Abstract
Objective:
To evaluate the sensitivity of quantitative EEG (QEEG) for electrographic seizure identification in the intensive care unit (ICU).
Methods:
Six-hour EEG epochs chosen from 15 patients underwent transformation into QEEG displays. Each epoch was reviewed in 3 formats: raw EEG, QEEG + raw, and QEEG-only. Epochs were also analyzed by a proprietary seizure detection algorithm. Nine neurophysiologists reviewed raw EEGs to identify seizures to serve as the gold standard. Nine other neurophysiologists with experience in QEEG evaluated the epochs in QEEG formats, with and without concomitant raw EEG. Sensitivity and false-positive rates (FPRs) for seizure identification were calculated and median review time assessed.
Results:
Mean sensitivity for seizure identification ranged from 51% to 67% for QEEG-only and 63%–68% for QEEG + raw. FPRs averaged 1/h for QEEG-only and 0.5/h for QEEG + raw. Mean sensitivity of seizure probability software was 26.2%–26.7%, with FPR of 0.07/h. Epochs with the highest sensitivities contained frequent, intermittent seizures. Lower sensitivities were seen with slow-frequency, low-amplitude seizures and epochs with rhythmic or periodic patterns. Median review times were shorter for QEEG (6 minutes) and QEEG + raw analysis (14.5 minutes) vs raw EEG (19 minutes; p = 0.00003).
Conclusions:
A panel of QEEG trends can be used by experts to shorten EEG review time for seizure identification with reasonable sensitivity and low FPRs. The prevalence of false detections confirms that raw EEG review must be used in conjunction with QEEG. Studies are needed to identify optimal QEEG trend configurations and the utility of QEEG as a screening tool for non-EEG personnel.
Classification of evidence review:
This study provides Class II evidence that QEEG + raw interpreted by experts identifies seizures in patients in the ICU with a sensitivity of 63%–68% and FPR of 0.5 seizures per hour.
Electrographic seizures occur in 8%–48% of critically ill patients1–8 and can be found in any critical care setting.3 Minimizing delay to diagnosis of nonconvulsive status epilepticus is critical as therapeutic interventions are most effective when initiated early.9,10
Increasing awareness of electrographic seizures has led to a growing demand for continuous EEG (cEEG) monitoring, but this is a labor and time-intensive process. To facilitate interpretation of prolonged EEG recordings, several quantitative EEG (QEEG) tools have been developed. QEEG is the visual representation of statistically transformed raw EEG signals. The most commonly used QEEG tool is compressed spectral array (CSA), which consists of a color display representing power in various frequency bands. Other QEEG techniques display EEG data based on amplitude (amplitude-integrated EEG [aEEG]; envelope trend), rhythmicity (rhythmicity spectrogram), or spectral symmetry (asymmetry index and spectrogram). These tools are used to highlight significant electrographic events on cEEG and identify subtle EEG changes over prolonged periods of time. There have been few studies assessing the sensitivity of QEEG for seizure identification and most are single-center, pediatric studies,11–21 or focused on the utility of single trends, such as aEEG22 or CSA.23 A systematic assessment of the accuracy of a panel of QEEG trends used in daily clinical practice is lacking.
METHODS
This study evaluated the sensitivity of a panel of commonly applied QEEG techniques, with and without the ability to see the corresponding raw EEG, for identification of seizures in critically ill patients when used by experts from multiple institutions.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review board at Emory University and was granted a waiver of informed consent.
cEEG recordings.
Using a clinical database, we identified cEEG recordings performed in patients admitted to the intensive care unit (ICU) between 2008 and 2010 for any of the following indications: treatment of refractory status epilepticus, suspicion of seizures, or management of intracranial pressure. Six-hour EEG epochs from 15 patients with and without seizures were selected by one of the authors (H.A.H.) to represent a variety of EEG findings commonly encountered in the critical care setting such as electrographic seizures, rhythmic delta activity, and periodic discharges. Digital EEG recordings were obtained using commercially available CT/MRI compatible electrodes that were placed according to the International 10–20 system.
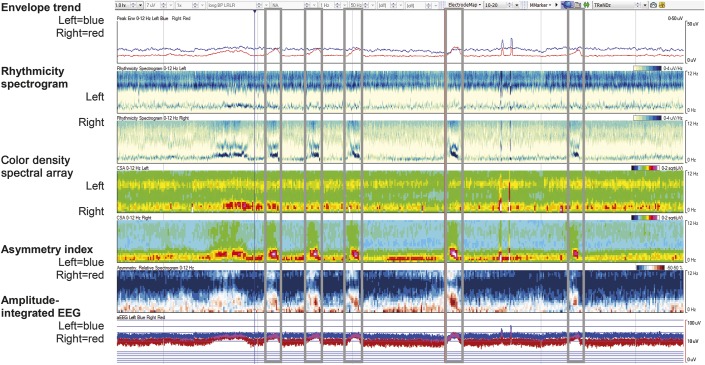
All 15 EEG recordings (standardized 16-channels displayed, longitudinal bipolar montage, sampling rate 500 Hz) were analyzed with QEEG tools available in the Insight II EEG review software version 11 (Persyst Inc., Prescott, AZ). Specific QEEG tools included in the analysis for review were seizure probability, envelope trend, CSA, rhythmicity spectrogram, asymmetry spectrogram, and aEEG (figure 1; table e-1 at Neurology.org). One hour of QEEG data was displayed per screen on a 24-inch high-resolution (1,600 × 1,200 pixels) monitor, such that 4.4 horizontal pixels represented 10 seconds of raw EEG data.
Figure 1. Example of a 1-hour quantitative EEG (QEEG) panel without automated seizure detection (SzD) as viewed by the QEEG and QEEG + raw reviewers.
All QEEG analyses are displayed as hemispheric averages with blue representing the left hemisphere and red representing the right hemisphere. Frequency scale ranges from 0 to 12 Hz. This recording contained 5 electrographic seizures (see gray boxes).
cEEG review.
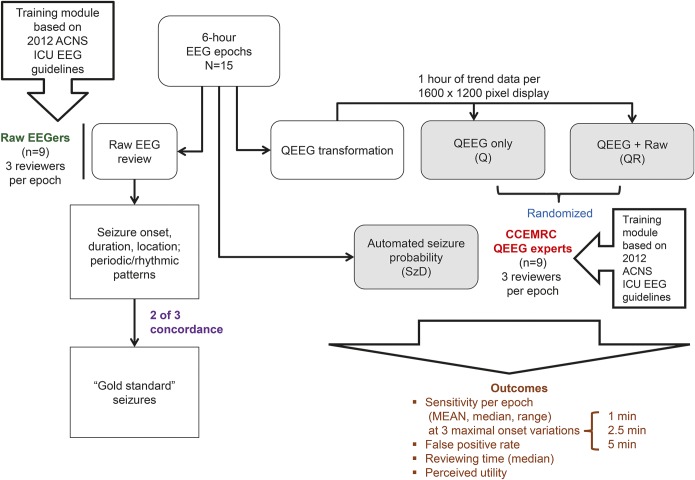
Each EEG epoch was reviewed in 3 formats: raw EEG without QEEG (R), QEEG with raw EEG (QR), and QEEG alone (Q) (figure 2). All 9 raw EEG reviewers were board-eligible or board-certified epileptologists selected from members of the Critical Care EEG Monitoring Research Consortium (CCEMRC). Each completed a training module24 based on the 2012 version of the American Clinical Neurophysiology Society (ACNS) standardized ICU EEG nomenclature25 with the aim of improving agreement on use of the following terms: seizures, evolution, periodic discharges, and rhythmic delta activity. Based on published criteria,25–28 electrographic seizures were defined as a paroxysmal change in EEG background lasting longer than 10 seconds with evolution in morphology, frequency, or spatial distribution.
Figure 2. Study methodology: EEG formatting and review algorithm.
ACNS = American Clinical Neurophysiology Society; CCEMRC = Critical Care EEG Monitoring Research Consortium; ICU = intensive care unit; QEEG = quantitative EEG; SzD = seizure detection algorithm.
Each R epoch was reviewed by 3 raw EEG reviewers who were asked to mark the onset and offset of all seizures as well as the maximal extent of seizure propagation: generalized (>8 channels), hemispheric (5–8 channels), or focal (≤4 channels).29 They were also asked to mark any rhythmic or periodic patterns (as defined by the ACNS standardized critical care EEG terminology [25]). A gold standard seizure was defined as a seizure marked by 2 out of 3 of the raw reviewers with at least 50% overlap in seizure duration (figure e-1).
A separate panel of 9 QEEG experts were selected to review the Q and QR epochs, with 3 reviewers assigned to review each epoch. These QEEG experts had at least 1 year of experience using QEEG in high-volume clinical practices. Epochs were assigned such that no reviewer reviewed the same epoch in both Q and QR formats. Reviewers were instructed to mark onsets of any events on the QEEG that they thought were probable seizures. Q reviewers did not have access to raw EEG. QR reviewers had access to the entire raw EEG but were encouraged to only review the raw EEG corresponding to QEEG areas of interest. None of the reviewers had access to patient video, as we did not aim to have reviewers distinguish clinical from purely electrographic seizures.
Seizure identification was considered positive if onset was marked on a QEEG panel within either 1 or 2.5 minutes of the seizure onset determined by the raw EEG reviewers (termed maximal onset variation). Because the inherent limitations of screen resolution would artifactually lower sensitivity in the QEEG arm if using a maximal onset variation of just 1 minute, we allowed up to 2.5 minutes onset variation from the time of the seizure onset determined by the raw EEG reviewers.
For each Q and QR epoch, the sensitivity for seizure identification was averaged among the 3 reviewers. Subsequently, the mean and median sensitivity across all epochs was calculated. Sensitivities were computed in this way for both maximal onset variations. False-positive rates (FPRs) for seizure identification for the Q and QR groups were also calculated.
Automated seizure detection.
We assessed the sensitivity and FPR of a proprietary automated seizure detection algorithm (SzD) (seizure probability, Insight II version 11, Persyst, Inc.), with the threshold for seizure detection set to a value of 1.0. None of the Q or QR reviewers had access to the SzD display (figure 1).
Review time and rating of QEEG utility.
The time required by each R, Q, and QR reviewer to review each epoch was recorded. In addition, Q and QR reviewers were also asked to rate the utility of each QEEG technique on a Likert scale ranging from 1 to 5 (1 = least useful).
Statistical analysis.
Means, medians, and interquartile ranges for sensitivities and FPR were reported for the Q, QR, and SzD group, for 2 maximal onset variations (1 and 2.5 minutes). Median reviewing times and ranges for each epoch as well as overall were also reported for the Raw, Q, and QR reviewers. A nonparametric Friedman test of differences among repeated measures (Matlab; Mathworks, Natick, MA) was conducted to determine statistical significance.
RESULTS
Seizure characteristics.
Across 15 epochs, there were on average 10.5 gold standard seizures per epoch (total 126; range 0–49); 32% of seizures were generalized, 36% hemispheric, 28% focal, and the remaining 4% were marked as indeterminate.
Sensitivity and FPRs for seizure identification.
Mean sensitivity for Q = 67% and QR = 68% was using the 2.5-minute maximal onset variation time. As expected, sensitivity declined with decreasing maximal onset variation: Q = 51% and QR = 63% when the maximal onset variation allowed was 1 minute (table 1). The mean FPRs across all epochs were 1/h for Q reviewers and 0.5/h for QR reviewers (table 1).
Table 1.
Characteristics of individual epochs, sensitivity (using a maximal onset variation of 2.5 and 1 minute), and false-positive rates
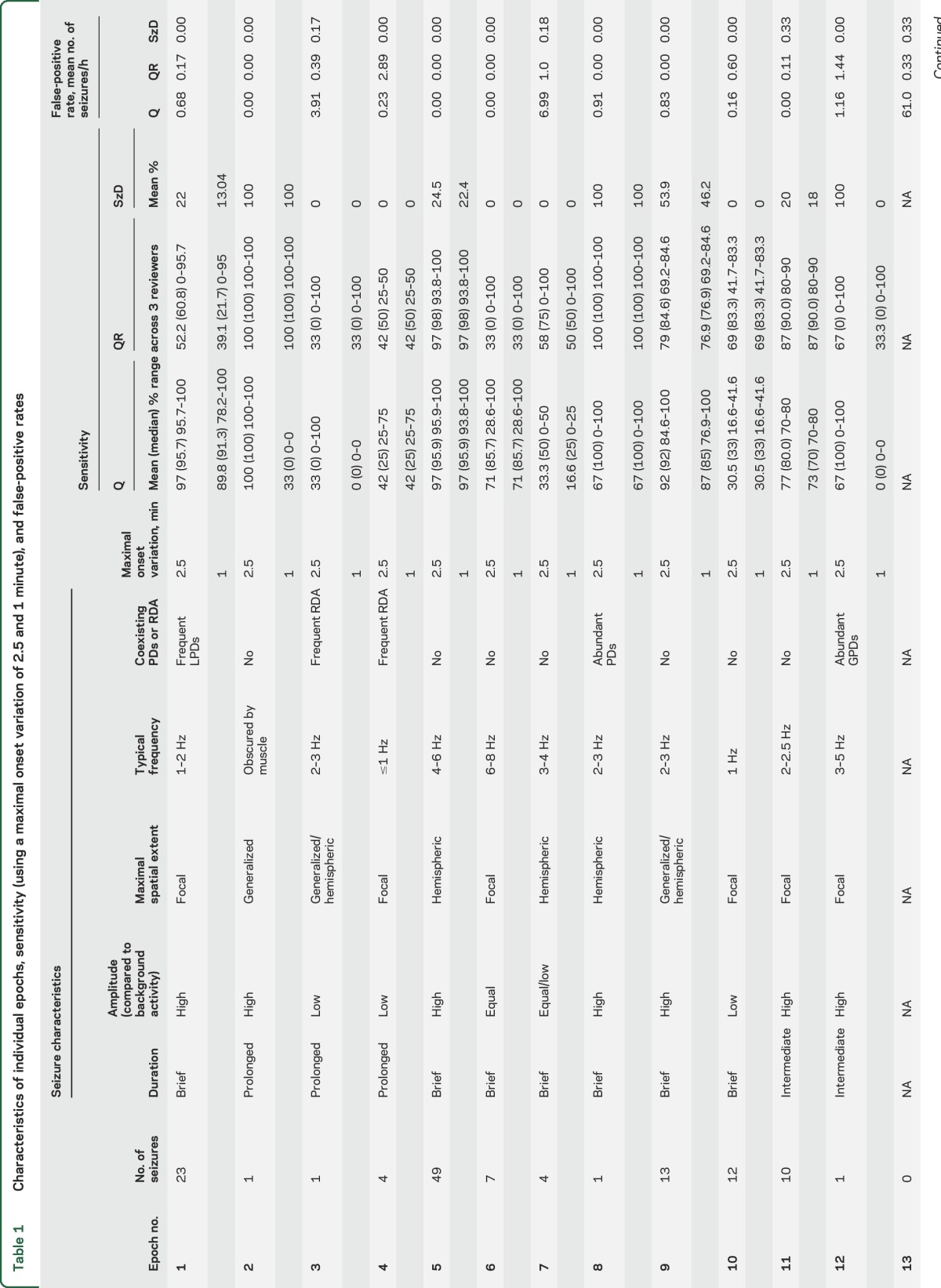
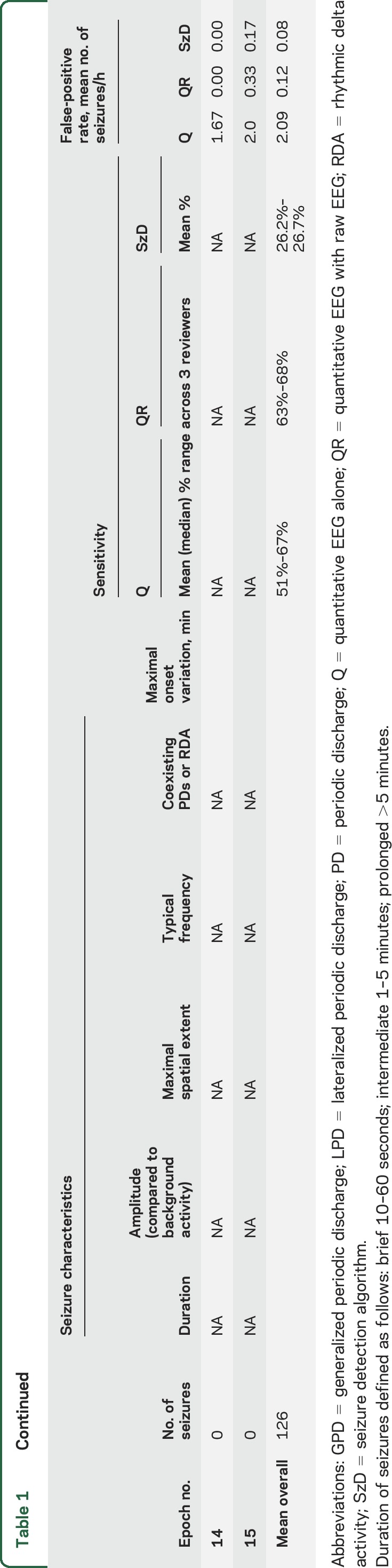
Compared to visual identification of seizures in Q and QR groups, SzD had a mean sensitivity of 27% and 25% when allowing maximal onset variations of 2.5 and 1 minute, respectively; mean FPR was 0.07/h.
Factors influencing sensitivity and FPRs for seizure identification.
Compared to gold standard seizures detected on raw EEG review, the Q and QR review showed significant variability in sensitivity. This was primarily due to differing characteristics of the individual raw EEG recordings (table 1; figures e-1–e-3). The highest sensitivities were seen in samples with frequent, hemispheric seizures (epochs 5, 9, 11: Q sensitivity 97%, 92%, and 77%, and QR 97%, 79%, and 87%, respectively). These epochs also had few or no false-positive detections, which may reflect the infrequent occurrence of artifact and periodic patterns in these epochs.
Lower sensitivity was seen in epochs with low-frequency, slowly evolving, low-amplitude seizures, but sensitivities improved when reviewers had access to raw EEG (epoch 4: mean Q = 42%, median 25%; mean QR = 42%, median 50%; epoch 10: mean Q = 30.5%, QR = 69%).
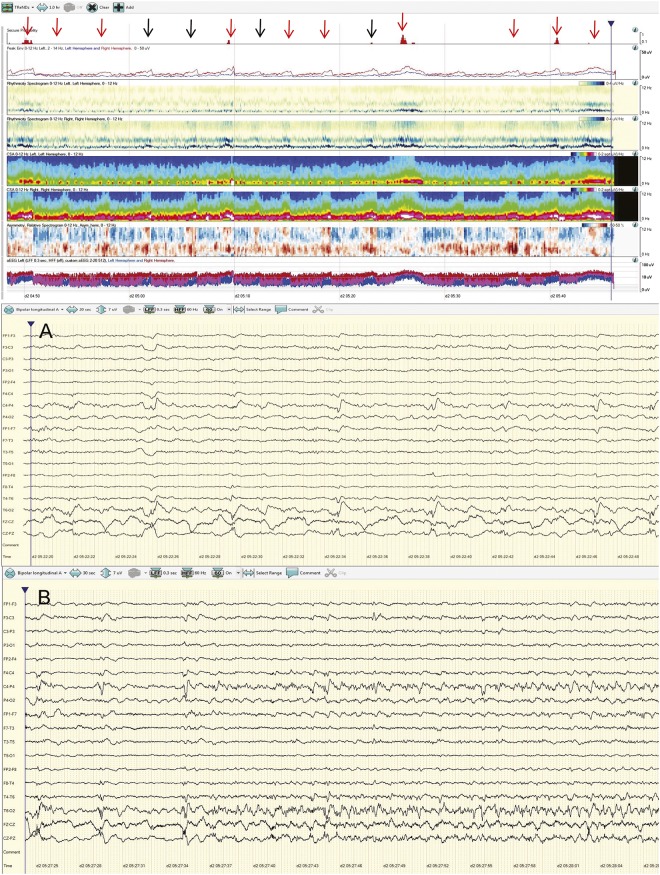
Of epochs that demonstrated lower sensitivity on Q than QR, some epochs (e.g., 1 and 4) also had poor agreement among raw reviewers. Epoch 1 contained frequent, lateralized periodic discharges (LPDs) over the right temporal region that were clearly distinguished from background activity. However, at times the LPDs evolved to electrographic seizures with a QEEG signature very similar to the LPDs (figure 3). Consequently, 1 of 3 R reviewers labeled all electrographic seizures as LPDs alone, leading to poor raw EEG agreement for this epoch. Epoch 4 had occasional prolonged seizures in addition to frequent runs of rhythmic delta activity that did not meet ictal criteria as a source of poor agreement among raw reviewers. These are good examples of the controversial ictal-interictal continuum for which there is considerable variability in interrater agreement.
Figure 3. Epoch 1: Periodic discharges mimicking electrographic seizures on quantitative EEG (QEEG).
This epoch contained frequent, brief lateralized periodic discharges (LPDs) over the right temporal region that were occasionally nonevolving (black arrows), but often evolved into electrographic seizures (red arrows). The QEEG signature seen at the time of nonevolving periodic discharges (black arrow/event A; raw EEG panel A) is very similar to the QEEG pattern seen during the ictal pattern (red arrow/event B, raw EEG panel B). One of 3 raw EEG without QEEG reviewers labeled all electrographic seizures as LPDs alone. Automated seizure detection identified very few of the electrographic seizures. (Note that seizure detection algorithm trend [“seizure probability” at the top of the figure] is included here for comparison, but was not visible to quantitative EEG alone or quantitative EEG with raw EEG reviewers).
On the other hand, some epochs demonstrated good agreement on raw EEG review but Q sensitivity was still suboptimal even with concomitant raw EEG. In epoch 6 (figure e-2), seizures were brief and low-amplitude compared to background. Although this raw EEG pattern resulted in a subtle QEEG signal, it was stereotyped and characteristic of an ictal pattern. This is an example of the power of identifying an initial signature ictal pattern, which can lead to rapid identification of subsequent ictal events of similar morphology.
With certain epochs, the QR group displayed lower sensitivity compared to Q group (epochs 1, 7, 9). These epochs had a predominance of periodic patterns that might have led the reviewer to change impression from seizure to periodic patterns upon reviewing the raw EEG.
Review time and rating of QEEG utility.
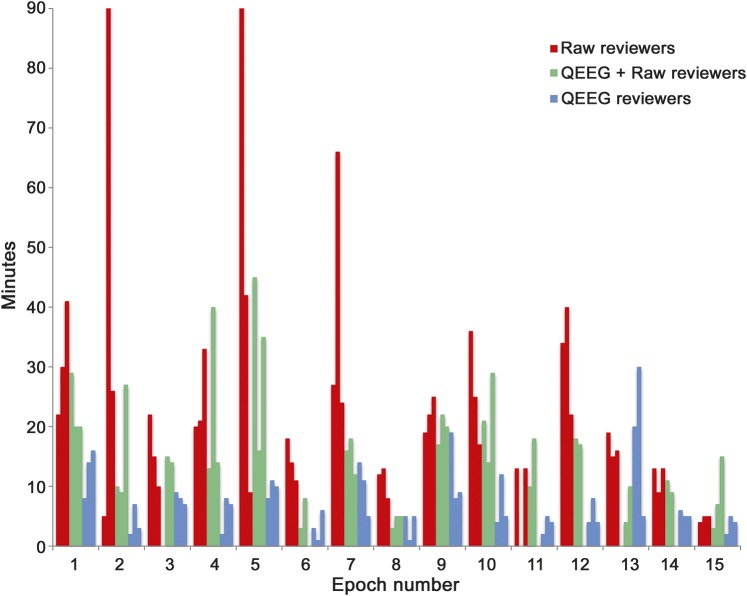
Median EEG review times (figure 4) were shorter for QEEG alone (6 minutes) and QR (14.5 minutes) compared to raw EEG review (19 minutes; p = 0.00003). Based on self-reported Likert scale ratings, CSA and rhythmicity spectrogram were perceived as the most helpful QEEG techniques for visual identification of seizures.
Figure 4. Comparison of reviewing time for reviewers when using raw EEG without quantitative EEG, quantitative EEG with raw EEG, and quantitative EEG alone.
Note that epochs 2 and 5 required significantly more time for raw review by one reviewer. Both of these epochs contained prolonged seizures that were marked as such by 2 of the raw EEG reviewers. However, one reviewer annotated both of these epochs as containing multiple, short seizures. We suspect that the additional review time was required by this reviewer in order to distinguish and annotate each seizure.
DISCUSSION
This multicenter study provides Class II evidence that a panel of multiple QEEG trends viewed by experts can be used to identify seizures in critically ill adults with reasonable sensitivity and low FPR and significantly shortens review time compared to comprehensive raw EEG interpretation.
A prior study23 investigating the sensitivity of a single QEEG trend (CSA) in 113 adults (39 with seizures) found a median sensitivity of 94.2% of seizures per recording. Although our study had lower sensitivity, our FPR was lower, as the aim of our study was to assess the accuracy of seizure identification by experts, and not just the performance of QEEG as a screening tool.
Other studies evaluating QEEG sensitivity were performed in pediatric patients. One study12 reported a median sensitivity of 83.3% of seizures identified per recording using color density spectral array (CDSA) and 81.5% using aEEG. Missed seizures fell into the following categories: low voltage (<75 μV), short duration (<1 minute), focal, or seizures that occurred in the context of abundant interictal epileptiform discharges. Our study confirms that epochs with low-frequency and low-amplitude seizures had lower sensitivities.
In another single-center study,11 experienced QEEG readers were significantly more accurate than inexperienced readers, particularly when reviewing the envelope trend (87% vs 52%). Our study supports similar seizure detection rates by experienced QEEG reviewers. Despite the fact that the QEEG panel utilized in our study consisted of a larger number of QEEG trends (5 trends vs 2 or fewer in prior studies), the accuracy of seizure identification was no better than prior studies. This reflects the complexity and variation in our EEG samples: seizures of different morphology and spatial extent may be best identified using one QEEG trend display over another.
This study incorporated several methodologic details in an attempt to answer specific clinical questions. We recruited several experienced electroencephalographers who routinely utilize QEEG from various centers across the CCEMRC. Our methods were more stringent compared to real-life practice, as we asked QEEG reviewers to mark only probable or likely seizures in an effort to ascertain a more realistic FPR. It was our expectation that expert reviewers would be more discerning with better differentiation of nonictal patterns (such as mechanical artifact and arousal patterns) from seizures. We also chose to withhold access to concurrent video recording in order to provide a more focused evaluation of the EEG interpretation alone, without clinical bias. Allowing access to the video recording may have decreased the rate of false-positive detections by allowing proper identification of seizure-mimicking artifacts, but would have come at the expense of increased review time. Despite these stringent conditions, sensitivities for seizure identification were comparable to several of the aforementioned studies, and FPRs were much lower. EEG data was selected by only one investigator, which may introduce selection bias; however, the EEG epochs were intended to contain seizures of various frequencies, durations, and locations in order to replicate the diversity of seizure patterns seen in daily clinical practice. In addition, some epochs contained abundant rhythmic and periodic patterns, which are known to be difficult to differentiate from electrographic seizures.
Prior studies have shown low to moderate interrater agreement for detection of electrographic seizures in critically ill patients, even among experts30,31; hence, our analysis required raw EEG agreement among at least 2 reviewers to determine gold standard seizures. It follows, however, that some reduction in QEEG sensitivity may arise from suboptimal interrater agreement on some QEEG epochs due to the nature of the patterns they contain, and not necessarily a limitation of using QEEG itself. This is supported by the fact that epochs with abundant rhythmic or periodic patterns as well as periods of reactivity exhibited not only a higher FPR overall but also a wider range of FPRs among reviewers, suggesting interrater disagreement in QEEG interpretation. This highlights an important observation that periodic patterns may frequently resemble seizures on QEEG and accurate distinctions can be difficult even with concomitant raw EEG review, especially when periodic patterns evolve into seizures. This supports findings from a prior study of interrater agreement of the ACNS ICU EEG terminology, where interrater agreement for evolution of periodic patterns was only fair (21%).32
An important feature of our study was the assessment of an automated seizure detection algorithm (SzD). Sensitivity of SzD was much lower compared with human identification with Q or QR review. Similar to human review, lower sensitivities for SzD were seen in epochs with low amplitude, slowly evolving seizures, or with abundant periodic patterns. Adjusting the manufacturer's default settings for the SzD algorithm may have increased its sensitivity, at the expense of a higher FPR. Our results suggest that automated seizure detection in the ICU setting will require further advances to improve sensitivity in the ICU setting before approaching the performance of expert QEEG users.
QEEG analysis saves significant review time, either alone or in conjunction with raw EEG, corroborating the results of another recent study by Moura et al.33 They reported a sensitivity of 87.3% for seizure detection using CDSA and significant time-savings comparing QEEG to conventional EEG review (8 ± 4 minutes for CSA-guided review vs 38 ± 17 minutes for conventional review; p < 0.005). Similar to this study,33 our study demonstrates that time-savings of QEEG over raw EEG review was greater for epochs with no or few seizures, compared to epochs with multiple seizures. This may partly have been due to instructions to mark all seizures, no matter how brief, which is not usually done in routine practice.
Prior research suggests that although QEEG displays are a useful screening tool for seizure identification, there is potential for false-positives, especially when used by inexperienced personnel.23,34 Our study demonstrates that expert review of a panel of QEEG trends leads to lower FPRs with acceptable sensitivity similar to prior studies. However, intermittent raw EEG assessment is still necessary to confirm seizures suspected on QEEG. Hence, we recommend that QEEG be used as a screening tool to guide directed raw EEG review by an experienced neurophysiologist, in order to maximize sensitivity while minimizing false-positive detections. Additional guidance on the use of QEEG in clinical practice can be found in the ACNS Consensus Statement on Continuous EEG in Critically Ill Adults and Children.35
This study demonstrates reasonable overall sensitivity of QEEG for seizure detection but is variable based on the electrographic pattern. Sensitivity is highest for frequent seizures of higher amplitude than background activity and propagation beyond initial onset. Lower sensitivities were seen for brief, low-amplitude, focal seizures. Knowing which patterns are identified less readily on QEEG allows the reviewer to understand the limitations of QEEG review. What remains to be determined is the clinical impact, if any, of failing to detect brief, focal, and infrequent electrographic events. Further investigations are needed to optimize the use of QEEG as a screening tool for identification of seizures as well as other rhythmic and periodic patterns that may not clearly meet seizure criteria but may still be of clinical significance. Efforts should include investigating which trend combinations as well as time, frequency, and amplitude scales provide a panel that optimally displays various seizure types and improves interrater agreement. Finally, research is needed to evaluate the feasibility of using QEEG in real time at the bedside with interpretation performed by personnel with no formal EEG training. Although automated seizure detection software is rapidly evolving with advances in artifact rejection to reduce false detections, improvements in sensitivity are still needed. These advances in the use of QEEG in the ICU setting have potential for improving outcomes of critically ill patients by reducing time to accurate recognition and treatment of electrographic seizures.
Supplementary Material
GLOSSARY
- ACNS
American Clinical Neurophysiology Society
- aEEG
amplitude-integrated EEG
- CCEMRC
Critical Care EEG Monitoring Research Consortium
- CDSA
color density spectral array
- cEEG
continuous EEG
- CSA
compressed spectral array
- FPR
false-positive rate
- ICU
intensive care unit
- LPD
lateralized periodic discharge
- Q
quantitative EEG alone
- QEEG
quantitative EEG
- QR
quantitative EEG with raw EEG
- R
raw EEG without quantitative EEG
- SzD
seizure detection algorithm
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Critical Care EEG Monitoring Research Consortium, Nicholas S. Abend, Chinasa Nwankwo, Jeff Politsky, Susan T. Herman, Tobias Loddenkemper, Linda Huh, Jessica Carpenter, Stephen Hantus, Jan Claassen, Aatif M. Husain, Nicolas Gaspard, David Gloss, Eva K. Ritzl, Tennille Gofton, Joshua Goldstein, Sara Hocker, Ann Hyslop, Korwyn Williams, Xiuhua Bozarth, Courtney J. Wusthoff, Andres Fernandez, Jerzy P. Szaflarski, Andreas Kramer, Brandon Foreman, Pearce Korb, Leslie Rudzinski, Rup Sainju, Jennifer Hopp, Ram Mani, Kathryn A. Davis, Giridhar P Kalamangalam, Kan Ding, Mark S. Quigg, Kevin F Haas, Adam Ostendorf, Deepti Zutshi, and Kim Pargeon
AUTHOR CONTRIBUTIONS
Hiba Arif Haider: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision, obtaining funding. Rosana Esteller: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Cecil David Hahn: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Michael Brandon Westover: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. Jonathan J. Halford: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Jongwoo Lee: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Mouhsin M. Shafi: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, Nicolas Gaspard: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Susan T. Herman: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, review of EEG data samples. Elizabeth E. Gerard: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Lawrence J. Hirsch: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. Joshua Andrew Ehrenberg: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data. Suzette M. LaRoche: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
H. Haider: Supported by an NINDS R25 Research Education Training Grant. R. Esteller, C. Hahn, M. Westover, J. Halford, J. Lee, M. Shafi, N. Gaspard, S. Herman, E. Gerard, L. Hirsch, J. Ehrenberg, and S. LaRoche report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 2.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg 2009;109:506–523. [DOI] [PubMed] [Google Scholar]
- 3.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750–1755. [DOI] [PubMed] [Google Scholar]
- 4.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009;37:2051–2056. [DOI] [PubMed] [Google Scholar]
- 5.Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol 2004;61:1090–1094. [DOI] [PubMed] [Google Scholar]
- 6.Vespa PM, Miller C, McArthur D, et al. . Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 7.Vespa PM, O'Phelan K, Shah M, et al. . Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–1446. [DOI] [PubMed] [Google Scholar]
- 8.Vespa PM, Nuwer MR, Nenov V, et al. . Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999;91:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology 1993;43:483–488. [DOI] [PubMed] [Google Scholar]
- 10.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res 1998;814:179–185. [DOI] [PubMed] [Google Scholar]
- 11.Akman CI, Micic V, Thompson A, Riviello JJ Jr. Seizure detection using digital trend analysis: factors affecting utility. Epilepsy Res 2011;93:66–72. [DOI] [PubMed] [Google Scholar]
- 12.Stewart CP, Otsubo H, Ochi A, Sharma R, Hutchison JS, Hahn CD. Seizure identification in the ICU using quantitative EEG displays. Neurology 2010;75:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abend NS, Dlugos D, Herman S. Neonatal seizure detection using multichannel display of envelope trend. Epilepsia 2008;49:349–352. [DOI] [PubMed] [Google Scholar]
- 14.Bourez-Swart MD, van Rooij L, Rizzo C, et al. . Detection of subclinical electroencephalographic seizure patterns with multichannel amplitude-integrated EEG in full-term neonates. Clin Neurophysiol 2009;120:1916–1922. [DOI] [PubMed] [Google Scholar]
- 15.El-Dib M, Chang T, Tsuchida TN, Clancy RR. Amplitude-integrated electroencephalography in neonates. Pediatr Neurol 2009;41:315–326. [DOI] [PubMed] [Google Scholar]
- 16.Rennie JM, Chorley G, Boylan GB, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed 2004;89:F37–F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah DK, de Vries LS, Hellstrom-Westas L, Toet MC, Inder TE. Amplitude-integrated electroencephalography in the newborn: a valuable tool. Pediatrics 2008;122:863–865. [DOI] [PubMed] [Google Scholar]
- 18.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics 2007;120:770–777. [DOI] [PubMed] [Google Scholar]
- 19.Toet MC, van der Meij W, de Vries LS, Uiterwaal CS, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 2002;109:772–779. [DOI] [PubMed] [Google Scholar]
- 20.Pensirikul AD, Beslow LA, Kessler SK, et al. . Density spectral array for seizure identification in critically ill children. J Clin Neurophysiol 2013;30:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topjian AA, Fry M, Jawad AF, et al. . Detection of electrographic seizures by critical care providers using color density spectral array after cardiac arrest is feasible. Pediatr Crit Care Med 2015;16:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitzschke R, Muller J, Engelhardt R, Schmidt GN. Single-channel amplitude integrated EEG recording for the identification of epileptic seizures by nonexpert physicians in the adult acute care setting. J Clin Monit Comput 2011;25:329–337. [DOI] [PubMed] [Google Scholar]
- 23.Williamson CA, Wahlster S, Shafi MM, Westover MB. Sensitivity of compressed spectral arrays for detecting seizures in acutely ill adults. Neurocrit Care 2014;20:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACNS Nomenclature Training Module, Critical Care EEG Monitoring Research Consortium [online]. Available at: http://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc/education. Accessed April 3, 2016. [Google Scholar]
- 25.Hirsch LJ, LaRoche SM, Gaspard N, et al. . American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch LJ, Brenner RP, Drislane FW, et al. . The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol 2005;22:128–135. [DOI] [PubMed] [Google Scholar]
- 27.Beniczky S, Hirsch LJ, Kaplan PW, et al. . Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54(suppl 6):28–29. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia 2007;48(suppl 8):39–41. [DOI] [PubMed] [Google Scholar]
- 29.S RS, S OS, Husain AM. Seizure burden score: a quantitative description of seizure intensity in continuous EEG recordings. Presented at the 4th London-Innsbruck Colloquium on Status Epilepticus and Acute Seizures, 2013, Salzburg, Austria.
- 30.Ronner HE, Ponten SC, Stam CJ, Uitdehaag BM. Inter-observer variability of the EEG diagnosis of seizures in comatose patients. Seizure 2009;18:257–263. [DOI] [PubMed] [Google Scholar]
- 31.Halford JJ, Shiau D, Desrochers JA, et al. . Inter-rater agreement on identification of electrographic seizures and periodic discharges in ICU EEG recordings. Clin Neurophysiol 2015;126:1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB, Critical Care EEGMRC. Interrater agreement for critical care EEG terminology. Epilepsia 2014;55:1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura LM, Shafi MM, Ng M, et al. . Spectrogram screening of adult EEGs is sensitive and efficient. Neurology 2014;83:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topjian AA, Fry M, Jawad AF, et al. . Detection of electrographic seizures by critical care providers using color density spectral array after cardiac arrest is feasible. Pediatr Crit Care Med 2015;16:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman ST, Abend NS, Bleck TP, et al. . Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol 2015;32:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.