Abstract
The F1-ATPase, the soluble part of FoF1-ATP synthase, is a rotary molecular motor consisting of α3β3γδε. The γ and ε subunits rotate relative to the α3β3δ sub-complex on ATP hydrolysis by the β subunit. The ε subunit is known as an endogenous inhibitor of the ATPase activity of the F1-ATPase and is believed to function as a regulator of the ATP synthase. This inhibition by the ε subunit (ε inhibition) of F1-ATPase from thermophilic Bacillus PS3 was analyzed by single molecule measurements. By using a mutant ε subunit deficient in ATP binding, reversible transitions between active and inactive states were observed. Analysis of pause and rotation durations showed that the ε inhibition takes a different path from the ADP-Mg inhibition. Furthermore, the addition of the mutant ε subunit to the α3β3γ sub-complex was found to facilitate recovery of the ATPase activity from the ADP-Mg inhibition. Thus, it was concluded that these two inhibitions are essentially exclusive of each other.
Keywords: ATP synthase, ADP inhibited form, regulation
The FoF1-ATPase/synthase (FoF1) is a rotary molecular motor that consists of water-soluble ATP-driven F1 and membrane-embedded H+- or Na+-driven Fo and couples ATP synthesis/hydrolysis and ion flow1,2. Its rotation has been studied by direct observation at the single-molecule level and details of the rotary catalytic mechanism have been revealed3–5. The F1-ATPase (α3β3γδε) hydrolyzes ATP into ADP and Pi and the hydrolysis of one ATP drives a discrete 120° rotation of the γε subunits relative to the other subunits. The 120° step can be resolved into an 80° sub-step initiated by the ATP-binding and a 40° sub-step initiated by the release of one of the reaction products, Pi. This sequence of events can be summarized as “ATP-binding dwell”, “80° stepping rotation”, “catalytic dwell”, and “40° stepping rotation”.
The ε subunit, the smallest subunit of F1-ATPase, is known as an endogenous inhibitor of the ATPase activity of bacterial and chloroplast F1. Although the inhibition by the ε subunit (ε inhibition) is common, the mechanism behind it varies considerably among species. In F1-ATPases from Escherichia coli (EF1) and from chloroplast (CF1), when F1-ATPase is separated from Fo, the ε subunit functions as a dissociative inhibitor: The ε subunit inhibits the ATPase activity just by the binding and the inhibition is controlled by dissociation/association of the ε subunit from the F1-ATPase6–8. Consequently, the presence of excess ε subunit strengthens the inhibition. In contrast, the ε subunit of F1-ATPase from thermophilic Bacillus PS3 (TF1) does not dissociate from the TF1 complex and, thus, excess ε hardly affects the ATPase activity9. The ε inhibition in TF1 is controlled by a change of the conformation of the ε subunit. When the ε subunit is in an extended-state conformation, it inserts its C-terminal α helices into the cavity of the α3β3 ring and inhibits the ATPase activity10–12. When the ε subunit is in an intermediate-state conformation, in which the C-terminal α helices of the ε subunit are withdrawn out of the α3β3 ring, the ATPase activity is not inhibited. This uninhibited state of the ATPase is stabilized by the ATP binding to the ε subunit, which is called folded-state conformation, with its C-terminal α helices folded into a helix-turn-helix conformation. This mechanism has been supported by a number of biochemical studies10–13.
The F1-ATPase is also known to be inhibited by ADP-Mg trapped in the catalytic site(s), which has been termed as the ADP-Mg inhibition (or the ADP inhibition). All the F1-ATPases thus far examined have been shown to be subject to this mode of inhibition. Recovery from the ADP-Mg inhibited state is accelerated by the binding of ATP to the non-catalytic site(s) on the α subunits14,15. Single-molecule study has shown that the ADP-Mg inhibition can be observed as pauses of the rotation16. The pause angle of the γ subunit during the ADP-Mg inhibition is the same as that of the catalytic dwell, namely, 80° from the ATP-binding dwell.
Several previous studies have proposed that the ε inhibition stabilizes the ADP-Mg inhibited state17–19. However, the ε inhibition is effective even in the presence of a detergent, lauryldimethylamine oxide (LDAO), which is known to weaken the ADP-Mg inhibition12. Our previous study indicated that the ε subunit greatly reduces the affinity of ADP-Mg to the catalytic sites: the ε subunit may actually counteract the ADP-Mg inhibition20.
In order to examine the relationship between the ε inhibition and the ADP-Mg inhibition, we have carried out single-molecule analysis of the effects of the ε subunit on the rotation of TF1 and measurement of the ATPase activity of TF1 in the present study. Previously, observation of repetitive transitions between active and ε inhibited states was limited to a very low concentration of ATP (200 nM), because the wild-type ε subunit binds ATP very strongly at room temperature19. In the present study, use of a mutant ε subunit deficient in ATP binding made the observation possible even with 200 μM ATP, allowing us to carry out detailed analyses. The results indicated that the ε inhibition and the ADP-Mg inhibition are essentially exclusive of each other.
Materials and Methods
Materials
A mutant (αC193S, βHis10 at N-terminus, and γS107C) α3β3γ sub-complex of TF1-ATPase (TF1(−ε)) was labeled at a cystein on the γ subunit by biotin-PEAC5-maleimide (Dojin, Japan) as described16. The ΔNC mutant α3β3γ sub-complex of TF1-ATPase, which contains αK175A/T176A mutations in addition to the above mutations, was prepared as described14,16. Wild-type and R126A mutant ε subunits of TF1 were prepared as described13,21. The nucleotide content of the ε subunit was estimated by UV absorption spectrum to be less than 0.05 mol of ATP/mol of ε22. The α3β3γε sub-complex used in the ATPase measurement was reconstituted from the α3β3γ sub-complex and the ε subunit prior to use by simple mixing at an approximate molar ratio of α3β3γ:ε = 1:10. ATP was purchased from Wako Chemicals. Phosphoenolpyruvate was purchased from Sigma-Aldrich. Streptavidin coated magnetic beads (0.73 μm) were purchased from Seradyn (Indianapolis). Pyruvate kinase and lactate dehydrogenase were purchased from Roche Diagnostics. Other chemicals were of the highest grades available.
Rotation assay
A flow cell was constructed from a bottom cover glass coated with Ni2+-NTA, an uncoated top cover glass, and spacers of ca. 100 μm thickness16. F1 at 5.4 nM (with or without 90 nM εWT or 120 nM εR126A) in 50 mM MOPS-KOH (pH 7.0) and 50 mM KCl was infused into the flow cell. After 2 min, the flow cell was washed with buffer A (50 mM MOPS-KOH (pH 7.0), 50 mM KCl, 2 mM MgCl2 and 1% bovine serum albumin) and the magnetic beads were infused. After 10 min, the unbound beads were washed out with buffer A and buffer A containing various concentrations of ATP-Mg and an ATP-regenerating system (50 μg/ml of pyruvate kinase and 2.5 mM phosphoenolpyruvate) was infused. Although the binding of TF1(−ε) and ε is known to be strong enough9, excess ε subunit (1 μM) was also included to avoid possible loss of the ε subunit. Rotating beads (or broken pieces thereof) were observed in the bright field mode on an inverted microscope (IX-71, Olympus) with a 100× objective. The images were recorded at the video rate (30 fps) by a full-frame charge-coupled device camera equipped with a high speed shutter (FC-300M, TAKEX, Tokyo) and recorded on a digital video recorder (DSR-45A, Sony, Tokyo). The shutter speed was set to 1/30 s. The images of the beads were analyzed by a custom-made software (Ryohei Yasuda, Duke University, Durham, NC)3.
Pause analysis
The pausing periods longer than 1 s were defined as the inactive state. The rotating periods between successive inactive states were defined as the active state. The durations of the inactive and the active states were measured.
ATPase assay
ATPase activity was measured spectrophotometrically with an ATP-regenerating system coupled to NADH oxidation at 25°C23. The assay mixture consisted of 50 mM MOPS-KOH (pH 7.0), 50 mM KCl, 2.5 mM phosphoenolpyruvate, 2 mM MgCl2, 0.2 mM NADH, 50 μg/ml pyruvate kinase, 50 μg/ml lactate dehydrogenase, and various concentrations of ATP-Mg. The reaction was initiated by the addition of 1.7 to 6.8 nM of biotinylated subunit complexes of TF1 to the assay mixture, and the changes in the absorbance at 340 nm were measured on a spectrophotometer V-550 (JASCO, Tokyo). The ATPase activity was calculated by the kinetics during 1010–1110 s after the initiation of the reaction. For measurement with TF1(ΔNC), the concentration of the TF1(ΔNC) was 36 nM.
Other methods
The concentration of ε was determined by the Bradford method24 with a correction factor of 0.5422. The concentration of F1 was determined by UV absorption with a molar extinction coefficient of F1, 154,000 at 280 nm.
Results and Discussion
Reversible inhibition of TF1 by the ε subunit
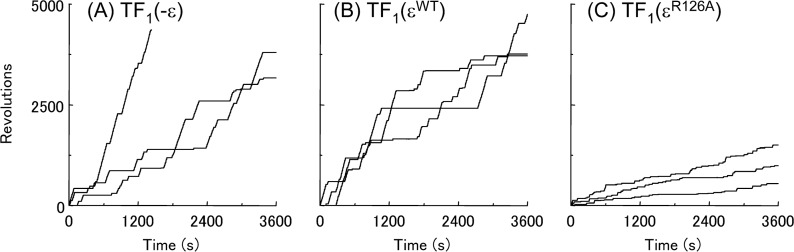
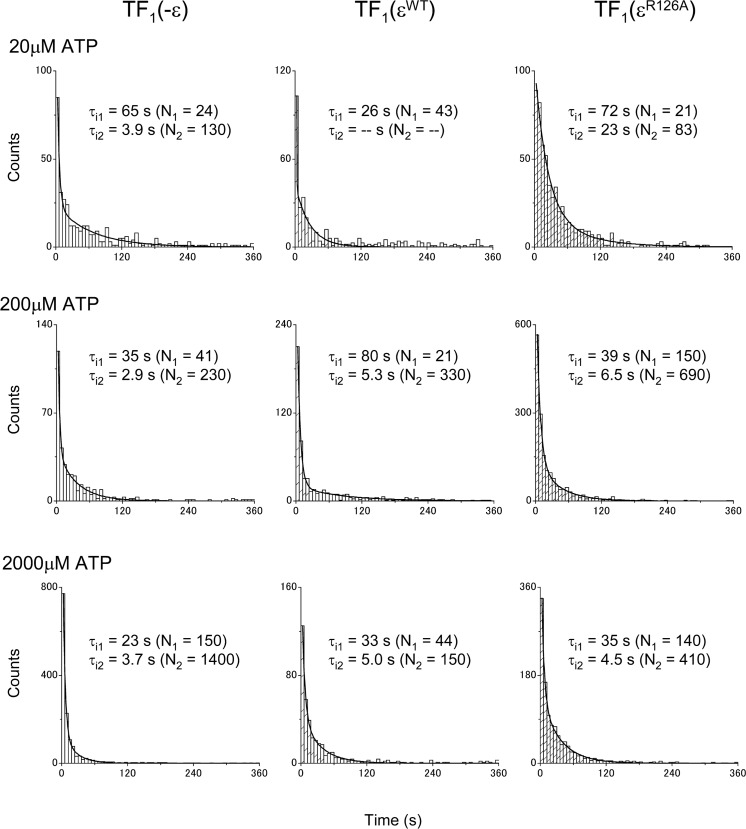
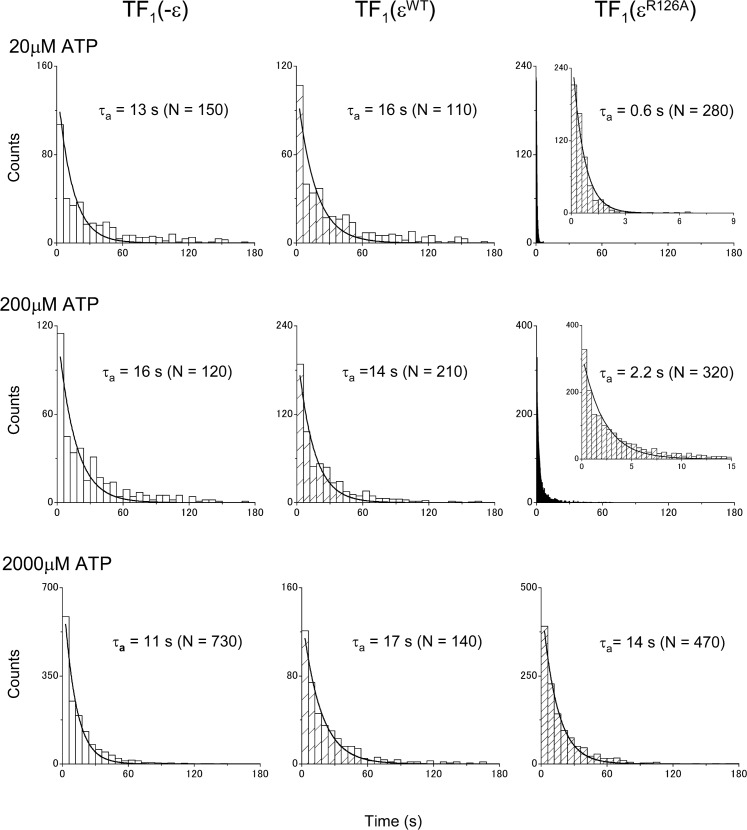
It has been proposed that ATP binding to the ε subunit of TF1 stabilizes its folded-state conformation, with which TF1 is in the ATPase-active state13. As shown in Figure 1, TF1 with the wild-type ε subunit (TF1(εWT)) showed essentially the same rotational behavior as the TF1 lacking ε subunit (TF1(−ε)). Because the ATP binding to the wild-type ε subunit is very strong with a Kd in the order of μM at room temperature12,13, once the ATPase is activated, it does not return to the ε inhibited state, when the ATP concentration is higher than the Kd. Use of a mutant ε subunit (εR126A), which is essentially deficient in ATP binding with a Kd larger than 1 mM13, allowed observation of reversible transition of TF1 into the ε inhibited state even with high concentrations of ATP (200 μM). The overall rotational rate of the TF1(εR126A) was clearly slower (Fig. 1). In a previous study, it was only at extremely low concentrations of ATP that such transitions were observed with TF1(εWT)19. It should be noted that reversible transitions, such as observed here with the mutant εR126A, are physiologically relevant, because the ATP binding to the wild-type ε subunit is weak at 65°C12, a physiological temperature of the Bacillus PS3. The repetitive transitions between the active and the inactive states allowed us to evaluate the lifetimes of the states. The distributions of the durations of the inactive state (pauses longer than 1 s) and of the active state (the state in between successive inactive states) are shown in Figures 2 and 3, respectively. Their lifetimes were estimated by fitting each panel of the data with a function with one or two exponentials (Figs. 2 and 3 and Table 1). The lifetimes of the inactive state (τi1 and τi2) were essentially the same among TF1(−ε), TF1(εWT), and TF1(εR126A) for various concentrations of ATP (Fig. 2 and Table 1). The inactive state observed with TF1(−ε) was the ADP-Mg inhibition, because the ATPase lacked the ε subunit. The inactive state observed with TF1(εWT) must have been also the ADP-Mg inhibition, because εWT must have been in the folded-state conformation with bound ATP at the ATP concentrations employed.
Figure 1.
Time-course of the rotation of beads attached to TF1(−ε), TF1(εWT) and TF1(εR126A). Typical examples of time-course of bead rotation at 200 μM ATP with (A) TF1(−ε), (B) TF1(εWT) and (C) TF1(εR126A) are shown. Data with similar bead sizes are shown.
Figure 2.
Distribution of inactive state durations. The durations of the inactive state were measured and their distributions are shown. Samples used are indicated on the top of each column. The ATP concentrations were 20 μM, 200 μM, and 2 mM, respectively from the top to the bottom rows. Each distribution was fitted with the sum of two exponential functions: y = N1 exp (−t/τi1) + N2 exp (−t/τi2). The best-fit values of τi1, τi2, N1, and N2 are shown on each panel. Solid lines show the best-fit curves. τi2 and N2 for TF1(εWT) with 20 μM ATP could not be obtained due to no convergence.
Figure 3.
Distribution of active state durations. The durations of the active state were measured and their distributions are shown. Samples used are indicated on the top of each column. The ATP concentrations were 20 μM, 200 μM, and 2 mM, respectively from the top to the bottom rows. Each distribution was fitted with a single exponential function: y = N exp (−t/τa). The best-fit values of τa and N are shown on each panel. Solid lines show the best-fit curves. Insets in TF(εR126A) with 20 and 200 μM are enlargements along the x-axis.
Table 1.
Time constants for active/inactive state durations
| Sample, ATP (μM) | active (τa, s) | inactive1a (τi1, s) | inactive2b (τi2, s) |
|---|---|---|---|
| TF1(−ε), 20 | 13 | 65 | 3.9 |
| TF1(−ε), 200 | 16 | 35 | 2.9 |
| TF1(−ε), 2000 | 11 | 23 | 3.7 |
| TF1(εWT), 20 | 16 | 26 | –c |
| TF1(εWT), 200 | 14 | 80 | 5.3 |
| TF1(εWT), 2000 | 17 | 33 | 5.0 |
| TF1(εR126A), 20 | 0.6 | 72 | 23 |
| TF1(εR126A), 200 | 2.2 | 39 | 6.5 |
| TF1(εR126A), 2000 | 14 | 35 | 4.5 |
Inactive1 and inactive2 represent slower and faster time constants, respectively.
Not determined due to no convergence.
Two exponentials were necessary to fit the data of the inactive state durations. The lifetimes were basically in agreement with those previously reported for the ADP-Mg inhibition16. The short lived inactive state (corresponding to τi2) was also observed as in a previous study. Although a slightly larger value was obtained for τi2 with TF1(εR126A) at 20 μM ATP (Fig. 2 and Table 1), we focus only on τi1 here, as in the previous study16. In contrast to the relative insensitivity of the lifetimes of the inactive state to the presence and the kind of the ε subunit or to the ATP concentration, the lifetime of the active state (τa) for TF1(εR126A) was different from that of TF1(−ε) or TF1(εWT) and depended on the ATP concentration (Fig. 3 and Table 1). It was shorter than that with TF1(−ε) or TF1(εWT) by over 10 fold at 20 μM ATP. As the ATP concentration was raised, the lifetime became longer, reaching the same level with TF1(−ε) or TF1(εWT) at 2 mM ATP. According to the previously proposed model that the ε subunit stabilizes the ADP-Mg inhibition18,19 and the ADP-Mg inhibition is prerequisite for the ε inhibition19, the lifetime of the active state should not be shortened by the presence of the ε subunit, while the lifetime of the inactive state could be affected. Thus, this has led us to consider an alternative scheme where the ε inhibition and the ADP-Mg inhibition are the parallel paths (Fig. 4). With this scheme, it can be estimated that most (ca. 86%) of the inhibited states of the TF1(εR126A) with 200 μM ATP must be by the ε. Distinction between the two inhibited states, apart from kinetic arguments, is currently not feasible, because the angular position of the γ subunit in the ε inhibition and that in the ADP-Mg inhibition are the same (data not shown), which supports a previous proposal17,19.
Figure 4.
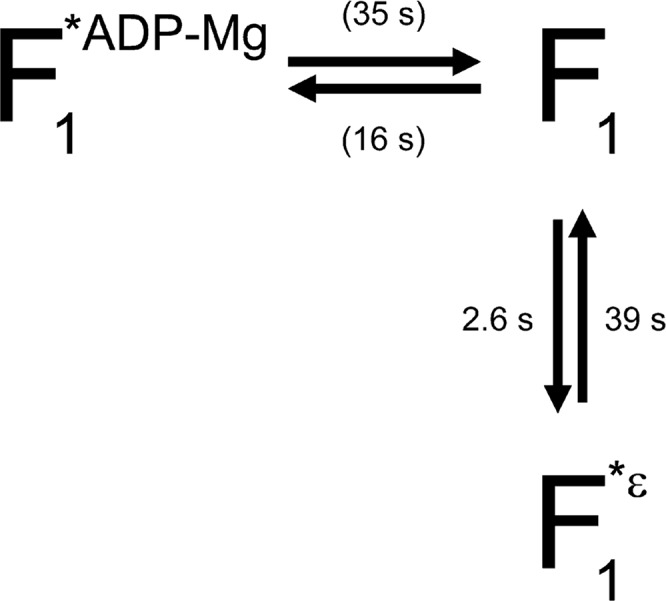
Schematic model of the relationship between ε inhibition and ADP-Mg inhibition. F1, F1*ε and F1*ADP-Mg represent F1 in active state, ε inhibited state and ADP-Mg inhibited state, respectively. The time constant for each path at 200 μM ATP is shown. The time constant for recovery from ε inhibition was taken from τi1 for TF1(εR126A). Values shown in parentheses, which represent time constants for the ADP-Mg inhibition, are from τa and τi1 for TF1(−ε). The time constant for the ε inhibition is calculated as 1/(1/τa(TF1(εR126A)) − 1/τa(TF1(−ε))) assuming that τa(TF1(εR126A)) contained contributions both from the ε inhibition and from the ADP-Mg inhibition (τa(TF1(−ε))).
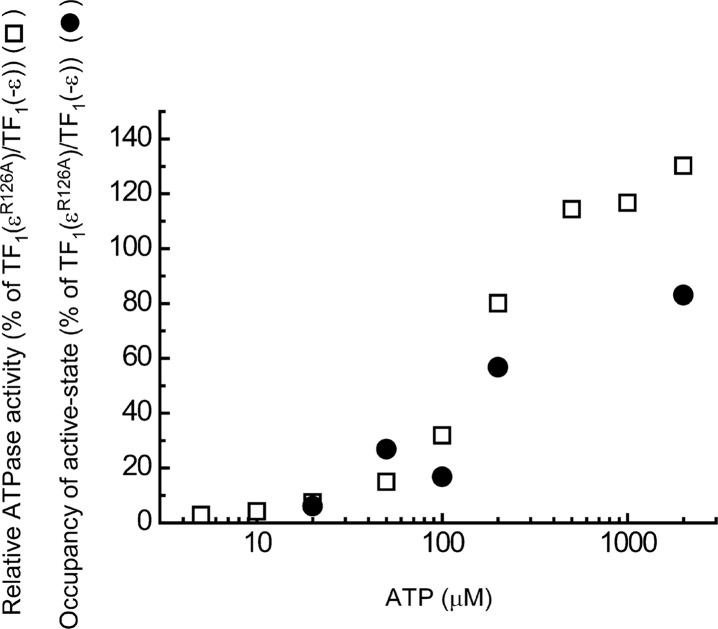
The kinetic constants obtained by our analysis (Table 1) are not far from those expected from biochemical experiments12. The occupancy of the active-state ((sum of durations of the active states) / (sum of durations of the inactive and the active states)) was calculated for TF1(εR126A) and divided by that of TF1(−ε). This ratio had a dependence on the ATP concentration which was similar to the ATP dependence of the relative ATPase activity of TF1(εR126A) to TF1(−ε) (Fig. 5). The agreement shows that the results by the two methods are consistent. However, our values are different from those reported by Tsumuraya et al.19. According to their report, the ε subunit prolonged the duration of the inactive state by 6 fold (from 110 s to 670 s) and the active state was not affected significantly (from 300 to 330 s) at 200 nM ATP19. On the basis of the reported apparent second-order rate constant for activation12, the activation of the ε inhibited TF1(εWT) is estimated to take about 3 days with 200 nM ATP and should not have been observable at the ATP concentration. As Tsumuraya et al. pre-incubated TF1(εWT) in a high concentration of ATP, which was necessary to observe the rotation of TF1(εWT) at low concentrations of ATP, the ε subunit in their study may have retained ATP and what was observed may have been the differences in the ADP-Mg inhibition between TF1(−ε) and TF1(εWT with bound ATP) rather than those in the ε inhibition.
Figure 5.
Comparison of relative ATPase activity and occupancy of the active state. The occupancy of the active state was the sum of the durations in the active state divided by the same sum plus the sum for the inactive state. The obtained occupancy for TF1(εR126A) was divided by that for TF1(−ε) and plotted against the ATP concentration (closed circles). ATPase activity of TF1(εR126A) was divided by that of TF1(−ε) and plotted against the ATP concentration (open squares).
Effect of ε subunit on ATPase activity of TF1(ΔNC)
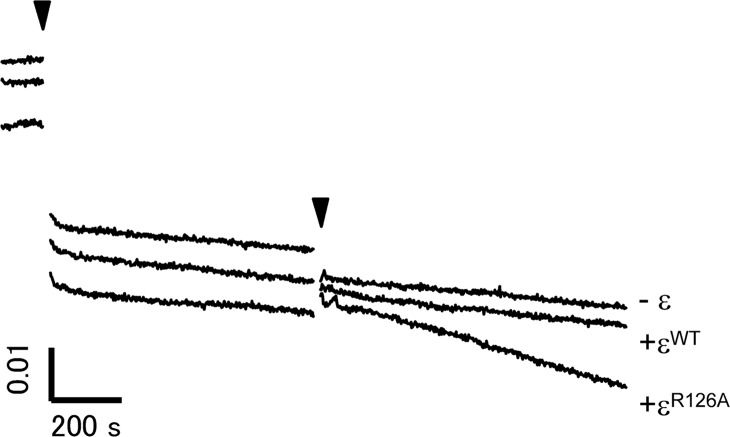
TF1 comprising the K175A/T176A double mutant of the α subunit, TF1(NC), lacks the nucleotide binding ability of the non-catalytic sites on α subunits and its ADP-Mg inhibition is known to be very strong15 (recovery from F1*ADP-Mg hardly occurs). To examine further the relationship between the ε inhibition and the ADP-Mg inhibition, the effect of the ε subunit on the ATPase activity of TF1(ΔNC) was examined. If the ε inhibition is through stabilization of the ADP-Mg inhibition, addition of ε should result in even stronger inhibition. The results are shown in Figure 6. TF1(ΔNC) showed a very low ATPase activity, as previously reported15. Although no effect on the ATPase activity was observed with εWT, when εR126A was added, recovery of the ATPase activity was observed, contrary to the above prediction (Fig. 6). Because the activation is specific to the mutant ε that is essentially deficient in ATP binding, it is likely that the extended-state conformation of the C-terminal domain is involved in TF1(ΔNC)’s escape from ADP-Mg inhibition. The extended-state conformation of the ε subunit is known to reduce the affinity of the catalytic sites of TF1 to ADP by 1 to 3 orders of magnitude20. The conformational change of the ε subunit in the ADP-Mg inhibited F1 (F1*ADP-Mg in Fig. 4) from the intermediate- to the extended-states may facilitate direct escape from the ADP-Mg inhibited state (F1*ADP-Mg) to the ε inhibited state (F1*ε) (a route not shown in Fig. 4), resulting in the recovery of the ATPase activity of TF1(ΔNC) though weak (Fig. 6).
Figure 6.
Activation of TF1(ΔNC) by the addition of εR126A. The ATPase activity of TF1(ΔNC) was measured by the decrease of absorbance at 340 nm using the NADH-coupled ATP-regenerating system at 50 μM ATP. The reaction was initiated by the addition of TF1(ΔNC) lacking ε at the time indicated by the left arrowhead. εWT, εR126A (360 nM), or buffer (−ε), was added at the time indicated by the right arrowhead. Vertical and horizontal bars denote 0.01 absorbance and 200 s, respectively.
Comparison with F1-ATPases from other sources
Nakanishi-Matsui et al. have reported that the ε subunit of EF1 made frequent pauses during observation of the rotation in the ms order time scale25. This is consistent with the EF1 ε subunit being a non-competitive inhibitor that reduces the Vmax value of the EF1 (−ε)26,27. Konno et al. have reported results of single-molecule analysis on the ε inhibition of cyanobacterial F1-ATPase17. The inhibition was in an on/off fashion accompanying association and dissociation of the ε subunit. The position of the γ subunit arrested in the ε inhibited state was the same as that in the ADP-Mg inhibited state. From these results, the authors argued that the ε inhibition might be effective through stabilizing the ADP-Mg inhibition.
However, the present results show that the two modes of inhibition are not closely related and, therefore, it may be a mere coincidence that the γ subunit stops at the same angular position in the two inhibited states. Conclusion must await the determination of the structure of F1-ATPase with a ε subunit in the extended-state conformation.
In summary, the current study has shown that the ε inhibition takes a different path from the ADP-Mg inhibition. Use of the mutant ε subunit deficient in ATP binding allowed us to carry out precise analyses on the ε inhibition under various conditions. Further analysis, e.g., in the presence of high concentrations of ADP or Pi18, with the mutant ε subunit will clarify the rather complicated regulatory mechanisms of the bacterial ATP synthase.
Acknowledgments
We thank Shigeyuki Kato for preparation of ε subunit, Kunio Arai for his contribution to the very early stage of the research, Dr. Ryo Hanai for critical reading of the manuscript, and the members of Kato-Yamada’s laboratory for their help. We also thank Dr. Ryohei Yasuda for the development of image analyzing software. Y. Hirono-Hara was supported by a research fellowship of the Japan Society for the Promotion of Science for Young Scientists. This work was supported in parts by Grants-in-Aid for Scientific Research on Priority Areas (No. 18074002) and for Young Scientists (B) (No. 18770118) from the Ministry of Education, Culture, Sports, Science and Technology and Rikkyo University Grant for the Promotion of Research (to Y. K.-Y.) and Rikkyo University Special Fund for Research (to T. H.).
References
- 1.Boyer PD. The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida M, Muneyuki E, Hisabori T. ATP synthase—a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001;2:669–677. doi: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda R, Noji H, Yoshida M, Kinosita K, Jr, Itoh H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature. 2001;410:898–904. doi: 10.1038/35073513. [DOI] [PubMed] [Google Scholar]
- 4.Adachi K, Oiwa K, Nishizaka T, Furuike S, Noji H, Itoh H, Yoshida M, Kinosita K., Jr Coupling of rotation and catalysis in F1-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. doi: 10.1016/j.cell.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe R, Iino R, Noji H. Phosphate release in F1-ATPase catalytic cycle follows ADP release. Nat. Chem. Biol. 2010;6:814–820. doi: 10.1038/nchembio.443. [DOI] [PubMed] [Google Scholar]
- 6.Laget PP, Smith JB. Inhibitory properties of endogenous subunit epsilon in the Escherichia coli F1 ATPase. Arch. Biochem. Biophys. 1979;197:83–89. doi: 10.1016/0003-9861(79)90222-4. [DOI] [PubMed] [Google Scholar]
- 7.Sternweis PC, Smith JB. Characterization of the inhibitory epsilon subunit of the proton-translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1980;19:526–531. doi: 10.1021/bi00544a021. [DOI] [PubMed] [Google Scholar]
- 8.Ort DR, Oxborough K. In situ regulation of chloroplast coupling factor activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:269–291. [Google Scholar]
- 9.Kato Y, Matsui T, Tanaka N, Muneyuki E, Hisabori T, Yoshida M. Thermophilic F1-ATPase is activated without dissociation of an endogenous inhibitor, epsilon subunit. J. Biol. Chem. 1997;272:24906–24912. doi: 10.1074/jbc.272.40.24906. [DOI] [PubMed] [Google Scholar]
- 10.Kato-Yamada Y, Yoshida M, Hisabori T. Movement of the helical domain of the epsilon subunit is required for the activation of thermophilic F1-ATPase. J. Biol. Chem. 2000;275:35746–35750. doi: 10.1074/jbc.M006575200. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Murakami T, Iino R, Suzuki J, Ono S, Shirakihara Y, Yoshida M. FoF1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of epsilon subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 2003;278:46840–46846. doi: 10.1074/jbc.M307165200. [DOI] [PubMed] [Google Scholar]
- 12.Iino R, Murakami T, Iizuka S, Kato-Yamada Y, Suzuki T, Yoshida M. Real time monitoring of conformational dynamics of the epsilon subunit in F1-ATPase. J. Biol. Chem. 2005;280:40130–40134. doi: 10.1074/jbc.M506160200. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Yoshida M, Kato-Yamada Y. Role of the epsilon subunit of thermophilic F1-ATPase as a sensor for ATP. J. Biol. Chem. 2007;282:37618–37623. doi: 10.1074/jbc.M707509200. [DOI] [PubMed] [Google Scholar]
- 14.Milgrom YM, Ehler LL, Boyer PD. ATP binding at noncatalytic sites of soluble chloroplast F1-ATPase is required for expression of the enzyme activity. J. Biol. Chem. 1990;265:18725–18728. [PubMed] [Google Scholar]
- 15.Matsui T, Muneyuki E, Honda M, Allison WS, Dou C, Yoshida M. Catalytic activity of the alpha3beta3gamma complex of F1-ATPase without noncatalytic nucleotide binding site. J. Biol. Chem. 1997;272:8215–8221. doi: 10.1074/jbc.272.13.8215. [DOI] [PubMed] [Google Scholar]
- 16.Hirono-Hara Y, Noji H, Nishiura M, Muneyuki E, Hara KY, Yasuda R, Kinosita K, Jr, Yoshida M. Pause and rotation of F1-ATPase during catalysis. Proc. Natl. Acad. Sci., USA. 2001;98:13649–13654. doi: 10.1073/pnas.241365698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konno H, Murakami-Fuse T, Fujii F, Koyama F, Ueoka-Nakanishi H, Pack CG, Kinjo M, Hisabori T. The regulator of the F1 motor: inhibition of rotation of cyanobacterial F1-ATPase by the epsilon subunit. EMBO J. 2006;25:4596–4604. doi: 10.1038/sj.emboj.7601348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feniouk BA, Suzuki T, Yoshida M. Regulatory interplay between proton motive force, ADP, phosphate, and subunit epsilon in bacterial ATP synthase. J. Biol. Chem. 2007;282:764–772. doi: 10.1074/jbc.M606321200. [DOI] [PubMed] [Google Scholar]
- 19.Tsumuraya M, Furuike S, Adachi K, Kinosita KJ, Jr, Yoshida M. Effect of epsilon subunit on the rotation of thermophilic Bacillus F1-ATPase. FEBS Lett. 2009;583:1121–1126. doi: 10.1016/j.febslet.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Yasuno T, Muneyuki E, Yoshida M, Kato-Yamada Y. Modulation of nucleotide binding to the catalytic sites of thermophilic F1-ATPase by the epsilon subunit: implication for the role of the epsilon subunit in ATP synthesis. Biochem. Biophys. Res. Commun. 2009;390:230–234. doi: 10.1016/j.bbrc.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 21.Hisabori T, Kato Y, Motohashi K, Kroth-Pancic P, Strotmann H, Amano T. The regulatory functions of the gamma and epsilon subunits from chloroplast CF1 are transferred to the core complex, alpha3beta3, from thermophilic bacterial F1. Eur. J. Biochem. 1997;247:1158–1165. doi: 10.1111/j.1432-1033.1997.01158.x. [DOI] [PubMed] [Google Scholar]
- 22.Kato-Yamada Y, Yoshida M. Isolated epsilon subunit of thermophilic F1-ATPase binds ATP. J. Biol. Chem. 2003;278:36013–36016. doi: 10.1074/jbc.M306140200. [DOI] [PubMed] [Google Scholar]
- 23.Stiggall DL, Galante YM, Hatefi Y. Preparation and properties of complex V. Methods Enzymol. 1979;55:308–315. doi: 10.1016/0076-6879(79)55036-8. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi-Matsui M, Kashiwagi S, Hosokawa H, Cipriano DJ, Dunn SD, Wada Y, Futai M. Stochastic high-speed rotation of Escherichia coli ATP synthase F1 sector: the epsilon subunit-sensitive rotation. J. Biol. Chem. 2006;281:4126–4131. doi: 10.1074/jbc.M510090200. [DOI] [PubMed] [Google Scholar]
- 26.Dunn SD, Zadorozny VD, Tozer RG, Orr LE. Epsilon subunit of Escherichia coli F1-ATPase: effects on affinity for aurovertin and inhibition of product release in unisite ATP hydrolysis. Biochemistry. 1987;26:4488–4493. doi: 10.1021/bi00388a047. [DOI] [PubMed] [Google Scholar]
- 27.Iino R, Hasegawa R, Tabata KV, Noji H. Mechanism of inhibition by C-terminal alpha-helices of the epsilon subunit of Escherichia coli FoF1-ATP synthase. J. Biol. Chem. 2009;284:17457–17464. doi: 10.1074/jbc.M109.003798. [DOI] [PMC free article] [PubMed] [Google Scholar]