Abstract
Human studies and some animal work have shown more docosahexaenoic acid (DHA) and arachidonic acid (ARA) was accumulated or converted from precursors in females compared to males. This study explored in-depth the effect of gender on fatty acid composition and polyunsaturated fatty acid metabolism in rats fed one of two well-defined diets containing 10% total fat. One diet contained 15% of linoleic acid (LA) and 3% of α-linolenic acid (ALA) of the total fatty acids (LA+ALA diet), while the other diet contained 15% LA and 0.05% ALA (LA diet). At the age of 20 weeks, all animals were orally administered a single dose of a mixture of deuterium-labeled LA and ALA. Caudal venous blood was then drawn at 0, 2, 4, 8, 12, 24, 48, 96 and 168 h. The concentrations of the deuterated precursors and their metabolites in plasma total lipids were quantified by GC/MS negative chemical ionization. Endogenous fatty acids were quantified by GC/FID analysis. When expressed as the percentage of oral dosage, female rats accumulated more precursors and more products, deuterated DHA and deuterated n-6 docosapentaenoic acid (2H5-DPAn-6), in plasma than did male rats in both the LA+ALA diet and the LA diet. For the endogenous non-labeled PUFA, greater concentrations of DHA and DPAn-6 were similarly observed in female rats compared to males within each diet. A lower concentration of non-labeled ARA was observed only in female rats fed the LA+ALA diet. In summary, greater endogenous and exogenous DHA and DPAn-6 was observed in female rat plasma and this was independent of dietary ALA status.
Keywords: Essential fatty acid, alpha-linolenic acid, linoleic acid, DHA, DPA, omega-3, omega-6, gender, stable isotope, GC/MS
1. INTRODUCTION
In 1929, Burr and Burr [1] first discovered that n-6 polyunsaturated fatty acids (PUFA) are necessary for proper growth and development of mammals and thus termed them essential fatty acids (EFAs). Ever since, much research has been conducted on all aspects of EFAs including possible gender differences. Since the first study by Loeb and Burr [2], which reported a gender difference in EFA requirements, a series of studies in the 1960s by Lyman and Ostwald [3, 4] observed that female rats utilized EFAs more efficiently, especially arachidonic acid (ARA), an n-6 PUFA. This series of studies suggested that the gender difference in EFA metabolism was strongly related to sex and gonadal hormones, mainly estrogen and testosterone [3–6]. It was found that increased hormone levels were correlated with changes in liver microsomal phospholipid fatty acid composition, and possibly involving the underlying mechanism of calcium uptake activity [7, 8]. But these differences were not observed in an in vitro rat microsomal system using a radio isotopic technique [9].
It was not until the late 1970s that the n-3 PUFAs were considered to be essential nutrients in mammals [10–13]. During the past decade, the gender differences in n-3 PUFAs conversion and fatty acid compositions in human subjects has been investigated following the report by Burdge et al [14–17]. Several reviews [18–20] indicate that docosahexaenoic acid (DHA) levels were significantly higher for both endogenous fatty acid composition and conversion from the isotopic precursor α-linolenic acid (ALA). This increase in DHA in females has been reported to be closely related to hormone levels [21–23] probably through an up-regulation of the mRNA expression of FADS2, the gene that encodes the Δ6-desaturase in human primary hepatocytes [24] and in rat liver [25, 26].
However, gender differences reported in many previous studies were observed under dietary circumstances where 18:3n-3, DHA and other highly unsaturated fatty acids (HUFA) were consumed. The 20- and 22-carbon fatty acids with three or more double bonds are categorized as HUFA. Thus, it was unclear whether the higher DHA observed in females was due to direct dietary intake or de novo synthesis from dietary 18:3n-3, or both. Furthermore, studies of gender during the last decade have focused upon n-3 PUFA without attention to n-6 PUFAs. This study investigated the possible gender effect on both the n-3 and n-6 fatty acid compositions as well as de novo synthesized PUFAs in rats using two well-defined diets containing only linoleic acid (LA) and ALA for dietary PUFA content. One diet contained a high level (3 wt% of total fatty acids) of 18:3n-3 (LA+ALA diet), while the other contained an extremely low level, with only 0.04 wt% of 18:3n-3 (LA diet); both diets included 15 wt% of 18:2n-6 but no long chain PUFA (LC PUFA). Stable isotope tracer techniques coupled with mass spectrometry negative-chemical ionization analysis (GC/MS NCI) were employed to quantify the de novo synthesis of deuterated LC PUFA metabolized from deuterated C18 PUFAs.
2. MATERIALS and METHODS
2.1. Animal and diet
All animal procedures were carried out in accordance with the NIH Animal Care and Welfare guidelines under the animal study proposal #LMBB-YL-11, which was approved by the Animal Care and Use Committee, National Institute on Alcohol Abuse and Alcoholism, NIH. The animal facility was under conventional conditions with controlled temperature 22 °C and illumination (12 h light-dark cycles); food and water were provided ad libitum. The animals in this study came from the second generation of in-house breeding, as described in a previous report [27]. Briefly, the time-pregnant female, Long-Evans hooded rats on the third day of gestation were obtained from Charles River Laboratory (Portage, MI, USA), and they were immediately placed on one of two pelleted custom diets. One diet was high in n-3 PUFA and contained 15% of 18:2n-6, 3.1% of 18:3n-3 and 1.3% 22:6n-3, while the other was extremely low in n-3 PUFA and contained 15% of 18:2n-6, 0.04% of 18:3n-3 without 22:6n-3 (LA diet). The offspring were weaned onto the same diet as the mother at three weeks of age. At 16 wks of age, animals on the high n-3 PUFA diet were switched to the LA+ALA diet, which included 15% of 18:2n-6 and 3.1% of 18:3n-3 but with no DHA nor other HUFA. These animals consumed the LA+ALA diet for 4 weeks and then groups were separated according to gender and designated as group HM (male) and group HF (female), n = 6 for each group. The animals on the LA as group LM (male) and group LF diet continued to consume the same LA diet and were designated (female), n = 6 for each group. All diets contained 10 g of fat per 100 g diet and were modified from the AIN-93G formulation [28]. The compositions of the two experimental diets and the corresponding study groups are presented in Table 1.
TABLE 1.
Nutrient composition of experimental diets and study groups
| Ingredienta | Amount (g/kg of diet) |
|
|---|---|---|
| Protein (20%) | ||
| Casein (vitamin free) | 200 | |
| Carbohydrate (60%) | ||
| Cornstarch | 150 | |
| Dextrose | 199.5 | |
| Maltose-dextrin | 150 | |
| Sucrose | 100 | |
| Others (10%) | ||
| Cellulose | 50 | |
| Mineral & Salt Mix | 35 | |
| Vitamin Mix | 10 | |
| L-Cystine | 3 | |
| Choline Bitartrate | 2.5 | |
| tert-butylhydroquinone | 0.02 | |
| Fat (10%) | 100 | |
| LA+ALA Diet | LA Dietb | |
| HCO | 77.5 | 81 |
| Safflower Oil | 17.7 | 19 |
| Flaxseed Oil | 4.8 | - |
|
Fatty Acid Compositionc (wt% of total fatty acids) |
||
| Saturates | 77.2 | 80.85 |
| Monounsaturates | 4.3 | 3.92 |
| 18:2n-6 | 15.3 | 15.13 |
| 18:3n-3 | 3.12 | 0.04 |
| Study Groups | ||
| HM (male) | + | |
| HF (female) | + | |
| LM (male) | + | |
| LF (female) | + | |
2.2. Isotope and chemicals
Deuterium-labeled ethyl linolenate (2H5-17, 17, 18, 18, 18-18:3n-3) and deuterium-labeled ethyl linoleate (2H5-17, 17, 18, 18, 18-18:2n-6) were obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA); isotopic purity was greater than 95% for the former and 98% for the latter. A dual labeled isotope, deuterated and carbon-13 labeled gamma-linolenate ethyl ester (7,8-13C2, 99%; 2,2,3,3,4,4,5,5,6-2H9, 98%-18:3n-6) was used as an internal standard for the quantification of the labeled fatty acids and their in vivo metabolites. Its chemical purities were greater than 95%. Olive oil and boron trifluoride-methanol solution (BF3 14% in methanol) were acquired from Sigma-Aldrich Chemical (St. Louis, MO); methanol was obtained from Burdick & Jackson (Muskegon, MI); hexane and chloroform were from EMD Chemicals Inc. (Gibbstown, NJ); butylated hydroxytoluene (BHT) was from Acros (Geel, Belgium). Standard docosatrienoic ethyl ester (22:3n-3) and reference standards GLC–462 containing 28 fatty acid methyl esters (FAME) were purchased from Nu-Chek Prep (Elysian, MN). All chemicals were commercially purchased and used without further purification.
2.3. Administration of isotopes and sampling
At the age of 20 wk, fasting animals were administrated by gavage a single oral dose of an oil mixture of 2H5-18:3n-3 ethyl ester (12.5 mg/kg BW) and 2H5-18:2n-6 ethyl ester (62.5 mg/kg BW) in olive oil as the vehicle. Caudal vein blood (0.2–0.3 mL) was drawn from each animal at 2, 4, 8, 12, 24, 48, 96 and 168 h after administration. Control samples, at 0 h, were taken two days prior to the initiation of the study. The coefficient of variance for the sampling time was less than 5% for all animals during the entire experimental period. Plasma was separated shortly after collection at 1,750 g × 5 min in Eppendorf microcentrifuge, then was frozen and stored at − 80 °C until analysis.
2.4. Total lipid extraction and fatty acid analysis
Plasma total lipids were extracted with chloroform: methanol (2:1) according to Folch [29]. Briefly, 100 µl of rat plasma was mixed with 1 mL of methanol contained 50 µg BHT and then extracted twice with 2 mL of chloroform. Dual-labeled 18:3n-6 (150 ng per sample) and 23:0 ethyl esters (10 µg) were added to each sample prior to lipid extraction as internal standards for isotopic and unlabeled fatty acids, respectively. The chloroform total lipid extract was split into two aliquots. One half was dried under a stream of nitrogen and transmethylated by 14% of BF3 in methanol, as described by Morrison and Smith [30] and modified by Salem et al [31]. Signals of fatty acid methyl esters were acquired using GC/FID. The other half of each sample was dried and hydrolyzed with 1 mL of 5% KOH in methanol and derivatized with pentafluorobenzyl bromide and diisopropylamine in acetonitrile (1:10:1000, v/v/v; PFB reagent) to form the fatty acid PFB ester for GC/MS NCI analysis of the deuterated PUFA [32, 33].
2.5. Instrumentation
2.5.1. Gas chromatography
An Agilent 6890N Plus LAN system fast gas chromatograph coupled with flame ionization detector and a 7683 Series Injector (Agilent Technologies, Inc., Santa Clara, CA) were employed to acquire the chromatograms of non-labeled fatty acid methyl esters. GC parameters were slightly modified from a previous report [34], as detailed in Lin et al [35]. Briefly, a fused-silica, narrow-bore, high-efficiency DB-FFAP capillary column (15 m length × 0.1 mm ID × 0.1 µm film thickness) was used for chromatographic separation of FAME with hydrogen as the carrier gas at a constant pressure of 355 kPa. A GC ChemStation B.01.02 along with a built-in integrator was employed for data acquisition and peak integration.
2.5.2. Gas chromatography/mass spectrometry
An Agilent 5973 MSD equipped with an Agilent 6890 GC Plus LAN system (Agilent Technologies, Inc., Santa Clara, CA) was employed for the quantification of the deuterated fatty acids, as previously reported [33]. Briefly, an aliquot of 1 µl fatty acid pentafluorobenzyl ester per sample was injected onto a DB-FFAP capillary column (30 m length × 0.25 mm ID × 0.25 µm film thickness) interfaced directly into the chemical ion source in negative mode with methane as the reagent gas and helium as carrier gas. Selected ion monitoring (SIM) for the base peaks (M-PFB) of the isotopic fatty acids was carried out with continuous monitoring in data acquisition and quantified. Selected mass over charge (m/z) values monitored by GC/MS NCI are summarized in Table 2 for the fatty acids of interest along with those of the internal standards.
TABLE 2.
Mass over charge (m/z) of the selected pentafluorobenzyl ester ions of GC/MS NCI
| Fatty acids | Un- labeled |
Labeled |
|---|---|---|
| 1H5- | 2H5- | |
| n-3 | ||
| 18:3n-3 | 277 | 282 |
| 20:5n-3 | 301 | 306 |
| 22:5n-3 | 329 | 334 |
| 22:6n-3 | 327 | 332 |
| n-6 | ||
| 18:2n-6 | 279 | 284 |
| 18:3n-6 | 277 | 282 |
| 20:2n-6 | 307 | 312 |
| 20:3n-6 | 305 | 310 |
| 20:4n-6 | 303 | 308 |
| 22:4n-6 | 331 | 336 |
| 22:5n-6 | 329 | 334 |
| Internal standards | 13C2, 2H9- | |
| 23:0 | 353 | n/a |
| 18:3n-6 | 277 | 288 |
Footnote:
GC/MS NCI indicates gas chromatography/mass spectrometry negative chemical ionization
2.6. Calculation and statistics
The fatty acids were quantified by comparing the integrated area of the fatty acid peaks on the gas chromatograms with those of the corresponding internal standards. The amount of the unlabeled endogenous fatty acids was expressed either as a concentration (mg/mL of plasma), or as a percentage of total fatty acids by weight (wt%). Automated data processing was applied to the calculation of the unlabeled fatty acids, as detailed in a previous report [35]. The concentrations of the isotopic fatty acids were expressed as the parts per thousand (permille) of the initial isotope doses (‰dose) per mL of plasma. Areas under the curves (AUC0→168h, ‰dose/mL×h) were calculated by integrating the area under the time-course curves starting from 0 to 168 h using a trapezoid estimation method [36]. All data were expressed as mean ± SEM unless indicated in the text.
Repeated measures ANOVA were applied to assess the effects of gender over the time course of the above parameters related to the deuterated fatty acids using SPSS 13.0 for Windows (SPSS Inc, Chicago, IL). A two-sided, pairwise Student’s t test was applied to determine significant differences in fatty acid compositions between genders (Windows Excel 2003). Significance level was set at a P value of 0.05 or lower.
Because of the drastically different levels of ALA between two diets, the direct comparisons of plasma labeled or non-labeled PUFA concentrations of two genders were made only within each diet, but not comparing the two diets.
3. RESULTS
3.1. Animal condition
During the entire experiment, all animals appeared healthy and behaved normally. The rat body weights (mean ± SEM, g) at the beginning of isotope administration were 735 ± 41 and 436 ± 24 for the LA+ALA group males and females (HM, HF), respectively; while they were 752 ± 33 and 444 ± 42 for the LA group males and females (LM, LF), respectively. There were no significant differences between the body weights of animals fed the two diets, within each gender. Compared to the LA+ALA diet group, animals in the LA diet group exhibited harder caudal skin and slightly more aggressive behavior during blood sampling, and consumed about 2 g/d more of their diet, which might account for a tendency towards increased body weight.
3.2. Greater DHA and DPAn-6 but less ARA in female fatty acid content
The concentrations (µg/mL) of the unlabeled, endogenous fatty acids of plasma total lipids are presented in Table 3. A summary of various fatty acids indices in wt% of total fatty acids is also presented. In the LA+ALA diet group, n-3 PUFAs were significantly higher in female rats (group HF) than in males (group HM). Individually, 18:3n-3 was higher by 25% (27 vs. 22; P < 0.05), 20:5n-3 was higher by 12% (28 vs. 25; P < 0.05), and 22:6n-3 was higher by 18% (193 vs. 163; P < 0.001), while there was no statistically significant difference for 22:5n-3 (15 µg/mL in both genders). For the counterpart n-6 PUFAs, 18:3n-6 and 22:5n-6 were significantly higher in female – by 16% and 22%, respectively. However, the most abundant n-6 PUFA, 20:4n-6, was significantly lower by 19% (636 vs. 783; P < 0.001) in females in the LA+ALA diet group. Similarly, with regards to the immediate precursors of 20:4n-6, 20:3n-6 was lower in females by 29% (25 vs. 36; P < 0.001), while the primary precursor 18:2n-6 was identical comparing the genders (446 vs. 447). Surprisingly, the minor PUFA 20:3n-9 had the largest difference comparing genders as it was 39% lower in female rats. For MUFAs, 18:1n-9 and 16:1n-7 appeared about 25% and 13% higher in females, even though there was no statistically significant differences. 18:0 was significantly higher in females by 17% (P < 0.01), and 16:0 was not significantly different. The complete fatty acid compositions of plasma total lipids is presented in wt% of total fatty acids in Supplement Table 1.
TABLE 3.
The concentrations and compositions of fatty acids in rat plasma
| Group | HF | HM | LF | LM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | ||||||||||
| Diet | LA+ALA Diet | LA Diet | ||||||||||||
| Fatty Acids | Fatty Acid Concentrations (µg/mL plasma) | |||||||||||||
| 10:0 | 4.2 | ± | 0.2 | 5.0 | ± | 0.5 | 5.4 | ± | 1.0 | 4.7 | ± | 0.4 | ||
| 12:0 | 74.2 | ± | 13.6 | 62 | ± | 10 | 83 | ± | 14 | 95 | ± | 18 | ||
| 14:0 | 91.5 | ± | 15.2 | 70 | ± | 9 | a1 | 105 | ± | 14 | 105 | ± | 16 | |
| 16:0 | 663 | ± | 60 | 610 | ± | 41 | 754 | ± | 53 | 793 | ± | 67 | ||
| 18:0 | 535 | ± | 14 | 457 | ± | 12 | a2 | 666 | ± | 10 | 554 | ± | 26 | a2 |
| 20:0 | 3.1 | ± | 0.2 | 3.1 | ± | 0.2 | 3.4 | ± | 0.2 | 3.6 | ± | 0.2 | ||
| 24:0 | 6.0 | ± | 0.3 | 7.3 | ± | 0.3 | b1 | 6.3 | ± | 0.2 | 7.0 | ± | 0.3 | |
| 16:1n-7 | 141 | ± | 14 | 125 | ± | 12 | 152 | ± | 12 | 172 | ± | 18 | ||
| 18:1n-9 | 552 | ± | 60 | 441 | ± | 37 | 736 | ± | 93 | 657 | ± | 42 | ||
| 18:1n-7 | 92 | ± | 8 | 112 | ± | 10 | 120 | ± | 12 | 168 | ± | 18 | b1 | |
| 20:1n-9 | 5.8 | ± | 0.6 | 6.0 | ± | 0.6 | 9.0 | ± | 0.9 | 9.7 | ± | 0.8 | ||
| 24:1n-9 | 4.2 | ± | 0.2 | 6.6 | ± | 0.5 | b2 | 5.2 | ± | 0.2 | 6.5 | ± | 0.3 | b2 |
| 18:2n-6 | 446 | ± | 25 | 447 | ± | 25 | 420 | ± | 27 | 433 | ± | 35 | ||
| 18:3n-6 | 7.5 | ± | 0.5 | 6.3 | ± | 0.2 | a1 | 9.5 | ± | 0.8 | 7.6 | ± | 0.4 | a1 |
| 20:2n-6 | 5.2 | ± | 0.8 | 5.4 | ± | 0.4 | 4.7 | ± | 0.5 | 5.8 | ± | 0.5 | b1 | |
| 20:3n-6 | 25.3 | ± | 1.4 | 36 | ± | 1 | b3 | 18.2 | ± | 1.2 | 34.6 | ± | 2.6 | b3 |
| 20:4n-6 | 636 | ± | 19 | 783 | ± | 14 | b3 | 961 | ± | 25 | 1068 | ± | 26 | |
| 22:4n-6 | 8.1 | ± | 0.9 | 7.6 | ± | 0.6 | 25.8 | ± | 1.7 | 23.9 | ± | 1.8 | ||
| 22:5n-6 | 7.4 | ± | 0.5 | 5.8 | ± | 0.2 | a2 | 188 | ± | 5 | 121 | ± | 4 | a3 |
| 18:3n-3 | 27.3 | ± | 2.8 | 21.8 | ± | 2.6 | a1 | 2.7 | ± | 0.7 | 2.4 | ± | 0.4 | |
| 20:5n-3 | 28.2 | ± | 2.2 | 25.1 | ± | 1.4 | a1 | 1.0 | ± | 0.2 | 0.9 | ± | 0.05 | |
| 22:5n-3 | 15.4 | ± | 0.9 | 14.7 | ± | 0.9 | 1.4 | ± | 0.1 | 1.6 | ± | 0.1 | ||
| 22:6n-3 | 193 | ± | 4.3 | 163 | ± | 4.4 | a3 | 23 | ± | 0.3 | 17 | ± | 0.7 | a3 |
| 20:3n-9 | 5.15 | ± | 0.27 | 8.44 | ± | 0.41 | b3 | 10.8 | ± | 0.62 | 19.1 | ± | 0.83 | b3 |
| TFA (mg/mL) | 3.69 | ± | 0.19 | 3.54 | ± | 0.13 | 3.93 | ± | 0.24 | 4.47 | ± | 0.24 | ||
| Summary (% wt of total fatty acids) | ||||||||||||||
| Σ Saturates | 36.0 | ± | 1.41 | 33.3 | ± | 1.05 | a2 | 35.3 | ± | 1.28 | 33.9 | ± | 1.32 | a2 |
| Σ MUFA | 22.7 | ± | 2.22 | 20.3 | ± | 1.62 | a1 | 22.4 | ± | 1.74 | 23.3 | ± | 1.28 | |
| Σ n-6 PUFA | 31.0 | ± | 1.18 | 36.5 | ± | 1.11 | b3 | 38.5 | ± | 1.06 | 38.7 | ± | 1.05 | |
| Σ n-3 PUFA | 7.13 | ± | 0.29 | 6.27 | ± | 0.12 | a2 | 0.72 | ± | 0.03 | 0.55 | ± | 0.03 | a3 |
| n-6/n-3 PUFA | 4.35 | ± | 0.06 | 5.82 | ± | 0.14 | b3 | 53.8 | ± | 2.01 | 71.6 | ± | 3.00 | b3 |
| n-3%/HUFA | 25.9 | ± | 0.56 | 19.5 | ± | 0.60 | a3 | 2.07 | ± | 0.05 | 1.49 | ± | 0.04 | a3 |
| AA/DHA | 3.31 | ± | 0.10 | 4.84 | ± | 0.18 | b3 | 42.4 | ± | 1.6 | 64.9 | ± | 2.4 | b3 |
Footnote: Data were expressed in Mean ± SEM (n=6); significant difference was tested by paired t-test for genders but not for diets; significance was set at P<0.05 (1), 0.01 (2), and 0.001 (3).
indicates fatty acid was significantly higher in female rats;
as significantly lower in females.
A similar pattern of differences comparing the two genders was observed among the LA diet groups for plasma 22:6n-3 and 22:5n-6. 22:6n-3 was higher by 38% (P < 0.001) and 22:5n-6 was higher by 56% in females (P < 0.001). As expected, the overall concentrations of 22:6n-3 among the LA group was much lower (17–23 µg/mL) and 22:5n-6 was much higher (121–188 µg/mL) in comparison to those in the LA+ALA group. 18:3n-6 was higher by 24% while 20:3n-6 was significantly lower in females by about 50% (P < 0.001), even though the concentrations of both were close to those in LA+ALA group. 20:3n-9 in females was about half of that in male rats (P < 0.001). The remaining PUFAs, including 18:3n-3, 20:5n-3, 22:5n-3, 18:2n-6, and 20:4n-6, showed no significant differences comparing genders in the LA diet.
In addition to the concentrations, the ratio of 22:6n-3 to its precursor 22:5n-3 was higher in females in comparison to the males (16.4 vs. 10.6) fed the LA diet, in which 18:3n-3 was extremely low (0.04% of total fatty acids). However, the ratio of 22:6n-3 to 22:5n-3 was closer among males and females in the LA+ALA diet (12.5 vs. 11.1) when dietary 18:3n-3 reached 3% of total fatty acids.
3.3. Higher deuterated n-3 PUFA in female rat plasma
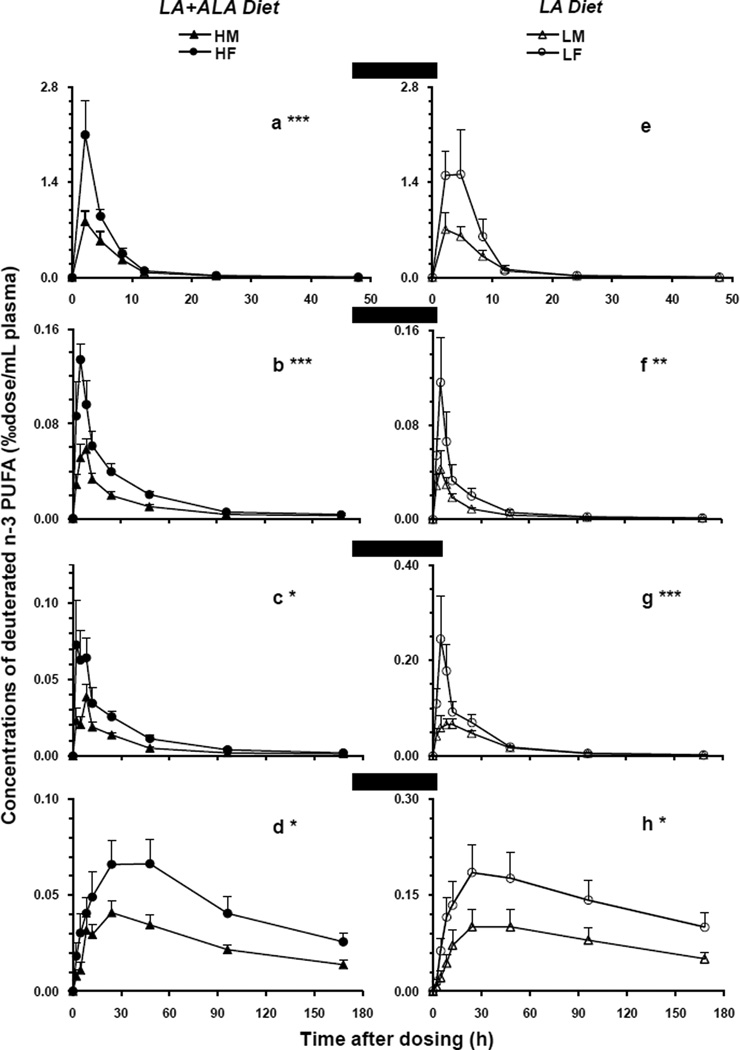
The appearance and disappearance of plasma 2H5-18:3n-3 and its major metabolites (‰dose/mL) was plotted in Figure 1 as a function of time over 168 h (50 h for 2H5-18:3n-3) after a single oral dosing. On the left panel, which shows the LA+ALA diet groups, the precursor 2H5-18:3n-3 reached peaks of 0.8 and 2.1 in group HM and HF, respectively, at the first sampling time point (2 h). And, then the deuterated precursor decreased sharply over time, almost disappearing at 24 h (0.003), and eventually became undetectable after 96 h (Figure 1a). Thus, the maxima of plasma 2H5-18:3n-3 in females was more than twice that in male rats. These accounted for 3.7 and 2.4% of the initial oral dosages for female and male rat per plasma compartment, respectively. The time-course curves of the precursors comparing genders were significantly different (P < 0.001) in LA+ALA diet.
Figure 1.
The plasma time-course curves of the deuterated n-3 precursor (2H5-18:3n-3) and its metabolites in male and female rats fed LA+ALA diet (left) or LA diet (right). The concentrations of the labeled fatty acids (‰dose/mL plasma) were presented on the y-axis as a function of time at 0, 2, 4, 8, 12, 24, 48, 96, and 168 h after a single oral dose of the isotope (50 h for 2H5-18:3n-3). Values were expressed as the mean ± SEM (n=6). Repeated measures ANOVA was applied to ascertain significant differences for time-course curves between genders within each diet. The significance level was set at a P value of 0.05*, 0.01**, or 0.001***. (a/e) 2H5-18:3n-3, (b/f) 2H5-20:5n-3, (c/g) 2H5-22:5n-3, and (d/h) 2H5-22:6n-3. HM (▲) and HF (●) indicate male and female animals fed the LA+ALA diet; LM (▲) and LF (○) indicate male and female animals fed the LA diet.
Similar patterns were observed for the deuterated n-3 PUFA metabolites derived from 2H5-18:3n-3. As presented in figure 1b, 2H5-20:5n-3 reached a mean peak of 0.13 ‰dose/mL at 4 h in females, and then decayed quickly to 0.10 at 8 h and to 0.06 at 12 h. For the males, the maxima was about half of that of females and at the later time of 8 h. The 2H5-20:5n-3 remained detectable for both genders at the final sampling point, 168 h after dosing. For 2H5-22:5n-3 (1c), female rats reached a plateau of 0.073 between 2 h and 8 h, then decayed slowly while males peaked at 8 h at 0.039. For the end product, 2H5-22:6n-3 (1d), female rats reached a peak of 0.066 at 24 h, while males reached a peak of 0.041 at the same time, with the female group value higher by about 62%. 2H5-DHA decayed slowly and was detectable at 168 h for both genders with values of about 35% of the maxima.
On the right panel, which shows the LA diet groups (Fig 1 e–h), similar patterns were observed for all the deuterated n-3 PUFA, except the 2H5-18:3n-3 (1e) which, although quite difference in appearance, did not exhibit statistical significance. The time-course curves of three metabolites were significantly different comparing genders (P < 0.05 or lower). The maxima of 2H5-18:3n-3 was about 2.7% and 2.1% of the initial dosage for females and males, respectively. The peak concentrations (‰dose/mL) of 2H5-20:5n-3 (1f) was 0.12 at 4 h in females in comparison with 0.04 (P < 0.01) in males. Plasma 2H5-22:5n-3 (1g) in females reached 0.25 at 4 h while in males was only 0.066 between 8 to 12 h, about a four-fold difference. As for the end product, the maxima of 2H5-22:6n-3 (1h) in female rats was 0.19 at 24 h, higher by about 84% than that in males at 0.10.
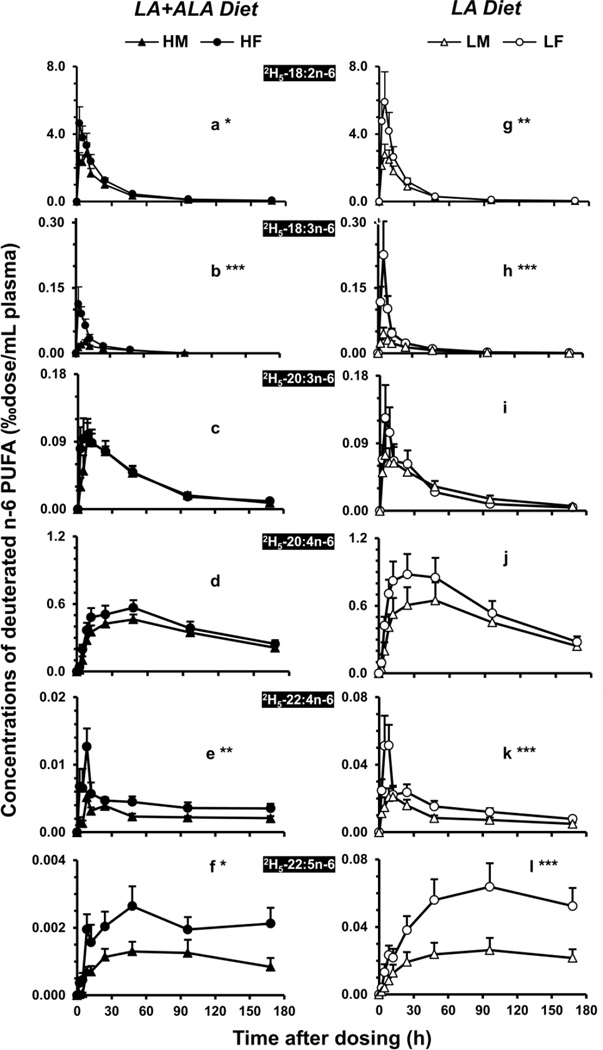
3.4. Higher deuterated DPAn-6, 22:4n-6, and 18:2n-6 in female rat plasma
The appearance and disappearance of 2H5-18:2n-6 and its metabolites among male and female plasma for the two dietary groups are presented in Figure 2. The time-course curves of n-6 PUFA comparing genders were significantly different for 2H5-18:2n-6 (2a; P < 0.05), 2H5-18:3n-6 (2b; P < 0.001), 2H5-22:4n-6 (2e; P < 0.01), and 2H5-22:5n-6 (2f; P < 0.01). On the left panel, the LA+ALA diet groups, 2H5-18:2n-6 (2a) exhibited a maximal concentration (‰dose/mL) of 4.6 at 2 h in female and 2.8 at 8 h in males. These values accounted for a similar value for the percentage of the initial dosage, 8%. 2H5-18:2n-6 showed a slower decay and elimination in both genders than 2H5-18:3n-3 and could be detected at 168 h after dosing. 2H5-18:3n-6 (2b) peaked at the same time as its precursor and was significantly higher in females than in males (P < 0.001). There was no significant differences observed comparing the two genders for the appearance and disappearance of 2H5-20:3n-6 (2c) or2H5-20:4n-6 (2d). Among n-6 PUFAs, the end product 2H5-22:5n-6 (2f) exhibited the greatest difference comparing genders (P < 0.05) with maxima of 0.0026 in females vs. 0.0013 in males, at the 48 h after dose.
Figure 2.
The plasma time-course curves of the deuterated n-6 precursor (2H5-18:2n-6) and its metabolites in male and female rats fed the LA+ALA diet (left) or the LA diet (right). (a/g) 2H5-18:2n-6 and its metabolites (b/h) 2H5-18:3n-6, (c/i) 2H5-20:3n-6, (d/j) 2H5-20:4n-6, (e/k) 2H5-22:4n-6, and (f/l) 2H5-22:5n-6. See legend to Figure 1 for more details.
On the right panel of Figure 2 (2g–2l), among the LA diet groups, deuterated n-6 PUFAs showed a similar pattern compared to the LA+ALA groups. Female rats accumulated significantly greater precursor (2g) and metabolites including 2H5-18:3n-6 (2h), 2H5-22:4n-6 (2k), and 2H5-22:5n-6 (2l). The maxima of 2H5-18:2n-6 was 5.9 vs. 2.8 ‰dose/mL among females and males, which accounted for 10.5 and 8.3% of the initial dosages, respectively. The peak maxima for the end product 2H5-22:5n-6 (2l) was about 2.5-fold in female plasma relative to that of males, 0.064 vs. 0.026. Their time-course curves showed a significant difference comparing two genders (P < 0.001).
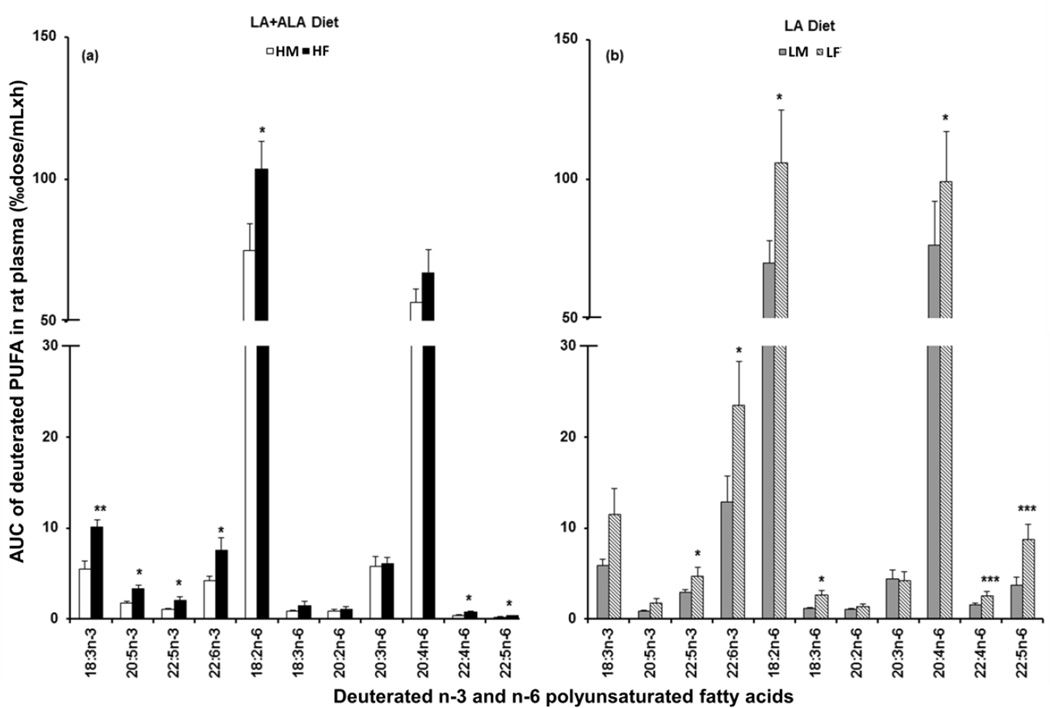
3.5. Area under the time-course curves of deuterated PUFA
A more global parameter relative to the magnitudes of the peak maxima were also calculated, the areas under the time-course curves for the major PUFAs, as presented in Figure 3. On the LA+ALA diet (3a), the AUC of individual n-3 PUFA was about 2-fold greater in female rats than in male (P < 0.05), including 2H5-18:3n-3 (10 vs. 5.5), 2H5-20:5n-3 (3.3 vs. 1.7), 2H5-22:5n-3 (2.1 vs. 1.0), and 2H5-22:6n-3 (7.6 vs. 4.2). Similarly for the precursor and C22 n-6 PUFA metabolites, a significantly greater AUC (P < 0.05) was observed in females for 2H5-18:2n-6 (103 vs. 75), 2H5-22:4n-6 (0.72 vs. 0.41), and 2H5-22:5n-6 (0.35 vs. 0.18). However, no significant difference was observed for 2H5-18:3n-6, 2H5-20:3n-6, and 2H5-20:4n-6 comparing genders for the LA+ALA diet. While on the LA diet (3b), a significant difference between female and male rats was observed for 2H5-22:5n-3 (4.7 vs. 2.9) and 2H5-22:6n-3 (24 vs. 13) in n3 PUFAs and for 2H5-18:2n-6 (106 vs. 70), 2H5-18:3n-6 (2.6 vs. 1.1), 2H5-20:4n-6 (99 vs. 76), 2H5-22:4n-6 (2.6 vs. 1.5) and 2H5-22:5n-6 (8.7 vs. 3.7) in the n-6 PUFAs, respectively. A somewhat greater value for the AUC was observed for 2H5-18:3n-3 and 2H5-20:5n-3 in females, but this did not reach statistical significance.
Figure 3.
Accumulations of the labeled n-3 and n-6 PUFA in rat plasma were presented as the area under the time-course curves over 0 to 168 h (AUC0→168, ‰dose/mL×h). The gender difference was compared in the LA+ALA diet (a) and in the LA diet (b). A two-sided, pairwise Student’s t test was applied to detect significant differences between genders within each diet (P < 0.05*, 0.01**, or 0.001***). See legend to Figure 1 for more details.
4. DISCUSSION
This study explored the variable of gender on both the endogenous PUFA concentrations and the de novo deuterated LC PUFA synthesis in rats fed diets high or extremely low in ALA with constant LA and no HUFA. Our results indicate that greater plasma concentrations of both the endogenous DHA and DPAn-6 and metabolized 2H5-DHA and 2H5-DPAn-6 were observed in female rats in both types of diet. However, it was noteworthy that the female rats exhibited a greater sensitivity and accommodation to extremely low dietary ALA.
Our findings of greater accumulation of 22:6n-3 and 22:5n-6 in female rat plasma are in general agreement with a variety of observations related to DHA tissue content in rats [25, 37–40]. Similarly in human subjects, higher erythrocyte DHA levels were observed in women of childbearing age by Smit et al [17] and Magnusardottir et al [23], as well as in postmenopausal women after hormone replacement therapy [21, 22]. This higher DHA among females was observed in liver phospholipids, but not in triacylglycerols in adult rats [37, 39]. Higher DPAn-6 was reported by Smit et al [17] in human female erythrocytes and by Kitson et al in rats [39]. In the parallel stable isotope tracer study, there was a greater accumulation of de novo synthesized 2H5-22:6n-3 and 2H5-22:5n-6 in female rat plasma relative to that of males. These observations agree with the results obtained in human studies. Burge et al. reported that 13C-U-DHA was synthesized in young women [14] but it was undetectable in plasma lipid classes in young men [15]. Pawlosky [16] reported a greater synthesized rate from 2H5-22:5n-3 to 2H5-22:6n-3 in female than in male. In this animal study, the accumulation of 2H5-22:5n-3 was particularly enhanced in female rat plasma when that diet was extremely low in 18:3n-3. It was apparent that 2H5-22:5n-3 was more important than downstream precursors in the formation of 2H5-22:6n-3. The female rat might supply more 2H5-22:5n-3 for the formation of end product 2H5-22:6n-3. And this could result in greater accumulation of 2H5-22:6n-3 in female than in male animals. This finding supported a report by Millar et al [41], which suggests that 22:5n-3 may be a reservoir for the synthesis of the major long-chain n-3 fatty acids in human, both for 20:5n-3 and 22:6n-3. It is possible that the observed higher 22:4n-6 in n-6 PUFA plays a similar role as 2H5-22:5n-3 for the end product 22:5n-6 accumulation.
By contrast, ARA was significantly lower in female rat plasma when dietary 18:3n-3 was high, but was similar in females and males when the diet was extremely low in 18:3n-3. This comparison suggested that during a prolonged deficiency of dietary 18:3n-3, female rats accumulated relatively more ARA than those fed the adequate level of dietary 18:3n-3 to meet their physiological needs in addition to higher DHA and DPAn-6 than do males. This supported the observation of a 1960s report in which female rats gained more ARA when diets were devoid of both n-6 and n-3 EFA [3], and a recent study [22] when diets were high in 18:2n-6 (~52% of total fatty acids), but very low n-3 EFA (~0.55%). In addition, 24:1n-9, 20:3n-6 and 20:3n-9 were significantly lower and 18:0 higher in female rat plasma relative to males for both diets (P < 0.01). This is in partial agreement with a report by Marks et al [42] that several monounsaturated fatty acids were significantly higher in male rat liver and plasma phospholipids, even though there were no differences in hepatic Stearoyl-CoA desaturase 1 expression. However, Marks et al [42] also observed 16:0 was significantly lower in female rats, but in our study no significant gender difference in 16:0 in either diet.
This study indicates that the gender differences in the end products of both n-3 and n-6 PUFA in rats are independent of the status of dietary 18:3n-3 since the differences were observed in animals fed diets either high or extremely low in ALA. Though no comparable research has been reported for 22:5n-6, this observation is in basic agreement with a human cohort study reported by Welch et al [43] that the gender difference of the n-3 PUFA is independent of the status of dietary n-3 PUFA, occurring either in fish-eaters or non-fish-eaters. Similarly, Giltay et al observed a gender difference of DHA in human subjects under controlled diets with constant ALA and no n-3 HUFA [21]. When dietary 18:3n-3 was extremely low (0.04 wt%), our study found that the differences were more notable as the female rats exhibited a higher ratio of 22:6n-3 to 22:5n-3 compared to the males. Extier et al [25] reported a similar gender effect based on a rat model fed on diets with high 18:2n-6 (22 wt% of total fatty acids) and repletion of n-3 PUFA (0.3%). This is also in agreement with Pawlosky et al [16] who reported that DHA formation was stimulated in women on a beef-based diet (higher n-6 PUFA), but not on a fish diet (higher n-3 PUFA). The conclusion from the systematic review by Lohner et al [20] also suggests that the gender difference in human plasma DHA mainly presented in populations with low intake of dietary n-3 HUFA, and this could be extenuated by supplying large amount of fish oil (8 g/day) [44]. Thus, female mammals might present a greater sensitivity to the status of dietary n-3 PUFA and better accommodation an extremely low dietary n-3 PUFA level.
This study describes gender differences in endogenous and de novo accumulation of synthesized DHA and DPAn-6 in rats under well-defined diets containing only C18 PUFAs. These stable isotopic studies indicates that female rats retained more deuterated C18 precursors after an oral administration and could subsequently produce more end products in both n-3 and n-6 PUFAs, which disappeared much slower than their precursors in plasma. This phenomena could also be interpreted as a greater lipoprotein transportation of metabolized HUFA from the liver into the circulation system in females than in males. In addition to possible metabolic differences, the deposit of PUFAs in white adipose tissues and viscera and catabolism of PUFAs might also contribute to this gender difference. A further investigation in the liver, the primary site of fatty acid metabolism in mammals, as well as other viscera, would be important to pursue in future studies. Future compartmental kinetic analysis of the time-course curves would be helpful to calculate precisely the kinetic parameters of PUFA metabolism from C18 to subsequent metabolites, C20 and C22 PUFA, in rats.
In summary, our results demonstrated that female rats accumulated more endogenous and de novo synthesized DHA and DPAn-6 than did males, and this is possibly through both the greater conversion of de novo synthese of DHA and DPAn-6 from C18 precursors and the greater transportation of end products from liver to the circulatory system. This difference was more notable under the circumstance of extremely low dietary ALA.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Dr. Lee Chedester and Dr. Raouf Kechrid for their expert assistance with the animal work. This project was funded by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH.
This project was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
ABBREVIATIONS
- ALA
18:3n-3, α-linolenic acid
- ARA
20:4n-6, arachidonic acid
- AUC
area under the time-course curve
- BF3
boron trifluoride
- BHT
butylated hydroxytoluene
- DPAn-3
22:5n-3, n-3 docosapentaenoic acid
- DPAn-6
22:5n-6, n-6 docosapentaenoic acid
- DHA
22:6n-3, docosahexaenoic acid
- EFA
essential fatty acid
- EPA
20:5n-3, eicosapentaenoic acid
- FID
flame ionization detector
- GC
gas liquid chromatography
- HUFA
highly unsaturated fatty acid
- LA
18:2n-6, linoleic acid
- LC PUFA
long chain polyunsaturated fatty acid
- MS
mass spectrometry
- MUFA
monounsaturated fatty acids
- NCI
negative chemical ionization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 2.Loeb HG, Burr GO. A study of sex differences in the composition of rats, with emphasis on the lipid component; sex difference in susceptibility to essential fatty acid deficiency with high and low fat diets. J Nutr. 1947;33:541–551. doi: 10.1093/jn/33.5.541. [DOI] [PubMed] [Google Scholar]
- 3.Lyman RL, Ostwald R, Bouchard P, Shannon A. Effect of sex and gonadal hormones on rat plasma lipids during the development of an essential fatty acid deficiency. Biochem J. 1966;98:438–450. doi: 10.1042/bj0980438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostwald R, Bouchard P, Miljanich P, Lyman RL. Influence of sex and gonadal hormones on rat-liver and carcass lipids during the development of an essential fatty acid deficiency. Biochem J. 1965;97:485–499. doi: 10.1042/bj0970485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyman RL, Shannon A, Ostwald R, Miljanich P. Effect of estradiol and testosterone on the fatty acids of plasma cholesteryl esters and phospholipids in the castrated rat. Can J Biochem. 1964;42:365–376. doi: 10.1139/o64-044. [DOI] [PubMed] [Google Scholar]
- 6.Ostwald R, Lyman RL. Influence of sex and gonadal hormones on lipid metabolism in essential fatty acid-deficient rats. Lipids. 1968;3:199–210. doi: 10.1007/BF02531187. [DOI] [PubMed] [Google Scholar]
- 7.Moore L, Chen T, Knapp HR, Jr, Landon EJ. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975;250:4562–4568. [PubMed] [Google Scholar]
- 8.Moore L, Knapp HR, Jr, Landon EJ. An effect of estradiol and testosterone on the calcium pump activity and phospholipid fatty acid composition of rat liver microsomes. Endocrinology. 1977;100:1516–1520. doi: 10.1210/endo-100-6-1516. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen K, Gan MV, Holman RT. Sex differences in the metabolism of fatty acids in vitro. Biochim Biophys Acta. 1969;187:19–25. doi: 10.1016/0005-2760(69)90128-3. [DOI] [PubMed] [Google Scholar]
- 10.Tinoco J, Williams MA, Hincenbergs I, Lyman RL. Evidence for nonessentiality of linolenic acid in the diet of the rat. J Nutr. 1971;101:937–945. doi: 10.1093/jn/101.7.937. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MA, Sinclair AJ. The limitations of whole tissue analysis to define linolenic acid deficiency. J Nutr. 1972;102:1315–1321. doi: 10.1093/jn/102.10.1315. [DOI] [PubMed] [Google Scholar]
- 12.Tinoco J, Babcock R, Hincenbergs I, Medwadowski B, Miljanich P, Williams MA. Linolenic acid deficiency. Lipids. 1979;14:166–173. doi: 10.1007/BF02533868. [DOI] [PubMed] [Google Scholar]
- 13.Salem N., Jr . Omega-3 fatty acids: Molecular and biochemical aspects. In: Spiller GA, Scala J, editors. Current topics in nutrition and disease: New protective roles for selected nutrients. New York: Alan R Liss; 1989. pp. 109–228. [Google Scholar]
- 14.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 15.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 16.Pawlosky R, Hibbeln J, Lin Y, Salem N., Jr N-3 fatty acid metabolism in women. Br J Nutr. 2003;90:993–994. doi: 10.1079/bjn2003985. discussion 94-5. [DOI] [PubMed] [Google Scholar]
- 17.Smit EN, Fokkema MR, Boersma ER, Muskiet FA. Higher erythrocyte 22:6n-3 and 22:5n-6, and lower 22:5n-3 suggest higher delta-4-desaturation capacity in women of childbearing age. Br J Nutr. 2003;89:739–740. doi: 10.1079/BJN2003851. [DOI] [PubMed] [Google Scholar]
- 18.Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids. 2006;75:161–168. doi: 10.1016/j.plefa.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Childs CE, Hoile SP, Burdge GC, Calder PC. Changes in rat n-3 and n-6 fatty acid composition during pregnancy are associated with progesterone concentrations and hepatic fads2 expression. Prostaglandins Leukot Essent Fatty Acids. 2012;86:141–147. doi: 10.1016/j.plefa.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Lohner S, Fekete K, Marosvolgyi T, Decsi T. Gender differences in the long-chain polyunsaturated fatty acid status: Systematic review of 51 publications. Ann Nutr Metab. 2013;62:98–112. doi: 10.1159/000345599. [DOI] [PubMed] [Google Scholar]
- 21.Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 22.Giltay EJ, Duschek EJ, Katan MB, Zock PL, Neele SJ, Netelenbos JC. Raloxifene and hormone replacement therapy increase arachidonic acid and docosahexaenoic acid levels in postmenopausal women. J Endocrinol. 2004;182:399–408. doi: 10.1677/joe.0.1820399. [DOI] [PubMed] [Google Scholar]
- 23.Magnusardottir AR, Steingrimsdottir L, Thorgeirsdottir H, Gunnlaugsson G, Skuladottir GV. Docosahexaenoic acid in red blood cells of women of reproductive age is positively associated with oral contraceptive use and physical activity. Prostaglandins Leukot Essent Fatty Acids. 2009;80:27–32. doi: 10.1016/j.plefa.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Sibbons CM, Brenna JT, Lawrence P, Hoile SP, Clarke-Harris R, Lillycrop KA, Burdge GC. Effect of sex hormones on n-3 polyunsaturated fatty acid biosynthesis in hepg2 cells and in human primary hepatocytes. Prostaglandins Leukot Essent Fatty Acids. 2014;90:47–54. doi: 10.1016/j.plefa.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Extier A, Langelier B, Perruchot MH, Guesnet P, Van Veldhoven PP, Lavialle M, Alessandri JM. Gender affects liver desaturase expression in a rat model of n-3 fatty acid repletion. J Nutr Biochem. 2010;21:180–187. doi: 10.1016/j.jnutbio.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Kitson AP, Marks KA, Shaw B, Mutch DM, Stark KD. Treatment of ovariectomized rats with 17beta-estradiol increases hepatic delta-6 desaturase enzyme expression and docosahexaenoic acid levels in hepatic and plasma phospholipids. Prostaglandins Leukot Essent Fatty Acids. 2013;89:81–88. doi: 10.1016/j.plefa.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N., Jr An n-3 fatty acid deficiency impairs rat spatial learning in the barnes maze. Behav Neurosci. 2009;123:196–205. doi: 10.1037/a0013801. [DOI] [PubMed] [Google Scholar]
- 28.Reeves PG, Nielsen FH, Fahey GC., Jr Ain-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 29.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 30.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron tri-fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 31.Salem N, Jr, Reyzer M, Karanian J. Losses of arachidonic acid in rat liver after alcohol inhalation. Lipids. 1996;31(Suppl):S153–S156. doi: 10.1007/BF02637068. [DOI] [PubMed] [Google Scholar]
- 32.Pawlosky RJ, Sprecher HW, Salem N., Jr High sensitivity negative ion gc-ms method for detection of desaturated and chain-elongated products of deuterated linoleic and linolenic acids. J Lipid Res. 1992;33:1711–1717. [PubMed] [Google Scholar]
- 33.Lin YH, Pawlosky RJ, Salem N., Jr Simultaneous quantitative determination of deuterium- and carbon-13-labeled essential fatty acids in rat plasma. J Lipid Res. 2005;46:1974–1982. doi: 10.1194/jlr.M500128-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Masood A, Stark KD, Salem N., Jr A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res. 2005;46:2299–2305. doi: 10.1194/jlr.D500022-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Lin YH, Salem N, Jr, Wells EM, Zhou W, Loewke JD, Brown JA, Lands WE, Goldman LR, Hibbeln JR. Automated high-throughput fatty acid analysis of umbilical cord serum and application to an epidemiological study. Lipids. 2012;47:527–539. doi: 10.1007/s11745-012-3661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh KC, Kwan KC. Comparison of numerical integrating algorithms by trapezoidal, lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 37.Burdge GC, Slater-Jefferies JL, Grant RA, Chung WS, West AL, Lillycrop KA, Hanson MA, Calder PC. Sex, but not maternal protein or folic acid intake, determines the fatty acid composition of hepatic phospholipids, but not of triacylglycerol, in adult rats. Prostaglandins Leukot Essent Fatty Acids. 2008;78:73–79. doi: 10.1016/j.plefa.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67:19–27. doi: 10.1017/S0029665108005983. [DOI] [PubMed] [Google Scholar]
- 39.Kitson AP, Smith TL, Marks KA, Stark KD. Tissue-specific sex differences in docosahexaenoic acid and delta6-desaturase in rats fed a standard chow diet. Appl Physiol Nutr Metab. 2012;37:1200–1211. doi: 10.1139/h2012-103. [DOI] [PubMed] [Google Scholar]
- 40.Ghasemifard S, Hermon K, Turchini GM, Sinclair AJ. Metabolic fate (absorption, beta-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. Br J Nutr. 2015;114:684–692. doi: 10.1017/S0007114515002457. [DOI] [PubMed] [Google Scholar]
- 41.Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, Turchini GM, Cameron-Smith D, Sinclair AJ. A short-term n-3 dpa supplementation study in humans. Eur J Nutr. 2013;52:895–904. doi: 10.1007/s00394-012-0396-3. [DOI] [PubMed] [Google Scholar]
- 42.Marks K, Kitson A, Stark K. Hepatic and plasma sex differences in saturated and monounsaturated fatty acids are associated with differences in expression of elongase 6, but not stearoyl-coa desaturase in sprague–dawley rats. Genes & Nutrition. 2013;8:317–327. doi: 10.1007/s12263-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch AA, Shakya-Shrestha S, Lentjes MA, Wareham NJ, Khaw KT. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results from the epic-norfolk cohort. Am J Clin Nutr. 2010;92:1040–1051. doi: 10.3945/ajcn.2010.29457. [DOI] [PubMed] [Google Scholar]
- 44.Metherel AH, Armstrong JM, Patterson AC, Stark KD. Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fatty Acids. 2009;81:23–29. doi: 10.1016/j.plefa.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin YH, Shah S, Salem N., Jr Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J Nutr Biochem. 2011;22:758–765. doi: 10.1016/j.jnutbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.