Abstract
Sleep is important for regulating many physiologic functions that relate to metabolism. Because of this, there is substantial evidence to suggest that sleep habits and sleep disorders are related to diabetes risk. In specific, insufficient sleep duration and/or sleep restriction in the laboratory, poor sleep quality, and sleep disorders such as insomnia and sleep apnea have all been associated with diabetes risk. This research spans epidemiologic and laboratory studies. Both physiologic mechanisms such as insulin resistance, decreased leptin, and increased ghrelin and inflammation and behavioral mechanisms such as increased food intake, impaired decision-making, and increased likelihood of other behavioral risk factors such as smoking, sedentary behavior, and alcohol use predispose to both diabetes and obesity, which itself is an important diabetes risk factor. This review describes the evidence linking sleep and diabetes risk at the population and laboratory levels.
Keywords: Sleep, Diabetes, Insomnia, Obesity, Circadian
Introduction
Insufficient sleep duration is recognized as an important unmet public health problem [1] and has been included as a national health priority in Healthy People 2020 [2]. Many previous studies have associated habitual short sleep duration to important adverse cardiometabolic outcomes, including weight gain, obesity, diabetes, cardiovascular disease, and stress. A proposed mechanism of these relationships is that insufficient sleep duration triggers dysregulation of metabolism [3–7] and increased immune response [8, 9], resulting in appetite dysregulation and cardiometabolic disease [4, 7].
The pathways between short sleep and diabetes are unclear. Several studies have used partial sleep deprivation protocols to elicit sleep loss and study metabolic consequences. These studies typically involve recruitment of young, healthy volunteers who typically sleep 8–9 h to undergo an acute sleep restriction of 4–6 h over a period of days to weeks. One common finding is that sleep deprivation is associated with insulin resistance and glucose intolerance [10–12], described in more detail below. These findings have come from studies that have employed continuous glucose monitoring [13] as well as challenge paradigms such as glucose tolerance tests [14]. This has led to a large number of studies that have aimed to explore this relationship in more detail and discern potential mechanistic pathways [11, 15–17]. Parallel to these laboratory findings, several epidemiologic studies have examined self-reported short sleepers relative to obesity and diabetes risk [18–22], frequently finding overlapping patterns.
The present review addresses the following issues: (1) population trends linking habitual insufficient sleep with diabetes risk, (2) potential physiologic mechanisms linking sleep loss with diabetes risk, (3) potential behavioral mechanisms linking sleep loss with diabetes risk, (4) increased prevalence of diabetes in obstructive sleep apnea and treatment implications, and (5) importance of circadian control of metabolism in the relationship between sleep and diabetes. We finish with recommendations for future work and conclusions.
Insufficient Sleep and Diabetes Risk: Population Trends
Definition of Insufficient Sleep
Conflicting Definitions and Operationalizations
There are currently hundreds of studies that have examined habitual sleep duration as an independent variable in relation to outcomes such as cardiometabolic disease morbidity and mortality. Although there is an emerging consensus regarding how sleep duration should be defined, there are many ways that sleep duration has been operationalized in population-level studies. First, although the most common approach is to use retrospective self-report survey items as these are most feasible for population-level research, other studies have used prospective self-report, e.g., sleep diaries, single-timepoint objective measures, e.g., polysomnography, and prospective objective measures, e.g., actigraphy. Since there is no direct measure of sleep in humans, all of these estimates assess somewhat different aspects of sleep and there is still no gold standard measure of habitual sleep duration. Because of this, varying study methodologies needs to be considered during evaluation.
Within measurement approaches (e.g., survey items, sleep diary, actigraphy), methods may also vary. For example, some survey measures use time estimates to compute sleep duration, some ask for “typical” or “weekday” (i.e., modal) sleep duration, whereas others ask for “average” (i.e., mean or median) sleep duration; also, some items specifically ask about night-time sleep only and some assess 24-h sleep. There is a lack of consensus on how to operationalize sleep duration as a variable. Some studies evaluate it as a continuous variable, assuming that effects are continuous and linear. Other studies evaluated sleep duration as a three-level variable (short, normal, and long) to account for nonlinear and potential threshold effects. Still others have used a four-factor approach (very short, short, normal, and long) to further clarify nonlinear effects, while some have used more categories, with a reference in the middle of the distribution. These varying approaches make comparisons across studies difficult. With these qualifications in mind, several important conclusions can be drawn from the existing literature regarding sleep and diabetes risk.
Consensus Recommendations
Recently, a panel convened jointly by the American Academy of Sleep Medicine and Sleep Research Society released recommendations for sleep duration in adults. This recommendation stated that 7 or more hours of sleep was likely necessary to maintain optimal health and functioning [23, 24]. In a companion document [25, 26], it was revealed that for the domain of metabolic health in particular, there was consensus that less than 7 h was inappropriate, that 7–9 h was probably appropriate, but that there was uncertainty of the appropriateness of >9 h of sleep. In a parallel effort by the National Sleep Foundation, a similar consensus panel also recommended at least 7 h of sleep for optimal health, though this group did achieve consensus that more than 9 h was not appropriate [27, 28]. Echoing these recommendations, the American Thoracic Society also released a statement that its consensus panel also warns that insufficient sleep duration (which they define as 6 h or less) is likely associated with poor health, including diabetes [29].
Prevalence of Insufficient Sleep
Population Prevalence Estimates
Based on the consensus statement released by the American Academy of Sleep Medicine and Sleep Research Society, the Centers for Disease Control and Prevention released prevalence data for insufficient sleep using the 2014 Behavioral Risk Factor Surveillance System. This report [30•] suggested that approximately one third of US adults do not get the recommended amount of sleep. Specifically, using the item, “On average, how many hours of sleep do you get in a 24-hour period?,” the distribution of sleep duration of the population is shown in Fig. 1. These estimates are consistent with other studies. For example, Krueger and Friedman [31] used data from the National Health Interview Survey to estimate that the prevalence of <7 h (i.e., ≤6 h since hours were only measured in whole numbers) is 28.3 %. Grandner and colleagues estimated this value to be higher, at 39.92 %, in the 2007–2008 wave of the National Health and Nutrition Examination Survey [32].
Fig. 1.
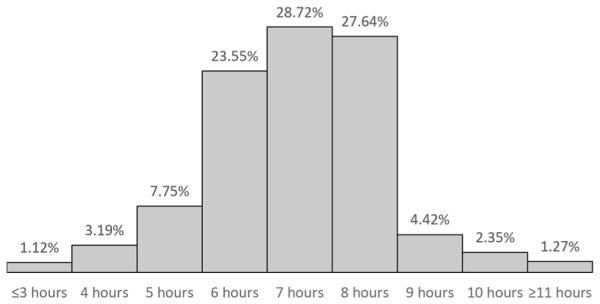
Distribution of sleep duration in the USA, using the 2014 BRFSS
Although there are claims made regarding the increased prevalence of sleep duration in society, available evidence suggests that sleep duration has not actually decreased much in recent years. For example, Bin and colleagues investigated global trends in sleep duration across 15 countries since the 1960s [33]. Overall, they found little to no evidence that sleep duration is shortening worldwide, especially in the USA. Among four US-based studies that employed similar methodology, one found a slight increase in sleep time, one found that the proportion of people sleeping 6 h or less decreased, but two found that the proportion has increased. Therefore, although a claim of increased prevalence of short sleep is not well-supported by the available data, the exceedingly high prevalence of short sleep duration is sufficiently alarming.
Social and Demographic Factors in Sleep Duration
It should be noted that sleep duration is differentially distributed in the population. For example, women are more likely to be short sleepers than men [34]. Also, racial/ethnic minorities—especially African-Americans—are more likely to be short sleepers [34, 35•, 36]. These effects are independent of socioeconomics, though poverty itself is a short sleep risk factor [34, 35•]. These patterns are especially relevant since there are gender, racial/ethnic, and socioeconomic differences in rates of diabetes prevalence, and sleep may be playing a role. In particular, sleep duration may play a role in racial/ethnic disparities in obesity and diabetes [35•, 36]. The social-ecological model of sleep and health (Fig. 2) attempts to bring these factors together to a coherent model that describes the role of sleep at the interface of upstream social-environmental determinants and downstream health consequences.
Fig. 2.
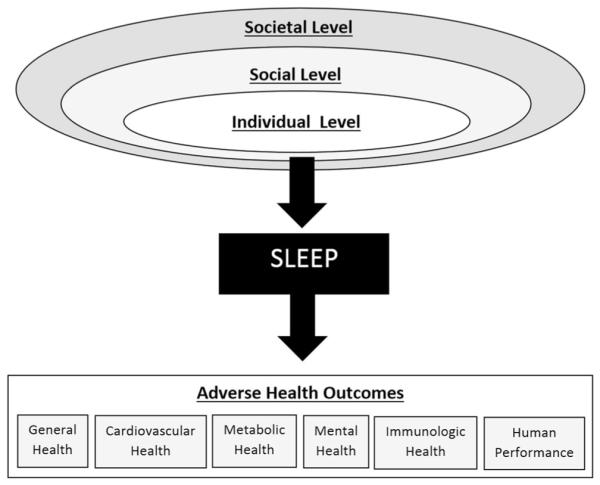
Social-ecological model of sleep and health (adapted from Grandner et al. [179], with permission from Elsevier)
Epidemiologic Studies of Sleep and Diabetes Risk
Short Sleep Duration and Diabetes
Compared to other traditional diabetes risk factors, such as being overweight, family history of diabetes, and physical inactivity, overwhelming evidence from meta-analyses indicate that curtailed sleep duration, such as <5 h per day (very short sleep) and <6 h per day (short sleep) durations, is equally predictive of diabetes, with relative risks of 1.48 (95 % CI 1.25, 1.76) and 1.18 (95 % CI 1.10, 1.26), respectively [37]. The cardiometabolic consequences of short sleep duration may be stronger in prediabetes, a precursor condition to diabetes characterized by impaired fasting glucose and glucose tolerance, and a blood sugar of 5.6 and 6.9 mmol/L. In one study, researchers found that individuals who reported short sleep duration (≤5 h) had a 2.06 higher odds of prediabetes (95 % CI 1.00–4.22) compared to individuals who reported 7 h of sleep [38].
The relationship between short sleep duration and diabetes and diabetes outcomes is well-established across different contexts and within different groups. Although men and women are affected by the negative effects of short sleep duration on diabetes, it is more pronounced in men [39]. Consistently, racial/ethnic minorities in the USA are another group affected by the negative effects of short sleep duration on diabetes [40]. In a nationally representative sample, Zizi and colleagues found that black and white short sleepers (≤5 h) were 91 % more likely to report a diabetes diagnosis compared to those who slept on average 6–8 h per day [41]. However, Jackson and colleagues found that the relationship between short sleep duration and diabetes was stronger among whites compared to blacks [42]. These two sets of findings highlight that short sleep and its impact on diabetes cut across sociodemographic factors and is a national issue that requires urgent attention. Although the putative association between short sleep and diabetes is well evidenced, the causal mechanisms that undergird this association are not fully understood. Meta-analytical evidence indicate that sleep duration, specifically short sleep duration, is associated with hemoglobin A1c (HbA1c), a marker of glucose metabolism [43]. In sum, the aforementioned evidence indicates that the relationship between short sleep duration and diabetes and diabetes outcomes may be mediated by unhealthy glucose levels and insulin resistance.
Short Sleep Duration and Obesity
Short sleep duration is associated with current and future obesity [44]. Previous evidence indicates that the relationship between short sleep and obesity is mediated by several factors which includes, but is not limited to, increased appetite/dietary intake, physical activity, and/or thermoregulation. A meta-analysis of 16 cross-sectional studies indicates that short sleepers are more likely to have nontraditional eating habits that deviate from the standard three-meals-a-day regimen, generally resulting in eating very high caloric foods at rare periods of the day. Over time, these poor eating and sleeping patterns can lead to chronic health conditions, such as obesity, cardiovascular disease, and cardiometabolic conditions. To address this pattern, it is imperative to understand the causal pathway that leads short sleepers to unhealthy dietary intake behaviors. One possible explanation is the mediating effect of leptin and ghrelin, which are hormones responsible for the physiological drives of satiation and increased appetite, respectively. Short sleepers compared to average sleepers generally have reduced leptin, elevated ghrelin, and increased body mass index [45]. Other possible pathways include increased opportunity to eat, increased fatigue, and altered thermoregulation [46••].
Potential Physiologic Mechanisms Linking Sleep Loss and Diabetes Risk
Insulin Resistance
In a landmark study by Spiegel and colleagues, 11 healthy men were assessed in the laboratory following 12 or 4 h in bed. Insulin and glucose measures were assessed using an intravenous glucose tolerance test at 5 and 10 h after meals. The results showed rather strikingly that the sleep deprivation condition was associated with higher baseline insulin and glucose and, as a result, higher homeostatic model assessment (HOMA) values, an indicator of insulin resistance [14]. This study was followed up by several others, which showed similar findings—that sleep deprivation was associated with increased insulin sensitivity and could potentially lead to insulin resistance [37, 47–49]. These studies have been seen as the strongest evidence physiologically linking sleep loss to the increased rates of diabetes observed in epidemiologic studies. Follow-up studies are examining the role of processes upstream of insulin signaling to better understand mechanisms by which sleep loss may induce insulin resistance. For example, sleep restriction may alter insulin signaling in adipocytes and this may be driving insulin resistance [50].
Leptin and Ghrelin
Several studies have suggested that the metabolic hormones leptin and ghrelin are implicated in the association between sleep and diabetes risk. Leptin is a hormone secreted by adipose tissue that is involved with signaling of feelings of satiety. Sleep laboratory studies have shown that acute sleep deprivation decreases leptin [13, 51], which would presumably result in decreased satiety. Ghrelin, on the other hand, is a hormone that is secreted by the stomach in a pulsatile manner, prior to meals, stimulating hunger. In laboratory studies, acute sleep deprivation has resulted in increased ghrelin, perhaps indicating increase physiologic hunger [12, 13, 17, 52]. This combination of increased hunger and decreased satiety may increase eating, which may predispose individuals to obesity and diabetes. Studies have shown concomitant changes to energy balance [53, 54] and subjective experiences of hunger [13] that are consistent with this hypothesis. Further, disruptions in leptin and ghrelin may also result in dysregulation of insulin and glucose, which have been explored in a number of other studies relative to sleep loss [17, 50, 55–58].
Leptin was proposed as early as 60 years ago [59] but the specific protein and gene coding regions were discovered via studies of obese mice [60]. Similar to other hormones, its secretion is pulsatile, and it has a distinct circadian rhythm, with elevations in the evening and early morning [61]. Leptin is a hormone that is primarily secreted by white adipose tissue; for this reason, it has been studied as a signal for adiposity [62, 63]. Its primary functions, though, seem to be in signaling to the hypothalamus the level of current energy reserves for the purpose of adjusting food intake and energy expenditure [63, 64]. In the case of depleted energy stores, hypoleptinemia signals activate food intake via upregulation of agouti-related protein and neuropeptide Y in the arcuate nucleus and orexin and melanin-concentrating hormone in the lateral hypothalamus. Hypoleptinemia signals also activate food intake via downregulation of pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript in the arcuate nucleus and brain-derived neurotrophic factor in the ventro-medial hypothalamus [64]. Leptin also acts in the ventrotegmental area to regulate reward experiences of and motivation for feeding, as well as in the brainstem to promote satiety [64].
A study in rats documented the nighttime rise in leptin but did not see alterations due to 5-h sleep restriction, despite showing increases in ghrelin and food intake [65]. Another study in rats, however, did show that sleep restriction lasting up to 4 weeks resulted in consistently lower leptin levels and concominant food intake [66]. Similarly, Rosa Neto and colleagues showed leptin decreases due to sleep deprivation [67], as did Martins and colleagues [68]. A study in rats showed that exogenous leptin suppresses rapid eye movement (REM) but enhances slow wave sleep [69].
Different results are seen in mice. Husse and colleagues found that total sleep deprivation was associated with elevated leptin that persisted after recovery [70]. Further, Laposky and colleagues found that leptin-deficient mice showed marked sleep dysregulation, suggesting a causal relationship between leptin and sleep regulation [71, 72].
In humans, Rasmussen and colleagues [73] studied sleep and leptin levels before and after dramatic weight loss (mean BMI = 41 to 27), compared to a nonobese control group (mean BMI = 23). The nonobese group demonstrated mean polysomnographic sleep of 448 min; the obese group slept less with a mean 360 min, which increased to 385 min following weight loss. Regarding 24-h leptin, the obese group demonstrated higher mean leptin secretion (35 μg/L) compared to the nonobese group (12 μg/L, p < 0.01). Following weight loss, leptin decreased significantly, to 17 μg/L, which was no longer significantly different from the nonobese group. Thus, it seems that the reduction in fat mass led to reductions in leptin production, and this effect outweighed any effect related to sleep duration changes.
Spiegel and colleagues studied leptin secretion changes as a result of sleep deprivation to 4 h. They found that sleep deprivation reduced mean levels of leptin by 19 %, reduced maximal levels by 26 %, and reduced rhythm amplitude by 20 % [51]. Similarly, the same group performed another study, where they studied sleep restriction for 2 days [13]. They found an 18 % decrease in leptin levels. Studies by Pejovic and colleagues [74] and Omisade and colleagues [75] both showed increased leptin following one night of sleep deprivation. Mullington and colleagues found reductions in leptin amplitude following sleep restriction [76]. However, one night of sleep deprivation did not lead to alterations in leptin levels in two studies [77, 78]. Similarly, in a study of eight individuals in a sleep restriction protocol that reduced sleep opportunity by one third for 8 days, Calvin and colleagues found no differences in leptin levels versus nine individuals who did not receive sleep restriction [79]. In a study of 11 healthy adults in a 5.5-h sleep deprivation condition, Nedeltcheva and colleagues also did not find a difference in leptin levels, despite showing increased snacking [80]. St-Onge and colleagues found that sleep deprivation to 4 h for 4 days was also not associated with leptin changes [48], while Reynolds and colleagues [55] and Simpson and colleagues [81] found that sleep restriction to 4 h for five nights resulted in increased leptin levels, a finding that was replicated by Markwald and colleagues in a longer protocol [53].
Several studies have evaluated leptin levels associated with habitual sleep duration. In a pediatric sample, Boeke and colleagues [82] found that chronic curtailed sleep was associated with lower leptin levels in girls at age 7, especially in girls with more adiposity. Among adolescents, though, lower leptin was seen among shorter-sleeping males only. No other relationships were found. In a sample of children ages 8–11, Hart and colleagues found that a sleep intervention for 3 weeks reduced energy intake and weight, but demonstrated lower leptin values [83]. In another study of 8–11-year olds, Kjeldsen and colleagues found that less sleep was associated with lower leptin values, but only in analyses adjusted only for body fat; after including other covariates, these relationships were no longer significant [84]. In samples of adolescents, Al-Disi and colleagues [85] and Martinez-Gomez and colleagues [86] found no relationship between sleep duration and leptin, though a study of male university students did show a relationship between sleep and leptin, with slightly higher leptin among short sleepers [87].
An analysis of the Wisconsin Sleep Cohort, which included 1024 adult volunteers, found that polysomnographic sleep was associated with lower leptin levels [45]. Similarly, in a subsample of the Women’s Health Initiative, short sleep duration was also associated with lower leptin levels [88]. In a sample of adults in the Quebec Family Study, Chaput and colleagues found that leptin levels were lower than expected among short sleepers, based on predicted values [89]. Charles and colleagues found that police officers sleeping 5–7 h had lower leptin values than those sleeping at least 8 h [90]. Similarly, a population-level study of adults in Brazil found that short sleep was associated with lower leptin levels in normal-weight individuals [91].
In contrast to the studies above reporting lower leptin levels with sleep short sleep duration, a report from the Cleveland Family Study, however, found that shorter sleep duration was associated with higher leptin levels [92], and a study from Taiwan found that hyperleptinemia was associated with shorter sleep duration [93]. Also, both Knutson and colleagues [94] and Littman and colleagues [95] found no association between sleep and baseline leptin. Further, Littman and colleagues found that those who increased sleep duration over time showed decreased leptin, relative to those that reduced sleep time [95]. One study evaluated baseline leptin levels in subjects with insomnia [96]. The insomnia subjects demonstrated less polysomnographic total sleep time, but no differences in leptin were found. In another study of sleep quality, this time in depressed adults, Hafner and colleagues found that poor sleep quality was associated with lower leptin, but only in normal-weight women over age 59 [97].
Given the circadian patterning of leptin, there may be an interacting role of light exposure. For example, Figueiro and colleagues found that when kept in dim light conditions (<0.5 lx), subjects in a 5-h sleep condition had reduced morning leptin levels relative to an 8-h sleep condition. However, when subjects underwent a 5-h sleep condition but were given 60 lx of either red, green, or blue light, leptin levels were significantly higher [98]. On the other hand, Garaulet and colleagues report on a CLOCK gene variant that predisposes to sleep reduction and metabolic effects, but does not seem to impact leptin values [99].
Laboratory studies have shown that sleep deprivation of normal sleepers results in decreased leptin [92, 100] and elevated ghrelin [101, 102]. Epidemiologic studies have replicated this finding, demonstrating associations between habitual short sleep duration and both decreased leptin and elevated ghrelin [45]. Ghrelin, produced in the stomach and pancreas, regulates hunger. It is a 28-amino acid peptide that was only recently discovered (in 1999) and named because of its potential role in growth hormone function, as the endogenous ligand for growth hormone secretagogue receptor 1-a (GHSR) [103]. It is primarily secreted by the stomach and its actions in the hypothalamus are most relevant to this discussion [103]. Through the GHSR pathway, ghrelin has unique orexigenic and adipogenic effects at the level of the hypothalamus [103–107].
Ghrelin has a well-characterized circadian rhythm, with increased secretion during the day, particularly before mealtimes, and marked suppression at night. Animal studies have shown that this suppression can be attenuated with restricted feeding [65]. Several studies have shown that ghrelin promotes slow wave sleep in humans [108].
Sleep deprivation may increase ghrelin. This has been seen in animal models [68, 109]. In humans, a now classic study by Spiegel and colleagues evaluated the effects of two nights of sleep curtailment to 4 h in bed in 12 healthy, young men. Total ghrelin levels after two nights of 4 h of sleep were 28 % higher, relative to a rested condition [13]. This one finding is frequently cited and has instigated several additional studies of ghrelin in sleep restriction. For example, Benedict and colleagues report that an acute sleep deprivation protocol was associated with significantly increased morning ghrelin concentrations [77]. Other studies, though have not seen changes in ghrelin [79, 80], and one study found that 24-h ghrelin decreased during sleep restriction for 5 days [53]. Sample characteristics may play a role—St-Onge found that sleep restriction was associated with increased ghrelin in men but not women [48, 110]. In contrast to these studies that employed partial sleep deprivation, few studies have been done with total sleep deprivation, but one study found elevated ghrelin levels [111].
A few studies of ghrelin associated with sleep abnormalities have also been conducted. In night eating syndrome patients, ghrelin levels were lower among those with night eating syndrome, relative to controls [112]. Motivala and colleagues found that nighttime ghrelin was lower among insomnia patients versus controls [96].
Age may also play a role. In a study of 8–11-year olds, a reduction in sleep time by 1.5 h did not produce a change in ghrelin [83]. In a study of 14–18-year-old girls, Al-Disi and colleagues found that shorter sleep duration was associated with elevated ghrelin levels [85], especially in the nonobese subsample. In a study of Danish children, Kjeldsen and colleagues, though, found that ghrelin levels were positively associated with higher sleep durations [84]. In a study of Japanese university students, no association between sleep and ghrelin levels was found [87].
In adults in the general population, actigraphically measured sleep was not associated with ghrelin in a Brazilian population sample [91] or a sample of postmenopausal, sedentary, overweight women [95]. In data from the Wisconsin Sleep Cohort Study, which was a prospective cohort study that included middle-aged adults, shorter habitual sleep duration was associated with increased ghrelin [45].
Inflammatory Cytokines
Here we briefly describe the potential role of two inflammatory cytokines in the relationship of sleep deprivation with diabetes—tumor necrosis factor (also referred to as tumor necrosis factor α or TNF-α) and interleukin-6 (IL-6). TNF-α is a cytokine that is produced by activated macrophages. It has many roles as a proinflammatory molecule, including stimulation of the acute phase reaction. It is also involved with systematic inflammation. TNF-α was given its name when it was discovered to induce apoptosis in tumor cells [113]. TNF-α promotes several related immune cell functions [114]. The primary functions of TNF-α involve activation of the nuclear factor kappa (NFk) B and mitogen-activated protein (MAP) kinase pathways. NFkB is a protein complex that controls DNA transcription and is involved in cellular responses to stressors, and MAP kinase is involved in many cellular processes, including response to stressors and gene expression. Taken together, these roles promote immune response and cell regulation.
Although several studies have evaluated relationships between TNF-α and sleep, some of these results are inconclusive. For example, one study found that sleep restriction to 6 h across 12 nights led to elevated 24-h TNF-α levels among men but not women [115]. Another study of one night of sleep restriction found increases in TNF-α messenger RNA expression [116]. Not all studies showed positive findings. In a study where sleep was restricted to two 2-h naps, no elevations in TNF-α were seen [117]. Another study of sleep restriction for 10 days found no changes in the levels of the TNF-α p55 receptor, which is a critical component for many of the functions of TNF-α [118]. It is possible to show increases in TNF-α without increased levels of the receptor, though, as this was seen in one study of total sleep deprivation [119].
Some of the inconsistent findings may in part be due to individual differences in vulnerability to sleep loss and the different receptor types studied [9]. Only one study has investigated TNF-α in a population setting. In the Cleveland Family Study, shorter polysomnographic sleep (which may not be related to habitual sleep) was associated with elevated TNF-α, such that each hour of sleep was associated with an 8 % increase in TNF-α [120]. Taken together, these studies suggest that sleep may play a regulatory role in TNF-α function.
Like TNF-α, IL-6 is a proinflammatory molecule. It is secreted by T-cells and macrophages, and like TNF-α, it plays an important role in both the acute phase response and chronic inflammation. It also plays a role in body temperature regulation and stimulation of energy utilization in adipose tissue, where 15–30 % of IL-6 is expressed. Interestingly, IL-6 release is 2–3 times greater in visceral, as opposed to subcutaneous, adipose tissue [121]. This may be one reason why IL-6 levels are associated with obesity [122]. It should be noted that IL-6 can have anti-inflammatory processes, since it inhibits TNF-α and IL-1, and promotes IL-10, whic his anti-inflammatory.
IL-6 is characterized by a circadian rhythm. It is highest at night, with a noted elevation around sleep onset [123]. IL-6 is then suppressed by slow wave sleep, which characterizes much of the early parts of sleep [123]. Sleep deprivation delays (pushes back) the nighttime peak of IL-6, which may be the reason for demonstrated undersecretion at night and oversecretion during the day [123]. If IL-6 is administered exogenously, slow wave sleep and REM sleep toward the beginning of the night are suppressed, with a rebound later in the night [123]. Interestingly, even though sleep deprivation in the laboratory may lead to nighttime undersecretion, a study of night shift work showed increased secretion at night [124].
Several laboratory studies have examined the effects of experimental sleep restriction on IL-6. Haack and colleagues found that after 12 days in the laboratory, where sleep was restricted to 4 h (versus 8 h), IL-6 levels were elevated by 62 % [118]. Similarly, in another 12-day laboratory study (this time with sleep restricted to 6 h), IL-6 secretion was again elevated [115]; since 24-h sampling was available, it was shown that differences were found between 18:00 and 24:00, and 3:30 and 6:00. A more recent study found that after five nights of sleep restriction to 4 h in 13 healthy young men, an increase in IL-6 was observed, which did not normalize after a limited recovery opportunity [125].
Several studies have also examined habitual sleep. In the Cleveland Family Study, elevated IL-6 (7 % increase per hour) was associated with self-reported long sleep duration [120]. In a Taiwanese cohort, long sleep duration was also associated with elevated IL-6 [126]. Although these studies did not find associations with short sleep, a study of Alzheimer’s caregivers and controls found that actigraphic sleep duration was negatively correlated with IL-6 [127], and a study of mothers found that self-reported short sleep of <5 h was associated with elevated IL-6 at 3 years postpartum [128].
Habitual short sleep duration has been associated with increased risk of cardiovascular disease [4, 7], though mechanisms for this relationship are unknown. Proposed mechanisms involve increased neurobehavioral stress response, increased oxidative stress, and increased inflammation. All of these proposed mechanisms are partially supported by the finding of increased inflammatory biomarkers associated with short sleep duration. This finding has been seen in the context of laboratory studies of sleep deprivation of normal sleepers, as well as population-based studies of habitual short sleepers [4]. Specifically, elevations in IL-6 and TNF-α associated with short sleep have been demonstrated in both population [120, 128] and laboratory [115, 116, 118, 125] studies, but these have not been evaluated in verified short sleepers.
Potential Behavioral Mechanisms Linking Sleep Loss and Diabetes Risk
In addition to physiologic pathways linking sleep loss and diabetes risk, there are a number of potential indirect pathways by which behavioral mechanisms may be playing a role. Sleep loss may lead to the consumption of more calories during the 24-h period, impaired decision-making that could lead an individual to make more unhealthy food choices, and increased likelihood of other unhealthy behaviors that can increase diabetes risk.
Increased Caloric Intake
Several studies have shown that sleep loss can increase caloric intake across the 24-h period. St-Onge and colleagues showed that otherwise healthy individuals, when restricted to 5 h of sleep opportunity, consumed approximately 500 kcal per day compared to a well-rested condition [54]. This finding was replicated and extended by Markwald and colleagues, who showed that this increase in caloric intake was associated with increased energy expenditure at night during the period where the individual was typically asleep [53]. Also, this study showed that the increased caloric intake occurred exclusively at night, while morning caloric intake was slightly reduced. In a large study by Spaeth and colleagues, this finding was replicated in a more diverse sample [129, 130]. The Spaeth study additionally showed that this increased energy intake was associated with about 1 kg of weight gain across 5 days, though the weight gain was disproportionately experienced by Black/African-American men, followed by non-Hispanic White men and Black/African-American women. Non-Hispanic White women showed the least weight gain. In a separate laboratory study, increased food intake following sleep deprivation was restricted to calorie-dense snacks [80]. Thus, sleep loss may increase energy intake at night, especially from energy-dense sources, resulting in weight gain and increased diabetes risk [131].
Impaired Decision-Making
Unhealthy eating is also a product of unhealthy food choices. Making healthy food choices can be difficult, and this can be even more difficult under conditions of sleep loss. Sleep loss impairs executive function [132–136]. In particular, sleep loss may impair an individual’s ability to make healthy choices by limiting their ability to accurately weigh risks and benefits of an action [137] and promote hedonic decision-making [138]. These could lead to an unhealthy eating pattern that may increase risk for diabetes.
Increased Likelihood of Other Unhealthy Behaviors
Habitual short sleep duration is associated with other unhealthy behaviors that may increase diabetes risk. Increased sedentary lifestyle is associated with habitual short sleep duration [139, 140], perceived insufficient sleep [141], and general sleep disturbances [142]. Smoking is also associated with both short sleep duration [140] and poor sleep quality [143]. Individuals with sleep difficulties are also more likely to consume excessive alcohol [144, 145]. Taken together, sleep difficulties such as insufficient sleep duration and poor sleep quality may lead to unhealthy behaviors that are themselves diabetes risk factors.
Diabetes and Obstructive Sleep Apnea
Overlapping Prevalence of Diabetes and Sleep Apnea
Type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA) are very prevalent in the obese population. Combining the data from the five NHANES studies showed that of the three variables age, race, and body mass index (BMI), BMI was the greatest contributor to the prevalence of diabetes [146]. T2DM in obesity is characterized by both insulin resistance and impaired insulin secretion. OSA, independent of obesity, has been shown to induce insulin resistance, although the exact mechanism is unclear [147].
A wide variation exists in the prevalence of OSA in patients with T2DM. This variation stems from the differences in the study population and definitions for respiratory disturbance index (RDI) to measure sleep breathing disorders [148]. Foster et al. noted an 86 % prevalence of OSA in his obese diabetic population [149]. Similarly, Aronsohn et al. reported a 77 % prevalence of OSA in their patients with T2DM. They also noted poorer glycemic controls with increasing severity of OSA [150]. Brooks et al. noted the prevalence of OSA in his diabetic population to be 70 %, although this study was limited by its size and a screening questionnaire of excessive sleepiness and snoring before recruitment [151]. Einhorn et al. noted a slightly lower prevalence of 36 % [152]. Heffner in his retrospective study of 16,066 persons with diabetes noted that only about 18 % of the population was diagnosed with OSA, a number significantly lower than that of the estimated prevalence in the studies mentioned above [153]. Similarly, many cross-sectional and prospective studies have shown a higher prevalence of T2DM in patients with OSA [154–156], confirming this bidirectional relationship of these chronic medical conditions.
Obstructive Sleep Apnea and Obesity
Obesity is one of the strongest risk factors for the development of OSA with a proportionally increasing prevalence with increasing BMI [157]. While the prevalence of OSA in the general population is estimated to be 0.15–0.3 %, the prevalence in the obese population is estimated to be 19–31 % [158]. Increasing BMI alters the upper airway mechanics contributing to the upper airway obstruction. Several mechanisms such as increased pharyngeal fat deposition, airway edema, alterations in the leptin signaling pathways, as well as reduction of the functional residual capacity, have been incriminated as the reasons for the development and progression of OSA [159, 160]; details of the pathophysiology are beyond the scope of this review.
Sleep Apnea and Insulin Resistance
Insulin resistance is characterized by decreased peripheral insulin responsiveness, resulting in decreased glucose uptake and glucose intolerance. Sleep apnea has been shown to be independently associated with increased insulin resistance [161], irrespective of obesity, body fat distribution, and age [162, 163]. The pathophysiology behind this association is believed to be due to the effects of sleep apnea on sleep, namely intermittent hypoxia (IH) and sleep fragmentation which trigger activation of the sympathetic nervous system, oxidative stress, systemic inflammation, dysregulation of the appetite-regulating hormones, and activation of the hypothalamic-pituitary-adrenal axis. These mechanisms contribute in the progression toward insulin resistance [164].
Activation of the sympathetic nervous system by IH associated with sleep apnea is thought to play a major role in the development of insulin resistance by reducing insulin sensitivity, insulin-mediated glucose uptake, and reduction of insulin secretion. Activation of the sympathetic nervous system could increase cortisol synthesis by activation of the hypothalamic-pituitary-adrenal axis. Cortisol increases glucose production, decreases peripheral glucose uptake, and inhibits insulin secretion from the pancreatic beta cells. Reactive oxygen species (ROS), generated due to oxidative stress secondary to IH, may worsen insulin resistance by inhibiting insulin-induced energy substrate uptake in muscle and adipose tissue. Low levels of appetite regulating hormones such as leptin and adiponectin have been associated with higher insulin resistance. Sleep fragmentation due to sleep apnea, resulting in sleep deprivation and loss of REM sleep, may also contribute to reduced glucose tolerance [164].
Sleep Apnea, Oxidative Stress, and Inflammation
IH associated with sleep apnea may result in oxidative stress and release of ROS, which contributes to a proinflammatory state [164]. ROS may inhibit the uptake of glucose in peripheral tissues and suppress insulin secretion [164]. Oxidative stress has been linked to pancreatic beta cell proliferation and cell death [165]. Treatment with continuous positive airway pressure (CPAP) therapy has been shown to attenuate oxidative stress [166]. Moderate to severe OSA is associated with endothelial dysfunction, increased arterial stiffness, and elevated serum inflammatory markers [167]. A baseline proinflammatory state is seen in diabetic patients as well, with elevated levels of circulating inflammatory cytokines, such as interleukin-6 and tumor necrosis factor alpha, which have been shown to play a role in the pathogenesis of insulin resistance and T2DM [164]. The exact mechanism of action of chronic inflammation in the pathogenesis of insulin resistance in sleep apneic patients is not known.
Treatment of Sleep Apnea
The potential mechanism of CPAP therapy on glycemic control is incompletely understood. Elimination of intermittent hypoxia and sleep fragmentation with CPAP therapy may result in improved glycemic control [168]. Observational studies have shown the beneficial effect of CPAP therapy on insulin resistance in patients with OSA and T2DM [151, 169].There are a limited number of randomized control trials (RCT) to evaluate the effect of CPAP therapy on glucose control in diabetic patients, with inconsistent results due to short-term follow-up and small sample sizes.
Martinez-Ceron and colleagues in a recently concluded RCT to evaluate the effect of CPAP therapy on OSA patients with suboptimally controlled diabetes mellitus showed improved insulin resistance and glycemic control, measured by HbA1c, over a period of 24 weeks. These benefits were not limited to patients with severe OSA. This improvement was thought to be secondary to reversal of the proinflammatory status attributed to CPAP therapy [170].
The optimal duration of CPAP therapy lacks clarity as well. It has been reported that glycemic control in T2DM is associated with apneas and hypopneas related to REM sleep rather than nonrapid eye movement (NREM) sleep [171]. Extension of CPAP use for up to 7 h was associated with greater reduction in HbA1c levels when compared to patients with 4 h of CPAP use [171].
In conclusion, CPAP use is associated with improved insulin sensitivity and glycemic control. These beneficial effects may be related to longer duration of CPAP use and greater compliance with CPAP therapy in patients with suboptimal control of diabetes.
Future Directions
There are several key directions for future research. For epidemiologic studies, perhaps the most pressing issue is a lack of phenotypic clarity regarding sleep habits associated with diabetes risk. Current studies stress the issue of insufficient sleep, yet estimates of insufficient sleep rely on habitual sleep duration. Not only is there a lack of objective, or even validated subjective, measures of sleep duration at the population level, sleep duration does not capture sleep need, which may vary across individuals, as does resilience to sleep loss. Further, the potentially high rate of confounding with insomnia needs to be clarified. In short, although the association between insufficient sleep and diabetes risk is relatively well-established, important characteristics and qualifications still need to be discerned.
From a physiologic standpoint, two main future directions are apparent from the literature. First, laboratory studies need to better operationalize real-world sleep loss so that relevant mechanistic links that increase population risk for diabetes are separated from apparent links that merely reflect an acute reaction to a laboratory setting. Questions remain as to whether pathways such as leptin, ghrelin, insulin, and inflammation reflect real-world causal pathways linking habitual short sleep duration and diabetes. Second, the role of circadian physiology needs to be explored in more detail. Existing basic research is showing that many metabolic processes are under circadian control and that circadian gene transcription patterns can influence insulin signaling and adipocyte function [172••, 173]. Also, in animal models, circadian patterns of food intake differentially impact aspects of weight regulation [173–178]. Currently, though, almost none of these studies have not made the translational step to human research.
Conclusions
Sleep is important for many physiologic processes, and many of these processes are involved in regulation of metabolism. Perhaps because of this, insufficient sleep and sleep disorders have been identified as novel and important risk factors for the development of diabetes. This is particularly alarming since insufficient sleep is experienced by approximately one third of the US population and sleep apnea—a sleep disorder highly prevalent among middle-aged and older adults—is present in three quarters or more of persons with diabetes. Increased recognition of the importance of sleep in the understanding of and management of diabetes is warranted. Current literature suggests both physiologic and behavioral pathways linking sleep and diabetes risk, which are also related to obesity risk. An overall schematic of this is presented in Fig. 3. Future research is needed to clarify the epidemiologic links between diabetes and specific sleep phenotypes, as well as to discern mechanistic links between sleep and metabolic dysfunction.
Fig. 3.
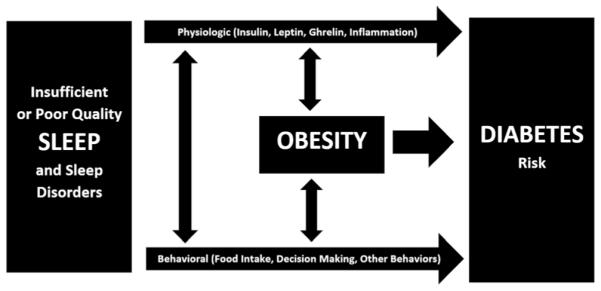
The role of physiologic and behavioral pathways and obesity in linking insufficient sleep duration and/or poor sleep quality and sleep disorders to diabetes risk
Acknowledgments
This work was supported by K23HL110216.
Footnotes
Conflict of Interest Michael A. Grandner, Azizi Seixas, Safal Shetty, and Sundeep Shenoy declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of Importance
•• Of major importance
- 1.Colten HR, Altevogt BM, Institute of Medicine Committee on Sleep Medicine and Research . Sleep disorders and sleep deprivation: an unmet public health problem. xviii. Institute of Medicine, National Academies Press; Washington: 2006. p. 404. [PubMed] [Google Scholar]
- 2.Office of Disease Prevention and Health Promotion . Healthy People 2020 objective topic areas. US Department of Health and Human Services; Washington: 2011. [Google Scholar]
- 3.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14(4):402–12. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14:239–47. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10(1):43–7. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Kavuru M. Sleep and metabolism: an overview. Int J Endocrinol. 2010:2010. doi: 10.1155/2010/270832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16(2):137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34(11):2454–7. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cauter E. Sleep disturbances and insulin resistance. Diabet Med. 2011;28(12):1455–62. doi: 10.1111/j.1464-5491.2011.03459.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–8. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–93. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman NG, Izci-Balserak B, Schopfer E, Jackson N, Rattanaumpawan P, Gehrman PR, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261–70. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33(7):956–61. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2008;30(12):1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 23.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–4. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–52. doi: 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–83. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirshkowitz M, Whiton K, Alpert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–43. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Hirshkowitz M, Whiton K, Alpert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, et al. An official American Thoracic Society statement: the importance of healthy sleep. Recommendations and future priorities. Am J Respir Crit Care Med. 2015;191(12):1450–8. doi: 10.1164/rccm.201504-0767ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–41. doi: 10.15585/mmwr.mm6506a1. This report provides the first nationwide, county-level estimates of short sleep duration based on the newly released consensus statement. [DOI] [PubMed] [Google Scholar]
- 31.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169(9):1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev. 2012;16(3):223–30. doi: 10.1016/j.smrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–11. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. This review represents a comprehensive overview of the issues around sleep disparities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013;79:7–15. doi: 10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2015;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabet Med. 2013;30(6):676–80. doi: 10.1111/dme.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 40.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19(5):351–7. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Zizi F, Pandey A, Murrray-Bachmann R, Vincent M, McFarlane S, Ogedegbe G, et al. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. Am J Med. 2012;125(2):162–7. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson CL, Redline S, Kawachi I, Hu FB. Association between sleep duration and diabetes in black and white adults. Diabetes Care. 2013;36(11):3557–65. doi: 10.2337/dc13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SW, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.02.001. doi:10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–59. doi: 10.3945/an.115.008623. This review provides information regarding connections between sleep and dietary intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandner MA. Addressing sleep disturbances: an opportunity to prevent cardiometabolic disease? Int Rev Psychiatry. 2014;26(2):155–76. doi: 10.3109/09540261.2014.911148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9(1):73–80. doi: 10.5664/jcsm.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463(1):139–60. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 52.Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6(6):341–7. [PubMed] [Google Scholar]
- 53.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7(7):e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darukhanavala A, Booth JN, 3rd, Bromley L, Whitmore H, Imperial J, Penev PD. Changes in insulin secretion and action in adults with familial risk for type 2 diabetes who curtail their sleep. Diabetes Care. 2011;34(10):2259–64. doi: 10.2337/dc11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padilha HG, Crispim CA, Zimberg IZ, De-Souza DA, Waterhouse J, Tufik S, et al. A link between sleep loss, glucose metabolism and adipokines. Braz J Med Biol Res. 2011;44(10):992–9. doi: 10.1590/s0100-879x2011007500113. [DOI] [PubMed] [Google Scholar]
- 58.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140(901):578–96. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 61.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 63.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45(11):1455–62. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 64.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: The role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152(2):93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1071–9. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 66.Barf RP, Desprez T, Meerlo P, Scheurink AJ. Increased food intake and changes in metabolic hormones in response to chronic sleep restriction alternated with short periods of sleep allowance. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R112–7. doi: 10.1152/ajpregu.00326.2011. [DOI] [PubMed] [Google Scholar]
- 67.Rosa Neto JC, Lira FS, Venancio DP, Cunha CA, Oyama LM, Pimentel GD, et al. Sleep deprivation affects inflammatory marker expression in adipose tissue. Lipids Health Dis. 2010;9:125. doi: 10.1186/1476-511X-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martins PJ, Fernandes L, de Oliveira AC, Tufik S, D’Almeida V. Type of diet modulates the metabolic response to sleep deprivation in rats. Nutr Metab (Lond) 2011;8(1):86. doi: 10.1186/1743-7075-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8(3):197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 70.Husse J, Hintze SC, Eichele G, Lehnert H, Oster H. Circadian clock genes Per1 and Per2 regulate the response of metabolism-associated transcripts to sleep disruption. PLoS One. 2012;7(12):e52983. doi: 10.1371/journal.pone.0052983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2059–66. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen MH, Wildschiodtz G, Juul A, Hilsted J. Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss. Obesity (Silver Spring) 2008;16(7):1516–21. doi: 10.1038/oby.2008.249. [DOI] [PubMed] [Google Scholar]
- 74.Pejovic S, Vgontzas AN, Basta M, Tsaoussoglou M, Zoumakis E, Vgontzas A, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19(4):552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99(5):651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 76.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15(9):851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 77.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schioth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93(6):1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 78.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 79.Calvin AD, Carter RE, Adachi T, Macedo P, Albuquerque FN, van der Walt C, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144:79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12(1):47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boeke CE, Storfer-Isser A, Redline S, Taveras EM. Childhood sleep duration and quality in relation to leptin concentration in two cohort studies. Sleep. 2014;37(3):613–20. doi: 10.5665/sleep.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hart CN, Carskadon MA, Considine RV, Fava JL, Lawton J, Raynor HA, et al. Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics. 2013;132(6):e1473–80. doi: 10.1542/peds.2013-1274. [DOI] [PubMed] [Google Scholar]
- 84.Kjeldsen JS, Hjorth MF, Andersen R, Michaelsen KF, Tetens I, Astrup A, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond) 2014;38(1):32–9. doi: 10.1038/ijo.2013.147. [DOI] [PubMed] [Google Scholar]
- 85.Al-Disi D, Al-Daghri N, Khanam L, Al-Othman A, Al-Saif M, Sabico S, et al. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J. 2010;57(10):915–23. doi: 10.1507/endocrj.k10e-145. [DOI] [PubMed] [Google Scholar]
- 86.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, et al. Sleep duration and emerging cardio-metabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12(10):997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Matsumoto Y, Toyomasu K, Uchimura N. Assessment of physical and mental health in male university students with varying sleep habits. Kurume Med J. 2011;58(4):105–15. doi: 10.2739/kurumemedj.58.105. [DOI] [PubMed] [Google Scholar]
- 88.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, et al. Short sleep duration is associated with decreased serum leptin, increased energy intake, and decreased diet quality in post-menopausal women. Obesity (Silver Spring) 2013;22:E55–61. doi: 10.1002/oby.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 90.Charles LE, Gu JK, Andrew ME, Violanti JM, Fekedulegn D, Burchfiel CM. Sleep duration and biomarkers of metabolic function among police officers. J Occup Environ Med. 2011;53(8):831–7. doi: 10.1097/JOM.0b013e31821f5ece. [DOI] [PubMed] [Google Scholar]
- 91.Moraes W, Poyares D, Zalcman I, de Mello MT, Bittencourt LR, Santos-Silva R, et al. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 2013;14(4):312–8. doi: 10.1016/j.sleep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34(2):147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koo M, Lai NS, Chiang JK. Short duration of sleep is associated with hyperleptinemia in Taiwanese adults. J Clin Sleep Med. 2013;9(10):1049–55. doi: 10.5664/jcsm.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G, Study NSE. No association between leptin levels and sleep duration or quality in obese adults. Obesity (Silver Spring) 2011;19(12):2433–5. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Littman AJ, Vitiello MV, Foster-Schubert K, Ulrich CM, Tworoger SS, Potter JD, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31(3):466–75. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 96.Motivala SJ, Tomiyama AJ, Ziegler M, Khandrika S, Irwin MR. Nocturnal levels of ghrelin and leptin and sleep in chronic insomnia. Psychoneuroendocrinology. 2009;34(4):540–5. doi: 10.1016/j.psyneuen.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hafner S, Baumert J, Emeny RT, Lacruz ME, Thorand B, Herder C, et al. Sleep disturbances and depressed mood: a harmful combination associated with increased leptin levels in women with normal weight. Biol Psychol. 2012;89(1):163–9. doi: 10.1016/j.biopsycho.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Figueiro MG, Plitnick B, Rea MS. Light modulates leptin and ghrelin in sleep-restricted adults. Int J Endocrinol. 2012;2012:530726. doi: 10.1155/2012/530726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garaulet M, Sanchez-Moreno C, Smith CE, Lee YC, Nicolas F, Ordovas JM. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111 T/C SNP and weight loss. PLoS One. 2011;6(2):e17435. doi: 10.1371/journal.pone.0017435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 101.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10(Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 104.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 105.Ueno H, Shiiya T, Nakazato M. Translational research of ghrelin. Ann N Y Acad Sci. 2010;1200:120–7. doi: 10.1111/j.1749-6632.2010.05509.x. [DOI] [PubMed] [Google Scholar]
- 106.Heppner KM, Tong J. Regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur J Endocrinol. 2014;171:R21–32. doi: 10.1530/EJE-14-0183. [DOI] [PubMed] [Google Scholar]
- 107.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 108.Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, et al. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab. 2003;284(2):E407–15. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 109.Vetrivelan R, Fuller PM, Yokota S, Lu J, Saper CB. Metabolic effects of chronic sleep restriction in rats. Sleep. 2012;35(11):1511–20. doi: 10.5665/sleep.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17(3):331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 112.Allison KC, Ahima RS, O’Reardon JP, Dinges DF, Sharma V, Cummings DE, et al. Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. J Clin Endocrinol Metab. 2005;90(11):6214–7. doi: 10.1210/jc.2005-1018. [DOI] [PubMed] [Google Scholar]
- 113.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14(3-4):185–91. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 114.Jain S, Mills PJ. Cytokines, chronic stress, and fatigue. In: Fink G, editor. Encyclopedia of stress. 1. 2nd Academic; Oxford: 2007. pp. 698–704. [Google Scholar]
- 115.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 116.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 117.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 118.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56(2):318–24. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 122.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 123.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9(6):603–14. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 124.Khosro S, Alireza S, Omid A, Forough S. Night work and inflammatory markers. Indian J Occup Environ Med. 2011;15(1):38–41. doi: 10.4103/0019-5278.82996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21(11):799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.von Kanel R, Ancoli-Israel S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, et al. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56(1):41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Mantzoros CS. Maternal short sleep duration is associated with increased levels of inflammatory markers at 3 years postpartum. Metabolism. 2011;60(7):982–6. doi: 10.1016/j.metabol.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100(2):559–66. doi: 10.3945/ajcn.114.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shechter A, Grandner MA, St-Onge MP. The role of sleep in the control of food intake. Am J Lifestyle Med. 2014;8(6):371–4. doi: 10.1177/1559827614545315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jackson ML, Croft RJ, Kennedy GA, Owens K, Howard ME. Cognitive components of simulated driving performance: sleep loss effects and predictors. Accid Anal Prev. 2013;50:438–44. doi: 10.1016/j.aap.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 133.Jackson ML, Gunzelmann G, Whitney P, Hinson JM, Belenky G, Rabat A, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17(3):215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7(9):e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muto V, Shaffii-le Bourdiec A, Matarazzo L, Foret A, Mascetti L, Jaspar M, et al. Influence of acute sleep loss on the neural correlates of alerting, orientating and executive attention components. J Sleep Res. 2012;21(6):648–58. doi: 10.1111/j.1365-2869.2012.01020.x. [DOI] [PubMed] [Google Scholar]
- 136.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96(4):564–82. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Killgore WD, Grugle NL, Killgore DB, Leavitt BP, Watlington GI, McNair S, et al. Restoration of risk-propensity during sleep deprivation: caffeine, dextroamphetamine, and modafinil. Aviat Space Environ Med. 2008;79(9):867–74. doi: 10.3357/asem.2259.2008. [DOI] [PubMed] [Google Scholar]
- 138.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180(10):997–1006. doi: 10.1093/aje/kwu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL. Smoking, screen-based sedentary behavior, and diet associated with habitual sleep duration and chronotype: data from the UK Biobank. Ann Behav Med. 2016 doi: 10.1007/s12160-016-9797-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grandner MA, Jackson NJ, Izci-Balserak B, Gallagher RA, Murray-Bachmann R, Williams NJ, et al. Social and behavioral determinants of perceived insufficient sleep. Front Neurol. 2015;6:112. doi: 10.3389/fneur.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grandner MA, Patel NP, Perlis ML, Gehrman PR, Xie D, Sha D, et al. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. J Public Health. 2011;19(5):463–74. doi: 10.1007/s10389-011-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jaehne A, Unbehaun T, Feige B, Lutz UC, Batra A, Riemann D. How smoking affects sleep: a polysomnographical analysis. Sleep Med. 2012;13(10):1286–92. doi: 10.1016/j.sleep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 144.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, Verschuren WM. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: the MORGEN study. Eur J Prev Cardiol. 2014;21(11):1367–75. doi: 10.1177/2047487313493057. [DOI] [PubMed] [Google Scholar]
- 146.Menke A, Rust KF, Fradkin J, Cheng YJ, Cowie CC. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med. 2014;161(5):328–35. doi: 10.7326/M14-0286. [DOI] [PubMed] [Google Scholar]
- 147.Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131(5):1387–92. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 148.Redline S, Kapur VK, Sanders MH, Quan SF, Gottlieb DJ, Rapoport DM, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000;161(2):369–74. doi: 10.1164/ajrccm.161.2.9904031. [DOI] [PubMed] [Google Scholar]
- 149.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79(6):1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 152.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13(4):355–62. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 153.Heffner JE, Rozenfeld Y, Kai M, Stephens EA, Brown LK. Prevalence of diagnosed sleep apnea among patients with type 2 diabetes in primary care. Chest. 2012;141(6):1414–21. doi: 10.1378/chest.11-1945. [DOI] [PubMed] [Google Scholar]
- 154.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 156.Lindberg E, Theorell-Haglöw J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest. 2012;142(4):935–42. doi: 10.1378/chest.11-1844. [DOI] [PubMed] [Google Scholar]
- 157.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 158.Shetty S, Parthasarathy S. Obesity hypoventilation syndrome. Curr Pulmonol Rep. 2015;4(1):42–55. doi: 10.1007/s13665-015-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fogel RB, Malhotra A, White DP. Sleep. 2: pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(2):159–63. doi: 10.1136/thorax.2003.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 162.Makino S, Handa H, Suzukawa K, Fujiwara M, Nakamura M, Muraoka S, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64(1):12–9. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 163.Theorell-Haglow J, Berne C, Janson C, Lindberg E. Obstructive sleep apnoea is associated with decreased insulin sensitivity in females. Eur Respir J. 2008;31(5):1054–60. doi: 10.1183/09031936.00074907. [DOI] [PubMed] [Google Scholar]
- 164.Martinez Ceron E, Casitas Mateos R, Garcia-Rio F. Sleep apneahypopnea syndrome and type 2 diabetes. A reciprocal relationship? Arch Bronconeumol. 2015;51(3):128–39. doi: 10.1016/j.arbres.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 165.Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46(6):783–90. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 166.Alonso-Fernandez A, Garcia-Rio F, Arias MA, Hernanz A, de la Pena M, Pierola J, et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64(7):581–6. doi: 10.1136/thx.2008.100537. [DOI] [PubMed] [Google Scholar]
- 167.Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, et al. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martinez-Ceron E, Fernandez-Navarro I, Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev. 2016;25:121–30. doi: 10.1016/j.smrv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 169.Harsch IA, Schahin SP, Bruckner K, Radespiel-Troger M, Fuchs FS, Hahn EG, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71(3):252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 170.Martinez-Ceron E, Barquiel B, Bezos AM, Casitas R, Galera R, Garcia-Benito C, et al. Effect of CPAP on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–85. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 171.Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–63. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172••.Perelis M, Ramsey KM, Marcheva B, Bass J. Circadian transcription from beta cell function to diabetes pathophysiology. J Biol Rhythms. 2016;31(4):323–36. doi: 10.1177/0748730416656949. This thorough review describes some of the current state of the science in the relationship between basic circadian physiology and metabolic regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, et al. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38(12):1849–60. doi: 10.5665/sleep.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27(2):69–83. doi: 10.1016/j.tem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347(6227):1265–9. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hatori M, Panda S. Response of peripheral rhythms to the timing of food intake. Methods Enzymol. 2015;552:145–61. doi: 10.1016/bs.mie.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 178.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20(6):1006–17. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grandner MA, Patel NP, Hale L, Moore M. Mortality associated with sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


