Abstract
Adipose tissue (AT) is home to an abundance of immune cells. With chronic obesity, inflammatory immune cells accumulate and promote insulin resistance and the progression to type 2 diabetes mellitus (T2DM). In contrast, recent studies have highlighted the regulation and function of immune cells in lean, healthy adipose tissue, including those associated with type 2 or “allergic” immunity. Although traditionally activated by infection with multicellular helminthes, AT type 2 immunity is active independently of infection, and promotes tissue homeostasis, adipose tissue “browning”, and systemic insulin sensitivity, protecting against obesity-induced metabolic dysfunction and T2DM. In particular, group 2 innate lymphoid cells (ILC2s) are integral regulators of AT type 2 immunity, producing the cytokines IL-5 and IL-13, promoting eosinophils and alternatively activated macrophages, and cooperating with and promoting AT regulatory T (Treg) cells. In this review, we focus on the recent developments in our understanding of ILC2 cells and type 2 immunity in adipose tissue metabolism and homeostasis.
Keywords: group 2 innate lymphoid cells, adipose tissue, metabolism, diabetes, type 2 immunity
Introduction
Over the past several decades there has been a remarkable population shift in body habitus, with over one third of individuals in many high-income countries classified as obese, and rates of overweight and obese individuals rising worldwide [1]. Obesity predisposes to certain types of cancer, cardiovascular disease, liver disease, infertility, sleep apnea, and metabolic diseases such as insulin resistance and type 2 diabetes mellitus (T2DM). T2DM is a particularly devastating consequence of chronic obesity, resulting in persistently elevated blood glucose and damage to the eyes, kidneys, nerves, and vascular system. Interventions aimed at restricting food consumption or increasing exercise have achieved limited success in preventing T2DM, indicating that novel approaches will be required to counteract obesity and diabetes.
With obesity, adipose tissue (AT) expands, initially retaining relatively normal metabolic and vascular function and modest levels of immune cell activation [2, 3]. Elements of this early AT inflammatory response may be beneficial during aging [4] and normal AT remodeling during periods of feeding and fasting [5]. However, with chronic obesity, AT expansion becomes dysregulated, leading to adipocyte hypoxia and death, enhanced cytokine and chemokine secretion, dysregulation in fatty acid fluxes, and pronounced alterations in adipose immune cells [6]. This AT dysfunction is accompanied by ectopic lipid deposition and chronic low-grade inflammation in many tissues, including liver, pancreas, brain and skeletal muscle, eventually promoting systemic insulin resistance and the progression to T2DM [7, 8]. The first association between obesity and inflammation was described over 20 years ago [9], linking the production of tumor necrosis factor-α (TNF-α) by AT to insulin resistance and diabetes in multiple experimental models of rodent obesity. Since that seminal work, numerous studies have implicated the coordinate regulation of inflammatory cytokines (IL-1β, TNF-α, IL-6, INF-γ), signaling cascades (NF-κB, JNK, inflammasome) and leukocytes (macrophages, T cells, NK cells, neutrophils, mast cells) to mediate “metabo-inflammation”, a form of chronic, low-grade inflammation [3, 10–13]. Ultimately, systemic inflammatory cytokines have been shown to blunt insulin signaling in target tissues such as liver and muscle [7], leading to insulin resistance and the progression towards T2DM.
In contrast, the immune cells present in healthy AT are less well understood. These AT-resident leukocytes are also surprisingly abundant, and recent studies have described their metabolically beneficial roles, protecting against obesity-driven metabolic disease [13, 14]. In visceral AT, which surrounds the intestines and peritoneal cavity, there is a particularly numerous type of innate lymphoid cell (ILC) that produces the cytokines IL-5 and IL-13, hallmarks of a type 2 or “allergic” immune response. These group 2 innate lymphoid cells (ILC2s) are early mediators of protective type 2 immune responses during parasitic helminth infection, as well as pathologic allergic immune responses in the settings of asthma and atopic dermatitis [15]. However, ILCs can also promote tissue repair and homeostasis, including basal adipose tissue health and function [16]. Here we review our current understanding of the regulation and metabolic impact of AT ILC2s and associated type 2 immune responses.
Group 2 innate lymphoid cells
Innate lymphoid cells (ILCs) represent a growing family of immune cells with roles in tissue homeostasis and the initiation of inflammatory responses. ILCs are morphologically similar to B lymphocytes and T lymphocytes, but lack recombination activating gene (RAG)-dependent antigen receptors and do not express common cell lineage markers, including CD3, CD4, CD8, B220, CD11b, CD11c, and Gr1 [17]. In contrast to most T lymphocytes, ILCs are developmentally deployed to tissues, reacting to a wide array of stimuli to secrete effector cytokines. These properties allow ILCs and other innate-like lymphocytes (i.e. NKT, γδ T cells, B1 B cells) to play fundamental roles in early inflammatory responses during infection, but perhaps more importantly to coordinate with tissue macrophages and regulate the “physiologic inflammation” that occurs during normal tissue development, function, and remodeling [18]. The ILC family is divided into three groups: group 1 (ILC1 and NK cells), group 2 (ILC2) and group 3 (ILC3, LTi) based both on the phenotypical characteristics and the functional resemblance of their cytokine-secretion profile to that of their analogous T helper cell subsets (Th1, Th2, Th17) [19–21]. In this review we focus on the metabolic impact of ILC2s and type 2 immune cells in AT, although the potential roles of innate immune cells in other metabolic organs could also be significant and are currently poorly understood.
In 2001, a population of non-B, non-T cells was described that secrete type-2 cytokines in response to IL-25 [22]. In 2010, multiple groups further characterized these cells in mice [23–25] and humans [26], initially calling these cells innate type 2 helper cells, natural helper cells, or nuocytes, but by consensus now named group 2 innate lymphoid cells (ILC2). ILC2s express high levels of the transcription factor GATA-binding protein-3 (GATA3) and the retinoic acid receptor-related orphan receptor alpha (RORα) and require IL-7 for their development. ILC2s produce key T helper 2 (Th2) cell-associated cytokines, including IL-5, IL-9 and IL-13, as well as amphiregulin (Areg), a ligand for the epidermal growth factor receptor (EGFR). ILC2s are distributed developmentally throughout mucosal (skin, lung, gastrointestinal tract) and non-mucosal tissues (adipose tissues, liver, female reproductive tract, mesenteric lymph node) and locally replicate at these sites [27, 28]. ILC2s respond to epithelial signals such as IL-25, IL-33, thymic stromal lymphopoeitin (TSLP), as well as the lymphokines IL-2 and IL-7, lipid mediators, nutrients, and hormones. ILC2s coordinate innate type 2 immune responses through the production of type 2 cytokines IL-5 and IL-13, which are required for accumulation of eosinophils and the maintenance of alternatively activated macrophages (AAMs) [29]. During adaptive immune responses, such as those directed against helminth infections and certain allergens, ILC2 cooperate with CD4+ T helper 2 (Th2) cells, together producing type 2 cytokines that regulate AAM, eosinophilia, goblet cell hyperplasia and smooth muscle contractility. While these type 2 immune responses are critical to restricting helminth infections and neutralizing toxins, dysregulation of ILC2 and type 2 immunity promotes a wide spectrum of pathologic allergic disorders, including asthma, allergy, and atopic dermatitis [29].
ILC2s and type 2 immunity in tissue homeostasis and repair
ILC2s can also promote tissue remodeling and repair. For example, in a mouse model of H1N1 influenza virus-induced lung damage, lung-resident ILC2s restore lung epithelial barrier integrity and airway repair through the production of Areg [30]. In a mouse model of intestinal damage and inflammation, the epithelial cytokine IL-33 can promote ILC2 Areg production, leading to the resolution of colitis and promoting epithelial repair [31]. Indeed, type 2 immune responses are known to promote wound repair and tissue regeneration following infection or injury [32], suggesting that ILC2s may be key organizers of these beneficial tissue responses. Recent data suggest that ILC2s are distributed throughout adipose depots, including in fat-associated lymphoid clusters (FALCs) [23], and are important modulators of AT immune composition and adipocyte function [33, 34], including the balance of white and beige adipocytes [14]. Many of these “non-canonical” ILC2 functions are shared with tissue Treg cells, including Areg production and the ability to modulate AT homeostasis [35, 36]. To understand the metabolic impact of AT ILC2s, we first review the structure and function of AT.
Adipose Tissues: White, Brown, and Beige
Metabolism is regulated by the coordinate actions of the small intestine, pancreas, liver, muscle, brain, and AT, which together regulate energy uptake and storage and communicate fuel availability [37]. AT is present in discrete locations in the body, including under the skin (subcutaneous), surrounding deep organs (visceral), and in the bone marrow [38–40], accounting for 15–20% of body mass in lean healthy humans. Excess visceral AT is particularly associated with obesity-induced metabolic dysfunction [3]. AT is composed of lipid-laden adipocytes supported in a framework of collagen fibers and other extracellular matrix (ECM) proteins. Adipocyte precursors, fibroblasts, endothelial cells, and nerves contribute to AT growth and remodeling in response to feasting and famine, hormonal milieu, and aging [41]. AT is also a robust endocrine organ, releasing a wide range of signaling hormones or “adipokines”, including leptin and adiponectin, which modulate the metabolic responses of liver, muscle, brain, and pancreas, and which may directly impact immune cell function [42, 43].
Based on histological criteria, two types of AT are recognized: white adipose tissue (WAT) and brown adipose tissue (BAT). Althought both AT classes are involved in energy balance, they are functionally distinct, with unique developmental origins and opposing roles in body energetics [39, 40, 44, 45]. WAT stores excess energy as triglycerides whereas BAT expresses uncoupling protein 1 (UCP1) and is specialized in the dissipation of energy through the production of heat. WAT is the most abundant type of AT in adult mammals, while BAT is typically located in the interscapular and paraspinal regions in infants and small mammals [46]. Studies have identified functional BAT in a substantial proportion of adult humans, with high quantities of adult BAT associated with lower body weight; with increasing age, BAT decreases and body weight and insulin resistance increases [47]. There is a recently described third type of AT, composed of “beige” or “brite” (brown-in-white) adipocytes, a brown-like adipocyte that has been shown to increase in number with prolonged exposure to cold, and is interspersed within the subcutaneous WAT depots of rodents [48–50]. Beige AT expressing UCP1 can be induced to generate heat [51] and perform other beneficial metabolic functions, including the secretion of adipokines and the disposal of pathogenic lipids [52]. Recent studies have found that BAT present in adult humans most strongly resembles beige AT in mice [52, 53]. Increasing the quantity and function of beige AT in mice and humans protects against insulin resistance and type 2 diabetes [52], suggesting that pathways and cells that activate AT “browning” may be powerful tools to combat metabolic dysfunction.
Type 2 immune cells in healthy fat
Immune cells associated with type 2 immunity are prevalent in lean AT. In this section, we review the cells and cytokines that orchestrate this type 2 immune “module”. Macrophages are readily identified in lean AT and include a subset of anti-inflammatory “M2” or alternatively activated macrophages (AAMs), characterized by IL-10 secretion, arginase 1 and CD206 (mannose receptor) expression [54]. Siamon Gordon and colleagues originally defined AAMs as macrophages generated by IL-4 or IL-13 signaling, contrasting them with classically activated or “M1” macrophages, which are primed by IFN-γ or TLR signaling and are associated with anti-viral and anti-bacterial responses [55]. AAMs are vital components of immune responses against helminthes, as well as fibrotic immune responses associated with allergic pathology. The term AAM has been more broadly applied to macrophages polarized by other alternative signals, including immune complexes and IL-10. In reality, macrophages are heterogeneous with a range of tissue functions, although the concept of the AAM is useful to describe one end of this functional spectrum [56]. In mouse models, reduction of AAM numbers in the AT, either via genetic manipulation [57] or as a naturally occurring phenomenon of obesity [58], has been shown to promote insulin resistance, suggesting that AAMs can be protective against metabolic disease. AT AAMs appear to be involved in AT remodeling, restricting classical inflammation, and promoting AT browning [59]. Although these studies indicate the involvement of AAMs in several metabolic processes, the precise in vivo function(s) of AAM require further study.
ILC2s and eosinophils are also enriched in lean AT, collectively producing IL-4 and IL-13, suggesting that these cells are “upstream” regulators of AT AAMs (Figure 1). IL-4 competent eosinophils are conspicuous in AT, and both IL-4/IL-13 and eosinophils are required for optimal AT AAM maintenance [60]. ILC2-derived IL-5 supports the accumulation and maintenance of AT-resident eosinophils, and loss of eosinophils or IL-5 has been shown to exacerbate insulin resistance in mouse models of obesity [33, 60] (Figure 1). Natural killer T (NKT) cells can also be activated to produce IL-4/IL-13 in certain circumstances, and may augment ILC2s and eosinophils to support AAMs and Treg cells, protecting against obesity-induced insulin resistant and diabetes [61, 62]. Although rare in resting AT, CD4+ Th2 cells have been shown to accumulate in AT after helminth infection or with aging, and may be a further source of type 2 cytokines in these contexts [63, 64]. ILC2s and eosinophils can also induce AT browning, in part independently of AAMs, suggesting that these cells may perform other AAM-independent functions in AT. Together, ILC2 are the dominant in vivo source of murine IL-5 and IL-13 [33, 60], cytokines that are essential to the maintenance of tissue AAMs and eosinophils. These findings position ILC2s as key orchestrators of type 2 immune responses in AT and elsewhere.
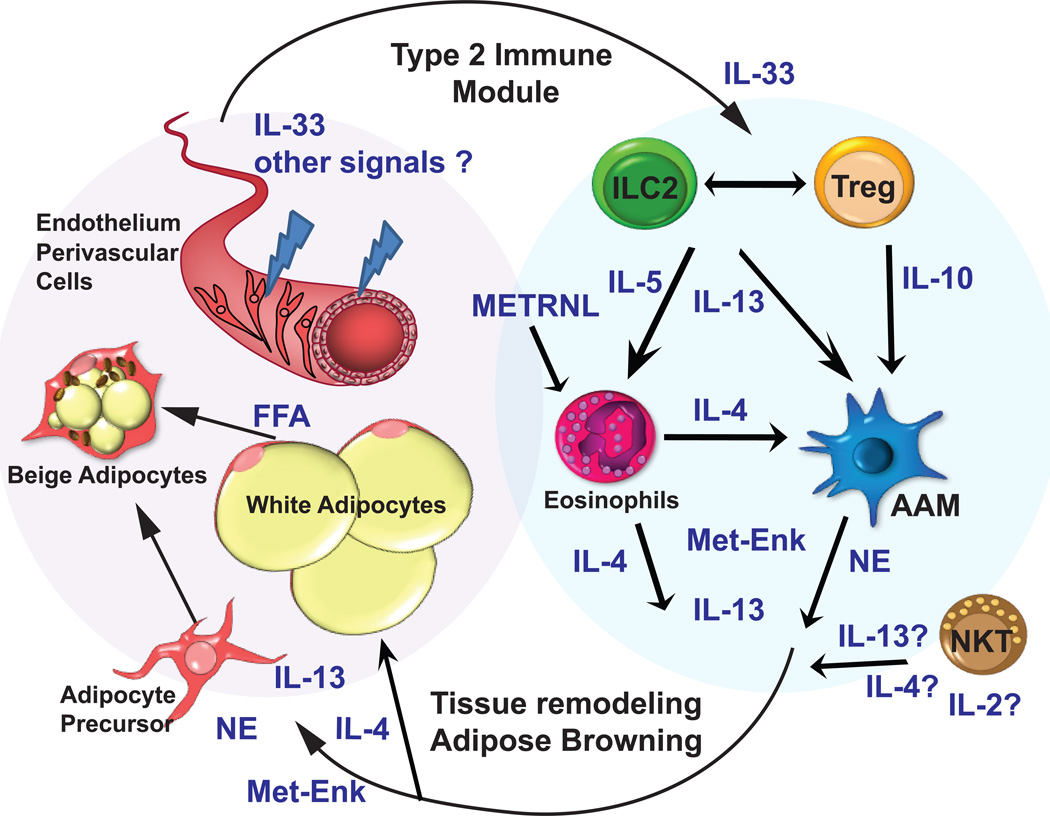
Figure 1. Model of the interaction of type 2 immunity and healthy adipose tissue.
Adipose tissue ILC2s produce IL-5 and IL-13, whereas Treg cells produce IL-10, and together these cells and cytokines coordinate recruitment and maintenance of eosinophils and AAMs right). ILC2s and NKT cells are required for Treg-cell maintenance in the AT, and NKT cells may be an additional source of IL-4 and IL-13. Eosinophis can produce IL-4 and are required for optimal AAM maintenance. Together, this “regulated” type 2 immune response (right) acts coordinately on the adipose tissue to promote browning and other adipose tissue remodeling (left). IL-4 and/or IL-13 promote adipocyte precursors to replicate and undergo beige differentiation. Met-Enk and AAM-derived norepinephrine (NE) further support beige adipocytes, as well as free fatty acid release from white adipocytes. In turn, adipose endothelium and supporting cells release IL-33, and other unknown chemokines and cytokines, that support the numbers and function of ILC2s and Treg cells. ILC2: group 2 innate lymphoid cells; Treg: regulatory T cell; AAM: alternatively activated macrophage; NE: norepinephrine; FFA: free fatty acids; Met-Enk: methionine encephalin.
Signals that regulate AT ILC2s and type 2 immunity
In this section, we review the signals that promote ILC2 residence in the AT and their activation (Figure 2). We focus on Interleukin-33 (IL-33), a major AT cytokine that regulates ILC2s to impact AT metabolic function [65–67]. IL-33 is a member of the IL-1 cytokine family, and is produced and stored in the nucleus of diverse non-hematopoietic cells. Upon release, IL-33 binds the heterodimeric receptor T1/ST2 (IL1RL1) and IL1RAP on target cells, activating down-stream canonical NF-κB signaling pathways [68]. Resting AT contains ample IL-33, which is expressed in this tissue by subsets of endothelial cells, fibroblast-like cells, and possibly other sources [65, 69]. Under normal or HFD feeding, mice deficient in IL-33 or the IL-33 receptor ST2 gain more weight, have increased whole-body adiposity and display impaired insulin secretion and glucose homeostasis [67, 70, 71]. Furthermore, mice lacking either IL-33 or ST2 are characterized by diminished ILC2 activation and decreased numbers of eosinophils, AAMs, and Treg cells in AT [33, 63, 70, 71]. Conversely, administration of IL-33 is metabolically beneficial, reducing adiposity, AT inflammation, and fasting glucose levels and improving glucose and insulin tolerance in obese mice [67, 70–72]. IL-33 drives the accumulation and activation of AT Treg cells and ILC2s [69–72], promoting the accumulation of eosinophils and AAMs [33, 65] (Figure 1, Figure 2). Nevertheless, many questions remain, including the relevant cellular sources of AT IL-33, the direct and indirect targets of IL-33, and the physiologic stimuli that trigger IL-33 production, release, and cleavage.
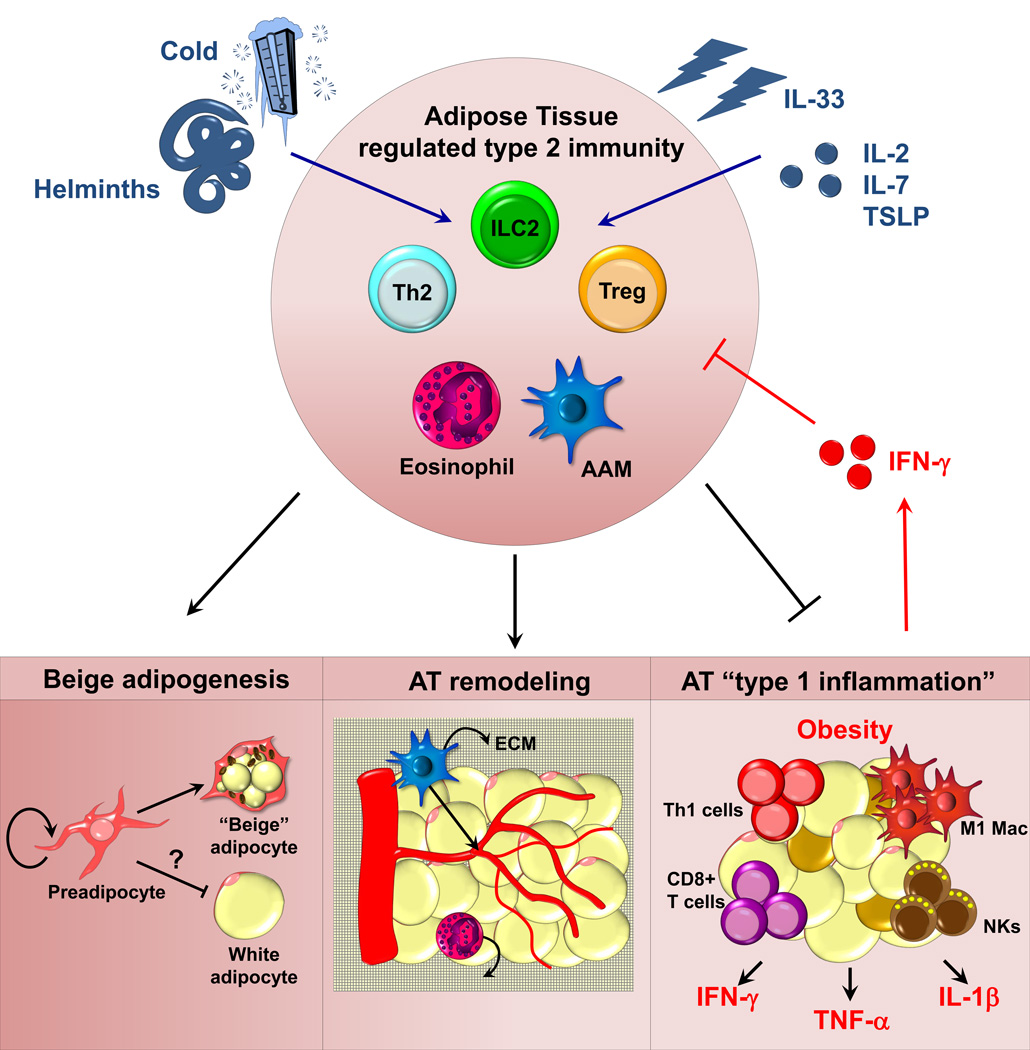
Figure 2. Model of regulators of adipose tissue homeostatic immune cells.
Positive signals enhancing numbers or function of “regulated” type 2 immunity within the AT are depicted in blue. IL-33 directly activates and supports ILC2 and Treg cells. Helminths and cold enhance responses through currently unknown mechanisms. IL-2, IL-7, and TSLP are speculated to be positive signals in AT. Signals that restrict this AT immune module are noted in red. Obesity-associated IFN-γ directly represses ILC2 and possibly other cells noted, including Treg cells. On bottom, three possible mechanisms by which type 2 immunity could impact AT function are illustrated: 1) promoting AT browning 2) promoting AT physiologic remodeling or 3) restricting excess or maladaptive “type 1 inflammation”. NK: nature killer cells; M1 Mac: M1 macrophage; ECM: extracellular matrix.
AT-resident ILC2s are present in mice lacking IL-33 signaling, suggesting that the residence of ILC2s in AT is regulated by other cytokines and factors [63]. AT ILC2s also express CD25 (IL-2Rα), CD127 (IL-7Rα), as well as receptors for IL-9, IL-25 and TSLP [73]. Both IL-7 and TSLP are abundant in AT [74, 75] and can cooperate with IL-33 to support ILC2 survival and function in vitro [76] (Figure 2). Adipose NKT cells have been shown to produce IL-2 [61], a cytokine that promotes ILC2 and Treg-cell proliferation and cytokine release [77, 78]. Furthermore, IL-33 induces the upregulation of CD25 on ILC2s, which may help ILC2s become more sensitive to T-cell-derived IL-2, suggesting the possibility of cooperation between IL-2 and IL-33 in promoting ILC2s in the AT [63]. Besides cytokines, AT ILC2s may also be regulated by lipid mediators. For example, lung ILC2s express receptors for cysteinyl leukotrienes and prostaglandins, both of which can regulate ILC2 function [79, 80]. ILC2s also express a number of chemokines and integrins, including α4β7, CCL1, CCR4, and CCR8. Little is known about the precise signals that permit ILC2 trafficking to AT, or elsewhere, during development. AT ILC2s express receptors for type 1 (IFN-α, IFN-β) and type 2 interferons (IFN-γ) and IL-27, all of which can potently repress ILC2 function and proliferation [63, 81], although their precise function(s) in AT metabolism are unclear.
The “regulated” type 2 immune response in AT
AT displays a confluence of innate type 2 immune cells with Treg cells [33, 57, 60, 71, 82–84]. Although this cellular combination is particularly active in AT, similar “regulated” type 2 immune responses are associated with chronic helminth infection, as well as tissue damage and repair, suggesting a coordinated regulation and interaction. In this section, we review the role of AT Treg cells and their interaction with type 2 immunity.
Treg cells are abundant in the visceral AT of lean mice, where they have a distinct transcriptome and antigen-receptor repertoire [82, 83]. Similar to ILC2s, Treg cells rapidly populate AT after birth, replicating locally [69]. AT Treg cells express high levels of CD25 and IL-10, as well as the transcription factors GATA3 and PPARγ, and the IL-33 receptor. They uniquely depend on these molecules, as mice lacking IL-33, IL-33R, or Treg-cell PPARγ expression have a profound loss of Treg cells specifically within the AT, and evidence of increased AT inflammation [69, 71, 85]. IL-33-driven expansion of ILC2s and/or Treg cells has been shown to revert the chronic inflammatory processes that drive obesity and improve insulin resistance in mouse models [71, 72]. Moreover, IL-2 administration expands both Treg cells and ILC2s, and both are augmented by endogenous tissue IL-33 [63, 78]. Together, these data suggest that AT-Treg cells share many features with ILC2s, including responding to similar cytokines (IL-2, IL-33), expressing similar transcriptional programs (GATA3, PPARγ), producing shared signaling molecules (Areg), and residing in similar perivascular locations [63, 78], and may cooperate with type 2 immune responses to maintain AT metabolic homeostasis as well as tissue homeostasis and repair at other sites.
During aging, AT undergoes physiological remodeling which can be accompanied by adiposity and metabolic dysregulation. In mice, AT Treg cells accumulate until 6–9 months of age, but decline again as the animal ages further [63, 86]. Recently, Bapat et al. [4] analyzed AT Treg cells in aging mice and showed that their accumulation paradoxically promoted AT insulin resistance. Mice deficient in Treg cells within the AT, accomplished by the deletion of PPARγ from FoxP3+ Treg cells, were leaner with age and more insulin sensitive [4]. Although this report did not determine the precise mechanism by which AT Treg cells promote age-related insulin resistance in mice, and indeed some of their results contrast with previous observations [82], the authors suggest that excess AT Treg cells could suppress local "healthy" AT inflammation, leading to a failure of tissue remodeling [4]. This work did not analyze AT ILC2s, but indicates that immune cells within the AT can interact in context-dependent manners to impact metabolic health, suggesting appropriate immune balance may be key to AT health and function.
ILC2 function in the context of type 2 immunity in AT homeostasis
Despite the evidence supporting an impact of ILC2s and regulated type 2 immunity in AT and whole animal metabolism, the precise mechanisms are only beginning to be explored. Possible models for ILC2s and related type 2 immune cells impacting AT metabolic function include: 1) supporting AT browning 2) promoting adipose tissue remodeling or 3) limiting excessive “type 1” inflammatory responses. In this section, we will address each of these hypotheses (Figure 2, bottom).
Eosinophils, AAMs, and ILC2s have all been implicated in cold-induced AT browning in mice [84, 87, 88] (Figure 2). AAMs were initially shown to promote adaptive thermogenesis in BAT, where macrophage catecholamine production (norepinephrine) in response to IL-4/IL-13 signaling was shown to be required for this response [88]. Optimal cold-induced subcutaneous AT browning required the type 2 cytokines IL-4/IL-13, AAM catecholamine production, and eosinophils, and exogenous administration of stabilized IL-4 was also shown to promote AT browning [84]. In parallel, another study implicated Meteorin-like, an exercise-induced muscle-derived “myokine”, which acts via eosinophils to induce AT browning [84, 87]. Most recently, administration of IL-33 has been shown to promote AT-resident ILC2s activation, WAT browning and increased caloric expenditure in mice [66, 70]. Adoptive transfer of ILC2s from IL-33-treated mice into wildtype or ILC2-deficient Rag2−/− γc−/− mice also induce browning of WAT, demonstrating a central role for IL-33 and ILC2 in this phenomena [70]. One study found that ILC2s express both proprotein convertase subtilisin/kexin type 1 (Pcsk1), an endopeptidase involved in processing prohormones into an active form, as well as its target proenkephalin A (Penk) [70]. One product of Penk processing is the opioid-like peptide methionine-enkephalin (Met-Enk), which can be produced by ILC2s and is increased after IL-33 stimulation (Figure 1). Administering Met-Enk led to increased numbers of UCP1+ beige adipocytes and increased energy consumption [70]. However, studies definitively identifying ILC2 as the relevant source of AT Met-Enk are required. In a second study, IL-33 administration led to ILC2-derived IL-13 production, which acted on PDGFRα+ adipose precursors to promote their proliferation and commitment to a beige fate [66]. Further, adipose precursor-specific deletion of IL-4Rα, the co-receptor for IL-4 and IL-13, showed that IL-33 induced IL-4/IL-13 acts directly on PDGFRα+ adipose precursor, bypassing the eosinophil-AAM-norepinephrine axis [66]. A possible unified model is one in which type 2 immunity regulates adipose browning by first generating and priming adipocyte precursors via IL-4/-13 stimulation, then possibly promoting their differentiation through Met-Enk signaling, and finally supporting thermogenic activation through macrophage-derived catecholamine production (Figure 1). As AT browning is regulated by multiple direct and indirect pathways [89], the precise mechanisms and metabolic relevance of type 2 immunity-directed browning in both rodent models and humans are fundamental and unanswered questions.
AT remodeling is a crucial and dynamic process during the life of a human. With abundant food supply, AT rapidly grows, requiring the formation of extracellular matrix (ECM) and angiogenesis, two processes known to facilitate adipogenesis and healthy AT expansion [6]. During extended fasting, AT must be able to contract and release lipids, likely activating similar processes. At other tissue sites, eosinophils and AAMs are intimately involved in tissue development and remodeling, as they produce matrix metalloproteinases (MMPs), which cleave ECM components, thereby regulating ECM abundance, composition and structure [90]. AAMs secrete vascular endothelial factor (VEGF) which promote angiogenesis and vascular remodeling [91]. Engulfing and removal of damaged adipocytes by AT macrophages is also required for tissue remodeling and differentiation of new adipocytes at sites of adipocyte loss [92]. As the dominant upstream regulators of AAMs and eosinophils in AT [33], ILC2s may also control AT tissue remodeling. In line with this possible role, ILC2-derived IL-13 acts on PDGFRα+ adipose progenitors, promoting their proliferation [66]. Interestingly, during high-fat feeding in adult mice, PDGFRα+ adipose progenitors have been shown to interact with AT macrophages, forming an adipogenic cell niche, which appears to be critical to the generation of new adipocytes and remodeling in adult AT [93]. As “healthy” classical inflammation has also been implicated in AT remodeling [94] future studies are required to delineate the precise cellular and molecular contributes of AT immune cells to tissue remodeling.
Excess AT inflammation during obesity contributes to insulin resistance, suggesting that cells and signals restricting classical inflammation could promote metabolic benefit. Therefore, the regulated type 2 immune response in AT may also impact metabolic function via limiting excess classical inflammation. In support of this model, activation of AT-regulated type 2 immunity via the administration of IL-33 in obese mice was shown to almost completely reduce classically activated or “M1” macrophages accumulation and inflammatory cytokine production in visceral AT [67, 72]. Mice deficient in AAMs display increased evidence of AT inflammatory cytokines [57]. Similarly, eliminating AT Treg cells led to increased evidence of visceral AT inflammation and insulin resistance in mice [83, 95]. Nevertheless, further definitive studies are required in mice and humans.
Interactions of ILC2s and other immune cells within AT
There is an intricate crosstalk between ILC2s, Treg cells, mast cells, and Th2 cells during type 2 immune responses to helminths, as well as those associated with tissue homeostasis and repair. IL-33 is one signal that coordinates these interactions, although ILC2 MHC class II expression, cytokines (IL-2, IL-4, IL-9, IL-13), or unknown signals could also participate in these interactions [65]. In this section we focus on the interactions between ILC2, Treg and Th2 cells, the most abundant type 2-associated immune lymphocytes in AT [63]. ILC2s and Treg cells have been shown to localize to similar perivascular areas of the AT under resting conditions [61, 63] and in multiple tissues after induction by IL-33 or helminth infection (Figure 2) [63], suggesting these cells may interact in vivo. The costimulatory protein ICOS is highly expressed in AT Treg cells, whereas ICOS ligand (ICOSL) is expressed in AT ILC2s [63]. After administration of IL-33 or infection with N. brasiliensis, in mice, ICOSL+ ILC2s and ICOShi Treg cells have been shown to accumulate in tissues and persist for prolonged periods [63]. In these scenarios, ILC2-intrinsic IL-33 signaling and ICOSL expression promoted Treg-cell accumulation via ICOSL-ICOS interactions and other unknown pathways. Together, these findings suggest that AT ILC2s are required for optimal Treg-cell maintenance. In mice treated with α-galactosylceramide, activated AT-resident NKT cells have also been shown to support AT Treg cells expansion and suppressor activity via production of IL-2 [61], likely providing additional support. Whether AT Treg cells exert a repressive effect on ILC2s within the AT is not known, although a recent study in a mouse model of allergic lung inflammation suggest that Treg cells can dampen IL-5 and IL-13 production by lung-resident ILC2 after allergen challenge, promoting rapid resolution of inflammation [96]. One possibility is that AT Treg cells compete with ILC2s for signals, such as IL-2 or IL-33, as similar competitive interactions have been described for NK cells and Treg cells [97]. Further studies examining the precise relationships between ILC2s and tissue Treg cells are warranted.
ILC2s and Th2 cells are also intricately coordinated during type 2 immune responses. As the number of Th2 cells increases dramatically in AT after helminth infections, these interactions may be important to the metabolic effects of type 2 immunity. Tissue Th2 cells can display innate-like function, responding directly to activating IL-33 [98], suggesting these cells may be able to functionally complement ILC2 activities in AT. Conversely, the cells and signals that might restrict AT ILC2s and Th2 cells are poorly defined. One candidate is NK cells, which along with ILC2s are the dominant innate lymphoid population present in resting AT. NK cells contribute to the early stages of obesity-induced AT inflammation, producing IFN-γ to help polarize macrophages to an inflammatory M1 phenotype [3, 99]. IFN-γ has been shown to directly repress ILC2 activation and proliferation [63], and regulated type 2 immune responses within the AT have been shown to decline during states of chronic obesity, suggesting a possible NK-cell-mediated inhibition of ILC2s in AT. Further studies are warranted to determine the biologic significance of these complex cellular interactions and overlapping functions.
Helminth infections and type 2 immunity
Type 2 immunity in the AT is active in young mice, but appears to wane with obesity or extended age [63]. Although human AT is less well described, ILC2s and Treg cells are both present and their cellular numbers decline with obesity [14]. Therefore, loss of the homeostatic AT immune responses could contribute to the development of obesity and T2DM. Approaches to amplify existing AT type 2 immunity may be viable therapeutic approaches to target obesity and T2DM [64]. In this section, we review how helminths, potent natural inducers of regulated type 2 immune responses, alter AT and whole animal metabolism.
Helminths can invade and damage epithelial and endothelial barriers at target tissues, such as skin, lung, and intestine. Damaged cells initiate innate anti-parasite type 2 immune responses, including activation of ILC2s [32, 100]. Eventually, antigen-presenting cells engage adaptive immune responses in lymphoid tissues, generating Th2 cells and elevated IgE. Treg cells, Breg cells, and other regulatory processes are also induced by helminths and can limit excessive immune responses in models of infection, inflammation, and autoimmunity [32, 101]. As such, immune responses to helminths have a striking resemblance to endogenous immune activation in homeostatic AT. Epidemiologic data suggest decreasing exposure to gastrointestinal helminths and Schistosoma species correlates with increasing prevalence of inflammatory diseases, including the incidence of T2DM [102–104], suggesting activation of type 2 immunity by helminths may be metabolically beneficial in humans.
In mice, transient helminth infections have both therapeutic and preventative effects against obesity and diabetes. These effects can be partially reproduced with the intra-peritoneal injection of helminth-derived products such as S. mansoni-soluble egg antigens [105], and are associated with the prolonged induction of type 2 immune response in the AT in mice [60, 105, 106]. After infection with the parasite N. brasiliensis, AT ILC2s, Th2 cells and eosinophils have been shown to increase and remain elevated for many months after helminth expulsion [33, 60, 63]. These findings are particularly striking as helminthes such as N. brasiliensis do not directly contact adipose tissue, suggesting indirect effects on AT type 2 immune cell trafficking or expansion. These findings suggest AT retains a prolonged “metabolic memory” of prior infections which may lead to lasting metabolic alterations for the organism. However, helminth infection promotes type 2 immune responses not only in AT but in several tissues, including the liver and intestine. Recent studies have highlighted the intricate relationship between helminth infection, altered intestinal microbiome, and type 2 immunity [107], and further studies are required to understand the specific metabolic contributions of helminth-induced AT, liver, or intestinal alterations.
ILCs in other metabolic tissues
In addition to visceral and subcutaneous AT, ILC2s and type 2 immune responses are distributed through many metabolically relevant tissues in mice. In particular, the intestine supports an abundant population of ILC2s and eosinophils. Intestinal ILC2s co-express IL-5 and IL-13 and this is enhanced after feeding in mice [27]. IL-13 and IL-5 production by ILC2s was shown to be suppressed following fasting, and is regulated in part by the circadian clock through vasoactive intestinal peptide (VIP) and its receptor VPAC2 on ILC2s [27]. Malnutrition elicited by vitamin A deficiency in mice is associated with an imbalance in the proportion and response of intestinal ILC2s and ILC3s, which resulted in defective ILC3-dependent antibacterial immunity [108] and expansion of interleukin-13 (IL-13)–producing ILC2s that promoted enhanced resistance to nematode infection in mice [108]. ILC2s in the intestine require IL-25 from intestinal epithelial tuft cells for basal cytokine secretion and optimal activation during helminth infection [100]. In turn, ILC2s produce IL-13 that support tuft cell differentiation, IL-25 production, and goblet cell expansion [100]. These findings reinforce the concept of ILC2s intimately interacting with tissue-resident progenitor cells to impact tissue function, although the metabolic impact of this intestinal type 2 circuit is currently unknown.
Activated ILC2s can also have either beneficial or detrimental effects on liver homeostasis. In mouse models of adenovirus-induced hepatitis, ILC2s have been shown to promote liver protection, possibly through the ability of IL-33-dependent ILC2s to limit TNF-α production from hepatic T cells and macrophages [109]. In contrast, in mouse models of liver fibrosis, the expansion of liver-resident ILC2s by IL-33 induces a pro-fibrotic response via IL-13, which triggers activation and trans-differentiation of hepatic stellate cells via the IL-4Rα/STAT6 signaling pathway [110]. The metabolic effects of ILC2s and type 2 immunity in the liver, the primary site of glucose formation (gluconeogenesis), require further studies.
ILC2 are also abundant in lung tissue and can promote murine models of asthma [29]. Counterintuitively, ILC2 and the “regulated” type 2 immune module are repressed in obese AT, whereas obesity is associated with increased asthma risk and severity [111]. The precise etiologies of obesity-related asthma are unclear and likely multifactorial, but one possibility is that non-allergic immunity is increased with obesity. Indeed, lung IL-17+ ILC3 are increased in murine models of obesity-related asthma and may contribute to this link [112].
Conclusions
Originally described in the context of anti-helminth immunity, ILC2s now appear to have a role in the control of tissue homeostasis, including AT and whole body metabolism. ILC2s are present in metabolic tissues, particularly AT in both humans and mice, and impaired ILC2 response are associated with metabolic disturbances in obese individuals and mice. Restoration of ILC2 responses in the AT promotes whole-body insulin sensitivity, and glucose and lipid homeostasis. Rather than acting in isolation, ILC2s detect signals such as IL-33 and coordinate a response with Treg cells, AAMs, and eosinophils. One intriguing result of activation of this AT type 2 immune module is AT browning, a process strongly associated with metabolic benefit in the setting of obesity and T2DM. However, as type 2 immune responses are involved in tissue development and remodeling, it is likely that remodeling pathways also account for some of the metabolic effects of ILC2s, and remain poorly explored. Stimulating AT ILC2s and type 2 immune processes with activating signals, such as IL-33, or with helminth/helminth-derived products may offer therapeutic avenues for metabolic disorders, although careful consideration is warranted. Further studies are required, particularly in humans, to confirm findings in rodents and test the applicability to therapeutics that target obesity and type 2 diabetes.
Acknowledgments
We thank Drs. S. Van Dyken, A.V. Molofsky, and S. Nozzari for comments on the manuscript. This work was supported by K08DK101604 (ABM) from the NIH, UCSF REAC Grant (ABM), and the UCSF Department of Laboratory Medicine.
Abreviations
- AT
adipose tissue
- BAT
brown adipose tissue
- WAT
white adipose tissue
- ILC2
group 2 innate lymphoid cell
- Treg
Foxp3+ regulatory T cell
- T2DM
type 2 diabetes mellitus
- AAM
alternatively activated macrophage
Footnotes
Conflict of interest:
The authors declare no financial or commercial conflict of interest.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 4.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asterholm IW, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metabolism. 2014:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 11.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 13.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 14.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mjosberg J, Spits H. Type 2 innate lymphoid cells-new members of the "type 2 franchise" that mediate allergic airway inflammation. Eur J Immunol. 2012;42:1093–1096. doi: 10.1002/eji.201242549. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker JA, Barlow JL, McKenzie ANJ. Innate lymphoid cells - how did we miss them? Nature Reviews Immunology. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 18.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2015;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 19.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Montaldo E, Juelke K, Romagnani C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur J Immunol. 2015;45:2171–2182. doi: 10.1002/eji.201545598. [DOI] [PubMed] [Google Scholar]
- 22.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 23.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 24.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 27.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Moltke J, Locksley RM. I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr Opin Immunol. 2014;31:58–65. doi: 10.1016/j.coi.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- 38.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308:E1085–E1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 43.Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689–702. doi: 10.1038/nrcardio.2012.148. [DOI] [PubMed] [Google Scholar]
- 44.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 45.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 46.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 47.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 49.Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. 2014;6:33–42. doi: 10.4252/wjsc.v6.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu HC, et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guigas B, Molofsky AB. A worm of one's own: how helminths modulate host adipose tissue function and metabolism. Trends Parasitol. 2015;31:435–441. doi: 10.1016/j.pt.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 72.Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J Immunol. 2015;194:4777–4783. doi: 10.4049/jimmunol.1500020. [DOI] [PubMed] [Google Scholar]
- 73.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Lucas S, Taront S, Magnan C, Fauconnier L, Delacre M, Macia L, Delanoye A, et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PLoS One. 2012;7:e40351. doi: 10.1371/journal.pone.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turcot V, Bouchard L, Faucher G, Garneau V, Tchernof A, Deshaies Y, Perusse L, et al. Thymic stromal lymphopoietin: an immune cytokine gene associated with the metabolic syndrome and blood pressure in severe obesity. Clin Sci (Lond) 2012;123:99–109. doi: 10.1042/CS20110584. [DOI] [PubMed] [Google Scholar]
- 76.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 77.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Gool F, Molofsky AB, Morar MM, Rosenzwajg M, Liang HE, Klatzmann D, Locksley RM, Bluestone JA. Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901 e893. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duerr CU, McCarthy CDA, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nature Immunology. 2016;17:65-+. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Medicine. 2009;15:930–U137. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu ZQ, Zhao WH. The IL-33/ST2 axis is specifically required for development of adipose tissue-resident regulatory T cells. Cell Mol Immunol. 2015;12:521–524. doi: 10.1038/cmi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Natl Acad Sci U S A. 2015;112:482–487. doi: 10.1073/pnas.1423486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elias I, Franckhauser S, Bosch F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte. 2013;2:109–112. doi: 10.4161/adip.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 93.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18:355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asterholm IW, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte Inflammation Is Essential for Healthy Adipose Tissue Expansion and Remodeling. Cell Metabolism. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23:431–437. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194:863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gasteiger G, Rudensky AY. Interactions between innate and adaptive lymphocytes. Nat Rev Immunol. 2014;14:631–639. doi: 10.1038/nri3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Jr, Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, Glasner A, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 100.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2015 doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finlay CM, Walsh KP, Mills KH. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–230. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 102.Wiria AE, Djuardi Y, Supali T, Sartono E, Yazdanbakhsh M. Helminth infection in populations undergoing epidemiological transition: a friend or foe? Semin Immunopathol. 2012;34:889–901. doi: 10.1007/s00281-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 103.Wiria AE, Wammes LJ, Hamid F, Dekkers OM, Prasetyani MA, May L, Kaisar MM, et al. Relationship between carotid intima media thickness and helminth infections on Flores Island, Indonesia. PLoS One. 2013;8:e54855. doi: 10.1371/journal.pone.0054855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 105.Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, Ozir-Fazalalikhan A, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29:3027–3039. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 106.Yang Z, Grinchuk V, Smith A, Qin B, Bohl JA, Sun R, Notari L, et al. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect Immun. 2013;81:1905–1914. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, Piersigilli A, et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity. 2015;43:998–1010. doi: 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, Sun J. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 112.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]