Abstract
Migraine is the third most common disease worldwide, the most common neurological disorder, and one of the most common pain conditions. Despite its prevalence, the basic physiology and underlying mechanisms contributing to the development of migraine is still poorly understood and development of new therapeutic targets is long overdue. Until recently, the major contributing pathophysiological event thought to initiate migraine was cerebral and meningeal arterial vasodilation. However, the role of vasodilation in migraine is unclear and recent findings challenge its necessity. While vasodilation itself may not contribute to migraine, it remains possible that vessels play a role in migraine pathophysiology in the absence of vasodilation. Blood vessels consist of a variety of cell types that both release and respond to numerous mediators including growth factors, cytokines, adenosine triphosphate (ATP), and nitric oxide (NO). Many of these mediators have actions on neurons that can contribute to migraine. Conversely, neurons release factors such as norepinephrine and calcitonin gene-related peptide (CGRP) that act on cells native to blood vessels. Both normal and pathological events occurring within and between vascular cells could thus mediate bi-directional communication between vessels and the nervous system, without the need for changes in vascular tone. This review will discuss the potential contribution of the vasculature, specifically endothelial cells, to current neuronal mechanisms hypothesized to play a role in migraine. Hypothalamic activity, cortical spreading depression (CSD), and dural afferent input from the cranial meninges will be reviewed with a focus on how these mechanisms can influence or be impacted by blood vessels. Together, the data discussed will provide a framework by which vessels can be viewed as important potential contributors to migraine pathophysiology, even in light of the current uncertainty over the role of vasodilation in this disorder.
1.1 Introduction
Migraine headache is the most common neurological disorder and one of the most common pain conditions. It is characterized by recurrent multiphasic symptoms, which include episodes of unilateral pulsating head pain. The entire sequence of migraine symptoms can last many hours to days and can vary greatly among patients. There are four distinct phases of migraine: the premonitory phase, aura phase, headache phase, and postdrome phase (Charles, 2013). Up to 80% of individuals who suffer from migraines experience premonitory symptoms hours or even days leading up to the headache attack (Becker, 2013). Premonitory symptoms including excessive yawning, food cravings, mood changes, fatigue, sore neck, and confusion among others, and are considered a reliable predictor of an impending migraine (Blau and MacGregor, 1994; Becker, 2013). Toward the end of the premonitory phase before the onset of headache, about 15-30% of migraine suffers report visual disturbances known as aura (Goadsby, 2012). Patients with aura can experience geometric patterns (e.g. fortification spectra), light oscillations of varying intensities, or partial vision loss (e.g. scotoma). The headache phase of migraine, which typically follows aura, is characterized by moderate to severe unilateral throbbing head pain that persists for longer than 4 hours but can last up to 72 hours. Additional symptoms associated with the headache phase include nausea, vomiting, enhanced sensitivity to light, sound and smell, and cutaneous allodynia (Piovesan et al., 2003; Goadsby, 2009a; Levy, 2010; Giamberardino, 2003; Burstein et al., 2004). After the headache subsides, many patients report postdrome symptoms such as cognitive impairments, fatigue, and changes in mood that can persist for 18-24 hours post-headache (Goadsby, 2009a).
Two distinct clinical states of migraine have been identified: episodic and chronic migraine. Episodic migraines are characterized as 14 or fewer headache days per month, while chronic migraine is characterized by 15 or more headache days per month for more than 3 months where at least 8 of the headaches meet the criteria for migraine (Headache Classification Committee of the International Headache Society, 2013). Clinical and epidemiological observations demonstrate that episodic migraine can progress to chronic migraine at a rate of 2.5% annually (Bigal et al., 2008). The migraine phases combined can last several days, and given their severity, migraines have a significant impact on overall quality of life by negatively affecting physical, social, and occupational function. According to the World Health Organization's Global Burden of Disease Study analysis of data collected from 1990 to 2010, migraine headache is the third most prevalent disease in the world (Vos et al., 2012). Additionally, a recent review of 4 national surveillance studies on migraine reinforces what is commonly known in the general population and also reported consistently throughout the literature, that migraine is 2-3 times more prevalent in females than males (Smitherman et al., 2013). Despite the prevalence, the basic physiology and underlying factors contributing to the development of migraine headache is still poorly understood.
Unlike other pain states, migraine sufferers report multiple distinct triggers. These triggers are innocuous in healthy patients which suggests that the sensitivity to different triggers in migraine is due to maladaptive changes within the nervous system. The higher incidence of migraine headache in females compared to males strongly suggests a role for female hormones in the onset of migraine. Before puberty, the annual incidence of migraine between males and females is similar (approximately 4%) (Bille, 1997), but at puberty, rates in females increase to 18% while only increasing to 6% in males (Lipton et al., 2001). Incidence remains higher in females until post-menopause. Further, approximately 50% of female migraineurs have attacks related to specific times of their menstrual cycle (Martin, 2004) with up to 20% of female migraineurs experiencing what are termed “pure menstrual” migraines, which by definition occur between days -2 and +3 from the onset of menstruation (Brandes, 2006). This implicates changes in hormone levels as an important migraine trigger in females. Besides changes in sex hormones, the most commonly reported migraine trigger is stress (Spierings et al., 1997; Martin and MacLeod, 2009; Houle and Turner, 2013). As many as 80% of patients indicate that stress is their primary trigger for migraine (Kelman, 2007). The physiological stress response involves the activation of the hypothalamic-pituitary-adrenocortical axis (HPA) and the autonomic nervous system. Possible mechanisms whereby activation of these systems evoke headache include stress hormone-mediated activation/sensitization of afferent nociceptors, changes in descending inhibitory control and alterations in immune system responses, among others (Burstein and Jakubowski, 2005; Imbe et al., 2006; Meng and Cao, 2007; Maier, 2003; Sauro and Becker, 2009). Recent reports also show that the peak susceptibility to migraine is in the 18-24 hours after a stressful event and therefore stress itself may not be the trigger, rather the consequences of the stressful event such as sleep disturbances, changes in diet, or other physiological changes that occur after the resolution of stress (Kemper et al., 2001; Lipton et al., 2014; Goadsby, 2014). Other common migraine triggers include alcohol, environmental irritants, exercise, changes in the weather, improper duration of sleep and intense sensory stimuli (Kelman, 2007). Patients who identify their individual triggers are often successful at reducing the frequency of migraine attacks by consciously decreasing their exposure to the trigger (Martin et al., 2014). Despite such interventions, migraine remains highly prevalent.
Since the discovery of elevated levels of the 5-HT metabolite 5-HIAA (5-hydroxyindoleacetic acid) in the urine of patients during migraine attacks (Sicuteri, 1972), the role 5-HT plays in migraine has been hotly debated. Although it is unclear whether levels increase, decrease, or remain unchanged during migraine attacks, the general consensus in the migraine field is that 5-HT plays an important role in this disorder (Dussor, 2014). In fact, the most widely utilized pharmacological agents for the acute treatment of migraine are 5-HT based. Triptans are a family of serotonin (5-HT) -1B, -1D, and -1F agonists which include sumatriptan, zolmitriptan, rizatriptan, eletriptan, and naritriptan. They are taken at the onset of migraine symptoms in order to terminate attacks or at the very least to decrease headache intensity/duration. Triptans alone account for up to 80% of medications prescribed for migraine (Diener et al., 2011). Unfortunately, less than 50% of patients taking oral triptans are pain-free at 2 hours, and 30% have a reoccurrence of headache within 24 hours (Ferrari et al., 2001; Goadsby and Sprenger, 2010). Although they are the only drugs specifically developed to treat migraine headache, the exact mechanism by which triptans reduce migraine pain is unknown. They possess vasoconstrictive properties and therefore are generally not prescribed in patients with cardiovascular disease or abnormal blood pressure (Dodick et al., 2004). Moreover, repeated dosing with triptans can lead to a phenomenon known as medication-overuse headache (MOH) (Kristoffersen and Lundqvist, 2014). MOH is characterized as a headache occurring on more than 15 days per month with regular overuse (more than 3 months) of one or more drugs that can be taken for acute and/or symptomatic treatment of headache (Headache Classification Committee of the International Headache Society, 2013). Therefore, triptans are not recommended for daily use and are not prescribed as a migraine prophylactic. As a preventative measure to decrease the frequency of migraine headaches, patients will often be prescribed antiepileptics such as topiramate, beta-blockers such as propranolol, or anti-depressants such as amitriptyline off-label. However, these drugs can cause severe adverse effects including nausea, vomiting, weight gain, decreased cognition, and withdrawal symptoms upon discontinuation of the medication (Edvinsson and Linde, 2010; Stovner et al., 2009). Furthermore, migraine prophylactics only decrease the frequency of headache by about 50% in 40-50% of patients (Tfelt-Hansen and Olesen, 2012).
The development of new migraine compounds is long overdue. Recently, several studies have shown that selective 5-HT-1F agonists (e.g. lasmitidan (Nelson DL et al., 2010) have efficacy in several clinical trials as an abortive migraine treatment (for review see (Tfelt-Hansen and Olesen, 2012; Hoffmann and Goadsby, 2014). In addition, targeting calcitonin gene-related peptide (CGRP) directly has been shown to be a promising therapy. CGRP was found to be significantly elevated in the plasma of patients during acute migraines (Goadsby et al., 1990) and administration of CGRP to migraineurs can trigger attacks (Lassen et al., 2002). This led to the hypothesis that CGRP-based therapeutics may have efficacy for migraine. In a phase 3 clinical study, the CGRP-receptor antagonist telcagepant was more effective than placebo for the reduction in migraine symptoms; unfortunately elevated liver enzymes were detected and further development of this compound was halted (Ho et al., 2008). More recently, phase 2 clinical trials have shown that monoclonal antibodies to CGRP and the CGRP receptor significantly reduce the number of migraine headache days (Dodick et al., 2014a, Dodick et al., 2014b; Bigal et al., 2015a, Bigal et al., 2015b). Importantly, the CGRP antibodies by design are extremely specific, have long half-lives and are unlikely to cause liver toxicity given that they are not subject to hepatic metabolism (Mitsikostas and Rapoport, 2015). Currently, these antibodies are in phase 3 clinical trials for management and prevention of episodic and chronic migraine headache.
For many decades, the major contributing pathophysiological event thought to initiate migraine was cerebral and meningeal arterial vasodilation (Goadsby, 2009a; Shevel, 2011). Support for the vascular migraine hypothesis grew, in part, due to the use of the vasoconstrictor ergotamine, an ergot alkaloid that was found to reduce temporal artery pulsations and relieve headache pain in migraine patients (Graham, 1938; Drummond and Lance, 1983). Furthermore, other vasoconstrictors were soon discovered to abort migraine attacks including noradrenaline and 5-HT (Ostfeld and Wolff, 1955; Kimball et al., 1960; Anthony et al., 1967). Since ergotamine was a non-selective vasoconstrictor with affinity for 5-HT, noradrenaline and dopamine receptors, more selective 5-HT receptor agonists were developed to reduce the side-effect profile of perspective migraine therapies (for review see Humphrey, 2007). This resulted in the eventual development of the 5-HT-1B/1D agonist, sumatriptan, which was shown to cause vasoconstriction and effectively reduced migraine symptoms (Humphrey et al., 1990). Although there is a strong correlation between migraine pathology and the associated vasculature, other studies have concluded that vasodilation is an epiphenomenon and does not contribute to migraine directly (Olesen, 1990; Goadsby, 2009b). For example, vasoactive intestinal polypeptide (VIP) has been shown to dilate cranial arteries to a similar extent as pituitary adenylate cyclase activating peptide (PACAP-38), however VIP does not produce migraine in migraineurs (Rahmann et al., 2008) while PACAP-38 does (Amin et al., 2012). In addition, vasoactive substances known to trigger migraine, such as the NO donor nitroglycerine (NTG; Thomsen et al., 1994) and sildenafil (Kruuse et al., 2003) were reported not to cause cerebral and meningeal blood vessel dilation in migraineurs during attacks (Schoonman et al., 2008). Although this may be true for NTG, CGRP administration, which also triggers migraine in migraineurs, has been shown to cause blood vessel dilation at time points where headaches occur (Asghar et al., 2010). Discrepancies reported in the literature may be due at least in part to the use of different vasodilators (and the differential downstream effects of nitric oxide vs. CGRP) and/or different methods to detect changes in the vasculature. However, a recent study showed no significant changes in blood vessels during spontaneous migraines in humans (Amin et al., 2013) leading the authors to conclude that vasodilation is not the cause of migraine. Consequently, attention within the migraine field has largely shifted to the role that changes in the nervous system play in migraine pathophysiology. Moreover, it is likely that migraine is a consequence of dysfunctional neuronal networks (Edvinsson et al., 2012) as certain neurological symptoms of migraine cannot be explained solely by the vascular model of headache (e.g. VIP does not trigger migraines, but causes vasodilation).
Although the migraine field seems poised to discard the vascular hypothesis, this is primarily based on an unclear role of vasodilation in migraine. Before vessels are completely discarded however, it seems appropriate to further discuss whether vessels might contribute to migraine in the absence of vasodilation. The cells comprising blood vessels (e.g. endothelial and smooth muscle cells) do not merely exist to dilate and constrict blood vessels, and they may make an important contribution to migraine without a change in vascular diameter. Consequently, migraine symptoms may arise from a combination of dilation-independent vascular events (Tietjen and Khubchandani, 2015) and neurogenic mechanisms interacting throughout the brain and within the trigeminovascular system in the meninges (Levy, 2010). The purpose of the remainder of this review is to discuss the potential contribution of the vasculature to current neuronal mechanisms hypothesized to play a role in migraine including altered hypothalamic activity, cortical spreading depression (CSD), and dural afferent input from the cranial meninges. Focusing on dilation-independent mechanisms may help determine whether the vascular hypothesis as a whole should be laid to rest, or whether the field should simply move beyond vasodilation as the cause of migraine.
1.2 Blood vessel anatomy
Before discussing the potential role of blood vessels in several neuronal mechanisms thought to contribute to migraine, it is important to briefly review the anatomy of the blood vessel (see figure 1). The inner-most layer of the blood vessel, the tunica intima, is comprised of endothelial cells surrounded by a subendothelial layer of connective tissue and internal elastic lamina which provides a flexible barrier between the endothelium and the inner smooth muscle cell layer. The middle layer, the tunica media, is comprised of smooth muscle cells, connective tissue and a thick elastic band called the external elastic lamina, which separates the middle and outer layers. The outermost layer, tunica adventitia, consists of nerve fibers, fibroblasts, perivascular adipose tissue and collagen. Vascular smooth muscle cells within the tunica media have been shown to regulate vascular tone primarily in response to sympathetic nervous system innervation (e.g. adrenergic receptor activation), NO, as well as the local synthesis, uptake and release of 5-HT (Green, 200; Ni et al., 2008). The contribution of smooth muscle cells and their contribution to vascular tone in migraine has largely overshadowed the diverse functions of another major vascular cell type, endothelial cells. Endothelial cells directly contact the circulating blood in the lumen and also control vessel function. They express a variety proteins including growth factors (e.g. vascular endothelial cell growth factors, VEGF) (Breier and Risau, 1996), coagulants/anticoagulants (Stern et al., 1985), lipoproteins (e.g. low density lipoprotein, LDL) (Sawamura et al., 1997), and junction proteins (e.g. platelet endothelial cell adhesion molecule, PECAM-1) (Albelda et al., 1991) as well as metabolites (e.g. NO and 5-HT) (Palmer et al., 1988; Green, 2006), hormones (e.g. endothelin-1) (Yanagisawa et al., 1988), and cytokines (tumor necrosis factor, TNF-α) (Mantovani et al., 1997). Thus, endothelial cells are involved in the regulation a variety of functions including cell-cell barrier maintenance, vascular tone, vascular remodeling, immune surveillance, blood coagulation, and nutrient uptake among others (for review see Cines et al, 1998). Taken together, these studies demonstrate that the blood vessel does not simply provide a conduit for the movement of blood that can constrict and dilate; rather it coordinates a much more complicated web of signaling between multiple cell-types. Dysregulation of any part of this vascular signaling process may contribute to migraine pathology. The remainder of this review will highlight the potential contribution of vascular endothelial signaling to neuronal events thought to contribute to migraine.
Fig. 1.
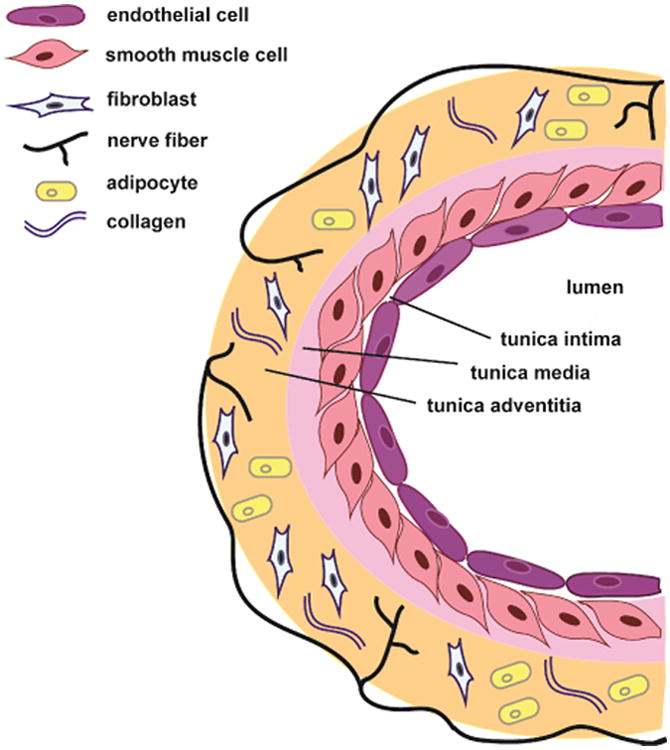
Anatomy of a blood vessel. Bloods vessels are comprised of 3 layers: the tunica intima, tunica media, and tunica adventitia. The innermost layer, the tunica intima, is comprised of a single layer of endothelial cells. The middle layer, the tunica media is predominately comprised of smooth muscle cells. The outermost layer, the tunica adventitia, consists of nerve fibers, fibroblasts, perivascular adipose tissue and collagen. Compared to smaller vessels (as depicted here), large vessels have increased tunica intima/media/adventitia thick- ness due to increased numbers of cells in each layer.
2.1 Hypothalamus
The location of origin of migraines within the nervous system is unknown but many have speculated that this complex brain disorder may be driven by the hypothalamus. The hypothalamus, located at the base of the brain with widespread connections throughout the central nervous system, is involved in maintenance of homeostasis by controlling the endocrine system and coordinating activity within the autonomic nervous system. It regulates many physiological functions including food intake, energy balance, responses to stress, and circadian rhythms. Additionally, the hypothalamus is involved in the processing of trigeminal nociceptive signaling (Malick et al., 2000; Holland and Goadsby, 2007), a type of afferent sensory input critical for the pain phase of migraine (reviewed below). Many migraine patients experience premonitory symptoms related to dysfunction of the aforementioned systems including sleep disturbances, changes in wakefulness, mood, appetite and/or thirst. Additionally, functional imaging studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) showed hypothalamic activation during spontaneous migraine (Afridi et al., 2005; Denuelle et al., 2007). Moreover, Goadsby and colleagues found increased activity in the posterolateral hypothalamus among other brain areas during the premonitory phase of NTG-triggered migraines (Maniyar et al., 2014). These data suggest that the hypothalamus plays a central role in the initiation/progression of migraine.
Hypothalamic regulation of hormonal cycles in women may contribute to the cyclic nature of migraine. As previously mentioned, migraine headache is three times more prevalent in females compared to males after puberty (Lipton et al., 2014). An explanation for the sexual dimorphism of migraine may be attributed to, among other events, estrogen regulation of hypothalamic networks that control the menstrual cycle (Brandes, 2006). During the follicular phase of the menstrual cycle, the amount of estrogens (e.g. estradiol) secreted by the growing follicle dramatically increase and act as a positive stimulus for hypothalamic-pituitary-mediated surges in polypeptides involved the progression of the menstrual cycle (Kelly et al., 2005). Evidence suggests that estradiol-mediated activation of estrogen receptors expressed by endothelial cells (Venkov et al., 1996) augments endothelial nitric oxide synthase (eNOS) activity (Caulin-Glaser et al., 1997; Simoncini and Genazzani, 2000; Martin and Behbehani, 2006) resulting in the rapid increase in NO release in human female endothelial cells, an effect that was antagonized by the 17β-estradiol antagonist, ICI 164, 384 (Caulin-Glaser et al., 1997). Moreover, there is a positive correlation between the incidence of migraine and expression of biomarkers of endothelial cell activation in women (Tiejen, GE et al., 2009). These data suggest that the increased incidence of migraine in women may be due in part to the effects of hypothalamic regulation of female hormones such as estradiol on endothelial cells.
Given the multitude of factors known to cause migraine, stress being the most common (Kelman, 2007), autonomic related symptoms may reflect normal hypothalamic responses to environmental triggers. During stress, the PVN of the hypothalamus releases hormones including vasopressin, oxytocin and corticotrophin–releasing hormone, altering the balance between parasympathetic and sympathetic tone. Importantly, hypothalamic neurons can regulate parasympathetic preganglionic neurons in the superior salivatory nucleus (SSN) and sympathetic preganglionic neurons in the spinal intermediolateral nucleus (Moulton et al., 2014). Although data assessing the role of the autonomic nervous system in migraine is conflicting, a majority of studies report sympathetic hypofunction and enhanced cranial parasympathetic tone in migraineurs between attacks (Shechter et al., 2002; Peroutka, 2004; Avnon et al., 2003). Activation of the SSN stimulates the release of acetylcholine, VIP, PACAP, and NO from postganglionic parasympathetic neurons in the sphenopalatine ganglion (SPG) (Uddman et al., 1999; Edvinsson et al., 2001) resulting in the local release of inflammatory mediators that can activate meningeal nociceptors (Burstein and Jakubowski, 2005, Csati et al., 2012a, Csati et al., 2012b). Therefore, the interaction between parasympathetic and trigeminal sensory systems following hypothalamic activation of the SSN may facilitate the progression of migraine.
PACAP, VIP and NO activate a variety of alternative signaling pathways in vessels independent of vasodilation that may contribute to migraine pathophysiology. The vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide-receptor-1 (VPAC1R) and VPAC2 receptors are expressed in various cell types throughout the body (Wei and Mojsov, 1996) including endothelial cells (Steinhoff et al., 1999; Borsani et al., 2013) and show equal affinity for VIP and PACAP (Zhou et al., 2002). In a model of ischemia, VIP increased the expression and secretion of VEGF in endothelial cells (Yang et al., 2009, Yang et al., 2013) leading to increased angiogenesis (Potente et al., 2011). Considering the mediators of angiogenesis are thought to contribute to the development of chronic inflammation (Ribatti et al., 2007), angiogenic signaling may also contribute to inflammatory pain. During angiogenesis, VEGF recruits immune cells such as macrophages and neutrophils to the site of tissue injury where they produce various inflammatory cytokines including interleukins and chemokines thought to be involved in the pathology of pain (Miller et al., 2009; Kiguchi et al., 2012; Selvaraj et al., 2015). The release of the highly permeable gaseous molecule NO may also contribute to nociceptive neurotransmission. Under inflammatory conditions, vessel and nociceptor eNOS and VEGF immunofluorescence increases (Borsani E et al., 2013). Moreover, an eNOS inhibitor, L-N(5)-(1-iminoethyl)ornithine (L-NIO), was found to attenuate inflammatory hyperalgesia (Borsani et al., 2013). In addition, PACAP not only leads to de novo formation of new blood vessels, but also plasma protein extravasation (neurogenic inflammation) in human skin (Seeliger et al., 2010). Taken together these data suggest that hypothalamic regulation of parasympathetic tone may activate vascular endothelial cell signaling pathways known to contribute to inflammatory pain. Therefore, it is possible that the hypothalamus contributes to migraine via endothelial cell-dependent signaling pathways independent of vasodilation. How this leads to the specific set of symptoms characteristic of migraine, and not other pain states, is unclear.
3.1 Cortical Spreading Depression
Cortical Spreading Depression (CSD) is the widespread depolarization of neuronal and glial membranes due to sudden loss of membrane resistance and ionic gradients. Characterized as a brief burst of activity in the cortex that leads to the inhibition of all spontaneous and evoked synaptic activity within cerebral grey matter (for review see (Eikermann-Haerter and Ayata, 2010; Pietrobon and Moskowitz, 2014), CSD is thought to cause massive K+ (Grafstein, 1956) and glutamate (Van Harreveld, 1959) efflux which contributes to the depolarization of adjacent brain tissue. The signal propagates as a wave at a velocity of about 2-5 mm/min across the cortical surface (Leao, 1945; Grafstein, 1956; Ochs, 1962; Aitken et al., 1998; Smith et al., 2006) and is associated with numerous physiological changes in the cortex including alterations in intra and extracellular ion concentrations, neurotransmitter release and changes in blood flow and oxygen levels (Somjen, 2001; Pietrobon and Moskowitz, 2014).
CSD may contribute to the pathophysiology of several diseases including migraine (Bolay and Moskowitz, 2005; Charles and Baca, 2013). It has been proposed that since the rate of aura spread across the surface of the primary visual cortex during migraine corresponds to the propagation velocity of CSD (Lashley, 1941; Leao, 1945; Milner, 1959), that the wave of neuronal excitation/inhibition may contribute to reported migraine symptoms (i.e. aura). Moreover, imaging studies show patterns of changes in blood flow in the cortex of human migraine patients corresponds to the mean cortical velocity of perceived aura across the visual field (Hansen et al., 2013; Charles and Baca, 2013).
Although CSD is thought to be the underlying basis of aura, whether it contributes to headache is less clear. Headache classically follows aura across the phases of migraine but headache is thought to be mediated by nociceptive trigeminal afferents innervating the meninges (discussed below) and not due to direct cortical events. While Leao first proposed that CSD could evoke pain neurotransmission via activation of trigeminal afferents (Leao, 1944), later studies found that CSD can activate meningeal nociceptors (Moskowitz and Macfarlane, 1993; Zhang et al., 2010) as well as second-order neurons in the spinal trigeminal nucleus, a brainstem region that processes nociceptive information (Moskowitz et al., 1993; Zhang et al., 2011a). Moskowitz and colleagues have shown expression of the neuronal marker c-fos in the trigeminal nucleus caudalis in a rat model of CSD. They also found that sumatriptan inhibited c-fos expression in this region, but did not alter the induction of CSD (Moskowitz et al., 1993). Taken together, these data suggest that CSD does not likely cause headache directly, rather it may contribute to headache via activation/sensitization of meningeal afferents of the trigeminal nerve by substances released as a result of CSD.
During CSD, the demand for energy increases dramatically and in order to restore ionic gradients and neuronal function, cerebral blood flow (CBF) also increases (Shinohara et al., 1979). In agreement with this observation, others have reported that after the depolarization wave, CBF and oxygen levels transiently increase (Lukyanova and Bures, 1967; Piilgaard and Lauritzen, 2009). This increase in CBF is followed by a persistent decrease in blood flow (Lambert and Michalicek, 1994; Ayata et al., 2004) and oxygen levels (Piilgaard and Lauritzen, 2009) leading to a period of tissue hypoxia (Lukyanova and Bures, 1967; Lacombe et al., 1992; Otori et al., 2003; Takano et al., 2007). Therefore, in order to restore metabolic balance, cortical neurons including interneurons and astroglia are thought to release of a variety of neurotransmitters including NO, carbon monoxide, adenosine, hydrogen ions, potassium ions, and lipoxygenase products known to alter cerebral vascular tone (Cauli et al., 2004; Vaucher et al., 2000; Zonta et al., 2003; Filosa et al., 2006; Koehler et al., 2006; Busija et al., 2008). Independent of vascular tone, these signaling molecules also directly affect endothelial cell signaling pathways (De Caterina et al., 1995; Jozkowicz et al., 2003; Erlinge and Burnstock, 2008; Dalvi et al., 2015; Mark et al., 2001). Moreover, the close association between cerebral blood vessels and neurons (Hawkins and Davis, 2005) facilitates 2-way communication between cortical neurons and endothelial cells comprising the blood vessels. Just as endothelial cells respond to substances released by neurons, neurons can respond to substances released by endothelial cells. For example, arterial endothelial cells synthesize and store peptides such as CGRP (Cai, et al., 1993; Doi et al., 2001; Luo et al., 2008). Endothelial cell release of CGRP may contribute to increased neuronal excitability within the cortex (Tozzi et al., 2012) in a similar manner as has been reported for astrocyte-mediated release of glutamate (Seidel et al., 2016). Ayata and colleagues have suggested that neuronal hyperexcitability due to neurovascular dysfunction enhances susceptibility to ischemic CSD-like depolarizations during mild changes in metabolic demand (Eikermann-Haerter et al., 2012; von Bornstadt et al., 2015). Thus, due to neurovascular mechanisms that contribute to neuronal hyperexcitability (e.g. endothelial cell release of CGRP), sensory stimulation in a specific cortical region that would otherwise go undetected in non-migraine patients (e.g. somatosensory events) may trigger a CSD event and aura in migraineurs.
4.1 Meningeal afferents
As previously mentioned, the pain phase of migraine likely requires activation of trigeminal nociceptors innervating the cranial meninges (Burstein et al., 2015). Trigeminal nociceptors are pain-sensing neurons that bifurcate from the cell body located in the trigeminal ganglion (TG), sending an axon branch to innervate intracranial and extracranial tissue and another axon branch to synapse on second order neurons in the trigeminal nucleus caudalis (TNC) or trigeminocervial complex (TCC) (Strassman et al., 1994; Hoskin et al., 1999). Stimulation of dura mater near blood vessels and sinuses has been shown to produce pain in humans that closely mirrors the sites of pain commonly reported during migraine (e.g. behind the eye) (Penfield, 1940; Ray, 1940). Although, numerous studies have reported that dural afferents are sensitive to noxious mechanical (Kaube et al., 1992; Strassman et al., 1996; Levy and Strassman, 2002) and chemical (Sarchielli et al., 2001; Perini et al., 2005) stimuli, it is still unclear how these neurons are activated during migraine. Meningeal arteries and veins including their extensive capillary network supply blood to the dura (Fricke B et al., 2001). Considering the close association between trigeminal afferents and cerebral/dural vasculature (Mayberg et al., 1981, Mayberg et al., 1984) it is possible that cells comprising blood vessels can sensitize and/or directly activate meningeal afferents leading to headache.
During migraine, intracranial and circulating levels of various inflammatory mediators, which are known to sensitize primary afferent nociceptors are elevated (Sarchielli et al., 2001; Perini et al., 2005). Thought to be a consequence of neurogenic inflammation in the meninges, immune cells including dural mast calls and macrophages release a host of proflammatory mediators including 5-HT, histamine, prostaglandins, and cytokines (Mekori and Metcalfe, 2000; Levy, 2009; Reuter et al., 2001), which are known to sensitize meningeal nociceptors (Levy et al., 2007; Zhang et al., 2007, Zhang et al., 2011b, Zhang et al., 2012, Yan et al., 2012). Neuronal receptors are thought to mediate the sensitizing actions of inflammatory cytokines (Yan et al., 2012; Nicol et al., 1997; Czeschik et al., 2008), however other non-neuronal cell types may also be involved as ablation of neuronal TNF receptors in the ganglia does not inhibit peripheral sensitization (Parada et al., 2003). Levy and colleagues have shown that local application of TNF-α to the meninges evokes TNF receptor-mediated activation of p38 MAP kinase in dural blood vessels, and that the p38 antagonist SB203580 inhibits TNF-α-mediated meningeal afferent sensitization (Zhang et al., 2011b). Other factors released by endothelial cells may also contribute to meningeal afferent sensitization as Levine and colleagues have recently reported that endothelial cell-mediated release of endothelin-1 (ET-1), a potent vasodilator and mediator elevated in human plasma at the onset of migraine attacks (Kallela et al., 1998), sensitizes nociceptors to mechanical-stimuli via endothelial cell-mediated release of ATP leading to hyperalgesia (Joseph et al., 2011, Joseph et al., 2014, Joseph et al., 2015). Further, sumatriptan and a β-adrenergic receptor antagonist, ICI118551, inhibited ET-1-induced hyperalgesia (Joseph and Levine, 2013). Although, the vasoconstrictive properties of ET-1 mediated primarily by ETA receptors are not associated with changes in cerebral blood flow during CSD (Goadsby et al., 1996), others have reported that ET-1 mediates neurogenic inflammation in rat dura mater via ETB receptors (Brandli et al., 1996) and that gene variants encoding both ET receptor subtypes are associated with migraine in humans (Tzourio et al., 2001; Lemos et al., 2011; Tikka-Kleemola et al., 2009). While promising, results from a clinical trial indicate that the mixed ETA/ETB antagonist, Bosentan, was not efficacious for aborting migraine attacks and therefore, it is unclear what role ET release from endothelial cells may have in migraine pathophysiology (May et al., 1996). In addition, c-type natriuretic peptide (CNP), an endothelium-derived hyperpolarizing factor (EDHF) which is secreted from endothelial cells (Lumsden et al., 2010; Moyes et al., 2014) induces thermal hyperalgesia in mice (Loo et al., 2012). This CNP-induced thermal hypersensitivity is mediated by PKC phosphorylation-dependent potentiation of TRPV1 via the natriuretic peptide receptor (NPR)-C on peripheral sensory neurons (Loo, L et al., 2013). These new data suggest that cells comprising blood vessels (e.g. endothelial cells) can contribute to afferent sensitization via the release of factors such as ET-1 and CNP. Admittedly, these studies have been conducted in tissues outside of the head and are thus not directly relevant to migraine but they could provide important information on mechanisms that may similarly occur within the brain and meninges. It is also possible that during a migraine attack, changes in metabolic demand or other stimuli such as mechanical stimulation (Bodin and Burnstock, 2001) can cause neurons and other vascular cell types to release ATP. Activation of purinergic receptors located on endothelial cells causes endothelial cell-mediated release of NO, which diffuses readily to smooth muscle cells and leads to subsequent vasodilation (Burnstock, 2015). In addition to NO-mediated vasodilation however, NO released from endothelial cells may also mediate meningeal afferent sensitization, as NO donors that are known to cause headache in migraineurs (Olesen and Jansen-Olesen, 2000) have recently been shown to promote delayed meningeal nociceptor sensitization as well as ERK phosphorylation in meningeal arteries (Zhang et al., 2013). This latter study also showed that blockade of ERK phosphorylation inhibited NTG-mediated afferent sensitization. Further, new data from Levine and colleagues implicate a role for mast cells and endothelial cells in NTG-induced hyperalgesia (Ferrari et al., 2016). The activation of purinergic receptors on endothelial cells also stimulates proflammatory pathways (Erlinge and Burnstock, 2008) including the release of interleukins (Seiffert et al., 2006) known to sensitize meningeal afferents (Zhang et al., 201; Yan et al., 2012). Purinergic receptors have been shown to mediate increases in endothelial cell surface expression of intercellular adhesion molecule-1 (ICAM-1) (Seiffert et al., 2006) and vascular cell adhesion molecule-1 (VCAM-1) (Seye et al., 2004), which are important for the recruitment of immune cells such as neutrophils (Dawicki et al., 1995) and monocytes (Seye et al., 2003) to endothelial cells. The release of proflammatory mediators by the recruited immune cells further amplifies meningeal afferent sensitization. Taken together, vasodilation may be an epiphenomena that has previously overshadowed concurrent endothelial cell-mediated signaling pathways contributing to sensitization of meningeal afferents and migraine pain.
Just as endothelial cell-mediated signaling influences primary afferent nociceptors, nociceptor-mediated signaling can also influence blood vessels (see figure 2). The release of vasoactive neuropeptides such as substance P and CGRP from meningeal afferents (Edvinsson et al., 1983; Ebersberger et al., 1999; Harrison and Geppetti, 2001) causes vasodilation (Brain and Grant, 2004; Smillie and Brain, 2011) and plasma protein extravasation (PPE) in the dura (Markowitz et al., 1987; O'Shaughnessy and Connor, 1994; Moussaoui et al., 1993), the latter due to alterations in blood vessel permeability. Enhanced blood flow and leakage of plasma constituents protects the brain and meninges by quickly diluting and clearing out noxious stimuli. However, the increase in vascular permeability may also allow for cytokines and other proflammatory mediators secreted and recruited by the endothelial cells to readily move through the vessel and potentiate meningeal nociceptor activation (Figure 2). In addition to sensory neurons, sympathetic neurons originating from the superior cervical ganglion (SCG) also innervate the meninges (Keller, JT et al., 1989; Andres, KH et al., 1987). The release of sympathetic neurotransmitters such as neuropeptide Y and norepinephrine from sympathetic fibers can directly act on vessels in the meninges (Edvinsson and Uddman, 1981; Edvinsson et al., 1983; Edvinsson, 1985; Keller, JT and Marfurt, CF, 1991). For example, NPY has been shown to contribute to ET-1 release in human endothelial cells (Abdel-Samad, et al., 2012) as well as increased adhesion of leukocytes to endothelial cells (Sung et al., 1991). Additionally, norepinephrine has been shown to induce endothelial cell IL-6 production (Stohl et al., 2012; Gornikiewicz et al., 2000). Thus, bi-directional communication between meningeal nerve fibers (sensory and sympathetic) and endothelial cells comprising the associated vasculature may facilitate the headache phase of migraine, further suggesting that endothelial cells play a central role in the pathology of migraine.
Fig. 2.
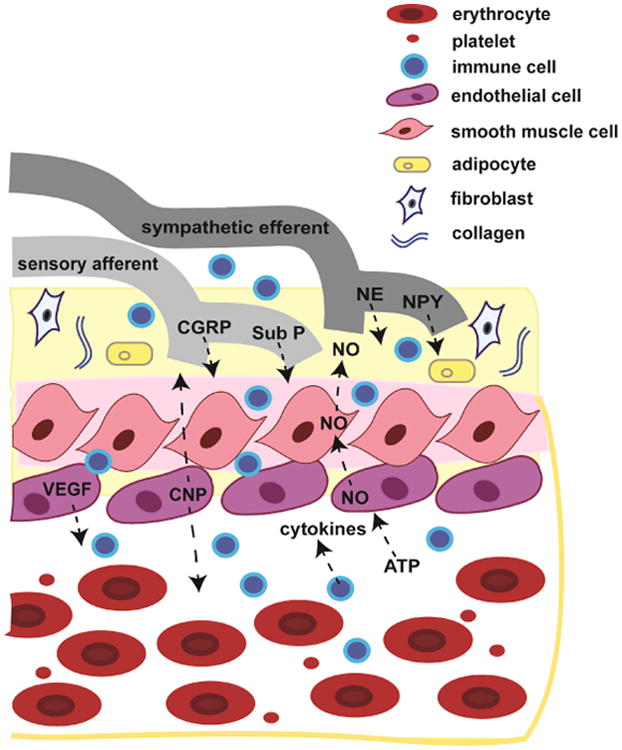
Bidirectional signaling between meningeal nerve fibers, immune cells and cells comprising the associated blood vessels. Meningeal sensory afferents originating from the trigeminal ganglia innervate the meningeal vasculature and release vasoactive neuropeptides including substance P (Sub P) and calcitonin gene- related peptide (CGRP). In addition, sympathetic efferents from the superior cervical ganglion release neurotransmitters including neu- ropeptide Y (NPY) and norepinephrine (NE) that can act on vessels in the meninges. Conversely, cells comprising the blood vessel as well as those in the vascular lumen can influence meningeal sensory afferents. Endothelial cells can release c-type natriuretic peptide (CNP) and potentiate sensory afferent neuronal firing. During angio- genesis, endothelial cells release vascular endothelial cell growth factor (VEGF), which recruits immune cells such as macrophages and neutrophils. The recruited immune cells infiltrate the nearby tissue and release cytokines known to sensitize sensory afferents. In addition, changes in metabolic demand and other stimuli such as shear stress can cause the release of adenosine triphosphate (ATP) from multiple cell types within the vessel. Endothelial cell purinergic receptor activation causes the release and diffusion of nitric oxide (NO) throughout the vessel and surrounding tissue resulting in a wide range of effects including sensory afferent sensitization.
Meningeal afferents express numerous ion channels including transient receptor potential (TRP) channels, acid-sensing ion channels (ASICS), glutamate-gated channels, ATP-gated channel, and K+ channels that when activated may contribute to the pain of migraine (for revew see Yan and Dussor, 2014). Among the stimuli capable of activating or sensitizing dural afferents are capsaicin (via TRPV1), mustard oil (via TRPA1), hypotonic solutions (via TRPV4), or an inflammatory soup (Strassman et al., 1996; Bove and Moskowitz, 1997; Wei et al., 2011; Edelmayer et al., 2012). Vascular endothelial cells also express a number of channels including the recently identified mechano-sensing channel, Piezo2 (Ferrari et al., 2015), sodium channels such as Nav1.7 (Rice et al., 2015), and various TRP channels including TRPA1 (Earley, 2012) and TRPV4 (Yao and Garland, 2005). Therefore, just as nociceptors detect numerous noxious stimuli including changes in temperature, pH, and pressure, recent reports suggest that endothelial cells express the machinery to detect similar noxious stimuli. Of late, Levine and colleagues demonstrated that Piezo2 channels expressed on endothelial cells mediate inflammatory hyperalgesia (Ferrari et al., 2015). It is possible that Piezo2 channels located on endothelial cells are able to detect mechanical forces such as shear stress within the vessel leading to the release of substances capable of activating meningeal afferents. Additionally, a situation could exist for example where activators of TRPA1 including environmental irritants (e.g. cigarette smoke, chlorine gas) which are well-known migraine triggers (for review see Dussor et al., 2014) are released into the blood and initiate signaling between meningeal nociceptors and endothelial cells. The full extent to which different ion channels expressed on endothelial cells contribute to processes culminating in nociceptor sensitization is currently unknown. However, bidirectional signaling between meningeal nociceptors and endothelial cells could further amplify an inflammatory process leading to a positive feedback loop potentiating nociceptive signals to the CNS and causing headache.
5.1 Conclusions
Before the migraine field abandons the theory of a vascular contribution to the disorder, it is important to consider that the cells comprising the blood vessel may contribute to the initiation and progression of migraine attacks independent of vasodilation. The strong positive correlation between the effects of migraine triggers (e.g. NO, PACAP-38, and CGRP) and migraine therapies (e.g. ergotamine, triptans) on changes in blood vessel diameter have contributed greatly to the idea that vasodilation plays a critical role in migraine. However, recent reports indicate that during spontaneous migraine there is little to no dilation of vessels. And importantly, the reverse is also true; blood vessel dilation does not always produce a migraine. But the unclear role of blood vessel dilation during migraine attacks is not evidence of a lack of communication between vessels and surrounding neuronal structures, it may simply be evidence of a lack of vasodilation in migraine pathophysiology. Alternatively, blood vessel dilation may be an epiphenomenon masking a chain of events leading to the development of migraine hours later. Although there may be no vasodilation during the migraine attack, vessels may dilate at time points well before the attack and this may leave a series of signaling events within the vessel in its wake. Of particular interest, CNP a potent vasodilator released by endothelial cells was recently reported to cause no change in the diameter of cerebral arteries in guinea pigs and humans (Gus, et al., 2015), but produced a pain phenotype via potentiation of TRPV1 on peripheral sensory neurons (Loo et al., 2012). Although, CNP did not produce migraine in healthy volunteers (Gus, et al., 2015), it has yet to be tested in migraineurs (this is especially relevant since NO/CGRP do not produce migraines in healthy volunteers, but do in migraineurs). These recent developments point to the possibility that signaling events within/between vascular cells, not the presence of vasodilation, may be critical to the progression of the attack. Ultimately, whether CNP and other known headache agents cause cerebral vasodilation may be irrelevant. Blood vessels are more than just a conduit for blood that can contract and dilate; they are comprised of a variety of cell types that generate numerous molecules and mediators important for intra- and intercellular processes. Endothelial cells mediate immune cell recruitment and downstream inflammatory signaling pathways, which may be critical to the pathophysiology of migraine. They also express a variety of channels and receptors (e.g. TRP channels and purinergic receptors) thought to be involved in the detection of noxious stimuli, and along with neurons, endothelial cells may potentiate the responses to noxious stimuli. Any of these processes may be critical for the development of a migraine attack regardless of whether vasodilation is present.
In order to further determine whether vessels play a role in migraine, several important questions should be answered. First, it is important to determine whether vasodilation occurs at any time point before the onset of migraine e.g. during the interictal phase leading up to an attack or during the premonitory phase. Prior reports assessing changes in vessel diameter were performed after the onset of migraine or at time points immediately following administration of the trigger (where there is clearly vasodilation from NO or CGRP). As mentioned above, there may be no vasodilation at the peak of a migraine attack, but prior vasodilation may contribute to a cascade of signaling events that long outlasts the changes in vessel diameter. Determining whether or not vasodilation is present even at very early time points, or whether the signaling cascade is the only relevant component, is important to know before the dilation hypothesis is discounted. Second, migraine triggers that may not produce cerebral vasodilation (e.g. sildenafil, Kruuse et al., 2003) should be examined more closely to better understand their mechanisms. If indeed these triggers act independently of dilation at any time point, this would provide clear evidence that vasodilation is not necessary and the more relevant mechanisms downstream of these triggers can be identified. Finally, mediators like CNP produce hypersensitivity in preclinical models but may not cause cerebral vasodilation in humans. This peptide should be examined more closely for its ability to trigger attacks in migraineurs. CNP may represent an important, endothelial-derived molecule that is capable of triggering attacks independent of vasodilation. Understanding how CNP and potentially other vessel-derived mediators promote attacks may provide important insights into dilation-independent vascular contributions to migraine. Ultimately, there are many connections between vessels and migraine and many potential mechanisms by which they can contribute to the disorder. While it may be reasonable at this point to discard vasodilation as a direct cause of migraine, it seems far too premature to completely eliminate vessels from the list of factors contributing to the pathophysiology of migraine.
Highlights.
Migraine is the 3rd most common and 8th most disabling disease on earth.
Neuronal mechanisms play a key role in migraine pathophysiology.
Human studies have lead to increased scrutiny of the vascular theory of migraine.
Vasodilation may not be necessary or sufficient for migraine.
Vascular endothelial cells may contribute to migraine without vasodilation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Samad D, Perreault C, Ahmarani L, Avedanian L, Bkaily G, Magder S, D'Orleans-Juste P, Jacques D. Differences in neuropeptide Y-induced secretion of endothelin-1 in left and right human endocardial endothelial cells. Neuropeptides. 2012;46:373–382. doi: 10.1016/j.npep.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- Aitken PG, Tombaugh GC, Turner DA, Somjen GG. Similar propagation of SD and hypoxic SD-like depolarization in rat hippocampus recorded optically and electrically. J Neurophysiol. 1998;80:1514–1521. doi: 10.1152/jn.1998.80.3.1514. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Guo S, Hougaard A, Hansen AE, Schytz HW, van der Geest RJ, de Koning PJ, Larsson HB, Olesen J, Ashina M. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia. 2012;32:140–149. doi: 10.1177/0333102411431333. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJ, Larsson HB, Olesen J, Ashina M. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–461. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Andres KH, von During M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- Anthony M, Hinterberger H, Lance JW. Plasma serotonin in migraine and stress. Arch Neurol. 1967;16:544–552. doi: 10.1001/archneur.1967.00470230096013. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Kapijimpanga T, van der Geest RJ, van der Koning P, Larsson HB, Olesen J, Ashina M. Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology. 2010;75:1520–1526. doi: 10.1212/WNL.0b013e3181f9626a. [DOI] [PubMed] [Google Scholar]
- Avnon Y, Nitzan M, Sprecher E, Rogowski Z, Yarnitsky D. Different patterns of parasympathetic activation in uni- and bilateral migraineurs. Brain. 2003;126:1660–1670. doi: 10.1093/brain/awg158. [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Becker WJ. The premonitory phase of migraine and migraine management. Cephalalgia. 2013;33:1117–1121. doi: 10.1177/0333102412437390. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, Loupe PS, Burstein R, Newman LC, Lipton RB. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015a;14:1081–1090. doi: 10.1016/S1474-4422(15)00249-5. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC, Burstein R, Loupe PS, Ma Y, Yang R, Silberstein SD. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015b;14:1091–1100. doi: 10.1016/S1474-4422(15)00245-8. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Bille B. A 40-year follow-up of school children with migraine. Cephalalgia. 1997;17:488–491. doi: 10.1046/j.1468-2982.1997.1704488.x. discussion 487. [DOI] [PubMed] [Google Scholar]
- Blau JN, MacGregor EA. Migraine and the neck. Headache. 1994;34:88–90. doi: 10.1111/j.1526-4610.1994.hed3402088.x. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bolay H, Moskowitz MA. The emerging importance of cortical spreading depression in migraine headache. Rev Neurol (Paris) 2005;161:655–657. doi: 10.1016/s0035-3787(05)85108-2. [DOI] [PubMed] [Google Scholar]
- Borsani E, Giovannozzi S, Cocchi MA, Boninsegna R, Rezzani R, Rodella LF. Endothelial nitric oxide synthase in dorsal root ganglia during chronic inflammatory nociception. Cells Tissues Organs. 2013;197:159–168. doi: 10.1159/000342518. [DOI] [PubMed] [Google Scholar]
- Bove GM, Moskowitz MA. Primary afferent neurons innervating guinea pig dura. J Neurophysiol. 1997;77:299–308. doi: 10.1152/jn.1997.77.1.299. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- Brandli P, Loffler BM, Breu V, Osterwalder R, Maire JP, Clozel M. Role of endothelin in mediating neurogenic plasma extravasation in rat dura mater. Pain. 1996;64:315–322. doi: 10.1016/0304-3959(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Breier G, Risau W. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 1996;6:454–456. doi: 10.1016/0962-8924(96)84935-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic Signalling and Endothelium. Curr Vasc Pharmacol. 2015 doi: 10.2174/1570161114666151202204948. [DOI] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol. 2005;493:9–14. doi: 10.1002/cne.20688. [DOI] [PubMed] [Google Scholar]
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol. 2008;86:379–395. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WQ, Dikranian K, Bodin P, Turmaine M, Burnstock G. Colocalization of vasoactive substances in the endothelial cells of human umbilical vessels. Cell Tissue Res. 1993;274:533–538. doi: 10.1007/BF00314550. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- Charles A. The evolution of a migraine attack - a review of recent evidence. Headache. 2013;53:413–419. doi: 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9:637–644. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Csati A, Tajti J, Kuris A, Tuka B, Edvinsson L, Warfvinge K. Di stribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience. 2012a;202:158–168. doi: 10.1016/j.neuroscience.2011.10.055. [DOI] [PubMed] [Google Scholar]
- Csati A, Tajti J, Tuka B, Edvinsson L, Warfvinge K. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion -- interaction with the sensory system. Brain Res. 2012b;1435:29–39. doi: 10.1016/j.brainres.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Dalvi S, Nguyen HH, On N, Mitchell RW, Aukema HM, Miller DW, Hatch GM. Exogenous arachidonic acid mediates permeability of human brain microvessel endothelial cells through prostaglandin E2 activation of EP3 and EP4 receptors. J Neurochem. 2015;135:867–879. doi: 10.1111/jnc.13117. [DOI] [PubMed] [Google Scholar]
- Dawicki DD, McGowan-Jordan J, Bullard S, Pond S, Rounds S. Extracellular nucleotides stimulate leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol. 1995;268:L666–673. doi: 10.1152/ajplung.1995.268.4.L666. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- Diener HC, Holle D, Dodick D. Treatment of chronic migraine. Curr Pain Headache Rep. 2011;15:64–69. doi: 10.1007/s11916-010-0159-x. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J investigators ALDs. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014a;13:1100–1107. doi: 10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014b;13:885–892. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Martin VT, Smith T, Silberstein S. Cardiovascular tolerability and safety of triptans: a review of clinical data. Headache. 2004;44(Suppl 1):S20–30. doi: 10.1111/j.1526-4610.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- Doi Y, Kudo H, Nishino T, Kayashima K, Kiyonaga H, Nagata T, Nara S, Morita M, Fujimoto S. Synthesis of calcitonin gene-related peptide (CGRP) by rat arterial endothelial cells. Histol Histopathol. 2001;16:1073–1079. doi: 10.14670/HH-16.1073. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Lance JW. Extracranial vascular changes and the source of pain in migraine headache. Ann Neurol. 1983;13:32–37. doi: 10.1002/ana.410130108. [DOI] [PubMed] [Google Scholar]
- Dussor G. Serotonin, 5HT1 agonists, and migraine: new data, but old questions still not answered. Curr Opin Support Palliat Care. 2014;8:137–142. doi: 10.1097/SPC.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci. 2014;5:1085–1096. doi: 10.1021/cn500083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Elsas T, Suzuki N, Shimizu T, Lee TJ. Origin and Co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53:221–228. doi: 10.1002/jemt.1086. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–655. doi: 10.1016/S0140-6736(10)60323-6. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Rosendal-Helgesen S, Uddman R. Substance P: localization, concentration and release in cerebral arteries, choroid plexus and dura mater. Cell Tissue Res. 1983;234:1–7. doi: 10.1007/BF00217397. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Villalon CM, MaassenVanDenBrink A. Basic mechanisms of migraine and its acute treatment. Pharmacol Ther. 2012;136:319–333. doi: 10.1016/j.pharmthera.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Characterization of the contractile effect of neuropeptide Y in feline cerebral arteries. Acta Physiol Scand. 1985;125:33–41. doi: 10.1111/j.1748-1716.1985.tb07690.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Tfelt-Hansen P, Skarby T, Gjerris F, Olesen J. Presence of alpha-adrenoceptors in human temporal arteries. Comparison between migraine patients and controls. Cephalalgia. 1983;3:219–224. doi: 10.1046/j.1468-2982.1983.0304219.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Adrenergic, cholinergic and peptidergic nerve fibres in dura mater--involvement in headache? Cephalalgia. 1981;1:175–179. doi: 10.1046/j.1468-2982.1981.0104175.x. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr Neurol Neurosci Rep. 2010;10:167–173. doi: 10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, Zheng Y, Qin T, Kurth T, Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125:335–345. doi: 10.1161/CIRCULATIONAHA.111.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Green P, Levine JD. Contribution of Piezo2 to endothelium-dependent pain. Mol Pain. 2015;11:65. doi: 10.1186/s12990-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Levine JD, Green PG. Mechanisms mediating Nitroglycerin-induced Delayed Onset Hyperalgesia in the Rat. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fricke B, Andres KH, Von During M. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech. 2001;53:96–105. doi: 10.1002/jemt.1074. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA. Referred muscle pain/hyperalgesia and central sensitisation. J Rehabil Med. 2003:85–88. doi: 10.1080/16501960310010205. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Pathophysiology of migraine. Neurol Clin. 2009a;27:335–360. doi: 10.1016/j.ncl.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. The vascular theory of migraine--a great story wrecked by the facts. Brain. 2009b;132:6–7. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol. 2012;15:S15–22. doi: 10.4103/0972-2327.99993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ. Stress and migraine: something expected, something unexpected. Neurology. 2014;82:1388–1389. doi: 10.1212/WNL.0000000000000349. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Adner M, Edvinsson L. Characterization of endothelin receptors in the cerebral vasculature and their lack of effect on spreading depression. J Cereb Blood Flow Metab. 1996;16:698–704. doi: 10.1097/00004647-199607000-00021. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010;9:285–298. doi: 10.1016/S1474-4422(10)70005-3. [DOI] [PubMed] [Google Scholar]
- Gornikiewicz A, Sautner T, Brostjan C, Schmierer B, Fugger R, Roth E, Muhlbacher F, Bergmann M. Catecholamines up-regulate lipopolysaccharide-induced IL-6 production in human microvascular endothelial cells. FASEB J. 2000;14:1093–1100. doi: 10.1096/fasebj.14.9.1093. [DOI] [PubMed] [Google Scholar]
- Grafstein B. Mechanism of spreading cortical depression. J Neurophysiol. 1956;19:154–171. doi: 10.1152/jn.1956.19.2.154. [DOI] [PubMed] [Google Scholar]
- Graham J, Wolff HG. Mechanism of migraine headache and action of ergotamine tartrate. Arch NeurPsych. 1938;39:737–763. [Google Scholar]
- Green AR. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;147(Suppl 1):S145–152. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Goetze JP, Jeppesen JL, Burnett JC, Olesen J, Jansen-Olesen I, Ashina M. Effect of natriuretic peptides on cerebral artery blood flow in healthy volunteers. Peptides. 2015;74:33–42. doi: 10.1016/j.peptides.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Baca SM, Vanvalkenburgh P, Charles A. Distinctive anatomical and physiological features of migraine aura revealed by 18 years of recording. Brain. 2013;136:3589–3595. doi: 10.1093/brain/awt309. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM group MKPs. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Goadsby PJ. Emerging targets in migraine. CNS Drugs. 2014;28:11–17. doi: 10.1007/s40263-013-0126-2. [DOI] [PubMed] [Google Scholar]
- Holland P, Goadsby PJ. The hypothalamic orexinergic system: pain and primary headaches. Headache. 2007;47:951–962. doi: 10.1111/j.1526-4610.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- Hoskin KL, Zagami AS, Goadsby PJ. Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: a comparative study of monkey and cat. J Anat. 1999;194(Pt 4):579–588. doi: 10.1046/j.1469-7580.1999.19440579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle TT, Turner DP. Natural experimentation is a challenging method for identifying headache triggers. Headache. 2013;53:636–643. doi: 10.1111/head.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PP. The discovery of a new drug class for the acute treatment of migraine. Headache. 2007;47(Suppl 1):S10–19. doi: 10.1111/j.1526-4610.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- Humphrey PP, Feniuk W, Perren MJ. Anti-migraine drugs in development: advances in serotonin receptor pharmacology. Headache. 1990;30:12–16. doi: 10.1111/j.1526-4610.1990.hed30s1012.x. discussion 24-18. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Ferrari LF, Levine JD. Homocysteine-induced attenuation of vascular endothelium-dependent hyperalgesia in the rat. Neuroscience. 2015;284:678–684. doi: 10.1016/j.neuroscience.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Levine JD. ATP release mechanisms of endothelial cell-mediated stimulus-dependent hyperalgesia. J Pain. 2014;15:771–777. doi: 10.1016/j.jpain.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Role of endothelial cells in antihyperalgesia induced by a triptan and beta-blocker. Neuroscience. 2013;232:83–89. doi: 10.1016/j.neuroscience.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- Kallela M, Farkkila M, Saijonmaa O, Fyhrquist F. Endothelin in migraine patients. Cephalalgia. 1998;18:329–332. doi: 10.1046/j.1468-2982.1998.1806329.x. [DOI] [PubMed] [Google Scholar]
- Kaube H, Hoskin KL, Goadsby PJ. Activation of the trigeminovascular system by mechanical distension of the superior sagittal sinus in the cat. Cephalalgia. 1992;12:133–136. doi: 10.1046/j.1468-2982.1992.1203133.x. [DOI] [PubMed] [Google Scholar]
- Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- Keller JT, Marfurt CF, Dimlich RV, Tierney BE. Sympathetic innervation of the supratentorial dura mater of the rat. J Comp Neurol. 1989;290:310–321. doi: 10.1002/cne.902900210. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen signaling in the hypothalamus. Vitam Horm. 2005;71:123–145. doi: 10.1016/S0083-6729(05)71005-0. [DOI] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Kemper RH, Meijler WJ, Korf J, Ter Horst GJ. Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia. 2001;21:549–557. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12:55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kimball RW, Friedman AP, Vallejo E. Effect of serotonin in migraine patients. Neurology. 1960;10:107–111. doi: 10.1212/wnl.10.2.107. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen ES, Lundqvist C. Medication-overuse headache: a review. J Pain Res. 2014;7:367–378. doi: 10.2147/JPR.S46071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuse C, Thomsen LL, Birk S, Olesen J. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–247. doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- Lacombe P, Sercombe R, Correze JL, Springhetti V, Seylaz J. Spreading depression induces prolonged reduction of cortical blood flow reactivity in the rat. Exp Neurol. 1992;117:278–286. doi: 10.1016/0014-4886(92)90137-f. [DOI] [PubMed] [Google Scholar]
- Lambert GA, Michalicek J. Cortical spreading depression reduces dural blood flow--a possible mechanism for migraine pain? Cephalalgia. 1994;14:430–436. doi: 10.1046/j.1468-2982.1994.1406430.x. discussion 393-434. [DOI] [PubMed] [Google Scholar]
- Lashley K. Patterns of Cerebral Integration indicated by the scotomas of migraine. Archives of Neurology and Psychiatry. 1941;46:331–339. [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Leao A. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Leao Aa, M RS. Propogation of spreading cortical depression. J Neurophysiol. 1945;8:33–45. [Google Scholar]
- Lemos C, Neto JL, Pereira-Monteiro J, Mendonca D, Barros J, Sequeiros J, Alonso I, Sousa A. A role for endothelin receptor type A in migraine without aura susceptibility? A study in Portuguese patients. Eur J Neurol. 2011;18:649–655. doi: 10.1111/j.1468-1331.2010.03239.x. [DOI] [PubMed] [Google Scholar]
- Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- Levy D. Migraine pain and nociceptor activation--where do we stand? Headache. 2010;50:909–916. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Buse DC, Hall CB, Tennen H, Defreitas TA, Borkowski TM, Grosberg BM, Haut SR. Reduction in perceived stress as a migraine trigger: testing the “let-down headache” hypothesis. Neurology. 2014;82:1395–1401. doi: 10.1212/WNL.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Scher AI. Epidemiology and economic impact of migraine. Curr Med Res Opin. 2001;17(Suppl 1):s4–12. doi: 10.1185/0300799039117005. [DOI] [PubMed] [Google Scholar]
- Loo L, Shepherd AJ, Mickle AD, Lorca RA, Shutov LP, Usachev YM, Mohapatra DP. The C-type natriuretic peptide induces thermal hyperalgesia through a noncanonical Gbetagamma-dependent modulation of TRPV1 channel. J Neurosci. 2012;32:11942–11955. doi: 10.1523/JNEUROSCI.1330-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanova LD, Bures J. Changes in pO2 due to spreading depression in the cortex and nucleus caudatus of the rat. Physiol Bohemoslov. 1967;16:449–455. [PubMed] [Google Scholar]
- Lumsden NG, Khambata RS, Hobbs AJ. C-type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr Pharm Des. 2010;16:4080–4088. doi: 10.2174/138161210794519237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ, Li D, Deng HW, Li YJ. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept. 2008;150:66–72. doi: 10.1016/j.regpep.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol. 2000;84:2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232–241. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Introna M. Endothelial activation by cytokines. Ann N Y Acad Sci. 1997;832:93–116. doi: 10.1111/j.1749-6632.1997.tb46240.x. [DOI] [PubMed] [Google Scholar]
- Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–1058. [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483–495. doi: 10.1016/j.cpr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Martin PR, Reece J, Callan M, MacLeod C, Kaur A, Gregg K, Goadsby PJ. Behavioral management of the triggers of recurrent headache: a randomized controlled trial. Behav Res Ther. 2014;61:1–11. doi: 10.1016/j.brat.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Martin VT. Menstrual migraine: a review of prophylactic therapies. Curr Pain Headache Rep. 2004;8:229–237. doi: 10.1007/s11916-004-0057-1. [DOI] [PubMed] [Google Scholar]
- Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis--part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- May A, Gijsman HJ, Wallnofer A, Jones R, Diener HC, Ferrari MD. Endothelin antagonist bosentan blocks neurogenic inflammation, but is not effective in aborting migraine attacks. Pain. 1996;67:375–378. doi: 10.1016/0304-3959(96)03137-5. [DOI] [PubMed] [Google Scholar]
- Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- Meng ID, Cao L. From migraine to chronic daily headache: the biological basis of headache transformation. Headache. 2007;47:1251–1258. doi: 10.1111/j.1526-4610.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P. Note on a possible correspondence between scotomas of migraine and spreading depression of Leao. Electroencephalogr Clin Neurophysiol. 1959;10:705. doi: 10.1016/0013-4694(58)90073-7. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Rapoport AM. New players in the preventive treatment of migraine. BMC Med. 2015;13:279. doi: 10.1186/s12916-015-0522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One. 2014;9:e95508. doi: 10.1371/journal.pone.0095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui SM, Philippe L, Le Prado N, Garret C. Inhibition of neurogenic inflammation in the meninges by a non-peptide NK1 receptor antagonist, RP 67580. Eur J Pharmacol. 1993;238:421–424. doi: 10.1016/0014-2999(93)90879-m. [DOI] [PubMed] [Google Scholar]
- Moyes AJ, Khambata RS, Villar I, Bubb KJ, Baliga RS, Lumsden NG, Xiao F, Gane PJ, Rebstock AS, Worthington RJ, Simone MI, Mota F, Rivilla F, Vallejo S, Peiro C, Sanchez Ferrer CF, Djordjevic S, Caulfield MJ, MacAllister RJ, Selwood DL, Ahluwalia A, Hobbs AJ. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. doi: 10.1172/JCI74281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 2008;154:663–674. doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy CT, Connor HE. Investigation of the role of tachykinin NK1, NK2 receptors and CGRP receptors in neurogenic plasma protein extravasation in dura mater. Eur J Pharmacol. 1994;263:193–198. doi: 10.1016/0014-2999(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Ochs S. The nature of spreading depression in neural networks. International Reviews in Neurobiology. 1962;4:1–69. [Google Scholar]
- Olesen J. Headache diagnosis and vascular pathophysiology. Rinsho Shinkeigaku. 1990;30:1317–1322. [PubMed] [Google Scholar]
- Olesen J, Jansen-Olesen I. Nitric oxide mechanisms in migraine. Pathol Biol (Paris) 2000;48:648–657. [PubMed] [Google Scholar]
- Ostfeld AM, Wolff HG. Arterenol (norepinephrine) and vascular headache of the migraine type; studies on headache. AMA Arch Neurol Psychiatry. 1955;74:131–136. doi: 10.1001/archneurpsyc.1955.02330140015003. [DOI] [PubMed] [Google Scholar]
- Otori T, Greenberg JH, Welsh FA. Cortical spreading depression causes a long-lasting decrease in cerebral blood flow and induces tolerance to permanent focal ischemia in rat brain. J Cereb Blood Flow Metab. 2003;23:43–50. doi: 10.1097/01.WCB.0000035180.38851.38. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Penfield Wa, M F. Dural Headache and innervation of the dura mater. Arch NeuroPsych. 1940;44:43–75. [Google Scholar]
- Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, Bussone G, Toso V. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Migraine: a chronic sympathetic nervous system disorder. Headache. 2004;44:53–64. doi: 10.1111/j.1526-4610.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci. 2014;15:379–393. doi: 10.1038/nrn3770. [DOI] [PubMed] [Google Scholar]
- Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- Piovesan EJ, Young BW, Werneck LC, Kowacs PA, Oshinsky ML, Silberstein SD. Recurrent extratrigeminal stabbing and burning sensation with allodynia in a migraine patient. Cephalalgia. 2003;23:231–234. doi: 10.1046/j.1468-2982.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Ray Ba, W HG. Experimental studies on headache. Pain-sensitive structures of the head and their significance. Arch Surg. 1940;41:813. [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, Theoharides TC, Waeber C, Moskowitz MA. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. doi: 10.1124/pr.59.2.3. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albrecht PJ, Wymer JP, Black JA, Merkies IS, Faber CG, Waxman SG. Sodium channel Nav1.7 in vascular myocytes, endothelium, and innervating axons in human skin. Mol Pain. 2015;11:26. doi: 10.1186/s12990-015-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001;57:132–134. doi: 10.1212/wnl.57.1.132. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Tognoloni M, Russo S, Vulcano MR, Feleppa M, Mala M, Sartori M, Gallai V. Variations in the platelet arginine/nitric oxide pathway during the ovarian cycle in females affected by menstrual migraine. Cephalalgia. 1996;16:468–475. doi: 10.1046/j.1468-2982.1996.1607468.x. [DOI] [PubMed] [Google Scholar]
- Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49:1378–1386. doi: 10.1111/j.1526-4610.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain. 2008;131:2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Buddenkotte J, Schmidt-Choudhury A, Rosignoli C, Shpacovitch V, von Arnim U, Metze D, Rukwied R, Schmelz M, Paus R, Voegel JJ, Schmidt WE, Steinhoff M. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010;177:2563–2575. doi: 10.2353/ajpath.2010.090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel JL, Escartin C, Ayata C, Bonvento G, Shuttleworth CW. Multifaceted roles for astrocytes in spreading depolarization: A target for limiting spreading depolarization in acute brain injury? Glia. 2016;64:5–20. doi: 10.1002/glia.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, Kuner R. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell. 2015;27:780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- Shechter A, Stewart WF, Silberstein SD, Lipton RB. Migraine and autonomic nervous system function: a population-based, case-control study. Neurology. 2002;58:422–427. doi: 10.1212/wnl.58.3.422. [DOI] [PubMed] [Google Scholar]
- Shevel E. The extracranial vascular theory of migraine--a great story confirmed by the facts. Headache. 2011;51:409–417. doi: 10.1111/j.1526-4610.2011.01844.x. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Dollinger B, Brown G, Rapoport S, Sokoloff L. Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science. 1979;203:188–190. doi: 10.1126/science.758688. [DOI] [PubMed] [Google Scholar]
- Sicuteri F. Headache as possible expression of deficiency of brain 5-hydroxytryptamine (central denervation supersensitivity) Headache. 1972;12:69–72. doi: 10.1111/j.1526-4610.1972.hed1202069.x. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Raloxifene acutely stimulates nitric oxide release from human endothelial cells via an activation of endothelial nitric oxide synthase. J Clin Endocrinol Metab. 2000;85:2966–2969. doi: 10.1210/jcem.85.8.6853. [DOI] [PubMed] [Google Scholar]
- Smillie SJ, Brain SD. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides. 2011;45:93–104. doi: 10.1016/j.npep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Smith JM, Bradley DP, James MF, Huang CL. Physiological studies of cortical spreading depression. Biol Rev Camb Philos Soc. 2006;81:457–481. doi: 10.1017/S1464793106007081. [DOI] [PubMed] [Google Scholar]
- Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427–436. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Spierings EL, Sorbi M, Maassen GH, Honkoop PC. Psychophysical precedents of migraine in relation to the time of onset of the headache: the migraine time line. Headache. 1997;37:217–220. doi: 10.1046/j.1526-4610.1997.3704217.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, McGregor GP, Radleff-Schlimme A, Steinhoff A, Jarry H, Schmidt WE. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept. 1999;80:49–55. doi: 10.1016/s0167-0115(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Stern D, Nawroth P, Handley D, Kisiel W. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985;82:2523–2527. doi: 10.1073/pnas.82.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl LL, Zang JB, Ding W, Manni M, Zhou XK, Granstein RD. Norepinephrine and adenosine-5′-triphosphate synergize in inducing IL-6 production by human dermal microvascular endothelial cells. Cytokine. 2013;64:605–612. doi: 10.1016/j.cyto.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovner LJ, Tronvik E, Hagen K. New drugs for migraine. J Headache Pain. 2009;10:395–406. doi: 10.1007/s10194-009-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14:3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Sung CP, Arleth AJ, Feuerstein GZ. Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ Res. 1991;68:314–318. doi: 10.1161/01.res.68.1.314. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J. Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs. 2012;26:375–382. doi: 10.2165/11630590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol. 1994;1:73–80. doi: 10.1111/j.1468-1331.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA. Migraine and biomarkers of endothelial activation in young women. Stroke. 2009;40:2977–2982. doi: 10.1161/STROKEAHA.109.547901. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Khubchandani J. Vascular biomarkers in migraine. Cephalalgia. 2015;35:95–117. doi: 10.1177/0333102414544976. [DOI] [PubMed] [Google Scholar]
- Tikka-Kleemola P, Kaunisto MA, Hamalainen E, Todt U, Gobel H, Kaprio J, Kubisch C, Farkkila M, Palotie A, Wessman M, Kallela M. Genetic association study of endothelin-1 and its receptors EDNRA and EDNRB in migraine with aura. Cephalalgia. 2009;29:1224–1231. doi: 10.1111/j.1468-2982.2009.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, Bonsi P, Picconi B, Cupini LM, Materazzi S, Geppetti P, Sarchielli P, Calabresi P. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A. 2012;109:18985–18990. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser MG, Alperovitch A. Association between migraine and endothelin type A receptor (ETA -231 A/G) gene polymorphism. Neurology. 2001;56:1273–1277. doi: 10.1212/wnl.56.10.1273. [DOI] [PubMed] [Google Scholar]
- Uddman R, Tajti J, Moller S, Sundler F, Edvinsson L. Neuronal messengers and peptide receptors in the human sphenopalatine and otic ganglia. Brain Res. 1999;826:193–199. doi: 10.1016/s0006-8993(99)01260-3. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J Neurochem. 1959;3:300–315. doi: 10.1111/j.1471-4159.1959.tb12636.x. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–171. [PubMed] [Google Scholar]
- Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94:727–733. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- von Bornstadt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, Sandow N, Kura S, Eikermann-Haerter K, Endres M, Boas DA, Moskowitz MA, Lo EH, Dreier JP, Woitzik J, Sakadzic S, Ayata C. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85:1117–1131. doi: 10.1016/j.neuron.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Edelmayer RM, Yan J, Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31:1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Multiple human receptors for pituitary adenylyl cyclase-activating polypeptide and vasoactive intestinal peptide are expressed in a tissue-specific manner. Ann N Y Acad Sci. 1996;805:624–627. doi: 10.1111/j.1749-6632.1996.tb17531.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Dussor G. Ion channels and migraine. Headache. 2014;54:619–639. doi: 10.1111/head.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- Yang J, Shi QD, Song TB, Feng GF, Zang WJ, Zong CH, Chang L. Vasoactive intestinal peptide increases VEGF expression to promote proliferation of brain vascular endothelial cells via the cAMP/PKA pathway after ischemic insult in vitro. Peptides. 2013;42:105–111. doi: 10.1016/j.peptides.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Yang J, Zong CH, Zhao ZH, Hu XD, Shi QD, Xiao XL, Liu Y. Vasoactive intestinal peptide in rats with focal cerebral ischemia enhances angiogenesis. Neuroscience. 2009;161:413–421. doi: 10.1016/j.neuroscience.2009.03.052. [DOI] [PubMed] [Google Scholar]
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1beta and IL-6 on intracranial meningeal nociceptors. Cephalalgia. 2012;32:66–72. doi: 10.1177/0333102411430848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann Neurol. 2013;73:741–750. doi: 10.1002/ana.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011a;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011b;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S. PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci. 2002;3:423–439. doi: 10.2174/1389203023380576. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


