Abstract
Constitutive β-catenin/Tcf activity, the primary transforming events in colorectal carcinoma, occurs through induction of the Wnt pathway or APC gene mutations that cause familial adenomatous polyposis. Mice carrying Apc mutations in their germ line (ApcMin) develop intestinal adenomas. Here, the crossing of ApcMin with cyclin D1−/− mice reduced the intestinal tumor number in animals genetically heterozygous or nullizygous for cyclin D1. Decreased tumor number in the duodenum, intestines, and colons of ApcMin/cyclin D1+/− mice correlated with reduced cellular proliferation and increased differentiation. Cyclin D1 deficiency reduced DNA synthesis and induced differentiation of colonic epithelial cells harboring mutant APC but not wild-type APC cells in vivo. In previous studies, the complete loss of cyclin D1 through homozygous genetic deletion conveyed breast tumor resistance. The protection of mice, genetically predisposed to intestinal tumorigenesis, through cyclin D1 heterozygosity suggests that modalities that reduce cyclin D1 abundance could provide chemoprotection.
β-Catenin is an important structural component of submembranal cell-cell adhesion sites and contributes to the canonical Wnt signaling pathway (9, 20). The β-catenin/Tcf pathway governs diverse processes, including cellular fate and positioning, cell proliferation, and survival (6). Induction of Wnt signaling stabilizes β-catenin, resulting in nuclear translocation and the formation of a transcriptional activation complex with members of the Tcf/LEF-1 family (8, 16, 30). The destruction complex regulating β-catenin abundance includes the adenomatous polyposis coli (APC) protein (47, 59), axin/conductin (7, 15), and glycogen synthase kinase 3β (45). In this complex, phosphorylation by glycogen synthase kinase 3β targets β-catenin to the ubiquitin-proteasome pathway for degradation (1, 17, 49, 70). Mutations in APC, axin/conductin, and β-catenin have been associated with cancer and the induction of constitutive nuclear β-catenin/Tcf complexes (9, 22, 31, 46). Activation of β-catenin induces target genes, including c-Myc and cyclin D1 through a canonical Tcf site and WISP1 through a CRE site, and the activity of several nuclear receptors (52).
The induction of constitutive β-catenin/Tcf activity by the Wnt pathway comprises the primary transforming events in colorectal carcinoma (CRC) (10). Mutations in the APC gene are responsible for familial adenomatous polyposis, and inactivation of both APC alleles occurs frequently in sporadic colorectal adenomas. Mice carrying Apc mutations in their germ line (ApcMin) develop intestinal adenomas. The ApcMin allele is a fully penetrant dominant mutation resulting from exposure to ethylnitrosourea. Mice heterozygous for ApcMin develop a reproducible number of multiple adenomas throughout the intestine (58). Wnt activation and several signaling pathways stabilize β-catenin and enhance its transcriptional activity by preventing phosphorylation of the N-terminal domain.
Cyclin D1 protein abundance is elevated in human adenocarcinomas and adenomatous polyps of the colon (4, 5). As the cyclin D1 gene is not amplified in human colon cancers but is overexpressed, it has been hypothesized that oncogenic signals induce cyclin D1 abundance, thereby contributing to the CRC tumor phenotype. We previously demonstrated that cyclin D1 is induced by activation of the Wnt signaling pathway through a LEF1 binding site in the cyclin D1 promoter (27, 55). The transcriptional activation of the cyclin D1 gene was inhibited by the wild-type APC (APCwt) or by axin proteins (55). Antisense cyclin D1 abolished SW480 colon cancer cell tumor growth in nude mice (4), further suggesting a role for cyclin D1 in colonic tumor growth.
Mice with the cyclin D1 gene homozygously deleted are resistant to breast tumorigenesis induced by mammary gland-targeted ErbB2 or Ras (71). Surprisingly, however, increased breast tumorigenesis was observed in cyclin D1−/− mice when they were crossed to mice expressing activated β-catenin targeted to the mammary gland (44). These findings suggested that cyclin D1 may play distinct roles depending upon the oncogenic signals. Alternatively, cyclin D1 may regulate progenitor stem cell population expansion, and these stem cells may in turn affect tumor proclivity in a target organ. The mechanism by which cyclin D1 loss mediates a response to oncogenic signals in vivo remains to be fully understood. Cyclin D1 encodes a regulatory subunit that together with the cyclin-dependent kinase partner forms a holoenzyme that phosphorylates the retinoblastoma (pRB) protein. Although cyclin D1 promotes G1 phase cell cycle progression in cultured cells, cyclin D1-overexpressing tumors do not necessarily display higher proliferative indices (39, 53). Recent studies have identified cdk binding-independent functions of cyclin D1 (66). In this regard, cyclin D1 has been shown to inhibit CEBPβ and peroxisome proliferator-activated receptor gamma (PPARγ), which are known to function in a common signaling pathway regulating cellular differentiation (23, 67). Cyclin D1 has been shown to either promote or inhibit differentiation in cultured cells. In vivo studies of cellular differentiation in cyclin D1-deficient mice have not been conducted to date. Although the tumor resistance of cyclin D1−/− mice raises the prospect that cyclin D1 induction may have therapeutic value, reduction rather than complete inactivation of cyclin D1 is a more likely practical outcome of such therapies. To date, formal evidence that a reduction, rather than a complete loss, of cyclin D1 conveys tumor resistance remains to be established.
We investigated the role of cyclin D1 in colonic epithelial cell differentiation, proliferation, and tumor formation induced by activation of the β-catenin pathway, using ApcMin and cyclin D1-deficient mice. Cyclin D1 deficiency reduced intestinal polyp formation. Activation of β-catenin signaling reduced the formation of goblet cells in a cyclin D1-independent manner. Reduction of cyclin D1 abundance in the presence of activated β-catenin signaling increased cellular differentiation and decreased DNA synthesis in colonic epithelium. The loss of a single allele of cyclin D1 was sufficient to convey relative tumor resistance, altered cellular differentiation, and DNA synthesis in vivo. Mice nullizygous for cyclin D1, which have abolished cyclin D1 abundance, are resistant to breast tumorigenesis induced by Ras or ErbB2. Therapeutic modalities to date, however, only reduce rather than abolish cyclin D1 abundance. The present studies are the first to demonstrate that reducing, rather than abolishing, cyclin D1 protects against a genetic predisposition to tumorigenesis.
MATERIALS AND METHODS
Mouse handling and breeding.
Animal care was conducted in accordance with the standards set forth by the Institute for Animal Studies of the Albert Einstein College of Medicine. The cyclin D1−/− mice (56) and ApcMin mice (32) were previously described. The genetic backgrounds of the ApcMin and cyclin D1 null mice are as follows.
The ApcMin mice were generated by first treating C57BL/6J males with the mutagen ethylnitrosourea, followed by mating to AKR/J females. From this breeding a female exhibiting an abnormal circling behavior was selected to breed with a B6 male to ascertain whether this behavior was inheritable. Some progeny from this mating displayed an adult-onset anemia. The anemic mice were found to develop intestinal polyps. Later publications identified the Min mutation as due to a truncation in the APC gene (58). The Min mutation was maintained by backcrossing into B6 females; the mice were at their ninth backcross generation as of the original publication (32). Homozygosity for the mutation is lethal, and it is transmitted as an autosomal dominant trait.
The cyclin D1 null mice were generated by gene targeting in embryonic stem (ES) cells, which deleted part of exon 1 and all of exons 2 and 3 while replacing this region with a neo cassette. A 129/Sv mouse genomic library was used to isolate the proper fragments used in the targeting. Once constructed, the target vector was electroporated into D3 129/Sv ES cells. Cyclin D1 heterozygote ES cells were identified and injected into blastocyst-stage C57BL/6 mouse embryos. The chimeric progeny were bred to C57BL/6 mice. Cyclin D1 heterozygotes were bred from this cross. The animals used in this study are from the breeding of the ApcMin mice to the cyclin D1 null mice.
Genotyping.
A 1-cm section of tail was removed to prepare genomic DNA (3). Tail sections were incubated overnight at 50 to 55°C in 700 μl of a proteolytic solution (50 mM Tris [Sigma] [pH 8.0], 100 mM EDTA [Sigma], and 0.5% sodium dodecyl sulfate [Fisher]) plus 20 μl of 20-mg/ml proteinase K solution (GibcoBRL). DNA was purified by phenol-chloroform (Sigma) extraction and ethanol precipitation, followed by resuspension in 250 μl of 0.1× TE (1× TE is 10 mM Tris plus 1 mM EDTA). PCR analysis of genotypes for the ApcMin mice (58) and cyclin D1−/− mice (2) has been described previously.
Polyp characterization and statistical analysis.
Mice were euthanatized by placing them into a CO2-saturated tank. Intestines were removed (stomach to rectum) and perfused with phosphate-buffered saline to wash out intestinal lumens. Intestines were then sectioned at the duodenum-jejunum junction and ileum-cecum junction. Longitudinal incisions were made along the length of the sections. Tissues were aligned onto graph paper and flattened out (to determine intestinal length, polyp position, and polyp size), followed by fixation in 10% buffered formalin (Fisher) for 48 h. The intestinal sections were then visually inspected and scored for polyp position (distance) and size (x, y, and z) data by using a dissecting microscope (SMZ-2B; Nikon) at a magnification of ×6 with back lighting. This method allowed for the identification of opaque polyps as small as 0.5 mm in diameter due to the greater cell density of the polyp tissue. Representative samples were removed either for freezing or for fixation and processing. Significant differences in tumor characteristics between genotypes were determined by using the Kruskal-Wallis model. Significant genotype-specific differences in tumor characteristics were determined by using the Student t test and the Kruskal-Wallis model.
Western blots.
The abundances of β-catenin, PPARγ1, Tcf4, and guanine nucleotide dissociation inhibitor (GDI) proteins were determined by Western blot analysis as previously described (65). Antibodies included anti-Tcf4 (6H5-3; Upstate), anti-PPARγ1 (H-100; Santa Cruz), and anti-β-catenin (clone 14; Transduction Labs of BD Biosciences). The GDI antibody was a generous gift from Perry Bickel, Washington University, St. Louis, Mo. At the time of dissection, segments of intestine from either the mid-duodenal or distal colon were removed and snap frozen in liquid nitrogen for lysate preparation. Prior to removal, intestinal segments were first visually inspected for the presence or absence of polyps. Tissue was homogenized (PowerGen Model 125; Fisher Scientific) in 1.5 volumes of lysate buffer (50 mM HEPES, 150 mM NaCl, 1.0 mM EDTA, 1.0 mM EGTA [pH 7.2]). Typical analysis was performed by loading 50 μg of protein and resolving in a 10% acrylamide gel. Proteins were transferred onto a nitrocellulose filter for blotting. Densitometry was used to quantitate protein signal levels (ImageQuant software; Molecular Dynamics).
Immunohistochemistry.
Paraffin-embedded sections were stained and visualized by using a diaminobenzidine method (LSAB+, peroxidase) as detailed in a protocol provided by the manufacturer (Dako Corp., Carpenteria Calif.). Antibodies used were anti-cyclin D1 (Ab-3; NeoMarkers), anti-β-catenin (clone 14; Transduction Labs of BD Biosciences), and anti-PPARγ1 (H-100; Santa Cruz) (24). Briefly, after deparaffinization, antigens were retrieved by microwave irradiation in a 0.01 M (pH 6.0) trisodium citrate buffer. The slides were washed and incubated with primary antibodies for 1 h at room temperature, followed by incubation with secondary antibodies. Positive signals were revealed with the diaminobenzidine chromogen under the conditions recommended by the supplier. The slides were then counterstained with Harris hematoxylin (Fisher Scientific). In vivo bromodeoxyuridine (BrdU) labeling was conducted as previously described (34). Briefly, at 3 h prior to sacrifice, mice were injected intraperitoneally with 100 μl of BrdU (10 mg/ml) per 10 g of body weight. (For ApcMin mice [n = 13 mice], there were a total of 147 crypts [4 cyclin D1+/+ mice, 49 crypts; 4 cyclin D1+/− mice, 34 crypts; 5 cyclin D1−/− mice, 64 crypts], and for Apcwt mice [n = 12], there were a total of 196 crypts [4 cyclin D1+/+ mice, 66 crypts; 4 cyclin D1+/− mice, 64 crypts; 4 cyclin D1−/− mice, 66 crypts].) Statistical analyses were performed by using the Mann-Whitney U test, and significant differences were established as those with P values <0.05.
Goblet cell quantification.
Distal colon samples were analyzed for goblet cells, which were identified by using Alcian blue stain (69) (a method for staining mucosubstances). After deparaffinization, tissues were immersed for 3 min in a 3% acetic acid solution. The tissues were then immersed in 1% Alcian blue in 3% acetic acid (pH 2.5) for 30 min. Tissues were rinsed with water, counterstained with 0.1% Nuclear Fast Red for 5 min, and washed for 1 min with water. Finally, the tissues were dehydrated and mounted. The mean number of goblet cells was determined from four animals of each background, using an average of 10 complete crypt units from each animal. Statistical analyses were performed by using the Mann-Whitney U test, and significant differences were established as those with P values of <0.05.
Plasmids, cell lines, transfections, and reporter assays.
The reporter plasmids for the (AOX)3LUC (acyl coenzyme A oxidase triple PPARγ response element [PPRE]), the PPARγ1 promoter-reporter, and the expression plasmids for β-catenin (55, 65), cyclin D1 (67), and Muc2 Luc (63) were previously described.
Cell culture, DNA transfection, and luciferase assays were performed as previously described (68). The cyclin D1−/− 3T3, cyclin D1 wt 3T3, and CaCo2 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum and 1% penicillin-streptomycin. Cells were plated in 12-well dishes (Falcon) and seeded at 50 to 60% confluency the night before transfections were performed. Cells were transfected by using Superfect or Polyfect reagent (Qiagen). The medium was changed after 3 h, and the luciferase activity was determined at 24 to 36 h posttransfection. Comparisons were made between the effect of transfecting an active expression vector(s) and the effect of an equal amount of vector cassette. Renilla luciferase (TK-LUC) was cotransfected (1:10 ratio with the reporter) into all wells as an internal control for transfection efficiency. In each experiment a dose response was determined with 300 and 600 ng of expression vector or equal moles of empty expression vector cassette and the reporter plasmids (4.8 μg). Luciferase assays were performed at room temperature with an Autolumat LB 953 (EG&G Berthold); the initial 10 s of the reaction was used to assess luciferase content, with the values expressed in arbitrary light units. Statistical analyses were performed by using the Mann-Whitney U test, and significant differences were established as those with P values of <0.05. Luciferase buffers and reagents were purchased from Promega.
ChIP assay.
Chromatin immunoprecipitation (ChIP) analysis was performed according to a protocol provided by Upstate Biotechnology under modified conditions. 3T3 cells (106) were grown in Dulbecco's modified Eagle's medium with 10% serum. The cells were cross-linked by adding 1.1% formaldehyde buffer containing 100 mM sodium chloride, 1 mM EDTA-Na (pH 8.0), 0.5 mM EGTA-Na, and Tris-HCl (pH 8.0) directly to the culture medium for 10 min at 37°C. The medium was aspirated. Cells were washed twice with ice-cold phosphate-buffered saline containing 10 mM dithiothreitol and protease inhibitors, lysed with warm 1% sodium dodecyl sulfate lysis buffer, and then incubated for 10 min on ice. The cell lysates were sonicated to shear DNA to lengths of between 200 and 1,000 bp, and the samples were diluted 10-fold in ChIP dilution buffer. To reduce nonspecific background, the cell pellet suspension was precleared with 60 μl of salmon sperm DNA-protein A-agarose 50% slurry (Upstate Biotechnology) for 2 h at 4°C with agitation. Chromatin solutions were precipitated overnight at 4°C with antibodies to acetyl histone H3, acetyl histone H3 (lys 9) (Upstate), PPARγ (E8), and HDAC1 (H11) (Santa Cruz) with rotation. For a negative control, rabbit immunoglobulin G was immunoprecipitated by incubating the supernatant fraction for 1 h at 4°C with rotation; 60 μl of salmon sperm DNA-protein A-agarose slurry was added, left for 2 h at 4°C with rotation to collect the antibody-histone complex, and washed extensively according to the manufacturer's protocol. Input and immunoprecipitated chromatin was incubated at 65°C overnight to reverse cross-links. After proteinase K digestion for 1 h, DNA was extracted by using a Qiagen spin column kit. Precipitated DNAs were analyzed by PCR. The primers 5′-AAACCCCTCCTCTCTGCCTC-3′ and 5′-CCTCGGAGGAGGAGTAGGAG-3′ were used for PCR to identify PPARγ-responsive elements in the mouse LPL promoter.
RESULTS
Cyclin D1 deficiency reduces the number of intestinal polyps formed in ApcMin mice.
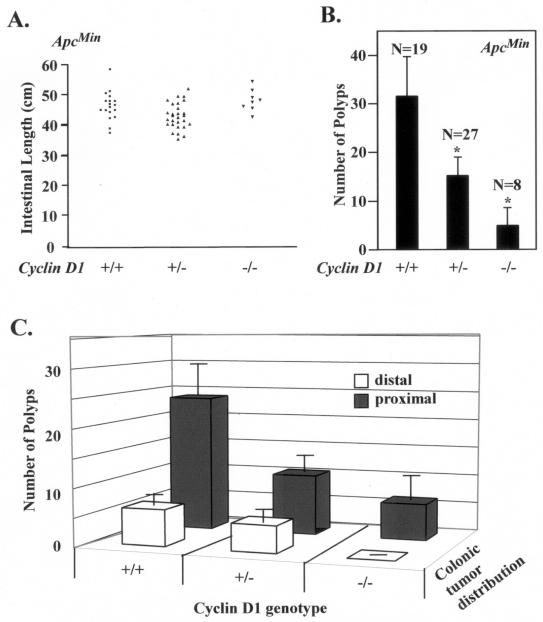
In order to examine the functional requirement for cyclin D1 in APC-mediated β-catenin signaling, we crossed ApcMin mice, which possess a constitutively active β-catenin signaling pathway (58), with mice lacking cyclin D1 (2, 56). Male and female mice were closely watched for a moribund appearance, as originally reported, and were sacrificed at 33 weeks. The mean intestinal lengths were similar for ApcMin mice of each cyclin D1 genotype (ApcMin/cyclin D1+/+, 47.6 ± 1.1 cm; ApcMin/cyclin D1+/−, 45.2 ± 0.76 cm; and ApcMin/cyclin D1−/−, 49.5 ± 1.4 cm) (Fig. 1A). cyclin D1wt mice in this study had a mean tumor load of 32 ± 8, similar to that in the original report on ApcMin (29 tumors per animal). To assess the effect of the cyclin D1 genotype on the distribution of tumors, the intestine was divided into anatomical segments (stomach-duodenum, jejunum-ileum, and cecum-colon) and scored for polyp number, location, and size. A total of 54 mice (19 cyclin D1+/+, 27 cyclin D1+/−, and 8 cyclin D1−/− mice) were analyzed for polyp number, location, and size. Compared with the ApcMin/cyclin D1+/+ mice, the number of colonic polyps was reduced 50% in the cyclin D1+/− mice (ApcMin/cyclin D1+/+, 32 ± 8 per animal; ApcMin/cyclin D1+/−, 15.2 ± 3.9 polyps per animal [n = 27 mice]). A further 67% reduction in polyp number was found in the ApcMin/cyclin D1−/− mice (n = 8 mice; 5 ± 3.8 polyps per animal) compared to the cyclin D1+/− mice (Fig. 1B). The majority of colonic polyps appeared in the distal colon. No polyps were detected in the proximal colons of ApcMin/cyclin D1−/− mice (Fig. 1C). Together these studies demonstrate that the loss of either one or both alleles of cyclin D1 causes a significant decrease in the number of colonic adenomas (P < 0.05). The adenomas were polypoid or broad base raised with distorted hyperplastic and dysplastic crypts. Formal histological evaluation of the adenomas throughout the gastrointestinal tracts of ApcMin mice of each cyclin D1 genotype revealed no differences in gross histological characteristics or grade of adenomas between ApcMin mice of each cyclin D1 genotype (data not shown).
FIG. 1.
Cyclin D1 deficiency reduces colonic polyp formation. (A) Scatter plot showing the distribution of intestinal lengths as determined for the cyclin D1 wt, heterozygous, or nullizygous genotypes. (B) Polyp formation in ApcMin mice genetically deficient for cyclin D1. The mean number of colon polyps for each cyclin D1 genotype in the ApcMin genetic background is shown. Asterisks indicate P values <0.01. (C) Mean number of proximal and distal colon polyps for each cyclin D1 genotype. Error bars indicate standard errors of the means.
ApcMin inhibition of colonic epithelial cell differentiation is cyclin D1 dependent.
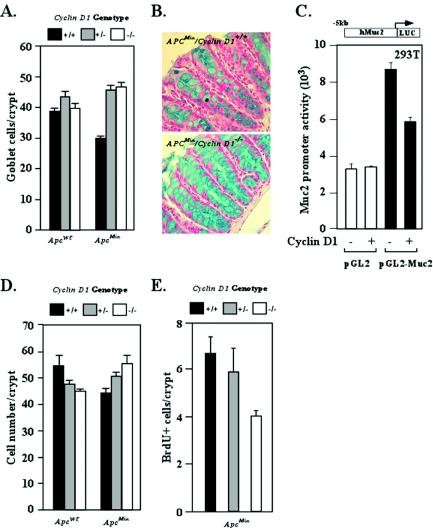
In view of the reduction in colonic polyp number in the ApcMin/cyclin D1-deficient mice, we examined colonic epithelial cell differentiation and DNA synthesis. Alcian blue staining of goblet cells was used as a marker of intestinal cellular differentiation. In vivo labeling was performed with BrdU to assess cellular proliferation in the gastrointestinal tract. The number of goblet cells in each crypt was reduced by 25% in ApcMin compared with Apcwt mice (Fig. 2A and B). The mucin abundance in each cell was increased dramatically, indicating that cyclin D1 may play a negative role in regulation of mucin synthesis. To examine further the possibility that cyclin D1 may directly regulate mucin expression, the promoter of the mucin-2 gene was linked to a luciferase reporter (pGL2-Muc2) and tested in transient-transfection assays. Cyclin D1, compared to the vector control, repressed activity of the Muc2 promoter by 30%. The control luciferase reporter without the Muc2 promoter was not affected by cyclin D1 (Fig. 2C).
FIG. 2.
Intestinal cellular proliferation and differentiation in ApcMin mice genetically deficient for cyclin D1. (A) Goblet cells were stained with Alcian blue and counted. The number is shown as mean and standard error of the mean/crypt for each cyclin D1 genotype in the Apcwt and ApcMin genetic backgrounds (data comparisons are based on the following: ApcMin mice, total of 20 animals [8 cyclin D1+/+ mice, 80 crypts counted; 4 cyclin D1+/− mice, 22 crypts counted; 8 cyclin D1−/− mice, 75 crypts counted]; Apcwt mice, total of 11 animals [4 cyclin D1+/+ mice, 40 crypts; 3 cyclin D1+/− mice, 24 crypts; 4 cyclin D1−/− mice, 33 crypts]). (B) Analysis of colon epithelial goblet cells by Alcian blue staining in ApcMin mice by cyclin D1 genotype, with two cross-sectioned representative examples shown. (C) 293T cells were transfected with Muc2 promoter-driven luciferase reporter plasmid (pGL2-Muc2). Cyclin D1 cotransfection represses reporter activity in a Muc2 promoter-specific manner. pGL2 served as a reporter control. (D) Total colon epithelial cell counts per crypt in each genotype. (E) BrdU-positive cells per crypt.
In the Apcwt mice, cyclin D1 deficiency did not affect goblet cell number. In contrast, in the ApcMin mice, the number of goblet cells per crypt was increased approximately 50% in cyclin D1-deficient mice compared with the ApcMin/cyclin D1+/+ mice, consistent with a model in which cyclin D1 inhibits differentiation induced by ApcMin (Fig. 2A) (n = 15 mice). Importantly, this alteration in number of goblet cells per crypt was observed in mice that were heterozygous for cyclin D1. The number of cells per crypt was reduced by approximately 22% in ApcMin compared with Apcwt mice. Cyclin D1 deficiency reduced the number of cells per crypt in Apcwt mice (Fig. 2D), suggesting that cyclin D1 abundance contributes to final cell number of normal intestinal crypts. Consistent with the reduction in colonic polyp formation in cyclin D1-deficient mice, in vivo BrdU labeling was reduced approximately 35% in the colonic crypts of the ApcMin/cyclin D1−/− compared with the ApcMin/cyclin D1+/+ mice (n = 23 mice) (Fig. 2E).
Cyclin D1 heterozygosity reduces duodenum polyp formation and DNA in ApcMin mice.
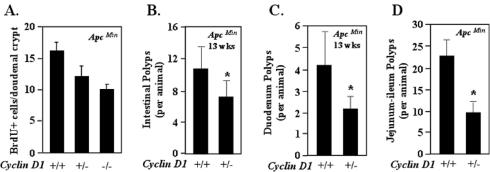
To determine whether cyclin D1 regulated DNA synthesis in the presence of ApcMin throughout the enteric epithelium, in vivo BrdU analysis of the duodenum was conducted. As with the colonic epithelium in the presence of ApcMin, cyclin D1 deficiency reduced labeling in the duodenal epithelium (Fig. 3A). In vivo BrdU labeling (n = 12 mice; average of 40 crypts/mouse examined) demonstrated a reduction in the mean number of BrdU-staining cells in the duodenal crypts of the ApcMin/cyclin D1+/− and ApcMin/cyclin D1−/− mice compared with the ApcMin/cyclin D1+/+ mice (Fig. 3A). To determine whether alterations in the number of polyps and cellular proliferation occurred at earlier time points, we examined mice at 13 weeks of age (Fig. 3B) (n = 9 mice; 5 cyclin D1+/+ and 4 cyclin D1+/−). These ApcMin/cyclin D1+/− mice demonstrated a 25% decrease in polyp number (ApcMin/cyclin D1+/+, 10.8 ± 2.78 polyps; ApcMin/cyclin D1+/−, 7.25 ± 2.02 polyps). A reduction in polyp number was also observed in the duodena (Fig. 3C) and ilea and jejuna (Fig. 3D) of ApcMin/cyclin D1+/− compared with ApcMin/cyclin D1+/+ mice. Compared with their wt littermates, mice either heterozygous or nullizygous for cyclin D1 showed a reduction in the total number of polyps and adenomas. Importantly, consistent with the findings for colonic epithelium, ApcMin mice heterozygous for cyclin D1 showed reduced polyps throughout the gastrointestinal tract and reduced intestinal polyps (Fig. 1B) (polyps were reduced by 50% in ApcMin/cyclin D1+/− mice [ApcMin/cyclin D1+/+, 32; ApcMin/cyclin D1+/−, 15.2; P = 0.001]), and a significant reduction in jejunum-ileum polyps was observed with the loss of a single cyclin D1 allele (P = 0.0019) (ApcMin/cyclin D1+/+, 22.8 ± 3.3; ApcMin/cyclin D1+/−, 9.7 ± 1.8) (Fig. 3D).
FIG. 3.
Cyclin D1 heterozygosity reduces duodenal polyp number in ApcMin mice. (A) In vivo BrdU staining of the duodenum (n = 9 animals), shown as mean and standard error of the mean/crypt, in ApcMin mice by cyclin D1 genotype (13 weeks). (B to D) Mean numbers of polyps in ApcMin/cyclin D1wt and heterozygote mice (data are based on comparisons from nine mice [four cyclin D1+/+ and five cyclin D1+/−]) overall (B), in the duodenum (C), and in the jejunum-ileum (D). Asterisks indicate P values <0.05.
Cyclin D1 heterozygosity reduces cyclin D1 abundance and increases PPARγ without affecting β-catenin/Tcf abundance in ApcMin colonic epithelium.
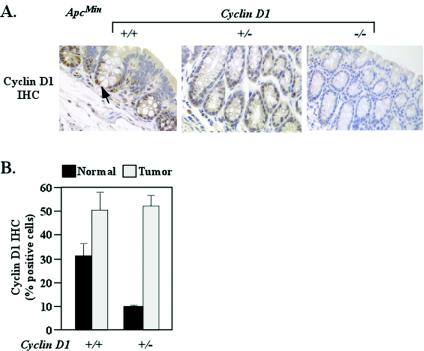
To investigate the molecular mechanisms by which cyclin D1 regulated the development of polyps in ApcMin mice, we assessed the abundances of cyclin D1 and β-catenin/Tcf, as each of these proteins has been shown to regulate colonic epithelial cellular growth in cultured cells (4, 50). Cyclin D1 is a downstream target induced by β-catenin/Tcf signaling (55). Tissue sections of colonic adenoma and normal mucosa were immunostained for cyclin D1 (n = 24 mice; 33 weeks). Cyclin D1 immunoreactivity was nuclear, and the distribution was similar between wt and heterozygous genotypes and was absent in the cyclin D1−/− mice (n = 24 mice). The percentage of cells staining positive for cyclin D1 was decreased in the normal mucosa of the ApcMin/cyclin D1+/− mice (9.3% ± 0.7%) compared with the ApcMin/cyclin D1+/+ mice (32% ± 4.7%) (Fig. 4) (n = 24). Cyclin D1 was overexpressed in adenoma tissue, and there was no difference in the number of adenomatous cells staining positive between wt and heterozygous cyclin D1 genotypes (50% ± 8.2% versus 51.7% ± 4.8%) (Fig. 4B).
FIG. 4.
Cyclin D1 abundance in ApcMin/cyclin D1-deficient intestinal epithelium. (A) Cyclin D1 immunoreactivity in nonadenomatous colon mucosa for each cyclin D1 genotype (n = 24 animals; eight tissue preparations from each genotype). IHC, immunohistochemistry. (B) Bar graph comparing levels of cyclin D1 immunoreactivity in colon mucosa and polyps from cyclin D1 wt and heterozygote mice (n = 24; means and standard errors of the means are shown).
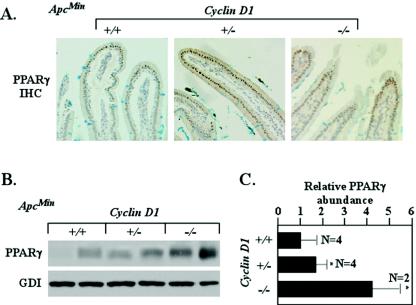
PPARγ1 mutations have been identified in human colon cancer, and growth of human colon tumors in nude mice was inhibited by PPARγ ligands (12, 50, 51). In contrast, PPARγ ligands increased polyp numbers in ApcMin mice in some but not all studies (25, 37, 48), which perhaps is related in part to the finding that PPARγ inhibits β-catenin abundance only in the presence of wt APC (14). Together these studies suggest that the molecular genetic determinants of PPARγ abundance and function may be important in determining mechanisms of colonic tumorigenesis. PPARγ expression is induced during colonic epithelial cell differentiation (29, 71), and activation of Wnt signaling inhibits differentiation and PPARγ expression. It was hypothesized, therefore, that PPARγ abundance may be reduced in ApcMin intestinal epithelium compared with Apcwt intestinal epithelium. In 33-week-old mice, PPARγ1 immunoreactivity was located with similar subcellular distributions in ApcMin and Apcwt mice, with reduced relative staining in the ApcMin mice (Fig. 5A and data not shown). As cyclin D1 repressed PPARγ expression in Apcwt cells in culture and in vivo (67), we investigated whether cyclin D1 loss induced PPARγ expression in vivo in ApcMin mutant cells. Comparison was made between ApcMin mice that were cyclin D1+/+, cyclin D1+/−, or cyclin D1−/−. Increased PPARγ1 abundance was detected by Western blotting in cyclin D1-deficient mice compared with cyclin D1 wt mice (n = 10) (Fig. 5B). Relative PPARγ abundance was increased with cyclin D1 deficiency. ApcMin/cyclin D1+/+ levels was set at 1 (±0.74). PPARγ1 levels in the ApcMin/cyclin D1+/− colon were 70% higher (1.68 ± 0.54), with a fourfold increase in the ApcMin/cyclin D1−/− mice (4.2 ± 1.2) (Fig. 5C). Thus, ApcMin inhibits PPARγ abundance, consistent with the reduced differentiation markers of Alcian blue (Fig. 2A). Cyclin D1 abundance significantly affected only the reduced PPARγ abundance found in the ApcMin epithelium. Thus, the genetic inhibition of cyclin D1 partially reversed the reduced levels of PPARγ found in ApcMin mice.
FIG. 5.
Genetic cyclin D1 deficiency increases PPARγ abundance in ApcMin intestinal epithelium. (A) Immunohistochemical (IHC) staining of duodenal epithelia of ApcMin mice. (B) Representative Western blot analysis of PPARγ1 in colonic epithelium in ApcMin mice (comparisons were made with 10 animals [4 cyclin D1+/+, 4 cyclin D1+/−, and 2 cyclin D1−/−] for each cyclin D1 genotype, two representative examples are shown for each genotype, and each lane represents lysate preparations made from individual animals with GDI as a loading control). (C) Analysis of mean PPARγ1 protein levels by densitometry (wt set to 1; mean and standard error of the mean; 13 weeks), normalized to GDI loading control. Asterisks indicate P values <0.05.
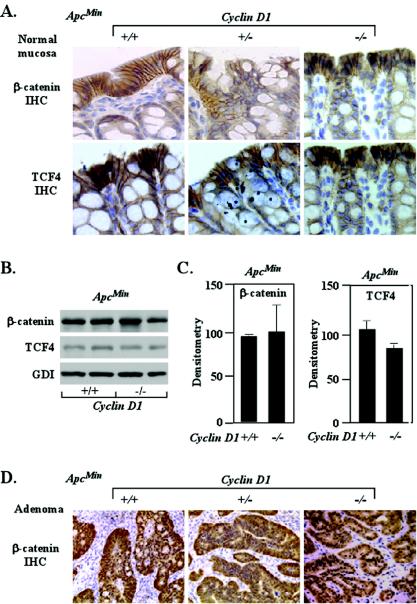
As the present studies suggested that cyclin D1 inhibited the differentiation and promoted proliferation of ApcMin colonic epithelium, we assessed the role of cyclin D1 in regulating β-catenin and Tcf4 abundance in ApcMin mice. The relative abundance and localization of β-catenin in the colonic epithelia of ApcMin mice were not altered by cyclin D1 deficiency (n = 24) (Fig. 6A). β-Catenin abundance, determined by Western blotting, was unaltered between genotypes (Fig. 6B and C). The Tcf4 levels in the ApcMin/cyclin D1−/− colonic epithelium were not significantly different from the ApcMin/cyclin D1+/+ levels (Fig. 5B and C). The β-catenin immunoreactivity of ApcMin tumors was also not altered by cyclin D1 deficiency (Fig. 6D). These studies suggest that cyclin D1 does not function as an upstream regulator of β-catenin signaling and are consistent with previous studies indicating that cyclin D1 is a downstream target of β-catenin.
FIG. 6.
β-Catenin/Tcf expression in intestinal epithelium in ApcMin mice genetically deficient for cyclin D1. (A) Immunohistochemistry (IHC) of β-catenin and Tcf4 in normal colon from ApcMin mice for each cyclin D1 genotype (one representative example of each, from an assessment based on 24 animals and eight tissue preparations from each genotype, is shown). The image of normal mucosa for cyclin D1+/+ mice also contains adenomatous tissue. (B) Two representative examples are displayed, each prepared from individual animals. (C) Mean data from immunohistochemistry Western analysis for β-catenin and Tcf4 normalized to GDI loading control (wt set to 100) (n = 10). Error bars indicate standard errors of the means. (D) Immunohistochemistry of β-catenin in colon adenoma from ApcMin mice for each cyclin D1 genotype.
Cyclin D1 inhibits β-catenin-induced PPARγ1 promoter activation.
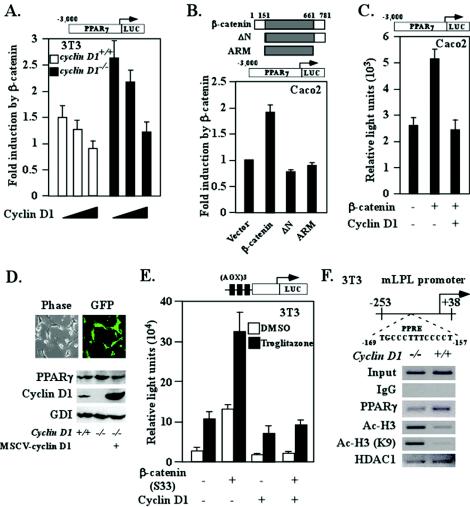
The present studies showed, first, reduced PPARγ abundance in ApcMin colonic epithelium associated with a reduction in goblet cell number and Alcian blue staining. Second, PPARγ abundance was increased in the normal colonic epithelium of ApcMin mice upon loss of cyclin D1. The reduction in PPARγ abundance in ApcMin colonic epithelium may be due to the repression of PPARγ expression or function by activation of β-catenin signaling. Alternatively, as PPARγ is induced during colonic epithelial cell differentiation (28, 60), the decrease in PPARγ may be secondary to ApcMin inhibition of colonic epithelial cell differentiation. We hypothesized that the increased levels of PPARγ in the ApcMin/cyclin D1−/− mice may have been due to the loss of a repressor of PPARγ expression in ApcMin cyclin D1−/− cells. We investigated the role of cyclin D1 in the increased PPARγ1 expression in the ApcMin/cyclin D1−/− colonic epithelium. The PPARγ1 promoter was analyzed in reporter gene assays to determine whether activation of β-catenin signaling reduced PPARγ expression directly, and we also assessed whether cyclin D1 regulates PPARγ in the presence or absence of an activating β-catenin/Tcf signal pathway. As the ApcMin mutation induces β-catenin/Tcf signaling, we determined the effect of constitutive activation of the β-catenin signaling on the PPARγ1 promoter by using the stabilized mutant β-catenin S33 (Fig. 7A). Transfection experiments were carried out with cyclin D1−/− 3T3 cells, with data normalized for transfection efficiency by using renilla luciferase. The fold induction of PPARγ1 promoter activity without β-catenin cotransfection was set as 1 (the activity of the PPARγ1 promoter was higher [nearly threefold] in cyclin D1−/− cells than in wt 3T3 cells [954 versus 333 light units]). PPARγ LUC reporter activity was induced nearly threefold by β-catenin S33 in cyclin D1−/− cells, with an approximately twofold difference found in 3T3 cyclin D1+/+ cells (1.6- ± 0.36-fold; P = 0.09; n = 6) and a threefold difference found in 3T3 cyclin D1−/− cells (2.85- ± 0.54-fold; P = 0.007; n = 6). Cotransfection of an expression plasmid for cyclin D1 reduced β-catenin-induced PPARγ1 promoter activity in 3T3 cells (from 1.6- to 0.78-fold [±0.12-fold]; P = 0.03) and reduced β-catenin-induced activity by 80% in cyclin D1−/− 3T3 cells (from 2.85- to 1.4-fold [±0.18-fold]; P = 0.014) (Fig. 7A). Cyclin D1 expression by cotransfection inhibited β-catenin induction of PPARγ promoter activity in the CaCo2 colon cancer cell line (Fig. 7C). The function domain of β-catenin required for PPARγ1 induction was mapped to its N terminus, since either the N-terminal deletion mutant or an ARM domain of β-catenin alone did not fulfill the transactivation function of β-catenin (Fig. 7B). Further experimentation was conducted by using retroviral transduction and provided high efficiency of gene transfer (100%). Transduction of cyclin D1−/− mouse embryo fibroblasts with cyclin D1 reduced endogenous PPARγ expression (Fig. 7D).
FIG. 7.
Cyclin D1 inhibits β-catenin-dependent signaling to the PPARγ1 promoter. (A) Cyclin D1 (150 or 300 ng) and β-catenin S33 (450 ng) were cotransfected with the PPARγ1 promoter. The luciferase reporter data are means and standard errors of the means. (B) CaCo2 colon cancer cells were cotransfected with the PPARγ1 promoter reporter and DNA encoding wt β-catenin and mutants. The luciferase reporter data are means and standard errors of the means. (C) CaCo2 cells were transfected with cyclin D1 and/or β-catenin together with the PPARγ1 promoter. The luciferase reporter data are presented as means and standard errors of the means from three independent experiments. (D) Cyclin D1 null mouse embryo fibroblasts were infected with cyclin D1 virus. Lysates were prepared and subjected to Western blot assay for PPARγ and cyclin D1. GDI served as a loading control. MSCV, murine stem cell virus. (E) The PPARγ-responsive reporter (AOX)3LUC was coexpressed with an expression vector for PPARγ1 (120 ng) and 150 or 300 ng of cyclin D1 in the presence of the PPARγ1 ligand (troglitazone) in SW480 colon cancer cells. The luciferase reporter data are means and standard errors of the means; all experiments were repeated six times. DMSO, dimethyl sulfoxide. (F) ChIP assays were performed on cyclin D1+/+ and cyclin D1−/− 3T3 cells, and immunoprecipitation was conducted with antibodies as indicated. The final DNA extractions were amplified by using pairs of primers to the PPRE region of the mouse LPL gene. IgG, immunoglobulin G.
NIH 3T3 cells express low but detectable levels of PPARγ and express wt β-catenin. Cotransfection of the β-catenin S33 expression vector (450 ng) induced (AOX)3-LUC reporter activity fivefold, and this activity was repressed 80% by cyclin D1 cotransfection. The coexpression of β-catenin/S33 and PPARγ augmented (AOX)3-LUC reporter activity 25-fold, and this activity was repressed 80% by cyclin D1 cotransfection. Thus, activated β-catenin enhances PPARγ signaling, which in turn is antagonized by cyclin D1 (Fig. 7E).
As cyclin D1 inhibited PPARγ expression in ApcMin colonic epithelium in vivo and inhibited PPARγ-responsive gene expression, ChIP assays were performed to examine possible mechanisms by which cyclin D1 might regulate local chromatin structure at an endogenous PPRE. Cyclin D1 deficiency enhanced recruitment of acetylated H3 at the PPRE of the LPL promoter (Fig. 7F). This finding is consistent with previous findings that histone H3-K9/14 acetylation associates with the promoter region of activated genes (36). These studies suggest that the abundance of cyclin D1 may regulate PPARγ signaling by altering local chromatin structure and may facilitate, directly or indirectly, the recruitment of proteins with corepressor activity to the promoters of target genes (19, 35, 41).
DISCUSSION
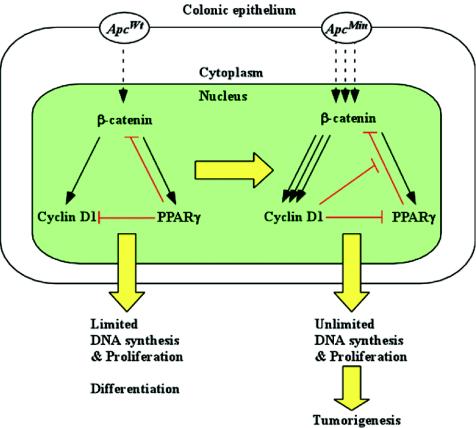
The present studies are novel, first in showing that cyclin D1 heterozygosity is a tumor-resistant genotype. In the present studies, cyclin D1 loss reduced the rate of gastrointestinal tumor formation induced by inactivation of the Apc gene. Cyclin D1 abundance in adenoma induced by ApcMin was increased approximately 50%. The absence of a single cyclin D1 allele reduced the number of polyps formed in the jejunum, ileum, and colon. A reduction in polyp formation was observed as early as 13 weeks. Second, through analyzing DNA synthesis and markers of differentiation in vivo, the present studies demonstrate the requirement for cyclin D1 as a regulator of both intestinal epithelial cell proliferation and differentiation in the presence of a specific activated oncogenic signaling pathway. Thus, cyclin D1 inhibited epithelial cell differentiation only in the presence of an activated Apc/β-catenin signaling pathway. ApcMin colonic epithelium displayed reduced goblet cell numbers, consistent with a role for Wnt signaling as an inhibitor of cellular differentiation. Cyclin D1 deficiency increased colonic epithelial cell differentiation in the presence of a mutant Apc gene. Together these studies suggest that cyclin D1 functions to inhibit differentiation and promote DNA synthesis in the presence of an activated Apc/β-catenin signaling pathway (Fig. 8). Activated Apc/β-catenin signaling induces cyclin D1, and here cyclin D1 did not affect β-catenin abundance, placing cyclin D1 downstream of β-catenin.
FIG. 8.
Cyclin D1 function in ApcMin CRC growth control. In cell with an Apcwt background, PPARγ provides negative feedback repression for β-catenin/Tcf4 signaling pathway-induced cell proliferation. Mutation of the Apc gene results in abnormal β-catenin translocation and overexpression of cyclin D1 in the nucleus. Overexpression of cyclin D1 further mediates ApcMin-dependent inhibition of colonic epithelial cell differentiation as assessed by goblet cell formation and ApcMin-dependent DNA synthesis.
Activation of Wnt/β-catenin signaling inhibits differentiation of adipocytes (43) and here enteric cell differentiation, as evidenced by the reduction in goblet cell number in the enteric crypts of the ApcMin mice. Cyclin D1 deficiency enhanced goblet cell formation, correlating with a reduction in tumor formation. Reduced representation of goblet cells is characteristic of many aberrant crypt foci of both humans and rodents (38, 40) and is considered to constitute early preneoplastic lesions (11, 40, 57). Furthermore, several CRC chemopreventive agents promote a differentiated cellular phenotype. Together these studies suggest that cyclin D1 deficiency may contribute to tumor resistance by promoting colonic epithelial cell differentiation.
As several million patients are prescribed the PPARγ agonist thiazolidinedione (TZD) for treatment of diabetes, it is important to understand the molecular genetic mechanisms by which PPARγ regulates cellular proliferation and differentiation. The present studies provide further insight into these signaling pathways in vivo. During a molecular survey of candidate genes previously implicated in the progression of CRC growth, we observed increased abundance of PPARγ1 in the ApcMin/cyclin D1−/− intestinal and colonic epithelia. These findings for gastrointestinal epithelium are consistent with recent studies showing that cyclin D1 antagonized PPARγ function and repressed PPARγ expression in fibroblasts (67). As PPARγ expression is induced during CRC differentiation, the induction of PPARγ in the ApcMin/cyclin D1−/− columnar epithelium compared with the ApcMin/cyclin D1+/+ mice is consistent with features of a more differentiated phenotype upon loss of cyclin D1. In previous studies, PPARγ1 was the predominant PPAR expressed in colonic epithelium (26). PPARγ agonists (TZD or 15-deoxy-Δ12,14-prostaglandin J2 [15d-PGJ2]) inhibited colonic tumor growth in some but not all studies (50). In recent studies, induction of transgenic antisense cyclin D1 induced PPARγ expression in the livers of transgenic mice (67). Furthermore, cyclin D1 deficiency was shown to promote adipocyte differentiation in response to PPARγ ligands, consistent with a physiological role for cyclin D1 as an inhibitor of PPARγ function (67).
Recent studies have suggested that β-catenin-dependent proliferation is regulated by PPARγ (14). Members of the PPAR family, which also includes PPARα and PPARδ, are classified as ligand-activated nuclear receptors (42). Ligands for PPARγ are members of two classes of molecules termed eicosanoids. These include 15d-PGJ2 or the TZD class. PPARγ agonists (TZD or 15d-PGJ2) inhibited the growth of implanted colonic tumors which contain mutations in the APC protein (33, 42, 50, 65, 67), and mutations in the nuclear receptor PPARγ were reported in human colon cancer (51). PPARγ is an inhibitor of cyclin D1 expression and cellular proliferation (65). Chemical carcinogen-induced intestinal tumorigenesis is increased in mice that are heterozygous for PPARγ, consistent with evidence that PPARγ may function as a cell type-specific tumor suppressor (42).
The present studies underscore the importance of cyclin D1 as an inhibitor of PPARγ abundance in vivo. Furthermore, cyclin D1 expression in cyclin D1−/− cells through viral transduction inhibited endogenous PPARγ expression. Cyclin D1 repressed PPARγ activity and promoter activity in the presence of an activating β-catenin in reporter gene studies with CaCo2 cells. Cyclin D1 inhibited β-catenin S33-induced PPARγ promoter activity and repressed (AOX)3LUC reporter activity induced by PPARγ ligands. Together these studies are consistent with a model in which cyclin D1 functions both as a key upstream inhibitor of PPARγ function and as a key downstream effector of Apc/β-catenin-induced proliferation and differentiation (Fig. 8). Induction of PPARγ transactivation by β-catenin adds to the growing list of non-Tcf sites that are regulated by activated β-catenin. These sequences include those binding CREB, retinoic acid receptor α, the androgen receptor, and unknown sites within the promyelocytic leukemia promoter (52, 54, 61). As cyclin D1 expression is induced by activated β-catenin/Tcf signaling, these studies suggest that the abundance of cyclin D1 may in turn repress a subset of genes that promote cellular differentiation. ChIP assays suggest that cyclin D1 abundance regulates local chromatin structure at an endogenous PPRE. Previous studies demonstrated a connection between acetylation at histone H3, lysine 9, an open chromatin structure, and active gene transcription (36). The present studies showed that cyclin D1 deficiency correlates with increased acetylation at these residues of histone H3 at an endogenous PPRE. Although the mechanism by which cyclin D1 regulates local chromatin structure at a PPRE to mediate associated gene repression remains to be determined, these studies extend previous observations in which increased PPARγ-responsive gene expression was seen in cyclin D1−/− cells (67).
The present studies demonstrate that intestinal and colonic tumor formation induced by ApcMin was reduced upon the loss of a single cyclin D1 allele. The cyclin D1 abundance was reduced by approximately 50% in the ApcMin/cyclin D1+/− colonic epithelium, suggesting that the relative abundance of cyclin D1 is a key determinant of tumor onset. The loss of a single allele of the cyclin-dependent kinase inhibitor p21CIP1 also promoted Apc-initiated intestinal tumor formation (69), and the loss of a single p27KIP1 allele (13), or p53, which is a regulator of p21CIP1 expression, also promotes tumor formation (64). The length of the gastrointestinal tract was not significantly altered in the cyclin D1−/− mice, and previous reports suggest that the intestine is unaffected in p21CIP1−/− mice. It is likely that the Apc mutation functions collaboratively with quantitative changes in the relative abundance of cyclin D1 or the cdk inhibitor p21CIP1 in the induction of tumor formation. Recent studies with cultured CRC cell lines suggest that the induction of c-Myc by β-catenin/Tcf represses p21CIP1 and may thereby regulate intestinal epithelial proliferation (62). The allele-dependent function of cyclin D1 in the present studies may reflect the clinical observations of a graded correlation between the abundance of either p21CIP1 or cyclin D1 and CRC clinical prognosis (72). The increase in PPARγ abundance upon loss of a single cyclin D1 allele is of interest, as PPARγ is a suppressor of colon carcinogenesis and haploinsufficiency at the PPARγ locus can increase sensitivity to chemical carcinogenesis (14).
The present studies underscore distinct roles for cyclin D1 in colonic versus mammary epithelium in the presence of an activating β-catenin signaling pathway. Cyclin D1−/− mice are relatively resistant to mammary tumor formation induced by the oncogenes ErbB2 and Ras (71). Surprisingly, cyclin D1−/− mice were not resistant to Wnt-induced mammary tumor formation (71). Mammary gland-targeted stabilized ΔN89-β-catenin induced precocious alveolar development and mammary tumors (18), consistent with a role for β-catenin in mammary epithelial cellular proliferation. Furthermore, cyclin D1 deficiency enhanced the tumorigenic phenotype of mammary gland-targeted ΔN89-β-catenin mice (44). Cyclin D1 is required for normal cellular differentiation in the murine mammary gland. As β-catenin plays a role in expanding progenitor cell compartments (21, 62), we had hypothesized that early division in the alveolar lineage may involve cyclin D1-independent stimulation οf β-catenin targets, whereas later differentiation by β-catenin may be inhibited by cyclin D1 (44). In the present studies, in the colonic epithelium, cyclin D1 inhibited differentiation and promoted proliferation only in the presence of Apcmut and not in the presence of Apcwt. Cyclin D1 has been considered a logical target for cancer therapy. The distinct role for cyclin D1 in colonic versus epithelial tumor progression induced by activated β-catenin signaling underscores the importance of delineating the tissue-specific function of a candidate therapeutic target.
Acknowledgments
We thank P. Sicinski and R. Weinberg for cyclin D1−/− mice, R. Kucherlapati for ApcMin mice, and M. Caparas for assistance with the manuscript.
This work was supported in part by grants R01CA70896, R01CA75503, R01CA86072, and R01CA86071 (to R.G.P.) and R03AG20337 (to C.A.). R.G.P was a recipient of the Irma T. Hirschl and Weil Caulier award and was the Diane Belfer Faculty Scholar in Cancer Research. Work conducted at the Lombardi Comprehensive Cancer Center was supported by the NIH Cancer Center Core grant (NIH P30 CA51008-13).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. [DOI] [PubMed] [Google Scholar]
- 3.Albanese, C., A. Reutens, M. D'Amico, B. Boumediene, M. Fu, T. Link, R. Nicholson, R. A. Depinho, and R. G. Pestell. 2000. Sustained mammary gland directed ponasterone A-inducible expression in transgenic mice. FASEB J. 14:877-884. [DOI] [PubMed] [Google Scholar]
- 4.Arber, N., Y. Doki, E. K.-H. Han, A. Sgambato, P. Zhou, N.-H. Kim, T. Delohery, M. G. Klein, P. R. Holt, and I. B. Weinstein. 1997. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 57:1569-1574. [PubMed] [Google Scholar]
- 5.Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1994. The PRAD-1/cyclin D1 oncogene product accumulates in a subset of colorectal carcinoma. Int. J. Cancer 58:568-573. [DOI] [PubMed] [Google Scholar]
- 6.Batlle, E., J. T. Henderson, H. Beghtel, M. M. W. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell 111:251-263. [DOI] [PubMed] [Google Scholar]
- 7.Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596-599. [DOI] [PubMed] [Google Scholar]
- 8.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ze'ev, A., and B. Geiger. 1998. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr. Opin. Cell Biol. 10:629-639. [DOI] [PubMed] [Google Scholar]
- 10.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 11.Bird, R. P. 1987. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 37:147-151. [DOI] [PubMed] [Google Scholar]
- 12.Brockman, J. A., R. A. Gupta, and R. N. Dubois. 1998. Activation of PPARgamma leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology 115:1049-1055. [DOI] [PubMed] [Google Scholar]
- 13.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girnun, G. D., W. M. Smith, S. Drori, P. Sarraf, E. Mueller, C. Eng, P. Nambiar, D. W. Rosenberg, R. T. Bronson, W. Edelmann, R. Kucharlapati, F. J. Gonzalez, and B. M. Spiegelman. 2002. APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc. Natl. Acad. Sci. USA 99:13771-13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart, M. J., R. de los Santos, I. N. Albert, B. Rubinfeld, and P. Polakis. 1998. Downregulation of beta-catenin by human axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol. 8:573-581. [DOI] [PubMed] [Google Scholar]
- 16.Huber, O., C. Bierkamp, and R. Kemler. 1996. Cadherins and catenins in development. Curr. Opin. Cell Biol. 8:685-691. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imbert, A., R. Eelkema, S. Jordan, H. Feiner, and P. Cowin. 2001. ΔN89β-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J. Cell Biol. 153:555-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenuwein, T. 2001. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol. 11:266-273. [DOI] [PubMed] [Google Scholar]
- 20.Kemler, R. 1993. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 9:317-321. [DOI] [PubMed] [Google Scholar]
- 21.Kielman, M. F., M. Rindapaa, C. Gaspar, N. van Poppel, C. Breukel, S. van Leeuwen, M. M. Taketo, S. Roberts, R. Smits, and R. Fodde. 2002. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat. Genet. 32:594-605. [DOI] [PubMed] [Google Scholar]
- 22.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 23.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 24.Lee, R. J., C. Albanese, R. J. Stenger, G. Watanabe, G. Inghirami, G. K. I. Haines, M. Webster, W. J. Muller, J. S. Brugge, R. J. Davis, and R. G. Pestell. 1999. pp60v-src induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways: a role for cAMP response element-binding protein and activating transcription factor-2 in pp60v-src signaling in breast cancer cells. J. Biol. Chem. 274:7341-7350. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre, A. M., I. Chen, P. Desreumaux, J. Najib, J. C. Fruchart, K. Geboes, M. Briggs, R. Heyman, and J. Auwerx. 1998. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat. Med. 4:1053-1057. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre, M., B. Paulweber, L. Fajas, J. Woods, C. McCrary, J. F. Colombel, J. Najib, J. C. Fruchart, C. Datz, H. Vidal, P. Desreumaux, and J. Auwerx. 1999. Peroxisome proliferator-activated receptor gamma is induced during differentiation of colon epithelium cells. J. Endocrinol. 162:331-340. [DOI] [PubMed] [Google Scholar]
- 27.Lin, S.-Y., W. Xia, J. C. Wang, K. Y. Kwong, B. Spohn, Y. Wen, R. G. Pestell, and M.-C. Hung. 2000. Beta-catenin, a novel prognostic marker for breast cancer: its role in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. USA 97:4262-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansen, A., H. Guardiola-Diaz, J. Rafter, C. Branting, and J. A. Gustafsson. 1996. Expression of the peroxisome proliferator-activated receptor (PPAR) in the mouse colonic mucosa. Biochem. Biophys. Res. Commun. 222:844-851. [DOI] [PubMed] [Google Scholar]
- 29.Mariadason, J. M., D. Arango, G. A. Corner, M. J. Aranes, K. A. Hotchkiss, W. Yang, and L. H. Augenlicht. 2002. A gene expression profile that defines colon cell maturation in vitro. Cancer Res. 62:4791-4804. [PubMed] [Google Scholar]
- 30.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 31.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of β-catenin Tcf-signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 32.Moser, A. R., H. C. Pitot, and W. F. Dove. 1990. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247:322-324. [DOI] [PubMed] [Google Scholar]
- 33.Mueller, E., M. Smith, P. Sarraf, T. Kroll, A. Aiyer, D. S. Kaufman, W. Oh, G. Demetri, W. D. Figg, X. P. Zhou, C. Eng, B. M. Spiegelman, and P. W. Kantoff. 2000. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc. Natl. Acad. Sci. USA 97:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muraoka, R. S., A. E. Lenferink, B. Law, E. Hamilton, D. M. Brantley, L. R. Roebuck, and C. L. Arteaga. 2002. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 22:2204-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama, J.-I., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. S. Grewal. 2001. Role of histone H3 lysine 9 methylation in heterochromatin assembly and epigenetic gene silencing. Science 292:110-113. [DOI] [PubMed] [Google Scholar]
- 36.Nicolas, E., C. Roumillac, and D. Trouche. 2003. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol. Cell. Biol. 23:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niho, N., M. Takahashi, T. Kitamura, Y. Shoji, M. Itoh, T. Noda, T. Sugimura, and K. Wakabayashi. 2003. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 63:6090-6095. [PubMed] [Google Scholar]
- 38.Otori, K., K. Sugiyama, T. Hasebe, S. Fukushima, and H. Esumi. 1995. Emergence of adenomatous aberrant crypt foci (ACF) from hyperplastic ACF with concomitant increase in cell proliferation. Cancer Res. 55:4743-4746. [PubMed] [Google Scholar]
- 39.Oyama, T., K. Kashiwabara, K. Yoshimoto, A. Arnold, and F. Koerner. 1998. Frequent overexpression of the cyclin D1 oncogene in invasive lobular carcinoma of the breast. Cancer Res. 58:2876-2880. [PubMed] [Google Scholar]
- 40.Pretlow, T. P., B. J. Barrow, W. S. Ashton, M. A. O'Riordan, T. G. Pretlow, J. A. Jurcisek, and T. A. Stellato. 1991. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res. 51:1564-1567. [PubMed] [Google Scholar]
- 41.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferase. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 42.Rosen, E. D., and B. M. Spiegelman. 2001. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 276:37731-37734. [DOI] [PubMed] [Google Scholar]
- 43.Ross, S. E., N. Hemati, K. A. Longo, C. N. Bennett, P. C. Lucas, R. L. Erickson, and O. A. MacDougald. 2000. Inhibition of adipogenesis by Wnt signaling. Science 289:950-953. [DOI] [PubMed] [Google Scholar]
- 44.Rowlands, T. M., I. V. Pechenkina, S. J. Hatsell, R. G. Pestell, and P. Cowin. 2003. Dissecting the roles of beta-catenin and cyclin D1 during mammary development and neoplasia. Proc. Natl. Acad. Sci. USA 100:11400-11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272:1023-1026. [DOI] [PubMed] [Google Scholar]
- 46.Rubinfeld, B., P. Robbins, M. El-Gamil, I. Albert, E. Porfiri, and P. Polakis. 1997. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 275:1790-1792. [DOI] [PubMed] [Google Scholar]
- 47.Rubinfeld, B., B. Souza, I. Albert, O. Muller, S. H. Chamberlain, F. R. Masiarz, S. Munemitsu, and P. Polakis. 1993. Association of the APC gene product with beta-catenin. Science 262:1731-1734. [DOI] [PubMed] [Google Scholar]
- 48.Saez, E., P. Tontonoz, M. C. Nelson, J. G. Alvarez, U. T. Ming, S. M. Baird, V. A. Thomazy, and R. M. Evans. 1998. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat. Med. 4:1058-1061. [DOI] [PubMed] [Google Scholar]
- 49.Salomon, D., P. A. Sacco, S. G. Roy, I. Simcha, K. R. Johnson, M. J. Wheelock, and A. Ben-Ze'ev. 1997. Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J. Cell Biol. 139:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarraf, P., E. Mueller, D. Jones, F. J. King, D. J. DeAngelo, J. B. Partridge, S. A. Holden, L. B. Chen, S. Singer, C. Fletcher, and B. M. Spiegelman. 1998. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat. Med. 4:1046-1052. [DOI] [PubMed] [Google Scholar]
- 51.Sarraf, P., E. Mueller, W. M. Smith, H. M. Wright, J. B. Kum, L. A. Aaltonen, A. de la Chapelle, B. M. Spiegelman, and C. Eng. 1999. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol. Cell 3:799-804. [DOI] [PubMed] [Google Scholar]
- 52.Shah, S., M. J. Pishvaian, V. Easwaran, P. H. Brown, and S. W. Byers. 2002. The role of cadherin, beta-catenin, and AP-1 in retinoid-regulated carcinoma cell differentiation and proliferation. J. Biol. Chem. 277:25313-25322. [DOI] [PubMed] [Google Scholar]
- 53.Shoker, B. S., C. Jarvis, M. P. Davies, M. Iqbal, D. R. Sibson, and J. P. Sloane. 2001. Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br. J. Cancer. 84:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shtutman, M., J. Zhurinsky, M. Oren, E. Levina, and A. Ben-Ze'ev. 2002. PML is a target gene of β-catenin and plakoglobin, and coactivates β-catenin-mediated transcription. Cancer Res. 62:5947-5954. [PubMed] [Google Scholar]
- 55.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. G. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 57.Siu, I., D. R. Robinson, S. Schwartz, H.-J. Kung, T. G. Pretlow, R. B. Petersen, and T. P. Pretlow. 1999. The identification of monoclonality in human aberrant crypt foci. Cancer Res. 59:63-66. [PubMed] [Google Scholar]
- 58.Su, L. K., K. W. Kinzler, B. Vogelstein, A. C. Preisinger, A. R. Moser, C. Luongo, K. A. Gould, and W. F. Dove. 1992. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256:668-670. [DOI] [PubMed] [Google Scholar]
- 59.Su, L. K., B. Vogelstein, and K. W. Kinzler. 1993. Association of the APC tumor suppressor protein with catenins. Science 262:1734-1737. [DOI] [PubMed] [Google Scholar]
- 60.Thomson, A. B., K. Doring, M. Keelan, and G. Armstrong. 1997. Nutrient uptake into undifferentiated and differentiated HT-29 cells in culture. Can. J. Physiol. Pharmacol. 75:351-356. [DOI] [PubMed] [Google Scholar]
- 61.Truica, C. I., S. Byers, and E. P. Gelmann. 2000. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 60:4709-4713. [PubMed] [Google Scholar]
- 62.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A.-P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 63.Velcich, A., L. Palumbo, L. Selleri, G. Evans, and L. Augenlicht. 1997. Organization and regulatory aspects of the human intestinal mucin gene (MUC2) locus. J. Biol. Chem. 272:7968-7976. [DOI] [PubMed] [Google Scholar]
- 64.Venkatachalam, S., Y.-P. Shi, S. N. Jones, H. Vogel, A. Bradley, D. Pinkel, and L. A. Donehower. 1998. Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J. 17:4657-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, C., M. Fu, M. D'Amico, C. Albanese, J. N. Zhou, M. Brownlee, M. P. Lisanti, V. K. Chatterjee, M. A. Lazar, and R. G. Pestell. 2001. Inhibition of cellular proliferation through IκB kinase-independent and peroxisome proliferator-activated receptor gamma-dependent repression of cyclin D1. Mol. Cell. Biol. 21:3057-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, C., Z. Li, M. Fu, T. Bouras, and R. G. Pestell. 2004. Signal transduction mediated by cyclin D1: from mitogens to cell proliferation: a molecular target with therapeutic potential, p. 217-237. In R. Kumar (ed.), Cancer treatment research. Kluwer Academic Publisher, Dordrecht, The Netherlands. [DOI] [PubMed]
- 67.Wang, C., N. Pattabiraman, M. Fu, J. N. Zhou, T. Sakamaki, C. Albanese, Z. Li, K. Wu, J. Hulit, P. Neumeister, P. M. Novikoff, M. Brownlee, P. Scherer, J. G. Jones, K. D. Whitney, L. A. Donehower, E. L. Harris, T. Rohan, D. C. Johns, and R. G. Pestell. 2003. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol. Cell. Biol. 23:6159-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe, G., A. Howe, R. J. Lee, C. Albanese, I. W. Shu, A. N. Karnezis, L. Zon, J. Kyriakis, K. Rundell, and R. G. Pestell. 1996. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc. Natl. Acad. Sci. USA 93:12861-12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, W., C., J. Mathew, A. Velcich, W. Edelmann, R. Kucherlapati, M. Lipkin, K. Yang, and L. H. Augenlicht. 2001. Targeted inactivation of the p21WAF1/cip1 gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosa. Cancer Res. 61:565-569. [PubMed] [Google Scholar]
- 70.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10:1443-1454. [DOI] [PubMed] [Google Scholar]
- 71.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]
- 72.Zirbes, T. K., S. E. Baldus, S. P. Moenig, S. Nolden, D. Kunze, S. T. Shafizadeh, P. M. Schneider, J. Thiele, A. H. Hoelscher, and H. P. Dienes. 2000. Prognostic impact of p21/waf1/cip1 in colorectal cancer. Int. J. Cancer 89:14-18. [DOI] [PubMed] [Google Scholar]