Abstract
Neurofeedback (NFB) involves a brain-computer interface that allows users to learn to voluntarily control their cortical oscillations, reflected in the electroencephalogram (EEG). Although NFB is being pioneered as a noninvasive tool for treating brain disorders, there is insufficient evidence on the mechanism of its impact on brain function. Furthermore, the dominant rhythm of the human brain is the alpha oscillation (8–12 Hz), yet its behavioral significance remains multifaceted and largely correlative. In this study with 34 healthy participants, we examined whether during the performance of an attentional task, the functional connectivity of distinct fMRI networks would be plastically altered after a 30-min session of voluntary reduction of alpha rhythm (n=17) versus a sham-feedback condition (n=17). We reveal that compared to sham-feedback, NFB induced an increase of connectivity within the salience network (dorsal anterior cingulate focus), which was detectable 30 minutes after termination of training. This increase in connectivity was negatively correlated with changes in 'on-task' mind-wandering as well as resting state alpha rhythm. Crucially, there was a causal dependence between alpha rhythm modulations during NFB and at subsequent resting state, not exhibited by the sham group. Our findings provide neurobehavioral evidence for a temporally direct, plastic impact of NFB on a key cognitive control network of the brain, suggesting a promising basis for its use to treat cognitive disorders under physiological conditions.
Keywords: electroencephalogram (EEG), neurofeedback, brain-computer interface (BCI), brain plasticity, functional MRI (fMRI), functional connectivity, alertness, salience network, dorsal anterior cingulate (dACC), mind wandering, auditory oddball, attention, cognitive control
1. Introduction
EEG neurofeedback (NFB) is a brain-computer interface (BCI) method that enables users to gain voluntary control of their cortical oscillations by receiving moment-to-moment feedback from their electroencephalogram (EEG) (Kamiya et al. 1969). As such, it holds promise for modifying abnormal brain oscillations in various disorders, such as ADHD and epilepsy (Heinrich et al. 2007). Most NFB involves multiple sessions repeated on at least a weekly basis, whose effects generally accumulate over time, reputedly as a result of long-term changes in the brain (Sterman et al. 1970). However, evidence of a temporally direct impact of NFB on brain plasticity remains crucial for it to be recognized as a ground-breaking approach that is veritably safe, inexpensive, and accessible.
Recently, lasting changes in cortical plasticity were detected for the first time in the direct aftermath of NFB, using transcranial magnetic stimulation (TMS) (Ros et al. 2010). Inspired by this discovery we asked whether fMRI would be able to capture the early neuromodulatory effects of NFB, while harnessing its high spatial-resolution in order to expose the causal effects of NFB on brain functional networks and behavior. For NFB we considered voluntary control of the alpha (8–12 Hz) rhythm, based on its prevalence in the human EEG and our previous finding that its amplitude can be readily attenuated (desynchronized) by naïve participants (Ros et al. 2010). Alpha rhythm synchronization or desynchronization, respectively, generally reflects the inhibition or excitation of sensory cortex (Romei et al. 2008; Haegens et al. 2011) which frequently appears during internally versus externally-directed attention (Cooper et al. 2003). Recent simultaneous EEG-fMRI studies have attempted to correlate the alpha rhythm with the activity of temporally-coherent fMRI networks: revealing alpha synchronization to be positively associated with both the task-negative 'default-mode network' (DMN) (Hlinka et al. 2010; Mantini et al. 2007; Jann et al. 2009) and task-positive 'salience network' (Sadaghiani et al. 2010) connectivity. Behaviorally, the activation of the DMN has been shown to coincide with mind-wandering plus lapses in sensory attention (Christoff et al. 2009; Mason et al. 2007; Weissman et al. 2006); while in contrast, salience-network activation has been linked to the successful performance of sensory attention tasks (Kiehl et al. 2005; Sadaghiani et al. 2009; Langner et al. 2012). In order to disentangle these seemingly conflicting functional correlates of alpha rhythm, we sought to examine via NFB to what extent alpha desynchronization would modulate the connectivity of these networks, together with attentional function. To do so, we undertook separate fMRI recordings of participants immediately before and after NFB, during the performance of an auditory attention task containing random mind-wandering probes. Based on the prevailing evidence, we hypothesized that successful alpha desynchronization would lead to greater plastic alterations in DMN and/or salience network, which would individually correlate with reduced mind-wandering behavior.
2. Methods
2.1 Participants and experimental design
After approval of the study by the Research Ethics Board of University of Western Ontario, Canada, a total of 34 right-handed participants (mean age: 32.6, SD: 10.7, 24 women, 10 men) were recruited in the study. All participants were recruited from the neighborhood of the university scanning center and were carefully screened for the presence of neurological or psychiatric disorders during a structured SCID-I-Interview at the Psychiatry Department. Prior to the study, written informed consent was obtained from each participant. Upon arrival to the examination facility, participants were randomized to one of two experimental groups: EEG-neurofeedback (NFB, n=17) or sham-neurofeedback (SHAM, n=17). Experimental procedures were identical in every way for the two groups, except that SHAM group participants did not receive veridical feedback from their EEG activity, but rather were re-played EEG signal from a previously recorded session of a NFB-successful participant (their real EEG activity was nevertheless recorded for offline analysis). The overall experimental protocol of 3 sequential parts that occurred within the same daytime visit: MRI scan before neurofeedback (~30 min), EEG neurofeedback (~30 min), and MRI scan after neurofeedback (~30 min). No adverse effects were reported by any participant either before or after NFB or SHAM.
2.2 fMRI Paradigm
Participants underwent a total of 2 identical, pre-and-post MRI sessions: the first session directly preceded neurofeedback, and the second scan directly followed it. More specifically, given the time required for setup of EEG recording, neurofeedback started ~30 minutes after completion of the first fMRI scan. Since we were particularly interested in the plasticity of neurofeedback effects, we made note of the elapsed time between the end of neurofeedback and the beginning of the second fMRI scan for every participant (mean ± SD = 24 min ± 2). Participants were instructed to keep their eyes open, remain motionless as much as possible and not to think of anything in particular. Following a localizer and anatomical scan (~10 min), participants completed an auditory oddball fMRI task (details of MRI data acquisition in next section). The task consisted of one 6 minute run of 181 auditory stimuli presented with a computer presentation system (E-Prime 2.0, Psychology Software Tools Inc., USA), by means of sound attenuating MRI-compatible headphones (Serene Sound System, Resonance Technology Inc., CA, USA). Participants had to identify the pseudo-random occurrence of 1000 and 2000 Hz long-tone sine stimuli (500 ms, target) within a sequence of short-tone sine stimuli (200 ms, non-target): pressing Button 1 for the former and no response for the latter. The interstimulus interval (ISI) was 2 seconds and the probability of long-tone vs. short-tone stimulus occurrence was 20% vs. 80%. The traditional approach for assessing levels of mind-wandering (Mason et al. 2007; Christoff et al. 2009) is to engage the participant with a low-attention task, during which “thought” probes occurring at random intervals interrogate the participant whether they were "on-task" (attentive) or "off-task" (mind-wandering). For example, Christoff et al. (Christoff et al. 2009) used a visual task where participants had to identify a target number within a sequence of random digits while a thought-probe question was presented during 5% of the trials. We adapted the protocol by Christoff et al. for the present experiment by implementing an auditory oddball as the low-attention task, whilst additionally inserting a ring tone as a thought probe stimulus at a probability of 3% (approx. 1 probe every 50–70 seconds). Upon hearing the telephone ring, participants were instructed to ask themselves the question “Was your mind wandering at the time of the ring?”, and reply “Yes” or “No” via the keypad. Mind-wandering was described to each participant as “having any thoughts that are not related to the task”. Lastly, we recorded the trial-by-trial reaction time (RT) to oddball target stimuli as well as mind-wandering probes during the task.
2.3 fMRI acquisition
All MRI data were acquired using a Magnetom Verio 3.0 Tesla scanner (Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phase array head coil. Whole-brain BOLD functional images were obtained with gradient echo (EPI) sequence, with 3000 ms repetition time [TR]; 20 ms time of echo [TE]; 90 degree flip angle; 256 mm field of view [FOV]; and 2 × 2 × 2 voxel resolution (mm). Sampling consisted of 60 interleaved slices, 2mm thick, no gap, parallel to the anterior-posterior commissure (AC-PC) line. The first four (extra) images in each run were automatically discarded by the scanner to allow the magnetization to reach equilibrium. The functional time-series consisted of 120 consecutive image volumes obtained over 6 minutes. Anatomical images were obtained using a T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence: (TR / TE / TI = 2000ms / 4 ms/ 900 ms; flip angle = 9°; FOV = 256mm × 256 mm; 1mm isotropic resolution; 176 slices, no gap, GRAPPA acceleration=2). Image pre-processing was performed in SPM8 (www.fil.ion.ucl.ac.uk/spm/), and included slice-timing correction, motion correction, spatial normalization and smoothing using a FWHM (full-width half-maximum) Gaussian filter of 8mm. Motion correction was performed by aligning (within-subject) each time-series to the first image volume using a least-squares minimization and a 6-parameter (rigid body) spatial transformation. Data were normalized using the unified segmentation on T1 image pipeline (Ashburner and Friston, 2005) which can improve the accuracy of spatial normalization and thus inter-subject comparisons. This involves four steps: coregistering the functional volumes to their respective anatomical images using 12 parameter affine alignment, segmenting the anatomical images into gray and white matter, normalizing the anatomical volumes to the T1 gray-matter template, and applying the same transformation to the functional volumes. During the latter, process images were resliced to 3 mm isotropic resolution in Montreal Neurological Institute (MNI) space.
2.4 fMRI connectivity analysis
The overall connectivity dynamics of fMRI behavioral experiments are difficult to study due to a lack of well-understood brain-activation models plus inter-subject variability (Allen et al. 2011). A strength of independent component analysis (ICA) is that it is model-free and thus makes no underlying assumptions about the spatiotemporal time-course of individual fMRI activations. Previous work has also revealed a correspondence of temporally-coherent networks across behavioral tasks and resting-state conditions (Calhoun et al. 2008). Hence, group spatial independent component analysis (group-ICA) was implemented using the GIFT toolbox (v1.3i, http://mialab.mrn.org/) in Matlab 7.6 (Mathworks Inc., MA, USA). Here, we performed group-ICA on the pooled auditory oddball dataset (both experimental groups, pre and post sessions). This approach allows for unique time courses for each subject, but assumes common group maps, ensuring that independent component (IC) spatial maps match across all participants as well as conditions. Importantly, this particular group-ICA approach was recently shown to accurately capture inter-subject, and hence inter-group, differences in IC amplitude as well as spatial extent (Allen et al. 2011). Accordingly, we used the Infomax algorithm to extract a total of 20 independent components (ICs), based on recent methodological studies reporting good reproducibility with this number in GIFT (Rosazza et al. 2011), which was confirmed by dimension estimation using the minimum description length (MDL) criteria. For each IC, its time course corresponded to the waveform of a specific pattern of coherent brain activity, and the intensity of this pattern across voxels was expressed in the associated z-score spatial map. Hence, the z-scores reflected the degree to which a given voxel’s time series was coupled to the time series of a specific component, scaled by the standard deviation of the error term. All ICs were manually inspected for the presence of obvious artifacts (e.g. edges, ventricles, white matter) and a final subset of 8 artifact-free ICs corresponding to intrinsic connectivity networks (ICNs) were identified according to the templates described previously (Damoiseaux et al. 2006). To ensure component reliability, we ran ICA a total of 20 times with boot-strapping and random starting points (ICASSO method), resulting in all identified ICNs meeting the average intra-cluster similarity > 0.9 threshold. For all subjects pre and post sessions, z-score spatial maps (n=17) of each ICN were imported into SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) for a one-sample t-test, corrected for family-wise error (FWE) at P < 0.05, providing a statistical cut-off for the visualization of each ICN. For within-group contrasts, paired t-tests were conducted on z-score spatial maps corresponding to pre and post sessions. Using WFU Pickatlas (http://fmri.wfubmc.edu/software/PickAtlas), we employed region-of-interest (ROI) analyses for FWE-correction based on the hypothesized effects in the salience and DMN networks. According to the findings of Sadaghiani et al (Sadaghiani et al. 2010), Brodmann Area (BA) 24 + 32 masks were used to include the entire anterior cingulate area; while BA10 (frontal pole), BA31 (posterior cingulate), BA7 (precuneus), BA39 (occipitoparietal) were used as the DMN nodes. In order to relate pre-to-post fMRI connectivity changes to individual EEG and mind wandering measures, we calculated the post-minus-pre z-score map (T2 - T1) manually for each participant, which subsequently acted as the dependent variable in a multiple regression analysis. In this more data driven analysis we employed FWE < 0.05 small volume correction (SVC), where the (orthogonal) contrast of maximal network connectivity (T2 + T1) was used to determine the ROI centre of a 10mm-radius sphere. Hence, the salience network ROI centre featured in the dorsal ACC (−2, 12, 38 for NFB; 8, 18, 36 for SHAM) and the default-mode network ROI centre featured in the precuneus (0, −68, 34 for NFB; 2, −68, 32 for SHAM). In order to examine between-group effects, we carried out a 2 × 2 mixed ANOVA (Group × Session) setting the threshold for interaction at FWE < 0.05 SVC (one-tailed).
2.5 EEG neurofeedback paradigm
The EEG neurofeedback training session took place outside of the MRI scanner. Immediately before and after the training session, participants completed Spielberger's State Anxiety Inventory and Thayer's Activation-Deactivation Checklist questionnaires. All participants wore a multi-channel EEG cap which passively recorded their whole-scalp activity (see section below). In parallel, we placed an additional electrode on top of the cap, bridging with the Pz channel, which was specifically used for neurofeedback. This electrode was connected to a ProComp+ amplifier (Thought Technology, Canada) interfacing with EEGer 4.2 neurofeedback software (EEG Spectrum Systems, CA). We reasoned that averaging the global alpha signal from multiple cortical areas would lead to a mixing of local cortical dynamics and therefore would not be as effective a signal for neurofeedback control. We settled for site Pz (parietal cortex), where the alpha rhythm is commonly maximal (Ergenoglu et al. 2004). Separate ground and reference electrodes were placed on the right and left earlobes, respectively. Each session consisted of a 3-min baseline, followed by 30 minutes of continuous neurofeedback, and lastly a 3-min post-baseline (all in 'eyes open' condition). During (feedback-free) baseline recordings, participants were asked to relax with their eyes open and gaze at a blank wall. Sham group participants did not receive veridical feedback from their EEG activity, but were re-played EEG signal from a previously recorded session of a NFB-successful participant (their whole-scalp EEG activity was nevertheless recorded). For the purpose of online NFB training, the EEG signal was IIR (infinite impulse response) band-pass filtered to extract alpha (8–12 Hz) amplitude with an epoch size of 0.5 seconds. The protocol was set-up so that participants were rewarded upon suppression of their absolute alpha amplitude. For both NFB and SHAM participants, the amplitude threshold for reward was initially set so that their alpha amplitude would temporally occur circa 60% of the time below the initial 3-min baseline average (i.e. they received negative-feedback 40% of the time). In cases where the participant achieved disproportionately larger (> 80%) or lower (< 40%) rates of reward during feedback, this reward ratio was re-applied at the beginning of each training period based on the EEG of the preceding 30 seconds. Hence, the NFB paradigm ensured that both NFB and SHAM participants were exposed to the same sensory stimuli and frequencies of reward. Visual feedback was clearly displayed on a 20" monitor via a dynamic bar graph on the center of the screen whose height was proportional to real-time alpha amplitude fluctuations. On the same screen, participants also interacted with a "SpaceRace" game. Here, participants were told that the space-ship moved forward through space when they were “in-the-zone” of their target brain activity (i.e. alpha lower than threshold), increasing their points in the game, and which fell back to stationary when they were “out-of-the-zone” (i.e. alpha higher than threshold). The aim of the training was to use the feedback they received during the game to learn to keep the spaceship travelling through space. Participants of both groups (NFB, SHAM) were not given any explicit instructions or mental strategies by the experimenter on how to achieve control over their spaceship, but were told to be guided by the visual feedback process. Moreover, they were not informed on the type of EEG parameter or frequency that was being rewarded. The 30-min session was divided into 10 three-minute periods. Each participant had a small break (of 10 seconds) between each 3-minute period, during which their score for the preceding periods was displayed. After completing the feedback training session, NFB and SHAM participants were asked to note down what strategy, if any, in their experience was most successful for gaining points during the game.
2.6 EEG recording
Scalp voltages were recorded using a 19 Ag/AgCl electrode cap according to the 10–20 international system: Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2. (Electro-cap International, Inc. www.electro-cap.com). The ground electrode was placed on the scalp, at a site equidistant between Fpz and Fz. Electrical signals were amplified with the Mitsar 21-channel EEG system (Mitsar-201, CE0537, Mitsar, Ltd. http://www.mitsar-medical.com) and all electrode impedances were kept under 5 kΩ. For online recording, electrodes were referenced to linked earlobes, and then the common average reference was calculated off-line before further analysis. The EEG was recorded continuously, digitized at a sampling rate of 250 Hz, and stored on hard disk for off-line analysis. EEG data were then filtered with a 0.5–40 Hz bandpass filter off-line.
2.7 EEG pre-processing
Following EEG recording, all EEG data were imported into the Matlab toolbox EEGLAB v9 (http://sccn.ucsd.edu/eeglab/). We used ICA decomposition to first remove stereotypical artifacts, since the Infomax algorithm has previously been shown to be capable of reliably separating eye activities, such as blinking and lateral eye movement (e.g. Jung et al., 2000). Subsequent artifact rejection methods consisted of the exclusion of epochs with large amplitudes (over ±80µV), direct-current bias, physiologically irresolvable dipoles and muscular activity of frontal and temporal muscles defined by fast activity over 20 Hz.
2.8 EEG neurofeedback spectral analysis
EEG spectral amplitudes were calculated offline via Short Time Fourier Transform (STFT) in 4-second epochs (50% overlapping with Hanning window) in each of the following bandwidths: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), low beta (12–18 Hz), and high beta (18–25Hz). Higher frequencies (gamma >25 Hz) were not analyzed as they may easily be contaminated by muscle artifact throughout the extended NFB session. We chose to capture the full alpha (8–12 Hz) bandwidth, reflective of the NFB protocol, which was designed to anticipate the high inter-individual variability of the alpha rhythm distribution found in clinical populations (Llinás et al. 1999). We primarily analysed local alpha amplitude data of the feedback electrode (Pz), in addition to global alpha amplitudes for EEG-fMRI large-scale network analyses. For exploratory EEG analyses, amplitude data was additionally averaged across the following cortical ROIs: Frontal (Fp1 + Fp2 + F7 + F3 + Fz + F4 + F8), Central (C3 + Cz + C4), Temporal (T3 + T4 + T5 + T6), Parietal (P3 + Pz + P4), and Occipital (O1 + O2).
For NFB and SHAM as between-group factors, a GROUP × TIME repeated measures ANOVA was conducted on absolute amplitudes. Post hoc paired t-tests corresponding to pre and post sessions were then conducted at a threshold of P < 0.05 corrected. For all bandwidths, the normalized training EEG change for each participant was estimated by the ratio of the average EEG amplitude during all ten training periods and the first baseline EEG, and designated as 'training EEG change'. Likewise, the normalized change in the baseline EEG amplitude was expressed by the ratio of the second divided by the first baseline, and designated as 'resting EEG change'. Statistical z-score estimates of divergence in the regression coefficients between NFB and SHAM groups were computed by dividing the differences between coefficients by their standard error (Paternoster et al. 1998).
3. Results
3.1 Baseline differences between NFB and SHAM groups
Independent two-sample t-tests did not reveal any statistically significant (P < 0.05) differences between NFB and SHAM groups for age (t = 0.5), gender (t = 0.7), hours of sleep (t = −0.4), or time of day during testing (t = −0.3). Likewise, there were no significant baseline differences between groups in frequency of mind-wandering (t = 0.1), oddball stimulus reaction time (t = − 0.1), reaction time to mind-wandering probe (t = −0.9); nor mean EEG alpha amplitude at frontal (t = −0.7), temporal (t = −0.9), central (t = −0.8), occipital (t = −0.7) or parietal (t = −0.4) electrodes. In addition, independent two-sample t-tests on baseline fMRI connectivity maps disclosed no statistically-corrected (FWE < 0.05) differences between NFB and SHAM groups for either the salience or the default-mode network.
3.2 EEG neurofeedback time-course and topography
During NFB and SHAM protocols, participants attempted to control either real-time or false (pre-recorded) alpha amplitude, respectively, which was recorded and fed-back from midline parietal cortex (electrode Pz) during a 30-min feedback training session. In order to analyze the time-course, the session was subdivided into ten equal periods of 3 min each. A feedback-free, eyes-open, resting baseline was also recorded for 3 min prior to and following the end of feedback (periods 0 and 11 respectively).
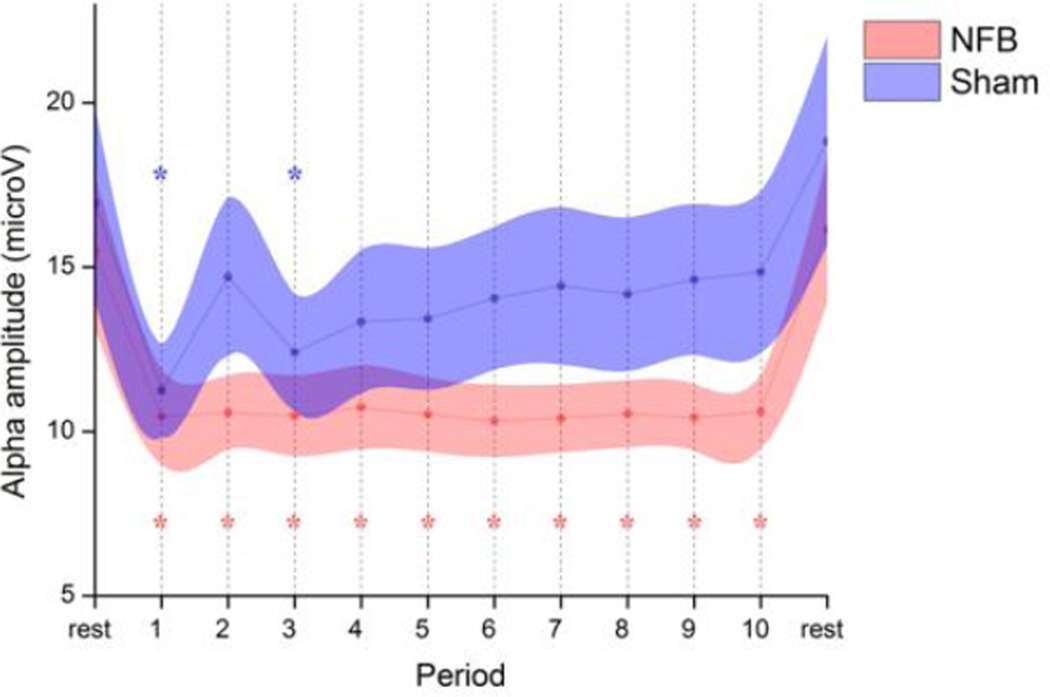
As can be seen from Fig 1, NFB participants were successful in reducing their target alpha amplitude across all training periods at parietal electrode Pz. The SHAM group on the other hand, after an initial drop, experienced a recovery to near-baseline levels across time. The opening drop exhibited by the SHAM group may have reflected a focusing of attention related to the unsuccessful search for a suitable cognitive strategy. A repeated measure ANOVA (GROUP × PERIOD, 2 × 12) revealed a main effect for PERIOD (F11,352 = 22.2, P < 0.01) and no overall effect for GROUP (F1,32 = 1.3, n.s.). Importantly, there was a significant GROUP × PERIOD interaction (F11,352 = 2.0, P = 0.03), indicating a significantly different alpha desynchronization between groups. Post-hoc paired Dunnett's test comparisons with the first resting period revealed a significant reduction for all training periods in the NFB group (P < 0.05 corrected), whilst only periods 1 and 3 were significant for the SHAM group. No significant changes were detected between the two resting periods for either group. Interestingly, within-subject amplitude correlations between theta, alpha, and beta bands during NFB were consistently positive within a statistically significant range of 0.5 < r < 0.7, suggesting a broader effect of NFB training on flanking frequency bands.
Fig 1. Time-course of mean alpha (8–12 Hz) amplitude for NFB and SHAM groups at feedback electrode Pz.
The session began with a 3-min resting baseline, followed by 30-min of feedback (periods 1–10) from midline parietal cortex (Pz). Periods significantly different from baseline are indicated with an asterisk (P<0.05). Shaded areas represent SEM.
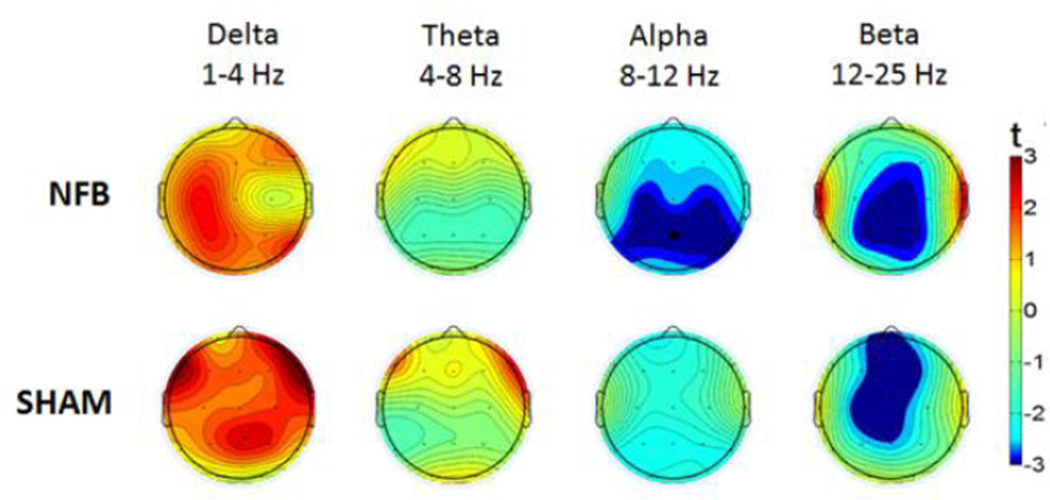
To investigate these relationships further, we constructed topographic plots representing the statistical change across the whole-scalp between the resting period and the mean amplitude of all training periods; these are depicted in Fig 2 for NFB and SHAM groups in each frequency band. Paired t-tests revealed a significant global alpha amplitude reduction (collapsed across all electrodes) during NFB (t = 2.7, P < 0.05 corrected), which was absent in the SHAM group (t = 1.9, n.s.). Given that we primarily hypothesized alpha changes at the Pz feedback site and/or globally, the reported t-tests can be considered exploratory for all other band-widths or cortical locations (t>|3|, P<0.05 uncorrected).
Fig 2. Topographic plots of mean EEG amplitude change during feedback (relative to rest).
Upper and lower panels represent NFB and SHAM groups, with successive EEG bandwidths featuring from left to right. Dark red and dark blue colors indicate statistically significant positive and negative changes (P < 0.05), respectively.
3.3 EEG resting state activity
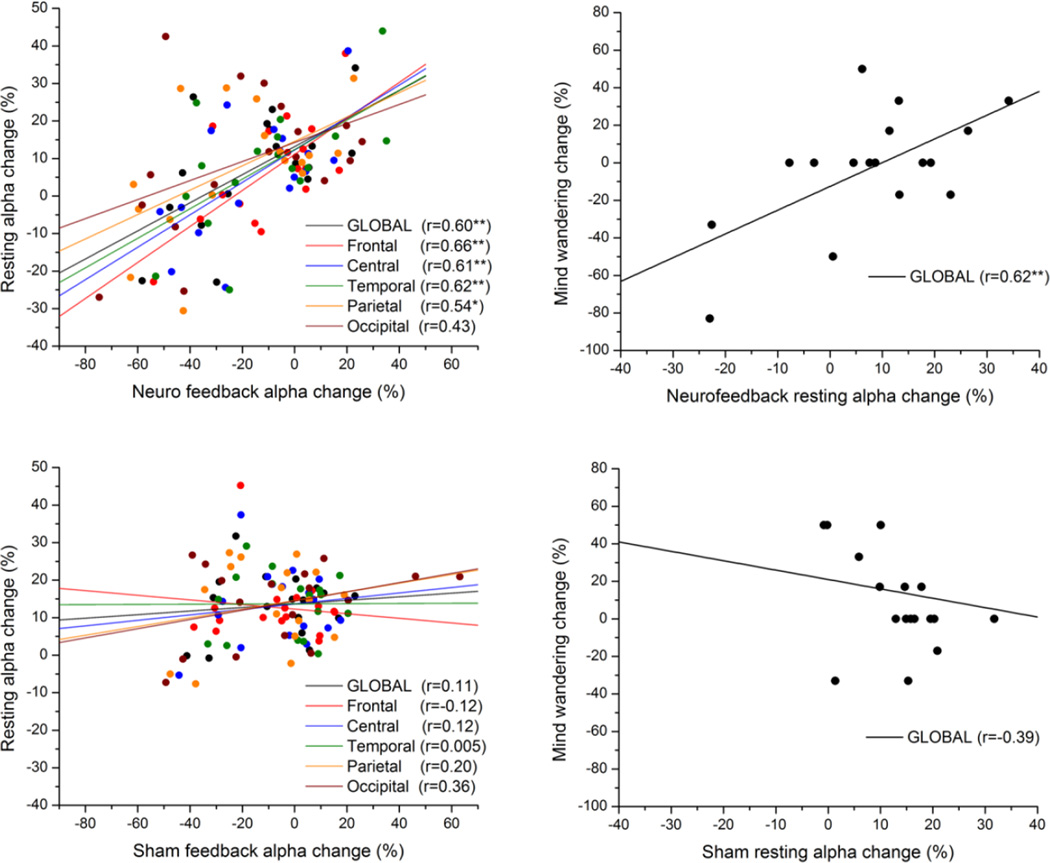
Our previously published results (Ros et al. 2010) had pointed to a causal influence of dynamic EEG changes during neurofeedback training on subsequent EEG resting state activity. We interrogated this effect in the current dataset, which benefited from a sham-feedback control group. For both groups, we correlated the mean amplitude change during feedback and the respective change in resting state amplitude after feedback. We utilized % signal change (relative to the first resting period) in order to normalize alpha synchronization change between participants. As can be seen in Fig 3, apart from the occipital lobe, resting state changes were significantly predicted by and were positively correlated with changes during NFB; yet this effect was absent in all lobes during sham-feedback. We directly tested the hypothesis that global alpha resting state change was more dependent on NFB than SHAM (i.e. greater regression coefficient). Given the significance of the global effect (z = 1.69, P < 0.05), we concentrated on global alpha changes in further large-scale fMRI network analyses.
Fig 3.
Scatter-plot of mean alpha amplitude change across electrodes during feedback vs. resting state (post-feedback), for NFB (upper-left panel) and SHAM (lower-left panel) groups. The anatomical location of each subgroup of electrodes is represented by a different colour (see legend). Correlation of global alpha change with mind-wandering change (right panels). Linear regression lines pertaining to each subgroup are in their respective colors. * P < 0.05, **P <0.01.
For the NFB group, we identified a significant positive correlation between global resting alpha change and mind-wandering change (r = 0.62, p < 0.01), which was nonsignificant (r = − 0.39, n.s.) for the SHAM group; for global alpha, there was a significant difference between NFB and SHAM regression coefficients with respect to mind-wandering (z = 2.9, p < 0.01). An analogous effect was seen for alpha at the feedback-electrode Pz (NFB: r = 0.61; SHAM: r = − 0.41; difference P < 0.01). Exploratory analyses on flanking bandwidths revealed a significant univariate correlation between resting theta change and mind-wandering change for the NFB group (r = 0. 52, P = 0.03) but not for the SHAM group (r = −0.15, n.s.). The relationship between mind-wandering and the delta or beta bands was nonsignificant for both groups. To investigate the shared variance (multicollinearity) between multiple EEG bands and mind-wandering change, we conducted a multivariate regression with all band-widths as independent variables: this yielded the NFB group resting alpha change as the only significant regressor (r = 0.62, p < 0.01).
3.4 fMRI network connectivity
Pooling the NFB and SHAM data, and from the total of 20 components extracted by the ICA decomposition, we were able to reliably identify 8 anatomically-circumscribed, intrinsic connectivity networks (ICNs) compatible with current literature (Damoiseaux et al. 2006): default-mode (C7), cingulate (C11), motor (C5), visual (C2), auditory (C17), rostral PFC (C9), right and left fronto-parietal (C15, C16) networks. Prior to further statistical analyses, masks were created corresponding to each ICN using a one-sample t-test (P < 0.01 FWE). The principal goal of subsequent analyses was to examine whether, pre-to-post neurofeedback, any reliable fMRI connectivity changes could be detected within circumscribed ICNs, and how these changes were related to differences in EEG and mind-wandering measures. Our primary hypotheses were derived from previous reports linking alpha synchronization with i) the salience network (Sadaghiani et al. 2010), and ii) the default-mode network (Jann et al. 2009; Hlinka et al. 2010).
3.4.1 Salience Network
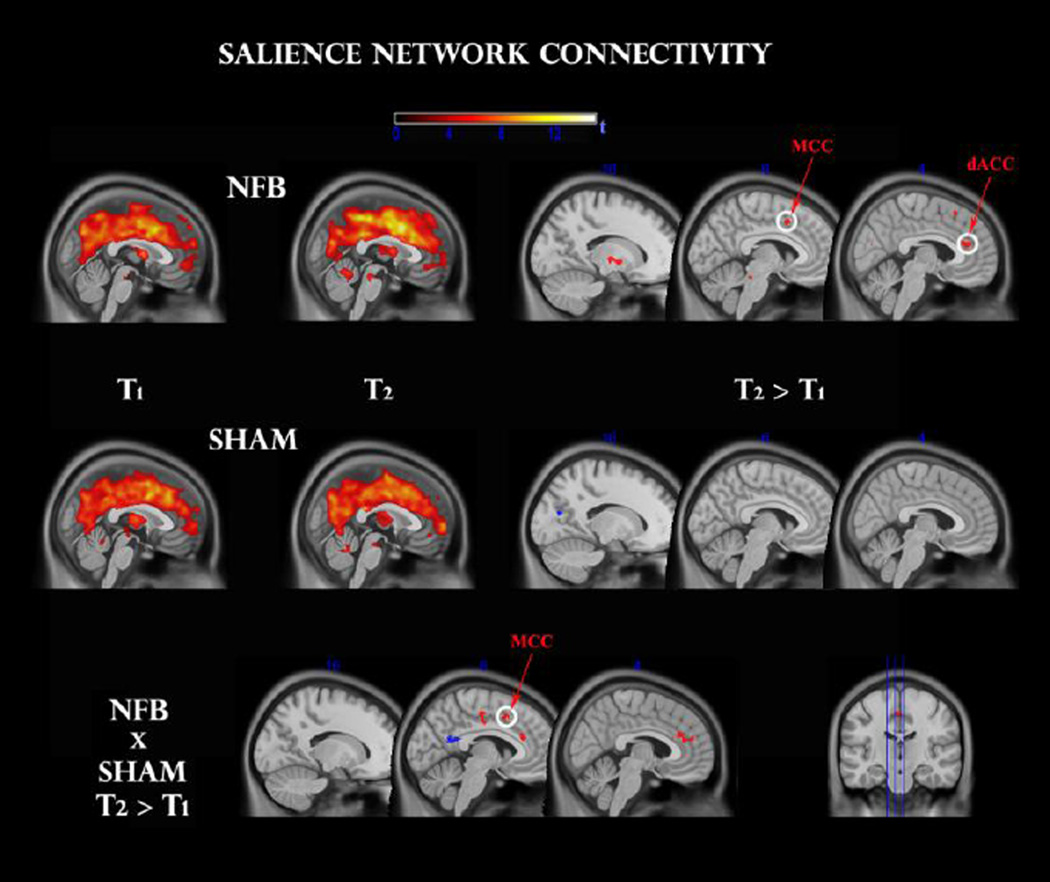
A one-sample t-test within pre (T1) and post (T2) sessions of each group revealed a coherent cingulo-opercular network of activation during the oddball task, with maximal connectivity at dorsal anterior (dACC), as well as bilateral insular, thalamic, basal ganglia, cerebellar and ponto-mesencephalic regions (see Fig 4, top panel). This is consistent with a network of areas previously reported to be responsible for salience detection (Seeley et al. 2007) and intrinsic alertness (Clemens et al. 2011).
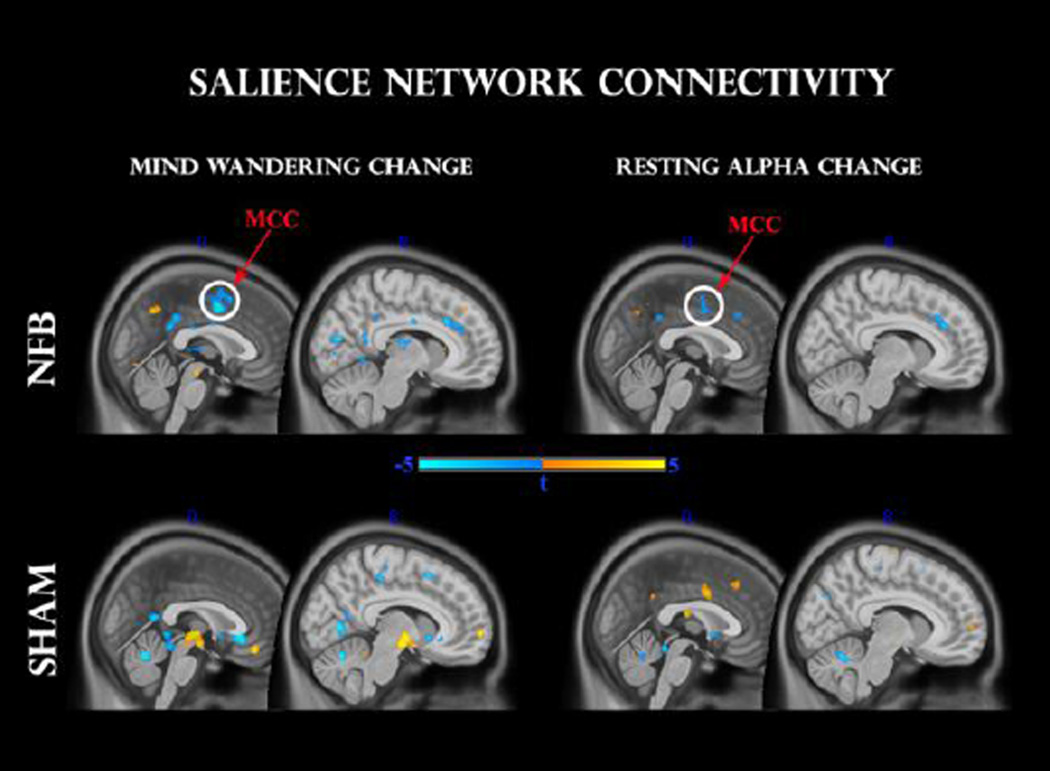
Fig 4.
Functional connectivity change within the salience network, before (T1) and after (T2) feedback, for NFB (top panel) and SHAM (middle panel) groups. Clusters surviving the family-wise error (FWE < 0.05) correction are circled in white. Other clusters were thresholded at p < 0.001 uncorrected. A TIME × GROUP interaction (bottom panel) reveals a significant modulation in comparable regions. dACC: dorsal anterior cingulate cortex; MCC: midcingulate cortex.
For the NFB group, a paired t-test indicated significantly increased functional connectivity after neurofeedback in the dACC (t = 6.0, 55 voxels) and MCC (t = 5.27, 15 voxels) clusters at FWE < 0.05, as seen in Fig 4. Exploratory analyses (P < 0.001 uncorrected) additionally revealed up-regulation of left thalamus (t = 4.5, 20 voxels), left medial globus pallidus (t = 5.6, 20 voxels), and (most likely) left locus coeruleus (t = 4.0, 15 voxels). No significant effects were detected for the SHAM group at this statistical and cluster-extent threshold. In order to contrast these effects with the SHAM group directly, we additionally conducted a mixed repeated-measures ANOVA, and observed a significant GROUP × TIME interaction (P < 0.05, voxels > 15): NFB group changes were more positive once again for the ACC (t = 2.0, 25 voxels), MCC (t = 2.5, 25 voxels) and globus pallidus (t = 3.0, 50 voxels); of which the MCC cluster survived a small-volume correction (FWE < 0.05).
3.4.2 Default-Mode Network
No statistically significant group effects (FWE P < 0.05) were found within the default-mode network after feedback, for either NFB or SHAM groups.
3.5 EEG vs fMRI connectivity vs mind-wandering
3.5.1 Salience Network
In this analysis we separately regressed global resting-alpha change, as well as mind wandering change, against individual z-score connectivity change maps in the salience network. In order to explore their intersection we searched for common voxels which passed the P < 0.001 uncorrected threshold with both regressors. As can be seen in Fig 5 for the NFB group, both mind-wandering and alpha change correlated negatively with connectivity in sizeable clusters (k > 25) of the dorsal ACC (t = −4.4 and t = −4.0 respectively) and MCC (t = −6.0, t = −4.1 respectively), with the latter cluster passing the small-volume corrected threshold (FWE < 0.05) for both mind-wandering and alpha change. Hence, individual changes in alpha as well as mind-wandering were negatively associated with connectivity differences in the salience network. Interestingly for the SHAM group, and opposite to the relationship seen with the NFB group, a positive correlation was observed between resting alpha change and functional connectivity within a proximal cluster within the MCC (t = 4.2, 40 voxels) at p < 0.001 uncorrected. On the other hand, negatively correlated clusters with changes in mind-wandering were located predominantly in white matter areas, with exploratory p < 0.001 clusters in posterior cingulate (t = −4.2) and subgenual cingulate (t = −4.5) regions. Positive correlations were found in the medial orbital gyrus (t = 5.4) and right brainstem (t = 5.3). However, none of these clusters coincided with regions that were significantly correlated with resting EEG changes.
Fig 5.
Regression analysis between pre-to-post changes in salience network functional connectivity and mind-wandering change (left panel), as well as resting alpha change (right panel). Upper and lower panels indicate NFB and SHAM groups, respectively. Clusters surviving the family-wise error (FWE < 0.05) correction are circled in white. MCC: mid-cingulate cortex.
3.5.2 Default-Mode Network
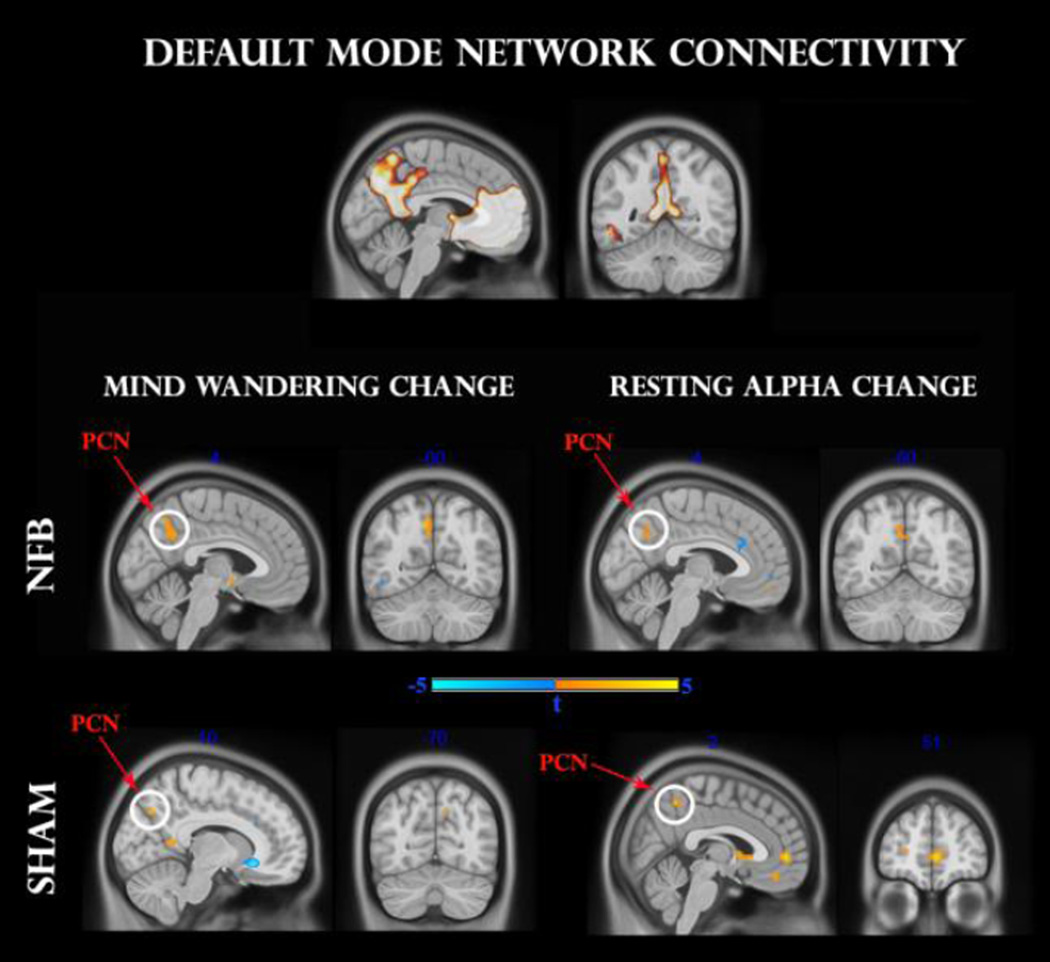
As for the salience network, we identified clusters of DMN connectivity that mutually correlated with changes in resting state alpha synchronization and frequency of mind-wandering. As shown in Fig 6 for the NFB group, both mind-wandering and alpha change correlated positively with connectivity in sizeable clusters (k > 25) of the precuneus (t = 3.7 and t = 3.6, respectively), passing the small-volume corrected threshold (FWE < 0.05). This positive relationship was mirrored by the SHAM group, albeit by smaller clusters (k > 10) within the precuneus (t = 4.1 and t = 3.8, respectively). Moreover for the SHAM group only, exploratory analyses (P < 0.001) identified a more extensive positive correlation with resting state alpha change in a region of the medial prefrontal cortex (t = 5.0). Hence, both NFB and SHAM groups remained consistent with numerous reports of a positive association between alpha synchronization and DMN connectivity (Hlinka et al. 2010; Mantini et al. 2007; Jann et al. 2009).
Fig 6.
Regression analysis between pre-to-post changes in default-mode network (DMN) functional connectivity and mind-wandering change (left panel), as well as resting alpha change (right panel). The upper panel designates the DMN mask used for analysis. Middle and lower panels indicate NFB and SHAM groups, respectively. Clusters surviving the family-wise error (FWE < 0.05) correction are circled in white. Pcn: precuneus; mPFC: medial prefrontal cortex.
3.6 Mind-wandering and oddball task
For the NFB group, pre-to-post RT change to mind-wandering probes was positively correlated with change in mind-wandering frequency (r = 0.58, p = 0.1), while no reliable relationship was evident for the SHAM group (r = −0.24, n.s.). Correlations between RT change to mind-wandering probes and oddball-targets were not significant for either NFB (r = −0.28, n.s) or SHAM (r = −0.15, n.s.). Paired t-tests revealed there were no significant pre-to-post differences in mind-wandering frequency for NFB (t = 0.4, n.s.) or SHAM (t = −1.4, n.s.). Likewise, no significant differences were evident in pre-to-post RT to mind-wandering probes for NFB (t = − 1.5, n.s.) or for SHAM (t = 0.5, n.s.); nor RT to oddball-targets for NFB (t = −0.8, n.s.) or for SHAM (t = 0.3, n.s.).
3.7 Anxiety vs resting state alpha
There was no significant correlation between changes in global resting alpha amplitude and state anxiety following NFB (r = 0.3, n.s.) or SHAM (r = 0.3, n.s.), demonstrating that alpha reduction was not significantly related to changes in anxiety.
3.8 Participants’ self-report on neurofeedback
No adverse effects were reported by any participant either before or after NFB or SHAM. At the completion of the experiment participants were interrogated on what, if any, cognitive strategy they employed that seemed to lead to greater success in the neurofeedback game. Analysis of the data suggest that the predominant strategy reported by NFB participants was that of focused visual attention (12/17 or >70%), while the SHAM group presented no consistent strategy (threshold 4/17, or >23%).
4. Discussion
Plastic modulation of fMRI network connectivity
Our general objective was to examine whether a single session of EEG neurofeedback (NFB) could modify brain network dynamics beyond the time frame of the training session. Indeed, our results indicate that at around 30 minutes after training, NFB induced a statistically significant up-regulation of functional connectivity within the dACC/MCC of the salience network in the experimental but not in the sham group. Hence utilizing fMRI and a placebo-control group we extend the findings of Ros et al. (Ros et al. 2010) demonstrating that the adult cortex is sufficiently plastic that a mere half-hour of targeted volitional activity (i.e. NFB) is capable of intrinsically reconfiguring the brain's functional activity to last above and beyond - and at least as long as- the time period of training itself. Recent real-time fMRI studies have reported functional connectivity changes during NFB proper (Rota et al., 2011, Hamilton et al 2011), with the exception of Hampson et al. who found altered brain dynamics in the 5-min resting period across multiple NFB sessions (Hampson et al. 2011). However, this relatively brief elapsed time following NFB remains insufficient to substantiate LTP-like (long-term potentiation) brain plasticity effects, which last beyond approx. 20 min (Schulz & Fitzgibbons 1997). Hence, our observations provide a temporally direct association between NFB and plastic modulation of brain functional networks, forming an important link with emerging evidence of longer-term (> 1 week) functional connectivity changes after multiple NFB training sessions, either via EEG (Coben & Padolsky 2007) or fMRI (Yoo et al. 2007).
Our results are crucially strengthened by the finding that the mean increase of connectivity within salience network regions-of-interest (ROIs) coincided with a major cluster that negatively correlated with individual changes of resting-state alpha; while the latter measure was in turn predicted by the degree of alpha reduction during NFB, directly echoing the NFB protocol (alpha desynchronization). This overall concordance was absent from the sham group, where resting EEG amplitude change was not significantly predicted by the degree of individual EEG control during NFB. This outcome is consistent with Hebbian forms of neural plasticity whereby sustained (de)correlation of synaptic activities (directly reflected by EEG amplitudes) could shift population dynamics to increasingly more (de)synchronized states (Tass & Hauptmann 2007). Lastly, for the NFB group, we observed significant dACC/MCC clusters from the GROUP × TIME interaction which coincided with clusters that regressed negatively with EEG alpha synchronization, which was not the case for the sham group.
EEG correlations with fMRI connectivity and mind-wandering
NFB effects were found to be tightly coupled to individual changes in internal task-unrelated thoughts (i.e. mind-wandering). There was firstly a significant correlation, absent from the sham group, between resting-state alpha change and frequency of self-reported mind-wandering. Moreover, greater resting state alpha reductions were associated with lower reaction times to the mind-wandering probe. This corroborates an earlier report linking mind-wandering behavior with increased alpha amplitude (Moore et al. 2012). Secondly, fMRI connectivity differences in the dACC/MCC region correlated negatively with changes in mind-wandering, consistent with a separate report of enhanced salience network activity during awareness of mind-wandering (Hasenkamp et al. 2011). Importantly, the same region coincided with a large cluster negatively associated with resting alpha changes. The sham group did not exhibit this overall congruence between EEG, fMRI connectivity and mind-wandering change, as individually there was no significant correlation between EEG resting state (in any band) and mind-wandering change. Hence, for the NFB group, our result of a negative correlation between global resting alpha and salience network connectivity confirms the same anatomical location but is of opposite sign to the relationship observed by Sadaghiani et al (Sadaghiani et al. 2010). Intriguingly, in the sham group, we also observed a positive relation between resting alpha change and a comparable region of the dACC/MCC. How to reconcile these results? It is interesting to note that the alpha rhythm has been observed to quantitively follow an inverted-U function in proportion with arousal (Ota et al. 1996), compatible with its familiar decrease during transitions to drowsiness/sleep or high alertness. In the sham group, this phenomenon could resolve the negative correlation seen between alpha and mind-wandering, reported elsewhere (Braboszcz & Delorme 2011), together with evidence that EEG-BOLD coupling may vary between different behavioral states (Schölvinck et al. 2010). iewed speculatively from this framework, NFB may be seen to have acted more towards the right-side of the inverted-U (higher arousal), whilst sham within the left (lower arousal)(Ota et al. 1996).
In accordance with previous work, we found a positive relationship between changes in alpha synchronization and functional connectivity/activity in the default-mode network (DMN) (Hlinka et al. 2010; Mantini et al. 2007; Jann et al. 2009). Here, NFB and sham group participants displayed the strongest positive correlations with precuneal and mPFC functional connectivity, respectively. Moreover, in both groups, positive precuneal connectivity change was associated with higher frequency of mind-wandering, consistent with previous online thought-sampling investigations (Christoff et al. 2009; Mason et al. 2007; Hasenkamp et al. 2011).
Relationship with intrinsic alertness and attention
As an intervention NFB possesses a notable advantage over correlational designs in that it is able to preferentially test for 'cause and effect'. Yet, behavioral interventions are usually faced with the problem of dissociating stimulus-dependent (extrinsic) vs. stimulus-independent (intrinsic) effects. However a NFB paradigm uniquely permits the same external stimuli and frequencies of reward to be used across all participants. Hence, participants' entrained neuronal (EEG) differences may be considered as resulting minimally from external factors, and can instead be regarded as being driven by the modulation of internal, stimulus-independent brain states (Poulet & C. C. H. Petersen 2008). We would thus like to propose the existence of mechanisms that modulate the brain's 'intrinsic alertness' (Clemens et al. 2011; Sadaghiani et al. 2010), operating independently of -and not driven by- external factors, in view of our finding of a significant three-way correspondence between individual NFB changes in alpha network oscillations, fMRI salience network connectivity, and mind-wandering behavior. Furthermore, while visual stimuli were used for feedback and posterior alpha rhythms have been implicated in visual processing (Ergenoglu et al. 2004; Romei et al. 2008), our attention task involved auditory perception. Hence, the fact that no exploratory functional connectivity alterations were observed in either visual or auditory networks once again points to a potential cross-modal or sensory-independent role of global alpha rhythms in regulating alertness (Schürmann et al. 2000). As a result, our data indicate for the first time that the intrinsic, stimulus-independent effect of tonic alpha desynchronization is reflected in amplified dACC/MCC connectivity specifically within the salience network, strikingly coinciding with regions involved with supramodal alertness (Langner et al. 2012). The salience network has previously been implicated in salience detection (Seeley et al. 2007) and cognitive control (Dosenbach et al. 2006), while the dACC/MCC has been found to activate during thought suppression (Wyland et al. 2003), selective attention (Weissman et al. 2005), stimulus anticipation (Aarts et al. 2008) and emotional arousal (McRae et al. 2008).
In support of our findings, a growing body of evidence has linked alertness, attention, and/or arousal, on the one hand, and alpha desynchronization on the other. Trial-by-trial variations in sensory detection (Ergenoglu et al. 2004; Haegens et al. 2011) and subjective attentional state (J. Macdonald et al. 2011) are found to be associated with peristimulus EEG alpha desynchronization, while the excitability (Romei et al. 2008) and neuronal spike rate (Haegens et al. 2011) of sensory cortices is heightened. Secondly, concurrent reduction of theta, alpha, and beta amplitudes (comparable to the broader attenuation observed during our NFB protocol) appears to be a distinctive signature during alerting (Fan et al. 2007) as well as selective attention (Fries et al. 2001).
Alpha rhythm has been shown to globally attenuate upon eyes opening whilst correlating negatively with skin conductance, a classic measure of sympathetic arousal (Barry et al. 2007). Moreover, administration of caffeine, an often used stimulant to boost alertness, induces global reductions in alpha synchronization and increased galvanic skin response (Barry et al. 2005). Conversely, momentary cognitive lapses during a sustained-attention tasks (such as simulated driving) are found to be associated with increases in alpha (Huang et al. 2008). In sum, evidence suggests that higher alpha synchronization reflects inhibition of sensory cortical areas (Romei et al. 2008; Haegens et al. 2011), acting as a functional correlate of internally versus externally-directed attention (Cooper et al. 2003). Hence, given its impact on the salience network and mind-wandering, alpha-desynchronizing NFB may be seen as facilitating external or bottom-up attentional drive, characterized by suppressed internally-generated activity and cortical fluctuations typical of tonic, vigilant brain states (Harris & Thiele 2011; Schroeder & Lakatos 2009). Indeed, our self-report data suggest that the overwhelming strategy reported by NFB participants was that of focused visual attention (12/17 or >70%), while the SHAM group presented no predominant strategy. The alpha reduction during NFB thus likely reflected a selective visual attention strategy consistent with the parietal site of feedback and its induced topography. Hence, a more integrated account of our findings is proposed whereby increased dACC connectivity, indirectly induced by NFB, could be representative of enhanced tonic alertness/error monitoring demands in order to maintain task-set and attentional engagement (Weissman et al. 2005; Dosenbach et al. 2006). Within this framework, our findings draw interesting parallels with the effects of attention-based meditation training, which include the strengthening of dACC connectivity after focused attention (Manna et al. 2010) and during mindfulness (Kilpatrick et al. 2011).
Implications for brain disorders and potential physiological mechanisms
It is fascinating to speculate what physiological mechanisms could be responsible for the functional reconfiguration observed in the salience network. Conceivably, NFB regulation of the EEG, which mainly comprises of summed post-synaptic potentials (Nunez 2000), may act to directly modulate gross synaptic activity (Crochet et al. 2006) plus internal brain states (Poulet & C. C. H. Petersen 2008), which could result from the release of neuromodulators acting along diffuse pathways (Castro-Alamancos & Calcagnotto 2001). Alpha rhythms have been reported to be distinctly affected by the lesion and pharmacological blockade of noradrenergic pathways (Rougeul-Buser & Buser 1997) as well as modulated by cholinergic agonists (Lörincz et al. 2008). Regardless of the cellular mechanisms that subserve its effects, the current NFB protocol may have a significant therapeutic prospect in brain disorders exhibiting blunted dACC or salience network function. The pervasive role of this large-scale network and its dACC node have been linked to a wide range of pathophysiologies (Menon 2011), with reports of altered function in ADHD (Bush 2011), addiction (Goldstein et al. 2010), major depression (Menon 2011), schizophrenia (Menon 2011), and PTSD (Daniels et al. 2010). In particular, a therapeutic study that observed improvements in ADHD symptoms reported normalization of dACC activation following multiple NFB sessions (Lévesque et al. 2006). Likewise, the noradrenergic stimulant methylphenidate has been seen to increase dACC activation (Rubia et al. 2011) and cortical disinhibition (Schneider et al. 2011) in proportion to the clinical response of patients with attentional deficits. Intriguingly, in an earlier investigation we found that alpha desynchronization also leads to a lasting enhancement of cortical disinhibition (Ros et al. 2010). Furthermore, children with ADHD specifically show impaired reduction of parietal alpha rhythms during preparatory visual attention (Mazaheri et al. 2010). Hence, our findings provide direct, sham-controlled support for alpha-desynchronization NFB as a potentially novel protocol to modulate the crucial anatomical regions and cortical mechanisms implicated in ADHD (Bush 2011; Lévesque et al. 2006). Its evident strength is that it may be rapidly learned by naïve subjects, demonstrating a tangible impact after one session; data from our recently completed study with clinical (PTSD) patients are supportive of this view (Kluetsch et al., in preparation).
Limitations
There are several limitations related to our study. Firstly, although we detected group changes in salience network coupling, we did not find an overall difference in post-NFB behavioral measures, such as frequency of mind-wandering or reaction-time. This could be related to the high baseline performance of participants and/or the relative ease of the oddball task. Inspection of our data actually indicates that more than a third of experimental participants (NFB n=6, SHAM n=6) performed at ceiling during the initial baseline session (with 100% absence of mind-wandering). Thus in a significant proportion of participants, our task turned out to be insensitive to the improvements we hypothesized. Secondly, since we did not perform simultaneous EEG-fMRI, we could not ascertain the relationship between task-evoked EEG and BOLD change, nor able to evaluate possible pre-to-post group differences of the former. Thirdly, it is evident from exploratory analyses that the NFB protocol did not alter alpha amplitude selectively (we observed relative reductions in flanking bands). The alpha band was nevertheless the most significantly suppressed and correlated most robustly with reduction in mind-wandering and negatively with salience network coupling. However, a separate study conducted to explicitly address the alpha-band specificity of the NFB protocol would be appropriate by up/down-training other EEG bands, given that our observations only provide evidence of its sensitivity to alpha changes. Naturally, our results do not preclude additional spectral patterns from being associated with increased mind-wandering, such as low alpha-high theta (Braboszcz & Delorme 2011), which frequently corresponds to early states of drowsiness. Lastly, changes in salience network connectivity could be argued to be due to a self-regulation effect that potentially discriminated the NFB and sham groups. Here, self-report data indicate that the majority (>80%) of the sham participants did attempt to self-regulate during the session and were uncertain as to whether they were part of the sham group. Furthermore, taking into consideration the tight correlations with mind-wandering and the previously observed association between alpha rhythm and the salience network (Sadaghiani et al. 2010), self-regulation alone does not seem to be a plausible account for the observed outcome. Finally, it would have been fascinating to explore whether the current short-term effects may have generalized to longer time-scales (> 1 month) following the repeated application of the NFB protocol; we look forward to future studies investigating this relationship.
5. Conclusion
To conclude, we have provided the first neuroimaging evidence that alpha-band desynchronization can directly induce a plastic reinforcement of dACC connectivity within the salience network, which individually correlates with decreases in mind-wandering. Functional coupling within this network appears to be critical for cognitive control while its dysfunction has been implicated in a range of brain disorders (Menon 2011). Hence, as a special application of brain-computer interface technology, EEG-based NFB may offer the unique opportunity to induce anatomically-specific brain changes under physiological conditions, drastically reducing adverse effects. Our sham-controlled study offers promising neurobehavioral support for the use of EEG neurofeedback as a safe, inexpensive, and accessible tool for modulating brain function in health and disease.
Highlights.
Alpha (8–12 Hz) synchronisation was reduced through voluntary control, utilising real-time feedback from the EEG (neurofeedback).
fMRI salience network connectivity was found to be enhanced after neurofeedback, during a sensory attention task (auditory oddball).
This enhancement was detectable 30 minutes after neurofeedback, indicating an induction of cortical plasticity.
fMRI connectivity increases were subsequently correlated with decreased mind wandering and resting-state alpha amplitude.
Acknowledgments
We thank The Foundation for Neurofeedback and Applied Neuroscience, in particular H. John Fisher, for generously providing the neurofeedback hardware and software. We are similarly grateful to Suzy Southwell, Melody Chow and Richard Neufeld for their technical support and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. The Journal of Neuroscience. 2008;28(18):4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Ea, et al. Capturing inter-subject variability with group independent component analysis of fMRI data: A simulation study. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, et al. Caffeine effects on resting-state arousal. Clinical Neurophysiology. 2005;116(11):2693–2700. doi: 10.1016/j.clinph.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Barry RJ, et al. EEG differences between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology. 2007;118(12):2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. NeuroImage. 2011;54(4):3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl Ka, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: in vitro and in vivo. Journal of Neurophysiology. 2001;85(4):1489–1497. doi: 10.1152/jn.2001.85.4.1489. [DOI] [PubMed] [Google Scholar]
- Christoff K, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens B, et al. Revealing the Functional Neuroanatomy of Intrinsic Alertness Using fMRI: Methodological Peculiarities. PloS one. 2011;6(9):e25453. doi: 10.1371/journal.pone.0025453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coben R, Padolsky I. Assessment-Guided Neurofeedback for Autistic Spectrum Disorder. Journal of Neurotherapy. 2007;11(1):5–23. [Google Scholar]
- Cooper NR, et al. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. International Journal of Psychophysiology. 2003;47(1):65–74. doi: 10.1016/s0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- Crochet S, et al. Synaptic plasticity in local cortical network in vivo and its modulation by the level of neuronal activity. Cerebral Cortex. 2006;16(5):618–631. doi: 10.1093/cercor/bhj008. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JK, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. Journal of Psychiatry & Neuroscience. 2010;35(4):258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenoglu T, et al. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Research. Cognitive brain research. 2004;20(3):376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Fan J, et al. The relation of brain oscillations to attentional networks. The Journal of Neuroscience. 2007;27(23):6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, et al. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, et al. Alpha Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(48):19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, et al. Biofeedback of Real-Time Functional Magnetic Resonance Imaging Data from the Supplementary Motor Area Reduces Functional Connectivity to Subcortical Regions. Brain Connectivity. 2011;1(1):91–98. doi: 10.1089/brain.2011.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nature reviews. Neuroscience. 2011 Sep 12; doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, et al. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Heinrich H, Gevensleben H, Strehl U. Annotation: neurofeedback - train your brain to train behaviour. Journal of Child Psychology and Psychiatry. 2007;48(1):3–16. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- Hlinka J, et al. Slow EEG pattern predicts reduced intrinsic functional connectivity in the default mode network: An inter-subject analysis. NeuroImage. 2010;53(1):239–246. doi: 10.1016/j.neuroimage.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Huang R-S, et al. Tonic and phasic electroencephalographic dynamics during continuous compensatory tracking. NeuroImage. 2008;39(4):1896–1909. doi: 10.1016/j.neuroimage.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Jann K, et al. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. NeuroImage. 2009;45(3):903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Kamiya J, Callaway E, Yeager CL. Visual evoked responses in subjects trained to control alpha rhythms. Psychophysiology. 1969;5(6):683–695. doi: 10.1111/j.1469-8986.1969.tb02871.x. [DOI] [PubMed] [Google Scholar]
- Kiehl Ka, et al. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25(3):899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Kilpatrick La, et al. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. NeuroImage. 2011;56(1):290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, et al. Staying responsive to the world: modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Human Brain Mapping. 2012;33(2):398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neuroscience Letters. 2006;394(3):216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Lörincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. The Journal of Neuroscience. 2008;28(3):660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J, Mathan S, Yeung N. Trial-by-Trial Variations in Subjective Attentional State are Reflected in Ongoing Prestimulus EEG Alpha Oscillations. Frontiers in Psychology. 2011 May 2;:1–16. doi: 10.3389/fpsyg.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Research Bulletin. 2010;82(1–2):46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Mantini D, et al. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences. 2007;104(32):13170. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, et al. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biological psychiatry. 2010;67(7):617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- McRae K, et al. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41(2):648–655. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moore A, et al. Regular, brief mindfulness meditation practice improves electrophysiological markers of attentional control. Frontiers in human neuroscience. 2012 Feb 6;:18. doi: 10.3389/fnhum.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL. Toward a quantitative description of large-scale neocortical dynamic function and EEG. The Behavioral and brain sciences. 2000;23(3):371–398. doi: 10.1017/s0140525x00003253. discussion 399–437. [DOI] [PubMed] [Google Scholar]
- Ota T, Toyoshima R, Yamauchi T. Measurements by biphasic changes of the alpha band amplitude as indicators of arousal level. International Journal of Psychophysiology. 1996;24(1–2):25–37. doi: 10.1016/s0167-8760(96)00048-7. [DOI] [PubMed] [Google Scholar]
- Paternoster R, et al. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36(4):859–866. [Google Scholar]
- Poulet JFa, Petersen CCH. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454(7206):881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Romei V, et al. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cerebral cortex. 2008;18(9):2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T, et al. Endogenous control of waking brain rhythms induces neuroplasticity in humans. The European Journal of Neuroscience. 2010;31(4):770–778. doi: 10.1111/j.1460-9568.2010.07100.x. [DOI] [PubMed] [Google Scholar]
- Rosazza C, et al. Functional Connectivity during Resting-State Functional MR Imaging: Study of the Correspondence between Independent Component Analysis and Region-of-Interest-Based Methods. American Journal of Neuroradiology. 2011;1:1–8. doi: 10.3174/ajnr.A2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeul-Buser A, Buser P. Rhythms in the alpha band in cats and their behavioural correlates. International Journal of Psychophysiology. 1997;26(1–3):191–203. doi: 10.1016/s0167-8760(97)00764-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, et al. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biological psychiatry. 2011;70(3):255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, et al. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. The Journal of Neuroscience. 2010;30(30):10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. The Journal of Neuroscience. 2009;29(42):13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MKF, et al. Effects of long-acting methylphenidate in adults with attention deficit hyperactivity disorder: a study with paired-pulse transcranial magnetic stimulation. Neuropsychobiology. 2011;64(4):195–201. doi: 10.1159/000326693. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends in Neurosciences. 2009;32(1):9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Fitzgibbons JC. Differing mechanisms of expression for short- and long-term potentiation. Journal of Neurophysiology. 1997;78(1):321–334. doi: 10.1152/jn.1997.78.1.321. [DOI] [PubMed] [Google Scholar]
- Schölvinck ML, et al. Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann M, et al. Electroencephalogram alpha (8–15 Hz) responses to visual stimuli in cat cortex, thalamus, and hippocampus: a distributed alpha network? Neuroscience letters. 2000;292(3):175–178. doi: 10.1016/s0304-3940(00)01456-7. [DOI] [PubMed] [Google Scholar]
- Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterman MB, Howe RC, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167(921):1146–1148. doi: 10.1126/science.167.3921.1146. [DOI] [PubMed] [Google Scholar]
- Tass P, Hauptmann C. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. International Journal of Psychophysiology. 2007;64(1):53–61. doi: 10.1016/j.ijpsycho.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Weissman DH, et al. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;15(2):229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, et al. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wyland CL, et al. Neural correlates of thought suppression. Neuropsychologia. 2003;41(14):1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Yoo S-S, et al. Functional magnetic resonance imaging-mediated learning of increased activity in auditory areas. Neuroreport. 2007;18(18):1915–1920. doi: 10.1097/WNR.0b013e3282f202ac. [DOI] [PubMed] [Google Scholar]