Abstract
BACKGROUND
Cancer and sepsis have surprisingly similar immunologic responses and equally dismal long term consequences. In cancer, increased myeloid-derived suppressor cells (MDSCs) induce detrimental immunosuppression, but little is known about the role of MDSCs after sepsis. Based on our chronic sepsis animal models, we hypothesized that after sepsis in humans, MDSCs will be persistently increased, functionally immunosuppressive, and associated with adverse clinical outcomes.
METHODS
Blood was obtained from 74 patients within 12 hours of severe sepsis/septic shock (SS/SS), and at set intervals out to 28 days, as well as in 18 healthy controls. MDSCs were phenotyped for cell surface receptor expression and enriched by cell sorting. Functional and genome-wide expression analyses were performed. Multiple logistic regression analysis was conducted to determine if increased MDSC appearance was associated with in-hospital and long-term outcomes.
RESULTS
After SS/SS, CD33+CD11b+HLA-DR−/low MDSCs were dramatically increased out to 28 days (p<0.05). When co-cultured with MDSCs from SS/SS patients, antigen-driven T-cell proliferation and TH1/TH2 cytokine production were suppressed (p<0.05). Additionally, septic MDSCs had suppressed HLA gene expression and upregulated ARG1 expression (p<0.05). Finally, SS/SS patients with persistent increased percentages of blood MDSCs had increased nosocomial infections, prolonged ICU stays, and poor functional status at discharge (p<0.05).
CONCLUSION
After SS/SS in humans, circulating MDSCs are persistently increased, functionally immunosuppressive, and associated with adverse outcomes. This novel observation warrants further studies. As observed in cancer immunotherapy, MDSCs could be a novel component in multimodality immunotherapy targeting detrimental inflammation and immunosuppression after SS/SS to improve currently observed dismal long-term outcomes.
Keywords: Myeloid derived suppressor cell, MDSC, sepsis, systemic inflammatory response syndrome, SIRS, compensatory anti-inflammatory response syndrome, CARS, persistent inflammation/immunosuppression and catabolism syndrome, PICS
INTRODUCTION
The Agency for Healthcare Research and Quality (AHRQ) recently ranked sepsis as the most expensive condition treated in US hospitals with estimated costs exceeding $20 billion dollars1. Additionally, sepsis is common in hospitalized surgical patients; it occurs ten times more frequently than pulmonary embolism and myocardial infarction combined2. Improved early care over the past decade has decreased in-hospital mortality from severe sepsis/septic shock (SS/SS) from over 35% to less than 15%2. Unfortunately, many early survivors now progress to a state of chronic critical illness (CCI; defined by intensive care unit (ICU) stays ≥ 14 days) 8, 9, 5 and a substantial portion of these individuals develop persistent inflammation, immunosuppression and catabolism syndrome (PICS), for which current interventions are ineffective6. These patients are often discharged to long term acute care (LTAC) facilities and suffer indolent death5, 7. CCI after SS/SS has surprisingly similar metabolic, immunologic and phenotypic manifestations to that of patients with advanced cancer8. Central to the immunologic response in chronic sepsis and cancer is the expansion of myeloid-derived suppressor cells (MDSCs) which is integral to the “emergency myelopoietic” host response that is aimed at preserving innate immunity.
MDSCs are a heterogeneous group of immature myeloid cells and their expansion may be protective in the short term9. They are poor phagocytes and can prevent over activation of the immune system by secreting IL-10 or TGFβ. However, their prolonged presence can induce persistent inflammation (via nitric oxide, myeloperoxidase and reactive oxygen species production), and cause immunosuppression (via inhibition of T-cell proliferation, elaboration of anti-inflammatory cytokines and poor antigen presentation)6, 10, 11. MDSCs are commonly reported in cancer, and modulating MDCSs is now a vital component in multimodality therapy offering prolonged relief for cancers with traditionally dismal outcomes12, 13. However, the role of MDSCs in the host response to SS/SS is not well-defined3, 6, 14. In the laboratory, we modelled chronic sepsis and were the first to observe MDSC expansion after sepsis in rodents15. Although prior studies have displayed MDSC expansion after human sepsis, they were limited to the acute phase after sepsis and did not examine the potential role that MDSCs play in the adverse clinical outcomes after sepsis16, 17. The specific aims of the current study, therefore, were to: (1) determine whether the expansion of MDSCs is persistent after SS/SS in surgical patients; (2) identify the genomic and functional status of these MDSCs; and (3) determine whether persistent MDSC expansion is associated with adverse outcomes consistent with the development of PICS.
METHODS
Patient Selection
This three year observational study of surgical patients ended in January 2015. The cohort consists of SS/SS patients in the surgical ICUs at UFHealth in Gainesville Florida. SS/SS was defined by the American College of Chest Physicians/Society of Critical Care Medicine Conference criteria18. Patients greater than 18 years old were included. Patients were excluded if their expected survival was less than 48 hours, they had severe head injury, were receiving chronic corticosteroid or immunosuppressive therapies, or if they were transferred from another hospital. The study was approved by the UFHealth Institutional Review Board, and signed informed consent was obtained from the subject or their legally-appointed representative. Informed consent was also obtained from healthy control (HC) subjects (n=18) for blood sampling.
Data and Sample Collection
Clinical and laboratory variables were either uploaded directly into the REDCap™ electronic case report form from the EPIC™ electronic medical record or entered manually. Blood was collected within 12 hours of diagnosis of SS/SS and on days 1, 4, 7, 14, 21 and 28, or until discharge from the ICU or death.
Phenotypic Analysis
Whole blood was stained using antibodies as previously described19. MDSCs were characterized as CD33+CD11b+HLA-DR−/low; subsets of monocytic-MDSCs were classified as CD14+ and granulocytic-MDSCs were classified as CD14− CD15+ (Supplemental Figure 1). Cells were analyzed using LSRII Flow Cytometer (Becton Dickinson). MDSC populations were also evaluated for their IL4Rα expression. Total leukocyte numbers were determined from clinical laboratory measurements in the SS/SS patients.
Cell Sorting and T-cell suppression
MDSCs were enriched as CD33+CD11b+HLA-DR−/low by cell sorting (FACSAria™, Becton Dickinson). Nucleic acids were extracted from enriched MDSCs as previously described19.
CD3+ T-cells were isolated from HC subjects with the Negative Pan T-cell Isolation Kit (Miltenyi, San Diego, CA) and stained with Cell Trace Violet (Life Tech, Grand Island, NY). MDSCs and stained T-cells were counted and 106 cells were plated at a 2:1, 1:1, and 0.5:1 MDSC to T-cell ratio, and stimulated at a 1:1 ratio with Human T-Activator CD3/CD28 Dynabeads (Life Tech) and IL-2 (LifeTech). After four days of culture at 37° C and 5% CO2, cells were stained with PE-CD4 and FITC-CD8 (Becton Dickinson), and Live/Dead dye-Far Red (Life Tech). CellTrace Violet was measured in viable CD4+ and CD8+ T-cells.
Cytokine Analysis
T-cell suppression assay supernatants underwent cytokine analysis. Concentrations of IL-4, IFN-γ and IL-10 were determined using Milliplex kits (Millipore) on the Luminex® MAGpix Multiplex reader.
Genomic Analysis
RNA was extracted from lysates using QIAGEN Rneasy™ Mini Kit (Qiagen). RNA integrity was assessed with an Agilent 2100 Bioanalyzer (Santa Clara, CA, USA). RNA was labeled using Nugen (NuGEN Technologies, Inc.) Ovation Pico WTA® System V2. Resulting cDNA was labeled, fragmented using Nugen Encore® Biotin Module, hybridized onto GeneChip® Human Transcriptome Array 2.0 (Affymetrix, Santa Clara, CA) and processed following manufacturer’s instructions.
Gene expression profile analysis
Nucleic acids were extracted and mRNA abundance levels were determined using the Affymetrix HTA 2.0 microarray. Extracted RNA from days 7 and 14 in the SS/SS patient cohort underwent genome-wide expression analysis. Data across these days were indistinguishable; therefore, data were pooled. Microarray expression was normalized using RMA™ as implemented in Partek (Partek Inc, St Louis MO). Significant genes (p<0.001) were identified with class prediction tools implemented in BRB-ArrayTools™ Version: 4.2.1 - Stable Release, developed by Richard Simon & BRB-ArrayTools Development Team (http://linus.nci.nih.gov/BRB-ArrayTools.html). The ability of genes significant at p<0.001 to distinguish between HC and SS/SS patients was determined using leave-one-out-cross-validation studies and Monte Carlo simulations. Gene Ontology™ and BioCarta™ Pathway analysis were conducted using BRB ArrayTools.
Statistical Analysis
Mixed model analysis of longitudinal changes in circulating MDSCs was performed to account for correlations among repeated measurements for each patient. Total and percentage of MDSCs, granulocytic-MDSCs and monocytic-MDSCs from SS/SS patients were compared to HC subjects. Kruskal-Wallis test was used to compare patients who died within 14 days of ICU admission to those that survived longer than 14 days; survivors were further categorized by their ICU length of stay (LOS) (<14 days or ≥14 days). Patients with only one blood sample collected (n=2) or that expired within seven days (n=5) were excluded, resulting in a total of 67 patients.
Patients were recognized as having one of three distinct temporal patterns of MDSC expression: “sustained low”, “decreasing”, or “sustained high.” Patients were considered to have a “sustained low” MDSC expression pattern if MDSC levels were less than or equal to 30% of live cells and two standard deviations (SD) above the control mean, as measured by flow cytometry. Patients with a declining percentage of MDSCs, indicated by a negative slope over time, were classified as “decreasing” MDSC pattern. The remaining patients had either persistently elevated MDSCs or an increasing percentage of MDSC as indicated by a positive slope; these patients were categorized as “sustained high” MDSC. These three distinct expression patterns were then evaluated to determine their predictive value.
The primary outcome of interest was functional status at discharge and the secondary outcome was the incidence of nosocomial infections as an indicator of adverse in-hospital outcomes. Functional status at the time of discharge was determined to be either “successful” or “poor” based on disposition placement. Disposition was “successful” if patients were discharged to home, an inpatient rehabilitation facility or a skilled nursing facility (SNF). Discharge to another inpatient hospital, hospice, LTAC, or death were considered to be “poor” dispositions. The secondary outcome of interest was the presence of at least one site with nosocomial infection.
Multivariate logistic regression models were fit where the MDSC pattern category was the main predictor of interest, adjusting for variables significant in bivariate analysis at 0.1 level among baseline characteristic variables shown in Supplemental Table 1. Model discrimination was assessed using the area under the receiver operating characteristic curve (AUC). Statistical analysis was performed using SAS (v.9.3, Cary, NC).
Using the percentage of MDSCs may underestimate the difference between HC and SS/SS patients since SS/SS patients often have a leukocytosis. As a sensitivity analysis we also repeated statistical modeling with the absolute number of MDSCs. Previous modeling utilized the percentage of HC MDSCs to define the “low sustained” group; however, HC analysis did not include complete blood count and the absolute number of HC MDSCs could not be calculated. Therefore, we used cubic polynomial models to predict the corresponding log absolute MDSC counts for the “low sustained” group. This corresponded to an absolute MDSC count less than 4583 cells/μL and was defined as “low sustained”. “Decreasing” was again defined as a negative slope indicating a decreasing number of MDSCs, and the remaining patients were categorized as “sustained high.”
RESULTS
Supplemental Table 1 lists demographics, comorbidities, illness severity and outcome of the 67 included study patients of which 26 (39%) were female with a mean age of 60 years. Sixty-two (93%) of these patients had a least one co-morbid condition and the median number of comorbidities was four. The primary infection for the SS/SS cohort was pneumonia (n=22; 33%), blood stream infection (n=8; 12%) and urinary tract infection (n=6; 9%). Thirty-seven (55%) patients presented in septic shock and the median APACHE II score was 23 [24 (36%) had APACHE II score >25]. Eight (12%) patients died during hospitalization; the primary causes of death were multiple organ failure (2), pulmonary embolism (2), sepsis (1), hypoxia (1), cardiac dysfunction (1), and withdrawal of life sustaining therapy (1). Thirty-five (52%) patients had at least one nosocomial infection; the most common infections were pneumonia (n=22, 33%), blood stream infection (n=8, 12%), and urinary tract infection (n=6, 9%). Sixty-one (91%) patients required mechanical ventilation and 11 (16%) patients required dialysis. The median of ventilator free ICU days was five and ICU-free days was two. Thirty-nine (58%) patients were in the ICU ≥14 days. Fifty-nine (88%) patients were discharged alive and their top three dispositions included home (n=19; 33%), LTAC (n=13; 22%), and SNF (n=10; 17%).
MDSCs increase acutely and remain present chronically in SS/SS patients
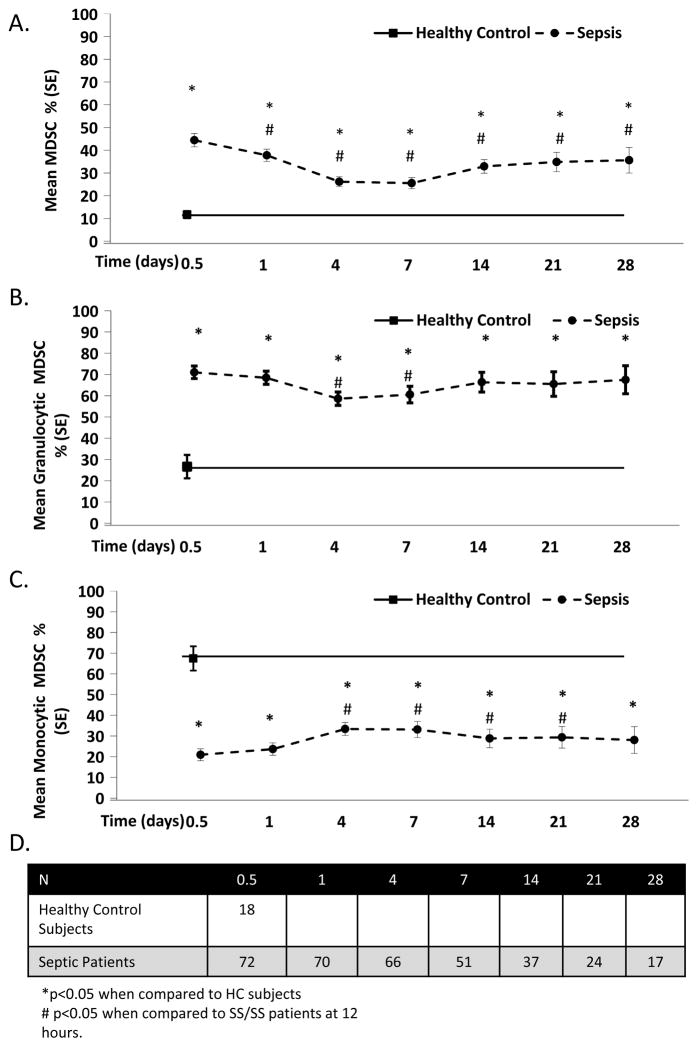
Figure 1A demonstrates that the percentage of MDSCs dramatically increases in SS/SS patients when compared to HC subjects over the 28 day study period. In patients who remained hospitalized for 28 days, MDSC levels remained significantly elevated over the entire ICU stay. Additionally, IL-4Rα, another cell surface marker associated with MDSCs, was significantly elevated at 24 hours and remained elevated through 28 days20 (Supplemental Figure 3).
Figure 1. Percentage MDSCs in Patient with Severe Sepsis/Septic Shock over 28 Days.
A. MDSC percentages are significantly elevated in severe sepsis/septic shock (SS/SS) patients when compared to healthy controls (HC) at all time-points. SS/SS patients have an immediate elevation in MDSCs (CD33+ CD11b+ HLA-DR−/low) within hours of clinical symptoms that persists for at least 28 days. B. Elevated levels of MDSCs in SS/SS patients are predominantly granulocytic (CD33+ CD11b+ HLA-DR−/low CD14− CD15+). C. SS/SS patients have a significant decrease in monocytic-MDSCs (CD33+ CD11b+ HLA-DR−/lowCD15+ CD14+) compared to HC subjects. D. Total number of patients per time point. The HC subjects had a single blood draw (n=18). The study cohort decreased as patients were discharged or expired.
Figures 1B and 1C depict the percentage of circulating granulocytic and monocytic-MDSCs after SS/SS. In HC subjects, the majority of MDSCs are monocytic in nature. After SS/SS, that ratio inverts as the percentage of granulocytic-MDSCs become significantly elevated at all time points when compared to HC subjects. Conversely, the percentage of monocytic-MDSCs was significantly decreased at all time points when compared to HC subjects.
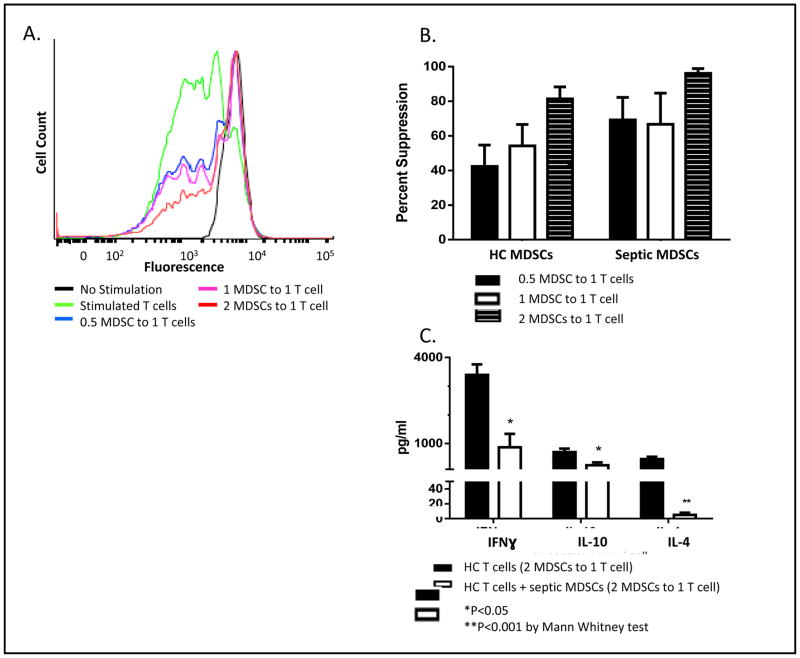
MDSCs suppress ex vivo T-cell production
Figure 2 demonstrates that anti-CD3/CD28 stimulated HC T-cells readily proliferate; however, HC T-cell proliferation is suppressed by MDSCs from SS/SS patients in a dose-dependent fashion as the ratio of SS/SS MDSCs to HC T-cells increases (Figure 2A) (p<0.01). When CD4+ or CD8+ T-cells (identified by cell surface staining) are incubated at a ratio of 2 SS/SS MDSCs to 1 HC T-cell, the percent suppression exceeded greater than 90% in both subpopulations (as compared to T-cells in the absence of MDSCs; data not shown). Similar to our work in septic rodents21, T-cells from SS/SS patients failed to proliferate in response to stimulation, even in the absence of added MDSCs (data not shown). Additionally, Figure 2B illustrates that HC MDSCs produce a dose dependent suppression of HC T-cells, which is not significantly different from SS/SS MDSCs. This suggests that HC MDSCs also have immunosuppressant properties, but are present in much reduced numbers.
Figure 2. MDSC Effect on T-cell Proliferation.
A. A single healthy control (HC) subject’s T-cell suppression data, representative of our data set, is shown here as a histogram. HC CD3+ T-cells were stimulated with IL-2 and anti-CD3/CD28 in the absence and presence of MDSCs. Unstimulated HC T-cells do not actively replicate (black peak); however, stimulated HC T-cells replicate and fluorescence intensity is divided between daughter cells during each replication cycle; this results in decreased fluorescence and multiple peak shifts towards the left (green line). The height of this peak signals frequency of T-cells undergoing replication. However, HC T-cells incubated with MDSCs from severe sepsis/septic shock (SS/SS) patients are significantly suppressed in a dose-dependent fashion and remain non-replicating. B. MDSCs from either HC subjects (n=4) or SS/SS patients (n=4) were incubated with HC T-cells in increasing ratios. SS/SS MDSCs are not significantly more suppressive than HC MDSCs, suggesting that HC MDSCs also have immunosuppressive capacity. C. MDSCs suppress T-cell cytokine production. HC T-cells were incubated with SS/SS MDSCs or HC MDSC (2 MDSCs to 1 T-cell) and stimulated with IL-2 and anti-CD3/CD28 beads. SS/SS MDSCs significantly suppressed the ability of HC T-cells to produce INFγ, IL -10, and IL-4.
MDSCs suppress T-cell cytokine production
Stimulation of T-cells via the T-cell receptor is commonly used to evaluate the polarity of their response through their cytokine production (TH1 versus TH2). Not surprisingly, T-cells from SS/SS patients express significantly lower levels of IFNγ, IL-10 and, IL-4 when compared to HC T-cells (p <0.05) (data not shown). Ex-vivo, co-culture of MDSCs from SS/SS patients (2:1 MDSCs:T-cell) suppress IFNγ, IL-10 and IL-4 production (Figure 2C) (p <0.05). Interestingly, the suppression of cytokine production affected both TH1 (IFNγ) and TH2 (IL-10, IL-4) cytokines from HC T-cells (Figure 2C), and replicated the suppression displayed by T-cells isolated from SS/SS patients (data not shown).
Genomic Analysis
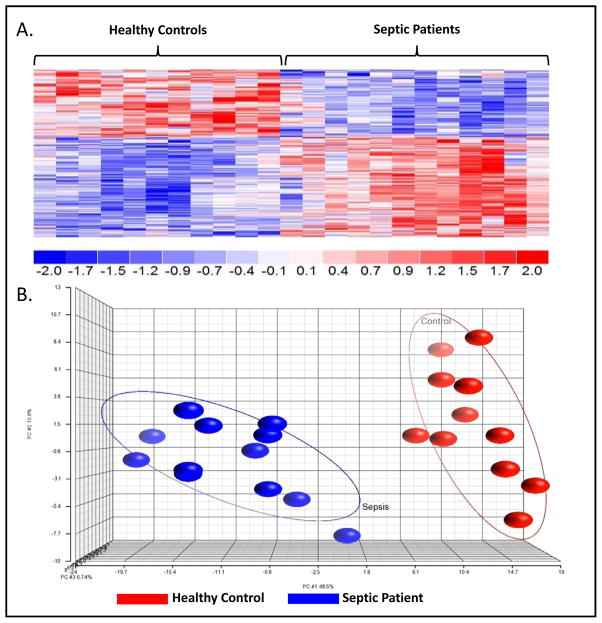
Figure 3 depicts the gene transcriptional patterns of enriched MDSCs from SS/SS patients and HC subjects. There were 288 probe sets corresponding to 257 genes that had significant differences (p<0.001) in expression when comparing HC and SS/SS patients. Individual gene analysis of enriched MDSCs from SS/SS patients was consistent with suppressed HLA gene expression (reduced CD74, and HLA-DOA/-DQA1/-DQA2/-DQB/-DRB1/DRB3/-DRB4) and upregulated arginase-1 (ARG1) expression (p<0.001). These changes are consistent with decreased antigen presentation and a depletion of arginine, an amino acid essential to T-cell function22. Further transcriptomic analysis revealed expected significant upregulation (HP and CYBB) and downregulation (CCR3) of MDSC-associated immunity genes. Canonical Pathway and Causal Network Analysis further supported these pathway alterations, as well as a pattern of simultaneous low grade inflammation with immune suppression (p<0.001) (Supplemental Figure 2). Gene Ontology transcriptomic analysis revealed significant increases in the “nitric oxide (NO) biosynthesis” and the “regulation of reactive oxygen species (ROS) production” pathways, consistent with a chronic inflammatory role for MDSCs, as well as decreased “detection of bacterium,” consistent with impaired MDSC innate immune function.
Figure 3. Severe Sepsis/Septic Shock Patient and Healthy Control Subject Gene Expression.
Using a false discovery adjusted probability of <0.001 and a two-fold difference in expression, the temporal pattern of the expression of the sepsis responsive genes differed between MDSCs from the healthy control (HC) subjects and severe sepsis/septic shock (SS/SS) patients. A. There was a significant difference in the expression of 257 genes between MDSCs isolated from SS/SS patients and HC subjects (p <0.001). B. Principle Component Analysis (PCA) of the gene expression from the 257 genes demonstrates the overall differences in the patterns of gene expression. Gene expression patterns from HC subjects (red) are tightly grouped to the right and the SS/SS patients (blue) are tightly grouped to the left. The tight grouping and lack of overlap of data points reflects the significant difference between the two groups.
MDSCs are associated with adverse in-hospital outcomes
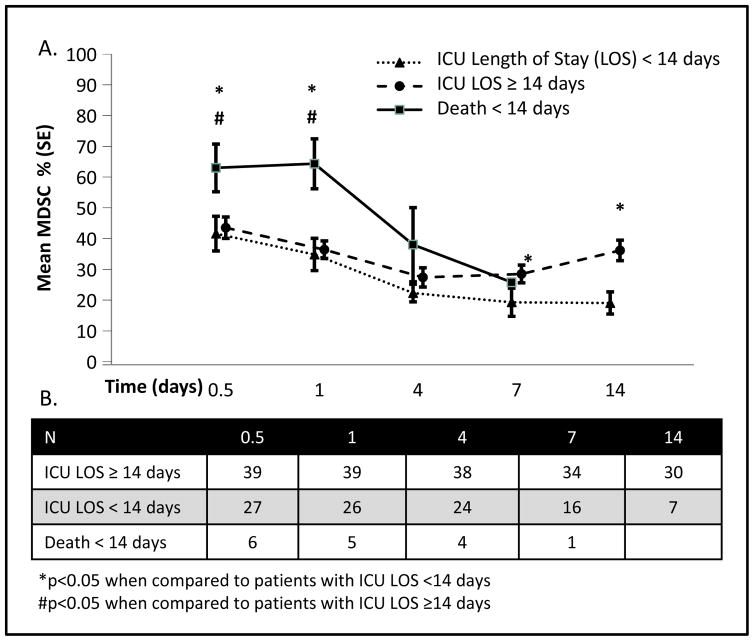
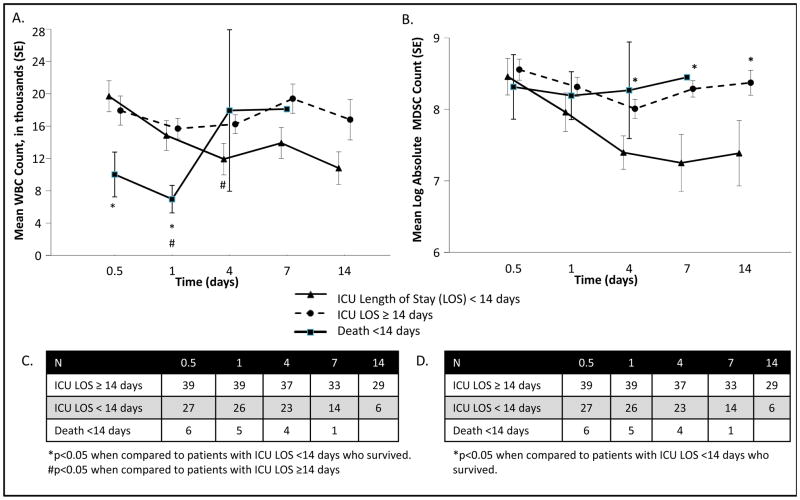
Figure 4 reveals that patients with early mortality had the highest initial percentage of MDSCs at both 12 and 24 hours when compared to patients surviving longer than 14 days (p<0.05). Patients with longer ICU LOS (≥14 days) had a greater percentage of MDSCs throughout their hospital course; on hospital day 7 the percentage of MDSCs became significantly elevated when compared to their counterparts. Those patients who were in the ICU for longer than 14 days had an increased percentage of MDSCs beginning on day 4, while those patients with ICU stays less than 14 days had a steady decrease in their MDSC population. The absolute number, rather than the percentage, of MDSCs were reanalyzed as predictors of early death and length of ICU stay. Interestingly, the percentage of MDSCs in the blood was more predictive than the absolute number of MDSCs. Although patients with early mortality (<14 days) had significantly higher percentages of MDSCs, the absolute numbers of MDSCs were similar because of a relative leukopenia in this population (Figure 5). When evaluating ICU LOS, however, higher absolute numbers of MDSCs were also predictive of longer ICU LOS at 7 and 10 days, mirroring what was seen when evaluating percentage of MDSCs (data not shown), however, this did not provide pertinent additional information.
Figure 4. Increased Circulating MDSCs in Severe Sepsis/Septic Shock Patients with Subacute Mortality.
A. Patients who succumb to early mortality (<14 days) have significantly elevated percentages of MDSCs when compared to patients that survive greater than 14 days at both the 12 and 24 hour time points from onset of clinical sepsis. MDSC levels then sharply decline until death. Patients that survive longer than 14 days have similar levels of MDSCs; however, MDSCs are slightly elevated at all time-points in those patients that remain in the ICU longer than 14 days. At 14 days, patients that will continue to remain in the ICU have significantly elevated percentages of MDSCs compared to those patients who are transferred out of the ICU. B. Total number of patients per time point.
Figure 5. Percentage of MDSCs is more Clinically Prognostic than Absolute Number of MDSCs.
A. Patients that succumb to early mortality (<14 days) have a relative leukopenia during the first 24 hours when compared to those who survive greater than 14 days. Patients with ICU LOS greater than or equal to 14 days have a persistently higher leukocytosis when compared to those patients with ICU LOS less than 14 days. B. Patients with early mortality have similar absolute numbers of MDSCs as survivors because the relative leukopenia is offset by a significantly greater percentage of MDSCs (Figure 4). Patients with shorter ICU LOS (<14 days) have lower absolute numbers of MDSCs, this is statistically significant at days 4, 7 and 14 and correlates with the data reported on percentage of MDSCs. C & D. Total number of patients per time point.
MDSC pattern is associated with incidence of nosocomial infection and patient discharge status
Table 1A containing a prediction model for poor disposition, demonstrates that “sustained high” MDSC percentages are associated with poor functional status at the time of discharge, as indicated by significantly increased odds of “poor” dispositions. Multivariate logistic regression analysis identified septic shock status and APACHE score on admission as independent risk factors; however, the association remained significant after adjustment for APACHE score and septic shock status indicators. In fact, “sustained high” MDSCs percentages were a strongly predictive model, with an AUC of 0.79 (95% CI 0.68–0.90) (Table 1A). Importantly, in Table 1B, a prediction model for nosocomial infection, patients with “sustained high” MDSC percentages were also more likely to have at least one subsequent nosocomial infection (OR 6.67 95% CI 1.32–33.69) compared to patients with “sustained low” MDSC percentages (Table 1B). In addition, patients who developed nosocomial infections had more ventilator days (18 vs. 5 days; p<0.01) longer ICU stays, (25 vs. 13 days; p<0.01) and longer total hospital stays (35 vs. 15 days; p<0.01) when compared to those patients who did not develop nosocomial infections. Again, repeating the analysis using absolute numbers of MDSCs, rather than percentage of MDSCs, did not alter the demonstrated association of MDSCs to this outcome (data not shown).
Table 1. MDSC Pattern is Associated with Functional Status at the Time of Discharge and Rate of Nosocomial Infection.
A. Patients were categorized into three MDSC expression patterns: “sustained low,” “decreasing,” and “sustained high” and as having either poor or successful dispositions. Patients were recognized as having distinct temporal patterns of MDSC expression: “sustained low”, “decreasing”, or “sustained high”. Patients were considered to have a “sustained low” MDSC expression pattern if MDSC levels were less than or equal to 30% of the total leukocyte count. Patients with a declining percentage of MDSCs, as indicated by a negative slope over time, were classified as “decreasing” MDSC pattern. The remaining patients had either persistently elevated MDSCs or an increasing percentage of MDSC as indicated by a positive slope; these patients were categorized as “sustained high” MDSC. Patients with “sustained high” levels of MDSCs have almost eight times the odds of having a poor disposition when compared with patients with “sustained low” MDSCs. This relationship remains significant after adjustment for APACHE II Score and presence of septic shock (OR 7.33). B. Patients with “sustained high” levels of MDSCs had almost seven times the odds of having nosocomial infections when compared with patients with “sustained low” MDSCs. Significant association remained after adjustment for age and number of comorbidities (OR 8.4). This suggests that high levels of circulating MDSC may make patients more vulnerable to nosocomial infection during hospitalization.
| A. Disposition Status | Unadjusted OR(95% CI) | Adjusted OR (95% CI) (AUC=0.79 95% CI 0.68–0.90) |
|---|---|---|
|
| ||
| Decreasing vs. Sustained Low | 2.80 (0.77, 10.18) | 2.70 (0.64, 11.29) |
| Sustained High vs. Sustained Low | 7.88 (1.56, 39.76)a | 7.33 (1.25, 43.12) a |
| Septic Shock | 3.61 (1.28, 1.19) a | 2.49 (0.79, 7.88) |
| Apache Score (per 1 unit increase) | 1.12 (1.04, 1.22) a | 1.12 (1.02, 1.22) a |
| B. Incidence of Nosocomial Infection | Unadjusted OR (95% CI) | OR (95% CI) (AUC=0.73 95% CI 0.61–0.85) |
|---|---|---|
|
| ||
| Decreasing vs. Sustained Low | 2.24 (0.69, 7.26) | 3.16 (0.86, 11.57) |
| Sustained High vs. Sustained low | 6.67 (1.32, 33.69) a | 8.40 (1.48, 47.53) a |
| Age (per 1 unit increase) | 0.97 (0.94, 1.01) | 0.98 (0.95, 1.02) |
| Number of comorbidities (per 1 unit increase) | 0.79 (0.61, 1.02) | 0.78 (0.58, 1.05) |
Abbreviations. OR, odds ratio; CI, confidence interval; AUC, area under the receiver operating characteristics curve.
p<0.05
DISCUSSION
Sepsis remains a major unsolved problem and is the most expensive condition treated in U.S hospitals. There are over 1 million cases of sepsis per year in the United States accounting for 9% of the overall annual deaths23, 24, and septic shock has an estimated mortality of greater than 50%2, 25, 26. Additionally, the incidence of sepsis is increasing, especially in the elderly where those who survive the acute phase of sepsis go on to suffer dismal long term outcomes2, 25, 26. Furthermore, despite basic laboratory data and early phase clinical testing, numerous promising immunomodulatory interventions have uniformly failed in definitive Phase III clinical trials. This begs the question of whether we understand, or oversimplify, the underlying pathophysiology. Our comprehension of sepsis has evolved; fulminant death from an overwhelming SIRS response (where blocking early pro-inflammation made sense) has been replaced by a paradigm of simultaneous inflammation and immune suppression5. However, we now recognize that it is the prolonged dysregulation, and the failure to return to immunologic homeostasis that identifies the patients who experience dismal long term outcomes5, 27. Additionally, advances in early recognition and clinical management of SS/SS have decreased in-hospital mortality2, with the unfortunate consequence of creating a new phenotype of patients that progress into a futile trajectory of CCI and PICS5. The clinical course of PICS is characterized by manageable organ dysfunctions, recurrent nosocomial infections and dramatic loss of lean body mass despite aggressive nutritional interventions5, 7, 28–30. These patients are often discharged to LTAC facilities where they commonly experience progressive declines in cognitive and functional status, sepsis recidivism requiring re-hospitalization31, and ultimately suffer an indolent death5, 7. Based on our chronic sepsis models and emerging information from other chronic inflammatory diseases, including cancer8, we proposed persistent MDSC expansion as a unifying mechanism to explain the chronic inflammation, immune suppression and catabolism seen in PICS patients5, 32, 33.
In this study we have revealed that MDSCs, identified by their cell surface receptor expression of CD33+CD11b+HLA-DR−/low and upregulation of IL-4Rα, are markedly expanded after SS/SS in surgical patients out to 28 days post-sepsis. While other investigators have demonstrated elevated MDSCs after human sepsis16, 17, our study is unique in several ways. First, prior studies have focused on the acute phase of sepsis16, 17, while we have documented chronic expansion of MDSCs out 28 days. Second, we have shown that the ratio of granulocytic to monocytic-MDSCs reverses after SS/SS compared to HC subjects. While this is consistent with other chronic inflammatory diseases12, 13, it has not been previously documented in human sepsis. Third, while other investigators have used a density gradient to collect heterogeneous populations of monocytic-cells11, 12, we have directly isolated MDSCs using fluorescence-activated cell sorting. This methodology properly identifies MDSCs and allows interrogation of both transcriptional and functional status, which confirmed that these cells are truly immunosuppressive. Finally, and perhaps most importantly, we have demonstrated that a sustained increased presence of circulating MDSCs is associated with adverse outcomes (increased nosocomial infections, prolonged ICU stays, and poor functional status at discharge) that are consistent with PICS. Of note, we identified different temporal patterns of MDSC expression, rather than just overall numbers at any time point, in order to better incorporate personalized medicine into our analysis34; this revealed that those patients whose MDSC pattern reflected ‘an inability to return to homeostasis after their infection’ were more likely to have an adverse clinical disposition.
The main limitation of this study was that it was performed at a single institution in a limited number of patients. The total number of study patients could be increased by including multiple institutions; however, the level of complexity and required personnel to consistently perform the sophisticated analyses required places limitations on widespread institution participation. Another limitation of this study was incomplete data at later time points as patients were discharged; collection of blood post hospital discharge would allow for a more robust generalizable analysis. Additionally, although we performed multivariate logistic regression analysis, control samples from more aged individuals34, 35 as well as individuals undergoing minor operations could have been of use to prevent further confounding variables in the analysis.
In conclusion, we found that circulating MDSCs are persistently increased in surgical patients after SS/SS, functionally immunosuppressive, and associated with adverse long term outcomes consistent with PICS. These novel observations warrant further confirmative studies. Of note, MDSCs are phenotypically plastic, which allows them a diverse functionality in response to their environmental conditions, but also creates the potential for future immunomodulating therapies22. As observed in cancer immunotherapy36, targeting MDSCs could become a component in multimodality therapy aimed at reversing detrimental inflammation and immunosuppression after SS/SS to improve currently observed dismal long-term morbidity and mortality37.
Supplementary Material
Cells were stained with the following antibodies: CD33 APC, HLA-DR FITC, CD11b AF700, CD14 PB, and CD15 PE-Cy7. MDSCs were characterized as CD33+ CD11b+ HLA-DR−/low with subsets of Monocytic-MDSCs are classified as CD14+ and Granulocytic MDSCs are classified as CD14− CD15+. The cells were analyzed using an LSR II Flow Cytometer. A. Flow cytometry dot plot of viable cells as determined by Sytox Blue. Cells were then gated as double positive for CD33 and CD11b. B. Cells were gated as HLA-DR+ or HLA-DR−/low based on isotype control. Subsets of those populations were then gated as CD124 high or low. C. Monocytic and granulocytic MDSCs were then differentiated as CD14+ monocytic and CD14− CD15+ granulocytic cells.
In severe sepsis/septic shock (SS/SS) patients, gene ontology pathway analysis demonstrated that several pathways involved in nitric oxide (NO) biosynthesis and bacterial detection had a greater change in expression from healthy controls (HC) in the subacute periods (days 7 and 14). In the heat maps shown, dark blue represents upregulation, whereas light blue represents down regulation. A. SS/SS patients with corresponding higher numbers of MDSCs had increased levels of NO biosynthesis which has been demonstrated to impair T-cell function B. There were significant differences in expression of genes involved in bacterial detection in MDSCs from SS/SS patients compared to HC subjects.
At time points one, four and seven days, IL-4 receptor-α appearance is significantly elevated in severe sepsis/septic shock(SS/SS) patients when compared to healthy control (HC) subjects as identified by flow cytometry.
Isotype controls were performed for all included fluorochromes (CD33 APC, HLA-DR FITC, CD11b Alexa Fluor 700, CD14 Pacific Blue, CD124 PE, CD274 BV650, and CD15 PE-Cy7). Red peaks represent the isotype control and the blue overlay represents the positive fluorochrome.
Supplemental Table 1. Summary of Baseline Characteristics and Hospital Outcomes for All Cohorts and by Reported Outcomes. The patient cohort was primarily male (female, n=26; 39%), aged 60 with four comorbidities. The most common comorbidities were hypertension (n=33; 49%), hypercholesterolemia/hyperlipidemia (n=22; 33%), and diabetes (n=19; 28%). Thirty-seven (55%) patients had septic shock and the average APACHE Score on admission was 23. Average inhospital mortality was 12% and total ICU LOS was 16 days. 52% (n=35) had at least one nosocomial infection and 43% (n=29) had poor discharge status indicating poor functional status as discharge. Additionally, we evaluated for the impact of malignancy (n= 12; 18%). There was no difference in percentage of total MDSCs, granulocytic-MDSCs or monocytic-MDSCs at any time point between those with history of malignancy and those without.
Acknowledgments
This work was supported by grant R01 GM40586-27 awarded by the NIGMS. PAE was supported by P30 AG028740 from the National Institute on Aging and by the NIH NIGMS grant 1 R01 GM113945-01. AMM was supported by NIH NIGMS grant R01 GM105893-01A1. BES and BM were supported by a training grant in burn and trauma research (T32 GM-08431). Finally, PAE, TOB, AMM, FAM, SCB, BB, and LLM were all supported by P50 GM111152-01 (NIGMS).
The Sepsis and Clinical Research Center Investigators comprised of: Ricardo Ungaro, BS; Dina C. Nacionales, MD1; M. Cecilia Lopez, BS2; Jennifer Lanz, ARNP1; Ruth Davis, RN1; Juan C. Mira, MD1; David Holden, BS1; Patrick Verdugo, BS1; Mark Segal, MD3; Azra Bihorac, MD4; Christiaan Leeuwenburgh, PhD5; and Henry V. Baker, PhD2. Departments of Surgery1, Molecular Genetics and Microbiology2, Nephrology3, Anesthesia4, Aging and Geriatrics5, University of Florida College of Medicine; Gainesville, Florida
Footnotes
Conflict of Interest and Financial Disclosure Statement: No conflict of or competing interests have been declared.
References
- 1. [Accessed Feb. 17, 2016];National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. 2011 Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf. [PubMed]
- 2.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Jama. 1997;278(3):234–40. [PubMed] [Google Scholar]
- 3.Rimmele T, Payen D, Cantaluppi V, et al. Immune Cell Phenotype and Function in Sepsis. Shock. 2016;45(3):282–91. doi: 10.1097/SHK.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson JE, Cox CE, Hope AA, et al. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–54. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17(3–4):281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–30. doi: 10.1097/TA.0b013e3182ab1ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371(4):380–3. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 9.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015:1–9. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuenca AG, Moldawer LL. Myeloid-derived suppressor cells in sepsis: friend or foe? Intensive Care Med. 2012;38(6):928–30. doi: 10.1007/s00134-012-2575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darcy CJ, Minigo G, Piera KA, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;18(4):R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janols H, Bergenfelz C, Allaoui R, et al. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96(5):685–93. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 18.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25(11):1789–95. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 20.Damuzzo V, Pinton L, Desantis G, et al. Complexity and challenges in defining myeloid- derived suppressor cells. Cytometry B Clin Cytom. 2015;88(2):77–91. doi: 10.1002/cyto.b.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scumpia PO, Delano MJ, Kelly-Scumpia KM, et al. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110(10):3673–81. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Pribis JP, Rodriguez PC, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. 2014;259(1):171–8. doi: 10.1097/SLA.0b013e31828611f8. [DOI] [PubMed] [Google Scholar]
- 23.Clermont G, Angus DC, Kalassian KG, et al. Reassessing the value of short-term mortality in sepsis: comparing conventional approaches to modeling. Crit Care Med. 2003;31(11):2627–33. doi: 10.1097/01.CCM.0000094233.35059.81. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34(8):1084–93. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 25.Starr ME, Ueda J, Yamamoto S, et al. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med. 50(2):371–80. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 27.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probst C, Pape HC, Hildebrand F, et al. 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury. 2009;40(1):77–83. doi: 10.1016/j.injury.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Probst C, Zelle BA, Sittaro NA, et al. Late death after multiple severe trauma: when does it occur and what are the causes? J Trauma. 2009;66(4):1212–7. doi: 10.1097/TA.0b013e318197b97c. [DOI] [PubMed] [Google Scholar]
- 30.Sasser SM, Varghese M, Joshipura M, et al. Preventing death and disability through the timely provision of prehospital trauma care. Bull World Health Organ. 2006;84(7):507. doi: 10.2471/blt.06.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang DW, Tseng CH, Shapiro MF. Rehospitalizations Following Sepsis: Common and Costly. Crit Care Med. 2015;43(10):2085–93. doi: 10.1097/CCM.0000000000001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueno V, Sant'Anna OA, Lord JM. Ageing and myeloid-derived suppressor cells: possible involvement in immunosenescence and age-related disease. Age (Dordr) 2014;36(6):9729. doi: 10.1007/s11357-014-9729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nacionales DC, Szpila B, Ungaro R, et al. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195(5):2396–407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nacionales DC, Gentile LF, Vanzant E, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. 2014;192(2):612–22. doi: 10.4049/jimmunol.1302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meirow Y, Kanterman J, Baniyash M. Paving the Road to Tumor Development and Spreading: Myeloid-Derived Suppressor Cells are Ruling the Fate. Front Immunol. 2015;6:523. doi: 10.3389/fimmu.2015.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manjili MH. Phenotypic plasticity of MDSC in cancers. Immunol Invest. 2012;41(6–7):711–21. doi: 10.3109/08820139.2012.673670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were stained with the following antibodies: CD33 APC, HLA-DR FITC, CD11b AF700, CD14 PB, and CD15 PE-Cy7. MDSCs were characterized as CD33+ CD11b+ HLA-DR−/low with subsets of Monocytic-MDSCs are classified as CD14+ and Granulocytic MDSCs are classified as CD14− CD15+. The cells were analyzed using an LSR II Flow Cytometer. A. Flow cytometry dot plot of viable cells as determined by Sytox Blue. Cells were then gated as double positive for CD33 and CD11b. B. Cells were gated as HLA-DR+ or HLA-DR−/low based on isotype control. Subsets of those populations were then gated as CD124 high or low. C. Monocytic and granulocytic MDSCs were then differentiated as CD14+ monocytic and CD14− CD15+ granulocytic cells.
In severe sepsis/septic shock (SS/SS) patients, gene ontology pathway analysis demonstrated that several pathways involved in nitric oxide (NO) biosynthesis and bacterial detection had a greater change in expression from healthy controls (HC) in the subacute periods (days 7 and 14). In the heat maps shown, dark blue represents upregulation, whereas light blue represents down regulation. A. SS/SS patients with corresponding higher numbers of MDSCs had increased levels of NO biosynthesis which has been demonstrated to impair T-cell function B. There were significant differences in expression of genes involved in bacterial detection in MDSCs from SS/SS patients compared to HC subjects.
At time points one, four and seven days, IL-4 receptor-α appearance is significantly elevated in severe sepsis/septic shock(SS/SS) patients when compared to healthy control (HC) subjects as identified by flow cytometry.
Isotype controls were performed for all included fluorochromes (CD33 APC, HLA-DR FITC, CD11b Alexa Fluor 700, CD14 Pacific Blue, CD124 PE, CD274 BV650, and CD15 PE-Cy7). Red peaks represent the isotype control and the blue overlay represents the positive fluorochrome.
Supplemental Table 1. Summary of Baseline Characteristics and Hospital Outcomes for All Cohorts and by Reported Outcomes. The patient cohort was primarily male (female, n=26; 39%), aged 60 with four comorbidities. The most common comorbidities were hypertension (n=33; 49%), hypercholesterolemia/hyperlipidemia (n=22; 33%), and diabetes (n=19; 28%). Thirty-seven (55%) patients had septic shock and the average APACHE Score on admission was 23. Average inhospital mortality was 12% and total ICU LOS was 16 days. 52% (n=35) had at least one nosocomial infection and 43% (n=29) had poor discharge status indicating poor functional status as discharge. Additionally, we evaluated for the impact of malignancy (n= 12; 18%). There was no difference in percentage of total MDSCs, granulocytic-MDSCs or monocytic-MDSCs at any time point between those with history of malignancy and those without.