Abstract
Objective:
To evaluate if cognitive reserve (CR) contributes to interindividual differences in frontal gray matter density (GMD) and executive impairment that underlie heterogeneity in the disease course of confirmed frontotemporal lobar degeneration (FTLD) pathology.
Methods:
Fifty-five patients with autopsy confirmation or a pathogenic mutation consistent with underlying tau (FTLD-tau) or TDP-43 (FTLD-TDP) pathology and 90 demographically comparable healthy controls were assessed with T1 MRI and neuropsychological measures (Mini-Mental State Examination, letter fluency, forward digit span, Rey complex figure, and Boston Naming Test). CR was indexed using a composite measure of education and occupation. We used t tests to identify reduced GMD in patients with FTLD relative to controls, regression analyses to relate reduced GMD to CR index, and correlations to relate regions of GMD associated with CR to performance on neuropsychological measures.
Results:
Patients with FTLD demonstrated impairment on neuropsychological measures. Patients with FTLD exhibited reduced bilateral frontotemporal GMD relative to controls, consistent with the known anatomic distribution of FTLD pathology. Higher CR index was associated with superior letter fluency and with GMD in right dorsolateral prefrontal cortex, orbitofrontal cortex, rostral frontal cortex, and inferior frontal gyrus. Furthermore, we found that higher GMD in frontal regions associated with CR was associated with superior letter fluency.
Conclusions:
Executive control and verbal ability assessed by letter fluency in FTLD is mediated in part by CR and frontal GMD. The identification of factors influencing cognitive and anatomic heterogeneity in FTLD suggests that CR should be considered in symptom detection, prognosis, and treatment.
Frontotemporal lobar degeneration (FTLD) is a pathologic spectrum of progressive neurodegenerative conditions affecting the frontal and temporal lobes that are associated with executive, social, and language impairments.1 The disease course of FTLD is highly variable across individuals, including age at onset,2 rate of decline,3 and survival.4 While some biologic mechanisms have been proposed to account for this heterogeneity,5,6 environmental factors contributing to disease course remain obscure.
Cognitive reserve (CR) theories suggest environmental factors including education and occupation provide a reserve against the clinical manifestation of neurodegenerative disease despite significant pathologic burden.7 Individuals with high CR may compensate for dementia-associated neurodegeneration by increasing recruitment of frontal executive resources to improve cognitive performance and delay symptom detection.8,9 In patients with FTLD, access to frontal executive resources is compromised early in disease course due to frontal tau or TDP-43 pathology. Prior research on CR in FTLD spectrum disorders is largely limited to patients with behavioral variant frontotemporal dementia (bvFTD) with unconfirmed FTLD pathology, and has largely focused on brain metabolism and other functional measures.10–12 There is a thus a need for investigation of CR in FTLD spectrum patients with confirmed FTLD pathology using structural measures of brain integrity.
Recent evidence from patients with autopsy-confirmed FTLD suggests that higher occupational attainment is associated with longer survival from symptom onset.13 From this perspective, patients with FTLD with higher occupational attainment may detect dementia symptoms earlier than lower occupational attainment patients due to increased frontally mediated work demands, therefore giving the impression that they are surviving longer. This is in contrast with traditional CR accounts based on Alzheimer disease (AD) that suggest that higher CR factors such as education and occupational attainment are associated with shorter survival.
Critically, since AD has a neuroanatomic distribution of disease distinct from FTLD, including less frontal lobe disease, CR may function differently in patients with FTLD. However, to our knowledge, the anatomic and cognitive profiles associated with CR in patients with confirmed FTLD remain unknown. Given the frontal distribution of disease in FTLD, we hypothesized that CR in patients with FTLD would be associated with less severe disease in terms of frontal cortex gray matter (GM) and frontally mediated cognitive performance.
METHODS
Participants.
We report 55 patients recruited from the Penn Frontotemporal Degeneration Center at the University of Pennsylvania and clinically diagnosed by a board-certified neurologist (M.G., D.I.) using published criteria for a clinical syndrome associated with FTLD pathology.14–17 Inclusion criteria consisted of postmortem neuropathologic diagnosis or genetic screening (supplemental data at Neurology.org), an antemortem T1-weighted MRI scan, neuropsychological assessment, and known occupational status and years of education. Patients' clinical syndrome included bvFTD (n = 34), amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (ALS-FTD, n = 7), nonfluent-agrammatic primary progressive aphasia (n = 6), corticobasal syndrome (CBS, n = 4), semantic-variant primary progressive aphasia (n = 1), progressive supranuclear palsy (PSP, n = 2), and ALS with mild cognitive impairment (n = 1). Disease duration was defined as time of symptom onset, based on caregiver report of the earliest clinical feature, until time of MRI acquisition. Disease duration can alternatively be calculated as months between first diagnosis and clinical appointment and post hoc analyses confirm similar results when using this calculation (all p < 0.05).
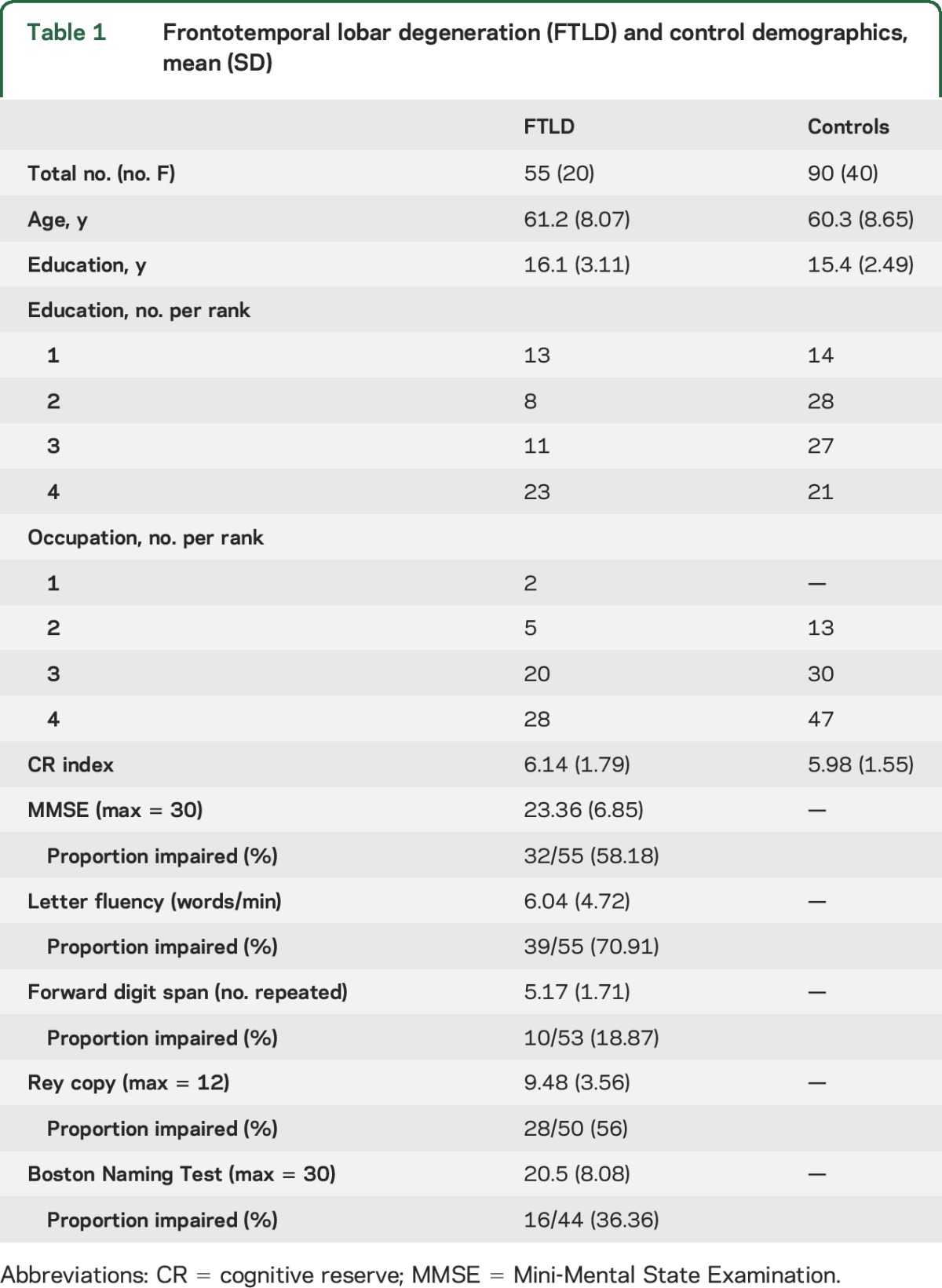
To identify regions of significant GM atrophy in FTLD, we recruited 90 demographically comparable healthy controls who self-reported a negative history for neurologic or psychiatric disease and scored Mini-Mental State Examination (MMSE) >27.18 Demographic features of patients with FTLD and controls are summarized in table 1. There was no significant difference in age, education, sex, or CR index (see below) between controls and patients (all p values > 0.05).
Table 1.
Frontotemporal lobar degeneration (FTLD) and control demographics, mean (SD)
Standard protocol approvals, registrations, and patient consents.
All patients and controls participated in an informed consent procedure approved by an institutional review board convened at the University of Pennsylvania.
Cognitive reserve index.
We assessed CR using a composite measure of education and occupation similar to previous reports.10,19 Education was recorded in years and ranked with a score ranging from 1 to 4: (1) ≤12 years (primary or secondary education; n = 13); (2) >12 and <16 (some postsecondary education; n = 8); (3) 16 years (college education; n = 11); and (4) >16 years (graduate education; n = 23). Occupation was ranked on a 1- to 4-point scale based on US census categories: (1) unskilled laborers (n = 2); (2) operative and service workers (n = 5); (3) managers, administrators, clerical, and sales (n = 20); (4) professional and technical workers (n = 28). We report a CR index as the sum of education and occupation ranks.
Neuropsychological assessment.
Neuropsychological assessments including letter fluency,20 MMSE,21 forward digit span,22 Rey figure copy,23 and Boston Naming Test24 were performed approximately 2 months within date of scan (mean 2.20, SE 0.53; range 0–16) (see appendix e-1 for details of assessments). Forward digit span was unavailable for 2 patients, Rey copy was unavailable for 5 patients, and Boston Naming Test was unavailable for 11 patients.
In addition to reporting raw patient performance on neuropsychological assessments, we report the proportion of patients impaired on each task relative to published normative data of healthy controls matched to the mean age and mean education of our patients where available21,20,25; we report patient performance on Rey copy relative to normative data based on healthy controls recruited by the Penn Frontotemporal Degeneration Center who were matched to patient mean age and education (n = 22 [50% female]; age, years: mean 61.81, SD 4.97; education, years: mean 15.73, SD 2.37). We defined patient impairment on each task as performance ≥1.96 SD, equivalent to p < 0.05, below normative data from healthy controls.
We used linear regression to relate performance on neuropsychological measures to CR index including age at assessment as a nuisance covariate. We report one-tailed p values as we predicted higher CR index to relate to better performance on neuropsychological measures.
Neuroimaging analyses.
High-resolution volumetric MRIs were acquired and processed for all participants as described in appendix e-1. We used randomise software in FSL to perform nonparametric, permutation-based statistical analyses.26 Permutation-based statistical testing is robust to concerns regarding multiple comparisons since, rather than a traditional assessment of 2 sample distributions, this method assesses a true assignment of factors (e.g., group, CR index) to GM relative to many (e.g., 10,000) random assignments. We adopt a priori statistical thresholds similar to prior reports27 that include FWE correction for GM t tests and, to minimize Type II error (not observing a true regression result), uncorrected p values for GM regressions.
Analysis 1.
First, we evaluated regions of reduced gray matter density (GMD) in patients with FTLD relative to controls using a nonparametric t test. For this analysis, we constrained t tests using an explicit mask restricted to include only high probability GM (>0.5). We report clusters surviving p < 0.001 (family-wise error) threshold and cluster extent of >50 adjacent voxels relative to 10,000 random permutations.
Analysis 2.
Second, we conducted 2 regression analyses to evaluate the relationship between CR index and GMD in patients (analysis 2a) and in controls (analysis 2b), and restricted both analyses to a mask defined by regions of reduced GMD in patients relative to controls from analysis 1. This focused our interpretation of CR in the context of GM regions affected by FTLD; it would be difficult to interpret how regions of GMD not significantly different from controls contribute to cognitive function of patients with FTLD. Table e-1 illustrates whole-brain analyses that do not include an explicit mask of reduced GMD.
We report clusters that survive a p < 0.05 (uncorrected) threshold and cluster extent threshold of >50 adjacent voxels relative to 10,000 random permutations. Analysis 2a (patients) included disease duration and age at MRI as nuisance covariates and analysis 2b (controls) included age at MRI as a nuisance covariate in an effort to control for factors associated with individual differences in GM but not specifically associated with CR.
Analysis 3.
Finally, we used Pearson correlations to examine regions associated with CR in patients relative to performance on neuropsychological measures. For each patient, we extracted the mean GMD in each region identified as associated with CR index from analysis 2a. We then correlated mean GMD with performance on each neuropsychological test and report Bonferroni-corrected p values.
RESULTS
Neuropsychological assessment.
Patients demonstrated most impairment on letter fluency (70.91% impaired) consistent with compromised frontal resources relative to other domains including attention on forward digit span (18.87% impaired), visuospatial function on Rey copy (56% impaired), nonspecific global cognitive impairment on MMSE (58.18% impaired), and language deficits on the Boston Naming Test (36.36% impaired) (table 1). Regression analyses indicated that higher CR index was associated with better performance on letter fluency (95% CI −0.015, 1.38; t = 1.96, p = 0.028), but not MMSE, forward digit span, Rey copy, or Boston Naming Test (all p > 0.05).
Neuroimaging results.
Analysis 1.
Patients with FTLD had reduced GMD relative to controls throughout bilateral frontal and temporal lobes, consistent with the known pattern of GM disease associated with FTLD pathology (table 2, figure 1).
Table 2.
Results of a t test showing regions of reduced gray matter density (GMD) in frontotemporal lobar degeneration (FTLD) patients relative to healthy controls (analysis 1) and results of regression analyses in patients (analysis 2a) and controls (analysis 2b) showing a positive relationship between GMD and cognitive reserve (CR) in regions from analysis 1
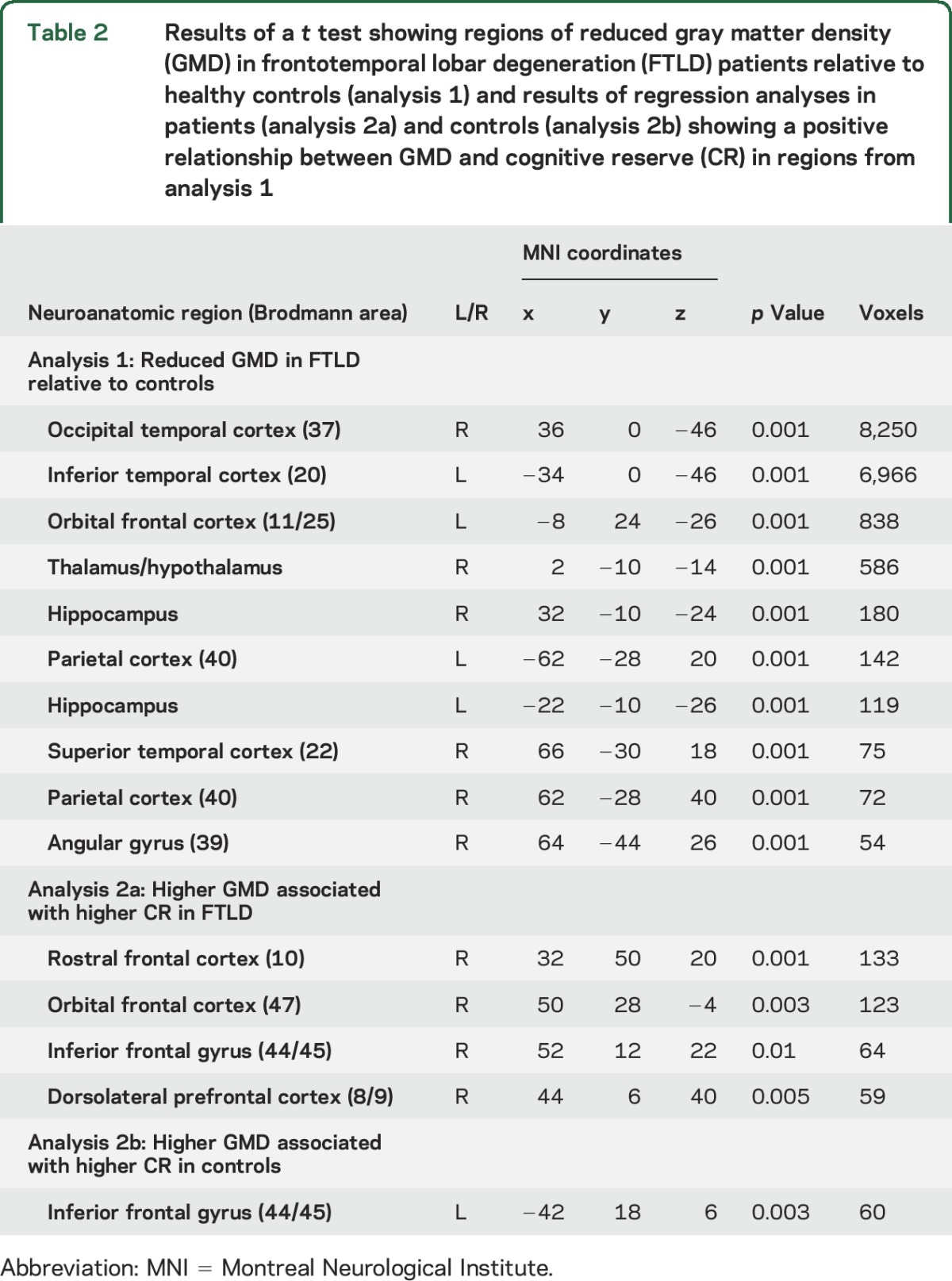
Figure 1. Higher cognitive reserve (CR) in patients with frontotemporal lobar degeneration (FTLD) and higher gray matter density (GMD) in diseased frontal lobe regions.
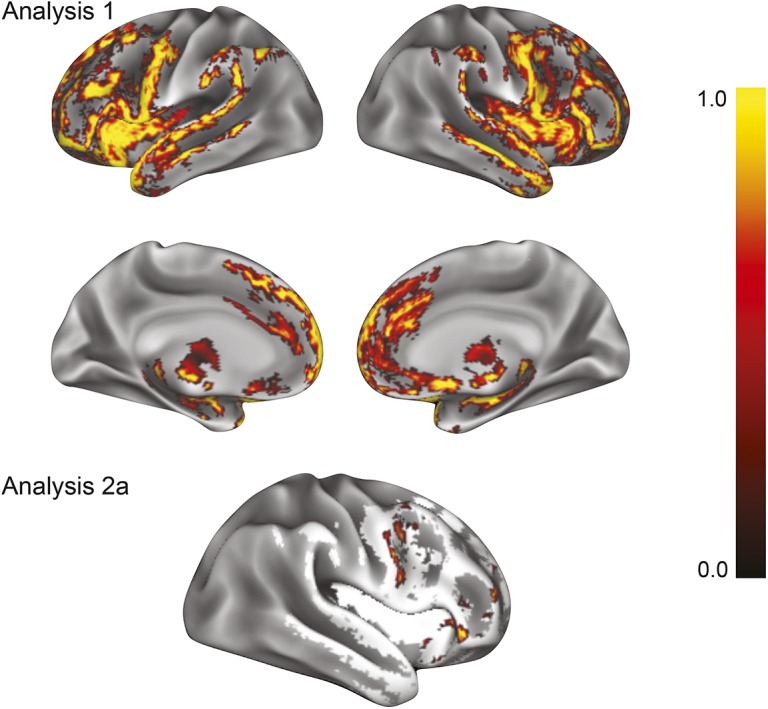
Analysis 1: Results of a nonparametric t test show regions of reduced GMD in patients with FTLD (n = 55) relative to demographically comparable controls (n = 90). Analysis 2a: Results of a regression analysis in patients with FTLD (n = 55) restricted to regions of reduced GMD from analysis 1 (white regions) demonstrate that higher GMD in the right dorsolateral prefrontal cortex, rostral frontal cortex, orbital frontal cortex, and inferior frontal gyrus is associated with higher CR index. Color bar represents 1 − p value with yellow representing highest significance.
Analysis 2a.
By restricting analysis to regions where patients exhibited reduced GMD relative to controls, we next found that higher CR index in patients was associated with higher GMD in right frontal cortex, including rostral, orbital, inferior, and dorsolateral prefrontal regions (table 2, figure 1). Thus, a patient with a higher CR index exhibited higher frontal GMD in these diseased regions in comparison to a patient with a lower CR index. We found no association between lower CR index and higher GMD in any region of reduced GMD relative to controls (not shown).
Analysis 2b.
To examine the specificity of the relationship between CR index and GMD in FTLD, we performed a comparable analysis in controls restricted to the same GM regions as the regression analysis in patients. We found that higher CR index in controls was associated with higher GMD in the left inferior frontal gyrus only (table 2, figure e-1).
Analysis 3.
In patients, frontal GMD related to higher CR index was also associated with superior performance on letter fluency (p < 0.05), but not MMSE, forward digit span, Rey copy, or Boston Naming Test (all p > 0.05) (table 3, figure 2). Other regions of frontal GMD from analysis 2 were not related to superior performance on any neuropsychological task (all p > 0.05) (not shown).
Table 3.
In patients with frontotemporal lobar degeneration, mean gray matter density in regions positively associated with cognitive reserve from analysis 2a are also positively associated with performance on letter fluency, but not on Mini-Mental State Examination (MMSE), forward digit span, Rey copy, or the Boston Naming Test (analysis 3)
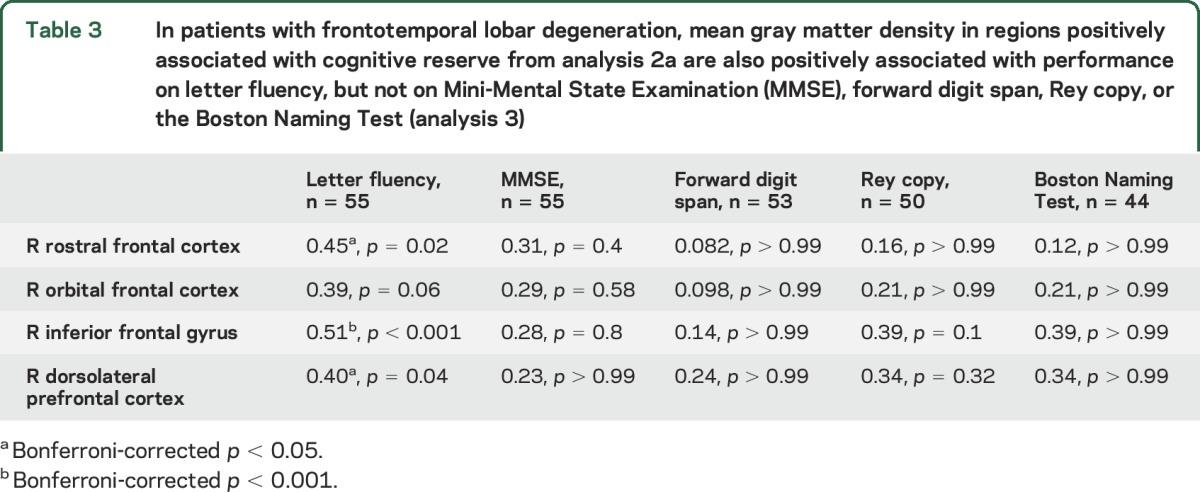
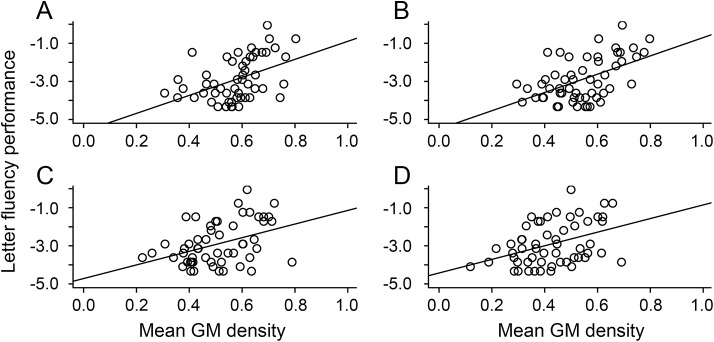
Figure 2. Higher gray matter density (GMD) associated with higher cognitive reserve (CR) and superior letter fluency.
Analysis 3: Mean GMD in regions associated with higher CR from analysis 2a are also associated with superior performance on letter fluency in patients with frontotemporal lobar degeneration (n = 55), including right rostral frontal cortex (A), inferior frontal gyrus (B), orbital frontal cortex (C), and dorsolateral prefrontal cortex (D).
Post hoc analyses.
While our primary analyses use a CR index, we performed post hoc analyses to evaluate education and occupational attainment alone; we observed findings largely convergent with our CR index analyses (see appendix e-1, table e-2, and figure e-2).
To evaluate whether right frontal GMD associated with CR is partially driven by sources of heterogeneity in our cohort, we also performed post hoc logistic regression analyses evaluating neuropathologic, genetic, and clinical subgroups. First, we observed that mean GMD in right rostral, orbital, inferior, and dorsolateral prefrontal regions were not significant predictors of tau vs TDP-43 pathologic subgroups (all factors p > 0.10). Second, we observed that each of these 4 GM regions also were not significant predictors of genetic mutation or sporadic forms of disease (all factors p > 0.10). Finally, we evaluated whether the 4 GM regions associated with CR were significant predictors of bvFTD clinical phenotype vs other phenotypes, since bvFTD is the predominant phenotype in our cohort. This analysis revealed that one region, right dorsolateral prefontal cortex (p = 0.048), was more reduced in bvFTD relative to other phenotypes; however, all remaining regions did not differ (all p > 0.05). Together, these findings are suggestive that these sources of heterogeneity are not likely confounding our observed findings related to CR in FTLD.
DISCUSSION
Our results suggest that CR contributes to interindividual differences in reduced frontal GMD and executive impairment that underlie heterogeneity in the disease course of FTLD. In an analysis restricted to frontal and temporal lobe regions of reduced GMD relative to controls, patients with higher CR exhibited higher GMD in right frontal cortical regions compared to patients with lower CR. Moreover, we demonstrate that regions related to CR in FTLD appear to be specific: CR in control participants was only related to higher GMD in the left IFG. In patients with FTLD, higher frontal GMD in right frontal cortical regions was associated with superior performance on letter fluency, a neuropsychological measure of executive control and verbal ability indicative of frontal lobe integrity. These findings are consistent with a reserve model linking preserved frontal anatomic integrity and superior strategic processing to the prolonged survival of patients with FTLD who have higher education and occupational attainment.13
Our findings are consistent with other evidence suggesting that CR is an environmental factor influencing the spectrum of disease in FTLD. Prior neuroimaging studies of patients with bvFTD with unconfirmed FTLD pathology indicate CR may counteract the onset of dementia,10–12 while a recent survival analysis in patients with autopsy-confirmed FTLD pathology by our group indicates that higher occupational attainment is associated with prolonged survival from symptom onset.13 These studies suggest that, despite the commonality of frontotemporal-predominant neurodegeneration, patients with FTLD appear to exhibit heterogeneous disease course that is determined in part by environmental factors related to CR. It is imperative to identify and understand potential environmental contributors to the rate of decline in FTLD, as these may serve as prognostic markers and eventual therapeutic targets.
Our analysis of CR in control participants demonstrates specificity of higher GMD in right frontal cortex associated with CR in patients with FTLD. Several other studies have investigated CR in healthy controls, demonstrating a positive relationship between CR and structural brain integrity. For example, one study demonstrates that in healthy older controls, higher years of education are positively associated with GM volume in the superior temporal gyrus, insula, and anterior cingulate cortex.28 While we similarly found a positive association between CR index and GMD in the left IFG in our healthy control group, this region did not overlap with any region of higher GMD associated with higher CR index in our patient group. We interpret this to suggest that our findings of higher GMD associated with higher CR in the right rostral and orbital frontal cortex, dorsolateral prefrontal cortex, and inferior frontal gyrus are specific to patients with FTLD. Future work is necessary to evaluate the potential role of left IFG in CR in healthy controls, but limited neuropsychological data in the current control cohort preclude our ability to evaluate the behavioral consequences of this association.
To our knowledge, no prior studies have examined the association between CR and heterogeneity in neuroanatomical structure and cognitive function in patients with a clinical FTLD syndrome due to confirmed FTLD-tau or FTLD-TDP pathology. Some studies report that patients with clinically diagnosed FTD who have higher education and occupation exhibit evidence of greater frontal disease as measured by regional cerebral glucose utilization and regional cerebral blood flow.10–12 These studies have been interpreted to suggest that CR confers compensatory benefit such that individuals with FTD who have higher CR can better withstand accumulating frontotemporal pathology than individuals with lower CR, and therefore delay symptom presentation. This resembles findings reported in AD.8,9 However, differences in patient population and study design necessitate cautious interpretation of these results. For example, as many as 16.7% of patients with FTD-related syndromes have AD neuropathology.29 Furthermore, the relationship between higher CR and greater frontotemporal pathology is difficult to interpret in this work in the absence of neuropsychological data. For example, it is unclear if for the same severity of frontotemporal pathology, patients with FTD with higher CR demonstrate better cognitive or behavioral function than patients with FTD with lower CR. Future research is needed to provide a more detailed assessment of how structural GM, functional imaging, and neuropsychological measures interact in the context of CR.
Several mechanisms may underlie the association between higher CR index and higher right frontal cortex GMD and superior letter fluency in patients with FTD. One possible mechanism is interindividual differences in genetic predisposition. For example, genetic studies in healthy twins suggest that there is a genetic contribution to normal variation in human cognitive function and brain morphology, including frontal cortex GM volume.30 Another possibility is that higher frontal GM and superior cognitive function in patients with FTD with higher CR may be a function of earlier stages of disease. For example, patients with higher education and occupational attainment who are more dependent on frontally mediated executive functions may be more sensitive to the emergence of cognitive difficulty, leading to clinical symptom detection at an earlier stage of disease. Either of these proposed mechanisms could result in patients with higher CR exhibiting relatively higher frontal GM and superior letter fluency compared to patients with lower CR. Longitudinal structural and functional neuroimaging and neuropsychological assessment in controls and patients with FTD are necessary to evaluate individual differences in rates of disease progression underlying heterogeneity in FTLD disease course related to education and occupation as proxies of CR.
Our findings contribute to a growing body of evidence for factors thought to influence heterogeneity in FTLD disease course, and suggest the consideration of both biological and environmental factors. Others suggest that risk alleles in single nucleotide polymorphisms, including rs1768208 in the myelin oligodendrocyte basic protein (MOBP) gene31 and rs1990622 in the TMEM106B gene,5 are associated with greater pathology and earlier age at onset and death in FTLD. Moreover, recent evidence indicates that epigenetic factors like C9orf72 promoter hypermethylation are associated with reduced neuronal loss and reduced GM atrophy in frontal cortex.6 While genetic factors may contribute to heterogeneity in the disease course of FTLD, our exploratory analyses suggest that genetic status is not a confounder of the current observations. Future research should investigate interactions between environmental factors, like CR, and biological factors, like genetics and epigenetics, on clinical heterogeneity in FTLD.
Several potential caveats should be considered in the current study. We assessed CR using a composite index of education and occupation; however, future research should also consider other environmental factors implicated in CR such as midlife leisure activities.32 Our patient cohort self-reported predominant white race and most received a college education; thus, more racially and educationally diverse samples are needed in future studies of CR in patients with FTLD. Another caveat to consider is that the current study cohort included several different clinical phenotypes with a majority of our sample comprising bvFTD and the remaining consisting of primary progressive aphasias and movement disorders (e.g., CBS, PSP). While future research is necessary to stratify by clinical phenotype, our post hoc analyses suggested that only one GM region, right dorsolateral prefrontal cortex, was related to bvFTD. Importantly, regions implicated in CR that are most likely to be shared across clinical phenotypes, such as right inferior frontal gyrus, did not show differences in GMD. Other sources of heterogeneity in our patient cohort including presence/absence of a genetic mutation and FTLD-tau/FTLD-TDP pathology also did not appear to contribute to GM differences in regions related to CR, though future studies must address these distinct groups.
With these caveats in mind, our research suggests that CR is an environmental factor contributing to heterogeneity in executive control and verbal ability of patients with known FTLD pathology mediated by neuroanatomic structure. These findings stimulate investigation into additional environmental contributors to disease course, and suggest their importance in prognostic considerations and treatment trials in patients with FTLD.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- bvFTD
behavioral variant frontotemporal dementia
- CBS
corticobasal syndrome
- CR
cognitive reserve
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- GM
gray matter
- GMD
gray matter density
- MMSE
Mini-Mental State Examination
- PSP
progressive supranuclear palsy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.P., M.G., C.T.M.: drafting/revising the manuscript for content. K.P., L.M., M.G., C.T.M.: study concept/design. K.P., C.O., K.T., K.F., V.V.D., M.G., E.B.L., J.Q.T., V.M.-Y.L., D.J.I., C.M.: analysis/interpretation of data. K.P., C.O., K.T., K.F., C.M.: performed statistical analysis. J.Q.T., V.M.-Y.L., M.G., C.M.: obtained funding.
STUDY FUNDING
Supported by the NIH (AG043503, AG017586, AG032953, AG010124, NS044266, NR014777), Dana Foundation, Wyncote Foundation, and the Arking Family Fund.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 2014;129:469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 2005;62:925–930. [DOI] [PubMed] [Google Scholar]
- 3.Josephs KA, Whitwell JL, Weigand SD, et al. Predicting functional decline in behavioural variant frontotemporal dementia. Brain 2011;134:432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology 2003;61:349–354. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher MD, Suh E, Grossman M, et al. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol 2014;127:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan CT, Russ J, Wood EM, et al. C9orf72 promoter hypermethylation is neuroprotective: neuroimaging and neuropathologic evidence. Neurology 2015;84:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol 2008;63:112–118. [DOI] [PubMed] [Google Scholar]
- 9.Bosch B, BartrEs-Faz D, Rami L, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer's disease. Cortex 2010;46:451–461. [DOI] [PubMed] [Google Scholar]
- 10.Borroni B, Premi E, Agosti C, et al. Revisiting brain reserve hypothesis in frontotemporal dementia: evidence from a brain perfusion study. Dement Geriatr Cogn Disord 2009;28:130–135. [DOI] [PubMed] [Google Scholar]
- 11.Perneczky R, Diehl-Schmid J, Drzezga A, Kurz A. Brain reserve capacity in frontotemporal dementia: a voxel-based 18F-FDG PET study. Eur J Nucl Med Mol Imaging 2007;34:1082–1087. [DOI] [PubMed] [Google Scholar]
- 12.Spreng RN, Drzezga A, Diehl-Schmid J, Kurz A, Levine B, Perneczky R. Relationship between occupation attributes and brain metabolism in frontotemporal dementia. Neuropsychologia 2011;49:3699–3703. [DOI] [PubMed] [Google Scholar]
- 13.Massimo L, Zee J, Xie SX, et al. Occupational attainment influences survival in autopsy-confirmed frontotemporal degeneration. Neurology 2015;84:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009;10:131–146. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19.Premi E, Gazzina S, Bozzali M, et al. Cognitive reserve in Granulin-related frontotemporal dementia: from preclinical to clinical stages. PLoS One 2013;8:e74762–e74768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177. [PubMed] [Google Scholar]
- 21.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA 1993;269:2386–2391. [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV). San Antonio, TX: Pearson; 2008. [Google Scholar]
- 23.Libon DJ, Rascovsky K, Gross RG, et al. The Philadelphia Brief Assessment of Cognition (PBAC): a validated screening measure for dementia. Clin Neuropsychol 2011;25:1314–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan E, Goodglass H. Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 25.Shirk SD, Mitchell MB, Shaughnessy LW. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther 2011;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ash S, Menaged A, Olm C, et al. Narrative discourse deficits in amyotrophic lateral sclerosis. Neurology 2014;83:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenaza-Urquijo EM, Landeau B, La Joie R, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 2013;83:450–457. [DOI] [PubMed] [Google Scholar]
- 29.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulshoff Pol HE, Schnack HG, Posthuma D, et al. Genetic contributions to human brain morphology and intelligence. J Neurosci 2006;26:10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan CT, Toledo JB, Avants BB, et al. Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol Aging 2014;35:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001;57:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.