflicts to disclose.
REFERENCES
- Avissar S, Nechamkin Y, Barki-Harrington L, Roitman G, Schreiber G. Differential G protein measures in mononuclear leukocytes of patients with bipolar mood disorder are state dependent. J Affect Disord. 1997;43:85–93. doi: 10.1016/s0165-0327(96)01400-0. [DOI] [PubMed] [Google Scholar]
- Avissar S, Barki-Harrington L, Nechamkin Y, Roitman G, Schreiber G. Elevated dopamine receptor-coupled G(s) protein measures in mononuclear leukocytes of patients with schizophrenia. Schizophr Res. 2001a;47:37–47. doi: 10.1016/s0920-9964(00)00038-4. [DOI] [PubMed] [Google Scholar]
- Avissar S, Roitman G, Schreiber G. Differential effects of the antipsychotics haloperidol and clozapine on G protein measures in mononuclear leukocytes of patients with schizophrenia. Cell Mol Neurobiol. 2001b;21:799–811. doi: 10.1023/A:1015164423918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP, Maestrini E, Consortium IMGSoA Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry. 2003;8:916–924. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Pratt SI. Psychosocial rehabilitation and quality of life for older adults with serious mental illness: recent findings and future research directions. Curr Opin Psychiatry. 2009;22:381–385. doi: 10.1097/YCO.0b013e32832c9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Ebstein RP, Biederman J, Stern R, Berman M, van Praag HM. The effect of L-dopa and propranolol on human CSF cyclic nucleotides. Psychopharmacology. 1978;58:307–310. doi: 10.1007/BF00427396. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- Bickle J. The molecules of social recognition memory: implications for social cognition, extended mind, and neuroethics. Conscious Cogn. 2008;17:468–474. doi: 10.1016/j.concog.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Cowburn RF, Ohm TG, Bogdanovic N, Fastbom J. A quantitative autoradiographic study of [3H]cAMP binding to cytosolic and particulate protein kinase A in post-mortem brain staged for Alzheimer’s disease neurofibrillary changes and amyloid deposits. Brain Res. 1999;818:383–396. doi: 10.1016/s0006-8993(98)01307-9. [DOI] [PubMed] [Google Scholar]
- Bowers MB, Jr, Study RE. Cerebrospinal fluid cyclic AMP and acid monoamine metabolites following probenecid: studies in psychiatric patients. Psychopharmacology. 1979;62:17–22. doi: 10.1007/BF00426029. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Countryman RA, Neve RL, Colombo PJ. Hippocampal overexpression of mutant creb blocks long-term, but not short-term memory for a socially transmitted food preference. Learn Mem. 2005;12:12–17. doi: 10.1101/lm.85005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Cabanero M, Laje G, Detera-Wadleigh S, McMahon FJ. Association study of phosphodiesterase genes in the sequenced treatment alternatives to relieve depression sample. Pharmacogenet Genomics. 2009;19:235–238. doi: 10.1097/FPC.0b013e328320a3e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 2016 doi: 10.1016/j.neuron.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Chang A, Li PP, Warsh JJ. Altered cAMP-dependent protein kinase subunit immunolabeling in post-mortem brain from patients with bipolar affective disorder. J Neurochem. 2003;84:781–791. doi: 10.1046/j.1471-4159.2003.01605.x. [DOI] [PubMed] [Google Scholar]; J Neurochem. 2003;85(1):286. Erratum appears in. [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci U S A. 2011;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Chu JY, Chow BK. Vasopressin-independent mechanisms in controlling water homeostasis. J Mol Endocrinol. 2009;43:81–92. doi: 10.1677/JME-08-0123. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon H, Darlington T, Pimentel R, Smith KR, Huff CD, Hu H, Jerominski L, Hansen J, Klein M, Callor WB, Byrd J, Bakian A, Crowell SE, McMahon WM, Rajamanickam V, Camp NJ, McGlade E, Yurgelun-Todd D, Grey T, Gray D. Genetic risk factors in two Utah pedigrees at high risk for suicide. Transl Psychiatry. 2013;3:e325. doi: 10.1038/tp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin J. Science and commerce. Gene tests for psychiatric risk polarize researchers. Science. 2008;319:274–277. doi: 10.1126/science.319.5861.274. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633:297–304. doi: 10.1016/0006-8993(94)91552-0. [DOI] [PubMed] [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, Lord C, Orlich F, King B, Harwood R. The peer relationships of girls with ASD at school: comparison to boys and girls with and without ASD. J Child Psychol Psychiatry. 2014;55:1218–1225. doi: 10.1111/jcpp.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Seidel EM, Eickhoff SB, Kellermann T, Gur RC, Schneider F, Habel U. Neural correlates of social approach and withdrawal in patients with major depression. Soc Neurosci. 2011;6:482–501. doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AP, Richards SH, Greaves CJ, Campbell JL. Interventions targeting social isolation in older people: a systematic review. BMC Pub Health. 2011;11:647. doi: 10.1186/1471-2458-11-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32:149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Biederman J, Rimon R, Zohar J, Belmaker RH. Cyclic GMP in the CSF of patients with schizophrenia before and after neuroleptic treatment. Psychopharmacology. 1976;51:71–74. doi: 10.1007/BF00426324. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Edmunds CE, Simpson LJ, Sale JE. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol Cell. 2008;30:519–529. doi: 10.1016/j.molcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, Morton EA, Tan J, Berrettini WH, Li H, Abel T, Brodkin ES. Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res. 2012;228:299–310. doi: 10.1016/j.bbr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Feleder C, Tseng KY, Calhoon GG, O’Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Fields A, Li PP, Kish SJ, Warsh JJ. Increased cyclic AMP-dependent protein kinase activity in postmortem brain from patients with bipolar affective disorder. J Neurochem. 1999;73:1704–1710. doi: 10.1046/j.1471-4159.1999.731704.x. [DOI] [PubMed] [Google Scholar]
- Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- Garver DL, Johnson C, Kanter DR. Schizophrenia and reduced cyclic AMP production: evidence for the role of receptor-linked events. Life Sci. 1982;31:1987–1992. doi: 10.1016/0024-3205(82)90037-6. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Cramer H, Beckmann H. Low CSF concentrations of cyclic GMP in schizophrenia. Br J Psychiatry. 1983;142:288–291. doi: 10.1192/bjp.142.3.288. [DOI] [PubMed] [Google Scholar]
- Ghose S, Chin R, Gallegos A, Roberts R, Coyle J, Tamminga C. Localization of NAAG-related gene expression deficits to the anterior hippocampus in schizophrenia. Schizophr Res. 2009;111:131–137. doi: 10.1016/j.schres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco D, McCabe R, Kallert T, Hansson L, Fiorillo A, Priebe S. Friends and symptom dimensions in patients with psychosis: a pooled analysis. PLoS One. 2012;7:e50119. doi: 10.1371/journal.pone.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: lessons from voles. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Hattab J, Meir D, Ebstein RP, Belmaker RH. Plasma cyclic AMP and cyclic GMP in childhood-onset psychoses. J Autism Dev Disord. 1984;14:159–164. doi: 10.1007/BF02409658. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Wang L, Wachi C, Daudi S, Csernansky J, Marlow-O’Connor M, Keedy S, Torres I. Structural pathology underlying neuroendocrine dysfunction in schizophrenia. Behav Brain Res. 2011;218:106–113. doi: 10.1016/j.bbr.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, Kelly MP, Kelley C, Graf R, Langen B, Logue S, Brennan J, Jiang L, Charych E, Egerland U, Liu F, Marquis KL, Malamas M, Hage T, Comery TA, Brandon NJ. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther. 2009;331:574–590. doi: 10.1124/jpet.109.155994. [DOI] [PubMed] [Google Scholar]
- Gregor T, Fujimoto K, Masaki N, Sawai S. The onset of collective behavior in social amoebae. Science. 2010;328:1021–1025. doi: 10.1126/science.1183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurguis GN, Turkka J, George DT, Linnoila M. Beta-adrenoreceptor coupling to GS protein in alcohol dependence, panic disorder, and patients with both conditions. Neuropsychopharmacology. 1997;16:69–76. doi: 10.1016/S0893-133X(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Blakeley JE, Antai-Otong D, Vo SP, Orsulak PJ, Petty F, Rush AJ. Adrenergic receptor function in panic disorder. II. Neutrophil beta 2 receptors: Gs protein coupling, effects of imipramine treatment and relationship to treatment outcome. J Psychiatr Res. 1999a;33:309–322. doi: 10.1016/s0022-3956(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Vo SP, Blakeley J, Orsulak PJ, Rush AJ. Characteristics of norepinephrine and clonidine displacement of [3H]yohimbine binding to platelet alpha2-adrenoreceptors in healthy volunteers. Psychiatry Res. 1999b;85:305–314. doi: 10.1016/s0165-1781(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Marwick K, McKirdy J, Sussmann J, Romaniuk L, Johnstone EC, Wan HI, McIntosh AM, Lawrie SM. Hippocampal function in schizophrenia and bipolar disorder. Psychol Med. 2010;40:761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- Hawton A, Green C, Dickens AP, Richards SH, Taylor RS, Edwards R, Greaves CJ, Campbell JL. The impact of social isolation on the health status and health-related quality of life of older people. Qual Life Res. 2011;20:57–67. doi: 10.1007/s11136-010-9717-2. [DOI] [PubMed] [Google Scholar]
- Hegde S, Capell WR, Ibrahim BA, Klett J, Patel NS, Sougiannis AT, Kelly MP. Phosphodiesterase 11A4 (PDE11A4) in hippocampus is required for the consolidation of social but not non-social memories. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.106. http://dx.doi.org/10.1038/npp.2016.1106. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Kumashiro H, Yashima Y, Kaneko M, Numata Y, Oshima N, Watanabe A. Plasma cyclic AMP level in psychiatric diseases of childhood. Folia Psychiatr Neurol Jpn. 1980;34:9–16. doi: 10.1111/j.1440-1819.1980.tb01508.x. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson PH, Finger EN, Magliaro BC, Smith SM, Converso A, Sanderson PE, Mullins D, Hyde LA, Eschle BK, Turnbull Z, Sloan H, Guzzi M, Zhang X, Wang A, Rindgen D, Mazzola R, Vivian JA, Eddins D, Uslaner JM, Bednar R, Gambone C, Le-Mair W, Marino MJ, Sachs N, Xu G, Parmentier-Batteur S. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-py ran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d] pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology. 2011;61:665–676. doi: 10.1016/j.neuropharm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Jager R, Russwurm C, Schwede F, Genieser HG, Koesling D, Russwurm M. Activation of PDE10 and PDE11 phosphodiesterases. J Biol Chem. 2012;287:1210–1219. doi: 10.1074/jbc.M111.263806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr E, Eikeseth S, Eldevik S, Aase H. Frequency and latency of social interaction in an inclusive kindergarten setting: a comparison between typical children and children with autism. Autism. 2007;11:349–363. doi: 10.1177/1362361307078134. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU, Maier W, Schild HH, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Ji L, Chauhan V, Flory MJ, Chauhan A. Brain region-specific decrease in the activity and expression of protein kinase A in the frontal cortex of regressive autism. PLoS One. 2011;6:e23751. doi: 10.1371/journal.pone.0023751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kafka MS, van Kammen DP. Alpha-Adrenergic receptor function in schizophrenia. Receptor number, cyclic adenosine monophosphate production, adenylate cyclase activity, and effect of drugs. Arch Gen Psychiatry. 1983;40:264–270. doi: 10.1001/archpsyc.1983.01790030034004. [DOI] [PubMed] [Google Scholar]
- Kafka MS, van Kammen DP, Bunney WE., Jr Reduced cyclic AMP production in the blood platelets from schizophrenic patients. Am J Psychiatry. 1979;136:685–687. doi: 10.1176/ajp.136.5.685. [DOI] [PubMed] [Google Scholar]
- Kafka MS, Kleinman JE, Karson CN, Wyatt RJ. Alpha-adrenergic receptors and cyclic AMP production in a group of schizophrenic patients. Hillside J Clin Psychiatry. 1986;8:15–24. [PubMed] [Google Scholar]
- Kaiya H. Second messenger imbalance hypothesis of schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 1992;46:33–38. doi: 10.1016/0952-3278(92)90056-o. [DOI] [PubMed] [Google Scholar]
- Kaiya H, Ofuji M, Nozaki M, Tsurumi K. Platelet prostaglandin E1 hyposensitivity in schizophrenia: decrease in cyclic AMP formation and in inhibitory effects on aggregation. Psychopharmacol Bull. 1990;26:381–384. [PubMed] [Google Scholar]
- Kang WH. Assaying the SCF platelets cyclic nucleotides of schizophrenics and analyzing its correlation with pathopschological factors. Zhonghua shen jing jing shen ke za zhi = Chin J Neurol Psychiatry. 1990;23(266–268):318. [PubMed] [Google Scholar]
- Kanof PD, Johns C, Davidson M, Siever LJ, Coccaro EF, Davis KL. Prostaglandin receptor sensitivity in psychiatric disorders. Arch Gen Psychiatry. 1986;43:987–993. doi: 10.1001/archpsyc.1986.01800100081011. [DOI] [PubMed] [Google Scholar]
- Kanof PD, Davidson M, Johns CA, Mohs RC, Davis KL. Clinical correlates of platelet prostaglandin receptor subsensitivity in schizophrenia. Am J Psychiatry. 1987;144:1556–1560. doi: 10.1176/ajp.144.12.1556. [DOI] [PubMed] [Google Scholar]
- Kanof PD, Coccaro EF, Johns CA, Davidson M, Siever LJ, Davis KL. Cyclic-AMP production by polymorphonuclear leukocytes in psychiatric disorders. Biol Psychiatry. 1989;25:413–420. doi: 10.1016/0006-3223(89)90194-7. [DOI] [PubMed] [Google Scholar]
- Kelley DJ, Bhattacharyya A, Lahvis GP, Yin JC, Malter J, Davidson RJ. The cyclic AMP phenotype of fragile X and autism. Neurosci Biobehav Rev. 2008;32:1533–1543. doi: 10.1016/j.neubiorev.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP. Putting together the pieces of phosphodiesterase distribution patterns in the brain: a jigsaw puzzle of cyclic nucleotide regulation. In: Brandon NJ, West AR, editors. Cyclic nucleotide phosphodiesterases in the central nervous system: from biology to disease. John Wiley & Sons Inc; New Jersey: 2014. [Google Scholar]
- Kelly MP. Does phosphodiesterase 11A (PDE11A) hold promise as a future therapeutic target? Curr Pharm Des. 2015;21:389–416. doi: 10.2174/1381612820666140826114941. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Cheung YF, Favilla C, Siegel SJ, Kanes SJ, Houslay MD, Abel T. Constitutive activation of the G-protein subunit Galphas within forebrain neurons causes PKA-dependent alterations in fear conditioning and cortical Arc mRNA expression. Learn Mem. 2008;15:75–83. doi: 10.1101/lm.723708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Logue SF, Brennan J, Day JP, Lakkaraju S, Jiang L, Zhong X, Tam M, Sukoff Rizzo SJ, Platt BJ, Dwyer JM, Neal S, Pulito VL, Agostino MJ, Grauer SM, Navarra RL, Kelley C, Comery TA, Murrills RJ, Houslay MD, Brandon NJ. Phosphodiesterase 11A in brain is enriched in ventral hippocampus and deletion causes psychiatric disease-related phenotypes. Proc Natl Acad Sci U S A. 2010;107:8457–8462. doi: 10.1073/pnas.1000730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Adamowicz W, Bove S, Hartman AJ, Mariga A, Pathak G, Reinhart V, Romegialli A, Kleiman RJ. Select 3′,5′-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal. 2014;26:383–397. doi: 10.1016/j.cellsig.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Kelsoe J. In: Method to predict response to treatment for psychiatric illnesses. Office UPT, editor. US20100233702 A1. The regents of the University of California; Oakland, CA, USA: 2010. p. 1. [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kondo N, Sakai M, Kuroda Y, Kiyota Y, Kitabata Y, Kurosawa M. General condition of hikikomori (prolonged social withdrawal) in Japan: psychiatric diagnosis and outcome in mental health welfare centres. Int J Soc Psychiatry. 2013;59:79–86. doi: 10.1177/0020764011423611. [DOI] [PubMed] [Google Scholar]
- Launay J, Dunbar RI. Playing with strangers: which shared traits attract us most to new people? PLoS One. 2015;10:e0129688. doi: 10.1371/journal.pone.0129688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Terranova ML, Sedowofia K, Clayton R, Manning A. A mouse model of early social interactions after prenatal drug exposure: a genetic investigation. Psychopharmacology. 1994;113:388–394. doi: 10.1007/BF02245214. [DOI] [PubMed] [Google Scholar]
- Lee JM, Kim SH, Jang DP, Ha TH, Kim JJ, Kim IY, Kwon JS, Kim SI. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. NeuroImage. 2004;22:831–840. doi: 10.1016/j.neuroimage.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Lerer B, Ebstein RP, Shestatsky M, Shemesh Z, Greenberg D. Cyclic AMP signal transduction in posttraumatic stress disorder. Am J Psychiatry. 1987;144:1324–1327. doi: 10.1176/ajp.144.10.1324. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenberg N, Harvey BH, Brand L, Wegener G, Brink CB. Chronic treatment with the phosphodiesterase type 5 inhibitors sildenafil and tadalafil display anxiolytic effects in Flinders Sensitive Line rats. Metab Brain Dis. 2012;27:337–340. doi: 10.1007/s11011-012-9284-z. [DOI] [PubMed] [Google Scholar]
- Locke J, Ishijima EH, Kasari C, London N. Loneliness, friendship quality and social networks of adolescents with high-functioning autism in an inclusive school setting. J Res Special Educ Needs. 2010;10:74–81. [Google Scholar]
- Lukas M, Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Luo HR, Wu GS, Dong C, Arcos-Burgos M, Ribeiro L, Licinio J, Wong ML. Association of PDE11A global haplotype with major depression and antidepressant drug response. Neuropsychiatr Dis Treat. 2009;5:163–170. doi: 10.2147/ndt.s4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memo M, Kleinman JE, Hanbauer I. Coupling of dopamine D1 recognition sites with adenylate cyclase in nuclei accumbens and caudatus of schizophrenics. Science. 1983;221:1304–1307. doi: 10.1126/science.6310753. [DOI] [PubMed] [Google Scholar]
- Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, Zheng Y, Diffenderfer KE, Zhang J, Soltani S, Eames T, Schafer ST, Boyer L, Marchetto MC, Nurnberger JI, Calabrese JR, Odegaard KJ, McCarthy MJ, Zandi PP, Alba M, Nievergelt CM, Pharmacogenomics of Bipolar Disorder S. Mi S, Brennand KJ, Kelsoe JR, Gage FH, Yao J. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99. doi: 10.1038/nature15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Byrne M, Hodges A, Lawrie SM, Owens DG, Johnstone EC. Schizotypal components in people at high risk of developing schizophrenia: early findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2002;180:179–184. doi: 10.1192/bjp.180.2.179. [DOI] [PubMed] [Google Scholar]
- Nesvaderani M, Matsumoto I, Sivagnanasundaram S. Anterior hippocampus in schizophrenia pathogenesis: molecular evidence from a proteome study. Aust N Z J Psychiatry. 2009;43:310–322. doi: 10.1080/00048670902721103. [DOI] [PubMed] [Google Scholar]
- Ofuji M, Kaiya H, Nozaki M, Tsurumi K. Platelet prostaglandin E1 hyposensitivity in schizophrenia: reduction of prostaglandin E1- or forskolin-stimulated cyclic AMP response in platelets. Life Sci. 1989;45:2135–2140. doi: 10.1016/0024-3205(89)90079-9. [DOI] [PubMed] [Google Scholar]
- Pathak G, Ibrahim BA, McCarthy SA, Baker K, Kelly MP. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacology. 2015;95:434–447. doi: 10.1016/j.neuropharm.2015.04.026. [DOI] [PubMed] [Google Scholar]
- Pathak G, Agostino MJ, Bishara K, Capell WR, Fisher JL, Hegde S, Ibrahim BA, Pilarzyk K, Sabin C, Tuczkewycz T, Wilson S, Kelly MP. PDE11A negatively regulates lithium responsivity. Mol Psychiatry. doi: 10.1038/mp.2016.155. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr Res. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Pompili M, Lester D, Innamorati M, Tatarelli R, Girardi P. Assessment and treatment of suicide risk in schizophrenia. Expert Rev Neurother. 2008;8:51–74. doi: 10.1586/14737175.8.1.51. [DOI] [PubMed] [Google Scholar]
- Rahman S, Li PP, Young LT, Kofman O, Kish SJ, Warsh JJ. Reduced [3H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem. 1997;68:297–304. doi: 10.1046/j.1471-4159.1997.68010297.x. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, Brunberg JA, Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. 2001;108:79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- Rametti G, Segarra N, Junque C, Bargallo N, Caldu X, Ibarretxe N, Bernardo M. Left posterior hippocampal density reduction using VBM and stereological MRI procedures in schizophrenia. Schizophr Res. 2007;96:62–71. doi: 10.1016/j.schres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Reidpath DD, Chan KY, Gifford SM, Allotey P. ‘He hath the French pox’: stigma, social value and social exclusion. Sociol Health Illn. 2005;27:468–489. doi: 10.1111/j.1467-9566.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Ebert D, Hennig J, Olbrich HM. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res. 2008;99:155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, Harkavy-Friedman J, Malaspina D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009a;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009b;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah CR, Forsberg CG, Kang JQ, Veenstra-VanderWeele J. Letting a typical mouse judge whether mouse social interactions are atypical. Autism Res. 2013;6:212–220. doi: 10.1002/aur.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar-Gold H, Gur R, Wagner S. Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PLoS One. 2013;8:e65085. doi: 10.1371/journal.pone.0065085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Jones KA, Woolfrey KM, Burgdorf J, Russell TA, Kalmbach A, Lee H, Yang C, Bradberry MM, Wokosin D, Moskal JR, Casanova MF, Waters J, Penzes P. Social, communication, and cortical structural impairments in Epac2-deficient mice. J Neurosci. 2012;32:11864–11878. doi: 10.1523/JNEUROSCI.1349-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoesz BM, Hare JF, Snow WM. Neurophysiological mechanisms underlying affiliative social behavior: insights from comparative research. Neurosci Biobehav Rev. 2013;37:123–132. doi: 10.1016/j.neubiorev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton LA, Christensen L, Blacher J. Friendship quality in adolescents with and without an intellectual disability. J Appl Res Intellect Disabil JARID. 2013;26:522–532. doi: 10.1111/jar.12051. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. REVIEW: oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM, McCann SM, Licinio J. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci U S A. 2006;103:15124–15129. doi: 10.1073/pnas.0602795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, Penzes P. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Xu X, Tian Q, Zhang J, Qian K, Wang YX, Petralia RS, Tu W, Zhu LQ, Wang JZ, Lu Y. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron. 2012;73:774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1511085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN. The neurobiology of human social behaviour: an important but neglected topic. J Psychiatry Neurosci JPN. 2008;33:391–392. [PMC free article] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Siu KP, Warsh JJ. Postmortem cerebral cortex Gs alpha-subunit levels are elevated in bipolar affective disorder. Brain Res. 1991;553:323–326. doi: 10.1016/0006-8993(91)90843-k. [DOI] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Siu KP, Kamble A, Hornykiewicz O, Warsh JJ. Cerebral cortex Gs alpha protein levels and forskolin-stimulated cyclic AMP formation are increased in bipolar affective disorder. J Neurochem. 1993;61:890–898. doi: 10.1111/j.1471-4159.1993.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Young LT, Li PP, Kamble A, Siu KP, Warsh JJ. Mononuclear leukocyte levels of G proteins in depressed patients with bipolar disorder or major depressive disorder. Am J Psychiatry. 1994;151:594–596. doi: 10.1176/ajp.151.4.594. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, Wang H, Xu J, Li L. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011;192:84–90. doi: 10.1016/j.pscychresns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Zhou X, Lindsay H, Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014;42:e91. doi: 10.1093/nar/gku310. [DOI] [PMC free article] [PubMed] [Google Scholar]