Estimates of obesity prevalence based on the 2013–2014 National Health and Nutrition Examination Survey average almost 40%, corresponding to a 7% increase from the 1999–2000 cycle.1 Similar alarming trajectories are reported worldwide,2 along with increasing concerns for related health risks. The traditional concept that obesity results from a mere imbalance between energy intake and energy consumption has evolved to incorporate novel modulating factors, which may be targeted to develop preventative strategies. One such example is the potential role of disruption of circadian rhythms. In this brief review, the physiology of circadian control of body weight and metabolism, and the implications of altered circadian rhythms for obesity risk in humans will be discussed, together with potential countermeasures that may mitigate such consequences. The focus is on evidence accumulated from human studies. For a comprehensive review on the molecular mechanisms underlying the clock in metabolism and obesity, we direct the interested readers to Eckel-Mahan and Sassone-Corsi.3
Historical Perspective
Circadian systems have evolved as biological mechanisms to facilitate adaptation to the daily change from day to night, with related changes in activity and other behaviors. The intrinsic oscillatory system enables organisms to optimally synchronize their physiological and behavioral rhythms to the 24-hour solar day.
Although humankind has been aware of the existence of periodicity in natural phenomena, the origins of chronobiology as a science are relatively recent and can be traced back to the seventeenth century, with the landmark studies of Jean-Jacques de Mairan, a French astronomer. De Mairan noted that the daily leaf movements of the Mimosa plant, synchronous with the day-night cycle, persisted even in the absence of light exposure. Based on these observations, he hypothesized an endogenous botanic rhythm driving the plant movements. Confirmation of this was provided by the Swiss botanist Augustus Pyramus de Candolle, who showed that the period of such internal rhythmicity not only persisted under conditions of constant illumination, but was shorter than 24 hours, suggesting that an internal oscillator was responsible for the cycling. Such initial inferences of endogenously generated rhythms, with a period deviant from 24 hours in free-running conditions (i.e., in the absence of environmental temporal cues) were then extended to animals. The field of chronobiology expanded exponentially in the twentieth century, when the term circadian (derived from the latin expression circa diem - approximately a day) was coined.4 Additional properties of the internal, self-sustaining oscillator were described, such its innateness, its ability to yield temporal information thus serving as a clock, and to be synchronized (entrained) by external cues (Zeitgeber, the German word for time giver), with the dominant time giver in nature being the light-dark cycle. In the meantime, the neuroanatomy of circadian biology began to be unraveled with the identification of the central pacemaker in the suprachiasmatic nucleus (SCN) in the hypothalamus.5,6 The subsequent discovery of a multitude of autonomous timekeepers in the body, and even in single cells, has helped unmask the multioscillatory nature of circadian rhythms, while investigation of genetic components has led to major advances in understanding the molecular genetic substrate of the endogenous circadian system.
Overview of the Circadian Timing System
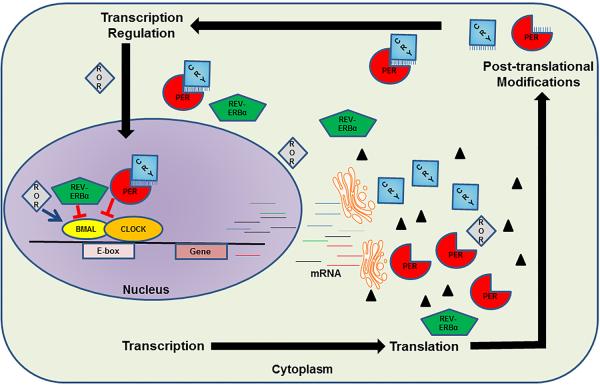
The molecular circadian system consists of core proteins including circadian locomotor output cycles kaput (CLOCK), brain and muscle Arnt-like protein 1 (BMAL1), period (PER), and cryptochrome (CRY). These proteins, via transcription-translational feedback loops, generate independent circadian oscillations within each cell (Figure 1). Simplistically, CLOCK and BMAL1 are transcriptional factors which form a heterodimer that binds to the promoter of several target genes including PER and CRY isoforms. Once PER and CRY proteins are formed, they undergo posttranslational modifications, form heterodimers, enter the nucleus and cause termination of CLOCK-BMAL1 mediated transcription of genes. At the cellular level, this rhythmicity of gene transcription and translation is responsible for appropriate timing of protein synthesis, protein degradation, DNA damage repair, DNA synthesis, and mitochondrial bioenergetics.7 For instance, oxidative stress is anticipated during the day when DNA synthesis is inactive, and the expression of DNA repair machinery is increased. On the contrary, at nighttime, inactivity and low stress are anticipated, and therefore DNA synthesis is active. Furthermore, the metabolic status of the cell regulates BMAL1 transcription via reverse-erb alpha (REV-ERBα, a repressor) and the opposing retinoic acid orphan receptors (RORα and β, activators).
Figure 1.
Simplified schematic depicting the molecular organization of the cellular circadian clock. (Copyright – Mayo Foundation).
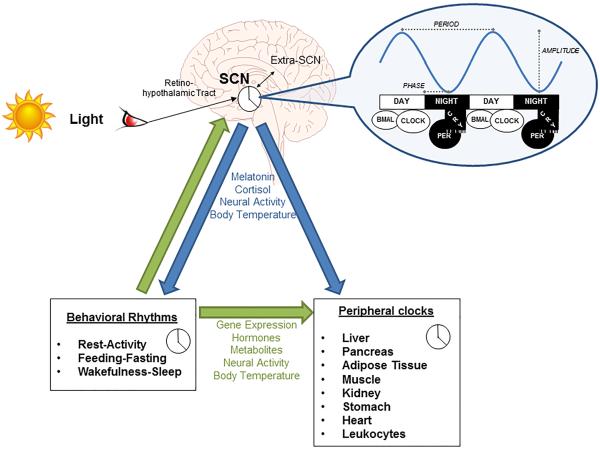
Akin to cellular temporal organization, synchronization of different organs such as heart, liver, pancreas, adipose tissue, and gastrointestinal tract would be optimal for integrated function of the whole organism. The recent discovery of intrinsic circadian fluctuations within these and other peripheral tissues further speaks to the ubiquitous nature of circadian systems.8 Overall, the intricate molecular mechanisms that tick away in each cell of different tissues, have different independent rhythms modulated by the master clock in the brain. In mammals, the SCN in the anterior hypothalamus is responsible for gathering the light-input from the external environment and coordinates the peripheral clocks present in each organ and cell. Via the retino-hypothalamic tract, the light input is transmitted from the ganglion cells in the retina to the SCN. The master clock then conveys this information to cells and tissues throughout the body and synchronizes them via neural and humoral signaling, including melatonin and cortisol (Figure 2). This synchronization is essential for the temporal organization within cells, organs and systems, to optimize resources and maintain homeostasis.
Figure 2.
Structural and functional organization of the circadian timing system. SCN = suprachiasmatic nucleus. (Copyright – Mayo Foundation).
The endogenous rhythm of the SCN, as well as those of the peripheral clocks, exhibits an oscillatory period slightly different than 24 hours.9 However, in natural conditions the internal clock is entrained to the 24-hour day, primarily by the light-dark cycle. Hence in humans, day is the normal active time during which wakefulness, feeding, activity and stress are anticipated. Night, on the other hand, is normally associated with sleep, fasting, inactivity, and lowering of stress. Importantly, while the cycling in sleep-wakefulness, activity-rest, and fasting-feeding is tightly regulated by the central pacemaker, these behaviors may also act as time givers themselves. Although the molecular and physiological pathways are not completely understood, behavioral cycles may modulate circadian rhythm’s phase, amplitude and period in central and peripheral clocks by timing and resetting rhythmic gene expression, hormone release, metabolites, temperature, and neuronal activity. For instance, physical activity has been shown in humans to phase-shift melatonin onset and alter its circulating levels, reflecting master clock resetting.10 In addition to gating light signals, the sleep/wake schedule influences the secretory pattern of hormones including that of the circadian markers cortisol and of melatonin,11 while direct and indirect projections to the SCN are sent from neuronal circuits controlling arousal and sleep states. Timing and duration of sleep/wake episodes may also regulate circadian oscillations in the periphery, for instance by affecting rhythms of transcripts in the blood transcriptome.12 At the cellular level, metabolites (derived from feeding/fasting behavior) such as glucose, AMP/ATP, NAD/NADH can alter CLOCK/BMAL expression via regulation of ROR and REV-ERB.
The individual circadian characteristics are determined by the interplay between genetic and environmental factors. Inter-individual variations in temporal preferences for routine activities and sleep define the chronotypes, which reflect different phases of entrainment. Morning and evening chronotypes, often referred to as “lark” and “owl” patterns, respectively, are characterized by different peak times in circadian rhythms (i.e., acrophases), with morning chronotypes exhibiting earlier (advanced) acrophases and evening types exhibiting later (delayed) acrophases, as showed by markers of circadian rhythms such as core body temperature, melatonin and cortisol secretion. Accordingly, “larks” are predisposed to earlier wake-up times and bedtimes, and perform at their best in the morning hours. Conversely, “owls”, who manifest a preference for delayed sleep timing, usually getting to bed very late at night and awakening later in the day, are physically and mentally more active towards the evening.
Circadian Control of Body Weight
As with other biological processes, the circadian system is responsible for synchronizing energy homeostasis with the day-night cycle, and thus is critical for control of body weight and for general metabolic health. The SCN orchestrates the circadian control of metabolism both via direct mechanisms, as through melatonin and cortisol release, and indirectly by timing feeding schedule, activity, and sleep, which in turn feed back to the central clock via the pathways mentioned above and depicted in Figure 2.
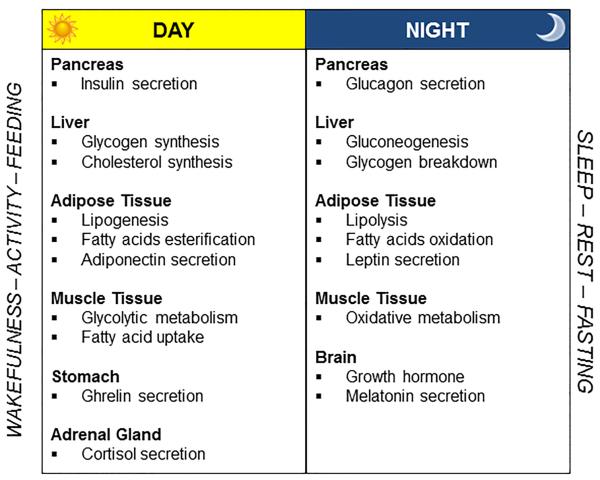
During the daytime, when individuals are active and consume food, glucose is metabolized and fat stored in the adipose tissue (Figure 3). Glycogen synthesis is stimulated by increased insulin secretion, which also fosters lipid synthesis and esterification of fatty acids in adipose tissue, possibly via activation of lipoprotein lipase in adipocytes.13 Appetite regulatory hormones promote eating during daytime hours, as indicated by increased levels of the appetite promoting ghrelin, and decreased leptin, the satiety hormone,14,15 while energy expenditure is elevated.16 Consistent with this oscillatory pattern, gut activity is enhanced during the daytime.
Figure 3.
Day/night pattern of metabolic function. (Copyright – Mayo Foundation).
Fat mobilization is instead prominent at nighttime, resulting in higher levels of circulating fatty acids in the bloodstream. Nocturnal lipolysis is elicited by enhanced levels of growth hormone.17 Additional lipolytic effects are mediated by reduced insulin secretion, which would also cause the liver to convert glycogen into glucose, which is released into the bloodstream. Elevation in glucagon during the night further stimulates gluconeogenesis. Variations in glucose homeostasis in terms of lower glucose tolerance and insulin sensitivity occur at night.18,19 Endocrine regulation of appetite is modulated so as to inhibit eating behavior during the biological night, primarily via elevated leptin.14,15 In addition to physical inactivity, energy expenditure at night is attenuated.20
Although metabolic compounds and functions may manifest fluctuations across the 24-hours, it is important to point out that whether such oscillatory dynamics are directly governed by the master clock or are rather modulated by external cues such as sleep or food intake, remains for the most part controversial. For example, Scheer et al21 demonstrated that diurnal excursions in insulin and leptin are not driven by the central pacemaker but rather by food intake. The influence of fasting-feeding status is consistent with the role of food intake as time giver. The feeding cycle is a powerful zeitgeber for peripheral timekeepers, capable of entraining rhythms of local clocks and clock-controlled genes in tissues and organs such as pancreas, liver, and adipose tissue. Postulated synchronizing mechanisms comprise food metabolites such as glucose and fatty acids, appetite-related hormones such as ghrelin, cellular redox state, and body temperature, and cellular signaling pathways including SIRT1s, PPARs, AMPK,3 underscoring the relevance of mealtime for metabolic regulation/health. In humans, it has been recently shown that fasting/feeding regulates the phase of adipocyte mRNA expression of clock core genes and of energy metabolism genes, such as genes controlling cholesterol biosynthesis and glucose transport.22 Other than timing of food, the macronutrient composition of meals may also modulate expression of both central and peripheral clocks. Switching from a high-carbohydrate, low-fat diet to a low-carbohydrate, high-fat regimen causes phase-delay and increases amplitude of cortisol rhythm (indicative of effects on the central pacemaker) and alters gene expression in blood monocytes, including the PER family and genes implicated in the regulation of energy and fat metabolism, such as SIRT1, ACOX3, and IDH3A.23
Of note, biological oscillations with longer periods synchronous with seasonal changes also exist and are particularly critical for fat metabolism. Fluctuations in human body weight occur across the seasons, with greater fat accumulation, mostly in the abdominal region, achieved during winter months.24,25 Circannual rhythm in body weight results from seasonal variations in physical activity, energy intake, and nutrient composition.25 Such circannual rhythms in humans are a manifestation of residual influences of hibernation and seasonal photoperiodism. In natural settings, daylight duration provides important information regarding seasonal variations. Longer daylight duration (summer-like photoperiod) allows an organism to gather and store excess energy in anticipation of winter months ahead, when food may be scarce and hibernation may occur to preserve energy. In other mammals, these changes in body weight would have an advantage in that they would promote survival. However, in humans in the modern environment of excess food and comfortable shelter throughout the year, this no longer remains an appropriate strategy. In fact, with artificial lengthening of daytime caused by long exposures to lights from electronic devices, these mechanisms may contribute to promoting obesity, by prolonging the time available for gathering and storing energy.
Consequences of Circadian Disruption
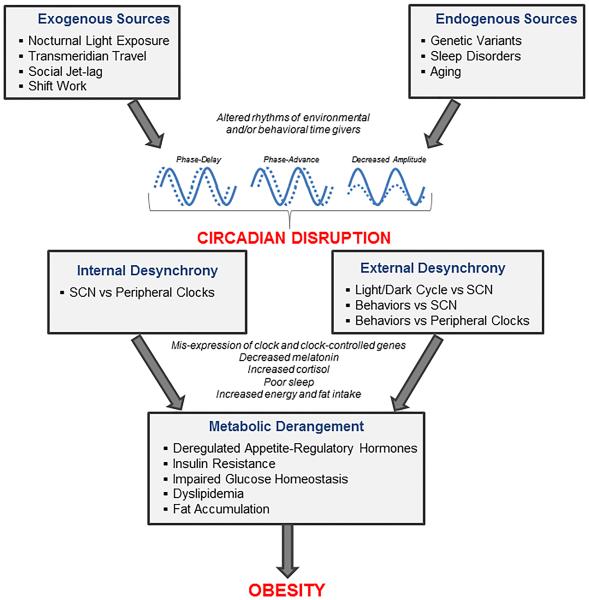
Internal synchronization of physiological activities and behavioral outputs with the external environment is crucial for adequate functioning and ultimately for survival of organisms, including humans. Threats to circadian health may arise from exogenous factors, including nocturnal light exposure, shift work, transmeridian travel, and social jet lag, and from endogenous factors, like genetic variants, aging, and sleep disorders (Figure 4). Challenges to circadian synchrony may compromise well-being and generate substantial health risks, including for obesity.
Figure 4.
Potential mechanistic pathway linking circadian disruption to obesity. (Copyright – Mayo Foundation).
Exogenous Sources of Chronodisruption
Similar to other organisms, human rhythms have been timed according to the natural light-dark cycle for thousands of years. However, in modern society such synchronization is increasingly compromised by the constant environmental illumination, which extends the photoperiod and masks the change between light and darkness.
Extended exposure to artificial light is indeed the primary determinant of circadian disruption. While it was originally thought that only bright light (2,500-10,000 lux) could significantly affect the central pacemaker, later experiments have showed that even lower intensity light (200-500 lux), as is typically used for indoor illumination, can reset the human clock.26 Light exposure induces phase-shift in several physiological and behavioral rhythms, with the magnitude and direction (advance vs delay) of the phase shifts depending upon several factors, including the intensity of light, duration of exposure, biological rhythm being considered, and timing of exposure relative to the phase.27 For instance, exposure to light in the morning advances the phase of the circadian rhythm of melatonin and plasma cortisol, while light exposure in the evening delays onset of melatonin and drop of core body temperature. Nocturnal light exposure has been associated with increased body weight and waist circumference,28 perhaps via delayed onset and total secretion of melatonin. As melatonin is implicated in glucose and lipid metabolism primarily via insulin regulation, its suppression may directly provoke metabolic dysfunction and fat accumulation. However, considering that melatonin is responsible for entraining biological processes via direct communication with the SCN, such effects are most likely a consequence of the circadian desynchronization that melatonin suppression would cause. Circadian entrainment by light cycling is further weakened by the rising popularity of electronic light-emitting devices, which are extensively used in the evening before sleep.29 The light spectrum radiated by these devices is mainly composed of short wavelengths, which are most effective in blunting melatonin onset and delaying circadian phase,27 thus causing delayed bedtime and poor sleep.
In addition to light pollution, in the present “24-hour society”, the night is no longer devoted to rest and sleep. Activities which were traditionally confined to the biological day, such as work and feeding, now take place even at night, provoking a mismatch between behaviors and intrinsic circadian rhythms. Individuals may delay and restrict their sleep window during the week, and then engage in compensatory “catch-up” sleep over the weekends. This phenomenon, termed “social jet-lag”30 because of its similarity with the effects of transmeridian travel, reflects the uncoupling between biological and social clocks, and is thought to favor circadian desynchronization by shifting bedtimes and waking times. Social jet-lag, quantified as the difference between timings of sleep during workdays and free days, is increasingly pervasive, may occur in one- to two-thirds of the population,30 and has gained increasing attention. Emerging evidence also suggests that social jet-lag predisposes to excess body weight and fat mass accumulation that, along with inflammation and glucose dysregulation,30,31 may also favor later development of cardiometabolic disease.
Another form of circadian disruption that can be frequently encountered in industrialized societies is that related to shift work. This occupation schedule, involving approximately 28% of the workforce in the US,32 provokes a misalignment between the rest-activity cycle and the internal circadian rhythms, which remain locked to the external natural light-dark cycle. In spite of the chronic experience of reversed photoperiods, there is little evidence that adaptation may occur. Failure to adapt is presumably due to exposure to diurnal light in the morning during commuting, which, together with inconsistent sleep times, would preclude adaptive resetting of the internal clock by inhibiting the phase-delay shifting required for circadian re-alignment, as indicated by unadjusted melatonin rhythm.33 Due to sustained exposure to circadian mismatch and consequent molecular and physiological perturbations, shift workers may experience significant adverse health effects. Indeed, this work schedule has been linked to a myriad of medical conditions such as cancer, diabetes, and cardiovascular diseases,34 with the evidence for obesity risk being particularly compelling. Cross-sectional and prospective studies identify shift work as an independent predictor of excess body weight,35-37 and indicate that abdominal obesity, the obesity phenotype carrying the greatest risk, is prominent.36 These harmful effects of shift work are conceivably a function of circadian disruption. Due to nocturnal lighting exposure, levels of urinary 6-sulfatoxymelatonin, the main melatonin metabolite, are lower in rotating night shift workers38 than daytime workers. In addition to the disruptive impact of nocturnal light, the temporal re-arrangement of meals/snacking throughout the 24-hour period is thought to negatively affect control of body weight. Shift workers report overall greater caloric intake, with increased fat intake and snack consumption at night.39-41 Energy intake during the biological night, when the circadian clock is set for a fasted state and biological rhythms are timed accordingly, contributes to fat accumulation and metabolic dysfunction. In this respect, there is growing evidence that timing of calorie ingestion is implicated in weight control, with consuming most of the total daily calorie in the evening hours predisposing to obesity and metabolic dysfunction.42,43 Consistently, the response to meals consumed at night in both real and simulated shift workers indicates impaired lipid and glucose tolerance.44 Altered hormonal patterns may underlie appetite dysregulation,40 while elevated cortisol, which favors visceral fat deposition, may play a role in the high prevalence of abdominal obesity.45 Experimental simulations of shift work schedules confirm observational data and provide causative evidence that circadian perturbation promotes metabolic derangement which may ultimately lead to weight gain.46,47 These studies also suggest that, in addition to environmental desynchronization, an internal desynchronization between the SCN and peripheral clocks (such as liver and pancreas), may occur and plausibly be involved in the adverse health effects of shift work, with a primary causal role exerted by the reverse rest-activity cycle. Bearing in mind that peripheral oscillators are more sensitive to entrainment by mealtime than the SCN, it follows that internal dissonance may ensue when the feeding schedule is repeatedly not aligned with the central pacemaker rhythm, in turn presumably contributing to metabolic dysregulation. Although supportive mechanistic evidence in humans is lacking, animal studies corroborate the hypothesis that uncoupling between central and peripheral clocks may arise from reverse feeding, and lead to dyslipidemia and increased adiposity, for instance by phase-shifting expression of several clock-controlled genes critically implicated in hepatic lipogenesis like FAS, HMGCR, and DGAT1.48
Disrupted circadian rhythms not only promote excess body weight, but also hamper weight loss. By considering outcomes in bariatric patients, Ketchum et al49 found that the magnitude of weight loss achieved after gastric bypass surgery was lower in shift workers compared to non-shift workers, suggesting that these patients may need additional postoperative care and education on healthy “timing” habits.
Another candidate mechanism though which circadian misalignment may compromise health involves the sleep schedule. In combination with homeostatic sleep pressure, the circadian system governs timing and duration of sleep primarily via melatonin. During the biological day, when melatonin is low, the circadian arousal drive opposes the sleep propensity. Sleep during the daytime is thus qualitatively and quantitatively deteriorated compared to nocturnal sleep. This explains why daytime sleep, as occurring in shift workers, is often disrupted and curtailed.40 The resulting accumulation of sleep debt conceivably yields additional harmful influences, as discussed below. On the other hand, sleep also modulates the timekeeping system by inhibiting access to light. Hence, inadequate sleep, in terms of sleep fragmentation, sleep disorders, or sleep deficiency, may in turn lead to circadian disruption. Sleep curtailment, usually achieved by delaying bedtime, accompanies unhealthy routines comprising extensive use of electronic media and snacking in the evening, and is increasingly widespread.29 Growing evidence links insufficient sleep to increased morbidity and mortality, as recently reviewed elsewhere.50,51 Sleep deficiency enhances vulnerability to weight gain and obesity, mostly abdominal obesity.52,53 It has been estimated that, for each hour of sleep lost, body mass index may increase by 1.22 kg/m2.54 Observational data also relate inadequate sleep to unhealthy, obesogenic lifestyle habits that may favor fat accumulation.55 Laboratory studies on experimental sleep curtailment, in the setting of ad libitum access to food, provide mechanistic insight on this association, showing that the anticipated energy need related to extended wakefulness is greatly exceeded by a larger calorie intake.16 Rather than being merely due to an increased time opportunity to eat, neuroendocrine responses evoked by sleep loss are thought to contribute to the overeating and weight gain.56 Importantly, experimentally superimposing circadian misalignment upon sleep deprivation exacerbates these adverse metabolic effects.57 Irrespective of sleep duration, other sleep dimensions such as sleep fragmentation and sleep disorders including insomnia and sleep apnea have also been implicated in obesity and metabolic risk, with circadian perturbation as a possible factor in the equation.58
Endogenous Sources of Chronodisruption
Polymorphisms in circadian core genes and related haplotypes have been implicated in risk of obesity. There is evidence suggestive that the CLOCK 3111T/C single-nucleotide polymorphism predisposes to excess body weight and yields resistance to weight loss.59,60 PER2 polymorphisms have been associated with abdominal obesity, which seems the obesity phenotype more closely linked to aberrant circadian rhythms.61 Variants of the CLOCK gene have also been related to calorie intake and nutrient composition, with carriers reporting more calories, fat and carbohydrate consumption.62 Similar associations with dietary regimens have been found in PER3 5/5 genotypes.63
As aforementioned, individual circadian preferences have been associated with distinct risk patterns. Evening chronotypes are more prone to weight gain and fat mass deposition,64,65 along with more frequently reporting poor dietary habits, sedentary lifestyles, and sleep difficulties.64,66 In addition, because individual chronotypes lie in the continuum between morning and evening types, those at the tails of the distribution, who manifest extreme morning or evening chronotypes, may experience a substantial mismatch between their internal circadian rhythms and the light-dark cycle, hence experiencing the consequent detrimental health effects. Accumulating data indicate that individual chronotypes may interact with work schedules so as to modify the health hazards associated with shift work. In this regard, morning types are more vulnerable and less prone to adaptation than evening types.67
Although innate, the circadian timing system undergoes considerable modifications with aging. While circadian evening preference dominates childhood and adolescence, in adulthood a gradual transition towards morning preferences begins, which then become prominent in older age. This phase-advance shift in the elderly is associated with attenuated amplitude of circadian rhythms and deteriorated sleep.68 However, it is unclear whether the elderly are more or less sensitive to the deleterious influences of imposed circadian perturbations, with studies reporting less tolerance for shift work69 but more resilience to jet-lag.70 On the other hand, there is a consistent body of literature indicating that vulnerability to chronodisruption may be greater in children and adolescents. The physiological tendency to evening chronotype, combined with heavy nighttime electronic use, results in later bedtimes and chronic sleep debt.29 This pattern, in conjunction with unhealthy eating behaviors, may ultimately favor weight gain.71,72
Potential Countermeasures
Regardless of its exogenous or endogenous origin, circadian disruption may be susceptible to correction, with mitigation of its adverse metabolic influence. Given that light is the primary synchronizing agent, phototherapy is an effective strategy to reset the circadian pacemaker. Since the clock-resetting function of light is modulated by the timing of exposure relative to the phase of the rhythm, as discussed above, either phase-advance or phase-delay can be evoked, with light exposure in the morning hours causing phase-advance and light exposure in the evening causing phase-delay. While phototherapy originally involved prolonged exposure to continuous bright light (2-8 hours), shorter sessions of intermittent lighting can elicit circadian phase-shifts of similar magnitude.73 Phototherapy has been successfully applied in shift workers to shift circadian rhythms and promote entrainment to the rest-activity/sleep-wake cycles. High-intensity lighting in the work environment during the shifts, in combination with limited exposure to morning light at the end of the shift, and during commuting, and darkness during daytime sleep opportunities, have been proven to favor circadian adaptation.74,75 Regarding shift work, potential health risks may be conceivably reduced by preferentially assigning late night assignments to owl chronotypes, and ensuring that lark chronotypes have schedules that start earlier in the morning.
As a critical component of the circadian machinery, melatonin can also be targeted for resetting the clock. Similarly to photic stimulation, phase-shifting effects vary depending on the biological timing, with administration of exogenous melatonin in the later afternoon/early evening inducing phase-advances and facilitating sleep onset.76 Due to its phase-shifting properties, melatonin is often used to alleviate the consequences of jet lag during transmeridian travel and may also be beneficial for recovering from “social” jet lag or shift-work. In spite of these advantages, it is important to note that melatonin supplementation has been shown to acutely impair glucose tolerance,77 with this effect possibly modified by genetic variants in MTNR1B.78 Therefore, caution should be exercised prior to administration of this hormone in individuals at high-metabolic risk.
Given the link between timing of food intake and obesity/metabolic risk, the feeding schedule can be manipulated to enhance weight control and metabolic health. Support for this concept arises from a weight loss intervention in women with metabolic syndrome, which showed that a high-calorie breakfast compared to a high-calorie dinner resulted in greater weight loss, and improved metabolic profile.79
In spite of the indisputable benefits of increased physical activity in weight management, adequate timing of exercise should be cautiously considered based on its phase-shifting ability. Exercise during the daytime and nighttime advances and delays circadian rhythms, respectively, by modulating melatonin onset.80,81 Exercising at the appropriate circadian time may hence ensure no alteration of circadian rhythms and possibly amplify health benefits.
Treating sleep disorders and thus normalizing sleep pattern may aid in resetting the clock. Sleeping within the individual circadian window can ensure that sleep needs are met and may protect from weight gain. Indeed, short sleepers who extend their sleep time to a healthy duration (7-8 hours), manifest reduced weight and lower fat deposition compared to those maintaining short sleep.82 Also, timing and content of food intake, physical activity, and sleep may be modulated to counteract genetic circadian backgrounds and genetic variants that predispose to obesity.83
Future Perspectives
While the molecular and physiological components of the timekeeping system are relatively well characterized in animals, understanding of this process in humans is limited. Since a thorough delineation of the mechanisms and pathways underlying circadian regulation is essential for elucidating health implications of circadian disruption, laboratory-based, mechanistic investigations in human subjects are needed. The relevance of the circadian pacemaker to physiological regulation is further underscored by its implications for medical therapy. Efficacy and predictability of pharmacological treatment are attenuated by uneven responsiveness to drugs throughout the day, which reflect daily variations in physiological rhythms of targeted biological systems. The potential benefits of chronotherapy are increasingly recognized in the clinical setting, especially for hypertension management.84 Whether timing of administration of pharmacological weight control, such as orlistat, may relate to more effective weight reduction, is currently unknown. Given the individual variability in circadian phases and the health implications, individual chronotypes should be taken into account as well. In the era of individualized and precision medicine, tailored treatment to each person’s internal clock is an intriguing concept that warrants investigation. In this respect, a pioneering attempt to implement a chronotype-adjusted shift schedule in a real work environment may yield considerable benefits, including improved sleep pattern, enhanced wellbeing, and reduced social jet-lag.85 Whether this individualized occupational schedule may also favor weight control and metabolic health has yet to be determined.
Aside from isolated evidence on the benefits of normalizing circadian profile, there is a lack of randomized, controlled studies informing as to whether restoring a healthy circadian pattern is, first, a feasible, efficacious and sustainable intervention, and second, may prevent fat accumulation and perhaps facilitate weight loss and improve cardiometabolic status. Identification of the relevant environmental, behavioral, and biological variables is also of great importance.
Conclusion
Circadian rhythms exert a profound impact on health, by synchronizing activities from the molecular through to behavioral outputs. When the proper function of this highly-controlled circadian machinery, vital for survival, is compromised, health hazards may arise. As circadian disruption is becoming increasingly pervasive in our 24-hour society, understanding the sources and implications of circadian disruption are critical for developing effective strategies to buffer any health consequences.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL114676, R01 HL114024, and R01 HL065176 to V.K.S., and American Heart Association grant 16SDG27250156 to N.C.
Footnotes
Disclosures
The Mayo Foundation has received a gift from the Phillips Respironics Foundation for the study of sleep and cardiovascular disease. V.K.S. served as a consultant for Respicardia, ResMed, Sorin Inc., U-Health, Philips, Dane Garvin, Ronda Grey, and Glaxo Smith Kline and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. The other authors report no conflicts.
References
- 1.Ogden C, Carroll M, Fryar C, Flegal K. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halberg F. Physiologic 24-hour periodicity: general and procedural considerations with reference to the adrenal cycle. Z Vitam Horm Fermentforsch. 1959;10:225–296. [PubMed] [Google Scholar]
- 5.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 6.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes Rev. 2009;10:25–36. doi: 10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 10.Buxton OM, L'Hermite-Baleriaux M, Hirschfeld U, VanCauter E. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12:568–574. doi: 10.1177/074873049701200611. [DOI] [PubMed] [Google Scholar]
- 11.Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One. 2012;7:e30037. doi: 10.1371/journal.pone.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci USA. 2014;111:E682–E691. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arasaradnam MP, Morgan L, Wright J, Gama R. Diurnal variation in lipoprotein lipase activity. Ann Clin Biochem. 2002;39:136–139. doi: 10.1258/0004563021901883. [DOI] [PubMed] [Google Scholar]
- 14.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JOL, Frystyk J. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab. 2005;90:741–746. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- 16.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi Y, Kipnis D, Daughaday W. Growth hormone secretion during sleep. J Clin Invest. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Cauter E, Desir D, Decoster C, Fery F, Balasse EO. Nocturnal decrease in glucose-tolerance during constant glucose-infusion. J Clin Endocrinol Metab. 1989;69:604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 19.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loboda A, Kraft WK, Fine B, et al. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pivovarova O, Jurchott K, Rudovich N, Hornemann S, Ye L, Mockel S, Murahovschi V, Kessler K, Seltmann AC, Maser-Gluth C, Mazuch J, Kruse M, Busjahn A, Kramer A, Pfeiffer AF. Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J Clin Endocrinol Metab. 2015;100:2291–2302. doi: 10.1210/jc.2014-3868. [DOI] [PubMed] [Google Scholar]
- 24.Visscher TLS, Seidell JC. Time trends (1993-1997) and seasonal variation in body mass index and waist circumference in the Netherlands. Int J Obes. 2004;28:1309–1316. doi: 10.1038/sj.ijo.0802761. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Olendzki BC, Li W, Hafner AR, Chiriboga D, Hebert JR, Campbell M, Sarnie M, Ockene IS. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur J Clin Nutr. 2006;60:519–528. doi: 10.1038/sj.ejcn.1602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boivin DB, Czeisler CA. Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport. 1998;9:779–782. doi: 10.1097/00001756-199803300-00002. [DOI] [PubMed] [Google Scholar]
- 27.Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 28.Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98:337–344. doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 29.National Sleep Foundation . 2011 Sleep in America Poll: communications technology in the bedroom. National Sleep Foundation; Washington, DC: 2011. [Google Scholar]
- 30.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes. 2015;39:842–848. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the US: data from the 2010 National Health Interview Survey. Am J Ind Med. 2013;56:647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumont M, Benhaberou-Brun D, Paquet J. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001;16:502–511. doi: 10.1177/074873001129002178. [DOI] [PubMed] [Google Scholar]
- 34.Wang XS, Armstrong MEG, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond) 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity. 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 36.Peplonska B, Bukowska A, Sobala W. Association of rotating night shift work with BMI and abdominal obesity among nurses and midwives. PLoS One. 2015;10:e0133761. doi: 10.1371/journal.pone.0133761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao I, Bogossian F, Turner C. Does maintaining or changing shift types affect BMI? A longitudinal study. J Occup Environ Med. 2012;54:525–531. doi: 10.1097/JOM.0b013e31824e1073. [DOI] [PubMed] [Google Scholar]
- 38.Hansen AM, Garde AH, Hansen J. Diurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiol Int. 2006;23:1203–1215. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 39.de Assis MAA, Kupek E, Nahas MV, Bellisle F. Food intake and circadian rhythms in shift workers with a high workload. Appetite. 2003;40:175–183. doi: 10.1016/s0195-6663(02)00133-2. [DOI] [PubMed] [Google Scholar]
- 40.Schiavo-Cardozo D, Lima MMO, Pareja JC, Geloneze B. Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol. 2013;79:807–811. doi: 10.1111/cen.12114. [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse J, Buckley P, Edwards B, Reilly T. Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol Int. 2003;20:1075–1092. doi: 10.1081/cbi-120025536. [DOI] [PubMed] [Google Scholar]
- 42.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 43.Bandin C, Scheer FAJL, Luque AJ, Avila-Gandia V, Zamora S, Madrid JA, Gomez-Abellan P, Garaulet M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes. 2015;39:828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 44.Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol Int. 2004;21:937–947. doi: 10.1081/cbi-200037171. [DOI] [PubMed] [Google Scholar]
- 45.Manenschijn L, van Kruysbergen RGPM, de Jong FH, Koper JW, van Rossum EFC. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011;96:E1862–E1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 46.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101:1066–1074. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FAJL. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherji A, Kobiita A, Damara M, Misra N, Meziane H, Champy MF, Chambon P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci USA. 2015;112:E6691–E6698. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ketchum ES, Morton JM. Disappointing weight loss among shift workers after laparoscopic gastric bypass surgery. Obes Surg. 2007;17:581–584. doi: 10.1007/s11695-007-9100-8. [DOI] [PubMed] [Google Scholar]
- 50.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11:81–89. doi: 10.1016/j.jsmc.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endo. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 52.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES, Li CY, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity. 2014;22:598–607. doi: 10.1002/oby.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Appelhans BM, Janssen I, Cursio JF, Matthews KA, Hall M, Gold EB, Burns JW, Kravitz HM. Sleep duration and weight change in midlife women: the SWAN Sleep Study. Obesity. 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep. 2010;33:753–757. doi: 10.1093/sleep/33.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 57.Leproult R, Holmback U, Van Canter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Depner CM, Stothard ER, Wright KP., Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:1–9. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, Maj M. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Garaulet M, Corbalan MD, Madrid JA, Morales E, Baraza JC, Lee YC, Ordovas JM. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes. 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garaulet M, Corbalan-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, Ordovas JM. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110:917–921. doi: 10.1016/j.jada.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garaulet M, Esteban Tardido A, Lee YC, Smith CE, Parnell LD, Ordovas JM. SIRT1 and CLOCK 3111T> C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int J Obes. 2012;36:1436–1441. doi: 10.1038/ijo.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bienertova-Vasku J, Novak J, Zlamal F, Lipkova J, Stastny J, Forejt M, Jackowska A, Vasku A. The PER3 VNTR polymorphism is a predictor of dietary composition in the Central European population. Biol Rhythm Res. 2014;45:747–757. [Google Scholar]
- 64.Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, Yoo HJ, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Shin C, Kim NH. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100:1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 65.Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol Int. 2013;30:682–690. doi: 10.3109/07420528.2013.782311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen H, Broms U, Mannisto S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29:920–927. doi: 10.3109/07420528.2012.699128. [DOI] [PubMed] [Google Scholar]
- 67.Nagashima S, Masutani E, Wakamura T. Food intake behavior and chronotype of Japanese nurses working irregular shifts. Int J Psychol Stud. 2014;6:107–116. [Google Scholar]
- 68.Duffy JF, Zitting KM, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin. 2015;10:423–434. doi: 10.1016/j.jsmc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koller M. Health risks related to shift work - an example of time-contingent effects of long-term stress. Int Arch Occup Environ Health. 1983;53:59–75. doi: 10.1007/BF00406178. [DOI] [PubMed] [Google Scholar]
- 70.Waterhouse J, Edwards B, Nevill A, Carvalho S, Atkinson G, Buckley P, Reilly T, Godfrey R, Ramsay R. Identifying some determinants of "jet lag," and its symptoms: a study of athletes and other travellers. Brit J Sport Med. 2002;36:54–60. doi: 10.1136/bjsm.36.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berkey CS, Rockett HRH, Colditz GA. Weight gain in older adolescent females: the internet, sleep, coffee, and alcohol. J Pediatr. 2008;153:635–639. doi: 10.1016/j.jpeds.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kjeldsen JS, Hjorth MF, Andersen R, Michaelsen KF, Tetens I, Astrup A, Chaput JP, Sjodin A. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes. 2014;38:32–39. doi: 10.1038/ijo.2013.147. [DOI] [PubMed] [Google Scholar]
- 73.Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 74.James FO, Walker CD, Boivin DB. Controlled exposure to light and darkness realigns the salivary cortisol rhythm in night shift workers. Chronobiol Int. 2004;21:961–972. doi: 10.1081/cbi-200035944. [DOI] [PubMed] [Google Scholar]
- 75.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J Physiol Endocrinol Metab. 2001;281:E384–E391. doi: 10.1152/ajpendo.2001.281.2.E384. [DOI] [PubMed] [Google Scholar]
- 76.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 77.Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–1719. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garaulet M, Gomez-Abellan P, Rubio-Sastre P, Madrid JA, Saxena R, Scheer FA. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism. 2015;64:1650–1657. doi: 10.1016/j.metabol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21:2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 80.Miyazaki T, Hashimoto S, Masubuchi S, Honma S, Honma KI. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am J Physiol Regul Integr Comp Physiol. 2001;281:R197–205. doi: 10.1152/ajpregu.2001.281.1.R197. [DOI] [PubMed] [Google Scholar]
- 81.Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 82.Chaput JP, Despres JP, Bouchard C, Tremblay A. Longer sleep duration associates with lower adiposity gain in adult short sleepers. Int J Obes. 2012;36:752–756. doi: 10.1038/ijo.2011.110. [DOI] [PubMed] [Google Scholar]
- 83.Dashti HS, Follis JL, Smith CE, et al. Gene-environment interactions of circadian-related genes for cardiometabolic traits. Diabetes Care. 2015;38:1456–1466. doi: 10.2337/dc14-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hermida RC, Ayala DE, Fernández JR, Portaluppi F, Fabbian F, Smolensky MH. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 2011;24:383–391. doi: 10.1038/ajh.2010.217. [DOI] [PubMed] [Google Scholar]
- 85.Vetter C, Fischer D, Matera JL, Roenneberg T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. 2015;25:907–911. doi: 10.1016/j.cub.2015.01.064. [DOI] [PubMed] [Google Scholar]