Significance
Ca2+ and CO2 are fundamental biological signaling molecules in microbes, animals, and plants. Although Ca2+ was proposed to act as a second messenger in CO2 signaling in guard cells of terrestrial plants, the role of Ca2+ in CO2 signal transduction pathways in aquatic photosynthetic organisms remains largely unknown. We show here that a chloroplast Ca2+-binding protein, CAS, changes its localization in response to environmental CO2 conditions and regulates the expression of nuclear-encoded limiting-CO2–induced genes, including two key bicarbonate transporters. These findings led us to propose a model for the participation of Ca2+ signals in chloroplast-regulated CO2 signal transduction of aquatic photosynthetic organisms and help us to further understand the role of Ca2+ in CO2 signal transduction in eukaryotes.
Keywords: acclimation, bicarbonate transporter, calcium signaling, photosynthesis, pyrenoid
Abstract
Aquatic photosynthetic organisms, including the green alga Chlamydomonas reinhardtii, induce a CO2-concentrating mechanism (CCM) to maintain photosynthetic activity in CO2-limiting conditions by sensing environmental CO2 and light availability. Previously, a novel high-CO2–requiring mutant, H82, defective in the induction of the CCM, was isolated. A homolog of calcium (Ca2+)-binding protein CAS, originally found in Arabidopsis thaliana, was disrupted in H82 cells. Although Arabidopsis CAS is reported to be associated with stomatal closure or immune responses via a chloroplast-mediated retrograde signal, the relationship between a Ca2+ signal and the CCM associated with the function of CAS in an aquatic environment is still unclear. In this study, the introduction of an intact CAS gene into H82 cells restored photosynthetic affinity for inorganic carbon, and RNA-seq analyses revealed that CAS could function in maintaining the expression levels of nuclear-encoded CO2-limiting–inducible genes, including the HCO3– transporters high-light activated 3 (HLA3) and low-CO2–inducible gene A (LCIA). CAS changed its localization from dispersed across the thylakoid membrane in high-CO2 conditions or in the dark to being associated with tubule-like structures in the pyrenoid in CO2-limiting conditions, along with a significant increase of the fluorescent signals of the Ca2+ indicator in the pyrenoid. Chlamydomonas CAS had Ca2+-binding activity, and the perturbation of intracellular Ca2+ homeostasis by a Ca2+-chelator or calmodulin antagonist impaired the accumulation of HLA3 and LCIA. These results suggest that Chlamydomonas CAS is a Ca2+-mediated regulator of CCM-related genes via a retrograde signal from the pyrenoid in the chloroplast to the nucleus.
Carbon dioxide (CO2) is a key environmental signal for physiological responses in many organisms (1). For photosynthetic organisms, CO2 is essential for survival. In vascular plants, guard cells in leaves control the opening and closure of stomata in response to environmental CO2 concentrations, with these events controlled by protein kinase HT1 (2) and carbonic anhydrase (3). In aquatic conditions, the CO2 diffusion rate is ∼10,000-fold lower compared with that in air (4). Therefore, aquatic photosynthetic organisms, including microalgae, are frequently exposed to limiting-CO2 stress. To acclimate to this stress, most microalgae possess a CO2-concentrating mechanism (CCM) to increase CO2 concentrations around the CO2-fixation enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and to maintain adequate photosynthetic efficiency.
The eukaryotic CCM has been studied in the model green alga Chlamydomonas reinhardtii (5). CCM1/CIA5 was identified as a zinc-finger–type regulatory factor for the induction of most limiting-CO2–induced genes, including HLA3 (high-light activated 3), LCIA (low-CO2–inducible gene A), and LCIB (low-CO2–inducible gene B) (6–9). HLA3 is an ATP-binding cassette transporter localized to the plasma membrane and associated with HCO3– transport from the outside of cells into the cytosol (10–13). LCIA is a possible anion channel localized to the chloroplast envelope and is associated with inorganic carbon (Ci) (CO2 and HCO3–) uptake into the chloroplast stroma in cooperation with HLA3 (12, 14). LCIB is a chloroplast soluble protein whose localization is associated with distinct CO2-acclimation states, including high-CO2 (HC) (5 to 0.5%), low-CO2 (LC) (0.03 to 0.5%), and very-low-CO2 (VLC) (<0.03%) (15). In HC and LC conditions, LCIB is dispersed throughout the chloroplast stroma and is essential for the survival in LC conditions (11, 16, 17). In contrast, in VLC conditions, LCIB is localized as a ring-like structure in the vicinity of the pyrenoid (14, 17), where Rubisco is enriched for CO2-fixation. Although the function of LCIB in each CO2-acclimation state remains to be elucidated, it is proposed that LCIB functions not only in LC conditions but also in VLC conditions for CO2 uptake (14).
In addition to CO2, Ca2+ also plays a role in the regulation of photosynthesis (18) and could mediate CO2 signal transduction (19). As a molecular component related to the Ca2+ signal, a thylakoid Ca2+-binding protein, CAS, has been shown to mediate the transient elevation of cytosolic Ca2+ concentration ([Ca2+]cyt), as well as stromal Ca2+ concentration ([Ca2+]stro), in guard cells of Arabidopsis thaliana and to regulate plant immune responses and stomatal closure (20–22). In Chlamydomonas, an ortholog of CAS (Cre12.g497300) was also detected in the thylakoid membrane fraction (23, 24). It was proposed that CAS is required for photoacclimation by regulating induction of light-harvesting complex stress-related protein 3 (LHCSR3) (25) and for forming a super complex for photosynthetic cyclic electron flow (CEF) in anaerobic conditions (26). From Chlamydomonas insertion mutant library screening experiments, we previously isolated a mutant strain, H82, in which a hygromycin resistance gene cassette was inserted into CAS (27). H82 cells showed decreased Ci affinity and did not accumulate HLA3 or LCIA in LC conditions.
In this study, we show the suborganellar localization of CAS in the chloroplast in vivo and its Ca2+-binding activity in vitro. Furthermore, using complemented strains of H82, the link between CAS and regulation of the CCM is elucidated by examining the expression patterns of HLA3 and LCIA in response to CO2 and Ca2+ changes. From these results, we propose the existence of chloroplast-mediated regulation of the CCM by Ca2+-binding protein CAS in parallel with regulation by CCM1/CIA5.
Results
Complementation of H82 Mutant Phenotype by CAS Gene.
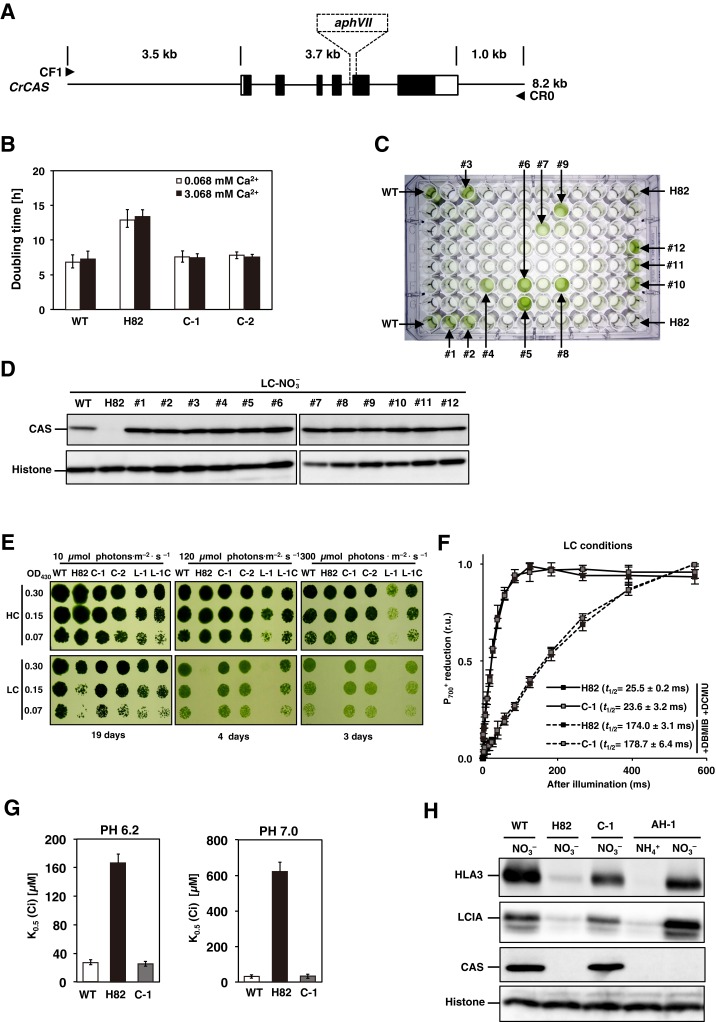
To examine whether disruption of CAS by insertion of a hygromycin resistance gene cassette is responsible for the phenotype of H82, an 8.2-kb PCR-amplified genomic DNA fragment containing the WT CAS gene with its own predicted promoter was cotransformed into H82 cells with a paromomycin resistance gene cassette, aphVIII (Fig. S1A). From 774 paromomycin-resistant transformants, two strains showing similar growth rates to that of WT cells in LC conditions were isolated and designated as C-1 and C-2 (Fig. S1B). In parallel, 12 strains from 92 paromomycin resistance transformants generated by transforming a plasmid pTY2b-CAS to H82 exhibited recovered growth rate (Fig. S1C) and accumulated CAS (Fig. S1D) in LC conditions. Then, C-1 was used for further analyses. The HC-requiring phenotype of H82 cells was dependent on light intensity (Fig. S1E and SI Results and Discussion), and the addition of excess Ca2+ did not recover the retarded growth rate of the H82 cells (Fig. S1B and SI Results and Discussion).
Fig. S1.
Photosynthetic characterization of CAS insertion mutant (H82) and its complemented strains. (A) Schematic representation of the fragment introduced into H82 cells for complementation and the insertion of the aphVII cassette in the genome of strain H82. The solid rectangles indicate the exons of the CAS gene. The open rectangles represent the 5′ UTR (Left) and 3′ UTR (Right). The fragment was amplified using primers CF1 and CR0. The broken lines indicate the insertion of aphVII into the genome of the H82 mutant. (B) The doubling time of WT, H82, and its complemented strains (C-1 and C-2) in low-CO2 (LC) conditions at pH 7.0. The respective doubling times of 7.0, 7.6 h, and 7.8 h of WT, C-1, and C-2 cells were shorter than the 13.0 h for H82 in LC conditions. The optical density at 730 nm was measured at four time points (0, 12, 24, and 36 h) for calculating the doubling time. High-CO2 (HC)-grown WT cells were centrifuged and resuspended by fresh HSM medium in the presence of the indicated concentration of CaCl2 and switched to LC conditions. (C) Growth phenotype of WT, H82, and 92 paromomycin-resistance transformants obtained by transforming plasmid pTY2b-CAS into H82 cells in LC conditions for 24 h. Twelve strains showing similar growth rates with WT were designated as #1 to #12. (D) Accumulation of CAS in the WT and obtained 12 complemented strains under LC conditions for 12 h in the presence of . (E) Spot test of WT, H82, complemented strains of H82 (C-1 and C-2), and pgrl1 mutant (L-1) cells, as well as its complemented strain (L-1C) for growth in HC and LC conditions. Cells grown to logarithmic phase were diluted to the indicated optical density (OD430: 0.3, 0.15, and 0.07); then, 3 µL of cell suspension was spotted onto six HSM agar plates and incubated in HC or LC chambers with the indicated light intensity for the indicated periods. (F) reduction kinetics of H82 and C-1 cells grown in LC conditions. The kinetics were measured after actinic light illumination for 10 s and then switching to dark periods. These curves show the kinetics of reduction after cessation of actinic light exposure. The kinetics were recorded in the presence of 10 μM dichlorophenyl-dimethylurea (DCMU) (solid line) or 10 μM DCMU and 2 μM 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) (broken line). The time periods required for half reduction of (t1/2) were calculated. Data in all measurements are mean values ± SD from three biological replicates. (G) Inorganic carbon (Ci) affinity of WT, H82, and C-1 grown in LC conditions for 12 h. Photosynthetic oxygen evolution was measured in external dissolved Ci concentrations at pH 6.2 or pH 7.0. The K0.5 (Ci) values were calculated as the Ci concentration required for half maximum oxygen-evolving activity. Data from all experiments show mean values ± SD from three biological replicates. (H) Accumulation of LCIA, HLA3, and CAS in WT, H82, C-1, and AH-1 cells. Cells were grown in HSM medium with (HSM-) or NO3− (HSM-NO3−) as the nitrogen source in LC conditions for 12 h. Histone H3 was used as a loading control. (I and J) Accumulation of HLA3, LCIA, and LHCSR3 in WT, H82, and C-1. HC-grown cells were transferred to LC conditions for 20 min, 2 h, 4 h, or 12 h before sampling. Cells were illuminated in 120 µmol photons∙m–2∙s–1. Histone H3 was used as a loading control. To compare the accumulation levels of LHCSR3 in H82 cells with that of WT, aliquots of total cell protein corresponding to 1 µg of chlorophyll were loaded in the lane designated ×1, and the same amount of protein was serially diluted 2 to 16 times and loaded in the lanes designated ×2, ×4, ×8, and ×16, respectively. (K) Time course of fragments per kilobase of exon per million fragments mapped (FPKM) values of LCIA, HLA3, and LCIB in WT, H82, and C-1 cells. Each strain grown in HC (0 h) conditions was transferred to LC conditions for 0.3 or 2 h. FPKM values were calculated from two biological replicates.
Because it was reported that CEF activity increases in LC conditions possibly for increased ATP demand for ABC-type bicarbonate transporter HLA3 in CCM (12, 28) and that CAS is associated with the CEF complex formation in anaerobic conditions (26), it was possible that CAS-dependent CEF might contribute to CCM. However, CEF activity was not affected by the lack of CAS protein in the LC conditions at 120 µmol photons·m–2·s–1 that we examined (Fig. S1F and SI Results and Discussion). This result coincides with the previous finding that down-regulation of CAS did not impair CEF activity in aerobic conditions (26).
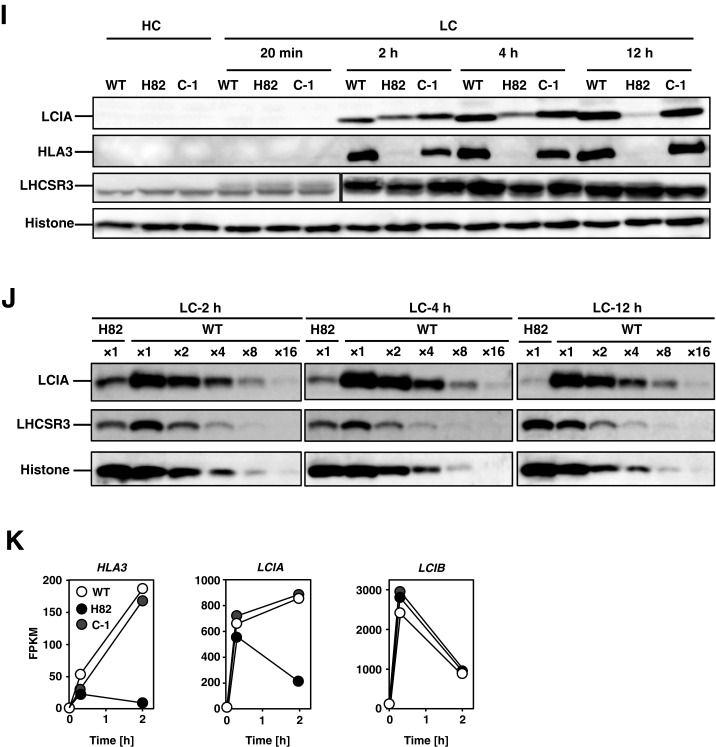
To evaluate induction of the CCM in LC conditions, photosynthetic affinities for Ci were evaluated by measuring the rates of photosynthetic oxygen (O2) evolution of WT, H82, and C-1 cells. At pH 7.8 (ratio of :CO2 = 28:1), the Ci concentration required for half maximal velocity [K0.5 (Ci)] in C-1 cells was 58 ± 8 µM, which was similar to that of 50 ± 8 µM in WT cells and ∼19 times lower than that of 1,087 ± 113 µM in H82 cells (Fig. 1A and Table S1). Even at pH 6.2 (:CO2 = 0.7:1) and pH 7.0 (:CO2 = 4.3:1), the respective K0.5 (Ci) values of C-1 were 6.5-fold and 19.7-fold lower than that of H82 cells (Fig. S1G and Table S1). Because maximum rates of photosynthesis (Vmax) of the three strains WT, H82, and C-1 were similar with each other at each pH condition (Table S1), the decreased Ci affinity in H82 cells could be partly explained by a defect in Ci uptake activity. The accumulation and fixation of [14C]-labeled Ci in H82 cells were 0.13 mM and 0.34 nmol per microliter of sorbitol impermeable space (SIS) after 80 s of illumination, respectively, which was lower than 0.22 mM and 1.34 nmol μL SIS–1 of C-1 cells (Fig. 1 B and C).
Fig. 1.
Characterization of CAS insertion mutant H82 and its complemented strain C-1. (A) Inorganic carbon (Ci) affinity of WT, H82, C-1, and transgenic H82 cells containing inducible genes of HLA3 and LCIA (AH-1) grown in medium with or as nitrogen sources in low-CO2 (LC) conditions for 12 h. Photosynthetic O2-evolving activity was measured in external dissolved Ci concentrations at pH 7.8, and the Ci concentration required for half maximal velocity [K0.5 (Ci)] was calculated. Data in all experiments are mean values ± SD from three biological replicates. *P < 0.01 by Student’s t test. (B and C) Accumulation (B) and fixation (C) of Ci in WT, H82, and C-1 cells. Cells were grown in LC conditions for 12 h, and the intracellular Ci concentration and carbon fixation were measured using a silicone-oil layer method. SIS, sorbitol impermeable space. (D) Accumulation of CAS, LCIA, HLA3, and LCIB in WT, H82, and C-1 cells. Cells were grown in high-CO2 (HC) or LC conditions for 12 h. Histone H3 was used as a loading control.
Table S1.
Photosynthetic parameters of WT and transformant cells
| pH | Strain name | Growth conditions | Vmax of O2-evolving activity, µmol O2⋅mgChl–1⋅h–1 | K0.5 (Ci), µM |
| 6.2 | WT | LC-NH4+ for 12 h | 296 ± 16 | 28 ± 3 |
| H82 | LC-NH4+ for 12 h | 253 ± 32 | 167 ± 13 | |
| C-1 | LC-NH4+ for 12 h | 300 ± 23 | 25 ± 4 | |
| C-2 | LC-NH4+ for 12 h | 303 ± 26 | 25 ± 2 | |
| 7.0 | WT | LC-NH4+ for 12 h | 230 ± 22 | 31 ± 3 |
| H82 | LC-NH4+ for 12 h | 214 ± 11 | 618 ± 84 | |
| C-1 | LC-NH4+ for 12 h | 228 ± 22 | 34 ± 2 | |
| C-2 | LC-NH4+ for 12 h | 227 ± 9 | 32 ± 1 | |
| 7.8 | WT | HC-NH4+ | 248 ± 6 | 235 ± 12 |
| LC-NH4+ for 2 h | 235 ± 2 | 72 ± 9 | ||
| LC-NH4+ for 2 h, 0.5 mM BAPTA | 222 ± 9 | 144 ± 14 | ||
| LC-NH4+ for 2 h, 0.5 mM BAPTA and 0.75 mM CaCl2 | 240 ± 5 | 63 ± 12 | ||
| LC-NH4+ for 2 h, 0.1% DMSO (mock) | 253 ± 15 | 59 ± 18 | ||
| LC-NH4+ for 2 h, 50 µM W-7 | 168 ± 18 | 157 ± 22 | ||
| LC-NH4+ for 2 h, 75 µM W-7 | 152 ± 21 | 235 ± 48 | ||
| LC-NH4+ for 2 h, 50 µM W-5 | 235 ± 2 | 52 ± 6 | ||
| LC-NH4+ for 2 h, 75 µM W-5 | 236 ± 17 | 57 ± 13 | ||
| LC-NH4+ for 12 h | 233 ± 24 | 50 ± 8 | ||
| LC-NO3– for 12 h | 197 ± 5 | 44 ± 16 | ||
| H82 | LC-NH4+ for 12 h | 217 ± 24 | 1,087 ± 113 | |
| LC-NO3– for 12 h | 249 ± 17 | 793 ± 91 | ||
| C-1 | LC-NH4+ for 12 h | 239 ± 29 | 58 ± 8 | |
| LC-NO3– for 12 h | 205 ± 11 | 35 ± 7 | ||
| AH-1 | LC-NH4+ for 12 h | 207 ± 19 | 1,008 ± 70 | |
| LC-NO3– for 12 h | 207 ± 11 | 430 ± 66 | ||
| C-2 | LC-NH4+ for 12 h | 231 ± 21 | 59 ± 5 | |
| FN-1 | LC-NH4+ for 12 h | 233 ± 4 | 53 ± 4 | |
| CL-1 | LC-NH4+ for 12 h | 241 ± 14 | 40 ± 13 |
The data are shown ±SD, which were obtained from three independent experiments. BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N,N'-tetraacetic acid; HC, high-CO2 (aerated with 5.0%); K0.5 (Ci), Ci concentration required for half of Vmax; LC, low-CO2 (aerated with 0.04%); Vmax, maximum O2-evolving activity.
In addition to recovery of the accumulation of CAS and photosynthetic affinities for Ci in C-1, LC-induced accumulation of HLA3 and LCIA was also restored (Fig. 1D). To further evaluate the contribution of the defect in the accumulation of HLA3 and LCIA to the decreased Ci affinity in H82 cells, strain AH-1 was generated, in which two recombinant plasmids, pTY2b-LCIA and pTY2b-HLA3 (12), were introduced into H82 cells and in which HLA3 and LCIA could be induced by switching the nitrogen source from NH4+ to NO3– (Fig. S1H). Although the K0.5 (Ci) value of 1,008 ± 70 µM in AH-1 cells was similar to that of 1,087 ± 113 µM in H82 cells in conditions, that of AH-1 cells decreased to 430 µM from 793 ± 91 µM in H82 cells by expression of both HLA3 and LCIA in NO3– conditions (Fig. 1A and Table S1). However, the K0.5 (Ci) value in AH-1 cells was still ∼10-fold higher than those in WT and C-1 cells. These results suggested that decreased Ci affinity in H82 cells was partially caused by a defect in the accumulation of HLA3 and LCIA, but other additional factors could be responsible for the HC-requiring phenotype of H82 cells.
CAS-Dependent Regulation of Nuclear-Encoded LC-Inducible Genes.
To evaluate the cause of decreased Ci affinity of H82 cells other than HLA3 and LCIA, genes whose expression was affected by the CAS mutation were screened by RNA-seq analysis (Table S2). WT, H82, and C-1 cells were grown in HC or LC conditions for 0.3 and 2 h, and the respective transcriptome profiles were compared. After exposure to HC and LC conditions for 0.3 h, no gene other than CAS was affected by the mutation. In contrast, in LC conditions for 2 h, the expression levels of 44 genes in addition to CAS were significantly different [FDR (false discovery rate) < 0.05] in H82 cells compared with those in WT and C-1 cells (Table S3). Among them, the expression levels of 13 genes were decreased more than fourfold by the CAS mutation, including the following: HLA3; LCIA; DNJ31 encoding putative DnaJ-like chaperonin; CAH4 and CAH5 encoding mitochondrial carbonic anhydrases; PPP30 encoding type 2C protein phosphatase; chloroplast carrier protein 1 (CCP1) and CCP2 encoding putative chloroplast envelope membrane proteins; low-CO2–inducible gene D (LCID); LHCSR3.1 and LHCSR3.2 encoding LHCSR3; and two unknown genes (Cre12.g541550 and Cre26.g756747). Eventually, the transcriptional abundances of these 12 genes, except for gene Cre12.g541550, were decreased by the CIA5 mutation (9).
Table S2.
Summary of RNA-seq samples and sequence alignment
| Sample | Sequencer | Read length, bp | Total no. of clean bases, Gb | Total no. of clean reads | Reads with paired-end alignments to 1 output stream(s) | Percentage of total reads |
| WT-HC-1 | Illumina HiSeq 2500 | 100 | 8.35 | 47265130 | 27609316 | 58.41 |
| WT-HC-2 | Illumina HiSeq 2500 | 100 | 8.21 | 45637471 | 29885519 | 65.48 |
| WT-LC-20min-1 | Illumina HiSeq 2500 | 100 | 9.68 | 58266403 | 23808590 | 40.86 |
| WT-LC-20min-2 | Illumina HiSeq 2500 | 100 | 8.32 | 46300307 | 29881377 | 64.54 |
| WT-LC-2h-1 | Illumina HiSeq 2500 | 100 | 8.57 | 47940877 | 31067123 | 64.80 |
| WT-LC-2h-2 | Illumina HiSeq 2500 | 100 | 10.01 | 57546663 | 29885519 | 51.93 |
| H82-HC-1 | Illumina HiSeq 2500 | 100 | 8.34 | 46983516 | 30403878 | 64.71 |
| H82-HC-2 | Illumina HiSeq 2500 | 100 | 7.59 | 42574570 | 27063569 | 63.57 |
| H82-LC-20min-1 | Illumina HiSeq 2500 | 100 | 9.50 | 53019310 | 34222317 | 64.55 |
| H82-LC-20min-2 | Illumina HiSeq 2500 | 100 | 10.35 | 57397611 | 38222209 | 66.59 |
| H82-LC-2h-1 | Illumina HiSeq 2500 | 100 | 7.91 | 43967647 | 28932872 | 65.80 |
| H82-LC-2h-2 | Illumina HiSeq 2500 | 100 | 6.79 | 37781602 | 24637228 | 65.21 |
| C1-HC-1 | Illumina HiSeq 2500 | 100 | 6.42 | 38382258 | 16394246 | 42.71 |
| C1-HC-2 | Illumina HiSeq 2500 | 100 | 7.16 | 40414944 | 25098869 | 62.10 |
| C1-LC-20min-1 | Illumina HiSeq 2500 | 100 | 6.71 | 37685632 | 23073042 | 61.23 |
| C1-LC-20min-2 | Illumina HiSeq 2500 | 100 | 7.40 | 40831129 | 27740665 | 67.94 |
| C1-LC-2h-1 | Illumina HiSeq 2500 | 100 | 8.32 | 47822947 | 29906711 | 62.54 |
| C1-LC-2h-2 | Illumina HiSeq 2500 | 100 | 8.45 | 46527856 | 29890701 | 64.24 |
Sequencing details and alignment summary are provided for each individual sample lane. In each condition, sequencing data were obtained from two biological replicates.
Table S3.
Genes differentially expressed in H82 cells with an FDR < 0.05, compared with those in WT and C-1 cells in LC conditions for 2 h
| Gene ID* | Gene name | Average FPKM in WT | Average FPKM in H82 | Average FPKM in C-1 | Description of gene product | q value (WT LC 2 h vs. H82 LC 2 h) | q value (C-1 LC 2 h vs. H82 LC 2 h) | q value (WT LC 2 h vs. C-1 LC 2 h) | WT LC 2 h FPKM/H82 LC 2 h FPKM | C-1 LC 2 h FPKM/H82 LC 2 h FPKM | ||||||
| HC | LC 0.3 h | LC 2 h | HC | LC 0.3 h | LC 2 h | HC | LC 0.3 h | LC 2 h | ||||||||
| Cre02.g097800 | HLA3 | 2.1 | 55.0 | 152.3 | 2.5 | 22.4 | 7.0 | 1.9 | 35.4 | 154.3 | Plasma membrane-localized ATP-binding cassette transporter associated with HCO3– uptake | 2.14.E−03 | 2.14.E−03 | 9.98.E−01 | 21.8 | 22.1 |
| Cre12.g497300 | CAS | 367.7 | 152.1 | 264.9 | 6.0 | 6.1 | 7.1 | 99.4 | 95.2 | 89.2 | Thylakoid membrane-localized calcium-binding protein containing Rhodanese-like domain | 2.14.E−03 | 2.14.E−03 | 3.92.E−02 | 37.4 | 12.6 |
| Cre03.g204577 | DNJ31 | 3.0 | 4.0 | 13.2 | 2.6 | 2.9 | 2.9 | 3.0 | 3.3 | 26.6 | DnaJ-like chaperonin | 2.14.E−03 | 2.14.E−03 | 2.13.E−01 | 4.6 | 9.3 |
| Cre05.g248400 | CAH4 | 7.4 | 756.1 | 2,335.8 | 3.0 | 499.4 | 296.1 | 6.4 | 712.7 | 2,329.5 | Mitochondrial carbonic anhydrase, CA4 | 2.14.E−03 | 2.14.E−03 | 9.98.E−01 | 7.9 | 7.9 |
| Cre05.g248450 | CAH5 | 8.5 | 749.9 | 2,134.9 | 3.4 | 485.7 | 289.7 | 6.4 | 694.1 | 2,243.7 | Mitochondrial carbonic anhydrase, CA5 | 2.14.E−03 | 2.14.E−03 | 9.98.E−01 | 7.4 | 7.7 |
| Cre07.g334750 | PPP30 | 2.4 | 16.4 | 15.2 | 2.2 | 16.0 | 3.2 | 1.6 | 33.0 | 20.2 | Type 2C protein phosphatase containing Stage II sporulation protein E (SpoIIE) (PF07228) domain | 5.41.E−03 | 2.14.E−03 | 8.71.E−01 | 4.8 | 6.4 |
| Cre06.g309000 | LCIA | 2.5 | 626.8 | 798.5 | 2.1 | 533.6 | 151.1 | 0.6 | 911.0 | 866.5 | Chloroplast envelope-localized possible anion channel associated with inorganic carbon uptake; nitrite transporter (NAR1) homolog | 2.14.E−03 | 2.14.E−03 | 9.98.E−01 | 5.3 | 5.7 |
| Cre04.g223300 | CCP1 | 3.1 | 466.2 | 632.6 | 2.4 | 308.7 | 137.7 | 0.7 | 428.7 | 700.0 | Low-CO2–inducible chloroplast envelope protein (LIP36-1) | 2.14.E−03 | 2.14.E−03 | 9.97.E−01 | 4.6 | 5.1 |
| Cre12.g541550 | — | 7.7 | 6.7 | 24.3 | 33.6 | 15.5 | 6.8 | 48.3 | 27.0 | 34.4 | † | 2.14.E−03 | 2.14.E−03 | 4.55.E−01 | 3.6 | 5.1 |
| Cre26.g756747 | — | 1.3 | 157.5 | 41.0 | 1.0 | 137.8 | 13.6 | 0.4 | 279.4 | 67.1 | † | 2.14.E−03 | 2.14.E−03 | 2.34.E−01 | 3.0 | 4.9 |
| Cre04.g222800 | LCID | 10.5 | 39.3 | 61.9 | 5.8 | 30.0 | 17.6 | 4.2 | 59.2 | 82.2 | Homolog of low-CO2–inducible protein B (LCIB) | 2.14.E−03 | 2.14.E−03 | 6.97.E−01 | 3.5 | 4.7 |
| Cre08.g367400 | LHCSR3.2 | 1.6 | 245.1 | 164.3 | 2.0 | 236.3 | 46.0 | 1.7 | 340.6 | 208.8 | Stress-related chlorophyll a/b binding protein 3 | 2.14.E−03 | 2.14.E−03 | 8.02.E−01 | 3.6 | 4.5 |
| Cre08.g367500 | LHCSR3.1 | 1.6 | 290.1 | 191.8 | 1.9 | 263.9 | 49.7 | 2.9 | 341.6 | 223.2 | Stress-related chlorophyll a/b binding protein 3 | 2.14.E−03 | 2.14.E−03 | 9.39.E−01 | 3.9 | 4.5 |
| Cre04.g222750 | CCP2 | 5.5 | 150.4 | 199.3 | 3.4 | 151.7 | 69.9 | 3.4 | 234.5 | 280.6 | Low-CO2–inducible chloroplast envelope protein (LIP36-2) | 2.14.E−03 | 2.14.E−03 | 6.49.E−01 | 2.9 | 4.0 |
| Cre06.g281600 | LCI23 | 4.2 | 36.0 | 86.3 | 5.4 | 53.2 | 28.6 | 6.6 | 69.8 | 104.8 | Low-CO2–inducible septin-like protein | 2.14.E−03 | 2.14.E−03 | 8.91.E−01 | 3.0 | 3.7 |
| Cre02.g088551 | — | 5.2 | 14.0 | 22.2 | 4.1 | 12.5 | 8.9 | 5.9 | 22.3 | 32.1 | † | 2.14.E−03 | 2.14.E−03 | 4.27.E−01 | 2.5 | 3.6 |
| Cre11.g467617 | LCI19 | 4.2 | 15.2 | 28.0 | 3.8 | 16.2 | 8.8 | 3.5 | 19.3 | 29.5 | Low-CO2–inducible gamma hydroxybutyrate dehydrogenase | 2.14.E−03 | 2.14.E−03 | 9.98.E−01 | 3.2 | 3.4 |
| Cre09.g399400 | TGL15 | 11.6 | 8.7 | 15.2 | 1.4 | 2.9 | 3.6 | 1.9 | 4.9 | 12.0 | Triacylglycerol lipase | 2.14.E−03 | 2.14.E−03 | 7.20.E−01 | 4.2 | 3.3 |
| Cre05.g234652 | — | 17.7 | 29.3 | 50.5 | 13.5 | 32.5 | 17.4 | 17.3 | 47.6 | 55.9 | Predicted protein with CobW domain | 2.14.E−03 | 2.14.E−03 | 9.76.E−01 | 2.9 | 3.2 |
| Cre10.g439700 | CGL28 | 6.5 | 56.5 | 58.4 | 6.4 | 64.5 | 25.7 | 4.8 | 122.9 | 82.2 | RNA binding protein | 3.88.E−03 | 2.14.E−03 | 5.99.E−01 | 2.3 | 3.2 |
| Cre09.g391726 | — | 5.5 | 17.0 | 16.2 | 14.8 | 48.1 | 30.6 | 62.0 | 116.0 | 96.9 | † | 2.86.E−02 | 2.14.E−03 | 2.14.E−03 | 0.5 | 3.2 |
| Cre17.g720950 | SRD3 | 14.4 | 89.7 | 28.2 | 11.4 | 91.5 | 14.1 | 17.2 | 223.0 | 43.5 | Putative 3-oxo-5-alpha-steroid 4-dehydrogenase | 1.29.E−02 | 2.14.E−03 | 2.81.E−01 | 2.0 | 3.1 |
| Cre02.g080800 | — | 2.8 | 8.2 | 10.6 | 2.7 | 9.7 | 4.6 | 2.1 | 14.8 | 14.1 | † | 2.14.E−03 | 2.14.E−03 | 6.04.E−01 | 2.3 | 3.1 |
| Cre03.g162800 | LCI1 | 2.6 | 2,144.6 | 2,496.1 | 2.3 | 1,403.0 | 927.0 | 1.5 | 1,833.1 | 2,674.8 | Plasma membrane-localized protein associated with inorganic carbon transport | 2.18.E−02 | 1.61.E−02 | 9.98.E−01 | 2.7 | 2.9 |
| Cre03.g168100 | — | 7.4 | 18.5 | 32.9 | 6.6 | 17.1 | 17.0 | 8.9 | 33.0 | 46.9 | † | 3.02.E−02 | 2.14.E−03 | 4.75.E−01 | 1.9 | 2.8 |
| Cre03.g151650 | SMM7 | 2.5 | 24.2 | 16.1 | 1.8 | 27.3 | 5.1 | 1.8 | 35.4 | 13.4 | Lysine methyltransferase | 2.14.E−03 | 2.14.E−03 | 8.26.E−01 | 3.2 | 2.6 |
| Cre10.g463370 | — | 6.8 | 2.5 | 3.8 | 2.7 | 1.8 | 1.3 | 3.3 | 2.4 | 3.2 | Putative smooth-muscle-myosin-light-chain kinase | 8.15.E−03 | 2.79.E−02 | 9.30.E−01 | 3.0 | 2.6 |
| Cre02.g077750 | FAP211 | 2.7 | 3.1 | 4.1 | 4.5 | 5.1 | 9.0 | 5.8 | 13.0 | 22.9 | Flagellar-associated protein | 2.14.E−03 | 2.14.E−03 | 2.14.E−03 | 0.5 | 2.5 |
| Cre03.g183300 | — | 19.8 | 46.3 | 18.6 | 10.5 | 42.9 | 7.9 | 14.5 | 59.3 | 19.3 | Predicted protein with starch-binding domain | 2.45.E−02 | 2.36.E−02 | 9.98.E−01 | 2.4 | 2.5 |
| Cre03.g189350 | — | 80.3 | 69.2 | 129.6 | 50.2 | 54.1 | 47.2 | 53.3 | 79.7 | 112.0 | † | 2.14.E−03 | 2.14.E−03 | 9.12.E−01 | 2.8 | 2.4 |
| Cre06.g295450 | HPR1 | 65.8 | 158.6 | 144.4 | 39.6 | 211.7 | 71.8 | 61.7 | 283.8 | 166.2 | Hydroxypyruvate reductase | 3.49.E−02 | 1.50.E−02 | 9.49.E−01 | 2.0 | 2.3 |
| Cre05.g241950 | ASC2 | 217.0 | 191.7 | 374.2 | 174.9 | 184.6 | 152.9 | 239.0 | 287.3 | 351.3 | Voltage-dependent anion-selective channel | 6.84.E−03 | 2.45.E−02 | 9.98.E−01 | 2.5 | 2.3 |
| Cre09.g398500 | — | 60.6 | 35.3 | 66.2 | 10.7 | 16.8 | 22.7 | 14.7 | 28.8 | 51.7 | † | 2.14.E−03 | 5.41.E−03 | 7.53.E−01 | 2.9 | 2.3 |
| Cre09.g404750 | SRR2 | 8.7 | 6.3 | 11.5 | 3.3 | 3.9 | 5.6 | 2.7 | 5.5 | 12.1 | Scavenger receptor cysteine-rich (SRCR) protein | 6.84.E−03 | 2.14.E−03 | 9.98.E−01 | 2.1 | 2.2 |
| Cre06.g281450 | SRR22 | 12.3 | 8.6 | 12.6 | 3.3 | 3.9 | 5.5 | 3.2 | 5.4 | 11.5 | Scavenger receptor cysteine-rich (SRCR) protein | 2.14.E−03 | 9.41.E−03 | 9.71.E−01 | 2.3 | 2.1 |
| Cre14.g621650 | MCT1 | 72.9 | 93.2 | 101.6 | 48.0 | 87.5 | 48.0 | 56.7 | 99.6 | 98.9 | Acyl-carrier-protein, S-malonyltransferase | 1.80.E−02 | 3.02.E−02 | 9.98.E−01 | 2.1 | 2.1 |
| Cre07.g343050 | — | 7.7 | 16.6 | 26.0 | 7.9 | 16.0 | 13.7 | 6.5 | 14.8 | 28.0 | † | 2.94.E−02 | 1.18.E−02 | 9.93.E−01 | 1.9 | 2.1 |
| Cre12.g531900 | — | 33.4 | 41.0 | 48.6 | 16.6 | 36.7 | 22.8 | 26.7 | 47.8 | 45.3 | † | 1.80.E−02 | 3.11.E−02 | 9.98.E−01 | 2.1 | 2.0 |
| Cre09.g398250 | — | 60.3 | 33.2 | 42.2 | 10.6 | 17.3 | 19.9 | 15.8 | 30.3 | 39.1 | † | 1.61.E−02 | 2.79.E−02 | 9.95.E−01 | 2.1 | 2.0 |
| Cre01.g030350 | CGL41 | 2.2 | 3.5 | 3.5 | 3.4 | 6.5 | 8.9 | 4.0 | 4.0 | 17.4 | Predicted RbcX protein | 2.14.E−03 | 3.71.E−02 | 2.14.E−03 | 0.4 | 2.0 |
| Cre17.g710300 | — | 18.3 | 10.0 | 4.6 | 20.8 | 23.3 | 11.6 | 19.9 | 21.1 | 22.6 | Pherophorin (DUF3707) | 2.14.E−03 | 3.84.E−02 | 2.14.E−03 | 0.4 | 2.0 |
| Cre13.g573250 | — | 30.8 | 32.1 | 25.8 | 13.0 | 25.4 | 13.3 | 17.6 | 41.6 | 24.5 | Thiosulfate sulfurtransferase with Rhodanese-like domain | 1.80.E−02 | 4.84.E−02 | 9.98.E−01 | 1.9 | 1.8 |
| Cre02.g093750 | NRX2 | 11.2 | 9.8 | 7.6 | 8.7 | 18.9 | 17.1 | 7.4 | 14.2 | 8.9 | Nucleoredoxin 2 | 2.14.E−03 | 2.45.E−02 | 8.99.E−01 | 0.4 | 0.5 |
| Cre12.g546550 | FEA1 | 277.5 | 36.5 | 4.7 | 136.0 | 26.5 | 17.2 | 172.6 | 65.9 | 6.4 | Fe-assimilating protein; High-CO2 inducible protein H43 | 2.14.E−03 | 9.41.E−03 | 6.27.E−01 | 0.3 | 0.4 |
| Cre05.g244400 | — | 1.6 | 2.4 | 2.1 | 3.1 | 6.7 | 6.2 | 2.1 | 3.4 | 1.9 | Nucleotide-diphospho-sugar transferase | 2.94.E−02 | 2.94.E−02 | 9.97.E−01 | 0.4 | 0.3 |
Gene ID, phytozome gene accession number.
Gene product that is not annotated.
In Chlamydomonas, accumulation of LHCSR3, which is essential for energy-dependent quenching (qE), is shown to be dependent on CAS in high-light condition (25). Because its function during LC acclimation related to CAS is unclear, we examined LHCSR3 accumulation in LC conditions in a time-dependent manner (Fig. S1I). After shifting to LC conditions, significant accumulation of LHCSR3 was detected within 2 h in WT cells, supporting the previous report that LHCSR3 is induced by CO2-limiting stress (29, 30). Although the accumulation of LHCSR3 in H82 cells was twofold lower than that in WT and C-1 cells in LC at 2 h or 4 h, similar accumulation levels of LHCSR3 were detected in each strain at 12 h (Fig. S1J). These results suggested that CAS regulates LHCSR3 accumulation at an early stage of CCM induction. In contrast, other CCM-related genes, including HLA3 and LCIA, were transiently induced at the mRNA levels at 0.3 h in H82 cells, but their mRNA levels could not be maintained at the same levels as in WT and C-1 cells at 2 h in contrast to LCIB (Fig. S1K), suggesting that CAS could be required for maintaining the mRNA levels of HLA3 and LCIA after the initial induction of these genes by CCM1/CIA5.
Requirement of Ca2+ for LC-Induced Accumulation of HLA3 and LCIA.
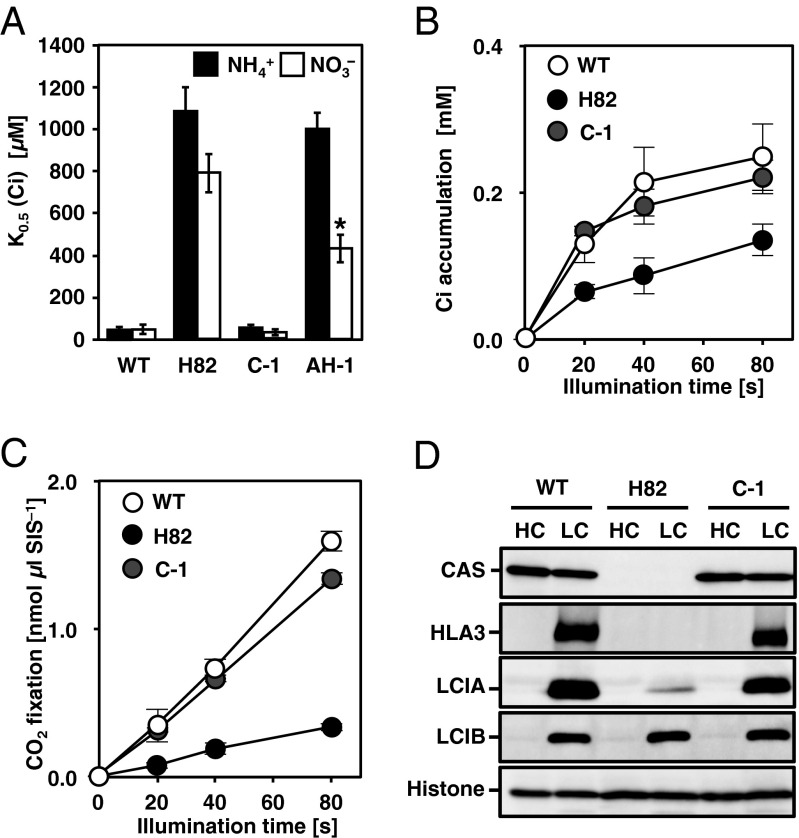
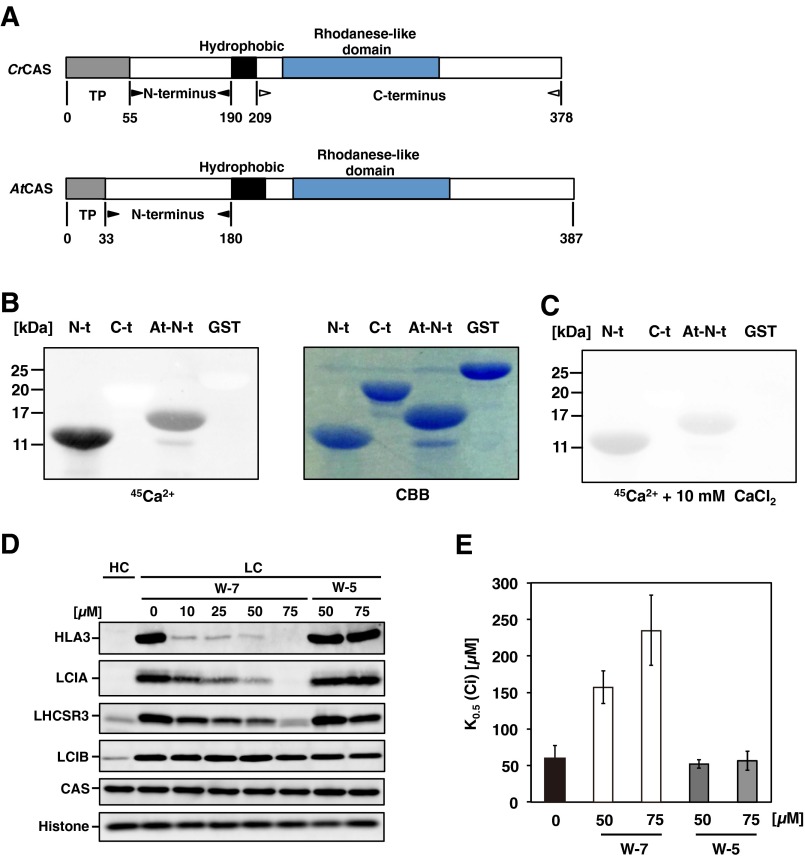
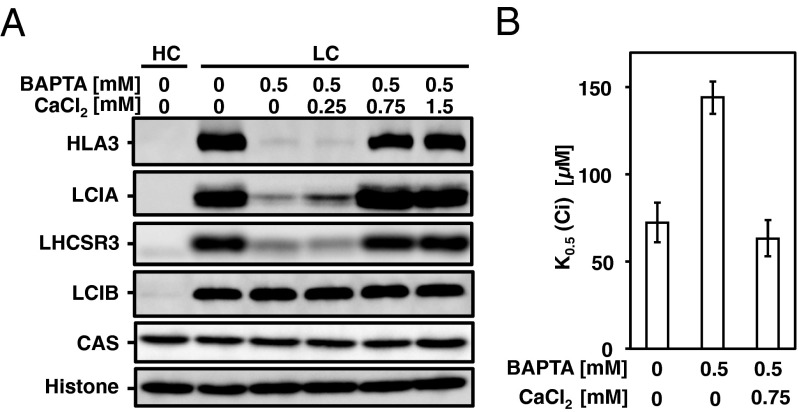
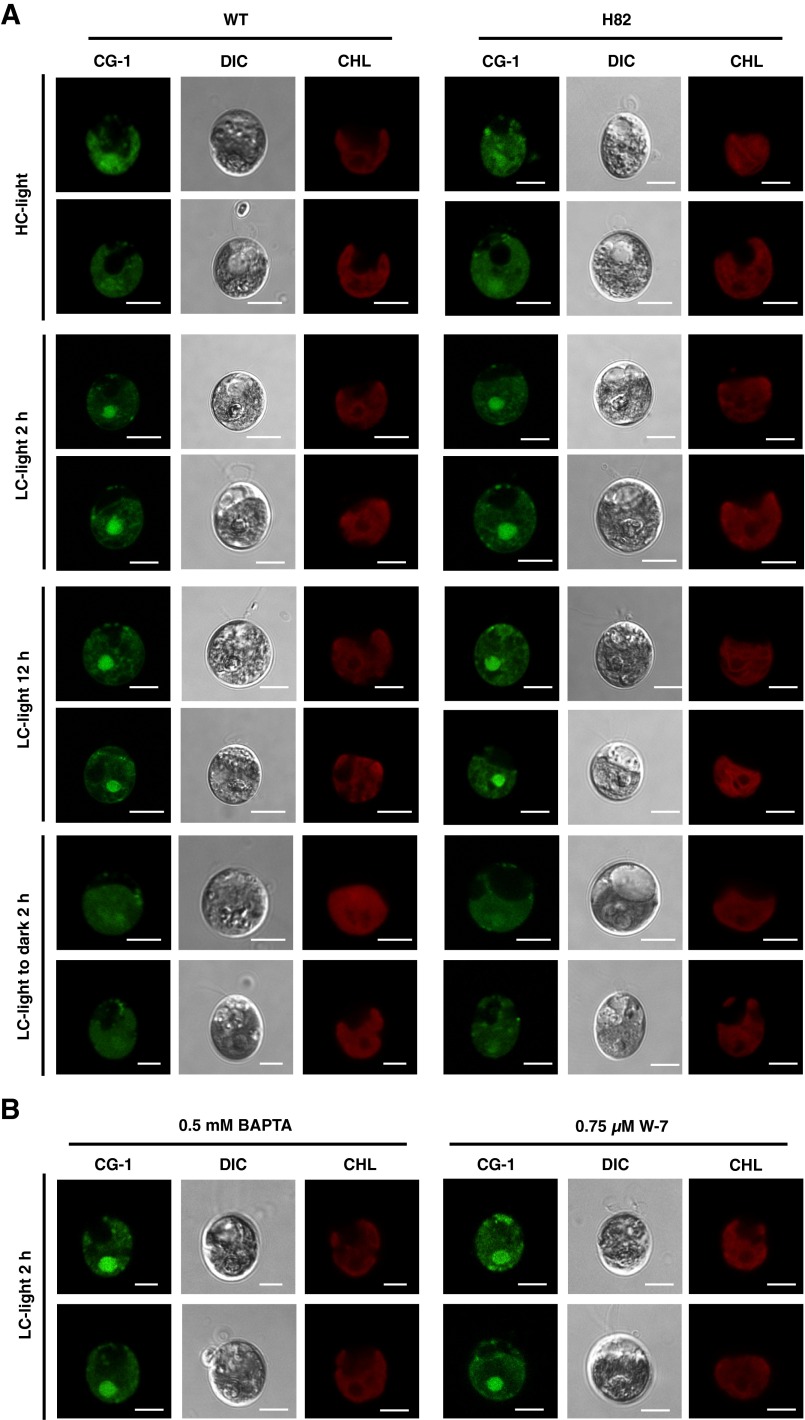
As in the case of AtCAS, the N terminus of CrCAS also has Ca2+-binding activity (Fig. S2 A–C and SI Results and Discussion). To know the link between Ca2+ signal via CAS and regulation of transporters, the accumulation of HLA3 and LCIA in WT cells grown in the presence of 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA), a membrane-impermeable Ca2+-specific chelator (31), was measured in LC conditions. The accumulation of HLA3 and LCIA was dramatically decreased in the presence of 0.5 mM BAPTA (Fig. 2A) as in the case of LHCSR3 (25, 30), and concomitantly K0.5 (Ci) increased twofold (72 ± 9 µM to 144 ± 14 µM) (Fig. 2B and Table S1). Considering that the addition of 0.75 mM CaCl2 rescued the accumulation of HLA3 and LCIA and decreased Ci affinity, extracellular Ca2+ is necessary for the accumulation of these transporters and for the photosynthetic Ci affinity. To further examine the regulation of these transporters by an intracellular Ca2+ signal, the impact of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7), a membrane-permeable calmodulin antagonist, on the accumulation of HLA3 and LCIA and photosynthetic Ci affinity was examined. In the presence of W-7, concentration-dependent effects of decreased accumulation of HLA3 and LCIA were observed, as in the case of LHCSR3 (Fig. S2D) (25, 30), and concomitantly K0.5 (Ci) increased from 59 ± 18 µM (mock) to 235 ± 48 µM in the presence of 75 µM W-7 (Fig. S2E and Table S1). In contrast, addition of N-(6-aminohexyl)-1-naphthalenesulfonamide hydrochloride (W-5), a biologically inactive calmodulin antagonist, did not show any significant effects. As a control, LCIB was normally induced in the presence of both chemicals. These results suggested that a calmodulin-mediated Ca2+ signal could play roles in the LC-induced accumulation of HLA3 and LCIA.
Fig. S2.
Ca2+-binding activity of CrCAS in vitro, and accumulation of HLA3 as well as LCIA, inorganic carbon (Ci) affinity in the presence of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) or 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA). (A) The N terminus and C terminus are separated by a hydrophobic sequence. The gray, solid, and blue rectangles indicate the transit peptide, hydrophobic sequence, and rhodanese-like domain, respectively. The numbers below the illustration represent the positions from the initial amino acid. The transit peptide of AtCAS was predicted using the ChloroP program. (B) 45Ca blotting analysis of the N and C termini (N-t and C-t) of the CAS protein. Purified recombinant N terminus, C terminus, N terminus of AtCAS (At-N-t), and GST with the same number of molecules were loaded, stained with Coomassie Brilliant blue (CCB) (B, Right), and probed with 45Ca in the absence (B, Left) or presence (C) of 10 mM cold CaCl2. kDa, kilodalton. (D) Accumulation of HLA3, LCIA, LHCSR3, LCIB, and CAS in WT cells in the presence of W-7 or N-(6-aminohexyl)-1-naphthalenesulfonamide hydrochloride (W-5). (E) Photosynthetic oxygen (O2) evolution was measured in external dissolved Ci concentrations at pH 7.8. WT cells were grown in the presence of the indicated chemicals at the specified concentrations in low-CO2 (LC) conditions for 2 h. Data in all experiment indicate mean value ± SD from three biological replicates.
Fig. 2.
The effects of extracellular calcium (Ca2+) chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA) on the accumulation of HLA3, LCIA, LHCSR3, LCIB, and CAS (A) and on inorganic carbon (Ci) affinity in low-CO2 (LC) conditions at pH 7.8 (B). Histone H3 was used as a loading control. High-CO2 (HC)-grown WT cells were centrifuged, resuspended by fresh high-salt medium supplemented with 20 mM 3-(N-morpholino)propanesulfonic acid (HSM) medium in the presence of the indicated chemicals and cultured in LC conditions for 2 h. For the culturing medium without any indications, HSM liquid medium contained 0.068 mM CaCl2. Data in all experiment indicate mean value ± SD from three biological replicates.
Relocation of Thylakoid Membrane-Localized CAS by LC in Light.
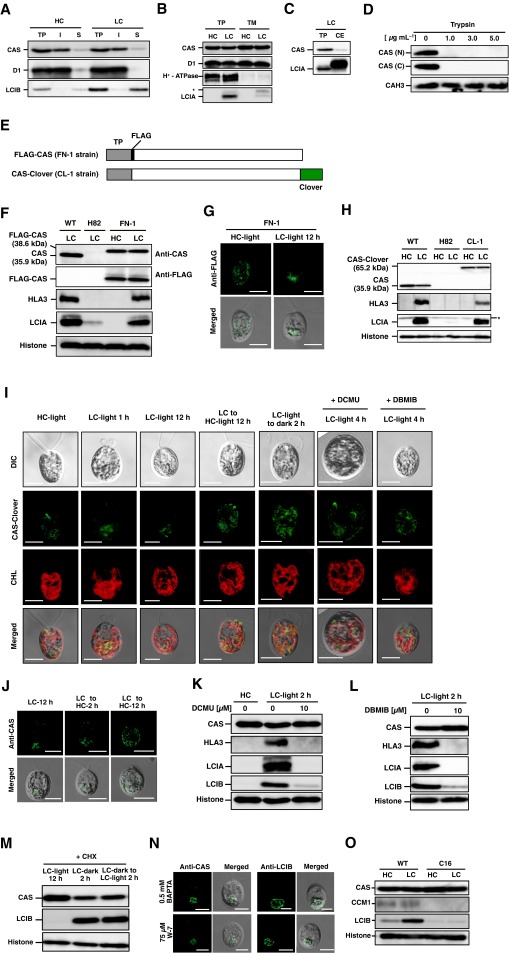
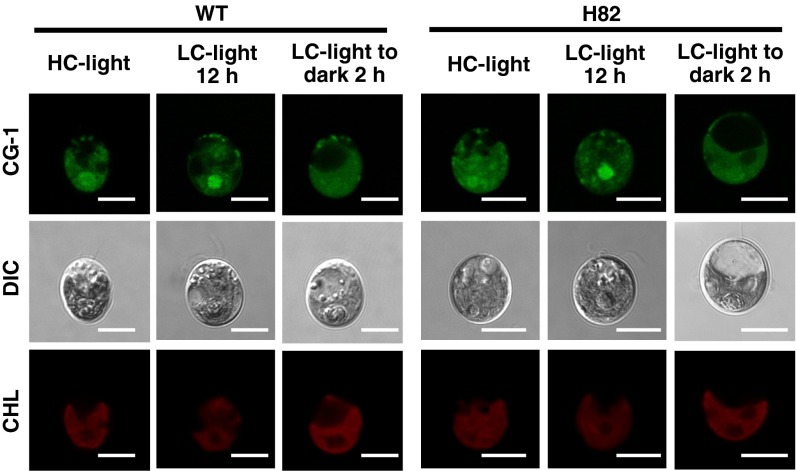
To examine the localization of CAS, total protein, soluble/insoluble, chloroplast envelope, and thylakoid membrane fractions were isolated and probed with antibodies against CAS, thylakoid membrane-localized D1, soluble protein LCIB, plasma membrane-localized H+-ATPase (12, 32), and chloroplast envelop-localized LCIA (12) (Fig. S3 A–C). CAS was mainly detected in the insoluble and thylakoid membrane fractions where D1 was enriched, as shown in the previous proteome analyses (23, 24). Similar to the case of AtCAS, both the N and C terminus of CrCAS were exposed to the stromal side of thylakoid membrane (Fig. S3D and SI Results and Discussion). Next, to elucidate the detailed subcellular localization of CAS in vivo, an indirect immunofluorescence assay using an anti-CAS antibody was performed (Fig. 3A). In HC conditions with light illumination at 120 µmol photons·m–2·s–1 (HC-light), fluorescent signals were observed as dispersed speckles in the chloroplast. In contrast, after shifting to LC conditions in light (LC-light), the fluorescent signals were aggregated in the pyrenoid after 2 h, and the aggregation was observed as tubule-like structures inside the pyrenoid at 12 h. These localization patterns were consistent with the results using a complemented strain FN-1 expressing FLAG-tag fused CAS and an anti-FLAG antibody (Fig. S3 E–G). Furthermore, to observe CAS localization in living cells, we also generated a complemented strain, CL-1, expressing exogenous CAS tagged with Clover, a Chlamydomonas-adapted modified GFP (33) (Fig. S3 E and H). Fluorescent signals of CAS-Clover were also distinctly aggregated and observed as a tubule-like structure in the pyrenoid in LC-light conditions at 12 h (Fig. S3I). The aggregated tubule-like signals were dispersed throughout the chloroplast when transferred to HC-light conditions (Fig. S3 I and J).
Fig. S3.
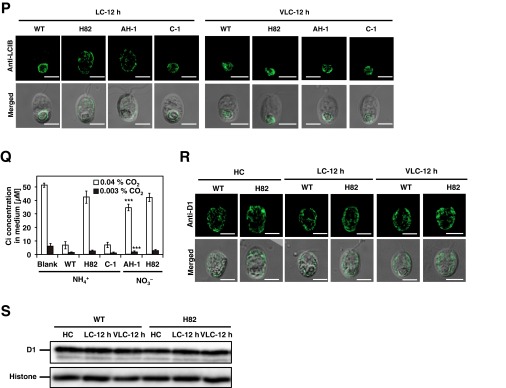
Subcellular localization and topology of CrCAS, the accumulation of CAS, HLA3, LCIA, and LCIB in the presence of dichlorophenyl-dimethylurea (DCMU), 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB), cycloheximide (CHX), or the CCM1 insertion mutant, localization of LCIB in the CAS insertion mutant, and inorganic carbon (Ci) concentration in growth medium. (A–C) Immunoblotting analysis in soluble, insoluble, thylakoid membrane, and chloroplast envelope fractions with antibodies against CAS, D1, LCIB, H+-ATPase, and LCIA. Cells were grown in high-CO2 (HC) or low-CO2 (LC) conditions for 12 h. Asterisks indicate nonspecific bands. CE, chloroplast envelope fraction; I, insoluble fraction; S, soluble fraction; TM, thylakoid membrane fraction; TP, total protein. (D) Topology of CrCAS. The thylakoid fraction (0.05 mg of chlorophyll per milliliter) isolated from CC-400 cells was suspended in a digesting buffer containing trypsin at the indicated concentrations or no protease. The proteins were separated by 15% SDS/PAGE and probed by N terminal (N) or C terminal (C) terminal-specific anti-CAS antibodies or an anti-CAH3 antibody. (E) Schematic representation of recombinant CAS proteins in complemented strains (FN-1 and CL-1). The gray, solid, open, and green rectangles indicate the transit peptide (TP), CAS, FLAG-tag, and Clover-tag, respectively. Clover-tag was connected using flexible linker with 18-bp nucleotide acid (CCGCCGCGCCGGGGCCCG). (F) Accumulation of LCIA, HLA3, and CAS in WT, H82, and FN-1 cells was recorded. Cells were grown in LC conditions for 12 h. (G) Localization of FLAG-CAS in FN-1 using an indirect immunofluorescence assay with an antibody against FLAG as the primary antibody. Cells grown in HC conditions were shifted to LC conditions for 12 h in the light. Each image is placed with the flagella facing upward on the panel. “Merged” indicates the merger of a fluorescence image with its differential interference contrast (DIC) images. (Scale bars: 5 µm.) (H) Accumulation of LCIA, HLA3, and CAS in WT, H82, and its complemented strain expressing Clover-tagged CAS (CL-1). Cells were grown in LC conditions for 12 h. (I) Localization of CAS-Clover fluorescence in CL-1 strain. (Scale bars: 5 µm.) (J) Changes of CAS localization in cells from LC to HC in time course in WT cells by an indirect immunofluorescence assay. LC-acclimated cells were shifted to HC conditions for 2 and 12 h in the light before sampling. (Scale bars: 5 µm.) (K and L) Accumulation of proteins in WT cells grown in the ethanol (mock, solvent used for inhibitors), DCMU (10 µM), and DBMIB (10 µM). HC-grown WT cells were centrifuged, resuspended by fresh HSM medium containing DCMU (10 µM) or DBMIB (10 µM), and switched to LC conditions for 2 h. (M) The effect of CHX on the accumulation of CAS and LCIB. For CHX treatment, cells grown in HC-light conditions were transferred to LC-light conditions for 12 h. Cells acclimated to LC-light conditions were subjected to LC-dark conditions for 2 h, and then the dark-acclimated cells were transferred to LC-light conditions for 2 h. The chemical was added to the medium when light or CO2 conditions were changed. (N) Localization of CAS and LCIB in the presence of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) or 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA) using an indirect immunofluorescence assay. WT cells were grown in LC conditions for 2 h. The chemical was added to fresh medium before shifting from HC to LC conditions. (Scale bars: 5 µm.) (O) Accumulation of CAS in WT and C16 cells grown in HC or LC conditions for 12 h in light. (P) Localization of LCIB in WT, H82, AH-1, and C-1 cells using an indirect immunofluorescence assay. HC-grown cells were transferred to LC or very LC (VLC) conditions for 12 h. AH-1 cells were cultured in HSM medium with NO3– (HSM-NO3–) as the nitrogen source. (Scale bars: 5 µm.) (Q) Measurement of Ci concentrations in the HSM medium with NH4+ (HSM-NH4+) as the nitrogen source or HSM-NO3– medium after culturing cells in 0.04% or 0.003% CO2 for 12 h. Blank means the Ci concentrations in culture medium without cells. Data in all experiments are mean values ± SD from three biological replicates. ***P = 0.07 by Student’s t test. (R) Localization of D1 in HC-, LC-, and VLC-grown WT and H82 cells using an indirect immunofluorescence assay. (Scale bars: 5 µm.) (S) Accumulation of D1 in HC-, LC-, and VLC-grown WT and H82 cells.
Fig. 3.
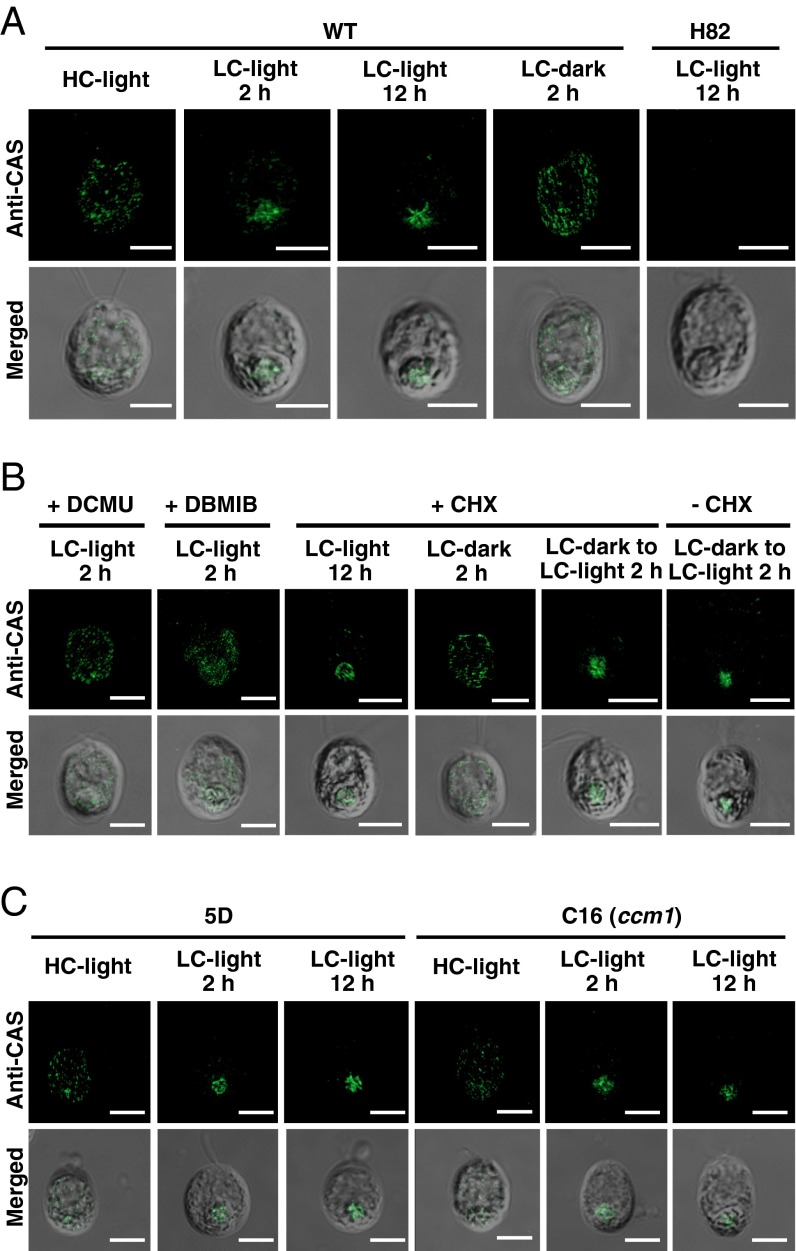
Subcellular localization of CAS. (A) Localization of CAS in WT and H82 cells was assessed using an indirect immunofluorescence assay with an anti-CAS antibody. Cells grown in high-CO2 (HC) conditions were shifted to low-CO2 (LC) conditions for 2 h or 12 h in light, and then the LC-acclimated cells (12 h) were transferred from light to dark for 2 h in the LC condition. (B) The effect of dichlorophenyl-dimethylurea (DCMU) (10 µM), 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) (10 µM), or cycloheximide (CHX) (10 µg⋅mL–1) on the localization of CAS in WT cells. The cells cultured in each condition used in A were also used for these drug treatments. The HC-grown cells were incubated under LC-light conditions in the presence of DCMU or DBMIB for 2 h. For CHX treatments, HC-grown cells were transferred to LC-light conditions in the presence of CHX for 12 h. The LC-light–acclimated cells were subjected to LC-dark conditions in the presence of CHX for 2 h. The LC-dark–acclimated cells were transferred to LC-light conditions with or without CHX for 2 h. (C) Localization of CAS in a CCM1 insertion mutant, C16, and its parental 5D strain using an indirect immunofluorescence assay. Cells grown in HC conditions were shifted to LC-light conditions for 2 h or 12 h. For all panels, each image is placed with the flagella facing upward on the panel. Merged images show the fluorescence image superimposed with its differential interference contrast image. (Scale bars: 5 µm.)
Changing localization in the chloroplast in response to CO2 was also shown in the case of LCIB, and light as well as CO2 could affect its localization (17). Similarly, the aggregated fluorescent signals of CAS in LC-acclimated cells became diffuse after transferring from LC-light to LC conditions in the dark (LC-dark) within 2 h (Fig. 3A and Fig. S3I). Considering the fact that the localization of CAS to the pyrenoid (as well as the accumulation of HLA3, LCIA, and LCIB) was inhibited in the presence of dichlorophenyl-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) (Fig. 3B and Fig. S3 I, K, and L) and that these inhibitors also suppress the CCM (34), the localization of CAS to the pyrenoid could be important for regulation of the CCM in LC-light conditions where the CCM was active.
Next, to determine whether the aggregation of CAS in LC-light is associated with de novo protein synthesis, the effect of cycloheximide (CHX) was examined. By switching from HC-light to LC-light conditions or from LC-light to LC-dark conditions, a change in the localization of fluorescent signals derived from CAS was observed in the presence of CHX (Fig. 3B). These results suggested that the LC-induced CAS relocation was not associated with de novo protein synthesis, in contrast to the case of LCIB (35). Moreover, the addition of CHX inhibited the accumulation of LCIB but not CAS in LC conditions (Fig. S3M), and the addition of BAPTA and W-7 did not affect CAS localization (Fig. S3N). Because the accumulation of CAS and its relocation in response to CO2 were not impaired in strain C16, a CCM1 insertion mutant (6) (Fig. 3C and Fig. S3O), relocation of CAS was regulated by external CO2 concentration irrespective of CCM1/CIA5 function. Additionally, the impaired photosynthetic Ci affinity in H82 cells caused poor Ci consumption in the culture medium, affecting the localization of LCIB in LC conditions (Fig. S3 P–S and SI Results and Discussion).
Increased Ca2+ Concentrations in the Pyrenoid in LC-Light Conditions.
Because CAS had low Ca2+-binding affinity (20), a high Ca2+ concentration should be required to bind Ca2+. This aspect of CAS raised the possibility that the subcellular regions where free Ca2+ is enriched could be related to CAS localization. To test this hypothesis, we monitored the fluorescence of Calcium Green-1, AM, a Ca2+-sensitive fluorescent dye, in WT and H82 cells (Fig. 4 and Fig. S4). In both HC and LC conditions, apparent fluorescent signals were detected in regions that overlapped with chlorophyll. Notably, distinct high levels of fluorescent signals were observed in the region of the pyrenoid in both WT and H82 cells, especially in LC-light conditions, thereby implying that free Ca2+ might be concentrated in the pyrenoid. These increased fluorescent signals in LC-light conditions were not impaired by the CAS mutation (Fig. 4 and Fig. S4A) as well as BAPTA and W-7 (Fig. S4B). In contrast, the fluorescent signals in the pyrenoid were decreased after shifting from LC-light to LC-dark conditions for 2 h. Additionally, the signal intensities of Ca2+ indicator in chloroplast were slightly stronger in H82 than in WT cells after switching to LC conditions for 12 h (Fig. 4 and Fig. S4A), suggesting a higher free Ca2+ concentration in the chloroplast of H82 cells. Considering that depletion of CAS protein does not affect the total cellular Ca2+ content (25), increased free Ca2+ could be caused by the redistribution of internal Ca2+.
Fig. 4.
Calcium Green-1, AM fluorescence in WT and H82 cells. Cells were grown in high-CO2 (HC) or low-CO2 (LC) conditions for 12 h in light, or cells in LC-light were transferred to LC-dark conditions for 2 h, and then incubated with Calcium Green-1, AM (CG-1) at room temperature for 30 min. Fluorescence images derived from CG-1 treatments and chlorophyll (CHL) are shown. Each image is placed with the flagella facing upward on the panel. DIC, differential interference contrast image. (Scale bars: 5 µm.)
Fig. S4.
Calcium Green-1, AM fluorescence in WT and H82 cells (A), the effect of 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA) or N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) on the fluorescence of Calcium Green-1, AM (B). (A) Cells were grown in high-CO2 (HC) or low-CO2 (LC) conditions for 2 h or 12 h in light. Cells in LC-light (12 h) were transferred to LC-dark conditions for 2 h. (Scale bars: 5 µm.) (B) Cells were grown in LC-light conditions for 2 h. The chemical being tested was added to fresh medium before shifting from HC to LC conditions. CG-1 and CHL represent fluorescence derived from Calcium Green-1, AM and chlorophyll, respectively. Each image is placed with the flagella facing upward on the panel. DIC, differential interference contrast image. (Scale bars: 5 µm.)
SI Results and Discussion
High CO2-Requiring Phenotype of H82 Cells Is Partially Rescued in Dim Light.
It was proposed that the CAS interacts with a proton gradient regulation-like 1 protein (PGRL1) in a CEF complex in anaerobic conditions (26), and a PGRL1 insertion mutant exhibited a light-dependent HC-requiring phenotype in spots tests (39). To examine whether the HC-requiring phenotype of the CAS insertion mutant H82 was dependent on light intensity, the growth rate was measured on agar plates at different light intensities (Fig. S1E). When exposed to 0.04% CO2 and dim light (10 µmol photons·m–2·s–1), H82 cells showed a slower growth rate than complemented strains (C-1 and C-2), but the growth rate of H82 cells in dim light was increased, compared with that of it in either mild light (120 µmol photons·m–2·s–1) or strong light (300 µmol photons·m–2·s–1) conditions. As a control, the growth rate of PGRL1 mutant (L-1) was equivalent to that of its complemented strain (L-1C) in dim light but was retarded in mild light and strong light conditions. These results indicated that light intensity had an effect on the HC-requiring phenotype of H82 cells and that they were more sensitive to CO2-limiting stress than the L-1 strain in dim light. In the presence of 5% CO2, L-1 cells showed obviously retarded growth compared with L-1C cells in strong light, in which H82 cells showed a similar growth rate to that of C-1 and C-2 cells. These results indicated that L-1 cells were more sensitive to high light stress than H82 cells.
It was reported that the defects in photoacclimation by down-regulated CAS could be rescued by the addition of excess Ca2+ (3.06 mM) in the culture medium (25). To examine whether the addition of excess Ca2+ could also rescue the retarded growth rate of H82 cells in LC conditions, the doubling times of WT, H82, and its complemented strains were measured in HSM medium (0.068 mM Ca2+) or with 45-times higher levels of Ca2+ (3.06 mM). However, the addition of excess Ca2+ did not affect the growth rate of H82 cells, as well as that of WT and the complemented strains (Fig. S1B). Considering that chelating the extracellular Ca2+ decreased the Ci affinity of WT cells in LC conditions (Fig. 2B), the accumulation level of CAS could be important for restoring the growth of H82 cells by the addition of excess Ca2+.
Cyclic Electron Flow Activity Is Not Inhibited by the Lack of CAS in LC Conditions.
It was reported that photosynthetic cyclic electron flow (CEF) is increased in CO2-limiting conditions (28), as well as in anaerobic conditions where down-regulation of CAS resulted in a strong inhibition of CEF activity (26). To investigate whether disruption of CAS also impaired CEF activity in LC conditions, we measured light-induced absorption changes at 705 nm, which reflect the redox state of P700, in H82 and C-1 cells. The P700 of the cells was oxidized by actinic light illumination whereas was reduced by CEF after cessation of actinic light illumination (dark-recovery) in the presence of dichlorophenyl-dimethylurea (DCMU), which blocks electrons from PSII. The dark-recovery kinetics were significantly slowed down in the presence of 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB), which blocks electrons in CEF (40) (Fig. S1F), suggesting that both H82 and C-1 strains have CEF activity. In LC conditions for 12 h, H82 cells showed a comparable half-time (t1/2) of rereduction to C-1 strain after cessation of the actinic light in the presence of DCMU (Fig. S1F), suggesting that the CEF is not affected by the lack of CAS protein, at least in the LC conditions we examined, which was consistent with the previous report that the down-regulation of CAS did not impaired the CEF activity in aerobic conditions (26).
Ca2+-Binding Activity of Chlamydomonas CAS.
Arabidopsis CAS (AtCAS) has Ca2+-binding sites in its N terminus (20). To evaluate the Ca2+-binding activity of Chlamydomonas CAS (CrCAS) with that of AtCAS, the recombinant proteins for the N and C terminus of CrCAS and N terminus of AtCAS were prepared (Fig. S2A). When blotting with 45Ca, a radioactive signal was detected with the N terminus of CrCAS, as in the case of the N terminus of AtCAS, but not the C terminus of CrCAS (Fig. S2B). After adding an extra 10 mM CaCl2 to the binding solution, the radioactive signal decreased (Fig. S2C), presumably because of competition with 45Ca for the Ca2+-binding site of CAS. These results indicated that the N terminus of CrCAS has Ca2+-binding activity.
CAS Is Exposed to the Stromal Side of the Thylakoid Membrane.
To determine the topology of CrCAS in the thylakoid membrane, intact thylakoid membranes were isolated, treated with trypsin, and analyzed by immunoblotting using two anti-CAS antibodies that could specifically detect the N-terminal or C-terminal domains of CAS. Both the N terminus and C terminus of CAS were degraded by trypsin and were not detected (Fig. S3D). This result suggested that CAS could be anchored to the thylakoid membrane, and both the Ca2+-binding domain in the N terminus and the rhodanese-like domain in the C terminus were exposed to the stromal side, which was similar to the case of AtCAS (20). In contrast, a thylakoid lumen-localized carbonic anhydrase, CAH3 (41), was not affected significantly by trypsin.
Poor Ci Consumption in the Culture Medium of H82 Locked the Mutant Cells in LC State and Caused Different LCIB Location.
To further examine alternative causes of decreased Ci affinity of H82 cells, LCIB localization in LC-light conditions was focused on because previous reports showed that aberrant LCIB localization occasionally led to decreased photosynthetic Ci affinity (35). As in the previous report (16, 17, 42), indirect immunofluorescence signals derived from an anti-LCIB antibody were observed as a ring-like structure around the pyrenoid in WT and C-1 cells aerated with ordinary air (0.04% CO2) (Fig. S3P). This aggregated localization of LCIB was not affected in the presence of 1,2-bis(o-aminophenoxy)ethane-N,N,N,N′-tetraacetic acid (BAPTA), a membrane-impermeable Ca2+-specific chelator (31), or N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7), a membrane-permeable calmodulin antagonist (Fig. S3N). In contrast, fluorescent signals were not aggregated but dispersed mainly along the chloroplast envelope in H82 and AH-1 cells (Fig. S3P). These results suggested two possibilities. First, CAS directly regulates LCIB relocation, or second, the CO2 acclimation state in H82 cells is locked in the low-CO2 (LC) state where LCIB is dispersed in the chloroplast but not in very-low-CO2 (VLC) state where LCIB shows a ring-like structure around the pyrenoid. To examine these possibilities, we measured the Ci concentrations in medium without cells or that cultured with WT, H82, and C-1 cells aerated with ordinary air for 12 h (Fig. S3Q). Although the Ci concentrations in medium without cells was 51.3 µM, the Ci concentrations in medium cultured with WT and C-1 cells were decreased to 6.8 µM and 7.2 µM, respectively, corresponding to the VLC state (12, 14, 15), and a large portion of Ci could be removed from the medium by active Ci uptake of cells. In contrast, that of H82 and AH-1 cells was 42.3 µM and 38.1 µM, which were in the range of the LC state (14, 15). These results suggested that the aberrant localization of LCIB in H82 cells could be caused by remaining Ci concentrations in the cultured medium, which were not at a sufficiently low level to induce the aggregated localization of LCIB around the pyrenoid. Actually, when H82 and AH-1 cells were cultured with 0.003% CO2 for 12 h, in which the Ci concentrations were 1.8 µM and 2.9 µM, respectively, these cells also showed a proper ring-like structure of LCIB around the pyrenoid (Fig. S3P). To compare the localization of photosynthetic proteins with the dynamic relocation of the LCIB and CAS in response to CO2 and light (Fig. 3 and Fig. S3 I, J, and P), the accumulation and fluorescence signals of the photosystem II reaction center D1 were observed. Neither the accumulation nor the localization of D1 was affected by the CO2 concentrations and by the defect of CAS (Fig. S3 R and S) although we cannot rule out the possibility that other photosynthesis-related proteins other than D1 could change their localization in response to CO2 and light. This point should be clarified in the further analyses.
Tentative Model for the Function of CAS in Regulation of CCM.
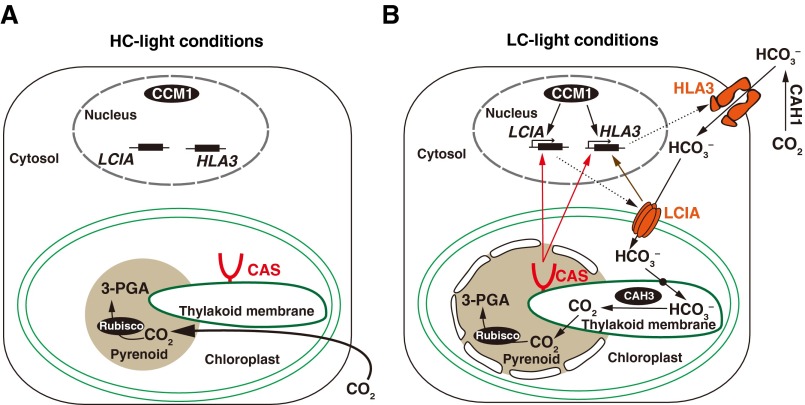
Based on the results obtained in this study, we proposed a function of CAS for CO2- and Ca2+-mediated regulation of the CCM in Chlamydomonas cells (Fig. S5). In HC conditions, Chlamydomonas cells can obtain sufficient amounts of CO2 for photosynthesis by the passive diffusion of extracellular CO2 (Fig. S5A). In LC-light conditions, reduced extracellular Ci concentrations cause an internal CO2-limiting stress and decreased photosynthetic activity (Fig. S5B). To acclimate to this stress, CCM1/CIA5 rapidly induces the expression of CCM-related genes, including HLA3 and LCIA, within 0.3 h (9). In parallel, in response to the LC stress in light, Ca2+-binding protein CAS changes its localization into the pyrenoid. This change in localization could be dependent on the photosynthetic electron transfer because of the inhibition by DCMU and DBMIB (Fig. 3B). Then, CAS mediates a retrograde signal from the chloroplast to the nucleus to maintain the expression of HLA3 and LCIA. It is possible that the rhodanese-like domain in the C-terminal regions could be related to signal transduction. Considering that AtCAS was proposed to mediate the transient elevation of [Ca2+]cyt and [Ca2+]stro (22) and that perturbation of intracellular Ca2+ homeostasis impaired the accumulation of HLA3 and LCIA (Fig. 2A and Fig. S2D), the internal Ca2+ signal could be mediated by CAS as a retrograde signal. In addition, extracellular Ca2+ is necessary for the accumulation of HLA3 and LCIA (Fig. 2A). Finally, cells obtain concentrated Ci by transporter-mediated uptake of and increase CO2 concentrations around Rubisco in LC conditions.
Fig. S5.
A tentative model illustrates how CAS protein regulates the expression of HLA3 and LCIA during the CCM induction by shifting from high-CO2 (HC)-light (A) to low-CO2 (LC)-light (B) conditions. The red structure indicates the CAS protein. The CAS-meditated pathways involved in regulating expression of HLA3 and LCIA are shown as red arrows. The LCIA-mediated pathway involved in regulating expression of HLA3 is indicated as brown arrows. CAH, carbonic anhydrase; PGA, phosphoglyceric acid.
Discussion
In this study, we identified a Ca2+-binding protein as a regulator of the CCM by characterization of a CAS insertion mutant, H82, and its complemented strain, C-1. RNA-seq analyses of these strains revealed that CAS is required for maintaining the expression of 13 nuclear-encoded LC-induced genes after induction (Table S3). Of those genes regulated by CAS, HLA3 and LCIA, whose accumulation was decreased in H82 cells (Fig. 1D), are involved in uptake for operation of the CCM (11–14). However, simultaneous expression of HLA3 and LCIA could only partially rescue the decreased Ci affinity of H82 cells (Fig. 1A), suggesting that some of the 13 CO2-limiting–inducible genes other than HLA3 and LCIA could contribute to the operation of the CCM in Chlamydomonas cells.
Thylakoid-membrane–localized CAS changed its localization from dispersed in HC-light or LC-dark conditions, where the CCM was inactive, to an aggregated tubule-like structure in the pyrenoid in LC-light conditions, where the CCM is active (Fig. 3A and Fig. S3I), which means that the aggregation of CAS to the pyrenoid in response to the availability of environmental light and LC is important for regulation of the CCM. Considering that some of the thylakoid membrane penetrates the pyrenoid, which are termed pyrenoid tubules (36), it is possible that CAS could be localized in or along the pyrenoid tubules in LC-light conditions and could change its localization reversibly in response to CO2 conditions. The change in CAS localization was independent of de novo protein synthesis (Fig. 3B), which was unlike the case of LCIB (35), suggesting that aggregated CAS is not newly synthesized and that CAS itself could move in response to light and CO2 conditions. This distinct localization of CAS could be explained by the thylakoid membrane remodeling observed previously in varying light conditions (37) although the actual mechanism for the change in localization requires further analysis.
Previously, based on the fact that the accumulation of chloroplast envelope-localized LCIA is required for the expression of HLA3, unknown retrograde signals from the chloroplast to nuclear gene have been suggested to support the CCM (12). This study further revealed a regulatory pathway related to chloroplast-retrograde signaling from the thylakoid- and/or pyrenoid-tubule–localized CAS to nuclear genes because CAS was essential to maintain the expression of 13 genes possibly important for operation of the CCM, including both HLA3 and LCIA. Additionally, the fact that accumulation of CCM1 was not inhibited by the loss of CAS protein (27), and vice versa (Fig. S3O), and that CAS could relocate to pyrenoid tubules in response to LC-light irrespective of CCM1 suggest that CCM1 and CAS could function in parallel to each other in the regulation of CCM, including HCO3– transporters (Fig. S5 and SI Results and Discussion).
When chelating external Ca2+ by application of BAPTA, the accumulation of HLA3 and LCIA was decreased, and the addition of CaCl2 restored accumulation (Fig. 2A). Because BAPTA cannot permeate the plasma membrane, it remains in the extracellular space (31) and prevents the elevation of both [Ca2+]cyt and [Ca2+]stro (22), meaning that the elevation of intracellular Ca2+ is required for the accumulation of these two HCO3– transporters. In terrestrial plants, the observation that CO2-induced changes in [Ca2+]cyt and stomatal closing were attenuated by chelating or without adding external Ca2+ (19) leads to a hypothesis for the participation of [Ca2+]cyt in CO2 signal transduction in guard cells. Considering that CAS also mediates transient elevation of [Ca2+]cyt and [Ca2+]stro in Arabidopsis (21, 22), it is possible that the regulation of these transporters by CAS could be through Ca2+ signals resulting from the influx of [Ca2+]ext into Chlamydomonas cells.
Moreover, with the use of a Ca2+ indicator, free Ca2+ could be concentrated in the pyrenoid, especially in LC-light conditions (Fig. 4 and Fig. S4A). Because the Ca2+-binding characteristics of CAS involve low affinity and high capacity (20), the coexistence of CAS and higher concentrations of Ca2+ in the pyrenoid could be important to activate CAS function. A rhodanese-like domain conserved in the C terminus of CAS is thought to exhibit a regulatory function rather than an enzymatic one (18), which might account for the consequent signal transduction followed by binding of Ca2+ at its N terminus. Furthermore, because the chloroplastic Ca2+ has been shown to be important for the chloroplast metabolism and the function of the thylakoid (18), where CAS protein is localized (Fig. S3B), it is possible that the absence of CAS could cause damage on thylakoid or pyrenoid structure in H82 cells in LC conditions. Of note, stronger fluorescent signals derived from a Ca2+ indicator in the pyrenoid in LC-light conditions was also observed in H82 cells (Fig. 4 and Fig. S4A), which was not affected by perturbation of intracellular Ca2+ homeostasis (Fig. S4B), suggesting that the change in Ca2+ concentration is not directly regulated by CAS and could act upstream of the Ca2+ signal for regulation of the CCM.
In addition to HLA3 and LCIA, the mRNA abundance and accumulation of LHCSR3 were significantly decreased by the impairment of CAS in LC conditions at 2 h (Table S3 and Fig. S1 I and J), supporting the previous finding that CAS knock-down Chlamydomonas strains showed decreased levels of LHCSR3 accumulation (25). Considering that the accumulation of HLA3, LCIA, and LHCSR3 was simultaneously decreased by the application of BAPTA or W-7 in LC at 2 h, the expression of these proteins could be regulated by CAS protein or Ca2+ signal in a similar way.
In contrast to CCM1/CIA5, CAS is highly conserved in vascular plants, as well as green eukaryotic algae, over the course of evolution. Our findings showed that chloroplast-mediated retrograde signaling pathways via CAS were already developed in the green algae lineage, which could throw light on understanding the cross-talk between Ca2+- and CO2-dependent signal transduction pathways in photosynthetic organisms. Furthermore, considering that the induction of the CCM is dependent on limiting-CO2 conditions, as well as on light intensity (29), the relationship between CO2-limiting stress and high-light stress could be clarified by further analyses of this CAS mutant. The trigger of relocation of CAS into the pyrenoid, as well as Ca2+ in response to changes in the availability of CO2 and light, could be a key regulatory factor for the CO2-sensing mechanism in photosynthetic eukaryotes.
Materials and Methods
C. reinhardtii strain C-9 (mt–) was used as a WT line (6). CCM1 insertion mutant C16, as well as its parental 5D strain (6), and a PGRL1 insertion mutant (38) were characterized previously. For the complementation assay, the DNA genomic fragment was amplified using specific primers (Table S4). The genotypes of strains used in this study are listed in Table S5. In physiological assays, cells were cultured in Tris acetate-phosphate liquid medium for preculture and subcultured in modified high-salt medium () supplemented with 20 mM 3-(N-morpholino)propanesulfonic acid (Mops) to midlog phase (OD730 0.3 to 0.5) for photoautotrophic growth. For all culture conditions without other conditions specified, cells were cultured at 25 °C with illumination at 120 µmol photons∙m–2∙s–1.
Table S4.
Sequences of primers used in this study
| Primer name | Sequence |
| CF1 | 5′-CTGACACACGTTACCACACAGCACCAC-3′ |
| CR0 | 5′-ATCCGTAACCACTTCGCGCTCGTTC-3′ |
| GST-CAS-F1 | 5′-CCCGGGTCGACTCGAGGATGAGCTGGACTCCACTGTGG-3′ |
| GST-CAS-R1 | 5′-GATGCGGCCGCTCGAGCGAGCGGGGGGCGGGCAG-3′ |
| GST-CAS-F2 | 5′-CCCGGGTCGACTCGAGCGCGGCTACGCCGGCGAC-3′ |
| GST-CAS-R2 | 5′-GATGCGGCCGCTCGAGCTCGCCCAGAGTGACGGGGT-3′ |
| GST-AtCAS-F | 5′-ATTCCCGGGTCGACTCGAGGTTTCACTTCCAACATCAACTTCAATCATCTCT-3′ |
| GST-AtCAS-R | 5′-ACGATGCGGCCGCTCGAGCGTATCCATGGTCGATGAAGCAATC-3′ |
| FLAG-CAS-F1 | 5′-ATCGATAAGCTTGATATCGACTACAAGGACGACGACGACAAG GATGAGCTGGACTCCACTGTGG-3′ |
| FLAG-CAS-F2 | 5′-ATCGATAAGCTTGATGGGGCTACTCGAACGCATGAG-3′ |
| FLAG-CAS-R1 | 5′-CTGCAGGAATTCGATGTGATGGAACATGGCAAGGGGCA-3′ |
| FLAG-CAS-R2 | 5′-GTCCTTGTAGTCGATGTCGTGGTCCTTGTAGTCGCCGTCGTGGTCCTTGTAGTCAGCGCGGGCAGCAGACGCCT-3′ |
| CAS-Clover-F | 5′-TTTGCAGGATGCATATGCAGCTTGCTAACGCTCCT-3′ |
| CAS-Clover-R | 5′-CGATGACGTCAGATCTCGAGCGGGGGGCGGGCAG-3′ |
| pTY2b-CAS-F | 5′-AGTCACTCGAGATCTGGGGCTACTCGAACGCAT-3′ |
| pTY2b-CAS-R | 5′-CTTGATATCGAATTCTTACGAGCGGGGGGCGGG-3′ |
Table S5.
Genetic information of strains used in this study
| Name of strain | Genotype | Promoter and 3′ UTR used for gene expression | Source |
| C-9 | WT | N.A. | (6) |
| H82 | cas::aphVII | N.A. | (27) |
| C-1 | cas:: aphVII, CAS | Native promoter and 3′ UTR of CAS | This study |
| C-2 | cas:: aphVII, CAS | Native promoter and 3′ UTR of CAS | This study |
| #1–#12 | cas:: aphVII, CAS | Tandemly duplicated enhancer elements of NIA1 fused with minimal β2-tubulin promoter and RbcS2 3′ UTR in plasmid pTY2b | Ref. 12 and this study |
| AH-1 | cas:: aphVII, HLA3, LCIA | Tandemly duplicated enhancer elements of NIA1 fused with minimal β2-tubulin promoter and RbcS2 3′ UTR in plasmid pTY2b | Ref. 12 and this study |
| FN-1 | cas:: aphVII, FLAG-CAS | Native promoter and 3′ UTR of CAS | This study |
| CL-1 | cas:: aphVII, CAS-Clover | Hsp70A fused with RbcS2 (AR) promoter and RbcS2 3′ UTR in plasmid pOpt_Clover_Hyg | Ref. 33 and this study |
| 5D | nit1-305, cw-15 | N.A. | (6) |
| C16 | ccm1:: Nia1 | N.A. | (6) |
| L-1 | pgrl1:: aphVIII | N.A. | (38) |
| L-1C | pgrl1:: aphVIII, PGRL1 | Native promoter and 3′ UTR of PGRL1 | (38) |
N.A., not applicable.
Additional information is described in the SI Materials and Methods.
SI Materials and Methods
Strains and Culture Conditions.
For maintenance, Chlamydomonas strains were cultured at 22 °C with light intensity below 10 μmol photons·m–2·s–1 on Tris-acetate-phosphate (TAP) agar plates. For physiological and biochemical experiments, a 5-mL volume of cells was precultured mixotrophically in TAP medium with shake at 25 °C with 60 μmol photons·m–2·s–1 for 24 h. Then, cells were centrifuged at 600 × g, and pellets were resuspended in 50 mL of modified high-salt medium supplemented with 20 mM 3-(N-morpholino)propanesulfonic acid (Mops) (HSM-NH4+) in high-CO2 (HC) (5% CO2) conditions at 120 μmol photons·m–2·s–1. For LC induction, HC-acclimated cells were centrifuged, resuspended in 50 mL of fresh HSM medium, and cultured in low-CO2 (LC) (0.04% CO2) conditions at 120 μmol photons·m–2·s–1 for indicated time periods. For induction of HLA3 and LCIA in the AH-1 strain, the HC-acclimated cells were centrifuged, washed with a small amount of HSM (NO3–) medium, resuspended in 50 mL of HSM (NO3–) medium, and cultured in HC conditions for 24 h. In all experiments, cell density was kept in midlog phase (approximately OD730 = 0.4) before harvesting the cells for analyses.
Plasmid Construction.
To generate the expression plasmid for CAS fused with FLAG sequence with its own promoter, two genomic fragments were amplified by primer set FLAG-CAS-F2/R2 for promoter and transit peptide sequence and by FLAG-CAS-F1/R1 for the remaining sequence of CAS. The FLAG sequence was included in primers FLAG-CAS-F1 and FLAG-CAS-R2. These two fragments were then simultaneously cloned into pBlueScript II SK(+) using an In-Fusion reaction (Clontech). In the case of expression plasmid for Clover-tagged CAS, the genomic fragments (Fig. S3E) of CAS were amplified using primer set CAS-Clover-F/R and cloned into the pOpt_Clover_Hyg plasmid (33). To generate recombinant plasmid pTY2b-CAS plasmid, the genomic fragments of CAS were amplified using primer set pTY2b-CAS-F/R and cloned into the pTY2b-aphVIII vector (12). In this plasmid, the transcription was driven by tandemly duplicated enhancer elements of the nitrate reductase gene (NIA1), as well as the minimal promoter of the β2-tubulin gene (TUB2), and terminated by 3′ UTR of the small subunit of Rubisco (RBCS2). The N-terminal chloroplast transit peptide was determined as amino acids 1 to 54 of CrCAS, as described previously (23). All PCR reactions were performed using Prime STAR GXL DNA Polymerase (Takara), and the nucleotide sequences were verified by sequencing. The fragments containing the promoter, tagged CAS sequence, and 3′ UTR were amplified and cotransformed into H82 cells with a paromomycin resistance gene cassette, aphVIII. In the case of Flag-tagged CAS, the native promoter of the CAS gene was used. The nucleotide sequences of primers are listed in Table S4.
Transformation and Complementation of H82.
Genomic sequences of CAS with its own predicted promoter (∼3.5 kb upstream the 5′ UTR) were amplified using the primer sets CF1 and CF0 (Table S4) and introduced into H82 cells with the paromomycin resistance gene (aphVIII) cassette. Alternatively, a recombinant plasmid pTY2b-CAS-aphVIII was introduced into H82 cells. Transformation of cells was performed by electroporation using a NEPA 21 electroporator (NEPAGENE). The transformants were incubated at 25 °C for 12 h with gentle shaking under an illumination of less than 1.5 μmol photons·m–2·s–1 and then spread over TAP plates containing 10 μg⋅mL–1 paromomycin. After culturing at 25 °C in 80 μmol photons·m–2·s–1 for 6 d, colonies that appeared were subjected to the growth test screening as described previously (27). Briefly, cells were cultured in HC conditions at 120 μmol photons·m–2·s–1 to logarithmic phase in a 96-well microtiter plate containing liquid HSM medium, diluted to optical density OD700 = 0.03 to 0.05, and then transferred to LC conditions for 24 h. OD700 was measured using a microtiter plate reader XFluor4 (TECAN), and transformants showing similar OD700 with that of WT were selected as complemented candidates.
Photosynthetic Oxygen Evolution and Ci Uptake.
For evaluating the affinity for inorganic carbon (Ci), the rate of dissolved Ci-dependent photosynthetic oxygen (O2) evolution was measured. Cells harvested after growth in HC and LC conditions were suspended in Ci-depleted 20 mM Mes-NaOH buffer (pH 6.2), Mops-NaOH buffer (pH 7.0), and Hepes-NaOH buffer (pH 7.8) at 10 µg⋅mL–1 chlorophyll. Photosynthetic O2 evolution was measured by applying a Clark-type O2 electrode (Hansatech Instruments), as described preciously (29). Ci uptake was measured using the silicone oil centrifugation method, as described previously (12). Each value was corrected by evaluating the cell volume as sorbitol impermeable space (SIS) using [14C]sorbitol and 3H2O.
Immunoblotting Analysis.
Extracted total proteins suspended in SDS loading buffer containing 50 mM Tris⋅HCl (pH 8.0), 25% (vol/vol) glycerol, 2% (wt/vol) SDS, and 0.1 M DTT were incubated at 37 °C for 30 min and subsequently centrifuged at 13,000 × g for 10 min. The supernatant was loaded onto an SDS-polyacrylamide gel electrophoresis (SDS/PAGE) gel for the separation of proteins. Next, proteins were transferred to a Fluoro Trans polyvinylidene fluoride (PVDF) (Pall Life Science) membrane using a semidry blotting system. Membranes were blocked with 5% (wt/vol) skim milk powder (Wako) in PBS. Blocked membranes were washed with PBS containing 0.1% (vol/vol) Tween 20 (PBS-T) and treated with the following antibodies: anti-LCIA (1:5,000 dilution), anti-HLA3 (1:1,000 dilution), anti-LCIB (1:5,000 dilution), anti-CAS (1:5,000 dilution), anti-LHCSR3 (1:5,000 dilution), anti-Histone H3 (1:20,000 dilution), anti-D1 (1:20,000 dilution), anti–H+-ATPase (1:2,500 dilution), and anti-FLAG (1:10,000 dilution). The purified antibody recognizing the C terminus of CAS was generated using the peptide sequence Cys-RTGTTSTRRLPAPRS, and this antibody was used for most of the analyses. The purified antibody recognizing the N terminus of CAS was generated using the peptide sequence Cys-DELDSTVESVVGAVKATG (this study), and it was used only for determining the topology of the N terminus of CAS. Antibodies against D1 (cat no. AS05084A; Agrisera) and H+-ATPase [plasma membrane-localized P2-type proton transporting ATPase (PMH1)] (cat no. AS07260; Agrisera) were purchased from Agrisera. To recognize the primary antibody, a horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Life Technologies) or goat anti-mouse IgG antibody (Life Technologies) was used as a secondary antibody in a dilution of 1:10,000.
RNA-Sequencing Assay.
Total RNA was extracted from cells cultured in HC and LC for 0.3-h conditions or LC for 2-h conditions, using an RNeasy Plant Mini Kit (QIAGEN) in accordance with the manufacturer’s protocol. Each mRNA was obtained from 10 μg of total RNA using Dynabeads Oligo(dT)25 (Life Technologies). The cDNA libraries were prepared using a NEBNext mRNA library prep kit for Illumina (NEB), as described previously (43). Sequencing was performed using a HiSeq 2500 system. The resulting reads were aligned to v5.5 of the Chlamydomonas reinhardtii genome annotation, downloaded from the Joint Genome Institute. Alignments were performed using the Tophat program. The FPKM (fragments per kilobase of exon per million mapped fragments) values were calculated using the Cufflinks program, and significantly differentially expressed transcripts were identified using the Cufdiff program, as described previously (44).
Indirect Immunofluorescence Assay of CAS and Fluorescence Analysis of CAS-Clover.
Cultured cells in midlogarithmic phase were collected by centrifugation and rinsed with PBS buffer twice. Then, 100 µL of each cell suspension was spotted onto poly-l-lysine-treated glass slides (Poly-Prep Slides; Sigma) and air-dried for 5 min at room temperature. Cells were fixed with 4% (wt/vol) formaldehyde in PBS for 20 min at room temperature and then incubated in 100% ice-cold methanol at –30 °C for 20 min to remove chlorophyll. Immunofluorescence staining was performed as described previously (17). Purified antibodies against CAS (C terminus), LCIB, and FLAG were used at dilutions of 1:500, 1:200, 1:500, respectively. After washing, Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies) or Alexa Fluor 488 goat anti-mouse IgG (Abcam) was used with 1:500 and 1:2,000 dilution, respectively. For analysis of Clover fluorescence, CL-1 cells grown in the indicated conditions were used for observations. Finally, digital fluorescence and transmission images were acquired using a confocal fluorescence microscope (TCS SP8; Leica) with a 488-nm laser line and then deconvoluted using Huygens Essential software (Scientific Volume Imaging B.V.).
Expression of Recombinant Protein in Escherichia coli.
A full-length cDNA of CAS from C. reinhardtii or from Arabidopsis thaliana was synthesized using SuperScript III Reverse Transcriptase (Life Technologies). The transit peptide of CrCAS was determined as described previously (23). The transit peptide of AtCAS was predicted using the ChloroP program. The N-terminal and C-terminal sequences were amplified using specific primers (Table S4) and cloned into the XhoI site of a pGEX-6p-1 vector using the In-Fusion reaction (Clontech). Recombinant GST-tagged proteins and GST were expressed in E. coli BL21 (DE3) cells (Life Technologies) by the addition of 0.5 mM isopropylthio-beta-d-galactoside at 20 °C for 6 h. The recombinant proteins were loaded on GSTrap FF columns and cleaved on-column with PreScission Protease (GE Healthcare) for 24 h in accordance with the manufacturer’s instructions. Protein concentrations were measured using a protein assay kit (Bio-Rad) by plotting from the standard curve of BSA.
Calcium-Binding Assay.
Each recombinant protein of 0.42 nmol was separated by 15% SDS/PAGE and transferred onto Fluoro Trans PVDF (Life Science) by electroblotting. GST was used as negative control. The membranes were washed with buffer containing 5 mM MgCl2, 60 mM KCl, and 10 mM Mes-KOH (pH 6.5) and then incubated in the same buffer with the addition of 45CaCl2 (370 MBq⋅mL–1; PerkinElmer) to 74 kBq·mL–1 for 10 min, and rinsed in 50% (vol/vol) ethanol for 5 min. The protein-bound 45Ca was exposed to an imaging plate (Fuji) for 24 h and visualized by autoradiography.
Subcellular Fractionation.
The C-9 strain was used to isolate the soluble and insoluble fractions. Cells grown in HC or LC conditions for 12 h were collected. To separate into soluble and insoluble fractions, cells were disrupted by sonication and centrifuged for 5 min at 2,000 × g to remove the unbroken cells. The supernatants were centrifuged at 60,000 × g for 1 h at 4 °C to obtain soluble (supernatant) and insoluble fractions (pellet).
For isolation of thylakoid membranes in small-scale samples, 100 mL of C-9 cells were cultured in HC or LC conditions for 12 h and harvested by centrifugation at 600 × g for 5 min at room temperature. The cells were disrupted by vortexing with 800 mg of Zirconia/Silica beads (0.5-mm diameter; BioSpec Products) for 5 min. To remove unbroken cells and chloroplasts, the supernatants were centrifuged at 760 × g for 2 min at 4 °C and then collected at 5,000 × g for 15 min. The isolation of thylakoid membranes by discontinuous sucrose gradient centrifugation was performed as described previously (12).
To isolate chloroplast envelopes, the intact chloroplast fraction was isolated from 10 L of strain CC-400 cells acclimated to LC conditions. By Percoll gradient centrifugation, the thylakoid fraction and intact chloroplast fraction were obtained from the 20 to 45% (vol/vol) and 45 to 65% (vol/vol) interfaces, respectively. The chloroplast envelopes were isolated from the intact chloroplast fraction, as described previously (12).
Isolation of Thylakoids and Protease Treatment.
The fraction from 20 to 45% (vol/vol) of Percoll gradient centrifugation was used for the isolation of thylakoids for determining the topology of CAS. The isolation of thylakoid membranes by discontinuous sucrose gradient centrifugation was performed as reported previously (12).
The thylakoid fraction (0.05 mg chlorophyll⋅mL–1) was suspended in reaction buffer containing trypsin (Sigma) at the indicated concentrations and incubated on ice for 30 min. Proteolysis was stopped by adding SDS loading buffer and heating at 100 °C for 5 min. The samples were analyzed after denaturing gel electrophoresis and immunoblotting and then probed using antibodies against CAS (N terminal), CAS (C terminal), or CAH3 (41).
Fluorescence Distribution of Calcium Indicator.
HC- or LC-acclimated cells were collected at 600 × g for 5 min and washed once using fresh HSM medium. Then cells were adjust to 1 × 107 cells per milliliter and incubated in Ca2+-free HSM medium with 1 mM EGTA, 15 µM Calcium Green-1, AM, and 0.02% pluronic F-127 at room temperature for 30 min. The EGTA was added to minimize interference coming from the binding of free Ca2+ to the extracellular hydrolyzed Ca2+ indicator. The Calcium Green-1, AM signal was generated by excitation at 488 nm and emission between 500 and 550 nm. Digital fluorescence and transmission images were acquired using a confocal fluorescence microscope (TCS SP8; Leica).
Measurement of Ci Concentration in the Culture Medium.
Cell suspensions were centrifuged at 1,000 × g for 5 min to remove cells from the supernatant. Subsequently, 20 μL of the supernatant was injected directly into the gas-stripping column of the gas chromatograph through a hypodermic needle. Ci concentration was measured using a gas chromatograph (GC-8A; Shimadzu) with a methanizer (MTN-1; Shimadzu), as described previously (29).
Acknowledgments
We thank Gilles Peltier and Xenie Johnson (Commissariat à l'Energie Atomique/Centre National de la Recherche Scientifique) for providing the pgrl1 mutant and its complemented strain; Göran Samuelsson (Umeå University) for the anti-CAH3 antibody; and Michael Hippler (University of Münster) for the anti-LHCSR3 antibody. We also thank Kosuke Tsuda for technical assistance. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 25120714 and 16H04805 (to H.F.) and 25840109 (to T.Y.); the Japan Science and Technology Agency (JST) Advanced Low Carbon Technology Research and Development Program (ALCA) (H.F.); JST Core Research for Evolutional Science and Technology (CREST) (Y.T. and S.-i.O.); and a Cooperative Research grant of the Genome Research for BioResource, NODAI Genome Research Center, Tokyo University of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq raw data (Tables S2 and S3) in this paper have been deposited in the DNA Data Bank of Japan (DDBJ) Sequence Read Archive (DRA) (accession no. DRA004677).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606519113/-/DCSupplemental.
References
- 1.Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT. Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci. 2014;71(5):831–845. doi: 10.1007/s00018-013-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto M, et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol. 2006;8(4):391–397. doi: 10.1038/ncb1387. [DOI] [PubMed] [Google Scholar]
- 3.Hu H, et al. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol. 2010;12(1):87–93, sup pp 1–18. doi: 10.1038/ncb2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones HG. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. 2nd Ed Cambridge Univ Press; Cambridge UK: 1992. [Google Scholar]
- 5.Wang Y, Stessman DJ, Spalding MH. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015;82(3):429–448. doi: 10.1111/tpj.12829. [DOI] [PubMed] [Google Scholar]
- 6.Fukuzawa H, et al. Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA. 2001;98(9):5347–5352. doi: 10.1073/pnas.081593498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang Y, Zhang J, Weeks DP. The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2001;98(9):5341–5346. doi: 10.1073/pnas.101534498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura K, et al. Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2004;135(3):1595–1607. doi: 10.1104/pp.104.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang W, et al. Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell. 2012;24(5):1876–1893. doi: 10.1105/tpc.112.097949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Im CS, Grossman AR. Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J. 2002;30(3):301–313. doi: 10.1046/j.1365-313x.2001.01287.x. [DOI] [PubMed] [Google Scholar]
- 11.Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3- transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2009;106(14):5990–5995. doi: 10.1073/pnas.0812885106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamano T, Sato E, Iguchi H, Fukuda Y, Fukuzawa H. Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2015;112(23):7315–7320. doi: 10.1073/pnas.1501659112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao H, Wang Y, Fei X, Wright DA, Spalding MH. Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii. Plant J. 2015;82(1):1–11. doi: 10.1111/tpj.12788. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Spalding MH. Acclimation to very low CO2: Contribution of limiting CO2 inducible proteins, LCIB and LCIA, to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol. 2014;166(4):2040–2050. doi: 10.1104/pp.114.248294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vance P, Spalding MH. Growth, photosynthesis, and gene expression in Chlamydomonas over a range of CO2 concentrations and CO2/O2 ratios: CO2 regulates multiple acclimation states. Can J Bot. 2005;83(7):796–809. [Google Scholar]
- 16.Wang Y, Spalding MH. An inorganic carbon transport system responsible for acclimation specific to air levels of CO2 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2006;103(26):10110–10115. doi: 10.1073/pnas.0603402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamano T, et al. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 2010;51(9):1453–1468. doi: 10.1093/pcp/pcq105. [DOI] [PubMed] [Google Scholar]
- 18.Hochmal AK, Schulze S, Trompelt K, Hippler M. Calcium-dependent regulation of photosynthesis. Biochim Biophys Acta. 2015;1847(9):993–1003. doi: 10.1016/j.bbabio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca(2+) sensing in guard cells. Nature. 2003;425(6954):196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- 21.Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008;53(6):988–998. doi: 10.1111/j.1365-313X.2007.03390.x. [DOI] [PubMed] [Google Scholar]
- 22.Nomura H, et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun. 2012;3:926. doi: 10.1038/ncomms1926. [DOI] [PubMed] [Google Scholar]
- 23.Allmer J, Naumann B, Markert C, Zhang M, Hippler M. Mass spectrometric genomic data mining: Novel insights into bioenergetic pathways in Chlamydomonas reinhardtii. Proteomics. 2006;6(23):6207–6220. doi: 10.1002/pmic.200600208. [DOI] [PubMed] [Google Scholar]
- 24.Terashima M, Specht M, Naumann B, Hippler M. Characterizing the anaerobic response of Chlamydomonas reinhardtii by quantitative proteomics. Mol Cell Proteomics. 2010;9(7):1514–1532. doi: 10.1074/mcp.M900421-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petroutsos D, et al. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. Plant Cell. 2011;23(8):2950–2963. doi: 10.1105/tpc.111.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terashima M, et al. Calcium-dependent regulation of cyclic photosynthetic electron transfer by a CAS, ANR1, and PGRL1 complex. Proc Natl Acad Sci USA. 2012;109(43):17717–17722. doi: 10.1073/pnas.1207118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Yamano T, Kajikawa M, Hirono M, Fukuzawa H. Isolation and characterization of novel high-CO2-requiring mutants of Chlamydomonas reinhardtii. Photosynth Res. 2014;121(2-3):175–184. doi: 10.1007/s11120-014-9983-x. [DOI] [PubMed] [Google Scholar]
- 28.Lucker B, Kramer DM. Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth Res. 2013;117(1-3):449–459. doi: 10.1007/s11120-013-9932-0. [DOI] [PubMed] [Google Scholar]
- 29.Yamano T, Miura K, Fukuzawa H. Expression analysis of genes associated with the induction of the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2008;147(1):340–354. doi: 10.1104/pp.107.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruyama S, Tokutsu R, Minagawa J. Transcriptional regulation of the stress-responsive light harvesting complex genes in Chlamydomonas reinhardtii. Plant Cell Physiol. 2014;55(7):1304–1310. doi: 10.1093/pcp/pcu068. [DOI] [PubMed] [Google Scholar]
- 31.Polisensky DH, Braam J. Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 1996;111(4):1271–1279. doi: 10.1104/pp.111.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell AM, Coble AJ, Cohen LD, Chng TH, Russo KM. Identification and DNA sequence of a new H+-ATPase in the unicellular green alga Chlamydomonas reinhardtii. J Phycol. 2001;37(4):536–542. [Google Scholar]
- 33.Lauersen KJ, Kruse O, Mussgnug JH. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl Microbiol Biotechnol. 2015;99(8):3491–3503. doi: 10.1007/s00253-014-6354-7. [DOI] [PubMed] [Google Scholar]
- 34.Badger MR, Kaplan A, Berry JA. Internal inorganic carbon pool of Chlamydomonas reinhardtii-evidence for a carbon-dioxide concentrating mechanism. Plant Physiol. 1980;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamano T, Asada A, Sato E, Fukuzawa H. Isolation and characterization of mutants defective in the localization of LCIB, an essential factor for the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Photosynth Res. 2014;121(2-3):193–200. doi: 10.1007/s11120-013-9963-6. [DOI] [PubMed] [Google Scholar]
- 36.Engel BD, et al. Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife. 2015;4:e04889, and correction (2015) 4:e11383. doi: 10.7554/eLife.04889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuartzman SG, et al. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell. 2008;20(4):1029–1039. doi: 10.1105/tpc.107.055830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolleter D, et al. Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell. 2011;23(7):2619–2630. doi: 10.1105/tpc.111.086876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang KV, et al. Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell. 2014;26(7):3036–3050. doi: 10.1105/tpc.114.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alric J, Lavergne J, Rappaport F. Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii (I) aerobic conditions. Biochim Biophys Acta. 2010;1797(1):44–51. doi: 10.1016/j.bbabio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson J, et al. A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998;17(5):1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duanmu D, Wang Y, Spalding MH. Thylakoid lumen carbonic anhydrase (CAH3) mutation suppresses air-Dier phenotype of LCIB mutant in Chlamydomonas reinhardtii. Plant Physiol. 2009;149(2):929–937. doi: 10.1104/pp.108.132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujiwara T, et al. A nitrogen source-dependent inducible and repressible gene expression system in the red alga Cyanidioschyzon merolae. Front Plant Sci. 2015;6:657. doi: 10.3389/fpls.2015.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]