Abstract
Background/Objective
The obesity epidemic appears to be driven in large part by our modern environment inundated by food cues, which may influence our desire to eat. While insulin decreases food intake in both animals and humans, the effect of insulin on motivation for food in the presence of food cues is not known. Therefore, the aim of this study was to evaluate the effect of an intravenous insulin infusion on the brain response to visual food cues, hunger and food craving in non-obese human subjects.
Subjects/Methods
Thirty-four right-handed healthy non-obese subjects (19F/15M, age: 29±8 yrs.; BMI: 23.1±2.1 kg/m2) were divided in two groups matched by age, and BMI: the Insulin Group (18 subjects) underwent a hyperinsulinemic-euglycemic-clamp, and the control group (16 subjects) received an intravenous saline infusion, while viewing high and low-calorie food and non-food pictures during a functional MRI scan. Motivation for food was determined via analogue scales for hunger, wanting and liking ratings.
Results
Food images induced brain responses in the hypothalamus, striatum, amygdala, insula, ventromedial prefrontal cortex (PFC), dorsolateral PFC, and occipital lobe (whole brain correction, P<0.05). Wanting (P<0.001) and liking (P<0.001) ratings were significantly higher for the food than the non-food images, but not different between insulin and saline infusion groups. Hunger ratings increased throughout the MRI scan and correlated with preference for high-calorie food pictures (r=0.70; P<0.001). However neither brain activity nor food craving were affected by hyperinsulinemia or hormonal status (leptin and ghrelin levels) (P=NS).
Conclusion
Our data demonstrate that visual food cues induce a strong response in motivation/reward and cognitive-executive control brain regions in non-obese subjects, but that these responses are not diminished by hyperinsulinemia per se. These findings suggest that our modern food cue saturated environment may be sufficient to overpower homeostatic hormonal signals, and thus contribute to the current obesity epidemic.
Introduction
The United States is currently facing an obesity epidemic, with more than two-thirds of adults being overweight or obese(1). Obesity prevalence has steadily increased in the last four decades(2); which has been ascribed to our modern western lifestyle with easy access to and consumption of high-calorie (HC) foods together with diminished physical activity(3, 4), leading to accumulation of fat stores and hence obesity. In addition, we are continuously surrounded by food-related stimuli (especially visual stimuli) aimed at influencing our desire to eat(5). Viewing of food commercials has been reported to increase food intake in both children and adults(6). Furthermore, increased television(7) and outdoor advertisements(8) have been associated with higher prevalence of adiposity. Thus, such food-related stimuli may serve to promote food cue reactivity and food intake leading to weight gain and obesity.
Circulating nutrients and hormones can directly modulate specific central nervous system (CNS) neurons conveying information about peripheral energy status(9, 10). The CNS receives input about fuel availability (glucose and fatty acid levels) and changes in circulating hormones emanating from the gut, beta cell and adipose depot. The CNS integrates these internal (homeostatic) and external signals (time of the day, food availability, and social context) to eventually determine motivation for food and eating behavior. Specific CNS regions appear to play an important role in this process including: the hypothalamus (feeding centers), limbic system (desire to eat/pleasure), striatum (regulation of motivation and generation of rewarding feelings/sensations), frontal cortex (motivation and executive-control), and motor areas (seeking food behavior and driving the physical act of eating)(11). However, how the CNS specifically processes metabolic and hormonal signals from the periphery and environmental cues to ultimately determine food intake is not well established.
A variety of hormones including insulin, leptin, GLP-1 and ghrelin influence eating behavior directly(9, 10). Circulating insulin in response to food ingestion, in particular, has been reported to influence food intake in animals(12–14). Specifically, insulin acts as a satiety hormone by binding to insulin receptors on neurons regulating feeding, such as the arcuate nucleus of the hypothalamus to promote weight loss(15). In human studies, intranasal insulin has been reported to increase satiety and decrease food intake(16, 17). It is noteworthy, however, that peripheral delivery of insulin using the hyperinsulinemic-clamp technique has yielded opposite results on eating behavior(18). However, these studies were not done in the context of food cues, which are ubiquitous in real-life settings. Thus, the effect of circulating insulin on food motivation and intake in the presence of a visual food stimuli remains to be determined in humans.
This study was undertaken to assess insulin’s capacity to modify the CNS response to a visual food stimulus. For this purpose, we used functional magnetic resonance imaging (fMRI) while participants viewed HC and low-calorie (LC) food and non-food (NF) pictures and then responded to food motivation visual analog rating scales (VAS); a well-established methodological approach for evaluating CNS responses and eating behavior in humans(19–22). Previous studies in healthy individuals have shown that visualization of HC food pictures affects neuronal activation of the dorsal/ventral striatum, orbital frontal cortex and insula(22); brain areas associated with reward motivation, emotional decision, and taste perception, respectively(23). However, how insulin per se acts in this setting remains uncertain. Therefore, this study tested the hypothesis that insulin would diminish brain responses to food pictures in healthy non-obese individuals.
Materials and Methods
Study Participants
Thirty-four right-handed, non-obese, healthy subjects participated in this study. Subjects were randomly assigned to two groups that were age, body mass index (BMI), hemoglobin A1c, and education-matched (Supplemental Table 1). The 1) Insulin Group (INS): 18 subjects (8 males / 10 females, age: 30±7 years; BMI: 23.6±2.1 kg/m2) underwent a hyperinsulinemic-euglycemic clamp and 2) Saline Group (SAL): 16 subjects (7 males / 9 females, age: 29±8 years; BMI: 22.4±2.0 kg/m2) received an intravenous saline infusion (SAL group) while in a MRI scanner. Prior to the study, participants had a screening visit at the Yale Hospital Research Unit. Subjects with any major medical disorder, that consumed a vegetarian diet or with contraindications for a MRI scan were excluded. Women were studied either in the follicular or the luteal phase of their menstrual cycle and the date of their last menstrual cycle and use of birth control pill was recorded (no statistically significant group differences were observed, P=0.313; Supplemental Table 2). All participants had a hemoglobin A1c (5.3±0.3%) within the normal range(24) and gave written informed consent. The Human Investigation Committee from the Human Research Protection Program at Yale University approved this study.
Study Design
Participants that qualified for the study were invited to come to the Yale Magnetic Resonance center for the fMRI session in conjunction with an insulin-clamp or saline infusion. At 10:30 am, all subjects were fed a small meal consisting of a turkey sandwich, apple, and artificially sweetened, zero calorie soda. Participants were instructed to eat the whole meal with the goal to keep them in a neither hungry nor full state, as previously described(21). Before the study, a catheter was inserted into an antecubital vein for infusion of insulin and glucose. A second catheter was placed in the opposite arm for blood sampling and kept patent with an isotonic saline infusion. One hour prior to the scan, they received orientation about the fMRI task and completed a brief practice task using a portable computer (15 min). At 12:30 pm, subjects were transferred to the MRI suite for the combined fMRI-infusion procedure (figure 1). Inside the MRI scanner participants received a continuous intravenous (IV) infusion of either insulin (2 mU/kg.min) or saline (75ml/hour). The INS group received a variable glucose infusion with the goal to maintain plasma glucose at ~95 mg/dL, as previously described.(25) Blood samples were taken for glucose and hormone measurements at baseline (prior to starting the IV infusions) and before and after each blood oxygen level-dependent (BOLD) session. While in the MRI scanner, BOLD acquisitions were obtained while subjects looked at images of HC and LC food and NF (neutral) pictures. The pictures were presented through a liquid crystal display panel mounted on the head coil. The fMRI-infusion studies lasted approximately 2 hours.
Figure 1. Study Procedure (Event-related fMRI design).
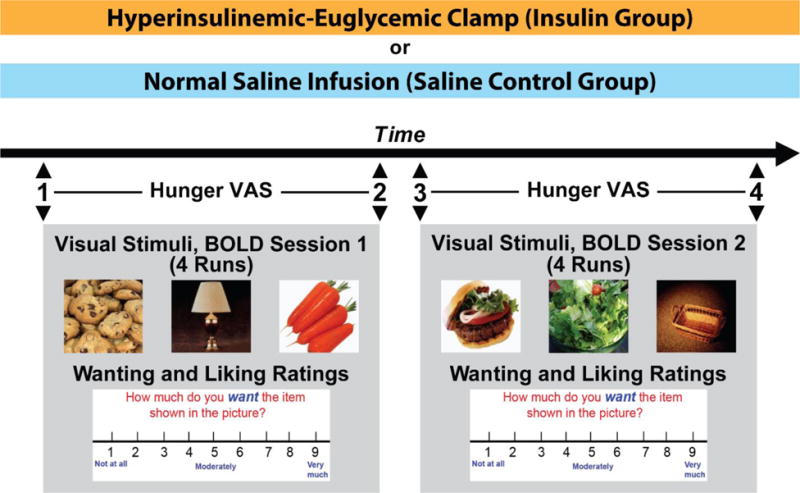
34 subjects underwent either a hyperinsulinemic-euglycemic clamp (n=18) or a normal saline infusion (n=16) while in an MRI scanner. The scan consisted of two sessions of functional MRI using blood oxidation level dependent (BOLD) signaling. During each session, 4 runs of 21 pictures (7 High Calorie Food (HC), 7 Low Calorie Food (LC), and 7 Non-Food (NF)) in random order were projected onto a screen in the scanner. Right after a picture presentation (6 seconds), subjects were shown a visual analog scale and given 3 seconds each to rate their wanting and liking for the item in the picture on a scale of 1 (“not at all”) to 9 (“very much”). Immediately prior to and immediately after each session (time points 1, 2, 3 and 4), the subjects were presented with a rating scale and asked to rate their hunger on a scale of 1 (“not at all hungry”) to 9 (“extremely hungry”).
Visual Stimuli
E-Prime software (Psychological Software Tools Inc.) was used for presentation of visual stimuli that consisted of 168 pictures of food (56 HC; 56 LC) and 56 NF pictures. The task was divided in 2 sessions, and each session comprised of 4 functional runs. Each run consisted of 21 HC food (7), LC food (7), and NF (7) pictures. Levels of emotional valence were commensurable across condition (HC vs LC), runs and sessions. The description of the pictures, emotional valence, and calorie content of the food pictures have been described previously(26). Each picture was shown only once and presented in a randomized order (Figure 1).
Functional MRI task
Event-related fMRI design was used for the visual stimuli task, where each trial consisted of 3 events: picture viewing, rating, and inter-stimulus interval. The first event was the presentation of either food or NF pictures for 6 seconds in the computer screen. Subsequently after the presentation of each picture, VAS of liking and wanting ratings were displayed in the computer screen. The first VAS asked the volunteers to rate “how much do you like the item shown in the picture?” and the second VAS asked “how much do you want the item shown in the picture?”. Participants selected a number from 1 to 9 on the VAS (1=“not at all”; 9=“very much”) using a MRI-compatible button box. Finally, to relax between the trials, a blank screen with a fixation cross was presented with jittered inter-trial time interval from 3–9 sec (average = 6 seconds). For hunger ratings, a similar VAS was performed before and after each BOLD session (approximate time-points: 15, 45, 65 and 120 minutes).
Functional MRI Acquisition
A 3.0 Tesla Siemens Trio MRI scanner was used for the fMRI signal acquisition. Functional images were collected with a standard quadrature head coil, using a T2*-sensitive gradient-recalled single shot echo planar pulse sequence. For the anatomical images of the functional slice locations, spin echo imaging was obtained in the axial plane parallel to the AC-PC line (TR=300 msec, TE=2.46 msec, bandwidth=310 Hz/pixel, flip angle=60°, field of view=220×220 mm, matrix=256×256, 32 slices with slice thickness=4mm and no gap). A single-shot gradient echo planar imaging (EPI) sequence was used for BOLD signal. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired (TR=2,000 msec, TE=25 msec, bandwidth=2520 Hz/pixel, flip angle=85°, field of view=220×220 mm, matrix=64×64, 32 slices with slice thickness=4mm and no gap, 198 measurements). A high-resolution 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was used to acquire the sagittal images for the multi-subject registration (TR=2530 msec; echo time (TE)=2.4 ms; bandwidth=238 Hz/pixel; flip angle (FA)=9°; slice thickness=1mm; field of view=256 × 256 mm; matrix=256 × 256).
Biochemical Analysis
The plasma glucose concentration was determined by the glucose oxidase method (YSI, Yellow Springs, OH). Plasma insulin, ghrelin, leptin, and glucagon (Millipore, St. Charles, MO), and cortisol (Diagnostic Products Corp, Los Angeles, CA) were measured by double-antibody radioimmunoassay.
Statistical Analysis
Subject Characteristics and Metabolic Parameters
Statistical analyses were performed using IBM SPSS version 19.0. All values represent the mean ± standard deviation (SD), unless otherwise specified. Comparisons within subjects were determined by paired-sample t-test, and between subjects by independent-sample t-test for equality of means. Two-way repeated-measure ANOVAs were performed to identify between-group differences in food motivation ratings for liking, wanting, and hunger. Then, within each group, one-way repeated-measure ANOVA was conducted. Correlations between eating hunger and food preference were determined by Pearson correlation.
Functional MRI Analysis
The digital data (Digital Imaging and Communication in Medicine (DICOM)) was converted to analyze data by XMedCon(27) where the first 3 images were discarded to enable the signal to achieve steady-state equilibrium between radio-frequency pulsing and relaxation leaving 195 images per slice per trial for analysis. Data were motion corrected using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5), and were discarded if linear motion was greater than 1.5 mm or rotation was greater than 2 degrees. For individual subject data analysis, General Linear Model (GLM) was used to determine the regions with changes in signal in response to the visual task (HC, LC or NF image). The functional images were spatially smoothed with a 6 mm Gaussian kernel to account for variations in the location of activation across subjects. The output maps were normalized beta-maps, which were in the acquired space (3.44mm × 3.44mm × 4mm). The Yale BioImage Suite software package (http://www.bioimagesuite.org/)(28) was used to calculate two linear and one non-linear registration. These three registrations were concatenated and applied as one registration to bring the data into a common reference brain space. The Colin27 Brain(29) in the Montreal Neurological Institute (MNI) space(30) was used as the reference brain. Data were converted to AFNI format (http://afni.nimh.nih.gov)(31)and a 2×3 ANOVA was used for group analysis (INS × SAL) and task (HC, LC, NF pictures) with task as the within-subjects fixed-effect factor, group (INS, SAL) as the between-subjects factor, and subject as the random-effect factor. To examine the relationship between food motivation ratings, hormone level, and brain response, whole brain correlation analysis with task-related brain activity were conducted with hormone levels and eating behavior ratings using BioImageSuite. To correct for multiple comparisons, we used family-wise errors (FWE) correction determined by Monte Carlo simulation using the AFNI AlphaSim program.
Results
Metabolic Profile
The hormone levels in INS and SAL groups are shown in Table 1. Baseline glucose levels were comparable in both INS and SAL groups (INS: 85±11 mg/dL and SAL: 79±12 mg/dL; P=NS) and increased slightly during the pre-infusion MRI scan (INS: 93±4 mg/dL vs SAL: 89±4; P<0.05). As expected, during the euglycemic-hyperinsulinemic clamp (INS group) insulin levels increased significantly (P<0.001), while they decreased in the control SAL group (P<0.001). In both groups leptin levels increased slightly; whereas ghrelin levels decreased (805±296 to 716±253 ng/mL, P<0.05) in the INS group and increased in the SAL group (896±374 to 1021±429 ng/mL; P<0.001). Insulin, glucagon and ghrelin levels were statistically different at the end of the fMRI study when comparing both groups (mixed repeated-measures ANOVA, P<0.005).
Table 1.
Plasma hormone levels at baseline and at the end of the fMRI Scan
| Insulin Group | Saline Group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | End First Session | End of Study | Baseline | End First Session | End of Study | ANOVA | |
| Insulin (uU/mL) | 26 ± 15 | 151 ± 38 | 168 ± 47* | 22 ± 12 | 13 ± 6 | 11 ± 6* | P<0.001 |
| Glucagon (pg/mL) | 66 ± 23 | 40 ± 18 | 36 ± 15* | 66 ± 16 | 65 ± 17 | 55 ± 15* | P=0.001 |
| Cortisol (ug/dL) | 14 ± 9 | 11 ± 5 | 12 ± 5 | 16 ± 8 | 13 ± 8 | 12 ± 7* | P=0.53 |
| Leptin (ng/mL) | 6.9 ± 4.7 | 7.5 ± 5.0 | 8.6 ± 6.1* | 6.7 ± 4.8 | 6.9 ± 4.7 | 7.5 ± 5.4 | P=0.12 |
| Ghrelin (ng/mL) | 805 ± 296 | 755 ± 278 | 716 ± 253* | 896 ± 374 | 981 ± 367 | 1021 ± 429* | P<0.001 |
| GH | 5 ± 4 | 5 ± 4 | 7 ± 5 | 7 ± 10 | 9 ± 9 | 8 ± 8 | P=0.47 |
Data are expressed as mean ± SD.
Indicates significance at P<0.05 for a paired T-test comparing baseline versus end of study within each group. Repeated-measures ANOVA was significant for insulin, glucagon and ghrelin, between groups at a P<0.05.
Food Motivation Measurements
Hunger Ratings
As shown in Figure 2, hunger ratings increased significantly in both groups (repeated-measures ANOVA: INS: [F(3, 51)=8.051, P<0.01] and SAL: [F(3, 45)=26.314, P<0.001]. Mixed repeated-measures ANOVA analysis showed no group differences for hunger by time interaction [F(3, 96)=1.333, P=0.27]. In both groups participants reported a progressive increase in hunger (from 2.8±2.0 to 5.7±2.7; P<0.001]. Compared to baseline, the ratings at the end of the first session were significantly increased, and thereafter sustained throughout the task (Figure 2a).
Figure 2. Eating Behavior Ratings and Hunger Correlations.
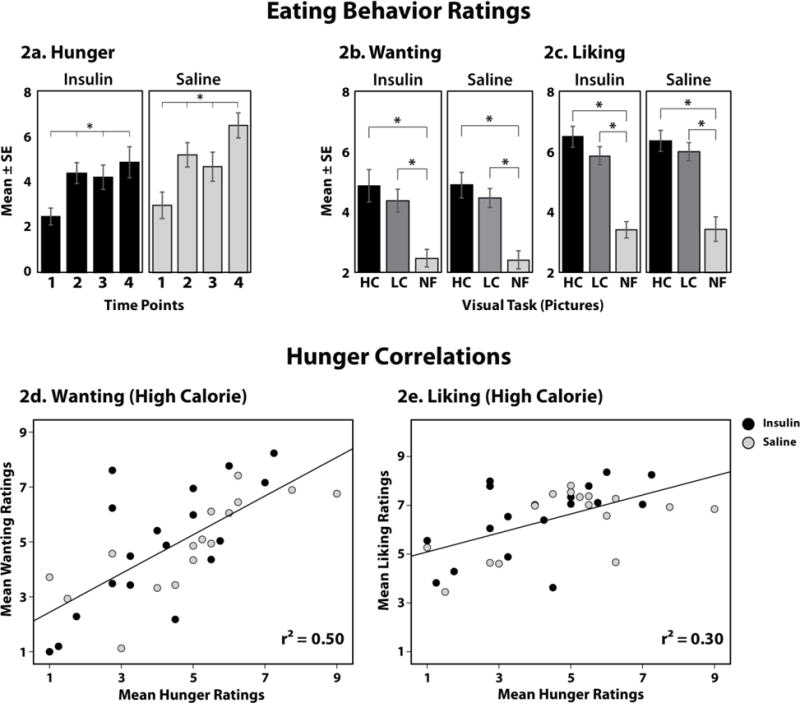
(2a.) Mean hunger ratings for each group at each of the 4 time points during the study. (2b.) Mean wanting and liking ratings for High Calorie (HC), Low Calorie (LC), and Non-Food (NF) images in both groups. (2d–e.) Correlation graphs for Mean Hunger ratings with Mean Wanting (2d) and Mean Liking ratings (2e) during high calorie foods by group. There were no significant group differences by mixed repeated-measures ANOVA. P<0.05.
Wanting and Liking Rating
Wanting and liking ratings were obtained for each picture presented during the BOLD sessions; and their mean ratings are shown in Figures 2b and 2c, respectively. Both groups demonstrated a significant difference across conditions for both wanting (INS: [F(2, 34)=18.01, P<0.001] and SAL: [F(2, 27)=32.71, P<0.001]); and liking (INS: [F(2, 34)=29.42, P<0.001], and SAL: [F(2, 28)=41.00, P<0.001]), with greater wanting and liking ratings for the food (HC and LC) versus NF images (P<0.05); no statistical differences were detected between HC and LC images. However, there were no differences in wanting and liking ratings when comparing between the INS and SAL groups (mixed repeated-measures ANOVA for wanting [F(2, 64)=0.028, P=0.97] and liking [F(2, 62)=0.101, P=0.90] ratings in response to the visual stimuli).
Correlation Analysis
Hunger X Food Motivation Ratings
To examine relationships between hunger and specific food preference, correlation analyses were conducted. There was a strong positive correlation between mean hunger and mean wanting ratings for HC (r=0.71; P<0.001) (Figure 2d) and LC food pictures (r=0.48; P<0.005) (not shown). Hunger also correlated with liking HC (r=0.55; P=0.001) food pictures (Figure 2e), but not liking LC food pictures (r=0.07; P=NS). The correlation between hunger and wanting ratings for HC food pictures was significant for each of the groups, and not affected by the insulin infusion (INS: r=0.71; P=0.001; SAL: r=0.57; P=0.02).
Food Motivation X Metabolic Profile
There were no significant correlations between hunger, wanting and liking ratings and the measured glucose and hormone levels or hormonal changes during the infusion studies (P=NS). In the INS group, but not the SAL group, mean leptin levels correlated negatively with both hunger (r= −0.52; P=0.03) and wanting HC (r= −0.53; P=0.02); but not with liking (P=NS). Furthermore, the increment in leptin levels during the MRI studies had no effect on food motivation (P=NS). The two groups were matched by gender, and the results were not affected by co-varying for gender.
Whole-Brain Response
Group X Task effect
A group (INS, SAL) × task (HC, LC, NF) ANOVA showed no group difference or Group × Task interaction effect in response to visual cues between the INS and SAL groups after whole brain FWE correction at P<0.05. The two groups had a similar brain response to visual food cues despite their distinct metabolic profile. There were also no sex-interactions (“sex by group”, “sex by “task”; or “sex by group by task”). Thus, t-test results of food task effects were presented in both groups together.
Food task effect
The whole brain contrast map for food condition (HC and LC combined) relative to the NF condition, including both the INS and SAL groups (all 34 subjects) showed that food condition increased activity in the striatum (dorsal, ventral), ventromedial prefrontal cortex (VmPFC), dorsolateral PFC (DLPFC), insula, amygdala, hypothalamus, and occipital lobe compared to the NF condition (Figure 3a). A similar brain response was observed when the whole brain contrast included only the HC (Figure 3b) and LC food pictures relative to the NF pictures (supplemental Figures 1 and 2). The whole brain contrast maps for each individual group (INS and SAL) are presented in supplemental Figures 1 and 2.
Figure 3. Differences between Food and Non-Food Conditions.
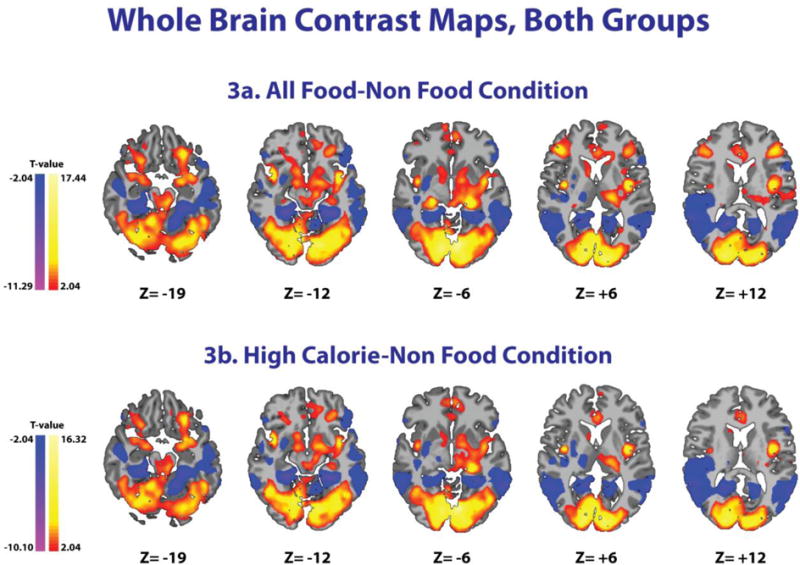
Whole brain contrast maps for all 34 subjects (Insulin and Saline groups) showing increased activity in red/yellow and decreased activity in blue/purple. Increased activity can be seen in the striatum (dorsal, ventral), ventromedial prefrontal cortex, dorsolateral prefrontal cortex, insula, amygdala, hypothalamus, and occipital lobe for both All Food and High Calorie conditions relative to the Non-Food condition. MNI coordinates were used. T-test, FWE correction at P<0.05.
Whole Brain Correlations
Hormones and food motivation ratings did not correlate with any major brain areas when both groups were analyzed together. Hunger ratings in the INS, but not the SAL group, were associated with brain responses in the medial PFC (mPFC), hypothalamus, and caudate (Figure 4); which are known areas involved in food motivation and feeding behavior(11). To better understand group differences, Fisher’s z transformation was implemented to convert correlation coefficient to z scores. Using these z-scores, there were no group differences with correction for multiple comparisons. These results suggest that the observed hunger correlation patterns may also be continuous in SAL, but more apparent in INS group.
Figure 4. Whole brain, voxel-based correlation analysis.
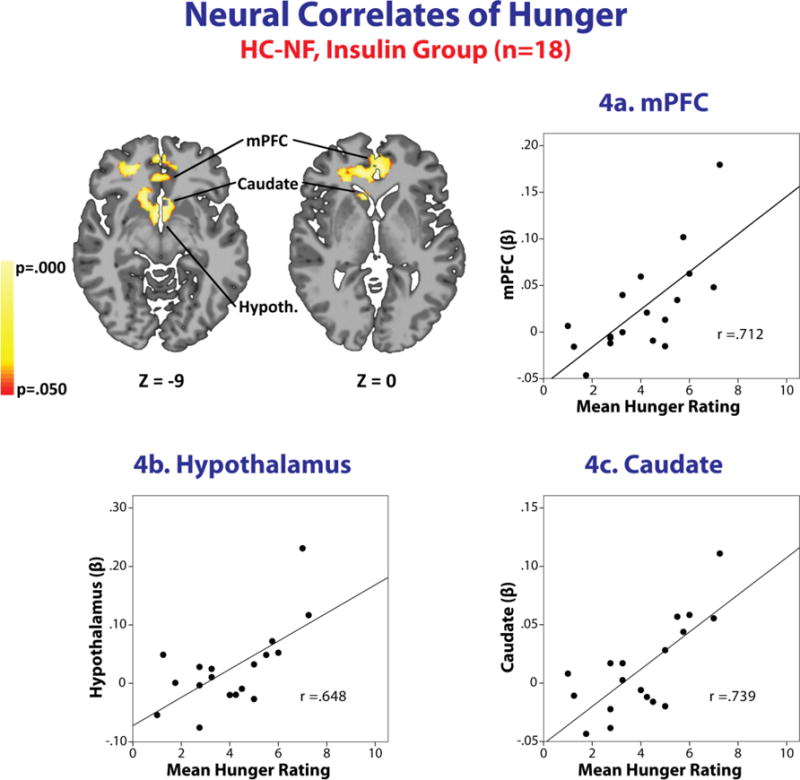
Brain images and region-specific correlation scatterplots showing correlations between activation in the medial Prefrontal Cortex (3a.), Hypothalamus (3b.), and Caudate (3c.) and the mean hunger rating for the Insulin group (n=18) in the High Calorie – Non-Food condition. Increased activation in each region is positively and strongly associated with an increase in mean hunger rating in this condition (P < 0.05), Monte Carlo gray matter smoothing. There were no outliers in these associations. MNI coordinates were used (whole brain corrected at P<0.05).
Discussion
This study demonstrates that in non-obese human subjects, in a neither hungry nor full state, visual food stimuli relative to NF stimuli induced brain responses in the hypothalamus, striatum (dorsal, ventral), amygdala, insula, VmPFC, DLPFC, and occipital lobe, as measured by fMRI. As expected, in the INS group, insulin increased to levels seen in non-diabetic obese individuals after a meal(32); while ghrelin (an orexigenic hormone) decreased. In contrast, insulin decreased and ghrelin levels increased in the SAL group. Despite these disparate hormonal profiles, there were no group differences in brain responses to visual food stimuli (supplemental Figure 1 and 2). Food motivation (determined by hunger, liking and wanting ratings) was also not affected by hyperinsulinemia (Figure 2). Hunger increased similarly in both groups (Figure 2a) and was associated with an increased preference for HC food pictures (Figure 2d). These results suggest that visual food cues have a strong effect on motivation for food in non-obese individuals independent of substantial changes in the hormonal homeostatic milieu.
Our findings are consistent with previous neuroimaging food cue studies(19, 33–35). Food images increase activity in key brain areas implicated in food motivation and intake; including the hypothalamus, striatum, insula, and cortical regions (36, 37). Although, many earlier studies have taken into account the feeding status, i.e. hungry versus satiated, few studies have assessed the metabolic and hormonal changes linked to the participants’ response to external (environment) signals. In particular, based of animal studies, insulin appears to play a major role in modulating eating behavior, since it is secreted by the pancreas in response to food intake, crosses the blood brain barrier, and binds to insulin receptors in brain regions known to modulate feeding(38). Furthermore, intranasal insulin in humans has been reported in some studies to decrease food intake(16, 17, 39) and increase cerebral blood flow in areas involved in eating behavior(40). However, in other studies, intranasal insulin was not found to affect hunger ratings, motivation to eat or brain activity in the presence of food stimuli, in keeping with the current data (41).
The hormonal levels observed in the INS group were consistent with those seen in an acute post-meal state, i.e. increases in insulin and leptin and decreases in ghrelin. Nevertheless, there were no group differences in relation to the brain response to food pictures. These results differ from previous studies examining the effect of ghrelin on the brain response to food pictures; where fasting ghrelin, but not post-glucose load levels, correlated with brain activity in a variety of eating-related brain regions(42). Moreover, an acute intravenous infusion of ghrelin increased neuronal response to food pictures(43). We speculate that visual food cues are a particularly powerful stimulus promoting food motivation and eating behavior, which may only be further potentiated by changes in plasma ghrelin, whereas it cannot be lessened by peripheral delivery of insulin. In support of this possibility, both our self-report and brain data indicates that viewing HC food pictures plays a crucial role in increased hunger during the task. Greater hunger response was significantly associated with increased wanting and liking of HC food stimuli specifically. In addition, the brain response to HC food in the medial PFC, striatum and hypothalamus positively correlated with increased hunger ratings specifically in the INS group. These regions are associated with food-related motivation and reward responses(11), suggesting the direct impact of palatable food cues on reactivity of specific brain regions linked to feeding behavior, particularly in the setting of hyperinsulinemia. Given that we are constantly surrounded by food cues in daily life, our data are consistent with the hypothesis that environmental factors exert a particularly pronounced effect on our eating habits, favoring consumption of calorie-dense food independent of the homeostatic milieu. In keeping with this view it has been reported that food advertising influences food preference(6), increases food intake(7) and may lead to weight gain(8, 44); and television food commercials relative to NF commercials induce a greater brain response(45). The brain’s ability to respond to a greater extent to external food stimuli over internal homeostatic signals may have been fundamental for survival in times of scarcity and food abundance.
One of the limitations of this study is that insulin was given peripherally, rather than directly into the CNS. Insulin has been reported to decrease food intake when given by the intranasal route(39), perhaps because intranasal insulin likely causes higher and more localized levels of insulin in feeding centers of the brain. Nonetheless, in this study, we induced similar increases in insulin as are observed in obese individuals after a meal(32), which cause significant increases of insulin in cerebrospinal fluid(46). Although we cannot refute the possibility that the timing when the fMRI scans were performed (between 30–120 minutes after initiating IV insulin infusion) may not have allowed sufficient time for insulin to reach adequate levels in the CNS; there was no time-effect on brain responses between the first and second scan sessions (repeated-measures ANOVA, P=NS). Furthermore, Page et al(47) demonstrated that changes in circulating insulin during the first hour after oral glucose (75g), induced changes in cerebral blood flow in the caudate and putamen. We also observed that blood glucose levels were slightly but significantly lower in the SAL in comparison to the INS group. Although reductions in blood glucose levels have been shown to promote hunger and to affect brain response to visual food cues(48), we did not observe any differences in our two groups. In fact, we would have expected that the lower glucose levels in the SAL group would have further stimulated hunger in comparison to the INS group.
Another limitation of the study is that we did not measure actual food intake. The use of VAS to determine food motivation, such as hunger and food wanting and liking, are subjective ratings that may not be sensitive enough to identify subtle effects of hyperinsulinemia on eating behavior. Insulin has also been shown to effect food palatability(39), most likely through its effect on the reward dopaminergic system(49), but we did not evaluate food preference in this study. Therefore we cannot exclude that hyperinsulinemia does not alter actual food consumption and food palatability.
Although a within-subject study design could have strengthened our research study, we chose a between-subject design to avoid repeated exposure and habituation to the visual stimuli. We also were not able to obtain a direct measurement of insulin sensitivity during the fMRI study. Even though we performed the hyperinsulinemic-euglycemic clamp in the INS group, these studies were not performed in the fasting state precluding the use of the glucose infusion rate as an accurate measurement of peripheral insulin sensitivity. However, we do not expect that insulin resistance affected our results in these healthy BMI- and age-matched non-obese subjects. Finally, because of the small number of subjects on each group (supplemental table 1), we were not able to evaluate the effect of gender on our results. Although intranasal insulin has been reported to affect eating behavior in men, but not in women(16, 17), when the two groups were analyzed together, we did not observe any sex-interaction by group or by task on brain response. In addition, the two groups were well matched by sex, making it unlikely that gender played a major role in our results.
In conclusion, our data demonstrate that food pictures activate CNS regions involved in reward and motivation-executive control of eating behavior. Furthermore, in the presence of visual food stimuli, neither hyperinsulinemia nor other induced hormonal changes affected hunger, food preference ratings, and brain activation. These results suggest that an environment inundated with food stimuli can supersede homeostatic signals and alter brain control of energy balance; promoting food intake and weight gain. This might, at least in part, explain how the modern western lifestyle is contributing to the current obesity epidemic.
Supplementary Material
Acknowledgments
We thank Ellen Hintz, Karen Allen, Anne O’Connor, Darlene Tempesta, Gina Solomon, Hedy Sarofin, Terry Hickey, Kristen A. Tsou, Edward Gaiser, Christian Schmidt, Ralph Jacob, Mikhail Smolgovsky, Irene Chernyak, Codruta Todeasa, and Aida Groszmann for their assistance as well as the subjects who participated in this study. We acknowledge support for the Bioimagesuite software used in the fMRI analysis from the National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering (NIBIB) under grant 1 R01 EB006494-01.
Grant Support:
This work was supported by in part by National Institutes of Health (NIH)/NIDDK grants (Grant Numbers R01 DK20495, T32 DK 07058, P30 DK045735, K23 DK098286-02, R01-DK099039), NIH/NIAAA (K08 AA023545-02), the Yale Center for Clinical Investigation supported by the Clinical and Translational Science Award (CTSA) (UL1 TR000142) from the National Center for Advancing Translational Science (NCATS), NIH Roadmap Common Fund (UL1-DE019586), and NIH/National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R01 EB006494-01).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.F CD, C MD, O CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2009–2010. NCHS Health E-Stat. 2014 [Google Scholar]
- 3.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–6. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 4.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen DA. Obesity and the built environment: changes in environmental cues cause energy imbalances. Int J Obes (Lond) 2008;32(Suppl 7):S137–42. doi: 10.1038/ijo.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28(4):404–13. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreyeva T, Kelly IR, Harris JL. Exposure to food advertising on television: associations with children’s fast food and soft drink consumption and obesity. Econ Hum Biol. 2011;9(3):221–33. doi: 10.1016/j.ehb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Lesser LI, Zimmerman FJ, Cohen DA. Outdoor advertising, obesity, and soda consumption: a cross-sectional study. BMC Public Health. 2013;13:20. doi: 10.1186/1471-2458-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz MW, Porte D. Diabetes, obesity, and the brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 10.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 11.De Silva A, Salem V, Matthews PM, Dhillo WS. The use of functional MRI to study appetite control in the CNS. Experimental diabetes research. 2012;2012:764017. doi: 10.1155/2012/764017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–5. [Google Scholar]
- 13.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain research bulletin. 1984;12(5):571–5. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- 14.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–9. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 15.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44(2):147–51. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- 16.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93(4):1339–44. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 17.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53(11):3024–9. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 18.Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34(9):826–31. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 19.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 20.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135(5):1014–8. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 21.Page KA, Seo D, Belfort-Deaguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121:4161–9. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharmuller W, Ubel S, Ebner F, Schienle A. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett. 2012;518(2):106–10. doi: 10.1016/j.neulet.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 26.Page KA, Seo D, Belfort-Deaguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–9. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolf E. XMedCon - An open-source medical image conversion toolkit. European Journal of Nuclear Medicine. 2003;30(suppl 2):S246. TP39. [Google Scholar]
- 28.Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011;9(1):69–84. doi: 10.1007/s12021-010-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Nuclear Science Symposium and Medical Imaging Conference; San Francisco, USA. 1993. pp. 1813–7. [Google Scholar]
- 31.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 32.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81(2):442–8. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 34.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21(4):1790–7. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PM, Ferguson AV. Neurophysiology of hunger and satiety. Developmental disabilities research reviews. 2008;14(2):96–104. doi: 10.1002/ddrr.13. [DOI] [PubMed] [Google Scholar]
- 37.Salem V, De Silva A, Matthews PM, Dhillo WS. Imaging the neuroendocrinology of appetite. Adipocyte. 2012;1(1):68–72. doi: 10.4161/adip.19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848(1–2):114–23. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 39.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes. 2012;61(4):782–9. doi: 10.2337/db11-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling TM, Ferreira de Sa DS, Westerhausen R, Strelzyk F, Larra MF, Hallschmid M, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Human brain mapping. 2014;35(5):1944–56. doi: 10.1002/hbm.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira de Sa DS, Schulz A, Streit FE, Turner JD, Oitzl MS, Blumenthal TD, et al. Cortisol, but not intranasal insulin, affects the central processing of visual food cues. Psychoneuroendocrinology. 2014;50:311–20. doi: 10.1016/j.psyneuen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, et al. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2013;18(5):855–62. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- 43.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7(5):400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 44.McClure AC, Tanski SE, Gilbert-Diamond D, Adachi-Mejia AM, Li Z, Li Z, et al. Receptivity to television fast-food restaurant marketing and obesity among U.S. youth. Am J Prev Med. 2013;45(5):560–8. doi: 10.1016/j.amepre.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci. 2014;9(7):932–8. doi: 10.1093/scan/nst059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64(1):190–4. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 47.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309(1):63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58:448–52. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


