Abstract
Background: Flavonoids may have beneficial cerebrovascular effects, but evidence from racially and geographically representative cohorts in comprehensive flavonoid databases is lacking. Given racial and geographic disparities in stroke incidence, representative cohort studies are needed.
Objectives: We evaluated the association between flavonoid intake and incident ischemic stroke in a biracial, national cohort using updated flavonoid composition tables and assessed differences in flavonoid intake by sex, race, and region of residence.
Methods: We evaluated 20,024 participants in the REGARDS (REasons for Geographic and Racial Differences in Stroke) study, a biracial prospective study. Participants with stroke history or missing dietary data were excluded. Flavonoid intake was estimated by using a Block98 food frequency questionnaire and the USDA’s Provisional Flavonoid Addendum and Proanthocyanidin Database. Associations between quintiles of flavonoid intake and incident ischemic stroke were evaluated by using Cox proportional hazards models, adjusting for confounders.
Results: Over 6.5 y, 524 acute ischemic strokes occurred. Flavanone intake was lower in the Southeastern United States but higher in blacks than in whites. After multivariable adjustment, flavanone intake was inversely associated with incident ischemic stroke (HR: 0.72; 95% CI: 0.55, 0.95; P-trend = 0.03). Consumption of citrus fruits and juices was inversely associated with incident ischemic stroke (HR: 0.69; 95% CI: 0.53, 0.91; P-trend = 0.02). Total flavonoids and other flavonoid subclasses were not associated with incident ischemic stroke. There was no statistical interaction with sex, race, or region for any flavonoid measure.
Conclusions: Greater consumption of flavanones, but not total or other flavonoid subclasses, was inversely associated with incident ischemic stroke. Associations did not differ by sex, race, or region for the association; however, regional differences in flavanone intake may contribute to regional disparities in ischemic stroke incidence. Higher flavanone intake in blacks suggests that flavanone intake is not implicated in racial disparities in ischemic stroke incidence.
Keywords: ischemic stroke, flavonoid, plant-based diet, polyphenol, epidemiology
Introduction
Flavonoids are bioactive, polyphenolic compounds widely distributed in plants. Flavonoids are believed to be implicated in the beneficial properties of plant-based diets toward cardiovascular health, including stroke (1). Proposed cardioprotective mechanisms for flavonoids include antioxidant and anti-inflammatory action, modulation of lipid metabolism and platelet function, and attenuation of hypertension (2). Consistent with proposed biological mechanisms, results from epidemiologic studies suggest a protective effect for flavonoids against cardiovascular disease mortality (1, 3, 4), incident coronary heart disease (5, 6), and incident stroke (7), as well as other indicators of cardiovascular risk, such as arterial stiffness, incident hypertension, and type 2 diabetes (8–11). Although dietary flavonoid research has progressed over the past decade, existing literature is limited by incomplete dietary flavonoid information and lack of geographic and racial/ethnic diversity of studied populations, especially in the context of incident stroke.
A lack of comprehensive dietary flavonoid composition tables has especially limited previous research. The use of diet composition tables with missing or incomplete information of flavonoid concentration in some foods or mixed dishes may have led to underestimation of flavonoid intake. Conversely, imputation of values in cooked dishes from raw foods, without adjusting for processing losses, may have led to overestimation. To address these limitations, the USDA released the Provisional Flavonoid Addendum. The Provisional Flavonoid Addendum provides flavonoid values for an expanded number (>7000) of foods and beverages and uses more precise estimation methods for flavonoid content in mixed and cooked dishes (12).
A second limitation of existing literature is a general lack of racial and regional diversity in the previous studies. Black Americans and residents of the Southeastern United States, a region also known as the Stroke Belt, bear a higher burden of stroke risk than whites and those living elsewhere in the United States (13, 14). Because traditional risk factors account for only 50% of the racial disparity in stroke, a variety of nontraditional risk factors, including dietary habits, may contribute to these disparities (15, 16). A Southern-style diet, characterized by high intakes of meats and fried foods, is associated with greater stroke risk, and those with high adherence to a Southern-style diet are more often black or residents of the Southeastern United States (17). In contrast, high adherence to a plant-based diet is associated with lower stroke risk (17–20). Differences in dietary flavonoid intake may help explain racial and regional differences in stroke, but to our knowledge, only a few previous studies compared dietary flavonoid consumption by race (21, 22) and none by region in the United States.
The REGARDS11 (REasons for Geographic and Racial Differences in Stroke) study is a national, biracial prospective cohort study designed to investigate racial and regional disparities in stroke. Using the USDA’s expanded flavonoid database, the Provisional Flavonoid Addendum, and data from REGARDS, we evaluated the association between total flavonoid and flavonoid subclass intakes and incident acute ischemic stroke. Given the REGARDS study’s unique design, we also assessed differences in flavonoid consumption by race and region.
Methods
Participants.
The REGARDS study is a prospective cohort study designed to study racial and geographic differences in stroke. Between 2003 and 2007, 30,239 community-dwelling adults, ≥45 y old and living in the continental United States, were recruited into the study. The study design has been described in detail previously (23). Participants were oversampled from the Southeastern region of the United States, referred to as the Stroke Belt, including Alabama, Arkansas, Georgia, Louisiana, Mississippi, Tennessee, North Carolina, and South Carolina. Within the Stroke Belt, the coastal plains regions of Georgia, North Carolina, and South Carolina, often referred to as the Stroke Buckle, experience an even higher rate of stroke mortality than the rest of the Stroke Belt. Self-reported race and sex were balanced by design, resulting in a cohort with 56% Stroke Belt residents and 44% residents of the remaining contiguous lower 48 states, 42% black, and 55% female participants (24). Information about age, self-reported race (non-Hispanic black or white), region of residence (Stroke Belt, Stroke Buckle, or non–Stroke Belt), education, income, smoking status, physical activity, baseline health status, and medical history, including stroke risk factors such as hypertension, diabetes, and obesity status, was collected by computer-assisted telephone interview. Trained health care professionals conducted in-home visits to perform standardized measures of risk factors and collect blood and urine samples. The Institutional Review Boards of all participating universities approved this study. Written informed consent was obtained from all participants.
Dietary assessment.
A self-administered Block98 FFQ was left with study participants at the in-home visit. The Block98 FFQ is a 107-item questionnaire, developed by NutritionQuest and has been validated in populations similar to REGARDS (25, 26). Although not specifically designed to assess flavonoid intake, this FFQ inquires about intakes of flavonoid-rich foods, including fruits, vegetables, tea, and wine, and has been used to demonstrate a diet-disease relation for flavonoid intake and breast cancer outcomes (27).
Flavonoid intake.
Flavonoid intakes of interest were total flavonoid intake, anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, isoflavones, and proanthocyanidins. Flavonoid content was obtained from the USDA Database for the Proanthocyanidin Content of Selected Foods and the USDA’s Provisional Flavonoid Addendum to the USDA Food and Nutrient Database for Dietary Studies (FNDDS), 4.1 (12, 28). Supplemental Table 1 summarizes the flavonoid compounds included in each database. The newly released Provisional Flavonoid Addendum contains data for 29 flavonoids in 6 flavonoid subclasses for 7174 foods and beverages in the FNDDS, 4.1, and accounts for processing and cooking effects better than previous USDA flavonoid databases (12). In contrast, the USDA Database for the Proanthocyanidin Content of Selected Foods, released in 2004, had not been updated at the time of analysis (28). The Provisional Flavonoid Addendum and Proanthocyanidin Database are largely complementary, although they contain overlapping information about flavan-3-ols, identified as monomers in the Proanthocyanidin Database. Given methodological differences between databases, when a food was included in both databases, and if a flavan-3-ol value (Addendum) and monomer value (Proanthocyanidin Database) were both available, only the flavan-3-ol value from the Provisional Flavonoid Addendum was used. Thearubigin intake, which is exclusively from black tea, was excluded from flavan-3-ol and total flavonoid estimates in the main analysis, although it was included in sensitivity analyses because there is no consensus on analytic estimation methods for thearubigins (29). The association between daily tea consumption (defined as mean intake of ≥1 cup of tea or 240 mL daily), which can be considered a marker of thearubigin consumption, was examined separately.
Food items on the Block98 FFQ were linked to the Provisional Flavonoid Addendum and Proanthocyanidin Database by using 8-digit FNDDS and 5-digit standard reference food codes, respectively. The sum of flavonoid compounds was calculated for each flavonoid subclass. For combined items on the FFQ, such as “apples or pears,” a weighted average of flavonoids was calculated by using population-based weighted intakes consistent with the Block98 FFQ. Estimated daily flavonoid intake per participant equaled the reported amount (grams) of food consumed multiplied by the flavonoid content of the corresponding food (expressed as mg flavonoid/100 g of food) and summed across foods.
Stroke ascertainment.
Telephone surveillance every 6 mo was used to assess vital status and gather information about reasons for hospitalization, including stroke, transient ischemic attack, and stroke symptoms. Medical records were retrieved for suspected strokes, followed by central physician adjudication of stroke events. Stroke is defined as 1) focal neurologic deficit lasting >24 h or 2) nonfocal neurological symptoms with brain imaging consistent with stroke (24). The National Death Index was queried annually to identify stroke deaths that might not have been hospitalized. Incident strokes occurring up to 30 September 2013 were included.
Statistical analyses.
All analyses were conducted in SAS version 9.3 (SAS Institute). We categorized flavonoid intakes into quintiles to avoid assumptions about the dose-response relation and because quintiles offered good separation of highest and lowest intake with ample power. We summarized baseline characteristics by quintiles of flavonoid intake. Given methodological differences in the creation of the Provisional Flavonoid Addendum (12) and the Proanthocyanidin Database, 2 total flavonoid intake variables were tested by using 2 different definitions. One included only flavonoid values found in the Provisional Flavonoid Addendum (total flavonoid I), and another added proanthocyanidin intake (total flavonoid II). Correlations between nutrients were explored by using Pearson and Spearman correlations.
Cox proportional hazards models were used to examine the association between quintiles of flavonoid intake and risk of incident acute ischemic stroke (AIS). Participants were censored at date of stroke, loss to follow-up, or last stroke adjudication, whichever occurred first. After verification of the proportional hazards assumption, models were built sequentially. Model I was an unadjusted model. Model II included continuous age, continuous energy intake, demographic variables (sex, self-reported race, and region of residence), annual income (categorized as <$20,000, $20,000–34,999, $35,000–74,999, ≥$75,000, or refused), education (categorized as less than high school, high school, some college, or college graduate or higher), physical activity (none, 1–3 times/wk, or ≥4 times/wk), smoking status (never, former, or current smoker), history of coronary artery disease at baseline, and an age-race interaction term. The age-race interaction term was included in the model to account for differences in stroke incidence between blacks and whites at younger ages (24). Model III included a modified Mediterranean diet adherence score, excluding fruit, vegetable, and alcohol intake components to avoid overadjustment (30). Statistical interaction by demographic variables of self-reported race (non-Hispanic black or white), sex (men or women), and region (Stroke Belt and Stroke Buckle combined compared with non–Stroke Belt as well as Stroke Belt, Stroke Buckle, or non–Stroke Belt, separately) was tested by using likelihood ratio tests. Trend tests were conducted by assigning each quintile its median value and modeling the exposure as a continuous variable. Analyses involving total flavonoids and flavan-3-ols were repeated including thearubigins to facilitate comparison with other flavonoid studies that include them. We also considered alternate categorizations of flavonoid intake, including quartiles and deciles, to verify findings using quintiles. Sensitivity analysis with the use of multiply imputed missing covariates was conducted to assess possible bias due to 1) reporting of income as “refused” (n = 2291) and 2) exclusion of participants with missing information on 3 covariates: smoking (n = 78), education (n = 291), physical activity (n = 9), or some combination (n = 11). Using the Monte Carlo Markov chain method for missing at-random assumptions and the available covariate data, we created 5 imputed datasets with PROC MI in SAS. Parameter estimates from each imputed dataset were pooled by using PROC MIANALYZE. P < 0.05 was considered statistically significant in all analyses.
Results
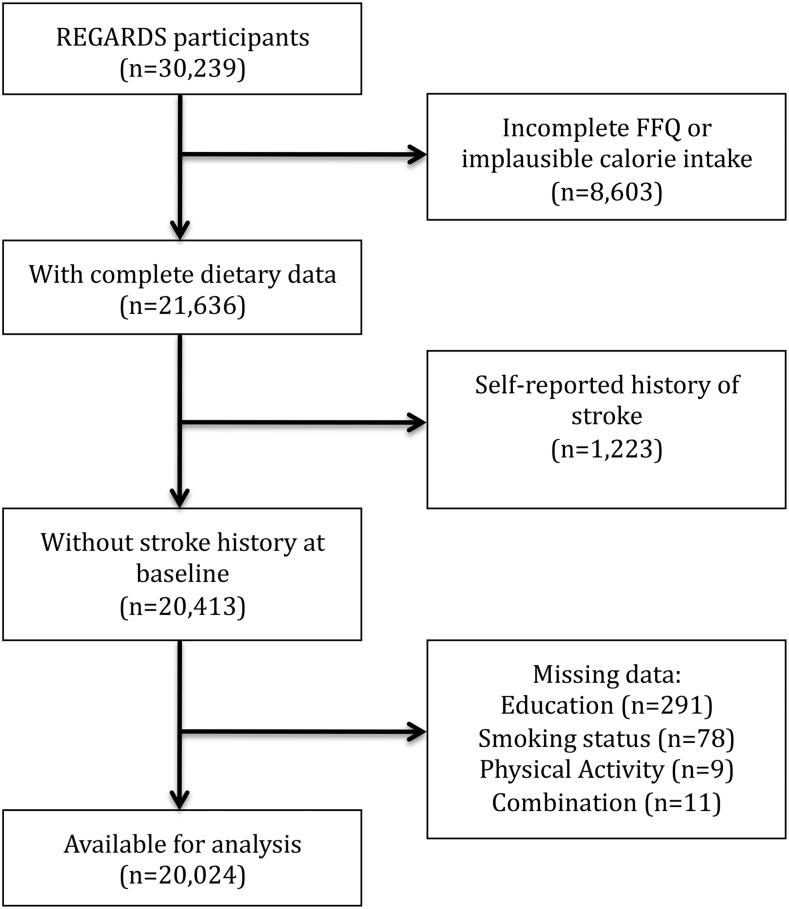
Complete dietary information with plausible energy intake (500–5000 kcal/d) was available for 21,636 participants. After excluding participants with a history of stroke (n = 1223) or missing information on key covariates (n = 389), 20,024 participants (56% women), remained for this analysis. The study flow diagram is shown in Figure 1. During a mean follow-up of 6.5 y, 524 incident AIS events were observed, 250 in women and 274 in men. There were no differences by important characteristics except that FFQ returners were more often black (61.3% compared with 38.7%), were less educated (20% compared with 10% with less than a high school education), and had lower income (24.0% compared with 15.8% earned <$20,000/y). Median intake of vegetables was higher in white participants (195 g/d; IQR: 128–291 g/d) compared with black participants (150 g/d; IQR: 96–237 g/d) (P < 0.0001), although median fruit intake was lower among white participants (205 g/d; IQR: 100–346 g/d) than black participants (278 g/d; IQR: 141–455 g/d) (P < 0.0001). Differences in median intake of vegetables comparing those living outside of the Stroke Belt (181 g/d; IQR: 118–278 g/d), in the Stroke Belt (175 g/d; IQR: 111–273 g/d), and in the Stroke Buckle were small (182 g/d; IQR: 118–281 g/d) (P < 0.0001). Median intake of fruits was higher for those living outside of the Stroke Belt (248 g/d; IQR: 125–401 g/d) than those in the Stroke Belt (208 g/d; IQR: 101–356 g/d) or in the Stroke Buckle (212 g/d; IQR: 103–360 g/d) (P < 0.0001).
FIGURE 1.
Study flow diagram for the inclusion of participants free from history of stroke at baseline enrollment in the REGARDS Study. REGARDS, REasons for Geographic and Racial Differences in Stroke.
The top food sources of flavonoids, by subclass, are shown in Supplemental Table 2. Individuals with higher total flavonoid intake were more likely to be white, be more educated, be less sedentary, have higher household income, and never have smoked Table 1. The distribution of baseline characteristics did not differ by using total flavonoid II. The distribution of sex, race, and region of residence are shown in Supplemental Table 3. Total flavonoid and flavonoid subclass intakes were higher among white participants than their black counterparts except for flavanones. Anthocyanidin, flavanone, and isoflavone intakes tended to be lower among those living in the Stroke Belt and Stroke Buckle than those living elsewhere. The Spearman rank correlation between total flavonoid intake and energy intake was low (ρ = 0.24, P < 0.001); therefore, energy-adjusted residuals were not used, but total energy intake was included in final models.
TABLE 1.
Baseline characteristics for 20,024 stroke-free participants in the REGARDS study 2003–2007 by quintile of total flavonoid intake (total I)1
| Total flavonoid I intake |
||||||
| Q1 (n = 4005) | Q2 (n = 4005) | Q3 (n = 4005) | Q4 (n = 4004) | Q5 (n = 4005) | P2 | |
| Median (range) intake, mg/d | 34.3 (≤50.8) | 66.6 (50.9–83.4) | 102.9 (83.5–127.0) | 156.9 (127.1–208.3) | 296.8 (≥208.4) | |
| Age, y | 63.6 ± 9.1 | 64.4 ± 9.2 | 65.1 ± 9.2 | 65.4 ± 9.4 | 64.7 ± 9.1 | 0.002 |
| Energy intake, kcal/d | 1400 ± 571 | 1600 ± 648 | 1750 ± 689 | 1850 ± 699 | 1960 ± 789 | <0.001 |
| Female | 2288 (57.1) | 2241 (56.0) | 2224 (55.5) | 2213 (55.3) | 2287 (57.1) | 0.34 |
| White | 2394 (59.8) | 2485 (62.1) | 2580 (64.4) | 2782 (69.5) | 3124 (78.0) | <0.001 |
| Region3 | <0.001 | |||||
| Stroke Belt | 1426 (35.6) | 1288 (32.2) | 1323 (33.0) | 1378 (34.4) | 1480 (37.0) | |
| Stroke Buckle | 765 (19.1) | 831 (20.8) | 805 (20.1) | 901 (22.5) | 1070 (26.7) | |
| Non-Belt | 1814 (45.3) | 1886 (47.1) | 1877 (46.9) | 1664 (43.1) | 1397 (36.3) | |
| Physical activity | <0.001 | |||||
| None | 1539 (38.4) | 1322 (33.0) | 1202 (30.0) | 1133 (28.3) | 1216 (30.4) | |
| 1–3 times/wk | 1441 (35.9) | 1494 (37.3) | 1540 (38.5) | 1535 (38.3) | 1467 (36.6) | |
| ≥4 times/wk | 1025 (25.6) | 1189 (29.7) | 1263 (31.5) | 1336 (33.4) | 1322 (33.0) | |
| Smoking status | <0.001 | |||||
| Never | 1608 (40.2) | 1774 (43.6) | 1908 (47.6) | 1928 (48.2) | 1948 (48.6) | |
| Former | 1603 (40.2) | 1629 (42.3) | 1651 (41.2) | 1660 (41.5) | 1575 (39.3) | |
| Current | 794 (19.8) | 569 (14.2) | 454 (11.1) | 427 (10.4) | 515 (12.0) | |
| Education | <0.001 | |||||
| <High school | 487 (12.2) | 372 (9.3) | 333 (8.3) | 304 (7.6) | 324 (8.1) | |
| High school graduate | 1176 (29.4) | 1039 (25.9) | 904 (22.6) | 935 (23.4) | 1016 (25.4) | |
| Some college | 1141 (28.5) | 1107 (27.6) | 1077 (26.9) | 1055 (26.4) | 1130 (28.2) | |
| College graduate | 1201 (30.0) | 1487 (37.1) | 1691 (42.2) | 1710 (42.7) | 1535 (38.3) | |
| Annual income | <0.001 | |||||
| Refused | 454 (11.3) | 486 (12.1) | 457 (11.4) | 439 (11.0) | 455 (11.4) | |
| <$20,000 | 752 (18.8) | 599 (15.0) | 566 (14.1) | 538 (13.4) | 570 (14.2) | |
| $20,000–$34,999 | 1016 (25.4) | 940 (23.5) | 896 (22.4) | 989 (24.7) | 944 (23.6) | |
| $35,000–$74,999 | 1193 (29.8) | 1279 (31.9) | 1352 (33.8) | 1270 (31.7) | 1278 (31.9) | |
| ≥$75,000 | 590 (14.7) | 701 (17.5) | 734 (18.3) | 768 (19.2) | 758 (18.9) | |
| Hypertension | 2214 (55.3) | 2290 (57.2) | 2218 (55.4) | 2260 (56.5) | 2170 (54.2) | 0.22 |
| Diabetes | 775 (19.9) | 721 (18.7) | 712 (18.4) | 647 (16.7) | 663 (17.2) | 0.09 |
| BMI, kg/m2 | 29.4 ± 6.3 | 29.3 6.1 | 28.9 ± 6.0 | 28.9 ± 6.0 | 28.8 ± 6.0 | 0.01 |
| CAD history4 | 404 (10.1) | 383 (9.6) | 379 (9.5) | 416 (10.4) | 431 (10.8) | 0.25 |
| mMed score5 | 2.8 ± 1.3 | 3.0 ± 1.3 | 3.1 ± 1.3 | 3.1 ± 1.3 | 3.1 ± 1.3 | <0.001 |
Values are means ± SDs or n (%) unless otherwise indicated. Total flavonoid I is the sum of anthocyanidin, flavan3-ol, flavanone, flavone, flavonol, and isoflavone intakes. CAD, coronary artery disease; mMed score, modified Mediterranean diet score; Q, quintile; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Data were analyzed by using ANOVA and chi-square tests for continuous and categorical variables, respectively.
Stroke Belt includes Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee. Stroke Buckle includes coastal plains of Georgia, North Carolina, and South Carolina. Non-Belt includes remaining area in lower 48 contiguous states. Regions are mutually exclusive.
Cardiovascular disease history: self-reported or electrocardiogram-detected at baseline.
Range: 0–6 (excludes fruit, vegetable, and alcohol intake).
Associations between flavonoid intake and incident AIS are shown in Table 2. There was an inverse association between flavanone intake and risk of AIS emerging at the second quintile in multivariable-adjusted models with a statistically significant linear trend. In unadjusted models, a significant inverse association with isoflavone intake compared extreme quintiles of intake, although this was not significant in multivariable-adjusted models. There were no consistent associations between total flavonoid, anthocyanidin, flavan-3-ol, flavone, flavonols, or proanthocyanidins and stroke. There was no >5% change in the HR after additional adjustment for the modified Mediterranean dietary score across all flavonoid intake measures. When thearubigin intake was included in calculations of total flavonoid and flavan-3-ol intake, results were materially unchanged (data not shown). There was no statistically significant association between daily tea consumption and incident ischemic stroke (HR: 1.09; 95% CI: 0.89, 1.34).
TABLE 2.
HRs and 95% CIs for incident AIS by quintile of flavonoid intake in the REGARDS study1
| Quintile | Median (range) intake, mg/d | AIS, n | Model I2 | Model II3 | Model III4 |
| Total flavonoid I5 | |||||
| 1 | 34.3 (≤50.8) | 112 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 66.6 (50.9–83.4) | 116 | 1.00 (0.77, 1.30) | 0.96 (0.74, 1.25) | 0.96 (0.74, 1.26) |
| 3 | 102.9 (83.5–127.0) | 106 | 0.91 (0.70, 1.18) | 0.93 (0.71, 1.22) | 0.93 (0.71, 1.22) |
| 4 | 156.9 (127.1–208.3) | 82 | 0.69 (0.52, 0.92) | 0.73 (0.55, 0.98) | 0.74 (0.55, 0.98) |
| 5 | 296.8 (≥208.4) | 108 | 0.93 (0.72, 1.22) | 0.94 (0.71, 1.26) | 0.95 (0.72, 1.27) |
| P-trend6 | 0.40 | 0.62 | 0.62 | ||
| Anthocyanidin | |||||
| 1 | 3.5 (≤4.8) | 126 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.9–7.8) | 98 | 0.76 (0.58, 0.98) | 0.72 (0.55, 0.95) | 0.73 (0.58, 0.95) |
| 3 | 9.6 (7.9–11.8) | 93 | 0.73 (0.56, 0.95) | 0.72 (0.54, 0.94) | 0.72 (0.55, 0.95) |
| 4 | 14.6 (11.9–18.4) | 91 | 0.70 (0.54, 0.92) | 0.70 (0.53, 0.93) | 0.71 (0.53, 0.94) |
| 5 | 25.3 (≥18.5) | 116 | 0.90 (0.70–1.16) | 0.93 (0.70, 1.23) | 0.94 (0.71, 1.25) |
| P-trend6 | 0.92 | 0.67 | 0.67 | ||
| Flavan-3-ol | |||||
| 1 | 7.9 (≤12.4) | 119 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 17.8 (12.5–24.0) | 92 | 0.75 (0.57, 0.98) | 0.79 (0.60, 1.10) | 0.79 (0.60, 1.04) |
| 3 | 35.4 (24.1–52.6) | 120 | 0.97 (0.76, 1.26) | 1.05 (0.81, 1.36) | 1.06 (0.81, 1.37) |
| 4 | 87.1 (52.7–119.6) | 86 | 0.69 (0.52, 0.91) | 0.71 (0.54, 0.95) | 0.72 (0.54, 0.95) |
| 5 | 221.0 (≥119.7) | 107 | 0.88 (0.68, 1.14) | 0.97 (0.73, 1.27) | 0.97 (0.74, 1.28) |
| P-trend6 | 0.77 | 0.93 | 0.93 | ||
| Flavanone | |||||
| 1 | 2.1 (≤3.9) | 117 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.0–9.9) | 100 | 0.84 (0.64, 1.10) | 0.88 (0.68, 1.16) | 0.89 (0.68, 1.16) |
| 3 | 16.4 (10.0–23.7) | 95 | 0.80 (0.61, 1.04) | 0.82 (0.62, 1.08) | 0.82 (0.62, 1.08) |
| 4 | 34.8 (23.8–47.9) | 104 | 0.86 (0.66, 1.12) | 0.80 (0.61, 1.05) | 0.80 (0.61, 1.05) |
| 5 | 58.1 (≥48.0) | 108 | 0.90 (0.69, 1.17) | 0.73 (0.55, 0.95) | 0.72 (0.55, 0.95) |
| P-trend6 | 0.82 | 0.03 | 0.03 | ||
| Flavone | |||||
| 1 | 0.2 (≤0.3) | 122 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 0.5 (0.4–0.6) | 103 | 0.82 (0.63, 1.06) | 0.88 (0.68, 1.15) | 0.90 (0.69, 1.17) |
| 3 | 0.8 (0.7–0.9) | 94 | 0.74 (0.57, 0.97) | 0.87 (0.66, 1.14) | 0.88 (0.67, 1.17) |
| 4 | 1.1 (1.0–1.5) | 115 | 0.90 (0.69, 1.16) | 1.08 (0.82, 1.42) | 1.11 (0.84, 1.46) |
| 5 | 2.1 (≥1.6) | 90 | 0.70 (0.53, 0.92) | 0.94 (0.70, 1.26) | 0.97 (0.72, 1.31) |
| P-trend6 | 0.49 | 0.88 | 0.88 | ||
| Flavonol | |||||
| 1 | 6.7 (≤8.9) | 108 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 11.3 (9.0–13.5) | 129 | 1.17 (0.90, 1.51) | 1.19 (0.92, 1.55) | 1.20 (0.92, 1.56) |
| 3 | 15.9 (13.6–18.9) | 92 | 0.80 (0.61, 1.06) | 0.89 (0.67, 1.19) | 0.90 (0.68, 1.21) |
| 4 | 22.6 (19.0–27.7) | 91 | 0.80 (0.60, 1.05) | 0.91 (0.68, 1.22) | 0.93 (0.69, 1.25) |
| 5 | 35.8 (≥27.8) | 104 | 0.92 (0.70, 1.21) | 1.16 (0.86, 1.57) | 1.19 (0.88, 1.61) |
| P-trend6 | 0.72 | 0.79 | 0.79 | ||
| Isoflavone | |||||
| 1 | 0.10 (≤0.18) | 119 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 0.25 (0.19–0.33) | 113 | 0.93 (0.72, 1.20) | 1.05 (0.81, 1.37) | 1.07 (0.82, 1.39) |
| 3 | 0.43 (0.34–0.56) | 101 | 0.82 (0.63, 1.07) | 0.97 (0.74, 1.28) | 1.00 (0.76, 1.32) |
| 4 | 0.73 (0.57–1.02) | 97 | 0.79 (0.60, 1.03) | 1.01 (0.76, 1.34) | 1.01 (0.79, 1.42) |
| 5 | 1.68 (≥1.03) | 94 | 0.76 (0.58, 0.99) | 1.06 (0.79, 1.44) | 1.06 (0.84, 1.59) |
| P-trend6 | 0.37 | 0.11 | 0.11 | ||
| Proanthocyanidin | |||||
| 1 | 33.8 (≤46.8) | 112 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 58.7 (46.9–70.9) | 101 | 0.87 (0.67, 1.14) | 0.91 (0.70, 1.20) | 0.92 (0.70, 1.21) |
| 3 | 84.0 (71.0–98.7) | 102 | 0.88 (0.67, 1.15) | 0.92 (0.70, 1.22) | 0.94 (0.71, 1.25) |
| 4 | 116.9 (98.8–141.3) | 111 | 0.95 (0.73, 1.24) | 1.04 (0.78, 1.37) | 1.07 (0.80, 1.42) |
| 5 | 181.5 (≥141.4) | 98 | 0.83 (0.64, 1.09) | 0.89 (0.65, 1.23) | 0.93 (0.67, 1.28) |
| P-trend6 | 0.23 | 0.80 | 0.80 | ||
| Total flavonoid II7 | |||||
| 1 | 79.8 (≤109.6) | 120 | 1.00 (—) | 1.00 (—) | 1.00 (—) |
| 2 | 139.3 (109.7–168.6) | 94 | 0.92 (0.75, 1.29) | 0.74 (0.56, 0.97) | 0.74 (0.57, 0.98) |
| 3 | 200.2 (168.7–237.6) | 108 | 0.95 (0.73, 1.24) | 0.83 (0.63, 1.09) | 0.84 (0.64, 1.10) |
| 4 | 284.2 (237.7–346.9) | 113 | 0.77 (0.57, 1.01) | 0.87 (0.66, 1.15) | 0.89 (0.67, 1.17) |
| 5 | 452.5 (≥347.0) | 89 | 0.92 (0.71, 1.21) | 0.73 (0.65, 1.01) | 0.74 (0.55, 1.01) |
| P-trend6 | 0.08 | 0.19 | 0.19 |
HRs (95% CIs) were estimated by using Cox proportional hazards models. AIS, acute ischemic stroke; REGARDS, REasons for Geographic and Racial Disparities in Stroke.
Unadjusted model.
Adjusted for age, energy, sex, race, region of residence, education, income, exercise, smoking status, self-report of coronary artery disease at baseline, and age × race interaction term.
Adjusted for age, energy, sex, race, region of residence, education, income, exercise, smoking status, self-report of coronary artery disease at baseline, age × race interaction term, and modified Mediterranean diet score.
Sum of anthocyanidin, flavan-3-ol, flavanone, flavone, flavonol, and isoflavone intakes.
Test for trend by using median values for each quintile, modeled as a continuous variable.
Sum of total flavonoid I and proanthocyanidin intakes.
Because citrus fruits and juices are the main dietary source of flavanones, vitamin C and potassium were added to model II to account for their potential beneficial effect. In the total cohort, comparing extreme quintiles of flavanone intake, the HR was unchanged by adding vitamin C (HR: 0.76; 95% CI: 0.53, 1.11) or potassium (HR: 0.74; 95% CI: 0.55, 0.98). When considering quintiles of citrus fruit and juice intake as the exposure instead of flavanone intake, the highest quintile of citrus fruits and juices intake was inversely associated with incident AIS in the total cohort in multivariable-adjusted models with the same covariates as model III in the main analysis (quintiles HR2vs1: 0.89; 95% CI: 0.68, 1.16; HR3vs1: 0.74; 95% CI: 0.56, 0.97; HR4vs1: 0.82; 95% CI: 0.63, 1.07; HR5vs1: 0.69; 95% CI: 0.53, 0.91; P-trend = 0.02). Median intakes of citrus fruits and juices for each quintile in ascending order were 5.1, 21.0, 60.8, 142.3, and 266.9 g/d. In post hoc analysis, effect estimates for comparisons of extreme quantiles were similar to results from quintiles (quartiles HR4vs1: 0.77; 95% CI: 0.61, 0.98 and deciles HR10vs1: 0.76; 95% CI: 0.51, 1.12). There was a 5% decrease in RR of incident ischemic stroke for each additional 10-g of flavanone consumed (HR: 0.95; 95% CI: 0.92, 0.98). There was no statistically significant interaction by sex, self-reported race, or region of residence in flavonoid analyses (all P-interaction > 0.40) Stratified analyses are in Tables 3 and 4, and Supplemental Table 4. Tests for statistical interaction by region of residence did not differ when the Stroke Belt and Stroke Buckle were considered separately or combined and are presented in Table 3 as combined. Results from sensitivity analyses by using multiply imputed missing covariates were materially unchanged from the complete case analysis.
TABLE 3.
HRs (95% CIs) for incident AIS by quintile of flavonoid intake for participants in the REGARDS study, stratified by self-reported race1
| White (n = 13,365) |
Black (n = 6659) |
||||||
| Quintile | Median (range) intake, mg/d | AIS, n | Model I2 | Model III3 | AIS, n | Model I2 | Model III3 |
| Total flavonoid I4 | |||||||
| 1 | 34.3 (≤50.8) | 69 | 1.00 (—) | 1.00 (—) | 41 | 1.00 (—) | 1.00 (—) |
| 2 | 66.6 (50.9–83.4) | 66 | 0.90 (0.64, 1.26) | 0.88 (0.63, 1.24) | 45 | 1.10 (0.67, 1.56) | 0.87 (0.56, 1.30) |
| 3 | 102.9 (83.5–127.0) | 71 | 0.92 (0.66, 1.28) | 0.91 (0.65, 1.28) | 38 | 1.00 (0.64, 1.52) | 0.99 (0.63, 1.56) |
| 4 | 156.9 (127.1–208.3) | 60 | 0.72 (0.51, 1.01) | 0.68 (0.47, 0.97) | 29 | 0.88 (0.45, 1.21) | 0.92 (0.57, 1.49) |
| 5 | 296.8 (≥208.4) | 84 | 0.91 (0.66, 1.26) | 0.98 (0.70, 1.36) | 21 | 0.92 (0.62, 1.72) | 0.83 (0.39, 1.30) |
| P-trend5 | 0.63 | 0.95 | 0.50 | 0.39 | |||
| Anthocyanidin | |||||||
| 1 | 3.5 (≤4.8) | 75 | 1.00 (—) | 1.00 (—) | 51 | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.9–7.8) | 67 | 0.88 (0.63, 1.22) | 0.83 (0.59, 1.15) | 31 | 0.58 (0.37, 0.90) | 0.58 (0.37, 0.91) |
| 3 | 9.6 (7.9–11.8) | 61 | 0.78 (0.55, 1.09) | 0.76 (0.54, 1.08) | 32 | 0.66 (0.43, 1.03) | 0.67 (0.42, 1.05) |
| 4 | 14.6 (11.9–18.4) | 64 | 0.80 (0.58, 1.21) | 0.80 (0.56, 1.13) | 27 | 0.55 (0.35, 0.88) | 0.57 (0.35, 0.92) |
| 5 | 25.3 (≥18.5) | 83 | 0.97 (0.71, 1.32) | 1.00 (0.71, 1.42) | 33 | 0.83 (0.53, 1.28) | 0.84 (0.51, 1.38) |
| P-trend5 | 0.86 | 0.61 | 0.70 | 0.87 | |||
| Flavan-3-ol | |||||||
| 1 | 7.9 (≤12.4) | 71 | 1.00 (—) | 1.00 (—) | 48 | 1.00 (—) | 1.00 (—) |
| 2 | 17.8 (12.5–24.0) | 62 | 0.79 (0.56, 1.11) | 0.84 (0.59, 1.18) | 30 | 0.69 (0.44, 1.09) | 0.71 (0.45, 1.13) |
| 3 | 35.4 (24.1–52.6) | 71 | 0.86 (0.62, 1.19) | 0.91 (0.64, 1.28) | 49 | 1.20 (0.81, 1.79) | 1.33 (0.88, 2.00) |
| 4 | 87.1 (52.7–119.6) | 59 | 0.65 (0.65, 0.92) | 0.68 (0.48, 0.97) | 27 | 0.77 (0.48, 1.24) | 0.79 (0.49, 1.28) |
| 5 | 221.0 (≥119.7) | 87 | 0.86 (0.63, 1.17) | 0.98 (0.71, 1.36) | 20 | 0.90 (0.53, 1.52) | 0.90 (0.52, 1.53) |
| P-trend5 | 0.77 | 0.50 | 0.75 | 0.64 | |||
| Flavanone | |||||||
| 1 | 2.1 (≤3.9) | 88 | 1.00 (—) | 1.00 (—) | 29 | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.0–9.9) | 61 | 0.74 (0.55, 1.03) | 0.79 (0.57, 1.09) | 39 | 1.03 (0.64, 1.66) | 1.10 (0.68, 1.78) |
| 3 | 16.4 (10.0–23.7) | 67 | 0.88 (0.62, 1.21) | 0.91 (0.66, 1.25) | 28 | 0.62 (0.37, 1.05) | 0.67 (0.40, 1.12) |
| 4 | 34.8 (23.8–47.9) | 66 | 0.89 (0.54, 1.23) | 0.82 (0.59, 1.14) | 38 | 0.77 (0.47, 1.25) | 0.77 (0.47, 1.26) |
| 5 | 58.1 (≥48.0) | 68 | 0.91 (0.46, 1.25) | 0.70 (0.50, 0.97) | 40 | 0.84 (0.52, 1.36) | 0.76 (0.47, 1.24) |
| P-trend5 | 0.87 | 0.10 | 0.12 | 0.23 | |||
| Flavone | |||||||
| 1 | 0.2 (≤0.3) | 77 | 1.00 (—) | 1.00 (—) | 45 | 1.00 (—) | 1.00 (—) |
| 2 | 0.5 (0.4–0.6) | 65 | 0.71 (0.51, 0.98) | 0.80 (0.57, 1.11) | 38 | 1.02 (0.66, 1.57) | 1.07 (0.68, 1.64) |
| 3 | 0.8 (0.7–0.9) | 60 | 0.60 (0.43, 0.83) | 0.74 (0.52, 1.04) | 34 | 1.06 (0.68, 1.66) | 1.20 (0.75, 1.90) |
| 4 | 1.1 (1.0–1.5) | 76 | 0.69 (0.50, 0.95) | 0.89 (0.64, 1.25) | 39 | 1.46 (0.95, 2.24) | 1.70 (1.06, 2.67) |
| 5 | 2.1 (≥1.6) | 72 | 0.64 (0.46, 0.88) | 0.92 (0.65, 1.31) | 18 | 0.73 (0.42, 1.34) | 0.94 (0.52, 1.68) |
| P-trend5 | 0.07 | 0.86 | 0.50 | 0.98 | |||
| Flavonol | |||||||
| 1 | 6.7 (≤8.9) | 64 | 1.00 (—) | 1.00 (—) | 44 | 1.00 (—) | 1.00 (—) |
| 2 | 11.3 (9.0–13.5) | 80 | 1.10 (0.78, 1.50) | 1.11 (0.79, 1.55) | 49 | 1.30 (0.87, 1.96) | 1.33 (0.88, 2.02) |
| 3 | 15.9 (13.6–18.9) | 60 | 0.71 (0.50, 1.00) | 0.80 (0.56, 1.16) | 32 | 0.99 (0.63, 1.54) | 1.09 (0.68, 1.72) |
| 4 | 22.6 (19.0–27.7) | 65 | 0.72 (0.51, 1.01) | 0.86 (0.59, 1.23) | 26 | 0.95 (0.58, 1.56) | 1.05 (0.63, 1.75) |
| 5 | 35.8 (≥27.8) | 81 | 0.82 (0.59, 1.14) | 1.11 (0.77, 1.60) | 23 | 1.18 (0.71, 1.95) | 1.34 (0.76, 2.38) |
| P-trend5 | 0.11 | 0.72 | 0.95 | 0.35 | |||
| Isoflavone | |||||||
| 1 | 0.10 (≤0.18) | 68 | 1.00 (—) | 1.00 (—) | 51 | 1.00 (—) | 1.00 (—) |
| 2 | 0.25 (0.19–0.33) | 72 | 0.96 (0.69, 1.34) | 1.12 (0.82, 1.61) | 41 | 0.89 (0.59, 1.34) | 0.95 (0.63, 1.45) |
| 3 | 0.43 (0.34–0.56) | 68 | 0.84 (0.60, 1.18) | 1.04 (0.76, 1.53) | 33 | 0.81 (0.52, 1.25) | 0.89 (0.56, 1.41) |
| 4 | 0.73 (0.57–1.02) | 74 | 0.87 (0.63, 1.21) | 1.30 (0.91, 1.85) | 23 | 0.65 (0.40, 1.06) | 0.68 (0.40, 1.17) |
| 5 | 1.68 (≥1.03) | 68 | 0.85 (0.61, 1.19) | 1.52 (0.95, 2.24) | 26 | 0.61 (0.38, 0.98) | 0.66 (0.38, 1.17) |
| P-trend5 | 0.37 | 0.15 | 0.03 | 0.19 | |||
| Proanthocyanidin | |||||||
| 1 | 33.8 (≤46.8) | 67 | 1.00 (—) | 1.00 (—) | 45 | 1.00 (—) | 1.00 (—) |
| 2 | 58.7 (46.9–70.9) | 64 | 0.80 (0.57, 1.14) | 0.86 (0.61, 1.22) | 37 | 1.00 (0.65, 1.56) | 1.01 (0.65, 1.58) |
| 3 | 84.0 (71.0–98.7) | 76 | 0.88 (0.63, 1.13) | 0.96 (0.68, 1.35) | 26 | 0.83 (0.51, 1.35) | 0.84 (0.51, 1.38) |
| 4 | 116.9 (98.8–141.3) | 72 | 0.81 (0.58, 1.22) | 0.92 (0.65, 1.32) | 39 | 1.33 (0.87, 2.04) | 1.42 (0.89, 2.27) |
| 5 | 181.5 (≥141.4) | 71 | 0.81 (0.58, 1.13) | 0.95 (0.61, 1.40) | 27 | 0.83 (0.51, 1.34) | 0.85 (0.48, 1.51) |
| P-trend5 | 0.37 | 0.83 | 0.40 | 0.64 | |||
| Total flavonoid II6 | |||||||
| 1 | 79.8 (≤109.6) | 74 | 1.00 (—) | 1.00 (—) | 45 | 1.00 (—) | 1.00 (—) |
| 2 | 139.3 (109.7–168.6) | 56 | 0.70 (0.49, 0.98) | 0.67 (0.47, 0.95) | 38 | 0.86 (0.56, 1.33) | 0.93 (0.60, 1.44) |
| 3 | 200.2 (168.7–237.6) | 72 | 0.78 (0.57, 1.08) | 0.76 (0.54, 1.06) | 42 | 1.00 (0.64, 1.54) | 1.10 (0.71, 1.71) |
| 4 | 284.2 (237.7–346.9) | 79 | 0.85 (0.62, 1.17) | 0.85 (0.61, 1.20) | 27 | 0.96 (0.62, 1.49) | 0.80 (0.48, 1.33) |
| 5 | 452.5 (≥347.0) | 69 | 0.68 (0.48, 0.98) | 0.73 (0.51, 1.05) | 22 | 0.76 (0.45, 1.28) | 0.92 (0.53, 1.61) |
| P-trend5 | 0.13 | 0.39 | 0.42 | 0.33 | |||
HRs (95% CIs) were estimated by using Cox proportional hazards models. AIS, acute ischemic stroke; REGARDS, REasons for Geographic and Racial Disparities in Stroke.
Unadjusted model.
Adjusted for age, energy, sex, race, region of residence, education, income, exercise, smoking status, self-report of coronary artery disease at baseline, age × race interaction term, and modified Mediterranean diet score.
Sum of anthocyanidin, flavan-3-ol, flavanone, flavone, flavonol, and isoflavone intakes.
Test for trend by using median values for each quintile, modeled as a continuous variable.
Sum of total flavonoid I and proanthocyanidin intakes.
TABLE 4.
HRs (95% CIs) for incident AIS by quintile of flavonoid intake for participants in the REGARDS study, stratified by residence in the Stroke Belt1
| Stroke Belt and Stroke Buckle (n = 11,267) |
Non–Stroke Belt (n = 8757) |
||||||
| Quintile | Median (range) intake, mg/d | AIS, n | Model I2 | Model III3 | AIS, n | Model I2 | Model III3 |
| Total flavonoid I4 | |||||||
| 1 | 34.3 (≤50.8) | 60 | 1.00 (—) | 1.00 (—) | 50 | 1.00 (—) | 1.00 (—) |
| 2 | 66.6 (50.9–83.4) | 55 | 0.91 (0.63, 1.31) | 0.90 (0.62, 1.31) | 56 | 1.05 (0.72, 1.54) | 1.03 (0.54, 1.34) |
| 3 | 102.9 (83.5–127.0) | 55 | 0.91 (0.63, 1.31) | 0.89 (0.61, 1.29) | 54 | 1.00 (0.68, 1.47) | 0.96 (0.53, 1.25) |
| 4 | 156.9 (127.1–208.3) | 47 | 0.70 (0.48, 1.02) | 0.67 (0.45, 1.00) | 42 | 0.86 (0.57, 1.29) | 0.81 (0.65, 1.44) |
| 5 | 296.8 (≥208.4) | 70 | 0.96 (0.68, 1.35) | 0.99 (0.69, 1.43) | 35 | 0.85 (0.55, 1.31) | 0.85 (0.70, 1.51) |
| P-trend5 | 0.30 | 0.91 | 0.75 | 0.68 | |||
| Anthocyanidin | |||||||
| 1 | 3.5 (≤4.8) | 73 | 1.00 (—) | 1.00 (—) | 53 | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.9–7.8) | 58 | 0.81 (0.57, 1.14) | 0.77 (0.54, 1.09) | 40 | 0.69 (0.46, 1.04) | 0.67 (0.44, 1.01) |
| 3 | 9.6 (7.9–11.8) | 45 | 0.64 (0.44, 0.93) | 0.64 (0.44, 0.94) | 48 | 0.84 (0.57, 1.23) | 0.82 (0.54, 1.01) |
| 4 | 14.6 (11.9–18.4) | 45 | 0.64 (0.44, 0.93) | 0.63 (0.43, 0.93) | 46 | 0.53 (0.53, 1.16) | 0.80 (0.52, 1.21) |
| 5 | 25.3 (≥18.5) | 66 | 1.00 (0.71, 1.40) | 1.00 (0.68, 1.46) | 50 | 0.54 (0.54, 1.17) | 0.86 (0.55, 1.30) |
| P-trend5 | 0.71 | 0.63 | 0.41 | 0.69 | |||
| Flavan-3-ol | |||||||
| 1 | 7.9 (≤12.4) | 61 | 1.00 (—) | 1.00 (—) | 58 | 1.00 (—) | 1.00 (—) |
| 2 | 17.8 (12.5–24.0) | 45 | 0.73 (0.49, 1.07) | 0.75 (0.51, 1.11) | 47 | 0.79 (0.53, 1.15) | 0.84 (0.57, 1.24) |
| 3 | 35.4 (24.1–52.6) | 61 | 0.94 (0.66, 1.34) | 1.01 (0.70, 1.46) | 59 | 1.02 (0.71, 1.46) | 1.09 (0.75, 1.59) |
| 4 | 87.1 (52.7–119.6) | 48 | 0.65 (0.44, 0.94) | 0.67 (0.45, 0.98) | 38 | 0.76 (0.50, 1.14) | 0.77 (0.50, 1.18) |
| 5 | 221.0 (≥119.7) | 72 | 0.90 (0.64, 1.27) | 1.00 (0.70, 1.43) | 35 | 0.82 (0.54, 1.24) | 0.89 (0.57, 1.37) |
| P-trend5 | 0.96 | 0.43 | 0.60 | 0.62 | |||
| Flavanone | |||||||
| 1 | 2.1 (≤3.9) | 78 | 1.00 (—) | 1.00 (—) | 39 | 1.00 (—) | 1.00 (—) |
| 2 | 6.3 (4.0–9.9) | 58 | 0.77 (0.55, 1.09) | 0.81 (0.58, 1.14) | 42 | 0.96 (0.62, 1.49) | 1.03 (0.67, 1.60) |
| 3 | 16.4 (10.0–23.7) | 51 | 0.72 (0.51, 1.02) | 0.71 (0.50, 1.02) | 44 | 0.94 (0.61, 1.48) | 1.02 (0.66, 1.56) |
| 4 | 34.8 (23.8–47.9) | 43 | 0.63 (0.43, 0.91) | 0.58 (0.39, 0.84) | 61 | 1.21 (0.81, 1.81) | 1.16 (0.77, 1.74) |
| 5 | 58.1 (≥48.0) | 57 | 0.87 (0.62, 1.23) | 0.69 (0.49, 0.99) | 51 | 0.98 (0.65, 1.48) | 0.82 (0.53, 1.25) |
| P-trend5 | 0.50 | 0.08 | 0.71 | 0.31 | |||
| Flavone | |||||||
| 1 | 0.2 (≤0.3) | 66 | 1.00 (—) | 1.00 (—) | 1.00 (—) | 1.00 (—) | |
| 2 | 0.5 (0.4–0.6) | 60 | 0.93 (0.65, 1.31) | 1.03 (0.72, 1.46) | 56 | 0.70 (0.47, 1.04) | 0.75 (0.50, 1.13) |
| 3 | 0.8 (0.7–0.9) | 43 | 0.65 (0.44, 0.95) | 0.80 (0.54, 1.19) | 43 | 0.84 (0.58, 1.23) | 0.96 (0.65, 1.43) |
| 4 | 1.1 (1.0–1.5) | 63 | 0.96 (0.68, 1.35) | 1.18 (0.81, 1.71) | 51 | 0.83 (0.57, 1.21) | 1.03 (0.68, 1.55) |
| 5 | 2.1 (≥1.6) | 55 | 0.76 (0.53, 1.08) | 1.04 (0.71, 1.54) | 52 | 0.63 (0.41, 0.96) | 0.88 (0.56, 1.41) |
| P-trend5 | 0.25 | 0.77 | 35 | 0.10 | 0.90 | ||
| Flavonol | |||||||
| 1 | 6.7 (≤8.9) | 59 | 1.00 (—) | 1.00 (—) | 49 | 1.00 (—) | 1.00 (—) |
| 2 | 11.3 (9.0–13.5) | 65 | 1.10 (0.77, 1.55) | 1.15 (0.80, 1.64) | 64 | 1.26 (0.87, 1.82) | 1.24 (0.85, 1.81) |
| 3 | 15.9 (13.6–18.9) | 47 | 0.74 (0.51, 1.10) | 0.84 (0.56, 1.25) | 45 | 0.88 (0.59, 1.31) | 0.98 (0.64, 1.50) |
| 4 | 22.6 (19.0–27.7) | 51 | 0.77 (0.53, 1.11) | 0.88 (0.59, 1.31) | 40 | 0.84 (0.55, 1.27) | 0.97 (0.62, 1.52) |
| 5 | 35.8 (≥27.8) | 65 | 0.92 (0.65, 1.31) | 1.19 (0.80, 1.76) | 39 | 0.91 (0.60, 1.38) | 1.16 (0.73, 1.87) |
| P-trend5 | 0.43 | 0.71 | 0.23 | 0.91 | |||
| Isoflavone | |||||||
| 1 | 0.10 (≤0.18) | 68 | 1.00 (—) | 1.00 (—) | 51 | 1.00 (—) | 1.00 (—) |
| 2 | 0.25 (0.19–0.33) | 64 | 0.92 (0.65, 1.29) | 1.09 (0.77, 1.54) | 49 | 0.93 (0.63, 1.34) | 1.05 (0.71, 1.56) |
| 3 | 0.43 (0.34–0.56) | 53 | 0.78 (0.54, 1.10) | 0.97 (0.67, 1.40) | 48 | 0.88 (0.60, 1.31) | 1.06 (0.71, 1.60) |
| 4 | 0.73 (0.57–1.02) | 55 | 0.83 (0.58, 1.18) | 1.12 (0.76, 1.65) | 42 | 0.74 (0.49, 1.11) | 0.99 (0.64, 1.54) |
| 5 | 1.68 (≥1.03) | 47 | 0.78 (0.54, 1.13) | 1.18 (0.76, 1.82) | 47 | 0.73 (0.49, 1.08) | 1.15 (0.72, 1.83) |
| P-trend5 | 0.37 | 0.25 | 0.10 | 0.64 | |||
| Proanthocyanidin | |||||||
| 1 | 33.8 (≤46.8) | 68 | 1.00 (—) | 1.00 (—) | 44 | 1.00 (—) | 1.00 (—) |
| 2 | 58.7 (46.9–70.9) | 48 | 0.68 (0.47, 0.98) | 0.72 (0.50, 1.05) | 53 | 1.18 (0.79, 1.76) | 1.22 (0.81, 1.83) |
| 3 | 84.0 (71.0–98.7) | 60 | 0.86 (0.61, 1.21) | 0.92 (0.64, 1.32) | 52 | 0.91 (0.59, 1.38) | 0.96 (0.62, 1.50) |
| 4 | 116.9 (98.8–141.3) | 56 | 0.79 (0.56, 1.13) | 0.88 (0.59, 1.28) | 55 | 1.20 (0.81, 1.78) | 1.37 (0.89, 2.11) |
| 5 | 181.5 (≥141.4) | 55 | 0.79 (0.56, 1.13) | 0.86 (0.56, 1.32) | 43 | 0.89 (0.59, 1.36) | 1.01 (0.62, 1.65) |
| P-trend5 | 0.47 | 0.87 | 0.51 | 0.83 | |||
| Total flavonoid II6 | |||||||
| 1 | 79.8 (≤109.6) | 67 | 1.00 (—) | 1.00 (—) | 53 | 1.00 (—) | 1.00 (—) |
| 2 | 139.3 (109.7–168.6) | 50 | 0.75 (0.52, 1.08) | 0.74 (0.51, 1.07) | 44 | 0.76 (0.51, 1.14) | 0.75 (0.50, 1.12) |
| 3 | 200.2 (168.7–237.6) | 53 | 0.73 (0.51, 1.05) | 0.71 (0.49, 1.02) | 55 | 1.01 (0.69, 1.47) | 1.00 (0.67, 1.49) |
| 4 | 284.2 (237.7–346.9) | 59 | 0.81 (0.57, 0.15) | 0.80 (0.55, 1.16) | 54 | 0.99 (0.68, 1.45) | 0.98 (0.65, 1.49) |
| 5 | 452.5 (≥347.0) | 58 | 0.74 (0.52, 1.06) | 0.77 (0.52, 1.15) | 31 | 0.65 (0.42, 1.01) | 0.67 (0.41, 1.10) |
| P-trend5 | 0.27 | 0.41 | 0.16 | 0.21 | |||
HRs (95% CIs) were estimated by using Cox proportional hazards models. The Stroke Belt includes Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee. The Stroke Buckle includes coastal plains of Georgia, North Carolina, and South Carolina. The Non–Stroke Belt includes remaining area in lower 48 contiguous states. Regions are mutually exclusive. AIS, acute ischemic stroke, REGARDS, REasons for Geographic and Racial Disparities in Stroke.
Unadjusted model.
Adjusted for age, energy, sex, race, region of residence, education, income, exercise, smoking status, self-report of coronary artery disease at baseline, age × race interaction term, and modified Mediterranean diet score.
Sum of anthocyanidin, flavan-3-ol, flavanone, flavone, flavonol, and isoflavone intakes.
Test for trend using median values for each quintile, modeled as a continuous variable.
Sum of total flavonoid I and proanthocyanidin intakes.
Discussion
To our knowledge, this is the first study in a biracial US population to evaluate the association of total flavonoid and 7 flavonoid subclass intakes with incident AIS with the use of an extensive contemporary database, the USDA’s Provisional Flavonoid Addendum (12). This expanded flavonoid database provides detailed information on flavonoid content across 6 of 7 flavonoid subclasses for a large number of foods and beverages, including multi-ingredient and processed foods, which enables a more comprehensive evaluation of flavonoid intake and incident AIS than has been done before. Our study suggests that specific subclasses of flavonoids are more relevant to incident AIS than total flavonoid intake. Higher flavanone intake was associated with a 28% decreased RR of incident AIS after adjustment for multiple covariates. Adjustment for a modified Mediterranean diet quality score did not meaningfully alter results, which suggests that the association between flavanone intake and ischemic stroke is independent of overall dietary quality. These findings were robust for different categorizations of flavanone intake. There was a similar inverse association for higher intakes of citrus fruits and juices, the primary dietary source of flavanones. These findings are consistent with the Nurses’ Health Study, which found a 19% reduction in risk of incident AIS associated with high flavanone intake, by using a previous version of the USDA flavonoid databases (7). An inverse, although nonsignificant, association between flavanone intake and stroke mortality has also been described (3, 4). Furthermore, consumption of fruits, especially citrus fruits, an important source of flavanones, has been associated with fewer AIS events (19, 31–33).
Flavanones are associated with improved microvascular reactivity, lower blood pressure, improved flow-mediated dilation, and improved lipid profiles (34, 35). Naringenin and hesperitin, both flavanone compounds, are potential candidates for neuroprotection because they cross the blood-brain barrier in animal models, inhibiting inflammatory signaling and cytokine production (36, 37). These salutary vascular and anti-inflammatory effects, paired with their ability to cross the blood-brain barrier, make flavanones biologically plausible candidates for neuroprotection.
After multivariable adjustment, we found no statistically significant association between total flavonoid or flavonoid subclass intakes other than for flavanones. Greater intakes of anthocyanidins, flavan-3-ols, proanthocyanidins, and total flavonoids have been inversely associated with stroke events in men and women (5–19% risk reduction), although they failed to reach statistical significance (3, 7). However, in a meta-analysis, all of these flavonoid subclasses were found to have a 10–13% relative reduction of risk of combined fatal and nonfatal cardiovascular disease (38). Differences between the effect estimates obtained from our study and meta-analyses could be that this study focused on adjudicated incident AIS rather than combined fatal and nonfatal cardiovascular disease in the meta-analysis.
In this study, flavones and flavonols were not associated with incident AIS. This lack of an association is consistent with the preponderance of previous epidemiologic studies of incident stroke and stroke mortality (1, 3, 7). However, in two previous meta-analyses, flavonols were inversely associated with incident AIS or combined fatal and nonfatal cardiovascular events (38, 39). Conflicting findings from observational studies and meta-analyses may be to the result of differences in statistical power and different outcome definitions.
Although there was no significant effect modification by sex, race, or region of residence, black participants reported lower intakes of all flavonoid subclasses except flavonones than their white counterparts reported. These differences are consistent with previous studies of flavonoid subclass intakes in US adults (21). Furthermore, the lower intake of flavonoids except for flavanones was consistent with lower average intake of vegetables among black participants in this study. Given the inverse association between flavonone intake and incident stroke and that black participants in this study consumed more flavanones than their white counterparts, it is unlikely that differences in flavanone intake explain racial disparities in stroke. Racial disparities may be related to nonflavonoid nutrients that are deficient in diets otherwise low in fruits and vegetables. Regional disparities in stroke may be related to lower flavanone intake in residents of the Stroke Belt and Stroke Buckle. One published study addressed modification of the association between isoflavone intake and blood pressure by race, which suggests stimulation of endothelial nitric oxide synthase in the setting of relative nitric oxide deficiency as a potential mechanism to explain a stronger beneficial association between dietary isoflavone and blood pressure in black participants than in white participants (11). Other flavonoid subclasses have been implicated in nitric oxide modulation (40), but to our knowledge, no other studies in humans have been published that allow comparison of results.
Strengths of this study include the large sample size and prospective design, including racially and geographically diverse participants with excellent ascertainment of stroke outcomes. Furthermore, this study used the most comprehensive flavonoid database available to assess dietary flavonoid intake in the US population. There are limitations to this study worth mentioning. By using a comprehensive flavonoid database with a detailed FFQ, our study likely captured the most significant sources of dietary flavonoids. The Provisional Flavonoid Addendum was recently used to characterize flavonoid intake and important dietary sources of flavonoids in the US population in the What We Eat in America, NHANES 2007–2008 study (41). Important dietary sources were defined as those that contributed ≥5% of the intake of a flavonoid subclass or total flavonoids. The only important dietary source of flavonoids not specifically listed on the Block98 FFQ or included as a part of mixed dishes or aggregate FFQ items was soy protein powder, which contributed 32% of isoflavone intake. While this is a limitation for isoflavone analyses, the contribution of isoflavone intake to total flavonoid intake in the United States was very low. Four other important dietary sources of flavonoids, red and purple vegetables (8% of anthocyanidins), sweet peppers (9% of flavones), celery and squash (8% of flavones), and onions (5% of flavonols), were included in the Block98 as a part of mixed dishes or aggregate FFQ items. These four foods were all represented in the USDA recipes used to develop nutrient descriptions for the 7174 foods and beverages in the Provisional Flavonoid Addendum. Furthermore, because onions, sweet peppers, red and purple vegetables, and celery and squash are often consumed as a part of mixed dishes rather than in isolation, using standard recipes to estimate their intake may improve measurement rather than relying on respondents to correctly report the proportionate amount of a vegetable, such as onion, consumed as a part of a dish. Second, dietary assessment was conducted only at baseline; therefore, changes in dietary intake of flavonoids were not assessed. Because this analysis was conducted in a dietary subsample, selection bias is a concern. However, the return rate for the FFQ in the REGARDS study was 70%, which is higher than other self-administered questionnaire return rates. Incident AIS rates were the same comparing those who did and did not return the FFQ, and they were similar in the majority of characteristics. Nonreturners were more likely to be black, less educated, and poorer. Because these characteristics were not found to modify the association between flavonoid intake and risk incident AIS, suggesting homogeneity for the association across strata, bias is unlikely to result from the exclusion of those participants. There was also no difference between results obtained from the complete case analysis compared with analyses with imputed values for missing covariates. Although we considered many potential confounders, residual and unmeasured confounding is always a concern in observational studies. Finally, because of multiple comparisons, statistical significance should be interpreted cautiously and in the context of existing literature.
We found that a higher reported dietary intake of flavanones is associated with a 28% lower risk of incident AIS. Moreover, greater citrus intake, the predominant source of flavanones, is associated with a reduction in the risk of AIS. While there was no effect modification by race or region of residence, lower flavanone intake in the Stroke Belt and Stroke Buckle may contribute to excess stroke burden in residents of the Stroke Belt and Stroke Buckle. Lower vegetable and flavonoid consumption, except for flavanones, in black participants suggests that nonflavonoid dietary factors may be more important than flavonoids to explain the excess stroke risk in this group.
Acknowledgments
MEG, SEJ, TJH, WM, and VV designed the research; MEG, SEJ, TJH, and AA conducted the research; MEG analyzed the data and had primary responsibility for the content; and MEG and VV wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AIS, acute ischemic stroke; FNDDS, Food and Nutrient Database for Dietary Studies; REGARDS, REasons for Geographic and Racial Differences in Stroke
References
- 1.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev 2012;70:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weseler AR, Bast A. Pleiotropic-acting nutrients require integrative investigational approaches: the example of flavonoids. J Agric Food Chem 2012;60:8941–6. [DOI] [PubMed] [Google Scholar]
- 3.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 2012;95:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013;127:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr 2015;114:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy A, Rimm EB, O’Reilly EJ, Logroscino G, Kay C, Chiuve SE, Rexrode KM. Dietary flavonoids and risk of stroke in women. Stroke 2012;43:946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 2012;96:781–8. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy A, O’Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson SI, Steffen LM, Swett K, Smith C, Burke L, Zhou X, Shikany JM, Rodriguez CJ. Dietary total isoflavone intake is associated with lower systolic blood pressure: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Clin Hypertens (Greenwich) 2016;18:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebastian RS, Goldman JD, Martin CL, Steinfeldt LC, Enns CW, Moshfegh AJ. Flavonoid Values for USDA Survey Foods and Beverages 2007–2008: Provisional Flavonoid Addendum to the USDA Food and Nutrient Database for Dietary Studies, 4.1, and Flavonoid Intake Files from What We Eat in America (WWEIA), National Health and Nutrition Examination Survey (NHANES) 2007–2008. Beltsville (MD): USDA Agriculture Research Service; 2014. [Google Scholar]
- 13.Casper ML, Wing S, Anda RF, Knowles M, Pollard RA. The shifting stroke belt: changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke 1995;26:755–60. [DOI] [PubMed] [Google Scholar]
- 14.Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH. Factors explaining excess stroke prevalence in the US Stroke Belt. Stroke 2009;40:3336–41. [DOI] [PubMed] [Google Scholar]
- 15.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke 2011;42:3369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region have independent and synergistic effects on dietary intakes in black and white women. Nutr J 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judd SE, Gutierrez OM, Newby PK, Howard G, Howard VJ, Locher JL, Kissela BM, Shikany JM. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in black Americans. Stroke 2013;44:3305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsivgoulis G, Psaltopoulou T, Wadley VG, Alexandrov AV, Howard G, Unverzagt FW, Moy C, Howard VJ, Kissela B, Judd SE. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke 2015;46:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999;282:1233–9. [DOI] [PubMed] [Google Scholar]
- 20.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Vance TM, Chun OK. Estimated intake and major food sources of flavonoids among US adults: changes between 1999–2002 and 2007–2010 in NHANES. Eur J Nutr 2016;55:833–43. [DOI] [PubMed] [Google Scholar]
- 22.Bai W, Wang C, Ren C. Intakes of total and individual flavonoids by US adults. Int J Food Sci Nutr 2013;65:9–20. [DOI] [PubMed] [Google Scholar]
- 23.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 24.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 26.Caan BJ, Slattery ML, Potter J, Quesenberry CP Jr, Coates AO, Schaffer DM. Comparison of the Block and the Willett self-administered semiquantitative food frequency questionnaires with an interviewer-administered dietary history. Am J Epidemiol 1998;148:1137–47. [DOI] [PubMed] [Google Scholar]
- 27.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol 2007;165:514–23. [DOI] [PubMed] [Google Scholar]
- 28.USDA Agricultural Research Service. USDA database for the proanthocyanidin content of selected foods. Beltsville (MD): Agricultural Research Service Nutrient Data Laboratory; 2004. [Google Scholar]
- 29.Zamora-Ros R, Knaze V, Romieu I, Scalbert A, Slimani N, Clavel-Chapelon F, Touillaud M, Perquier F, Skeie G, Engeset D, et al. Impact of thearubigins on the estimation of total dietary flavonoids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Clin Nutr 2013;67:779–82. [DOI] [PubMed] [Google Scholar]
- 30.Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013;80:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnsen SP, Overvad K, Stripp C, Tjonneland A, Husted SE, Sorensen HT. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr 2003;78:57–64. [DOI] [PubMed] [Google Scholar]
- 32.Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliovaara M, Jarvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr 2009;102:1075–83. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K; Jichi Medical School Cohort Study Group. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol 2011;21:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr 2011;93:73–80. [DOI] [PubMed] [Google Scholar]
- 35.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab 2011;96:E782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 2004;36:592–604. [DOI] [PubMed] [Google Scholar]
- 37.Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JP. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys 2009;484:100–9. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 2014;111:1–11. [DOI] [PubMed] [Google Scholar]
- 39.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr 2010;140:600–4. [DOI] [PubMed] [Google Scholar]
- 40.Duarte J, Francisco V, Perez-Vizcaino F. Modulation of nitric oxide by flavonoids. Food Funct 2014;5:1653–68. [DOI] [PubMed] [Google Scholar]
- 41.Sebastian RS, Wilkinson Enns C, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, Moshfegh AJ. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US Adults. J Nutr 2015;145:1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]