Abstract
In long-term depression (LTD) at synapses in the adult brain, synaptic strength is reduced in an experience-dependent manner. LTD thus provides a cellular mechanism for information storage in some forms of learning. A similar activity-dependent reduction in synaptic strength also occurs in the developing brain and there provides an essential step in synaptic pruning and the postnatal development of neural circuits. Here we review evidence suggesting that LTD and synaptic pruning share components of their underlying molecular machinery and may thus represent two developmental stages of the same type of synaptic modulation that serve different, but related, functions in neural circuit plasticity. We also assess the relationship between LTD and synaptic pruning in the context of recent findings of LTD dysregulation in several mouse models of autism spectrum disorder (ASD) and discuss whether LTD deficits can indicate impaired pruning processes that are required for proper brain development.
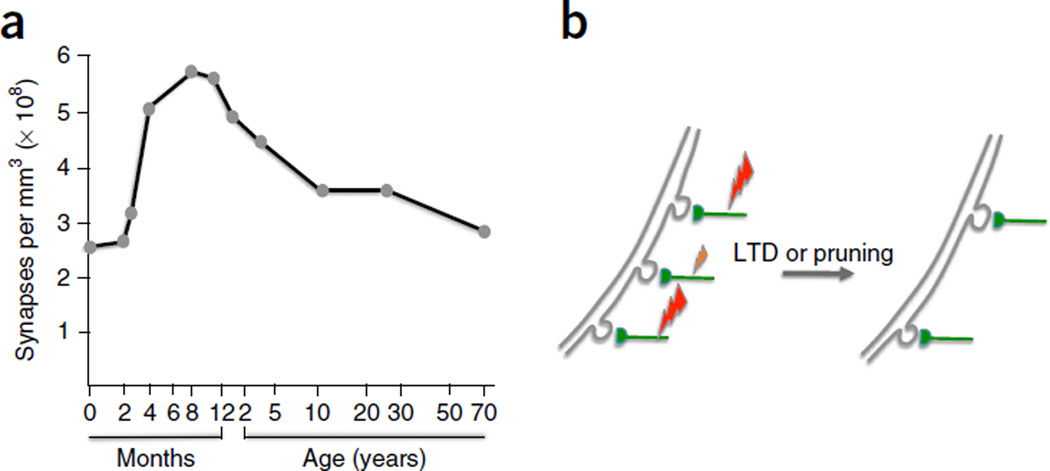
The density of synaptic connections undergoes dramatic changes during nervous system development: for example, in the human cortex a sharp increase in synaptic density during the first 1–2 years after birth is followed by a prolonged period of competitive, activity-dependent synapse elimination that reduces synaptic density by about 50% and ultimately results in the typical microarchitecture of the mature cortex (Fig. 1a)1. Synaptic pruning occurs subsequent to a period of axonal pruning that takes place during the first months after birth. In primates, about 70% of callosal axons are eliminated postnatally2. These numbers illustrate the enormous extent of connectivity modifications in the developing brain.
Figure 1.
Experience-dependent pruning shapes the cortical circuit architecture. (a) Synaptic density as a function of age in the human primary visual cortex. (b) The stabilization or elimination of cortical spines depends on the level of input activity and is controlled by Hebbian plasticity rules. Large and small lightning bolts symbolize synaptic input strength. LTD at weakly active synapses may result in synapse and spine pruning. Both LTD and pruning are activity-dependent processes that require activity at a threshold level. Panel a adapted from ref. 1, Elsevier.
Developmental synaptic pruning is a phenomenon that is well-known from observations at the developing neuromuscular junction (NMJ) in rodents: at birth, each muscle fiber receives synapses from approximately ten motor nerve axons3, which are eliminated—except for one—during the second postnatal week4. The stabilization and loss of synaptic contacts are preceded respectively by corresponding strengthening and weakening of synaptic efficacy5. Elimination of weaker inputs can be prevented by laser removal of the strong input6. These observations suggest that axon withdrawal follows a competitive process at the level of synapses reminiscent of synaptic competition in bidirectional synaptic plasticity—that is, long-term potentiation (LTP) and LTD—in the mature CNS (for review, see refs. 7–9). In his book The Organization of Behavior, Donald Hebb proposed his famous rule for synaptic modifications in neuronal assemblies10:
When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased.
A more popular version of this rule reads “neurons that fire together wire together.” While Hebb’s rule does not explicitly mention the weakening of synapses, this effect was subsequently suggested by Stent11 on the basis of the work of Hubel and Wiesel that described plasticity during the critical period in the visual cortex of kittens12. In fact, these and subsequent studies on plasticity in the visual cortex have inspired early suggestions of an essential overlap between molecular pathways involved in LTD and synaptic pruning (see refs. 8,13). We will begin this review by describing the cortical findings that support this suggestion and outline the implications.
There are two good reasons to now revisit the idea of a role of LTD-like molecular pathways in synaptic pruning, more than 15 years after it was initially presented. First, a recent focus on synaptopathies in developmental brain disorders14,15 has resulted in a new interest in synaptic dysfunction in autism spectrum disorder (ASD), particularly in deficits in synapse maturation and pruning. Brain overgrowth is one of the earliest signs in some forms of ASD16. Both brain hyperconnectivity17 and hypoconnectivity18,19 have been found in children with autism. The resulting change in excitatory drive and activity patterns, as well as cascading changes in network function via plasticity mechanisms, might explain why autism often is associated with either hyper- or hyposensitivity to sensory input20. Unexpectedly, one of the most consistent findings in ASD mouse models is a dysregulation of LTD, which has been observed across different genetic abnormalities and across different brain areas (see below). We propose here that LTD dysregulation reflects alterations in synaptic pruning that prevent proper development of neuronal circuits.
A second important reason to re-evaluate the relationship between LTD and synaptic pruning is that additional details about the molecular pathways involved have become available. It is well established that changes in synaptic strength can be associated with structural plasticity21. However, the data now available enable a molecule-by-molecule comparison of plasticity processes in LTD and synaptic pruning that had not been possible before. Most of the new molecular data have been obtained from recordings in the mouse or rat cerebellum. Therefore, while we will also compare synaptic depression mechanisms at different developmental stages at the NMJ and cortical synapses, we will build our argument largely on findings from cerebellar synapses. In the context of synaptic pruning deficits in autism, this is not an obvious choice. However, it is at glutamatergic synapses onto cerebellar Purkinje cells that the molecular pathways underlying both LTD, at parallel fiber (PF) synapses, and developmental synaptic pruning, at climbing fiber (CF) synapses, have been described at a level of detail that is not available from other CNS synapses. In fact, CF synapses are one of the few types of synapses in the CNS where developmental synaptic pruning can be readily assessed using electrophysiological techniques and where the number of inputs can be quantified on the basis of the number of discrete all-or-none steps in synaptic responses. At most other CNS synapses it is more difficult to isolate responses evoked by single presynaptic neurons, and thus this quantification method cannot be readily applied. In addition, cerebellar synaptic abnormalities have been studied in diverse ASD mouse models, motivated by frequent observations of cerebellar dysfunction associated with autism (for review, see refs. 22,23). Thus, cerebellar circuits are optimally suited to study how the same molecular machinery is used for synaptic depression mechanisms in the developing and the adult brain and how pathological alterations in this machinery affect circuit function and behavior (Box 1).
Box 1. Outlook.
The intimate relationship between LTD and synaptic pruning suggested here raises a series of new questions and challenges: frst, the paradox seems to exist that in autism LTD often is enhanced or even saturated, but post-mortem analysis of human brains points toward reduced pruning113–115. The cerebellar studies allow us to take a closer look. In Fmr1 knockout mice, enhanced LTD is accompanied by an acceleration of CF elimination132. In contrast, in patDp/+ mice (and likely in Nlgn3 mutant mice)133,135 LTD is impaired and CF elimination is delayed136. Thus, these mouse studies suggest that in ASD synaptic plasticity and pruning can either be too strong or too weak (see also ref. 127). Similarly, decreased network connectivity has been reported in autism (for example, refs. 18,19), although most studies show increased connectivity. Future work will have to examine the consequences of these opposing abnormalities for brain development and function. Moreover, studies are needed of how alterations in synapse formation and/or maintenance (not discussed here) add to network connectivity changes that result from abnormalities in synaptic pruning.
Second, recent fndings suggest that FMRP is required for the degradation and elimination of synapses through its interaction with myocyte enhancer factor-2 (MEF2)144. This fnding suggests that abnormal regulation of mRNA translation in autism may not only affect LTD, but also subsequent steps in synaptic pruning, along with many more cellular processes. It will be important to test whether MEF2 acts in LTD and which additional MEF2-controlled pathways might be relevant in the context of ASD.
Third, we need to determine which autism symptoms can be explained by LTD dysregulation and defcits in synaptic pruning. We have recently shown that, in a mouse model for the human 15q11–13 duplication, LTD dysregulation may contribute to the impairment of a form of associative motor learning (EBC) that is affected in individuals with autism136. This study provides a rare demonstration of an ASD-typical behavioral alteration that is a direct consequence of LTD dysregulation. Moreover, EBC is conserved throughout vertebrate evolution and thus can be used as a biomarker that allows direct comparison of motor defcits between mice and humans (see ref. 145). Beyond the cerebellum and motor behaviors, such a link between synaptic dysfunction and behavioral defcits has so far not be established, likely because of the complexity of cortical circuits and the behaviors that these circuits control. The question thus remains whether defcits in LTD and synaptic pruning may cause cognitive dysfunction and abnormalities in social behaviors by preventing the proper development of cortical circuits.
Fourth, we need to better understand plasticity processes in the developing and adult brain, beyond their importance in autism. It is remarkable that the scale of developmental plasticity is dramatically larger than the scale of adult synaptic plasticity. What are the factors that limit adult plasticity? It has been suggested that critical periods for developmental plasticity are controlled by the maturation of the local inhibitory network25 and that developmental changes in the degree of synapse or circuit plasticity result from the relative availability of plasticity molecules146. However, it remains to be determined why critical periods are not absolute and synapse elimination can be observed following LTD in the adult CNS, and how critical time windows can be extended or reopened for clinical intervention147.
Fifth, one of the most intriguing recent fndings in synaptic plasticity is that in the adult brain LTD can be followed by the elimination of weak synapses102–104. It is well known that the number and morphology of spines can change in adult brain plasticity148,149. However, a less established fnding is that synapses weakened in LTD are subject to disconnection and elimination. How does synapse elimination in the adult brain relate to spine plasticity? Most importantly, can the removal of weak synapses contribute to information storage and learning?
Pruning in the visual cortex following monocular deprivation
The work of Hubel and Wiesel on connectivity changes during the critical period in the primary visual cortex of kittens12 has laid the groundwork for subsequent studies on synaptic pruning and its relationship to LTD. For this reason, we will begin with a discussion of activity-dependent pruning events in the primary visual cortex. In the adult visual cortex, the majority of neurons clustered in a cortical column respond preferentially to the stimulation of one eye, a phenomenon known as ocular dominance. The adult neuronal connectivity pattern results from developmental synaptic rewiring during the critical period including the elimination of synapses serving the nondominant eye. The degree of plasticity during the critical period is remarkably high, as demonstrated by Hubel and Wiesel in their classic monocular deprivation studies: if one eye is deprived of visual input during the critical period, neurons lose responsiveness to this eye, and the ocular dominance segregation is heavily weighted toward the intact eye12. Synaptic depression initiated by monocular deprivation is an active process that requires residual activity in the visually deprived retina24 and acts in synergy with the inhibitory GABAergic system25 that further promotes the shrinkage of ‘depressed’ spines and the survival of ‘potentiated’ ones26. Thus, synaptic depression during developmental circuit plasticity shares activity dependence with LTD in the mature cortex. These findings, together with the emerging similarities in the molecular pathways involved (see below), have led to the notion that the mechanisms underlying synaptic pruning resemble those found in LTD (for review, see refs. 8,13). Indeed, both bidirectional synaptic plasticity in the mature brain and synapse stabilization or pruning in the developing brain seem to adhere to the extended Hebb rule10,11 and similarly describe activity-dependent ‘learning’ mechanisms in neuronal circuits.
It was first suggested by Bienenstock, Cooper and Munro that synaptic depression at inputs from the deprived eye initiates the loss of responsiveness to visual stimulation27. This hypothesis has been experimentally supported: first, monocular deprivation by lid suture, which preserves residual activity in the retina, leads to a more pronounced depression of responses to input from the deprived eye than monocular inhibition by intraocular injection of tetrodotoxin. Thus, the reduction in responsiveness is facilitated by ongoing activity, which is a feature shared with activity-dependent, homosynaptic LTD24. Second, synaptic depression triggered by monocular deprivation occludes subsequent LTD induction28, suggesting that monocular deprivation initiates LTD and that LTD marks an early step in synaptic pruning.
The molecular pathways involved in LTD induction in the visual cortex and in deprivation-induced synaptic pruning show a strong overlap. NMDA receptor activation is required not only for LTP29,30, but also for homosynaptic LTD30,31. Larger calcium transients are required for LTP induction than for LTD32, suggesting that the amplitude of NMDA receptor responses and the associated calcium transients determine whether the synapses are potentiated or depressed. Just as in LTD, the ocular dominance shift following monocular deprivation also is NMDA receptor-dependent33,34. While acute metabotropic glutamate receptor 5 (mGluR5) inhibition has no appreciable effect on NMDA-receptor-dependent LTD or ocular dominance plasticity, both processes are impaired by chronic mGluR5 inhibition, suggesting that mGluR5 receptors are not directly involved in the respective induction pathways but that, during a critical period, mGluR5 activation is required to obtain some type of permissive condition shared by LTD and synaptic pruning35. Finally, both molecular deprivation and LTD cause the same changes in AMPA receptor phosphorylation and membrane expression: namely, decreased phosphorylation of GluA1 at Ser845, increased phosphorylation of GluA2 at Ser880 and reduced surface expression of GluA1 and GluA2 subunits28. These findings support the view that in the developing cortex LTD-like molecular mechanisms provide the initial steps in synaptic pruning (for review, see refs. 8,13). It is noteworthy that LTP also shares molecular events with pruning, such as the need for NMDA receptor activation and calcium signaling29,30, reflecting the activity dependence of these circuit-forming processes. However, the molecular pathways involved in LTP on the one hand and LTD and pruning on the other diverge further downstream in the signaling pathways— most notably at the level of GluA1 and GluA2 phosphorylation28—and thus LTP presents a plasticity phenomenon that even molecularly is clearly distinct from depression and synaptic pruning.
The study of dendritic spine plasticity offers an additional, ‘postsynaptic’ perspective on pruning and its relationship to LTD. The turnover rate of spines is high in the cortex of young and young adult rodents, but the lifetime of dendritic spines increases with age36–38. However, just as is the case for synaptic plasticity, spine plasticity persists in adulthood, enabling experience-dependent structural circuit plasticity following the redistribution of synaptic strength21 (Fig. 1b). Both processes are tightly related. For example, at hippocampal synapses LTD is accompanied by spine shrinkage, and both processes depend on the activation of NMDA receptors39. Similarly, the elimination of dendritic spines in the primary sensory cortex is activity and NMDA receptor dependent40, as are LTD and pruning in the visual cortex. Therefore, the results obtained from spine plasticity studies in cortical areas other than the visual cortex support the claim that LTD and pruning share elements of their underlying molecular pathways.
The molecular machinery of synaptic depression: cerebellum
LTD was first described as a cellular correlate of learning in the cerebellum. Here, LTD at PF synapses onto Purkinje cells may reduce the level of inhibition that the GABAergic Purkinje cells impose on their target cells in the cerebellar and vestibular nuclei, thus promoting activity in downstream motor and non-motor control pathways. LTD is a postsynaptic form of plasticity that can be triggered by PF and CF coactivation (for review, see ref. 41). LTD is reversed by postsynaptic LTP that results from PF activation alone (for review, see ref. 42). Both LTD and LTP contribute to a distributed cerebellar memory engram that involves several plasticity sites and mechanisms43,44.
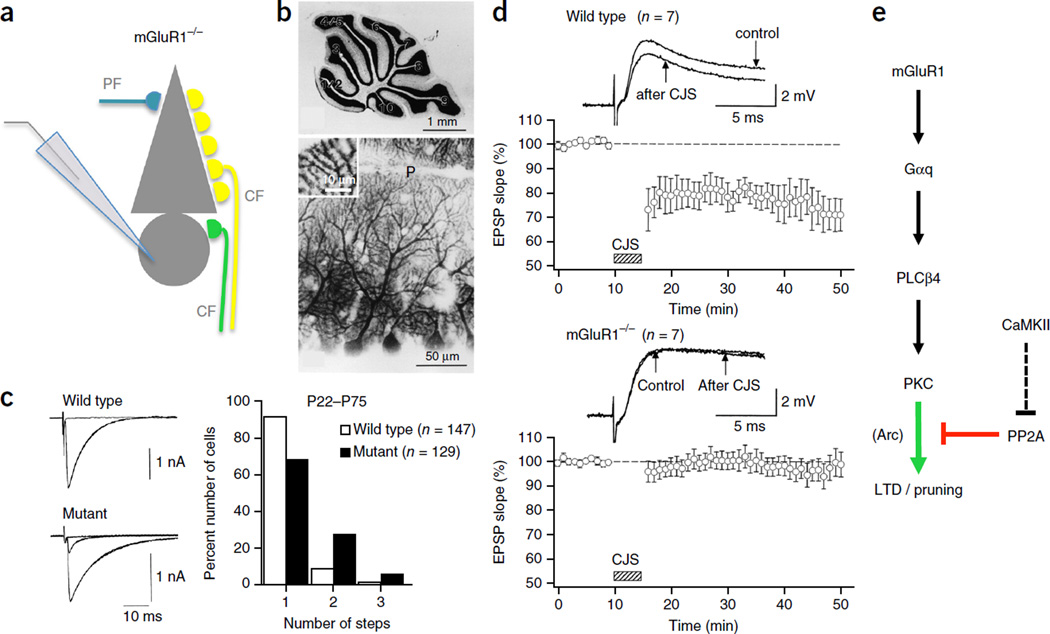
LTD induction depends on the activation of type 1 metabotropic glutamate receptors (mGluR1)45–47 (Fig. 2; for review, see ref. 48). Group I mGluRs (consisting of mGluR 1 and 5; only mGluR1 is expressed at appreciable levels in Purkinje cells) couple to Gαq proteins that activate a signaling cascade including phospholipase Cβ4 (PLCβ4) and protein kinase C (PKC; for review, see ref. 49). The activation of mGluR1 receptors specifically promotes LTD, but is not needed for LTP induction50. Consistent with the involvement of an mGluR1 signaling cascade, LTD is absent from Gαq knockout mice (Gnaq−/− mice)51 and from PLCβ4 knockout mice (Plcb4−/− mice)52. PLC produces diacylglycerol and inositol-1,4,5-trisphosphate (IP3) from phosphatidylinositol-4,5-bisphosphate, triggering the subsequent release of calcium from IP3-sensitive intracellular stores. Both consequences of PLC activity—production of diacylglycerol and calcium release from IP3-sensitive stores—promote the activation of PKC (for review, see ref. 49). LTD is indeed prevented by PKC inhibitors53,54, and it is absent from transgenic mice that express the pseudosubstrate PKC inhibitor PKC19–31 selectively in Purkinje cells55. Subsequent studies showed that the phosphorylation of GluA2 AMPA-type glutamate receptor subunits at Ser880 by PKCα is a critical step in GluA2 endocytosis and LTD56,57. Purkinje cells only weakly express GluA1 subunits58. Thus, GluA2 at Ser880 is the main PKC target in cerebellar LTD. In summary, PF LTD requires the activation of an mGluR1–PLCβ4–PKCα signaling cascade that provides an early step in GluA2 endocytosis, which removes functional AMPA receptors from the postsynaptic density and mediates LTD.
Figure 2.
Impaired CF synapse elimination and deficient PF LTD in mGluR1 knockout mice. (a) Schematic of the patch-clamp configuration used to record from an mGluR1 knockout Purkinje cell (PC) that is illustrated with multiple CF innervation. (b) Gross anatomy of the cerebellum (top, Nissl staining) and morphology of PC dendrites (bottom, calbindin immunostaining) are normal in mGluR1 knockout mice. (c) Persistent multiple CF innervation in adult mGluR1 knockout mice (P22–P75). CF-mediated EPSCs (left) and frequency distribution histogram showing the number of discrete CF EPSC steps at increasing stimulus strength (right), representing the number of CF inputs. (d) LTD at PF PC synapses is deficient in adult mGluR1 knockout mice. In wild-type mice, EPSPs elicited by PF stimulation undergoes LTD after conjunctive PF and CF stimulation (CJS) at 1 Hz for 5 min (top). In contrast, PF EPSPs are not depressed by CJS in mGluR1 knockout mice (bottom). All values are shown as mean ± s.e.m. (e) mGluR1 signaling pathway. CaMKII activation contributes to LTD through an indirect blockade of PP2A. CaMKII may similarly contribute to CF synaptic pruning, but this has not yet been verified. mGluR1, type 1 metabotropic glutamate receptor; Gαq, G-protein αq; PLCβ4, phospholipase Cβ4; PKC, protein kinase C; αCaMKII, α isoform of calcium/calmodulin-dependent kinase II; PP2A, protein phosphatase 2A; Arc, activity-regulated cytoskeleton-associated protein (also known as Arg3.1). Panels b,c adapted from ref. 73, Elsevier; d from ref. 47, AAAS.
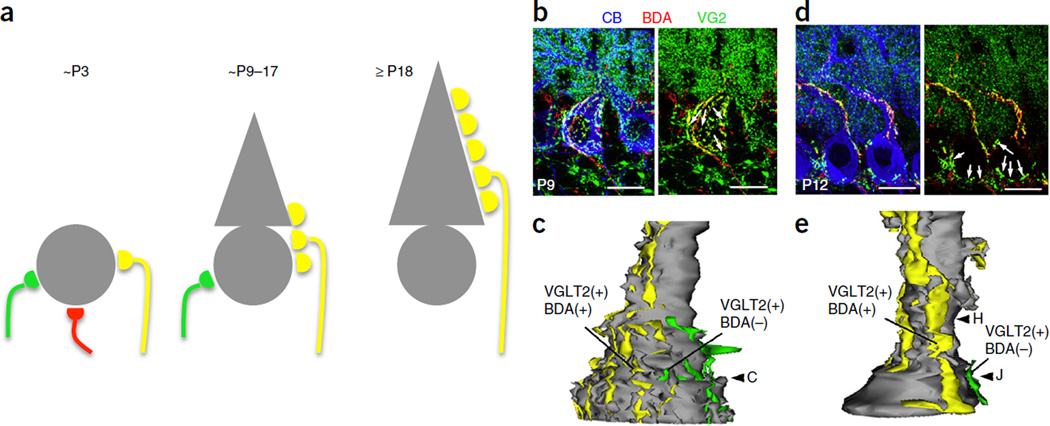
One of the best-studied examples of developmental synaptic pruning is the elimination of surplus CF inputs in the cerebellum. Purkinje cells are contacted at birth by usually three to five CFs, which are eliminated until, by the end of the third postnatal week, only one CF input remains in most Purkinje cells59. CF pruning is an active process and consists of distinct phases (for review, see ref. 60). During the first postnatal week, differences in synaptic input strength of several CFs innervating the soma become noticeable, with a single CF input emerging that can be identified as the future ‘winner’ CF input on the basis of response strength and morphology; this is the functional differentiation phase61. It seems that the one CF input is selectively strengthened whose activity is most closely related to the occurrence of calcium spikes and calcium influx through P/Q-type voltage-gated calcium channels in the Purkinje cell dendrite62,63. Subsequently the winner CF input begins to translocate to the dendrite, while maintaining synapses on the soma (Fig. 3)64,65, a step that also involves anterograde signaling by C1q-like 1 (C1ql1)66.
Figure 3.
Developmental CF synapse elimination in the rodent cerebellum. (a) Schemes representing CF innervation of Purkinje cells (PCs) at three stages of postnatal development. At around P3, the PC soma is innervated by multiple CFs with similar synaptic strengths. At around P9 to P17, a single winner CF extends its innervation territory from the soma to the growing PC dendrite, whereas the loser CFs maintain synapses on the soma. After P18, most of the somatic CF synapses are eliminated and a single winner CF innervates the PC, forming synapses on spines located on the primary dendrite. (b,d) Triple fluorescence labeling at P9 (b) and P12 (d) for BDA (biotinylated dextran amine, a tracer labeling a CF subset), VGluT2 (type 2 vesicular glutamate transporter, a CF terminal marker) and CB (calbindin, a PC marker). (b) At P9, the soma is innervated by BDA and VGluT2 double-positive CFs (yellow puncta) and BDA-negative and VGluT2-positive CF terminals (arrows, green puncta), indicating innervation by two different CFs. (d) At P12 the PC dendrites are innervated by BDA and VGluT2 double-positive CFs (yellow puncta) and the somata are contacted by BDA-negative and VGluT2-positive CFs (arrows, green puncta), indicating single strong CF inputs on PC dendrites and additional weak CF inputs on the somata. (c,e) Three-dimensionally reconstructed image of CF innervation from serial electron microscopic analysis of a PC at P9 (c) and P12 (e). Scale bars in b and d, 10 µm. Panels b–e adapted from ref. 64, Elsevier.
The weaker CF inputs, which are characterized by a lower probability of multivesicular release61, do not translocate to the dendrite and will be withdrawn in two distinct phases of CF elimination, an early and a late, that occur largely in parallel with the translocation of the winner CF to the dendrite64. This form of developmental plasticity is competitive, as reversal of the fate of a loser CF input has been observed following ablation of the emerging winner CF input65. The early phase of CF synapse elimination starts at around postnatal day (P) 7, just after the functional differentiation phase, and is independent of proper PF–Purkinje cell synapse formation67.
The late phase of CF synapse elimination starts at around P12 and continues until around P17. This phase marks the ongoing synaptic competition process at CF synapses, and it critically depends on the proper formation and activity of PF synapses67 and inhibitory basket cell synapses68 onto Purkinje cells. Thus, as in the visual cortex, where the critical period for developmental plasticity is defined by the proper excitation–inhibition balance26, CF synapse elimination also involves GABAergic signaling. Several signaling molecules involved in the late phase have been identified, as described below. Eventually, the elimination of loser CF synapses is completed by retraction, likely involving engulfment by glial cells for final digestion (for review, see ref. 60). Observations at cortical synapses show that microglia are indeed involved in synaptic pruning during development and thus contribute to the clearance of cellular material even in the uninjured brain69. Deficits in microglia recruitment (Cx3cr1 knockout mice)70,71 or in the tagging of synapses for removal by microglia (C1q knockout mice)72 result in impaired synaptic pruning, enhanced seizure rates72 and autism-resembling social behavior deficits71.
The molecular events involved in CF pruning, specifically the late phase of CF elimination, have been characterized in detail in a series of studies using genetically modified mice. In mutant mice lacking mGluR1 receptors (Grm1−/− mice), the regression of surplus CFs slows at the end of the second postnatal week and remains incomplete even after P22, an age at which the elimination process is typically finished (Fig. 2)73,74. Purkinje cell–specific expression of an alpha isoform of mGluR1 (mGluR1α) transgene rescues proper CF elimination47. These data show that mGluR1 receptors are crucial to CF synapse elimination. Similarly, impairment of CF elimination at the end of the second postnatal week has been observed in mutant mice lacking the G protein Gαq (Gnaq−/− mice)75, in mice lacking PLCβ4 (Plcb4−/− mice)52,76, and in mice lacking PKCγ (Prkcg−/− mice)77. Moreover, CF elimination is impaired in mice expressing the pseudosubstrate PKC inhibitor PKC19–31 (ref. 55). These findings show that the developmental elimination of surplus CF inputs depends, just as does LTD at PF synapses, on an mGuR1–Gαq–PLCβ4–PKC signaling cascade (Table 1; for review, see ref. 48). These results also show that the overlap in the molecular machinery involved is not complete: while CF elimination is impaired in Prkcg−/− mice, these mice show no LTD deficit54. Rather, LTD induction depends on the activation of PKCα56,57. The recent discovery of PF synapse elimination during cerebellar development completes the picture: PF synaptic pruning is impaired in mutant mice lacking mGluR1 or PKCγ78, suggesting that the same mGluR1–PKCγ signaling cascade that mediates CF synaptic pruning acts in PF synapse elimination as well. The specific roles of PKCα and PKCγ in synapse depression and elimination, respectively, remain to be determined.
Table 1.
LTD and synaptic pruning: overlap in molecular machinery
| Signaling pathway | LTD (PF) | Synaptic pruning (CF) | References |
|---|---|---|---|
| mGluR1 | X | X | 45,46,73 |
| Gαq | X | X | 51,75 |
| PLCβ4 | X | X | 52,76 |
| PKC | X | X | 55,57,77 |
| αCaMKII | X | X | 79 |
| Arc | X | X | 91,92 |
Both LTD at PF synapses and the developmental elimination of surplus CF synapses are impaired (X) in genetically modifed mice that, in most cases, were originally studied to examine the relationship between LTD and motor learning. The left column shows the targeted molecules.
In addition to the involvement of PKC, LTD depends on the activation of α-calcium/calmodulin-dependent protein kinase II (aCaMKII). In αCaMKII null mice (Camk2a−/− mice), LTD is absent, while LTP is unaffected79. More recently, it has been shown that CaMKII promotes LTD through an indirect pathway that involves the negative regulation of phosphodiesterase 1 (PDE1), the subsequent facilitation of cGMP–protein kinase G signaling and the downregulation of protein phosphatase 2A (PP2A)80. PP2A is part of the LTP induction pathway81 and might counteract the effects of PKC activation in LTD82. In Camk2a−/− mice, the developmental elimination of surplus CFs is impaired79 (Table 1), suggesting that the CaMKII-mediated removal of the LTD-brake mechanism exerted by PP2A acts in synaptic pruning as well. In hippocampal neurons, CaMKII translocation to the postsynaptic density has been linked to the activation of NMDA-type glutamate receptors and the resulting nanodomain calcium transients83. Purkinje cells express NMDA receptors containing the juvenile GluN2D subunit at extrasynaptic sites during the first postnatal week84, but their role at this early developmental stage remains unknown. Only at the end of the third postnatal week do Purkinje cells begin to express functional NMDA receptors at their CF input sites, and full expression levels are only reached ∼2 months after birth85,86. In ≥2-month-old mice, LTD induction at PF synapses depends on the activation of NMDA receptors87. In contrast, while NMDA receptor activation in the cerebellum is required for CF elimination88,89, the developmental onset of NMDA receptors expressed in Purkinje cells comes too late to permit a role in CF elimination. Thus, Purkinje cell NMDA receptors provide an exception to the notion that the molecular machineries involved in PF LTD and CF synaptic pruning are largely overlapping.
Finally, the immediate early gene product Arc, which promotes the endocytosis of AMPA receptor subunits and may contribute to the stabilization of recently adjusted synaptic weights (for review, see ref. 90), is required for the late phase of LTD91, as well as for the removal of surplus CF synapses from Purkinje cell somata during the late phase of the elimination process92,93 (Table 1).
These examples show that the molecular machineries involved in LTD and CF pruning are largely overlapping, suggesting that they represent related cellular processes. This notion holds for the initial synapse competition involved. The actual removal of loser synapses is guided by additional signaling factors. This may be the reason why in some mouse models (for example, Camk2a−/−) CF elimination is only delayed, and is eventually completed in adult mice79, suggesting that impairment of the initial synaptic competition process does not always fully prevent pruning.
LTD at climbing fiber synapses: the missing link
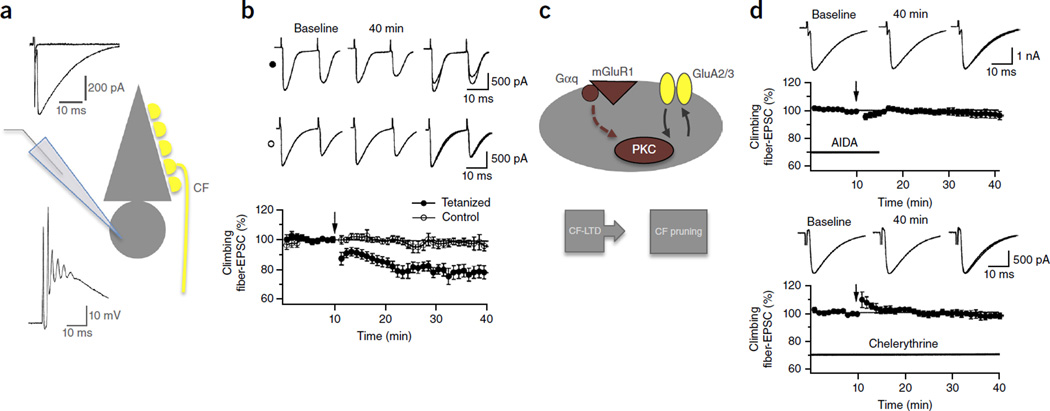
Our analysis so far, with the exception of a recent study on PF synapse elimination78, was based on the comparison of LTD at PF synapses and synaptic pruning at CF synapses. These are useful examples because at these synapses LTD and synaptic pruning have been studied in rich molecular detail. An obvious disadvantage of this approach is that we are comparing these plasticity phenomena at two different synapses. However, LTD has also been described at CF synapses in P14–30 rats (Fig. 4)94,95. CF LTD results from brief CF activation at 5 Hz and is, just like PF LTD, postsynaptically induced and expressed96. While CF LTD has not been characterized in as much molecular detail as its counterpart at PF synapses, it is known that CF LTD requires a rise in calcium transients for its induction as well as the activation of mGluR1 receptors and PKC (Fig. 4)94. Thus, the available data suggest that the same mGluR1–PKC signaling cascade that triggers PF LTD also induces CF LTD. This notion is further supported by the observation that LTD at both types of synapses is facilitated by the release of corticotropin-releasing factor from CF terminals and the activation of its receptors, which are G protein coupled and promote the activation of PKC97,98. Thus, it seems that the overlap in the molecular machineries even holds true when restricting the comparison to LTD and synaptic pruning at CF synapses and, similarly, LTD and elimination at PF synapses, although the available information on molecular mechanisms underlying CF LTD and PF synaptic pruning, respectively, is sparse. In young (P4–11) rodents, both LTD and LTP have been observed at CF synapses. LTP at relatively strong CF synapses establishes the winner CF input in the competitive elimination process, while LTD at weaker synapses initiates the removal of loser CF inputs99,100. Thus, activity-dependent bidirectional synaptic plasticity seems to provide the basis for the competitive selection process at the very beginning of CF input maturation.
Figure 4.
LTD at climbing fiber synapses depends on mGluR1–PKC signaling. (a) Recording configuration. Inset traces show typical CF responses recorded in voltage-clamp mode (CF EPSC; upper left) and current-clamp mode (complex spike; lower left). (b) CF LTD results from CF stimulation at 5 Hz for 30 s (n = 15; filled dots), but the EPSC amplitudes remain stable in the absence of tetanization (n = 5; open dots). (c) Model scheme of molecular events involved in CF LTD and CF pruning. (d) Top: CF LTD is absent in the presence of the group 1 mGluR antagonist AIDA (1 mM), which was bath-applied at the time indicated by the horizontal bar (n = 6). Bottom: CF LTD is also prevented in the presence of the bath-applied PKC inhibitor chelerythrine (10 µM; n = 5). Arrows indicate the time point of tetanization. All values are shown as mean ± s.e.m. Panels b,d adapted from ref. 94, Elsevier.
The observation that LTD (in young adult or adult) and synaptic pruning are impaired when the same signaling pathways are blocked suggests that the two plasticity phenomena share the same underlying molecular machinery. An alternative possibility, however, is that the two plasticity processes are interdependent: that is, PF LTD might depend on completed CF elimination or, alternatively, CF pruning might depend on the ability of PF synapses to undergo LTD. The former scenario seems unlikely as there is no indication that PF LTD depends on completion of the developmental elimination of surplus CF inputs. In P5–8 rats, at an age when Purkinje cells are innervated by PF and CF synapses but CF elimination is far from being completed (which occurs at ∼P18), paired PF and CF activation induces PF LTD, just as it does in the mature cerebellum101. In contrast, it is conceivable that proper CF elimination depends on an intact mGluR1–PKC signaling cascade for LTD induction and synapse elimination at PF synapses. Experimental support for this notion comes from the observation that CF elimination is impaired when NMDA receptors are pharmacologically blocked88,89. As functional NMDA receptors are only expressed in Purkinje cells ∼8 weeks after birth85,86, these NMDA receptors cannot be directly involved in CF synaptic pruning and have instead been assigned to mossy fiber-granule cell synapses89, which control activity of the PF input. A plausible scenario is that translocation of a winner CF input to the dendrite61,64,65 is facilitated by certain levels of activity in the mossy fiber–granule cell pathway that allow mGluR1-dependent removal of PF synapses from intermediate portions of the dendrite78 (for review, see ref. 60).
What then is the evidence that suggests that mGluR1–PKC signaling at the CF synapses themselves is crucial for the elimination of surplus CF synapses? Observations at several types of synapses support the notion that LTD and synaptic pruning represent distinct phases of a continuous depression and disconnection process. For example, in the adult cerebellum PF LTD can be followed by elimination of PF synapses that were depressed in a motor learning task102. Similarly, synaptic depression followed by elimination has been observed at the NMJ5 and at excitatory synapses onto CA1 hippocampal pyramidal cells103,104. Most importantly, in the immature cerebellum weak CF synapses are disconnected after undergoing LTD61,99,100, and CF LTD from P14 onwards has been found to depend on mGluR1–PKC signaling94. These results demonstrate, first, that mGluR1-dependent LTD exists at CF synapses at a postnatal age preceding completion of the elimination process (≤P18), and second, that synaptic depression (weak synapses) marks CF synapses for elimination. Thus, it seems that LTD mechanisms at CF inputs play a role in the developmental elimination of surplus CF synapses.
Synapse elimination and LTD at the neuromuscular junction
Excitatory synapses onto cerebellar Purkinje cells provide an excellent test case for the comparative analysis of mechanisms underlying LTD and synaptic pruning because at these synapses both processes have been extensively studied. At NMJ synapses, a developmental pruning process takes place that strongly resembles the elimination of surplus CF inputs in the cerebellum: at birth, each muscle fiber is innervated by approximately ten motor neurons3, which are subsequently eliminated until only one winner input remains at ∼P16–18 (ref. 4). Since LTD-like depression has been observed at the developing105 and adult NMJ106, we can address the question of how findings at NMJ synapses compare to the findings in the cerebellum. Synaptic depression at the NMJ is induced by repetitive depolarization and can be blocked by the calcium chelator BAPTA107. Photolytic calcium uncaging triggers LTD at these synapses as well108. Together, these findings suggest that LTD at the NMJ depends on postsynaptic depolarization and subsequent calcium elevation. Synaptic depression requires nitric oxide signaling at the developing109 and mature NMJ106. Moreover, LTD at the developing NMJ requires CaMKII activation while a form of potentiation depends on the activation of protein phosphatase 2B (calcineurin)110, pointing toward similar rules governing bidirectional synaptic plasticity, as found in the cerebellum (for review, see ref. 42), and toward an activity-dependent synapse competition that marks synaptic inputs for stabilization and elimination. Such a competition between multiple synaptic inputs is clearly demonstrated by the finding that laser removal of the stronger synaptic inputs rescues synapses that otherwise would have been eliminated6. Importantly, at the NMJ a loss of postsynaptic acetylcholine receptors and synapse weakening precedes synapse elimination5,111. Moreover, synapse elimination can be experimentally induced by blockade of neurotransmission with α-bungarotoxin112. These findings demonstrate that, as at cerebellar and hippocampal synapses, LTD and synaptic pruning at the NMJ represent plasticity processes along a continuous time axis, suggesting a shared underlying molecular machinery.
LTD dysregulation and synaptic pruning deficits in autism
The intimate relationship between LTD and synaptic pruning enables circuit plasticity in the developing and mature brain, and it therefore is also of interest in developmental brain disorders, such as autism. Indeed, synaptic dysfunction and abnormal synaptic pruning are characteristic features of ASD (for review, see refs. 15,113). Synaptic function cannot be assessed in post-mortem tissue analysis, but it has been reported that, in comparison to that in healthy subjects, the density of dendritic spines is enhanced in the cortex of individuals with autism, pointing toward deficits in spine pruning114,115 (for review, see ref. 113). Remarkably, dysregulation of LTD is another consistent observation in mouse models of autism across different genetic abnormalities and across different brain areas (Table 2; see below). It is because of these findings that the involvement of LTD-like mechanisms in synaptic pruning becomes important to autism research as well.
Table 2.
LTD dysregulation and alterations in CF pruning in ASD mouse models
| Mouse | Brain area | LTD | CF elimination (age tested) | References |
|---|---|---|---|---|
| Fmr1 knockout | Hippocampus | Enhanced | NA | 119 |
| Cerebellum | Enhanced | Accelerated (P21–48) | 132 | |
| Nlgn3 knockout | Cerebellum | Impaired* | Normal (2–3 months) | 133 (*but see 134) |
| Nlgn3-R451C | Cerebellum | ND | Delayed (P11–17; >P17) | 135 |
| Nlgn1−/−Nlgn2−/−Nlgn3−/− | Cerebellum | Normal | Normal | 134 |
| Syngap+/− | Hippocampus | Enhanced | NA | 124 |
| eIF4E transgenic | Hippocampus | Enhanced | NA | 122 |
| Striatum | Enhanced | NA | 122 | |
| Tsc2+/− | Hippocampus | Reduced | NA | 127 |
| 15q11–13 duplication | Cerebellum | Impaired | Delayed (P10–12; 2 months) | 136 |
LTD dysregulation has been observed in several mouse models of autism and across various brain areas. Developmental elimination of surplus CF inputs has been tested in all cerebellar studies shown. This table focuses on synaptic pruning, omitting spine pruning or changes in synapse formation and/or maintenance, to restrict the overview to phenomena that can be directly compared between the different mouse models. Note that in Nlgn1−/−Nlgn2−/−Nlgn3−/− triple-knockout mice the density of CF terminals contacting the distal Purkinje cell dendrite is reduced (ref. 134), which is a potential cause for the absence of both LTD enhancement/saturation and defcits in CF elimination.
ND, not determined; NA, not applicable.
The most comprehensive theoretical framework on LTD deficits and their involvement in ASD is provided by the metabotropic glutamate receptor (mGluR) theory of fragile X syndrome developed by Bear and colleagues. Fragile X syndrome is a form of inherited mental retardation that often is accompanied by ASD features and is caused by a mutation in the FMR1 gene, which encodes a repressor of mRNA translation, FMRP (fragile X mental retardation protein)116. Neuronal and circuit hyperexcitability contribute to fragile X syndrome phenotypes (for review, see ref. 117). In both fragile X patients and Fmr1 knockout mice, spines on pyramidal cells are longer than normal and appear immature, pointing toward a role in spine maturation and pruning118. This abnormality in spine maturation is accompanied by a pronounced dysregulation of LTD. In the hippocampus of Fmr1 knockout mice, mGluR5-dependent LTD is enhanced119. Group I mGluRs (mGluR1 and mGluR5) activate local mRNA translation120. It was thus proposed in the mGluR theory of fragile X syndrome that in fragile X the lack of the translation repressor protein FMRP leads to excessive protein synthesis downstream of group I mGluR activation, including synthesis of proteins that promote the induction of LTD (for review, see ref. 121). Remarkably, transgenic mice that overexpress the eukaryotic translation initiation factor 4E (eIF4E), which is regulated by FMRP, similarly show ASD-like behavioral alterations, enhanced spine density, enhanced mGluR-LTD in the hippocampus and, in addition, enhanced tetanization-evoked LTD in the striatum122. Similarly, hippocampal mGluR-LTD is enhanced in mice with a deletion for the eIF4E–binding protein 2 gene (Eif4ebp2), which encodes 4E-BP2, a suppressor of mRNA translation initiation123. Hippocampal mGluR-LTD is furthermore enhanced in Syngap+/− mice124. In these mice, extracellular signal-related kinases 1 and 2 (ERK1/2) signaling is enhanced, which may increase eIF4E cap-dependent translation (for review, see ref. 125). Local cap-dependent mRNA translation is also controlled by mammalian target of rapamycin (mTOR), which in turn is negatively regulated by the tuberous sclerosis complex proteins TSC1 and TSC2 (TSC shows ASD comorbidity; for review, see ref. 126). Indeed, hippocampal LTD is significantly reduced in Tsc2+/− mice127. These observations point toward a remarkable convergence of ASD-related abnormalities in the molecular network for translation control115,127 (for review, see ref. 125). The mGluR theory of fragile X syndrome has been further validated by the observation that in animal models the inhibition of mGluR5 can reduce the severity of several fragile X symptoms128,129. Clinical therapies based on the inhibition of mGluR5 have been tested, and although these trials were not successful, regulation of dendritic mRNA translation by the mGluR5 and mTOR pathways and their interactions remains one of the most promising targets for intervention119.
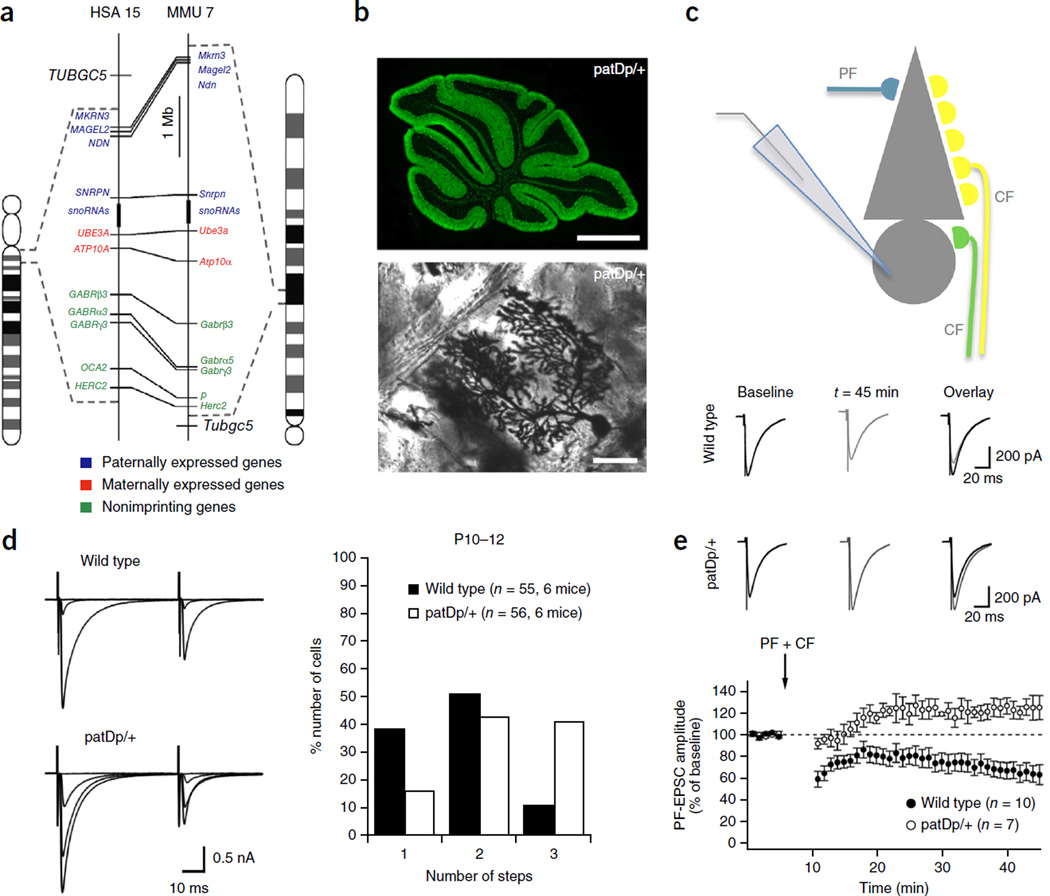
In the mGluR theory of fragile X, enhanced LTD is seen as a readout of dysregulated local mRNA translation, which ultimately contributes to specific fragile X symptoms. LTD dysregulation is a surprisingly common feature across different ASD mouse models with a wide range of genetic aberrations (see below). Thus, it is of interest to look more closely at LTD deficits in autism and to ask the question whether they occur in parallel with abnormal synaptic pruning. For this consideration, it is useful to return to cerebellar synapses, where both LTD and synaptic pruning can be readily studied and have been characterized in detail. Initial evidence for a cerebellar involvement in autism was met with skepticism, but it now seems that cerebellar abnormalities contribute to motor problems in ASD and might even contribute to the abnormal development of cortical circuits22,23. In fact, recent reports demonstrated that mice with a Purkinje cell–specific deletion of the TSC genes Tsc1 or Tsc2 show ASD-like alterations in social behaviors, suggesting a cerebellar contribution to cognitive impairment in ASD130,131. In Fmr1 knockout mice, Purkinje cell spines are elongated and appear immature, and LTD is enhanced132, as in the hippocampus119. Remarkably, the developmental elimination of surplus CF inputs is not delayed, but rather accelerated132. Fortunately, cerebellar LTD and CF elimination have been tested in other ASD mouse models, too, allowing direct comparisons. Cerebellar abnormalities have also been found in neuroligin-3 (Nlgn3) knockout mice. In these mice, mGluR-dependent LTD at PF synapses is impaired133. The loss of LTD using standard induction protocols may result from either blockade of molecular pathways required for LTD induction or from LTD saturation. In both cases the LTD mechanism becomes unavailable for further circuit plasticity. In Nlgn3 knockout mice LTD is saturated, possibly as a result of enhanced expression of mGluR1 (ref. 133; but see ref. 134). CF innervation was only tested in adult (2–3 month old) mice, and it was found to be normal133. In contrast, CF elimination has been studied during postnatal development in Nlgn3-R451C knock-in mice. In these mice, CF elimination appears normal at >P17 but is impaired at P11–17 (ref. 135). These findings raise the possibility that, in Nlgn3 knockout mice too, CF elimination is delayed during development, but this scenario remains to be experimentally confirmed. In Purkinje cell–specific triple neuroligin-1, neuroligin-2 and neuroligin-3 knockout mice, mGluR LTD and CF elimination are not affected, which may result from an accompanying reduction in the density of CF synapses on distal parts of the Purkinje cell dendrite134. It is conceivable that in these mice the reduction of the total CF input both prevents the survival of weaker CF synapses and prevents LTD saturation as a result of the reduced CF response. Thus, while LTD impairment and delayed CF pruning have been observed in mice with altered neuroligin signaling, it remains to be investigated under which conditions synaptic development and plasticity are affected and how the severity of these impairments depends on accompanying synaptic alterations in the circuit. Finally, in a mouse model (patDp/+ mice) for the human 15q11–13 duplication (Dup15q syndrome), a copy number variation that is the most frequent genetic abnormality in autism, LTD is impaired and LTP is induced instead (Fig. 5)136. Recent findings suggest that spine calcium transients evoked by PF and CF coactivation, the synaptic activation pattern typically used for LTD induction, are reduced in patDp/+ mice (C.P. and C.H., unpublished data), pointing toward a blockade of LTD induction pathways. CF elimination is delayed in patDp/+ mice, and a mild impairment persists into adulthood (Fig. 5)136. Thus, LTD dysregulation and abnormal synaptic pruning are observed together in cerebellar ASD mouse model studies.
Figure 5.
LTD dysregulation and impaired CF pruning in a mouse model of the human 15q11–13 duplication. (a) Schematic showing the corresponding regions of human chromosome 15 (left) and mouse chromosome 7 (right). Conserved linkage groups are shown by connecting lines between the human and mouse chromosomes. Genes that in wild-type mice are paternally expressed, maternally expressed and non-imprinting are labeled with blue, red and green, respectively. Dotted lines indicate the borders of the duplication regions. (b) Calbindin staining of a sagittal cerebellar section (top; scale bar: 1 mm) and Golgi staining of a Purkinje cell (PC) obtained from patDp/+ mice (bottom; scale bar: 50 µm). (c) Patch-clamp recording configuration. (d) CF pruning is impaired in patDp/+ mice. Left: typical traces showing discrete CF EPSC steps (holding potential: −10 mV) in slices from a P11 wild-type mouse (top) and a P11 patDp/+ mouse. Right: percentage of P10–12 wild-type (n = 55, from six mice) and patDp/+ (n = 56; from six mice) PCs showing one, two and three CF EPSC steps, which are taken as a measure of the number of CF inputs. (e) PF LTD dysregulation in patDp/+ mice. Top: typical PF EPSC traces before and after application of the tetanization protocol. PF LTD is induced in wild-type mice, but is absent from patDp/+ mice. Bottom: time graph showing PF LTD in wild-type mice (n = 10), and a potentiation in patDp/+ mice (n = 7). Arrow: time of tetanization. Error bars are mean ± s.e.m. Panels a,b,d,e adapted from ref. 136, Nature Publishing Group.
The focus on the cerebellum has an additional advantage in ASD research beyond the ability to measure synaptic pruning. In individuals with autism, delay eyeblink conditioning (EBC) is affected132,137,138. This is a form of motor learning that is mediated, at least partially, by cerebellar LTD (for review, see ref. 139). In cerebellar mouse studies, EBC impairment is seen alongside abnormal LTD (Fmr1 mice, ref. 132; patDp/+ mice, ref. 136; not tested in Nlgn3 knockout mice). It is conceivable that these findings reveal an ASD-relevant behavioral alteration that is a direct consequence of LTD dysregulation (see also Box 1; for comparison to motor deficits in Angelman syndrome, see ref. 140).
Motor learning deficits and cerebellar synaptopathies might facilitate the identification of molecular ASD biomarkers. A biomarker candidate is IGF-1, which is already used as a marker for cerebellar degeneration and is involved in CF pruning141. Moreover, understanding the regulatory factors that stabilize neurons in their mature state might be an important step to identify pharmaceutical targets that could restore developmental plasticity and enable the formation of normal connectivity patterns in ASD patient brains. One such identified stabilization mechanism that helps to maintain CF mono-innervation in adult mice is provided by the retinoic acid–related orphan receptor-α (RORα). Deletion of RORα in Purkinje cells by postnatal week 4 leads to the reappearance of immature Purkinje cell features, including multiple CF innervation142. RORα is also mutated in the staggerer mouse, which shows multiple CF innervation and is emerging as a mouse model of autism143.
Summary
Synaptic dysfunction is widely recognized as one of the contributing pathologies in autism and other brain developmental disorders. However, it often is difficult to pinpoint how specific synaptic alterations contribute to ASD symptoms. The focus on the cerebellum, an evolutionary conserved brain area with a simple circuit structure, has helped to provide a molecule-by-molecule comparison of the molecular machinery controlling LTD and developmental synaptic pruning, and to demonstrate the almost complete overlap in the underlying processes. This cerebellar approach has been useful in the study of autism, as the developmental elimination of surplus CF inputs to Purkinje cells provides one of the few examples of experimentally accessible synaptic pruning in the CNS. Moreover, the link between LTD dysregulation and EBC deficits provides a compelling example of an ASD-typical behavioral alteration resulting from a synaptic dysfunction that has been observed in multiple mouse models of autism.
Acknowledgments
We thank B. Kasthuri and members of the Hansel laboratory for comments. M.K. is supported by a Grant-in-Aid for Scientific Research (25000015) from JSPS, Japan, and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from AMED, Japan. C.H. is supported by the Simons Foundation (SFARI 203507 and 311232) and the National Institutes of Health (NS62771).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 2.LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J. Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapia JC, et al. Pervasive synaptic branch removal in the mammalian neuromuscular system at birth. Neuron. 2012;74:816–829. doi: 10.1016/j.neuron.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Redfern PA. Neuromuscular transmission in new-born rats. J. Physiol. (Lond.) 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- 6.Turney SG, Lichtman JW. Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: evidence for synaptic competition and its mechanism. PLoS Biol. 2012;10:e1001352. doi: 10.1371/journal.pbio.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 8.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat. Rev. Neurosci. 2012;13:798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- 10.Hebb DO. The Organization of behavior. New York: Wiley; 1949. [Google Scholar]
- 11.Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc. Natl. Acad. Sci. USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesel TN, Hubel DH. Effects of visual deprivation on the morphology and physiology of cells in the cat’s lateral geniculate body. J. Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 13.Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J. Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Grant SG. Synaptopathies: diseases of the synaptome. Curr. Opin. Neurobiol. 2012;22:522–529. doi: 10.1016/j.conb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 2012;4:a009886. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. J. Am. Med. Assoc. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 17.Supekar K, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5:738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostofsky SH, et al. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Martino A, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markram H, Rinaldi T, Markram K. The intense world syndrome-an alternative hypothesis for autism. Front. Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 22.Fatemi SH, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. This perspective paper argues that autism-related deficits in cerebellar output may prevent the proper development of neocortical circuits, providing an example of developmental diaschisis
- 24.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 25.Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 26.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog. Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 27.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. This study demonstrates that the same AMPA receptor subunit phosphorylation and dephosphorylation steps occur in LTD and ocular dominance plasticity in the visual cortex, thus providing strong cortical evidence for an overlap in molecular pathways involved in LTD and synaptic pruning
- 29.Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J. Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansel C, Artola A, Singer W. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. Eur. J. Neurosci. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 33.Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- 34.Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J. Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidorov MS, Kaplan ES, Osterweil EK, Lindemann L, Bear MF. Metabotropic glutamate receptor signaling is required for NMDA receptor-dependent ocular dominance plasticity and LTD in visual cortex. Proc. Natl. Acad. Sci. USA. 2015;112:12852–12857. doi: 10.1073/pnas.1512878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu. Rev. Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 41.Ito M, Yamaguchi K, Nagao S, Yamazaki T. Long-term depression as a model of cerebellar plasticity. Prog. Brain Res. 2014;210:1–30. doi: 10.1016/B978-0-444-63356-9.00001-7. [DOI] [PubMed] [Google Scholar]
- 42.Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 43.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 44.Gao Z, van Beugen BJ, De Zeeuw CJ. Distributed synergistic plasticity and cerebellar learning. Nat. Rev. Neurosci. 2012;13:619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 45.Aiba A, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 46.Conquet F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 47.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 48.Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Phil. Trans. R. Soc. Lond. B. 2008;363:2173–2186. doi: 10.1098/rstb.2008.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knöpfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. Cerebellum. 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- 50.Belmeguenai A, et al. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J. Neurophysiol. 2008;100:3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartmann J, et al. Distinct roles of Gαq and Gα11 for Purkinje cell signaling and motor behavior. J. Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto K, Miyata M, Watanabe M, Kano M. Roles of phospholipase Cbeta4 in synapse elimination and plasticity in developing and mature cerebellum. Mol. Neurobiol. 2001;23:69–82. doi: 10.1385/MN:23:1:69. [DOI] [PubMed] [Google Scholar]
- 53.Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, et al. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC γ mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 55.De Zeeuw CI, et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- 56.Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 57.Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCalpha confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–594. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 58.Baude A, Molnár E, Latawiec D, McIlhinney RA, Somogyi P. Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum. J. Neurosci. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J. Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cell. Mol. Life Sci. 2013;70:4667–4680. doi: 10.1007/s00018-013-1405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto K, et al. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc. Natl. Acad. Sci. USA. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawamura Y, et al. Spike timing-dependent selective strengthening of single climbing fibre inputs to Purkinje cells during cerebellar development. Nat. Commun. 2013;4:2732. doi: 10.1038/ncomms3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63:106–118. doi: 10.1016/j.neuron.2009.06.008. This paper characterizes how the synaptic competition of climbing fiber inputs takes place at the Purkinje cell soma and how the strongest CF input invades the growing dendrite, while weaker inputs are removed
- 65.Carrillo J, Nishiyama N, Nishiyama H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J. Neurosci. 2013;33:7641–7653. doi: 10.1523/JNEUROSCI.4561-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kakegawa W, et al. Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron. 2015;85:316–329. doi: 10.1016/j.neuron.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto K, et al. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. NeuroScience. 2009;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 68.Nakayama H, et al. GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron. 2012;74:384–396. doi: 10.1016/j.neuron.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 69.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 70.Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 72.Chu Y, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kano M, et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 74.Levenes C, Daniel H, Jaillard D, Conquet F, Crépel F. Incomplete regression of multiple climbing fibre innervation of cerebellar Purkinje cells in mGLuR1 mutant mice. Neuroreport. 1997;8:571–574. doi: 10.1097/00001756-199701200-00038. [DOI] [PubMed] [Google Scholar]
- 75.Offermanns S, et al. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc. Natl. Acad. Sci. USA. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kano M, et al. Phospholipase cbeta4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc. Natl. Acad. Sci. USA. 1998;95:15724–15729. doi: 10.1073/pnas.95.26.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kano M, et al. Impaired synapse elimination during cerebellar development in PKC γ mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 78. Ichikawa R, et al. Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination. Proc. Natl. Acad. Sci. USA. 2016;113:2282–2287. doi: 10.1073/pnas.1511513113. This paper is the first to demonstrate mGluR1-PKC dependent parallel fiber synapse elimination during cerebellar development
- 79.Hansel C, et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Kawaguchi SY, Hirano T. Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J. Physiol. (Lond.) 2013;591:1707–1730. doi: 10.1113/jphysiol.2012.245787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J. Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Launey T, Endo S, Sakai R, Harano J, Ito M. Protein phosphatase 2A inhibition induces cerebellar long-term depression and declustering of synaptic AMPA receptor. Proc. Natl. Acad. Sci. USA. 2004;101:676–681. doi: 10.1073/pnas.0302914101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 84.Misra C, Brickley SG, Wyllie DJ, Cull-Candy SG. Slow deactivation kinetics of NMDA receptors containing NR1 and NR2D subunits in rat cerebellar Purkinje cells. J. Physiol. (Lond.) 2000;525:299–305. doi: 10.1111/j.1469-7793.2000.t01-1-00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piochon C, et al. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J. Neurosci. 2007;27:10797–10809. doi: 10.1523/JNEUROSCI.2422-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Renzi M, Farrant M, Cull-Candy SG. Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J. Physiol. (Lond.) 2007;585:91–101. doi: 10.1113/jphysiol.2007.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J. Neurosci. 2010;30:15330–15335. doi: 10.1523/JNEUROSCI.4344-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 89.Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J. Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith-Hicks C, et al. SRF binding to SRE 6.9 in the Arc promoter is essential for LTD in cultured Purkinje cells. Nat. Neurosci. 2010;13:1082–1089. doi: 10.1038/nn.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mikuni T, et al. Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron. 2013;78:1024–1035. doi: 10.1016/j.neuron.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawata S, et al. Global scaling down of excitatory postsynaptic responses in cerebellar Purkinje cells impairs developmental synapse elimination. Cell Rep. 2014;8:1119–1129. doi: 10.1016/j.celrep.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 95.Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber->Purkinje neuron synapses: role of metabotropic glutamate receptors. J. Neurosci. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen Y, Hansel C, Linden DJ. Glutamate release during LTD at cerebellar climbing fiber-Purkinje cell synapses. Nat. Neurosci. 2002;5:725–726. doi: 10.1038/nn895. [DOI] [PubMed] [Google Scholar]
- 97.Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22:763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 98.Schmolesky MT, De Ruiter MM, De Zeeuw CI, Hansel C. The neuropeptide corticotropin-releasing factor regulates excitatory transmission and plasticity at the climbing fibre-Purkinje cell synapse. Eur. J. Neurosci. 2007;25:1460–1466. doi: 10.1111/j.1460-9568.2007.05409.x. [DOI] [PubMed] [Google Scholar]
- 99.Bosman LW, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J. Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohtsuki G, Hirano T. Bidirectional plasticity at developing climbing fiber-Purkinje neuron synapses. Eur. J. Neurosci. 2008;28:2393–2400. doi: 10.1111/j.1460-9568.2008.06539.x. [DOI] [PubMed] [Google Scholar]
- 101.Arata A, Ito M. Purkinje cell functions in the in vitro cerebellum isolated from neonatal rats in a block with the pons and medulla. Neurosci. Res. 2004;50:361–367. doi: 10.1016/j.neures.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 102. Wang W, et al. Distinct cerebellar engrams in short-term and long-term motor learning. Proc. Natl. Acad. Sci. USA. 2014;111:E188–E193. doi: 10.1073/pnas.1315541111. This study shows that in the adult cerebellum motor learning can be associated with an elimination of parallel fiber synapses
- 103.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 104.Wiegert JS, Oertner TG. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. USA. 2013;110:E4510–E4519. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lo YJ, Poo MM. Activity-dependent synaptic competition in vitro: heterosynaptic suppression of developing synapses. Science. 1991;254:1019–1022. doi: 10.1126/science.1658939. [DOI] [PubMed] [Google Scholar]
- 106.Etherington SJ, Everett AW. Postsynaptic production of nitric oxide implicated in long-term depression at the mature amphibian (Bufo marinus) neuromuscular junction. J. Physiol. (Lond.) 2004;559:507–517. doi: 10.1113/jphysiol.2004.066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lo YJ, Lin YC, Sanes DH, Poo MM. Depression of developing neuromuscular synapses induced by repetitive postsynaptic depolarizations. J. Neurosci. 1994;14:4694–4704. doi: 10.1523/JNEUROSCI.14-08-04694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cash S, Dan Y, Poo MM, Zucker R. Postsynaptic elevation of calcium induces persistent depression of developing neuromuscular synapses. Neuron. 1996;16:745–754. doi: 10.1016/s0896-6273(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 109.Wang T, Xie Z, Lu B. Nitric oxide mediates activity-dependent synaptic suppression at developing neuromuscular synapses. Nature. 1995;374:262–266. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]
- 110.Wan J, Poo M. Activity-induced potentiation of developing neuromuscular synapses. Science. 1999;285:1725–1728. doi: 10.1126/science.285.5434.1725. [DOI] [PubMed] [Google Scholar]
- 111.Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J. Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 113.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 115. Tang G, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. Using post-mortem histology, this study demonstrates deficits in spine pruning in autistic individuals. This developmental deficit is linked to hyperactive mTOR signaling
- 116.Verheij C, et al. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363:722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- 117.Contractor A, Klyachko VA, Portera-Cailliau C. Altered neuronal and circuit excitability in Fragile X syndrome. Neuron. 2015;87:699–715. doi: 10.1016/j.neuron.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Comery TA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc. Natl. Acad. Sci. USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 122. Santini E, et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. This paper shows that genetic interference with translation control pathways causes enhanced protein synthesis and autism-like synaptic and behavioral phenotypes
- 123.Aguilar-Valles A, et al. Inhibition of group I metabotropic glutamate receptors reverses autistic-like phenotypes caused by deficiency of the translation repressor eIF4E binding protein 2. J. Neurosci. 2015;35:11125–11132. doi: 10.1523/JNEUROSCI.4615-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barnes SA, et al. Convergence of hippocampal pathophysiology in Syngap +/− and Fmr1 −/y mice. J. Neurosci. 2015;35:15073–15081. doi: 10.1523/JNEUROSCI.1087-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Santini E, Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Sci. Signal. 2014;7:re10. doi: 10.1126/scisignal.2005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat. Rev. Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. Characterizing synaptic plasticity and levels of protein synthesis in Tsc2 +/− and Fmr1 /y mice, this paper shows that synaptic dysfunction caused by autism-relevant mutations can fall at opposite ends of the alteration spectrum and can be rescued in mice that carry both mutations
- 128.Dölen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J. Physiol. (Lond.) 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsai PT, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reith RM, et al. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2013;51:93–103. doi: 10.1016/j.nbd.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 132.Koekkoek SK, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 133.Baudouin SJ, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- 134.Zhang B, et al. Neuroligins sculpt cerebellar Purkinje-cell circuits by differential control of distinct classes of synapses. Neuron. 2015;87:781–796. doi: 10.1016/j.neuron.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lai E, et al. An autism-associated neuroligin-3 mutation impairs developmental synapse elimination in the cerebellum. Soc. Neurosci. Abstract. 2013:718.03. doi: 10.3389/fncir.2021.676891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Piochon C, et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 2014;5:5586. doi: 10.1038/ncomms6586. This study demonstrates deficits in cerebellar LTD and synaptic pruning in a mouse model for the human 15q11–13 duplication. Moreover, the paper shows impaired delay eyeblink conditioning, a form of motor learning that requires an intact cerebellum and that is affected in autistic individuals. As LTD is one of several plasticity types that contributes to eyeblink conditioning, this study is a rare example of a synaptic dysfunction that can be linked to an autism-typical behavioral alteration
- 137.Sears LL, Finn PR, Steinmetz JE. Abnormal classical eye-blink conditioning in autism. J. Autism Dev. Disord. 1994;24:737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- 138.Oristaglio J, et al. Children with autism spectrum disorders show abnormal conditioned response timing on delay, but not trace, eyeblink conditioning. NeuroScience. 2013;248:708–718. doi: 10.1016/j.neuroscience.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Freeman JH. Cerebellar learning mechanisms. Brain Res. 2015;1621:260–269. doi: 10.1016/j.brainres.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bruinsma CF, et al. Dissociation of locomotor and cerebellar deficits in a murine Angelman syndrome model. J. Clin. Invest. 2015;125:4305–4315. doi: 10.1172/JCI83541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kakizawa S, Yamada K, Iino M, Watanabe M, Kano M. Effects of insulin-like growth factor I on climbing fibre synapse elimination during cerebellar development. Eur. J. Neurosci. 2003;17:545–554. doi: 10.1046/j.1460-9568.2003.02486.x. [DOI] [PubMed] [Google Scholar]
- 142.Chen XR, et al. Mature Purkinje cells require the retinoic acid-related orphan receptor-α (RORα) to maintain climbing fiber mono-innervation and other adult characteristics. J. Neurosci. 2013;33:9546–9562. doi: 10.1523/JNEUROSCI.2977-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sarachana T, Hu VW. Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder. Mol. Autism. 2013;4:14. doi: 10.1186/2040-2392-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pfeiffer BE, et al. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015;350:aab3897. doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lohmann C, Kessels HW. The developmental stages of synaptic plasticity. J. Physiol. (Lond.) 2014;592:13–31. doi: 10.1113/jphysiol.2012.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 149.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]