Abstract
Possible effects of clothianidin seed-treated oilseed rape on honey bee colonies were investigated in a large-scale monitoring project in Northern Germany, where oilseed rape usually comprises 25–33 % of the arable land. For both reference and test sites, six study locations were selected and eight honey bee hives were placed at each location. At each site, three locations were directly adjacent to oilseed rape fields and three locations were situated 400 m away from the nearest oilseed rape field. Thus, 96 hives were exposed to fully flowering oilseed rape crops. Colony sizes and weights, the amount of honey harvested, and infection with parasites and diseases were monitored between April and September 2014. The percentage of oilseed rape pollen was determined in pollen and honey samples. After oilseed rape flowering, the hives were transferred to an extensive isolated area for post-exposure monitoring. Total numbers of adult bees and brood cells showed seasonal fluctuations, and there were no significant differences between the sites. The honey, which was extracted at the end of the exposure phase, contained 62.0–83.5 % oilseed rape pollen. Varroa destructor infestation was low during most of the course of the study but increased at the end of the study due to flumethrin resistance in the mite populations. In summary, honey bee colonies foraging in clothianidin seed-treated oilseed rape did not show any detrimental symptoms as compared to colonies foraging in clothianidin-free oilseed rape. Development of colony strength, brood success as well as honey yield and pathogen infection were not significantly affected by clothianidin seed-treatment during this study.
Keywords: Colony development, Field study, Neonicotinoid, Plant protection product, Pollinator, Seed-treatment
Introduction
The Western honey bee, Apis mellifera, is economically the most valuable pollinator of crop monocultures worldwide and yields of some fruit, seed and nut crops are estimated to decrease by more than 90 % without these pollinators (Klein et al. 2007). As abundance of wild bees in agricultural fields is often insufficient, managed honey bee hives are indispensable to ensure sufficient crop pollination. Furthermore, the global stock of domesticated honey bees is growing more slowly than agricultural demand for pollination (Aizen and Harder 2009). In recent years, honey bees have suffered from high colony mortality, including colony collapse disorder, overwinter or seasonal colony losses (vanEngelsdorp et al. 2008; Neumann and Carreck 2010; Smith et al. 2013). Honey bee colonies are exposed to multiple and varying stressors (Potts et al. 2010) including habitat loss, malnutrition, parasites and pathogens, and plant protection products (PPPs). In particular, systemically acting PPPs of the neonicotinoid class of compounds are often held responsible for honey bee colony losses (Sánchez-Bayo 2014; Goulson et al. 2015; Pisa et al. 2015). Like the botanical insecticide nicotine, neonicotinoids act as agonists at nicotinic acetylcholine receptors in the insect central nervous system (for reviews, see, Tomizawa and Casida 2005; Jeschke et al. 2013). Neuroactive neonicotinoids are commonly used as seed dressings in a variety of crops including oilseed rape (OSR) and are taken up systemically by the growing plant and distributed to all tissues (Elbert et al. 2008). The systemic activity of neonicotinoids makes them effective as a seed dressing, providing protection to crops in their more vulnerable early stages of growth. This reduces the number of foliar insecticide applications required, which are often applied at much higher application rates and generally result in an increased exposure of non-target organisms (Cutler et al. 2014; Pisa et al. 2015).
Due to the concerns about the impact of neonicotinoids on honey bees and other pollinators, the use of the three neonicotinoids imidacloprid, clothianidin, and thiamethoxam has been temporarily suspended in the European Union as seed treatment, soil application, and foliar treatment in crops attractive to bees (European Commission 2013). Various laboratory and semi-field studies that link poor overall condition of bee colonies to widespread use of neonicotinoid PPPs have been criticized (Cresswell and Thompson 2012; Guez 2012; Carreck and Ratnieks 2014; Godfray et al. 2014) for not using field realistic doses or for subjecting bees in the laboratory exclusively to food spiked with neonicotinoids. Thus, a key question is how neonicotinoids influence bees in real-world agricultural landscapes (Schmuck and Lewis 2016, this issue).
To examine potential effects of clothianidin seed dressing on pollinators under common agricultural practice we initiated a comprehensive monitoring project in 2013. This large-scale field study aimed to investigate possible side effects of clothianidin-dressed OSR seeds at the landscape level on various pollinators under actual agricultural conditions (Heimbach et al. 2016, this issue). Monitoring studies at the landscape level (Cutler and Scott-Dupree 2007; Pilling et al. 2013; Cutler and Scott-Dupree 2014; Cutler et al. 2014; Rundlöf et al. 2015) have not been widely performed within the registration procedure of PPPs in the European Union. Their use has been proposed for some time as a valuable tool to improve risk assessment by increasing realism (e.g., Liess et al. 2005). This project consisted of four different pollinator studies performed in the project area at the same time: a honey bee monitoring study (this paper), a mason bee (Osmia bicornis) monitoring study (Peters et al. 2016, this issue), a bumble bee (Bombus terrestris) monitoring study (Sterk et al. 2016, this issue), and a residue analysis of pollen and nectar from foraging honey bees in tunnel tents as well as of pollen, nectar, and honey of free-flying honey bees, bumble bees, and mason bees (Rolke et al. 2016, this issue).
For the honey bee study described here, eight honey bee hives were installed at each of twelve study locations, six in the reference (R) site and six in the test (T) site, and thus a total of 96 colonies was monitored. The objectives of the study were to investigate (1) the short-term effects of the OSR seed dressing on honey bee colonies exposed at the R and T site (e.g., population development, honey production, Varroa infestation levels etc.) to OSR at flowering and (2) post-exposure effects of the OSR seed dressing on honey bee colonies after transfer to four locations in a post-exposure monitoring area, where they were observed until autumn.
Materials and methods
Study locations
The study was conducted at two neighboring study sites in the vicinity of Sternberg, Northern Germany during OSR flowering (exposure phase). Each study site covered an area of approximately 65 km2 with a diameter of 9 km. In autumn 2013, Elado®-dressed OSR seeds (10 g clothianidin and 2 g β-cyfluthrin/kg seed) were drilled in all study fields at the test (T) site, whereas Elado®-free OSR seeds were drilled at the reference (R) site. For a detailed description of seed treatment, OSR fields, and planting, see Heimbach et al. 2016 (this issue). Six study locations were selected at the R site (Fig. 1a) and another six at the T site (Fig. 1b). Study locations were located in the center of each study site at least 3 km away from the outer edge to exclude a potential movement of foraging honey bees outside the study sites. The requirements for separation of reference and test conditions limit the possibility for true statistical replication which would be desirable under ideal conditions (Hurlbert 1984) but is hardly feasible for large-scale honey bee monitoring studies (Pilling et al. 2013). Since a possible treatment effect could be confounded with site differences, the study sites were cautiously selected to be as similar as possible (Heimbach et al. 2016, this issue). In addition, the applied mixed effects models (see “Data analysis”) are a common tool to address non-independence of data (Zuur et al. 2009). Three out of the six study locations per study site were established at the edge of an OSR field and the other three were situated in 400 m distance to the nearest OSR field (Figs. 1a and b). During the post-exposure phase, the study was continued at four locations in Erlensee, west-central Germany in an area without any agricultural or horticultural activities (Fig. S1A). These locations were chosen to be as close and similar to each other as possible.
Fig. 1.
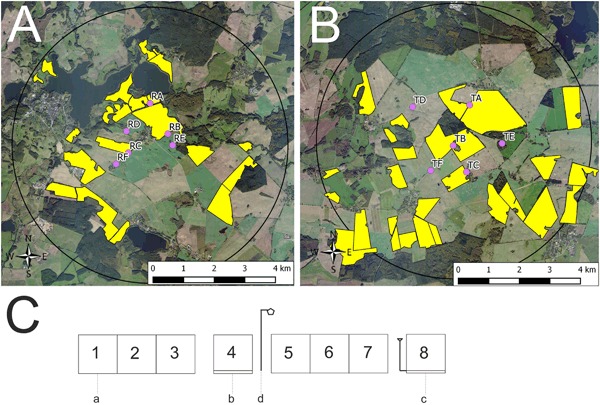
Study locations and arrangement of honey bee hives during the exposure phase. In a and b, yellow polygons indicate OSR fields. a Study locations at the reference site. Locations RA, RB, and RC were established at the edge of an OSR field and locations RD, RE, and RF were situated in 400 m distance to the nearest OSR field. b Study locations at the test site. Locations TA, TB, and TC were established at the edge of an OSR field and locations TD, TE, and TF were situated in 400 m distance to the nearest OSR field. c Arrangement of honey bee hives at the study locations during the exposure phase. a = honey bee hive, b = honey bee hive on a hive scale, c = honey bee hive on a hive scale connected to a rain gauge, d = anemometer
Honey bee colony handling and management
Honey bee (Apis mellifera carnica) colonies maintained according to normal beekeeping practice, disease-free, and queen-right were used. On 23–24 May 2013, test colonies were produced, each consisting of two brood combs, two food combs, and six empty combs and comb foundations. All combs were marked with numbers. Queen cells were added to the newly formed test colonies. All queens were offspring (F1) from the same mother queen. Newly emerged queens were allowed to perform mating flights and were individually marked. Developing colonies were inspected every 2–3 weeks and, when necessary, the hives were expanded or food was added. The colonies were overwintered in one box (brood chamber) each. When delivered to the study sites at the beginning of OSR full flowering on day after placement (DAP) -1 (21 April 2014), the hives consisted of the brood chamber including ten bee-covered combs. The setup of the hives took place on two consecutive days, at study locations RA, RB, RC, RE, RF, TA, TB, TC, and TF on DAP 0 and at study locations CD, TD, and TE on DAP 1 (Figs. 1a and 1b). Eight honey bee hives were placed at each location (Fig. 1c). In front view, the three hives on the left (1–3) were placed together on a metal frame followed by a single hive (4) on an individual metal frame, which was equipped with a hive scale. To the right, the same arrangement was repeated for hives 5–8. An anemometer was placed between hives 4 and 5. After set-up, all hives were marked with a colony code. The colony code consisted of the location designation (RA to RF and TA to TF for reference and test locations, respectively) and the position of the hive at the location (1–8). The exposure phase lasted 28 days. Honey bee hives were expanded during OSR blossom by adding honey supers, as necessary. Whenever honey supers were added during the study, a queen excluder was placed on top of the brood chamber. The exposure phase ended on 20 May 2014 (DAP 28) when 40–90 % of pods have reached final size.
At the end of OSR flowering, hives were loaded on a truck and transported to the four post-exposure study locations in Erlensee, west-central Germany (Fig. S1A). Every post-exposure location received two colonies from each exposure location resulting in 24 hives (including six hives on hive scales) at each post-exposure location, 12 from the R site and 12 from the T site (Fig. S1B). The selection and order of placement was randomized (Table S1). For the post-exposure phase, the data comparison of experimental groups was continued according to their grouping during the exposure phase, although colonies were re-grouped during the post-exposure phase. The post-exposure phase started on 26 May 2014 (DAP 34) and lasted for 123 days until 26 September 2014 (DAP 157). On DAP 36, each colony was fed with 2.5 kg of sucrose paste (Apifonda, Südzucker AG, Germany). On DAP 84, each colony was fed with 5 l of sucrose fructose glucose syrup (ApiInvert, Südzucker AG, Germany) and on DAP 108 and DAP 115 each colony was fed with 7 l ApiInvert. Treatment of honey bee colonies to control Varroa mites started on DAP 101 with the application of four Bayvarol® strips (Bayer AnimalHealth, Leverkusen, Germany; active substance: flumethrin), which were left inside the hives until the end of the study.
Colony endpoint measures
Each colony was continuously monitored with four assessments during the exposure phase and three assessments during the post-exposure phase. For individual assessment dates, see Table S2 (exposure phase) and Table S3 (post-exposure phase). During each assessment, the following parameters were recorded based on the Liebefeld method (Imdorf et al. 1987): estimated number of adult bees, estimated area covered with capped brood, and estimated area covered with open brood. All estimations were performed by one beekeeper. For the second assessment, bee number and brood area estimates were validated by weighing three combs per hive with and without bees and automatic photo evaluation of combs (software HoneybeeComplete, WSC Scientific, Heidelberg, Germany), respectively, showing high correlation and no bias in relation to experimental groups (data not shown). At every study location, two hives were placed on hive scales (CAPAZ GSM 200, CAPAZ, Oberkirch, Germany). These hive scales continuously measured the weight of the hive and recorded local weather data (temperature, humidity). One hive scale per study location was connected to a rain gauge. Recordings were taken in an interval of 1 h. From these data, a daily mean was calculated. The daily mean value of DAP 3 was used as the starting point for weight gain analysis because all colonies were provided with their first honey super at this time point.
Harvest of spring honey took place immediately after the exposure phase (DAP 33). Honey from each colony was extracted separately and weighed. Samples were taken for residue analysis (see Rolke et al. 2016, this issue) and for palynological analysis. Summer honey was harvested on DAP 84. Pollen collected by bees was sampled in order to study foraging activities of bees and to identify and classify pollen sources. Pollen samples were taken twice from all experimental colonies at two different sampling days during OSR flowering (DAP 15 and DAP 19/23). Bottom mount pollen traps including collection grids were added to the hives the day before sampling. On the following day, pollen traps were removed and the entire pollen yield was transferred to plastic bottles. Samples were stored at −20 °C until analysis. Palynological analysis of spring honey and bee-collected pollen grains was performed by the Bee Research Institute Celle (Germany) as described by von der Ohe et al. (2004).
To assess honey bee mortality and infestation with Varroa mites, the naturally occurring fall of dead bees and mites was recorded using flat plastic trays (340 mm × 340 mm × 55 mm) covered with metal grids which were placed onto the bottom board of the hives. First introduction of the plastic trays was during the 1st assessment (Table S2). Numbers of dead bees on the grids and dead mites on the trays were counted during each of the following assessments (Table S2). Flumethrin-induced mite fall was assessed continuously every 7 days. After each counting, plastic trays and grids were cleaned and immediately reintroduced. On DAP 100 (immediately before flumethrin treatment) and on DAP 147/148 (after 6 weeks of flumethrin treatment) infestation of worker bees with phoretic mites was recorded using the icing sugar method according to Dietemann et al. (2013). For the investigation of bee diseases, adult bees were taken from the honey supers at two different time points during the exposure phase (DAP 4–7, DAP 24–28) and once at the end of the post-exposure phase (DAP 153–155). For Nosema diagnosis, a semi-quantitative microscopic examination of a bulk sample of about 20 bees was carried out as described in detail by Topolska and Hartwig (2005). Samples for virus diagnostics were transferred to the Institute for Bee Research Hohen Neuendorf e.V. (Germany) for analysis. Total RNA of 10 bee heads per sample was extracted using standard methods following the manufacturer’s protocol (RNeasy Mini Kit, Qiagen, Hilden, Germany). Qualitative one-step RT-PCR for the detection of deformed wing virus (DWV), sacbrood virus (SBV), acute bee paralysis virus (ABPV), chronic bee paralysis virus (CBPV), and Kashmir bee virus (KBV) was performed according to standard protocols (One-step-RT-PCR Kit, Qiagen). Primer details and the length of the resulting amplicon as well as the temperature schemes used for each virus are given in Tables S4 and S5. Primer sequences for the detection of DWV, SBV, KBV, and ABPV were obtained from the literature (Stoltz et al. 1995; Benjeddou et al. 2001; Bakonyi et al. 2002; Genersch 2005; Yue et al. 2006; Maori et al. 2007).
Local weather recordings
Local weather recordings were made as described in detail by Heimbach et al. 2016 (this issue). Briefly, temperature, humidity, quantity of rainfall, and wind (speed and direction) were measured over the entire study period (except between exposure and post-exposure phase) including the days of assessment. For temperature, humidity, and rainfall, one data point was generated per hour, whereas wind data were saved every 10 min. For each parameter, a daily mean was calculated. These daily means were then added up to achieve sums for each parameter.
Data analysis
LASSO (Least Absolute Shrinkage and Selection Operator; Hastie et al. 2009) were implemented to perform an automatic feature selection to find out the variables and their interactions that are important for the reproduction endpoints and various metrics for bee pests and diseases, accounting for co-linearity at the same time. The LASSO procedure resulted in a parsimonious model with few parameters which served as a basis for the final model. The final model presented in this paper is based on expert judgment because it is always important to consider the identity of variables that are being removed, and whether certain variables are more biologically meaningful, or whether certain variables are better representations of underlying processes. The study site is always included in the final model regardless of the measure of significance because this is what we were interested in. Some interaction terms selected by the automatic LASSO procedure, like the interaction between DAP and wind sum, were considered not biologically relevant and were replaced by their main fixed effects. Co-linearity still exists in the final model, but not for the study site factor (variance inflation factor approximately 1), which means the standard error estimates are not inflated for the treatment effect. For other weather variables, chunk tests of correlated variables are powerful because co-linear variables join forces in the overall multiple degree of freedom association test, instead of competing against each other as when variables were tested individually. However, we were not interested in the strength of meteorological effects and, therefore, further chunk tests were not performed. The standard errors of meteorological coefficient estimates should be interpreted with caution though.
A linear mixed model (LMM) was constructed using the log of the ratio between the number of bees and the number of bees at 1st assessment as the response variable, and study site, distance to OSR, DAP, DAP^2, and meteorological conditions (temperature sum, wind sum) as the explanatory variables. The ratio between the number of bees and the number of bees at 1st assessment was used to adjust for slight initial differences. The intercept in the result table is the estimated mean value of the dependent variable (e.g., “log (Number of bees/Number of bees at 1st assessment)”), in case all continuous variables are held at 0 (“DAP” = 0, “Temperature sum” = 0, etc., if the predictor “Temperature sum” is centered, then at the mean temperature sum) and all categorical variables are held at their reference levels. For the covariate “Study site” the reference level is “reference site”. For the covariate “Distance to OSR” the reference level is “edge”. The polynomial term “DAP^2” describes curvature in the data and is included because exploratory data analysis revealed a clear quadratic relationship between “DAP” and “Number of bees” (parabolic curve). Nested random effect terms were included in the model to address the random effects due to specific locations and individual hives. The same was done for numbers of capped and open worker brood cells.
Linear mixed effect models were fitted to the data of honey yield. The amount of spring honey was related to study site, distance to OSR, and meteorological conditions (temperature sum, humidity sum, rain sum, and wind sum). Random intercepts for locations were included to account for the study design. The amount of summer honey was related to study site and distance to OSR with location random effects.
For the pollen composition data, a beta regression model was fitted to the relative amount of OSR pollen with study site, distance to OSR, and meteorological conditions (temperature sum, humidity sum, rain sum, and wind sum) as explanatory variables. Because beta regression requires data to be strictly greater than 0 and smaller than 1, values of 100 % were corrected to 99.9999 % before fitting the model.
For metrics regarding parasites and diseases, a generalized linear mixed model (GLMM) with Poisson error distribution and log link function was constructed using the Varroa infestation level as the response variable and DAP, study site, distance to OSR, and meteorological conditions (temperature sum, humidity sum, rain sum, and wind sum) as explanatory variables. Location was included as random factor. GLMMs with binomial error distribution and logit link function were fitted to Nosema, DWV, SBV, and ABPV in relation to DAP, study site, distance to OSR, and meteorological conditions.
Statistical evaluation was conducted with the statistical software package “R” (version 3.0.1; R Development Core Team 2011). Feature selections were conducted using the package “glmnet” (Friedman et al. 2010). The predictor variables were centered and scaled before entering into analysis whenever necessary. GLMMs and LMMs were fitted to the data using the packages “lme4” (Bates et al. 2014) and “nlme” (Pinheiro et al. 2015). Residual plots, random effects plots, and augmented predictions plots were examined to validate model assumptions.
A minimum detectable difference (MDD) concept has been developed as an indicator of the power of a test a posteriori for aquatic mesocosm/microcosm studies (Brock et al. 2015). However, the MDD-calculation depends on the statistical analyses (or tests) applied to analyze the data. The calculation of the MDD for this monitoring study generalized (or extended) the MDD concept to suit the mixed model analysis. Augmented prediction confidence intervals were used as the basis for the derivation of the MDD and MDD%. Detailed information can be found in the Supplementary Material (Extension of the MDD concept).
Results
Number of adult bees, capped, and open brood cells
The same pattern of development, which is characterized by an increase followed by a decrease in numbers of adult honey bees, could be observed in all colonies during the study period (Fig. 2a). Data from assessment 1 (DAP 4–7) indicated that colonies had similar starting conditions at the beginning of the study. Until assessment 2 at DAP 11–13, the number of adult bees increased by approximately one-third. The increase continued for all colonies until the number of adult honey bees peaked between assessments 4 (DAP 24–28) and 5 (DAP 50–52) (Fig. 2a and Table S6). The variability between study locations was similar throughout the study. The increase in the number of adult bees was similar between the R and T sites (Table 1). Furthermore, no statistically significant differences occurred between study locations situated at the edge of OSR fields and located in 400 m distance to OSR fields (Table 1).
Fig. 2.
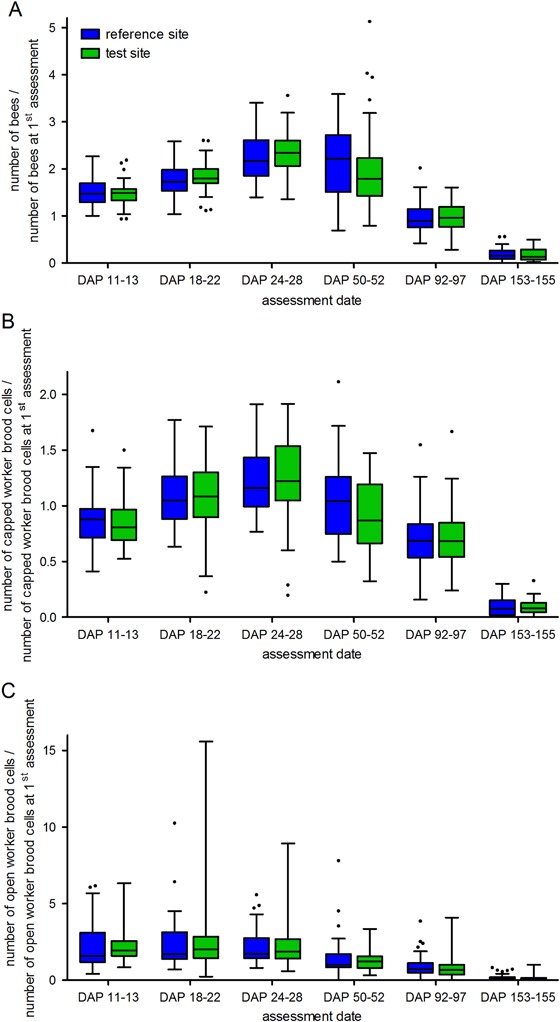
Development of the numbers of adult honey bees (a), capped brood cells (b), and open brood cells (c) in colonies located at study locations in the reference site (no clothianidin seed-dressing) and the test site (clothianidin seed-dressing). The box plots contain the 1st and 3rd quartiles, split by the median; traditional Tukey whiskers go 1.5 times the interquartile distance or to the highest or lowest point, whichever is shorter. Any data beyond these whiskers are shown as points. DAP day after placement
Table 1.
Summary of statistical results as obtained from a lognormal mixed model fitted to the log (number of bees/number of bees at 1st assessment), the log (number of capped worker brood cells/number of capped worker brood cells at 1st assessment), and the log (number of open worker brood cells/number of open worker brood cells at 1st assessment), respectively
| Log (number of bees/number of bees at 1st assessment) | Log (number of capped worker brood cells/number of capped worker brood cells at 1st assessment) | Log (number of open worker brood cells/number of open worker brood cells at 1st assessment) | |
|---|---|---|---|
| Intercept | 0.54 ± 0.06*** | 0.25 ± 0.14 | 0.10 ± 0.17 |
| DAP | 2.13 ± 0.49*** | −3.11 ± 1.05** | −3.61 ± 1.44* |
| DAP^2 | −0.43 ± 0.03 | −0.84 ± 0.07*** | −0.52 ± 0.09*** |
| Study site (test compared to reference) | 0.04 ± 0.07 | −0.01 ± 0.15 | 0.09 ± 0.17 |
| Distance to OSR (distant compared to edge) | −0.02 ± 0.07 | 0.11 ± 0.15 | 0.42 ± 0.17* |
| Temperature sum | −2.16 ± 0.49*** | 2.91 ± 1.04*** | 2.82 ± 1.43* |
| Wind sum | −0.28 ± 0.06*** | 0.14 ± 0.13 | 0.00 ± 0.18 |
Effect size ± SE is given for each parameter which contributed to the respective model. Positive values indicate an increase in relation to the respective reference value whereas negative values indicate a decrease. ***p < 0.001, **p < 0.01, *p < 0.05
At assessment 1, mean numbers of capped worker brood cells were comparable for R-site and T-site colonies. During the exposure phase, numbers of capped worker brood cells decreased slightly toward the assessment 2 and reached their maximum at assessment 4 (Fig. 2b). During the post-exposure phase, the number of capped worker brood cells decreased in the majority of colonies from both study sites. The variability between study locations was similar throughout the study. Marginal differences in the number of capped worker brood cells between the R and T sites were not statistically significant (Table 1). At most time points, study locations situated at the edge of OSR fields did not differ distinctively from those located at 400 m distance from the OSR fields. Simple Wald t-test showed the coefficient estimate for distance to the OSR fields was not significantly different from zero (Table 1).
Most colonies (49 colonies) showed an increase in the number of open worker brood cells during the first part of the exposure phase followed by a decrease toward the end of the exposure phase. This pattern was similar in all study locations and, hence, also for R and T site colonies (Fig. 2c). At the peak of open brood cells, numbers averaged 12,710 ± 3816 (mean ± SD; median: 13,300) at the R site and 12,115 ± 4685 (mean ± SD; median: 12,250) at the T site. The variability in the number of open worker brood cells was high at certain study locations (e.g., CC and TE) but overall similar (Fig. 2c). No statistically significant differences could be identified between the R site and T site locations or between study locations situated at the edge of OSR fields and located in 400 m distance to OSR fields (Table 1).
The %MDDs for the three reproduction-related endpoints were 15.2–21.4 % for the number of adult worker bees, 30.8–40.8 % for the number of capped worker brood cells, and 34.9–49.3 % for the number of open worker brood cells (Table 5). This indicates that in this monitoring study even relatively small effects on the number of bees as well as on numbers of capped and open worker brood cells could have been detected by the applied statistical analyses.
Table 5.
Summary of the MDD analysis
| Measures of bee development and health | MDD | %MDD |
|---|---|---|
| Number of adult worker bees | 269.7–4425 | 15.2–21.4 |
| Number of capped worker brood cells | 133.7–5337 | 30.8–40.8 |
| Number of open worker brood cells | 56.79–6665 | 34.9–49.3 |
| Spring honey yield | 2.0–2.0 | 7.8–8.5 |
| Percentage of Brassica napus pollen in spring honey | 5.2–5.3 | 7.2–7.6 |
| Summer honey yield | 3.1–3.2 | 27.7–43.2 |
| Naturally occurring fall of Varroa mites (DAP 11–92) | 0.70–4.9 | 73.3–690 |
| Flumethrin-induced fall of Varroa mites (DAP 108–150) | 16.6–44.1 | 67.9–69.7 |
| Varroa infestation level as determined by the icing sugar method | 4.3–15.0 | 23.4–24.5 |
| Nosema spores | 0.010–0.15 | 86.7–98.0 |
| DWV | 0.026–0.27 | 43.7–71.7 |
| SBV | 0–1.1 × 10−8 | 0–100.0 |
| ABPV | 2.6 × 10−6–0.34 | 52.3–100.0 |
Ranges for MDDs and relative MDDs (%MDD) for various endpoints are shown
Colony weight gain
In general, all measured colonies showed the same pattern of weight gain during the exposure phase (Fig. 3). From DAP 3 to DAP 9 a continuous increase of 23.18 ± 2.93 % in colony weight (R site, Figs. 3a and 3b) and 22.91 ± 3.41 % (T site, Figs. 3c and 3d) took place. Due to unfavorable weather conditions, the weight of the measured colonies changed only marginally from DAP 10 until DAP 21. Thereafter, colony weight increased again until DAP 27. Weight gain was very similar in R and T site colonies. During the post-exposure phase, the same 24 colonies were weighed continuously. Various apiarist operations influenced the colony weight during this period (feeding, adding/removing of honey supers, and bee escapes). However, as during the exposure phase, all measured colonies showed the same pattern of weight development during the post-exposure phase (data not shown). Weight development was similar in former R and T site colonies.
Fig. 3.
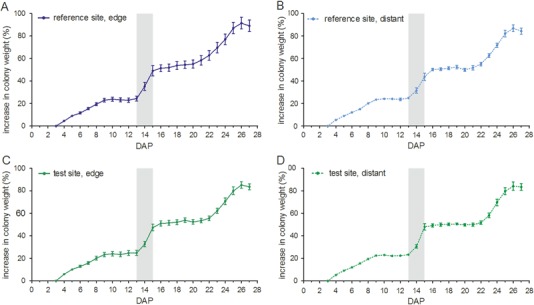
Comparison of percentage of increase in weight of colonies in experimental groups located at study locations in the reference site and the test site. Data are the means ± SEM. DAP day after placement
Honey yield
Spring honey yield of each individual colony was weighed and overall averaged 25.1 ± 3.6 kg per colony. Values for spring honey yield were combined for colonies of the three study locations within each experimental group (R edge, R distant, T edge, and T distant; Fig. 4a). There was clearly no effect of the clothianidin dressing on spring honey yield, but yields did differ according to the distance to the OSR fields (Fig. 4a, Table 2). Compared to colonies which were located at the edge of OSR fields, honey yield from colonies that were located 400 m distant from OSR fields was significantly lower (Table 2). This effect is consistent both for the R and the T site (Fig. 4a). Honey was harvested a second time (summer honey) approximately 1 month after placement of the colonies at the locations in Erlensee (DAP 84). Summer honey yield was very low with an average of 8.4 ± 3.9 kg per colony (Fig. 4b). The amount of harvested summer honey did not differ for colonies from the former R and T sites (Fig. 4b, Table 2). As for spring honey yield, the climatic parameters did not affect summer honey yield. A comparison of %MDD with the Difference (%) values (Table 5) indicates that the experimental design allowed for the determination of even relatively small effects on the yield of spring and summer honey if present.
Fig. 4.
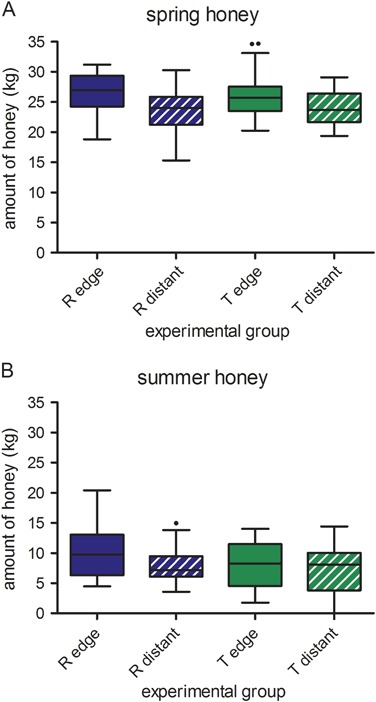
Amount of spring honey (a) and summer honey (b) harvested from colonies from different experimental groups. The box plots contain the 1st and 3rd quartiles, split by the median; traditional Tukey whiskers go 1.5 times the interquartile distance or to the highest or lowest point, whichever is shorter. Any data beyond these whiskers are shown as points. R reference site, T test site
Table 2.
Summary of statistical results as obtained from a LMM fitted to the amount of spring honey and summer honey, respectively
| Amount of spring honey | Amount of summer honey | |
|---|---|---|
| Intercept | 8.35 ± 63.03 | 8.34 ± 1.11*** |
| Study site (test compared to reference) | 0.25 ± 0.85 | −1.26 ± 1.08 |
| Distance to OSR (distant compared to edge) | −2.58 ± 0.89** | −1.19 ± 1.08 |
| Temperature sum | 0.08 ± 0.11 | |
| Humidity sum | 0.00 ± 0.02 | |
| Rain sum | −0.09 ± 0.13 | |
| Wind sum | −0.08 ± 0.06 |
Effect size ± SD is given for each parameter. Positive values indicate an increase in relation to the respective reference value whereas negative values indicate a decrease. For the parameter “Study site” the reference level is “reference site”. For the parameter “Distance to OSR” the reference level is “edge”. ***p < 0.001, **p < 0.01
Pollen composition
At least 50 % of pollen in spring honey samples from almost all experimental colonies originated from OSR (Fig. 5a). For R site colonies, the mean (±SD) percentage of OSR pollen in spring honey samples was 72.71 % ± 10.51 % and 69.96 % ± 10.39 % for edge and distant locations, respectively. For T site colonies, the mean (±SD) percentage of OSR pollen in spring honey samples was 79.61 % ± 7.48 % and 77.88 % ± 8.93 % for edge and distant locations, respectively. Colonies from the T site have on average a significantly higher proportion of OSR pollen in the spring honey samples than colonies from the R site but there was no significant difference between edge and distant study locations (Table 3). The relative frequency of pollen grains from other nectar sources was low (Fig. 5a). Among the pollen types other than OSR pollen, willow pollen was the most frequent in honey samples. The MDD results clearly indicate that the experimental design of the monitoring study allowed for the determination of relatively small effects on pollen composition in spring honey (Table 5).
Fig. 5.
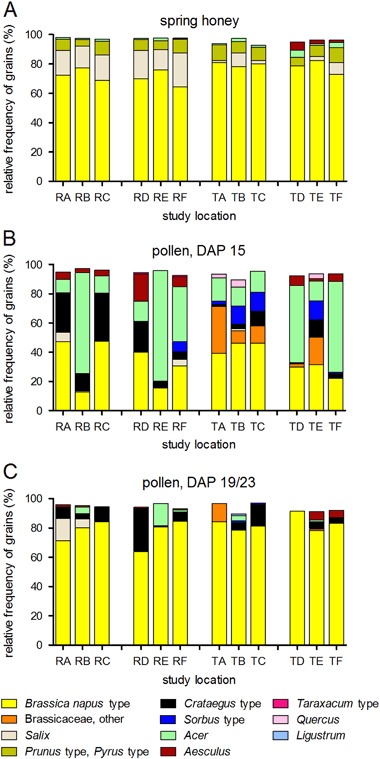
Percentage of various pollen grains in honey extracted from experimental colonies (a) as well as in pollen samples from experimental colonies at two sampling events (b: DAP +15; c: DAP +19/+23). DAP day after placement
Table 3.
Summary of statistical results as obtained from a beta regression model fitted to the relative frequency of Brassica napus pollen grains in spring honey
| Relative frequency of Brassica napus pollen grains | |
|---|---|
| Intercept | 15.10 ± 7.42* |
| Study site (test compared to reference) | 0.44 ± 0.10*** |
| Distance to OSR (distant compared to edge) | −0.13 ± 0.11 |
| Temperature sum | −0.01 ± 0.01 |
| Humidity sum | 0.00 ± 0.00* |
| Rain sum | 0.01 ± 0.01 |
| Wind sum | 0.00 ± 0.01 |
| Precision (phi)a | 23.68 ± 3.40*** |
Effect size ± SD is given for each parameter. Positive values indicate an increase in relation to the respective reference value whereas negative values indicate a decrease. All other parameters are as explained in the legend of Table 1. ***p < 0.001, *p < 0.05
a Precision (phi) is the precision component of the beta distribution, which is related to the variance of the observations. For fixed mean mu, the larger the phi the smaller the variance of the dependent variable
The percentage of OSR pollen in samples of bee-collected pollen was lower for the first sampling (DAP 15; 34.0 % ± 17.8 %) than for the second sampling (DAP 19/23; 80.2 % ± 12.0 %). This pattern was consistent for colonies from both the R and the T sites (Fig. 5b). For the first sampling (DAP 15, Fig. 5b), the mean (±SD) percentage of OSR pollen was 32.3 % ± 21.2 % and 35.8 % ± 13.5 % for R site colonies and T site colonies, respectively. For some R site study locations, the percentage of OSR pollen was highly variable. At some study locations, relatively high percentages of maple pollen (Acer) were recorded. For the second sampling (DAP 19/23, Fig. 5c), the mean (±SD) percentage of OSR pollen increased to 77.5 % ± 14.1 % and 82.8 % ± 8.8 % for R site colonies and T site colonies, respectively. In addition to OSR pollen, pollen of the Crataegus type was relatively abundant. Thus, for the second sampling, OSR became the major pollen source for honey bees both at the R site and the T site.
Pests and diseases
Honey bee mortality as determined by counting dead bees on a metal grid within the hive was very low in all colonies throughout the exposure phase and during most of the post-exposure phase (Fig. S2). From DAP 122 until the end of the post-exposure phase, up to 12 dead bees per day were detected in individual colonies although the median over all colonies remained low at ≤2 (Fig. S2). Fitting a general additive mixed model to the data revealed that the number of dead bees was equal for the R and the T site (data not shown).
During the exposure phase, naturally occurring daily fall of dead Varroa mites was low in the R site and in the T site (Fig. S3). As expected due to the annual population growth, daily mite fall (although highly variable) steadily increased during the post-exposure phase (Fig. S3). Flumethrin-induced mite fall was high with an average of 59.0 ± 27.1 mites per day at DAP 108 (Fig. S3B). These numbers increased even further during the subsequent assessments until they reached 64.5 ± 35.6 mites per day at DAP 129 (Fig. S3B). After the DAP 136 assessment, numbers decreased only slightly (Fig. S3B). Due to high Varroa infestation, 11 R-site colonies and 9 T-site colonies collapsed until DAP 155. Overall, the numbers of the Varroa mites fallen per day showed a high variability between colonies and study locations but were similar between the different experimental groups (Fig. S3).
On DAP 100 (immediately before the application of Bayvarol® strips) and on DAP 147/148, a second independent method, the icing sugar method (see “Colony endpoint measures”), was used to assess Varroa infestation levels (Fig. 6). At DAP 100, numbers of mites per approx. 500 bees are highly variable between colonies and study locations and ranged between 4 and 61 (Fig. 6a). There was no statistically significant difference depending on the study site (R and T) or the distance to OSR fields on mite load (Table 4). The Varroa mite load at DAP 147/148 was considerably higher at all study locations than at DAP 100 despite the continuous presence of Bayvarol® strips. At this time, the abundance of honey bees in five R-site and five T-site colonies was already too low to perform the icing sugar method. For the remaining colonies, mite load per approx. 500 bees ranged from 19 to 126 mites (Fig. 6b). There was no difference between R site colonies and T site colonies, nor between edge and distant locations.
Fig. 6.
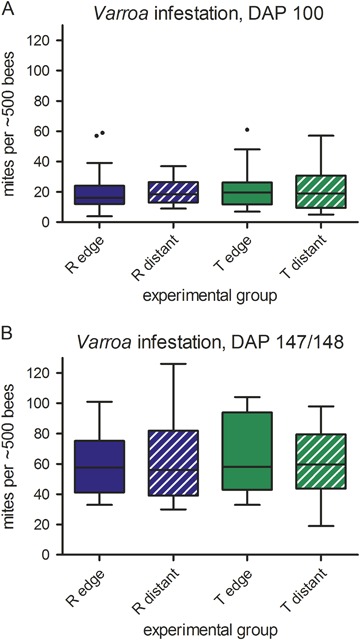
Number of Varroa mites per approx. 500 honey bees at DAP +100 (a) and at DAP +147/148 (b) in colonies belonging to different experimental groups during the exposure phase as determined by the icing sugar method. At DAP +147/148, sample sizes differ due to lost colonies (CA-1, CA-2, CB-3, CB-8, CF-4, TA-6, TC-4, TC-5, TD-6, TD-8), swarming (TB-4), and queen loss (TC-7). The box plots contain the 1st and 3rd quartiles, split by the median; traditional Tukey whiskers go 1.5 times the interquartile distance or to the highest or lowest point, whichever is shorter. Any data beyond these whiskers are shown as points. DAP day after placement
Table 4.
Results of GLMMs fitted to the numbers of Varroa mites per approx. 500 bees as well as of a logistical model applied to the presence/absence data of Nosema, DWV, SBV, and ABPV
| Varroa infestation level as determined by the icing sugar method | Nosema | DWV | SBV | ABPV | |
|---|---|---|---|---|---|
| Intercept | 3.48 ± 0.09*** | −3.90 ± 1.00*** | −3.21 ± 2.59 | −45.06 ± 13,448.37 | −7.71 ± 7.84 |
| DAP | 4.04 ± 3.53 | −8.01 ± 10.86 | 0.02 ± 0.05 | −6.52 ± 3,544.90 | 34.75 ± 42.91 |
| Study site (test compared to reference) | 0.03 ± 0.11 | −1.45 ± 0.95 | 0.12 ± 0.39 | 11.43 ± 9,508.04 | 0.23 ± 0.48 |
| Distance to OSR (distant compared to edge) | −0.02 ± 0.11 | 1.64 ± 1.04 | 0.41 ± 0.39 | −19.56 ± 8,816.63 | −0.44 ± 0.48 |
| Temperature sum | −2.37 ± 2.13 | 10.62 ± 8.03 | −0.19 ± 102.63 | −14.00 ± 24.92 | |
| Humidity sum | −1.61 ± 1.52 | 0.10 ± 38.56 | −12.20 ± 18.53 | ||
| Rain sum | 0.47 ± 0.26 | −1.91 ± 5.74 | 0.69 ± 1.99 | 0.50 ± 297.64 | −2.00 ± 2.16 |
| Wind sum | 0.22 ± 0.19 | −0.95 ± 3.08 | −0.40 ± 1.07 | 0.56 ± 314.03 | −1.85 ± 1.38 |
Effect size ± SD is given for each parameter. Positive values indicate an increase in relation to the respective reference value whereas negative values indicate a decrease. All other parameters are as explained in the legend of Table 1. ***p < 0.001
For naturally occurring (DAP 11–92) and flumethrin-induced (DAP 108–150) mite fall, %MDD ranged from 73.3 % to 690 % and from 67.9 % to 69.7 %, respectively (scattering due to DAP and distance to the nearest OSR field, Table 5). All %MDD greater than 100 % occurred on DAP ≤63 when the predicted number of mites fallen per day in both the R and T sites were very close to zero. For the Varroa infestation level as determined by the icing sugar method, the %MDD ranged from 23.4 % to 24.5 % (mean 24.0 %, Table 5) depending on DAP (100 or 147/148) and distance to the nearest OSR field. Thus, due to the experimental design of the monitoring study, small differences could have been identified as statistically significant.
Infestation with Nosema was relatively low. Nosema spores were detected in 12 of the 96 colonies in which 7 of the infested colonies showed only the lowest level of infestation (+, “few spores found”; see Topolska and Hartwig 2005). All colonies with an infestation with Nosema were tested positive only once, except colony RB-2, which was tested positive twice at DAP 7 and DAP 26. Medium (++) and high (+++) levels of infestation occurred usually at study locations where additional colonies were infested. There were slightly more colonies from the R site with Nosema infection. However, this difference between R and T sites was not statistically significant (Table 4). Likewise, there was no significant effect of the distance to the nearest OSR field (Table 4). The test power for Nosema infestation indicated by MDD is relatively low (Table 5); however, the infestation with Nosema in T site colonies was in fact slightly lower than in R site colonies. Thus, no effect of clothianidin seed dressing on Nosema infestation level could be identified.
The DWV was detected in honey bees from 7 out of 96 colonies (7.3 %) during the first sampling (“start of exposure”, Fig. 7b1) and from 3 out of 96 colonies (3.1 %) during the second sampling (“end of exposure”, Fig. 7b2). At the end of the post-exposure phase, bees from 39 out of 75 colonies (52.0 %) were positive for DWV (Fig. 7b3) and typical symptoms could be observed. The difference between R and T site colonies is marginal and not statistically significant (Table 4). The SBV did not occur in any of the colonies during the 1st and 3rd sampling, but in 3 out of 96 colonies (3.1 %) during the 2nd sampling at the end of the exposure phase (Fig. 7c). Despite the positive result in these three colonies, no symptoms of SBV infection at the colony level were observed. Due to the very limited numbers of SBV-infected colonies, a comprehensive statistical analysis was not possible which is indicated by the high standard errors of the parameter estimates (Table 4). At the “end of the post-exposure phase”, ABPV occurred in 34 out of 75 colonies (45.3 %; Fig. 7d3). There were no infections with CBPV or KBV detected in any of the experimental colonies. The %MDDs for the virus infections were 43.7–71.7 % for DWV, 0–100.0 % for SBV, and 52.3–100.0 % for ABPV (Table 5).
Fig. 7.
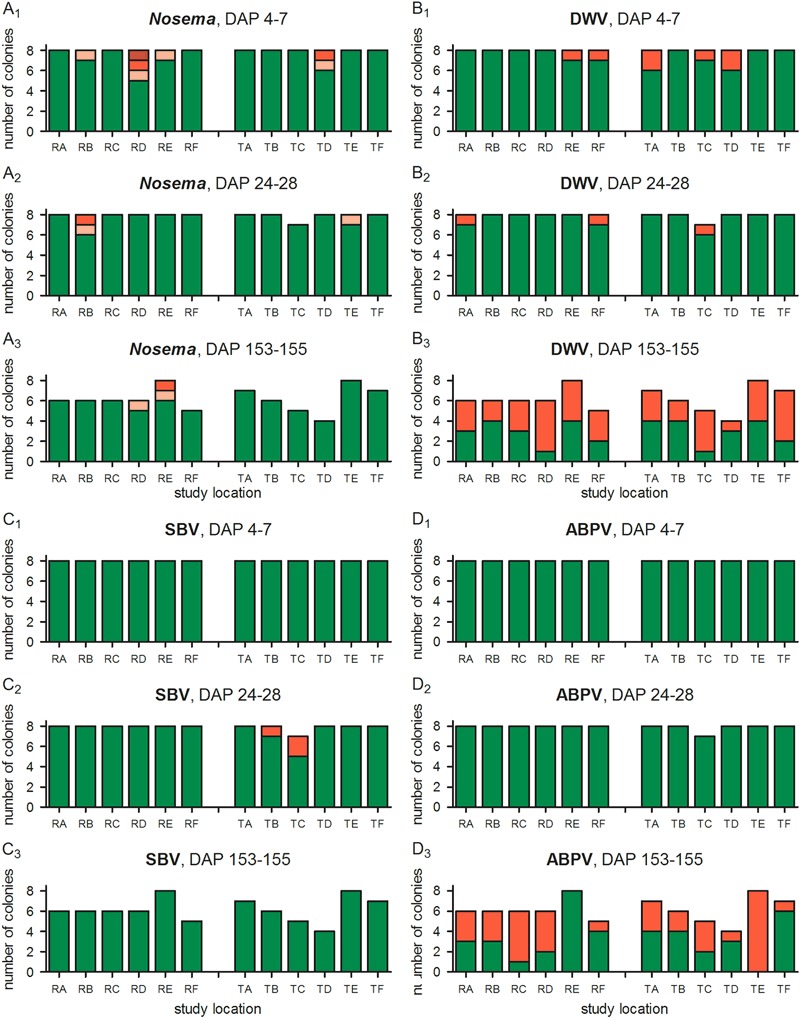
Occurrence of DWV, SBV, ABPV, and Nosema spp. in honey bee colonies at three different time points. For viruses, red bars indicate infection, green markings no infection. For Nosema, red bars indicate infection (light red: single or few spores not found in each field of vision; red: single or few spores found in each field of vision; dark red: numerous spores found in each field of vision), green markings indicate that no spores were found. Sample size was reduced during the second examination due to swarming of colony TB-4 and during the last examination further due to loss of colonies, these are left blank
Discussion
General
In the current study we attempted to use a realistic, worst-case scenario for honey bee exposure to clothianidin seed-treated OSR. During the bloom period (exposure phase), honey bee colonies were placed directly adjacent to OSR fields or 400 m away from the nearest OSR field, and then they were moved to a fall apiary for post-exposure monitoring. The honey bee colonies grew very well and showed a typical development during the exposure phase. During the post-exposure phase, colonies developed characteristically in respect to seasonal changes and the extensive hive locations. The choice of Varroa treatment by flumethrin turned out to be inefficient because of resistance, but this was not linked to exposure of clothianidin seed-treated OSR during blossom. Since no additional steps were taken to control Varroa and to avoid colony losses, both R-site and T-site colonies suffered from heavy infestation in the second half of September 2014. Thus, possible effects of clothianidin seed-treated OSR on weakened honey bee colonies could be evaluated.
Development of honey bee colonies during monitoring
It is widely accepted that foraging honey bees do not die immediately after visiting flowers in clothianidin-treated crops because residue levels in nectar and pollen are well below the acute oral LD50 (for recent reviews, see Godfray et al. 2014; Pisa et al. 2015). However, prolonged exposure of honey bees to sublethal doses of neonicotinoids is suspected to potentially lead to colony failure (Henry et al. 2012). In the majority of cases, sublethal effects were demonstrated in laboratory experiments and semi-field trials and are based on relatively high neonicotinoid exposure levels, e.g., in terms of the concentrations fed to honey bees, duration of exposure, and lack of choice (for a review, see Carreck and Ratnieks 2014). Within the context of this monitoring study, we could demonstrate, at least for clothianidin seed-treated winter OSR, that neonicotinoid concentrations in nectar and pollen are clearly lower under realistic outdoor conditions. Honey bees are exposed to low residue concentrations not only when freely foraging in the landscape but also when foraging under confined conditions in a tent (Rolke et al. 2016, this issue). This is consistent with earlier findings on clothianidin residues in OSR (Cutler and Scott-Dupree 2007; Pohorecka et al. 2012; Pilling et al. 2013; Cutler et al. 2014).
During this monitoring study, the number of dead bees, colony strength, brood development, and food storage levels were, on average, similar between R and T colonies. By monitoring the weight of colonies, it was evident, too, that there were no substantial losses of foraging bees exposed to clothianidin seed-treated OSR. So far, only very few studies have been conducted, in which the performance of honey bee colonies placed adjacent to fields treated or not treated with neonicotinoids are compared. In none of these studies, negative effects on honey bee colonies could be demonstrated. For example, in the 4-year study conducted by Pilling et al. (2013), honey bee colonies were placed beside thiamethoxam-treated or reference fields of maize (three replicates) or OSR (two replicates) for 5–8 days (first 3 years) or 19–23 days (4th year) to coincide with the crop flowering period. Pollen and nectar samples from test hives showed slightly higher concentrations of thiamethoxam residues compared to reference hives, but no differences in multiple measures of colony performance or overwintering survival were observed (Pilling et al. 2013). Two studies performed in Canada concluded that the potential harm from exposure to clothianidin in seed-treated OSR was negligible for honey bees (Cutler and Scott-Dupree 2007; Cutler et al. 2014). Comparable results were also obtained in a recent study by Rundlöf et al. (2015) who analyzed eight spring OSR fields sown with clothianidin-dressed OSR seeds and eight fields sown with untreated seeds across southern Sweden. The authors found no significant difference in honey bee colony growth between treated and reference fields (Rundlöf et al. 2015). Table S7 provides a comparison of experimental set-up details and results of relevant field studies mentioned here.
Attractiveness of OSR to honey bees
We found a significantly higher proportion of OSR pollen in spring honey samples from T site colonies compared to samples from R site colonies (Fig. 5a; Table 3). Thus, the results of our monitoring study may at first glance support the hypothesis that honey bees prefer nectar that contains low amounts of neonicotinoids. Evidence for such a preference was recently provided by Kessler et al. (2015) who found in choice experiments in the laboratory that bees were more likely to choose nectar containing imidacloprid or thiamethoxam (but not clothianidin). We believe, however, that the differences in pollen composition observed in the current monitoring study at the landscape level are more likely due to slight differences in the availability of alternative bee plants between the two study sites. In any case, we found no indications for the alternative hypothesis that honey bees are able to recognize and learn to avoid neonicotinoid-treated plants (for reviews, see Godfray et al. 2014; Pisa et al. 2015). Such an avoidance has recently been shown for pollinating flies and beetles (Easton and Goulson 2013).
In addition, both pollen sampling events confirmed the attractiveness of OSR pollen as a protein source for honey bees although the relative amount of OSR pollen in the bee pollen loads varied considerably depending on the local availability of alternative forage in the neighborhood of the study locations. Some plants were clearly highly attractive to honey bees, especially maple at certain study locations during the first sampling. This is not unexpected as honey bees have complex dietary requirements and are known to utilize a wide variety of pollen (and nectar) sources (e.g., Brodschneider and Crailsheim 2010; Garbuzov et al. 2015). We found no indications for preference or avoidance of OSR pollen from T site fields. This finding is in line with other field studies performed by Cutler et al. (2014) and Rundlöf et al. (2015), where the pollen extracted from pollen traps contained on average 88 and 57.8 % OSR pollen, respectively, with similar proportions for both reference and test fields. A lower percentage of 14 % OSR pollen pellets was reported by Garbuzov et al. (2015), who placed hives in a rural location near Brighton (UK) in 2012. In the study by Garbuzov et al. (2015), OSR was less than 4 % of the area within 6 km of the rural apiary, whereas OSR comprised 27 % of the arable land in the current study. In addition, hives were placed at the edge of OSR fields or 400 m distant from OSR field in the current study, whereas the closest OSR field was situated 700 m from the hives in the study performed by Garbuzov et al. (2015).
Residue analysis showed that clothianidin was absent in pollen and nectar samples from the R site (Rolke et al. 2016, this issue). This finding confirms that honey bees did not forage off-site and, thus, argue in favor of the study design. This is not trivial as, for example, Cutler et al. (2014), who detected clothianidin in pollen samples from reference hives, point to “the difficulty of conducting a perfectly controlled field study with free-ranging honey bees in real-world agroecosystems”. They argue further that this is especially true when conducting experiments with neonicotinoids, which are now widely used on a large number of crops and commodities (Cutler et al. 2014). Average concentrations of clothianidin was 0.50–0.97 μg/kg and 0.68–0.77 μg/kg, in pollen and nectar samples from T site colonies, respectively (Rolke et al. 2016, this issue). This indicates not only that T site colonies were indeed exposed to clothianidin but also that the exposure level is low under real agricultural field conditions.
Parasites and diseases
Many researchers assume the parasitic mite Varroa destructor to be the greatest threat to honey bees (for a review, see Rosenkranz et al. 2010). The mite acts as a disease vector and spreads RNA viruses such as DWV to the honey bees (for reviews, see Genersch and Aubert 2010; Le Conte et al. 2010). In our monitoring study, naturally occurring mite fall was initially low but increased exponentially during the second half of the post-exposure phase (Fig. S3A). Treatment with Bayvarol® strips did not show the expected effect, because of flumethrin resistance in the mite population as subsequently revealed by various tests (flumethrin contact test; test for altered voltage-gated sodium channel according to González-Cabrera et al. 2013). Numbers of Varroa mites in the hives therefore increased further (Fig. S3B and Fig. 6b). This very severe infestation with Varroa mites together with the concomitant incidence of Varroa-associated viruses (see below) considerably damaged the honey bee colonies which resulted in the loss of 11 R site colonies and 10 T site colonies by DAP 155. In order to evaluate potential differences in infestation levels between R and T site colonies, no additional treatment was applied to control Varroa infestation and to avoid colony losses. Despite the extreme conditions that the colonies were confronted with, no treatment group showed any advantage over the others which indicates that neither the exposure to clothianidin seed-treated OSR nor the distance to OSR fields had any effect on parasitization rate. This is in contrast to the results of a recent study on the effects of thiamethoxam-coated corn seeds on honey bee health (Alburaki et al. 2015), which is, at least to our knowledge, so far the only study investigating the effects of neonicotinoids on Varroa infestation levels. The results of Alburaki et al. (2015) showed significantly higher levels of Varroa infestation in colonies located in treated cornfields compared to those of reference fields.
In our monitoring study at the landscape level, the majority of samples taken during the exposure phase as well as at the end of the post-exposure phase were free of Nosema spores. We found no differences between the R and T site colonies and, thus, could not establish an effect of clothianidin seed-dressing on Nosema infection level. In contrast, various laboratory studies have shown that sublethal doses of neonicotinoids can lead to an increase in parasitic microsporidia (Nosema spp.) which may cause the death of bees (imidacloprid: Alaux et al. 2010; Pettis et al. 2012; thiacloprid: Vidau et al. 2011; Doublet et al. 2015). In these laboratory studies, test bees were heavily (experimentally) infected with Nosema spores (105–3.3 × 105 spores/bee) followed by an artificial maintaining of bees in small cages. In the current study, only naturally occurring infections were monitored and infected bees could potentially perform self-medication behavior (Gherman et al. 2014) or outside activities to limit contact in the hive with nest mates (Alaux et al. 2014). In this respect, a detail of the study of Pettis et al. (2012) is worth mentioning. Here, honey bee colonies were exposed to sub-lethal doses of imidacloprid. Subsequently, newly emerged bees from these colonies were challenged with Nosema spores and caged. Interestingly, individual caged bees showed a marked increase in Nosema spore production in the laboratory but the parent colonies in the field failed to show increased Nosema levels over time (Pettis et al. 2012).
Di Prisco et al. (2013) found that sublethal doses (21 ng/bee) of clothianidin can cause honey bee immune deficiency. In their study, the reduced immune defense was proven by an enhanced replication of DWV in honey bees bearing covert infections (Di Prisco et al. 2013). Interestingly, Di Prisco et al. (2013) also point out that “field studies are necessary to carefully evaluate their real impact under different environmental conditions”. In our monitoring study, infection of honey bees by viruses was very low during the exposure phase (Figs. 7b–d). At the end of the post-exposure phase, bees from many colonies of both the former R and T site were infected with DWV and ABPV. Since these viruses use Varroa mites as vectors (de Miranda et al. 2010; de Miranda and Genersch 2010), the high infection rate must be seen as a consequence of the high mite infestation of the colonies (see above). Thus, we found no evidence for clothianidin-induced immune deficiency under field conditions.
We conclude that Varroa mites plus viruses are the most “probable cause” of colony failure in the course of the current study. Neonicotinoid PPPs, in contrast, are “unlikely” as a factor of reduced survival. Thus, the outcome of the current study nicely mirrors the outcome of a causal analysis of observed declines in managed honey bees (Staveley et al. 2014).
Conclusion
Monitoring studies at the landscape level of this magnitude are complex and difficult to conduct and to interpret. Finding suitable study sites with sufficient distance between the reference and test areas, which are also separated from other potentially confounding PPP-treated alternative foraging sites, is very challenging. Furthermore, conducting such studies with sufficient true statistical replication is also difficult—or impossible. In our monitoring study, locations within each site are closer to each other than to any of the locations of the other site and, therefore, can be considered as “pseudoreplicates” (Hurlbert 1984). However, conditions at the project area were comparable for the R and T sites and differences in meteorological conditions were marginal (Heimbach et al. 2016, this issue). No alternative agricultural crops suitable for honey bees were cultivated in the study area during OSR flowering. Analyses of pollen, nectar and honey confirmed that OSR was the major food source of the bees during the exposure phase. Exposure of honey bees to clothianidin at the T site was verified by analysis of residues in nectar and pollen (Rolke et al. 2016, this issue). Colony development was very consistent. Harvested amounts of spring honey confirmed good conditions for the bees despite the numerous disturbances of the colonies by the beekeeper in the course of the study. Colony loss in the course of a medium or long term study due to “background disease” (varroosis in the case of the current study), queen loss, or swarming may be a potential drawback of such large-scale monitoring studies. A risk assessment based on laboratory data only, however, will not provide reliable information on the development and health status of honey bees following actual use of the PPP under real agricultural field conditions. This requires monitoring studies at the landscape level (Liess et al. 2005; Rundlöf et al. 2015). Indeed, no detrimental effects of clothianidin seed-treated OSR could be observed under the realistic exposure conditions of this study although various laboratory and semi-field studies (for recent reviews, see Belzunces et al. 2012; Godfray et al. 2014; Pisa et al. 2015) have reported sublethal effects in honey bees exposed to neonicotinoid insecticides.
Electronic supplementary material
Acknowledgments
Funding of all expenses for this study was through Bayer CropScience AG (Monheim, Germany). We thank the farmers and bee keepers from the Sternberg area for their cooperation and for many helpful discussions on the study design during multiple information events in Sternberg (Germany). Dr. Fred Heimbach (tier3 solutions GmbH, Leverkusen, Germany) acted as project coordinator of the entire monitoring project and Markus Persigehl (tier3 solutions GmbH, Leverkusen, Germany) as GLP study director for the honey bee monitoring study. Honey bee colonies were provided by the Imkerei Ullmann (Erlensee, Germany). We thank the bee keeper of the project, Sebastian Wiegand (Institut für Bienenkunde Oberursel, Germany), and many field assistants (tier3 solutions GmbH, Leverkusen, Germany) for their technical assistance in field and lab. Nosema diagnosis was performed by Beate Springer (Institut für Bienenkunde Oberursel, Germany). Prof. Dr. Hans-Toni Ratte (ToxRat Solutions GmbH, Alsdorf, Germany) kindly supervised the statistical power evaluation of this study. Finally, we thank the two anonymous reviewers for their careful reading of our manuscript and their many helpful comments and suggestions.
Funding
Funding of all expenses for this study was through Bayer CropScience.
Disclaimer
Although the study design was discussed with the sponsor, they had no role in the implementation, data collection, and interpretation of results.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Current address: Zoological Institute, Cologne Biocenter, University of Cologne, Cologne, Germany.
References
- Aizen MA, Harder LD. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr Biol. 2009;19:915–918. doi: 10.1016/j.cub.2009.03.071. [DOI] [PubMed] [Google Scholar]
- Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP, Le Conte Y. Interactions between Nosema microspores and a neonicotinoid weaken honey bees (Apis mellifera) Environ Microbiol. 2010;12:774–782. doi: 10.1111/j.1462-2920.2009.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux C, Crauser D, Pioz M, Saulnier C, Le Conte Y. Parasitic and immune modulation of flight activity in honey bees tracked with optical counters. J Exp Biol. 2014;217:3416–34124. doi: 10.1242/jeb.105783. [DOI] [PubMed] [Google Scholar]
- Alburaki M, Boutin S, Mercier PL, Loublier Y, Chagnon M, Derome N. Neonicotinoid-coated Zea mays seeds indirectly affect honey bee performance and pathogen susceptibility in field trials. PLoS ONE. 2015;10:e0125790. doi: 10.1371/journal.pone.0125790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi T, Farkas R, Szendroi A, Dobos-Kovacs M, Rusvai M. Detection of acute bee paralysis virus by RT-PCR in honey bee and Varroa destructor field samples: rapid screening of representative Hungarian apiaries. Apidologie. 2002;33:63–74. doi: 10.1051/apido:2001004. [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software arXiv. 2014;1406:5823. [Google Scholar]
- Belzunces LP, Tchamitchian S, Brunet JL. Neural effects of insecticides in the honey bee. Apidologie. 2012;43:348–370. doi: 10.1007/s13592-012-0134-0. [DOI] [Google Scholar]
- Benjeddou M, Leat N, Allsopp M, Davison S. Detection of acute bee paralysis virus and black queen cell virus from honey bees by reverse transcriptase pcr. Appl Environ Microbiol. 2001;67:2384–2387. doi: 10.1128/AEM.67.5.2384-2387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TC, Hammers-Wirtz M, Hommen U, Preuss TG, Ratte HT, Roessink I, Strauss T, Van den Brink PJ. The minimum detectable difference (MDD) and the interpretation of treatment-related effects of pesticides in experimental ecosystems. Environ Sci Pollut Res Int. 2015;22:1160–1174. doi: 10.1007/s11356-014-3398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodschneider R, Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010;41:278–294. doi: 10.1051/apido/2010012. [DOI] [Google Scholar]
- Carreck NL, Ratnieks FLW. The dose makes the poison: have “field realistic” rates of exposure of bees to neonicotinoid insecticides been overestimated in laboratory studies? J Apicult Res. 2014;53:607–614. doi: 10.3896/IBRA.1.53.5.08. [DOI] [Google Scholar]
- Cresswell JE, Thompson HM. Comment on “a common pesticide decreases foraging success and survival in honey bees”. Science. 2012;337:1453. doi: 10.1126/science.1224618. [DOI] [PubMed] [Google Scholar]
- Cutler GC, Scott-Dupree CD. Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. J Econ Entomol. 2007;100:765–772. doi: 10.1603/0022-0493(2007)100[765:ETCSCH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cutler CG, Scott-Dupree CD. A field study examining the effects of exposure to neonicotinoid seed-treated corn on commercial bumble bee colonies. Ecotoxicology. 2014;23:1755–1763. doi: 10.1007/s10646-014-1340-5. [DOI] [PubMed] [Google Scholar]
- Cutler GC, Scott-Dupree CD, Sultan M, McFarlane AD, Brewer L. A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ. 2014;2:e652. doi: 10.7717/peerj.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda JR, Cordoni G, Budge G. The acute bee paralysis virus-Kashmir bee virus-israeli acute paralysis virus complex. J Invertebr Pathol. 2010;103:S30–S47. doi: 10.1016/j.jip.2009.06.014. [DOI] [PubMed] [Google Scholar]
- de Miranda JR, Genersch E. Deformed wing virus. J Invertebr Pathol. 2010;103:S48–S61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Dietemann V, Nazzi F, Martin SJ, Anderson D, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B,Rosenkranz P, Ellis JD (2013) Standard methods for varroa research. In: Dietemann V, Ellis JD, Neumann P (eds). The COLOSS BEEBOOK, Vol. II: Standard methods for Apis mellifera pest and pathogen research. J Apicult Res 52(1): doi:10.3896/IBRA.1.52.1.09
- Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci U S A. 2013;110:18466–18471. doi: 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublet V, Labarussias M, de Miranda JR, Moritz RF, Paxton RJ. Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol. 2015;17:969–983. doi: 10.1111/1462-2920.12426. [DOI] [PubMed] [Google Scholar]
- Easton AH, Goulson D. The neonicotinoid insecticide imidacloprid repels pollinating flies and beetles at field-realistic concentrations. PLoS ONE. 2013;8:e54819. doi: 10.1371/journal.pone.0054819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert A, Haas M, Springer B, Thielert W, Nauen R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci. 2008;64:1099–1105. doi: 10.1002/ps.1616. [DOI] [PubMed] [Google Scholar]
- European Commission Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxamand imidacloprid, and prohibiting the use and sale of seeds treatedwith plant protection products containing those active substances. OJ L. 2013;139:12–26. [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Software. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzov M, Couvillon MJ, Schürch R, Ratnieks FLW. Honey bee dance decoding and pollen-load analysis show limited foraging on spring-flowering oilseed rape, a potential source of neonicotinoid contamination. Agric Ecosyst Environ. 2015;203:62–68. doi: 10.1016/j.agee.2014.12.009. [DOI] [Google Scholar]
- Genersch E. Development of a rapid and sensitive RT-PCR method for the detection of deformed wing virus, a pathogen of the honey bee (Apis mellifera) Vet J. 2005;169:121–123. doi: 10.1016/j.tvjl.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Genersch E, Aubert M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.) Vet Res. 2010;41:54. doi: 10.1051/vetres/2010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherman BI, Denner A, Bobiş O, Dezmirean DS, Marghitas LA, Schlüns H, Moritz RFA, Erler S. Pathogen-associated self-medication behavior in the honey bee Apis mellifera. Behav Ecol Sociobiol. 2014;68:1777–1784. doi: 10.1007/s00265-014-1786-8. [DOI] [Google Scholar]
- Godfray HC, Blacquière T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc Biol Sci. 2014;281:20140558. doi: 10.1098/rspb.2014.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- González-Cabrera J, Davies TG, Field LM, Kennedy PJ, Williamson MS. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS ONE. 2013;8:e82941. doi: 10.1371/journal.pone.0082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez D. Henry et al. (2012) homing failure formula, assumptions, and basic mathematics: a comment. Front Physiol. 2012;4:142. doi: 10.3389/fphys.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. New York: Springer; 2009. [Google Scholar]
- Henry M, Béguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Heimbach F, Russ A, Schimmer M, Born K (2016) Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: implementation of the monitoring project and its representativeness. Ecotoxicology. [DOI] [PMC free article] [PubMed]
- Hurlbert SH. Pseudoreplication and the design of ecological field experiments. Ecol Monogr. 1984;54:187–211. doi: 10.2307/1942661. [DOI] [Google Scholar]
- Imdorf A, Buehlmann G, Gerig L, Kilchenmann V, Wille H. Überprüfung der Schätzmethode zur Ermittlung der Brutfläche und der Anzahl Arbeiterinnen in freifliegenden Bienenvölkern. Apidologie. 1987;18:137–146. doi: 10.1051/apido:19870204. [DOI] [Google Scholar]
- Jeschke P, Nauen R, Beck ME. Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew Chem Int Ed Engl. 2013;52:9464–9485. doi: 10.1002/anie.201302550. [DOI] [PubMed] [Google Scholar]
- Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Stout JC, Wright GA. Bees prefer foods containing neonicotinoid pesticides. Nature. 2015;521:74–76. doi: 10.1038/nature14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Conte Y, Ellis M, Ritter W. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 2010;41:353–363. doi: 10.1051/apido/2010017. [DOI] [Google Scholar]
- Liess M, Brown C, Dohmen P, Duquesne S, Hart A, Heimbach F, Kreuger J, Lagadic L, Maund S, Reinert W, Streloke M, Tarazona JV. Effects of pesticides in the field. Brussels, Belgium: Society of Environmental Toxicology and Chemistry (SETAC); 2005. [Google Scholar]
- Maori E, Tanne E, Sela I. Reciprocal sequence exchange between non-retro viruses and hosts leading to the appearance of new host phenotypes. Virology. 2007;362:342–349. doi: 10.1016/j.virol.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Neumann P, Carreck NL. Honey bee colony losses. J Apicult Res. 2010;49:1–6. doi: 10.3896/IBRA.1.49.1.01. [DOI] [Google Scholar]
- Peters B, Gao Z, Zumkier U (2016) Large-scale monitoring of effects of clothianidin-dressed OSR seeds on pollinating insects in Northern Germany: effects on red mason bees (Osmia bicornis). Ecotoxicology [DOI] [PMC free article] [PubMed]
- Pettis JS, vanEngelsdorp D, Johnson J, Dively G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften. 2012;99:153–158. doi: 10.1007/s00114-011-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling E, Campbell P, Coulson M, Ruddle N, Tornier I. A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS ONE. 2013;8:e77193. doi: 10.1371/journal.pone.0077193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: linear and nonlinear mixed effects model R package version 3.1-121, http://CRAN.R-project.org/package=nlme. Accessed 15 Aug 2015
- Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck H, Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res Int. 2015;22:68–102. doi: 10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecka K, Skubida P, Miszczak A, Semkiw P, Sikorski P, Zagibajlo K, Teper D, Koltowski Z, Skubida M, Zdanska D, Bober A. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J Apicult Sci. 2012;56:115–134. [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing; 2011. [Google Scholar]
- Rolke D, Persigehl M, Peters B, Sterk G, Blenau W (2016) Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: residues of clothianidin in pollen, nectar and honey. Ecotoxicology [DOI] [PMC free article] [PubMed]
- Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J Invertebr Pathol. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Rundlöf M, Andersson GK, Bommarco R, Fries I, Hederström V, Herbertsson L, Jonsson O, Klatt BK, Pedersen TR, Yourstone J, Smith HG. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature. 2015;521:77–80. doi: 10.1038/nature14420. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F. Environmental science. The trouble with neonicotinoids. Science. 2014;346:806–807. doi: 10.1126/science.1259159. [DOI] [PubMed] [Google Scholar]
- Schmuck R, Lewis G (2016) Review of field and monitoring studies investigating the role of nitro-substituted neonicotinoid insecticides in the reported losses of honey bee colonies (Apis mellifera). Ecotoxicology [DOI] [PMC free article] [PubMed]
- Smith KM, Loh EH, Rostal MK, Zambrana-Torrelio CM, Mendiola L, Daszak P. Pathogens, pests, and economics: drivers of honey bee colony declines and losses. Ecohealth. 2013;10:434–445. doi: 10.1007/s10393-013-0870-2. [DOI] [PubMed] [Google Scholar]
- Staveley JP, Law SA, Fairbrother A, Menzie CA. A causal analysis of observed declines in managed honey bees (Apis mellifera) Hum Ecol Risk Assess. 2014;20:566–591. doi: 10.1080/10807039.2013.831263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk G, Peters B, Gao Z, Zumkier U (2016) Large-scale monitoring of effects of clothianidin-dressed OSR seeds on pollinating insects in Northern Germany: effects on large earth bumblebees (Bombus terrestris). Ecotoxicology [DOI] [PMC free article] [PubMed]
- Stoltz DB, Shen XR, Boggis C, Sisson G. Molecular diagnosis of Kashmir bee virus infection. J Apicult Res. 1995;34:153–160. doi: 10.1080/00218839.1995.11100900. [DOI] [Google Scholar]
- Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- Topolska G, Hartwig A. Diagnosis of Nosema apis infection by investigations of two kinds of samples: dead bees and live bees. J Apicult Res. 2005;49:75–79. [Google Scholar]
- vanEngelsdorp D, Hayes J, Jr, Underwood RM, Pettis J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE. 2008;3:e4071. doi: 10.1371/journal.pone.0004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidau C, Diogon M, Aufauvre J, Fontbonne R, Viguès B, Brunet JL, Texier C, Biron DG, Blot N, El Alaoui H, Belzunces LP, Delbac F. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honey bees previously infected by Nosema ceranae. PLoS ONE. 2011;6:e21550. doi: 10.1371/journal.pone.0021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ohe W, Persano-Oddo L, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004;35:S18–S25. doi: 10.1051/apido:2004050. [DOI] [Google Scholar]
- Yue C, Schröder M, Bienefeld K, Genersch E. Detection of viral sequences in semen of honey bees (Apis mellifera): evidence for vertical transmission of viruses through drones. J Invertebr Pathol. 2006;92:105–108. doi: 10.1016/j.jip.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Savaliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York, pp 574
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


