Abstract
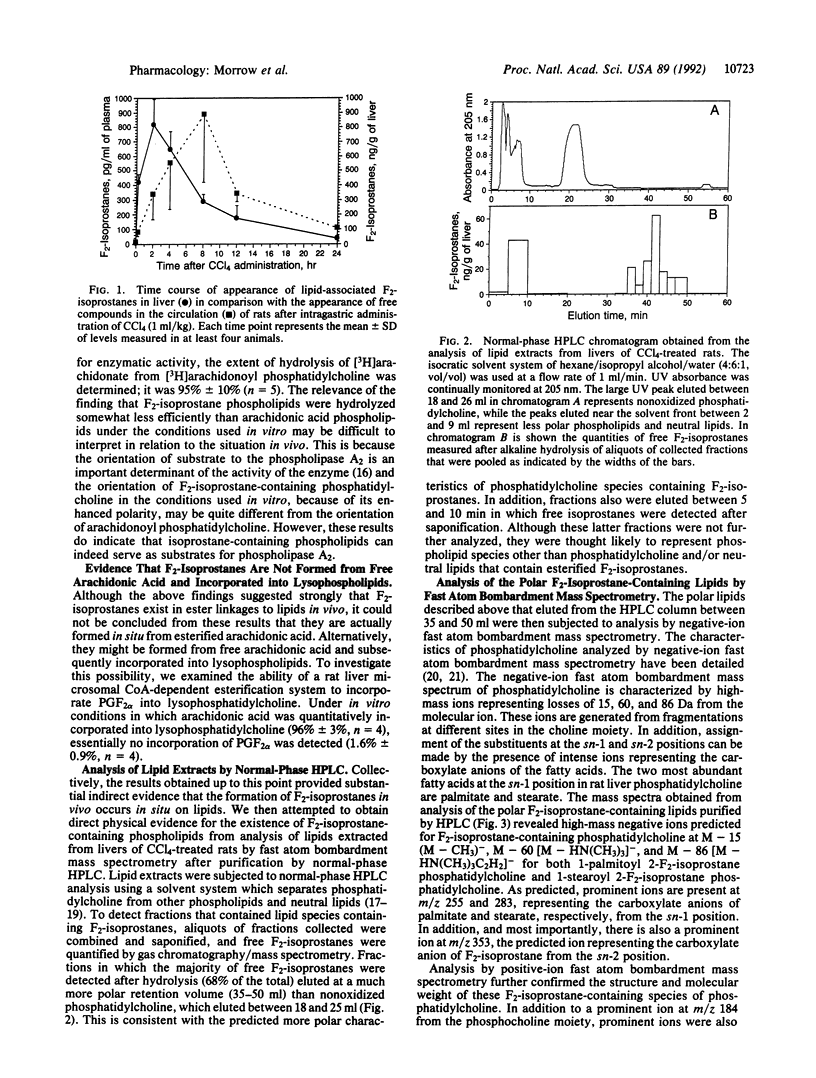
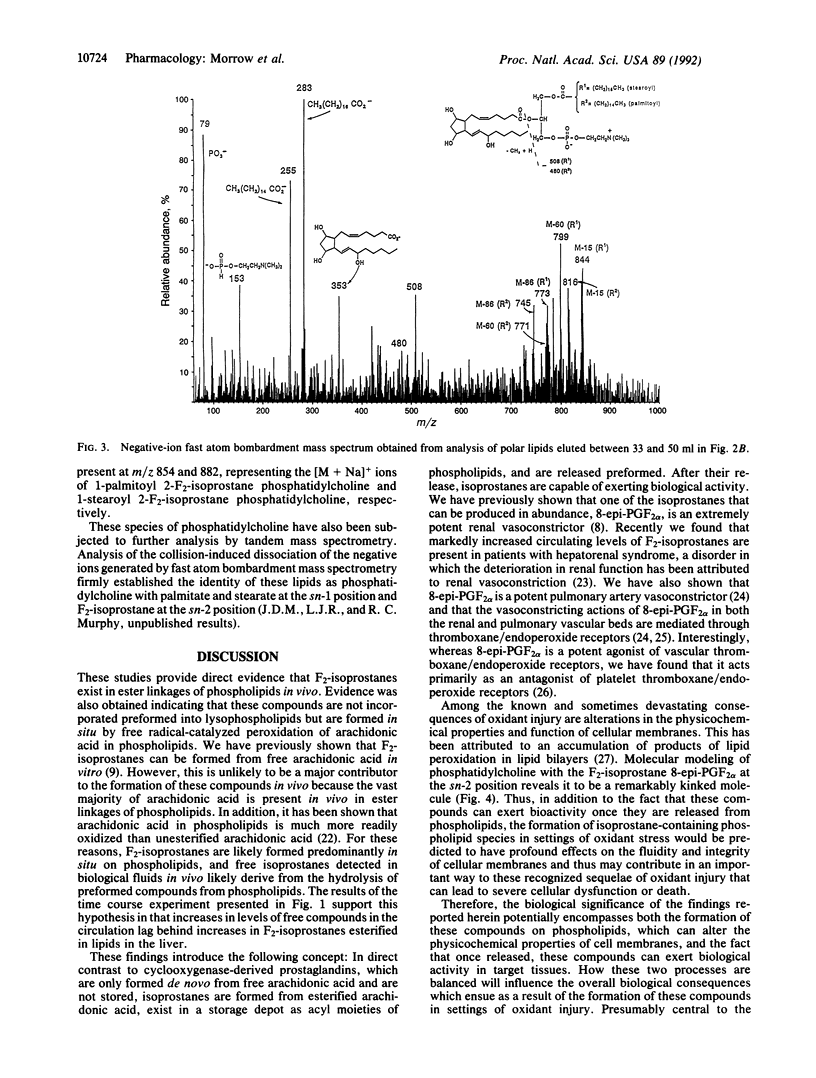
We recently reported the discovery of a series of bioactive prostaglandin F2-like compounds (F2-isoprostanes) that are produced in vivo by free radical-catalyzed peroxidation of arachidonic acid independent of the cyclooxygenase enzyme. Inasmuch as phospholipids readily undergo peroxidation, we examined the possibility that F2-isoprostanes may be formed in situ on phospholipids. Initial support for this hypothesis was obtained by the finding that levels of free F2-isoprostanes measured after hydrolysis of lipids extracted from livers of rats treated with CCl4 to induce lipid peroxidation were more than 100-fold higher than levels in untreated animals. Further, increased levels of lipid-associated F2-isoprostanes in livers of CCl4-treated rats preceded the appearance of free compounds in the circulation, suggesting that the free compounds arose from hydrolysis of peroxidized lipids. This concept was supported by demonstrating that free F2-isoprostanes were released after incubation of lipid extracts with bee venom phospholipase A2 in vitro. When these lipid extracts were analyzed by HPLC, fractions that yielded large quantities of free F2-isoprostanes after hydrolysis eluted at a much more polar retention volume than nonoxidized phosphatidylcholine. Analysis of these polar lipids by fast atom bombardment mass spectrometry established that they were F2-isoprostane-containing species of phosphatidylcholine. Thus, unlike cyclooxygenase-derived prostanoids, F2-isoprostanes are initially formed in situ on phospholipids, from which they are subsequently released preformed, presumably by phospholipases. Molecular modeling of F2-isoprostane-containing phospholipids reveals them to be remarkably distorted molecules. Thus, the formation of these phospholipid species in lipid bilayers may contribute in an important way to alterations in fluidity and integrity of cellular membranes, well-known sequelae of oxidant injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brash A. R., Ingram C. D., Harris T. M. Analysis of a specific oxygenation reaction of soybean lipoxygenase-1 with fatty acids esterified in phospholipids. Biochemistry. 1987 Aug 25;26(17):5465–5471. doi: 10.1021/bi00391a038. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Lane J. M. Ethane production and liver necrosis in rats after administration of drugs and other chemicals. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):467–478. doi: 10.1016/0041-008x(79)90400-9. [DOI] [PubMed] [Google Scholar]
- Chiariello M., Ambrosio G., Cappelli-Bigazzi M., Nevola E., Perrone-Filardi P., Marone G., Condorelli M. Inhibition of ischemia-induced phospholipase activation by quinacrine protects jeopardized myocardium in rats with coronary artery occlusion. J Pharmacol Exp Ther. 1987 May;241(2):560–568. [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Cordella-Miele E., Miele L., Beninati S., Mukherjee A. B. Stimulation of phospholipases A2 by transglutaminases. Adv Exp Med Biol. 1990;279:105–123. doi: 10.1007/978-1-4613-0651-1_7. [DOI] [PubMed] [Google Scholar]
- Ellingson J. S., Zimmerman R. L. Rapid separation of gram quantities of phospholipids from biological membranes by preparative high performance liquid chromatography. J Lipid Res. 1987 Aug;28(8):1016–1018. [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett T. L., Deems R. A., Dennis E. A. Activation, aggregation, inhibition and the mechanism of phospholipase A2. Adv Exp Med Biol. 1990;279:49–64. doi: 10.1007/978-1-4613-0651-1_4. [DOI] [PubMed] [Google Scholar]
- Hughes H., Smith C. V., Horning E. C., Mitchell J. R. High-performance liquid chromatography and gas chromatography-mass spectrometry determination of specific lipid peroxidation products in vivo. Anal Biochem. 1983 Apr 15;130(2):431–436. doi: 10.1016/0003-2697(83)90612-7. [DOI] [PubMed] [Google Scholar]
- Jensen N. J., Tomer K. B., Gross M. L. Fast atom bombardment and tandem mass spectrometry of phosphatidylserine and phosphatidylcholine. Lipids. 1986 Sep;21(9):580–588. doi: 10.1007/BF02534056. [DOI] [PubMed] [Google Scholar]
- Karara A., Dishman E., Falck J. R., Capdevila J. H. Endogenous epoxyeicosatrienoyl-phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J Biol Chem. 1991 Apr 25;266(12):7561–7569. [PubMed] [Google Scholar]
- Morrow J. D., Harris T. M., Roberts L. J., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990 Jan;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Minton T. A., Roberts L. J., 2nd The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992 Aug;44(2):155–163. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Roberts L. J., 2nd Quantification of noncyclooxygenase derived prostanoids as a marker of oxidative stress. Free Radic Biol Med. 1991;10(3-4):195–200. doi: 10.1016/0891-5849(91)90076-f. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Otamiri T., Tagesson C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am J Surg. 1989 Jun;157(6):562–566. doi: 10.1016/0002-9610(89)90699-5. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967 Jun;19(2):145–208. [PubMed] [Google Scholar]
- Sevanian A., Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med. 1985;1(4):263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Southorn P. A., Powis G. Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc. 1988 Apr;63(4):390–408. doi: 10.1016/s0025-6196(12)64862-9. [DOI] [PubMed] [Google Scholar]
- Stremler K. E., Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem. 1991 Jun 15;266(17):11095–11103. [PubMed] [Google Scholar]
- Takahashi K., Nammour T. M., Fukunaga M., Ebert J., Morrow J. D., Roberts L. J., 2nd, Hoover R. L., Badr K. F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992 Jul;90(1):136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]