Abstract
Background
The present interdisciplinary consensus review proposes clinical considerations and recommendations for anaesthetic practice in patients undergoing gastrointestinal surgery with an Enhanced Recovery after Surgery (ERAS) programme.
Methods
Studies were selected with particular attention being paid to meta‐analyses, randomized controlled trials and large prospective cohort studies. For each item of the perioperative treatment pathway, available English‐language literature was examined and reviewed. The group reached a consensus recommendation after critical appraisal of the literature.
Results
This consensus statement demonstrates that anaesthesiologists control several preoperative, intraoperative and postoperative ERAS elements. Further research is needed to verify the strength of these recommendations.
Conclusions
Based on the evidence available for each element of perioperative care pathways, the Enhanced Recovery After Surgery (ERAS ®) Society presents a comprehensive consensus review, clinical considerations and recommendations for anaesthesia care in patients undergoing gastrointestinal surgery within an ERAS programme. This unified protocol facilitates involvement of anaesthesiologists in the implementation of the ERAS programmes and allows for comparison between centres and it eventually might facilitate the design of multi‐institutional prospective and adequately powered randomized trials.
Editorial comment: what this article tells us.
This consensus paper includes a number of recommendations to enhance recovery in patients undergoing gastrointestinal surgery. Preoperatively, optimization of medical disease and cessation of smoking and alcohol intake are emphasized. Prevention of nausea and vomiting is important. Careful titration of anaesthetics and ensuring full recovery of neuromuscular blockade are recommended. During surgery, there should be normal values of arterial oxygen level, intraoperative temperature and glucose concentration. The article also includes recommendations regarding fluid therapy, opioid‐sparing analgesia and mobilization.
Over 234 million major surgical procedures are performed globally each year1 and despite advances in surgical and anaesthetic care, morbidity after abdominal surgery is still high2. Fast‐track or enhanced recovery after surgery (ERAS) clinical pathways have been proposed to improve the quality of perioperative care with the aim of attenuating the loss of functional capacity and accelerating the recovery process3.
The ERAS pathways reduce the delay until full recovery after major abdominal surgery by attenuating surgical stress and maintaining postoperative physiological functions. The implementation of the ERAS pathways has been shown to impact positively in reducing postoperative morbidity, and as a consequence, length of stay in hospital (LOSH) and its related costs4, 5, 6, 7, 8, 9.
In recent years, several studies have highlighted the impact of the anaesthetic management on postoperative morbidity and mortality10, 11, 12, 13. In view of the evidence that many elements of the ERAS programme published by the ERAS Society in 2009 are of related to anaesthetic care, it is imperative that guidelines on perioperative care include recommendations approved by an interdisciplinary team comprising anaesthesiologists and surgeons3.
As a follow‐up of the previous manuscript14 where the pathophysiological basis of the ERAS were analysed, this article represents an effort of the ERAS Society (www.erassociety.org) to present a consensus review of clinical considerations, including recommendations, for optimal anaesthesia care for patients undergoing gastrointestinal surgery within the ERAS programme. It is not the purpose of this manuscript to provide detailed information about each single ERAS element and for each type of gastrointestinal surgical procedure. Most of the ERAS elements have been already discussed extensively, specifically for different types of surgical procedures, as well the quality of evidence supporting each ERAS element15, 16, 17, 18, 19. It must be acknowledged that evidence supporting some of the ERAS elements still remains controversial.
Methods
An interdisciplinary group of physicians, anaesthesiologists and surgeons who are experts in the field of ERAS programmes were invited to participate in the preparation of this consensus statement.
Literature search
The authors met in October 2012 and the topics for inclusion were agreed upon and allocated. The principal literature search utilized MEDLINE, Embase and Cochrane databases to identify contributions related to the topic published between January 1966 and May 2014. Medical Subject Headings (MeSH) terms were used, as were accompanying entry terms for the patient group, interventions and outcomes. Key words included “‘anesthesia’’, “anaesthesia”, “analgesia”, “surgery”, “‘enhanced recovery’’ and “‘fast track’’. Reference lists of all eligible articles were checked for other relevant studies. Conference proceedings were not searched. Expert contributions came from within the ERAS Society Working Party.
Study selection, assessment and data analyses of the identified trials
Based on the literature search, titles and abstracts were screened by individual reviewers to identify reviews, case series, non‐randomized studies, randomized control studies, meta‐analyses and systematic reviews that were considered for each individual topic. Discrepancies in judgment were resolved by the senior author and during committee meetings of the ERAS Society Working Party.
Recommendations
Recommendations were made by the panel based on the evidence supporting each ERAS element. Specifically, “Strong recommendations” indicate that the panel was confident that the desirable effects of adherence to a recommendation outweighed the undesirable effects. “Weak recommendations” indicate that the desirable effects of adherence to a recommendation probably outweighed the undesirable effects, but the panel was less confident. Recommendations were based on the balance between desirable and undesirable effects, and on values and preferences.
Part A. Preoperative ERAS elements
An ERAS approach to preoperative evaluation
Pre‐admission risk stratification
Risk scoring systems have been used to try and identify which patients are at higher risk of death and complications from major surgery. Up to 80% of postoperative deaths come from this high‐risk group20. It is imperative not only to provide patients with an overview of the risk of surgery but also to select those patients for further investigation and optimization and decide which perioperative care pathway the patients should be on for resource allocation. In a major retrospective study in the USA, Khuri et al. analysed data on 105,951 patients undergoing a variety of different specialty major surgical procedures. The striking result was that if patients had a major complication within 30 days of surgery then it reduced median survival by 69% at 8 years21. Therefore, identification for risk factors for any major complication of surgery is also important.
Scoring systems for surgery
Many different scoring systems, some of them procedure‐specific, have been developed for patients undergoing surgery. The purpose of this section is to give an overview of the most common scoring system use in clinical practice beside the well known American Society of Anesthesiologists (ASA) physical status score.
POSSUM scores: in 1991, Copeland et al. described the POSSUM (Physiological and Operative Severity Scoring for the enUmeration of Mortality and morbidity) scoring system for general surgical patients22. This is a two part scoring system based on physiological assessment (12 variables) and operative severity (six variables). Each variable has a 1–4 point range depending on severity. The system predicts 30‐day risk for mortality (matrix for the 50% prediction of risk of mortality: specificity = 99.3% and sensitivity = 54.1%) and morbidity (matrix for the 50% prediction of risk of morbidity: specificity = 92.4% and sensitivity = 52.1%). The Portsmouth POSSUM (P‐POSSUM) better predicts postoperative mortality23, as the original POSSUM logistic regression equation over‐predicts mortality especially in low‐risk patients. POSSUM has been also modified slightly for different specialties such as colorectal24, oesophageal25 and vascular surgery26 to try and improve sensitivity and specificity for these specialties.
Assessing cardiac risk in non‐cardiac surgery
Cardiovascular risk can be predicted by multivariate risk incidences that include clinical and surgical criteria, and biological markers27, 28, 29. These tools have been incorporated in the recent ACC/AHA 2014 guidelines on perioperative cardiovascular evaluation and care for non‐cardiac surgery.30
The Lee index
The Lee Index is a modification of the original Goldman cardiac risk index31. It comprises six independent clinical determinants of major perioperative cardiac events:
History of ischaemic heart disease (IHD)
History of cerebrovascular disease
Heart failure
Preoperative insulin treatment for diabetes mellitus
Serum creatinine > 177 μmol/l
High‐risk type of surgery
All factors contribute 1 point equally to the index, and for patients with an index of 0, 1, 2 and 3 points the incidence of major cardiac complications is estimated at 0.4%, 0.9%, 7% and 11% respectively.31
Cardiovascular Risk Calculator
A similar tool to determine the postoperative probability of myocardial infarct or cardiac arrest has been validated by Gupta and colleagues in 211,410 patients undergoing surgery. It contains five independent predictors28 :
Type of surgery
Dependent functional status (inability to perform activities of daily living in the 30 days before surgery, partially independent or totally independent)
Abnormal serum creatinine
American Society of Anesthesiologists class (ASA)
Increasing age
More recently there has been increasing awareness that perioperative myocardial injury does not always present with any of the typical ischaemic features of chest pain, electrocardiogram changes, rhythm disturbance or heart failure. The VISION study measured troponins and showed a spectrum of results with 44% of troponin rises fulfilling the criteria for myocardial injury without fulfilling a traditional definition of perioperative myocardial infarction32.
Assessment of functional capacity
Estimating functional capacity is an important start of assessing a patient. Functional capacity is measured in metabolic equivalents (METs). One MET equals the basal metabolic rate at rest. Climbing one flight of stairs demands 4 METs and strenuous activity such as playing tennis or swimming is > 10 METS. The inability to perform 4 METS indicates poor functional capacity and is associated with an increased incidence of postoperative cardiac events.33 The presence of good functional capacity, even in the presence of stable IHD or other risk factors is associated with a good outcome.34 As patients poorly estimate their functional capacity, it is important to obtain an independent assessment using dynamic testing.
Dynamic Tests
Walk Tests—(2 min, 6 min, shuttle) All these tests measure the distance covered over a set period of time by the patient. They have been validated in clinical practice and are easy to administer.35, 36 Norms according to age and gender have been created. Although they correlated with cardiopulmonary testing, they have not been used to determine whether to operate or not on patients undergoing high‐risk surgery.
Cardiopulmonary Exercise Testing (CPET)
This is a dynamic non‐invasive objective test that evaluates the ability of a patient's cardiopulmonary system to adapt to a sudden increase in oxygen demand. The ramped exercise test is performed on a cycle ergometer with ECG monitoring and analysis of expired carbon dioxide and oxygen consumption, the later being directly related to oxygen delivery and a linear function of cardiac output when exercising. With increasing exercise, oxygen consumption will eventually exceed oxygen delivery. Aerobic metabolism becomes inadequate to meet the metabolic demands and blood lactate rises reflecting supplementary anaerobic metabolism. The value for oxygen consumption at this point is known as the anaerobic threshold (AT), expressed as ml/kg/min VO2 peak/max can also be measured. Both values have been used to try and predict the risk of complications. Older's original work in colorectal patients showed that if a patient's AT was less than 11 ml/kg/min, the patients was at higher risk of complications which was increased if there was the presence of ischaemic heart disease.37, 38 Snowden et al. showed that an AT cut‐off value of 10.1 ml/kg/min predicts complications better than an algorithm‐based activity assessment (Veterans Activity Questionnaire Index [VASI]).39 Similarly, in patients undergoing pancreatic, hepatic and vascular surgery and AT < 10 ml/kg/min predicts complications and early postoperative death40, 41, 42, 43. VO2 max has also been studied to predict outcome and has been shown to be a sensitive marker for cardiopulmonary complications in patients undergoing oesophageal resection44. Despite its high sensitivity, the specificity of the CPET is not high enough to identify patients with a significant preoperative risk correctly, as patients with low ATs can still undergo major surgery without complications.
Risk of acute kidney injury (AKI)
Approximately 1% of patients undergoing non‐cardiac surgery develop AKI, and it is associated with higher morbidity and mortality. Eleven preoperative risk factors (age 56 years or older, male sex, emergency surgery, intraperitoneal surgery, diabetes mellitus necessitating oral therapy, diabetes mellitus necessitating insulin therapy, active congestive heart failure, ascites, hypertension, mild preoperative renal insufficiency and moderate preoperative renal insufficiency) have been identified as independent predictors of AKI in patients undergoing non‐cardiac surgery. The risk of developing postoperative AKI can be stratified in five classes based on to the presence of these risks factors (General Surgery Acute Kidney Injury Risk Index).45
Summary and recommendations: preoperative scoring tools and functional capacity tests can be used to identify patients at risk of complications and to stratify perioperative risk (Table 1).
Table 1.
Scoring systems for surgery
| Test | Predicting | Scoring | Evidence level | Recommendation |
|---|---|---|---|---|
| P‐POSSUM | Mortality and Morbidity | 12 physiological and 6 operative variables | High | Strong |
| Lees index | Perioperative cardiac complications | 6 preoperative clinical factors | Moderate | Strong |
| Cardiovascular Risk Calculator | Myocardial Infarct or Cardiac Arrest | 4 preoperative clinical factors and 1 operative variable | Moderate | Strong |
| Shuttle Walk Test | Perioperative complications | Aerobic fitness | Moderate | Moderate |
| Shuttle Walk Test | Screening tool to proceed to CPET/echocardiography etc. | Aerobic fitness | Moderate | Strong |
| Cardiopulmonary Exercise testing (CPET) | Perioperative complications | Aerobic exercise – AT and VO2 max | Moderate | Strong |
| Cardiopulmonary Exercise testing (CPET) | Selecting patient's suitability for surgery | Aerobic exercise – AT and VO2 max | Moderate | Moderate |
| General Surgery Acute Kidney Injury Risk Index | Acute Kidney Injury | 11 preoperative clinical factors | Moderate | Moderate |
AT, anaerobic threshold; VO2, maximum oxygen consumption.
Recommendation grade
POSSUM: strong
Lee Index: strong
Cardiovascular Risk Calculator: strong
Walk tests: strong (to predict postoperative morbidity, but not to decide if operate or not)
CPET: strong
General Surgery Acute Kidney Injury Risk Index: strong
Optimization of pre‐existing health conditions
Alcohol
Alcohol abusers (defined by the World Health Organization as ingesting more than 36 g of ethanol or equivalent of 3 standard drinks/day) have an increased risk of perioperative bleeding and wound infection. Furthermore, alcohol impairs the metabolic stress response, cardiac and the immune function. The risk increases proportionately with the amount of alcohol ingested with an increased perioperative risk of 200–400% when ingestion exceeds 5 drinks or 60 g of ethanol per day. A minimum of 4 weeks abstinence is needed to reduce these risks, but 8–12 weeks may be needed for patients to return to normal. However, it is often a challenge to maintain abstinence in these patients even with replacement medical therapy. Patients with end stage liver failure due to cirrhosis are at extremely high risk and will need expert care for all types of procedures46, 47.
Smoking
Smokers often have comorbidities due to smoking such as chronic obstructive airways disease, emphysema, peripheral vascular and ischaemic heart disease and cerebrovascular disease that can increase the risk of perioperative complications independently. Smokers without these comorbidities still have an increased perioperative risk, mainly due to poor wound and tissue healing which can lead to wound infection48 as well as cardiopulmonary complications such as chest infection. Studies have been undertaken to assess whether short‐term abstinence from smoking can improve outcome. The cessation of smoking for 4 weeks prior to surgery has been shown to improve wound healing.48, 49, 50 The use of nicotine replacement therapy (NRT) and counselling facilitate preoperative smoking cessation.49 Other pharmacological interventions are also available. Varenicline, in combination with two preoperative 15‐minute standardized counselling sessions, started 1 week before surgery and followed up for 12 weeks, was shown to improve long‐term smoking abstinence (RR 1.45, 95% CI 1.01–2.07, P = 0.04) but not reduce postoperative complications in comparison with placebo. However, nausea occurred more frequently in patients treated with varenicline (13.3% vs. 3.7%, P = 0.004).51 Antidepressants such as bupropion also seem beneficial to improve smoking cessation, but limited data are available in the perioperative setting.52, 53
Preoperative anaemia
Haemoglobin is one of the main determinants of oxygen delivery. Preoperative anaemia is common and is an independent predictor of mortality and postoperative complications.54, 55 Haemoglobin levels should be corrected preoperatively, as it is common to expect a drop of haemoglobin concentrations due to blood loss and to the dilution effect of intravenous fluids. Correction of preoperative anaemia should take in consideration its aetiology.56, 57 Iron, folate, vitamin B12 supplements and/or erythropoietin should be used when appropriate. Medical management of preoperative anaemia takes time and should be planned at least 3–4 weeks before elective surgery. Although preoperative blood transfusion corrects anaemia rapidly and could be used in severely anaemic patients and/or in patients undergoing surgery with expected profound blood loss, caution should be used as it has been associated with increased mortality and morbidity.58, 59, 60 These effects seem to be dose‐dependent.58 The risk of transfusion‐related complications and the effect of blood transfusion on the immune system must be also considered.56, 57, 61 Evidence suggesting that normalizing preoperative haemoglobin levels prior to surgery reduces postoperative morbidity and mortality is lacking and studies evaluating the role of preoperative anaemia optimization are warranted.57, 62 Implementation of perioperative blood management protocols can reduce the risk of allogenic blood transfusions.56, 57
Cardiovascular risk reduction
It is not the intent of this manuscript to discuss in detail perioperative cardiovascular strategies to reduce cardiovascular risk. These interventions are extensively discussed in the recent ACC/AHA 2014 guidelines.30
Asthma, COPD and diabetes
Chronic conditions such as asthma, chronic obstructive airways disease63, diabetes mellitus64 malnutrition65, 66, 67 and frailty68 should be optimized prior to surgery.
Summary and recommendation: cessation of smoking and alcohol intake at least 4 weeks before surgery is recommended. Encouraging patients is not enough; pharmacological support and individual counselling should be offered to every patient who smokes and to alcohol abusers undergoing elective surgery. Optimization of medical conditions, such as cardiovascular diseases, anaemia, chronic obstructive airways disease, diabetes, nutritional status and frailty and should follow international recommendations.
Recommendation grade
Smoking cessation: high
Nicotine replacement therapy and counselling: high
Alcohol cessation: low
Medical optimization: strong
Pre‐anaesthetic medications
Patients undergoing major surgery are, as expected, anxious. Anxiety has also been shown in many studies to be the most common predictor for postoperative pain and positively correlates with postoperative pain intensity.69 Furthermore, preoperative pain is also a significant predictor for postoperative pain.70 Therefore, education and counselling, and preoperative analgesic and anxiolytic medication must be specifically addressed during the preoperative assessment of the patient. Short‐acting anxiolytics and analgesics can be administered to facilitate regional anaesthetic procedures and insertion of intravascular lines, provided they are used in adequate doses based on age and patients' comorbidities.71 Short‐acting benzodiazepines should be avoided in older patients (age > 60).72 Long‐acting sedatives and opioids should be avoided as they may hinder recovery, thus impairing postoperative mobilization and direct participation, resulting in prolonged length of stay.71
Summary and recommendation: long‐acting anxiolytic and opioids should be avoided as they may delay discharge. Short‐acting benzodiazepine should be avoided in the elderly.
Recommendation grade: strong
Preoperative fasting and carbohydrate loading
Although fasting guidelines of various anaesthesia societies support the safety of allowing clear fluids up to 2 h and solid food up to 6 h before the induction of anaesthesia, patients scheduled for elective surgery are commonly asked to fast from midnight. The evidence supporting this practice, with the belief to ensure an empty stomach before the induction of anaesthesia and decrease the risk of aspiration is lacking.73 On the contrary, it has been shown that fasting from midnight increases insulin resistance, patient's discomfort and potentially decreases intravascular volume, especially in patients receiving mechanical bowel preparation.74 In fact, functional intravascular deficit after fasting time, as indicated by guidelines75 or after 8 h fasting76 is minimally affected in patients undergoing elective surgeries without mechanical bowel preparation.75, 76 Results from two Cochrane meta‐analyses have shown that gastric content of patients following anaesthesia fasting guidelines is the same or lower of the gastric content of patients fasting after midnight.77, 78 Imaging studies have further supported the safety of allowing clear fluids up to 2 h before the induction of anaesthesia, showing complete gastric emptying with 90 min.79 Recently, the European and American Anesthesia Society have revised their fasting guidelines and have not changed their previous recommendations.80, 81 Preoperative treatment with oral complex carbohydrates (CHO) (maltodextrin) with a relatively high concentration (12.5%), with 100 g (800 ml) administered the night before of surgery and 50 g (400 ml) 2–3 h before induction of anaesthesia, reduces the catabolic state induced by overnight fasting and surgery. Indeed, overnight fasting before surgery inhibits insulin secretion and promotes the release of catabolic hormones such as glucagon and cortisol. By increasing insulin levels preoperative treatment with oral CHO reduces postoperative insulin resistance, maintains glycogen reserves, decreases protein breakdown and improves muscle strength.82 Faster surgical recovery and better postoperative well‐being still remains controversial83, 84. Delayed gastric emptying should be suspected in patients with documented gastroparesis, patients on prokinetic agents such as metoclopramide and/or domperidone, patients scheduled for gastrointestinal operations such oesophageal, gastric, fundoplication, paraesophageal hernia repair, gastro‐jejunostomy, in patients who underwent previous Whipple's procedure, in patients with achalasia and in patients with neurological diseases with dysphagia. Patients with diabetes with neuropathy and, less clearly, obese patients85 are considered to have delayed gastric emptying. However, gastric emptying after 300 ml of clear fluids 2–3 h before the induction of anaesthesia in obese patients has been shown to be similar to those of lean patients86, 87 and gastric emptying after CHO administration in patients with uncomplicated diabetes is normal.88, 89 The clinical relevance of preoperative CHO drinks in these specific populations remains to be established.
Summary and recommendation
Intake of clear fluids should be allowed until 2 h before induction of anaesthesia. Solids should be allowed until 6 h. Preoperative treatment with oral CHOs can be administered safely except in patients with documented delayed gastric emptying or gastrointestinal motility disorders and as well in patients undergoing emergency surgery.
Recommendation grade
Adherence to fasting guidelines (avoid overnight fasting): strong
Administration of preoperative CHOs: strong
Administration of preoperative CHOs in diabetic and obese patients: weak
Part B. Intraoperative and postoperative ERAS elements
Preventing and treating postoperative nausea and vomiting
Despite significant advances in our knowledge of PONV and the introduction of new agents, the overall incidence of PONV is currently estimated to be 20–30%. In high‐risk patients, the incidence in still as high as 70%,90 and it is one of the most unpleasant experiences in the perioperative period.91
There are many risk factors that predispose patients to PONV.92 The most widely used scoring system was developed by Apfel et al.,93 who created a simplified scoring system using only four risk factors – female gender, a history of motion sickness or PONV, non‐smoking status and the use of postoperative opioids.92
The multimodal approach to PONV within an ERAS programme contains the use of antiemetics and a total intravenous anaesthesia with propofol instead of inhalational agents. Avoidance of nitrous oxide is also important.94 Other factors like the reduction of preoperative fasting, carbohydrate loading and adequate hydration95, 96 and high inspired oxygen concentrations97 may influence the prevalence of PONV. The use of regional anaesthetic techniques and the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) as opioid‐sparing strategies may have an additional indirect influence on the prevalence of PONV.
Classes of antiemetics (serotonergic, dopaminergic, cholinergic and histaminergic) are based on the antagonism of different kinds of central receptors that are all involved in the pathophysiology of PONV and all have shown to be superior to placebo in the prevention of PONV.98 Newer drugs as the neurokinin‐1 receptor antagonists show encouraging results in initial trials.99 Unfortunately, none of the available pharmacological agents when used alone are effective in reducing the incidence of PONV by more than 25%. Antiemetic combinations are recommended for patients at higher risk of PONV. Combination therapy is more effective than monotherapy, and for high‐risk patients, combination with 2–3 antiemetics in addition to propofol based total intravenous anaesthetic (TIVA) has the greatest likelihood of reducing PONV.
Examples of antiemetic drugs are serotonin antagonists like ondansetron 4 mg i.v. or dopamine antagonists like droperidol 0.625–1.25 mg i.v. given at the end of surgery or a transdermal patch of scopolamine placed the evening prior to or 2 h before surgery. Dexamethasone 4–5 mg i.v. after induction of anaesthesia has also been shown to be effective, but its immunosuppressive effects on long‐term oncological outcome are unknown. Higher doses of dexamethasone have no additional effect and are associated to sleep disturbances. It should not be used in diabetic patients requiring insulin and not given prior to induction of anaesthesia due to perineal pain.
If PONV is present postoperatively, rescue therapy should be with an antiemetic from a different class unless the elapsed time from the previous antiemetic administration is greater than 6 h,100 After prophylactic administration of 4 mg ondansetron re‐dosing for established PONV was shown to be no more effective than placebo.101
Summary and recommendation: Aggressive PONV prevention strategy should be included in an ERAS protocol.102 All patients with 1–2 risk factors should receive as PONV prophylaxis a combination of two antiemetics. Patients with 3–4 risk factors should receive 2–3 antiemetics and total intravenous anaesthesia (TIVA) with propofol and opioid‐sparing strategies should be encouraged.93, 102
Recommendation grade: strong.
Standard anaesthetic protocol and depth of anaesthesia monitoring
Although there are no studies comparing general anaesthetic techniques for gastrointestinal surgery, it is sensible to assume that within the ERAS protocol efforts have to be made to minimize the impact of anaesthetic agents and techniques on organ function, and to facilitate rapid awakening from anaesthesia thus accelerating recovery of the patient's gastrointestinal and motor functions. As such particular attention can be drawn to the type of agents used and the monitoring of vital functions.
Traditionally the anaesthesiologist has relied on clinical signs to try and ensure appropriate depth of anaesthesia and avoidance of awareness but also avoiding overdose and the resultant depression of a patient's physiological status. Depth of anaesthesia can now be measured by many devices but in terms of clinical evaluation the data on Bispectral Index (BIS) far exceeds other devices.103 Recent focus has been on using depth of anaesthesia monitoring not just to avoid awareness during surgery but also to titrate the minimum amount of anaesthetic necessary to avoid complications.103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 This appears to have particular significance in the elderly population with cognitive dysfunction.117 Unfortunately BIS is not infallible. Many things can affect the BIS value, in particular neuromuscular relaxation, which is commonly used in anaesthesia. The specificity seems to be lower when using total intravenous anaesthesia (TIVA).106 There is also a lag time between EEG interpretation and the displayed BIS value.
When compared with clinical signs alone, BIS obtains lower rates of awareness during surgery.112, 113, 114, 116 Anaesthetic depth guided by BIS may also help reduce the amount of drug given,107, 116 with more rapid immediate recovery although the time to discharge home appears to be unaffected116. In Myles' study, 138 patients needed to have BIS monitoring to avoid one case of awareness.112 Avidan's studies104, 105 have demonstrated that maintaining anaesthetic depth with an end tidal concentration (EATC) between 0.7 and 1.3 MAC equivalents can prevent intraoperative awareness as effectively as anaesthesia guided by a BIS value between 40 and 60. The use of nitrous oxide, a N‐methyl‐D‐aspartate (NMDA) receptor antagonist, has been shown to reduce the risk of awareness118 with one study showing an NNT of 46,119 however, there were two cases of awareness in the ENIGMA study in patients having nitrous oxide.120 Recent studies have highlighted that patients with BIS levels < 45 under anaesthesia (reflecting increased suppression of brain activity) have an increased risk of death by up to 1.24‐fold (95% CI 1.06–1.44).121 Subsequent analysis suggests this may be a reflection of elderly patients who have multiple problems and cognitive dysfunction and may have a reduced life expectancy prior to surgery more likely to have low BIS values. More studies are needed to clarify this point. There is increasing interest in anaesthetic drugs and analgesic techniques. (e.g. morphine and thoracic epidural analgesia) and their effect on cancer outcome but there is currently not enough consistent data to support making specific recommendations.122, 123
Summary and recommendation: anaesthetic depth should be guided either maintaining an end tidal concentration of 0.7–1.3 MAC or BIS index between 40 and 60 with the aim not only to prevent awareness but also to minimize anaesthetic side effects and facilitate rapid awakening and recovery. Avoid too deep anaesthesia (BIS < 45), especially in elderly patients
Recommendation grade: strong
Neuromuscular blockade (NMB) and neuromuscular monitoring
This section discusses the importance of neuromuscular blockade and neuromuscular monitoring, and their potential implications specifically in the context of an ERAS programme. Neuromuscular blockade agents (NMBA) paralyse skeletal muscles, allowing optimal conditions for surgery. The level of NMB needed to obtain optimal surgical conditions can differ depending on the surgical approach. A deep NMB might be particularly useful when a laparoscopic approach is used.124, 125 A recent systematic review showed that during certain laparoscopic procedures deep NMB (e.g. Post‐Tetanic Count 1 or more; but Train of Four (TOF) Count of 0126) provide better surgical conditions than moderate NMB125, but limited evidence is available to support this practice.126 Moreover, the use of deep NMB during laparoscopic procedures, especially in countries where sugammadex is not available, may increase the risk of residual paralysis.126 Although moderate NMB certainly facilitates surgical work, the use of NMB might not be always necessary for patients undergoing open abdominal surgery. Indeed, an adequate level of anaesthesia without muscle relaxants can produce a good to excellent surgical field in approximately two‐third of patients undergoing radical retropubic prostatectomy.127 In the light of these considerations, the hypotheses that optimal NMB can potentially attenuate surgical stress by shortening the duration of surgery, and that it can facilitate the use of low pneumoperitoneum pressures, thereby reducing postoperative pain remain appealing, especially in the context of an ERAS programme. However, this needs to be tested in larger high‐quality trials.
At the end of surgery, it is important to restore neuromuscular function to preoperative levels and avoid residual muscle paralysis which can be responsible for respiratory insufficiency, hypoxia, aspiration into the lungs as well as distress for the patient.128 Similarly, it might impair early mobilization. To avoid residual muscle paralysis long‐acting NMBA should not be used.128 Hypothermia also influences neuromuscular function directly and prolongs duration of action and recovery time of NMBA significantly.129 Maintenance of normothermia is, therefore, essential to prevent residual paralysis.129
The use of NMBA must be guided by adequate assessment of neuromuscular block and appropriate monitoring. In healthy volunteers, it has been demonstrated that there is risk of pharyngeal dysfunction or aspiration if TOF < 0.9.130 Furthermore, three clinical trials131, 132, 133 have demonstrated that there is a greater proportion of hypoxaemic events and prolonged stay in the recovery room if TOF < 0.9. Even more experienced anaesthesiologists cannot clinically identify the degree of residual curarization.134 Several studies have shown that clinical tests and qualitative (visual or tactile) assessment of neuromuscular function (TOF, double burst suppression or tetanic stimulation) are not reliable and sufficient to detect residual curarization,128 even when sugammadex is used.135 Quantitative methods such as mechanomyography and acceleromyography provide more accurate information.136 Although mechanomyography remains the goal‐standard to measure neuromuscular function, its use in clinical practice remains limited136. On the contrary, acceleromyography can be used easily to measure neuromuscular function and avoid residual paralysis.136
There are three ways to avoid residual paralysis:
Waiting for a spontaneous recovery of neuromuscular function identified by a TOF>0.9. This approach might not be convenient for brief surgical procedures, as the effect of some NMBA can last longer than 4 h, even after a single dose administered at the beginning of surgery.137 Side effects of reversal agents are avoided.
Administering cholinesterase inhibitors. Side effects of cholinesterase inhibitors and antimuscarinic agents have to be considered.
Administering sugammadex. Sugammadex selectively revers the neuromuscular block induced by steroidal NMBA. Abrishami et al. demonstrated that sugammadex reverses neuromuscular block (rocuronium‐induced) faster than neostigmine and independent of the depth of the neuromuscular block.138 Sugammadex can be used at different dosages, 2, 4 or 16 mg/kg to reverse moderate, deep or recently induced block, respectively. Sugammadex reverses neuromuscular block 3–4 times faster than neostigmine, and the neuromuscular block is completely reversed after 5 min.
Summary and recommendations: It remains controversial if deep neuromuscular blockade during laparoscopic surgery improves operating conditions. Neuromuscular function should be always monitored when using NMBA to avoid residual paralysis. Long‐acting NMBA should be avoided. When NMBA are administered neuromuscular function should be monitored by using a peripheral nerve stimulator to ensure adequate muscle relaxation during surgery and optimal restoration of neuromuscular function at the end of surgery. A TOF ratio of 0.9 must be achieved to ensure adequate return of muscle function and thus preventing complications.
Recommendation grade: Monitoring neuromuscular function: strong.
Reversing neuromuscular blockade: strong.
Use of inspired oxygen
Oxygen is a highly reactive gas which is ubiquitous in anaesthetic practice. In cellular physiology the controlled oxidation of glucose to carbon dioxide with the concurrent reduction of oxygen to water is the basis for aerobic metabolism and production of energy. Therefore, one of the highest priorities of the anaesthesiologist is to try to ensure a patient does not become hypoxic to avoid interruption of cellular metabolism.
Oxygen is widely available in anaesthesia and has traditionally been added to increase the inspired fraction of oxygen above 21% to overcome hypoxia under anaesthesia caused by physiological changes such as pulmonary shunt. Although increasing the FiO2 is necessary to overcome hypoxia there has been increasing recognition that hyperoxia can cause damage due to the production of oxygen free radicals.
However, it has been suggested that high inspired oxygen concentration protects against the risk of surgical site infections. The PROXI trial, a multicentre RCT, found no differences between patients treated with a FiO2 30% vs. 80% in terms of SSI or pulmonary complications.139.A meta‐analysis including the PROXI trial showed that two subgroups of patients benefitted from high inspired oxygen therapy – those undergoing general anaesthesia and colorectal surgery.140 However a high‐heterogeneity was found among the studies included.140 The latest meta‐analysis including new nine RCTs (5001 patients) found a marginal reduction of SSI in patients undergoing colorectal surgery treated with high concentrations of oxygen vs. normal oxygen concentrations (RR 0.77, 95% CI 0.59–1.00, P = 0.03). The study also found that high oxygen concentrations reduce the incidence of late (24 h postoperatively) nausea and vomiting, but only in patients receiving volatile anaesthesia without antiemetic prophylaxis.97 Based on these data, it still remains unclear if high concentrations of oxygen protects against the risk of SSI.
On the con side was the long‐term follow‐up of patients included in the PROXI trial. This study showed a reduction in survival in patients with cancer who had received the higher inspired oxygen concentration.141 Unfortunately, the authors failed to report why patients died earlier than patients receiving normal inspired oxygen concentrations. Both this study and the analysis of outcomes of patients following cardiac arrest, which show a poorer neurological outcome in patients receiving a higher FiO2,142, 143 suggest that there can be harmful effects from receiving high inspired concentrations of oxygen.
Therefore, higher inspired oxygen concentrations of 80% may reduce surgical wound site infection especially in patients with colorectal cancer, but there may be deleterious effects on long‐term cancer outcomes. To reduce wound infection to a minimum the importance of other contributing factors such as maintaining patient's body temperature, cardiac output, glycaemic control, prophylactic antibiotics and minimizing surgical contamination should also be considered.
The short‐term use of high inspired oxygen concentrations is widely practised in anaesthesia to overcome hypoxic episodes and to pre‐oxygenate (de‐nitrogenate) the lungs prior to the induction of anaesthesia. Edmark and colleagues looked at differing inspired concentrations (60%; 80%; 100%) of oxygen for 5 min prior to the induction of anaesthesia.144 Computed tomography showed an increase in atelectasis in the 100% inspired oxygen group although patients took longer to desaturate. The use of 80% oxygen in a subgroup of the PROXI study and in a recent meta‐analysis also did not demonstrate any increased risk of pulmonary complications.97, 145
Summary and recommendations
The inspired fractional concentration of oxygen should be titrated to produce normal arterial oxygen levels and saturations. Prolonged periods of high inspired oxygen concentrations which result in hyperoxia should be avoided.
Recommendation grade: strong
100% inspired oxygen concentrations can be used for pre‐oxygenation prior to anaesthesia or for short periods to overcome hypoxia.
Recommendation grade: strong
Preventing intraoperative hypothermia
Perioperative hypothermia, defined as a core temperature below 36°C is a common adverse consequence of anaesthesia and surgery.146 The prevalence of inadvertent hypothermia ranges from 50% to 90%147 independently whether patients undergo laparoscopic or open surgery.148 Older adults are more prone to heat loss, whereas obesity has a protective effect.149
Hypothermia in most patients undergoing general anaesthesia is the result of an internal core‐to‐peripheral redistribution of body heat that usually reduces core temperature by 0.5–1.5°C in the first 30 min after induction of anaesthesia.150
Several meta‐analyses and RCTs have demonstrated that preventing inadvertent hypothermia during major abdominal surgery significantly reduces wound infections,151, 152 cardiac complications,151, 153 bleeding and transfusion requirements,153, 154 and improves immune function,151 the duration of post‐anaesthetic recovery155 and overall survival.156 Therefore, it makes sense to prevent the loss of body heat as also recommended by the ERAS society.
Use of active warming devices is highly recommended in all cases lasting more than 30 min151 and this can be achieved by using different warming devices (forced air warming systems, circulating water garments or warmed i.v. solutions). Combined strategies, and among the others preoperative warming, should be considered in vulnerable groups such as older patients with cardiorespiratory diseases, and surgery of long duration.147 Rewarming should be performed to a core temperature of 35.5–36.0°C before emergence from anaesthesia, and every effort should be made to avoid shivering by using meperidine 0.25–0.5 mg/kg. Alternatively clonidine 1–2 μg/kg i.v. can be used.
Summary and recommendation: Intraoperative hypothermia should be avoided by using active warming devices.
Recommendation grade: strong.
Surgical techniques
The short‐term benefits of laparoscopic vs. open surgery for abdominal surgery have been well established in the literature to date and include shorter length of stay, reduced postoperative morbidity, earlier passage of flatus and less narcotic analgesic requirements.157 However, long‐term outcomes have shown equivalence between laparoscopic and open surgery.158 The fact that laparoscopic practice has improved since these trials were initiated, further consolidates the role played by this technique as the preferable one for abdominal surgery. In the context of an enhanced recovery programme, the multicentre randomized LAFA study has shown positive benefits when laparoscopic resection is optimized within an ERAS protocol.5
The main goal of enhanced recovery strategy should not be based on the choice of laparoscopic vs. open, but less surgical invasiveness as the surgical technique should minimize wound trauma, tissue distraction and bleeding.
A recently updated Cochrane review comparing transverse with midline laparotomy incisions for abdominal surgery found less postoperative opiate analgesic use with transverse incisions159 but no differences in visual analogue pain scores reported by patients. Pooled data for spirometry after the operation showed that a transverse incision had less effect on vital capacity and FEV1. However, these benefits on pulmonary function did not result in reduced pulmonary complications or hospital stay. A trend towards a lower incidence of wound dehiscence was shown in the transverse incision group. Finally there was a reduction in incisional hernias with transverse incisions, but the studies showed a high variety of time to follow‐up.
A number of new minimally invasive surgical technologies have emerged over the past decade. A recent meta‐analysis of non‐randomized controlled trials has indicated that robotic total mesorectal excision (TME) did not reduce operation time, length of hospital stay, time to resume regular diet, postoperative morbidity or mortality160 and is a technique that requires evaluation through high‐quality randomized research. While single‐incision laparoscopic resections may improve recovery, no robust data have yet appeared and these techniques are at an early stage in their development.161 Furthermore, transvaginal and transrectal specimen extraction to avoid abdominal wounds has been described, but with little data on short‐ and long‐term results.162, 163 At this stage, no recommendation can be made on these procedures. However, the negative intraoperative pathophysiological consequences (e.g. head‐down‐position, longer operation time) have to be balanced to the benefits of the minimal‐invasive approaches and the use of an ERAS protocol.
Summary and recommendation: Laparoscopic surgery for gastrointestinal resections is recommended when the expertise is available. Transverse incisions for colonic resections should be preferred.
Recommendation grade:
Laparoscopic approach: strong;
Transverse incisions: low.
Nasogastric intubation
There is strong evidence that routine nasogastric decompression following elective laparotomy should be avoided.164 Prophylactic nasogastric tubes placed during surgery (to evacuate air) should be removed before reversal of anaesthesia. Fever, oropharyngeal and pulmonary complications are more frequent in patients with nasogastric tubes.164, 165, 166 Even death and other serious complications resulting from nasogastric tubes are reported.167, 168 Avoidance of nasogastric decompression is associated with an earlier return of bowel function164, 165, 166, 169 while gastroesophageal reflux is increased during laparotomy if nasogastric tubes are placed.170 Even in gastroduodenal and pancreatic surgery, there appears to be no evidence of a beneficial effect from the prophylactic use of nasogastric tubes.164, 171 However, the incidence of vomiting has been shown to be higher in patients without nasogastric tubes.164, 165, 166 Nevertheless, the benefits of routinely avoiding nasogastric intubations overcome the risks.
Delayed gastric emptying can occur in a small proportion of patients, leading to vomiting and fatal aspiration if not treated promptly by inserting a nasogastric tube.172, 173 The recognition and avoidance of this complication is essential. Teams should be taught to positively identify these changes, particularly when patients are failing to progress between 2 and 5 days after surgery.
Summary and recommendation: Prophylactic use of nasogastric tubes is not recommended for patients undergoing elective colorectal surgery, while its use in patients undergoing gastrectomy and oesophagectomy is still debatable. Patients with delayed gastric emptying after surgery should be treated by inserting a nasogastric tube.
Recommendation grade: strong.
Intraoperative glycaemic control
Blood glucose levels increase during and after elective surgery with the magnitude of hyperglycaemia depending upon the patient's metabolic state (fasting, fed, diabetes), the type of anaesthesia and analgesia and the severity of surgical tissue trauma.174
Strong evidence indicates that even moderate increases in blood glucose are associated with adverse outcomes.175, 176, 177 Patients with fasting glucose levels > 7 mmol/l or random blood glucose levels > 11.1 mmol/l on general surgical wards showed an 18‐fold increased in‐hospital mortality.175
More recent observations suggest that the quality of preoperative glycaemic control also is important. In fact elevated HbA1c levels have been found to be predictive of complications after cardiac and abdominal surgery.178, 179, 180, 181
Mere associations between two variables, i.e. glycaemia and clinical outcomes, do not prove a direct cause–effect relationship. At present there is insufficient evidence to demonstrate superiority of strict glycaemic control (blood glucose levels within a normal and narrow range) over conventional management in surgical patients. As in the ICU situation, it remains a balance between the benefits of bringing down glucose levels vs. the risks of hypoglycaemia. For the surgical patient on the ward, there is also the issue of the nursing staffing and their capacity to monitor patients on intensive insulin treatment to take into account. A review of the effect of glycaemic control on the incidence of surgical site infections was inconclusive, mainly because of the small number of studies (n = 5), the heterogeneity in patient populations, the route of insulin administrations, the definition of outcomes measures and the fact that glycaemic targets were different and/or were not achieved.182 Hence, to date, the optimal glucose level for enhancing clinical outcomes is unknown.
This uncertainty is reflected by the diversity of recommendations issued by Medical Associations concerning blood glucose control in critically ill and surgical patients.64, 183, 184, 185 Overall most of the Associations recommend treatment of random blood glucose concentrations > 10 mmol/l. A large randomized controlled trial of aggressive preservation of normoglycaemia vs. conventional glycaemic control is necessary to identify target blood glucose concentrations in patients undergoing major surgery.
In the meantime, it is important to emphasize that there are a range of elements in the ERAS protocol that will reduce insulin resistance and hence reduce the risk of hyperglycaemia and that should be employed.186 These include preoperative carbohydrates, an active mid thoracic epidural, early feeding and good pain control.
Summary and recommendation: Glucose concentrations should be kept as close to normal as possible without compromising safety. Employing perioperative treatments that reduce insulin resistance without causing hypoglycaemia is recommended.
Recommendation grade: strong.
Perioperative haemodynamic management
Preoperative period: preoperative hydration deficit can vary according to patients' comorbidities, preoperative fasting and use of preoperative mechanical bowel preparation (MBP). The avoidance of prolonged preoperative fasting,80, 81 MBP187, 188 and as well the administration of preoperative carbohydrate (CHO) drinks83 have substantially reduced intraoperative fluid requirements. However, when MBP is indicated fluid and electrolytes derangements occur even if patients are encouraged to drink.74, 189, 190 The replacement of preoperative intravascular deficits should be based on individualized intraoperative fluid administration strategies75 rather than administering fluid based on anecdotal “textbook recipes”.
Intraoperative period: intraoperative fluid therapy aims to administer balanced crystalloid solutions to cover the needs derived from the salt–water homoeostasis. This is in contrast to volume therapy where goal‐directed boluses of intravenous solutions are administered to treat objective evidence of hypovolaemia, and consequently improve intravascular volume and circulatory flow.
Intraoperative fluid therapy should aim to maintain a near‐zero fluid balance191 and substantial weight gain of more than 2.5 kg should be avoided.192. Intraoperative fluid requirements can be met with a basal crystalloid infusion rate of 3 ± 2 ml/kg/h (also called restrictive app‐roach11).192, 193, 194 Crystalloid excess increases the risk of pulmonary complications,193 prolonged ileus192, 195, 196 and delayed recovery.197
Crystalloid isotonic balanced solutions should be preferred and 0.9% saline solutions avoided.198, 199 Hyperchloraemia caused by the use of 0.9% saline solutions has been associated with kidney dysfunction200, 201, 202, prolonged hospital stay and increased 30‐day mortality (OR = 1.58, 95% CI 1.25–1.98).200
Intraoperative volume therapy should be performed by bolus administration of an intravenous solution based on objective measures of hypovolaemia. Goal‐directed fluid therapy (GDFT) aims to maintain central normovolaemia by utilizing changes in stroke volume measured by a minimally invasive cardiac output monitor to optimize the patients on their individual Frank–Starling curve.96, 203
Trans‐oesophageal Doppler (TOD)‐guided GDFT has been shown to reduce the length of hospital stay and postoperative complications in several RCTs of patients undergoing non‐cardiac surgery96, 204, 205, 206 and in a hospital quality improvement project.207 Similarly, GDFT based on pulse contour analysis and aiming to minimize stroke volume variations during the respiratory cycle of mechanically ventilated patients has also shown to decrease morbidity and accelerate recovery203, 208, 209, 210. These findings are in agreement with the results of 2 recent meta‐analysis209, 211.
However, the benefits of GDFT seem to be offset by the optimization of perioperative surgical care. In fact, in two recent RCTs, TOD‐guided GDFT showed no benefits on postoperative outcomes in low‐risk patients treated within an ERAS protocol.191, 212. These results could be also explained by a judicious fluid management in patients not treated with GDFT, as the amount of intravenous fluid received in patients randomized in these patients was significantly less than the amount received by the same population in previous studies.213
The benefits of GDFT become more clinically meaningful in high‐risk patients214, 215, and in patients undergoing surgery associated with larger intravascular fluid loss (blood loss and protein/fluid shift)213, 216. In the largest multicentre RCT (734 patients), Pearse et al. found a non‐significant trend towards decreased complications (36% vs. 43.4% respectively, P = 0.07) and 180‐day mortality (7.7% vs. 11.6% respectively, P = 0.08) in high‐risk patients receiving GDFT compared with patients receiving usual care.215 Auditing internal data (amount of intraoperative fluid given, surgical loss, complications, mortality, length of stay and readmission rate) is essential to determine if GDFT should be implemented as routine strategy to improve postoperative outcomes.213
Colloidal solutions have been mainly used to optimize stroke volume during GDFT.96, 204, 205, 206 Colloids improve circulatory flow to a greater extent,217, 218 produce better blood volume expansion and less interstitial space overload than crystalloids219 and could reduce the incidence of postoperative nausea and vomiting and postoperative pain.220 Recently, Yates et al. showed that in moderate–high‐risk patients GDFT with colloid boluses does not accelerate the recovery of bowel function, reduce complications or impair haemostasis compared with crystalloids.221 Recent data have suggested that the use of large volumes of colloids administered post‐resuscitation in critically ill patients can increase the risk of death and acute kidney injury (AKI) in critically ill patients,222, 223 but these results have not been consistently reproduced in the perioperative setting.224, 225 A recent study has found a dose‐dependent association between the volume of HES administered and the development of AKI. The Pharmacovigilance Risk Assessment Committee of the European Medicines Agency has recommended that HES should only be used for the treatment of hypovolaemia caused by acute blood loss when crystalloids alone are not considered sufficient and that it should be used at the lowest effective dose for the shortest period of time. It also states that treatment should be guided by continuous haemodynamic monitoring so that the infusion is stopped as soon as appropriate haemodynamic goals have been achieved. The committee also observed that there is a lack of robust long‐term safety data in patients undergoing surgical procedures and in patients with trauma.226 Moreover, the use of large volumes of colloids (2605 ± 512 ml) hydroxyethyl starch (HES) 130/0.4 during major urological procedures has shown to impair haemostasis and increase surgical blood loss compared with crystalloids.227 Nevertheless, crystalloid‐based GDFT can significantly increase the risk of fluid overload.227
Arterial hypotension should be treated with vasopressors when administering intravenous fluid boluses fails to significantly improve the stroke volume (stroke volume > 10%).13, 203 Inotropes should be considered in patients with reduced contractility (Cardiac Index < 2.5 l/min) to guarantee adequate oxygen delivery.203
Postoperative period. Early oral intake of fluids and solids following abdominal surgery should be encouraged171, 228, 229. If oral intake is tolerated, routine intravenous fluid administration should be discontinued after PACU discharge and restarted only if clinically indicated. In the absence of surgical losses to cover physiological needs patients should be encouraged to drink 25–35 ml/kg of water per day (1.75–2.75 l for an average person).11 After ensuring the patient is normovolaemic, hypotensive patients receiving epidural analgesia should be treated with vasopressors.230, 231
Summary and Recommendation: The goal of perioperative fluid therapy is to maintain fluid homeostasis avoiding fluid excess and organ hypoperfusion. Fluid excess leading to perioperative weight gain more than 2.5 kg should be avoided, and a perioperative near‐zero fluid balance approach should be preferred. The need of GDFT should be determined based on clinical and surgical factors. GDFT should be adopted especially in high‐risk patients and in patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift). Inotropes should be considered in patients with poor contractility CI < 2.5 l/min). 0.9% saline and saline‐based solutions should be avoided, with balanced solutions preferred. Colloids should be used to treat objective evidence of hypovolaemia. In patients receiving epidural analgesia, arterial hypotension should be treated with vasopressors after ensuring the patient is normovolaemic. In the absence of surgical losses, postoperative intravenous fluid should be discontinued and oral intake (1.5 l/day) encouraged.
Recommendation grade: GDFT: Strong in high‐risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift)
GDFT: low in low‐risk patients and in patients undergoing low‐risk surgery
Perioperative near‐zero fluid balance: moderate
Use of advanced haemodynamic monitoring: strong in high‐risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift)
Balanced crystalloids vs. 0.9% saline
Healthy volunteer studies have suggested that the excretion of an acute saline load is slower when compared with balanced crystalloid infusions232, 233, 234, and saline tends to overload the interstitial space to a greater extent, with a tendency to result in more oedema than balanced crystalloids.232 Mechanisms for excreting this saline excess are inefficient, depending on a slow and sustained suppression of the renin–angiotensin–aldosterone axis.219 In addition, 0.9% saline produces a hyperchloraemic acidosis, which along with renal oedema, can lead to a reduction in renal blood flow and renal cortical perfusion, even in healthy human volunteers.232
There are two relatively small randomized clinical trials in humans comparing 0.9% saline with Ringer's lactate in the perioperative period, showing that 0.9% saline caused more side effects.235, 236 One of these studies, involving patients undergoing renal transplantation, had to be stopped prematurely because, compared with none in those receiving Ringer's lactate, 19% of patients in the saline group had to be treated for hyperkalaemia and 31% for metabolic acidosis.235 In the other study, involving patients undergoing abdominal aortic aneurysm repair, those receiving saline needed more blood products and bicarbonate therapy.236 Three recent large observational studies200, 201, 202 have suggested that 0.9% saline, because of the high chloride content, may cause harm, especially to the kidney. In a study using a validated and quality assured database, evaluation of outcomes in 2,788 adults undergoing major open abdominal surgery who received only 0.9% saline and 926 who received only a balanced crystalloid on the day of surgery and showed that unadjusted in‐hospital mortality (5.6% vs. 2.9%) and the percentage of patients developing complications (33.7% vs. 23%) were significantly greater in the 0.9% saline group than in the balanced crystalloid group.202 Patients receiving 0.9% saline had significantly greater blood transfusion requirements and more infectious complications, and were 4.8 times more likely to require dialysis than those receiving balanced crystalloids. Another recent study provides support for chloride‐restrictive fluid strategies in critically ill patients.201 In an open‐label prospective sequential manner, 760 patients consecutively admitted to intensive care (30% of whom were admitted after elective surgery) received either traditional chloride‐rich solutions (0.9% sodium chloride, 4% succinylated gelatin solution or 4% albumin solution) or chloride‐restricted (Hartmann's solution, Plasma‐Lyte 148 or chloride‐poor 20% albumin). After adjusting for confounding variables, the chloride‐restricted group had decreased incidence of acute kidney injury [odds ratio 0.52 (95% CI 0.37–0.75), P < 0.001] and the use of renal replacement therapy [odds ratio 0.52 (95% CI 0.33–0.81), P = 0.004]. However, there were no differences in hospital mortality, hospital or ICU length of stay.201 A third study on 22,851 surgical patients with normal preoperative serum chloride concentration and renal function showed that the incidence of acute postoperative hyperchloraemia (serum chloride > 110 mmol/l) was 22%.200 Patients with hyperchloraemia were at increased risk of 30‐day postoperative mortality (3.0% vs. 1.9%; odds ratio 1.58 (95% CI 1.25–1.98) and had a longer median hospital stay [7.0 days (IQR 4.1–12.3) vs. 6.3 days (IQR 4.0–11.3)] than patients with normal postoperative serum chloride concentrations.200 Patients with postoperative hyperchloraemia were also more likely to have postoperative renal dysfunction.
There is a strong signal suggesting that 0.9% saline is harmful, particularly in the perioperative period when compared with balanced solutions199. However, there are currently no large‐scale randomized controlled trails that confirm this finding. Nevertheless, it may be preferable to use balanced crystalloids in the perioperative period and restrict the use of saline to patients who have alkalosis or have a hyperchloraemia secondary to conditions such as vomiting or high nasogastric tube aspirates, and in neurosurgical patients because of the relative hypo‐osmolarity of some of the balanced crystalloids.
Summary and Recommendations: 0.9% saline should be avoided and balanced crystalloids used in the preoperative period. The use of 0.9% saline should be restricted in hypochloraemic and acidotic patients.
Recommendation: strong
Pain management
Multimodal, evidence‐based and procedure‐specific analgesic regimens should be standard of care, with the aim to achieve optimal analgesia with minimal side effects and to facilitate the achievement of important ERAS milestones such as early mobilization and oral feeding (Table 2).237, 238
Table 2.
Non‐analgesic outcomes and current issues reported after abdominal surgery with different analgesic techniques
| Analgesia technique | Outcomes | ERAS | Control group | Complications/issues | |
|---|---|---|---|---|---|
| Laparotomy | TEA (low dose of LA and opioids) | ↓ PONV250 | – | SO | Hypotension, pruritus, bladder dysfunction248, 249 |
| ↑Recovery of bowel function244 | – | SO | |||
| ↓Insulin resistance246 | – | SO | |||
| ↓Respiratory complications247 | – | SO | |||
| ↑Health‐related quality of life353 | – | SO | |||
| = LOSH250 | – | SO | |||
| IT morphine | Health‐related quality of life354 | ✓ | SO | Respiratory depression, pruritus, bladder dysfunction265 | |
| IVLI | Anti‐inflammatory269 | – | SO | LA toxicity270 | |
| ↑Recovery of bowel function269 | – | SO | |||
| ↓LOSH269 | – | SO | |||
| = LOSH254 | ✓ | TEA | |||
| CWI LA | ↓/↑/= Recovery of bowel function275, 276, 277, 355 | ✓/– | SO;TEA | Ideal anatomic location not determined274 | |
| ↓/↑/= LOSH273, 275, 276 | – | SO;TEA | |||
| Abdominal trunks blocks | ↓Postoperative sedation284, 289 | – | SO | Timing, dose, volume of LA, technique297 | |
| ↓PONV283 | – | SO | |||
| Laparoscopy | TEA | ↑/=/↓ Recovery of bowel function243, 253, 254 | ✓/– | SO;IVLI;IT/TAP | Hypotension, pruritus, bladder dysfunction248, 249 |
| ↑/= LOSH253, 254 | ✓ | SO;IT;TAP | |||
| IT morphine | = Recovery of bowel function253, 268, 356 | ✓ | SO;TEA | Respiratory depression, pruritus, bladder dysfunction265 | |
| Facilitate mobilization356 | ✓ | TEA | |||
| ↓/= LOSH253, 268 | ✓ | SO;TEA | |||
| 23‐h LOSH after laparoscopic colectomy357 | ✓ | – | |||
| IVLI | Anti‐inflammatory269(↓ IL‐6, IL1‐R) | – | SO | LA toxicity270 | |
| ↑/= Recovery of bowel function254, 272 | ✓ | SO;TEA | |||
| = LOSH254 | ✓ | TEA | |||
| Abdominal trunksblocks | 23‐h LOSH after laparoscopic colectomy286 | ✓ | SO | Timing, dose and volume of LA, technique297 | |
| = LOSH295 | ✓ | SO | |||
| = LOSH, earlier urinary catheter removal296 | ✓ | TEA |
↓, decreasing; ↑, accelerating; =, no effect. SO, systemic opioids; TEA, thoracic epidural analgesia; IVLI, intravenous lidocaine infusion; CWI, continuous wound infusion; LA, local anaesthetic; LOSH, length of hospital stay in hospital; (ERAS), study within an ERAS programme.
Thoracic epidural analgesia (TEA)
TEA (T6‐T11) remains the gold standard for postoperative pain control in patients undergoing open abdominal surgery.239 It still remains unclear if epidural analgesia improves postoperative outcomes. Although the results of a large multicentre RCT failed to show a significant benefit of using epidural analgesia in association with general anaesthesia in reducing 30‐day mortality and postoperative morbidity in high‐risk patients240 a recent meta‐analysis of 9044 patients undergoing surgery with general anaesthesia and receiving epidural analgesia (4525 patients) found that epidural analgesia is associated with a 40% reduction of mortality.241 Initiation of neuroaxial blockade before surgery and its maintenance throughout surgery decreases the need for anaesthetic agents, opioids and muscle relaxants.242 Compared with parenteral opioids, epidural blockade has shown to provide better postoperative static and dynamic analgesia for the first 72 h,10, to accelerate the recovery of gastrointestinal function,243, 244, 245 to reduce insulin resistance246 and impact positively on cardiovascular and respiratory complications.241, 247. However, hypotension, urinary retention pruritus and motor blockade are common side effects.248 Although detrusor function can be impaired in patients receiving TEA, a recent RCT has shown that early removal of a urinary catheter (on postoperative day 1) does not increase the risk bladder recatheterization and urinary infection.249. Also TEA does not influence the duration of hospital stay.250
The same benefits have not been observed after laparoscopic procedures,59 especially in a context of an ERAS programme.251, 252, 253 However, TEA might still be valuable in patients at risk of respiratory complications, in those with high probability of conversion to laparotomy, or requiring transverse or Pfannenstiel‐like incisions.254 Furthermore, TEA may be useful to facilitate the recovery of bowel function even after laparoscopic colorectal surgery.243
Clinical management
Epidural blockade should be tested before surgery or in the immediate postoperative period (post‐anaesthesia care unit) to avoid non‐functioning epidurals and unnecessary opioid administration.255 The addition of opioids to local anaesthetic has shown to improve postoperative analgesia.248, 256 Although a paucity of studies have compared the analgesic efficacy of epidural solutions combining local anaesthetic with lipophilic opioids vs. those containing local anaesthetic combined with hydrophilic opioids, epidural solution containing morphine increase the risk of urinary retention.257, 258 However, the use of low dose of local anaesthetics (bupivacaine 0.1 mg/ml) and lipophilic opioids (e.g. fentanyl 3 μg/ml) seem to provide optimal analgesia with minimal side effects257. Epidural morphine (0.02 mg/ml) in adjunct to local anaesthetic can be preferred to lipophilic opioids to increase segmental analgesia spread and could be recommended for long midline incisions.259 Epidural infusions can be continued for 48‐72, gradually reducing infusion rates and until the recovery of gastrointestinal function. Adding adrenaline (1.5–2.0 μg/ml) to epidural mixture of local anaesthetic and fentanyl improves postoperative analgesia, especially during mobilization and coughing, and reduces pruritus and nausea.248, 256, 260, 261, 262 Evidence on the analgesic efficacy of epidural clonidine is inconclusive and the risk of hypotension and sedation is increased.263 Hypotension induced by epidural blockade should be treated with vasopressors as first choice provided the patient is not hypovolaemic. Orthostatic hypotension associated with postoperative epidural analgesia does not impair the ability to ambulate.264 Institutional policies on how to manage epidural side effects, terminate epidural infusions, and how transition to oral multimodal analgesia are recommended.
Intrathecal (IT) analgesia
IT morphine is a valuable analgesic technique to improve early postoperative analgesia265 and facilitates surgical recovery.266 However, compared with systemic opioids, the incidence of pruritus (OR 3.85, 95% CI 2.40–6.15) and respiratory depression (although rare) is increased (OR 7.86, 95% CI 1.54–40.3). Postoperative urinary retention is also slightly more frequent (OR 2.35, 95% CI 1.00–5.51).265 Hypotension in the first 12 h, especially in a context of an enhanced recovery pathway and a restrictive fluid management, has been also associated with the use of intrathecal hydromorphone (with bupivacaine or clonidine).267
In the light of these side effects, in the context of an multimodal analgesic regimen other regional anaesthesia technique could be favoured especially in elderly patients. Behind providing excellent analgesia,268 IT morphine seems an appealing technique to shorten hospital stay in low‐risk patients undergoing laparoscopic colorectal surgery with an ERAS protocol.253
Clinical management
Reported IT morphine dosage range between 200 and 250 μg in patients aged ≤ 75 years to μg 150 in patients > 75 years of age. Isobaric or hyperbaric bupivacaine (10–12.5 mg) have been used in conjunction with IT morphine.253, 268
Intravenous lidocaine (IVL) infusion
In view of its antinociceptive and anti‐inflammatory properties, systemic administration of IVL as adjuvant to systemic opioids has been shown to improve postoperative analgesia, reduce opioid consumption and speed surgical recovery.269, 270 Similar benefits have been observed after laparoscopic abdominal surgeries when compared with systemic opioids,271 but not when compared with TEA254, and especially in the absence of an ERAS programme.254, 272
Clinical management
A loading dose of 1.5 mg/kg (IBW) should be initiated 30 min before or at the induction of anaesthesia and continued until the end of surgery or in the recovery room (2 mg/kg/h IBW). The exact duration of the infusion providing optimal analgesia and facilitating also recovery remains unknown. Systemic toxicity is rare, but continuous cardiovascular monitoring is required.270
Continuous wound infusion (CWI) of local anaesthetic
CWI of local anaesthetic after open abdominal surgery has been shown to improve postoperative analgesia and reduce opioid consumption,273, 274 however the effect on the recovery of bowel function is unclear.273, 275 Two recent RCTs have compared the analgesic efficacy of CWI of local anaesthetic with TEA but the results are contrasting.276, 277 A recent feasibility study has compared the analgesic efficacy of CWI of local anaesthetic with epidural analgesia after laparoscopic abdominal surgery. Pain intensity was similar among patients receiving epidural and CWI of local anaesthetic.278
Despite promising results the analgesic efficacy of CWI of local anaesthetic remains inconclusive and several aspects related to this techniques need to be clarified. For example, although preperitoneal multihole catheters have consistently provided satisfactory analgesia, and subfascial catheters have provided better results than suprafascial catheters,279 the anatomical location associated with optimal recovery remains undetermined.274, 279 Furthermore, it remains to be established if the analgesic effect observed in different trials is mainly driven by the bolus of local anaesthetic commonly given at the end of surgery or by the infusion of local anaesthetic during the postoperative period.280
Clinical management
Preperitoneal continuous infusion of ropivacaine 0.2% (10 ml/h) for 48–72 h has been used in the majority of the studies. Other amide‐local anaesthetics have also been used. Systemic opioids are still required to control visceral pain.
Abdominal trunk blocks: transversus abdominis plane (TAP) block and rectus sheath block
Significant reduction of pain intensity and opioid consumption after ultrasound‐guided single‐shot TAP blocks has been observed but it is limited to the first 24 h after surgery.281, 282, 283 TAP blocks can also be performed by surgeons from the peritoneal cavity before closing the abdominal wall,284, 285 or laparoscopic guided.286, 287, 288 Few studies have reported a reduction of some of the opioids side effects such as nausea and vomiting283 or sedation,284, 289 but these results have not been reproduced consistently.281 Continuous infusion or intermittent administration of local anaesthetics through multihole catheters placed in the transversus abdominis plane have been used to improve and prolong opioid‐based postoperative analgesia up to 48–72 h after abdominal surgery, but the evidence supporting the analgesic efficacy of TAP‐infusion of local anaesthetic remains scarce and inconclusive.290, 291, 292 Niraj et al. found that epidural analgesia did not provide better visual analogue scores during coughing than intermittent local anaesthetic boluses through bilateral subcostal TAP catheters in the first 72 h after upper abdominal surgery.293 However, epidural failure rate were high (22%) and almost half of the TAP catheters had to be replaced in the postoperative period.
Similar benefits have been reported in abdominal laparoscopic procedures282, 294 and in a context of an ERAS programme.286, 295 Despite facilitating hospital discharge,286 bilateral single‐shot TAP blocks seem to do not reduce hospital stay after laparoscopic colorectal surgery.295 A recent RCT has shown that the analgesic efficacy of four‐quadrant TAP blocks in adjunct to bilateral posterior continuous TAP blocks, was not inferior to TEA after laparoscopic colorectal surgery.296
Clinical management
Optimal timing, choice of local anaesthetic, dosing and volumes remain unknown.297 However, it seems that a minimal volume of 15 ml is required to achieve satisfactory analgesia with single‐shot TAP block.297 Ropivacaine 0.2% (8–10 ml/h) can be infused for 48–72 h trough a multihole catheter. A bilateral infusion (8–10 ml/h each side) is required with a midline incision. Systemic opioids are needed to control visceral pain.
More studies that further validate the analgesic efficacy of TAP blocks are warranted.
Intraperitoneal local anaesthetic (IPLA)
The results of a meta‐analysis including eight RCTs have shown that IPLA after open abdominal surgery reduce postoperative pain scores but not opioid consumption. However, in the latest randomized control trial conducted in a context of an enhanced recovery programme, IPLA improved surgical recovery, reduced postoperative pain and opioid consumption in patients undergoing open colectomy and receiving thoracic epidural analgesia.298
IPLA has been shown to improve postoperative analgesia, reduce shoulder pain and opioid consumption after laparoscopic gastric surgery299.
Multimodal analgesia (MMA)
A MMA regimen based on routine use of NSAIDs, COX‐2 and acetaminophen (paracetamol) (PO or intravenously when available) should adopted if not contraindicated in patients undergoing open and laparoscopic abdominal procedures with the aim to reduce opioid consumption and their dose‐dependent side effects that impair recovery.300 NSAIDs and COX‐2 inhibitors have been shown to improve postoperative analgesia, reduce opioid consumption and some of their side effects by 30%.301 There have been recent concerns about the risk of anastomotic leakage and the use of NSAIDs or COX‐2 inhibitors after colorectal surgeries based on experimental, retrospective and case‐series studies.302. Large RCTs are needed to confirm these results. The risk of anastomotic leakage after bowel surgery was not significantly increased in a recent meta‐analysis of six RCTs (480 patients) of patients receiving at least one dose of NSAIDs or COX‐2 inhibitors within 48 h of surgery (Peto OR 2.16 [95% CI 0.85–5.53, P = 0.11])303. This effect seems to be molecule‐specific (diclofenac is associated with the highest risk)302 and class‐specific (risk of anastomotic leakage with NSAIDs, OR 2.13 [95% CI 1.24–3.65], P = 0.006, risk of anastomotic leakage with selective COX‐2 inhibitors OR 1.16 [95% CI 0.49–2.75] P = 0.741)304. Furthermore, the risk varies with duration of the treatment, and it is higher after 3 days or more of NSAIDs than after 1 or 2 days only304. Acetaminophen (paracetamol) has shown to improve postoperative analgesia, have an opioid‐sparing effect, but not reduce opioids side effects.305 However, a recent meta‐analysis has demonstrated that intravenous paracetamol reduces the risk of postoperative nausea and vomiting, but this effect seems more related to an improvement in postoperative pain rather than to a reduction in opioid consumption.306 Concerns have been raised about the cardiovascular risk and delayed bone healing associated with the use of NSAIDs and COX‐2 inhibitors307. Overall, the evidence is inconclusive307 and does not support the avoidance of short perioperative NSAIDs and COX‐2 inhibitors treatment in patients with low cardiovascular risk.307, 308 High‐dose of systemic steroids have also shown promising results309, 310, also in patients not undergoing gastrointestinal surgery.311, 312 Perioperative intravenous ketamine and gabapentinoids have also shown opioid‐sparing properties.313, 314 However, the risk of side effects such as dizziness and sedation should be considered. An opioid‐free ultimodal analgesic strategy based mainly on analgesic adjuvants would be appealing but more studies are warranted to establish the feasibility, efficacy and safety of such analgesic approaches.315 Wound infiltration with long‐acting multivesicular liposome formulation of bupivacaine as part of multimodal analgesic regimens has also shown promising results.316, 317 It must be acknowledged that most of the following recommendations come from studies not using enhanced recovery after surgery (ERAS) programmes. It might be possible that the well‐proven benefits of ERAS programmes might offset the reported advantages of different analgesic techniques.242 The synergistic effect of combining different analgesic medications remains unknown and the impact of MMA on long‐ term outcomes still remains to be determined318.
Summary and Recommendation: Analgesic techniques should aim to not only provide optimal pain control but also to facilitate the achievement of important milestones such as tolerance of oral intake, and early mobilization. Opioid side effects are dose‐dependent and delay recovery. Opioid‐sparing analgesic strategies, including regional analgesia techniques, should be implemented in a context of a multimodal analgesic regimen. Postoperative pain management should be procedure‐specific.
Recommendation grade: MMA: strong
Open abdominal surgery
TEA: strong for using it
IVLI: moderate for using it
CWI: weak for using it
TAP blocks: moderate for using it
Laparoscopic abdominal surgery
TEA: weak for using it
IVLI: moderate for using it
Intrathecal morphine: moderate for using it
TAP blocks: moderate for using it
Postoperative delirium
Postoperative delirium is increasingly recognized in surgical practice, particularly in the elderly population who have pre‐existing cognitive dysfunction. While delirium can be a symptom of a surgical or medical complication it is important to be recognized instantly.
The prevalence is underestimated and under‐diagnosed if no systematic monitoring is applied.319 It is defined as a condition of altered consciousness, orientation, memory, thought, perception, behaviour and possibly sleep pattern which develops acutely and shows a fluctuating clinical course.320 Delirium can be classified into three subtypes: the hyperactive delirium, the hypoactive delirium and a mixed form.321 Delirium as a symptom of acute cerebral dysfunction should not solely be perceived as a strictly binary phenomenon which is either present or absent. Detection of delirium also at pre‐delirium or sub‐syndromal levels could prevent further deterioration of cerebral function.
Undetected and untreated or delayed treatment of delirium does increase the rate of complications, the length of hospital stay as well as mortality322, 323 and is associated with long‐term cognitive dysfunction.324
Early detection in the postoperative setting is a prerequisite for finding and treating the underlying causes. Numerous validated Delirium Instruments have been validated for clinical use.325, 326
Delirium promoting factors such as prolonged preoperative fluid fasting times, deep anaesthesia time as well as disturbing the sleep–wake cycle and the use of sedatives and other delirogenic medications should be avoided.117, 327
If postoperative delirium is detected, the early symptomatic therapy based on pharmacological and non‐pharmacological measures, is associated with a decreased mortality323. Psychotic symptoms should be treated with neuroleptics. A systematic review that a low‐dose haloperidol therapy compared with a therapy with atypical neuroleptics has a similar effectiveness and side effect rate.328
If there is the necessity to apply substances with sedative properties, non‐benzodiazepines should be preferred (e.g. alpha‐2‐agonists) due to international guidelines for sedation. Benzodiazepines are known to be an independent risk factor for delirium and should therefore be avoided if possible.329
Summary and recommendation: Preventive measure as avoidance of prolonged fasting, deep anaesthesia, disturbance of sleep–wake cycle or delirogenic medications like benzodiazepines, atropine should be implemented. Systematic delirium screening and symptom‐oriented treatment should be performed and potential underlying medical causes should be ruled out.
Recommendation grade: strong.
Attenuation and treatment of postoperative ileus
Postoperative ileus (POI) is defined as a transient reduction of bowel motility that prevents effective transit of bowel content and tolerance of oral intake following surgical interventions.330 POI has been associated with prolonged hospital stay and higher risk of complications. POI can be classified in primary POI that occurs in the absence of surgical complications, and in secondary POI in the presence of surgical complications such as anastomotic leakage, abscess, peritonitis, etc.330 Primary POI is considered an inevitable consequence after abdominal surgery. However, its clinical presentation and duration can significantly vary among patients depending on the severity of the gastrointestinal dysfunction. Some patients can be totally asymptomatic and tolerate oral intake in the immediate postoperative period, while others experience gastrointestinal symptoms, cannot tolerate any oral intake for several days and might require insertion of a nasogastric tube (NGT).330 The definitions of primary POI remains elusive and many clinical trials still utilize personal definitions in view of the difficulty on how to clinically identify patients with a clinically relevant impairment of gastrointestinal dysfunction. In a recent study measuring the gastrointestinal transit after colorectal surgery, Van Bree et al. showed that the combination of tolerance of solid food and passage of stool best correlates with the recovery of gastrointestinal function (area under the curve 0.9, SE 0.04, 95% CI 0.79–0.95, P < 0.001), with a positive predictive value of 93% (95% CI 78–99).331 It also best predicts hospital stay.331 Others clinical indicators commonly used to assess POI, such as the time to first flatus, poorly correlate with the recovery of the gastrointestinal function.331 A list of clinical indicators commonly used in clinical practice to evaluate the recovery of the gastrointestinal function is reported in Fig. 1. Non‐ileus‐related nausea and intra‐abdominal surgical complications leading to secondary POI should be excluded.
Figure 1.
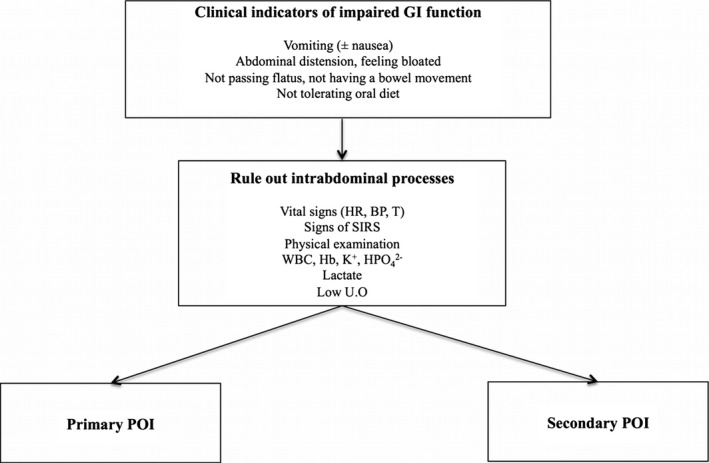
Identification of patients with primary or secondary postoperative Ileus (POI). SIRS, systemic inflammmatory response; WBC, white blood cell; Hb, hemoglobin; K+, potassium; HPO4 2−, phospate.
Due to its multifactorial pathogenesis several perioperative preventive strategies can be implemented to reduce the severity and duration of primary POI.332 Based on the results of a large retrospective study, it should be also considered that some patients might have a higher risk to develop prolonged primary POI (Table 3). These results need to be confirmed when adopting multiple interventions to attenuate postoperative gastrointestinal dysfunctions as in a context of an ERAS programme.333 Nasogastric decompression should be considered to prevent complications such as pulmonary aspiration and arrhythmias.164
Table 3.
Risk factors, prevention and management of primary POI
| Patients risk factors 333 |
|
| Intraoperative strategies to accelerate the recovery of gastrointestinal function |
| Postoperative strategies to accelerate the recovery of gastrointestinal function |
|
| Treatment of primary POI |
| NGT insertion332 |
Summary and recommendation: Primary POI is an inevitable consequence after gastrointestinal surgery and its pathogenesis is multifactorial. Multimodal preventing strategies should be adopted to facilitate the recovery of gastrointestinal function.
Recommendation grade: moderate
Early mobilization
Although the tradition of prolonged postoperative bed rest was abandoned over 75 years ago334 and the dangers of staying in bed acknowledged,335 modern surgical patients actually spend very little time out of bed.336 Early “enforced” or “structured” mobilization is a key component of virtually all ERAS programmes.16, 337 Patients cared for with the ERAS paradigms mobilize more and achieve independent mobilization earlier than those cared for without ERAS.7 Mobilization helps preserve muscle function and prevent complications associated with bed rest, but also aligns with the message of empowerment of patients to play an active role in their own recovery after surgery; this term is used instead of “convalescence”, which implies a passive process.
Protocols differ between pathways and there is no standard definition of early mobilization which may include exercising in bed, sitting out of bed, standing, walking in the room, walking in the hallway or exercising.338 Different successful pathways set different mobilization goals using different benchmarks such as time7 (hours out of bed, hours sitting or walking) or distance (e.g. number of times to walk a hallway or ward).339 These begin early, on the day of surgery, and increase each day to reach predetermined targets. There are no data to support the use of one plan over another or suggestion of a “dose–response” curve related to outcomes.
Unfortunately, there is little evidence available to guide how to best achieve early mobilization and even within established ERAS programmes adherence to mobilization targets may be quite low, suggesting a need for specific studies in this area.197 A review of the impact of early mobilization for medical and surgical patients found that the use of a more standardized and structured approach beginning as early as possible had the most favourable results.340 This begins in the preoperative setting with clear and explicit instructions detailing daily mobilization goals. These instructions are reinforced with written material which improves recall341 and which is brought by the patient to the hospital. Posters on the ward may help reinforce daily goals.342 Patients who begin an exercise programme in the preoperative period may also be more likely to be physically active postoperatively.343 Compliance may be improved by the use of a patient diary344 or when a pedometer is worn, which has been shown in other contexts to be associated with increased physical activity.345 Creation of separate ERAS “rehabilitation” wards344 or having a separate ward dining room may help337 but are not feasible in all settings. The absence of an in‐room entertainment system may promote increased walking.346 Having an audit tool available recording compliance with mobilization is important to identify and address barriers.
Achieving early mobilization on the ward requires integration between the patient and the various health care providers working in a multidisciplinary fashion form the beginning. Pain and drains inhibit ambulation.338 Ideally a dedicated pain service is involved in the ERAS team to optimize pain control and reduce side effects.337 Epidural analgesia provides excellent analgesia after open abdominal or thoracic surgery but it is associated with postoperative hypotension and with lower limb weakness if the epidural block is extended to the lumbar nerve roots.248 Epidural systems that reduce interference with ambulation should be used if possible. There is a tendency to bed rest patients experiencing orthostatic intolerance or hypotension, and to consider the epidural responsible for this effect. However, in patients with thoracic epidural analgesia hypotension is a relatively common side effect on postoperative day 1 but is often asymptomatic and does not predict the ability to walk.264 Furthermore, epidural analgesia is not associated with higher risk of orthostatic intolerance or hypotension than systemic opioids.347 Orthostatic intolerance seems to be more related to an impairment of the autonomic system and to an alteration of the baroreceptor reflex348, 349 rather than to other factors such as hypovolaemia,350 anaemia and pain.349 The underlying mechanisms are not yet fully understood.
Most pathways rely on nurses to assist with “enforcing” mobilization7 with physiotherapists involved in some programmes, suggesting an increased need for resources. Nurses should be involved in the creation of the mobilization plan from the beginning in order for the team to understand potential barriers to ambulation.351 Although there may be concern from nurses that ERAS will increase their daily workload related to these physical tasks, this has not been shown to be the case, perhaps because of increased patient independence.352
Summary and recommendation: Achievement of mobilization goals requires a multidisciplinary approach. Patients should be given written information setting daily targets for ambulation in hospital. Patients should be encouraged to increase their physical activity in the preoperative period. Patients should use a diary or pedometer to record their daily physical activity.
Recommendation grade: weak.
Comment
The practice of surgery and anaesthesia is continuously evolving and there is a need to offer the knowledge base for continuous training of those involved in the treatment of surgical patients. The ERAS Society (www.erassociety.org) was initiated by the former ERAS Study Group and was formed in 2010 to support these processes. The multidisciplinary Society participates in the improvement of perioperative care by developing new knowledge through research, education and also by being involved in the implementation of best practice.
The current manuscript presents a consensus review from the ERAS Society, discuss clinical considerations, and provide recommendations, for optimal anaesthesia care within the ERAS programme for patients undergoing gastrointestinal surgery. The quality of evidence supporting each ERAS element has been already evaluated according to the GRADE system and previously published15, 16, 17, 18, 19. The evidence‐based recommendations present the ERAS protocol interventions separately and overall, and are intended to be used by units undertaking to implement and upgrade to what the current literature shows to be best practice: the ERAS protocol. It must be acknowledged that, not being a systematic review, all articles quoted in the manuscript have been selected by the expert in each area, resulting in potential bias. Clinical considerations and recommendations for each of the ERAS elements are listed in Table 4.
Table 4.
ERAS elements: summary and recommendations
| Perioperative element | Summary and recommendation | Recommendation grade |
|---|---|---|
| Risk assessment | Preoperative scoring tools and functional capacity tests can be used to identify patients at risk of complications and to stratify perioperative risk. |
POSSUM: strong Lee Index: strong Cardiovascular Risk Calculator: strong Walk tests: strong CPET: strong General Surgery Acute Kidney Injury Risk Index: strong |
| Preoperative optimization | Cessation of smoking and alcohol intake at least 4 weeks before surgery is recommended. Encouraging patients is not enough; pharmacological support and individual counselling should be offered to every patient who smokes and to alcohol abusers undergoing elective surgery. Optimization of medical conditions, such as cardiovascular diseases, anaemia, COPD, nutritional status and diabetes should follow international recommendations. |
Smoking cessation: high NRT and counselling: high Alcohol cessation: low Medical optimization: strong Optimize preoperative anaemia reduces morbidity and mortality: moderate |
| Pre‐anaesthetic medication | Long‐acting anxiolytic and opioids should be avoided as they may delay discharge. Short‐acting benzodiazepine should be avoided in the elderly. | Strong. |
| Preoperative fasting and carbohydrates (CHOs) loading | Intake of clear fluids should be allowed until 2 h before induction of anaesthesia. Solids should be allowed until 6 h. Preoperative treatment with oral CHOs should be routinely administered except in patients with documented delayed gastric emptying or slow gastrointestinal motility and as well in patients undergoing emergency surgery. |
Adherence to fasting guidelines (avoid overnight fasting): strong Administration of preoperative CHOs:strong Administration of preoperative CHOs in diabetic and obese patients: weak |
| Preventing and treating postoperative nausea and vomiting (PONV) | Aggressive PONV prevention strategy should be included in an ERAS protocol102. All patients with 1–2 risk factors should receive a combination of two antiemetics. Patients with 3–4 risk factors should receive 2–3 antiemetics. Total intravenous anaesthesia (TIVA) with propofol and opioid‐sparing strategies should be encouraged. | Strong |
| Standard anaesthetic protocol | Anaesthetic depth should be guided either maintaining an end tidal concentration of 0.7–1.3 MAC or BIS index between 40 and 60 with the aim not only to prevent awareness but also to minimize anaesthetic side effects and facilitate rapid awakening and recovery. Avoid too deep anaesthesia (BIS < 45), especially in elderly patients | Strong |
| Neuromuscular blockade (NMB) and neuromuscular monitoring | It remains controversial if deep neuromuscular blockade during laparoscopic surgery improves operating conditions. Neuromuscular function should be always monitored when using NMBA to avoid residual paralysis. Long‐acting NMBA should be avoided. When NMBA are administered neuromuscular function should be monitored by using a peripheral nerve stimulator to ensure adequate muscle relaxation during surgery and optimal restoration of neuromuscular function at the end of surgery. A TOF ratio of 0.9 must be achieved to ensure adequate return of muscle function and thus preventing complications. |
Monitoring neuromuscular function: strong Reversing neuromuscular blockade: strong |
| Inspired Oxygen Concentration |
1) The inspired fractional concentration of oxygen should be titrated to produce normal arterial oxygen levels and saturations. Prolonged periods of high inspired oxygen concentrations which result in hyperoxia should be avoided. 2) 100% inspired oxygen concentrations can be used for pre‐oxygenation prior to anaesthesia or for short periods to overcome hypoxia. |
1) Strong 2) Strong |
| Preventing intraoperative hypothermia | Intraoperative hypothermia should be avoided by using active warming devices. | Strong. |
| Surgical techniques | Laparoscopic surgery for gastrointestinal surgery is recommended when the expertise is available. Transverse incisions for colonic resections can be preferred. |
Laparoscopic approach: strong Transverse incisions for colonic surgery: low |
| Nasogastric intubation | Prophylactic use of NGTs is not recommended for patients undergoing elective colorectal surgery, while its use in patients undergoing gastrectomy and oesophagectomy is still debatable. Patients with delayed gastric emptying after surgery should be treated by inserting a NGT. | Strong. |
| Intraoperative glycaemic control | Glucose levels should be kept as close to normal as possible without compromising safety. Employing perioperative treatments that reduce insulin resistance without causing hypoglycaemia is recommended. | Strong. |
| Perioperative haemodynamic management | The goal of perioperative fluid therapy is to maintain fluid homeostasis avoiding fluid excess and organ hypoperfusion. Fluid excess leading to perioperative weight gain more than 2.5 kg should be avoided, and a perioperative near‐zero fluid balance approach should be preferred. GDFT should be adopted especially in moderate–high‐risk patients. Inotropes should be considered in patients with poor contractility CI < 2.5 l/min). Colloids should not be used in septic patients and in patients with reduced renal function. Large amount of colloids can impair haemostasis. In patients receiving epidural analgesia arterial hypotension should be treated with vasopressors, ensuring the patient is normovolaemic. In the absence of surgical losses postoperative intravenous fluid should be discontinued and oral intake (1.5 l/day) encouraged. |
GDFT: Strong in high‐risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift) GDFT: low in low‐risk patients and in patients undergoing low‐risk surgery Perioperative near‐zero fluid balance: moderate Use of advanced hemodynamic monitoring: strong in high‐risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift) |
| Balanced crystalloids vs. 0.9% saline | 0.9% saline should be avoided and balanced crystalloid solution used in the preoperative period. The use of 0.9% saline should be restricted in hypochloraemic and acidotic patients. | Strong |
| Pain management | Analgesic techniques should aim to not only provide optimal pain control, but also to facilitate the achievement of important milestones such as tolerance of oral intake, and early mobilization. Opioids side effects are dose‐dependent and delay recovery. Opioid‐sparing analgesic strategies, including regional analgesia techniques, should be implemented in a context of a multimodal analgesic regimen. Postoperative pain management should be procedure‐specific |
MMA: strong Open abdominal surgery TEA: strong for using it IVLI: moderate for using it CWI: weak for using it TAP blocks: moderate for using it Laparoscopic abdominal surgery TEA: weak for using it IVLI: moderate for using it Intrathecal morphine: moderate for using it TAP blocks: moderate for using it |
| Postoperative Delirium | Preventive measure as avoidance of prolonged fasting, deep anaesthesia, disturbance of sleep‐wake cycle or delirogenic medications like benzodiazepines, atropine should be implemented. Systematic delirium screening and symptom‐oriented treatment should be performed and potential underlying medical causes should be ruled out. | Strong |
| Attenuation and treatment of postoperative ileus | Primary POI is an inevitable consequence after gastrointestinal surgery and its pathogenesis is multifactorial. Multimodal preventing strategies should be adopted to facilitate the recovery of gastrointestinal function. | Moderate |
| Early mobilization | Achievement of mobilization goals requires a multidisciplinary approach. Patients should be given written information setting daily targets for ambulation in hospital. Patients should be encouraged to increase their physical activity in the preoperative period. Patients should use a diary or pedometer to record their daily physical activity. | Weak. |
Feldheiser A, Aziz O, Baldini G, Cox BPBW, Fearon KCH, Feldman LS, Gan TJ, Kennedy RH, Ljungqvist O, Lobo DN, Miller T, Radtke FF, Ruiz Garces T, Schricker T, Scott MJ, Thacker JK, Ytrebø LM, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, Part 2: consensus statement for anaesthesia practice. Acta Anaesthesiologica Scandinavica 2016.
Conflicts of interest
Dr Olle Ljungqvist is founder, shareholder, board member Encare AB, Sweden; board member Nutricia A/S The Netherlands; he also receives speaker's honoraria from Fresenius Kabi, B/Braun, Nutricia, Merck. Dr Dileep N Lobo has received speaker's honoraria and unrestricted research funding from Fresenius Kabi, Baxter & BBraun, and has served on advisory boards of AbbVie & Baxter.
Funding
None.
References
- 1. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372: 139–44. [DOI] [PubMed] [Google Scholar]
- 2. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, Vallet B, Vincent JL, Hoeft A, Rhodes A. European Surgical Outcomes Study group for the Trials groups of the European Society of Intensive Care Medicine, the European Society of Anaesthesiology. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380: 1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH, Enhanced Recovery After Surgery G . Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Group recommendations. Arch Surg 2009; 144: 961–9. [DOI] [PubMed] [Google Scholar]
- 4. Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre‐Brook IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007; 245: 867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, Sprangers MA, Cuesta MA, Bemelman WA, LAFA Study Group . Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA‐study). Ann Surg 2011; 254: 868–75. [DOI] [PubMed] [Google Scholar]
- 6. Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbolle P, Hendel HW, Rosenberg J, Kehlet H. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg 2002; 89: 446–53. [DOI] [PubMed] [Google Scholar]
- 7. Basse L, Hjort Jakobsen D, Billesbolle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann Surg 2000; 232: 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast‐track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009; 136: 842–7. [DOI] [PubMed] [Google Scholar]
- 9. Serclova Z, Dytrych P, Marvan J, Nova K, Hankeova Z, Ryska O, Slegrova Z, Buresova L, Travnikova L, Antos F. Fast‐track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr 2009; 28: 618–24. [DOI] [PubMed] [Google Scholar]
- 10. Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra‐abdominal surgery. Cochrane Database Syst Rev 2005; (1): CD004088. [DOI] [PubMed] [Google Scholar]
- 11. Varadhan KK, Lobo DN. A meta‐analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69: 488–98. [DOI] [PubMed] [Google Scholar]
- 12. Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J. Meta‐analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg 2009; 96: 331–41. [DOI] [PubMed] [Google Scholar]
- 13. Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KG, Kalkman CJ. Intraoperative hypotension and 1‐year mortality after noncardiac surgery. Anesthesiology 2009; 111: 1217–26. [DOI] [PubMed] [Google Scholar]
- 14. Scott MJ, Baldini G, Fearon K, Feldheiser A, Feldman L, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker T, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand 2015; 59: 1212–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, Kassouf W, Muller S, Baldini G, Carli F, Naesheimh T, Ytrebo L, Revhaug A, Lassen K, Knutsen T, Aarsether E, Wiklund P, Patel HR. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr 2013; 32: 879–87. [DOI] [PubMed] [Google Scholar]
- 16. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O. Enhanced Recovery After Surgery Society, European Society for Clinical Nutrition and Metabolism, International Association for Surgical Metabolism and Nutrition. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS ®) Society recommendations. World J Surg 2013; 37: 259–84. [DOI] [PubMed] [Google Scholar]
- 17. Mortensen K, Nilsson M, Slim K, Schafer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K, Enhanced Recovery After Surgery G . Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS ®) Society recommendations. Br J Surg 2014; 101: 1209–29. [DOI] [PubMed] [Google Scholar]
- 18. Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, Ljungqvist O, Soop M, Ramirez J. Enhanced Recovery After Surgery Society, European Society for Clinical Nutrition and Metabolism, International Association for Surgical Metabolism and Nutrition. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS ®) Society recommendations. World J Surg 2013; 37: 285–305. [DOI] [PubMed] [Google Scholar]
- 19. Lassen K, Coolsen MM, Slim K, Carli F, deAguilar‐Nascimento JE , Schafer M, Parks RW, Fearon KC, Lobo DN, Demartines N, Braga M, Ljungqvist O, Dejong CH, Enhanced Recovery After Surgery Society fPC , European Society for Clinical N, Metabolism, International Association for Surgical M, Nutrition . Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS ®) Society recommendations. World J Surg 2013; 37: 240–58. [DOI] [PubMed] [Google Scholar]
- 20. Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, Grounds RM, Bennett ED. Identification and characterisation of the high‐risk surgical population in the United Kingdom. Crit Care 2006; 10: R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, Participants in the VANSQIP . Determinants of long‐term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005; 242: 326–41; discussion 41‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 1991; 78: 355–60. [DOI] [PubMed] [Google Scholar]
- 23. Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg 1998; 85: 1217–20. [DOI] [PubMed] [Google Scholar]
- 24. Tekkis PP, Poloniecki JD, Thompson MR, Stamatakis JD. Operative mortality in colorectal cancer: prospective national study. BMJ 2003; 327: 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tekkis PP, McCulloch P, Poloniecki JD, Prytherch DR, Kessaris N, Steger AC. Risk‐adjusted prediction of operative mortality in oesophagogastric surgery with O‐POSSUM. Br J Surg 2004; 91: 288–95. [DOI] [PubMed] [Google Scholar]
- 26. Neary WD, Heather BP, Earnshaw JJ. The Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM). Br J Surg 2003; 90: 157–65. [DOI] [PubMed] [Google Scholar]
- 27. Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg 2009; 208: 1009–16. [DOI] [PubMed] [Google Scholar]
- 28. Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, Esterbrooks DJ, Hunter CB, Pipinos II, Johanning JM, Lynch TG, Forse RA, Mohiuddin SM, Mooss AN. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124: 381–7. [DOI] [PubMed] [Google Scholar]
- 29. Lee TH, Goldman L. Letter by Lee and Goldman regarding article, “Development and validation of a risk calculator for prediction of cardiac risk after surgery”. Circulation 2012; 125: e385; author reply e86. [DOI] [PubMed] [Google Scholar]
- 30. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: 2215–45. [DOI] [PubMed] [Google Scholar]
- 31. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–9. [DOI] [PubMed] [Google Scholar]
- 32. Botto F, Alonso‐Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels‐Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schunemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, Devereaux PJ, Sessler DI, Walsh M, Guyatt G, McQueen MJ, Bhandari M, Cook D, Bosch J, Buckley N, Yusuf S, Chow CK, Hillis GS, Halliwell R, Li S, Lee VW, Mooney J, Polanczyk CA, Furtado MV, Berwanger O, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, Graham M, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Bhandari M, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish‐Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O'Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, Gerstein H, McDonald S, O'Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Cook D, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Mrkobrada M, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Villar JC, Cortes OL, Chaparro MS, Vasquez S, Castaneda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Chan MT, Choi GY, Gin T, Lit LC, Xavier D, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Abraham V, Afzal L, George P, Mala S, Schunemann H, Muti P, Vizza E, Wang CY, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Malaga G, Valderrama‐Victoria V, Loza‐Herrera JD, De Los Angeles Lazo M, Rotta‐Rotta A, Szczeklik W, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Paniagua P, Urrutia G, Maestre ML, Santalo M, Gonzalez R, Font A, Martinez C, Pelaez X, De Antonio M, Villamor JM, Garcia JA, Ferre MJ, Popova E, Alonso‐Coello P, Garutti I, Cruz P, Fernandez C, Palencia M, Diaz S, Del Castillo T, Varela A, de Miguel A, Munoz M, Pineiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Leuwer M, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sessler DI, Kurz A, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Nagele P, Blood J, Kalin M, Gibson D, Wildes T, Vascular events In noncardiac Surgery patIents cOhort evaluatioN Writing Group oboTVeInSpceI, Appendix 1 . The Vascular events In noncardiac Surgery patIents cOhort evaluatio NSIWG, Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatio NOC, Vascular events In noncardiac Surgery patIents cOhort evaluatio NVSI . Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology 2014; 120: 564–78. [DOI] [PubMed] [Google Scholar]
- 33. Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice G, American Society of E, American Society of Nuclear C, Heart Rhythm S, Society of Cardiovascular A, Society for Cardiovascular A, Interventions, Society for Vascular M, Biology, Society for Vascular S . ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 2007; 116: e418–99. [DOI] [PubMed] [Google Scholar]
- 34. Morris CK, Ueshima K, Kawaguchi T, Hideg A, Froelicher VF. The prognostic value of exercise capacity: a review of the literature. Am Heart J 1991; 122: 1423–31. [DOI] [PubMed] [Google Scholar]
- 35. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–23. [PMC free article] [PubMed] [Google Scholar]
- 36. Moriello C, Mayo NE, Feldman L, Carli F. Validating the six‐minute walk test as a measure of recovery after elective colon resection surgery. Arch Phys Med Rehabil 2008; 89: 1083–9. [DOI] [PubMed] [Google Scholar]
- 37. Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest 1999; 116: 355–62. [DOI] [PubMed] [Google Scholar]
- 38. Older P, Smith R, Hall A, French C. Preoperative cardiopulmonary risk assessment by cardiopulmonary exercise testing. Crit Care Resusc 2000; 2: 198–208. [PubMed] [Google Scholar]
- 39. Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, Manas DM. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg 2010; 251: 535–41. [DOI] [PubMed] [Google Scholar]
- 40. Ausania F, Snowden CP, Prentis JM, Holmes LR, Jaques BC, White SA, French JJ, Manas DM, Charnley RM. Effects of low cardiopulmonary reserve on pancreatic leak following pancreaticoduodenectomy. Br J Surg 2012; 99: 1290–4. [DOI] [PubMed] [Google Scholar]
- 41. Chandrabalan VV, McMillan DC, Carter R, Kinsella J, McKay CJ, Carter CR, Dickson EJ. Pre‐operative cardiopulmonary exercise testing predicts adverse post‐operative events and non‐progression to adjuvant therapy after major pancreatic surgery. HPB (Oxford) 2013; 15: 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Junejo MA, Mason JM, Sheen AJ, Moore J, Foster P, Atkinson D, Parker MJ, Siriwardena AK. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br J Surg 2012; 99: 1097–104. [DOI] [PubMed] [Google Scholar]
- 43. Hartley RA, Pichel AC, Grant SW, Hickey GL, Lancaster PS, Wisely NA, McCollum CN, Atkinson D. Preoperative cardiopulmonary exercise testing and risk of early mortality following abdominal aortic aneurysm repair. Br J Surg 2012; 99: 1539–46. [DOI] [PubMed] [Google Scholar]
- 44. Nagamatsu Y, Shima I, Yamana H, Fujita H, Shirouzu K, Ishitake T. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg 2001; 121: 1064–8. [DOI] [PubMed] [Google Scholar]
- 45. Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA Jr. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009; 110: 505–15. [DOI] [PubMed] [Google Scholar]
- 46. Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg 1999; 86: 869–74. [DOI] [PubMed] [Google Scholar]
- 47. Tonnesen H, Rosenberg J, Nielsen HJ, Rasmussen V, Hauge C, Pedersen IK, Kehlet H. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ 1999; 318: 1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg 2003; 238: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomsen T, Tonnesen H, Moller AM. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg 2009; 96: 451–61. [DOI] [PubMed] [Google Scholar]
- 50. Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 2014; 3: CD002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, Chapman KR, Chung F. A perioperative smoking cessation intervention with varenicline: a double‐blind, randomized, placebo‐controlled trial. Anesthesiology 2012; 117: 755–64. [DOI] [PubMed] [Google Scholar]
- 52. Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: a randomised controlled trial. Anaesthesia 2004; 59: 1053–8. [DOI] [PubMed] [Google Scholar]
- 53. Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2014; 1: CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leichtle SW, Mouawad NJ, Lampman R, Singal B, Cleary RK. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg 2011; 212: 187–94. [DOI] [PubMed] [Google Scholar]
- 55. Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, Soweid A, Hoballah JJ, Taher AT, Jamali FR. Preoperative anaemia and postoperative outcomes in non‐cardiac surgery: a retrospective cohort study. Lancet 2011; 378: 1396–407. [DOI] [PubMed] [Google Scholar]
- 56. Goodnough LT, Shander A. Patient blood management. Anesthesiology 2012; 116: 1367–76. [DOI] [PubMed] [Google Scholar]
- 57. Hare GM, Baker JE, Pavenski K. Assessment and treatment of preoperative anemia: Continuing Professional Development. Can J Anaesth 2011; 58: 569–81. [DOI] [PubMed] [Google Scholar]
- 58. Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30‐day mortality, surgical‐site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009; 208: 931–7, 37 e1‐2; discussion 38‐9. [DOI] [PubMed] [Google Scholar]
- 59. Halabi WJ, Kang CY, Nguyen VQ, Carmichael JC, Mills S, Stamos MJ, Pigazzi A. Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg 2014; 149: 130–6. [DOI] [PubMed] [Google Scholar]
- 60. Refaai MA, Blumberg N. The transfusion dilemma–weighing the known and newly proposed risks of blood transfusions against the uncertain benefits. Best Pract Res Clin Anaesthesiol 2013; 27: 17–35. [DOI] [PubMed] [Google Scholar]
- 61. Hare GM, Freedman J, David Mazer C. Review article: risks of anemia and related management strategies: can perioperative blood management improve patient safety? Can J Anaesth 2013; 60: 168–75. [DOI] [PubMed] [Google Scholar]
- 62. Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(Suppl 1): i41–59. [DOI] [PubMed] [Google Scholar]
- 63. Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, Weiss K, Owens DK, Aronson M, Barry P, Casey DE Jr, Cross JT Jr, Fitterman N, Sherif KD, Weiss KB, Clinical Efficacy Assessment Subcommittee of the American College of P . Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144: 575–80. [DOI] [PubMed] [Google Scholar]
- 64. Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail‐Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical E, American Diabetes A. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32: 1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lawson CM, Daley BJ, Sams VG, Martindale R, Kudsk KA, Miller KR. Factors that impact patient outcome: nutrition assessment. JPEN J Parenter Enteral Nutr 2013; 37: 30S–8S. [DOI] [PubMed] [Google Scholar]
- 66. McClave SA, Kozar R, Martindale RG, Heyland DK, Braga M, Carli F, Drover JW, Flum D, Gramlich L, Herndon DN, Ko C, Kudsk KA, Lawson CM, Miller KR, Taylor B, Wischmeyer PE. Summary points and consensus recommendations from the North American Surgical Nutrition Summit. JPEN J Parenter Enteral Nutr 2013; 37: 99S–105S. [DOI] [PubMed] [Google Scholar]
- 67. Miller KR, Wischmeyer PE, Taylor B, McClave SA. An evidence‐based approach to perioperative nutrition support in the elective surgery patient. JPEN J Parenter Enteral Nutr 2013; 37: 39S–50S. [DOI] [PubMed] [Google Scholar]
- 68. Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF, American College of Surgeons National Surgical Quality Improvement P, American Geriatrics S . Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012; 215: 453–66. [DOI] [PubMed] [Google Scholar]
- 69. Thompson T, Keogh E, French CC, Davis R. Anxiety sensitivity and pain: generalisability across noxious stimuli. Pain 2008; 134: 187–96. [DOI] [PubMed] [Google Scholar]
- 70. Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009; 111: 657–77. [DOI] [PubMed] [Google Scholar]
- 71. Walker KJ, Smith AF. Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev 2009; 9: CD002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lepouse C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post‐anaesthesia care unit. Br J Anaesth 2006; 96: 747–53. [DOI] [PubMed] [Google Scholar]
- 73. Maltby JR. Fasting from midnight–the history behind the dogma. Best Pract Res Clin Anaesthesiol 2006; 20: 363–78. [DOI] [PubMed] [Google Scholar]
- 74. Holte K, Nielsen KG, Madsen JL, Kehlet H. Physiologic effects of bowel preparation. Dis Colon Rectum 2004; 47: 1397–402. [DOI] [PubMed] [Google Scholar]
- 75. Bundgaard‐Nielsen M, Jorgensen CC, Secher NH, Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand 2010; 54: 464–9. [DOI] [PubMed] [Google Scholar]
- 76. Muller L, Briere M, Bastide S, Roger C, Zoric L, Seni G, de La Coussaye JE, Ripart J, Lefrant JY. Preoperative fasting does not affect haemodynamic status: a prospective, non‐inferiority, echocardio‐graphy study. Br J Anaesth 2014; 112: 835–41. [DOI] [PubMed] [Google Scholar]
- 77. Brady M, Kinn S, Ness V, O'Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev 2009; 4: CD005285. [DOI] [PubMed] [Google Scholar]
- 78. Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003; 4: CD004423. [DOI] [PubMed] [Google Scholar]
- 79. Lobo DN, Hendry PO, Rodrigues G, Marciani L, Totman JJ, Wright JW, Preston T, Gowland P, Spiller RC, Fearon KC. Gastric emptying of three liquid oral preoperative metabolic preconditioning regimens measured by magnetic resonance imaging in healthy adult volunteers: a randomised double‐blind, crossover study. Clin Nutr 2009; 28: 636–41. [DOI] [PubMed] [Google Scholar]
- 80. Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, Spies C, in't Veld B, European Society of Anaesthesiology . Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2011; 28: 556–69. [DOI] [PubMed] [Google Scholar]
- 81. American Society of Anesthesiologists C . Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2011; 114: 495–511. [DOI] [PubMed] [Google Scholar]
- 82. Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol 2009; 23: 401–9. [DOI] [PubMed] [Google Scholar]
- 83. Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta‐analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr 2013; 32: 34–44. [DOI] [PubMed] [Google Scholar]
- 84. Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 2014; 8: CD009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA. Delayed gastric emptying in the obese: an assessment using the non‐invasive (13)C‐octanoic acid breath test. Diabetes Obes Metab 2004; 6: 264–70. [DOI] [PubMed] [Google Scholar]
- 86. Harter RL, Kelly WB, Kramer MG, Perez CE, Dzwonczyk RR. A comparison of the volume and pH of gastric contents of obese and lean surgical patients. Anesth Analg 1998; 86: 147–52. [DOI] [PubMed] [Google Scholar]
- 87. Maltby JR, Pytka S, Watson NC, Cowan RA, Fick GH. Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non‐fasting obese patients. Can J Anaesth 2004; 51: 111–5. [DOI] [PubMed] [Google Scholar]
- 88. Breuer JP, von Dossow V, von Heymann C, Griesbach M, von Schickfus M, Mackh E, Hacker C, Elgeti U, Konertz W, Wernecke KD, Spies CD. Preoperative oral carbohydrate administration to ASA III‐IV patients undergoing elective cardiac surgery. Anesth Analg 2006; 103: 1099–108. [DOI] [PubMed] [Google Scholar]
- 89. Gustafsson UO, Nygren J, Thorell A, Soop M, Hellstrom PM, Ljungqvist O, Hagstrom‐Toft E. Pre‐operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand 2008; 52: 946–51. [DOI] [PubMed] [Google Scholar]
- 90. Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, Hooper VD, Kovac AL, Kranke P, Myles P, Philip BK, Samsa G, Sessler DI, Temo J, Tramer MR, Vander Kolk C, Watcha M. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2007; 105: 1615–28. [DOI] [PubMed] [Google Scholar]
- 91. Gan TJ. Postoperative nausea and vomiting–can it be eliminated? JAMA 2002; 287: 1233–6. [DOI] [PubMed] [Google Scholar]
- 92. Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg 2006; 102: 1884–98. [DOI] [PubMed] [Google Scholar]
- 93. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N, Investigators I. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Myles PS, Leslie K, Chan MT, Forbes A, Peyton PJ, Paech MJ, Beattie WS, Sessler DI, Devereaux PJ, Silbert B, Schricker T, Wallace S, the ATGftE‐IIi . The safety of addition of nitrous oxide to general anaesthesia in at‐risk patients having major non‐cardiac surgery (ENIGMA‐II): a randomised, single‐blind trial. Lancet 2014; 384: 1446–54. [DOI] [PubMed] [Google Scholar]
- 95. Holte K, Klarskov B, Christensen DS, Lund C, Nielsen KG, Bie P, Kehlet H. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double‐blind study. Ann Surg 2004; 240: 892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gan TJ, Soppitt A, Maroof M, el‐Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS. Goal‐directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97: 820–6. [DOI] [PubMed] [Google Scholar]
- 97. Hovaguimian F, Lysakowski C, Elia N, Tramer MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta‐analysis of randomized controlled trials. Anesthesiology 2013; 119: 303–16. [DOI] [PubMed] [Google Scholar]
- 98. Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev 2006; 3: CD004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, Habib AS, Knighton J, Carides AD, Zhang H, Horgan KJ, Evans JK, Lawson FC. A randomized, double‐blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg 2007; 104: 1082–9, tables of contents. [DOI] [PubMed] [Google Scholar]
- 100. Le TP, Gan TJ. Update on the management of postoperative nausea and vomiting and postdischarge nausea and vomiting in ambulatory surgery. Anesthesiol Clin 2010; 28: 225–49. [DOI] [PubMed] [Google Scholar]
- 101. Kovac AL, O'Connor TA, Pearman MH, Kekoler LJ, Edmondson D, Baughman VL, Angel JJ, Campbell C, Jense HG, Mingus M, Shahvari MB, Creed MR. Efficacy of repeat intravenous dosing of ondansetron in controlling postoperative nausea and vomiting: a randomized, double‐blind, placebo‐controlled multicenter trial. J Clin Anesth 1999; 11: 453–9. [DOI] [PubMed] [Google Scholar]
- 102. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo‐Deenadayalan S, Myles P, Nezat G, Philip BK, Tramer MR. Society for Ambulatory A. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014; 118: 85–113. [DOI] [PubMed] [Google Scholar]
- 103. Gelb AW, Leslie K, Stanski D, Shafer S. Monitoring the Depth of Anesthesia, Miller Anesthesia, 7th edn Philadelphia, PA: Elsevier, 2010. [Google Scholar]
- 104. Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, Karl L, Kamal S, Torres B, O'Connor M, Evers AS, Gradwohl S, Lin N, Palanca BJ, Mashour GA. Group B‐RR. Prevention of intraoperative awareness in a high‐risk surgical population. N Engl J Med 2011; 365: 591–600. [DOI] [PubMed] [Google Scholar]
- 105. Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C, Jacobsohn E, Evers AS. Anesthesia awareness and the bispectral index. N Engl J Med 2008; 358: 1097–108. [DOI] [PubMed] [Google Scholar]
- 106. Bowdle TA. Depth of anesthesia monitoring. Anesthesiol Clin 2006; 24: 793–822. [DOI] [PubMed] [Google Scholar]
- 107. Boztug N, Bigat Z, Akyuz M, Demir S, Ertok E. Does using the bispectral index (BIS) during craniotomy affect the quality of recovery? J Neurosurg Anesthesiol 2006; 18: 1–4. [DOI] [PubMed] [Google Scholar]
- 108. Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol 2006; 20: 81–99. [DOI] [PubMed] [Google Scholar]
- 109. Kakinohana M, Nakamura S, Miyata Y, Sugahara K. Emergence from propofol anesthesia in a nonagenarian at a Bispectral Index of 52. Anesth Analg 2005; 101: 169–70. [DOI] [PubMed] [Google Scholar]
- 110. Messner M, Beese U, Romstock J, Dinkel M, Tschaikowsky K. The bispectral index declines during neuromuscular block in fully awake persons. Anesth Analg 2003; 97: 488–91. [DOI] [PubMed] [Google Scholar]
- 111. Morimoto Y, Nogami Y, Harada K, Tsubokawa T, Masui K. Awareness during anesthesia: the results of a questionnaire survey in Japan. J Anesth 2011; 25: 72–7. [DOI] [PubMed] [Google Scholar]
- 112. Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B‐Aware randomised controlled trial. Lancet 2004; 363: 1757–63. [DOI] [PubMed] [Google Scholar]
- 113. Zhang C, Xu L, Ma YQ, Sun YX, Li YH, Zhang L, Feng CS, Luo B, Zhao ZL, Guo JR, Jin YJ, Wu G, Yuan W, Yuan ZG, Yue Y. Bispectral index monitoring prevent awareness during total intravenous anesthesia: a prospective, randomized, double‐blinded, multi‐center controlled trial. Chin Med J (Engl) 2011; 124: 3664–9. [PubMed] [Google Scholar]
- 114. Ekman A, Lindholm ML, Lennmarken C, Sandin R. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand 2004; 48: 20–6. [DOI] [PubMed] [Google Scholar]
- 115. Liao WW, Wang JJ, Wu GJ, Kuo CD. The effect of cerebral monitoring on recovery after sevoflurane anesthesia in ambulatory setting in children: a comparison among bispectral index, A‐line autoregressive index, and standard practice. J Chin Med Assoc 2011; 74: 28–36. [DOI] [PubMed] [Google Scholar]
- 116. Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev 2014; 6: CD003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chan MT, Cheng BC, Lee TM, Gin T. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013; 25: 33–42. [DOI] [PubMed] [Google Scholar]
- 118. Hopkins PM. Nitrous oxide: a unique drug of continuing importance for anaesthesia. Best Pract Res Clin Anaesthesiol 2005; 19: 381–9. [DOI] [PubMed] [Google Scholar]
- 119. Tramer M, Moore A, McQuay H. Omitting nitrous oxide in general anaesthesia: meta‐analysis of intraoperative awareness and postoperative emesis in randomized controlled trials. Br J Anaesth 1996; 76: 186–93. [DOI] [PubMed] [Google Scholar]
- 120. Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, Silbert BS, Pascoe E, Group ET . Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007; 107: 221–31. [DOI] [PubMed] [Google Scholar]
- 121. Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one‐year mortality after noncardiac surgery. Anesth Analg 2005; 100: 4–10. [DOI] [PubMed] [Google Scholar]
- 122. Ash SA, Buggy DJ. Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery? An update of available evidence. Best Pract Res Clin Anaesthesiol 2013; 27: 441–56. [DOI] [PubMed] [Google Scholar]
- 123. Fawcett WJ, Mythen MG, Scott MJ. Enhanced recovery: more than just reducing length of stay? Br J Anaesth 2012; 109: 671–4. [DOI] [PubMed] [Google Scholar]
- 124. Martini CH, Boon M, Bevers RF, Aarts LP, Dahan A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br J Anaesth 2014; 112: 498–505. [DOI] [PubMed] [Google Scholar]
- 125. Madsen MV, Staehr‐Rye AK, Gatke MR, Claudius C. Neuromuscular blockade for optimising surgical conditions during abdominal and gynaecological surgery: a systematic review. Acta Anaesthesiol Scand 2015; 59: 1–16. [DOI] [PubMed] [Google Scholar]
- 126. Kopman AF, Naguib M. Laparoscopic surgery and muscle relaxants: is deep block helpful? Anesth Analg 2015; 120: 51–8. [DOI] [PubMed] [Google Scholar]
- 127. King M, Sujirattanawimol N, Danielson DR, Hall BA, Schroeder DR, Warner DO. Requirements for muscle relaxants during radical retropubic prostatectomy. Anesthesiology 2000; 93: 1392–7. [DOI] [PubMed] [Google Scholar]
- 128. Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg 2010; 111: 129–40. [DOI] [PubMed] [Google Scholar]
- 129. Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology 2006; 104: 1070–80. [DOI] [PubMed] [Google Scholar]
- 130. Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg 2010; 111: 120–8. [DOI] [PubMed] [Google Scholar]
- 131. Butterly A, Bittner EA, George E, Sandberg WS, Eikermann M, Schmidt U. Postoperative residual curarization from intermediate‐acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth 2010; 105: 304–9. [DOI] [PubMed] [Google Scholar]
- 132. Murphy GS, Szokol JW, Franklin M, Marymont JH, Avram MJ, Vender JS. Postanesthesia care unit recovery times and neuromuscular blocking drugs: a prospective study of orthopedic surgical patients randomized to receive pancuronium or rocuronium. Anesth Analg 2004; 98: 193–200. [DOI] [PubMed] [Google Scholar]
- 133. Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, Nisman M. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology 2008; 109: 389–98. [DOI] [PubMed] [Google Scholar]
- 134. Fink H, Hollmann MW. Myths and facts in neuromuscular pharmacology. New developments in reversing neuromuscular blockade. Minerva Anestesiol 2012; 78: 473–82. [PubMed] [Google Scholar]
- 135. Kotake Y, Ochiai R, Suzuki T, Ogawa S, Takagi S, Ozaki M, Nakatsuka I, Takeda J. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg 2013; 117: 345–51. [DOI] [PubMed] [Google Scholar]
- 136. Claudius C, Viby‐Mogensen J. Acceleromyography for use in scientific and clinical practice: a systematic review of the evidence. Anesthesiology 2008; 108: 1117–40. [DOI] [PubMed] [Google Scholar]
- 137. Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003; 98: 1042–8. [DOI] [PubMed] [Google Scholar]
- 138. Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev 2009; 4: CD007362. [DOI] [PubMed] [Google Scholar]
- 139. Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Hogdall C, Lundvall L, Svendsen PE, Mollerup H, Lunn TH, Simonsen I, Martinsen KR, Pulawska T, Bundgaard L, Bugge L, Hansen EG, Riber C, Gocht‐Jensen P, Walker LR, Bendtsen A, Johansson G, Skovgaard N, Helto K, Poukinski A, Korshin A, Walli A, Bulut M, Carlsson PS, Rodt SA, Lundbech LB, Rask H, Buch N, Perdawid SK, Reza J, Jensen KV, Carlsen CG, Jensen FS, Rasmussen LS. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA 2009; 302: 1543–50. [DOI] [PubMed] [Google Scholar]
- 140. Togioka B, Galvagno S, Sumida S, Murphy J, Ouanes JP, Wu C. The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta‐analysis. Anesth Analg 2012; 114: 334–42. [DOI] [PubMed] [Google Scholar]
- 141. Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long‐term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow‐up of a randomized clinical trial. Anesth Analg 2012; 115: 849–54. [DOI] [PubMed] [Google Scholar]
- 142. Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation 2011; 123: 2717–22. [DOI] [PubMed] [Google Scholar]
- 143. Pilcher J, Weatherall M, Shirtcliffe P, Bellomo R, Young P, Beasley R. The effect of hyperoxia following cardiac arrest ‐ A systematic review and meta‐analysis of animal trials. Resuscitation 2012; 83: 417–22. [DOI] [PubMed] [Google Scholar]
- 144. Edmark L, Kostova‐Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology 2003; 98: 28–33. [DOI] [PubMed] [Google Scholar]
- 145. Staehr AK, Meyhoff CS, Rasmussen LS. Inspiratory oxygen fraction and postoperative complications in obese patients: a subgroup analysis of the PROXI trial. Anesthesiology 2011; 114: 1313–9. [DOI] [PubMed] [Google Scholar]
- 146. The Management of Inadvertent Perioperative Hypothermia in Adults. London: Royal College of Nursing (UK), 2008. [PubMed] [Google Scholar]
- 147. Moola S, Lockwood C. Effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment. Int J Evid Based Healthc 2011; 9: 337–45. [DOI] [PubMed] [Google Scholar]
- 148. Stewart BT, Stitz RW, Tuch MM, Lumley JW. Hypothermia in open and laparoscopic colorectal surgery. Dis Colon Rectum 2011; 42: 1292–5. [DOI] [PubMed] [Google Scholar]
- 149. Fernandes LA, Braz LG, Koga FA, Kakuda CM, Modolo NS, de Carvalho LR, Vianna PT, Braz JR. Comparison of peri‐operative core temperature in obese and non‐obese patients. Anaesthesia 2012; 67: 1364–9. [DOI] [PubMed] [Google Scholar]
- 150. Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008; 109: 318–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Esnaola NF, Cole DJ. Perioperative normothermia during major surgery: is it important? Adv Surg 2011; 45: 249–63. [DOI] [PubMed] [Google Scholar]
- 152. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334: 1209–15. [DOI] [PubMed] [Google Scholar]
- 153. Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277: 1127–34. [PubMed] [Google Scholar]
- 154. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008; 108: 71–7. [DOI] [PubMed] [Google Scholar]
- 155. Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, Narzt E, Lackner F. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997; 87: 1318–23. [DOI] [PubMed] [Google Scholar]
- 156. Moslemi‐Kebria M, El‐Nashar SA, Aletti GD, Cliby WA. Intraoperative hypothermia during cytoreductive surgery for ovarian cancer and perioperative morbidity. Obstet Gynecol 2012; 119: 590–6. [DOI] [PubMed] [Google Scholar]
- 157. Abraham NS, Byrne CM, Young JM, Solomon MJ. Meta‐analysis of non‐randomized comparative studies of the short‐term outcomes of laparoscopic resection for colorectal cancer. ANZ J Surg 2007; 77: 508–16. [DOI] [PubMed] [Google Scholar]
- 158. Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long‐term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev 2008; 34: 498–504. [DOI] [PubMed] [Google Scholar]
- 159. Brown SR, Goodfellow PB. Transverse verses midline incisions for abdominal surgery. Cochrane Database Syst Rev 2011; 4: CD005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gulla N, Noya G, Boselli C. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta‐analysis of short‐term outcome. Colorectal Dis 2012; 14: e134–56. [DOI] [PubMed] [Google Scholar]
- 161. Champagne BJ, Papaconstantinou HT, Parmar SS, Nagle DA, Young‐Fadok TM, Lee EC, Delaney CP. Single‐incision versus standard multiport laparoscopic colectomy: a multicenter, case‐controlled comparison. Ann Surg 2012; 255: 66–9. [DOI] [PubMed] [Google Scholar]
- 162. Alba Mesa F, Amaya Cortijo A, Romero Fernandez JM, Komorowski AL, Sanchez Hurtado MA, Fernandez Ortega E, Sanchez Margallo FM. Transvaginal sigmoid cancer resection: first case with 12 months of follow‐up–technique description. J Laparoendosc Adv Surg Techn A 2012; 22: 587–90. [DOI] [PubMed] [Google Scholar]
- 163. Cheung TP, Cheung HY, Ng LW, Chung CC, Li MK. Hybrid NOTES colectomy for right‐sided colonic tumors. Asian Journal Endosc Surg 2012; 5: 46–9. [DOI] [PubMed] [Google Scholar]
- 164. Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; 3: CD004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rao W, Zhang X, Zhang J, Yan R, Hu Z, Wang Q. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta‐analysis. Int J Colorectal Dis 2011; 26: 423–9. [DOI] [PubMed] [Google Scholar]
- 166. Cheatham ML, Chapman WC, Key SP, Sawyers JL. A meta‐analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg 1995; 221: 469–76; discussion 76‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yardley IE, Donaldson LJ. Patient safety matters: reducing the risks of nasogastric tubes. Clin Med 2010; 10: 228–30. [DOI] [PubMed] [Google Scholar]
- 168. Ronen O, Uri N. A case of nasogastric tube perforation of the nasopharynx causing a fatal mediastinal complication. Ear Nose Throat J 2009; 88: E17–8. [PubMed] [Google Scholar]
- 169. Johnson MD, Walsh RM. Current therapies to shorten postoperative ileus. Cleve Clin J Med 2009; 76: 641–8. [DOI] [PubMed] [Google Scholar]
- 170. Manning BJ, Winter DC, McGreal G, Kirwan WO, Redmond HP. Nasogastric intubation causes gastroesophageal reflux in patients undergoing elective laparotomy. Surgery 2001; 130: 788–91. [DOI] [PubMed] [Google Scholar]
- 171. Lassen K, Kjaeve J, Fetveit T, Trano G, Sigurdsson HK, Horn A, Revhaug A. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008; 247: 721–9. [DOI] [PubMed] [Google Scholar]
- 172. Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast‐track recovery programme after pancreatico‐ duodenectomy reduces delayed gastric emptying. Br J Surg 2008; 95: 1387–93. [DOI] [PubMed] [Google Scholar]
- 173. Berberat PO, Ingold H, Gulbinas A, Kleeff J, Muller MW, Gutt C, Weigand M, Friess H, Buchler MW. Fast track–different implications in pancreatic surgery. J Gastrointest Surg 2007; 11: 880–7. [DOI] [PubMed] [Google Scholar]
- 174. Schricker T, Lattermann R, Schreiber M, Geisser W, Georgieff M, Radermacher P. The hyperglycemic response to surgery: pathophysiology, clinical implications and modification by the anaesthetic technique. Clinical Intensive Care 1998; 9: 118–28. [Google Scholar]
- 175. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87: 978–82. [DOI] [PubMed] [Google Scholar]
- 176. Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 2010; 145: 858–64. [DOI] [PubMed] [Google Scholar]
- 177. Eshuis WJ, Hermanides J, van Dalen JW, van Samkar G, Busch OR, van Gulik TM, DeVries JH, Hoekstra JB, Gouma DJ. Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg 2011; 253: 739–44. [DOI] [PubMed] [Google Scholar]
- 178. Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010; 95: 4338–44. [DOI] [PubMed] [Google Scholar]
- 179. Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, Guyton RA, Thourani VH. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008; 136: 631–40. [DOI] [PubMed] [Google Scholar]
- 180. Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg 2009; 96: 1358–64. [DOI] [PubMed] [Google Scholar]
- 181. O'Sullivan CJ, Hynes N, Mahendran B, Andrews EJ, Avalos G, Tawfik S, Lowery A, Sultan S. Haemoglobin A1c (HbA1C) in non‐diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 2006; 32: 188–97. [DOI] [PubMed] [Google Scholar]
- 182. Kao LS, Meeks D, Moyer VA, Lally KP. Peri‐operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev 2009; July 8;(3): CD006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, deBoer MJ , Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba‐Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007; 28: 88–136. [DOI] [PubMed] [Google Scholar]
- 184. Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87: 663–9. [DOI] [PubMed] [Google Scholar]
- 185. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric S . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ljungqvist O, Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr 2012; 36: 389–98. [DOI] [PubMed] [Google Scholar]
- 187. Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta‐analysis. Int J Colorectal Dis 2012; 27: 803–10. [DOI] [PubMed] [Google Scholar]
- 188. Guenaga KF, Matos D, Wille‐Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011; CD001544. doi: 10.1002/14651858.CD001544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sanders G, Mercer SJ, Saeb‐Parsey K, Akhavani MA, Hosie KB, Lambert AW. Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. Br J Surg 2001; 88: 1363–5. [DOI] [PubMed] [Google Scholar]
- 190. Holte K. Pathophysiology and clinical implications of peroperative fluid management in elective surgery. Dan Med Bull 2010; 57: B4156. [PubMed] [Google Scholar]
- 191. Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SA, Hansen B, Moller DR, Lundbech LB, Andersen N, Berg V, Thomassen N, Andersen ST, Simonsen L. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near‐maximal stroke volume or zero fluid balance? Br J Anaesth 2012; 109: 191–9. [DOI] [PubMed] [Google Scholar]
- 192. Brandstrup B, Tonnesen H, Beier‐Holgersen R, Hjortso E, Ording H, Lindorff‐Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T . Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor‐blinded multicenter trial. Ann Surg 2003; 238: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, Kehlet H. Liberal or restrictive fluid administration in fast‐track colonic surgery: a randomized, double‐blind study. Br J Anaesth 2007; 99: 500–8. [DOI] [PubMed] [Google Scholar]
- 194. Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005; 103: 25–32. [DOI] [PubMed] [Google Scholar]
- 195. Chowdhury AH, Lobo DN. Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care 2011; 14: 469–76. [DOI] [PubMed] [Google Scholar]
- 196. Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002; 359: 1812–8. [DOI] [PubMed] [Google Scholar]
- 197. Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J. Enhanced Recovery After Surgery Study G. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011; 146: 571–7. [DOI] [PubMed] [Google Scholar]
- 198. Powell‐Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, Lewington AJ, Pearse RM, Mythen MG. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP). London: NHS National Library of Health, 2009. [Google Scholar]
- 199. Lobo DN, Awad S. Should chloride‐rich crystalloids remain the mainstay of fluid resuscitation to prevent'pre‐renal' acute kidney injury?: con. Kidney Int 2014; 86: 1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity‐matched cohort study. Anesth Analg 2013; 117: 412–21. [DOI] [PubMed] [Google Scholar]
- 201. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012; 308: 1566–72. [DOI] [PubMed] [Google Scholar]
- 202. Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, Kellum JA. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma‐Lyte. Ann Surg 2012; 255: 821–9. [DOI] [PubMed] [Google Scholar]
- 203. Feldheiser A, Conroy P, Bonomo T, Cox B, Garces TR, Spies C, Anaesthesia Working Group of the Enhanced Recovery After Surgery S, Enhanced Recovery After Surgery S . Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery. J Int Med Res 2012; 40: 1227–41. [DOI] [PubMed] [Google Scholar]
- 204. Conway DH, Mayall R, Abdul‐Latif MS, Gilligan S, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 2002; 57: 845–9. [DOI] [PubMed] [Google Scholar]
- 205. Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler‐optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006; 93: 1069–76. [DOI] [PubMed] [Google Scholar]
- 206. Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95: 634–42. [DOI] [PubMed] [Google Scholar]
- 207. Kuper M, Gold SJ, Callow C, Quraishi T, King S, Mulreany A, Bianchi M, Conway DH. Intraoperative fluid management guided by oesophageal Doppler monitoring. BMJ 2011; 342: d3016. [DOI] [PubMed] [Google Scholar]
- 208. Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010; 14: R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Benes J, Giglio M, Brienza N, Michard F. The effects of goal‐directed fluid therapy based on dynamic parameters on post‐surgical outcome: a meta‐analysis of randomized controlled trials. Crit Care 2014; 18: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal‐directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care 2005; 9: R687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K. Optimisation Systematic Review Steering G. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane Systematic Review. Br J Anaesth 2013; 111: 535–48. [DOI] [PubMed] [Google Scholar]
- 212. Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal‐directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg 2013; 100: 66–74. [DOI] [PubMed] [Google Scholar]
- 213. Miller TE, Roche AM, Mythen M. Fluid management and goal‐directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anaesth 2015; 62: 158–68. [DOI] [PubMed] [Google Scholar]
- 214. Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta‐analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high‐risk surgical patients. Anesth Analg 2011; 112: 1392–402. [DOI] [PubMed] [Google Scholar]
- 215. Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, Hinds C, Rowan K, Group OS . Effect of a perioperative, cardiac output‐guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014; 311: 2181–90. [DOI] [PubMed] [Google Scholar]
- 216. Mythen MG, Swart M, Acheson N, Crawford R, Jones K, Kuper M, McGrath JS, Horgan A. Perioperative fluid management: Consensus statement from the enhanced recovery partnership. Perioper Med (Lond) 2012; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Feldheiser A, Pavlova V, Bonomo T, Jones A, Fotopoulou C, Sehouli J, Wernecke KD, Spies C. Balanced crystalloid compared with balanced colloid solution using a goal‐directed haemodynamic algorithm. Br J Anaesth 2013; 110: 231–40. [DOI] [PubMed] [Google Scholar]
- 218. Verheij J, van Lingen A, Beishuizen A, Christiaans HM, de Jong JR, Girbes AR, Wisselink W, Rauwerda JA, Huybregts MA, Groeneveld AB. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med 2006; 32: 1030–8. [DOI] [PubMed] [Google Scholar]
- 219. Lobo DN, Stanga Z, Aloysius MM, Wicks C, Nunes QM, Ingram KL, Risch L, Allison SP. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three‐way crossover study in healthy volunteers. Crit Care Med 2010; 38: 464–70. [DOI] [PubMed] [Google Scholar]
- 220. Moretti EW, Robertson KM, El‐Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analgesia 2003; 96: 611–7. [DOI] [PubMed] [Google Scholar]
- 221. Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal‐directed fluid therapy in colorectal surgery. Br J Anaesth 2014; 112: 281–9. [DOI] [PubMed] [Google Scholar]
- 222. Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA, Investigators C. Australian, New Zealand Intensive Care Society Clinical Trials G. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367: 1901–11. [DOI] [PubMed] [Google Scholar]
- 223. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe‐Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J, Group ST , Scandinavian Critical Care Trials G . Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012; 367: 124–34. [DOI] [PubMed] [Google Scholar]
- 224. Gillies MA, Habicher M, Jhanji S, Sander M, Mythen M, Hamilton M, Pearse RM. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta‐analysis. Br J Anaesth 2014; 112: 25–34. [DOI] [PubMed] [Google Scholar]
- 225. Kashy BK, Podolyak A, Makarova N, Dalton JE, Sessler DI, Kurz A. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 2014; 121: 730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Agency EM. Hydroxyethyl‐starch solutions (HES) no longer to be used in patients with sepsis or burn injuries or in critically ill patients, 2013. [www document] http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Solutions_for_infusion_containing_hydroxyethyl_starch/European_Commission_final_decision/WC500162361.pdf (accessed 15 September 2015).
- 227. Rasmussen KC, Johansson PI, Hojskov M, Kridina I, Kistorp T, Thind P, Nielsen HB, Ruhnau B, Pedersen T, Secher NH. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg 2014; 259: 249–54. [DOI] [PubMed] [Google Scholar]
- 228. Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta‐analysis. J Gastrointest Surg 2009; 13: 569–75. [DOI] [PubMed] [Google Scholar]
- 229. Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC, Yu Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta‐analysis of randomized clinical trials. Dig Surg 2013; 30: 225–32. [DOI] [PubMed] [Google Scholar]
- 230. Holte K, Foss NB, Svensen C, Lund C, Madsen JL, Kehlet H. Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology 2004; 100: 281–6. [DOI] [PubMed] [Google Scholar]
- 231. Gould TH, Grace K, Thorne G, Thomas M. Effect of thoracic epidural anaesthesia on colonic blood flow. Br J Anaesth 2002; 89: 446–51. [PubMed] [Google Scholar]
- 232. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double‐blind crossover study on the effects of 2‐L infusions of 0.9% saline and plasma‐lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012; 256: 18–24. [DOI] [PubMed] [Google Scholar]
- 233. Lobo DN, Stanga Z, Simpson JAD, Anderson JA, Rowlands BJ, Allison SP. Dilution and redistribution effects of rapid 2‐litre infusions of 0.9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: a double‐blind crossover study. Clin Sci (Lond) 2001; 101: 173–9. [PubMed] [Google Scholar]
- 234. Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann's solution: a randomized double‐blind crossover study. Clin Sci (Lond) 2003; 104: 17–24. [DOI] [PubMed] [Google Scholar]
- 235. O'Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, Bennett‐Guerrero E. A randomized, double‐blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg 2005; 100: 1518–24. [DOI] [PubMed] [Google Scholar]
- 236. Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg 2001; 93: 817–22. [DOI] [PubMed] [Google Scholar]
- 237. PROcedure SPECific postoperative pain menagemenT (PROSPECT) [www document] http://www.postoppain.org/ (accessed 15 February 2013).
- 238. White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology 2010; 112: 220–5. [DOI] [PubMed] [Google Scholar]
- 239. Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, Lin EE, Liu SS. Efficacy of postoperative patient‐controlled and continuous infusion epidural analgesia versus intravenous patient‐controlled analgesia with opioids: a meta‐analysis. Anesthesiology 2005; 103: 1079–88; quiz 109‐10. [DOI] [PubMed] [Google Scholar]
- 240. Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, Collins KS, Group MATS. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002; 359: 1276–82. [DOI] [PubMed] [Google Scholar]
- 241. Popping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, Wenk M, Tramer MR. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta‐analysis of randomized controlled trials. Ann Surg 2014; 259: 1056–67. [DOI] [PubMed] [Google Scholar]
- 242. Carli F, Kehlet H, Baldini G, Steel A, McRae K, Slinger P, Hemmerling T, Salinas F, Neal JM. Evidence basis for regional anesthesia in multidisciplinary fast‐track surgical care pathways. Reg Anesth Pain Med 2011; 36: 63–72. [DOI] [PubMed] [Google Scholar]
- 243. Khan SA, Khokhar HA, Nasr AR, Carton E, El‐Masry S. Effect of epidural analgesia on bowel function in laparoscopic colorectal surgery: a systematic review and meta‐analysis. Surg Endosc 2013; 27: 2581–91. [DOI] [PubMed] [Google Scholar]
- 244. Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anaesthetics versus opioid‐based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 2000; 4: CD001893. [DOI] [PubMed] [Google Scholar]
- 245. Shi WZ, Miao YL, Yakoob MY, Cao JB, Zhang H, Jiang YG, Xu LH, Mi WD. Recovery of gastrointestinal function with thoracic epidural vs. systemic analgesia following gastrointestinal surgery. Acta Anaesthesiol Scand 2014; 58: 923–32. [DOI] [PubMed] [Google Scholar]
- 246. Uchida I, Asoh T, Shirasaka C, Tsuji H. Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg 1988; 75: 557–62. [DOI] [PubMed] [Google Scholar]
- 247. Popping DM, Elia N, Marret E, Remy C, Tramer MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta‐analysis. Arch Surg 2008; 143: 990–9; discussion 1000. [DOI] [PubMed] [Google Scholar]
- 248. Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta‐analysis. JAMA 2003; 290: 2455–63. [DOI] [PubMed] [Google Scholar]
- 249. Zaouter C, Wuethrich P, Miccoli M, Carli F. Early removal of urinary catheter leads to greater post‐void residuals in patients with thoracic epidural. Acta Anaesthesiol Scand 2012; 56: 1020–5. [DOI] [PubMed] [Google Scholar]
- 250. Marret E, Remy C, Bonnet F, Postoperative Pain Forum G . Meta‐analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg 2007; 94: 665–73. [DOI] [PubMed] [Google Scholar]
- 251. Rawal N. Epidural technique for postoperative pain: gold standard no more? Reg Anesth Pain Med 2012; 37: 310–7. [DOI] [PubMed] [Google Scholar]
- 252. Hubner M, Blanc C, Roulin D, Winiker M, Gander S, Demartines N. Randomized clinical trial on epidural versus patient‐controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg 2015; 261: 648–53. [DOI] [PubMed] [Google Scholar]
- 253. Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient‐controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011; 98: 1068–78. [DOI] [PubMed] [Google Scholar]
- 254. Wongyingsinn M, Baldini G, Charlebois P, Liberman S, Stein B, Carli F. Intravenous lidocaine versus thoracic epidural analgesia: a randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Reg Anesth Pain Med 2011; 36: 241–8. [DOI] [PubMed] [Google Scholar]
- 255. Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth 2012; 109: 144–54. [DOI] [PubMed] [Google Scholar]
- 256. Finucane BT, Ganapathy S, Carli F, Pridham JN, Ong BY, Shukla RC, Kristoffersson AH, Huizar KM, Nevin K, Ahlen KG, Canadian Ropivacaine Research G . Prolonged epidural infusions of ropivacaine (2 mg/mL) after colonic surgery: the impact of adding fentanyl. Anesth Analg 2001; 92: 1276–85. [DOI] [PubMed] [Google Scholar]
- 257. Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology 2011; 115: 181–8. [DOI] [PubMed] [Google Scholar]
- 258. Kim JY, Lee SJ, Koo BN, Noh SH, Kil HK, Kim HS, Ban SY. The effect of epidural sufentanil in ropivacaine on urinary retention in patients undergoing gastrectomy. Br J Anaesth 2006; 97: 414–8. [DOI] [PubMed] [Google Scholar]
- 259. Rawal N, Allvin R. Epidural and intrathecal opioids for postoperative pain management in Europe–a 17‐nation questionnaire study of selected hospitals. Euro Pain Study Group on Acute Pain. Acta Anaesthesiol Scand 1996; 40: 1119–26. [DOI] [PubMed] [Google Scholar]
- 260. Sakaguchi Y, Sakura S, Shinzawa M, Saito Y. Does adrenaline improve epidural bupivacaine and fentanyl analgesia after abdominal surgery? Anaesth Intensive Care 2000; 28: 522–6. [DOI] [PubMed] [Google Scholar]
- 261. Niemi G, Breivik H. The minimally effective concentration of adrenaline in a low‐concentration thoracic epidural analgesic infusion of bupivacaine, fentanyl and adrenaline after major surgery. A randomized, double‐blind, dose‐finding study. Acta Anaesthesiol Scand 2003; 47: 439–50. [DOI] [PubMed] [Google Scholar]
- 262. Niemi G, Breivik H. Epinephrine markedly improves thoracic epidural analgesia produced by a small‐dose infusion of ropivacaine, fentanyl, and epinephrine after major thoracic or abdominal surgery: a randomized, double‐blinded crossover study with and without epinephrine. Anesth Analg 2002; 94: 1598–605. [DOI] [PubMed] [Google Scholar]
- 263. Chan AK, Cheung CW, Chong YK. Alpha‐2 agonists in acute pain management. Expert Opin Pharmacother 2010; 11: 2849–68. [DOI] [PubMed] [Google Scholar]
- 264. Gramigni E, Bracco D, Carli F. Epidural analgesia and postoperative orthostatic haemodynamic changes. Eur J Anaesthesiol 2013; 30: 398–404. [DOI] [PubMed] [Google Scholar]
- 265. Meylan N, Elia N, Lysakowski C, Tramer MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta‐analysis of randomized trials. Br J Anaesth 2009; 102: 156–67. [DOI] [PubMed] [Google Scholar]
- 266. Levy BF, Scott MJ, Fawcett WJ, Day A, Rockall TA. Optimizing patient outcomes in laparoscopic surgery. Colorectal Dis 2011; 13(Suppl 7): 8–11. [DOI] [PubMed] [Google Scholar]
- 267. Hubner M, Lovely JK, Huebner M, Slettedahl SW, Jacob AK, Larson DW. Intrathecal analgesia and restrictive perioperative fluid management within enhanced recovery pathway: hemodynamic implications. J Am Coll Surg 2013; 216: 1124–34. [DOI] [PubMed] [Google Scholar]
- 268. Wongyingsinn M, Baldini G, Stein B, Charlebois P, Liberman S, Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth 2012; 108: 850–6. [DOI] [PubMed] [Google Scholar]
- 269. Marret E, Rolin M, Beaussier M, Bonnet F. Meta‐analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 2008; 95: 1331–8. [DOI] [PubMed] [Google Scholar]
- 270. Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, McIntyre LA, Nicole PC, Fergusson DA. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta‐analysis of randomized controlled trials. Can J Anaesth 2011; 58: 22–37. [DOI] [PubMed] [Google Scholar]
- 271. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010; 70: 1149–63. [DOI] [PubMed] [Google Scholar]
- 272. Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, Joris JL. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 2007; 106: 11–8; discussion 5‐6. [DOI] [PubMed] [Google Scholar]
- 273. Karthikesalingam A, Walsh SR, Markar SR, Sadat U, Tang TY, Malata CM. Continuous wound infusion of local anaesthetic agents following colorectal surgery: systematic review and meta‐analysis. World J Gastroenterol 2008; 14: 5301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006; 203: 914–32. [DOI] [PubMed] [Google Scholar]
- 275. Beaussier M, El'Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit JX, Azizi L, Gervaz P, Rohr S, Biermann C, Lienhart A, Eledjam JJ. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double‐blind, placebo‐controlled study. Anesthesiology 2007; 107: 461–8. [DOI] [PubMed] [Google Scholar]
- 276. Bertoglio S, Fabiani F, Negri PD, Corcione A, Merlo DF, Cafiero F, Esposito C, Belluco C, Pertile D, Amodio R, Mannucci M, Fontana V, Cicco MD, Zappi L. The postoperative analgesic efficacy of preperitoneal continuous wound infusion compared to epidural continuous infusion with local anesthetics after colorectal cancer surgery: a randomized controlled multicenter study. Anesth Analg 2012; 115: 1442–50. [DOI] [PubMed] [Google Scholar]
- 277. Jouve P, Bazin JE, Petit A, Minville V, Gerard A, Buc E, Dupre A, Kwiatkowski F, Constantin JM, Futier E. Epidural versus continuous preperitoneal analgesia during fast‐track open colorectal surgery: a randomized controlled trial. Anesthesiology 2013; 118: 622–30. [DOI] [PubMed] [Google Scholar]
- 278. Boulind CE, Ewings P, Bulley SH, Reid JM, Jenkins JT, Blazeby JM, Francis NK. Feasibility study of analgesia via epidural versus continuous wound infusion after laparoscopic colorectal resection. Br J Surg 2013; 100: 395–402. [DOI] [PubMed] [Google Scholar]
- 279. Ventham NT, O'Neill S, Johns N, Brady RR, Fearon KC. Evaluation of novel local anesthetic wound infiltration techniques for postoperative pain following colorectal resection surgery: a meta‐analysis. Dis Colon Rectum 2014; 57: 237–50. [DOI] [PubMed] [Google Scholar]
- 280. Moiniche S, Dahl JB. Wound catheters for post‐operative pain management: overture or finale? Acta Anaesthesiol Scand 2011; 55: 775–7. [DOI] [PubMed] [Google Scholar]
- 281. Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev 2010; CD007705. doi: 10.1002/14651858.CD007705.pub2. [DOI] [PubMed] [Google Scholar]
- 282. Siddiqui MR, Sajid MS, Uncles DR, Cheek L, Baig MK. A meta‐analysis on the clinical effectiveness of transversus abdominis plane block. J Clin Anesth 2011; 23: 7–14. [DOI] [PubMed] [Google Scholar]
- 283. Johns N, O'Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta‐analysis. Colorectal Dis 2012; 14: e635–42. [DOI] [PubMed] [Google Scholar]
- 284. Bharti N, Kumar P, Bala I, Gupta V. The efficacy of a novel approach to transversus abdominis plane block for postoperative analgesia after colorectal surgery. Anesth Analg 2011; 112: 1504–8. [DOI] [PubMed] [Google Scholar]
- 285. Owen DJ, Harrod I, Ford J, Luckas M, Gudimetla V. The surgical transversus abdominis plane block–a novel approach for performing an established technique. BJOG 2011; 118: 24–7. [DOI] [PubMed] [Google Scholar]
- 286. Favuzza J, Brady K, Delaney CP. Transversus abdominis plane blocks and enhanced recovery pathways: making the 23‐h hospital stay a realistic goal after laparoscopic colorectal surgery. Surg Endosc 2013; 27: 2481–6. [DOI] [PubMed] [Google Scholar]
- 287. Favuzza J, Delaney CP. Outcomes of discharge after elective laparoscopic colorectal surgery with transversus abdominis plane blocks and enhanced recovery pathway. J Am Coll Surg 2013; 217: 503–6. [DOI] [PubMed] [Google Scholar]
- 288. Keller DS, Stulberg JJ, Lawrence JK, Delaney CP. Process control to measure process improvement in colorectal surgery: modifications to an established enhanced recovery pathway. Dis Colon Rectum 2014; 57: 194–200. [DOI] [PubMed] [Google Scholar]
- 289. Brady RR, Ventham NT, Roberts DM, Graham C, Daniel T. Open transversus abdominis plane block and analgesic requirements in patients following right hemicolectomy. Ann R Coll Surg Engl 2012; 94: 327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Allcock E, Spencer E, Frazer R, Applegate G, Buckenmaier C 3rd. Continuous transversus abdominis plane (TAP) block catheters in a combat surgical environment. Pain Med 2010; 11: 1426–9. [DOI] [PubMed] [Google Scholar]
- 291. Kadam RV, Field JB. Ultrasound‐guided continuous transverse abdominis plane block for abdominal surgery. J Anaesthesiol Clin Pharmacol 2011; 27: 333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Bjerregaard N, Nikolajsen L, Bendtsen TF, Rasmussen BS. Transversus abdominis plane catheter bolus analgesia after major abdominal surgery. Anesthesiol Res Pract 2012; 2012: 596536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Niraj G, Kelkar A, Jeyapalan I, Graff‐Baker P, Williams O, Darbar A, Maheshwaran A, Powell R. Comparison of analgesic efficacy of subcostal transversus abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia 2011; 66: 465–71. [DOI] [PubMed] [Google Scholar]
- 294. De Oliveira GS Jr, Castro‐Alves LJ, Nader A, Kendall MC, McCarthy RJ. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta‐analysis of randomized controlled trials. Anesth Analg 2014; 118: 454–63. [DOI] [PubMed] [Google Scholar]
- 295. Walter CJ, Maxwell‐Armstrong C, Pinkney TD, Conaghan PJ, Bedforth N, Gornall CB, Acheson AG. A randomised controlled trial of the efficacy of ultrasound‐guided transversus abdominis plane (TAP) block in laparoscopic colorectal surgery. Surg Endosc 2013; 27: 2366–72. [DOI] [PubMed] [Google Scholar]
- 296. Niraj G, Kelkar A, Hart E, Horst C, Malik D, Yeow C, Singh B, Chaudhri S. Comparison of analgesic efficacy of four‐quadrant transversus abdominis plane (TAP) block and continuous posterior TAP analgesia with epidural analgesia in patients undergoing laparoscopic colorectal surgery: an open‐label, randomised, non‐inferiority trial. Anaesthesia 2014; 69: 348–55. [DOI] [PubMed] [Google Scholar]
- 297. Abdallah FW, Chan VW, Brull R. Transversus abdominis plane block: a systematic review. Reg Anesth Pain Med 2012; 37: 193–209. [DOI] [PubMed] [Google Scholar]
- 298. Kahokehr A, Sammour T, Shoshtari KZ, Taylor M, Hill AG. Intraperitoneal local anesthetic improves recovery after colon resection: a double‐blinded randomized controlled trial. Ann Surg 2011; 254: 28–38. [DOI] [PubMed] [Google Scholar]
- 299. Kahokehr A, Sammour T, Srinivasa S, Hill AG. Systematic review and meta‐analysis of intraperitoneal local anaesthetic for pain reduction after laparoscopic gastric procedures. Br J Surg 2011; 98: 29–36. [DOI] [PubMed] [Google Scholar]
- 300. Wu CL, Rowlingson AJ, Partin AW, Kalish MA, Courpas GE, Walsh PC, Fleisher LA. Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg Anesth Pain Med 2005; 30: 516–22. [DOI] [PubMed] [Google Scholar]
- 301. Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient‐controlled analgesia morphine side effects: meta‐analysis of randomized controlled trials. Anesthesiology 2005; 102: 1249–60. [DOI] [PubMed] [Google Scholar]
- 302. Klein M. Postoperative non‐steroidal anti‐inflammatory drugs and colorectal anastomotic leakage. NSAIDs and anastomotic leakage. Dan Med J 2012; 59: B4420. [PubMed] [Google Scholar]
- 303. Burton TP, Mittal A, Soop M. Nonsteroidal anti‐inflammatory drugs and anastomotic dehiscence in bowel surgery: systematic review and meta‐analysis of randomized, controlled trials. Dis Colon Rectum 2013; 56: 126–34. [DOI] [PubMed] [Google Scholar]
- 304. Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, Hulsewe KW, Luyer MD. Risk of anastomotic leakage with non‐steroidal anti‐inflammatory drugs in colorectal surgery. Br J Surg 2012; 99: 721–7. [DOI] [PubMed] [Google Scholar]
- 305. Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side‐effects and consumption after major surgery: meta‐analysis of randomized controlled trials. Br J Anaesth 2005; 94: 505–13. [DOI] [PubMed] [Google Scholar]
- 306. Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta‐analysis. Pain 2013; 154: 677–89. [DOI] [PubMed] [Google Scholar]
- 307. Mathiesen O, Wetterslev J, Kontinen VK, Pommergaard HC, Nikolajsen L, Rosenberg J, Hansen MS, Hamunen K, Kjer JJ, Dahl JB, Scandinavian Postoperative Pain A . Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand 2014; 58: 1182–98. [DOI] [PubMed] [Google Scholar]
- 308. Nussmeier NA, Whelton AA, Brown MT, Joshi GP, Langford RM, Singla NK, Boye ME, Verburg KM. Safety and efficacy of the cyclooxygenase‐2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology 2006; 104: 518–26. [DOI] [PubMed] [Google Scholar]
- 309. Vignali A, Di Palo S, Orsenigo E, Ghirardelli L, Radaelli G, Staudacher C. Effect of prednisolone on local and systemic response in laparoscopic vs. open colon surgery: a randomized, double‐blind, placebo‐controlled trial. Dis Colon Rectum 2009; 52: 1080–8. [DOI] [PubMed] [Google Scholar]
- 310. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side‐effects: systematic review and meta‐analysis. Br J Anaesth 2013; 110: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Lunn TH, Kristensen BB, Andersen LO, Husted H, Otte KS, Gaarn‐Larsen L, Kehlet H. Effect of high‐dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo‐controlled trial. Br J Anaesth 2011; 106: 230–8. [DOI] [PubMed] [Google Scholar]
- 312. de la Motte L, Kehlet H, Vogt K, Nielsen CH, Groenvall JB, Nielsen HB, Andersen A, Schroeder TV, Lonn L. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double‐blind, placebo‐controlled clinical trial. Ann Surg 2014; 260: 540–8; discussion 48‐9. [DOI] [PubMed] [Google Scholar]
- 313. Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev 2006; 1: CD004603. [DOI] [PubMed] [Google Scholar]
- 314. Weinbroum AA. Non‐opioid IV adjuvants in the perioperative period: pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res 2012; 65: 411–29. [DOI] [PubMed] [Google Scholar]
- 315. White PF. The changing role of non‐opioid analgesic techniques in the management of postoperative pain. Anesth Analg 2005; 101: S5–22. [DOI] [PubMed] [Google Scholar]
- 316. Candiotti KA, Sands LR, Lee E, Bergese SD, Harzman AE, Marcet J, Kumar AS, Haas E. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp 2014; 76: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel ®, a long‐acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res 2012; 5: 567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, Hansen MS, Kjer JJ, Mathiesen O, Scandinavian Postoperative Pain A . Post‐operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand 2014; 58: 1165–81. [DOI] [PubMed] [Google Scholar]
- 319. Brown TM, Boyle MF. Delirium. BMJ 2002; 325: 644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Tucker GJ. The diagnosis of delirium and DSM‐IV. Dement Geriatr Cogn Disord 1999; 10: 359–63. [DOI] [PubMed] [Google Scholar]
- 321. O'Keeffe ST. Clinical subtypes of delirium in the elderly. Dement Geriatr Cogn Disord 1999; 10: 380–5. [DOI] [PubMed] [Google Scholar]
- 322. Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo‐Summers LS, Inouye SK. Premature death associated with delirium at 1‐year follow‐up. Arch Intern Med 2005; 165: 1657–62. [DOI] [PubMed] [Google Scholar]
- 323. Heymann A, Radtke F, Schiemann A, Lutz A, MacGuill M, Wernecke KD, Spies C. Delayed treatment of delirium increases mortality rate in intensive care unit patients. J Int Med Res 2010; 38: 1584–95. [DOI] [PubMed] [Google Scholar]
- 324. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009; 180: 1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Radtke FM, Heymann A, Franck M, Maechler F, Drews T, Luetz A, Nachtigall I, Wernecke KD, Spies CD. How to implement monitoring tools for sedation, pain and delirium in the intensive care unit: an experimental cohort study. Intensive Care Med 2012; 38: 1974–81. [DOI] [PubMed] [Google Scholar]
- 326. Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anest Analg 2010; 111: 451–63. [DOI] [PubMed] [Google Scholar]
- 327. Radtke FM, Franck M, MacGuill M, Seeling M, Lutz A, Westhoff S, Neumann U, Wernecke KD, Spies CD. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur J Anaesthesiol 2010; 27: 411–6. [DOI] [PubMed] [Google Scholar]
- 328. Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev 2007; CD005594. [DOI] [PubMed] [Google Scholar]
- 329. Lonergan E, Luxenberg J, Areosa Sastre A, Wyller TB. Benzodiazepines for delirium. Cochrane Database Syst Rev 2009; 1: CD006379. [DOI] [PubMed] [Google Scholar]
- 330. Delaney CP, Kehlet H, Senagore A. Postoperative ileus: profiles, risk factors and definitions – a framework for optimizing surgical outcomes in patients undergoing major abdominal and colorectal surgery. Clinical Consensus Update in General Surgery 2006. Available at: http://www.clinicalwebcasts.com/updates/index.htm. (accessed 15 September 2015) [Google Scholar]
- 331. van Bree SH, Bemelman WA, Hollmann MW, Zwinderman AH, Matteoli G, El Temna S, The FO, Vlug MS, Bennink RJ, Boeckxstaens GE. Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Ann Surg 2014; 259: 708–14. [DOI] [PubMed] [Google Scholar]
- 332. Kehlet H. Postoperative ileus–an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 552–8. [DOI] [PubMed] [Google Scholar]
- 333. Chapuis PH, Bokey L, Keshava A, Rickard MJ, Stewart P, Young CJ, Dent OF. Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg 2013; 257: 909–15. [DOI] [PubMed] [Google Scholar]
- 334. Brieger GH. Early ambulation. A study in the history of surgery. Ann Surg 1983; 197: 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Brower RG. Consequences of bed rest. Critical Care Med 2009; 37: S422–8. [DOI] [PubMed] [Google Scholar]
- 336. Gatt M, Anderson AD, Reddy BS, Hayward‐Sampson P, Tring IC, MacFie J. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg 2005; 92: 1354–62. [DOI] [PubMed] [Google Scholar]
- 337. Kehlet H, Wilmore DW. Evidence‐based surgical care and the evolution of fast‐track surgery. Ann Surg 2008; 248: 189–98. [DOI] [PubMed] [Google Scholar]
- 338. Browning L, Denehy L, Scholes RL. The quantity of early upright mobilisation performed following upper abdominal surgery is low: an observational study. Aust J Physiother 2007; 53: 47–52. [DOI] [PubMed] [Google Scholar]
- 339. Henriksen MG, Jensen MB, Hansen HV, Jespersen TW, Hessov I. Enforced mobilization, early oral feeding, and balanced analgesia improve convalescence after colorectal surgery. Nutrition 2002; 18: 147–52. [DOI] [PubMed] [Google Scholar]
- 340. Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec 2012; 26: 87–94. [DOI] [PubMed] [Google Scholar]
- 341. Pitkethly M, Macgillivray S, Ryan R. Recordings or summaries of consultations for people with cancer. Cochrane Database Syst Rev 2008; 3: CD001539. [DOI] [PubMed] [Google Scholar]
- 342. Li C, Ferri LE, Mulder DS, Ncuti A, Neville A, Lee L, Kaneva P, Watson D, Vassiliou M, Carli F, Feldman LS. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery 2012; 152: 606–14; discussion 14‐6. [DOI] [PubMed] [Google Scholar]
- 343. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, Kaneva P, Augustin B, Wongyingsinn M, Gamsa A, Kim do J, Vassiliou MC, Feldman LS. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013; 27: 1072–82. [DOI] [PubMed] [Google Scholar]
- 344. Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, Kessels AG, Revhaug A, Kehlet H, Ljungqvist O, Fearon KC, von Meyenfeldt MF. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg 2007; 94: 224–31. [DOI] [PubMed] [Google Scholar]
- 345. Bravata DM, Smith‐Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296–304. [DOI] [PubMed] [Google Scholar]
- 346. Papaspyros S, Uppal S, Khan SA, Paul S, O'Regan DJ. Analysis of bedside entertainment services' effect on post cardiac surgery physical activity: a prospective, randomised clinical trial. Eur J Cardiothorac Surg 2008; 34: 1022–6. [DOI] [PubMed] [Google Scholar]
- 347. Moiniche S, Hjortso NC, Blemmer T, Dahl JB, Kehlet H. Blood pressure and heart rate during orthostatic stress and walking with continuous postoperative thoracic epidural bupivacaine/morphine. Acta Anaesthesiol Scand 1993; 37: 65–9. [DOI] [PubMed] [Google Scholar]
- 348. Kehlet H. Fast‐track surgery‐an update on physiological care principles to enhance recovery. Langenbecks Arch Surg 2011; 396: 585–90. [DOI] [PubMed] [Google Scholar]
- 349. Jans O, Bundgaard‐Nielsen M, Solgaard S, Johansson PI, Kehlet H. Orthostatic intolerance during early mobilization after fast‐track hip arthroplasty. Br J Anaeesth 2012; 108: 436–43. [DOI] [PubMed] [Google Scholar]
- 350. Bundgaard‐Nielsen M, Jans O, Muller RG, Korshin A, Ruhnau B, Bie P, Secher NH, Kehlet H. Does goal‐directed fluid therapy affect postoperative orthostatic intolerance?: A randomized trial. Anesthesiology 2013; 119: 813–23. [DOI] [PubMed] [Google Scholar]
- 351. Rehberg B. Why don't patients get out of bed postoperatively? Eur J Anaesthesiol 2013; 30: 395–6. [DOI] [PubMed] [Google Scholar]
- 352. Sjetne IS, Krogstad U, Odegard S, Engh ME. Improving quality by introducing enhanced recovery after surgery in a gynaecological department: consequences for ward nursing practice. Qual Saf Health Care 2009; 18: 236–40. [DOI] [PubMed] [Google Scholar]
- 353. Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health‐related quality of life after colonic surgery: results of a randomized trial. Anesthesiology 2002; 97: 540–9. [DOI] [PubMed] [Google Scholar]
- 354. Wodlin NB, Nilsson L, Kjolhede P. Health‐related quality of life and postoperative recovery in fast‐track hysterectomy. Acta Obstet Gynecol Scand 2011; 90: 362–8. [DOI] [PubMed] [Google Scholar]
- 355. Beaussier M, El'ayoubi H, Rollin M, Parc Y, Atchabahian A, Chanques G, Capdevila X, Lienhart A, Jaber S. Parietal analgesia decreases postoperative diaphragm dysfunction induced by abdominal surgery: a physiologic study. Reg Anesth Pain Med 2009; 34: 393–7. [DOI] [PubMed] [Google Scholar]
- 356. Virlos I, Clements D, Beynon J, Ratnalikar V, Khot U. Short‐term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. Br J Surg 2010; 97: 1401–6. [DOI] [PubMed] [Google Scholar]
- 357. Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23‐hour‐stay laparoscopic colectomy. Dis Colon Rectum 2009; 52: 1239–43. [DOI] [PubMed] [Google Scholar]
- 358. Vaughan‐Shaw PG, Fecher IC, Harris S, Knight JS. A meta‐analysis of the effectiveness of the opioid receptor antagonist alvimopan in reducing hospital length of stay and time to GI recovery in patients enrolled in a standardized accelerated recovery program after abdominal surgery. Dis Colon Rectum 2012; 55: 611–20. [DOI] [PubMed] [Google Scholar]
- 359. Yin Z, Sun J, Liu T, Zhu Y, Peng S, Wang J. Gum chewing: another simple potential method for more rapid improvement of postoperative gastrointestinal function. Digestion 2013; 87: 67–74. [DOI] [PubMed] [Google Scholar]


