Abstract
Recent cytogenetic and molecular investigations have improved our understanding of endometrial stromal tumors, including sarcomas (ESS), and helped redefine their classification into more pathogenetically meaningful categories. Because much more can be gained through such studies, we add information on another 22 ESS examined by karyotyping, PCR analysis, expression array analysis, and transcriptome sequencing. In spite of the known preference for certain pathogenetic pathways, we found considerable genetic heterogeneity in high‐grade (HG) as well as in low‐grade (LG) ESS. Not all HG tumors showed a YWHAE‐NUTM chimeric transcript and as many as six LGESS showed no hitherto known ESS‐related fusions. Among the transcripts identified by transcriptome sequencing and verified by Sanger sequencing, new variants of ZC3H7‐BCOR and its reciprocal BCOR‐ZC3H7 were identified as was involvement of the CREBBP and MLLT4 genes (both well known leukemia‐related genes) in two new fusions. FISH analysis identified a known EPC1‐PHF1 fusion which led to the identification of a new variant at the molecular level. The fact that around 70 genes were found differentially expressed, by microarray analysis, when comparing LGESS showing ESS‐related fusions with LGESS without such transcripts, underscores the biochemical importance of the observed genetic heterogeneity and hints that new subgroups/entities in LGESS still remain undiscovered. © 2016 The Authors. Genes, Chromosomes & Cancer Published by Wiley Periodicals, Inc.
INTRODUCTION
Endometrial stromal tumors (EST) are composed of cells resembling those of proliferative phase endometrial stroma. According to the most recent World Health Organization classification (WHO, 2014), four categories of EST exist depending on the tumors’ mitotic activity, vascular invasion, and prognosis: benign endometrial stromal nodules (ESN), low‐grade (LG) endometrial stromal sarcomas (ESS), high‐grade (HG) ESS, and undifferentiated endometrial/uterine sarcoma (UES/UUS). ESS, in its low‐ and high‐grade forms, account for <10% of uterine sarcomas (WHO, 2014).
About 60 ESS with chromosome abnormalities have been karyotyped and scientifically reported (Micci and Heim, 2015). Chromosomes 7 and 17 are recombined in the first genetic hallmark to be discovered in ESS, namely the translocation t(7;17)(p15;q11) (Fletcher et al., 1991; Sreekantaiah et al., 1991). Koontz et al. (2001) demonstrated that two zinc finger genes were fused by this translocation, the JAZF1 gene from chromosomal band 7p15 and SUZ12 (formerly known as JJAZ1) from 17q11. Chromosomal band 7p15‐21 was subsequently found rearranged with other partners than chromosome 17 in some ESS (Laxman et al., 1993; Iliszko et al., 1998; Gil‐Benso et al., 1999; Micci et al., 2003; Micci et al., 2006) suggesting that alternative, pathogenetically equivalent variant translocations exist. In the first such variant to be studied in molecular detail, a t(6;7), we found that JAZF1 was recombined with the PHF1 gene from chromosomal band 6p21 (Micci et al., 2006). Band 6p21 may also be rearranged in other ESS‐specific fusions with the EPC1 and MEAF6 genes from 10p11 and 1p34, respectively (Micci et al., 2006; Panagopoulos et al., 2012; Micci et al., 2014). Fusions of the JAZF1, SUZ12, and PHF1 genes appear to be frequent, although certainly not ubiquitous, in LGESS, but have also been found in other types of EST (Chiang et al., 2011).
Lately, another two fusion transcripts have also been identified in LGESS. ZC3H7B‐BCOR stems from an X;22‐translocation whereas MBTD1‐Cxorf67 is caused by a t(X;17) (Panagopoulos et al., 2013; Dewaele et al., 2014).
The cytogenetic literature contains altogether 19 cases with a t(10;17)(q22;p13), all of them belonging to the HGESS subtype with more aggressive clinical behavior. Lee et al. (2012a) described a YWHAE‐NUTM (previously known as YWHAE‐FAM22) chimeric fusion brought about by this rearrangement.
Gene expression profiles exist for 21 EST (Lee et al., 2012a; Davidson et al., 2013; Dewaele et al., 2014) of which six tumors were described as HGESS/UUS showing a 10;17‐translocation (Lee et al., 2012a; Dewaele et al., 2014), six were LGESS characterized by a specific 7;17‐translocation (Lee et al., 2012a; Dewaele et al., 2014), two cases were LGESS showing a t(X;17), and the remaining seven were LGESS with no karyotypic and/or genetic information (Davidson et al., 2013).
We wanted to increase the knowledge on ESS analyzing a series of 27 tumors (19 LGESS and eight HGESS) using different approaches that vary from standard techniques such as karyotyping and PCR to the more modern microarray and sequencing methodologies.
MATERIAL AND METHODS
Tumors
The material consisted of 27 samples from primary EST (Table 1) surgically removed at The Norwegian Radium Hospital. All tumors showed presence of endometrial differentiation. Eight of the tumors were diagnosed as HGESS whereas 18 were LGESS. HGESS are diagnosed based on morphology and the presence of diffuse (>70%) nuclear staining for cyclin D1 (WHO, 2014). Case 9 was an ESS showing areas with a mixture of LG and HG differentiation which changed from slide to slide. The case was described as not otherwise specified (NOS). The tumor biobank has been registered according to national legislation and the study has been approved by the Regional Committee for Medical Research Ethics South‐East, REK; project numbers S‐07194a and 2.2007.425.
Table 1.
Summary of the Data Obtained on 27 ESS Tumors by Karyotyping and Expression Analyses
| Casea | ESS subtype | RNA‐seq | Micro‐array | Karyotypeb | Fusion transcript |
|---|---|---|---|---|---|
| 1 | HG | yes | yes | 60∼70,add(1)(q32),i(6)(p10),dic(12;22)(p13;q13),add(19)(p13),+1∼2r,inc[cp9]/46,XX[10] |
YWHAE‐NUTM
ILF3‐CATSPERD RabGAP1L‐RBKS |
| 2 | HG | yes | yes | 53∼71 < 3n>,XXX,+i(1)(q10),+2,+2,‐3,‐4,‐5,‐6,+7,+7,‐8, add(9)(q13),‐11,‐12,‐13,‐14,‐15,‐15, −17,‐17,‐17,‐19,+20,+add(20)(p13),+21,+21,+11mar[cp14] |
GPR180‐DZIP1
WIPF2‐LASP1 |
| 3 | HG | yes | yes | 118∼125,del(1)(q11)x3,i(1)(q10)x2,add(2)(p13∼15),i(17)(q10),inc[cp5] |
RNF126‐NDUFA11
RPH3AL‐BZW2 |
| 4 | HG | yes | yes | nd | YWHAE‐NUTM |
| 5 | HG | yes | yes | nd | YWHAE‐NUTM |
| 6 | HG | yes | yes | nd |
YWHAE‐NUTM
MLL4‐RB1 |
| 7 | HG | yes | yes | nd | NRP2‐C2orf66 |
| 8 | HG | 46,XX,t(10;17)(q22;p13)[17]/46,XX[1] | YWHAE‐NUTM | ||
| 9 | NOS | yes | 46,X,?del(X)(p21)[2]/77∼80,XX,‐X,+3,‐6,+del(7)(p11),+8,+11,‐12,+16,‐18,+19,+20,+20, +21,+21[cp2]/46,XX[11] | EPC1‐PHF1 | |
| 10 | LG | yes | yes | 45,XX,‐10[16]/46,XX[1] | |
| 11 | LG | yes | 46,XX,t(7;17)(p15;q21)[10]/46,XX,idem,+13,‐15[3] | JAZF1‐SUZ12 | |
| 12 | LG | yes | 82∼90,XXXX,t(6;7)(q16;p15)x2,t(7;17)(p15;q12∼21)x2,add(11)(p15)x2,add(12)(q13), del(20)(q13),‐22,‐22,+mar[cp9] | JAZF1‐SUZ12 | |
| 13a | LG | yes | yes | 46,X,t(X;22)(p11;q13),t(3;4)(p23;q27)[8]/46,XX[8] | ZC3H7‐BCOR |
| 14 | LG | yes | yes | 46,XX,t(2;14)(q21;q24),‐3,add(3)(q21∼24),der(5)t(3;5)(q13∼21;p15),+del(5)(q13),der(6) t(5;6)(q13;q21),del(9)(p13),‐16,der(17)t(12;17)(q13;p13),‐22,+der(?;3)(?;p21),+mar[13] | GMPS‐SYNPR |
| 15 | LG | yes | yes | 47∼51,XX,+i(1)(q10),+2,+11,+13,+mar[cp9]/46,XX[6] | KDM2B‐CREBBP |
| 16a | LG | yes | 46,XX,t(1;6)(p32∼34;p21)[15] | MEAF6‐PHF1 | |
| 17a | LG | yes | yes | 42,X,dic(X;22)(p11;p11),der(1;9)(q25;p22∼24)ins(1;?)(q25;?),der(9)t(1;9)(q25;p22),‐10, der(13;15)(q10;q10),‐21,der(22)t(X;22)(p11;q13),+r[20] | ZC3H7‐BCOR |
| 18 | LG | yes | yes | nd | PXDN‐XIRP2 |
| 19 | LG | yes | yes | nd |
H2AFY‐HMHB1
WTIP‐BIRC6 |
| 20 | LG | yes | yes | nd |
ZC3H7B‐BCOR
c
BCOR‐ZC3H7B c |
| 21 | LG | yes | yes | nd | NAIP‐OCLN |
| 22a | LG | yes | 46,XX,inv(2)(p21q37),der(6)del(6)(p21)t(6;7)(q21;p15),der(7)t(6;7)(p21;p15)del(6)(q21)[11]/46,XX[4] | JAZF1‐PHF1 | |
| 23 | LG | yes | nd | JAZF1‐SUZ12 | |
| 24 | LG | yes | yes | 42∼45,XX,‐6,del(6)(q15),‐7,add(13)(q14),add(15)(q15),‐21,+r,+mar[cp9] | JAZF1‐PHF1 |
| 25a | LG | yes | 46,XX,inv(5)(p13∼p14q23∼q31)[13] | MEAF6‐PHF1 | |
| 26 | LG | yes | 45,XX,dic(7;14)(p11;p11),t(7;17)(p15;q21)[7] | JAZF1‐SUZ12 | |
| 27 | LG | 46,XX,ins(6;10)(p21;p?13p11),ins(10;6)(p11;p21p21),del(16)(q22)[18]/46,XX[1] | EPC1‐PHF1 c |
Case already published: cases 13 and 17 in Panagopoulos et al., 2013; case 16 in Panagopoulos et al., 2012; case 22 in Micci et al., 2006; case 25 in Micci et al., 2014.
nd: not done.
New variant of the fusion.
G‐banding and Karyotyping
The specimens intended for cytogenetic analysis (n = 18) were mechanically and enzymatically disaggregated and short‐term cultured as previously reported (Micci et al., 1999). The subsequent cytogenetic analysis and karyotypic description followed the recommendations of the International System for Human Cytogenetic Nomenclature (ISCN, 2009).
Molecular Investigations
RNA extraction
RNA was extracted from the tumors using Trizol reagent (Life Technologies) with a homogenizer (Omni THQ Digital Tissue Homogenizer, Kennesaw, GA). The RNA quality was evaluated using the Experion Automated Electrophoresis System (Bio‐Rad Laboratories, Hercules, CA). cDNA was synthesized using the iScript kit and random primers (Bio‐Rad Laboratories). All procedures were done according to the manufacturers’ recommendations.
Polymerase chain reaction (PCR)
Reverse transcriptase (RT) PCR was used to investigate the presence of known fusion transcripts in all the samples as well as to validate the results from RNA sequencing. The primers used are listed in Table 2. The conditions used for PCR reactions were as previously reported (Micci et al., 2006; Panagopoulos et al., 2012, 2013; Dewaele et al., 2014). All PCR products obtained were sent for direct sequencing (Sanger Sequencing) at GATC Biotech (http://www.gatc-biotech.com/en/sanger-services/lightrun-sequencing.html).
Table 2.
Overview of the Primers used in the PCR Assays for Detection of ESS‐specific Fusions
| Primer | Sequence 5′→3′ | Reported in |
|---|---|---|
| JAZF1‐357F | CCACAGCAGTGGAAGCCTTA | Micci et al., 2003 |
| EPC1‐1651F | CGCGGTGGAAGGGTCTTACTGGA | Micci et al., 2006 |
| MEAF6‐322F | CATTGGCAGGAGTTCAGGACCAGC | Panagopoulos et al., 2012 |
| ZC3H7B‐1190F | TGGACCCCTCCAAGAAGCTGGC | Panagopoulos et al., 2013 |
| ZC3H7B‐1250F | TGGACCCCTCCAAGAAGCTGGC | Present study |
| ZC3H7B‐1339F | TCGGAGACCCGGCTGGATGC | Present study |
| MBTD1_CXorf67_F | CTACAGCCTCCAGCATCACA | Dewaele et al., 2014 |
| X_17_nestedRT‐F1 | CATTTTGATGGATGGGAAGA | Dewaele et al., 2014 |
| X_17_nestedRT‐F2 | TGATCAGTGGGTAGACTGTGAGT | Dewaele et al., 2014 |
| YWHAEF1 | GCGGAGAACAGCCTAGTG | Present study |
| YWHAEF2 | CTTAATTCCCCTGACCGTGC | Present study |
| EPC1F4 | GGTGTATTGGATTTGCACGA | Present study |
| PHF1‐327R | AGCCCATCAGTCCATCTGGCCAG | Micci et al., 2006 |
| PHF1‐380R | GGACCAGACACACCTCCCTAGCACTG | Panagopoulos et al., 2012 |
| JJAZ1‐843R | CCGGGTTTTGTTTGATTGAGG | Micci et al., 2003 |
| BCOR‐3954R | TTGCCATCTGCTGCCGACACCT | Panagopoulos et al., 2013 |
| BCOR‐4201R | GAGGCAGCCTGGCAATCCTCTTCT | Present study |
| BCOR‐4048R | TGGGCGGAGAGCCGGAGAAC | Present study |
| MBTD1_CXorf67_R2 | CTCATCAGCTGACCCAGACA | Dewaele et al., 2014 |
| X_17_nestedRT‐R1 | CTCATCAGCTGACCCAGACA | Dewaele et al., 2014 |
| X_17_nestedRT‐R2 | CGCAGATTCAGGGCTTAGAC | Dewaele et al., 2014 |
| FAM22ABR1 | AGCCATCCTGTTCTGTCAC | Present study |
| FAM22ABR2 | GTGAACACAGACAGGGAGGT | Present study |
| PHF1R4 | TGGCAGTCCTGGTGATAAG | Present study |
Microarray
Twenty‐four samples were analyzed for gene expression. The microarray experiments were performed at the Norwegian Genomics Consortium in Oslo (http://oslo.genomics.no/) using the Illumina iScan which is based upon fluorescence detection of biotin‐labeled cRNA. The experiments were performed as previously described (Micci et al., 2013). Bead summary data was imported into GenomeStudio to remove control probes and to produce a text file containing the signal and detection P values per probe for all samples, as well as inputing missing data using the k‐nearest neighbor algorithm (Tech note: Inputing HumanHT‐12 Expression BeadChip Data; Illumina). The text file was imported into J‐Express Pro 2011, and signal intensity values were quantile normalized (Bolstad et al., 2003) and log transformed (base 2). Significance Analysis of Microarrays (SAM) (Tusher et al., 2001) was used to look for differentially expressed genes. To obtain manageable datasets, differentially expressed genes were defined by a fold change >2 and q value ¼ 0.
RNA sequencing
A total of 3 µg of RNA from 18 ESS with available RNA was sent for high‐throughput paired‐end RNA‐sequencing at the Norwegian Sequencing Centre, Ullevål Hospital (http://www.sequencing.uio.no/). The sequencing was performed using the Illumina HiSeq 2000 instrument. The Illumina software pipeline was used to process image data into raw sequencing data and only sequence reads marked as “passed filtering” were used in the downstream data analysis. The FASTQC software was used for quality control of the raw sequence data (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). An average number of 93 million sequence reads (range 32 to 180 millions) was obtained from transcriptome sequencing. We used the fusion discovery software FusionMap (release date 2012‐04‐16) (Ge et al., 2011) and the pre‐built Human B37 and RefGene from the FusionMap website (http://www.omicsoft.com/fusionmap/), as well as the Fusion Catcher software (version 0.99.3a beta‐April 15, 2014) with the associated ENSEMBL, UCSC, and RefSeq databases automatically downloaded by FusionCatcher (https://code.google.com/p/fusioncatcher/) (Kangaspeska et al., 2012) as an additional tool to detect fusion transcripts.
RESULTS
Karyotyping
The 18 samples which were cytogenetically analyzed all showed an abnormal karyotype (Table 1). Thirteen of them have not been reported before whereas five karyotypes have been published by our group (cases 13, 16, 17, 22, and 25) (Micci et al., 2006; Panagopoulos et al., 2012; Panagopoulos et al., 2013; Micci et al., 2014). Among the 13 new cases, four were HGESS, eight were LGESS, and one (case 9) was an ESS NOS. Only one HGESS (case 8) showed a 10;17‐translocation as the sole karyotypic abnormality. Cases 1 and 2 presented triploid karyotypes showing both structural and numerical aberrations, whereas case 3 had a near‐pentaploid karyotype with multiple structural rearrangements only few of which could be identified. None of the aberrations of these three HGESS showed involvement of any of the chromosomes, let alone chromosome bands, known to be involved in EST‐specific rearrangements. Among the eight LGESS, three tumors (cases 11, 12, and 26) showed the specific 7;17‐translocation either as the sole karyotypic aberration (case 11) or among other abnormalities (cases 12 and 26). Case 27 showed a 6;10‐ and a 10;6‐insertion. Case 10 had monosomy 10 as the sole aberration, whereas in the remaining three LGESS (cases 14, 15, and 24), a near‐diploid karyotype was found showing both numerical and structural aberrations. Case 9 had two unrelated clones, one with a possible del(X)(p21), the other, triploid, with both structural and numerical aberrations.
RT‐PCR
All tumors were tested for the fusions known to be associated with ESS. A summary of the results is shown in Table 1. The fusions were all confirmed by Sanger sequencing. PCR investigations confirmed the presence of all fusions previously published (Micci et al., 2006; Panagopoulos et al., 2012, 2013; Micci et al., 2014). The YWHAE‐NUTM fusion was found in five cases, all of them HGESS. The JAZF1‐SUZ12 fusion transcript was identified in four LGESS. A JAZF1‐PHF1 fusion was identified in one tumor (case 24). Case 9, the ESS NOS, showed a specific fusion between the EPC1 gene, mapping on 10p11, and the PHF1 gene, from 6p21. Fusion of the same genes was also found in case 27 but with a new variant, i.e., the junction involved exon 9 of the EPC1 (accession number NM_025209.3) (Fig. 1).
Figure 1.
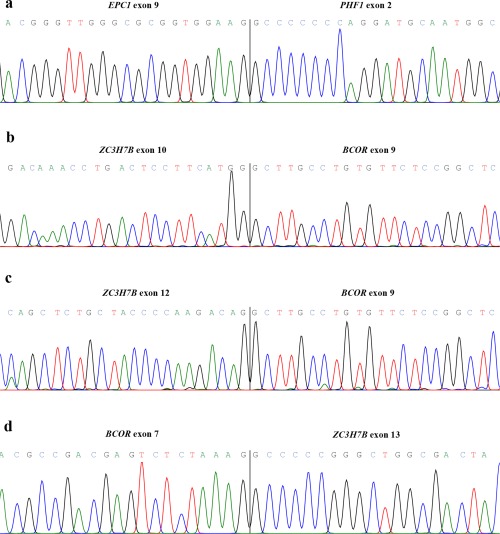
Partial chromatogram of the EPC1‐PHF1 fusion (a); ZC3H7B‐BCOR with involvement of exon 10 from ZC3H7B and exon 9 from BCOR (b); as well as fusion between exon 13 of ZC3H7B and exon 9 of BCOR (c); and the reciprocal BCOR‐ZC3H7B transcript (d). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RT‐PCR was used to validate those fusions obtained by sequencing analysis that showed higher seed count. An overview of the cases analyzed and the transcripts identified is provided in Table 1. In case 20, transcriptome sequencing (see below) identified the presence of a ZC3H7B‐BCOR and its reciprocal BCOR‐ZC3H7B fusion, previously described by Panagopoulos et al. (2013) as brought about by a X;22‐translocation. Sequencing of the amplified cDNA fragments showed two transcripts from the ZC3H7B‐BCOR fusion, one with involvement of exon 10 of ZC3H7B (accession number NM_017590.5) and exon 9 of BCOR (accession number NM_017745.5), the other showing a fusion between exon 12 of ZC3H7B and exon 9 of BCOR (Fig. 1). The reciprocal BCOR‐ZC3H7B fusion was between exon 7 of BCOR and exon 13 of ZC3H7B.
Microarray
The original microarray data can be found in the public database Gene Expression Omnibus (GEO; available at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE73361). The microarray analysis of 24 ESS was performed taking into consideration the pathological subclassification of the tumors so that the expression profiles of the HGESS (7 tumors) were compared to those of the LGESS (17 tumors). The ESS classified as NOS was grouped with the LG tumors since it showed an EPC1‐PHF1. We identified 514 differentially expressed unique genes in the comparison between the two groups, of which 187 genes were down‐regulated in the HG compared to the LG‐group of ESS and 327 genes were upregulated in the HG compared to the LG‐group of ESS (Supporting Information Table S1). The data were plotted using Correspondence Analysis (CA) (Fellenberg et al., 2001) to look for the greatest covariance between samples and genes so as to see if any array behaved differently compared to the others. The CA‐plot of the samples from the two groups (HG vs. LG) showed a distinct separation. Among the LGESS, several CA‐plots were also performed to compare different subgroups based on the karyotypic aberration and/or specific gene fusion present. In these analyses, False discovery rate (FDR) <0.05 was used as cutoff. The comparison between tumors showing t(7;17) and the LGESS samples without specific chromosomal rearrangement and/or gene fusion (referred to from now on as OTHER) revealed around 70 differentially expressed genes (Fig. 2), all other comparisons showed less than ten. The comparison between tumors showing a t(X;22) and OTHER identified eight differentially expressed genes. Furthermore, the comparisons between the group of ESS with 6p‐rearrangements versus t(X;22) or OTHER revealed no differentially expressed gene. A list of the genes and probes differentially expressed in each comparison can be found in Supporting Information.
Figure 2.
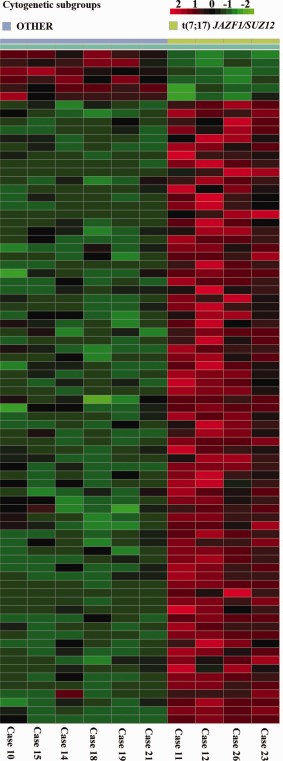
Image of the 74 differentially expressed genes between ESS carrying a t(7;17) and ESS without any known fusion transcript. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RNA Sequencing
The Fusion Map and Fusion Catcher programs were used to find fusion transcripts in the samples (Liu et al., 2016). We focused only on the common transcripts. Five out of the 18 ESS sequenced showed a previously described fusion: cases 13 and 17 had a BCOR‐ZC3H7B (Panagopoulos et al., 2013), cases 16 and 25 showed a MEAF6‐PHF1 (Panagopoulos et al., 2012; Micci et al., 2014), and in case 20 the ZC3H7B‐BCOR and its reciprocal BCOR‐ZC3H7B transcript were found. Validation of the fusions by PCR using specific primer combinations revealed the presence of three transcripts in the latter case (see Results for PCR analyses). Because most of the putative fusions detected by both programs/algoritms may be false positives, we selected 22 different fusions with the highest seed counts from 13 tumors to validate their actual presence by PCR using specific primer combinations. PCR reactions for seven transcripts did not show any amplification of the putative fusion, but the remaining 15 transcripts could be amplified by PCR and direct sequencing (Supporting Information Table S2). For all but two transcripts (H2AFY‐HMHB1 from case 19 and NAIP‐OCLN from case 21), the two genes were found to be in‐frame. The transcriptome sequencing did not detect any YWHAE‐NUTM fusion in the seven HGESS with NGS data.
DISCUSSION
Recent cytogenetic and molecular investigations have improved our understanding of EST and helped classify them into more meaningful categories. These tumors typically demonstrate relatively simple karyotypes with specific chromosomal rearrangements (the majority of ESN, LGESS, and YWHAE‐rearranged HGESS) or harbor complex cytogenetic aberrations lacking specific rearrangements (as in EUS/UUS). The 2014 WHO classification of EST (2014) incorporates molecular findings, and yet molecular testing is not routinely performed when these diagnoses are suspected. The molecular signature may be diagnostically helpful when dealing with cases of unusual location or morphology; furthermore, a better genetic characterization of these tumors may also provide prognostically and/or therapeutically relevant information. Because the cytogenetic and molecular literature on EST is still severely limited (Table 3), we add to the body of knowledge information on 27 ESS investigated using cytogenetic, PCR, expression array, and transcriptome sequencing analyses.
Table 3.
Overview of the ESS Cases Present in the Literature. The cases are grouped based on chromosomal rearrangements.
| Case | Karyotype | Reported by |
|---|---|---|
| 7;17‐rearrangements | ||
| 1 | 46,XX,t(7;13)(p15;q14),t(7;17)(p15;q11),del(11)(q21q23) | Fletcher et al., 1991 |
| 2 | 46,XX,t(7;13)(q11;p13),t(7;17)(p21;q12),del(11)(q13q21) | Sreekantaiah et al., 1991 |
| 3 | 46,XX,t(7;17)(p15‐21;q12‐21)/46,idem,‐7,+der(?)t(?;7) (?;q11)/45,idem,‐7,dic(15;22)(p11;p11),+der(?)t(?;7) | Dal Cin et al., 1992 |
| 4 | 46,XX,del(6)(q15),der(6)t(6;11)(p21;q11),add(7)(p21), t(7;17)(p15‐21;q12‐21),+9,‐11 | Pauwels et al., 1996 |
| 5 | 46,XX,der(7)t(7;16)(p14‐15;q22)t(7;9)(q22;q22),t(7;17) (p14‐21;q11‐21),der (9)t(7;9)(q22;q22),del(16)(q22)/ 47,idem,del(3)(p13p23),+mar | Hennig et al., 1997 |
| 6 | 42‐44,X,‐X,der(2)t(2;7)(p23;p15)t(2;15)(q35;q15),add(4) (p16),del(7)(p13p15),der(7)t(7;17)(p14;q12),add(8)(q24), −10,del(11)(p11),t(11;13)(p15;q14),del(15)(q15),‐16, del(17)(q12),der(18)t(16;18)(p11;p11),add(19)(p13),‐20, add(21)(q22),‐22,+2‐3mar/78‐83,idemx2 | Iliszko et al., 1998 |
| 7 | 53‐55,X,‐X,del(1)(p32),+del(1)(p21),+der(1)t(1;3)(p32;p21), del(3)(p21),+der (3;15)(q10;q10),‐5,+6,+der(6)add(6) (p11)add(6)(q27),add(7)(p11),+add(7) (p21),+8,+8,‐11,‐13, der(13;21)(q10;q10),add(14)(p11),der(15)t(6;15) (p21;p12), der(17)t(3;17)(p21;p13)x2,+18,+18,add(19)(q13),‐20, der(21;21) (q10;q10),+2mar,dmin | Gil‐Benso et al., 1999 |
| 8 | 46,XX,t(7;13)(p15;p13),t(7;17)(p15;q21) | Koontz et al., 2001 |
| 9 | 46,XX,t(7;17)(p15;q21) | Koontz et al., 2001 |
| 10 | 46,XX,t(7;17)(p15;q21) | Koontz et al., 2001 |
| 11 | 45,XX,‐7,t(7;17)(p15;q21) | Koontz et al., 2001 |
| 12 | 46,XX,der(7)t(7;21)(p11‐12;q11‐21),t(7;17)(p15;q12),r(8), der(13)del(13)(?q12q14)del(13)(?q22) | Micci et al., 2003 |
| 13 | 46,XX,t(7;17)(p15;q11),del(9)(q22),add(19)(q13) | Satoh et al., 2003 |
| 14 | 45,XX,der(3)t(3;7)(p21;p13)t(7;17)(p15;q21),add(5)(q33), der(7)t(3;7),der(7)t(7;12)(p15;p13),add(9)(q34),der(12) t(12;22)(p13;q11),del(14)(q24),der(16)t(7;16)(p15;q24), del(17)(q21),‐22 | Regauer et al., 2008 |
| 6p21‐rearrangements | ||
| 1 | 46,XX,del(5)(q33),der(7)t(6;7)(p21;p21) | Laxman et al., 1993 |
| 2 | 46,XX,der(3)t(3;7)(p12;p12),der(6)t(3;6)(p12;p21)t(6;7) (q21;q22),der(7)t(6;7)(q12;p13)t(6;7)(p21;q22), inv(17)(p12q11)c | Hrynchak et al., 1994 |
| 3 | 38,XX,‐1,del(1)(q11),‐2,add(2)(p13),‐3,der(4;14)t(4;14) (q35;q11)add(4)(p12),add(6)(p21),add(7)(q22),del(7) (p11p13),‐8,‐9,add(9)(q34),‐10,add(10)(q24),‐11,‐11, ins(12;?)(q13;?),‐14,‐14,‐15,ins(15)(q22;?),add(16)(q22), add(17)(q11),‐18,der(18)t(7;18)(q11;p11),‐19,add(20)(p13), add(21)(p11),‐22,add(22) (p11),+6mar | Sonobe et al., 1999 |
| 4 | 46,XX,der(6)ins(6;7)(p21;q34q11)del(6)(p21),der(7)del(7) (p15)t(6;7)(p21;q11),dup(7)(p22p15) | Micci et al., 2003 |
| 5 | 46,XX,inv(2)(p21q37),der(6)del(6)(p21)t(6;7)(q21;p15), der(7)t(6;7)(p21;p15)del(6)(q21) | Micci et al., 2006 |
| 6 | 47,X,der(X)t(X;16),+add(2)(q21),ins(2;22)(q31;q11q13), der(3)ins(3;13)(p24;q?22q32)t(3;6)(q28;q22),del(6)(p11), der(6)t(3;6),der(7)t(6;7)(?;p15)t(6;15)t(3;15)t(3;13)t(13;15) t(6;15)t(X;6)t(X;6)t(3;6),der(14)t(1;14)(q25;q32) | Micci et al., 2006 |
| 7 | 46,XX,t(6;10;10)(p21;q22;p11) | Micci et al., 2006 |
| 8 | 46,XX,t(1;6)(p32‐34;p21) | Panagopoulos et al., 2012 |
| X;22‐rearrangements | ||
| 1 | 46,X,t(X;22)(p11;q13),t(3;4)(p23;q27) | Panagopoulos et al., 2013 |
| 2 | 42,X,dic(X;22)(p11;p11),der(1;9)(q25;p22‐24)ins(1;?) (q25;?),der(9)t(1;9),‐10,der(13;15)(q10;q10),‐21,der(22) t(X;22)(p11;q13),+r | Panagopoulos et al., 2013 |
| X;17‐rearrangements | ||
| 1 | 46,X,t(X;17)(p11;q23) | Amant et al., 2003 |
| 2 | 46,X,t(X;17)(p11;q21)/45,idem,dic(4;22)(p15;q13) | Dewaele et al., 2014 |
| Cytogenetics rearrangements not known to be associated with LGESS | ||
| 1 | 45,XX,‐10,der(19)ins(10;19)(p11;p13q13)/45,idem,del(1) (p21‐22)/45,idem,del(1)(p21p35) | Dal Cin et al., 1988 |
| 2 | 46,XX,del(7)(q22q32),del(12)(q14q22) | Havel et al., 1989 |
| 3 | 76‐118,XXXX,del(3)(p12p21),t(11;21)(q13;q22),inc | Fletcher et al., 1991 |
| 4 | 49,XX,+7,+8,+9,der(14)t(14;22)(p13;q12)/49,XX,+3,+7,+8,der(14) | Laxman et al., 1993 |
| 5 | 80,XX?,del(1)(p11),i(1)(p10),del(4)(q24),del(5)(p11), del(6)(q12),del(12)(p11),add(16)(q12),add(19)(q13),inc | Laxman et al., 1993 |
| 6 | 45‐48,XX,der(3)t(3;6)(q29;p21),der(6)t(3;6)(q21;q27), +i(19)(q10)/88‐93,idemx2 | Gunawan et al., 1998 |
| 7 | 48‐50,XX,+2,+7/49‐50,XX,+der(1;7)(q10;q10),+2,+7,+10 | Iliszko et al., 1998 |
| 8 | 45‐46,XX,del(6)(q21),del(12)(p13) | Iliszko et al., 1998 |
| 9 | 46,XX,+t(1;3)(p13;p25),i(8)(q10),dic(15;16)(p11;q13) | Iliszko et al., 1998 |
| 10 | 46,XX,del(6)(q22),add(20)(q13) | Gil‐Benso et al., 1999 |
| 11a | 46,XX,inv(5)(p13‐14q23‐31) | Micci et al., 2014 |
| t(10;17) | ||
| 1 | 46,XX,t(10;17)(q22;p13) | Leunen et al., 2003 |
| 2 | 45,XX,add(3)(p11),der(9)t(7;9)(q11;p24),del(10)(q11q26), t(10;17)(q22;p13),der(11)t(3;11)(p22;q22),‐13 | Micci et al., 2003 |
| 3 | 46,XX,t(10;17)(q22;p13),t(12;13)(q24;q14) | Regauer et al., 2008 |
| 4 | 47,XX,der(9)del(9)(p11)del(9)(q12),del(10)(q22),der(11) t(9;11)(p12;q12),der(17)t(10;17)(q22;p13),+19 | Amant et al., 2011 |
| 5 | 47,XX,der(9)del(9)(p11)del(9)(q12),del(10)(q22),der(11) t(9;11)(q12;q12),der(17)t(10;17)(q22;p13),+19 | Lee et al., 2012a |
| 6 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012a |
| 7 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012a |
| 8 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012a |
| 9 | 43,XX,der(5)t(5;21)(q35;q11),der(9;11)(q10;q10),‐10, t(10;17)(q22;p13),‐21 | Lee et al., 2012a |
| 10 | 44,XX,der(5)t(5;21)(q35;q11),der(9;11)(q10;q10),‐10, t(10;17)(q22;p13),‐21 | Lee et al., 2012b |
| 11 | 44,XX,t(10;17)(q22;p13),del(11)(q1?2),‐19,‐22 | Lee et al., 2012a |
| 12 | 44,XX,t(10;17)(q22;p13),del(11)(q1?2),‐19,‐22 | Lee et al., 2012b |
| 13 | 47,XX,+i(1)(q10),t(9;9)(p24;q11),‐16,add(17)(p13),+mar | Lee et al., 2012a |
| 14 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012b |
| 15 | 46,XX,t(4;10;17)(q12;q22;p13) | Lee et al., 2012b |
| 16 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012b |
| 17 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012b |
| 18 | 45,X,‐X,t(10;17;12)(q22;p11;q13),add(19)(p13) | Lee et al., 2012a |
| 19 | 45,X,‐X,t(10;17;12)(q22;p11;q13),add(19)(p13) | Lee et al., 2012b |
| 20 | 46,XX,t(10;17)(q22;p13) | Lee et al., 2012b |
| 21 | 47,XX,t(10;17)(q22;p13),‐11,+19,+mar | Lee et al., 2012b |
| Cytogenetics rearrangements not known to be associated with HGESS but with a YWHAE‐NUTM fusion | ||
| 1 | 55‐58,X,del(X)(p11),+i(1)(q10),+2,+3,+4,+del(6)(q21),+7, −9,+del(12)(q21), +15,+17,+22,add(22)(q12)x2,+2r | Lee et al., 2012a |
| 2 | 46,XX,inv(6)(p21q13) | Lee et al., 2012a |
| 3 | 46,X,del(X)(p22),?dup(1)(q42),+i(1)(q10),+2,+3,+4,t(4;7) (q21;p22),+7,‐9,+12,+der(17)t(5;17)(p11;p11),+22, add(22)(q13)x2,+2‐4mar | Lee et al., 2012a |
| 4 | 46,X,der(X)t(X;1)(p22;q24),dup(1)(q12q32) | Lee et al., 2012a |
Molecular analysis detected a MEAF6‐PHF1 fusion.
The karyotypic data on 13 of the ESS described here (Table 1) have not been reported before. Karyotypic complexity was observed in both HG and LG tumors. In five cases, a neat correspondence between karyotypic aberrations and molecular findings was identified, i.e., case 8 showed a 10;17‐translocation leading to a YWHAE‐NUTM fusion, cases 11, 12, and 23 had a specific t(7;17) and a JAZF1‐SUZ12 fusion, and case 27 showed two insertions, an ins(6;10) and an ins(10;6), leading to an EPC1‐PHF1 fusion. In case 9, on the other hand, a specific fusion was seen between the EPC1 gene, mapping on 10p11, and the PHF1 gene from 6p21, in spite of the fact that no rearrangement of these two chromosome bands was seen cytogenetically. Likewise, a JAZF1‐PHF1 fusion was identified in one tumor (case 24) which in its karyotype had no visible rearrangement of 7p15 and/or 6p21. Evidently, cases of ESS exist that by karyotyping are “false negatives,” i.e., the pathogenetically crucial gene‐level rearrangement takes place without visible change of the chromosomal morphology. The fact that some ESS may carry cryptic rearrangements leading to one of the known fusions characteristic of this tumor type has also previously been alluded to (Micci et al., 2014). Specific gene fusions may also possibly be hidden in rings and/or marker chromosome(s) that are part of incompletely described karyotypes.
The karyotypic data gave the impression of increasing complexity from LG to HG ESS, something that has also been seen by others. Flicker et al. (2015) performed aCGH on 30 EST showing an increasing number of copy number changes from ESN to LGESS and UES. In their study as well as in the present one, the chromosomal aberrations differed considerably among the groups, indicating that a linear tumor progression from one group to the next does not take place. Instead, classification into different entities based on genetic aberration pattern is more appropriate.
The microarray analysis identified 514 differentially expressed genes between HG and LGESS. Seventy‐six of these genes are already known to be associated with cancer. No hint as to which of them may be the most important in ESS is at hand. The comparison between LGESS having a t(7;17) and/or JAZF1‐SUZ12 fusion versus those showing 6p‐rearrangements and/or PHF1 involvement showed no differentially expressed genes. In contrast, the comparison between tumors with t(7;17) and those with t(X;22) showed nine genes that were differentially expressed. These results show once more that despite the presence of different fusions, these tumors are quite similar and belong to the same subgroup as far as phenotype and gene expression pattern are concerned. The JAZF1‐SUZ12, JAZF1‐PHF1, EPC1‐PHF1, MEAF6‐PHF1, ZC3H7B‐BCOR, and possibly also MBTD1‐Cxorf67 gene fusions (admittedly, the latter was not found in our comparison/series) appear to represent biologically and clinically equivalent oncogenic events in the tumorigenesis of LGESS.
JAZF1 is a transcriptional repressor and SUZ12, PHF1, and MBTD1 are members of the polycomb group protein family involved in transcriptional repression. ZC3H7B is involved in protein‐nucleic acid interactions, while BCOR is known to interact with polycomb group proteins; hence, ZC3H7B‐BCOR probably mediates its oncogenic effects through aberrant epigenetic regulation (Panagopoulos et al., 2013). EPC1 is part of the nucleosome acetyltransferase of histone H4 complex, whereas MEAF6 is part of histone acetyltransferase multi‐subunit complexes. EPC1‐PHF1 and MEAF6‐PHF1 are believed to alter acetylation patterns of histone proteins resulting in unravelling of the heterochromatin and aberration gene expression (Avvakumov and Cote, 2007; Panagopoulos et al., 2012). All these fusions appear to combine genes that are known to be involved in transcriptional regulation, i.e., polycomb group complex‐mediated and aberration methylation/acetylation; hence, their presumed oncogenic effects are probably mediated through altered transcriptional control in endometrial stromal progenitor cells.
In contrast, the comparison between 7;17‐associated tumors and those described as OTHER (six tumors; cases 10, 14, 15, 18, 19, and 21), the ones showing no characteristic chromosomal rearrangement and/or fusion genes, revealed around 70 differently expressed genes (Supporting Information Table 3). Little cytogenetic information is available on these tumors as only three of them were sent for cell culturing and karyotyping. Case 10 showed monosomy 10 as the sole aberration in the karyotype, whereas case 14 showed as many as twelve chromosomal aberrations with both numerical and structural rearrangements, and case 15 had a hyperdiploid stemline with aberrations that could only be partly described in a composite karyotype. The cytogenetic literature contains information on seven ESS with no visible rearrangements of the chromosomal bands known to be involved in ESS‐specific fusions, but no molecular information is available on these tumors allowing a classification based on molecular signature. We cannot but speculate that the said six cases of our series may represent a subgroup or variants of “classical” LGESS, despite their unequivocal histological classification.
Transcriptome sequencing was performed on the 18 tumors with available RNA. Surprisingly and alarmingly, none of the programs/algorithms used to screen the data for fusion transcripts, i.e., FusionMap and Fusion Catcher, detected the presence of YWHAE‐NUTM in the four HGESS positive for this fusion by PCR. We did not check, however, if such a transcript was nevertheless present in the raw data and retrievable by use of the “grep” command (Panagopoulos et al., 2014a,b), but assume that to be the case. In spite of its immense investigative and attraction value, the next generation sequencing technology regrettably yields results that may be both falsely negative and positive (Panagopoulos et al., 2014a).
The only tumor showing a known ESS‐related fusion transcript by NGS was case 20 in which a ZC3H7B‐BCOR and its reciprocal BCOR‐ZC3H7B were detected. However, PCR analyses confirmed the presence of variants of fusions compared to those previously reported by Panagopoulos et al. (2013). The three new transcripts fuse either exon 10 or exon 12 of ZC3H7B (accession number NM_017590.5) with exon 9 of BCOR (accession number NM_017745.5) in the two variants of the ZC3H7B‐BCOR, whereas the variant of BCOR‐ZC3H7B shows an in‐frame fusion between exon 7 of BCOR and exon 13 of ZC3H7B (Fig. 1).
Twenty‐two putative fusions were selected from those ranking highest based on the sequence analyses with both programs in the 13 cases examined. Amplification of cDNA with specific primers for each fusion confirmed the presence of the transcripts in 15 instances (Supporting Information Table 2). The seven transcripts that were not confirmed by PCR could be false positives/artefacts introduced during the RNA library preparation stage (Quail et al., 2008; Panagopoulos et al., 2014c) and/or the consequence of read‐through transcription which occurs when the RNA polymerase continues beyond the normal termination sequence into an adjacent gene (Nacu et al., 2011). Among these 15 transcripts, GMPS‐SYNPR (case 14) was particularly interesting since the two involved genes map to 3q25 and 3p14, respectively, and the karyotype showed two different rearrangements involving 3q. The correspondence between the molecular and karyotypic data adds credibility to the idea that this could be an important transcript in tumor development. Its function needs further investigation. The fusion is in‐frame and involves exon 12 from the GMPS gene and exon 3 from SYNPR (Supporting Information Table 2). GMPS (accession number NM_003875) encodes a guanine monophosphate synthetase whereas SYNPR (accession number NM_144642) codes for a synatoporin. None of these genes has been reported to be directly involved in cancer; however, a study by Reddy et al. (2014) showed that GMPS is required for USP7‐mediated stabilization of p53.
Case 15 showed another interesting fusion, between the lysine (K)‐specific demethylase 2B (KDM2B) on 12q24 and the CREB binding protein (CREBBP) on 16p13. The in‐frame fusion is between exon 22 of KDM2B (accession number NM_032590) and exon 2 of CREBBP (accession number NM__004380). The KDM2B gene (also known as Ndy1, FBXL10, and JHDM1B) encodes a member of the F‐box protein family which is characterized by an ∼40 amino acid motif (F‐box). The F‐box proteins constitute one of the four subunits of the ubiquitin protein ligase complex called SCFs (SKP1‐cullin‐F‐box) which functions in phosphorylation‐dependent ubiquitination. It has been found that KDM2B, an H3K36 histone demethylase implicated in bypassing cellular senescence and somatic cell reprogramming, is markedly overexpressed in human pancreatic ductal adenocarcinoma (PDAC), with levels increasing with disease grade and stage, and showing the highest expression in metastases (Tzatsos et al., 2013). KDM2B silencing abrogated tumorigenicity of PDAC cell lines exhibiting loss of epithelial differentiation, whereas KDM2B overexpression cooperated with KrasG12D to promote PDAC formation in mouse models. Gain‐ and loss‐of‐function experiments coupled to genome‐wide gene expression and ChIP studies revealed that KDM2B drives tumorigenicity through two different transcriptional pathways. KDM2B repressed developmental genes through cobinding with Polycomb group (PcG) proteins at transcriptional start sites, but also activated a module of metabolic genes, including mediators of protein synthesis and mitochondrial function, cobound by the MYC oncoprotein and the histone demethylase KDM5A. These results defined epigenetic programs through which KDM2B subverts cellular differentiation and drives the pathogenesis of an aggressive subset of PDAC (Tzatsos et al., 2013). The CREBBP gene is ubiquitously expressed and is involved in the transcriptional coactivation of many different transcription factors. First isolated as a nuclear protein that binds to cAMP‐response element binding protein (CREB), the gene is now known to play critical roles in embryonic development, growth control, and homeostasis by coupling chromatin remodeling to transcription factor recognition. The protein encoded by this gene has intrinsic histone acetyltransferase activity but also acts as a scaffold to stabilize additional protein interactions with the transcription complex. It acetylates both histone and non‐histone proteins. Chromosomal translocations targeting this gene have been associated with acute myeloid leukemia (AML) (Borrow et al., 1996; Panagopoulos et al., 2014b). The breakpoint identified in our case 15 is the same as that previously described (Panagopoulos et al., 2000) indicating that the fusion retains the oncogenic activities typical for AML (Borrow et al., 1996) also in this case of LGESS. This is the first time that CREBBP is found involved in a fusion transcript in solid tumors, and its importance in LGESS needs to be evaluated in larger series. The Mitelman Database for Chromosome Aberrations and Gene Fusions in Cancer (http://cgap.nci.nih.gov/Chromosomes/Mitelman) reports two ESS with alteration of chromosome 16 (Laxman et al., 1993; Iliszko et al., 1998) and one with 12q rearrangement (Havel et al., 1989), though with different breakpoint positions from those of the 6;12‐translocation we identified. These rearrangements may possibly lead to a cryptic KDM2B‐CREBBP fusion since the three tumors did not show any rearrangement typical of ESS.
The finding of similar, specific gene fusions in different tumor types is well known in cancer, and ESS‐related fusions are no exception. Recently, ZC3H7B‐BCOR, MEAF6‐PHF1, and EPC1‐PHF1 fusions have been found in ossifying fibromyxoid tumors (OFMT) (Antonescu et al., 2014) and a JAZF1‐PHF1 was found in a cardiac ossifying sarcoma (Schoolmeester et al., 2013). Furthermore, a new PHF1‐fusion variant, EP400‐PHF1, was also described in OFMT (Gebre‐Medhin et al., 2012; Endo et al., 2013). We do not have any explanation for such similarities between different mesenchymal tumors or the involvement of the CREBBP gene in leukemias and ESS; it seems that they are not tissue‐specific although their tumorigenic role is unquestionable. The same can be said for the MLLT4‐RB1 transcript found in case 6. The fusion is between the myeloid/lymphoid or mixed‐lineage leukemia gene (MLLT4 on 6q27) and the retinoblastoma 1 gene (RB1 in 13q14). The former gene is usually found rearranged through a t(6;11)(q27;q23) leading to an MLL‐AF6 in childhood AML; its finding is associated with poor disease outcome. The latter gene encodes a negative regulator of the cell cycle and was the first tumor suppressor gene discovered. This is the first time the two genes have been found together in a fusion transcript. Their role as well as the mechanism through which they act, being it the formation of a chimeric protein or loss of function for RB1, needs to be clarified.
The eight remaining fusions detected by the sequencing analysis and confirmed by PCR have not been reported before and their role in ESS needs further elucidation. Because these tumors were all classified as ESS by histology but showed no known ESS‐specific fusion, they may represent new subgroups/entities under the big umbrella of ESS highlighting the presence of genetic heterogeneity in these tumors. Such heterogeneity is present in both HGESS and LGESS. In the former group, it was demonstrated by the fact that not all HG showed YWHAE‐fusion neither in the present series nor in the previous report by Sciallis et al. (2014). Both studies found that whereas YWHAE‐rearranged ESS consistently shows HG cytomorphology, the reverse is not always true, i.e., not all uniform HGESS carry YWHAE‐rearrangements. Furthermore, there remains a small group of LGESS which lack genetic rearrangements of known ESS‐associated fusion genes, indicating that additional, still unknown fusion transcripts may be characteristic of this tumor type and will be identified in the future. The expression profile, showing around 70 differently expressed genes, hints to the possibility that these tumors may belong to a new genetic subgroup. Further studies are necessary to obtain a more complete picture of ESS tumorigenesis. Because each approach has its pros and cons, it is mandatory to use a combination of techniques to avoid skewness brought about by both false positive and false negative results.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
REFERENCES
- Antonescu CR, Sung YS, Chen CL, Zhang L, Chen HW, Singer S, Agaram NP, Sboner A, Fletcher CD. 2014. Novel ZC3H7B‐BCOR, MEAF6‐PHF1, and EPC1‐PHF1 fusions in ossifying fibromyxoid tumors–molecular characterization shows genetic overlap with endometrial stromal sarcoma. Genes Chromosomes Cancer 53:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvakumov N, Cote J. 2007. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene 26:5395–5407. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193. [DOI] [PubMed] [Google Scholar]
- Borrow J, Stanton VP, Jr , Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dube I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. 1996. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB‐binding protein. Nat Genet 14:33–41. [DOI] [PubMed] [Google Scholar]
- Chiang S, Ali R, Melnyk N, McAlpine JN, Huntsman DG, Gilks CB, Lee CH, Oliva E. 2011. Frequency of known gene rearrangements in endometrial stromal tumors. Am J Surg Pathol 35:1364–1372. [DOI] [PubMed] [Google Scholar]
- Davidson B, Abeler VM, Hellesylt E, Holth A, Shih I, Skeie‐Jensen T, Chen L, Yang Y, Wang TL. 2013. Gene expression signatures differentiate uterine endometrial stromal sarcoma from leiomyosarcoma. Gynecol Oncol 128:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaele B, Przybyl J, Quattrone A, Finalet FJ, Vanspauwen V, Geerdens E, Gianfelici V, Kalender Z, Wozniak A, Moerman P, Sciot R, Croce S, Amant F, Vandenberghe P, Cools J, Debiec‐Rychter M. 2014. Identification of a novel, recurrent MBTD1‐CXorf67 fusion in low‐grade endometrial stromal sarcoma. Int J Cancer 134:1112–1122. [DOI] [PubMed] [Google Scholar]
- Endo M, Kohashi K, Yamamoto H, Ishii T, Yoshida T, Matsunobu T, Iwamoto Y, Oda Y. 2013. Ossifying fibromyxoid tumor presenting EP400‐PHF1 fusion gene. Hum Pathol 44:2603–2608. [DOI] [PubMed] [Google Scholar]
- Fellenberg K, Hauser NC, Brors B, Neutzner A, Hoheisel JD, Vingron M. 2001. Correspondence analysis applied to microarray data. Proc Natl Acad Sci USA 98:10781–10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JA, Kozakewich HP, Hoffer FA, Lage JM, Weidner N, Tepper R, Pinkus GS, Morton CC, Corson JM. 1991. Diagnostic relevance of clonal cytogenetic aberrations in malignant soft‐tissue tumors. N Engl J Med 324:436–442. [DOI] [PubMed] [Google Scholar]
- Flicker K, Smolle E, Haybaeck J, Moinfar F. 2015. Genomic characterization of endometrial stromal sarcomas with array comparative genomic hybridization. Exp Mol Pathol 98:367–374. [DOI] [PubMed] [Google Scholar]
- Ge H, Liu K, Juan T, Fang F, Newman M, Hoeck W. 2011. FusionMap: Detecting fusion genes from next‐generation sequencing data at base‐pair resolution. Bioinformatics 27:1922–1928. [DOI] [PubMed] [Google Scholar]
- Gebre‐Medhin S, Nord KH, Moller E, Mandahl N, Magnusson L, Nilsson J, Jo VY, Vult von SF, Brosjo O, Larsson O, Domanski HA, Sciot R, Debiec‐Rychter M, Fletcher CD, Mertens F. 2012. Recurrent rearrangement of the PHF1 gene in ossifying fibromyxoid tumors. Am J Pathol 181:1069–1077. [DOI] [PubMed] [Google Scholar]
- Gil‐Benso R, Lopez‐Gines C, Navarro S, Carda C, Llombart‐Bosch A. 1999. Endometrial stromal sarcomas: Immunohistochemical, electron microscopical and cytogenetic findings in two cases. Virchows Arch 434:307–314. [DOI] [PubMed] [Google Scholar]
- Havel G, Wedell B, Dahlenfors R, Mark J. 1989. Cytogenetic relationship between uterine lipoleiomyomas and typical leiomyomas. Virchows Arch B Cell Pathol Incl Mol Pathol 57:77–79. [DOI] [PubMed] [Google Scholar]
- Iliszko M, Mandahl N, Mrozek K, Denis A, Pandis N, Pejovic T, Babinska M, Nedoszytko B, Debniak J, Emerich J, Hrabowska M, Bloomfield CD, Limon J. 1998. Cytogenetics of uterine sarcomas: Presentation of eight new cases and review of the literature. Gynecol Oncol 71:172–176. [DOI] [PubMed] [Google Scholar]
- ISCN . 2009. An international system for human cytogenetic nomenclature. In: Shaffer LG, Slovak ML, Campbell LJ, editors. Basel: Karger. [Google Scholar]
- Kangaspeska S, Hultsch S, Edgren H, Nicorici D, Murumagi A, Kallioniemi O. 2012. Reanalysis of RNA‐sequencing data reveals several additional fusion genes with multiple isoforms. PLoS One 7:e48745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, Van den Berghe H, Dal Cin P, Fletcher JA, Sklar J. 2001. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci USA 98:6348–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman R, Currie JL, Kurman RJ, Dudzinski M, Griffin CA. 1993. Cytogenetic profile of uterine sarcomas. Cancer 71:1283–1288. [DOI] [PubMed] [Google Scholar]
- Lee CH, Ou WB, Marino‐Enriquez A, Zhu M, Mayeda M, Wang Y, Guo X, Brunner AL, Amant F, French CA, West RB, McAlpine JN, Gilks CB, Yaffe MB, Prentice LM, McPherson A, Jones SJ, Marra MA, Shah SP, van de Rijn M, Huntsman DG, Dal Cin P, Debiec‐Rychter M, Nucci MR, Fletcher JA. 2012a. 14‐3‐3 fusion oncogenes in high‐grade endometrial stromal sarcoma. Proc Natl Acad Sci USA 109:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Mariño‐Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, Amant F, Gilks CB, van de Rijn M, Oliva E, Debiec‐Rychter M, Dal Cin P, Fletcher JA, Nucci MR. 2012b. The clinicopathologic features of YWHAE‐FAM22 endometrial stromal sarcomas: a histologically high‐grade and clinically aggressive tumor. Am J Surg Pathol 36:641–653. [DOI] [PubMed] [Google Scholar]
- Liu S, Tsai WH, Ding Y, Chen R, Fang Z, Huo Z, Kim S, Ma T, Chang TY, Priedigkeit NM, Lee AV, Luo J, Wang HW, Chung IF, Tseng GC. 2016. Comprehensive evaluation of fusion transcript detection algorithms and a meta‐caller to combine top performing methods in paired‐end RNA‐seq data. Nucleic Acids Res 44:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micci F, Heim S. 2015. Tumors of the female genital organs In: Heim S, Mitelman F, editors. Cancer Cytogenetics. Wiley Blackwell, West Sussex, pp. 447–480. [Google Scholar]
- Micci F, Teixeira MR, Dietrich CU, Saeter G, Bjerkehagen B, Heim S. 1999. Combined RxFISH/G‐banding allows refined karyotyping of solid tumors. Hum Genet 104:370–375. [DOI] [PubMed] [Google Scholar]
- Micci F, Walter CU, Teixeira MR, Panagopoulos I, Bjerkehagen B, Saeter G, Heim S. 2003. Cytogenetic and molecular genetic analyses of endometrial stromal sarcoma: Nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogenet 144:119–124. [DOI] [PubMed] [Google Scholar]
- Micci F, Panagopoulos I, Bjerkehagen B, Heim S. 2006. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res 66:107–112. [DOI] [PubMed] [Google Scholar]
- Micci F, Panagopoulos I, Haugom L, Dahlback HS, Pretorius ME, Davidson B, Abeler VM, Trope CG, Danielsen HE, Heim S. 2013. Genomic aberration patterns and expression profiles of squamous cell carcinomas of the vulva. Genes Chromosomes Cancer 52:551–563. [DOI] [PubMed] [Google Scholar]
- Micci F, Gorunova L, Gatius S, Matias‐Guiu X, Davidson B, Heim S, Panagopoulos I. 2014. MEAF6/PHF1 is a recurrent gene fusion in endometrial stromal sarcoma. Cancer Lett 347:75–78. [DOI] [PubMed] [Google Scholar]
- Nacu S, Yuan W, Kan Z, Bhatt D, Rivers CS, Stinson J, Peters BA, Modrusan Z, Jung K, Seshagiri S, Wu TD. 2011. Deep RNA sequencing analysis of readthrough gene fusions in human prostate adenocarcinoma and reference samples. BMC Med Genom 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I, Isaksson M, Lindvall C, Bjorkholm M, Ahlgren T, Fioretos T, Heim S, Mitelman F, Johansson B. 2000. RT‐PCR analysis of the MOZ‐CBP and CBP‐MOZ chimeric transcripts in acute myeloid leukemias with t(8;16)(p11;p13). Genes Chromosomes Cancer 28:415–424. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I, Micci F, Thorsen J, Gorunova L, Eibak AM, Bjerkehagen B, Davidson B, Heim S. 2012. Novel fusion of MYST/Esa1‐associated factor 6 and PHF1 in endometrial stromal sarcoma. PLoS One 7:e39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I, Thorsen J, Gorunova L, Haugom L, Bjerkehagen B, Davidson B, Heim S, Micci F. 2013. Fusion of the ZC3H7B and BCOR genes in endometrial stromal sarcomas carrying an X;22‐translocation. Genes Chromosomes Cancer 52:610–618. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I, Gorunova L, Bjerkehagen B, Heim S. 2014a. The “grep” command but not FusionMap, FusionFinder or ChimeraScan captures the CIC‐DUX4 fusion gene from whole transcriptome sequencing data on a small round cell tumor with t(4;19)(q35;q13). PLoS One 9:e99439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I, Torkildsen S, Gorunova L, Tierens A, Tjonnfjord GE, Heim S. 2014b. Comparison between karyotyping‐FISH‐reverse transcription PCR and RNA‐sequencing‐fusion gene identification programs in the detection of KAT6A‐CREBBP in acute myeloid leukemia. PLoS One 9:e96570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I, Thorsen J, Gorunova L, Micci F, Heim S. 2014c. Sequential combination of karyotyping and RNA‐sequencing in the search for cancer‐specific fusion genes. Int J Biochem Cell Biol 53:462–465. [DOI] [PubMed] [Google Scholar]
- Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin R, Swerdlow H, Turner DJ. 2008. A large genome center's improvements to the Illumina sequencing system. Nat Methods 5:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BA, van der Knaap JA, Bot AG, Mohd‐Sarip A, Dekkers DH, Timmermans MA, Martens JW, Demmers JA, Verrijzer CP. 2014. Nucleotide biosynthetic enzyme GMP synthase is a TRIM21‐controlled relay of p53 stabilization. Mol Cell 53:458–470. [DOI] [PubMed] [Google Scholar]
- Schoolmeester JK, Sukov WR, Maleszewski JJ, Bedroske PP, Folpe AL, Hodge JC. 2013. JAZF1 rearrangement in a mesenchymal tumor of nonendometrial stromal origin: Report of an unusual ossifying sarcoma of the heart demonstrating JAZF1/PHF1 fusion. Am J Surg Pathol 37:938–942. [DOI] [PubMed] [Google Scholar]
- Sciallis AP, Bedroske PP, Schoolmeester JK, Sukov WR, Keeney GL, Hodge JC, Bell DA. 2014. High‐grade endometrial stromal sarcomas: A clinicopathologic study of a group of tumors with heterogenous morphologic and genetic features. Am J Surg Pathol 38:1161–1172. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C, Li FP, Weidner N, Sandberg AA. 1991. An endometrial stromal sarcoma with clonal cytogenetic abnormalities. Cancer Genet Cytogenet 55:163–166. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsos A, Paskaleva P, Ferrari F, Deshpande V, Stoykova S, Contino G, Wong KK, Lan F, Trojer P, Park PJ, Bardeesy N. 2013. KDM2B promotes pancreatic cancer via Polycomb‐dependent and ‐independent transcriptional programs. J Clin Invest 123:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2014. WHO classification of tumors of female reproductive organs. Lyon: IARC WHO Classification of Tumours, No 6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


