Abstract
The serine–threonine protein kinase, protein kinase C-δ (PKCδ), is emerging as a bi-functional regulator of cell death and proliferation. Studies in PKCδ−/− mice have confirmed a pro-apoptotic role for this kinase in response to DNA damage and a tumor promoter role in some oncogenic contexts. In non-transformed cells, inhibition of PKCδ suppresses the release of cytochrome c and caspase activation, indicating a function upstream of apoptotic pathways. Data from PKCδ−/− mice demonstrate a role for PKCδ in the execution of DNA damage-induced and physiologic apoptosis. This has led to the important finding that inhibitors of PKCδ can be used therapeutically to reduce irradiation and chemotherapy-induced toxicity. By contrast, PKCδ is a tumor promoter in mouse models of mammary gland and lung cancer, and increased PKCδ expression is a negative prognostic indicator in Her2+ and other subtypes of human breast cancer. Understanding how these distinct functions of PKCδ are regulated is critical for the design of therapeutics to target this pathway. This review will discuss what is currently known about biological roles of PKCδ and prospects for targeting PKCδ in human disease.
Keywords: Protein kinase C, Signal transduction, Cancer, Apoptosis, Therapeutics
1. Introduction
The first identification of a protein kinase, phosphorylase kinase, and the elucidation of its role in glucose metabolism led to intense investigation of protein phosphorylation as a mechanism for regulation of biological processes (Fischer & Krebs, 1955; Krebs & Fischer, 1955). Since that discovery over 60 years ago, phosphorylation and dephosphorylation of proteins by protein kinases and phosphatases, respectively, have been shown to control virtually all cellular functions (Cohen, 2002). Indeed, it has been postulated that up to 30% of cell proteins may be post-translationally modified by phosphorylation on serine, threonine or tyrosine (Cohen, 2000). These dynamic events allow cells to respond rapidly to changes in the intra- and extracellular environment through modification of the activity, stability, and localization of proteins. In addition to kinases and phosphatases, signaling cascades can also include transmembrane receptors and a variety of cytoplasmic and nuclear anchoring and modifier proteins. As most protein kinases and phosphatases are widely expressed, dynamic remodeling of these multi-component signaling complexes may help to determine the specificity of substrate phosphorylation/dephosphorylation in response to diverse stimuli.
Early studies established the paradigm of regulation of protein kinase activity by cyclic nucleotide second messengers (Smith, White, Siegel, & Krebs, 1981). Thus, when it was identified nearly 40 years ago, protein kinase C (PKC) represented a unique type of protein kinase that was cyclic nucleotide-independent but Ca2+ and lipid-dependent (Inoue, Kishimoto, Takai, & Nishizuka, 1977; Takai, Kishimoto, Inoue, & Nishizuka, 1977). Further studies revealed a family of related isoforms that are conserved in mammals, and to a lesser extent in lower eukaryotes (Ono et al., 1988; Mellor & Parker, 1998). The identification of a lipid-regulated protein kinase family led to the exploration of membrane lipids as signaling molecules and was a turning point in our understanding of how the cell membrane transduces extracellular signals to regulate intracellular functions. Subsequent studies have identified additional lipid-regulated kinases, most notably the Akt isoforms, which together with PKC regulate intracellular signaling cascades that control many cellular processes.
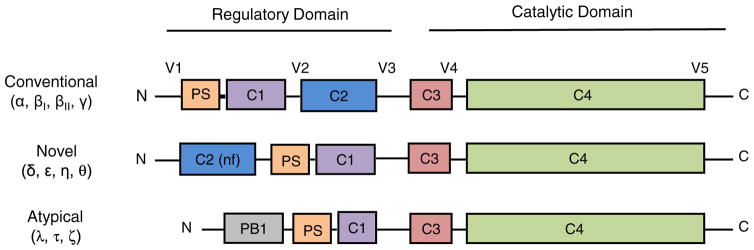
The PKC superfamily in mammals is comprised of 10 isoforms that are subdivided into conventional, novel, and atypical subfamilies based on their dependency on lipid second messengers, calcium and ion co-activators for activation (Fig. 1) (Newton, 2001). Most cells have multiple PKC isoforms. PKCα, -β, -δ, -ε, -λ/ι, and -ζ are expressed in many, or all tissues, while PKCθ, -γ, and -η have amore tissue specific expression pattern (Wetsel et al., 1992). Early attempts to understand the biological function of this kinase family used global regulators such as 12-O-tetradecanoylphorbol-13-acetate (TPA), which activates all conventional and novel PKC isoforms, and ATP-competitive kinase inhibitors, which have little or no isoform specificity. Hence, these approaches provided little insight into the function of specific PKC isoforms. The development of isoform-specific tools, including dominant inhibitory kinases, transgenic and knock-out mouse models, antisense/siRNA, and recently CRISPR/CAS9, has revealed unique roles for PKC isoforms in regulation of the DNA damage response, immune function, neural plasticity, cell migration, proliferation, survival, apoptosis, and metabolism (Reyland, 2009; Antal et al., 2015). Surprisingly, these studies suggest that PKC isoforms can have cell type specific functions, perhaps reflecting the interaction of kinases with unique anchoring proteins or regulatory pathways in a given cell type. Moreover, a given PKC isoform can participate in many different cellular pathways, and such “wiring” can change depending on the cellular environment. Given its central role in cell function, it seems likely that the alteration of PKC isoform expression, activity, or signaling could have important implications for human diseases, particularly in the context of cancer. This review will focus on PKCδ, a member of the novel isoform subfamily. We will explore the seemingly disparate biological functions of PKCδ, discuss how dysregulation of PKCδ may contribute to tissue damage and cancer, and explore prospects for targeting PKCδ in human disease.
Fig. 1.
PKC family members: Schematic representation of PKC domain composition among PKC isoform sub families. Pseudosubstrate (PS); lipid binding domain (C1-domain); Ca++ binding domain (C2-domain); ATP-binding domain (C3-domain); kinase domain (C4-domain); protein interaction domain (PB1). For novel PKC isoforms, the C2 domain is non-functional (nf). See text for additional information.
2. Physiologic regulation of PKC isoforms
A thorough understanding of how activation of PKC is regulated is critical as these steps represent potential targets for therapeutic intervention. Fig. 2 illustrates known mechanisms of PKCδ activation that will be discussed in the following sections. Regardless of the upstream signals, PKC activation is dependent on modification of the interaction of the C-terminal kinase core with a N-terminal regulatory domain (Newton, 2003). Early studies showed that allosteric activation of PKC is mediated by interaction with membrane lipids. Such “canonical” mechanisms of regulation have been well defined and known to be shared between PKC isoforms and other members of the closely related AGC kinase superfamily (Newton, 2003; Antal & Newton, 2014). However, it is also clear that PKC isoforms can function in a variety of subcellular sites and in response to stimuli that do not appear to induce hydrolysis of membrane phospholipid (Scott & Newton, 2012). A common theme of “non-canonical” activation is the modification of interactions between the C-terminal kinase core and the N-terminal regulatory domain via changes in intra- and intermolecular binding and kinase phosphorylation.
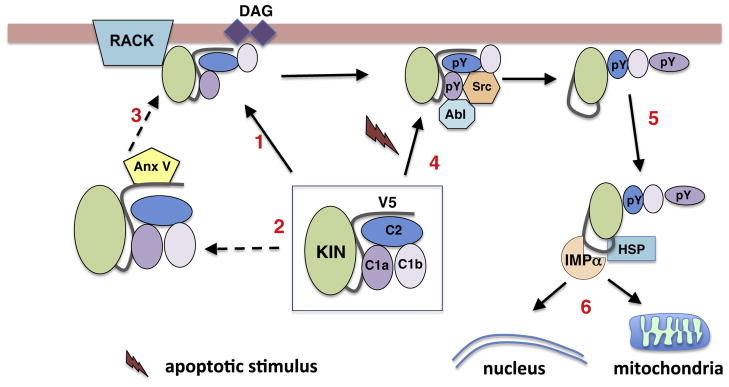
Fig. 2.
Mechanisms of PKCδ activation. PKCδ is maintained in an inactive state through a series of interactions between the kinase and regulatory domains (C1, C2a, and C1b). Activation can occur through multiple pathways. In response to generation of DAG PKCδ translocates to cellular membranes (arrow1). This may occur through interaction with scaffolding proteins such as RACKs (also AKAPs, C-KIPS, and p23/Tmp21; see text for more information). In some cases, translocation requires interactions with other cellular factors such as Annexin V (arrows 2 and 3). Non-canonical activation of PKCδ can result from upstream activation of Abl resulting in increased phosphorylation of Y155 (pY) in the C1a domain, which results in phosphorylation of Y64 (C2 domain) by Src kinase (arrow 4). This exposes a nuclear localization sequence that binds importin-α leading to nuclear import (arrows 5 and 6). Alternatively, PKC can traffic to mitochondria through interactions with HSP family members and Abl (arrow 6).
2.1. Canonical regulation
The catalytic domain of PKC is conserved between isoforms and includes the ATP-binding site, the kinase core, and the substrate-binding site (Fig. 1) (Gold, Barford, & Komander, 2006). The N-terminal regulatory domain is much less conserved and contains motifs that determine how specific isoforms are activated in response to extracellular signals. Regulatory modules in this domain include the pseudosubstrate motif and the diaclyglyerol (DAG) and Ca2+ cofactor binding C1 and C2 domains (Gold et al., 2006). The affinity of the C1 and C2 domains for DAG and Ca2+ determines the cofactor requirements for activation of specific PKC isoforms. PKCα, -β, and -γ make up the conventional PKC subfamily and contain functional C1 and C2 domains that bind DAG and Ca2+, respectively, while isoforms in the novel subfamily (PKCδ, -ε, -η, and -θ) have functional C1 domains and non-functional C2 domains (i.e., does not bind Ca2+), rendering these kinases dependent on DAG, but independent of Ca2+ for activation (Fig. 1).
Physiologic regulators of PKC, such as growth factor and G-protein-coupled receptors, stimulate the hydrolysis of membrane lipids by recruiting phospholipase C to the activated receptor (Kharait, Dhir, Lauffenburger, &Wells, 2006). Phospholipase C generates diaclyglyerol (DAG) and inositol-1,4,5-triphosphosphate (IP3) through hydrolysis of the membrane phospholipid, phosphoinositol. In response to increased generation of DAG, PKC localizes to the membrane via the C1 domain, enabling substrate binding and phosphorylation (Fig. 2, arrow 1) (Newton, 2001, 2003). The turnover of membrane phosphoinositol in response to extracellular signals is often referred to as the phosphoinositide (PI) cycle. Although first described in the 1950s, the significance of the PI cycle was not understood until much later when PKC was identified as the major cellular target for DAG.
2.2. Non-canonical regulation
For PKCδ, activation by alternative “non-canonical” pathways includes allosteric regulation via protein binding to the C1, C2, and/or V5 domains, tyrosine phosphorylation, and removal of the regulatory domain by caspase cleavage to generate a constitutively active kinase. A common theme of these activation mechanisms is the modification of interactions between the catalytic and regulatory domains. For example, tyrosine phosphorylation of PKCδ by non-receptor Src family tyrosine kinases, including Src, Fyn, and Lyn, can regulate redox-dependent activation of the kinase independent of lipids (Konishi et al., 2001; Rybin et al., 2004; Sumandea et al., 2008; Gong et al., 2015; Steinberg, 2015). Additional studies by Gong et al. show that redox-induced tyrosine phosphorylation of PKCδ can alter intramolecular interactions with the C2 domain and change substrate preference through a mechanism that involves dephosphorylation of Ser359 in the activation loop (Gong et al., 2015). Studies from our lab and others have demonstrated a role for tyrosine phosphorylation in activation of PKCδ in response to agonists (Rosenzweig, Aga-Mizrachi, Bak, & Sampson, 2004; Kajimoto, Sawamura, Tohyama, Mori, & Newton, 2010) and apoptotic stimuli(Yuan et al., 1998; Blass, Kronfeld, Kazimirsky, Blumberg, & Brodie, 2002; Humphries, Ohm, Schaack, Adwan, & Reyland, 2008; Lomonaco et al., 2008; Adwan, Ohm, Jones, Humphries, & Reyland, 2011; Gridling et al., 2014). Tyrosine residues shown to be important in the context of apoptosis include Y64, Y155, Y187, Y311, Y332, and Y512 (Konishi et al., 1997; Blass et al., 2002; Humphries et al., 2008; Gridling et al., 2014). Our studies show that phosphorylation of PKCδ at Y155 (C1a domain) and Y64 (C2 domain) exposes a cryptic nuclear localization signal (NLS) (V5 domain) through disruption of intramolecular contacts between the C2 and catalytic domains, allowing nuclear import (Adwan et al., 2011) (Fig. 2, arrows 4, 5 and 6; Fig. 3)).
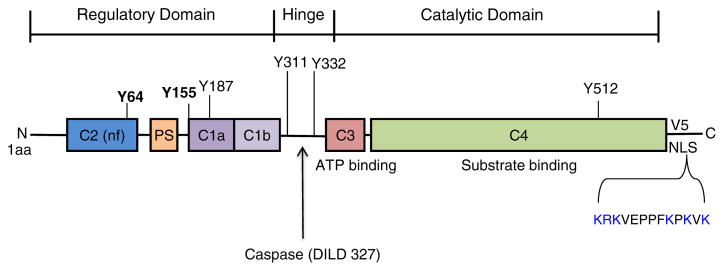
Fig. 3.
PKCδ: Functionally important motifs for apoptosis. Y64 and Y155 are known to be required for nuclear import in response to apoptotic signals. Other tyrosine residues known to be of functional importance are also shown. The nuclear localization signal (NLS) in the V5 region is highlighted in blue. Caspase cleavage in the hinge region releases a constitutively active, nuclear-targeted form of PKCδ. See text for details.
2.3. Subcellular targeting
In addition to the plasma membrane, there is evidence that “active” PKC can be targeted to many subcellular locations including the Golgi, ER, and nucleus. Studies by Gomel et al., which targeted exogenously expressed PKCδ to specific subcellular sites, revealed that cytoplasmic, nuclear, and mitochondrial targeting of PKCδ was pro-apoptotic, whereas targeting of PKCδ to the ER protected against etoposide and TNF-α-induced apoptosis (Gomel et al., 2007). Elegant studies by the Newton lab have used FRET-based fluorescence reporters to study the intracellular distribution of PKCδ in response to agonists (Kajimoto et al., 2010; Scott & Newton, 2012; Wu-Zhang, Schramm, Nabavi, Malinow, & Newton, 2012). These studies demonstrate recruitment of PKCδ to the mitochondria for regulation of metabolic respiration (Wu-Zhang et al., 2012) and UTP-induced nuclear translocation (Kajimoto et al., 2010). We have shown that the translocation of PKCδ from the cytoplasm to the nucleus is a highly regulated process that requires apoptosis-specific signals, including tyrosine phosphorylation of PKCδ and activation of caspase-3 (DeVries, Neville, & Reyland, 2002; Humphries et al., 2006).
Targeting of activated kinases to specific subcellular sites and/or substrates may depend on interaction with anchoring proteins. Such targeting proteins may function as scaffolds to assemble signaling complexes and/or disrupt intramolecular interactions to regulate kinase activation (Antal & Newton, 2014). DariaMochly-Rosen first proposed the existence of PKC isoform-specific anchoring proteins, or RACK’s (receptor for activated C-kinase) and her lab, and others have since identified a number of such proteins (Mochly-Rosen, 1995; Poole, Pula, Hers, Crosby, & Jones, 2004). They proposed that RACKs bind selectively to PKC isoforms upon activation and help to localize the active kinases to the membrane and possibly other intracellular sites. Other PKC binding proteins including C-KIPS (C-kinase interacting proteins) have been identified and may anchor PKC to sites where it can be activated and/ or direct PKC to specific subcellular compartments (Jaken & Parker, 2000). In addition, the A-kinase anchoring protein (AKAP) family of scaffold proteins has been shown to regulate localization of PKC to complexes that control NMDA and AMPA receptor function (Sanderson & Dell’Acqua, 2011).
Domains of PKCδ particularly important for facilitating protein–protein interactions include the C1 and C2 domains in the N-terminus and the highly flexible V5 domain in the C-terminal portion of the protein. Wang et al. have shown that interactions between the Golgi/ER protein p23/TMP21 and the C1 domain of PKCδ can modulate its apoptotic function by anchoring PKCδ to the perinuclear region (Wang, Xiao, & Kazanietz, 2011). Likewise, PKCδ–annexin V interactions mediated by the C2 domain can regulate membrane recruitment of PKCδ (Kheifets, Bright, Inagaki, Schechtman, & Mochly-Rosen, 2006; Hoque et al., 2014), and the binding of HSP25 to PKCδ has been shown to inhibit cell death (Lee et al., 2005) (see Fig. 2, arrows 2 and 3). Soltoff and colleagues have also identified a phosphotyrosine-binding domain within the C2 domain of PKC (Benes et al., 2005). The targeting of PKCδ to specific subcellular locales may explain the substrate specificity of different PKC isoforms as multiple isoforms can be activated downstream of a given signal. Importantly, the interaction of PKC with these anchoring proteins also suggests a level at which PKC activity may be modulated therapeutically.
3. Biological functions of PKCδ
PKCδ is a ubiquitously expressed kinase linked to the regulation of diverse cell and tissue functions. The current challenge is to understand how these seemingly disparate functions of PKCδ are specified, and whether they are mediated by the differential regulation of common signaling modules or pathways. Lessons from mouse models, particularly those in which the PKCδ gene has been disrupted, have been very informative in this regard, as has the manipulation of PKCδ expression and activity in vitro to test the contribution of PKCδ to specific signaling pathways.
3.1. Lessons from PKCδ knock-out and transgenic mice
Studies in PKCδ−/− mice have identified roles for this isoform in proliferation, immune function, apoptosis, and cell migration (Reyland, 2009). While loss of PKCδ has been reported to reduce fertility, post-natal development of PKCδ−/− mice appears to be normal (Ma, Baumann, & Viveiros, 2015), although subtle developmental phenotypes have been reported. For instance, we show a transient delay in mammary gland development in PKCδ−/− mice, including reduced ductal branching (Allen-Petersen et al., 2010). Stress and disease related phenotypes have also been reported. These include a link between PKCδ and the pathophysiology of nonalcoholic fatty liver disease (Greene et al., 2014; Zhang et al., 2014), endotoxin-induced lung injury (Chichger et al., 2012), and the expression of proinflammatory genes in vascular smooth muscle cells and pancreatic islet cells (Cantley et al., 2011; Ren et al., 2014).
Among the most significant phenotypes reported in PKCδ−/− mice are alterations in immune function. PKCδ−/− mice develop autoimmune disease as the mice age, supporting a role for PKCδ in self-antigen-induced B-cell tolerance (Mecklenbrauker, Saijo, Zheng, Leitges, & Tarakhovsky, 2002; Miyamoto et al., 2002; Banninger et al., 2011). Studies by Limnander et al. have shown that the PKCδ-dependent regulation of the Ca2+ sensor, STIM1, and Ca2+-dependent extracellular regulated kinase (ERK) activation is critical for the clonal deletion of auto-reactive B cells, suggesting a mechanism for the survival of auto-reactive B cells in these mice (Limnander et al., 2011). Interestingly, the expression of a dominant negative form of PKCδ specifically in T cells of transgenic mice also results in induction of lupus-like autoimmunity (Gorelik, Sawalha, Patel, Johnson, & Richardson, 2015). Recently two groups have described a similar phenotype associated with a rare loss-of-function mutation in the PRKCD gene in a human patient (Belot et al., 2013; Kuehn et al., 2013).
3.2. PKCδ and apoptosis
Studies from our lab and others have defined a critical role for PKCδ in the apoptotic response to DNA damage and cytotoxic stress (Majumder et al., 2001; Matassa, Carpenter, Biden, Humphries, & Reyland, 2001; Reyland, 2009; Basu & Pal, 2010). In vitro, salivary epithelial and smooth muscle cells isolated from PKCδ−/− mice are resistant to apoptotic stimuli (Leitges et al., 2001; Humphries et al., 2006). In vivo, PKCδ−/− mice are protected from irradiation-induced damage to the salivary gland and thymus and have a delay in mammary gland involution, a process driven by apoptosis (Humphries et al., 2006; Allen-Petersen et al., 2010). PKCδ can also contribute to apoptosis induced by death receptors including TRAIL and TNFα (Khwaja & Tatton, 1999; Gonzalez-Guerrico & Kazanietz, 2005; Yin, Sethi, & Reddy, 2010; Gordon, Anantharam, Kanthasamy, & Kanthasamy, 2012; Xu, Su, & Liu, 2012). Gonzalez-Guerrico et al. have shown that phorbol ester-induced apoptosis in LNCaP cells is mediated in part through PKCδ-dependent release of death receptor ligands (Gonzalez-Guerrico & Kazanietz, 2005). Likewise, PKCδ has been shown to regulate death receptor expression in response to ER stress (Xu et al., 2012) and is a downstream effector of TRAIL and TNFα-induced apoptosis (Gonzalez-Guerrico & Kazanietz, 2005; Yin et al., 2010; Gordon et al., 2012). The Mochly-Rosen lab has used tools based on RACKs to define a role for PKCδ in damage induced by ischemia and reperfusion in both the heart and the brain (Bright et al., 2004; Bright, Steinberg, & Mochly-Rosen, 2007; Churchill & Mochly-Rosen, 2007; Churchill, Qvit, & Mochly-Rosen, 2009; Churchill, Ferreira, Brum, Szweda, & Mochly-Rosen, 2010). Their studies show that the inhibition of PKCδ in mice prior to an experimentally induced ischemic event suppresses apoptosis and significantly reduces damage (Bright et al., 2004, 2007; Churchill &Mochly-Rosen, 2007; Churchill et al., 2009). Similar findings have recently been reported for ischemic injury to the lung (Kim et al., 2015, 2016).
The studies described above suggest a role for PKCδ as an integrator of damage signals upstream of the mitochondria. In support of this, our studies show that loss or inhibition of PKCδ suppresses early apoptotic events including loss of mitochondrial membrane potential and events downstream of the mitochondria such as caspase activation and DNA fragmentation (Reyland, Anderson, Matassa, Barzen, & Quissell, 1999; Matassa et al., 2001). Multiple mechanisms have been suggested by which PKCδ may control apoptosis including direct phosphorylation of substrates, regulation of transcription and mRNA processing, regulation of protein stability, and protein binding and sequestration. Potential substrates of PKCδ in apoptotic cells include heat shock proteins, transcription factors, kinases, DNA repair proteins, and Bcl-2 family members. For instance, PKCδ can promote apoptosis by suppressing phosphorylation of the pro-apoptotic protein, Bad (Murriel, Churchill, Inagaki, Szweda, & Mochly-Rosen, 2004), and through enhancing activation of Bax and Bak (Choi et al., 2006). PKCδ may also regulate cell death by binding to and sequestering proteins that either inhibit or promote apoptosis. For example, Masoumi et al. have shown that PKCδ can bind to Smac, an antagonist of inhibitor of activated proteases (IAPs) (Masoumi, Cornmark, Lonne, Hellman, & Larsson, 2012). Several reports suggest that PKCδ can regulate protein stability/degradation. PKCδ binds to Tap63 to increase its stability and promote apoptosis (Li et al., 2015), while PKCδ targets the antiapoptotic protein, Mcl-1, for degradation to trigger apoptosis (Sitailo, Tibudan, & Denning, 2006). In addition, PKCδ has been shown to regulate 3′ end processing of BIK mRNA to induce apoptosis through a mechanism that requires the Star-PAP processing complex and nuclear PIPKIα (Li et al., 2012). Finally, as discussed below, there is ample evidence that PKCδ can regulate cell survival pathways to induce apoptosis, including NF-κB, Akt and ERK (Lu, Liu, Yamaguchi, Miki, & Yoshida, 2009) as well as DNA damage-induced pathways.
3.2.1. Activation of PKCδ by pro-apoptotic signals
As PKCδ is a ubiquitously expressed kinase, its ability to activate apoptosis must be tightly regulated in order to prevent inappropriate cell death. Most studies indicate that specification of PKCδ function in the context of apoptosis is regulated by subcellular localization, and the translocation of PKCδ to specific subcellular areas in response to apoptosis stimuli is well documented. Many studies support a role for nuclear PKCδ in regulation of apoptosis (DeVries et al., 2002; DeVries-Seimon, Ohm, Humphries, & Reyland, 2007; Yoshida, 2008), however PKCδ has also been shown to translocate to the mitochondria in response to oxidative stress, ischemia and phorbol ester, where it presumably plays a direct role in regulating the apoptotic machinery (Li, Lorenzo, Bogi, Blumberg, & Yuspa, 1999; Majumder et al., 2000, 2001; Murriel et al., 2004; Qi & Mochly-Rosen, 2008). Additionally, the treatment of HeLa cells with ceramide also induces apoptosis and translocation of PKCδ to the Golgi (Kajimoto et al., 2004). Finally, splice variants of PKCδ have been identified that may play a role in modulating apoptosis (Patel, Song, & Cooper, 2006; Kim et al., 2011; Apostolatos et al., 2012). In particular, two splice variants, PKCδIX and PKCδII, both caspase-3 resistant, have been shown to inhibit apoptosis and/or promote survival (Patel et al., 2006, 2013; Kim et al., 2011; Apostolatos et al., 2012).
Our lab has shown that nuclear accumulation of PKCδ is critical for execution of the apoptotic program in response to DNA damage (DeVries et al., 2002). Key structural features important for the proapoptotic function of PKCδ in this context include a C-terminal nuclear localization signal (NLS), tyrosine residues in the regulatory domain, and a caspase cleavage site in the hinge region of the kinase (Fig. 3). Our studies support a model whereby nuclear translocation of PKCδ is initiated by tyrosine phosphorylation at Y155 and Y164 by Abl and Src tyrosine kinases, respectively, resulting in a conformational change that facilitates importin-α binding and nuclear import (see Fig. 2, arrows 4, 5, and 6) (Adwan et al., 2011; Wie, Adwan, DeGregori, Anderson, & Reyland, 2014). The sequential phosphorylation of PKCδ by Abl and Src is likely to be critical to cellular homeostasis as it assures that pro-apoptotic signaling by PKCδ is linked to activation of other cell death signals. Numerous other studies support a role for activation of PKCδ by Abl, and in some cases reciprocal activation of Abl by PKCδ (Yuan et al., 1998; Li et al., 2004; Lasfer et al., 2006). Activated caspase-3 is translocated to the nucleus with similar kinetics as PKCδ and can cleave nuclear PKCδ in the hinge region of the protein to generate the PKCδ catalytic fragment, a constitutively activated, nuclear-targeted form of PKCδ (Ghayur et al., 1996; DeVries-Seimon et al., 2007). While the PKCδ catalytic fragment is a potent inducer of apoptosis, whether it is functionally distinct from uncleaved PKCδ is not known, as both forms of PKCδ can induce apoptosis when overexpressed (DeVries-Seimon et al., 2007).
3.2.2. PKCδ and the DNA damage response
Many of the PKCδ substrates identified in apoptotic cells are nuclear proteins and suggest a role for PKCδ in the DNA damage response and/or DNA repair. Indeed, this proposed role for PKCδ fits well with the evidence that PKCδ functions as an apical regulator of apoptosis. Some studies place PKCδ upstream of the DNA damage sensors, DNA-dependent protein kinase, and ataxia telangiectasia mutated (ATM) and suggest a role for PKCδ in their activation in response to DNA damage (Bharti et al., 1998; Arango et al., 2012; Soriano-Carot, Quilis, Bano, & Igual, 2014). PKCδ has also been shown to function downstream of ATM activation (Li et al., 2004; LaGory, Sitailo, & Denning, 2010). Blocking PKCδ function inhibits phosphorylation of the ATM target, histone H2AX, a key regulator of the DNA damage response (Arango et al., 2012). PKCδ can also phosphorylate DNA-dependent protein kinase in cells exposed to genotoxins to inhibit DNA binding and suppress DNA double stranded break repair (Bharti et al., 1998). Further downstream, PKCδ phosphorylates the checkpoint protein Rad9 to regulate formation of the Rad9-Hus1-Rad1 (9-1-1) complex in response to DNA damage (Yoshida, Wang, Miki, & Kufe, 2003). Other studies support a role for PKC isoforms in cell cycle arrest (Watanabe et al., 1992) and specifically PKCδ in mediating G1 arrest through induction of p21 (Nakagawa et al., 2005), in S phase arrest (Santiago-Walker et al., 2005), and in the maintenance of the G2/M DNA damage checkpoint (LaGory et al., 2010). Finally, PKCδ may regulate the transcription of apoptotic genes and p21 through activation of p53 (Nakagawa et al., 2005; Perletti et al., 2005; Ryer et al., 2005; Yoshida, Liu, & Miki, 2006; Liu, Lu, Miki, & Yoshida, 2007; Saha, Adhikary, Kanade, Rorke, & Eckert, 2014).
3.2.3. Integration of PKCδ with cell survival pathways
Survival and death signaling are intimately linked such that cells can respond rapidly to environmental cues. During development, tissue patterning and remodeling occurs through orchestrated changes in proliferation and cell death. Likewise, growth factors and cell–cell contacts drive survival pathways such as mitogen-activated protein kinase (MAPK) and Akt in adult tissues, and disruption of these signals induces cell death. Thus, understanding how PKCδ interfaces with survival pathways is important for deciphering mechanisms of regulation of apoptosis by PKCδ. In one scenario, PKCδ could negatively regulate survival signaling in response to apoptotic signals, thus indirectly allowing apoptotic pathways to be activated. In this regard, PKCδ-dependent regulation of MAPK pathways (Efimova, Broome, & Eckert, 2004; Murriel et al., 2004; Blank et al., 2013), PI3K-Akt (Murriel et al., 2004), signal transducer and activator of transcription-1 (STAT-1) (DeVries, Kalkofen, Matassa, & Reyland, 2004), and NFκB (Ren et al., 2014; Dong et al., 2015) has been shown in many contexts. In a second scenario, PKCδ could directly regulate the DNA damage response and cell cycle arrest mechanisms, as discussed above, pushing a cell toward initiation of apoptotic pathways (Bharti et al., 1998; Yoshida et al., 2003; LaGory et al., 2010; Arango et al., 2012; Soriano-Carot et al., 2014). Evidence supports both scenarios, raising the possibility that PKCδ, or a PKCδ effector, may have a dual function in cell survival and cell death.
ERK kinases and other members of the MAPK family (c-Jun. N-terminal kinases (JNKs), and p38 MAPKs) are activated via cell surface receptors to regulate survival, proliferation, migration, and apoptosis (Zehorai, Yao, Plotnikov, & Seger, 2010; Plotnikov, Zehorai, Procaccia, & Seger, 2011). Studies have shown a role for PKCδ in regulation of all three MAPK pathways. PKCδ regulates ERK signaling downstream of growth factors, and in response to cell stress, including apoptotic stimuli (Efimova et al., 2004; Ryer et al., 2005; Choi et al., 2006; Hu, Kang, Philp, & Li, 2007; Symonds et al., 2011). Biphasic activation of ERK is often described in cells treated with DNA damaging agents (Zhuang & Schnellmann, 2006). In this regard, PKCδ has been shown to regulate the sustained activation of ERK, which is typically associated with cell death (Basu & Tu, 2005; Zhuang & Schnellmann, 2006; Lomonaco et al., 2008; Cagnol & Chambard, 2010). PKCδ has also been shown to enhance radiation-induced apoptosis via ERK activation and suppression of radiation-induced G2-M arrest (Lee et al., 2002). Other studies indicate that the activation of ERK in the context of DNA damage induces the G2M checkpoint (Tang et al., 2002; Wei, Xie, Tao, & Tang, 2010; Kolb, Greer, Cao, Cowan, & Yan, 2012). In addition to ERK, PKCδ also activates the JNK pathway in irradiation and chemotherapy-induced apoptosis (Yoshida, Miki, & Kufe, 2002). Primary cells derived from PKCδ−/− mice are deficient in JNK activation, and JNK activation in vivo in response to irradiation is reduced in these mice (Humphries et al., 2006). Finally, activation of p38δ downstream of PKCδ has been reported in many cell types (Efimova et al., 2004; Ryer et al., 2005; Choi et al., 2006; Blank et al., 2013; Saha et al., 2014). The activation of a PKCδ->p38δ pathway can regulate p53 activation, p21 expression (Ryer et al., 2005; Saha et al., 2014) and the subcellular localization of ERK (Efimova et al., 2004), potentially activating cell cycle arrest.
3.3. Dual role of PKCδ in Cancer
The ability of phorbol esters to promote tumors has been known for many years; however, it was not until the identification of PKC as the major phorbol ester “receptor” in the cell that the potential contribution of these kinases to human cancer was investigated (Castagna et al., 1982; Kikkawa, Takai, Tanaka, Miyake, & Nishizuka, 1983). In contrast to the pro-apoptotic function demonstrated in most non-transformed cells, numerous studies demonstrate a role for PKCδ in cancer cell proliferation and survival. Likewise, PKCδ has been shown to function as a tumor promoter in most (but not all) mouse models of cancer studied (Mauro et al., 2010; Symonds et al., 2011; Allen-Petersen, Carter, Ohm, & Reyland, 2014). Changes in PKCδ expression have been reported in some human tumors (Kahl-Rainer, Karner-Hanusch, Weiss, & Marian, 1994; D’Costa et al., 2006; Reno et al., 2008; Fukase et al., 2011) and increased PKCδ expression correlates with poor prognosis in specific subtypes of human breast cancer (McKiernan et al., 2008; Allen-Petersen et al., 2014). Notably, functional genomic alterations of PKCδ are rarely found (Antal & Newton, 2014). In fact, only two mutations in PKCδ associated with any disease have been identified (Belot et al., 2013; Kuehn et al., 2013). Thus, other factors such as PKCδ expression, localization, and/or the oncogenic context of tumor cells may be important for dictating the function of this kinase in cancer.
3.3.1. PKCδ as a tumor suppressor
As apoptosis is an important anti-tumor mechanism, PKCδ was initially proposed to be a tumor suppressor. Early studies showed that PKCδ overexpressing transgenic mice were resistant to certain types of chemically induced skin cancer (Reddig et al., 1999); however, these mice did get skin cancers with repeated exposure to UV irradiation (Aziz, Wheeler, Bhamb, & Verma, 2006). Similarly, Mischak et al. showed that overexpression of PKCδ could suppress proliferation of NIH 3T3 cells (Mischak et al., 1993), and similar findings have been reported in a variety of cancer cells. For instance, PKCδ negatively regulates the Wnt pathway to suppress proliferation of colon cancer cells (Hernández-Maqueda, Luna-Ulloa, Santoyo-Ramos, Castañeda-Patlán, & Robles-Flores, 2013), Gli1 activation and Hedgehog signaling in hepatocellular carcinoma(Cai, Li, Gao, Xie, & Evers, 2009), ERK activation in breast cancer cells, and the proliferation and migration of human malignant fibrous histocytoma cells (Fukase et al., 2011). PKCδ also regulates apoptosis in prostate cancer cells through a mechanismin dependent of caspase cleavage and that involves the p38MAPK pathway (Fujii et al., 2000; Tanaka, Gavrielides, Mitsuuchi, Fujii, & Kazanietz, 2003). In addition, there are reported roles for PKCδ in the negative regulation of cell cycle progression and activation of cell cycle arrest (Nakagawa et al., 2005; Santiago-Walker et al., 2005; LaGory et al., 2010). Such proapoptotic and anti-proliferative roles for PKCδ in vitro are consistent with a tumor suppressor function; however, experimental models of cancer in mice generally support a tumor promotional function for PKCδ. This may reflect in part the more complex milieu in vivo, which includes both tumor cells and stroma. The specific genetic lesions that drive cancer cells may also dictate PKCδ function, as discussed below.
3.3.2. PKCδ as a tumor promoter
Our lab has shown that lung tumors are dramatically reduced in urethane treated PKCδ−/− mice compared to PKCδ+/+ mice (Symonds et al., 2011), and that Her2/neu-induced tumorigenesis is reduced in PKCδ−/− mice (Allen-Petersen et al., 2014). Likewise, PKCδ supports the proliferation and survival of cancer stem cells (Chen, Forman, Williams, & Faller, 2014; Berardi et al., 2015) and helps to maintain c-Kit activation in colon cancer cells by promoting its recycling (Park et al., 2013). Other studies have demonstrated a synthetic lethal interaction between K-ras and PKCδ (Luo et al., 2009), and a recent report shows that the activation of PKCδ confers resistance to ALK inhibitors in ALK-dependent NSCLC cells (Wilson et al., 2015).
Our studies in KRAS mutant lung cancer suggest that PKCδ cooperates with other signaling circuits to drive specific tumor phenotypes. Thus, the function of PKCδ may be dictated by the specific oncogenic context of a cancer cell. Recently, several groups have defined subpopulations of KRAS mutant tumors based on their functional dependence on K-ras for survival (Singh et al., 2009; Collisson et al., 2011). We have previously shown that functional dependency on K-ras in lung cancer cells correlates with a pro-survival, pro-tumorigenic role for PKCδ (Symonds et al., 2011). By contrast, in the subset of KRAS mutant lung cancers that are no longer functionally dependent on K-ras, PKCδ regulates the apoptotic response to chemotherapeutic agents (Ohm et al., unpublished results), and PKCδ has been linked to survival pathways, particularly MAPK signaling cascades, through regulation of growth factor receptor signaling (Farshori, Shah, Arora, Martinez-Fuentes, & Catt, 2003; Iwabu, Smith, Allen, Lauffenburger, & Wells, 2004; Kharait et al., 2006; Xia & Land, 2007; Paugh et al., 2008). Our studies suggest that the pro-survival role of PKCδ in K-ras-dependent lung cancer cells is mediated through regulation of ERK, as the depletion of PKCδ results in decreased activation of ERK. However, the depletion of PKCδ does not suppress K-ras activation in K-ras-dependent lung cancer cells, indicating that PKCδ functions downstream of K-ras or in a collateral pathway (Symonds et al., 2011). Studies by Ueda et al. support this interpretation as they show that PKCδ regulates ERK signaling downstream of K-ras through activation of Raf (Ueda et al., 1996). PKCδ likely contributes to ERK activation by regulating the recycling of activated cell surface receptors as has been shown for ErbB2, KIT, and EGFR (Llado et al., 2004; Hu, Cheng, Pan, Wu, & Wu, 2013; Park et al., 2013; Bailey et al., 2014), and through ADAM-17-mediated ectodomain shedding of receptor tyrosine kinases (Kveiborg, Instrell, Rowlands, Howell, & Parker, 2011; Thorp et al., 2011). PKCδ may also support tumorigenesis via other survival pathways as studies from Xia et al. show that PKCδ is required for survival of NIH3T3 and pancreatic cancer cells expressing activated K-ras through a mechanism resulting in activation of Akt (Xia, Forman, & Faller, 2007; Xia, Chen, Forman, & Faller, 2009). We have shown that PKCδ is required for formation of an active signaling complex between Src and the Her2 receptor (Allen-Petersen et al., 2014). Similarly, PKCδ is important for the tissue colonization through formation of a signaling complex with Src, Akt, and CDCP1 (Casar et al., 2014). Still other studies have defined roles for PKCδ in the invasion and migration of tumor cells (Cerda et al., 2001; Jackson et al., 2005; Villar et al., 2007; Kho et al., 2009; Symonds et al., 2011). A novel substrate of PKCδ, BAG3, has recently been identified, and phosphorylation of BAG3 has been shown to regulate epithelial–mesenchymal transition in thyroid cancer cells (Li et al., 2013). Finally, studies from our lab and others have identified PKCδ as a potential regulator of tumor progression and metastasis through modulation of integrin αVβ3 regulated survival pathways (Putnam, Schulz, Freiter, Bill, & Miranti, 2009; Symonds et al., in press).
4. Targeting PKCδ
A major challenge in developing PKC-specific inhibitors is the very high degree of homology and conformation similarity between the different isoforms. This is particularly so for the kinase domains, and as this similarity extends to many other kinases, it becomes more challenging to target PKC in an isoform-specific manner. Another major challenge is that cells express multiple isoforms of PKC that are required for normal function, and each isoform can often regulate multiple functions. Therefore, inhibiting PKC can have significant off-target effects and also produce unwanted effects by on-target inhibition. As reviewed above, the activity of different PKC isoforms is in many cases tightly regulated by a series of inter- and intramolecular interactions that are accompanied by conformational changes in the enzyme leading to changes in subcellular localization and activity. One of the hallmarks of PKC activation is enzyme translocation to the plasma membrane, but also to the other cellular organelles. This translocation leads to the formation of newprotein–protein and protein–lipid interactions. Focusing on regions of the protein away from the active site, and which share much less sequence homology, has allowed development of novel reagents that can function either to activate or inhibit PKC activity in an isoform-specific manner (Mochly-Rosen, Das, & Grimes, 2012; Gower, Chang, & Maly, 2014).
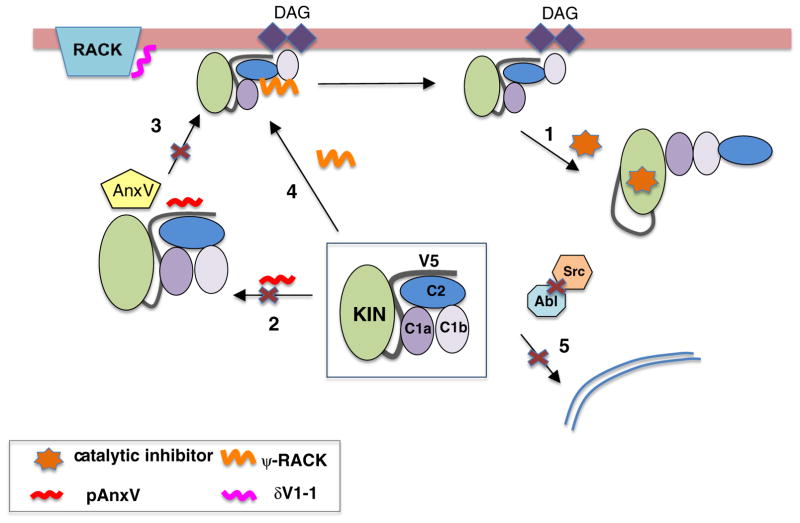
Fig. 4 summarizes potential mechanisms of targeting PKCδ therapeutically; these are discussed in more detail below. In general, such approaches can be classified according to their specific site of action (Mochly-Rosen et al., 2012). Agents that target catalytic activity of the kinase domain can either bind competitively to the ATP-binding site, or alternatively may compete for substrate binding (Fig. 4, arrow1). Approaches that target the regulatory domains may compete for binding (Fig. 4, arrows 2 and 3, Anx V peptide) or mimic the effects of DAG and lipid binding (Fig. 4, arrow4, Ψ-RACK), to either inhibit or activate the enzyme, respectively. Alternatively, reagents that target protein–protein interactions can be used to disrupt intramolecular regulatory interactions leading to increased activity, or to disrupt inter-molecular interactions to reduce activity (δV1-1). A final option that has recently been exploited uses knowledge of the specific pathways leading to non-canonical PKCδ activation by inhibiting upstream events such as tyrosine phosphorylation by Abl and Src to prevent PKCδ activation (Fig. 4, arrow 5).
Fig. 4.
Methods to target PKCδ activity. Activated PKC can be targeted with small molecule ATP-binding antagonists or substrate competitors (arrow1). Alternative strategies to overcome off-target effects have focused on disrupting intra- and intermolecular interactions. Peptides derived from inter-molecular binding partners, including Annexin V can disrupt interactions by binding to the C2 and V5 domains and prevent appropriate trafficking and activation (arrows 2 and 3). While peptides derived from PKCδ such as the δV1-1 peptide can bind to the target protein (e.g., RACKs) and prevent activation. Conversely, the pseudo-rack ψδ-RACK peptide derived from the C2-domain appears to disrupt regulatory interactions that maintain the kinase in the inactive state which leads to kinase activation (arrow 4). More recently targeting the upstream kinases (Abl and Src) required for non-canonical activation can also prevent PKCδ activation and protect against irradiation-induced cell death (arrow 5).
4.1. Targeting kinase activity
Significant efforts have been made to target PKC activity by discovering competitive inhibitors of ATP binding (Sobhia, Grewal, Bhat, Rohit, & Punia, 2012). The most well characterized of these are the bisindolylmaleimides (Wilkinson, Parker, & Nixon, 1993) exemplified by staurosporine, a non-specific pan-kinase inhibitor (Karaman et al., 2008). A number of efforts have focused on identifying derivatives; these include 7-hydroxystaurosporine (UCN-01) (Seynaeve, Kazanietz, Blumberg, Sausville, & Worland, 1994) and N-benzoyl-staurosporine (Midostaurin or PKC412) (Propper et al., 2001; Monnerat et al., 2004). However, these agents display significant off-target effects (Karaman et al., 2008). Sotrastaurin (AEB071), another potent PKC inhibitor (Ki 0.2–3.2 nM), shows some specificity for PKCθ, PKCα, and PKCβ compared to PKCδ, PKCε, and PKCη (Evenou et al., 2009), but it also shows a significant inhibition of many other kinases. Sotrastaurin inhibits T-cell activation by peptide–MHC and CD28 co-stimulation in a PKC-dependent manner (Evenou et al., 2009; Wagner et al., 2009) and is currently in clinical trials for liver transplantation (Matz et al., 2011) and renal transplantation (Kovarik et al., 2011).
Efforts to identify more isoform selective inhibitors of PKC led to the discovery of inhibitors that were selective for PKCβ. This included enzastaurin (Graff et al., 2005; Lee et al., 2008; Neri et al., 2008), which shows a 3- to 5-fold selectivity for PKCβ over other PKC isoforms but also inhibits other kinases. Enzastaurin is currently in clinical trials, either alone or in combination with conventional chemotherapies for malignant gliomas and pediatric brain tumors (Kilburn et al., 2015; Odia et al., 2015). Ruboxistaurin (RBX:LY333531) is another inhibitor shown to inhibit PKCβ 10–100× more selectively than other isoforms, and with minimal toxicity (Danis & Sheetz, 2009). Preliminary clinical trials suggested potential efficacy to reduce loss of vision in patients with diabetic retinopathy; however, subsequently it appears that the overall effect was very small (Deissler & Lang, 2016).
Efforts to develop novel PKCδ inhibitors using different scaffolds have met with limited success. A notable example is rottlerin, which was initially described as a selective inhibitor for PKCδ (Gschwendt et al., 1994). However, it was ultimately demonstrated that rottlerin could inhibit multiple kinases and was not selective for PKCδ (Soltoff, 2007). Recently, Faller and Williams et al. used computational pharmacophore modeling of the interactions of rottlerin and staurosporine with the kinase domains of PKC to develop novel PKCδ selective inhibitors (Chen et al., 2011, 2014; Takashima et al., 2014). These chimeric hybrid inhibitors were designed to retain the features of rottlerin that give it the apparent specificity over other PKC isozymes, while retaining features of staurosporine (and other general inhibitors) that make it a potent inhibitor. The third-generation compound (BJE106) targets PKCδ with an IC50 of 50 nM and shows a 1000-fold selectivity for PKCδ over PKCα (IC50 50 μM) (Takashima et al., 2014). These inhibitors were effective in suppressing growth of NRAS mutant melanoma cell lines, and this was mediated by caspase-dependent apoptosis. Furthermore, they showed that these inhibitors were also effective in inducing cell death in BRAF-mutant melanomas that had evolved resistance to BRAF inhibitors, suggesting a potential clinical application (Takashima et al., 2014).
A recurrent theme is that almost all kinase inhibitors that function by competing for ATP binding exhibit significant off-target effects (Karaman et al., 2008). To overcome these issues, an alternative approach has been developed that uses bisubstrate and bivalent inhibitors that target different regions of the kinase (reviewed in Gower et al., 2014). This approach was first developed for PKC by Ricourt et al., where they linked a pseudosubstrate peptide with a 5-isoquinolinylsulfonyl ATP-competitive inhibitor (Ricouart, Gesquiere, Tartar, & Sergheraert, 1991) and showed a 67-fold increase in potency versus the 5-isoquinolinylsulfonyl inhibitor alone. Subsequent efforts to target other isoforms of PKC show modest selectivity that was dependent on the identity of the pseudosubstrate sequence, which appears to steer the selectivity (van Ameijde et al., 2010).
4.2. Targeting the C1 regulatory domains
Exploration of PKC as a therapeutic target in cancer was initially based on its identification as the major cellular receptor for tumor-promoting phorbol esters (Castagna et al., 1982; Kikkawa et al., 1983; Ono et al., 1988; Newton, 1993). Binding to these phorbol esters occurs through interactions with the C1 domains, which are also involved in binding to DAG (Burns & Bell, 1991; Quest & Bell, 1994; Kazanietz, Barchi, Omichinski, & Blumberg, 1995). Subsequently, it was found that these same domains are involved in binding to the potent antitumor agent bryostatin-1. Bryostatin-1 is a partial agonist of several isoforms of PKC and leads to activation and translocation to the cell membrane. Paradoxically, bryostatin-1 can prevent PKCδ membrane translocation and down-regulation in prostate cancer cells (Szallasi et al., 1994; von Burstin, Xiao, & Kazanietz, 2010). In contrast to phorbol ester, bryostatin-1 was found to have little tumor promoter activity but instead could antagonize the effects of tumor promoters (Hennings et al., 1987; Szallasi et al., 1994). Bryostatin-1 subsequently was shown to significantly inhibit the growth of multiple cancer cell lines both in vitro and in vivo. It showed promising activity in phase I clinical trials; however, phase II trials as a monotherapy were disappointing (Propper et al., 1998; Zonder et al., 2001; Ajani et al., 2006). However, it has shown improved efficacy in combination therapy with other cytotoxic agents in advanced esophageal, gastric, or gastroesophageal cancers (Ajani et al., 2006; Ku et al., 2008; Barr et al., 2009) and in B-cell non-Hodgkin lymphoma (Barr et al., 2009). PKC activation also plays a critical role in Alzheimer’s disease (Savage et al., 1998) and AIDs (Roebuck, Gu, & Kagnoff, 1996), and bryostatin-1 is being investigated for the treatment of these diseases. The limited availability of bryostatin-1 and the complex synthesis has impeded more extensive studies. Significant efforts are underway to synthesize more accessible analogs with similar activity (Wender et al., 2011).
4.3. Disrupting protein–protein interactions to modulate PKC activity
Targeting PKC activity in an isoform-specific manner through the kinase or C1 regulatory domains has significant challenges because of the high degree of sequence and structural homology that exist between the different isoforms. Recognizing that different isoforms of PKC have isoform specific interactions, both through intramolecular regulatory interactions but also as part of inter-molecular targeting effects, led to efforts to understand the nature of these interactions. Early studies used peptides as tools to probe the mechanisms and functional consequences of these various protein–protein interactions, but it soon became apparent that peptides capable of mimicking or interfering with these important regulatory and scaffolding interactions could be useful of themselves as potential therapeutic molecules.
The Mochly-Rosen group pioneered the development of peptide agonists and antagonists as a way to modulate PKC activity in an isoform-specific manner (reviewed in Budas, Koyanagi, Churchill, & Mochly-Rosen, 2007; Churchill et al., 2009; Qvit & Mochly-Rosen, 2010). The activity of PKC is regulated via intramolecular interactions of the kinase domain with the regulatory domains that maintain the enzyme in an inactive state. Following activation, these regulatory domains become available to make interactions with scaffolding partners, or RACKs, that regulate the unique subcellular localizations (Mochly-Rosen, 1995; Budas et al., 2007). Mochly-Rosen and colleagues used a number of criteria to identify peptides that could potentially disrupt such protein–protein interactions (reviewed in Budas et al., 2007). They reasoned that scaffolding interactions with RACKs and other cellular partners should occur at specific sites, and that regions of PKC that shared homology with regions of the RACK could form similar interactions within the inactive state of the enzyme and function as auto-inhibitory sequences. Therefore, peptides derived from PKC that have sequence similarity to the RACK protein, termed pseudo-RACK (Ψ-RACK) peptides, could compete for the intramolecular interaction to activate the enzyme. Similarly, peptides derived from the RACK protein itself, or regions of PKC known to interact with the RACK, could prevent scaffolding interactions and thus inhibit PKC activity. This led to the identification of number of specific peptides that can function as isoform-specific inhibitors and which have potential therapeutic relevance. The δV1-1 peptide (aa8–17 of PKCδ, KAI-9803 or delcasertib), which has a thousand-fold selectivity for PKCδ over other isoforms, was shown in animal models to reduce infarct size when given during reperfusion following myocardial infarction (Inagaki et al., 2003; Qvit & Mochly-Rosen, 2014). However, the δV1-1 peptide did not show any significant effect in phase II trials in humans (Lincoff et al., 2014). A similar peptide derived from the C2 domain of PKCε, εV1–2 (aa 14–21, KAI-1678), has a 100-fold selectivity for PKCε over other enzymes and showed efficacy in animal models for prevention of heart failure (Inagaki, Koyanagi, Berry, Sun, & Mochly-Rosen, 2008) and for the inhibition of inflammatory pain (Sweitzer et al., 2004), but again did not show any significant effect in human clinical trials (Cousins, Pickthorn, Huang, Critchley, & Bell, 2013; Moodie, Bisley, Huang, Pickthorn, & Bell, 2013).
Other studies have targeted the interaction of PKCδ with HSP27 to sensitize cancer cells to apoptosis (Kim et al., 2007; Lee et al., 2011). A heptapeptide (HEPT) based on the PKCδ catalytic V5 (PKCδ-V5) region (FEQFLDI) was shown to inhibit the interaction of HSP27 with PKCδ and to restore HSP27-mediated radio- or chemoresistance in human lung and breast cancer cells (Kim et al., 2007; Lee et al., 2011). Fourteen treatments with HEPT (100mg/kg of body weight given intravenously) in mice did not induce toxicity during a 2-month examination (Lee et al., 2011). However, detailed in vivo studies of tumor targeting or therapeutic effects of the abolition of radio- and chemoresistance were not performed.
Scaffolding interactions with RACKs, AKAPs, and other cellular proteins clearly play important roles in regulating PKC activity and provide additional opportunities for targeting PKC in diseases. For example, the apoptotic activity of PKCδ in LnCaP cells in response to doxorobuicin and phorbol esters is negatively regulated by an association with the Golgi/ER protein p23/Tmp21 (Wang et al., 2011). The association of PKCδ with p23/TMP21 occurs through its C1b domain, and mutations in the C1b domain that disrupt this interaction, or alternatively knockdown of p23, dramatically increase cell death (Wang et al., 2011). Other C1 domains, including C1 domains in PKCε, PKCθ, and the RacGAP β also interact with p23/Tmp21 through their C1 domains (Wang & Kazanietz, 2010). This suggests that the discovery of peptides or small molecules that could disrupt this interaction could have potential therapeutic applications.
4.4. Inhibiting signaling pathways upstream of PKCδ activation
Patients undergoing cancer treatment will typically be subjected to chemo- and radiation therapy either as solo or combined modalities. Although the goal of such treatments is to eradicate the tumor, most patients also suffer debilitating damage to non-tumor tissue, which can impact their quality of life and in some cases limit the duration of therapy (Vissink et al., 2010). We have shown that PKCδ−/− mice are protected from IR-induced damage to the salivary gland, suggesting that the inhibition of PKCδ may be an attractive strategy for protection of non-tumor tissues (Humphries et al., 2006). In support of this, Arany et al. have shown that depletion of PKCδ in the salivary gland using siRNA-coupled nanoparticles can protect against IR-induced loss of salivary gland function (Arany et al., 2012; Arany, Benoit, Dewhurst, & Ovitt, 2013), while Pabla et al. report that loss of PKCδ leads to reduced damage to the kidneys of mice treated with cisplatin for GI cancer (Pabla et al., 2011).
As tyrosine phosphorylation initiates pro-apoptotic signaling by PKCδ, we proposed that inhibiting the tyrosine kinases that mediate this step may suppress apoptosis in the salivary gland. We show that phosphorylation of PKCδ at Y64 and Y155, nuclear accumulation of PKCδ, and apoptosis can be specifically inhibited by pre-treatment with tyrosine kinase inhibitors such as dasatinib and imatinib (Wie et al., 2014). Importantly, suppression of apoptosis in mice treated with irradiation to the head and neck is coupled with protection of salivary function in vivo (Wie and Reyland, unpublished data). Many tyrosine kinase inhibitors are FDA approved for cancer therapy; hence, these drugs could potentially be quickly transitioned into the clinic for use in patients receiving radiation therapy. Our findings provide a rationale for future clinical trials to investigate TKIs as a radioprotective therapy.
5. Conclusions
Over the past 40 years, our understanding of how the PKC family, and PKCδ specifically, is regulated has expanded tremendously. For instance, it is now appreciated that in addition to canonical lipid-regulated activation, many non-canonical mechanisms, including protein–protein interactions and tyrosine phosphorylation, regulate kinase activation. Furthermore, the ubiquitous expression of PKCδ suggests that its activation is highly regulated and that target specification must depend on interactions with other signaling and scaffolding proteins. Given the paucity of genetic alterations in PKCδ, altered expression, subcellular localization or activation of PKCδ may contribute to human diseases. Thus, strategies that directly alter PKCδ activity, or that regulate PKCδ activation, are likely to be useful therapeutically. The development of novel therapeutics for targeting PKCδ and other PKC isoforms may have wide applications for the treatment of cancer and other diseases.
Abbreviations
- PKC
Protein kinase C
- PKCδ
Protein kinase C-δ
- TPA
12-O-tetradecanoylphorbol-13-acetate
- DAG
Diaclyglyerol
- RACK
Receptor for activated C-kinase
- AKAP
A-kinase anchoring protein
- ATM
Ataxia telangiectasia mutated
- ERK
Extracellular regulated kinase
- MAPK
Mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Adwan TS, Ohm AM, Jones DN, Humphries MJ, Reyland ME. Regulated binding of importin-alpha to protein kinase C delta in response to apoptotic signals facilitates nuclear import. J Biol Chem. 2011;286:35716–35724. doi: 10.1074/jbc.M111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajani JA, Jiang Y, Faust J, Chang BB, Ho L, Yao JC, … Bleyer A. A multicenter phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Investig New Drugs. 2006;24:353–357. doi: 10.1007/s10637-006-6452-1. [DOI] [PubMed] [Google Scholar]
- Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene. 2014;33:1306–1315. doi: 10.1038/onc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Petersen BL, Miller MR, Neville MC, Anderson SM, Nakayama KI, Reyland ME. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis. 2010;1:e17. doi: 10.1038/cddis.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Newton AC. Tuning the signalling output of protein kinase C. Biochem Soc Trans. 2014;42:1477–1483. doi: 10.1042/BST20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, … Newton AC. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160:489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolatos A, Song S, Acosta S, Peart M, Watson JE, Bickford P, … Patel NA. Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem. 2012;287:9299–9310. doi: 10.1074/jbc.M111.313080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango D, Parihar A, Villamena FA, Wang L, Freitas MA, Grotewold E, Doseff AI. Apigenin induces DNA damage through the PKCdelta-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem Pharmacol. 2012;84:1571–1580. doi: 10.1016/j.bcp.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany S, Benoit DS, Dewhurst S, Ovitt CE. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther. 2013;21:1182–1194. doi: 10.1038/mt.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany S, Xu Q, Hernady E, Benoit DS, Dewhurst S, Ovitt CE. Pro-apoptotic gene knockdown mediated by nanocomplexed siRNA reduces radiation damage in primary salivary gland cultures. J Cell Biochem. 2012;113:1955–1965. doi: 10.1002/jcb.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MH, Wheeler DL, Bhamb B, Verma AK. Protein kinase C delta overexpressing transgenic mice are resistant to chemically but not to UV radiation-induced development of squamous cell carcinomas: a possible link to specific cytokines and cyclooxygenase-2. Cancer Res. 2006;66:713–722. doi: 10.1158/0008-5472.CAN-05-2684. [DOI] [PubMed] [Google Scholar]
- Bailey TA, Luan H, Tom E, Bielecki TA, Mohapatra B, Ahmad G, … Band H. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem. 2014;289:30443–30458. doi: 10.1074/jbc.M114.608992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banninger GP, Cha S, Said MS, Pauley KM, Carter CJ, Onate M, … Reyland ME. Loss of PKCdelta results in characteristics of Sjogren’s syndrome including salivary gland dysfunction. Oral Dis. 2011;17:601–609. doi: 10.1111/j.1601-0825.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr PM, Lazarus HM, Cooper BW, Schluchter MD, Panneerselvam A, Jacobberger JW, … Remick SC. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am J Hematol. 2009;84:484–487. doi: 10.1002/ajh.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. Scientific World Journal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Tu H. Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cdelta. Biochem Biophys Res Commun. 2005;334:1068–1073. doi: 10.1016/j.bbrc.2005.06.199. [DOI] [PubMed] [Google Scholar]
- Belot A, Kasher PR, Trotter EW, Foray AP, Debaud AL, Rice GI, … Bonnefoy N. Protein kinase cdelta deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65:2161–2171. doi: 10.1002/art.38008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Berardi DE, Flumian C, Rodriguez CE, Diaz Bessone MI, Cirigliano SM, Bal de Kier Joffe ED, … Todaro LB. PKCdelta inhibition impairs mammary cancer proliferative capacity but selects cancer stem cells, involving autophagy. J Cell Biochem. 2015 doi: 10.1002/jcb.25358. [DOI] [PubMed] [Google Scholar]
- Bharti A, Kraeft SK, Gounder M, Pandey P, Jin S, Yuan ZM, … Kharbanda S. Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Mol Cell Biol. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank VC, Bertucci L, Furmento VA, Pena C, Marino VJ, Roguin LP. A chimeric cyclic interferon-alpha2b peptide induces apoptosis by sequential activation of phosphatidylinositol 3-kinase, protein kinase Cdelta and p38 MAP kinase. Exp Cell Res. 2013;319:1471–1481. doi: 10.1016/j.yexcr.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–155. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Koyanagi T, Churchill EN, Mochly-Rosen D. Competitive inhibitors and allosteric activators of protein kinase C isoenzymes: a personal account and progress report on transferring academic discoveries to the clinic. Biochem Soc Trans. 2007;35:1021–1026. doi: 10.1042/BST0351021. [DOI] [PubMed] [Google Scholar]
- Burns DJ, Bell RM. Protein kinase C contains two phorbol ester binding domains. J Biol Chem. 1991;266:18330–18338. [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Cai Q, Li J, Gao T, Xie J, Evers BM. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem. 2009;284:2150–2158. doi: 10.1074/jbc.M803235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley J, Boslem E, Laybutt DR, Cordery DV, Pearson G, Carpenter L, … Biden TJ. Deletion of protein kinase Cdelta in mice modulates stability of inflammatory genes and protects against cytokine-stimulated beta cell death in vitro and in vivo. Diabetologia. 2011;54:380–389. doi: 10.1007/s00125-010-1962-y. [DOI] [PubMed] [Google Scholar]
- Casar B, Rimann I, Kato H, Shattil SJ, Quigley JP, Deryugina EI. In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated beta1 integrin and induction of FAK/PI3K/Aktmotility signaling. Oncogene. 2014;33:255–268. doi: 10.1038/onc.2012.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- Cerda SR, Bissonnette M, Scaglione-Sewell B, Lyons MR, Khare S, Mustafi R, Brasitus TA. PKC-delta inhibits anchorage-dependent and -independent growth, enhances differentiation, and increases apoptosis in CaCo-2 cells. Gastroenterology. 2001;120:1700–1712. doi: 10.1053/gast.2001.24843. [DOI] [PubMed] [Google Scholar]
- Chen Z, Forman LW, Miller KA, English B, Takashima A, Bohacek RA, … Faller DV. Protein kinase Cdelta inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr Relat Cancer. 2011;18:759–771. doi: 10.1530/ERC-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Forman LW, Williams RM, Faller DV. Protein kinase C-delta inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer. 2014;14:90. doi: 10.1186/1471-2407-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, … Harrington EO. Genetic disruption of protein kinase Cdelta reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L880–L888. doi: 10.1152/ajplung.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Kim MJ, Kang CM, Bae S, Cho CK, Soh JW, … Lee SJ. Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J Biol Chem. 2006;281:7049–7059. doi: 10.1074/jbc.M512000200. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Qvit N, Mochly-Rosen D. Rationally designed peptide regulators of protein kinase C. Trends Endocrinol Metab. 2009;20:25–33. doi: 10.1016/j.tem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, … Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MJ, Pickthorn K, Huang S, Critchley L, Bell G. The safety and efficacy of KAI-1678- an inhibitor of epsilon protein kinase C (epsilon PKC)-versus lidocaine and placebo for the treatment of postherpetic neuralgia: a crossover study design. Pain Med. 2013;14:533–540. doi: 10.1111/pme.12058. [DOI] [PubMed] [Google Scholar]
- Danis RP, Sheetz MJ. Ruboxistaurin: PKC-beta inhibition for complications of diabetes. Expert Opin Pharmacother. 2009;10:2913–2925. doi: 10.1517/14656560903401620. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2006;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- Deissler HL, Lang GE. The protein kinase c inhibitor: ruboxistaurin. Dev Ophthalmol. 2016;55:295–301. doi: 10.1159/000431204. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Kalkofen RL, Matassa AA, Reyland ME. Protein kinase Cdelta regulates apoptosis via activation of STAT1. J Biol Chem. 2004;279:45603–45612. doi: 10.1074/jbc.M407448200. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME. Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J Biol Chem. 2007;282:22307–22314. doi: 10.1074/jbc.M703661200. [DOI] [PubMed] [Google Scholar]
- Dong H, Li R, Yu C, Xu T, Zhang X, Dong M. Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCdelta/NF-kappaB pathway. Neuroscience. 2015;285:70–80. doi: 10.1016/j.neuroscience.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol Cell Biol. 2004;24:8167–8183. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, … Baier G. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther. 2009;330:792–801. doi: 10.1124/jpet.109.153205. [DOI] [PubMed] [Google Scholar]
- Farshori PQ, Shah BH, Arora KK, Martinez-Fuentes A, Catt KJ. Activation and nuclear translocation of PKCdelta, Pyk2 and ERK1/2 by gonadotropin releasing hormone in HEK293 cells. J Steroid Biochem Mol Biol. 2003;85:337–347. doi: 10.1016/s0960-0760(03)00226-7. [DOI] [PubMed] [Google Scholar]
- Fischer EH, Krebs EG. Conversion of phosphorylase b to phosphorylase a in muscle extracts. J Biol Chem. 1955;216:121–132. [PubMed] [Google Scholar]
- Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, … Kazanietz MG. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- Fukase N, Kawamoto T, Kishimoto K, Hara H, Okada Y, Onishi Y, … Akisue T. Protein kinase Cdelta in tumorigenesis of human malignant fibrous histiocytoma. Oncol Rep. 2011;26:1221–1226. doi: 10.3892/or.2011.1415. [DOI] [PubMed] [Google Scholar]
- Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, … Kufe D. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Barford D, Komander D. Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol. 2006;16:693–701. doi: 10.1016/j.sbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Gomel R, Xiang C, Finniss S, Lee HK, Lu W, Okhrimenko H, Brodie C. The localization of protein kinase Cdelta in different subcellular sites affects its proapoptotic and antiapoptotic functions and the activation of distinct downstream signaling pathways. Mol Cancer Res. 2007;5:627–639. doi: 10.1158/1541-7786.MCR-06-0255. [DOI] [PubMed] [Google Scholar]
- Gong J, Yao Y, Zhang P, Udayasuryan B, Komissarova EV, Chen J, … Steinberg SF. The C2 domain and altered ATP-binding loop phosphorylation at Ser(3)(5)(9) mediate the redox-dependent increase in protein kinase C-delta activity. Mol Cell Biol. 2015;35:1727–1740. doi: 10.1128/MCB.01436-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guerrico AM, Kazanietz MG. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J Biol Chem. 2005;280:38982–38991. doi: 10.1074/jbc.M506767200. [DOI] [PubMed] [Google Scholar]
- Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J Neuroinflammation. 2012;9:82. doi: 10.1186/1742-2094-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik G, Sawalha AH, Patel D, Johnson K, Richardson B. T cell PKCdelta kinase inactivation induces lupus-like autoimmunity in mice. Clin Immunol. 2015;158:193–203. doi: 10.1016/j.clim.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower CM, Chang ME, Maly DJ. Bivalent inhibitors of protein kinases. Crit Rev Biochem Mol Biol. 2014;49:102–115. doi: 10.3109/10409238.2013.875513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, … Thornton D. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- Greene MW, Burrington CM, Lynch DT, Davenport SK, Johnson AK, Horsman MJ, … Tirrell PC. Lipid metabolism, oxidative stress and cell death are regulated by PKC delta in a dietary model of nonalcoholic steatohepatitis. PLoS One. 2014;9:e85848. doi: 10.1371/journal.pone.0085848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridling M, Ficarro SB, Breitwieser FP, Song L, Parapatics K, Colinge J, … Rix U. Identification of kinase inhibitor targets in the lung cancer microenvironment by chemical and phosphoproteomics. Mol Cancer Ther. 2014;13:2751–2762. doi: 10.1158/1535-7163.MCT-14-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Hennings H, Blumberg PM, Pettit GR, Herald CL, Shores R, Yuspa SH. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987;8:1343–1346. doi: 10.1093/carcin/8.9.1343. [DOI] [PubMed] [Google Scholar]
- Hernández-Maqueda JG, Luna-Ulloa LB, Santoyo-Ramos P, Castañeda-Patlán MC, Robles-Flores M. Protein kinase C delta negatively modulates canonical Wnt pathway and cell proliferation in colon tumor cell lines. PLoS One. 2013;8:e58540. doi: 10.1371/journal.pone.0058540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Rentero C, Cairns R, Tebar F, Enrich C, Grewal T. Annexins - scaffolds modulating PKC localization and signaling. Cell Signal. 2014;26:1213–1225. doi: 10.1016/j.cellsig.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Hu CT, Cheng CC, Pan SM, Wu JR, Wu WS. PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2. Cell Signal. 2013;25:1457–1467. doi: 10.1016/j.cellsig.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Hu Y, Kang C, Philp RJ, Li B. PKC delta phosphorylates p52ShcA at Ser29 to regulate ERK activation in response to H2O2. Cell Signal. 2007;19:410–418. doi: 10.1016/j.cellsig.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene. 2008;27:3045–3053. doi: 10.1038/sj.onc.1210967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, … Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J Biol Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI, … Foster DA. Suppression of cell migration by protein kinase Cdelta. Oncogene. 2005;24:3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- Jaken S, Parker PJ. Protein kinase C binding partners. BioEssays. 2000;22:245–254. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kahl-Rainer P, Karner-Hanusch J, Weiss W, Marian B. Five of six protein kinase C isoenzymes present in normal mucosa show reduced protein levels during tumor development in the human colon. Carcinogenesis. 1994;15:779–782. doi: 10.1093/carcin/15.4.779. [DOI] [PubMed] [Google Scholar]
- Kajimoto T, Sawamura S, Tohyama Y, Mori Y, Newton AC. Protein kinase C {delta}-specific activity reporter reveals agonist-evoked nuclear activity controlled by Src family of kinases. J Biol Chem. 2010;285:41896–41910. doi: 10.1074/jbc.M110.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, Saito N. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem. 2004;279:12668–12676. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, … Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kazanietz MG, Barchi JJ, Jr, Omichinski JG, Blumberg PM. Low affinity binding of phorbol esters to protein kinase C and its recombinant cysteine-rich region in the absence of phospholipids. J Biol Chem. 1995;270:14679–14684. doi: 10.1074/jbc.270.24.14679. [DOI] [PubMed] [Google Scholar]
- Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343:848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Kheifets V, Bright R, Inagaki K, Schechtman D, Mochly-Rosen D. Protein kinase C delta (deltaPKC)-annexin V interaction: a required step in deltaPKC translocation and function. J Biol Chem. 2006;281:23218–23226. doi: 10.1074/jbc.M602075200. [DOI] [PubMed] [Google Scholar]
- Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Lee JH, … Kim KK. KITENIN recruits Dishevelled/PKC delta to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut. 2009;58:509–519. doi: 10.1136/gut.2008.150938. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Tatton L. Caspase-mediated proteolysis and activation of protein kinase Cdelta plays a central role in neutrophil apoptosis. Blood. 1999;94:291–301. [PubMed] [Google Scholar]
- Kikkawa U, Takai Y, Tanaka Y, Miyake R, Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983;258:11442–11445. [PubMed] [Google Scholar]
- Kilburn LB, Kocak M, Decker RL, Wetmore C, Chintagumpala M, Su J, … Blaney SM. A phase 1 and pharmacokinetic study of enzastaurin in pediatric patients with refractory primary central nervous systemtumors: a pediatric brain tumor consortium study. Neuro-Oncology. 2015;17:303–311. doi: 10.1093/neuonc/nou114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Lee HJ, Lee DH, Bae S, Soh JW, Jeoung D, … Lee YS. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res. 2007;67:6333–6341. doi: 10.1158/0008-5472.CAN-06-4344. [DOI] [PubMed] [Google Scholar]
- Kim JD, Seo KW, Lee EA, Quang NN, Cho HR, Kwon B. A novelmouse PKCdelta splice variant, PKCdeltaIX, inhibits etoposide-induced apoptosis. Biochem Biophys Res Commun. 2011;410:177–182. doi: 10.1016/j.bbrc.2011.04.096. [DOI] [PubMed] [Google Scholar]
- Kim H, Zhao J, Zhang Q, Wang Y, Lee D, Bai X, … Liu M. deltaV1-1 reduces pulmonary ischemia reperfusion-induced lung injury by inhibiting necrosis and mitochondrial localization of PKCdelta and p53. Am J Transplant. 2015;16:83–98. doi: 10.1111/ajt.13445. [DOI] [PubMed] [Google Scholar]
- Kim H, Zhao J, Zhang Q, Wang Y, Lee D, Bai X, … Liu M. deltaV1-1 reduces pulmonary ischemia reperfusion-induced lung injury by inhibiting necrosis and mitochondrial localization of PKCdelta and p53. Am J Transplant. 2016;16:83–98. doi: 10.1111/ajt.13445. [DOI] [PubMed] [Google Scholar]
- Kolb RH, Greer PM, Cao PT, Cowan KH, Yan Y. ERK1/2 signaling plays an important role in topoisomerase II poison-induced G2/M checkpoint activation. PLoS One. 2012;7:e50281. doi: 10.1371/journal.pone.0050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, … Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik JM, Neuhaus P, Cillo U, Weber M, Stitah S, Gatlik E, … Slade A. Sotrastaurin single-dose pharmacokinetics in de novo liver transplant recipients. Transpl Int. 2011;24:276–283. doi: 10.1111/j.1432-2277.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- Krebs EG, Fischer EH. Phosphorylase activity of skeletal muscle extracts. J Biol Chem. 1955;216:113–120. [PubMed] [Google Scholar]
- Ku GY, Ilson DH, Schwartz LH, Capanu M, O’Reilly E, Shah MA, … Schwartz GK. Phase II trial of sequential paclitaxel and 1 h infusion of bryostatin-1 in patients with advanced esophageal cancer. Cancer Chemother Pharmacol. 2008;62:875–880. doi: 10.1007/s00280-008-0677-y. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, … Oliveira JB. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121:3117–3125. doi: 10.1182/blood-2012-12-469544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveiborg M, Instrell R, Rowlands C, Howell M, Parker PJ. PKCalpha and PKCdelta regulate ADAM17-mediated ectodomain shedding of heparin binding-EGF through separate pathways. PLoS One. 2011;6:e17168. doi: 10.1371/journal.pone.0017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGory EL, Sitailo LA, Denning MF. The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint. J Biol Chem. 2010;285:1879–1887. doi: 10.1074/jbc.M109.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasfer M, Davenne L, Vadrot N, Alexia C, Sadji-Ouatas Z, Bringuier AF, … Reyl-Desmars F. Protein kinase PKC delta and c-Abl are required for mitochondrial apoptosis induction by genotoxic stress in the absence of p53, p73 and Fas receptor. FEBS Lett. 2006;580:2547–2552. doi: 10.1016/j.febslet.2006.03.089. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim SG, Kim HP, Kwon E, You J, Choi HJ, … Bang YJ. Enzastaurin, a protein kinase C beta inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Res. 2008;68:1916–1926. doi: 10.1158/0008-5472.CAN-07-3195. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Kim E-H, Seo WD, Choi TH, Cheon G-J, Lee Y-J, Lee Y-S. Heat shock protein 27-targeted heptapeptide of the PKCΔ catalytic V5 region sensitizes tumors with radio- and chemoresistance. Int J Radiat Oncol Biol Phys. 2011;80:221–230. doi: 10.1016/j.ijrobp.2010.11.069. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee DH, Cho CK, Bae S, Jhon GJ, Lee SJ, … Lee YS. HSP25 inhibits protein kinase C delta-mediated cell death through direct interaction. J BiolChem. 2005;280:18108–18119. doi: 10.1074/jbc.M501131200. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Soh JW, Dean NM, Cho CK, Kim TH, Lee SJ, Lee YS. Protein kinase Cdelta overexpression enhances radiation sensitivity via extracellular regulated protein kinase 1/2 activation, abolishing the radiation-induced G(2)-M arrest. Cell Growth Differ. 2002;13:237–246. [PubMed] [Google Scholar]
- Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, … Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Du ZX, Zong ZH, Liu BQ, Li C, Zhang Q, Wang HQ. PKCdelta-mediated phosphorylation of BAG3 at Ser187 site induces epithelial–mesenchymal transition and enhances invasiveness in thyroid cancer FRO cells. Oncogene. 2013;32:4539–4548. doi: 10.1038/onc.2012.466. [DOI] [PubMed] [Google Scholar]
- Li W, Laishram RS, Ji Z, Barlow CA, Tian B, Anderson RA. Star-PAP control of BIK expression and apoptosis is regulated by nuclear PIPKIalpha and PKCdelta signaling. Mol Cell. 2012;45:25–37. doi: 10.1016/j.molcel.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li C, Wu M, Chen Q, Wang Q, Ren J, Zhang Y. PKCdelta stabilizes TAp63 to promote cell apoptosis. FEBS Lett. 2015;589:2094–2099. doi: 10.1016/j.febslet.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang X, Rasheed N, Hu Y, Boast S, Ishii T, … Goff SP. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev. 2004;18:1824–1837. doi: 10.1101/gad.1223504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, … Weiss A. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat Immunol. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, … Investigators PA. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J. 2014;35:2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu ZG, Miki Y, Yoshida K. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol Cell Biol. 2007;27:8480–8491. doi: 10.1128/MCB.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado A, Tebar F, Calvo M, Moreto J, Sorkin A, Enrich C. Protein kinase Cdelta-calmodulin crosstalk regulates epidermal growth factor receptor exit from early endosomes. Mol Biol Cell. 2004;15:4877–4891. doi: 10.1091/mbc.E04-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonaco SL, Kahana S, Blass M, Brody Y, Okhrimenko H, Xiang C, … Brodie C. Phosphorylation of protein kinase Cdelta on distinct tyrosine residues induces sustained activation of Erk1/2 via down-regulation of MKP-1: role in the apoptotic effect of etoposide. J Biol Chem. 2008;283:17731–17739. doi: 10.1074/jbc.M801727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZG, Liu H, Yamaguchi T, Miki Y, Yoshida K. Protein kinase Cdelta activates RelA/p65 and nuclear factor-kappaB signaling in response to tumor necrosis factor-alpha. Cancer Res. 2009;69:5927–5935. doi: 10.1158/0008-5472.CAN-08-4786. [DOI] [PubMed] [Google Scholar]
- Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, … Elledge SJ. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Baumann C, Viveiros MM. Lack of protein kinase C-delta (PKCdelta) disrupts fertilization and embryonic development. Mol Reprod Dev. 2015 doi: 10.1002/mrd.22528. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Mishra NC, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D. Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ. 2001;12:465–470. [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, … Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- Masoumi KC, Cornmark L, Lonne GK, Hellman U, Larsson C. Identification of a novel protein kinase Cdelta-Smac complex that dissociates during paclitaxel-induced cell death. FEBS Lett. 2012;586:1166–1172. doi: 10.1016/j.febslet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- Matz M, Naik M, Mashreghi MF, Glander P, Neumayer HH, Budde K. Evaluation of the novel protein kinase C inhibitor sotrastaurin as immunosuppressive therapy after renal transplantation. Expert Opin Drug Metab Toxicol. 2011;7:103–113. doi: 10.1517/17425255.2011.540238. [DOI] [PubMed] [Google Scholar]
- Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, … Puricelli LL. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas. 2010;39:e31–e41. doi: 10.1097/MPA.0b013e3181bce796. [DOI] [PubMed] [Google Scholar]
- McKiernan E, O’Brien K, Grebenchtchikov N, Geurts-Moespot A, Sieuwerts AM, Martens JW, … Duffy MJ. Protein kinase Cdelta expression in breast cancer as measured by real-time PCR, western blotting and ELISA. Br J Cancer. 2008;99:1644–1650. doi: 10.1038/sj.bjc.6604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–865. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H, Pierce JH, Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, … Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerat C, Henriksson R, Le Chevalier T, Novello S, Berthaud P, Faivre S, Raymond E. Phase I study of PKC412 (N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor, combined with gemcitabine and cisplatin in patients with non-small-cell lung cancer. Ann Oncol. 2004;15:316–323. doi: 10.1093/annonc/mdh052. [DOI] [PubMed] [Google Scholar]
- Moodie JE, Bisley EJ, Huang S, Pickthorn K, Bell G. A single-center, randomized, double-blind, active, and placebo-controlled study of KAI-1678, a novel PKC-epsilon inhibitor, in the treatment of acute postoperative orthopedic pain. Pain Med. 2013;14:916–924. doi: 10.1111/pme.12088. [DOI] [PubMed] [Google Scholar]
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Oliva JL, Kothapalli D, Fournier A, Assoian RK, Kazanietz MG. Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cdelta-dependent induction of p21. J Biol Chem. 2005;280:33926–33934. doi: 10.1074/jbc.M505748200. [DOI] [PubMed] [Google Scholar]
- Neri A, Marmiroli S, Tassone P, Lombardi L, Nobili L, Verdelli D, … Sacchi S. The oral protein-kinase C beta inhibitor enzastaurin (LY317615) suppresses signalling through the AKT pathway, inhibits proliferation and induces apoptosis in multiple myeloma cell lines. Leuk Lymphoma. 2008;49:1374–1383. doi: 10.1080/10428190802078289. [DOI] [PubMed] [Google Scholar]
- Newton AC. Interaction of proteins with lipid headgroups: lessons from protein kinase C. Annu Rev Biophys Biomol Struct. 1993;22:1–25. doi: 10.1146/annurev.bb.22.060193.000245. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odia Y, Iwamoto FM, Moustakas A, Fraum TJ, Salgado CA, Li A, … Fine HA. A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas. J Neurooncol. 2015;127:127–135. doi: 10.1007/s11060-015-2020-x. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121:2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim WK, Song M, Park M, Kim H, Nam HJ, … Kim H. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clin Cancer Res. 2013;19:4961–4971. doi: 10.1158/1078-0432.CCR-13-0131. [DOI] [PubMed] [Google Scholar]
- Patel R, Apostolatos A, Carter G, Ajmo J, Gali M, Cooper DR, … Patel NA. Protein kinase C delta (PKCdelta) splice variants modulate apoptosis pathway in 3T3L1 cells during adipogenesis: identification of PKCdeltaII inhibitor. J Biol Chem. 2013;288:26834–26846. doi: 10.1074/jbc.M113.482638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NA, Song SS, Cooper DR. PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells. Gene Expr. 2006;13:73–84. doi: 10.3727/000000006783991890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh BS, Paugh SW, Bryan L, Kapitonov D, Wilczynska KM, Gopalan SM, … Kordula T. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22:455–465. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perletti G, Marras E, Dondi D, Osti D, Congiu T, Ferrarese R, … Tashjian AH., Jr p21(Waf1/Cip1) and p53 are downstream effectors of protein kinase C delta in tumor suppression and differentiation in human colon cancer cells. Int J Cancer. 2005;113:42–53. doi: 10.1002/ijc.20535. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC-interacting proteins: from function to pharmacology. Trends Pharmacol Sci. 2004;25:528–535. doi: 10.1016/j.tips.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Propper DJ, Macaulay V, O’Byrne KJ, Braybrooke JP, Wilner SM, Ganesan TS, … Harris AL. A phase II study of bryostatin 1 in metastatic malignant melanoma. Br J Cancer. 1998;78:1337–1341. doi: 10.1038/bjc.1998.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper DJ, McDonald AC, Man A, Thavasu P, Balkwill F, Braybrooke JP, … Twelves C. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–1492. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- Putnam AJ, Schulz VV, Freiter EM, Bill HM, Miranti CK. Src, PKCalpha, and PKCdelta are required for alphavbeta3 integrin-mediated metastatic melanoma invasion. Cell Commun Signal. 2009;7:10. doi: 10.1186/1478-811X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Mochly-Rosen D. The PKCdelta -Abl complex communicates ER stress to the mitochondria - an essential step in subsequent apoptosis. J Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- Quest AF, Bell RM. The regulatory region of protein kinase C gamma. Studies of phorbol ester binding to individual and combined functional segments expressed as glutathione S-transferase fusion proteins indicate a complex mechanism of regulation by phospholipids, phorbol esters, and divalent cations. J Biol Chem. 1994;269:20000–20012. [PubMed] [Google Scholar]
- Qvit N, Mochly-Rosen D. Highly specific modulators of protein kinase C localization: applications to heart failure. Drug Discov Today Dis Mech. 2010;7:e87–e93. doi: 10.1016/j.ddmec.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvit N, Mochly-Rosen D. The many hats of protein kinase Cdelta: one enzyme with many functions. Biochem Soc Trans. 2014;42:1529–1533. doi: 10.1042/BST20140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, … Verma AK. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- Ren J, Wang Q, Morgan S, Si Y, Ravichander A, Dou C, … Liu B. Protein kinase C-delta (PKCdelta) regulates proinflammatory chemokine expression through cytosolic interaction with the NF-kappaB subunit p65 in vascular smooth muscle cells. J Biol Chem. 2014;289:9013–9026. doi: 10.1074/jbc.M113.515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol. 2008;39:21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci (Landmark Ed) 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- Ricouart A, Gesquiere JC, Tartar A, Sergheraert C. Design of potent protein kinase inhibitors using the bisubstrate approach. J Med Chem. 1991;34:73–78. doi: 10.1021/jm00105a012. [DOI] [PubMed] [Google Scholar]
- Roebuck KA, Gu DS, Kagnoff MF. Activating protein-1 cooperates with phorbol ester activation signals to increase HIV-1 expression. AIDS. 1996;10:819–826. doi: 10.1097/00002030-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Rosenzweig T, Aga-Mizrachi S, Bak A, Sampson SR. Src tyrosine kinase regulates insulin-induced activation of protein kinase C (PKC) delta in skeletal muscle. Cell Signal. 2004;16:1299–1308. doi: 10.1016/j.cellsig.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Sabri A, Elouardighi H, Schaefer E, Steinberg SF. Stimulus-specific differences in protein kinase C delta localization and activation mechanisms in cardiomyocytes. J Biol Chem. 2004;279:19350–19361. doi: 10.1074/jbc.M311096200. [DOI] [PubMed] [Google Scholar]
- Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, … Liu B. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem. 2005;280:35310–35317. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- Saha K, Adhikary G, Kanade SR, Rorke EA, Eckert RL. p38delta regulates p53 to control p21Cip1 expression in human epidermal keratinocytes. J Biol Chem. 2014;289:11443–11453. doi: 10.1074/jbc.M113.543165. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL. Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem. 2005;280:32107–32114. doi: 10.1074/jbc.M504432200. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, … Scott RW. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Newton AC. Shedding light on local kinase activation. BMC Biol. 2012;10:61. doi: 10.1186/1741-7007-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seynaeve CM, Kazanietz MG, Blumberg PM, Sausville EA, Worland PJ. Differential inhibition of protein kinase C isozymes by UCN-01, a staurosporine analogue. Mol Pharmacol. 1994;45:1207–1214. [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem. 2006;281:29703–29710. doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, White HD, Siegel JB, Krebs EG. Cyclic AMP-dependent protein kinase I: cyclic nucleotide binding, structural changes, and release of the catalytic subunits. Proc Natl Acad Sci U S A. 1981;78:1591–1595. doi: 10.1073/pnas.78.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhia ME, Grewal BK, Bhat J, Rohit S, Punia V. Protein kinase C betaII in diabetic complications: survey of structural, biological and computational studies. Expert Opin Ther Targets. 2012;16:325–344. doi: 10.1517/14728222.2012.667804. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Soriano-Carot M, Quilis I, Bano MC, Igual JC. Protein kinase C controls activation of the DNA integrity checkpoint. Nucleic Acids Res. 2014;42:7084–7095. doi: 10.1093/nar/gku373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. Mechanisms for redox-regulation of protein kinase C. Front Pharmacol. 2015;6:128. doi: 10.3389/fphar.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, … Steinberg SF. Tyrosine phosphorylation modifies protein kinase C delta-dependent phosphorylation of cardiac troponin I. J Biol Chem. 2008;283:22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Wong SM, Tjolsen A, Allen CP, Mochly-Rosen D, Kendig JJ. Exaggerated nociceptive responses on morphine withdrawal: roles of protein kinase C epsilon and gamma. Pain. 2004;110:281–289. doi: 10.1016/j.pain.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, Reyland ME. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011;71:2087–2097. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Tan A-C, Reyland ME. PKCδ regulates integrin αVβ3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.7560. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi Z, Denning MF, Smith CB, Dlugosz AA, Yuspa SH, Pettit GR, Blumberg PM. Bryostatin 1 protects protein kinase C-delta from down-regulation in mouse keratinocytes in parallel with its inhibition of phorbol ester-induced differentiation. Mol Pharmacol. 1994;46:840–850. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- Takashima A, English B, Chen Z, Cao J, Cui R, Williams RM, Faller DV. Protein kinase Cdelta is a therapeutic target in malignant melanoma with NRAS mutation. ACS Chem Biol. 2014;9:1003–1014. doi: 10.1021/cb400837t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, … Ingram AJ. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK–ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- van Ameijde J, Poot AJ, vanWandelen LT, Wammes AE, Ruijtenbeek R, Rijkers DT, Liskamp RM. Preparation of novel alkylated arginine derivatives suitable for click-cycloaddition chemistry and their incorporation into pseudosubstrate and bisubstrate-based kinase inhibitors. Org Biomol Chem. 2010;8:1629–1639. doi: 10.1039/b922928k. [DOI] [PubMed] [Google Scholar]
- Villar J, Arenas MI, MacCarthy CM, Blanquez MJ, Tirado OM, Notario V. PCPH/ENTPD5 expression enhances the invasiveness of human prostate cancer cells by a protein kinase C delta-dependent mechanism. Cancer Res. 2007;67:10859–10868. doi: 10.1158/0008-5472.CAN-07-2041. [DOI] [PubMed] [Google Scholar]
- Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, … Reyland ME. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Burstin VA, Xiao L, Kazanietz MG. Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC) delta translocation and preventing PKCdelta-mediated release of tumor necrosis factor-alpha. Mol Pharmacol. 2010;78:325–332. doi: 10.1124/mol.110.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, von Matt P, Sedrani R, Albert R, Cooke N, Ehrhardt C, … Cottens S. Discovery of 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]pyrrole-2,5-dione (AEB071), a potent and selective inhibitor of protein kinase C isotypes. J Med Chem. 2009;52:6193–6196. doi: 10.1021/jm901108b. [DOI] [PubMed] [Google Scholar]
- Wang H, Kazanietz MG. p23/Tmp21 differentially targets the Rac-GAP beta2-chimaerin and protein kinase C via their C1 domains. Mol Biol Cell. 2010;21:1398–1408. doi: 10.1091/mbc.E09-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xiao L, Kazanietz MG. p23/Tmp21 associates with protein kinase Cdelta (PKCdelta) and modulates its apoptotic function. J Biol Chem. 2011;286:15821–15831. doi: 10.1074/jbc.M111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, … Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc Natl Acad Sci U S A. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Xie Y, Tao L, Tang D. Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cell Signal. 2010;22:1783–1789. doi: 10.1016/j.cellsig.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Wender PA, Baryza JL, Brenner SE, DeChristopher BA, Loy BA, Schrier AJ, Verma VA. Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity. Proc Natl Acad Sci U S A. 2011;108:6721–6726. doi: 10.1073/pnas.1015270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, … Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie SM, Adwan TS, DeGregori J, Anderson SM, Reyland ME. Inhibiting tyrosine phosphorylation of protein kinase Cdelta (PKCdelta) protects the salivary gland from radiation damage. J Biol Chem. 2014;289:10900–10908. doi: 10.1074/jbc.M114.551366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, … Garraway LA. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27:397–408. doi: 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular pharmacology of protein kinase Mzeta (PKMzeta) contrasts with its in vitro profile: implications for PKMzeta as a mediator of memory. J Biol Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Land H. Tumor suppressor p53 restricts Ras stimulation of RhoA and cancer cell motility. Nat Struct Mol Biol. 2007;14:215–223. doi: 10.1038/nsmb1208. [DOI] [PubMed] [Google Scholar]
- Xia S, Chen Z, Forman LW, Faller DV. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009;21:502–508. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Forman LW, Faller DV. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem. 2007;282:13199–13210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Su L, Liu X. PKCdelta regulates death receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis in human lung cancer cells. Mol Cancer Ther. 2012;11:2174–2182. doi: 10.1158/1535-7163.MCT-12-0602. [DOI] [PubMed] [Google Scholar]
- Yin S, Sethi S, Reddy KB. Protein kinase Cdelta and caspase-3 modulate TRAIL-induced apoptosis in breast tumor cells. J Cell Biochem. 2010;111:979–987. doi: 10.1002/jcb.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K. Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends Mol Med. 2008;14:305–313. doi: 10.1016/j.molmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Liu H, Miki Y. Protein kinase C delta regulates Ser46 phosphorylation of p53 tumor suppressor in the apoptotic response to DNA damage. J Biol Chem. 2006;281:5734–5740. doi: 10.1074/jbc.M512074200. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Miki Y, Kufe D. Activation of SAPK/JNK signaling by protein kinase Cdelta in response to DNA damage. J Biol Chem. 2002;277:48372–48378. doi: 10.1074/jbc.M205485200. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Wang HG, Miki Y, Kufe D. Protein kinase Cdelta is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. EMBO J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZM, Utsugisawa T, Ishiko T, Nakada S, Huang Y, Kharbanda S, … Kufe D. Activation of protein kinase C delta by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene. 1998;16:1643–1648. doi: 10.1038/sj.onc.1201698. [DOI] [PubMed] [Google Scholar]
- Zehorai E, Yao Z, Plotnikov A, Seger R. The subcellular localization of MEK and ERK–a novel nuclear translocation signal (NTS) paves a way to the nucleus. Mol Cell Endocrinol. 2010;314:213–220. doi: 10.1016/j.mce.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang J, Burrington CM, Davenport SK, Johnson AK, Horsman MJ, Chowdhry S, Greene MW. PKCdelta regulates hepatic triglyceride accumulation and insulin signaling in Lepr(db/db) mice. Biochem Biophys Res Commun. 2014;450:1619–1625. doi: 10.1016/j.bbrc.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- Zonder JA, Shields AF, Zalupski M, Chaplen R, Heilbrun LK, Arlauskas P, Philip PA. A phase II trial of bryostatin 1 in the treatment of metastatic colorectal cancer. Clin Cancer Res. 2001;7:38–42. [PubMed] [Google Scholar]