ABSTRACT
To better characterize the bacterial community members capable of biosurfactant production on leaves, we distinguished culturable biosurfactant-producing bacteria from nonproducers and used community sequencing to compare the composition of these distinct cultured populations with that from DNA directly recovered from leaves. Communities on spinach, romaine, and head lettuce leaves were compared with communities from adjacent samples of soil and irrigation source water. Soil communities were poorly described by culturing, with recovery of cultured representatives from only 21% of the prevalent operational taxonomic units (OTUs) (>0.2% reads) identified. The dominant biosurfactant producers cultured from soil included bacilli and pseudomonads. In contrast, the cultured communities from leaves are highly representative of the culture-independent communities, with over 85% of the prevalent OTUs recovered. The dominant taxa of surfactant producers from leaves were pseudomonads as well as members of the infrequently studied genus Chryseobacterium. The proportions of bacteria cultured from head lettuce and romaine leaves that produce biosurfactants were directly correlated with the culture-independent proportion of pseudomonads in a given sample, whereas spinach harbored a wider diversity of biosurfactant producers. A subset of the culturable bacteria in irrigation water also became enriched on romaine leaves that were irrigated overhead. Although our study was designed to identify surfactant producers on plants, we also provide evidence that most bacteria in some habitats, such as agronomic plant surfaces, are culturable, and these communities can be readily investigated and described by more classical culturing methods.
IMPORTANCE The importance of biosurfactant production to the bacteria that live on waxy leaf surfaces as well as their ability to be accurately assessed using culture-based methodologies was determined by interrogating epiphytic populations by both culture-dependent and culture-independent methods. Biosurfactant production was much more frequently observed in cultured communities on leaves than in other nearby habitats, such as soil and water, suggesting that this trait is important to life on a leaf by altering either the leaf itself or the interaction of bacteria with water. While pseudomonads were the most common biosurfactant producers isolated, this habitat also selects for taxa, such as Chryseobacterium, for which this trait was previously unrecognized. The finding that most epiphytic bacterial taxa were culturable validates strategies using more classical culturing methodologies for their study in this habitat.
INTRODUCTION
The phyllosphere is recognized to be a selective habitat in which epiphytic bacteria must be able to access limited and spatially heterogeneous nutrient supplies and endure frequent fluctuations in moisture availability on a water-repellent surface (1, 2). Recent culture-independent community analyses have confirmed the apparent selectivity of leaf surfaces, finding that leaf surfaces harbor less diverse communities than other environments, such as the immediate subtending soil (3, 4). Furthermore, plant species identity also appears to be a factor that can influence the composition of associated phyllosphere bacterial communities (5–9). While epiphytes are apparently distinct in their ability to grow and survive on leaves, their adaptations needed to thrive in this habitat remain largely unknown. Recently, we demonstrated that production of the biosurfactant syringafactin provides fitness benefits to Pseudomonas syringae on bean leaves (10). We have also observed a higher incidence of biosurfactant producers in the culturable bacterial communities on plants than in other habitats (11), suggesting that they contribute to the capacity to colonize the waxy hydrophobic leaf surface. Biosurfactants are biologically produced amphiphilic compounds that exhibit surface activity through the actions of their hydrophilic and hydrophobic groups. Epiphytic bacteria potentially use biosurfactants to increase the wettability of the leaf, to enhance diffusion of nutrients across the waxy cuticle, and/or aid in motility to favorable growth sites (2). Their many possible contributions to epiphytic fitness have led us to focus on the role of biosurfactants in bacterial colonization of leaves.
Despite the many potential roles of biosurfactants on leaves, only a few studies have examined their production in the phyllosphere (10, 12–17). Furthermore, these studies have focused only on their possible ecological role in specific strains and have not addressed the diversity or relative abundance of surfactant producers on leaf surfaces. A comprehensive examination of phyllosphere inhabitants likely would reveal strains and biosurfactants not normally encountered in other habitats. Although evidence from previous investigations link specific surfactants to particular activities, biosurfactants differ widely in their molecular structures, which could confer a myriad of properties and thus might lend them to contribute to very different functions. Furthermore, this structurally diverse class of compounds has broad industrial applications and considerable economic potential (18). Thus, in addition to better understanding their biological significance, an investigation of biosurfactant producers recovered from leaves might reveal new classes of surfactants for structure/functional analysis.
One inherent shortcoming of studies of the diversity of biosurfactant producers, as well as for any functional trait without a conserved genetic signature, is its reliance on the need to culture the bacteria. It is now widely recognized that cultured bacteria often are not representative of the community from which they are derived (19, 20); many bacteria are unculturable, and some culturable bacteria apparently are so rare in the community that they are difficult to detect with culture-independent methods. The cultivable community is clearly dictated to a great degree by the choice of medium and incubation conditions, which often differ from conditions in situ. However, agar surfaces likely approximate leaf surface conditions more than other environments, such as heterogeneous soil particles. While quantitative PCR-based methods suggest that as little as 0.1 to 5% of the bacteria on leaves are cultivable, a proportion similar to that in most other habitats (20), frequencies of 50% and higher have been recently described when more sophisticated methods are employed (21–23). Therefore, as part of our investigation into the diversity of biosurfactant producers, we also focused on identifying those bacterial taxa that might not be interrogated for lack of their culturability.
Previous studies have typically assessed biosurfactant production individually in selected cultured organisms. That strategy is quite laborious and thus usually restricts such examinations to relatively few bacterial isolates in any given study. Given the interest in biosurfactants, many studies have described new bacterial strains capable of their production (18). However, the majority of biosurfactant research to date has been focused on pseudomonads and bacilli (24), in part because they are frequently the most common biosurfactant-producing bacteria in environmental samples (11). The process by which novel biosurfactant producers can be determined is thus inefficient, requiring phenotyping of a large number of random isolates. Furthermore, unless one determines the phylogenetic identities of all of the strains tested, no information is obtained about the prevalence of a given biosurfactant-producing taxon in the examined environment, nor is it known whether this trait is characteristic of a given species. In this study, we have coupled a high-throughput assay that utilizes atomized droplets of oil to screen for biosurfactant production on agar plates (25) with a system in which large numbers of biosurfactant-producing and -nonproducing bacterial isolates cultured from leaves are separately pooled and community composition determined by high-throughput sequencing of their amplified rRNA genes. Additionally, we simultaneously characterized the total community recovered from leaves by sequencing ribosomal genes amplified from DNA recovered directly from leaves. Together, this strategy allowed us to identify even relatively uncommon biosurfactant-producing bacteria on plants, determine the relative abundance of different taxa of biosurfactant-producing organisms, calculate the frequency with which a given taxa could produce biosurfactants, and importantly, address the relative cultivability of various taxa on leaves of highly domesticated horticulture crops, in the respective production soil, and in the irrigation water sources from a major production region. We demonstrate that the genus Chryseobacterium, previously undocumented as a producer of biosurfactants, is an important and widespread biosurfactant producer on leaf surfaces, and that, unlike many other habitats, most bacterial taxa on the leaves of these plants had culturable representatives.
MATERIALS AND METHODS
Sample collection and processing.
Head lettuce, romaine, spinach, soil, and irrigation water samples were collected from commercial fields in the Salinas Valley of California on 25 April and 26 June 2012. To collect plant samples, entire lettuce and spinach plants were harvested at the base with a sterile scalpel, placed separately in polypropylene bags, and transported in coolers with ice packs. Corresponding soil and water samples were collected in 50-ml Falcon tubes. For each plant variety, four plant samples, one soil sample, and one water sample (when available) were randomly chosen for processing the same day. The water samples were from sources of water being used for irrigation. Plant samples were prepared by combining 1 to 2 leaves (head lettuce [Lactuca sativa var. capitata] and romaine [Lactuca sativa var. longifolia]; outer mature leaves were discarded, and only inner edible leaves were used) or 6 to 8 leaves (spinach [Spinacia oleracea] free of dirt or visible contaminants) from a single plant with 200 ml of washing buffer (10 mM KPO4 [pH 7.5]) in a 3.8-liter Ziplock bag. Soil samples consisted of 0.25 g of soil, which was added to 30 ml of washing buffer. The samples were sonicated (Branson 5510, 135 W, 42 KHz; Branson Ultrasonics Corp., Danbury, CT) for 5 min and agitated for 10 min. This method has been shown to remove from 60 to over 80% of all cells of epiphytic bacterial species from leaves, depending on the physical environment to which the leaves were previously exposed (26). Appropriate 10-fold dilutions were plated on 10% Trypticase soy agar (TSA; Difco) containing 1.5% agar and natamycin (21.6 μg/ml) and incubated for 4 days at 28°C. The remainder of the wash buffer or water sample was filtered on individual 0.2-μm Nalgene filter funnels (Thermo Scientific) and frozen for subsequent DNA isolation. In total, we processed 8 samples for each plant type, 6 samples for soil, and 4 samples for water. One head lettuce sample did not yield cultured bacteria (June) and thus was excluded from analyses that incorporated culture-dependent data.
Biosurfactant detection.
The atomized oil assay was performed as previously described (25); 100 randomly chosen bacterial colonies from dilution plates for each sample were evenly spotted onto TSA agar plates using sterile toothpicks and grown overnight before screening. An artist's airbrush was used to apply a fine mist of mineral oil droplets, and biosurfactant halos were visualized with an oblique source of bright light. Equivalent samples of bacterial colonies that either produced or did not produce biosurfactant were then separately collected from a given source with sterile toothpicks and transferred to a moistened filter paper (Whatman no. 1).
DNA extraction and barcoded pyrosequencing.
Filter papers (0.2 μm) from whole-leaf washes were cut into strips, added to bead-beating tubes, and processed with the PowerSoil DNA isolation kit (Mo Bio), according to the manufacturer's protocol. Whatman filter papers containing either biosurfactant-producing or -nonproducing bacteria from the atomized oil assay were added to a Falcon tube with 40 ml of washing buffer and 5 to 10 sterile glass beads and vortexed for 1 min. From this slurry, 4 ml was then centrifuged, and the pellet was added to the PowerSoil kit and processed as mentioned above. Triplicate PCRs were performed on extracted DNA, as described previously (27), amplifying a 16S region encompassing the hypervariable V5 to V7 regions with the primers 799f and 1492r, which exclude chloroplast amplification (28). PCR products for each sample were pooled and run on an electrophoresis gel. Bands corresponding to approximately 735 bp were excised and extracted from the gel (QIAquick). Final DNA concentrations were determined with a Qubit kit (Invitrogen). The final multiplex for a given plant species, soil, or water contained eight, six, or four, respectively, total community DNA samples and four pooled collections of biosurfactant-producing and -nonproducing bacterial colonies (one pool each of surfactant producers and nonproducers for each collection period). DNA of equal quantity for all samples was pooled to 15 ng/μl and sent to the W. M. Keck Center for Comparative and Functional Genomics (University of Illinois at Urbana, Champaign, IL) facility for downstream processing. Pyrosequencing was performed on 1/8th of a PicoTiter plate on the Roche GS-FLX+ system using software version 2.8 with acyclic flow pattern. This generated 101,951 high-quality reads.
Processing of pyrosequencing data.
Sequence analysis was performed using the Quantitative Insights Into Microbial Ecology (QIIME 1.8.0) software package (29). Prior to taxonomic and phylogenetic analysis, the following processing and filtering steps were performed using the standard QIIME pipeline (29): trimming of sequences to 480 bp and excluding shorter sequences, quality filtering (minimum average score of 25), assignment of sequences to samples based on their barcodes and barcode trimming, and clustering of sequences into operational taxonomic units (OTUs) at the 97% similarity level using UCLUST (30). Representative sequences of each OTU were aligned against the Greengenes core data set (31) with the PyNAST algorithm (32). Chimeras were detected using ChimeraSlayer (33) and excluded from downstream analysis. OTUs that were observed only once (singletons) were removed from the data set. This process narrowed our analysis to a total of 75,924 reads in 1,740 total OTU. Taxonomic classifications were assigned using UCLUST (30) or the RDP Classifier (34) at an 80% confidence threshold to enable comparison.
Statistical analyses.
For alpha- and beta-diversity analyses of individual samples (see Table 1 and Fig. 6, respectively), sequences were rarefied by randomly subsampling 200 reads per sample for 10 iterations. One head lettuce sample and two soil samples yielded fewer than 200 reads and were excluded from these analyses. In order to compare the cultured and uncultured (total) communities, individual samples were combined in the following manner: for culture-independent samples, each culture-independent sample was rarefied to 100 reads (the number of cultured colonies from each sample that were screened and pooled), and reads belonging to each sample type in each collection period were additively combined. For cultured samples, we combined the reads from each producing and nonproducing pair for each sample type in each collection period, rarefied to the physical number of colonies added to each pool. As an example, because 400 bacterial isolates were screened from spinach in June (cumulatively from four samples), and 119 of these produced surfactants, we added 119 reads from the cultured spinach surfactant-producing pool in June with 281 reads from the cultured spinach surfactant-nonproducing June pool to provide a balanced comparison against the combined 400 rarefied reads from the culture-independent June spinach samples. Because all samples within a comparison (replicates and corresponding culture pools; 4 to 6 samples) were required in order for comparisons to be calculated, we were unable to increase the subsample read size without reducing the total number of comparisons available for analysis. All rarefaction/pooling procedures were repeated for 10 iterations in order to mitigate random sampling bias. The communities presented in Fig. 2 are an average of the two collections (April and June) for each sample type.
TABLE 1.
Culture-based and culture-independent characterization of different agronomic habitatsa
| Parameter | Head lettuce | Romaine | Spinach | Soil | Water |
|---|---|---|---|---|---|
| Culture based | |||||
| % surfactant producers | 29 A | 28 AB | 34 A | 15 AB | 2 B |
| Population (log CFU/g) | 4.99 A | 5.19 A | 5.39 A | 6.78 B | 4.19 A |
| Culture independent | |||||
| Chao estimate | 57.6 A | 76.7 A | 91.0 A | 294.4 B | 86.5 A |
| Faith's phylodiversity | 1.76 A | 2.20 A | 2.52 A | 6.91 B | 3.42 A |
| Diversity (Chao estimate) of pooled cultured isolates | |||||
| Surfactant producers | 9.0b | 13 A | 21.1 A | 1.6b | NAb |
| Nonproducers | 37.5 A | 69.4b | 38.4 A | 41.9 A | 68.0b |
Values are averages; letters denote values that are significantly different as determined by Student's t test (P < 0.05) within row comparisons.
At least one of the two pools was excluded due to insufficient isolates (surfactant producers <80 or nonproducers <190) or low 16S rRNA gene reads, prohibiting statistical tests.
FIG 6.
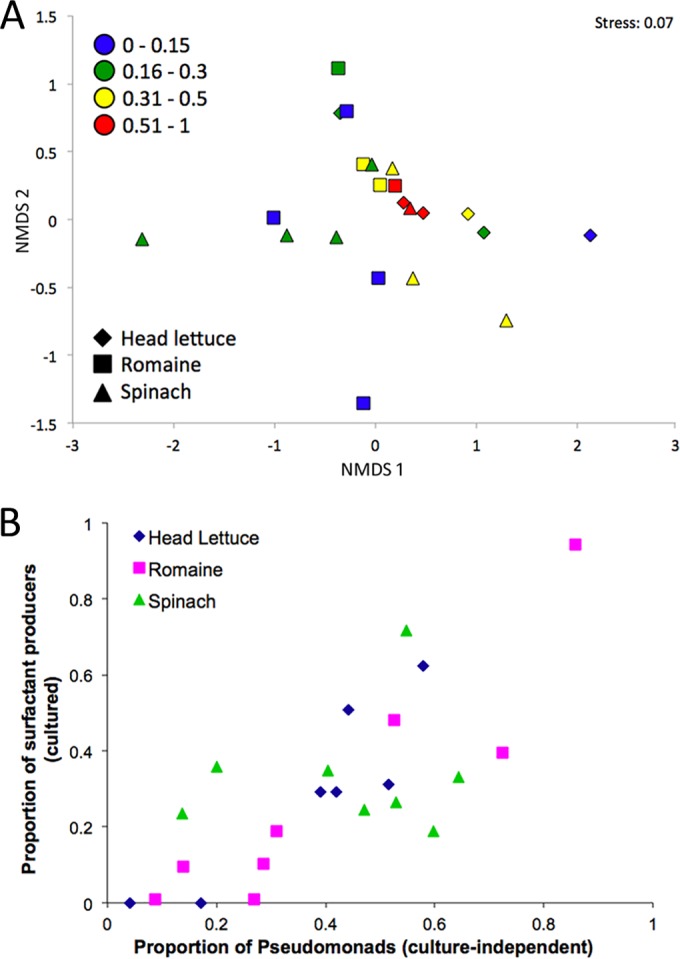
Correlations between frequency of biosurfactant production in cultured bacteria on leaves and culture-independent community structure. (A) Nonmetric multidimensional scaling (NMDS) plot based on weighted UniFrac distances comparing sequences of the total (culture-independent) bacterial communities on individual leaf samples differing in the proportion of surfactant-producing bacteria observed in the cultured community (proportions denoted by color). (B) Relationship of the proportion of pseudomonads in the total population of bacteria on leaves (abscissa) to the proportion of surfactant producers in the cultured community.
FIG 2.
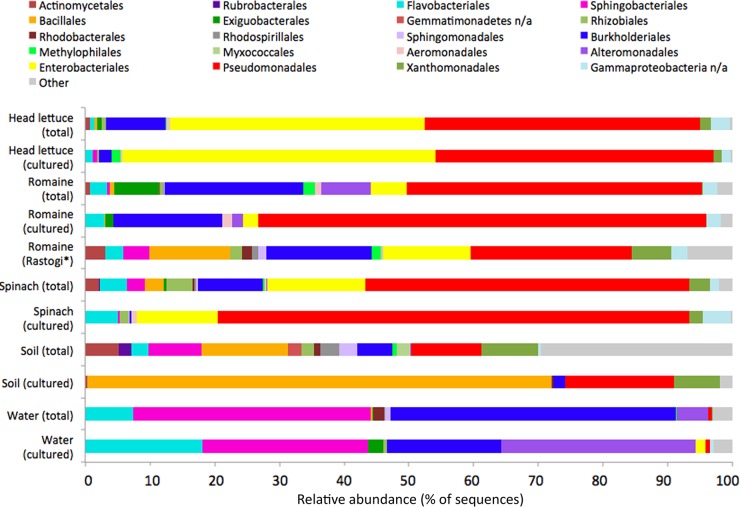
Composition of total and cultured bacterial communities on leaves and in associated water and soil samples. Culture-independent (total) communities are averages of 4 to 8 individual samples. Pooled cultured communities (cultured) reflect averages from two separate sampling times. Shown are orders that comprised at least 2% of the sequences in at least one sample of a given habitat. Unclassified orders are indicated by “n/a.” The culture-independent community of romaine as described previously by Rastogi et al. (27) is also included (“Rastogi*”).
The combined samples (rarefied to 200 reads each) were subsequently analyzed by calculating distance matrices (weighted and unweighted UniFrac, Bray-Curtis, binary, and abundance-based Jaccard) for each of the 10 iterations, and these were averaged and used to generate nonmetric multidimensional scaling plots (NMDS). Nonparametric tests were conducted using the R package vegan (35), accessed through the QIIME interface, by the functions adonis (PERMANOVA) and anosim (mantel). Student's t tests were performed for basic comparisons of collected data or beta-diversity metrics. Further statistical comparisons of collection and taxonomic data were performed in Excel and Statistica (StatSoft). Table S2 in the supplemental material was generated by averaging the 10 iterations of the combined subsamples and only including OTUs that had an average observation frequency in culture-independent samples of at least one (OTUs which represented at least 1% of the community on at least one leaf/soil/water sample had a reasonable chance of being cultured if they were indeed culturable, since 100 colonies were screened per sample). For these OTUs, it was determined what the “culturability” of each OTU was by comparing the relative proportion of that OTU in the culture-independent reads compared to that in the cultured reads. This table captured 96.8% of the cultured OTUs and 88.7% of the culture-independent OTUs. Surfactant production data were calculated by first extrapolating the estimated number of colonies of each OTU that were recorded as either producers or nonproducers (proportion of reads in a given sorted sample multiplied by the number of colonies placed in the sample) and then determining the overall percentage of estimated colonies that were assigned as producers.
Accession number(s).
The high-throughput sequencing data were submitted to the public Sequence Read Archive (SRA) of the NCBI under BioProject PRJNA309173.
RESULTS
Surfactant producers are prevalent on leaf surfaces.
To address surfactant production on leaves, we chose agricultural plants from commercial fields in the Salinas, CA growing region. On each of two dates, four replicate samples of the interior leaves from head lettuce, romaine lettuce, and spinach, as well as three adjacent soil and two irrigation water samples were examined. Bacterial cells were recovered by washing of leaves, and a portion of each sample was applied to a low-nutrient medium to culture as many cells as possible, while DNA recovered from cells directly washed from leaves or recovered from other samples was used to determine the total community composition by high-throughput sequencing of amplified small subunit ribosomal genes. The fraction of biosurfactant-producing bacteria was assessed by screening 100 randomly selected bacterial colonies using an atomized oil assay for each sample (Table 1). A higher proportion of the culturable bacteria on leaf surfaces exhibited biosurfactant production (30%) than those in soil (15%), while a significantly lower proportion (2%) of bacteria from water samples produced surfactants when tested on agar plates (Table 1). Screened colonies were then manually sorted to create pools of strains that either produced or did not produce biosurfactants from each habitat during each collection period. DNA was subsequently isolated from the pooled cultured strains to ascertain the composition of the surfactant-producing and -nonproducing members of the cultured community by high-throughput sequencing of amplified small subunit ribosomal genes.
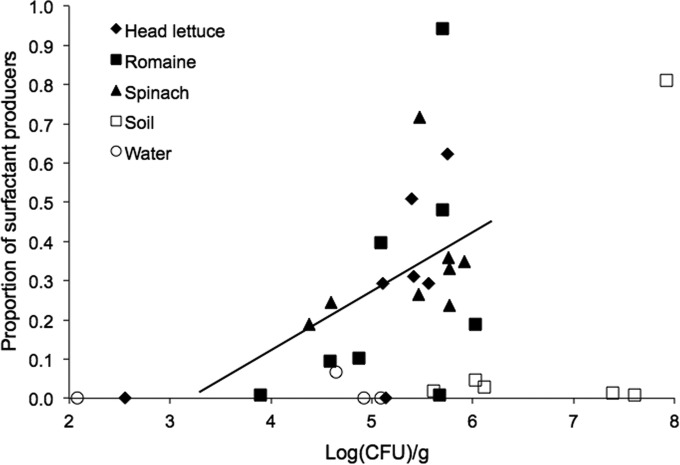
A positive correlation was observed between the frequency of surfactant production in the bacterial community and the total population size of culturable bacteria on a given leaf. This relationship was seen for each plant species as well as when leaves of all species were considered as a group (r2 = 0.24, P = 0.01) (Fig. 1). When soil and water samples were included in the correlation analysis, no such relationship was observed.
FIG 1.
Relationship between culturable bacterial population sizes in samples of leaves (filled symbols) and other habitats (open symbols) and the proportion of bacteria capable of biosurfactant production. A significant positive correlation was observed for phyllosphere samples (r2 = 0.24, P = 0.01). The line drawn represents the relationship y = 0.15x − 0.47.
Comparison of the culture-independent and culture-dependent communities of edible plants, soil, and water.
To determine the extent to which the epiphytic bacterial communities on these various plant species were culturable, community composition was assessed from DNA recovered directly from leaves (total bacteria), as well as DNA from the pooled cultured communities (see Materials and Methods). Chao and Faith's phylodiversity (PD) estimates calculated from the culture-independent approach revealed that the spinach leaves harbored more complex communities than interior romaine leaves, and that both were more complex than interior head lettuce leaves (Table 1). Similar to other studies (3, 36), we observed much higher diversity in soil samples than on leaf surfaces. The diversity of irrigation water as measured by Chao was similar to that of leaves, while estimates made using Faith's PD suggested that water communities were more phylogenetically diverse than those on the edible plants, although still substantially less than soil communities.
Not surprisingly, the compositions of the abundant taxonomic groups of bacteria identified using culture-independent analyses were much more similar on the various plant species studied compared to those of nearby soil and water (Fig. 2). Similar to other studies of edible leaf communities (27, 37), Pseudomonadales (primarily Pseudomonas) was the most dominant taxon observed on leaf surfaces. While on average they represented 46% of the bacteria on leaves, their proportion on individual leaf samples varied widely, ranging from 4 to 86%. Burkholderiales (14%) and Enterobacteriales (20%) were also commonly found on the leaves of all three plant species. In agreement with corresponding estimates of bacterial diversity, the fewest orders of epiphytic bacteria were observed on head lettuce, while the most were observed on spinach leaves. Furthermore, the plant species significantly influenced the leaf community, as measured by an anosim test based on unweighted UniFrac distance matrices (r = 0.16, P = 0.02). Seasonal dynamics have been reported to impact the phyllosphere community (27), and we also observed a significant influence of the sampling date on the leaf community by anosim (r = 0.24, P = 0.005). In contrast to leaf samples, we observed a much larger variety of bacterial taxa in agricultural soils, consistent with the relatively high Chao and PD values measured. These taxa were also more evenly distributed, lacking a dominant taxon, a common observation made in other unsaturated surface soils (38). Furthermore, we observed a low density of archaea (Nitrososphaerales, 3%) in soil samples, but we chose to exclude this from our analysis due to their inability to be cultured under our laboratory conditions.
A prior study by Rastogi et al. (27) provided a comprehensive culture-independent analysis of romaine epiphytes collected from the same growing region as that in our study but in a different year. The results of that study are compared to those of the current study shown in Fig. 2. Whereas we sampled only internal leaves from romaine plants, samples in the earlier study also included the most exterior leaves. All orders that were observed at a higher frequency in soil than on romaine leaves in this study also constituted a higher proportion of the community on spinach in this study, as well as on romaine leaves in the earlier study. These orders also constituted a lower relative proportion of the bacteria recovered from head lettuce. Thus, both the outer leaves of romaine lettuce and spinach appear to harbor bacteria of soil origin to a greater degree than those of the inner leaves of either romaine or head lettuce. In a comparison at the genus level of phylogenetic classification, Massilia was observed at a high relative abundance (5%) in the earlier study but was not found in our analysis. However, Rastogi et al. (27) used the RDP pipeline for phylogenetic analysis, while we used UCLUST. When the phylogenetic placement of our sequences was reassigned using the RDP Classifier, some of our unassigned OTUs previously grouped within Burkholderiales were reclassified as Massilia (data not shown). The only genera observed in the earlier study of romaine lettuce samples at a frequency of 1% prevalence or higher that we did not also find in this study, even when both data sets were analyzed with the RDP Classifier, were Duganella (2%) and Naxibacter (1%), neither of which were considered part of the “core” romaine community.
It was possible to address the extent to which cultured bacterial communities in different habitats reflected those determined by culture-independent methods. An accurate estimate of the composition of cultured bacterial communities was obtained by sequencing small subunit ribosomal amplicons in pools of large numbers of randomly collected biosurfactant-producing and -nonproducing bacterial strains. Because the number of colonies placed into each cultured pool was known, and similar volumes of each colony were introduced, we used weighted values to reconstruct the composition of overall cultured communities in a given sample. In this manner, we could compare the abundance of different culturable bacterial orders (representing both biosurfactant producers and nonproducers) with that determined from sequencing of amplicons from cells directly recovered from samples (Fig. 2). Overall, the relative abundance of a given bacterial order in cultured communities closely matched that of the total (culture-independent) communities on the leaves of a given plant species (Fig. 2). All orders of bacteria detected on plants at a frequency greater than 0.2% in culture-independent analyses were also present within the cultured communities. In contrast, the relative portion of different bacterial orders of cultured strains from soil often was very different from that estimated by direct DNA sequencing (Fig. 2). For example, Bacillales dominated the cultured community (72%) but represented only 13% of the sequences from DNA isolated directly from soil. Orders that comprised more than 1% of the uncultured soil population but which were not found in any cultures included Myxococcales, Rhodospirillales, Rubrobacterales, and an unidentified order belonging to Gemmatimonadetes. For agricultural water samples, the relative proportions of several dominant taxa differed substantially between cultured and total bacterial populations; several bacterial orders dominant in the culture-independent communities, such as the Aeromonadales, were much less abundant in the cultured bacterial community and vice versa (Fig. 2).
While at the level of order, bacterial cells on leaves appeared to be highly culturable, we examined culturability at a higher taxonomic resolution to determine if there were taxa that were less likely to be culturable. In order to estimate the culturability of the bacteria in our study, we calculated the ratio of the proportional incidence of occurrence of a given genus with that found in culture-independent analyses (Table 2), taking into context the habitat in which they were identified and the class in which they were found. For the genera examined (those with a prevalence of at least 0.2% in a given habitat), 60% of the genera from plants were cultured, 40% from the water samples, and 18% from soil. Additionally, more than half of the genera that were cultured from plants had very similar proportional representations in the cultured and total reads (incidence ratios close to one). We also distinguished these strains at the OTU level of classification for a given habitat and for their overall prevalence (Tables S1 and S2 in the supplemental material, respectively). Most of the common OTUs of culturable bacteria on leaves were also the most dominant in total bacterial communities, as determined by culture-independent assessment. For instance, the OTU corresponding to the surfactant-producing species Pseudomonas viridiflava accounted for 6% of culturable bacteria on leaves and also represented 13% of sequences of DNA directly isolated from leaves. Likewise, an OTU that includes Pseudomonas fluorescens was very dominant on leaves, accounting for 49% of the cultured bacteria and 30% of all sequences of directly isolated DNA. Overall, 40 OTU were detected at a frequency of at least 0.2% of the sequences determined from direct DNA isolation from leaves. Only six of these 40 OTU were not represented in the cultured bacteria from leaves (see Table S1). In contrast, 60 of the 76 OTU detected at a frequency of at least 0.2% of the sequences determined from direct DNA isolation from soil were not cultured. Some genera shown in Table 2 were eliminated at this resolution if their component OTUs cumulatively accounted for >0.2% of the sequences, but no OTU individually reached this prevalence. Four of the six OTU on leaves without culturable representatives were present at a very low frequency in the culture-independent community (less than 0.35%), and their lack of culturable representatives might reflect inadequate sampling for such relatively rare members. Interestingly however, an OTU representing the genus Alkanindiges (Pseudomonadales), an alkane degrader, was regularly observed in sequences from direct DNA isolated from leaves (1% of total sequences) but was not encountered in the cultured community. Likewise, an OTU representing Buchnera (Enterobacteriales), an obligate and unculturable aphid endosymbiont, was found only in one collection of spinach leaves assessed by the culture-independent method (at a high density up to 14% of sample sequences). Thus, while most bacterial taxa on leaves appear to be highly culturable, at lower phylogenetic levels, a few culture-resistant community members, presumably having specialized habitat requirements, can be identified. The relative cultivability of all OTUs that were observed at a frequency of at least 1% in at least one sample of DNA directly isolated from various habitats, calculated as a ratio of its frequency of occurrence in cultured cells of that same sample, is shown in Table S2.
TABLE 2.
Cultivability of different genera determined as the ratio of the proportional incidence of a given genus in the cultured bacteria from a given habitat with that found in bacteria culture-independent analysesa
| Taxon | No. by cultivability ratio |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plants |
Water |
Soil |
||||||||||
| 0 | <0.5 | ∼1 | >1.5 | 0 | <0.5 | ∼1 | >1.5 | 0 | <0.5 | ∼1 | >1.5 | |
| Acidimicrobiia | 2 | |||||||||||
| Actinobacteria | 2 | 4 | 1 | |||||||||
| Alphaproteobacteria | 1 | 2 | 9 | 2 | ||||||||
| Anaerolineae | 1 | |||||||||||
| Bacilli | 2 | 1 | 1 | 2 | ||||||||
| Betaproteobacteria | 1 | 2 | 1 | 1 | 1 | 8 | 2 | 1 | ||||
| Deltaproteobacteria | 2 | 8 | ||||||||||
| Fimbriimonadetes | 1 | |||||||||||
| Flavobacteriia | 1 | 1 | 1 | |||||||||
| Gammaproteobacteria | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | ||||
| Gemm-2 | 2 | |||||||||||
| Gemmatimonadetes | 4 | |||||||||||
| JL-ETNP-Z39 | 1 | |||||||||||
| Nitrospira | 1 | |||||||||||
| Pedosphaerae | 2 | |||||||||||
| Proteobacteria NA | 1 | 1 | ||||||||||
| Rubrobacteria | 1 | |||||||||||
| Solibacteres | 1 | |||||||||||
| Sphingobacteriia | 2 | 1 | 3 | 1 | 1 | 5 | ||||||
| Sva0725 | 1 | |||||||||||
| Thermoleophilia | 4 | |||||||||||
| TK17 | 2 | |||||||||||
Values reflect the number of genera observed in each class present in at least 0.2% of the culture-independent reads (by habitat) in columns corresponding to their cultivability ratios.
Comparison of biosurfactant-producing and -nonproducing bacteria.
The richness and diversity of surfactant-producing and -nonproducing bacterial communities was estimated by high-throughput sequencing of amplicons derived from pooled cultures sorted for this phenotype. A minimum of 80 and 190 isolates, respectively, in pools of surfactant producers and nonproducers, were used to generate Chao estimates of the total number of different taxa in each pool (Table 1); if insufficient isolates were obtained during culturing (such as surfactant producers from water), Chao estimates were excluded from this analysis. Spinach had a tendency to host more diverse populations of surfactant-producing bacteria than the other plant species examined, although there were not enough replications to determine statistical significance. In general, more phylogenetic diversity was observed in pools of bacteria that did not produce biosurfactants than in those that did (Table 1).
The taxonomic placement of those bacteria capable of producing biosurfactants differed somewhat between the different habitats (Fig. 3). The dominant surfactant-producing bacteria recovered from plants were Pseudomonadales (89% of all biosurfactant producers recovered from this habitat). In addition, Flavobacteriales, primarily Chryseobacterium, which has not previously been demonstrated to produce biosurfactant, was often isolated from plants (7% of isolates). While not frequently isolated, several strains of Xanthomonadales (1% of all biosurfactant-producing isolates from plants) and Enterobacteriales, specifically Erwinia spp. (1% of isolates) that produced biosurfactant, were recovered from plants. Curiously, unlike other orders that were consistently isolated from a given habitat over time, Exiguobacterales (Exiguobacterium) accounted for 5% of the sequences recovered from biosurfactant-producing bacteria cultured from romaine lettuce in June (and DNA signatures of this taxon were also observed on each romaine sample subjected to direct DNA sequencing in June, with an average frequency of 13%), but cultured representatives capable of biosurfactant production were not observed in any other leaf samples, and thus it may not be a common biosurfactant-producing taxon on leaves. In addition, very small numbers of Bacillales (Bacillus and Paenibacillus) were identified as cultured biosurfactant producers in a single surfactant pool from romaine leaves, yet they were commonly recovered in relatively high numbers from soil. A few additional biosurfactant-producing taxa were found rarely in leaf samples (Agrobacterium and Sphingobacterium), primarily from spinach. Due to our sampling process, it was impossible to determine if any taxa were erroneously introduced into our pools of surfactant-producing bacteria due to the occurrence of mixed colonies. Therefore, we have included in Table S2 in the supplemental material a column that includes the frequency at which a given cultured OTU was observed to be a surfactant producer (as opposed to a nonproducer) for all OTUs that occurred at a frequency of 1% or higher in at least one sample, with the presumption that a taxon that was frequently observed to produce surfactants is most likely a true surfactant producer. Additionally, we have created a large library of purified biosurfactant-producing strains, such as those of Agrobacterium, Chryseobacterium, and Sphingobacterium, many of which were taxa that were frequently identified as producers in this survey. For the most part, OTUs that were observed to produce surfactants at low frequencies (see Table S2) belonged to families and genera that also contained one or more OTUs that produced with high frequencies (or were observed in our strain library of biosurfactant producers). Furthermore, many cultured families and genera were never observed in the biosurfactant-producing pools, indicative of very little contamination from mixed colonies. However, one OTU belonging to the family Oxalobacteraceae was observed in our surfactant pools with only 1% frequency of being a surfactant producer, whereas the 8 other Oxalobacteraceae OTU (5 of which were cultured) were not observed to be surfactant producers. Therefore, this OTU was likely introduced as part of a mixed colony, and we make no claims on its ability to produce biosurfactants.
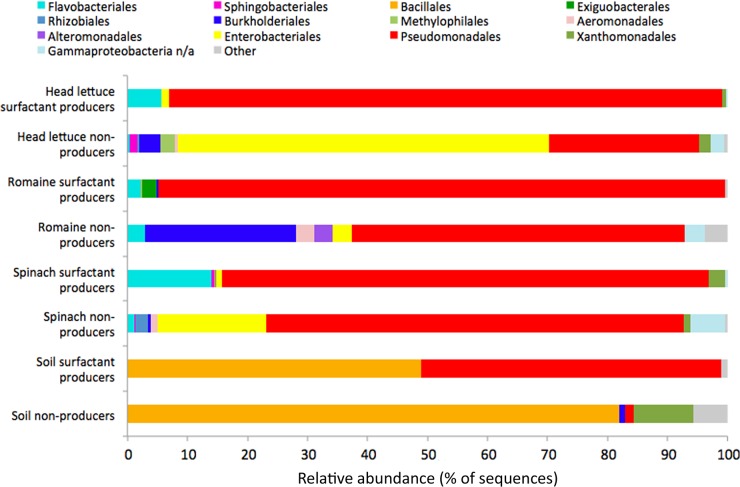
FIG 3.
Composition of cultured bacteria characterized as surfactant producers and nonproducers from various habitats. The bars shown are averages of the proportional incidence of isolation from two separate times of collection. The orders shown were observed in at least 1% of the amplicon sequences in at least one of the pools. Unclassified orders are indicated by “n/a.” Water samples are excluded due to the low number of surfactant producers recovered.
The incidence of culturable biosurfactant-producing bacteria in soil was much less than that of plants. These strains were primarily composed of Bacillales and Pseudomonadales. The incidence of biosurfactant-producing bacteria in irrigation water was also very low, with representatives of only the orders Exiguobacterales (60% of the reads recovered), Rhizobiales (Agrobacterium, 26%) and Burkholderiales (13%) recovered; only 7 colonies were pooled to obtain these values for water samples.
The predominant culturable plant-associated taxa incapable of producing biosurfactants (nonproducers) differed substantially from those capable of producing biosurfactants. The dominant cultured nonproducers recovered from plants were Pseudomonadales (47% overall), Enterobacteriales (29%), and Burkholderiales (13%). It was noteworthy that Pseudomonadales was a predominant taxon of both cultured biosurfactant-producing and nonproducing bacteria. It seemed possible that at a higher level of genetic resolution, biosurfactant-producing and -nonproducing Pseudomonadales might be distinguished from each other. When we examined the frequency with which each pseudomonad OTU was associated with biosurfactant production (see Table S2 in the supplemental material), we observed many more OTUs that included both surfactant-producing and -nonproducing representatives than OTUs that consisted solely of surfactant producers. Two predominant surfactant-producing Pseudomonas OTU were identified as examples at either end of the spectrum (primarily producer or mixture). One, characterized as Pseudomonas viridiflava, accounted for 9% of all culture-independent reads and had a 98% chance of being identified in culture as a surfactant producer. This OTU was observed on leaves of each plant species but was not observed in any soil or water samples. In contrast, another Pseudomonas OTU accounted for a staggering 23% of culture-independent reads and included strains that both produced and did not produce biosurfactants (44% chance of being identified in culture as a surfactant producer). While the definition of OTUs at a higher level of similarity (99%) identified more OTUs, each of which contained fewer representatives, cultured biosurfactant producers and nonproducers were still not unambiguously associated with a given OTU (data not shown). Thus, whereas biosurfactant production can be associated relatively unambiguously with a few OTUs (such as Pseudomonas viridiflava), it is clear that 16S rRNA signatures of the V5 to V7 hypervariable region are not predictive of this phenotype in most cases.
Examining underlying microbial structures.
The relative structure and beta diversity of cultured and total bacterial communities from various habitats were addressed using nonmetric multidimensional scaling (NMDS) plots. This abundance-based analysis, generated from weighted UniFrac distances, revealed that both cultured and uncultured bacterial communities from plants were distinct from those in either water or soil (Fig. 4A; see also Fig. S1 in the supplemental material). Whereas the separate collections of cultured soil bacteria clustered closer to each other than to total soil bacteria, cultured and total bacteria from head lettuce and romaine leaves were nearly coincident at a given sampling time (particularly in April), highlighting the effectiveness of our culturing method in preserving epiphytic community structure. Other analyses using Bray-Curtis and abundance-based Jaccard metrics revealed similar patterns.
FIG 4.
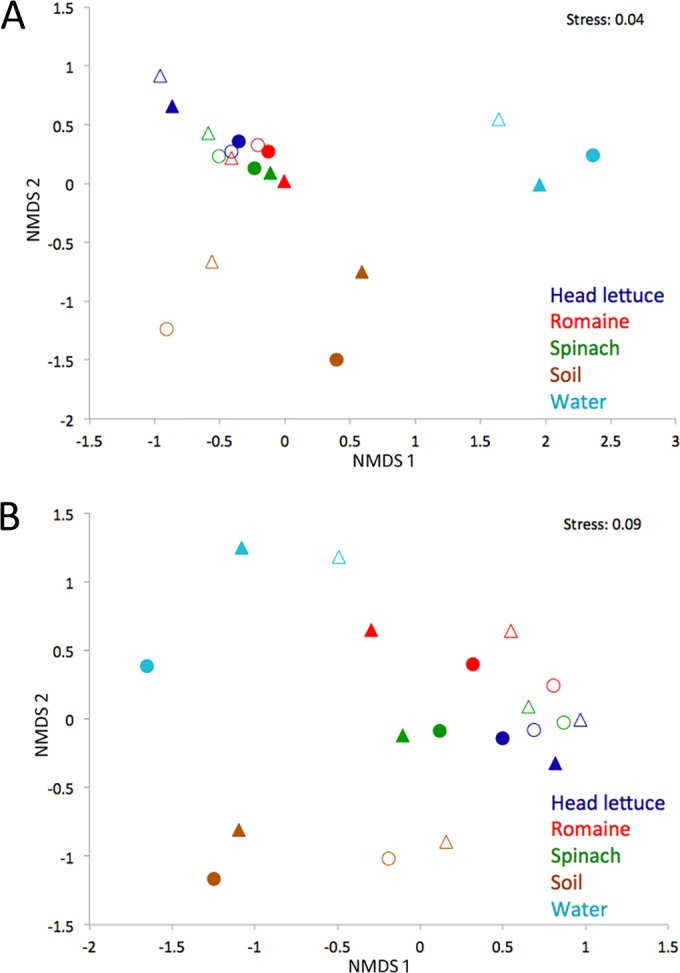
Nonmetric multidimensional scaling (NMDS) plots based on weighted UniFrac (A) and binary Jaccard (B) distance matrices comparing sequences of total bacteria (filled symbols) and cultured bacteria (open symbols) recovered from various habitats (color-coded) sampled in April (circles) or June (triangles).
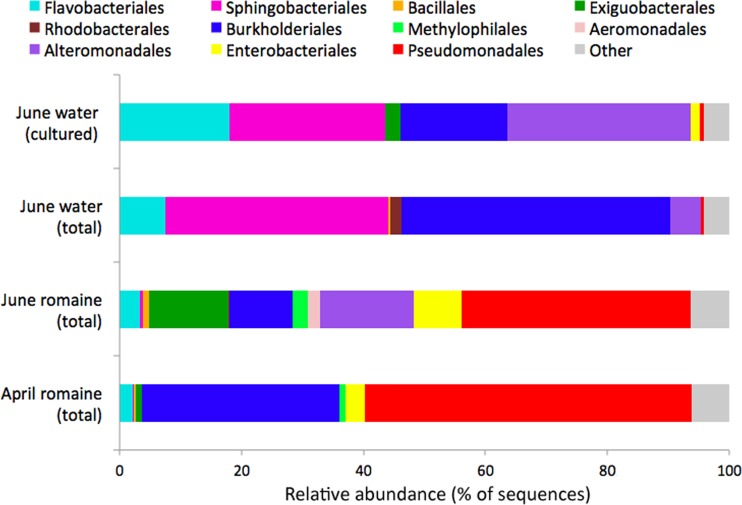
Because the majority of the leaf-associated bacterial communities were composed of relatively few OTUs (the most abundant 40 OTU accounted for 88.8% of all sequences), we also addressed the community structure with binary Jaccard matrices in order to incorporate less-common bacteria in our analysis (Fig. 4B). When each OTU was considered independent of its relative abundance, the communities in different habitats were slightly less distinguishable from one another. Overall, cultured bacteria from leaves all clustered together and differed most from those of total soil and water communities. Total bacteria from head lettuce leaves were most similar to cultured bacteria from leaf samples, while those from spinach were much less similar (Fig. 4B). Interestingly, the total bacterial community on one romaine lettuce collection (June) was positioned surprisingly close to that of the cultured bacterial community from water (June). We had already observed that a surfactant-producing Exiguobacterium strain was common both on these romaine lettuce leaves and in the nearby irrigation water. NMDS analysis, however, suggested that these samples shared other bacteria as well. When we expanded our binary Jaccard analysis to include individual leaf samples (instead of only the combined communities), we observed that the communities on romaine samples collected in June were significantly more similar to the cultured communities in water samples from June than that of total (culture-independent) communities in the June water samples (P < 0.05). Additionally, the communities on romaine leaves in June are significantly more similar to the cultured communities in water collected in June than are those on romaine leaves collected in April (P < 0.005). Although one might speculate that the bacteria on these romaine lettuce leaves are derived from irrigation water that might have been deposited onto leaves, it is important to note that the total bacteria on romaine clustered more with the cultured bacteria from water than from total water bacteria, suggesting that not all waterborne bacteria were equally present on leaves. Rather, the data suggest that specific immigrants (the ones that are easily cultured) from irrigation water subsequently flourished on these leaves. A more detailed analysis of the culturable and total bacterial communities on water and romaine lettuce provides support for the conjecture of selective enrichment of some taxa from water on leaves. As an example, bacteria belonging to orders, such as Alteromonadales, were present in the total bacterial community of water and were enriched in both the culturable water communities and the total bacterial community on sprinkle irrigated romaine (Fig. 5). On the other hand, Rhodobacterales were 2% of the total water community in June, but the corresponding OTUs belonging to this order were never observed in cultured water samples or on leaves wetted with that water. Thus, romaine leaves might be considered “green petri dishes” in that they appeared to enable the proliferation of culturable bacteria in irrigation water.
FIG 5.
Comparison of the cultured and total bacterial communities in irrigation water in June, as well as the total bacterial communities on romaine leaves in June and April.
Correlating underlying community structure with observable traits.
While we have demonstrated that when considered over many plant species and samples, cultured bacterial communities on leaves closely resemble those of total bacterial communities, we questioned whether ascertaining particular phenotypes of the cultured community would be predictive of the structure of the total bacterial communities on leaves. Since data for the proportional representation of biosurfactant producers on multiple independent samples of each of three plant species as well as total community composition were available, we determined the relationship of these factors using UniFrac distance-based NMDS plots and hierarchical clustering (Fig. 6A; see also Fig. S2 in the supplemental material). This analysis revealed that the individual leaf samples exhibiting the highest proportions of culturable surfactant-producing bacteria grouped together irrespective of the plants on which they were found, whereas those samples having the lowest incidence of surfactant-producing bacteria did not cluster together (Fig. 6A), apparently driven by the higher diversity of surfactant-nonproducing than surfactant-producing bacteria. Probably because of the high diversity of nonproducers, there was not a statistically significant relationship between the incidence of biosurfactant production and overall community structure, as determined using an adonis analysis.
Because the majority of the cultured surfactant producers from leaves are pseudomonads, we specifically tested for the predictive nature of biosurfactant-producing bacteria as an indicator for the prevalence of these organisms. A high correlation between surfactant production and the proportion of Pseudomonadales in a given sample was observed (r2 = 0.57, P < 0.005) (Fig. 6B). This relationship was very strong for head lettuce (r2 = 0.81, P < 0.03) and romaine leaves (r2 = 0.83, P = 0.01) but not for spinach leaves (r2 = 0.02, P > 0.95). It should also be noted that spinach harbored the most diverse surfactant producers, as well as the largest proportion of Pseudomonadales categorized as nonproducers (Fig. 3).
DISCUSSION
The majority of biosurfactant research to date has focused on the diverse products synthesized by pseudomonads and bacilli (24), and the identification of a broader diversity of biosurfactant-producing bacteria should help identify novel biosurfactants with potential new industrial applications. Our research has previously suggested that the phyllosphere is an environment that hosts particularly high frequencies of biosurfactant-producing bacteria (11). In the current study, we observed similarly high frequencies of biosurfactant-producing bacteria on leaf surfaces (∼30%) and provide comprehensive information on the identity of those bacteria that produce surfactants in the phyllosphere. As suggested from limited studies of other plant species (11, 16), we found pseudomonads to be the predominant biosurfactant-producing taxon on leaves. It was unexpected, however, that Chryseobacterium would be found to be a relatively common genus capable of biosurfactant production on all plant species, as it is a poorly studied taxon with no prior strains demonstrated to produce surfactant. Given the large number of studies that have attempted to isolate biosurfactant producers from other habitats, such as soil and water, Chryseobacterium strains with this phenotype might be common only on plants. Other genera from plants that were found to produce surfactants included Agrobacterium, Bacillus, Erwinia, Exiguobacterium, Paenibacillus, Rhodanobacter, and Sphingobacterium (see Table S2 in the supplemental material). While limited reports have appeared that note surfactant production in some of these taxa, more work will be needed to understand the significance of their biosurfactant production in the natural habitats in which they have been described. In order to address this issue in the future, we have established a large library of biosurfactant-producing isolates, including many representative isolates, such as Chryseobacterium spp., for further studies. Our current report uses one assay to broadly assess biosurfactant production, and future purification, tensiometric measurements, and chemical characterization will help elucidate how these surfactants differ in structure and function.
Our approach to also identify the bacteria incapable of biosurfactant production provided insight into the frequency with which biosurfactant production is exhibited by a given OTU (see Table S2 in the supplemental material). It is clear that biosurfactant production is not an invariant phenotype associated with a given taxon, and that strains with similar ribosomal sequences are physiologically diverse. However, some taxa are more frequently observed to produce surfactants than others; this type of information could allow us to infer levels of endogenous biosurfactant production on different plant species in future culture-independent studies of the phyllosphere.
Previously, we have observed a positive correlation between the incidence of surfactant-producing bacteria and the overall population size of Pseudomonas syringae on a given leaf of inoculated plants using mixed-inoculum experiments (10), suggesting that biosurfactant production increases the fitness of P. syringae on leaf surfaces. Similarly in this study, we observed a positive correlation between the relative abundance of surfactant-producing bacteria and total culturable bacterial population size. It thus appears that biosurfactants might be generally beneficial to bacteria on leaves. This hypothesis is also supported by the overall high frequency of surfactant producers encountered in the phyllosphere compared to other environments.
Considerable evidence suggests that most bacteria in most habitats remain uncultured (19). These observations have prompted many in the microbial ecology community to disregard community analyses that examine only those community members that are culturable. Unfortunately, only a few other studies have addressed the culturability of phyllosphere bacteria (22, 23, 39). The low incidence of culturability is particularly noteworthy in soil, where the apparent presence of a heterogeneous mixture of microenvironments with distinct chemical and physical restrictions presumably select for bacteria with distinct features capable of exploiting only localized regions of the soil (19). Indeed, in this study, we were only able to culture representatives of 16 of the 76 relatively abundant bacterial OTUs in the soil community. However, this study supports the findings of a few recent studies that reveal that not all habitats harbor bacteria that are similarly difficult to culture, since cultured representatives of 34 out of 40 relatively abundant (>0.2%) OTU on leaves could be recovered. Although this was surprising, it was not unprecedented; a recent study of phyllosphere bacteria using fluidic force microscopy demonstrated that 69 of 100 randomly chosen cells of clover epiphytes recovered from leaves were capable of growth in a low-nutrient medium (22), and high-throughput sequencing efforts in Arabidopsis similarly found nearly 50% of the relatively abundant (>0.1%) OTU determined by direct DNA sequencing to also be represented in a library of cultured strains from these same samples (23). Furthermore, a previous study on the apple phyllosphere sequenced 300 clones and found that 85% of them corresponded to known cultured OTUs, even though 4′,6-diamidino-2-phenylindole (DAPI) staining would have indicated that less than 1% of the cells were enumerated by culturing (39). Thus, while a relatively high proportion of the bacterial diversity on leaves apparently is culturable, it also suggests that a high proportion of the bacteria on leaves are dead at a given time in some settings, a finding consistent with the harsh environmental conditions and heterogeneous nutritional distribution expected on leaves and supported by some studies of the viability of particular taxa on leaves (40).
Although the leaf surface probably offers epiphytes a diversity of habitats differing in water availability and nutrient abundance, etc., it appears that much of the growth of epiphytes on leaves occurs at relatively few sites where nutrients are locally more abundant (41). It seems likely that these most favorable sites for bacterial growth provide primarily plant-produced sugars and other simple nutrients (42, 43). Furthermore, those bacteria that could thrive on the simple sugars, organic acids, and amino acids that might predominate on leaves (44) probably also can grow well on laboratory media consisting of low concentrations of similar nutrients, such as those used in this study. The high similarity in the community composition seen between those on romaine leaves and the bacteria that could be cultured from the irrigation water suggests that the converse may also be true: bacteria capable of growth on simple nutrients in culture might readily colonize leaves given other appropriate environmental conditions that would otherwise limit their growth. It is generally agreed that the leaf surface is a harsh environment with rapid fluctuations in bacterial population densities; it also would be expected that the high flux of UV irradiation and processes that would lead to cell lysis would quickly destroy genetic signatures of the bacteria upon death. Such rapid genetic degradation would cause the analysis of epiphytic communities by sequencing of total extractable DNA to resemble that of the culturable/viable bacteria, assuming that most of the bacteria on leaves could be cultured.
Similar to studies of other edible green vegetables, we found that the majority of bacteria on our leaf samples could be assigned to Pseudomonadales and Enterobacteriales (27, 45, 46). Although Methylobacterium spp. have been noted as important members of soybean, Arabidopsis, and clover leaves (3), they were only observed at low densities on a few of our leaf samples (primarily in April). Likewise, Sphingomonas species were also commonly found on other plants (3) but were quite uncommon on the plants studied here. While the most abundant bacterial taxa on leaves had a very high probability of being cultured, several taxa with apparently unique features that would make them unlikely to be easily cultured were also detected. Alkanindiges spp. were never cultured but were commonly observed as a small proportion of the total bacterial community from leaves, similar to other studies of lettuce (6, 27). It seems likely that this taxon would be common on leaves due to its apparent ability to catabolize the alkanes found in leaf surface waxes. As such, they may coexist with other leaf colonists because of nutritional niche partitioning and thus would not be cultured with our standard medium that lacks the apparently unique carbon sources that they consume. In contrast, the obligate aphid gut symbiont that was detected by culture-independent methods on the June spinach samples probably represents contamination that was likely associated with an insect infestation; our sampling methods would not have excluded small aphid body parts or gut fluid.
Other studies have used culture-independent methods to show that different plant species harbor somewhat distinct bacterial communities, including previous comparisons of head lettuce and romaine, as well as different ecotypes of a single plant species (Arabidopsis); this is perhaps due to varied leaf surface morphology, differential availability of soluble carbohydrates, and/or other compounds present on the leaf surface (6, 47). Similarly, we observed significant differences between the types of bacteria present on the different species of plants examined in this study. Head lettuce leaves harbored the least diverse bacterial community while also exhibiting the lowest dissimilarity between its total and culturable communities. Conversely, spinach harbored the most diverse communities, and compared to the other plants, its total bacterial communities were the most similar to soil communities (Fig. 4B). It is notable that the structure of leaves of these plant species vary, with head lettuce presumably providing a more protected environment for bacteria due to the closed nature of its canopy. Due to the overlapping leaves, epiphytes likely would be less influenced by external factors and sources of immigrant bacteria, such as soil. In contrast, romaine and spinach both have a more open canopy structure, and the short stature of spinach places its leaves in close proximity to soil, thereby facilitating immigration from this source. A comparison of the results of community analysis of romaine lettuce in this study and those of Rastogi et al. (27) provide further support for the impact of the proximity of soil on leaf bacterial communities. Whereas internal leaves of romaine lettuce were sampled in this study, Rastogi et al. (27) included the most exterior romaine lettuce leaves. While taxa common in soil were infrequent colonists of romaine lettuce in our study, they were relatively much more abundant on the leaves in the Rastogi et al. study, presumably reflecting immigration of soil or soilborne bacteria to the outermost leaves of their plants. The inner leaves studied here presumably would be resistant to such immigration. The nature of the external environment probably also strongly influences bacterial community composition on leaves, as factors, such as temperature, moisture, and light intensity and duration, would select for particular bacterial fitness traits or species but also influence host plants. Other studies have documented leaf community variability due to seasonal dynamics (27, 45, 48, 49). Likewise, we also observed significant differences in leaf community compositions on our agricultural samples collected from the same region but on different dates in April and June. Studies to isolate the particular environmental factors that can lead to seasonal changes in community composition should be fruitful.
Even if most dominant epiphytes are culturable, we were still surprised at the quantitative similarity of culturable epiphytic communities and those determined by culture-independent methodologies. This finding is in disagreement with many other phyllosphere studies that have suggested that the uncultured community is very different from that obtained by culturing. However, these discrepancies are probably due in large part to the different methodologies used in these studies. Some previous studies have used other methods, such as broth culturing, in which competition in vitro could quickly alter the composition of communities from those originally recovered from plants (50). Furthermore, some studies of epiphytic communities have been made on plants where epiphytic populations were likely very low (50, 51) and thus particularly subject to the effects of emigration from outside sources of bacterial inoculum, or from contaminating sequences present in the reagents and equipment used, a problem which has recently been shown to be acute in such a setting (52–54). In our study, we attempted to minimize the overrepresentation of bacteria capable of rapid growth by culturing the cells on solid media having low nutrient concentrations that presumably would not inhibit more oligotrophic colonists. Using this procedure, the only OTUs that would not be represented in the cultured community either would be unable to grow on plates or would require more than 4 days to produce a visible colony. (Very few such slow-growing colonies were observed, however.) Variation in cell size and the presence of extracellular compounds could have impacted the biomass of a given bacterium harvested and thus the proportion of its 16S rRNA gene signature in a pool in our process of determining culturable communities. Despite such possible sources of variation, the proportional representation of many taxa by culturing closely matched that determined by a culture-independent methodology (ratio close to 1, Table 2; see also Table S2 in the supplemental material). Although these conditions appear optimal for epiphytic bacteria, it is likely that different media and/or incubation periods would likely increase the apparent culturability of soil and water bacteria (55).
This study supports other recent studies that suggest that, contrary to popular belief, culture-based assays can be highly practical indicators of the true underlying community structure in certain natural habitats, such as leaves. Therefore, surveys of surfactant production or other traits of phyllosphere communities can provide a comprehensive assessment of the diversity of bacteria in the leaf habitat. These results also suggest that the large earlier body of work on phyllosphere microbiology that used culture-based methodologies is robust and can safely be used to guide new experiments using different technologies.
Supplementary Material
ACKNOWLEDGMENTS
We express our gratitude to the CA Leafy Greens Research Board and cooperating growers for facilitating farm access. We are grateful to Renee Koutsoukis for experimental assistance and Chris Wright at the W. M. Keck Center for Comparative and Functional Genomics for technical assistance. We also thank Despoina Lymperopoulou for critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01751-16.
REFERENCES
- 1.Hirano SS, Upper CD. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae–a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev 64:624–653. doi: 10.1128/MMBR.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindow SE, Brandl MT. 2003. Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt J. 2009. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knief C, Delmotte N, Chaffron S, Stark M, Innerebner G, Wassmann R, von Mering C, Vorholt J. 2012. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J 6:1378–1390. doi: 10.1038/ismej.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambais MR, Crowley DE, Cury J, Büll R, Rodrigues RR. 2006. Bacterial diversity in tree canopies of the Atlantic forest. Science 312:1917. doi: 10.1126/science.1124696. [DOI] [PubMed] [Google Scholar]
- 6.Hunter PJ, Hand P, Pink D, Whipps JM, Bending GD. 2010. Both leaf properties and microbe-microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (Lactuca species) phyllosphere. Appl Environ Microbiol 76:8117–8125. doi: 10.1128/AEM.01321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. 2010. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893. doi: 10.1111/j.1462-2920.2010.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel OM, Burch AY, Lindow SE, Post AF, Belkin S. 2011. Geographical location determines the population structure in phyllosphere microbial communities of a salt-excreting desert tree. Appl Environ Microbiol 77:7647–7655. doi: 10.1128/AEM.05565-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vokou D, Vareli K, Zarali E, Karamanoli K, Constantinidou H-IA, Monokrousos N, Halley J, Sainis I. 2012. Exploring biodiversity in the bacterial community of the Mediterranean phyllosphere and its relationship with airborne bacteria. Microb Ecol 64:714–724. doi: 10.1007/s00248-012-0053-7. [DOI] [PubMed] [Google Scholar]
- 10.Burch AY, Zeisler V, Yokota K, Schreiber L, Lindow SE. 2014. The hygroscopic biosurfactant syringafactin produced by Pseudomonas syringae enhances fitness on leaf surfaces during fluctuating humidity. Environ Microbiol 16:2086–2098. doi: 10.1111/1462-2920.12437. [DOI] [PubMed] [Google Scholar]
- 11.Burch A, Browne P, Dunlap C, Price N, Lindow SE. 2011. Comparison of biosurfactant detection methods reveals hydrophobic surfactants and contact-regulated production. Environ Microbiol 13:2681–2691. doi: 10.1111/j.1462-2920.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 12.Bunster L, Fokkema NJ, Schippers B. 1989. Effect of surface-active Pseudomonas spp. on leaf wettability. Appl Environ Microbiol 55:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrand P, Braun P, McRae K, Lu X. 1998. Role of the biosurfactant viscosin in broccoli head rot caused by a pectolytic strain of Pseudomonas fluorescens. Can J Plant Pathol 20:296–303. doi: 10.1080/07060669809500396. [DOI] [Google Scholar]
- 14.Hernandez-Anguiano AM, Suslow TV, Leloup L, Kado CI. 2004. Biosurfactants produced by Pseudomonas fluorescens and soft-rotting of harvested florets of broccoli and cauliflower. Plant Pathol 53:596–601. doi: 10.1111/j.0032-0862.2004.01048.x. [DOI] [Google Scholar]
- 15.Kruijt M, Tran H, Raaijmakers JM. 2009. Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J Appl Microbiol 107:546–556. doi: 10.1111/j.1365-2672.2009.04244.x. [DOI] [PubMed] [Google Scholar]
- 16.D'aes J, De Maeyer K, Pauwelyn E, Höfte M. 2010. Biosurfactants in plant-Pseudomonas interactions and their importance to biocontrol. Environ Microbiol Rep 2:359–372. [DOI] [PubMed] [Google Scholar]
- 17.Pauwelyn E, Huang C-J, Ongena M, Leclère V, Jacques P, Bleyaert P, Budzikiewicz H, Schafer M, Hofte M. 2013. New linear lipopeptides produced by Pseudomonas cichorii SF1-54 are involved in virulence, swarming motility, and biofilm formation. Mol Plant Microbe Interact 26:585–598. doi: 10.1094/MPMI-11-12-0258-R. [DOI] [PubMed] [Google Scholar]
- 18.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth T, Marchant R. 2010. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 19.Ritz K. 2007. The plate debate: cultivable communities have no utility in contemporary environmental microbial ecology. FEMS Microbiol Ecol 60:358–362. doi: 10.1111/j.1574-6941.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 20.Müller T, Ruppel S. 2014. Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol Ecol 87:2–17. doi: 10.1111/1574-6941.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa R, Yoshida S, Furuya N, Tsuchiya K, Tsushima S. 2011. Method for simple and rapid enumeration of total epiphytic bacteria in the washing solution of rice plants. Can J Microbiol 57:62–67. doi: 10.1139/W10-101. [DOI] [PubMed] [Google Scholar]
- 22.Stiefel P, Zambelli T, Vorholt JA. 2013. Isolation of optically targeted single bacteria by application of fluidic force microscopy to aerobic anoxygenic phototrophs from the phyllosphere. Appl Environ Microbiol 79:4895–4905. doi: 10.1128/AEM.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai Y, Müller DB, Srinivas G, Garrido-Oter R, Potthoff E, Rott M, Dombrowski N, Münch P, Spaepen S, Remus-Emsermann M, Hüttel B, McHardy A, Vorholt J, Schulze-Lefert P. 2015. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- 24.Raaijmakers J, de Bruijn I, Nybroe O, Ongena M. 2010. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 25.Burch A, Shimada B, Browne P, Lindow SE. 2010. Novel high-throughput detection method to assess bacterial surfactant production. Appl Environ Microbiol 76:5363–5372. doi: 10.1128/AEM.00592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien R, Lindow SE. 1989. Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology 79:619–627. doi: 10.1094/Phyto-79-619. [DOI] [Google Scholar]
- 27.Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JH. 2012. Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822. doi: 10.1038/ismej.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chelius MK, Triplett EW. 2001. The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol 41:252–263. doi: 10.1007/s002480000087. [DOI] [PubMed] [Google Scholar]
- 29.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Gonzalez A, Goodrich J, Gordon J, Huttley G, Kelley S, Knights D, Koenig J, Ley R, Lozupone C, McDonald D, Muegge B, Pirrung M, Reeder J, Sevinsky J, Turnbaugh P, Walters W, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 31.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen G. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander S, Sodergren E, Methe B, DeSantis TZ, The Human Microbiome Consortium, Petrosino JF, Knight R, Birren BW. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oksanen J, Blanchet G, Kindt R, Legendre P, Minchin P, O'Hara RB, Simpson GL, Solymos P, Stevens MH, Wagner H. 2015. vegan: community ecology package. R package version 2.2-1. http://CRAN.R-project.org/package=vegan.
- 36.Kim M, Singh D, Lai-Hoe A, Go R, Rahim RA, Ainuddin A, Chun J, Adams JM. 2012. Distinctive phyllosphere bacterial communities in tropical trees. Microb Ecol 63:674–681. doi: 10.1007/s00248-011-9953-1. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Velasco G, Welbaum GE, Boyer RR, Mane SP, Ponder MA. 2011. Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage described using pyrosequencing of 16S rRNA amplicons. J Appl Microbiol 110:1203–1214. doi: 10.1111/j.1365-2672.2011.04969.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Xia B, Treves DS, Wu L-Y, Marsh TL, O'Neill RV, Palumbo AV, Tiedje JM. 2002. Spatial and resource factors influencing high microbial diversity in soil. Appl Environ Microbiol 68:326–334. doi: 10.1128/AEM.68.1.326-334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yashiro E, Spear R, McManus P. 2011. Culture-dependent and culture-independent assessment of bacteria in the apple phyllosphere. J Appl Microbiol 110:1284–1296. doi: 10.1111/j.1365-2672.2011.04975.x. [DOI] [PubMed] [Google Scholar]
- 40.Monier J-M, Lindow SE. 2004. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl Environ Microbiol 70:346–355. doi: 10.1128/AEM.70.1.346-355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remus-Emsermann MN, Tecon R, Kowalchuk GA, Leveau JH. 2012. Variation in local carrying capacity and the individual fate of bacterial colonizers in the phyllosphere. ISME J 6:756–765. doi: 10.1038/ismej.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leveau JH, Lindow SE. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc Natl Acad Sci U S A 98:3446–3453. doi: 10.1073/pnas.061629598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vorholt JA. 2012. Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 44.Mercier J, Lindow SE. 2000. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl Environ Microbiol 66:369–374. doi: 10.1128/AEM.66.1.369-374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson IP, Bailey MJ, Fenlon JS, Fermor TR, Lilley AK, Lynch JM, McCormack PJ, McQuilken MP, Purdy KJ, Rainey PB, Whipps JM. 1993. Quantitative and qualitative seasonal changes in the microbial community from the phyllosphere of sugar beet (Beta vulgaris). Plant Soil 150:177–191. doi: 10.1007/BF00013015. [DOI] [Google Scholar]
- 46.Lopez-Velasco G, Carder PA, Welbaum GE, Ponder MA. 2013. Diversity of the spinach (Spinacia oleracea) spermosphere and phyllosphere bacterial communities. FEMS Microbiol Lett 346:146–154. doi: 10.1111/1574-6968.12216. [DOI] [PubMed] [Google Scholar]
- 47.Reisberg EE, Hildebrandt U, Riederer M, Hentschel U. 2013. Distinct phyllosphere bacterial communities on Arabidopsis wax mutant leaves. PLoS One 8:e78613. doi: 10.1371/journal.pone.0078613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redford AJ, Fierer N. 2009. Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb Ecol 58:189–198. doi: 10.1007/s00248-009-9495-y. [DOI] [PubMed] [Google Scholar]
- 49.Jackson CR, Denney WC. 2011. Annual and seasonal variation in the phyllosphere bacterial community associated with leaves of the southern magnolia (Magnolia grandiflora). Microb Ecol 61:113–122. doi: 10.1007/s00248-010-9742-2. [DOI] [PubMed] [Google Scholar]
- 50.Yang C-H, Crowley DE, Borneman J, Keen NT. 2001. Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci U S A 98:3889–3894. doi: 10.1073/pnas.051633898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shade A, McManus PS, Handelsman J. 2013. Unexpected diversity during community succession in the apple flower microbiome. mBio 4(2):e00602-12. doi: 10.1128/mBio.00602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurence M, Hatzis C, Brash DE. 2014. Common contaminants in next-generation sequencing that hinder discovery of low-abundance microbes. PLoS One 9:e97876. doi: 10.1371/journal.pone.0097876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay J, Singh K, Fern A, Kirton ES, He S, Woyke T, Lee J, Chen F, Dangl J, Tringe SG. 2015. Primer and platform effects on 16S rRNA tag sequencing. Front Microbiol 6:771. doi: 10.3389/fmicb.2015.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis KE, Joseph SJ, Janssen PH. 2005. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl Environ Microbiol 71:826–834. doi: 10.1128/AEM.71.2.826-834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.