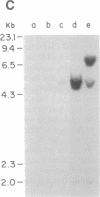
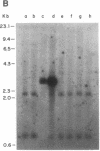
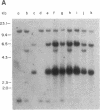
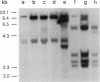
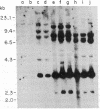
Abstract
Fourteen primary human lung tumor DNAs from smokers were analyzed for transforming activity by two DNA transfection assays. Activated protooncogenes were detected in 3 of 11 tumor DNAs by the NIH 3T3 focus assay, whereas activated protooncogenes were detected in 11 of 13 tumor DNAs by the NIH 3T3 cotransfection-nude mouse tumorigenicity assay. K- or NRAS genes activated by point mutation at codons 12 or 61 were detected in a large cell carcinoma, a squamous cell carcinoma, and 5 adenocarcinomas. An HRAS oncogene activated by a different mechanism was detected in an epidermoid carcinoma. One adenocarcinoma was found to contain an activated RAF gene. Two unidentified transforming genes were detected in a squamous cell carcinoma DNA and two adenocarcinoma DNAs. Eight of 10 lung adenocarcinomas that had formed metastases at the time of surgery were found to contain RAS oncogenes. No significant increase in metastasis was observed in the lung adenocarcinomas that contained one or more 6-kilobase EcoRI alleles of the LMYC gene. Overall, 12 of 14 (86%) of the lung tumor DNAs from smokers were found to contain activated protooncogenes. RAS oncogenes appear to play a role in the development of metastases in lung adenocarcinomas.
Full text
PDF
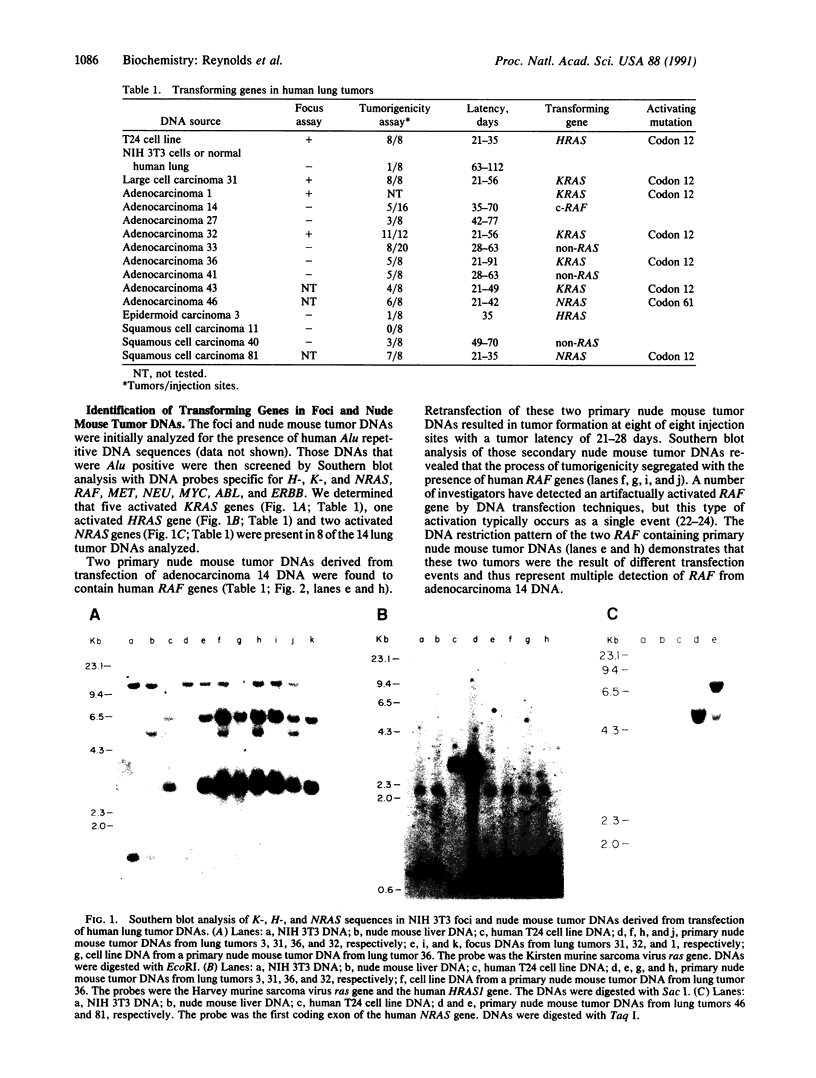


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Pirollo K. F., Zou Z. Q., Cheung H. Y., Lawler E. L., Garner R., White E., Bernstein W. B., Fraumeni J. W., Jr, Blattner W. A. Oncogenes in radioresistant, noncancerous skin fibroblasts from a cancer-prone family. Science. 1987 Aug 28;237(4818):1036–1039. doi: 10.1126/science.3616624. [DOI] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Broz S. D., Levinson A. D. Expression of the H-ras proto-oncogene is controlled by alternative splicing. Cell. 1989 Aug 11;58(3):461–472. doi: 10.1016/0092-8674(89)90427-3. [DOI] [PubMed] [Google Scholar]
- DOLL R., HILL A. B. A study of the aetiology of carcinoma of the lung. Br Med J. 1952 Dec 13;2(4797):1271–1286. doi: 10.1136/bmj.2.4797.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., Wright J. A., Jarolim L., Yanagihara K., Bassin R. H., Greenberg A. H. Transformation by oncogenes encoding protein kinases induces the metastatic phenotype. Science. 1987 Oct 9;238(4824):202–205. doi: 10.1126/science.3659911. [DOI] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding J. E. Smoking: health effects and control (1). N Engl J Med. 1985 Aug 22;313(8):491–498. doi: 10.1056/NEJM198508223130807. [DOI] [PubMed] [Google Scholar]
- Fukui M., Yamamoto T., Kawai S., Mitsunobu F., Toyoshima K. Molecular cloning and characterization of an activated human c-raf-1 gene. Mol Cell Biol. 1987 May;7(5):1776–1781. doi: 10.1128/mcb.7.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Activation of a c-K-ras oncogene by somatic mutation in mouse lymphomas induced by gamma radiation. Science. 1984 Sep 14;225(4667):1159–1162. doi: 10.1126/science.6474169. [DOI] [PubMed] [Google Scholar]
- Honkawa H., Masahashi W., Hashimoto S., Hashimoto-Gotoh T. Identification of the principal promoter sequence of the c-H-ras transforming oncogene: deletion analysis of the 5'-flanking region by focus formation assay. Mol Cell Biol. 1987 Aug;7(8):2933–2940. doi: 10.1128/mcb.7.8.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Takaku F., Nagao M., Sugimura T. Rat c-raf oncogene activation by a rearrangement that produces a fused protein. Mol Cell Biol. 1987 Mar;7(3):1226–1232. doi: 10.1128/mcb.7.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid U., Pfeifer A., Weichselbaum R. R., Dritschilo A., Mark G. E. The raf oncogene is associated with a radiation-resistant human laryngeal cancer. Science. 1987 Aug 28;237(4818):1039–1041. doi: 10.1126/science.3616625. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Shikama H., Imoto K., Izawa M., Naruke T., Okabayashi K., Nishimura S. Close correlation between restriction fragment length polymorphism of the L-MYC gene and metastasis of human lung cancer to the lymph nodes and other organs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2353–2356. doi: 10.1073/pnas.85.7.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R., Gallick G. E., Gutterman J. U. Differential expression of p21ras gene products among histological subtypes of fresh primary human lung tumors. Cancer Res. 1986 Mar;46(3):1530–1534. [PubMed] [Google Scholar]
- Liotta L. A. H-ras p21 and the metastatic phenotype. J Natl Cancer Inst. 1988 Jun 1;80(7):468–469. doi: 10.1093/jnci/80.7.468. [DOI] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W., Matheson D. W. GC-rich regions in genomes as targets for DNA alkylation. Carcinogenesis. 1988 Nov;9(11):2065–2072. doi: 10.1093/carcin/9.11.2065. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Martin C. N., Garner R. C., King M. M. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988 Dec 22;336(6201):790–792. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- Randerath E., Miller R. H., Mittal D., Avitts T. A., Dunsford H. A., Randerath K. Covalent DNA damage in tissues of cigarette smokers as determined by 32P-postlabeling assay. J Natl Cancer Inst. 1989 Mar 1;81(5):341–347. doi: 10.1093/jnci/81.5.341. [DOI] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Maronpot R. R., Anderson M. W., Aaronson S. A. Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):33–37. doi: 10.1073/pnas.83.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenhuis S., Slebos R. J., Boot A. J., Evers S. G., Mooi W. J., Wagenaar S. S., van Bodegom P. C., Bos J. L. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988 Oct 15;48(20):5738–5741. [PubMed] [Google Scholar]
- Rodenhuis S., van de Wetering M. L., Mooi W. J., Evers S. G., van Zandwijk N., Bos J. L. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987 Oct 8;317(15):929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Yuasa Y., Reynolds S. H., Aaronson S. A. Effects of two major activating lesions on the structure and conformation of human ras oncogene products. Proc Natl Acad Sci U S A. 1985 Jan;82(1):38–42. doi: 10.1073/pnas.82.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Cooper G. M. Activation of human raf transforming genes by deletion of normal amino-terminal coding sequences. Mol Cell Biol. 1987 Mar;7(3):1171–1179. doi: 10.1128/mcb.7.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman R. L. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968 Apr;68(2):153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990 Jul;5(7):1037–1043. [PubMed] [Google Scholar]
- Tefre T., Børresen A. L., Aamdal S., Brøgger A. Studies of the L-myc DNA polymorphism and relation to metastasis in Norwegian lung cancer patients. Br J Cancer. 1990 Jun;61(6):809–812. doi: 10.1038/bjc.1990.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr Molecular analysis of spontaneous mutations at the gpt locus in Chinese hamster ovary (AS52) cells. Mutat Res. 1989 Mar-May;220(2-3):241–253. doi: 10.1016/0165-1110(89)90028-6. [DOI] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Wiseman R. W., Stowers S. J., Miller E. C., Anderson M. W., Miller J. A. Activating mutations of the c-Ha-ras protooncogene in chemically induced hepatomas of the male B6C3 F1 mouse. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5825–5829. doi: 10.1073/pnas.83.16.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M., Candrian U., Maronpot R. R., Stoner G. D., Anderson M. W. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]