Abstract
The human head louse, Pediculus humanus capitis, is subdivided into several significantly divergent mitochondrial haplogroups, each with particular geographical distributions. Historically, they are among the oldest human parasites, representing an excellent marker for tracking older events in human evolutionary history. In this study, ancient DNA analysis using real-time polymerase chain reaction (qPCR), combined with conventional PCR, was applied to the remains of twenty-four ancient head lice and their eggs from the Roman period which were recovered from Israel. The lice and eggs were found in three combs, one of which was recovered from archaeological excavations in the Hatzeva area of the Judean desert, and two of which found in Moa, in the Arava region, close to the Dead Sea. Results show that the head lice remains dating approximately to 2,000 years old have a cytb haplogroup A, which is worldwide in distribution, and haplogroup B, which has thus far only been found in contemporary lice from America, Europe, Australia and, most recently, Africa. More specifically, this haplogroup B has a B36 haplotype, the most common among B haplogroups, and has been present in America for at least 4,000 years. The present findings confirm that clade B lice existed, at least in the Middle East, prior to contacts between Native Americans and Europeans. These results support a Middle Eastern origin for clade B followed by its introduction into the New World with the early peoples. Lastly, the presence of Acinetobacter baumannii DNA was demonstrated by qPCR and sequencing in four head lice remains belonging to clade A.
Introduction
The human louse, Pediculus humanus, is an obligatory haematophagous parasite that thrived exclusively on human blood for at least 5–7 million years ago [1, 2]. This species includes two ecotypes: the head louse (Pediculus humanus capitis De Geer) that lives and multiplies on the scalp, and the body louse (Pediculus humanus humanus Linnaeus), that lives and oviposits on clothes [3, 4, 5].
Until recently, only the body louse was assumed to act as a vector for at least three serious human diseases, namely epidemic typhus, trench fever and louse-borne relapsing fever caused by Rickettsia prowazekii, Bartonella quintana, and Borrelia recurrentis, respectively [6]. Body lice have also been shown to be able to host and possibly transmit the agent of plague, Yersinia pestis [5, 7]. Though head lice have been found in nature to carry the DNA of Bartonella quintana, Borrelia recurrentis, Acinetobacter baumannii and Yersinia pestis [5, 8, 9, 10, 11, 12, 13], and experimental infections have shown that these lice can also act as a vector of louse-borne diseases [14, 15], their epidemiological significance is still debated.
Mitochondrial DNA (mtDNA) has been widely used to study the genetic diversity of human lice, revealing the presence of several deeply divergent mtDNA clades or haplogroups named A, B and C [1, 2, 16, 17, 18]. Haplogroup A is the most common. It can be found throughout the world and includes both head and body lice [1, 2, 17, 18]. Clade B comprises only head lice. It is confined to the New Word, Europe and Australia and was recently reported from North and South Africa [2, 18, 19, 20]. Clade C, which only includes head lice, has been found in Ethiopia, Nepal and Thailand [13, 16]. Most recently, two additional novel clades were described in 2015 by Drali et al. and Ashfaq et al. [5, 20] besides the three classical recognized clades. The first novel clade is the clade D described by Drali et al. [5] and is referred as clade E in Ashfaq et al. [20]. This clade (clade D sensu Drali et al.) is the sister group of clade A and consist on lice from Ethiopia and Democratic Republic of the Congo and comprising both head and body lice [5]. The second novel clade is described only by Ashfaq et al. [20]. This clade is the sister group of clade C and consist on lice from Senegal and Mali, referred here as clade “E”. This clade comprises only head lice.
Pediculus humanus is probably one of the oldest and most intimate human parasites [21, 22] and is known to have a long-term co-evolutionary association with humans over millions of years [1]. As such it represents an excellent marker for tracking older events in human evolutionary history [4, 18].
Louse infestations are mentioned in the Bible as the third plague [21]. Lice have also been found in a variety of archaeological contexts around the word [21, 22, 23, 24]. These reports all indicate the long-time presence of lice throughout the world before the globalization initiated during Columbus’s era, as the result of early human migrants out of Africa [4]. For example, in the Old World, the earliest record of head lice goes back to the Neolithic age, roughly 9,000 years ago, obtained from an individual who lived in the Nahal Hemar cave in Israel [21]. In the New World, the oldest such record of head lice comes from an archaic human skeleton in Brazil, dated at more than 10,000 years old [22].
However, few molecular data on ancient lice are available. Indeed, ancient DNA offers a direct means to assess the past genetic structure and diversity of human lice, which can provide relevant information relating to the antiquity of migration patterns of humans, their lice and, therefore, the flow of louse-borne pathogens [25].
Thus, based on molecular analyses of head lice from Peruvian mummies, D. Raoult et al. showed that the worldwide clade A had a pre-Columbian presence in the American continent and likely had links to the Old World [17]. In 2013, A. Boutellis et al. confirmed this result and demonstrated that Clade B was also present in America for more than 4,000 years, prior to contact with Europe, suggesting an American origin for this haplogroup, followed by its dispersal to the Old World from America beginning in the 16th century [26]. Despite this finding, the precise source of this haplogroup prior to globalization remains unclear. However, other studies have suggested that could originate from Asia, which is reported to have populated the Americas [1, 18].
The analysis of ancient head louse eggs recovered from Israel dating from the Chalcolithic and early Islamic period, showed that these eggs may have belonged to people originating from West Africa, based on identification of the louse mitochondrial sub-clade C specific to that region [25].
In the present study, ancient DNA analysis of head lice remains dating from Roman period recovered from Israel was undertaken in order to: (1) identify their mitochondrial phylotypes, (2) reveal the phylogenetic relationship between ancient and contemporary human lice, and (3) look for louse-borne pathogens in these remains.
Materials and Methods
Ancient Head Lice Remains
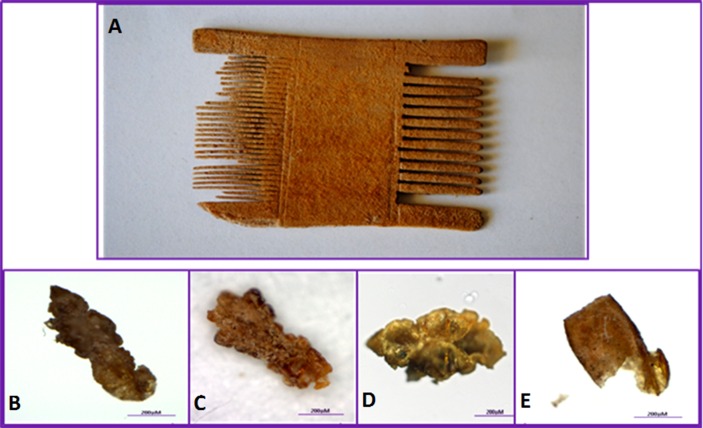
Head lice and their eggs were isolated from three louse combs from the Roman period (1st century AD to 6th century BC). Comb A and Comb B were recovered from archaeological excavations in Moa, in the Arava region, close to the Dead Sea, while Comb C was found in the Hatzeva area of the Judean desert (between Jericho and Dead Sea). In Moa, the excavations were conducted between 1981 and 1985 (permit no. A-1016/1981). The site appears to be a way station on the Spice Route connecting Petra to Gaza, where the remains of a caravanserai, a fortress and a temple were found. The excavation site in Hatzeva was a fortified road station from the Nabatean period in an agricultural settlement from the Early Arabic, late Roman period. The three combs were two-sided (Fig 1). From comb A, six lice parts and four eggs were isolated, from comb B, one entire specimen of male and nymph, respectively, eight lice parts and three eggs while from comb C only one entire nymph was isolated. All of the lice remains were preserved dry under sterile conditions. Morphological examination revealed that most of the eggs were embryonated. All examined combs are deposited in the Antiquities Authority in Jerusalem, Israel. Permission has been obtained from the Antiquities Authority to examine the combs and publish the results. All necessary permits were obtained for the described study, which complied with all relevant regulations.
Fig 1.
Recovery of ancient human head lice from a two-sided louse comb belonging to the Roman period (A) recovered from the Judean desert and Arava regions of Israel. In the lower part, entire specimens (B and C), the head and thorax of a head louse (D) and a damaged non-operculated egg (E) can be seen.
Ancient DNA Analysis
DNA extraction
Lice and eggs were rinsed twice in distilled water for 15 minutes and then crushed individually in sterile Eppendorf tubes. Two extractions blank were systematically co-extracted with the ancient lice samples during each extraction session. No more than five ancient samples were co-extracted at the same time. Finally, the total-DNA was extracted from seventeen head lice parts and seven nit/egg-parts using a phenol/chloroform/isoamyl protocol [27]. The extracted genomic DNA concentrations varied between 1.2 to 1.7 ng/ml for seven nit/egg-parts and between 1.4 to 2.4 ng/ml for seventeen head lice parts.
Identification of lice DNA and determination of lice clade
In order to decrease the possibility of contamination and false-positives, two sensitive sets of primers and probes for real-time quantitative polymerase chain reaction (qPCRs) were specifically designed for this study. Both qPCRs were designed in order to amplify all known clades of P. humanus, targeted 88-bp and 100-bp fragments of the cytochrome b gene (cytb) and the 12S ribosomal RNA (12S RNA), respectively. The design was performed with Primer3 software, version 4.0 (http://frodo.wi.mit.edu/primer3/), according to procedures described elsewhere [28]. The oligonucleotide sequences of the primers and probes were as follows: CytbF1 (5'- AGTGCTATTCCTRTTRTTGG-3'), CytbR1 (5'-AAYARYCGCTCTAAAGTAGG-3') and TaqMan probe (FAM-TGAGGAGGGTTTTCAGT- MGB) for the cytb gene, 12SF1 (5'- ATCTTACCTTTTAACTTTTGCT-3'), 12SR1 (5'- GCGTCTTGACTTGTACRTTA-3') and TaqMan probe (FAM- CTGGCACGTCGCTGTACTAA—MGB) for the 12S ARN gene.
PCR amplification was performed using a CFX96 Thermal Cycler (Bio-Rad Laboratories, Foster City, CA, USA) in a 20 μl reaction volume containing 5 μL of the DNA template, 10 μL of Eurogentec™ Probe PCR Master Mix (Eurogentec, Liège, Belgium), 0.5 μM of each primer and 0.5 μM of the FAM-labeled probe. The thermal cycling conditions included one incubation step at 50°C for two minutes and an initial denaturation step at 95°C for three minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and annealing extension at 60°C for 30 seconds.
At first, all the samples were screened in both qPCRs. Thereafter all those which were positive by at least one qPCR were subjected to conventional PCR targeting the 272 bps portion of cytb gene as described by Boutellis et al. [19].
All the products of the PCR amplification were checked on gel electrophoresis and then purified using NucleoFast 96 PCR plates (Macherey-Nagel EURL, Hoerdt, France) according to the manufacturer’s instructions. All products of both qPCRs and conventional PCR were sequenced with original primers on an ABI automated sequencer (Applied Biosystems, USA) with the BigDye Terminator v1.1 cycle (Applied Biosystems, Foster City, CA). The electropherograms obtained were assembled and corrected using ChromasPro software (Technelysium Pty, Queensland, Australia).
Detection of pathogens
To test for the presence of pathogens, qPCRs were performed using previously reported primers and probes targeting the 16S rRNA gene of Borrelia [29], the ITS intergenic spacer of Bartonella [8], the rpoB gene of Acinetobacter [9], the ompB gene of Rickettsia prowazekii and the pla gene of Yersinia pestis [27]. All qPCRs were performed using a CFX96 TMREAL-Time System C1000 Thermal Cycler (Bio-Rad Laboratories) and the Eurogentec Master Mix Probe PCR kit (Eurogentec).
Prevention of DNA contamination
In order to ensure that no contamination by modern DNA would interfere with the results, all pre-PCR and post-PCR procedures were performed respectively in a separate, clean room, free of louse DNA under a hood with air-capture, using autoclaved and UV treated material. Extractions and PCR amplification blanks were used as negative controls, in order to detect possible contamination by external DNA. No positive control was included in any experimental steps in order to minimize potential contamination. No amplifications were detected among the negative controls throughout the study.
Data Analysis
MEGA 6.06 was used for phylogenetic analyses and tree reconstruction with 500 bootstrap replicates using the maximum likelihood method under Kimura’s 2-parameter with complete deletion [30]
For comparison, the ancient DNA sequences obtained in this study were combined with the 30 cytb haplotypes reported by Drali et al. [31]. This dataset was complemented with newly available sequences in GenBank, after being assigned to haplotypes using DnaSP v5.10 [32]. Ancient lice sequences from 10,000 and 4,000 year-old Peruvian and Chilean mummies, respectively, were also included [17, 26]. Finally, a dataset that consisted of 49 haplotypes was created in which two are shared between contemporary and ancient lice. These haplotypes span 40 geographic locations (countries) on five continents (S1 Table). The novel haplotypes identified were deposited in GenBank under the following accession numbers: KX249763-KX249775.
In order to investigate the possible relationships among haplotypes, the median-joining (MJ) network using the Bandelt method was constructed using the NETWORK4.6 program (www.fluxus-engineering.com/sharenet.htm) [33].
For A. baumannii, the nucleotide sequences obtained in this study were aligned with the reference sequences available in public databases (GenBank) and a phylogenetic tree was also constructed using the maximum likelihood method under Kimura’s 2-parameter model implemented in MEGA 6.0 6, with 500 bootstrap replicates [30].
Results
In this study, we isolated DNA from twenty-four head lice parts and their eggs, P. h. capitis, from Roman-era remains retrieved in Israel. A 110-bp DNA fragment of the 12S RNA gene (qPCR) and 85-bp (qPCR) and 270-bp (conventional PCR) DNA fragments of cytb gene were targeted.
Detection of Lice DNA in Ancient Lice Samples
The qPCR targeting an 85-bp fragment of cytb gene was positive for all the 24 ancient DNA lice tested (32<Ct<35), while only 22 samples of the 24 tested were positive for a 110-bp fragment of the 12S RNA gene (33<Ct<36) (S1 Fig).
Determination of Lice Clade
To determine lice clade, all samples which were positive for at least one qPCR (24/24) were subjected to conventional PCR targeting a 272-bp fragment of the cytb gene coupled with sequencing (S2 Fig). To insure the reproducibility of the results, we also sequenced the products of both qPCRs, since the two shorts fragments amplified by both qPCRs can discriminate between all known clades of lice. Thus, at least one sequence per sample was successfully recovered from all the samples (24/24).
In comparison with previously well-defined lice mtDNA haplogroups A, C [16], B [1], D (sensu Drali et al.) [5] and E [20], all ten samples recovered from comb A belonged to mitochondrial clade B (41.6%). All lice samples from combs B (thirteen samples) and C (one sample) belonged to clade A (58.3%) (Table 1).
Table 1. Summary of ancient samples, DNA analyses and haplotypes assignment.
| Louse number (Lab code) | Part of lice amplified | PCR results | Haplogroup identity | Haplotype identity on the basis of partial cytb 270-bp | ||
|---|---|---|---|---|---|---|
| 12S (110-bp) | Cytb (80-bp) | Cytb (270-bp) | ||||
| Comb A | ||||||
| Romanic-HL1 | thorax /abdomen | + | + | + | B | Hap_B36 |
| Romanic-HL2 | thorax /abdomen | + | + | + | B | Hap_B36 |
| Romanic-HL3 | Thorax | + | + | + | B | Hap_B36 |
| Romanic-HL4 | Abdomen | + | + | + | B | Hap_B36 |
| Romanic-HL5 | Abdomen | + | + | + | B | Hap_B36 |
| Romanic-HL6 | Abdomen | + | + | + | B | Hap_B36 |
| Romanic-HN7 | non operculated egg | + | + | + | B | Hap_B36 |
| Romanic-HN8 | non operculated egg | + | + | + | B | Hap_B36 |
| Romanic-HN9 | operculated egg | NA | + | NA | B* | — |
| Romanic-HN10 | operculated egg | + | + | NA | B* | — |
| Comb B | ||||||
| Romanic-HL11 | entire male | + | + | + | A | Hap_A5 |
| Romanic-HL12 | entire nymph | + | + | + | A | Hap_A55 |
| Romanic-HL13 | leg/thorax/abdomen | + | + | + | A | Hap_A55 |
| Romanic-HL14 | thorax /abdomen | + | + | + | A | Hap_A5 |
| Romanic-HL15 | thorax /abdomen | + | + | + | A | Hap_A5 |
| Romanic-HL16 | thorax /abdomen | + | + | + | A | Hap_A5 |
| Romanic-HL17 | Thorax | + | + | + | A | Hap_A55 |
| Romanic-HL18 | Thorax | + | + | + | A | Hap_A5 |
| Romanic-HL19 | abdomen | + | + | + | A | Hap_A55 |
| Romanic-HL20 | abdomen | + | + | + | A | Hap_A5 |
| Romanic-HN21 | operculated egg | NA | + | NA | A* | — |
| Romanic-HN22 | non-operculated egg | + | + | + | A | Hap_A5 |
| Romanic-HN23 | non-operculated egg | + | + | NA | A* | — |
| Comb C | ||||||
| Romanic-HL24 | entire nymph | + | + | + | A | Hap_A56 |
| Total | 24 | 22/24 | 24/24 | 20/24 | 14(A)+10(B)/24 | 7(A5)+4(A55)+ 1(A56)+8(B36)/24 |
NA: not amplified
*- clades identified on the base of cytb qPCR product sequencing
Phylogenetic Analysis and Haplotype Assignment
For the phylogenetic analysis we used the maximum likelihood method (ML) (Fig 2) and a median-joining (MJ) network (Fig 3) analysis. Only the 272-bp sequences of the cytb gene (20/24) were analyzed and combined with all known modern and ancient haplotypes generated in our dataset.
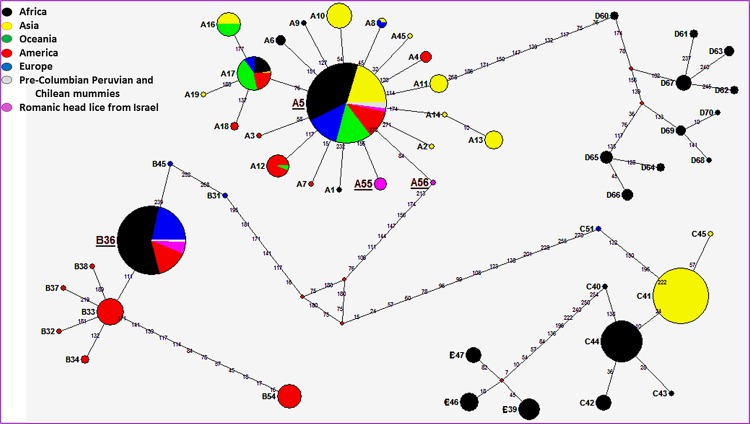
Fig 2. Cytb haplotype networks of contemporary and ancient human body and head lice.
Each circle area indicates a unique haplotype and variations in circle size are proportional to haplotype frequencies. Pie colors and sizes in circles represent the continents and the number of their sequence for a haplotype. The length of the links between nodes is proportional to mutational differences.
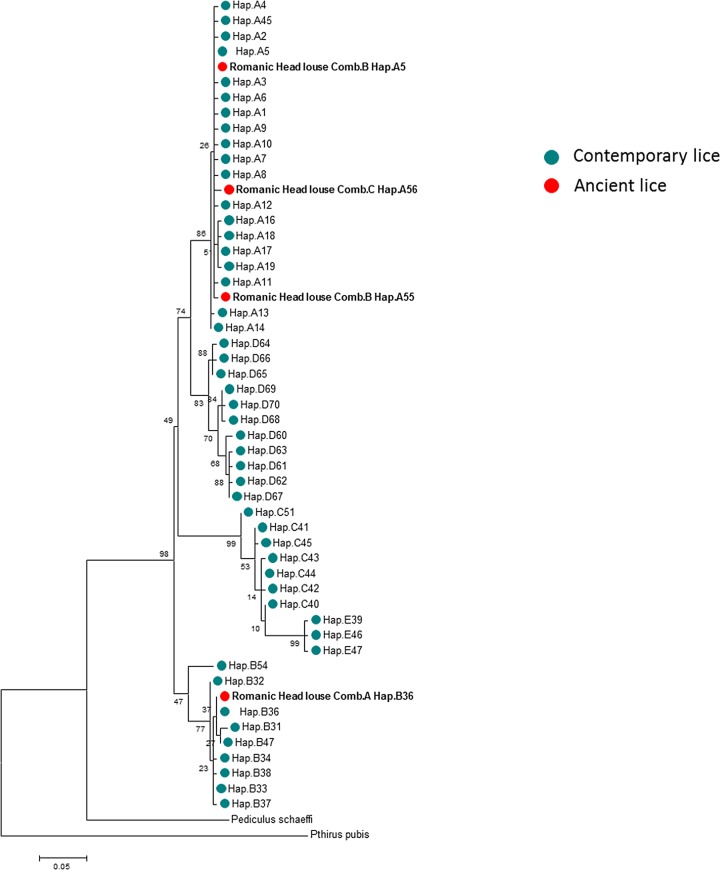
Fig 3. Maximum-likelihood (ML) phylogram of contemporary and ancient haplotypes of Pediculus humanus based on the partial 272-bp cytb gene with Pediculus schaeffi (KC241883) and Pthirus pubis (EU219990) as outgroups.
Within clade A (12/20), seven sequences (all from comb B) of these ancient head lice belonged to the widespread modern haplotype A5. This haplotype is the most prevalent worldwide (75% of locations and 45% of the 985 analyzed human lice) and is present in all continents. It occupies a central position in haplogroup A, as shown in the MJ network, and is also found in pre-Columbian Peruvian and Chilean mummies from the New World. The five remaining clade A sequences, four from comb B and one from comb C, yielded two novel haplotypes which were found to be unique, provisionally named here as A55 and A56, respectively. A thorough inspection of the haplotype network revealed that the A55 haplotype deviated from haplotype A5 by one mutation step while the A56 haplotype derived on haplotype A5 by two mutations steps. Nevertheless, the mutations that we found in both haplotypes A55 and A56 are synonymous, i.e. resulting in no amino acid changes, compared to the A5 haplotype. The sequences of these two novel haplotypes were deposited in GenBank under accession number: KX232678-KX232679.
Within the clade B ancient head lice, all eight sequences (all from comb A) belonged to the modern haplotype B36, which is the most common within the B-haplogroup (74% of the 184 analyzed haplogroup B human lice). This haplotype occupied a central position in the MJ network, and is found in Africa, Europe and America, as well as in pre-Columbian Chilean mummies.
Detection of Pathogens
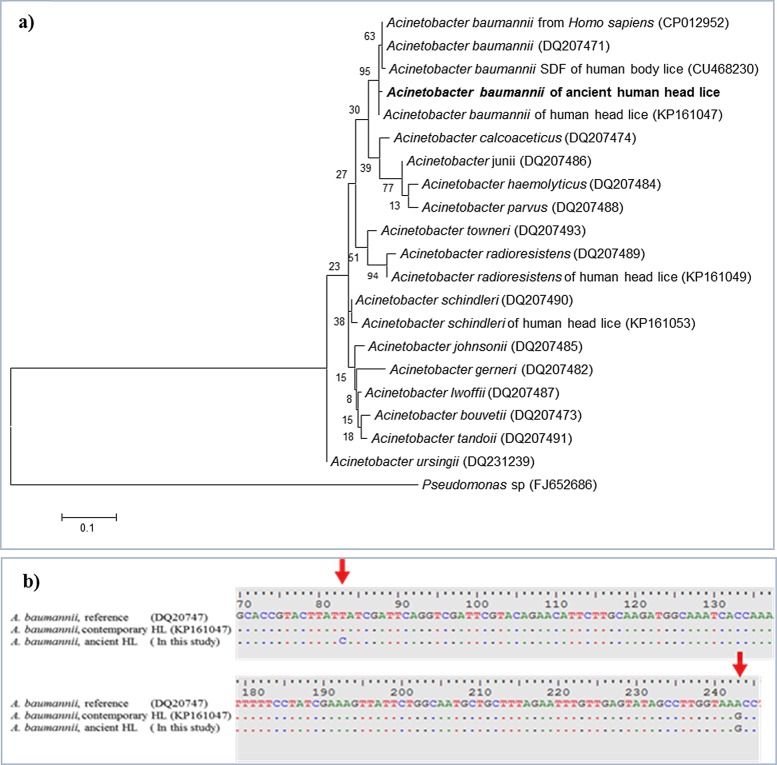
The qPCR targeting the rpoB gene of Acinetobacter was positive for four samples (4/24) of ancient lice DNA tested (34<Ct<36) from comb B. The qPCR products generated were directly sequenced. The four obtained sequences of the partial rpoB gene (182-bp) were 100% identical to one another and were identified as A. baumannii on the basis of a BLAST search. These sequences had 180 of 182 base positions in common (98.9% identity) with a reference strain of A. baumannii (GenBank accession number CP012952) and 181 of 182 base positions in common (99.4% similarity) with A. baumannii isolated from modern human head lice collected from elementary school children in Thailand (GenBank accession number KP161047). However, these ancient sequences have one mutation (position 81) that has not previously been described in modern A. baumannii (Fig 4B). The phylogenetic tree of this A. baumannii is shown in Fig 4A. All four A. baumannii positive ancient head lice belonged to clade A.
Fig 4. Acinetobacter baumannii from ancient head lice belonging to the Roman period.
a, Maximum-likelihood (ML) phylogenetic tree relationship based on 182-bp fragment rpoB gene of A. baumannii detected in ancient head lice was compared with the reference sequences strain, while Pseudomonas was used as an out group. Bootstrap values are indicated at the nodes. Bold indicates the taxonomic position of A. baumannii identified in this study. b, 182-bp of A. baumannii rpoB gene fragment sequenced from the four ancient head lice, exhibiting one mutation not present in the homologous gene sequence from its closest relative, the modern A. baumannii sequence in GenBank”.
Borrelia spp., Bartonella spp., Rickettsia prowazekii and Y. pestis DNA could not be identified in any of the 24 ancient head lice specimens tested.
Discussion
A paramount concern in studying ancient DNA is the prevention of its contamination by modern DNA [34]. Several elements support the authenticity of the results presented herein: qPCRs were designed specifically for this study, each experimental step was performed in separate rooms, free of lice and their DNA and no positive controls were used in the PCR examinations as recommended elsewhere [34]. Some of the sequences obtained were unique and presented mutations which have never previously been reported, lending greater confidence to the authenticity of the present results [34].
The mtDNA analysis in this study revealed that the head lice remains from approximately 2,000 years ago have cytb haplogroups A and B. To the best of our knowledge, the present study is the first to reveal the presence of a mitochondrial haplogroup B in a Middle Eastern region. It has thus far only been found in contemporary lice from America, Europe, Australia and, more recently, Africa (Algeria and South Africa) [2, 18, 19, 20]. Specifically, this haplogroup B has a B36 haplotype, the most common among B haplogroups [31]. Interestingly, this haplotype was also present for at least 4,000 years in South America before the arrival of European settlers [26].
Accordingly, the present findings show that clade B lice existed in the Mediterranean region long before the discovery of America, meaning that clade B lice did not originate from America as was previously thought but probably existed, at least in the Middle East, prior to contact between Native Americans and Europeans. These results are consistent with the hypothesis suggesting that this clade could originate from Asia, which is reported to have populated the Americas [1, 18].
The remaining samples had haplogroup A and yielded three haplotypes, in which two haplotypes (provisionally called A55 and A56 in this paper) were unique to the ancient head lice examined in this study, while haplotype A5, which is common worldwide, including in ancient head lice from Chilean and Peruvian mummies of the New World [17, 26, 31].
Prior research has suggested that the known lice clades evolved on different lineages of Homo, similarly to those known to date from 2.3 to 0.03 million years ago (MYA) [1, 20] and, accordingly, their geographic distribution can provide information regarding the evolutionary history of the lice as well as their human hosts [2]. Clade A lice most likely emerged in Africa and evolved on the host lineage that led to anatomically modern humans (Homo sapiens), showing signs of a recent demographic expansion out of Africa about 100,000 years ago, first to Eurasia and subsequently to Europe, Asia and the New World [1, 18]. Haplogroup B diverged from haplogroup A between 0.7 and 1.2 million years ago and may have evolved on archaic hominids, such as the Homo neanderthalensis, who expanded in Europe and Asia, and only became associated with modern humans during the period of overlap as the result of a recent host switch [1, 3, 4]. Thus, considering the present-day geographic distribution of the two haplotypes A5 and B36 and evidence of their long-time presence in the New World, along with our data from the Middle East, the most likely theory is that these two haplotypes were carried by early humans and migrated with them throughout the world before the globalization initiated during the time of Columbus. It seems that the B36 haplotype was originally present in archaic populations of the Middle East, and because this region was a passageway for Homo sapiens between Africa and the rest of the world [35], this haplotype could have switched to anatomically modern humans when they arrived, and migrated with them along with lice of haplotype A5 throughout the world including to America, where lice remained in situ for thousands of years until the second contact with European colonists in the 16th century [2].
Aside from their role as biological markers used to infer human evolutionary history, lice remains can provide information relating to past human sanitary conditions and diseases, because these lice are vectors of bacterial pathogens. Yet, genetic traces from pathogens can be identified in archaeological remains and ancient DNA has successfully been used to identify B. quintana in 4,000-year-old teeth [36] and was reported to occur in lice at the end of World War I [37]. Raoult et al. [34] showed that Napoleon’s soldiers in Vilnius were exposed to body lice containing B. quintana and that soldiers had evidence of infection with either R. prowazekii or B. quintana, concluding that louse-borne infectious diseases affected nearly one-third of Napoleon’s soldiers buried in Vilnius, and indicating that these diseases might have been a major factor in the French retreat from Russia.
This study reveals for the first time the presence of A. baumannii DNA in ancient human head lice remains belonging to clade A. Specifically, the rpoB sequence that was found has not been reported previously.
In recent years, A. baumannii was first isolated from the body lice of homeless people in Marseille (France) as well as from diverse countries worldwide [38]. A. baumannii DNA was also found in head lice collected from elementary school children in Paris belonging to clade A [9] and detected in body and head lice collected from healthy individuals from Ethiopia [10]. In 2015, S. Sunantaraporn et al. showed that A. baumannii could be detected in clade A and C head lice collected from elementary school children in Thailand [13].
Although A. baumannii was reported to occur in temporary head lice in several occasions, the clinical significance of this finding is unknown [9, 39]. More recently, A. baumannii was shown to cause nosocomial infections and severe community-acquired infections such as pneumonia, bacteremia, endocarditis, and meningitis, due to its increasing resistance to a wide range of antibacterial agents [40, 41].
Conclusion
The present work confirms that clade B lice existed, at least in the Middle East, prior to contacts between Native Americans and Europeans. Our results cannot support the previous hypothesis that clade B has an American origin and was imported from America to the Old World after its discovery by Christopher Columbus. We also identified the presence of nosocomial pathogen, A. baumannii in Roman-era head lice remains belonging to clade A.
Further study of the lice remains would be necessary to shed more light on the patterns of human migration worldwide, their lice and the flow of louse-borne pathogens at different times in history.
Supporting Information
1, 2 and 3 showed qPCR amplification targeted a 88-bp DNA fragment of cytb gene (24/24 positive with Ct varied between 32 to 38); 4 showed qPCR amplification targeted a 100-bp DNA fragment of 12S gene (22/24 positive with Ct varied between 32 to 38).
(PPTX)
(PPTX)
Haplotypes highlighted in blue are the newly identified haplotypes from sequences available in GenBank.
(XLSX)
Acknowledgments
We would like to thank Dr. Orit Shamir and Dr. Naama Sukenik from the Israel Antiquities Authority, as well as Professor Simcha Lev-Yadun from Haifa University, for their help in identifying and allowing us to examine the ancient louse combs for louse remains. We also thank IHU Méditerranée Infection for financially supporting the study.
Data Availability
All sequences of cytb haplotypes are available in GenBank under accession numbers KX232678-KX232679 and KX249763-KX249775.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Reed DL, Smith VS, Hammond SL, Rogers AR, Clayton DH. Genetic analysis of lice supports direct contact between modern and archaic humans. PLoS Biol 2004; 2:e340 10.1371/journal.pbio.0020340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Light JE, Allen JM, Long LM, Carter TE, Barrow L, Suren G, et al. Geographic distributions and origins of human head lice (Pediculus humanus capitis) based on mitochondrial data. J Parasitol 2008; 94:1275–1281. GE-1618[pii]; 10.1645/GE-1618.1 [DOI] [PubMed] [Google Scholar]
- 3.Ascunce MS, Toups MA, Kassu G, Fane J, Scholl K, Reed DL. Nuclear genetic diversity in human lice (Pediculus humanus) reveals continental differences and high inbreeding among worldwide populations. PLoS One 2013; 8:e57619 10.1371/journal.pone.0057619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutellis A, Abi-Rached L, Raoult D. The origin and distribution of human lice in the world. Infect Genet Evol 2014; 23:209–217. 10.1016/j.meegid.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 5.Drali R, Shako JC, Davoust B, Diatta G, Raoult D. A new clade of African body and head lice infected by Bartonella quintana and Yersinia pestis—Democratic Republic of the Congo. Am J Trop Med Hyg 2015; 93: 990–993. 10.4269/ajtmh.14-0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis 1999; 29:888–911. 10.1086/520454 [DOI] [PubMed] [Google Scholar]
- 7.Houhamdi L, Lepidi H, Drancourt M, Raoult D. Experimental model to evaluate the human body louse as a vector of plague. J Infect Dis 2006; 194:1589–1596. 10.1086/508995 [DOI] [PubMed] [Google Scholar]
- 8.Angelakis E, Diatta G, Abdissa A, Trape JF, Mediannikov O, Richet H, et al. Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg Infect Dis 2011; 17:2357–2359. 10.3201/eid1712.110453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvresse S, Socolovschi C, Berdjane Z, Durand R, Izri A, Raoult D, et al. No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary school children in Paris. Comp Immunol Microbiol Infect Dis 2011; 34:475–477. 10.1016/j.cimid.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Kempf M, Abdissa A, Diatta G, Trape JF, Angelakis E, Mediannikov O, et al. Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int J Infect Dis 2012; 16:e680–e683. 10.1016/j.ijid.2012.05.1024 [DOI] [PubMed] [Google Scholar]
- 11.Boutellis A, Mediannikov O, Bilcha KD, Ali J, Campelo D, Barker SC, et al. Borrelia recurrentis in head lice, Ethiopia. Emerg Infect Dis 2013; 19:796–798. 10.3201/eid1905.121480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangare AK, Boutellis A, Drali R, Socolovschi C, Barker SC, Diatta G, et al. Detection of Bartonella quintana in African body and head lice. Am J Trop Med Hyg 2014; 91:294–301. 10.4269/ajtmh.13-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunantaraporn S, Sanprasert V, Pengsakul Th, Phumee A, Boonserm, Tawatsin A, et al. Molecular survey of the head louse Pediculus humanus capitis in Thailand and its potential role for transmitting Acinetobacter spp. Parasit Vectors 2015; 8:127 10.1186/s13071-015-0742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberger J, Anderson JF. The transmission of Typhus fever, with especial reference to transmission by the head louse (Pediculus capitis). Public Health Reports (1896–1970) 1912; 27:297–307. 10.2307/4567527 [DOI] [Google Scholar]
- 15.Murray ES, Torrey SB. Virulence of Rickettsia prowazekii for head lice. Ann N Y Acad Sci 1975; 266: 25–34. 10.1111/j.1749-6632.1975.tb35086.x [DOI] [PubMed] [Google Scholar]
- 16.Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol 2003; 13:1414–1417. [DOI] [PubMed] [Google Scholar]
- 17.Raoult D, Reed DL, Dittmar K, Kirchman JJ, Rolain JM, et al. Molecular identification of lice from pre-Columbian mummies. J Infect Dis 2008; 197:535–543. 10.1086/526520 [DOI] [PubMed] [Google Scholar]
- 18.Ascunce MS, Fane J, Kassu G, Toloza AC, Picollo MI, Gonzalez-Oliver A, et al. Mitochondrial diversity in human head louse populations across the Americas. Am J Physiol Anthropol 2013; 152:118–129. [DOI] [PubMed] [Google Scholar]
- 19.Boutellis A, Bitam I, Fekir K, Mana N, Raoult D. Evidence that clade A and clade B head lice live in sympatry and recombine in Algeria. Med Vet Entomol 2015; 29: 94–98. 10.1111/mve.12058 [DOI] [PubMed] [Google Scholar]
- 20.Ashfaq M, Prosser S, Nasir S, Masood M, Ratnasingham S, Hebert PD. High diversity and rapid diversification in the head louse, Pediculus humanus (Pediculidae: Phthiraptera). Nature. Scientific Reports 2015; 5: 14188 10.1038/srep14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumcuoglu K. Pediculus and Pthirus In: Paleomicrobiology–Past Human Infections. Raoult D & Drancourt M (eds). Springer, Berlin: 2008; pp. 215–222. [Google Scholar]
- 22.Araujo A, Ferreira LF, Guidon N, Maues Da Serra FN, Reinhard KJ, et al. Ten thousand years of head lice infection. Parasitol Today 2000; 16:269 S0169–4758(00)01694-X[pii]. [DOI] [PubMed] [Google Scholar]
- 23.Rivera MA, Mumcuoglu K, Mathney RT, Matheny DG. Head lice eggs, Anthropophthirus capitis, from mummies of the Chinchorro tradition, Camarones 15-D, Northern Chile. Chungara, Revistade Antropologia Chilena 2008; 40:31–39. [Google Scholar]
- 24.Arriaza B, Orellana NC, Barbosa HS, Menna-Barreto RF, Araujo A, Standen V. Severe head lice infestation in an Andean mummy of Arica, Chile. J Parasitol 2012; 98:433–436. 10.1645/GE-2903.1 [DOI] [PubMed] [Google Scholar]
- 25.Drali R, Mumcuoglu KY, Yesilyurt G, Raoult D. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg 2015; 93:623–625. 10.4269/ajtmh.14-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutellis A, Drali R, Rivera MA, Mumcuoglu KY, Raoult D. Evidence of sympatry of clade A and clade B head lice in a pre-Columbian Chilean mummy from Camarones. PLoS One 2013; 8:e76818 10.1371/journal.pone.0076818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen-Hieu T, Aboudharam G, Signoli M, Rigeade C, Drancourt M, Raoult D. Evidence of a louse-borne outbreak involving typhus in Douai, 1710–1712 during the war of Spanish succession. PLoS One 2010; 5:e15405 10.1371/journal.pone.0015405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton B, Basu C. Real-time PCR (qPCR) primer design using free online software. Biochem Mol Biol Educ 2011; 39:145–154. 10.1002/bmb.20461 [DOI] [PubMed] [Google Scholar]
- 29.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, et al. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis 2011; 17:883–885. 10.3201/eid1705.100573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30:2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drali R, Abi-Rached L, Boutellis A, Djossou F, Barker SC, Raoult D. Host switching of human lice to new world monkeys in South America. Infect Genet Evol 2016; 39:225–231. 10.1016/j.meegid.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 32.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009; 25:1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 33.Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 1999; 16:37–48. [DOI] [PubMed] [Google Scholar]
- 34.Raoult D, Dutour O, Houhamdi L, Jankauskas R, Fournier PE, Ardagna Y, et al. Evidence for louse-transmitted diseases in soldiers of Napoleon's Grand Army in Vilnius. J Infect Dis 2006; 193:112–120. 10.1086/498534 [DOI] [PubMed] [Google Scholar]
- 35.Grugni V, Battaglia V, Hooshiar Kashani B, Parolo S, Al-Zahery N, et al. Ancient migratory events in the Middle East: New clues from the Y-chromosome variation of modern Iranians. PLoS One 2012; 7:e41252 10.1371/journal.pone.0041252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drancourt M, Tran-Hung L, Courtin J, Lumley H, Raoult D. Bartonella quintana in a 4000-year-old human tooth. J Infect Dis 2005; 191:607–611. 10.1086/427041 [DOI] [PubMed] [Google Scholar]
- 37.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev 1996; 9:273–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Scola B, Raoult D. Acinetobacter baumannii in human body louse. Emerg Infect Dis 2004; 10:1671–3. 10.3201/eid1009.040242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol 2012; 28:563–571. 10.1016/j.pt.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 40.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2006; 2:e7 10.1371/journal.pgen.0020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1, 2 and 3 showed qPCR amplification targeted a 88-bp DNA fragment of cytb gene (24/24 positive with Ct varied between 32 to 38); 4 showed qPCR amplification targeted a 100-bp DNA fragment of 12S gene (22/24 positive with Ct varied between 32 to 38).
(PPTX)
(PPTX)
Haplotypes highlighted in blue are the newly identified haplotypes from sequences available in GenBank.
(XLSX)
Data Availability Statement
All sequences of cytb haplotypes are available in GenBank under accession numbers KX232678-KX232679 and KX249763-KX249775.