Abstract
To serve vision, vertebrate rod and cone photoreceptors must detect photons, convert the light stimuli into cellular signals, and then convey the encoded information to downstream neurons. Rods and cones are sensory neurons that each rely on specialized ciliary organelles to detect light. These organelles, called outer segments, possess elaborate architectures that include many hundreds of light-sensitive membranous disks arrayed one atop another in precise register. These stacked disks capture light and initiate the chain of molecular and cellular events that underlie normal vision. Outer segment organization is challenged by an inherently dynamic nature; these organelles are subject to a renewal process that replaces a significant fraction of their disks (up to ~10%) on a daily basis. In addition, a broad range of environmental and genetic insults can disrupt outer segment morphology to impair photoreceptor function and viability. In this chapter, we survey the major progress that has been made for understanding the molecular basis of outer segment architecture. We also discuss key aspects of organelle lipid and protein composition, and highlight distributions, interactions, and potential structural functions of key OS-resident molecules, including: kinesin-2, actin, RP1, prominin-1, protocadherin 21, peripherin-2/rds, rom-1, glutamic acid-rich proteins, and rhodopsin. Finally, we identify key knowledge gaps and challenges that remain for understanding how normal outer segment architecture is established and maintained.
Keywords: photoreceptor, outer segment, cilia, membrane curvature, retinal degeneration, tetraspanin
1. Introduction
Vertebrate retinal photoreceptors are sensory neurons specialized to detect light and initiate the biological process of vision. They possess a dedicated and distinctive photosensory organelle evolutionarily derived from a primary non-motile cilium, referred to as an outer segment (OS). The ciliary basis of vertebrate vision originally spurred intense interest with the widespread application of biological transmission electron microscopy (TEM), and numerous elegant studies detailed the highly membranous and dynamic nature of rod and cone photoreceptor OSs (Anderson et al., 1978; Bok, 1982; LaVail, 1973; Rohlich, 1975; Young, 1967, 1976). These investigations revealed that the hundreds of stacked membranous disks comprising these organelles are renewed by opposed processes of disk morphogenesis and shedding. More recently, OS organelle architecture became a subject of renewed widespread attention, when it was discovered that humans suffer from a variety of inherited “ciliopathies” - syndromic diseases that often include problems with vision (Fliegauf et al., 2007). The molecular logic that unites these syndromes includes a set of several dozen genes that are important for ciliary structure and function. These diseases, and numerous other progressive retinal dystrophies illustrate that a wide range of insults can compromise rod and cone photoreceptor OS structure to cause various forms of retinopathy. This chapter reviews current knowledge and understanding of the molecules and mechanisms underlying OS architecture and identifies key questions remaining in this area. It focuses on OS-resident molecules with direct roles for structuring the OS organelles. Readers are referred to review articles on closely related topics not treated here, including, photoreceptor development (Brzezinski and Reh, 2015; Kennedy and Malicki, 2009; Swaroop et al., 2010), OS protein targeting (Pearring et al., 2013), OS phagocytosis (Kevany and Palczewski, 2010; Mazzoni et al., 2014), and photoreceptor cell biology (Molday and Moritz, 2015).
2. Photoreceptor morphology
2.1. A common plan for vertebrate photoreceptors
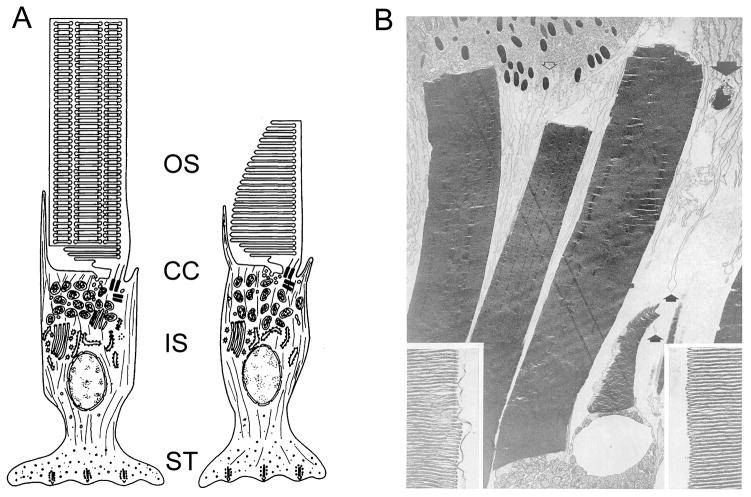
Retinal photoreceptors detect photons, transduce the light stimuli into cellular signals, and transmit the encoded information to downstream neurons. Vertebrate rod and cone photoreceptors each do so via a tripartite cellular architecture that includes, an OS that functions for phototransduction, a cell soma that houses the machinery required for cell viability and general housekeeping, and a synaptic terminal able to signal to second-order neurons. Photoreceptors represent the bulk of the neurons in the mammalian retina. Their OSs are elongated structure oriented along the axis of incoming light, and are packed tightly together in arrays that have evolved to satisfy competing needs for sensitivity and spatial resolution. Two photoreceptor subtypes serve distinct roles for visual perception. When photons are scarce, rod cells allow motion detection and the ultimate in light sensitivity. When more light is available, cone cells provide high-resolution color perception. Cone cells pre-date rods in evolutionary history, and are relatively abundant in retinas of cold-blooded species. In contrast, mammalian retinas are dominated by rod photoreceptors and typically possess relatively few cones. OSs are connected to the cell soma via a thin, eccentrically positioned bridge, called the connecting cilium (CC), which contains a (9+0) arrangement of doublet microtubules (Fig. 1A). The OS is essentially a very elaborate primary cilium, where the ciliary plasma membrane has been extensively amplified for sensitive light reception. The CC corresponds to the transition zone, present in all cilia, as the region between the basal bodies and the distal axoneme (Besharse and Horst, 1990; Horst et al., 1990; Rohlich, 1975). Purification of vertebrate OSs is facilitated by their exposure on the surface of isolated retinas and by their mechanically labile connection to the cell soma; ease of OS isolation played a major role for the detailed description of the phototransduction signaling cascade. It is important to note that the differing dimensions and organization of photoreceptors (and perhaps other factors) in the commonly studied vertebrate retinas (cow, frog, and mouse) result in unique subcellular fragmentation patterns during tissue homogenization. During homogenization, cow retinas tend to shed rod OSs that have broken away from their ISs at the CC (Papermaster, 1982). In contrast, although frog retinas shed isolated OSs, they also tend to generate OS-IS fragments that retain the IS ellipsoid (mitochondria-rich) region (Biernbaum and Bownds, 1985), and a similar scenario has been observed for mouse retinas (Gilliam et al., 2012). In every case, procedural details are critical to ensure significant OS enrichment, and the variability inherent in retinal homogenization means that quantitative assessments must be made to demonstrate purity and/or contamination levels. In addition to the CC itself, several unique structures are present on the distal photoreceptor inner segment (IS) that play important roles for the generation and support of OS architecture, including the periciliary ridge complex (Peters et al., 1983; Yang et al., 2010) and calycal processes (Borwein, 1985).
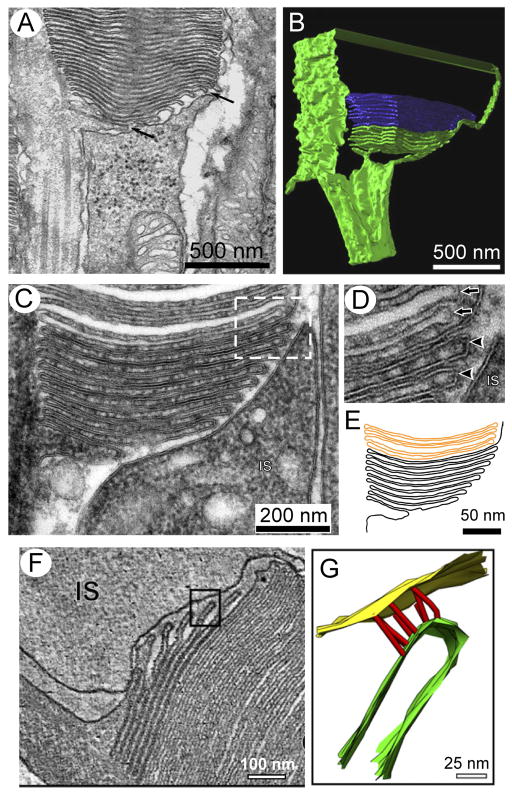
Figure 1. The OS in context.
A) Illustrations of rod (left) and cone (right) frog photoreceptors; adapted from (Bok, 1985). In each subtype, the light-sensitive OS is attached to the inner segment (IS) via the connecting cilium (CC). B) Transmission electron microscopy of adult frog OSs; three rod and one cone OSs are present. Apical processes (small arrows) and a phagosome containing shed disks (large arrow) are present. Insets show enlarged images of disk rims (left) and edges (right) from a cone OS. Reproduced with permission from (Kinney and Fisher, 1978a).
2.2. Rod and cone OS ultrastructure
2.2.1. Seminal investigations of adult and developing photoreceptors
A systematic investigation by Sjostrand was amongst the first to establish the “double membrane disk” as the fundamental structural unit of rod OSs in vertebrate species (Sjostrand, 1953). Adult guinea pig and perch retinal rods were shown to contain flattened membranous sacs (disks) of uniform dimension stacked atop one another with regular spacing; these disk membranes appeared to be completely internalized within an enclosing PM. The guinea pig rod OS was estimated to contain roughly 700 hundred disks, corresponding to a total surface area of 45 cm2 per million rods, a value similar to that observed for perch rods (46 cm2 per million rods), which display smaller diameters, but about twice as many disks (roughly 1400) per OS. Closer examination revealed that disks possess structurally resilient rim regions and a single deep indentation, where they are “incised” – structural features now known as incisures. Comparison of rod to cone OSs showed that cones display a basal-to-distal taper and possess disk membranes with a thicker, and more granular character. Sjostrand also emphasized that OSs are highly sensitive to fixation/embedding procedures; disk lumen expansion occurs readily in mildly affected samples and extensive disorganization (vesiculation) of disk membranes at the OS base takes place in less well-preserved samples. In a subsequent report, Sjostrand suggested that in contrast to rods, perch cone OS disks may be continuous with their OS plasma membrane (Sjostrand, 1959); these results were subsequently confirmed in other cold-blooded species (Moody and Robertson, 1960).
Cohen was one of the first to clearly demonstrate that mammalian rod and cone OSs each possess some disks that remain in continuity with the OS plasma membrane (Cohen, 1961). Many (perhaps all) disks in the basal third of the primate cone OS were found to be continuous with the plasma membrane. More distal regions of the primate cone OS also possess disks that are continuous with the plasma membrane, although their frequency is reduced. Primate rod OSs in contrast, possess only a few disks at their base that show continuity with the plasma membrane; the remainder of the rod disks are fully internalized. Essentially similar features are also found in the unusually large OSs present in frog photoreceptors; however, frog rods are also distinguished by multiple deep incisures, which create a flower-like appearance in TEM cross-sections (and appear as longitudinal striations in transverse sections). Frog cones show a particularly striking demonstration of disk-plasma membrane continuity (Nilsson, 1965). Essentially all cone OS disks show plasma membrane continuity and few, if any, are internalized as discrete compartments (Fig. 2A). In contrast, frog rod OSs (like mammalian rod OSs), possess only a few disks that are continuous with the plasma membrane, and these are only ever observed at the OS base. Akin to the findings for guinea pig, perch (Sjostrand, 1953), and monkey photoreceptors (Cohen, 1961), delayed fixation and/or retinal detachment causes artefactual basal disk vesiculation in frog photoreceptors (Nilsson, 1965).
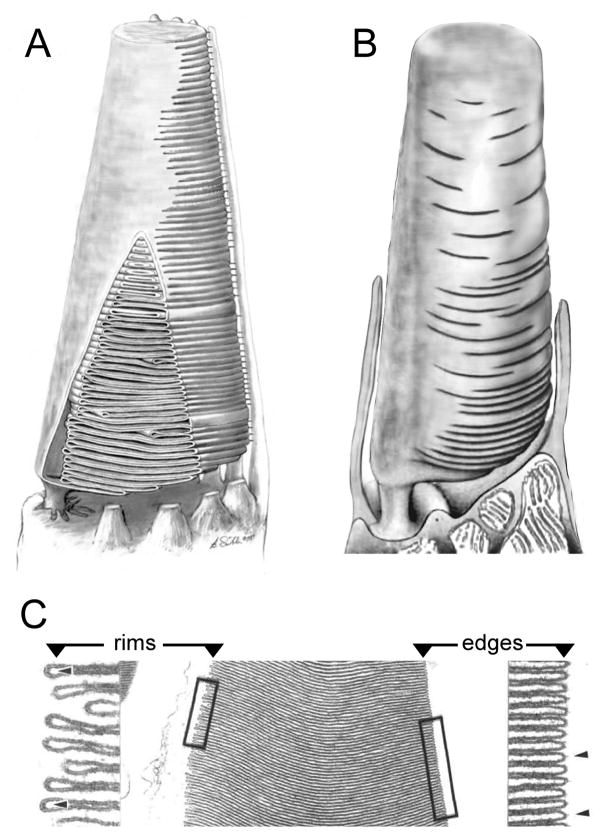
Figure 2. OS disk membrane topology varies by cell subtype, species, and disk age.
Renderings of cone photoreceptor OSs (A, B; not to scale). A) frog cone adapted from (Weber et al., 2011). Frog cone OSs possess a strong basal-to-distal taper. A portion of the plasma membrane has been cut away to reveal the internal structure. The plasma membrane opposite the eccentrically positioned cilium is pleated to form a large stack of disks that are further defined by the presence of rims, which partially bound the pleats and begin the process of defining a new compartment. The apertures that expose disk surfaces to the extracellular milieu extend about halfway around the disk boundaries, and are aligned along the length of the OS to those of adjacent disks. B) Mammalian cone adapted from (Anderson et al., 1978). Mammalian cone OSs possess more subtle basal-to-distal tapers than those present in frog cones. Like frog cone OSs, mammalian cone OSs also possess disks that remain in continuity with the plasma membrane. In this case however, the boundary of each mature disk is largely differentiated from the plasma membrane as a rim, so that the disks are largely internalized and only relatively small apertures remain open to the extracellular milieu. Moreover, these apertures are not aligned along the OS length, and make topological interpretation of sectioned tissue quite challenging. C) Transverse sections of frog cone OSs illustrate the distinction between U-shaped disk edges (right) and hairpin-shaped disk rims (left) that are present in the partially-internalized disks prevalent in cones. Adapted from (Corless and Fetter, 1987).
The topology of OS membranes has been a subject of frequent confusion and varied nomenclature in the literature, and deserves special mention. Careful examination of rod and cone disk membranes reveals the presence of two distinct types of disk boundaries. While fully internalized disks only possess “hairpin-shaped” boundaries, disks in continuity with the plasma membrane show both “hairpin-shaped” and “U-shaped” boundaries. These features are particularly well illustrated in longitudinal sections of frog cone OSs, in which nearly all disks are partially internalized, and therefore possess both types of boundaries (Fig. 2C). We shall use the term rim when referring to a hairpin-shaped boundary and edge when referring to a U-shaped boundary. Further, we shall use the term disks to refer to the entire stack of OS membranes oriented perpendicular to the photoreceptor cell axis, regardless of their degree of internalization. While disk rims are structural domains within individual disks, disk edges are shared between two adjacent disks. Finally, we use the term lamellae to refer to the central portions of disks, which may be bounded by rims and edges (or rims alone), depending on their extent of internalization. Assaying the extent of internalization for a given disk (or disks) is technically challenging, because three-dimensional reconstruction is required to do so rigorously. This issue is particularly important for evaluating mammalian cone OSs, because in contrast to frog cone OSs, the majority of disks are largely, but incompletely internalized. Figure 2B provides a rendering of a mammalian cone to illustrate this point; interested readers are referred to an excellent previous review for a more complete discussion of this topic (Anderson et al., 1978).
Authors of these seminal studies also noticed that the OSs of all species examined display intimate structural relationships with extracellular matrix material, and an adjacent retinal pigment epithelial (RPE) layer (Young and Bok, 1969). Rod OSs tips lie adjacent to a single layer of RPE cells, from which slender membranous processes extend (visible in Fig. 1B). These apical RPE processes typically sheath up to ~one-quarter of the full OS length in rods, and participate in the OS renewal process by phagocytosing disk packets. The number of photoreceptor OSs sheltered by a single RPE cell varies with species and geographical location within the retina; a density of up to 20:1 OS/RPE is documented in the primate retina (Snodderly et al., 2002). RPE-photoreceptor cell contacts are not mediated by junctional complexes; instead, only relatively weak adhesive forces hold the OSs in contact with the RPE. Although some adhesion molecules have been identified (Nandrot et al., 2006), this interaction is not yet completely defined. Cone OS tips are also sheathed by RPE apical processes; however, because cone OSs do not extend to the level of the RPE, the processes must travel farther to meet them. The central importance of the RPE-OS relationship was revealed by Young, who demonstrated that OSs undergo a regular renewal process (Young, 1967). This study used metabolic labeling in combination with autoradiography and conventional TEM to demonstrate that disks shed from the distal OS tip (and phagocytosed by the RPE) are replaced by new disk morphogenesis at the basal OS. Subsequent studies of OS renewal found that shedding occurs from both rod and cone OSs in all species examined (Nguyen-Legros and Hicks, 2000).
The dramatic ultrastructure of adult rod and cone OSs also prompted seminal investigations of OS morphogenesis in developing photoreceptors. Although by the first postnatal day, murine photoreceptor cells have differentiated, they lack outer segments, and project only what appears as a generic primary cilium into the subretinal space (Besharse et al., 1985; De Robertis, 1956; Liu and Williams, 2001). A ballooning of the ciliary plasma membrane at its distal tip then occurs, and (in the absence of fixation artifacts), an initial series of small well-organized disks are formed (Nilsson, 1964). Subsequent stages show an increasingly regular organization of flattened membranous disks. In some studies and species these initial disks are aligned along the long axis of the photoreceptor (Morrison, 1983; Obata and Usukura, 1992; Tokuyasu and Yamada, 1959). This arrangement may reflect evolutionary history, as a similar arrangement is present in ascidian ciliary photoreceptors (Lamb et al., 2007). Continued OS development results in the appearance of additional neatly-stacked disks that are oriented perpendicular to the photoreceptor axis; the new disks displace existing disks distally, and the OS eventually achieves its mature length and appearance.
2.2.2. Models for OS disk morphogenesis
Nilsson presented an early hypothesis for OS morphogenesis during photoreceptor development (Fig. 3), based on studies of the developing tadpole retina, a model that facilitates rapid retinal fixation in situ (Nilsson, 1964). The proposed sequence includes, plasma membrane invagination, followed by disk flattening, circumferential expansion, and a progressive translation towards the distal ciliary tip. A gradual separation of the flattened invaginations (disks) from the plasma membrane was proposed to occur during translation towards the distal ciliary tip. Investigation of several vertebrate species (including mammals), found largely similar structural features, but noted that patterns of disk enlargement are more consistent with growth via plasma membrane evagination (vs. invagination) (Anderson et al., 1978; Besharse et al., 1977; Carter-Dawson and LaVail, 1979; Kinney and Fisher, 1978a, b). These and similar studies resulted in a general hypothesis for OS disk morphogenesis in developing photoreceptors (Steinberg et al., 1980). Importantly, this model also accounts for the daily disk morphogenesis that occurs in adult photoreceptors, as part of the OS renewal process (Young, 1967).
Figure 3. Development of the vertebrate OS.
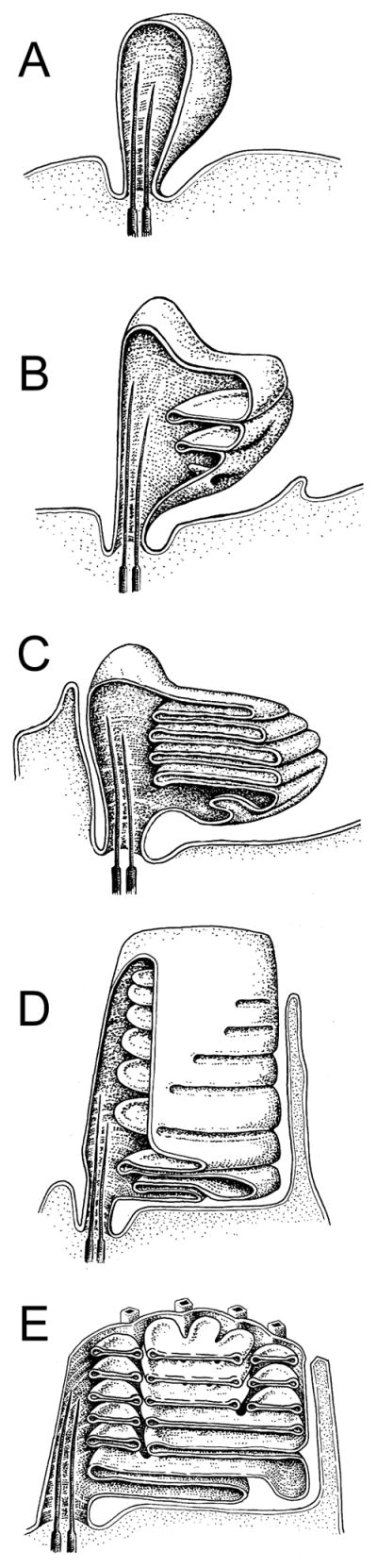
Renderings of ROS morphogenesis in Rana pipians tadpole; adapted from (Nilsson, 1964). Retinal pigment epithelium is omitted for clarity. A) Ballooning of the ciliary plasma membrane. B) Continued plasma membrane expansion and invagination forms initial nascent disk membranes. C) Expansion and displacement of existing disks by new disks create plasma membrane pleating and an initial stack of open disks. D) Disks are further differentiated by the gradual expansion of rims, which begin the process of internalization. E) Development of incisures in mature, completely internalized disks. Cone OS development was proposed to be similar, but rim expansion halts prior to complete disk internalization, and no incisures are formed.
The Steinberg, et al. model postulates that two sequential steps of plasma membrane growth contribute to disk morphogenesis (Fig. 4). During the first step (Fig. 4A), growth of the ciliary plasma membrane creates an evagination that grows away from the axoneme, to which it is anchored by its uppermost surface (Fig. 4A.1, arrowhead). Anchoring of the evagination’s lower surface to the axoneme allows a subsequent evagination to be initiated (Fig. 4A.2). Multiple evaginations must be present to form an internalized disk, because it is the surfaces of adjacent evaginations that become internalized as individual disks. During the second step (Fig. 4B), plasma membrane outgrowth occurs at the point where neighboring evaginations meet and are anchored to the axoneme by a disk rim. Addition of membrane surface area at this particular point advances the plasma membrane away from the axoneme in a bilateral manner (Fig. 4B.2, arrows). This extension of the plasma membrane allows a simultaneous advance of the highly curved rim domain away from its anchoring point - to initiate disk differentiation and internalization.
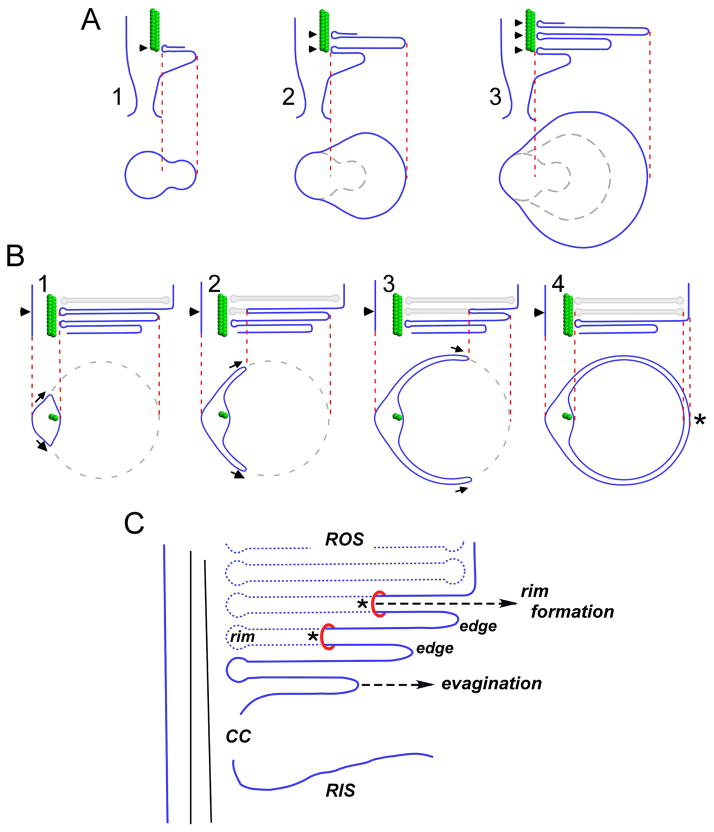
Figure 4. Steinberg et al. two-step (A, B) model for OS disk morphogenesis.
A) Step1 - growth of the ciliary plasma membrane creates an evagination with an upper surface anchored at an axonemal microtubule (A.1). Anchoring of the evagination lower surface allows for initiation of a new evagination (A.2). Evagination translation towards the cilium distal tip is accompanied by flattening and growth to mature disk diameter (A.3). At this stage, disks are bounded by edges; rims are absent, except for those regions immediately adjacent to the axonemal microtubules (arrowheads). Below: Tangential views sectioned through of each new evagination (arrowheads in longitudinal views). B) Step 2 - disk internalization begins by expansion of the rim region that anchors evaginations to the axoneme. Above: longitudinal views show expansion of the disk rim from left to right across one open disk (arrowheads). Below: tangential views sectioned through a single disk (arrowheads) undergoing internalization. The disk rim present at the axoneme advances bilaterally, in parallel with the enclosing plasma membrane at two growth points (arrows). Disk rim growth is proposed to occur by membrane addition. Completion of rim formation and disk internalization occurs opposite the axoneme (asterisk), and requires a membrane fission event to sever disk-plasma membrane continuity. Adapted from (Steinberg et al., 1980). C) A view of disk morphogenesis that emphasizes disk rim formation via membrane addition. Asterisks illustrate growth points, at which the addition of new plasma membrane allows rim formation to advance. Adapted from (Arikawa et al., 1992).
The Steinberg et al. two-step model is well supported by studies showing that disk differentiation by rim formation (assayed by the presence of peripherin-2/rds) is always accompanied by the appearance of an enclosing plasma membrane in both rods and cones (Arikawa et al., 1992; Burgoyne et al., 2015). The addition of new plasma membrane at specific growth points (Fig. 4C, asterisks) that is hypothesized to allow disk rim advance. The two-step model is also consistent with the documented sorting of membrane proteins into disk and plasma membrane domains that occurs in rod OSs (Molday and Molday, 1993). In at least one instance, a membrane protein can be specifically routed to the rod OS plasma membrane by preventing it from entering nascent disks (Nemet et al., 2014). It is important to note that the gradual process of disk internalization/differentiation from the plasma membrane does not represent a topological change as long as any amount of continuity is retained (as in Fig. 4B.3). Membrane fusion is therefore not, in principle, required for disk differentiation to proceed. In contrast, membrane fusion (more properly, fission) is only required as a final step in disk internalization - to disrupt a small neck of membrane and sever the continuity between the nascent disk and the plasma membrane. Regardless of whether rim formation occurs by membrane outgrowth or fusion, the distinction between disk rims and disk edges is a critical one for considering the molecular mechanisms underlying OS disk morphogenesis and ultrastructure.
An alternative view of disk membrane morphogenesis was suggested by an early study of developing kitten retinas, which observed irregular vesicular structures within the ballooning ciliary plasma membrane prior to the appearance of flattened disks (Tokuyasu and Yamada, 1959). This model suggests that small endocytic vesicles may fuse to form flattened disks. Models of disk formation by endosomal expansion (vs. plasma membrane evagination) have enjoyed limited but continued support (Chuang et al., 2007; Obata and Usukura, 1992), including a recent revival that argued for a similar “vesicular targeting” model (Sung and Chuang, 2010). A trio of recent publications have thoroughly examined the question of disk morphogenesis in numerous vertebrate species (discussed below), that together provide a compelling body of evidence that strongly upholds the plasma membrane evagination model for morphogenesis of vertebrate rod photoreceptors (Burgoyne et al., 2015; Ding et al., 2015; Pugh, 2015; Volland et al., 2015). Current thinking suggests that disk membrane morphogenesis in cones follows essentially similar principles (Mustafi et al., 2009).
2.2.3. OS disk patency and membrane tethering interactions
Beyond the question of how new disks form, early investigators also examined whether disks represent distinct intracellular compartments, or retain continuity with the plasma membrane such that disk lumens remain open to the extracellular space. Cohen was the first to examine the question of disk “openness” (or patency) using small molecule infiltration to identify disk lumens open to the extracellular milieu (Cohen, 1968, 1970). These studies reinforced the idea that most rod disks are fully internalized, but most cone disks retain continuity with the plasma membranes. Several studies of disk osmotic behavior support the concept that rod disks represent distinct intracellular compartments (Falk and Fatt, 1973; Heller et al., 1971). A number of subsequent studies used fluorescent tracers and light microscopy to ask similar questions in vivo, and reached similar conclusions (Laties et al., 1976; Matsumoto and Besharse, 1985; Yoshikami et al., 1974). Interestingly, one investigation found that rod OSs possess some open disks at their distal tips, as well as at their bases (Matsumoto and Besharse, 1985). Such distal rod disks, which display luminal communication with the extracellular environment, may indicate a “reversal” of the disk morphogenesis process, wherein disks may reestablish continuity with the plasma membrane as part of the shedding process. A more recent study of isolated intact photoreceptors calls into question the concept that once rod disks are fully internalized, they remain completely independent compartments for their entire lifetimes (Chen et al., 2002). This investigation found that, akin to a previous study (Matsumoto and Besharse, 1985), extracellular polar tracers preferentially label rod OS distal tips. Strikingly however, incubation after tracer washout resulted in relatively rapid dye propagation along the photoreceptor length. These results introduce the possibility that communication between disk lumens plays an as yet undefined physiological role for rod OSs.
Many early ultrastructural studies describe disks as “free floating” within the OS, but subsequent work by several groups revealed a network of cytoplasmic membrane-membrane tethers, variously described as “fibrils”, “filaments”, or “spacers” (Corless et al., 1987; Fetter and Corless, 1987; Kajimura et al., 2000; Nickell et al., 2007; Roof and Heuser, 1982; Usukura and Yamada, 1981). One class of tethers is extracellular and is anchored to disk edges and spans the gaps between the lamellae of individual disks (Fig. 5A). A second class is cytoplasmic and is anchored at disk rims and spans the gaps between adjacent disks (Fig. 5B). Finally, a third class of tethers, also cytoplasmic, bridges the gaps between disk rims and the OS plasma membrane (Fig. 5C). One study (Nickell et al., 2007) has also observed globular “spacers” distributed in a random fashion over disk surfaces (Fig. 5D).
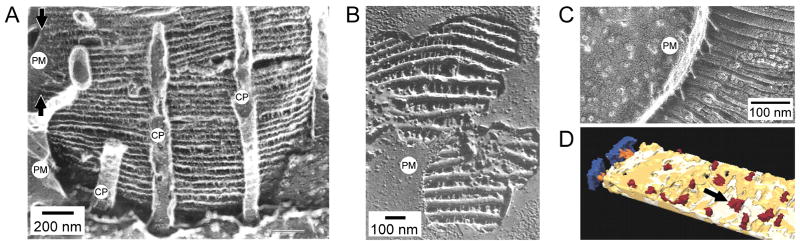
Figure 5. Tethering features that may contribute to OS architecture.
A) TEM image of a frog cone OS prepared by freeze-fracture deep-etch rotary-shadowing. Numerous regularly-organized axially-oriented tethers link adjacent disk edges. The leading edge of the OS plasma membrane (PM), the point at which disk internalization has halted, is indicated between the arrows (upper left). Several calycal processes (CP) are present in the image. Image adapted from (Fetter and Corless, 1987). B) Fracturing and removal of plasma membrane (PM) from a similarly prepared toad rod OS exposes regularly organized tethers linking adjacent disk rims. Image adapted from (Roof and Heuser, 1982). C) A similar preparation technique applied to a bovine rod OS protein reveals tethers between disk rims and the plasma membrane (PM). Image adapted from (Roof and Heuser, 1982). D) Cryoelectron microscopy of frozen vitrified mouse rod OSs finds tethers (orange) between disk rims and the plasma membrane (PM; blue) and “spacers” (red; arrow) distributed randomly upon the lamellar portion of the disk. Image adapted from (Nickell et al., 2007).
The functional importance of the various tethers is not known, but their presence is commonly assumed to indicate a role for organizing disk morphogenesis and/or disk stacking stability. It also seems likely that the numerous membrane-membrane tethers present in OSs contribute to their relatively high structural rigidity (Haeri et al., 2013). Detailed conceptual models for how tethering elements may contribute to disk morphogenesis and incisure development have been proposed (Corless and Fetter, 1987; Corless and Schneider, 1987), but remain to be tested and validated. Likewise, the molecular identity of OS membrane-membrane tethers remains to be rigorously demonstrated.
2.2.4. Recent investigations of OS ultrastructure and disk morphogenesis
Several studies have employed cryogenic electron tomography (cryo-ET) in conjunction with purified murine rod OSs to reexamine the dimensions of rod OS membrane architecture (Gilliam et al., 2012; Nickell et al., 2007). These studies largely confirm the bulk of the gross structural features originally documented for rod OSs via conventional TEM. Moreover, reasonably good quantitative agreement was seen for particular dimensions of OS substructures. The cryo-ET studies also describe novel observations that differed from prior findings. In one case, globular “spacers” that bridge cytoplasmic surfaces of adjacent disks were present – a feature presumed to contribute to the organization of disk stacks (Nickell et al., 2007). In contrast to an earlier report (Roof and Heuser, 1982), spacers were not restricted to disk rims, but were heterogeneous in size and randomly distributed on disk surfaces. A subsequent cryo-ET investigation made no mention of such interdisk “spacers”, but instead suggested that interdisk spacing may be governed by the repeat distance inherent in microtubule architecture (Gilliam et al., 2012). This latter study also observed that the nascent disks present at the OS base were invariably enveloped by the OS and not continuous with it, contributing to a dispute concerning the nature of nascent disk morphogenesis (discussed below). While the rapid freezing procedures used for cryo-ET sample preparation can preserve native biological structure, this approach requires substantial tissue disruption. Because OS ultrastructure is rapidly perturbed by detachment from the RPE and by delayed fixation, caution must be applied for interpreting images of isolated OS organelles.
Most recently, three independent studies have addressed the controversial question of basal OS ultrastructure and disk morphogenesis with a variety of independent approaches (Burgoyne et al., 2015; Ding et al., 2015; Volland et al., 2015). All support the Steinberg et al. hypothesis that new disks form via evagination of the ciliary plasma membrane. Critically, the new studies carefully document the value of preserving labile aspects of OS ultrastructure by perfusing animals with fixatives prior to dissection. Altogether, these studies offer a convincing demonstration that vesiculation and plasma membrane enclosure at the OS base are artifacts caused by inadequate fixation, as Nilsson had argued half a century ago. When proper precautions are implemented, a clear and reproducible visualization of basal disk continuity with the ciliary plasma membrane is observed in rod OSs from mice and other mammals (Fig. 6). By performing TEM tomography to analyze the three-dimensional organization of basal OS membranes in mice, monkeys, and cats, Volland et al. found up to 10 open disks in continuity with the ciliary plasma membrane, with quantitative variation among different species and according to the time of day. They also demonstrated that single longitudinal sections frequently give the appearance of vesicles and partial disks being completely enclosed by the OS plasma membrane, even though it is clear from three-dimensional analysis of serial tomograms that the partial disks are in fact continuous with the plasma membrane. Using conventional TEM and preferential labeling of OS membranes exposed to the extracellular space, (Ding et al., 2015) demonstrated the presence of open disks at the base of mouse rod OS, and also concluded that new disks are formed from membrane evaginations. Finally, (Burgoyne et al., 2015) combined conventional and cryo-immuno TEM to arrive at a largely similar set of conclusions. Importantly however, these authors also observed a novel ultrastructural feature – “fibers” linking the edges of nascent disks and the adjacent plasma membrane of the photoreceptor IS (illustrated in Fig. 6 F, G). Using immunogold labeling studies, these authors also reproduced the previously documented localization of protocadherin 21 at these sites (Rattner et al., 2001), and suggest that protocadherin 21 participates in these linkages (discussed below).
Figure 6. Organization of nascent rod OS disk membranes.
A) Evaginating basal disks of mouse rod OSs (arrows) show clear continuity with the ciliary plasma membrane when retinas are preserved via transcardial perfusion with fixative prior to dissection; adapted from (Volland et al., 2015). Arrows identify nascent disks continuous with the plasma. B) 3D rendering of an electron tomogram of the basal region of a monkey rod OS. Nascent disks, which show continuity with the ciliary plasma membrane are colored green, while fully internalized mature disks are colored blue; adapted from (Volland et al., 2015). C–E) In vivo perfusion with tannic acid-containing fixative highlights open disks; adapted from (Ding et al., 2015). The boxed region in C) is enlarged in D) and illustrated as a pseudo-colored tracing in E). Arrowheads mark nascent disks; arrows identify mature disks. The poor permeability of tannic acid through membranes results in enhanced staining of disks that retain continuity with the plasma membrane. F–G) Linkages connect evagination (nascent disk) edges with IS plasma membrane; adapted from (Burgoyne et al., 2015). F) Visualization of an OS base from a single tomogram slice. A 3D model of the tomographic data from the boxed area in F) is presented in G). The model illustrates fibers (red) linking an evagination (green) edge to the IS plasma membrane (yellow).
The recent confirmations that OS disk morphogenesis proceeds by a membrane evagination mechanism emphasize the knowledge gaps that remain for this subject. The details of how membrane evaginations form are not known; however, it has been noted that invaginations of varied shapes were often present in a bulge of the ciliary plasma membrane immediately proximal to the growing evaginations (Volland et al., 2015). This observation suggests that the initiation of a new evagination may involve a small precursor invagination. This invagination may be important for membrane reshaping to form a flattened structure that can evaginate, as well as for anchoring the incipient rim of the nascent disk. (Burgoyne et al., 2015) noted that the distance between distal and proximal membranes of each evagination is only ~11 nm, which is insufficient to accommodate the types of actin networks that occur in lamellipodia. They therefore suggest that the outward growth of the lamellae may be akin to an actin-independent blebbing mechanism. With tissue preserved by high-pressure freezing and freeze substitution, (Volland et al., 2015) observed that the extracellular distance between evaginations was so small (~4 nm) that intermolecular and surface forces between opposing membranes could come into play, potentially providing a force to drive a smaller evagination outward along the surface of its more mature neighbor (Israelachvili, 2011).
The recent validation of the membrane evagination hypothesis has led investigators to consider the process of nascent disk internalization by disk rim formation. (Volland et al., 2015) adhered to the concept originally proposed by Steinberg et al., that addition of membrane at growth points situated between evaginations allows the advance of rim formation and a progressive differentiation of disk from plasma membrane. In contrast, (Burgoyne et al., 2015) suggested that the leading edges of neighboring evaginations undergo membrane fusion to promote disk rim formation and internalization. Although the static ultrastructure of nascent disk membranes is consistent with both the growth point advance and membrane fusion models, we note that the growth point advance hypothesis offers a simple mechanism for the localization of rim proteins, including P/rds, rom-1 and ABCA4.
2.2.5. Significance of OS ultrastructure for function
The most obvious feature of OS architecture is the abundance of photopigment-filled membranous disks stacked along the axis of incoming light. This circumstance increases the sensory surface area by several orders of magnitude and provides for efficient photon capture. The differing shapes of rod and cone OSs, extent of disk internalization, segregation of disks into rim and lamellar domains, and the presence, number, and depth of disk incisures, have historically prompted questions as to the functional significance of these evolutionarily conserved structural features. (Caruso et al., 2006; Molday and Moritz, 2015). In most cases, these issues await definitive answers; however, OS membrane structure is well-documented to strongly affect diffusion within the rod OS. Several studies agree that the dense packing of “wall-to-wall” membranes within rod OS significantly impedes longitudinal diffusion within the cytoplasm and restricts the spread of signaling events (Calvert et al., 2010; Holcman and Korenbrot, 2004; Lamb et al., 1981; Olson and Pugh, 1993). In addition, the cytoplasmic microcompartments created by the tightly packed architecture possess dimensions that are small relative to the sizes of the protein macromolecules that function within them. An elegant investigation used transgenically expressed tandem repeat GFP reporters in X. laevis rods to show that this environment can sterically exclude macromolecules based on their physical dimensions (Najafi et al., 2012b). This important observation demonstrates that molecular processes within the OS cytoplasmic compartment (including phototransduction and other signaling events), must be considered in light of their microenvironment, which includes powerful contributions from steric exclusion and membrane surface potentials. Although the significance of rod disk division by incisures is not clear, it is believed to facilitate diffusion of cytoplasmic signaling molecules along the OS axis. It also restricts the diffusion of integral membrane proteins and molecules tethered to disk surfaces. A recent fluorescence microscopy study revealed that rhodopsin mobility across the lobed surfaces of X. laevis disks is highly heterogeneous. It further suggests that although the lobed structure slows long distance diffusion, fast local diffusion of rhodopsin occurs is retained each lobe (Najafi et al., 2012a). Although not yet untested, the close apposition of the lamellar membranes within individual disks is hypothesized to drive rim localization of some proteins via steric exclusion within disk lumens; if so, this mechanism would represent another example of how signaling can depend on OS ultrastructure (Tsybovsky et al., 2013). Finally, electrophysiological approaches in a pair of independent investigations have shown directly that rod photoresponses can vary depending on where a light stimulus is applied along the OS (Lamb et al., 1981; Schnapf, 1983). In each case, smaller and slower responses were elicited from the OS tip than the base; however, the differences were relatively modest. A subsequent study found a more robust positional effect of light stimulation, and concludes that the efficacy of rod responses can vary up to an order of magnitude, depending upon where along its length the OS is excited (Mazzolini et al., 2015). Altogether, studies to date demonstrate that OS architecture imposes unique constraints that shape the behavior of signaling molecules, and provide the environment required for phototransduction.
3. Molecular basis for OS architecture
3.1. Current concepts for cellular control of membranous organelle structure
3.1.1. Introduction
All eukaryotic cells must generate, maintain, and regulate their characteristic shapes and the morphologies of the specialized membranous organelles within them. Lipid bilayers alone tend to remain flat, because they possess inherent elastic properties, and therefore severe bilayer bending requires energy (Zimmerberg and Kozlov, 2006). Early studies showed that cytoskeletal elements can apply forces to cell membranes to produce large scale changes in cell morphology (Sheetz, 2001). Until recently however, the mechanisms by which high-curvature intracellular membranous organelles are shaped has remained obscure. The main historical debate has revolved around the question of whether membrane lipids or membrane proteins provide the majority of the energy needed for generating high curvature. Numerous investigations conducted over the past decade offer substantial evidence that specialized curvature-generating proteins are required to bend phospholipid bilayers, and that the contributions these proteins make outweigh those of the membrane lipids (Graham and Kozlov, 2010). Nonetheless, bilayer lipids are important for membrane shaping, and in some instances, particular lipid species act as essential ligands to regulate membrane-shaping proteins. Phospholipid composition can also affect the partitioning of curvature-generating protein domains into the bilayer, and the free energy from the phase change of partitioning can contribute to the work required for membrane remodeling. An arsenal of specialized methods has been developed for the study of curvature-generating proteins; biochemical, biophysical, imaging, and computational approaches have each been applied to a variety of model systems (McMahon and Boucrot, 2015; Shibata et al., 2009). A variety of recent investigations have elucidated the roles that particular proteins and lipid species can play for generating areas of high membrane curvature, and have revealed that multiple mechanisms exist for doing so (Fig. 7). Current thinking suggests that multiple molecular mechanisms often operate synergistically. We briefly summarize these findings here to provide a broader context for understanding how photoreceptor OS membrane architecture may be created and maintained. We also note that this area of cell biology/biophysics is developing rapidly and will likely undergo significant future revision.
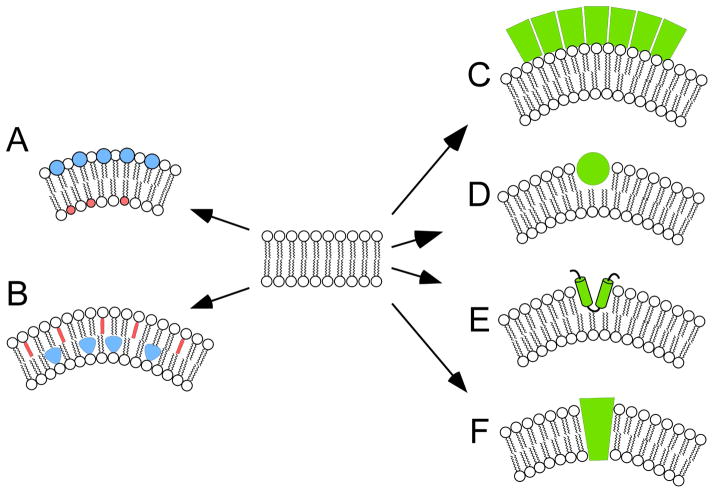
Figure 7. General mechanisms for the generation of membrane curvature.
Cells actively control membrane curvature using a variety of mechanisms. Although membrane lipid (A, B) and protein (C–F) composition can each contribute to curvature generation, the bulk of the energy required for shaping high curvature membranes is provided by specialized curvature-generating proteins. Mechanisms that contribute to membrane curvature generation include, A) phospholipid headgroup composition, B) phospholipid acyl chain composition, C) protein scaffolding, D) amphipathic helix insertion, E) hydrophobic loop insertion, F) conically-shaped transmembrane proteins.
3.1.2. Lipid contributions
The phospholipid bilayers that comprise cell membranes are inherently resistant to bending. Because the energy required to deform bilayers into high curvature geometries (i.e. - spherical vesicle diameters of <60 nm) exceeds that which is available from thermal energy at physiological temperatures, this tendency towards flatness must be countered by active mechanisms to deform the membrane from its “spontaneous curvature”. Quantitative treatments of the energetics involved commonly rely on Helfrich theory, which relates membrane elastic properties to the energetics of achieving particular shapes (Zimmerberg and Kozlov, 2006). Depending on their chemical structures, individual phospholipid molecules can promote curvature or planarity of membranes. “Conical” phospholipids, which possess headgroups with surface areas that are small relative to the area swept out by their acyl chains, are characterized by negative intrinsic spontaneous curvature values (Marsh, 2006). The incorporation of conical phospholipids into a bilayer introduces an inherent bending force (towards the headgroups), makes it more susceptible to deformation, and introduces bilayer surface defects, which can promote protein binding (Marsh, 2006; Rajamoorthi et al., 2005). Major species of conical phospholipids include phosphatidylethanolamines and lipids containing long polyunsaturated fatty acids. In contrast, “cylindrical” phospholipids possess headgroups with surface areas that are roughly similar to the area swept out by their acyl chains; these molecules are characterized by intrinsic spontaneous curvature values that are close to zero (Marsh, 2006). Major species of cylindrical phospholipids include phosphatidylcholines and lipids containing saturated fatty acids of moderate length. They promote tight headgroup packing, and bilayer planarity and rigidity. Cholesterol, a major component of eukaryotic membranes, generates similar effects (Strandberg et al., 2012). Although in principle, phospholipids alone (without protein contributions) could generate high membrane curvature via transverse bilayer asymmetry, the degree of phospholipid segregation thought to be required has not been documented in biological membranes (Bigay and Antonny, 2012; Zimmerberg and Kozlov, 2006). Nonetheless, because membrane curvature can stimulate lateral phospholipid redistribution in vitro (Callan-Jones et al., 2011), it is conceivable that curvature generation in vivo is likewise accompanied by the creation of phospholipid microdomains, and/or that the active creation of phospholipid microdomains enriched in particular species reduces the energy required for curvature generation.
3.1.3. Protein contributions
Studies of coated vesicles provide the first examples of protein-driven membrane curvature generation; these revealed a mechanism known as protein scaffolding. Coat proteins such as clathrin, COPI and COPII, polymerize into rigid curved cages that act as scaffolds for enclosing membranes of high curvature. These proteins do not interact with membranes directly, requiring a number of accessory proteins to mediate assembly of the coats onto the vesicles (Shibata et al., 2009). In contrast, a variety of peripheral membrane proteins do interact with membranes directly and employ scaffolding as a mechanism to generate curvature. Well-characterized examples include: dynamins, BAR domain superfamily proteins, caveolin, and ESCRT proteins; several different modes of membrane binding have been documented (McMahon and Boucrot, 2015; McMahon and Gallop, 2005). Amphiphysin, the best studied example, is a banana-shaped membrane-binding protein that uses binding energy to force membranes to conform to the curvature of the rigid protein scaffold (Peter et al., 2004).
Hydrophobic motif insertion represents a second major mechanism of membrane curvature generation, an action that is sometimes called wedging, because it has the effect of pushing apart the phospholipid headgroups of a single monolayer. In this case, a relatively shallow insertion into the outer part of the membrane induces local membrane curvature by increasing the surface area of only the outer monolayer leaflet. Since the surface area of the coupled inner monolayer remains constant, the bilayer is forced to adopt a positive curvature to accommodate the change (Campelo et al., 2008). To date, two protein structural motifs are documented to promote wedging - amphipathic helices and hydrophobic loops. Epsin is a well-studied example, in which amphipathic helix formation is induced and driven by insertion into the membrane (Ford et al., 2002; McMahon and Gallop, 2005). The amphipathic helix axis lies parallel to the membrane plane, and inserts to a limited depth and directly affects only the cytoplasmic monolayer. Primary sequence appears to be the main parameter governing membrane curvature generating activity; motifs with this capability generally include basic residues on their hydrophilic face (Drin and Antonny, 2010). Hydrophobic loops represent a second class of structural motif proposed to generate membrane curvature; these features are present as reticulon homology domains within the reticulon and DP1 (deleted in polyposis) families of proteins (Voeltz et al., 2006). Stretches of hydrophobic amino acids (typical ~31 residues) within reticulon homology domains insert into membranes and contribute to the structure of the high-curvature tubular network associated with the endoplasmic reticulum (Hu et al., 2008).
A third major mechanism associated with curvature-generating proteins is transmembrane modeling. Integral membrane proteins with intrinsically conical (or inverse conical shapes) can generate (and/or sense) membrane curvature in ways similar to those discussed above for hydrophobic motif insertion. The best characterized example of membrane curvature generation by an integral membrane protein is the FoF1 ATP synthase, which resides in the highly curved membranes of mitochondrial cristae. Recent cryo-ET studies demonstrate that the shape of enzyme monomers imparts an intrinsic kink in the surrounding bilayer (Baker et al., 2012), and dimerization conjoins kinks to generate substantial membrane curvature (Jiko et al., 2015).
A final major mechanism associated with curvature-generating proteins is polymerization. This process provides energy for driving unfavorable bilayer bending; however the means by which it does so depends upon the specific mechanism(s) at work. For example, polymerization can amplify the binding energy available to force the membrane to conform to a scaffold; alternatively, polymerization can align membrane-inserted wedges into an extended array (Shibata et al., 2009).
3.2. The connecting cilium and intraflagellar transport
As mentioned previously, the OS is an extraordinarily elaborate primary cilium. Cilia are structured around an axoneme that is based on microtubules that derive from a basal body. Thus, a tubulin-based cytoskeleton is fundamental for OS structure, and the 9+0 microtubule organization characteristic of all non-motile cilia also forms the basis for photoreceptor axonemes. OS axonemal microtubules contain acylated tubulin subunits (Sale et al., 1988), which stabilize the polymerized state. The precise locations of the axonemal microtubule distal ends vary among species; in frogs they appear to extend slightly more than halfway along the rod OS (Kaplan et al., 1987). The region known as the connecting cilium in photoreceptors corresponds to the transition zone of cilia in other cell types.
A variety of proteins are associated with the axoneme of the connecting cilium. The product of the MYO7A gene, which underlies Usher syndrome type 1B, a form of inherited deaf-blindness, was found to be localized between the axoneme and the ciliary plasma membrane, as well as along the periciliary plasma membrane (Liu et al., 1997; Williams, 2008). Since, this finding, the connecting cilium has become to be regarded as a “hotspot” for the localization of proteins encoded by retinal degeneration genes. Many of the retinal degenerations associated with these genes are syndromic, and are referred to as ciliopathies (Waters and Beales, 2011). In addition to the retina, they also affect tissues, such as the kidney (as in Senior-Løken Syndrome and nephronophthisis), brain (as in Joubert Syndrome and Bardet-Biedl Syndrome), and/or cochlea (as in Usher syndrome).
The connecting cilium provides the conduit for proteins to enter the OS from the IS, as well as for proteins leaving the OS to return to the IS. A large flux of proteins through the connecting cilium occurs when arrestin and transducin redistribute between the OS and IS, according to changes in ambient lighting (Pearring et al., 2013). Another event involves the distal movement of OS disk membrane proteins, the most abundant of which is rhodopsin. In a mouse rod photoreceptor, ~70 rhodopsin molecules are trafficked along the connecting cilium every second (Williams, 2002); in larger photoreceptors, such as those in frogs, the number is at least 10 times greater (Besharse and Wetzel, 1995).
Cilia (and flagella) contain an active transport system, known as intraflagellar transport (IFT), which is driven distally along the axonemal microtubules primarily by heterotrimeric kinesin-2 (Fig. 8), and basally by cytoplasmic dynein-2. Complexes of IFT proteins have been identified to associate with motors, moving up and down the ciliary axoneme as IFT “trains” (Cole et al., 1998; Kozminski et al., 1995; Pazour et al., 1999; Piperno and Mead, 1997; Porter et al., 1999). This complex of molecular motors and IFT proteins is important for the delivery of tubulin and other structural components of the axoneme (Hao et al., 2011; Pazour et al., 2000; Qin et al., 2004). Studies on mice in which the gene for the obligate motor subunit of heterotrimeric kinesin-2, KIF3A, was selectively knocked out in rod photoreceptors, showed that IFT was essential for development and maintenance of the OS, and photoreceptor viability (Jimeno et al., 2006a; Jimeno et al., 2006b; Marszalek et al., 2000). A comparable result was found in mice that possessed a hypomorphic allele of the gene encoding IFT88 (Pazour et al., 2002), and in zebrafish photoreceptors lacking IFT57 or IFT88 (Krock and Perkins, 2008).
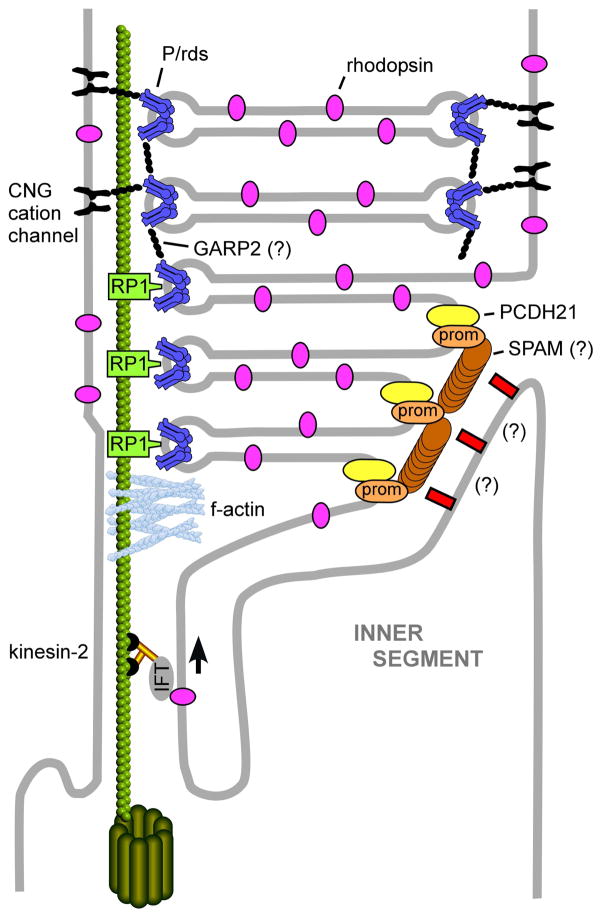
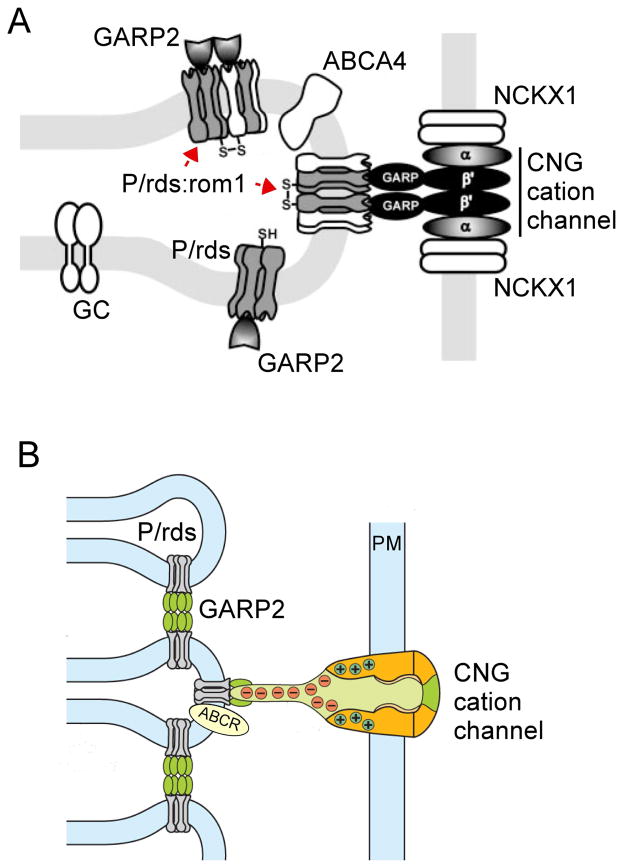
Figure 8. Distributions of OS-resident proteins with likely roles for organelle structure.
Schematic drawing (not to scale) of the basal rod OS shows the locations of the proteins discussed in this article. Kinesin-2 (black/gold) drives anterograde transport of IFT particles and cargo into the OS. Retrograde IFT transport (by dynein) is not illustrated. F-actin (light blue) is localized at the OS base in association with axonemal microtubules. These microfilaments are required for the initiation of new disk evaginations. Rhodopsin (magenta) is present in the ciliary plasma membrane, OS plasma membrane, and OS disk lamellar membranes. Rhodopsin abundance has a dose-dependent effect on disk diameter. Prominin-1 (prom; orange) is present at the edges of basal evaginations, where it interacts with PCDH21 and potentially SPAM. Prominin-1 may function to maintain disk edge membrane curvature. SPAM (brown) localization is putative, and based on its documented interaction with prominin-1 in Drosophila photoreceptors. SPAM function remains to be determined. PCDH21 (yellow) is present at the edges of basal evaginations, where it interacts with prominin-1. The identity (and interactions) of tethers (red rectangles), which link nascent disk edges and the IS plasma membrane remains to be determined. P/rds and P/rds-rom1 complexes (dark blue) are present in disk rim domains. P/rds likely plays a direct role for generating membrane curvature and may participate in disk-disk tethering. Rom-1 modulates P/rds function. RP1 (light green) is associated with axonemal microtubules at the IS-OS junction. RP1 may link nascent OS disks to the axoneme to govern their morphogenesis and stacking. The CNG cation channel (black) is present in the OS plasma membrane. In addition to its primary role for regulating OS permeability, the CNG cation channel contributes to OS structural stability by tethering the plasma membrane to disk rims via interaction with P/rds. GARP-2 (black) is present at OS disk rims. It may contribute to the long-term structural stability of OS disk stacking interactions and/or cGMP phosphodiesterase localization (not shown).
These studies also indicated that rhodopsin was transported by IFT. Rhodopsin was found to accumulate outside of the OS, prior to any defects in ciliary ultrastructure. Moreover, the ectopic rhodopsin was shown to underlie the cell death, because reduced rhodopsin expression in KIF3A-null mouse rods (Lopes et al., 2010) and in Ift88-mutant zebrafish rods (Tsujikawa and Malicki, 2004) rescued the cell death. Another OS protein, guanylate cyclase-1, was suggested to be transported by IFT, since ectopic guanylate cyclase-1 is detected in mice with deficient IFT88 (Insinna and Besharse, 2008), and it co-immunoprecipitates with IFT88 (Bhowmick et al., 2009). These results are consistent with a recent demonstration, that guanylate cycle-1 interacts with, and is co-transported and stabilized by rhodopsin (Pearring et al., 2015). The role of IFT in OS protein delivery has been questioned in studies by one group that had difficulty detecting significant defects in rhodopsin localization (Avasthi et al., 2009; Jiang et al., 2015). However, immunocytochemical studies of fixed tissue, showing end-state localization, have major drawbacks for studying dynamic cell biology, such as protein trafficking. The study of protein movements in live cells provides a more direct approach. Using fluorescence recovery after photobleaching, it was shown that movement of a rhodopsin-green fluorescent protein (GFP) fusion protein along the cilia of cultured epithelial cells was reduced significantly when KIF3A was reduced by shRNA. When fluorescence recovery after photobleaching was performed on the connecting cilia of rod photoreceptors in explants of rhodopsin-GFP mouse retinas; lack of KIF3A also resulted in a large decrease in the movement of rhodopsin (Trivedi et al., 2012).
How IFT propels proteins, such as rhodopsin, along the cilium is not yet known. However, single molecule tracking of SSTR3 or Smoothened (each of which are G-protein coupled receptors like rhodopsin), show that these proteins spend most of their time within cilia undergoing diffusion, with only ~20–30% of their time being associated with IFT particles (Milenkovic et al., 2015; Ye et al., 2013). These observations suggest quite transient and infrequent interactions with the IFT transport machinery.
3.3. OS-resident molecules that contribute to organelle structure
3.3.1. Lipids
The lipid profile of rod OS membranes is unique relative to the retina as a whole, and to other neural tissues (Fliesler and Anderson, 1983). The distinctive lipid composition confers properties that are important for optimal organelle function and likely contribute to OS architecture. Rod OSs contain at least four distinct membrane domains as defined by their unique protein compositions and morphology - the plasma membrane, the disk lamellae, the disk rim, and the nascent disks at the OS base. Because disk lamellae represent the vast majority of OS membrane surface area, their composition is most easily investigated. In only one other case (plasma membranes) has the lipid composition of a non-disk domain been reported. Recent studies have begun to elucidate how OS lipid composition is regulated; however, our knowledge of lipid distributions and dynamics remains rudimentary, as does understanding of how these factors contribute to OS structure and function.
Lipid synthesis and incorporation into OSs
OS lipids are primarily synthesized in the IS endoplasmic reticulum (Mercurio and Holtzman, 1982) and Golgi (van Meer et al., 2008) and are co-transported, at least in part, via transmembrane protein-containing vesicles destined for the OS (Rodriguez de Turco et al., 1997). Vesicle fusion at the IS periciliary ridge region (adjacent to the CC) allows for delivery of both proteins and phospholipids to the rod OS via the ciliary membrane (Besharse and Pfenninger, 1980; Papermaster et al., 1985). Molecules entering the ciliary compartment must surmount a diffusion barrier that regulates membrane protein traffic (Nachury et al., 2010). It is likely that additional trafficking pathways also exist, because lipid transport and delivery can occur independently of membrane protein trafficking. Inhibition of opsin trafficking to the OS (via brefeldin A and other pharmacological interventions) does not prevent lipid transport (Fliesler and Keller, 1997; Fliesler et al., 1995). Furthermore, turnover of rod OS lipids occurs at a substantially faster rate than does turnover of rod OS transmembrane proteins, suggesting that delivery rates may be different (Anderson et al., 1980a; Anderson et al., 1980b; Anderson et al., 1980c), and that mechanisms in addition to disk replacement must contribute to lipid loss. Rapid lipid turnover is particularly evident for a subpopulation of phosphatidylinositol (Anderson et al., 1980c). The mechanisms contributing to rapid lipid turnover in rod OSs remain to be defined; however, the prevalence of non-vesicular intracellular trafficking in other organelles and cell types suggests this as a possibility (Prinz, 2010; van Meer et al., 2008; Vance, 2015). One route for non-vesicular intracellular lipid transport is mediated by phospholipid transfer proteins, and although not well-studied in photoreceptors, one example has been documented (Dudley and Anderson, 1978). A recent report suggests that photoreceptors may possess a means to transport proteins from the OS to the cell body (Datta et al., 2015); additional studies would be required to assess the potential impact of this retrograde pathway on lipid trafficking.
Gross lipid composition
The ease with which rod OSs can be purified has facilitated extensive characterizations of their lipid composition (Anderson and Maude, 1970; Aveldano and Bazan, 1983; Fliesler and Anderson, 1983; Stone et al., 1979). In contrast, the low abundance and less accessible histological position of cone OSs have prevented rigorous assessments to date. The limited information that is available suggests that cone OS lipid composition may differ from that of rods (Agbaga, 2014). Lipid comprises approximately 50% of rod OS dry weight, and the majority (>90% by weight) are glycerophospholipids. The remainder is largely comprised of cholesterol and glycolipids, in amounts that depend slightly on species and investigation (Fliesler and Anderson, 1983). The phospholipids are dominated by phosphatidylcholine (PC; ~30–40 mol%), phosphatidylethanolamine (PE; ~30–40 mol%), phosphatidylserine (PS; ~10–12 mol%), and phosphatidylinositol (PI; ~1–2 mol%). In addition, non-sialylated sphingolipids and gangliosides comprise about 1 mol% each of OS lipids, a relatively low complement when compared to the lipid profile of the retina (Brush et al., 2010). Likewise, relatively little cholesterol (~10 mol% of total lipid) is present in purified OSs (Boesze-Battaglia et al., 1989; Fliesler and Schroepfer, 1982). In contrast, OS membranes possess an unusually high level of long chain polyunsaturated fatty acids, dominated by 22:6ω3 (also known as docosahexanoic acid), which can represent up to 50% of the total acyl chains (Anderson and Maude, 1970; Aveldano and Bazan, 1983; Stone et al., 1979).
Disk lipid composition and distribution
The lipid composition of rod OS disks largely mirrors that of unfractionated rod OS membranes (Boesze-Battaglia and Albert, 1989). This is an expected finding, because disk surface area in aggregate is very high relative to the OS plasma membrane surface area – roughly 1500:1 in rodents (Mayhew and Astle, 1997). In contrast, the surface area ratio of mammalian disk lamellae to rims is not radically different (~10:1 in rodents, calculated geometrically), so significant compositional differences may exist between these domains and unfractionated rod OS membranes. No evidence is yet available on this point and future studies will be required to address it. Given that lipids and fatty acids can exchange relatively rapidly between disks (Bibb and Young, 1974a, b), understanding how particular distributions are regulated will require an improved understanding of the main mechanisms that drive asymmetries. At least four processes are likely to contribute to shaping lipid distributions, including, 1) ATP-driven flippases, 2) lipid scramblases, 3) immobilized proteins with affinity for lipids, and 4) membrane curvature-driven sorting.
Akin to other eukaryotic cell membranes, rod OS disks possess transmembrane phospholipid asymmetry, displaying elevated concentrations of phosphatidylserine on their cytoplasmic leaflets (Hessel et al., 2000; Wu and Hubbell, 1993). ATP8A2, a ubiquitously expressed ATP-dependent phospholipid flippase helps drive this asymmetry (Coleman et al., 2009), but is not essential for establishing relatively normal OS architecture (Coleman et al., 2014). ABCA4, a photoreceptor-specific flippase, transports PE-retinal adducts which accumulate spontaneously in disk membranes as side reactions of the visual cycle (Beharry et al., 2004). ABCA4 flips these compounds from disk luminal leaflets to cytoplasmic faces for enzymatic processing and detoxification (Quazi et al., 2012); this activity is not required for OS structure, but is needed for long-term photoreceptor viability (Radu et al., 2008). The primary mechanism that acts in opposition to the ATP-driven flippases appears to be a constitutive phospholipid scramblase activity present in opsin, the G protein-coupled receptor responsible for initiating OS phototransduction (Goren et al., 2014; Menon et al., 2011). Importantly, opsin scramblase activity (and that of other G protein-coupled receptors) is enhanced by membrane packing defects, such as those present in cholesterol-poor membranes – such as OS disks. Because rhodopsin is present in such high densities in OS disk membranes (Palczewski, 2012), its sequestration of particular lipid species may also create transmembrane asymmetries; indeed, activated forms of rhodopsin affect PS mobility and distribution (Hessel et al., 2001). Although other OS proteins also bind particular phospholipids (Hessel et al., 2003), their relatively low abundance limits their potential impacts on the distributions of the major lipid classes. Finally, membrane curvature-driven lipid sorting can drive both lateral and transmembrane lipid asymmetries in membranes (Callan-Jones et al., 2011). Whether this mechanism contributes to lipid distributions in OS disks remains to be seen; however, the high curvature present in rim domains is of the magnitude required to drive lipid sorting, and suggests that further investigation is warranted.
In addition to the presence of transmembrane lipid asymmetry, there is substantial evidence for lateral heterogeneity within disk lamellar regions. Early evidence was provided by freeze-fracture EM studies; these data reveal cholesterol-rich “particle free patches” on mouse and frog OS disks that appear to exclude rhodopsin (Andrews and Cohen, 1979, 1983). These patches, typically 100–200 nm in diameter, are prevalent in basal disks, but show reduced frequency in more distal disks, and likely reflect the basal-to-distal cholesterol gradient present in OSs (discussed below). Particle-free patches were hypothesized to represent membrane regions of locally reduced fluidity that preferentially concentrate sphingomyelin and cholesterol. These very same molecules were later shown to spontaneously segregate into ordered membrane phases, also known as lipid rafts (Simons and Ikonen, 1997). Cholesterol-containing lipid microdomains in OS disks can affect phototransduction by affecting G-protein diffusion on the disk surface (Wang et al., 2008). Most investigations of lateral asymmetry in OS membranes have employed solubilization-based methods that identify “detergent-resistant membranes”. Caution is required when interpreting such studies, as the physiological significance of detergent-resistant membranes is unclear, and their relationship to rafts must be established on a case-by-case basis (Lichtenberg et al., 2005; Schuck et al., 2003).
An early study noted that lipid extraction from OS membranes is detergent, concentration, and light dependent (Aveldano, 1995). Detergent-resistant membranes isolated from rod OSs are enriched approximately 2-fold (vs. solubilized lipids) in cholesterol and sphingomyelin (Boesze-Battaglia et al., 2002). Akin to studies of membranes from other cell types, detergent-resistant membranes isolated from rod OSs using Triton X-100 are similarly enriched in saturated fatty acids (Martin et al., 2005); this same study also found that several proteins were preferentially associated with the detergent-resistant membrane fraction, including, caveolin-1, c-Src, and transducin-α. Several studies have found that the protein content of rod OS detergent-resistant membranes varies in response to light exposure (Nair et al., 2002; Senin et al., 2004; Wang et al., 2008). These results imply that lipid rafts may help shape rod cell photoresponses. The heterogeneity revealed by these biochemical studies of detergent-resistant membranes is consistent with cryo-ET analysis of frozen vitrified rod OSs, which shows areas of both high and low density rhodopsin packing (Nickell et al., 2007).
Plasma membrane lipid composition
The lipids of rod OS plasma membranes have significantly higher levels of saturation, cholesterol, and PC, than do OS disk membranes (Boesze-Battaglia and Albert, 1989). These factors generally thicken and stiffen membranes, and reduce packing defects, fluidity, and permeability - properties that are consistent with the barrier function required for a cell limiting membrane. OS plasma membranes also possess roughly two-fold more PS than do disks or typical mammalian plasma membranes (Boesze-Battaglia and Albert, 1992; van Meer et al., 2008). This composition predicts a high surface charge for the OS plasma membrane, which may be further heightened if PS is concentrated in the cytoplasmic leaflet, as is typical for eukaryotic plasma membranes. Little information is currently available on this point, or the possible role of elevated PS for the OS plasma membrane. The electrostatic charge imparted by PS to membranes of other cell types has the effect of recruiting and localizing specific peripheral signaling proteins (Bigay and Antonny, 2012). Interestingly, PS externalization on OS distal tips has recently been reported, and is hypothesized to mark disk packets for phagocytosis (Ruggiero et al., 2012).
OS basal-to-distal lipid distributions
The cholesterol content of unfractionated rod OSs is quite low relative to typical mammalian membranes (Fliesler and Schroepfer, 1982). Intriguingly, OS disk cholesterol varies approximately six-fold along the OS length. Although basal disks have a cholesterol composition similar to that of the plasma membrane (~30 mol%), they lose cholesterol as they age, and possess a much reduced cholesterol content (~5 mol%) by the time they reach the OS distal tip (Boesze-Battaglia et al., 1990; Boesze-Battaglia et al., 1989). The mechanism by which disk cholesterol is lost is not known; however, an exchange process driven by a relatively high PE content in disks and high PC content in plasma membrane has been proposed (Albert and Boesze-Battaglia, 2005). Transfer of cholesterol from disks to plasma membrane would presumably occur at disk rims, the point of maximal proximity between the two membranes, and may be mediated by one or more oxysterol-binding proteins, molecules that function for lipid transfer between intracellular membranous organelles. The significance of the rod OS cholesterol gradient remains to be demonstrated, but it may contribute to the axial variability in OS sensitivity noted by several laboratories. Several studies agree that basal disks in toad rod OSs are more sensitive and support faster responses that those more distally positioned (Baylor et al., 1979; Lamb et al., 1981; Schnapf, 1983). A more recent report likewise observes a phototransduction efficacy gradient (in frog rod OSs), but measures a somewhat larger (up to 2.5-fold) range (Mazzolini et al., 2015).
3.3.2. Proteins
3.3.2.1. Actin
Actin is a highly conserved, ubiquitous, and abundant protein that transitions between monomeric (g-actin) and filamentous (f-actin) forms under the control of ions, nucleotides, and actin-binding proteins. It performs essential cellular functions in a variety of structural capacities, and is well-documented to contribute to cell shape and motility (Dominguez and Holmes, 2011). Substantial f-actin is present in vertebrate photoreceptor ISs (Lewis et al., 1995), where it appears to be present in a submembrane cortical network, akin to that found in many eukaryotic cells types. In contrast, photoreceptor OSs appear to lack an actin cytoskeleton; they do, however, possess a network of actin microfilaments associated with the axoneme, at the OS basal region (Chaitin et al., 1984).
Structural properties and distribution
The actin monomer (g-actin) is composed of a 375 amino acid polypeptide chain that folds into a globular protein with two major domains (α/β), and is subject to a variety of posttranslational modifications. Numerous (angstrom-resolution) crystal structures of actin are available, and most were solved in combination with actin-binding proteins. Surprisingly, the actin monomer structure is affected only marginally by partner binding. Polymerization of g-actin into filaments (f-actin) creates two chains that wrap around each other to form a right-handed helix with a diameter of ~7 nm. Filament polarity is defined by plus (also known as barbed) and minus (also known as pointed) ends; the kinetics of subunit addition to the former are more rapid than the latter. Individual filaments can be assembled by actin-binding proteins into a variety of higher-order structures, including bundles and networks. Although actin immunoreactivity is present at several sites in photoreceptors (Lewis et al., 1995; Vaughan and Fisher, 1987), populations localized within the connecting cilium and basal OS in frogs (Chaitin et al., 1984) and various mammalian species (Chaitin and Bok, 1986) likely play specific roles for OS structure. Interestingly, phalloidin labeling of isolated photoreceptors from several species illustrates a single focal distribution of f-actin localized at the base of individual rod OSs (Vaughan and Fisher, 1987). TEM studies demonstrate a dense meshwork of f-actin within OS axonemes, from which distinct actin fibers originate and extend their plus ends towards the leading edge of nascent disk evaginations (Arikawa and Williams, 1989; Chaitin and Burnside, 1989).
Association with other proteins
In addition to self-association, actin participates in a very broad array of protein-protein interactions in eukaryotic cells. In photoreceptors, actin has been observed to interact with α-actinin (Arikawa and Williams, 1989), fascin 2 (Saishin et al., 2000), and tulp1 (Xi et al., 2005). Both α-actinin and fascin2 likely function for actin assembly and bundling and have been localized to OSs (Arikawa and Williams, 1989; Lin-Jones and Burnside, 2007; Saishin et al., 2000). The role of tulp1, likely a multifunctional protein, and its potential presence in OSs is not yet clear (Xi et al., 2005).
Human molecular genetics and animal models
Most mammals possess six actin-encoding genes. Isoform expression and distribution in photoreceptors have not been well characterized; however, it is likely that two cytoskeleton forms (ACTB and ACTG1) are present. Defects in the other four genes, which encode muscle actins, cause a variety of myopathies in humans (Tubridy et al., 2001). To date however, inherited defects in cytoplasmic forms have not been associated with retinal pathogenicity. Because gene knockouts are likely to be embryonic lethal in mice, photoreceptor-specific ablation would be needed to implement this type of approach.
Role for OS architecture
The presence of a discrete concentration of f-actin associated with the axonemes at the base of rod OSs suggests its possible participation in disk morphogenesis (illustrated in Fig. 8). Indeed, treatment of X. laevis photoreceptors with cytochalasin D, which disassembles actin filaments, profoundly impairs disk morphogenesis (Williams et al., 1988). Depolymerization of these actin microfilaments prevents the initiation of new disk membranes, resulting in excessive overgrowth of the basal-most evaginations in both rods and cones (Hale et al., 1996; Tian et al., 2014; Williams et al., 1988). In addition to demonstrating an essential role for f-actin in disk morphogenesis, these experiments support the evagination (vs. invagination) mechanism for new disk formation, because overgrown evaginations are not surrounded by a plasma membrane, as would have occurred if the growth occurred by invagination. Although the mechanistic details remain to be elucidated, it appears that f-actin is required for initiating new evaginations, but not for their continued expansion (Hale et al., 1996; Williams et al., 1988). Interestingly, existing disks continue their journey towards the distal OS tip even when the initiation of new evaginations are prevented by f-actin depolymerization (Kaplan, 1998).
3.3.2.2. RP1
RP1 is a photoreceptor-specific cytosolic protein that associates with photoreceptor ciliary axonemes via tubulin-binding doublecortin (DCX) domains, and is required for normal OS disk morphogenesis (Liu et al., 2002; Liu et al., 2004). Inherited defects in RP1 cause progressive retinal degenerations in humans.
Structural properties and distribution
The RP1 gene includes four exons that encode a ~240 kDa protein that is a member of the doublecortin family (Liu et al., 2002). The N-terminal half of the protein encodes two regions with DCX homology; previous studies of other proteins demonstrate that DCX domains can bind tubulin, stabilize microtubules, and function for neuronal migration (Horesh et al., 1999). RP1 is expressed in a photoreceptor-specific fashion in both rods and cones and is first detectable at ~p6 (Liu et al., 2002). Localization in mouse retina by immunohistochemistry/confocal microscopy reveals a highly restricted distribution within the photoreceptor cell layer - reactivity is confined exclusively to the IS-OS junction. Moreover, double-labeling studies demonstrate that RP1 largely co-localizes with the α-tubulin present in the photoreceptor axonemes (Liu et al., 2004).
Association with other proteins
Immunogold labeling of RP1 in murine retina shows the protein distributed along photoreceptor axonemes, primarily associated with the doublet microtubules (Liu et al., 2004). Co-precipitation analyses performed by the same study found that RP1 can bind to microtubules directly. Multiple protein regions appear to be involved in axonemal association, because an N-terminal DCX domain, and a C-terminal fragment lacking that domain, can each co-localize with axonemes independently. While the N-terminal DCX interaction is likely mediated via direct tubulin binding, the means by which the C-terminal fragment associates with axonemes remains to be determined. Like many microtubule-associated proteins (MAPs), RP1 promotes microtubule polymerization and stability, and elegant in vivo analyses show that loss of the DCX domain reduces photoreceptor axoneme length in murine retina (Liu et al., 2004). A more recent study using a spontaneously occurring L66P mutation, confirms these findings (Song et al., 2014). Although interactions with proteins other than tubulin have not been identified to date, the expression of retinal disease in murine RP1 models is strongly affected by the genetic background. Such findings suggest the possibility of additional protein-protein interactions (Liu et al., 2009).
Human molecular genetics and animal models
Mutations in the RP1 gene cause both autosomal recessive and autosomal dominant forms of retinitis pigmentosa (Bowne et al., 1999; Pierce et al., 1999). It has been estimated that 3–4% of RP cases are attributable to mutations in RP1 (Daiger et al., 2008). Of the several dozen RP1 pathogenic variants identified thus far, all are nonsense or frameshift mutations, and the vast majority leave the known tubulin-binding domains intact. These circumstances suggest that truncated gene products may participate in the etiology of some RP1-associated disease, and highlight the importance of investigating the mechanism(s) underlying inheritance patterns and pathogenicity. Several studies have addressed these questions using murine models in conjunction with human molecular genetic data. In one case, an inherited defect causing recessive human RP in humans was modeled by creation of a knock-in mouse containing a missense mutation (RP1-Q662X) predicted to truncate nearly 70% of the RP1 C-terminal protein coding sequence (Liu et al., 2012). The truncated gene product, which retains a full WT complement of N-terminal DXC tubulin-binding domains, is expressed, and correctly localized to photoreceptor axonemes. Although retinal degeneration is evident in RP1-Q662X homozygous mice, heterozygotes do not show evidence of disease, a result that mirrors the inheritance patterns of their human counterparts. Disease in RP1-Q662X homozygous mice can be alleviated and progression delayed by rescue with a WT RP1 transgene, confirming that the truncated RP1 gene products are not toxic. These results provide a proof-of-principle demonstration for the utility of gene therapy for RP1-associated human disease (Liu et al., 2012).
Role for OS architecture
Identification of RP1 as a MAP that is restricted to basal axonemal microtubules in both rods (Fig. 8) and cones, suggests the possibility that this molecule participates in linking rims of nascent OS disks to the photoreceptor axoneme (Liu et al., 2003). The most common pathogenic mutations in RP1 appear to generate progressive retinal disease by interrupting this linkage, thereby impairing disk morphogenesis and/or the proper stacking of newly completed disks. Identification and characterization of putative RP1 binding partner(s) localized to photoreceptor disk rims would be an important step towards validation of this hypothesis.
3.3.2.3. Prominin-1
Prominin-1 (also known as CD133 and AC133) is a penta-spanning integral membrane protein first discovered as a cell surface antigen on neuroepithelial progenitor cells (Miraglia et al., 1997; Weigmann et al., 1997). Subsequent work has found that prominin-1 is more broadly expressed in adult human tissues, and that it has utility as a cancer stem cell marker (Corbeil et al., 2001b). Interestingly, inherited defects in PROM1 cause retinal degeneration in humans (Maw et al., 2000). The protein is expressed in the vertebrate retina, is restricted to rod and cone photoreceptor OSs, and localizes to the edges of open disks (Han et al., 2012; Zacchigna et al., 2009). Studies indicate that this protein functions for OS disk morphogenesis and structural stability, but its mechanism of action remains to be determined.
Structural properties and distribution
Prominin-1 was identified independently by two groups, each working with non-retinal tissue. Yin et al. obtained a monoclonal antibody to a novel protein in a search for stem cell markers (Yin et al., 1997), while Weigman et al. obtained a monoclonal antibody to a novel protein in a search for cell surface markers (Weigmann et al., 1997). It was subsequently determined that the antibodies detected an identical protein (Corbeil et al., 1998). Prominin-1 is a ~120 kDa (~850 amino acids) transmembrane glycoprotein with relatively modest amino acid conservation across mammalian species (Corbeil et al., 2001a). No high-resolution structural data is available for prominin-1 (or closely related proteins), and current models are based largely on the primary sequence data. These predict a cleaved signal peptide, an extracellular N-terminus, five transmembrane domains, and a cytoplasmic C-terminus (Weigmann et al., 1997). Two large extracellular loops are located between transmembrane domains 2/3, and 4/5, comprising more than half of the amino acid sequence. A conserved cysteine-rich domain extends from the first transmembrane domain into the first cytosolic loop, and the extracellular loop regions contain several conserved leucine residues, six conserved cysteines, and multiple conserved sites for N-linked glycosylation. Protein electrophoretic mobility increases substantially after glycosidase treatment, suggesting that at least some of these latter sites possess carbohydrate modifications (Maw et al., 2000; Weigmann et al., 1997). An additional level of complexity is introduced by the presence of alternatively spliced protein variants that are expressed in a cell type-specific manner (Fargeas et al., 2004; Han and Papermaster, 2011).
Prominin-1 derives its name from its subcellular distribution on prominent cellular protrusions, including, microvilli, filipodia, and both motile and non-motile cilia (Corbeil et al., 2013; Weigmann et al., 1997). In one case, it has been further localized to cholesterol-rich membrane microdomains (Roper et al., 2000). Investigation of prominin-1 distribution in retina was prompted by identification of mutations that cause human retinal disease (discussed below). Immunogold labeling of mouse retina revealed prominin-1 is associated with the basal OS disks of rod photoreceptors (Maw et al., 2000). Subsequent studies have confirmed localization at the OS base, by applying immunohistochemistry and confocal microscopy to murine ocular cryosections (Yang et al., 2008; Zacchigna et al., 2009).
An investigation of prominin-1 in X. laevis retina demonstrated that this protein is present in both rod and cone photoreceptors, and that it is restricted to the edges of open OS disks in each cell type (Han et al., 2012). In frog rods, as in the mouse rods, prominin-1 labeling is confined to the basal regions of the OSs. Here, the ciliary plasma membrane has evaginated to create nascent disks that are in the process of being internalized. In a detailed and elegant analysis, Han et al. documented a mutually exclusive distribution of prominin-1 and peripherin/rds (P/rds) in the nascent disks. While prominin-1 occupies the U-shaped disk edges only, P/rds is restricted to the hairpin-shaped rims (discussed below). Similar results are observed for cones; however, because disks are never completely internalized in these organelles, patterns of prominin-1 reactivity extend along the entire length of the frog OSs. Close examination of murine rods and cones by these authors show consistent patterns of prominin-1 labeling.
Interestingly, a prominin-1 ortholog has also been identified in Drosophila using a screen for genes that function for invertebrate photoreceptor rhabdomere separation during eye development (Zelhof et al., 2006). In adult flies, the prominin gene product is localized to the rhabdomeric microvilli, the membrane protrusions packed with the photopigment rhodopsin. These results are consistent with those obtained in vertebrate photoreceptors, because both OSs and rhabdomeric microvilli are elaborated from the apical surface of polarized (neuro)epithelial cells. Taken together, these results suggest the possibility that vertebrate and invertebrate prominins may share a conserved function, a hypothesis strengthened by the observation that prominins localize to highly curved membrane protrusions in each case.
Association with other proteins
Prominin-1 has been reported to interact physically with protocadherin 21 (PCDH21) and β-actin (Yang et al., 2008). PCDH21 is a transmembrane protein important for OS structure, and has a distribution pattern in rod OSs at the edges of basal disks, which overlaps that of prominin-1 (discussed in more detail below). When PCDH21 is absent (i.e. - in knockout mice), prominin-1 distributes more broadly throughout the rod OS, a result suggesting that these proteins may interact physically (Yang et al., 2008). Furthermore, immunoprecipitation data from lysates of co-transfected HEK293 cells and from murine retinas, offer additional evidence that prominin-1 and PCDH21 can participate in a direct protein-protein interaction. Analogous immunoprecipitation data likewise suggest a direct protein-protein interaction between prominin-1 and β-actin (Yang et al., 2008).
Invertebrate prominin interacts genetically with several other genes, including eyes shut (eys), chaoptin, and crumbs (crb), during Drosophila eye development. All gene products localize on or adjacent to photoreceptor rhabdomeres, and the absence of or defects in any one component can compromise normal morphogenesis and structural stability (Gurudev et al., 2014; Husain et al., 2006; Nie et al., 2012; Zelhof et al., 2006). The interaction with eys is particularly interesting, because a defect in the vertebrate ortholog EYS, is responsible for the RP25 form of human autosomal retinitis pigmentosa (Abd El-Aziz et al., 2008; Collin et al., 2008). The protein encoded by the EYS gene is known as spacemaker (SPAM); it is an extracellular matrix protein of over 3,000 amino acid residues, with 21 EGF repeats in its N-terminal domain followed by 5 C-terminal lamG domains, and has been localized to the OS region of the porcine retina (Abd El-Aziz et al., 2008).
Human molecular genetics and animal models
Mutations in prominin-1 cause a broad range of retinal degenerations in humans. An initial report described a frameshift mutation causing premature termination (likely a null allele) in a consanguineous family, leading to an autosomal recessive early onset and severe retinal degeneration (Maw et al., 2000). Other than one case of polydactyly, there were no non-retinal symptoms reported. Other forms of disease were subsequently discovered, including a dominant variant (clinically diagnosed as Stargardt disease, cone-rod dystrophy, or macular dystrophy, depending on the family) associated with an Arg373Cys mutation (Yang et al., 2008). A detailed study of the Arg373Cys mutation in five different families likewise documented several retinal-specific disease phenotypes. These showed a variable involvement of rod photoreceptors, but all included bull’s eye maculopathy as a consistent clinical feature (Michaelides et al., 2010). Most recently, instances of autosomal recessive cone-rod dystrophy were reported to result from a 10-base pair deletion in intron 21 (and exon 22 skipping) of the PROM1 gene (Eidinger et al., 2015).
Several murine models for retinal disease caused by PROM1 mutations have been described to date. The first model investigates the impact of transgenic (rod photoreceptor–specific) expression of Arg373Cys mutant human prominin-1, on murine photoreceptor structure and function (Yang et al., 2008). Mutant protein expression reduced endogenous WT prominin-1 protein levels, was itself partially mislocalized, caused partial mislocalization of PCDH21, induced a progressive loss of photoreceptors, and disrupted OS disk morphogenesis.
Although prominin-1 is expressed relatively broadly outside the retina, phenotypic effects caused by genetic ablation of prominin-1 are retinal-specific (Zacchigna et al., 2009). Prominin-1 knockout mice are fertile, have normal lifespans, and show no gross anatomical or behavioral abnormalities (Zacchigna et al., 2009). These results suggest that a related protein, prominin-2, may provide functional redundancy outside the retina (Fargeas et al., 2003). Retinal development is essentially normal until p7 in homozygous knockouts; however, a progressive retinal degeneration begins at approximately p15, and two-thirds of the rod photoreceptors are lost by p90. Significant photoreceptor functional deficits are present at 1 month of age; both rod and cone function are reduced ~40% relative to age-matched WT animals, and nearly all responses are lost by 6 months of age in each cell type. OSs are elaborated from connecting cilia in both rods and cones, but are dysmorphic from the earliest time points examined (p12). TEM images generally show loosely stacked lamellar membranes oriented at roughly 90 degrees to those of WT photoreceptor disk membranes. Failure to develop normal OS organelles may be responsible for the mislocalization of rhodopsin that occurs in these animals. In contrast to homozygous null mice, heterozygous null animals show no obvious phenotypic effects. Altogether, the prominin-1 KO mouse phenotype closely resembles that produced by a null allele in human prominin-1 (Maw et al., 2000), and suggests this model can be a useful tool for understanding protein function in human health and disease.
Intriguingly, invertebrate prominin appears to be a bona fide ortholog of mammalian prominin-1, because the human gene (in combination with a human EYS gene) can complement a null prominin allele in Drosophila (Nie et al., 2012). The Drosophila null phenotype displays reduced spacing between photoreceptor rhabdomeres and light-dependent retinal degeneration (Husain et al., 2006; Nie et al., 2012; Zelhof et al., 2006). The “open” rhabdomere structure normally present in Drosophila is an evolutionary specialization of the invertebrate compound eye that provides increased sensitivity and signal-to-noise ratio for signaling, and depends upon several proteins, including prominin, chaoptin, SPAM, and crumbs for its structure. Parallel investigations of their function in vertebrate and invertebrate systems promises to be highly informative.
Role for OS architecture
It appears likely that prominin-1 is a part of the machinery and/or signaling apparatus required for OS disk edge formation and/or maintenance. It is restricted to the edges of open disks in both rods (Fig. 8) and cones, and OS morphogenesis is disturbed in its absence. In this regard, its reported interaction with PCDH21 and β-actin may support the participation of those proteins in ciliary membrane evagination, flattening, and disk rim formation. The presence of prominin-1 in the highly curved membrane protrusions present in a variety of cell types suggests that it may possess activity for membrane curvature generation and might thereby contribute to the shaping of OS disk edges. It may also, or alternatively, participate in extracellular scaffolding interactions that serve to tether disk edges together in support of disk stacking stability (Fetter and Corless, 1987). Such a role would represent a variant of the “adhesion capping” hypothesis proposed for Drosophila prominin by the SPAM protein (Gurudev et al., 2013). Because some vertebrate species (i.e. - humans) appear to possess a functional EYS gene, while others do not (i.e. – mice), detailed studies will be required to delineate the role of the SPAM protein and possible functional surrogates in vertebrate OSs (Abd El-Aziz et al., 2008).
3.3.2.4. Protocadherin 21 (PCDH21)
PCDH21 (also known as photoreceptor-specific cadherin; prCAD), is a member of the protocadherin family of proteins. Although canonical cadherin proteins play well-defined roles in cell-cell adhesion, protocadherins serve a more diverse array of biological functions (Sotomayor et al., 2014). Inherited defects in PCDH21 cause progressive retinal degenerations in humans.
Structural properties and distribution
PCDH21 was identified during a screen for genes with retinal-specific expression; it encodes a protein of ~860 amino acids that includes a signal peptide, six extracellular cadherin domain repeats, a single-pass transmembrane domain, and an intracellular C-terminus (Rattner et al., 2001). Expression appears to be restricted to the neural retina and pineal gland. In addition to the full-length ~120 kDa protein, two proteolytic fragments (~25 kDa and ~95 kDa) are detected in freshly dissected mouse retina, which are proposed to result from ectodomain shedding of the N-terminal cadherin repeat domain (Rattner et al., 2004). The protease responsible for the cleavage remains to be identified. The truncated PCDH21 C-terminal fragment (~25 kDa) that is retained in the OS membrane resists solubilization by treatment with gentle detergents. Two antibody-based approaches illustrate that PCDH21 is highly localized within the photoreceptor layer in murine retina. Immunohistochemical analysis in combination with confocal microscopy shows that PCDH21 is present in distinct puncta at the IS-OS junction of both rod and cone photoreceptors. PCDH21 expression is detectable by p2 and is already localized to the future site of disk morphogenesis - well before that process begins nearly 7 days later (Rattner et al., 2004). TEM analysis of immunogold labeling shows PCDH21 localized solely at the basal OS, and only along the edges of open disks (Rattner et al., 2004; Rattner et al., 2001). This timing and highly restricted distribution pattern suggests that PCDH21 may participate in disk morphogenesis. Because OS disks are subject to a daily axial displacement, the lack of PCHD21 labeling beyond the basal OS suggests a mechanism for retention at that location.
Association with other proteins
To date, PCDH21 has only been associated with one other protein, prominin-1 (Yang et al., 2008), and the distributions of these proteins overlap (discussed above). Immunoprecipitation experiments found that prominin-1 can be co-precipitated with PCDH21 from solubilized mouse retina, and from cultured cells co-transfected with both proteins. Thus, current evidence suggests that PCDH21 can engage in a direct association with prominin-1 in the basal membranes of nascent OS disks; the functional and mechanistic significance of this interaction remains to be demonstrated. PCDH21 is also hypothesised to interact directly with fibers tethering nascent disk edges to the adjacent IS plasma membrane (Burgoyne et al., 2015). Validation of this interesting idea, which currently rests solely on co-localization data, awaits the molecular identification of the fibers and the application of protein-protein interaction assay methods.
Human molecular genetics and animal models
Defects in the gene encoding human PCDH21, have been associated with autosomal recessive retinitis pigmentosa (RP65) and autosomal recessive cone-rod dystrophy (CORD15) by several studies (Duncan et al., 2012; Henderson et al., 2010; Nikopoulos et al., 2015; Ostergaard et al., 2010). Defects documented to date all reside within the N-terminal extracellular cadherin repeat domain, and include nonsense, missense, frameshift, and splice-site mutations. The recessive inheritance pattern in humans indicates that a reduced dosage of the gene product is adequate for retaining a measure of physiological function; a similar situation also appears to hold true for PCDH21 heterozygous knockout mice (Rattner et al., 2001). In contrast, and in accord with human disease, homozygous genetic ablation of PCDH21 (by deleting exons 10 – 17) perturbs OS structure. Homozygous PCDH21 knockout mice possess shortened, fragmented, and dysmorphic OSs that include membranous whorls and oversized disks (Rattner et al., 2001).
Role for OS architecture
A role for PCDH21 in cell-cell adhesion (analogous to other cadherins), has been questioned, because this protein does not localize at cell-cell junctions, does not appear to self-associate, and is mainly exposed to extracellular matrix (Rattner et al., 2004). The observation that heterozygous knockout retinas develop OSs of normal appearance implies that this protein may be present in stoichiometric excess, or may function via a catalytic mechanism. Membranes recognizable as malformed OSs are present in homozygous knockout retinas, so it seems likely that this protein plays a role for disk morphogenesis, rather than functioning as an essential building block for disk membrane architecture per se. Tethers between nascent disk edges and the adjacent IS plasma membrane have been documented recently by TEM, and are hypothesized to represent a heterotypic interaction of PCDH21 with an IS-localized protein (Burgoyne et al., 2015). Further investigation is required to evaluate this possibility. The observation that the only two proteins localized to nascent disk edges to date, PCDH21 and prominin-1 (Fig. 8), engage in a direct association (Yang et al., 2008) suggests that they may function cooperatively to orient adjacent nascent disk edges during the expansion of membrane evaginations and/or disk rim formation.
3.3.2.5. Peripherin-2/rds (P/rds)
P/rds is a tetraspanin protein that is required for normal OS morphogenesis and structure in mice, and causes a broad variety of inherited retinal diseases in humans, including retinitis pigmentosa and macular dystrophies (Goldberg, 2013; Stuck et al., 2016). A defect in P/rds is responsible for the retinal pathology in the well-studied retinal degeneration slow (rds) mouse model (Travis et al., 1992), and current thinking suggests that this protein can function directly for disk membrane morphogenesis.
Structural properties and distribution
P/rds is a ~39 kDa integral membrane glycoprotein that is part of the tetraspanin superfamily (Charrin et al., 2014; Goldberg, 2013). P/rds, like other tetraspanins commonly organize other proteins and/or membranes. Unlike most other tetraspanins however, P/rds is cell-type specific and resides within well-defined membrane domains that appear to lack dynamic protein exchange(Goldberg, 2006). Although P/rds localization at rod and cone disk rims has fueled speculation that it acts to structure these features (Arikawa et al., 1992; Molday et al., 1987), direct evidence for molecular function and mechanism has been lacking, and these remain open questions. P/rds is present in both open and closed (internalized) disks; however, it is only localized to disk rims and not disk edges (Arikawa et al., 1992; Ding et al., 2015). Incorporation of P/rds into nascent disks coincides with the transition of disk edge to disk rim.
P/rds functions as an oligomer and self-assembles via a multistep process that includes dimerization, tetramerization, and polymerization (Fig. 9A). Each step is essential for P/rds support of OS biogenesis; however, their mechanistic roles for protein function are not yet defined. P/rds self-assembly has been suggested as a potential energy source for driving unfavorable reactions required for disk morphogenesis (Goldberg, 2006). A portion of the P/rds protein in WT retinas is normally incorporated into non-covalent heterotetramers with rom1 (discussed in more depth below); rom-1 is a homologous tetraspanin that appears to regulate P/rds activity (Bascom et al., 1992; Loewen and Molday, 2000). Importantly, P/rds noncovalent homotetrameric self-assembly is sufficient to support relatively normal OS morphogenesis and structure in the absence of rom-1 (Clarke et al., 2000).
Figure 9. P/rds oligomerization, gross structure, and topology at rod OS disk rims.
A) P/rds dimers (modeled using CD81 monomers, described below) are likely assembled at the biosynthetic level and must undergo additional steps of oligomerization to function in support of OS morphogenesis. Adapted from (Goldberg, 2006). B) Left: 3D structure of the detergent-solubilized P/rds:rom1 heterotetramer. Arrows indicate a presumed annulus of detergent molecules bound at the transmembrane domains. Adapted from (Kevany et al., 2013). Right: 3D model of CD81, a homologous tetraspanin with a mass (26 kDa) somewhat smaller than that of P/rds (39 kDa). Image adapted from (Seigneuret, 2006). The dimensions determined for the P/rds:rom1 heterotetramer are consistent with a complex of four tetraspanin monomers. C) Topology of P/rds in the disk rim, showing hypothesized partitioning of the C-terminal amphipathic helix. The amphipathic helix is leashed to the P/rds transmembrane domain by a disordered region sufficient to bridge adjacent disks, potentially allowing trans insertion.
Noncovalent tetramers appear to be the minimal unit of P/rds function; all P/rds in photoreceptor OS membranes is present in this form (Goldberg and Molday, 1996b; Loewen and Molday, 2000). Noncovalent tetramers are formed by a dimerization of dimers (Goldberg et al., 1995; Loewen et al., 2001), mediated by determinants within the extracellular-2 domain (Ding et al., 2005; Goldberg et al., 2001). The subcellular sites for P/rds dimerization and tetramerization within photoreceptors remain to be rigorously established. Since steady-state P/rds levels are extremely low in WT photoreceptor ISs, determining subcellular assembly sites in normal photoreceptors is technically challenging. Nonetheless, this is a significant question, because defective tetramerization causes retinal degeneration (Kedzierski et al., 2001; Loewen et al., 2003). Several studies suggest that tetramerization can occur in the absence of OSs (Chakraborty et al., 2008; Goldberg et al., 1995), but additional studies are required to definitively resolve his question. The covalent assembly of P/rds tetramers into higher-order polymers is also mediated by the extracellular-2 domain. A single cysteine within this domain (C150) can form an intermolecular disulfide bond (Goldberg et al., 1998) that polymerizes tetramers into larger forms (Loewen and Molday, 2000). Polymerization is essential for OS morphogenesis; mutant P/rds lacking this capacity cannot support this process (Chakraborty et al., 2009).
In contrast to the highly structured extracellular-2 domain, the P/rds C-terminal domain is intrinsically disordered, and lacks stable secondary and tertiary structures in aqueous solutions (Ono et al., 2015; Ritter et al., 2005). The C-terminal domain is nonetheless essential for photoreceptor function and viability (Boon et al., 2008; Kohl et al., 1997). Intrinsically disordered proteins and intrinsically disordered regions lack a well-structured three-dimensional fold and defy the traditional view that protein function requires a stable structure, and fulfill a broad range of critical cellular roles (Dyson and Wright, 2005; Oldfield and Dunker, 2014). The functional significance of intrinsic disorder in the C-terminal domain is not known, though participation in P/rds trafficking is well-documented. In conjunction with a lipid anchor, the P/rds C-terminal domain alone is sufficient to direct proper trafficking of GFP to OS disk rims (Tam et al., 2004). That report and others (Salinas et al., 2013; Tian et al., 2014) have refined trafficking and localization determinants to a stretch of ~10 amino acids that lie within a disordered region. The mechanism by which this sequence directs P/rds to its OS destination remains to be determined, but interaction with an adaptor protein seems likely. Intrinsically disordered regions are well-suited to this role, as they often participate in high specificity/low affinity interactions. It is also possible that the plasticity in the C-terminal domain contributes to P/rds trafficking by regulating targeting sequence exposure.
Although flexible on their own, intrinsically disordered proteins and intrinsically disordered regions can acquire structure upon the binding of a target, and several investigations have shown that membrane mimetics can induce an amphipathic helix within the C-terminus central region (Boesze-Battaglia et al., 2003; Boesze-Battaglia et al., 2000; Ritter et al., 2005). A more recent study demonstrates that bona fide membranes can also interact with the amphipathic helix, and that membrane partitioning depends on phospholipid composition and on membrane curvature (Khattree et al., 2013). Interaction of the C-terminal amphipathic helix with membranes can promote membrane fusion in vitro (Boesze-Battaglia et al., 2003; Ritter et al., 2004), and similar activity has been observed in full-length P/rds (Boesze-Battaglia et al., 1998). The in vitro fusogenic activity observed for the P/rds C-terminus is hypothesized to be important for OS disk morphogenesis and shedding (Boesze-Battaglia et al., 1998; Edrington et al., 2007). The possible physiological significance of the in vitro fusogenic activity remains to be demonstrated.
More recent progress for understanding P/rds structure includes a protein model resolved to 18 angstroms, which is derived from single-particle TEM analysis of detergent-solubilized and purified bovine P/rds:rom-1 complexes (Kevany et al., 2013). Solubilized tetramers, which include an Amphipol annulus, resemble a tooth (Fig. 9B; left panel). By estimating the quantity of detergent bound, the transmembrane domain position, and the topological orientation, the authors propose an overall shape for the P/rds:rom-1 tetramer as a slightly flattened, somewhat conical cylinder of diameter ~5.5 nm and total length of ~11 nm, (which contains a 2:2 stoichiometry of P/rds and rom-1). These dimensions are in reasonably good agreement with a previous three-dimensional model of the CD81 tetraspanin monomer (Fig. 9B; right panel). A similar tetrameric arrangement of CD81 monomers would produce a transmembrane diameter of 5–6 nm and total length of ~9 nm (Seigneuret, 2006). These results indicate that P/rds tetramerization occurs via a “side-by-side” (parallel) association of two dimers; this configuration confirms conclusions drawn by a previous hydrodynamic study (Loewen et al., 2001), but argues against a “foot-to-foot” (series) model (Goldberg, 2006).
Association with other proteins
Although P/rds has been reported to bind a variety of proteins, only rom-1 and glutamic acid rich proteins (GARPs), have been validated by multiple laboratories, and confirmed as physiologically relevant to date (Bascom et al., 1992; Poetsch et al., 2001). Loss of rom-1 affects OS structure by subtly altering disk diameter, while loss of GARPs appears to reduce OS architectural stability (Clarke et al., 2000; Zhang et al., 2009). The molecular determinants for interaction of these proteins with P/rds are not yet rigorously determined, nor are their physiological functions. The significance of these proteins in relation to P/rds, and as independent actors is reviewed in additional detail below.
Human molecular genetics and animal models
A broad spectrum of human retinal diseases are caused by inherited defects in P/rds. Pathogenic mutations include nonsense, missense, and insertion/deletions that cause frameshifts; disease-causing mutations have been reported in nearly all protein domains (Boon et al., 2008). Additionally, autosomal dominant, recessive, and digenic inheritance patterns have all been well-documented to date (https://sph.uth.edu/retnet/). The most common clinical presentation of disease resulting from a P/rds mutation is a dominantly inherited progressive retinopathy, which causes significant loss of vision in the fourth or fifth decade of life. Significant variability in penetrance and phenotypic presentation is characteristic of these degenerations, and both central and peripheral dystrophies are well represented; this broad genetic and phenotypic heterogeneity has thus far confounded efforts to rationalize phenotype-genotype relationships. Instances of null mutations offer the simplest cases to interpret, and data from both humans and animals are available. In this regard, it is interesting to note that a heterozygous null defect in P/rds generates a peripheral retinal dystrophy in humans (Kajiwara et al., 1993) – a result consistent with the finding that a heterozygous null defect in P/rds (in rds mice) primarily affects rod photoreceptors (Cheng et al., 1997). A number of other mouse models for P/rds-associated inherited human retinal disease have been generated and include pathogenic missense, frameshift, and digenic mutations. In the main, the phenotypes observed are reported to be consistent with their corresponding human clinical presentations (Conley et al., 2010; Ding et al., 2004; Kedzierski et al., 1997; Kedzierski et al., 2001; McNally et al., 2002; Stricker et al., 2005; Stuck et al., 2014). Nonetheless, given the broad heterogeneity of disease associated with inherited defects in P/rds and the differences in mouse-human retinal anatomy and physiology, a reliable and comprehensive genotype-phenotype correlation scheme awaits an improved understanding of protein function, modifier gene and environmental effects, and the pathogenic process.
Role for OS architecture
The localization of P/rds at disk rims (Fig. 8), combined with the steep dose-dependence of OS structure on P/rds in murine models, strongly suggest that this protein functions in a direct fashion to structure OS membranes (Arikawa et al., 1992; Hawkins et al., 1985; Molday et al., 1987; Sanyal and Jansen, 1981). Efforts to understand more precisely how P/rds contributes to OS morphogenesis and structural maintenance have been hindered by technical challenges for assaying these processes. Because mature OS structure depends on OS morphogenesis, it is difficult to determine whether molecules required for morphogenesis also play a significant roles in mature disk stabilization.
Several lines of evidence (in addition to the two mentioned above), support the idea that P/rds contributes to disk morphogenesis by a direct participation in rim formation. First, P/rds is not present in disk edges, but is poised at membrane growth points - the putative locations needed for a self-assembling membrane morphogen to drive rim propagation (Arikawa et al., 1992; Corless and Fetter, 1987). Second, P/rds is well-documented to undergo extensive self-assembly (Goldberg and Molday, 1996b; Loewen and Molday, 2000), and inhibition of oligomerization compromises OS morphogenesis (Chakraborty et al., 2009; Kedzierski et al., 2001). Third, P/rds trafficking through the CC is preserved in rod cells of rhodopsin knockout mice and although normal OS morphogenesis is prevented, rudimentary OS domains, which contain disk-like structures (including high-curvature rims), are generated (Lee et al., 2006). No such structures are generated in mice which completely lack P/rds (Cohen, 1983); therefore, these data suggest that P/rds is sufficient and necessary for disk rim formation. Finally, the observation that cone OS structure can better tolerate reduced expression of P/rds (vs. rods), is consistent with the reduced need for complete disk rim closure in these organelles (Cheng et al., 1997).
The molecular mechanisms by which P/rds supports disk morphogenesis and OS structure remain controversial. P/rds was suggested to act as a membrane fusogen during OS morphogenesis (Boesze-Battaglia et al., 1998). This concept is awkward however, because the proposed fusogenic domain is cytoplasmic, yet disk internalization (via fusion) would require that it act upon extracellular membrane surfaces. An alternative proposal reported a “flattening” of pancreatic microsomes by in vitro transcribed WT protein (Wrigley et al., 2000). This approach suggested promise for routine assay of P/rds membrane sculpting activity, but subsequent reports have not been published, and our efforts to replicate the results have not been successful (A. Goldberg; personal observations). Because localization of the P/rds within the microsomes was not described, the mechanistic basis of the “flattening” remains unclear. A pair of more recent studies suggest that P/rds can participate in rim formation during disk morphogenesis by generating membrane curvature directly. The first investigation observes that full-length P/rds induces a high-curvature tubulovesicular membrane network when expressed in cultured cells, and that the isolated P/rds C-terminus can remodel large unilamellar phospholipid liposomes to generate smaller-diameter (high curvature) tubules and vesicles in vitro (Khattree et al., 2013). The second study finds that in vitro reconstitution of purified P/rds into phospholipid vesicles produces a population of “disk-like structures” with diameters of 40–100 nm. Periodicity observed in TEM micrographs of the negatively stained structures suggest that the reconstituted protein is packed into organized arrays (Kevany et al., 2013). Taken together, these latter two reports offer the first direct support for the notion that P/rds may participate in disk rim formation (and stabilization) by affecting membrane geometry directly.
In addition to its role(s) for nascent disk morphogenesis, the retention and abundance of P/rds in the rims of mature disks (2–4% of total OS membrane protein), suggests that it may function to stabilize these structures. Additional studies are required to investigate this possibility. In addition, since P/rds mediates tethering interactions between disk rims and the plasma membrane (Poetsch et al., 2001), it is conceivable that it also participates in tethering between the rims of adjacent disks. This possibility was first suggested by the finding that P/rds stoichiometry in disk rims is comparable to that of filamentous structures observed to tether disk rims together (Goldberg and Molday, 1996b; Roof and Heuser, 1982). The molecular composition of these filaments remains to be determined, and despite repeated efforts, P/rds is the only rim-localized protein discovered to date that is present in quantities consistent with this role (Kwok et al., 2008; Liu et al., 2007; Skiba et al., 2013). The cytoplasmic location of the intrinsically disordered P/rds C-terminus creates the interesting possibility that it participates in disk-disk tethering via “trans” amphipathic helix partitioning (Fig. 9C). This helix is leashed to the P/rds transmembrane core by an intrinsically disordered region predicted to be ~9 nm in length, which would be sufficient to bridge the ~7 nm gap that separates adjacent disk rims.
Finally, the ability of the P/rds C-terminus to promote membrane fusion in vitro (Edrington et al., 2007), combined with its localization in a topologically appropriate location (adjacent to the OS plasma membrane,) makes it plausible that P/rds may also participate in disk shedding. Future studies are required to determine whether it does so.
3.3.2.6. Rom-1
Rom-1 was discovered in a broad search for retina-specific transcripts potentially involved in retinal disease (Bascom et al., 1992). Cloning revealed it to be a P/rds homolog specifically expressed in photoreceptors, and a variety of subsequent studies found that rom-1 assembles with P/rds and slightly modifies its physiological function (Clarke et al., 2000). Although inherited defects in rom-1 do not by themselves cause monogenetic disease, rom-1 can act as a modifier gene for P/rds-associated disease.
Structural properties and distribution
Rom-1 is a ~37 kDa integral membrane protein that is ~35% identical to P/rds at the amino acid level (Bascom et al., 1992), and is likewise a tetraspanin superfamily member (Goldberg, 2013). The genomic organization of the genes encoding rom-1 and P/rds suggests that rom-1 was generated by an evolutionary duplication event (Bascom et al., 1993). Notably, rom-1 homologues do not appear to be present in all vertebrate genomes (Kedzierski et al., 1996). Nonetheless, like P/rds, rom-1 possesses cytoplasmic N- and C-termini, is assembled into non-covalent tetramers, and is present in both rod and cone photoreceptors (Bascom et al., 1992; Goldberg et al., 1995; Loewen and Molday, 2000; Moritz and Molday, 1996). Like P/rds, rom-1 distribution is highly restricted in photoreceptors; immunogold labeling studies find rom-1 strictly confined to OS disk rims of both rods and cones (Moritz and Molday, 1996). Unlike P/rds, rom-1 is not post-translationally modified by glycosylation (Bascom et al., 1992).
Association with other proteins
Rom-1 is capable of homomeric non-covalent self-assembly as well as heteromeric interaction with P/rds (Goldberg et al., 1995; Loewen and Molday, 2000; Moritz and Molday, 1996). The strength of the non-covalent rom-1 interaction with P/rds is less than that of P/rds homomeric interaction (Kedzierski et al., 1999). Rom-1 can also interact with P/rds via disulfide bonds (Loewen and Molday, 2000; Moritz and Molday, 1996). Association with P/rds likely drives rom-1 trafficking to OSs, as rom-1 does not contain an autonomous localization signal (Tam et al., 2004). Akin to P/rds, incorporation of rom-1 into tetrameric complexes appears to be mediated through its extracellular-2 domain (Goldberg and Molday, 1996a; Loewen et al., 2001). A detailed analysis of rom-1 and P/rds oligomerization found that rom-1 homotetramers are incapable of forming disulfide-mediated polymerized chains, and the presence of rom-1 in P/rds:rom-1 heterotetramers limits disulfide-mediated chain length (Loewen and Molday, 2000). The significance of these findings for physiological function remains to be established.
Human molecular genetics and animal models
There is no evidence that monogenic defects in rom-1 can cause human disease by themselves (Bascom et al., 1995). In contrast, several pathogenic mutations in rom-1, including, G80frameshift, L114frameshift, and G113E, cause digenic retinitis pigmentosa, when co-inherited with a L185P mutation in P/rds (Dryja et al., 1997; Kajiwara et al., 1994). The digenic pattern of disease inheritance was subsequently explained as a conditional defect in subunit assembly that causes a deficiency of tetrameric P/rds in vivo (Goldberg and Molday, 1996a; Kedzierski et al., 2001; Loewen et al., 2001). Intriguingly, rom-1 is not essential for establishing relatively normal OS architecture. Rom-1 knockout mice develop well-organized OSs that possess some disks with oversized diameters; this phenotype led to the proposal that rom-1 functions for regulating P/rds-mediated disk morphogenesis (Clarke et al., 2000). Rom-1 overexpression worsens photoreceptor pathology in the neural retina leucine zipper gene (Nrl) knockout mouse; the physiological significance of this result remains to be established (Chakraborty et al., 2012).
Role for OS architecture
Current evidence is consistent with the proposal that rom-1 modulates P/rds function for disk morphogenesis (Clarke et al., 2000). The demonstration that rom-1 limits the size of P/rds polymers (Loewen and Molday, 2000) suggests the interesting possibility that the polymerization of P/rds-containing tetramers is linked to the advance of rim formation during nascent disk internalization, and that disk circumference may be regulated in part by polymer length.
3.3.2.7. Glutamic acid rich proteins (GARPs)
GARPS are essential for rod photoreceptor function and viability; their loss causes retinal disease in humans and animals. A single gene (CNGB1), generates three different GARPs that are expressed in rods (but not cones), via alternative splicing (Colville and Molday, 1996). The largest and most critical of these is the β-subunit of the cyclic nucleotide-gated (CNG) cation channel. This channel mediates the cation conductance of the rod OS plasma membrane in response to light, and is therefore a required element of the phototransduction cascade to support vision. CNG-gated channels are heterotetrameric, and are composed of one β-subunit and three α-subunits (Zheng et al., 2002). Importantly, cone phototransduction also incorporates β-subunits into CNG cation channels; however, these are derived from a distinct gene (CNGB3) that does not include a GARP domain (Biel and Michalakis, 2007).
Structural properties and distribution
As mentioned, CNGB1 is a complex gene that expresses three differentially spliced GARP transcripts (Ardell et al., 2000); biochemical studies demonstrate that all three GARPs are present in rod, but not cone photoreceptors (Colville and Molday, 1996; Korschen et al., 1995). The longest polypeptide derived from CNGB1 is the β-subunit. Like other channel subunit homologs, the β-subunit encodes six transmembrane domains, a pore-lining “P loop”, and a cyclic nucleotide-binding region, which together function for channel transport function (Biel and Michalakis, 2007). In addition, the β-subunit also includes a novel N-terminal domain that includes subcellular targeting information and a proline and glutamic acid-rich region. This region is also expressed as two alternatively-spliced soluble cytoplasmic proteins, GARP1 and GARP2. Most study has focused on GARP2, which is relatively abundant in ROS (Batra-Safferling et al., 2006). GARP2 is a protein of 299 amino acids, which includes four proline-rich repeat domains (13–17 AAs each), but lacks the glutamic acid-rich regions present in other GARP variants (Korschen et al., 1999). The most striking feature of GARP2 is that it is an intrinsically disordered protein; it completely lacks stable tertiary structure in solution (Batra-Safferling et al., 2006). Although the proline-rich repeat domains of GARP2 (and GARP1) do possess limited α-helical structure, these proteins are otherwise highly plastic, as is the N-terminal GARP domain of the membrane bound CNG cation channel β-subunit. Localization of GARPs in bovine and murine retina by immunogold TEM microscopy finds them restricted to regions where the ROS plasma membrane comes into proximity with disk rims (Colville and Molday, 1996; Korschen et al., 1999).
Associations with other proteins
The intrinsic disorder present in GARPs has hindered elucidation of their interactions, because flexibility often fosters multiple binding partners, multivalent binding, and weak/transient interactions. One early study used immobilized peptides (corresponding to GARP proline-rich repeats), to suggest that GARPs can bind: guanylate cyclase, ABCA4, and PDE6, and may weakly self-assemble (Korschen et al., 1999). To date however, only GARP2 self-assembly and interaction with PDE6 have been confirmed by subsequent studies (Batra-Safferling et al., 2006; Pentia et al., 2006). Rather differing results were obtained by an investigation that used highly specific monoclonal antibodies in conjunction with detergent-solubilized ROS, to show that (all three) native GARP proteins associate with P/rds (Poetsch et al., 2001). The reasons for the disparate results are not yet clear, and it is important to note that they are not mutually exclusive. It is plausible that GARPs interact with different partners under different conditions, and also that some GARP interactions are labile and disrupted by detergents used for integral membrane protein solubilization. Additional studies are therefore needed. The challenges associated with documenting interactions of intrinsically disordered proteins (and GARPs) highlights the need for technical approaches that more closely model the native photoreceptor milieu. Several in cellulo and in vivo strategies illustrate how these strategies can be valuable complements to in vitro methods. An in vivo investigation concluded that individual GARP variants interact differentially with P/rds in X. laevis rod photoreceptors; whereas CNGB1 interactions were found to target/localize the channel, GARP2 interactions instead appear to participate in disk morphogenesis (Ritter et al., 2011). An in cellulo electrophysiological study observed that the CNG cation channel β-subunit N-terminal GARP domain and soluble GARP variants can each interact with CNG cation channel regions that regulate gating (Michalakis et al., 2011). Finally, transgenic overexpression of GARP2 in murine rods altered rod cell light responses by impacting phototransduction cascade gain and recovery; this finding demonstrates that despite its extreme plasticity, GARP2 can modulate phototransduction signaling in vivo (Sarfare et al., 2014).
Previously proposed models for GARP interactions in the rod OS are presented in Figure 10. In each case, GARPs are restricted to regions where disk rims lie alongside the OS plasma membrane, and interactions are hypothesized to scaffold signaling components. Figure 10A presents a model in which GARP interactions are viewed as primarily important for a tethering interaction between plasma membrane CNG cation channels and rim-localized P/rds (Poetsch et al., 2001). This association, which must bridge a gap between discontinuous membranes, is proposed to organize CNG cation channels within the OS plasma membrane. Channels are currently thought to be organized as a series of rings along the axis of the OS plasma membrane, with a regular spacing governed by the disk-disk stacking interval (Kaupp and Seifert, 2002). In addition to mediating tethering between disk rims and the plasma membrane, GARPs have also been proposed to mediate tethering between adjacent disk rims (Fig. 10B) - via interaction of GARP2 with P/rds (Batra-Safferling et al., 2006).
Figure 10. Disk rim complexes proposed to contribute to OS organization.
Models proposed for disk rim scaffolding of OS components, based on localization and protein-protein interaction data. A) Interactions between GARPs and P/rds are proposed to mediate fibril linkages between disk rims and the OS plasma membrane. Adapted from (Poetsch et al., 2001). B) An elaboration of the Poetsch et al. model suggests GARP2 interaction with P/rds mediates disk-disk stacking. Adapted from (Kaupp and Seifert, 2002).
An earlier proposal that GARPs function to organize a “transducisome” at disk rims (Korschen et al., 1999) appears larely untenable, as it is inconsistent with electrophysiological findings, and because some of the original study results have been called into question (Kaupp and Seifert, 2002). Nonetheless, several subsequent findings - including PDE6 localization at disk rims (Chen et al., 2008; Muradov et al., 2009), and tight binding of GARP2 by PDE6 (Pentia et al., 2006), support the idea that disk rims may act to dynamically scaffold one or more phototransduction enzymes. Given that GARP2 binds both PDE6 and P/rds, PDE6 scaffolding may occur via a PDE6-GARP2-P/rds based mechanism. Additional studies are needed to rigorously evaluate how significant such scaffolding is for photoreceptor function and viability, and whether other cascade components also participate.
Human molecular genetics and animal models
CNGB1 defects have been associated with autosomal recessive retinitis pigmentosa (RP45) by several studies. This inheritance pattern is consistent with other channelopathies, which frequently require homozygosity to manifest pathology, since ion transport rates in heterozygotes are adequate for physiological function. Defects documented to date include several that appear to modify channel transport function (Bareil et al., 2001; Kondo et al., 2004), and a missense mutation that replaces a conserved proline in the GARP domain (Fu et al., 2013). Additional study would be required to confirm pathogenicity of the mutations, and detail mechanisms underlying associated cases of retinal degeneration.
Role for OS architecture
Murine models in which one or all GARP variants are genetically ablated provide the basis for our current understanding of how GARPs contribute to OS structure. The channel βsubunit was observed to be dispensable for disk and OS morphogenesis; OS structure in young animals is only modestly affected by loss of the β-subunit alone (Huttl et al., 2005). This was a surprising finding. Because the β-subunit interaction with P/rds spans the sizable gap between the OS plasma membrane and disk rims (17–18 nm), their interaction was imagined to play a major role for structuring the spatial relationship between these discontinuous membranes. Although β-subunit loss does slightly erode OS membrane organization, the primary initial impact is a shortening of the OS layer, and many OS organelles retain evenly-stacked disks of relatively uniform diameters (Huttl et al., 2005). It is possible that β-subunit interactions with P/rds do contribute to the stability or fidelity of disk morphogenesis and stacking interactions, since OS structure does gradually erode in older animals; however, this may also result from secondary sequelae.
In contrast to loss of the β-subunit alone, genetic ablation of all three GARPs (via deletion of a core promoter sequence and exons 1 and 2 of CNGB1), creates a more significant ultrastructural phenotype. Pan-GARP knockout mice show perturbed OS structures at a young age (p20) that include irregularly shaped OSs and disks of unusually large diameter (Zhang et al., 2009). These results demonstrate that as an ensemble, GARPs are required for establishing normal OS structure. Because these consequences are not generated by loss of the β-subunit alone, the simple conclusion is that soluble GARP variants must play an essential role for OS structure. To date, the mechanisms by which they do so have not been determined. Although GARP2 has been proposed to mediate disk stacking interactions by interacting with P/rds to tether disk rims together (Batra-Safferling et al., 2006), the presence of evenly stacked disks in ROS from pan-GARP KO retinas indicates that GARP2 is not required to establish disk stacking (Gilliam et al., 2012; Zhang et al., 2009). It is possible that GARP2 participates as a stabilizing factor in such linkages after they have been established; this protein is present in numbers comparable to filamentous structures, which apparently mediate spacing of adjacent rims within disk stacks (Roof and Heuser, 1982).
3.3.2.8. Rhodopsin
Rhodopsin is a widely studied and well-documented member of the seven-transmembrane helix receptor superfamily (Hofmann and Palczewski, 2015; Palczewski, 2006). This photosensitive molecule is the major protein constituent of rod OS membranes (~90% by mass) and is present at millimolar intracellular concentrations (Liebman and Entine, 1968). Inherited defects in the human rhodopsin gene are responsible for a significant fraction (30–40%) of autosomal dominant retinitis pigmentosa cases (Malanson and Lem, 2009).
Structural properties and distribution
Rhodopsin has been the subject of intense scrutiny since its original identification nearly a century-and-a-half ago. This G-protein coupled receptor initiates photoreceptor intracellular signaling in response to light, and consists of the chromophore 11-cis retinal covalently coupled to the protein rod opsin. Rod opsin is a 35 kDa protein with an extracellular/intradiskal N-terminus, seven helical transmembrane domains connected by short intervening loops, and a cytoplasmic tail. A variety of posttranslation modifications, including disulfide formation, N-linked glycosylation, palmitoylation, acylation, and phosphorylation, are essential aspects of normal rhodopsin structure/function and targeting (Hofmann and Palczewski, 2015; Palczewski, 2006). Its crystal structure was solved to 2.8 angstroms in 2000, the first such structure for a G-protein coupled receptor (Palczewski et al., 2000). Upon light absorption, the 11-cis chromophore isomerizes to all-trans, resulting in protein structural changes that generate an active conformation that stimulates the G-protein transducin and initiates the visual transduction process.
Rhodopsin is a rod photoreceptor-specific protein. Although mainly localized to OSs, immunocytochemical studies find a lesser, but measurable amount of protein reactivity within intracellular IS membranes, likely reflecting the high demand and synthesis of this extremely abundant protein (Molday et al., 1987; Papermaster et al., 1985). The mechanisms that direct rhodopsin transport to OSs are still under investigation (Wang and Deretic, 2014). Within OSs, rhodopsin is concentrated in disk lamellae at high densities (~25,000 per μm2), but is excluded from disk rim domains (Molday et al., 1987; Palczewski, 2006). Rhodopsin is also present in OS plasma membranes; however, its concentration is approximately 50% lower than that of disk membranes (Molday and Molday, 1987). The structure of the rhodopsin within disk lamellae has been characterized by a variety of techniques. Although a seminal study suggested that it diffuses freely within the plane of the membrane (discussed below), more recent studies find several levels of higher-order structural organization. Protein dimers appear to be organized into paracrystalline arrays (Fotiadis et al., 2003; Liang et al., 2003) that are themselves are confined to nanodomains interspersed with domains containing little or no rhodopsin (Rakshit et al., 2015; Whited and Park, 2015). Most recently, “tracks” of rhodopsin dimers were observed by cryoelectron microscopy, and have been suggested to contribute to the uniformity of the rod cell single photon response (Gunkel et al., 2015).
Association with other proteins
Rhodopsin interacts with three major partners within the OS - the G-protein transducin, rhodopsin kinase, and arrestin – all players within the phototransduction cascade. Upon photoactivation, rhodopsin catalyzes the exchange of bound GDP for GTP on transducin, as the first step in the G-protein coupled cascade of vision (Arshavsky and Burns, 2012; Wensel, 2008). Rhodopsin is then phosphorylated at multiple sites within its cytoplasmic C-terminus by rhodopsin kinase, and binds arrestin, a two-step process that terminates signal transduction (Lamb and Pugh, 2006). In addition, rhodopsin interacts with guanylate cyclase-1 (GC-1), one of two guanylate cyclases documented in rod OSs; this interaction is necessary for GC-1 stability and delivery to rod OSs (Pearring et al., 2015).
Early investigations suggested that rhodopsin has a high rate of diffusion within the disk lamellae (Poo and Cone, 1974), consistent with its ability to rapidly catalyze GDP exchange on multiple transducin molecules. This observation implies that its interactions with other disk components are highly transient (Arshavsky and Burns, 2012). In contrast, several more recent studies indicate that rhodopsin self-associates in disk membranes and forms identifiable domains with restricted mobility (Fotiadis et al., 2003; Liang et al., 2003). The extensive organization of a molecule previously identified as freely diffusible has sparked considerable debate. Therefore, more recent investigations have aimed to reexamine rhodopsin diffusion (Govardovskii et al., 2009; Najafi et al., 2012a), and develop models that can reconcile phototransduction kinetics and structural information (Dell’Orco and Schmidt, 2008; Gunkel et al., 2015; Schoneberg et al., 2014).
Human molecular genetics and animal models
More than one hundred mutations in human rhodopsin can produce recessive and dominant forms of retinitis pigmentosa, as well as congenital stationary night blindness, which are respectively produced by: null alleles (Rosenfeld et al., 1992), gain of function mutations (Dryja et al., 1990), and constitutively signaling forms (Dryja et al., 1993). Several of the human mutations have been modeled in animals, providing insights into disease processes and the structural importance of rhodopsin for rod OSs.
The most common pathogenic mutation in rhodopsin, P23H, causes a biosynthetic defect associated with low expression, ER retention, and instability of mutant rhodopsin expressed heterologously in cultured cells (Kaushal and Khorana, 1994; Sung et al., 1991). Although numerous studies implicate a pathogenic mechanism associated with ER stress and/or proteasomal overload (Lin et al., 2007; Lobanova et al., 2013; Tam and Moritz, 2007; Tam et al., 2010), significant effects on rod OS structure are also well-documented. Transgenic P23H mice exhibit perturbed disk morphogenesis and shortened OSs, results that are consistent with reduced rhodopsin levels and dominant negative effects (Liu et al., 1997; Naash et al., 1993). In the complete absence of WT rhodopsin, the mutant protein fails to escape the photoreceptor IS and compromises cell viability (Frederick et al., 2001). A knock-in mouse study of P23H rhodopsin-GFP also finds this protein to be unstable, but capable of trafficking to the OS to some extent (Price et al., 2011). A subsequent investigation of P23H rhodopsin-GFP found that it can produce disk structural abnormalities, and that the extent of defects are closely correlated to the levels of mutant protein in the OSs (Price et al., 2012). A study using P23H knock-in mice confirms that this mutation reduces retinal protein levels significantly (10 to 100-fold), and generates shortened rod OSs and modest disorganization of disk membranes, particularly at the basal OSs (Sakami et al., 2011). Transgenic X. laevis provide additional insights into P23H behavior in vivo. In frog rods that express P23H rhodopsin-GFP, the fusion protein is detectable in OSs at a 1000-fold lower level than endogenous opsin, and creates both aggregated protein foci and disk structural defects, including vesiculotubular structures (Haeri and Knox, 2012). This same study found analogous structural defects in P23H transgenic mice. Interestingly, dark rearing can partially mask the biosynthetic defect and impact of P23H rhodopsin on X. laevis disk structure (Bogea et al., 2015; Tam and Moritz, 2007). Under these conditions, relatively high levels of protein are incorporated into OSs with minimal effect on disk organization. Subsequent light exposure triggers a massive vesiculation of disk membranes, possibly as a consequence of mutant opsin aggregation or partial specific volume increase. These results emphasize the importance of environmental conditions for influencing the P23H phenotype and indicate that caution is required for interpreting how animal model phenotypes may apply to human photoreceptor pathophysiology. Nonetheless, the combined results from P23H (and other) animal models for pathogenic defects in rhodopsin suggest that this protein may play a significant part in OS membrane architecture.
Role for OS architecture
Rhodopsin composes the vast majority of the total OS integral membrane protein, and rhodopsin knockout mice are unable to elaborate OS organelles (Humphries et al., 1997; Lem et al., 1999). This observation clearly demonstrates that, in addition to its well-studied function for phototransduction, this protein also acts as an essential structural “building block” for rod OS membrane architecture. Similar findings have been reported for Drosophila affected by NinaE mutations, which eliminate the Rh1 rhodopsin present in photoreceptors R1–R6 (Kumar and Ready, 1995). The extensive convoluted photosensitive membranes of the invertebrate rhabdomere never develop. Given this steep dependence of OS structure on rhodopsin, it is challenging to determine whether, and by what mechanism(s), this protein plays additional more subtle roles in OS membrane structure. Nonetheless, studies from several laboratories demonstrate that rhodopsin gene dosage and mutations can each impact OS membrane architecture and organelle morphology in predictable ways.
Several laboratories have examined the effects of varied rhodopsin gene dosage on murine photoreceptor structure. Liang et al. have noted that OSs in heterozygous null rhodopsin mice maintain WT levels of rhodopsin packing density, but exhibit reduced length and diameter, which results in reduced OS volumes (Liang et al., 2004). In contrast, transgenic overexpression of WT bovine rhodopsin in mouse photoreceptors by 1.4-fold results in a roughly equivalent increase in OS disk area, and reduced size of incisures, with no significant effect on rhodopsin packing density (Wen et al., 2009). Likewise, overexpression of WT human rhodopsin in mouse photoreceptors also increases OS volume; a roughly linear relationship is observed over a 4-fold range of rhodopsin expression (Price et al., 2012). Together, these three studies support the notion that rhodopsin acts as a structural building block for the OS organelle. Results from one study also suggest that the ratio of rhodopsin to rim-generating proteins (such as P/rds) is an important determinant of disk morphology (Wen et al., 2009). In this regard, it appears that rhodopsin can play a role for regulating incisure depth (and possibly number), by directly affecting disk surface area, and thereby the disk surface area-to-rim ratio. Importantly, a second study from the same laboratory found that incisure depth increased as disk diameter decreased in heterozygous rhodopsin knockout mice, lending significant support to this hypothesis (Makino et al., 2012).
4. Concluding remarks and future directions
Although the lipid compositions of OS disk and plasma membranes have been well-documented, additional studies are needed to refine understanding of heterogeneous lipid distributions and their significance for OS morphogenesis, structure, and function. Implementation and/or development of new experimental approaches and a deeper appreciation of lipid physical chemistry will be required to achieve a better understanding of how particular membrane geometries are formed, remodeled, and contribute to signaling processes. Likewise, to advance understanding of protein contributions to OS organelle structure, cryo-ET must be applied to document key protein structures, and to reveal how macromolecular structures are organized in membranes. New strategies will be required to assess the essential roles of intrinsically disordered proteins for OS structure, and innovative approaches must be developed to distinguish between molecules that function solely for organelle morphogenesis, and those that alternatively or additionally play roles downstream for maintaining structural stability. It will be equally important to develop an improved understanding of how the uniquely constrained OS cytoplasmic compartments and omnipresent membrane surfaces scaffold and regulate signaling molecules in support of phototransduction and other cellular processes.
Studies to date suggest that phospholipids and membrane proteins, delivered primarily (and perhaps exclusively) through the ciliary membrane, provide the raw materials for OS structure. The disk morphogenesis that occurs during initial OS development and during OS renewal in mature adult photoreceptors appears to occur though essentially similar processes, which include two or more sequential steps that take place at the OS base. First, a series of ciliary membrane evaginations, anchored to axonemal microtubules by their incipient rims, are initiated, flattened, and progressively expanded to their mature diameters. Second, apposing surfaces of adjacent evaginations are gradually internalized via rim advance at growth points. In some instances, a third step occurs; a membrane fission event breaks the continuity between disk and plasma membrane, creating a completely internalized disk.
Current information suggests that ciliary membrane evaginations may be anchored to axonemal microtubules via RP1-tubulin associations, potentially via interaction with incipient disk rims. The details of this important interaction remain to be elucidated. Although evagination initiation may be driven by growth of f-actin microfilaments anchored at the axoneme, continued evagination growth is more likely driven by membrane blebbing, perhaps guided by contact with the IS plasma membrane. Extracellular domains of disk edge-localized PCDH21 and prominin-1, potentially in association with SPAM, are likely candidates for this interaction. The identification of binding partner(s) for these proteins that reside on IS plasma membranes and/or calycal processes will be an important future goal. Notably, the mechanisms that underlie the translocation of nascent disk evaginations towards the OS distal tip are not yet defined, nor are the sorting mechanisms that govern where along the axoneme particular cargos depart from the IFT trains for their sites of function. Release of P/rds at the level of nascent disks would allow its incorporation into higher-order polymers that create incipient rims, then help advance rim formation and disk internalization. The mechanisms by which P/rds generates membrane curvature and the processes that regulate the extent of rim formation (i.e. – in rods vs. cones) remain to be determined. Rom-1 may play some role in this regard. Furthermore, the processes regulating the templating and mature size of disks remain to be defined, as do the molecular mechanisms underlying incisure initiation and maturation, and disk-disk stacking. It is possible that cytoplasmic tethering interactions of GARPs with P/rds are important for some or all of these processes. It is also possible that extracellular tethering interactions, involving prominin-1 (and SPAM-like proteins), contribute to disk templating and/or stacking interactions. Processes that incorporate rhodopsin and other OS proteins into disks and/or plasma membranes may also include significant regulatory aspects that shape OS architecture and remain to be described. Finally, the question remains as to what mechanisms are responsible for ensuring that the daily disk shedding from millions of retinal photoreceptors in a vertebrate retina occurs in a timely and geographically appropriate manner. The increasing ease with which vertebrate genomes can be manipulated, combined with revolutionary advances in high-resolution light and electron microscopic imaging, will surely bring answers to these and other fascinating questions in the coming decades.
Acknowledgments
We wish to thank Dr. Charles Lindemann for helpful discussions, and Breyanna Cavanaugh and Stefan Poag for illustrations. Related studies in the authors’ laboratories has been supported by grants from: the Oakland University Research Excellence Fund and NIH EY013246 (to AFXG), FFB-Canada NSERC RGPIN-2015-04326 and CIHR MOP-64400 (to OLM), and NIH EY013408 and EY024667 (to DSW).
ABBREVIATIONS
- GARP
glutamic acid-rich protein
- GFP
green fluorescent protein
- cryo-ET
cryogenic electron tomography
- CNG
cyclic nucleotide-gated
- IFT
intraflagellar transport
- IS
inner segment
- OS
outer segment
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- P/rds
peripherin-2/rds
- RPE
retinal pigment epithelial
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd El-Aziz MM, Barragan I, O’Driscoll CA, Goodstadt L, Prigmore E, Borrego S, Mena M, Pieras JI, El-Ashry MF, Safieh LA, Shah A, Cheetham ME, Carter NP, Chakarova C, Ponting CP, Bhattacharya SS, Antinolo G. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–1287. doi: 10.1038/ng.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga MM, DK, Brush RS, Lydic T, Conley SM, Naash MI, Gavin RE, Busik JV, Anderson RE. Differential composition of docosahexaenoic acid and very long chain polyunsaturated fatty acids in rod and cone photoreceptor membranes. Invest Ophthalmol Vis Sci. 2014;55:370. [Google Scholar]
- Albert AD, Boesze-Battaglia K. The role of cholesterol in rod outer segment membranes. Prog Lipid Res. 2005;44:99–124. doi: 10.1016/j.plipres.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- Anderson RE, Maude MB. Phospholipids of bovine outer segments. Biochem. 1970;9:3624–3628. doi: 10.1021/bi00820a019. [DOI] [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. Freeze-fracture evidence for the presence of cholesterol in particle-free patches of basal disks and the plasma membrane of retinal rod outer segments of mice and frogs. J Cell Biol. 1979;81:215–228. doi: 10.1083/jcb.81.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. Freeze-fracture studies of photoreceptor membranes: new observations bearing upon the distribution of cholesterol. J Cell Biol. 1983;97:749–755. doi: 10.1083/jcb.97.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell MD, Bedsole DL, Schoborg RV, Pittler SJ. Genomic organization of the human rod photoreceptor cGMP-gated cation channel beta-subunit gene. Gene. 2000;245:311–318. doi: 10.1016/s0378-1119(00)00023-8. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa K, Williams DS. Organization of actin filaments and immunocolocalization of alpha-actinin in the connecting cilium of rat photoreceptors. J Comp Neurol. 1989;288:640–646. doi: 10.1002/cne.902880410. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Marc RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–14298. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveldano MI. Phospholipid solubilization during detergent extraction of rhodopsin from photoreceptor disk membranes. Arch Biochem Biophys. 1995;324:331–343. doi: 10.1006/abbi.1995.0046. [DOI] [PubMed] [Google Scholar]
- Aveldano MI, Bazan NG. Molecular species of phosphatidylcholine, -ethanolamine, -serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J Lipid Res. 1983;24:620–627. [PubMed] [Google Scholar]
- Baker LA, Watt IN, Runswick MJ, Walker JE, Rubinstein JL. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1204935109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M. Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet. 2001;108:328–334. doi: 10.1007/s004390100496. [DOI] [PubMed] [Google Scholar]
- Bascom RA, Liu L, Heckenlively JR, Stone EM, McInnes RR. Mutation analysis of the ROM1 gene in retinitis pigmentosa. Hum Mol Genet. 1995;4:1895–1902. doi: 10.1093/hmg/4.10.1895. [DOI] [PubMed] [Google Scholar]
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Bascom RA, Schappert K, McInnes RR. Cloning of the human and murine ROM1 genes: genomic organization and sequence conservation. Hum Mol Genet. 1993;2:385–391. doi: 10.1093/hmg/2.4.385. [DOI] [PubMed] [Google Scholar]
- Batra-Safferling R, Abarca-Heidemann K, Körschen HG, Tziatzios C, Stoldt M, Budyak I, Willbold D, Schwalbe H, Klein-Seetharaman J, Kaupp UB. Glutamic acid-rich proteins of rod photoreceptors are natively unfolded. J Biol Chem. 2006;281:1449–1460. doi: 10.1074/jbc.M505012200. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. The membrane current of single rod outer segments. J Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Forestner DM, Defoe DM. Membrane assembly in retinal photoreceptors. III Distinct membrane domains of the connecting cilium of developing rods. J Neurosci. 1985;5:1035–1048. doi: 10.1523/JNEUROSCI.05-04-01035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977;196:536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Horst CJ. The photoreceptor connecting cilium a model for the transition zone. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. Plenum Press; New York: 1990. pp. 389–417. [Google Scholar]
- Besharse JC, Pfenninger KH. Membrane assembly in retinal photoreceptors I. Freeze-fracture analysis of cytoplasmic vesicles in relationship to disc assembly. J Cell Biol. 1980;87:451–463. doi: 10.1083/jcb.87.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Wetzel MG. Immunocytochemical localization of opsin in rod photoreceptors during periods of rapid disc assembly. J Neurocytol. 1995;24:371–388. doi: 10.1007/BF01189064. [DOI] [PubMed] [Google Scholar]
- Bhowmick R, Li M, Sun J, Baker SA, Insinna C, Besharse JC. Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic. 2009;10:648–663. doi: 10.1111/j.1600-0854.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C, Young RW. Renewal of fatty acids in the membranes of visual cell outer segments. J Cell Biol. 1974a;61:327–343. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C, Young RW. Renewal of glycerol in the visual cells and pigment epithelium of the frog retina. J Cell Biol. 1974b;62:378–389. doi: 10.1083/jcb.62.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Michalakis S. Function and dysfunction of CNG channels: insights from channelopathies and mouse models. Mol Neurobiol. 2007;35:266–277. doi: 10.1007/s12035-007-0025-y. [DOI] [PubMed] [Google Scholar]
- Biernbaum MS, Bownds MD. Frog rod outer segments with attached inner segment ellipsoids as an in vitro model for photoreceptors on the retina. The Journal of general physiology. 1985;85:83–105. doi: 10.1085/jgp.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev Cell. 2012;23:886–895. doi: 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD. Fatty acid composition of bovine rod outer segment plasma membrane. Exp Eye Res. 1989;49:699–701. doi: 10.1016/s0014-4835(89)80064-8. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp Eye Res. 1992;54:821–823. doi: 10.1016/0014-4835(92)90040-y. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with Triton X-100-resistant membrane rafts from rod outer segment disk membranes. J Biol Chem. 2002;277:41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Goldberg AFX, Dispoto J, Katragadda M, Cesarone G, Albert AD. A soluble peripherin/Rds C-terminal polypeptide promotes membrane fusion and changes conformation upon membrane association. Exp Eye Res. 2003;77:505–514. doi: 10.1016/s0014-4835(03)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Hennessey T, Albert AD. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J Biol Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Lamba OP, Napoli AA, Jr, Sinha S, Guo Y. Fusion between retinal rod outer segment membranes and model membranes: a role for photoreceptor peripherin/rds. Biochem. 1998;37:9477–9487. doi: 10.1021/bi980173p. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Stefano FP, Fenner M, Napoli AA., Jr A peptide analogue to a fusion domain within photoreceptor peripherin/rds promotes membrane adhesion and depolarization. Biochim Biophys Acta. 2000;1463:343–354. doi: 10.1016/s0005-2736(99)00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogea TH, Wen RH, Moritz OL. Light induces ultrastructural changes in rod outer and inner segments, including autophagy, in a transgenic Xenopus laevis P23H rhodopsin model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2015;56:7947–7955. doi: 10.1167/iovs.15-16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. Renewal of photoreceptor cells. Meth Enzymol. 1982;81:763–772. doi: 10.1016/s0076-6879(82)81102-6. [DOI] [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985;26:1659–1694. [PubMed] [Google Scholar]
- Boon CJ, Den Hollander AI, Hoyng CB, Cremers FP, Klevering BJ, Keunen JE. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res. 2008;27:213–235. doi: 10.1016/j.preteyeres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Borwein B. Scanning electron microscopy in retinal research. Scan Electron Microsc. 1985:279–301. [PubMed] [Google Scholar]
- Bowne SJ, Daiger SP, Hims MM, Sohocki MM, Malone KA, McKie AB, Heckenlively JR, Birch DG, Inglehearn CF, Bhattacharya SS, Bird A, Sullivan LS. Mutations in the RP1 gene causing autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1999;8:2121–2128. doi: 10.1093/hmg/8.11.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush RS, Tran JT, Henry KR, McClellan ME, Elliott MH, Mandal MN. Retinal sphingolipids and their very-long-chain fatty acid-containing species. Invest Ophthalmol Vis Sci. 2010;51:4422–4431. doi: 10.1167/iovs.09-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Reh TA. Photoreceptor cell fate specification in vertebrates. Development. 2015;142:3263–3273. doi: 10.1242/dev.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, Meschede IP, Burden JJ, Bailly M, Seabra MC, Futter CE. Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc Natl Acad Sci USA. 2015;112:15922–15927. doi: 10.1073/pnas.1509285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan-Jones A, Sorre B, Bassereau P. Curvature-driven lipid sorting in biomembranes. Cold Spring Harb Perspect Biol. 2011;3:a004648. doi: 10.1101/cshperspect.a004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Schiesser WE, Pugh EN., Jr Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol. 2010;135:173–196. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo F, McMahon HT, Kozlov MM. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Caruso G, Bisegna P, Shen L, Andreucci D, Hamm HE, DiBenedetto E. Modeling the role of incisures in vertebrate phototransduction. Biophys J. 2006;91:1192–1212. doi: 10.1529/biophysj.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitin MH, Bok D. Immunoferritin localization of actin in retinal photoreceptors. Invest Ophthalmol Vis Sci. 1986;27:1764–1767. [PubMed] [Google Scholar]
- Chaitin MH, Burnside B. Actin filament polarity at the site of rod outer segment disk morphogenesis. Invest Ophthalmol Vis Sci. 1989;30:2461–2469. [PubMed] [Google Scholar]
- Chaitin MH, Schneider BG, Hall MO, Papermaster DS. Actin in the photoreceptor connecting cilium: immunocytochemical localization to the site of outer segment disk formation. J Cell Biol. 1984;99:239–247. doi: 10.1083/jcb.99.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Conley SM, Nash Z, Ding XQ, Naash MI. Overexpression of ROM-1 in the cone-dominant retina. Adv Exp Med Biol. 2012;723:633–639. doi: 10.1007/978-1-4614-0631-0_80. [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Ding XQ, Conley SM, Fliesler SJ, Naash MI. Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones. Hum Mol Genet. 2009;18:797–808. doi: 10.1093/hmg/ddn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Ding XQ, Fliesler SJ, Naash MI. Outer segment oligomerization of Rds: Evidence from mouse models and subcellular fractionation. Biochem. 2008;47:1144–1156. doi: 10.1021/bi701807c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127:3641–3648. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang Y, Koutalos Y. Dynamic behavior of rod photoreceptor disks. Biophys J. 2002;83:1403–1412. doi: 10.1016/S0006-3495(02)73911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yoshida T, Bitensky MW. Light-induced translocation of cyclic-GMP phosphodiesterase on rod disc membranes in rat retina. Mol Vis. 2008;14:2509–2517. [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, Li S, Goto Y, Cao Y, Naash MI. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday LL, Rossant J, Szel A, Molday RS, Birch DG, McInnes RR. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- Cohen AI. The fine structure of the extrafoveal receptors of the Rhesus monkey. Exp Eye Res. 1961;1:128–136. doi: 10.1016/s0014-4835(61)80018-3. [DOI] [PubMed] [Google Scholar]
- Cohen AI. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J Cell Biol. 1968;37:424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI. Further studies on the question of the patency of saccules in outer segments of vertebrate photoreceptors. Vision Res. 1970;10:445–453. doi: 10.1016/0042-6989(70)90001-5. [DOI] [PubMed] [Google Scholar]
- Cohen AI. Some cytological and initial biochemical observations on photoreceptors in retinas of rds mice. Invest Ophthalmol Vis Sci. 1983;24:832–843. [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Zhu X, Djajadi HR, Molday LL, Smith RS, Libby RT, John SW, Molday RS. Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival. J Cell Sci. 2014;127:1138–1149. doi: 10.1242/jcs.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin RW, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, Zonneveld MN, Blokland EA, Strom TM, Hoyng CB, den Hollander AI, Cremers FP. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 2008;83:594–603. doi: 10.1016/j.ajhg.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville CA, Molday RS. Primary structure and expression of the human beta-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J Biol Chem. 1996;271:32968–32974. doi: 10.1074/jbc.271.51.32968. [DOI] [PubMed] [Google Scholar]
- Conley SM, Ding XQ, Naash MI. RDS in cones does not interact with the beta subunit of the cyclic nucleotide gated channel. Retinal Degenerative Diseases: Laboratory and Therapeutic Investigations. 2010;664:63–70. doi: 10.1007/978-1-4419-1399-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil D, Fargeas CA, Huttner WB. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochem Biophys Res Commun. 2001a;285:939–944. doi: 10.1006/bbrc.2001.5271. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Karbanova J, Fargeas CA, Jaszai J. Prominin-1 (CD133): molecular and cellular features across species. Prominin-1 (Cd133): New Insights on Stem & Cancer Stem Cell Biology. 2013;777:3–24. doi: 10.1007/978-1-4614-5894-4_1. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001b;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Weigmann A, Huttner WB. AC133 hematopoietic stem cell antigen: human homologue of mouse kidney prominin or distinct member of a novel protein family? Blood. 1998;91:2625–2626. [PubMed] [Google Scholar]
- Corless JM, Fetter RD. Structural features of the terminal loop region of frog retinal rod outer segment disk membranes: III. Implications of the terminal loop complex for disk morphogenesis, membrane fusion, and cell surface interactions. J Comp Neurol. 1987;257:24–38. doi: 10.1002/cne.902570104. [DOI] [PubMed] [Google Scholar]
- Corless JM, Fetter RD, Zampighi OB, Costello MJ, Wall-Buford DL. Structural features of the terminal loop region of frog retinal rod outer segment disk membranes: II. Organization of the terminal loop complex. J Comp Neurol. 1987;257:9–23. doi: 10.1002/cne.902570103. [DOI] [PubMed] [Google Scholar]
- Corless JM, Schneider TG. Patterns of interdisk connections within the lamellar domains of retinal rod outer segment disks: observations relevant to the axial propagation of incisures. Exp Eye Res. 1987;45:883–905. doi: 10.1016/s0014-4835(87)80104-5. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Sullivan LS, Gire AI, Birch DG, Heckenlively JR, Bowne SJ. Mutations in known genes account for 58% of autosomal dominant retinitis pigmentosa (adRP) Adv Exp Med Biol. 2008;613:203–209. doi: 10.1007/978-0-387-74904-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P, Allamargot C, Hudson JS, Andersen EK, Bhattarai S, Drack AV, Sheffield VC, Seo S. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2015;112:E4400–4409. doi: 10.1073/pnas.1510111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. Morphogenesis of the retinal rods; an electron microscope study. J Biophys Biochem Cytol. 1956;2:209–218. doi: 10.1083/jcb.2.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Orco D, Schmidt H. Mesoscopic Monte Carlo simulations of stochastic encounters between photoactivated rhodopsin and transducin in disc membranes. J Phys Chem B. 2008;112:4419–4426. doi: 10.1021/jp709963f. [DOI] [PubMed] [Google Scholar]
- Ding JD, Salinas RY, Arshavsky VY. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J Cell Biol. 2015;211:495–502. doi: 10.1083/jcb.201508093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Nour M, Ritter LM, Goldberg AF, Fliesler SJ, Naash MI. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum Mol Genet. 2004;13:2075–2087. doi: 10.1093/hmg/ddh211. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Stricker HM, Naash MI. Role of the second intradiscal loop of peripherin/rds in homo and hetero associations. Biochem. 2005;44:4897–4904. doi: 10.1021/bi048414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Berson EL, Rao VR, Oprian DD. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet. 1993;4:280–283. doi: 10.1038/ng0793-280. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Kajiwara K, Berson EL. Dominant and digenic mutations in the peripherin/RDS and ROM1 genes in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38:1972–1982. [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Dudley PA, Anderson RE. Phospholipid transfer protein from bovine retina with high activity towards retinal rod disc membranes. FEBS Lett. 1978;95:57–60. doi: 10.1016/0014-5793(78)80051-9. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Roorda A, Navani M, Vishweswaraiah S, Syed R, Soudry S, Ratnam K, Gudiseva HV, Lee P, Gaasterland T, Ayyagari R. Identification of a novel mutation in the CDHR1 gene in a family with recessive retinal degeneration. Arch Ophthalmol. 2012;130:1301–1308. doi: 10.1001/archophthalmol.2012.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Edrington TC, Lapointe R, Yeagle PL, Gretzula CL, Boesze-Battaglia K. Peripherin-2: an intracellular analogy to viral fusion proteins. Biochem. 2007;46:3605–3613. doi: 10.1021/bi061820c. [DOI] [PubMed] [Google Scholar]
- Eidinger O, Leibu R, Newman H, Rizel L, Perlman I, Ben-Yosef T. An intronic deletion in the PROM1 gene leads to autosomal recessive cone-rod dystrophy. Mol Vis. 2015;21:1295–1306. [PMC free article] [PubMed] [Google Scholar]
- Falk G, Fatt P. Changes in structure of the disks of retinal rods in hypotonic solutions. J Cell Sci. 1973;13:787–797. doi: 10.1242/jcs.13.3.787. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586–8596. doi: 10.1074/jbc.M210640200. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Joester A, Missol-Kolka E, Hellwig A, Huttner WB, Corbeil D. Identification of novel Prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J Cell Sci. 2004;117:4301–4311. doi: 10.1242/jcs.01315. [DOI] [PubMed] [Google Scholar]
- Fetter RD, Corless JM. Morphological components associated with frog cone outer segment disc margins. Invest Ophthalmol Vis Sci. 1987;28:646–657. [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Schroepfer GJ., Jr Sterol composition of bovine retinal rod outer segment membranes and whole retinas. Biochim Biophys Acta. 1982;711:138–148. doi: 10.1016/0005-2760(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Frederick JM, Krasnoperova NV, Hoffmann K, Church-Kopish J, Ruther K, Howes K, Lem J, Baehr W. Mutant rhodopsin transgene expression on a null background. Invest Ophthalmol Vis Sci. 2001;42:826–833. [PubMed] [Google Scholar]
- Fu Q, Wang F, Wang H, Xu F, Zaneveld JE, Ren H, Keser V, Lopez I, Tuan HF, Salvo JS, Wang X, Zhao L, Wang K, Li Y, Koenekoop RK, Chen R, Sui R. Next-generation sequencing-based molecular diagnosis of a Chinese patient cohort with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54:4158–4166. doi: 10.1167/iovs.13-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell. 2012;151:1029–1041. doi: 10.1016/j.cell.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–175. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Fales LM, Hurley JB, Khattree N. Folding and subunit assembly of photoreceptor peripherin/rds is mediated by determinants within the extracellular/intradiskal EC2 domain: implications for heterogeneous molecular pathologies. J Biol Chem. 2001;276:42700–42706. doi: 10.1074/jbc.M107511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF, Loewen CJ, Molday RS. Cysteine residues of photoreceptor peripherin/rds: role in subunit assembly and autosomal dominant retinitis pigmentosa. Biochem. 1998;37:680–685. doi: 10.1021/bi972036i. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Defective subunit assembly underlies a digenic form of retinitis pigmentosa linked to mutations in peripherin/rds and rom-1. Proc Natl Acad Sci USA. 1996a;93:13726–13730. doi: 10.1073/pnas.93.24.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: hydrodynamic evidence for a tetrameric quaternary structure. Biochem. 1996b;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Moritz OL, Molday RS. Heterologous expression of photoreceptor peripherin/rds and Rom-1 in COS-1 cells: assembly, interactions, and localization of multisubunit complexes. Biochem. 1995;34:14213–14219. doi: 10.1021/bi00043a028. [DOI] [PubMed] [Google Scholar]
- Goldberg AFX. Essential tetraspanin functions in the vertebrate retina. In: Berditchevski F, Rubinstein E, editors. Tetraspanins. Springer Science+Business Media; Dordretcht: 2013. pp. 321–344. [Google Scholar]
- Goren MA, Morizumi T, Menon I, Joseph JS, Dittman JS, Cherezov V, Stevens RC, Ernst OP, Menon AK. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat Commun. 2014;5:5115. doi: 10.1038/ncomms6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, Korenyak DA, Shukolyukov SA, Zueva LV. Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Mol Vis. 2009;15:1717–1729. [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Kozlov MM. Interplay of proteins and lipids in generating membrane curvature. Curr Opin Cell Biol. 2010;22:430–436. doi: 10.1016/j.ceb.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunkel M, Schoneberg J, Alkhaldi W, Irsen S, Noe F, Kaupp UB, Al-Amoudi A. Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure. 2015;23:628–638. doi: 10.1016/j.str.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Gurudev N, Florek M, Corbeil D, Knust E. Prominent role of prominin in the retina. Prominin-1 (Cd133): New Insights on Stem & Cancer Stem Cell Biology. 2013;777:55–71. doi: 10.1007/978-1-4614-5894-4_4. [DOI] [PubMed] [Google Scholar]
- Gurudev N, Yuan M, Knust E. Chaoptin, prominin, eyes shut and crumbs form a genetic network controlling the apical compartment of Drosophila photoreceptor cells. Biol Open. 2014;3:332–341. doi: 10.1242/bio.20147310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri M, Knox BE. Rhodopsin mutant P23H destabilizes rod photoreceptor disk membranes. PLoS One. 2012;7:e30101. doi: 10.1371/journal.pone.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri M, Knox BE, Ahmadi A. Modeling the flexural rigidity of rod photoreceptors. Biophys J. 2013;104:300–312. doi: 10.1016/j.bpj.2012.11.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale IL, Fisher SK, Matsumoto B. The actin network in the ciliary stalk of photoreceptors functions in the generation of new outer segment discs. J Comp Neurol. 1996;376:128–142. doi: 10.1002/(SICI)1096-9861(19961202)376:1<128::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Han Z, Anderson DW, Papermaster DS. Prominin-1 localizes to the open rims of outer segment lamellae in Xenopus laevis rod and cone photoreceptors. Invest Ophthalmol Vis Sci. 2012;53:361–373. doi: 10.1167/iovs.11-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Papermaster DS. Identification of three prominin homologs and characterization of their messenger RNA expression in Xenopus laevis tissues. Mol Vis. 2011;17:1381–1396. [PMC free article] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RK, Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41:701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Heller J, Ostwald TJ, Bok D. The osmotic behavior of rod photoreceptor outer segment discs. J Cell Biol. 1971;48:633–649. doi: 10.1083/jcb.48.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RH, Li Z, Abd El Aziz MM, Mackay DS, Eljinini MA, Zeidan M, Moore AT, Bhattacharya SS, Webster AR. Biallelic mutation of protocadherin-21 (PCDH21) causes retinal degeneration in humans. Mol Vis. 2010;16:46–52. [PMC free article] [PubMed] [Google Scholar]
- Hessel E, Heck M, Muller P, Herrmann A, Hofmann KP. Signal transduction in the visual cascade involves specific lipid-protein interactions. J Biol Chem. 2003;278:22853–22860. doi: 10.1074/jbc.M302747200. [DOI] [PubMed] [Google Scholar]
- Hessel E, Herrmann A, Muller P, Schnetkamp PP, Hofmann KP. The transbilayer distribution of phospholipids in disc membranes is a dynamic equilibrium evidence for rapid flip and flop movement. Eur J Biochem. 2000;267:1473–1483. doi: 10.1046/j.1432-1327.2000.01147.x. [DOI] [PubMed] [Google Scholar]
- Hessel E, Muller P, Herrmann A, Hofmann KP. Light-induced reorganization of phospholipids in rod disc membranes. J Biol Chem. 2001;276:2538–2543. doi: 10.1074/jbc.M009061200. [DOI] [PubMed] [Google Scholar]
- Hofmann L, Palczewski K. The G protein-coupled receptor rhodopsin: a historical perspective. Meth Mol Biol. 2015;1271:3–18. doi: 10.1007/978-1-4939-2330-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Korenbrot JI. Longitudinal diffusion in retinal rod and cone outer segment cytoplasm: the consequence of cell structure. Biophys J. 2004;86:2566–2582. doi: 10.1016/S0006-3495(04)74312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J, Reiner O. Doublecortin, a stabilizer of microtubules. Hum Mol Genet. 1999;8:1599–1610. doi: 10.1093/hmg/8.9.1599. [DOI] [PubMed] [Google Scholar]
- Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunological epitope. Cell Motil Cytoskel. 1990;17:329–344. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Husain N, Pellikka M, Hong H, Klimentova T, Choe KM, Clandinin TR, Tepass U. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev Cell. 2006;11:483–493. doi: 10.1016/j.devcel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M, Pfeifer A, Gerstner A, Yau KW, Biel M. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci. 2005;25:130–138. doi: 10.1523/JNEUROSCI.3764-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili JN. Intermolecular and Surface Forces. Academic Press; Oxford, UK: 2011. [Google Scholar]
- Jiang L, Wei Y, Ronquillo CC, Marc RE, Yoder BK, Frederick JM, Baehr W. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J Biol Chem. 2015;290:12765–12778. doi: 10.1074/jbc.M115.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiko C, Davies KM, Shinzawa-Itoh K, Tani K, Maeda S, Mills DJ, Tsukihara T, Fujiyoshi Y, Kuhlbrandt W, Gerle C. Bovine F1Fo ATP synthase monomers bend the lipid bilayer in 2D membrane crystals. Elife. 2015;4:e06119. doi: 10.7554/eLife.06119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno D, Feiner L, Lillo C, Teofilo K, Goldstein LS, Pierce EA, Williams DS. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci. 2006a;47:5039–5046. doi: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno D, Lillo C, Roberts EA, Goldstein LS, Williams DS. Kinesin-2 and photoreceptor cell death: requirement of motor subunits. Exp Eye Res. 2006b;82:351–353. doi: 10.1016/j.exer.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kajimura N, Harada Y, Usukura J. High-resolution freeze-etching replica images of the disk and the plasma membrane surfaces in purified bovine rod outer segments. J Electron Microsc (Tokyo) 2000;49:691–697. doi: 10.1093/oxfordjournals.jmicro.a023860. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Sandberg MA, Berson EL, Dryja TP. A null mutation in the human peripherin/RDS gene in a family with autosomal dominant retinitis punctata albescens. Nat Genet. 1993;3:208–212. doi: 10.1038/ng0393-208. [DOI] [PubMed] [Google Scholar]
- Kaplan MW. Disk membrane initiation and insertion are not required for axial disk displacement in Xenopus laevis rod outer segments. Curr Eye Res. 1998;17:73–78. doi: 10.1076/ceyr.17.1.73.5248. [DOI] [PubMed] [Google Scholar]
- Kaplan MW, Iwata RT, Sears RC. Lengths of immunolabeled ciliary microtubules in frog photoreceptor outer segments. Exp Eye Res. 1987;44:623–632. doi: 10.1016/s0014-4835(87)80134-3. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kaushal S, Khorana HG. Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochem. 1994;33:6121–6128. doi: 10.1021/bi00186a011. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- Kedzierski W, Moghrabi WN, Allen AC, Jablonski-Stiemke MM, Azarian SM, Bok D, Travis GH. Three homologs of rds/peripherin in Xenopus laevis photoreceptors that exhibit covalent and non-covalent interactions. J Cell Sci. 1996;109(Pt 10):2551–2560. doi: 10.1242/jcs.109.10.2551. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Nusinowitz S, Birch D, Clarke G, McInnes RR, Bok D, Travis GH. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 2001;98:7718–7723. doi: 10.1073/pnas.141124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski W, Weng J, Travis GH. Analysis of the rds/peripherin.rom1 complex in transgenic photoreceptors that express a chimeric protein. J Biol Chem. 1999;274:29181–29187. doi: 10.1074/jbc.274.41.29181. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–2138. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Tsybovsky Y, Campuzano ID, Schnier PD, Engel A, Palczewski K. Structural and functional analysis of the native peripherin-ROM1 complex isolated from photoreceptor cells. J Biol Chem. 2013;288:36272–36284. doi: 10.1074/jbc.M113.520700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattree N, Ritter LM, Goldberg AF. Membrane curvature generation by a C-terminal amphipathic helix in peripherin-2/rds, a tetraspanin required for photoreceptor sensory cilium morphogenesis. J Cell Sci. 2013;126:4659–4670. doi: 10.1242/jcs.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney MS, Fisher SK. The photoreceptors and pigment epithelim of the adult Xenopus retina: morphology and outer segment renewal. Proc R Soc Lond B Biol Sci. 1978a;201:131–147. doi: 10.1098/rspb.1978.0036. [DOI] [PubMed] [Google Scholar]
- Kinney MS, Fisher SK. The photoreceptors and pigment epithelium of the larval Xenopus retina: morphogenesis and outer segment renewal. Proc R Soc Lond B Biol Sci. 1978b;201:149–167. doi: 10.1098/rspb.1978.0037. [DOI] [PubMed] [Google Scholar]
- Kohl S, Christ-Adler M, Apfelstedt-Sylla E, Kellner U, Eckstein A, Zrenner E, Wissinger B. RDS/peripherin gene mutations are frequent causes of central retinal dystrophies. J Med Genet. 1997;34:620–626. doi: 10.1136/jmg.34.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Qin M, Mizota A, Kondo M, Hayashi H, Hayashi K, Oshima K, Tahira T, Hayashi K. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest Ophthalmol Vis Sci. 2004;45:4433–4439. doi: 10.1167/iovs.04-0544. [DOI] [PubMed] [Google Scholar]
- Korschen HG, Beyermann M, Muller F, Heck M, Vantler M, Koch KW, Kellner R, Wolfrum U, Bode C, Hofmann KP, Kaupp UB. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature. 1999;400:761–766. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]
- Korschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Muller F, Dose A, Godde M. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- Kwok MC, Holopainen JM, Molday LL, Foster LJ, Molday RS. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteom. 2008;7:1053–1066. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci. 2007;8:960–975. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, McNaughton PA, Yau KW. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5137–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- Laties AM, Bok D, Liebman P. Procion yellow: a marker dye for outer segment disc patency and for rod renewal. Exp Eye Res. 1976;23:139–148. doi: 10.1016/0014-4835(76)90197-4. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973;58:650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Matsumoto B, Fisher SK. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:2404–2416. [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Maeda T, Maeda A, Modzelewska A, Filipek S, Saperstein DA, Engel A, Palczewski K. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem. 2004;279:48189–48196. doi: 10.1074/jbc.M408362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Liebman PA, Entine G. Visual pigments of frog and tadpole (Rana pipiens) Vision Res. 1968;8:761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Burnside B. Retina-specific protein fascin 2 is an actin cross-linker associated with actin bundles in photoreceptor inner segments and calycal processes. Invest Ophthalmol Vis Sci. 2007;48:1380–1388. doi: 10.1167/iovs.06-0763. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Collin RW, Cremers FP, den Hollander AI, van den Born LI, Pierce EA. Expression of wild-type Rp1 protein in Rp1 knock-in mice rescues the retinal degeneration phenotype. PLoS One. 2012;7:e43251. doi: 10.1371/journal.pone.0043251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lyubarsky A, Skalet JH, Pugh EN, Jr, Pierce EA. RP1 is required for the correct stacking of outer segment discs. Invest Ophthalmol Vis Sci. 2003;44:4171–4183. doi: 10.1167/iovs.03-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Saveliev A, Pierce EA. The severity of retinal degeneration in Rp1h gene-targeted mice is dependent on genetic background. Invest Ophthalmol Vis Sci. 2009;50:1566–1574. doi: 10.1167/iovs.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteom. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhou J, Daiger SP, Farber DB, Heckenlively JR, Smith JE, Sullivan LS, Zuo J, Milam AH, Pierce EA. Identification and subcellular localization of the RP1 protein in human and mouse photoreceptors. Invest Ophthalmol Vis Sci. 2002;43:22–32. [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zuo J, Pierce EA. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci. 2004;24:6427–6436. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Williams DS. Coincident onset of expression of myosin VIIa and opsin in the cilium of the developing photoreceptor cell. Exp Eye Res. 2001;72:351–355. doi: 10.1006/exer.2000.0963. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu TH, Stowe S, Matsushita A, Arikawa K, Naash MI, Williams DS. Defective phototransductive disk membrane morphogenesis in transgenic mice expressing opsin with a mutated N-terminal domain. J Cell Sci. 1997;110(Pt 20):2589–2597. doi: 10.1242/jcs.110.20.2589. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Skiba NP, Arshavsky VY. Proteasome overload is a common stress factor in multiple forms of inherited retinal degeneration. Proc Natl Acad Sci U S A. 2013;110:9986–9991. doi: 10.1073/pnas.1305521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–5378. doi: 10.1074/jbc.275.8.5370. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Moritz OL, Molday RS. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J Biol Chem. 2001;276:22388–22396. doi: 10.1074/jbc.M011710200. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Moritz OL, Tam BM, Papermaster DS, Molday RS. The role of subunit assembly in peripherin-2 targeting to rod photoreceptor disk membranes and retinitis pigmentosa. Mol Biol Cell. 2003;14:3400–3413. doi: 10.1091/mbc.E03-02-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Jimeno D, Khanobdee K, Song X, Chen B, Nusinowitz S, Williams DS. Dysfunction of heterotrimeric kinesin-2 in rod photoreceptor cells and the role of opsin mislocalization in rapid cell death. Mol Biol Cell. 2010;21:4076–4088. doi: 10.1091/mbc.E10-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Wen XH, Michaud NA, Covington HI, DiBenedetto E, Hamm HE, Lem J, Caruso G. Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One. 2012;7:e37832. doi: 10.1371/journal.pone.0037832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanson KM, Lem J. Rhodopsin-mediated retinitis pigmentosa. Prog Mol Biol Transl Sci. 2009;88:1–31. doi: 10.1016/S1877-1173(09)88001-0. [DOI] [PubMed] [Google Scholar]
- Marsh D. Elastic curvature constants of lipid monolayers and bilayers. Chem Phys Lipids. 2006;144:146–159. doi: 10.1016/j.chemphyslip.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- Matsumoto B, Besharse JC. Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Invest Ophthalmol Vis Sci. 1985;26:628–635. [PubMed] [Google Scholar]
- Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Roper K, Weigmann A, Huttner WB, Denton MJ. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9:27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Astle D. Photoreceptor number and outer segment disk membrane surface area in the retina of the rat: stereological data for whole organ and average photoreceptor cell. J Neurocytol. 1997;26:53–61. doi: 10.1023/a:1018563409196. [DOI] [PubMed] [Google Scholar]
- Mazzolini M, Facchetti G, Andolfi L, Proietti Zaccaria R, Tuccio S, Treu J, Altafini C, Di Fabrizio EM, Lazzarino M, Rapp G, Torre V. The phototransduction machinery in the rod outer segment has a strong efficacy gradient. Proc Natl Acad Sci USA. 2015;112:E2715–2724. doi: 10.1073/pnas.1423162112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Safa H, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res. 2014;126:51–60. doi: 10.1016/j.exer.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Membrane curvature at a glance. J Cell Sci. 2015;128:1065–1070. doi: 10.1242/jcs.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- McNally N, Kenna PF, Rancourt D, Ahmed T, Stitt A, Colledge WH, Lloyd DG, Palfi A, O’Neill B, Humphries MM, Humphries P, Farrar GJ. Murine model of autosomal dominant retinitis pigmentosa generated by targeted deletion at codon 307 of the rds-peripherin gene. Hum Mol Genet. 2002;11:1005–1016. doi: 10.1093/hmg/11.9.1005. [DOI] [PubMed] [Google Scholar]
- Menon I, Huber T, Sanyal S, Banerjee S, Barre P, Canis S, Warren JD, Hwa J, Sakmar TP, Menon AK. Opsin is a phospholipid flippase. Curr Biol. 2011;21:149–153. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Gaillard MC, Escher P, Tiab L, Bedell M, Borruat FX, Barthelmes D, Carmona R, Zhang K, White E, McClements M, Robson AG, Holder GE, Bradshaw K, Hunt DM, Webster AR, Moore AT, Schorderet DF, Munier FL. The PROM1 mutation p.R373C causes an autosomal dominant bull’s eye maculopathy associated with rod, rod-cone, and macular dystrophy. Invest Ophthalmol Vis Sci. 2010;51:4771–4780. doi: 10.1167/iovs.09-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Zong X, Becirovic E, Hammelmann V, Wein T, Wanner KT, Biel M. The glutamic acid-rich protein is a gating inhibitor of cyclic nucleotide-gated channels. J Neurosci. 2011;31:133–141. doi: 10.1523/JNEUROSCI.4735-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic L, Weiss LE, Yoon J, Roth TL, Su YS, Sahl SJ, Scott MP, Moerner WE. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc Natl Acad Sci USA. 2015;112:8320–8325. doi: 10.1073/pnas.1510094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Molday RS, Molday L. Isolation and characterization of rod outer segment disk and plasma membranes. In: Hargrave PA, editor. Photoreceptor Cells. Elsevier; New York: 1993. pp. 131–150. [Google Scholar]
- Molday RS, Molday LL. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol. 1987;105:2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Moritz OL. Photoreceptors at a glance. J Cell Sci. 2015;128:4039–4045. doi: 10.1242/jcs.175687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MF, Robertson JD. The fine structure of some retinal photoreceptors. J Biophys Biochem Cytol. 1960;7:87–92. doi: 10.1083/jcb.7.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz OL, Molday RS. Molecular cloning, membrane topology, and localization of bovine rom-1 in rod and cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1996;37:352–362. [PubMed] [Google Scholar]
- Morrison JD. Morphogenesis of photoreceptor outer segments in the developing kitten retina. J Anat. 1983;136:521–533. [PMC free article] [PubMed] [Google Scholar]
- Muradov H, Boyd KK, Haeri M, Kerov V, Knox BE, Artemyev NO. Characterization of human cone phosphodiesterase-6 ectopically expressed in Xenopus laevis rods. J Biol Chem. 2009;284:32662–32669. doi: 10.1074/jbc.M109.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naash MI, Hollyfield JG, al-Ubaidi MR, Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci USA. 1993;90:5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr Biol. 2002;12:421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- Najafi M, Haeri M, Knox BE, Schiesser WE, Calvert PD. Impact of signaling microcompartment geometry on GPCR dynamics in live retinal photoreceptors. J Gen Physiol. 2012a;140:249–266. doi: 10.1085/jgp.201210818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Maza NA, Calvert PD. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci USA. 2012b;109:203–208. doi: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Sircar M, Finnemann SC. Novel role for alphavbeta5-integrin in retinal adhesion and its diurnal peak. Am J Physiol Cell Physiol. 2006;290:C1256–1262. doi: 10.1152/ajpcell.00480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet I, Tian GL, Imanishi Y. Submembrane assembly and renewal of rod photoreceptor cGMP-gated channel: insight into the actin-dependent process of outer segment morphogenesis. J Neurosci. 2014;34:8164–8174. doi: 10.1523/JNEUROSCI.1282-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000;196:245–313. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Mahato S, Mustill W, Tipping C, Bhattacharya SS, Zelhof AC. Cross species analysis of Prominin reveals a conserved cellular role in invertebrate and vertebrate photoreceptor cells. Dev Biol. 2012;371:312–320. doi: 10.1016/j.ydbio.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Nikopoulos K, Avila-Fernandez A, Corton M, Lopez-Molina MI, Perez-Carro R, Bontadelli L, Di Gioia SA, Zurita O, Garcia-Sandoval B, Rivolta C, Ayuso C. Identification of two novel mutations in CDHR1 in consanguineous Spanish families with autosomal recessive retinal dystrophy. Sci Rep. 2015;5:13902. doi: 10.1038/srep13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SE. Receptor cell outer segment development and ultrastructure of the disk membranes in the retina of the tadpole (Rana Pipiens) J Ultrastruct Res. 1964;11:581–602. doi: 10.1016/s0022-5320(64)80084-8. [DOI] [PubMed] [Google Scholar]
- Nilsson SE. The ultrastructure of the receptor outer segments in the retina of the leopard frog (Rana Pipiens) J Ultrastruct Res. 1965;12:207–231. doi: 10.1016/s0022-5320(65)80016-8. [DOI] [PubMed] [Google Scholar]
- Obata S, Usukura J. Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell Tissue Res. 1992;269:39–48. doi: 10.1007/BF00384724. [DOI] [PubMed] [Google Scholar]
- Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–584. doi: 10.1146/annurev-biochem-072711-164947. [DOI] [PubMed] [Google Scholar]
- Olson A, Pugh EN., Jr Diffusion coefficient of cyclic GMP in salamander rod outer segments estimated with two fluorescent probes. Biophys J. 1993;65:1335–1352. doi: 10.1016/S0006-3495(93)81177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Miyashita M, Ono Y, Okazaki H, Watanabe S, Tochio N, Kigawa T, Nishimura C. Comparison of residual alpha- and beta-structures between two intrinsically disordered proteins by using NMR. Biochim Biophys Acta. 2015;1854:229–238. doi: 10.1016/j.bbapap.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Ostergaard E, Batbayli M, Duno M, Vilhelmsen K, Rosenberg T. Mutations in PCDH21 cause autosomal recessive cone-rod dystrophy. J Med Genet. 2010;47:665–669. doi: 10.1136/jmg.2009.069120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287:1612–1619. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Papermaster DS. Preparation of retinal rod outer segments. Meth Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Salinas RY, Baker SA, Arshavsky VY. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res. 2013;36:24–51. doi: 10.1016/j.preteyeres.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Spencer WJ, Lieu EC, Arshavsky VY. Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. Elife. 2015;4 doi: 10.7554/eLife.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentia DC, Hosier S, Cote RH. The glutamic acid-rich protein-2 (GARP2) is a high affinity rod photoreceptor phosphodiesterase (PDE6)-binding protein that modulates its catalytic properties. J Biol Chem. 2006;281:5500–5505. doi: 10.1074/jbc.M507488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol. 1983;96:265–276. doi: 10.1083/jcb.96.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Quinn T, Meehan T, McGee TL, Berson EL, Dryja TP. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nat Genet. 1999;22:248–254. doi: 10.1038/10305. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001;276:48009–48016. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BA, Sandoval IM, Chan F, Nichols R, Roman-Sanchez R, Wensel TG, Wilson JH. Rhodopsin gene expression determines rod outer segment size and rod cell resistance to a dominant-negative neurodegeneration mutant. PLoS One. 2012;7:e49889. doi: 10.1371/journal.pone.0049889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BA, Sandoval IM, Chan F, Simons DL, Wu SM, Wensel TG, Wilson JH. Mislocalization and degradation of human P23H-rhodopsin-GFP in a knockin mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:9728–9736. doi: 10.1167/iovs.11-8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA. Lipid trafficking sans vesicles: where, why, how? Cell. 2010;143:870–874. doi: 10.1016/j.cell.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN., Jr Photoreceptor disc morphogenesis: The classical evagination model prevails. J Cell Biol. 2015;211:491–493. doi: 10.1083/jcb.201510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, Bok D, Travis GH. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49:3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by (2)H NMR and X-ray diffraction. J Am Chem Soc. 2005;127:1576–1588. doi: 10.1021/ja046453b. [DOI] [PubMed] [Google Scholar]
- Rakshit T, Senapati S, Sinha S, Whited AM, Park PS. Rhodopsin forms nanodomains in rod outer segment disc membranes of the cold-blooded Xenopus laevis. PLoS One. 2015;10:e0141114. doi: 10.1371/journal.pone.0141114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Chen J, Nathans J. Proteolytic shedding of the extracellular domain of photoreceptor cadherin. Implications for outer segment assembly. J Biol Chem. 2004;279:42202–42210. doi: 10.1074/jbc.M407928200. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Williams J, Cooke C, Savchenko A, Lyubarsky A, Pugh EN, Nathans J. A photoreceptor-specific cadherin is essential for the structural integrity of the outer segment and for photoreceptor survival. Neuron. 2001;32:775–786. doi: 10.1016/s0896-6273(01)00531-1. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Arakawa T, Goldberg AFX. Predicted and measured disorder in peripherin/rds, a retinal tetraspanin. Protein Pept Lett. 2005;12:677–686. doi: 10.2174/0929866054696217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Boesze-Battaglia K, Tam BM, Moritz OL, Khattree N, Chen SC, Goldberg AF. Uncoupling of photoreceptor peripherin/rds fusogenic activity from biosynthesis, subunit assembly, and targeting: a potential mechanism for pathogenic effects. J Biol Chem. 2004;279:39958–39967. doi: 10.1074/jbc.M403943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Khattree N, Tam B, Moritz OL, Schmitz F, Goldberg AF. In situ visualization of protein interactions in sensory neurons: glutamic acid-rich proteins (GARPs) play differential roles for photoreceptor outer segment scaffolding. J Neurosci. 2011;31:11231–11243. doi: 10.1523/JNEUROSCI.2875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Deretic D, Bazan NG, Papermaster DS. Post-Golgi vesicles cotransport docosahexaenoyl-phospholipids and rhodopsin during frog photoreceptor membrane biogenesis. J Biol Chem. 1997;272:10491–10497. doi: 10.1074/jbc.272.16.10491. [DOI] [PubMed] [Google Scholar]
- Rohlich P. The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell Tissue Res. 1975;161:421–430. doi: 10.1007/BF00220009. [DOI] [PubMed] [Google Scholar]
- Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Cowley GS, McGee TL, Sandberg MA, Berson EL, Dryja TP. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992;1:209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci USA. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saishin Y, Ishikawa R, Ugawa S, Guo W, Ueda T, Morimura H, Kohama K, Shimizu H, Tano Y, Shimada S. Retinal fascin: functional nature, subcellular distribution, and chromosomal localization. Invest Ophthalmol Vis Sci. 2000;41:2087–2095. [PubMed] [Google Scholar]
- Sakami S, Maeda T, Bereta G, Okano K, Golczak M, Sumaroka A, Roman AJ, Cideciyan AV, Jacobson SG, Palczewski K. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J Biol Chem. 2011;286:10551–10567. doi: 10.1074/jbc.M110.209759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale WS, Besharse JC, Piperno G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil Cytoskel. 1988;9:243–253. doi: 10.1002/cm.970090306. [DOI] [PubMed] [Google Scholar]
- Salinas RY, Baker SA, Gospe SM, III, Arshavsky VY. A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS One. 2013;8:e54292. doi: 10.1371/journal.pone.0054292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Sarfare S, McKeown AS, Messinger J, Rubin G, Wei H, Kraft TW, Pittler SJ. Overexpression of rod photoreceptor glutamic acid rich protein 2 (GARP2) increases gain and slows recovery in mouse retina. Cell Commun Sig. 2014;12:67. doi: 10.1186/s12964-014-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf JL. Dependence of the single photon response on longitudinal position of absorption in toad rod outer segments. J Physiol. 1983;343:147–159. doi: 10.1113/jphysiol.1983.sp014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneberg J, Heck M, Hofmann KP, Noe F. Explicit spatiotemporal simulation of receptor-G protein coupling in rod cell disk membranes. Biophys J. 2014;107:1042–1053. doi: 10.1016/j.bpj.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys J. 2006;90:212–227. doi: 10.1529/biophysj.105.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senin II, Hoppner-Heitmann D, Polkovnikova OO, Churumova VA, Tikhomirova NK, Philippov PP, Koch KW. Recoverin and rhodopsin kinase activity in detergent-resistant membrane rafts from rod outer segments. J Biol Chem. 2004;279:48647–48653. doi: 10.1074/jbc.M402516200. [DOI] [PubMed] [Google Scholar]
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Hu J, Kozlov MM, Rapoport TA. Mechanisms shaping the membranes of cellular organelles. Annu Rev Cell Dev Biol. 2009;25:329–354. doi: 10.1146/annurev.cellbio.042308.113324. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sjostrand FS. The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol. 1953;42:15–44. doi: 10.1002/jcp.1030420103. [DOI] [PubMed] [Google Scholar]
- Sjostrand FS. Fine structure of cytoplasm: the organization of membranous layers. Rev Mod Phys. 1959;31:301–318. [Google Scholar]
- Skiba NP, Spencer WJ, Salinas RY, Lieu EC, Thompson JW, Arshavsky VY. Proteomic identification of unique photoreceptor disc components reveals the presence of PRCD, a protein linked to retinal degeneration. J Proteome Res. 2013;12:3010–3018. doi: 10.1021/pr4003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Sandstrom MM, Leung IY, Zucker CL, Neuringer M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2815–2818. [PubMed] [Google Scholar]
- Song D, Grieco S, Li Y, Hunter A, Chu S, Zhao L, Song Y, DeAngelis RA, Shi LY, Liu Q, Pierce EA, Nishina PM, Lambris JD, Dunaief JL. A murine RP1 missense mutation causes protein mislocalization and slowly progressive photoreceptor degeneration. Am J Pathol. 2014;184:2721–2729. doi: 10.1016/j.ajpath.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Gaudet R, Corey DP. Sorting out a promiscuous superfamily: towards cadherin connectomics. Trends Cell Biol. 2014;24:524–536. doi: 10.1016/j.tcb.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Stone WL, Farnsworth CC, Dratz EA. A reinvestigation of the fatty acid content of bovine, rat and frog retinal rod outer segments. Exp Eye Res. 1979;28:387–397. doi: 10.1016/0014-4835(79)90114-3. [DOI] [PubMed] [Google Scholar]
- Strandberg E, Tiltak D, Ehni S, Wadhwani P, Ulrich AS. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamem.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Stricker HM, Ding XQ, Quiambao A, Fliesler SJ, Naash MI. The Cys214-->Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice. Biochem J. 2005;388:605–613. doi: 10.1042/BJ20041960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck MW, Conley SM, Naash MI. The Y141C knockin mutation in RDS leads to complex phenotypes in the mouse. Hum Mol Genet. 2014;23:6260–6274. doi: 10.1093/hmg/ddu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck MW, Conley SM, Naash MI. PRPH2/RDS and ROM-1: Historical context, current views and future considerations. Prog Retin Eye Res. 2016;52:47–63. doi: 10.1016/j.preteyeres.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190:953–963. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Schneider BG, Agarwal N, Papermaster DS, Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL. Dark rearing rescues P23H rhodopsin-induced retinal degeneration in a transgenic Xenopus laevis model of retinitis pigmentosa: a chromophore-dependent mechanism characterized by production of N-terminally truncated mutant rhodopsin. J Neurosci. 2007;27:9043–9053. doi: 10.1523/JNEUROSCI.2245-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15:2027–2037. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Campbell M, Keaney J, Farrar GJ, Humphries MM, Kenna PF, Humphries P. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum Mol Genet. 2010;19:4421–4436. doi: 10.1093/hmg/ddq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Ropelewski P, Nemet I, Lee R, Lodowski KH, Imanishi Y. An unconventional secretory pathway mediates the cilia targeting of peripherin/rds. J Neurosci. 2014;34:992–1006. doi: 10.1523/JNEUROSCI.3437-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Groshan KR, Lloyd M, Bok D. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- Trivedi D, Colin E, Louie CM, Williams DS. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric kinesin-2. J Neurosci. 2012;32:10587–10593. doi: 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Tsybovsky Y, Orban T, Molday RS, Taylor D, Palczewski K. Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter. Structure. 2013;21:854–860. doi: 10.1016/j.str.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubridy N, Fontaine B, Eymard B. Congenital myopathies and congenital muscular dystrophies. Curr Opin Neurol. 2001;14:575–582. doi: 10.1097/00019052-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Usukura J, Yamada E. Molecular organization of the rod outer segment - a deep-etching study with rapid freezing using unfixed frog retina. Biomed Res (Tokyo) 1981;2:177–193. [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Fisher SK. The distribution of F-actin in cells isolated from vertebrate retinas. Exp Eye Res. 1987;44:393–406. doi: 10.1016/s0014-4835(87)80173-2. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Volland S, Hughes LC, Kong C, Burgess BL, Linberg KA, Luna G, Zhou ZH, Fisher SK, Williams DS. Three-dimensional organization of nascent rod outer segment disk membranes. Proc Natl Acad Sci USA. 2015;112:14870–14875. doi: 10.1073/pnas.1516309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res. 2014;38:1–19. doi: 10.1016/j.preteyeres.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang X, Zhang L, He F, Zhang G, Jamrich M, Wensel TG. Activation-dependent hindrance of photoreceptor G protein diffusion by lipid microdomains. J Biol Chem. 2008;283:30015–30024. doi: 10.1074/jbc.M803953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber PW, Howle LE, Murray MM, Corless JM. A simplified mass-transfer model for visual pigments in amphibian retinal-cone outer segments. Biophys J. 2011;100:525–534. doi: 10.1016/j.bpj.2010.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XH, Shen L, Brush RS, Michaud N, Al-Ubaidi MR, Gurevich VV, Hamm HE, Lem J, Dibenedetto E, Anderson RE, Makino CL. Overexpression of rhodopsin alters the structure and photoresponse of rod photoreceptors. Biophys J. 2009;96:939–950. doi: 10.1016/j.bpj.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited AM, Park PS. Nanodomain organization of rhodopsin in native human and murine rod outer segment disc membranes. Biochim Biophys Acta. 2015;1848:26–34. doi: 10.1016/j.bbamem.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res. 2002;42:455–462. doi: 10.1016/s0042-6989(01)00228-0. [DOI] [PubMed] [Google Scholar]
- Williams DS. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433–441. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J Comp Neurol. 1988;272:161–176. doi: 10.1002/cne.902720202. [DOI] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem. 2000;275:13191–13194. doi: 10.1074/jbc.c900853199. [DOI] [PubMed] [Google Scholar]
- Wu G, Hubbell WL. Phospholipid asymmetry and transmembrane diffusion in photoreceptor disc membranes. Biochem. 1993;32:879–888. doi: 10.1021/bi00054a020. [DOI] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, Marmorstein AD, Crabb JW, Hagstrom SA. Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46:4754–4761. doi: 10.1167/iovs.05-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, McMillan DR, Liberman MC, Li T. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet. 2010;6:e1000955. doi: 10.1371/journal.pgen.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen H, Zhao C, Chen Y, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S, Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT, Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118:2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Breslow DK, Koslover EF, Spakowitz AJ, Nelson WJ, Nachury MV. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- Yoshikami S, Robinson WE, Hagins WA. Topology of the outer segment membranes of retinal rods and cones revealed by a fluorescent probe. Science. 1974;185:1176–1179. doi: 10.1126/science.185.4157.1176. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–725. [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Oh H, Wilsch-Brauninger M, Missol-Kolka E, Jaszai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB, Corbeil D, Dewerchin M, Vinckier S, Moons L, Carmeliet P. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29:2297–2308. doi: 10.1523/JNEUROSCI.2034-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof AC, Hardy RW, Becker A, Zuker CS. Transforming the architecture of compound eyes. Nature. 2006;443:696–699. doi: 10.1038/nature05128. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Molday LL, Molday RS, Sarfare SS, Woodruff ML, Fain GL, Kraft TW, Pittler SJ. Knockout of GARPs and the beta-subunit of the rod cGMP-gated channel disrupts disk morphogenesis and rod outer segment structural integrity. J Cell Sci. 2009;122:1192–1200. doi: 10.1242/jcs.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]