Abstract
Background
Vimentin is a member of the intermediate filament proteins and a canonical marker of the epithelial-mesenchymal transition (EMT), which is pivotal in tumorigenesis, metastasis and invasion in non-small cell lung cancer (NSCLC). The current meta-analysis aimed to investigate the associations between vimentin and prognosis and progression in NSCLC.
Methods
Databases with literature published in English, including PubMed, Web of Science, Embase, Science Direct, Wiley Online Library, Ovid, Cochrane Central Register of Controlled Trials, LILACS and Google Scholar, and the CNKI, VIP, CBM and WanFang databases in Chinese were used for the literature search. The key terms included (1) ‘vimentin’ OR ‘vim’ OR ‘vmt’ OR ‘vm’ OR ‘hel113’ OR ‘ctrct30’ and (2) ‘pulmon*’ OR ‘lung’ OR ‘alveolar’ and (3) ‘cancer’ OR ‘carcinoma’ OR ‘tumor’ OR ‘adenocarcinoma’ OR ‘squamous’ OR ‘neoplas*’ OR ‘malignan*’. The data were combined by random effect model and the H value and I2 were used to assess the heterogeneity. All the meta-analysis was conducted using Stata 12.0.
Results
Thirty-two qualified studies (4118 cases) were included in the current meta-analysis. Twelve studies with 1750 patients were included to assess the significance of vimentin in the overall survival (OS) of NSCLC; the pooled hazard ratio (HR) was 1.831 (confidence interval (CI): 1.315–2.550, P<0.001) in the univariate analysis and 1.266 (CI: 0.906–1.768, P = 0.167) in the multivariate analysis. Four studies with 988 cases were applicable to determine the significance of vimentin in the disease-free survival (DFS) of NSCLC; the pooled HR of the DFS was 1.224 (CI: 0.921–1.628, P = 0.164) in the univariate analysis and 1.254 (CI: 0.985–1.956, P = 0.067) in the multivariate analysis. Regarding the relationships between vimentin and clinicopathological factors, the pooled odds ratio (OR) with 3406 NSCLCs indicated that up-regulated vimentin was associated with smoking (OR = 1.359, CI: 1.098–1.683, P = 0.004), poor differentiation (OR = 2.133, CI: 1.664–2.735, P<0.001), an advanced TNM stage (OR = 3.275, CI: 1.987–5.397, P<0.001), vascular invasion (OR = 3.492, CI: 1.063–11.472, P = 0.039), lymph node metastasis (OR = 2.628, CI: 1.857–3.718, P<0.001), recurrence (OR = 1.631, CI: 1.052–2.528, P = 0.029) and pleural invasion (OR = 2.346, CI: 1.397–3.941, P = 0.001). There was no significant correlation between vimentin and age, gender, diameter, T stage, distant metastasis, or marginal invasion (P>0.05).
Conclusion
An overexpression of vimentin may predict the progression and an unfavorable survival of NSCLC. Vimentin may represent a helpful biomarker and a potential target for the treatment strategies of NSCLC. Additional, prospective studies with large samples are necessary to confirm the significance of vimentin in NSCLC.
Introduction
Lung cancer is considered the most frequent type of cancer and is regarded as the leading cause of cancer-related deaths worldwide [1]. In China, a similar trend is present in which the incidence and mortality of lung cancer has rapidly increased, and lung cancer currently ranks as the first type of dominating malignancies [2]. Non-small cell lung cancer (NSCLC), which comprises approximately 85%, is the predominant type of lung cancer. As a result of a deficiency in efficacious biomarkers for early diagnosis, the majority of NSCLC sufferers are diagnosed in an advanced stage [3, 4]. There is increasing evidence for therapeutic targets, such as EGFR, HER2, ALK, ROS1, BRAF, MET, VEGF, and FGFR1, which have received attention in clinical research. However, the prognosis for patients with NSCLC remains poor[5–7]. Thus, highly sensitive and specific biomarkers for the prediction of progression and prognosis are urgently demanded to improve the survival of patients with lung cancer.
Vimentin is a highly conserved intermediate filament protein with 57 KDa and is a member of the cytoskeletal proteins; it is observed in various cell types[8]. As an important marker of the EMT, vimentin is essential to the progression and prognosis of cancer through the EMT and the corresponding signaling pathways, which contribute to the tumorigenesis, metastasis, invasion and drug resistance of various cancers[9, 10]. Accumulating evidence indicates that vimentin is critical for the progression and prognosis of lung cancer [11–13]. However, according to published studies, the role of vimentin is inconsistent, and the function of vimentin remains controversial regarding whether it predicts a better or worse prognosis. Thus, the current meta-analysis aimed to determine the role of vimentin expression in the progression and prognosis of NSCLC.
Methods
Publication search
In this meta-analysis, databases with literature published in English, including PubMed, Web of Science, EMBASE, Science Direct, Wiley Online Library, Ovid, Cochrane Central Register of Controlled Trials, LILACS and Google Scholar, and the CNKI, VIP, CBM and WanFang databases in Chinese were used for the literature search. The studies qualified for the present meta-analysis were updated on October 31, 2015. The search terms were as follows: ‘vimentin’ OR ‘vim’ OR ‘vmt’ OR ‘vm’ OR ‘hel113’ OR ‘ctrct30’ and ‘pulmon*’ OR ‘lung’ OR ‘alveolar’ and ‘cancer’ OR ‘carcinoma’ OR ‘tumor’ OR ‘adenocarcinoma’ OR ‘squamous’ OR ‘neoplas*’ OR ‘malignan*’.
Selection criteria
The literature was screened according to the following criteria. The inclusion criteria for the primary studies included 1) patients with a diagnosis of primary or metastatic lung cancer confirmed by pathology, 2) the determination of vimentin protein or vimentin mRNA in the tissues of NSCLC patients using immunohistochemistry (IHC) or real-time reverse transcription-polymerase chain reaction (qRT-PCR), 3) investigation of the relation between vimentin expression and the overall survival (OS), disease free survival (DFS) or clinicopathological features (age, gender, tumor size, distant metastasis, subtype, grading, TNM stage, or lymph node metastasis),and the survival data may be directly or indirectly obtained, 4) publication in English or Chinese, and 5) when authors had several publications or reported data on the same patient population, only the most recent or largest sample study was included. The exclusion criteria for the primary studies included 1) reviews, letters, conference data, and case reports, 2) an overlap among articles or duplicate data, 3) the use of animals or cell lines, 4) insufficient data availability for the clinicopathological features, estimation of the HR or the 95% CI, 5) vimentin was combined with other markers or a positive EMT to predict the prognosis and progression in NSCLC, or 6) a poor study quality.
Data extraction
Two authors were responsible for independent data collection; when the two authors had inconsistent opinions regarding a study, a third author made the final decision. Data regarding the names of the first authors, year of publication, countries, numbers of patients, technology of detection and clinicopathological parameters, including age, gender, diameter, histology, smoking status, differentiation, T stage, TNM stages, vascular invasion, lymph node metastasis, distant metastasis, recurrence and marginal invasion, were extracted to calculate pooled ORs. Furthermore, the survival analysis type and HR with 95% CI were collected.
Quality assessment
Two authors (Zhihua Ye and Xin Zhang) independently evaluated the quality of the eligible studies using the Newcastle-Ottawa Scale (NOS)[14]. In the NOS, each study is assessed using three points: the selection of cohorts, the comparability of cohorts and the ascertainment of outcomes. Each study was able to be recoded with no more than one star of each item in the Selection and Outcomes section. Moreover, in the Comparability section, a maximum of two stars was recorded. The total stars for each study were calculated by the sum of the three points, which resulted in a maximum of nine stars. A study with more stars indicated a better quality.
Statistical analysis
All data analysis was conducted using Stata 12.0 and the metan package contributed to the combination of the data. Combined ORs and corresponding 95% CIs were used to evaluate the relationships between vimentin and the clinicopathological factors, including age, gender, tumor size, histology, smoking status, differentiation, T stage, TNM stages, vascular invasion, lymph node metastasis, distant metastasis, recurrence and marginal invasion. The prognostic significance of vimentin in the patients with lung cancer was appraised by pooled HRs with 95% CIs. The HRs and 95% CIs were determined directly from the univariate or multivariate survival analysis and indirectly from Kaplan–Meier survival curves as reported by Parmar[15]. The H value and its 95%CI was used to estimate the heterogeneity among the eligible studies[16–18]. Moreover, Q test (chi-squared test) and I2 statistic with its 95%CI were also reported[19]. Random-effects (RE) models were performed regarding of the heterogeneity since RE models are more conservative and can provide better estimates with wider confidence intervals[20, 21]. Furthermore, we conducted Begg’s test to assess the publication bias, and it was considered statistically significant with a P value less than 0.05.
Results
Study characteristics
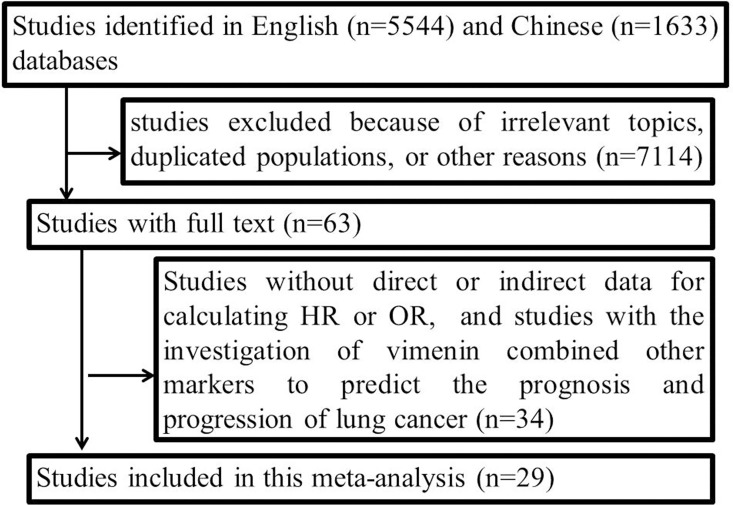
The flow chart of the literature search is shown in Fig 1. Thirty-two studies with 4118 cases were included with a closing date of October 31, 2015. Six studies (822 cases) only investigated the prognosis[11, 22–26], and eighteen studies (1946 cases) only assessed the correlation between vimentin and progression in NSCLC[13, 27–43]. Moreover, eight studies (1350 cases) provided data regarding both prognosis and progression in NSCLC[12, 44–50]. The detailed information of the studies is presented in Tables 1 and 2.
Fig 1. Flow chart of literature search.
Table 1. Characteristics of the included studies for the overall survival (OS) analysis.
| First author | Year | Country | Cancer | Stage | Follow up(M) | Case(N) | Treatment | Technique | Location of staining | Cut off | Overall Survival | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||||||||||
| HR (95% CI) | HR (95% CI) | ||||||||||||
| Che JJ | 2015 | China | SCC | I, IIIa | 70 | 103 | pulmonectomy | IHC | membrane | >0% | 2.08(1.34–3.21) | 1.918(1.107–3.324) | 8 |
| Hroyasu N | 2015 | Japan | AC | I- III | 38.1* | 101 | pulmonectomy | IHC | Cytoplasm | >median score | 2.720 (1.23–6.52) | 8 | |
| Terumasa S | 2015 | Japan | AC | Ia-IIIb | 63* | 239 | pulmonectomy | IHC | Cytoplasm | scores≥2 | 2.41(1.3–4.46) | 8 | |
| Xu MM | 2014 | China | NSCLC | I- III | 60 | 150 | pulmonectomy | IHC | Cytoplasm | >20% | 0.580(0.389–0.867) | 8 | |
| Kong FF | 2014 | China | NSCLC | I- IV | 60 | 68 | pulmonectomy | IHC | Cytoplasm | scores≥1 | 2.596(1.386–4.864) | 1.287(0.642–2.580) | 8 |
| Zhang X | 2014 | China | NSCLC | I- IIIa | 60 | 118 | pulmonectomy | IHC | Cytoplasm | score≥3 | 1.664(1.031–2.686) | 8 | |
| Guan XQ | 2014 | China | NSCLC | I-IV | 60 | 500 | pulmonectomy | IHC | Cytoplasm | score≥3 | 0.842(0.626–1.132) | 7 | |
| Chao Z | 2013 | China | NSCLC | I- IV | 39* | 119 | pulmonectomy | IHC | Cytoplasm | ≥10% | 2.21(1.21–4.039) | 1.012(0.52–1.968) | 8 |
| Zhang H | 2013 | China | SCC | I- IIIa | 51.5* | 204 | pulmonectomy | IHC | Cytoplasm | score≥4 | 1.435(1.086–1.896) | 1.17(0.877–1.561) | 8 |
| Frank R (a) | 2012 | America | NSCLC | Ia- IV | 24 | 57 | chemotherapy | IHC | Cytoplasm | ≥10% | 0.65(0.31–1.38) | 8 | |
| Frank R (b) | 2012 | America | NSCLC | Ia- IV | 24 | 38 | placebo | IHC | Cytoplasm | ≥10% | 2.32(1.09–4.94) | 8 | |
| Zhou H | 2012 | China | NSCLC | I- III | 41.5* | 52 | pulmonectomy | IHC | Cytoplasm | score≥3 | 3.45(1.66–7.11) | 7 | |
| Shi YL | 2011 | China | NSCLC | I-IV | 54 | 165 | pulmonectomy | IHC | Cytoplasm | score≥4 | 2.18(1.43–3.24) | 7 | |
| Ye T | 2009 | China | NSCLC | IIIa | 36 | 75 | pulmonectomy | IHC | Cytoplasm | score≥1 | 1.79(0.92–3.49) | 7 |
NSCLC: non-small cell lung cancer, SCC: squamous cell carcinoma, AC: adenocarcinoma, IHC: immunohistochemistry, NOS: Newcastle-Ottawwwa-Scale.
*: median follow-up time.
Table 2. Characteristics of the included studies for the disease-free survival (DFS) analysis.
| First author | Year | Country | Cancer | Stage | Follow up(M) | Case(N) | Treatment | Technique | Location of staining | Cut off | Disease-free Survival | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||||||||||
| HR (95% CI) | HR (95% CI) | ||||||||||||
| Hroyasu N | 2015 | Japan | AC | I- III | 38.1* | 101 | pulmonectomy | IHC | Cytoplasm | >median score | 1.72(1.00–2.99) | 8 | |
| Guan XQ | 2014 | China | NSCLC | I-IV | 60 | 500 | pulmonectomy | IHC | Cytoplasm | score≥3 | 1.015(0.800–1.287) | 8 | |
| Zhang H | 2013 | China | SCC | I- IIIa | 51.5* | 204 | pulmonectomy | IHC | Cytoplasm | score≥4 | 1.474(1.117–1.944) | 1.164(0.873–1.552) | 8 |
| Yasuhiro C | 2011 | Japan | NSCLC | I- III | 53.7* | 183 | pulmonectomy | IHC | NR | ≥50% | 1.326(0.634–2.770) | 1.144(0.535–2.443) | 8 |
NSCLC: non-small cell lung cancer, SCC: squamous cell carcinoma, AC: adenocarcinoma, IHC: immunohistochemistry, NOS: Newcastle-Ottawwwa-Scale.
*: median follow-up time.
Association of vimentin expression and OS
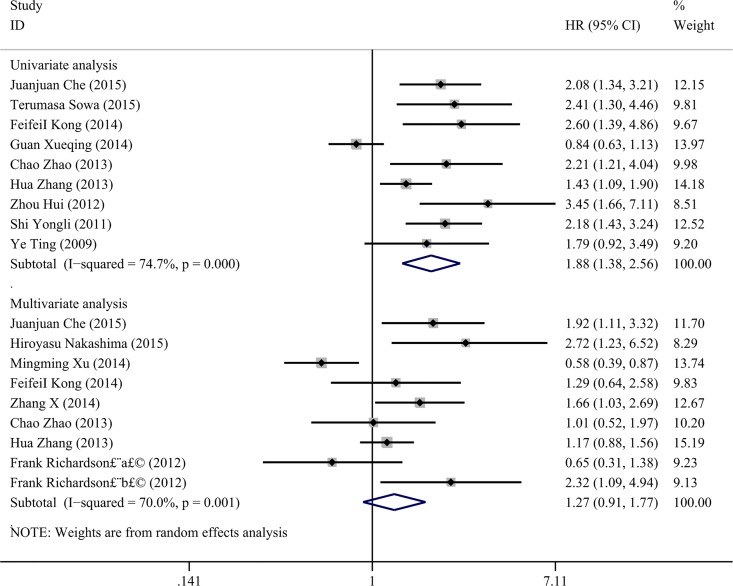
We initially focused on the relation between vimentin and OS in NSCLC; thirteen qualified studies with a total number of 1750 cases were included to estimate the significance of vimentin in the overall survival of NSCLC. Data for the HR and 95% CI for the overall OS analysis were extracted indirectly from survival curves and directly from univariate and multivariable analyses as described in the methods. The univariate analysis included nine studies with 1525 NSCLCs, and eight studies with 958 NSCLCs were included in the multivariable analysis. In the univariate analysis, a random-effect model was used with the heterogeneity test (P<0.001, I2 = 75%, 95%CI: 51%-87%, H = 2.0, 95%CI: 1.4–2.8), and the results of the pooled HR indicated that positive vimentin expression predicted an unfavorable OS (HR = 1.878, CI: 1.378–2.562, P<0.001, Fig 2). However, the pooled HR of the OS in the multivariable analysis was 1.266 with a 95% CI of 0.906 to 1.768 (P = 0.167) using the random-effect model with the heterogeneity test (P = 0.001, I2 = 70.0%, 95%CI: 40%-85%, H = 1.8, 95%CI: 1.3–2.6, Fig 2). In addition, an analysis of the subgroups was performed, and the results are shown in Table 3.
Fig 2. Forest plots of vimentin expression and OS rate in NSCLC via univariate and multivariate analyses.
Table 3. Subgroup analysis of HR in overall survival (OS) by univariate and multivariable analyses.
| Group | Study (N) | Heterogeneity test | HR (95% CI) | P | ||
|---|---|---|---|---|---|---|
| I2(95%CI) | H(95%CI) | P | ||||
| Overall survival(U) | 9 | 75%(51%-87%) | 2.0(1.4–2.8) | <0.001 | 1.878(1.378–2.562) | <0.001 |
| Country | ||||||
| China | 8 | 77%(53%-88%) | 2.1(1.5–2.9) | <0.001 | 1.831 (1.315–2.550) | <0.001 |
| Japan | 1 | - | - | - | - | |
| Sample | ||||||
| ≤200 | 6 | 0%(0%-75%) | 1.0(1.0–2.0) | 0.831 | 2.251(1.806–2.805) | <0.001 |
| >200 | 3 | 83%(49%-94%) | 2.4(1.4–4.3) | 0.002 | 1.352(0.807–2.264) | 0.252 |
| Cancer type | ||||||
| SCC | 2 | 55.7% | 0.160 | 1.724(1.060–2.802) | 0.028 | |
| AC | 1 | - | - | - | - | |
| Year | ||||||
| 2013–2015 | 6 | 80%(57%-91%) | 2.2(1.5–3.3) | <0.001 | 1.736(1.159–2.601) | 0.007 |
| 2009–2012 | 3 | 0.0%(0%-90%) | 1.0(1.0–3.1) | 0.851 | 2.229(1.638–3.034) | <0.001 |
| Overall survival(M) | 9 | 70%(40%-85%) | 2.0(1.3–2.6) | 0.001 | 1.266(0.906–1.768) | 0.167 |
| Region | ||||||
| China | 6 | 71%(31%-87%) | 1.8(1.2–2.8) | 0.005 | 1.168(0.814–1.676) | 0.4 |
| Japan and USA | 3 | 75%(18%-92%) | 2.0(1.1–3.6) | 0.018 | 1.584(0. 643–3.899) | 0.317- |
| Sample | ||||||
| ≤200 | 8 | 74%(47%-87%) | 2.0 (1.4–2.8) | <0.001 | 1.296(0.851–1.975) | 0.225 |
| >200 | 1 | - | - | - | - | |
| Cancer type | - | - | - | - | ||
| SCC | 2 | 58.9% | 0.119 | 1.414(0.883–2.265) | 0.149 | |
| AC | 1 | - | - | - | ||
| Year | ||||||
| 2013–2015 | 7 | 72%(38%-87%) | 1.9(1.3–2.8) | 0.002 | 1.273(0.886–1.830) | 0.191 |
| 2009–2012 | 2 | 81.9% | 0.019 | 1.226(0.352–4.267) | 0.748 | |
U: univariate analysis, M: multivariable analysis, SCC: squamous cell carcinoma, AC: Adenocarcinoma.
Association of vimentin expression and DFS
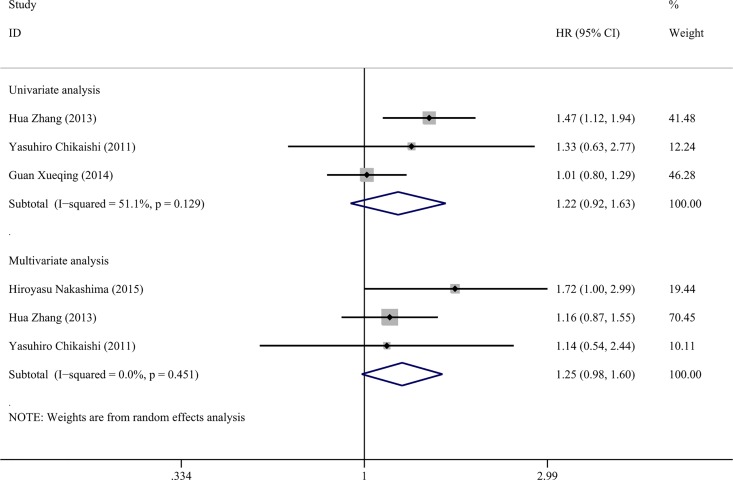
Four studies were included in the meta-analysis to assess the significance of vimentin in the DFS of NSCLC. Survival data were extracted from univariate analyses with 3 studies of 887 NSCLC cases included, as well as multivariable analyses with 3 studies of 488 NSCLC cases included. The pooled HR was 1.224 (CI: 0.921–1.628, P = 0.164, Fig 3) in the univariate analysis using the random-effect model with the heterogeneity test (P = 0.129, I2 = 51%, 95%CI: 0%-86%, H = 1.4, 95%CI: 1.0–2.7); in the multivariable analysis, the pooled HR was 1.254 (CI: 0.985–1.956, P = 0.067) via the random-effect model based on the heterogeneity test (P = 0.451, I2 = 0%, 95%CI: 0%-90%, H = 1.0, 95%CI: 1.0–3.1, Fig 3).
Fig 3. Forest plots of vimentin expression and DFS rate in NSCLC via univariate and multivariate analyses.
Correlations between vimentin and clinicopathological characteristics
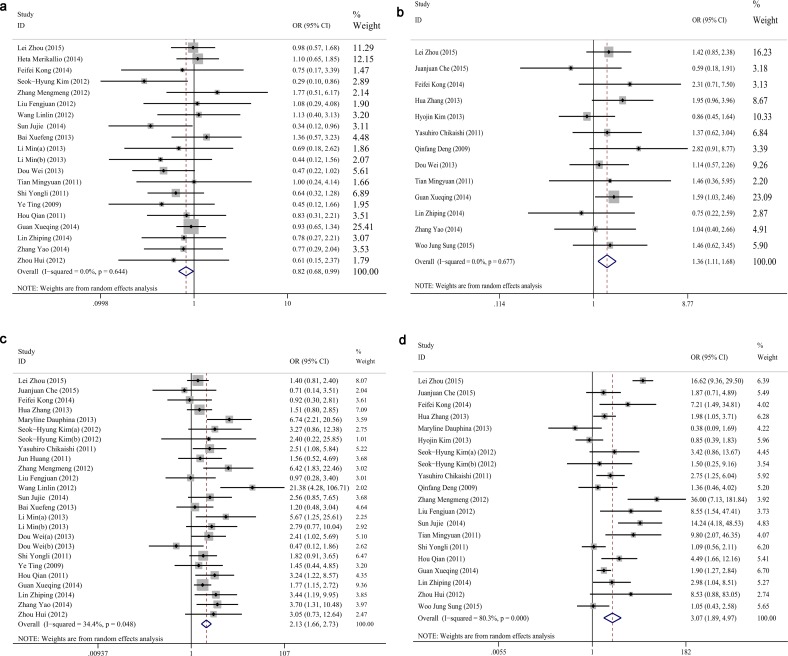
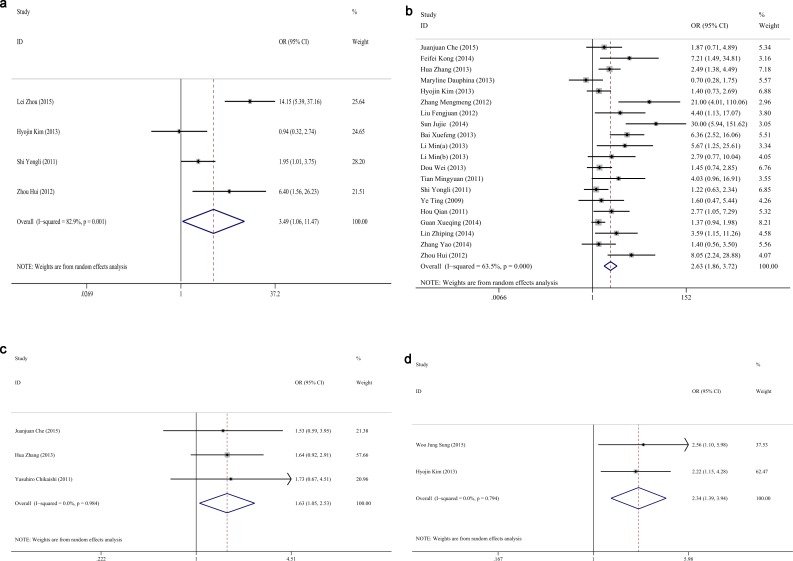
Finally, we analyzed the relationships between vimentin and the clinicopathological characteristics; twenty-six studies were analyzed with a sample size of 3296 NSCLCs. The pooled ORs of vimentin in various parameters are summarized in Table 4. The results of the pooled OR indicated that vimentin positivity was associated with the histology of adenocarcinoma (OR = 0.821, CI: 0.684–0.986, P = 0.034, Fig 4A), smoking (OR = 1.362, CI: 1.105–1.678, P = 0.003, Fig 4B), poor differentiation (OR = 2.133, CI: 1.664–2.735, P<0.001, Fig 4C), advanced TNM stage (OR = 3.275, CI: 1.987–5.397, P<0.001, Fig 4D), vascular invasion (OR = 3.492, CI: 1.063–11.472, P = 0.039, Fig 5A), lymph node metastasis (OR = 2.628, CI: 1.857–3.718, P<0.001, Fig 5B), recurrence (OR = 1.632, CI: 1.054–2.529, P = 0.028, Fig 5C) and pleural invasion (OR = 2.344, CI: 1.395–3.393, P = 0.001, Fig 5D). There was no significant correlation between vimentin and age, gender, diameter, T stage, distant metastasis, or marginal invasion (P>0.05).
Table 4. Analysis of relationships between vimentin and clinicopathological variables in NSCLC.
| Clinicalpathological Variable | No. of Studies | model | Cases (N) | Pooled OR | 95% CI | P value | Test for Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| P value | I2(95%CI) | H(95%CI) | ||||||||
| Age(≥ 60/<60) | 24 | Random effect | 2810 | 0.927 | 0.789 | 1.088 | 0.353 | 0.799 | 0%(0%-45%) | 1.0(1.0–1.3) |
| Gender(male/female) | 25 | Random effect | 3105 | 1.122 | 0.944 | 1.332 | 0.192 | 0.473 | 0%(0%-44%) | 1.0(1.0–1.3) |
| Diameter(>3.0cm/≤3.0 cm) | 11 | Random effect | 1166 | 1.317 | 0.919 | 1.888 | 0.133 | 0.123 | 34%(0%-68%) | 1.2(1.0–1.8) |
| Histology(SCC/AC) | 20 | Random effect | 2259 | 0.821 | 0.684 | 0.986 | 0.034 | 0.644 | 0%(0%-48%) | 1.0(1.0–1.4) |
| Smoking status(yes/no) | 12 | Random effect | 2050 | 1.362 | 1.105 | 1.678 | 0.004 | 0.677 | 0%(0%-57%) | 1.0(1.0–1.5) |
| Differentiation(poorly/moderate or well) | 25 | Random effect | 2856 | 2.133 | 1.664 | 2.735 | <0.001 | 0.048 | 34%(0%-60%) | 1.2(1.0–1.6) |
| T stage(T2 or T3/T1) | 6 | Random effect | 957 | 1.044 | 0.704 | 1.548 | 0.830 | 0.498 | 0%(0%-75%) | 1.0(1.0–2.0) |
| TNM stages(III or IV/I or II) | 20 | Random effect | 2661 | 3.069 | 1.894 | 4.974 | <0.001 | <0.001 | 80%(70%-87%) | 2.3(1.8–2.8) |
| Vascular invasion(yes/no) | 4 | Random effect | 722 | 3.492 | 1.063 | 11.472 | 0.039 | 0.001 | 83%(56%-93%) | 2.4(1.5–3.9) |
| Lymph node metastasis(yes/no) | 20 | Random effect | 2395 | 2.628 | 1.857 | 3.718 | <0.001 | <0.001 | 64%(41%-77%) | 1.7(1.3–2.1) |
| Recurrence(yes/no) | 3 | Random effect | 490 | 1.632 | 1.054 | 2.529 | 0.028 | 0.984 | 0%(0%-490%) | 1.0(1.0–3.1) |
| Distant metastasis | 2 | Random effect | 248 | 3.975 | 0.724 | 21.807 | 0.112 | 0.069 | 70% (-) | (-) |
| Marginal invasion | 2 | Random effect | 477 | 2.429 | 0.789 | 7.473 | 0.122 | 0.025 | 80% (-) | (-) |
| Pleural invasion | 2 | Random effect | 290 | 2.344 | 1.395 | 3.393 | 0.001 | 0.794 | 0% (-) | (-) |
SCC: squamous cell carcinoma, AC: Adenocarcinoma.
Fig 4. Forest plots of ORs for associations between vimentin and clinicopathological characteristics in NSCLC.
(a) Histology, (b) Smoking status, (c) Differentiation, and (d) TNM stages.
Fig 5. Forest plots of ORs for associations between vimentin and clinicopathological characteristics in NSCLC.
(a) Vascular invasion, (b) Lymph node metastasis, (c) Recurrence, and (d) Pleural invasion.
Publication bias and sensitivity analysis
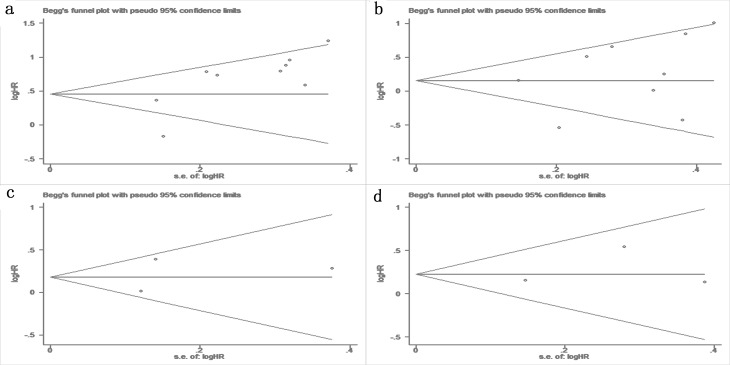
Begg’s test was used to examine the publication bias of the eligible studies, and no obvious publication bias was identified in the OS (P = 0.175 in univariate analysis, Fig 6A, P = 0.251 in multivariable analysis, Fig 6B) or the DFS (P>0.99 in both univariate and multivariable analyses, Fig 6C and 6D). Similar results of Egger’s test were identified in the majority of the clinicopathological characteristics; a significant publication bias was only identified in lymph node metastasis (P<0.001, data not shown).
Fig 6. Funnel plots for publication bias.
(a) OS in univariate analysis, (b) OS in multivariate analysis, (c) DFS in univariate analysis, and (d) DFS in multivariate analysis.
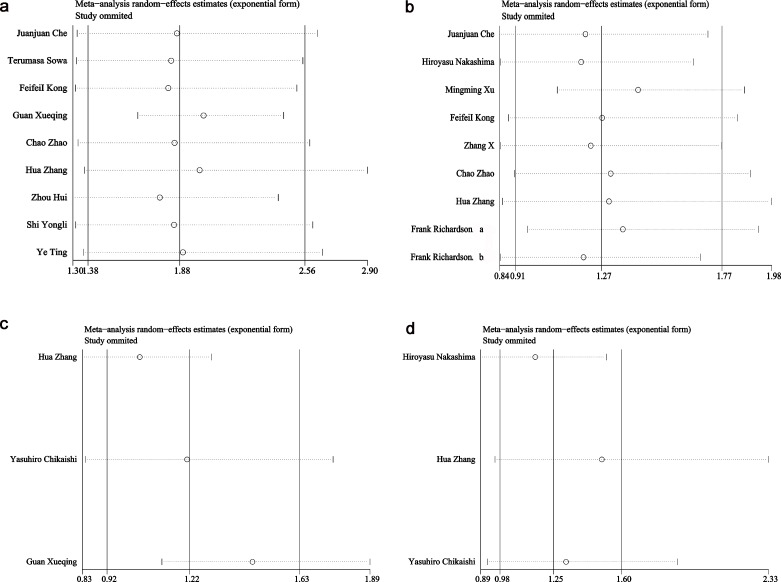
The results of the sensitivity analysis are shown in Fig 7. No significant changes were identified in the pooled results when a study was removed, which indicated that the results of this meta-analysis were reliable.
Fig 7. Sensitivity analyses of included studies.
(a) OS in univariate analysis, (b) OS in multivariate analysis, (c) DFS in univariate analysis, and (d) DFS in multivariate analysis.
Discussion
Metastasis, invasion and drug resistance lead to the failure of cancer treatments in patients with NSCLC. Thus, poor survival has remained an issue despite technological advances in treatments. Several biomarkers have been investigated to predict the progression and prognosis; however, stable and reliable markers have not been identified. Therefore, there is a critical demand to investigate novel biomarkers with high sensitivity and specificity with the goal to improve the survival of NSCLC.
Vimentin is an important component of the intermediate filament family and plays a vital role in the anchorage of organelles and cellular integrity[8]. Furthermore, vimentin,as a mesenchymal phenotype, is a sign of the epithelial-to-mesenchymal transition (EMT), which is essential to carcinogenesis[51]. Recently, increasing evidence has demonstrated that vimentin exerted a substantial influence on the progression and prognosis of cancer. In NSCLC, vimentin was involved in various aspects of cancer, including metastasis, drug resistance, EMT and recurrence. Moreover, the prognostic significance of vimentin has been investigated. However, the results among previous studies have been inconsistent.
The conclusion of several published studies indicated that vimentin was significant in predicting the prognosis and progression of NSCLC. However, inconsistent results have also been demonstrated in published studies. Therefore, a cumulative and combined analysis of these studies was necessary to determine the value of vimentin in the prognosis and progression of NSCLC. The current study comprises the first meta-analysis to assess the value of vimentin in predicting the progression and prognosis of NSCLC. In the univariate analysis of the OS, the pooled HR indicated that positive vimentin expression predicted a shorter OS. Nevertheless, in the multivariate analysis, in which the analysis removes confounding factors, the pooled HR of the OS failed to indicate a significance of vimentin in the prognosis of NSCLC. In this meta-analysis, univariate analysis and multivariate analysis showed inconsistent trend of prognostic values of vimentin expression in NSCLC. The conflict between the two analyses may depend on the less number and samples. Prospective studies with large sample sizes are required to confirm the value of vimentin in the prediction of prognosis and progression in NSCLC. Moreover, the pooled HR of the DFS suggested that vimentin was not an independent prognostic factor in the univariate or multivariate analyses. The current meta-analysis results also indicated that up-regulated vimentin predicted poor differentiation of cancer cells, advanced TNM stages, vascular invasion, lymph node metastasis and recurrence; moreover, in NSCLC with smoking or squamous cell carcinoma, vimentin expression was also up-regulated compared with cases with no smoking or adenocarcinoma. Another interesting finding of this paper that the overexpression of vimentin was no significant depended the age, gender, diameter, T stage, distant metastasis, or marginal invasion! But literature reviews suggest that overexpression of EMT-TFs factors (e.g.vimentin) have a significate correlated to another cancer, like MBC, liver cancer. The cause may be that although EMT-TFs factors (e.g.vimentin) have been demonstrated to be vital in the carcinogenesis of various cancers, the aberrant expression of vimentin and its mechanisms may be different in different cancers because of the heterogeneity of cancers. Thus, the associations between vimentin and clinicopathological characteristics in NSCLC may be inconsistent to other cancers, like MBC, liver cancer. Therefore, vimentin was closely related to the progression of NSCLC and predicted the progression and prognosis of NSCLC. However, prospective studies with large sample sizes are needed to confirm the significance of vimentin in NSCLC.
Regarding the relationships between vimentin and the progression and prognosis of NSCLC, evidence regarding the molecular mechanism supported the results of this meta-analysis. He et al reported that vimentin overexpression and the EMT were induced by metadherin (MTDH) via the Wnt/β-catenin pathway in lung cancer, which is essential to the invasion and metastasis of cancer progression and may support the results of this meta-analysis that vimentin was associated with vascular invasion and lymph node metastasis in NSCLC[52]. In addition, miRNA-214 up-regulated vimentin expression and promoted the EMT, which resulted in the metastasis of lung adenocarcinoma via suppressor-of-fused protein, and the decreased expression of miRNA-30c may induce the EMT and result in the invasion of NSCLC [53, 54]. Moreover, Wang et al demonstrated that 51 altered miRNAs were identified after the EMT was induced by transforming growth factor beta-1 in lung cancer in vitro, which indicated the potentially critical role of miRNAs in the regulation of vimentin and the EMT in lung cancer [55]. Cancer stem cells, which comprise a small proportion of tumor cells, are undifferentiated and responsible for tumor spreading. Recent studies have demonstrated that several markers of cancer stem cells in lung cancer, such as CD44, CD133 and ALDH1, are closely correlated with vimentin expression and the EMT in lung cancer[26, 56]. Zhao et al. demonstrated that increased vimentin expression and the EMT were induced by cigarette smoke extracts, which may provide an explanation for the high expression in NSCLC patients who smoke [57]. Thus, vimentin contributed to the progression of NSCLC through the EMT.
Four studies did not provide sufficient data to calculate the HR and CI of vimentin in the prediction of prognosis in NSCLC [29, 58–60]. These studies all indicated that vimentin has no significant value in the prediction of OS in lung cancer (all P>0.05). Furthermore, in the studies of Hyojin et al, the results also suggested vimentin did not correlate with DFS in lung adenocarcinoma, and Alex et al. reported that vimentin did not predict progression-free survival (PFS)[58]. The negative results, which were not combined, may influence the pooled results, and the limitation of this meta-analysis will be discussed in the limitation section.
In the past decades, evidence-based medicine has become increasingly valued. A meta-analysis, which combines all the existing evidence and accumulates the individual sample data, can obtain a more stable result and drive a more convincing conclusion, as compared to individual study with small sample size. In this present meta-analysis, we demonstrated that the overexpression of vimentin predicted a poor overall survival of NSCLC in the univariate analysis and up-regulated vimentin expression was associated with several clinicopathological factors, including histology of squamous cell carcinoma, smoking, poor differentiation, advanced TNM stages, vascular invasion, lymph node metastasis and recurrence in NSCLC. By accumulating the individual studies, the results of this meta-analysis were more convincing and could provide more powerful evidence for NSCLC treatment in clinical practice. The current study is the first investigation to focus on the correlations between vimentin and the prognosis and clinicopathological characteristics of lung cancer using a meta-analysis, and the results of this analysis are meaningful. Nevertheless, there were several limitations that must be considered. 1) One of the major limitations of this meta-analysis was that the publication bias tests were underpowered since the studies<10, and it may cause the combined results unstably and affect the conclusion[61]. 2) The studies that did not provide direct data or data that could be used to calculate the HR or were excluded, which may result in an inaccurate pooled HR and OR. 3) Moreover, only studies published in English or Chinese were included, which may result in a potential publication bias. 4) The studies did not assess completely equal cancer types and clinical stages of NSCLC. 5) Finally, there were no identical standards to define positive vimentin expression, which may contribute to the heterogeneity among the studies and thus attenuate the reliability of the pooled results.
Conclusions
The present meta-analysis demonstrates that the overexpression of vimentin predicts a poor overall survival of NSCLC in the univariate analysis but not the multivariate analysis. Vimentin has minimal value in predicting disease-free survival in NSCLC. However, up-regulated vimentin expression was associated with several clinicopathological factors, including histology of squamous cell carcinoma, smoking, poor differentiation, advanced TNM stages, vascular invasion, lymph node metastasis and recurrence in NSCLC. Overall, Vimentin is essential to progression in NSCLC, and vimentin expression in the tissues of patients with NSCLC predicts progression and prognosis. Nevertheless, additional, prospective studies with large sample sizes are required to confirm the value of vimentin in the prediction of prognosis and progression in NSCLC.
Supporting Information
(PPTX)
(DOCX)
(DOC)
(DOC)
Acknowledgments
The study was supported by the Fund of Guangxi Provincial Health Bureau Scientific Research Project (Z2013201, Z2014055), Natural Science Foundation of Guangxi, China (2015GXNSFCA139009) and the Fund of National Natural Science Foundation of China (NSFC 81360327, NSFC 81560469). American Journal Experts (AJE) provided professional scientific editing service on this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the Fund of Guangxi Provincial Health Bureau Scientific Research Project (Z2013201, Z2014055), Natural Science Foundation of Guangxi, China (2015GXNSFCA139009) and the Fund of National Natural Science Foundation of China (NSFC 81360327, NSFC 81560469). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. 10.3322/caac.21254 . [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual report on status of cancer in China, 2010. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2014;26(1):48–58. 10.3978/j.issn.1000-9604.2014.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brothers JF, Hijazi K, Mascaux C, El-Zein RA, Spitz MR, Spira A. Bridging the clinical gaps: genetic, epigenetic and transcriptomic biomarkers for the early detection of lung cancer in the post- National Lung Screening Trial era. BMC medicine. 2013;11:168 10.1186/1741-7015-11-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC cancer. 2013;13:158 10.1186/1471-2407-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–71. 10.3322/caac.21235 . [DOI] [PubMed] [Google Scholar]
- 6.Kumarakulasinghe NB, van Zanwijk N, Soo RA . Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology. 2015;20(3):370–8. 10.1111/resp.12490 . [DOI] [PubMed] [Google Scholar]
- 7.Parums DV. Current status of targeted therapy in non-small cell lung cancer. Drugs of today. 2014;50(7):503–25. 10.1358/dot.2014.50.7.2185913 . [DOI] [PubMed] [Google Scholar]
- 8.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and molecular life sciences: CMLS. 2011;68(18):3033–46. 10.1007/s00018-011-0735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6 10.1186/s40169-015-0048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology. 2012;17(7):1048–59. 10.1111/j.1440-1843.2012.02173.x . [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, Jiang SX, Sato Y, Hoshi K, Matsumoto T, Nagashio R, et al. Prevalent and up-regulated vimentin expression in micropapillary components of lung adenocarcinomas and its adverse prognostic significance. Pathol Int. 2015;65(4):183–92. 10.1111/pin.12257 . [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Liu J, Yue D, Gao L, Wang D, Zhang H, et al. Clinical significance of E-cadherin, beta-catenin, vimentin and S100A4 expression in completely resected squamous cell lung carcinoma. J Clin Pathol. 2013;66(11):937–45. 10.1136/jclinpath-2013-201467 . [DOI] [PubMed] [Google Scholar]
- 13.Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81(1):117–22. 10.1016/j.lungcan.2013.03.011 . [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 15.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815–34. . [DOI] [PubMed] [Google Scholar]
- 16.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Statistics in medicine. 1998;17(8):841–56. . [DOI] [PubMed] [Google Scholar]
- 17.Mittlbock M, Heinzl H. A simulation study comparing properties of heterogeneity measures in meta-analyses. Statistics in medicine. 2006;25(24):4321–33. 10.1002/sim.2692 . [DOI] [PubMed] [Google Scholar]
- 18.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PloS one. 2013;8(7):e69930 10.1371/journal.pone.0069930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. Bmj. 2007;335(7626):914–6. 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Statistics in medicine. 2001;20(6):825–40. 10.1002/sim.650 . [DOI] [PubMed] [Google Scholar]
- 21.Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: A simulation study. Statistical methods in medical research. 2012;21(4):409–26. 10.1177/0962280210392008 . [DOI] [PubMed] [Google Scholar]
- 22.Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu YF, et al. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pacific journal of cancer prevention: APJCP. 2014;15(9):4013–8. . [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, qin Q, Wang Q, Zhang J, Xu Y, Li W, et al. SCUBE3 overexpression predicts poor prognosis in non-small cell lung cancer. Bioscience trends. 2013;7(6):264–9. . [PubMed] [Google Scholar]
- 24.Zhang X, Wang Z, Kang Y, Li X, Ma X, Ma L. MCAM expression is associated with poor prognosis in non-small cell lung cancer. Clinical and Translational Oncology. 2014;16(2):178–83. 10.1007/s12094-013-1057-6 [DOI] [PubMed] [Google Scholar]
- 25.Richardson F, Young GD, Sennello R, Wolf J, Argast GM, Mercado P, et al. The evaluation of E-Cadherin and vimentin as biomarkers of clinical outcomes among patients with non-small cell lung cancer treated with erlotinib as second- or third-line therapy. Anticancer Res. 2012;32(2):537–52. . [PubMed] [Google Scholar]
- 26.Sowa T, Menju T, Sonobe M, Nakanishi T, Shikuma K, Imamura N, et al. Association between epithelial-mesenchymal transition and cancer stemness and their effect on the prognosis of lung adenocarcinoma. Cancer medicine. 2015. 10.1002/cam4.556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Yu L, Wu S, Feng Z, Song W, Gong X. Clinicopathological significance of KAI1 expression and epithelial-mesenchymal transition in non-small cell lung cancer. World J Surg Oncol. 2015;13:234 10.1186/s12957-015-0657-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merikallio H, T TT, Paakko P, Makitaro R, Kaarteenaho R, Lehtonen S, et al. Slug is associated with poor survival in squamous cell carcinoma of the lung. International journal of clinical and experimental pathology. 2014;7(9):5846–54. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Yoo SB, Sun P, Jin Y, Jheon S, Lee CT, et al. Alteration of the E-Cadherin/beta-Catenin Complex Is an Independent Poor Prognostic Factor in Lung Adenocarcinoma. Korean journal of pathology. 2013;47(1):44–51. 10.4132/KoreanJPathol.2013.47.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Kim JM, Shin MH, Kim CW, Huang SM, Kang DW, et al. Correlation of epithelial-mesenchymal transition markers with clinicopathologic parameters in adenocarcinomas and squamous cell carcinoma of the lung. Histology and histopathology. 2012;27(5):581–91. . [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Qiu Y, Chen G, Huang L, He J. The relationship between Bmi-1 and the epithelial-mesenchymal transition in lung squamous cell carcinoma. Med Oncol. 2012;29(3):1606–13. 10.1007/s12032-011-9998-5 . [DOI] [PubMed] [Google Scholar]
- 32.Deng QF, Zhou CC, Su CX. Clinicopathological features and epidermal growth factor receptor mutations associated with epithelial-mesenchymal transition in non-small cell lung cancer. Respirology. 2009;14(3):371–6. . [DOI] [PubMed] [Google Scholar]
- 33.Zhang M. The influence of Bmi-1 on the epithelial-mesenchymal transition in non-small cell lung cancer. 2012.
- 34.Liu F . Relationship of Egfl7 and Epithelial-Mesenchymal Transition in Patients with Non-small Lung Cancer. Progress in Modern Biomedicine. 2012;12(6):1110–4. [Google Scholar]
- 35.Wang L. The relationship betwwen the expression of EMT and 14-3-3 and the lung cancer. 2012.
- 36.Sun J. Expression of Twist in non-small cell cancer and its role in epithelial -mesenchymal transition. Chin J Cancer Prev Treat. 2014;21(16):1258–61. [Google Scholar]
- 37.Bai X. The study of EMT transition in non-small cell lung cancer. Journal Of Inner Mongolia Medical University. 2013;35(S1):1–6. [Google Scholar]
- 38.Li M. Correlation between EMT and chemoresistance in non-small cell lung cancer. Med Qilu. 2013;28(2):117–20. [Google Scholar]
- 39.Dou W. Clinical significance of epithelial-mesenchymal transition-related protein expressions in the carcinoma cells of non-small cell lung cancer 2013.
- 40.Tian M. Expressions of E-cadherin and Vimentin in Lung Cancer Tissue and Their Relationship to Epithelial-Mesenchymal Transition. Chin J Biologicals. 2011;24(9). [Google Scholar]
- 41.Hou Q. Preliminary study on the relationship between non-small cell lung cancer epithelial mesenchymal transition metastasis and its influencing factors. 2011.
- 42.Zhang Y. Correlation of MDM2 and epithelial-mesenchymal transition related biomarkers in non-small cell lung cancer. Jiangsu Med J. 2014;40(16):1915–7. [Google Scholar]
- 43.Lin. Correlation between GLI1 and epithelial mesenchymal transition (EMT) in non-small cell lung cancer. 2012.
- 44.Che J, Yang Y, Xiao J, Zhao P, Yan B, Dong S, et al. Decreased expression of claudin-3 is associated with a poor prognosis and EMT in completely resected squamous cell lung carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(8):6559–68. 10.1007/s13277-015-3350-1 . [DOI] [PubMed] [Google Scholar]
- 45.Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M, Guo YH, et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 2014;31(6):2660–8. 10.3892/or.2014.3129 . [DOI] [PubMed] [Google Scholar]
- 46.Guan X. Clinical significance of the expression of CD44 + / CD24 combined with E, Vimentin Cadherin in non-small cell lung cancer. Journal of Practical Oncology. 2014;28(4):5. [Google Scholar]
- 47.Zhou H. Correlation of hypoxia—inducible factor-2a expressionand angiogenesis, lymphangiogenesis and epithelial-mesenchymal transition in non-small cell lung cancer. 2012.
- 48.Shi Y. Expression and prognostic significance of epithelial-mesenchymal transition-related proteins in non-small cell lung cancer. 2011.
- 49.Ye T. The PrognosticSignifieance of EPithelialand Mesenchymal Proteinsin Resected StageIlla Non-Small CellLung CarCinoms. 2009.
- 50.Chikaishi Y, Uramoto H, Tanaka F. The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res. 2011;31(12):4451–6. . [PubMed] [Google Scholar]
- 51.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. American journal of respiratory cell and molecular biology. 2014;50(1):1–6. 10.1165/rcmb.2013-0314TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He W, He S, Wang Z, Shen H, Fang W, Zhang Y, et al. Astrocyte elevated gene-1(AEG-1) induces epithelial-mesenchymal transition in lung cancer through activating Wnt/beta-catenin signaling. BMC cancer. 2015;15:107 10.1186/s12885-015-1124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long H, Wang Z, Chen J, Xiang T, Li Q, Diao X, et al. microRNA-214 promotes epithelial-mesenchymal transition and metastasis in lung adenocarcinoma by targeting the suppressor-of-fused protein (Sufu). Oncotarget. 2015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Z, Xia Y, Wang P, Liu B, Chen Y. Low expression of microRNA-30c promotes invasion by inducing epithelial mesenchymal transition in non-small cell lung cancer. Molecular medicine reports. 2014;10(5):2575–9. 10.3892/mmr.2014.2494 . [DOI] [PubMed] [Google Scholar]
- 55.Wang HB, Zhang HJ, Su JM, Zhou WY, Wang HY, Chen XF. [Epithelial-mesenchymal transition (EMT) and its effect on microRNA expression in lung cancer]. Zhonghua zhong liu za zhi [Chinese journal of oncology]. 2011;33(8):590–3. . [PubMed] [Google Scholar]
- 56.Sung WJ, Park KS, Kwak SG, Hyun DS, Jang JS, Park KK. Epithelial-mesenchymal transition in patients of pulmonary adenocarcinoma: correlation with cancer stem cell markers and prognosis. International journal of clinical and experimental pathology. 2015;8(8):8997–9009. . [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y, Xu Y, Li Y, Xu W, Luo F, Wang B, et al. NF-kappaB-mediated inflammation leading to EMT via miR-200c is involved in cell transformation induced by cigarette smoke extract. Toxicological sciences: an official journal of the Society of Toxicology. 2013;135(2):265–76. 10.1093/toxsci/kft150 . [DOI] [PubMed] [Google Scholar]
- 58.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J . FoxQ1 overexpression influences poor prognosis in non-small cell lung cancer, associates with the phenomenon of EMT. PLoS One. 2012;7(6):e39937 10.1371/journal.pone.0039937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, et al. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(22):7430–7. 10.1158/1078-0432.CCR-08-0935 . [DOI] [PubMed] [Google Scholar]
- 60.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Canadian journal of surgery Journal canadien de chirurgie. 2001;44(3):180–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53(11):1119–29. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
(DOCX)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.