Abstract
Background
There are established guidelines for recommended dietary intake for hypertension treatment and cardiovascular disease prevention. Evidence is lacking for effective dietary patterns for kidney disease prevention.
Study Design
Prospective cohort study
Setting & Participants
Atherosclerosis Risk in Communities (ARIC) study participants with baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 (N=14,882)
Predictor
The Dietary Approaches to Stop Hypertension (DASH) diet score was calculated based on self-reported dietary intake of red and processed meat, sweetened beverages, sodium, fruits, vegetables, whole grains, nuts and legumes, and low-fat dairy products, averaged over two visits.
Outcomes
Cases were ascertained based on development of eGFR <60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline from baseline, an ICD-9/10 code for a kidney disease–related hospitalization or death, or end-stage renal disease from baseline through 2012.
Results
A total of 3,720 participants developed kidney disease during a median follow-up of 23 years. Participants with a DASH diet score in the lowest tertile were 16% more likely to develop kidney disease than those with the highest score tertile (HR, 1.16; 95% CI, 1.07-1.26; p for trend <0.001), after adjusting for socio-demographics, smoking status, physical activity, total caloric intake, baseline eGFR, overweight/obese status, diabetes status, hypertension status, systolic blood pressure, and anti-hypertensive medication use. Of the individual components of the DASH diet score, high intake of red and processed meat was adversely associated with kidney disease and high intake of nuts, legumes, and low-fat dairy products was associated with reduced risk of kidney disease.
Limitations
Potential measurement error due to self-reported dietary intake and lack of data on albuminuria
Conclusions
Consuming a DASH-style diet was associated with lower risk for kidney disease, independent of demographic characteristics, established kidney risk factors, and baseline kidney function. Healthful dietary patterns, such as the DASH diet, may be beneficial for kidney disease prevention.
Keywords: chronic kidney disease (CKD), diet, dietary protein, health promotion, kidney disease prevention, disease progression, incident kidney disease, modifiable risk factor, renal function, DASH diet score, food frequency questionnaire, dietary acid load
The Dietary Approaches to Stop Hypertension (DASH) diet, a dietary pattern that is high in fruits, vegetables, and low-fat dairy products, substantially decreases blood pressure.1 The addition of sodium reduction to the DASH diet further lowers blood pressure and reduces the risk of hypertension, type 2 diabetes, cardiovascular disease, stroke, and mortality.1-6 The DASH diet has been recommended by multiple clinical guidelines for health promotion and disease prevention.7-11
While treatment of traditional cardiovascular risk factors like hypertension and diabetes is the primary approach to prevent kidney disease, evidence for dietary approaches to prevent kidney disease are lacking. Current clinical guidelines focus primarily on dietary restriction of protein and sodium to prevent kidney disease progression, but the evidence supporting this suggestion is weak (graded as level 2B).12 A comprehensive approach, such as that prescribed in the DASH diet, may be more meaningful given that nutrients likely have additive or synergistic effects.13 Furthermore, dietary patterns rather than nutrient restriction may be easier to implement given the success of the DASH diet for the prevention and treatment of other chronic conditions.14
Previous research has demonstrated a significant association between the DASH diet and kidney function reduction in older Caucasian women.15,16 The objective of this study was to assess the longitudinal relationship between consuming a DASH-style diet with sodium reduction and subsequent risk of kidney disease in a more diverse general population sample, including African-American and Caucasian men and women. Elucidating this relationship could inform the use of dietary modification as a preventative strategy for kidney disease.
METHODS
Study Population and Design
We conducted a prospective analysis of the Atherosclerosis Risk in Communities (ARIC) study.17 The ARIC study is a community-based observational study of 15,792 middle-aged (45-64 years), predominantly African-American and Caucasian men and women. Study participants were enrolled in 1987-1989 from four US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Follow-up study visits occurred in 1990-1992 (study visit 2), 1993-1995 (study visit 3), 1996-1998 (study visit 4), and 2011-2013 (study visit 5). The Institutional Review Board (IRB) at each site approved the study protocol and study participants provided informed consent at each study visit (IRB #H.34.99.07.02.A1). After excluding participants with missing dietary intake data (n=18), implausibly low caloric intake (<600 kcal for men and <500 kcal for women; n=149), and implausibly high caloric intake (>4,200 kcal for men and >3,600 kcal for women; n=152), those with baseline estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or end-stage renal disease identified by linkage to the US Renal Data System (USRDS) registry (n=356), those who were neither African-American nor Caucasian (n=48), and those with missing covariates (n=187), our analytic sample size was 14,882 (Figure S1, available as online supplementary material).18
Measurement of Dietary Intake
Usual dietary intake was assessed at study visit 1 (baseline, 1987-1989) and visit 3 (1993-1995) using a semi-quantitative 66-item food frequency questionnaire, modified from the Willett questionnaire.19-21 The questionnaire was administered in person by a trained interviewer with visual representations of portions (glasses and measuring cups of different sizes). Participants reported how often, on average, they consumed each food item of a particular portion size in the preceding year. Nutrient intake was calculated by multiplying self-reported frequency of consumption and portion size by the nutritional content of each food item from US Department of Agriculture data sources. The reliability of these diet data was previously assessed in a randomly selected subset of participants from all four sites who repeated the food frequency questionnaire at a follow-up visit (study visit 2, 1990-1992; n=419).19 For the analysis, we incorporated the two measurements of dietary intake (baseline and visit 3) by using the cumulative average diet, which improves estimation of usual dietary intake relative to a single measurement.22 That is, for those who developed kidney disease or were censored between baseline and visit 3, the baseline dietary intake data are used. Otherwise, for those who developed kidney disease or were censored after visit 3, the mean of the values from baseline and visit 3 is used.
Definition of DASH Diet Score
We assessed the degree to which study participants followed a DASH-style diet with reduced sodium using two previously developed indices.4,16,23,24 Study participants were not advised to follow a DASH diet, nor had the DASH diet results been published by the time of dietary assessment, and study participants did not receive dietary counseling. The primary analysis used a score based primarily on food items: low intake of 1) red and processed meat, 2) sweetened beverages, and 3) sodium; and high intake of 4) fruits, 5) vegetables, 6) whole grains, 7) nuts and legumes, and 8) low-fat dairy (Table S1).4 Each component was scored from 1 to 5 based on ranked distribution in quintiles, which is ideally suited to this analysis since the food frequency questionnaire is designed to rank individuals on dietary intake rather than quantify absolute nutrient intake levels.
In sensitivity analyses, we used an alternative score based on nine nutrients: low intake of 1) saturated fat, 2) total fat, 3) cholesterol, and 4) sodium; and high intake of 5) protein, 6) fiber, 7) magnesium, 8) calcium, and 9) potassium (Table S2).16,23,24 For the purposes of our study, the food item-based score and the nutrient-based score were both analyzed as tertiles. A higher score signifies that a participant's dietary pattern more closely resembles a DASH-style diet. Mean levels of DASH diet scores and individual components of the DASH diet scores for the overall study population and by case status are presented in Table S3.
Ascertainment of Kidney Disease
Blood levels of creatinine were measured using the modified kinetic Jaffe method, standardized to the National Institute of Standards and Technology standard, and calibrated to account for laboratory drift.25,26 Kidney function was assessed using the 2009 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equationfor eGFR .27 Measurement of urine albumin-creatinine ratio was not available in this study and thus was not included in the composite outcome variable.
Kidney disease cases were ascertained by meeting at least one of the following criteria: 1) eGFR <60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline at any follow-up study visit relative to baseline; 2) kidney disease-related hospitalization or death based on International Classification of Disease (ICD)-9/10 codes identified through active surveillance and linkage to the National Death Index; or 3) end-stage renal disease (dialysis or transplantation) identified by linkage to the USRDS registry between baseline (study visit 1, 1987-1989) and December 31, 2012. This outcome was designed to mitigate potential selection bias by disease status and allow for more complete outcome ascertainment during periods of time between study visits. As a sensitivity analysis, cases of kidney disease were identified using visit-based measures exclusively, i.e. eGFR <60 mL/min/1.73 m2 at a subsequent study visit accompanied by ≥25% eGFR decline relative to baseline.
Measurement of Covariates
At the baseline study visit, demographic characteristics (age, sex, race), socioeconomic status (education level), health behaviors (physical activity, smoking), and health history (diagnosed disease, medication use) were ascertained using a structured questionnaire administered by trained interviewers. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared using measurements taken while participants were wearing light clothing without shoes. Three seated measurements of blood pressure were taken by a certified technician using a random-zero sphygmomanometer after resting for five minutes. The average of the second and third blood pressure readings was used in the analysis. Fasting blood specimens were collected from participants during the baseline study visit. Blood levels of glucose were measured by the modified hexokinase/glucose-6-phosphate dehydrogenase method.
Overweight or obese status was defined as BMI ≥25 kg/m2. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current anti-hypertensive medication use in the preceding two weeks. Diabetes was defined as fasting blood glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, self-reported history of diagnosed diabetes, or current diabetes medication use in the preceding two weeks.
Statistical Analysis
Descriptive statistics (means, proportions) were used to characterize the study population with respect to baseline demographic and clinical factors according to tertile of DASH diet score. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between DASH diet score and kidney disease, incorporating time to event. The minimally adjusted regression model (model 1) included demographic characteristics (age, sex, race-center), socioeconomic status (education level), health behaviors (physical activity, smoking), and total caloric intake (the standard method for energy adjustment).22,28,29 In model 2, we additionally adjusted for baseline kidney function (eGFR modeled as two linear spline terms with one knot at 90 mL/min/1.73 m2). In model 3, we additionally adjusted for comorbidities relevant to dietary behavior and kidney disease risk (overweight/obese status, diabetes status, hypertension status, systolic blood pressure, use of angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs]). Effect modification by demographic factors (sex, race), socioeconomic status (education level), and clinical characteristics (overweight/obese status, diabetes status, hypertension status) was assessed by conducting stratified analyses and tests of interaction. In a sensitivity analysis, we performed the same analyses using the alternative, nutrient-based DASH diet score. In addition, we investigated the relationship between the individual components of each score and risk of kidney disease, modeling all factors together in the fully adjusted model (model 3). Due to the expected underestimation of dietary sodium intake from the food frequency questionnaire, as a sensitivity analysis, we modified both DASH diet indices to exclude sodium. Tests for linear trend were conducted using quantiles as ordinal variables (tertiles for the total scores, quintiles for components of the primary DASH diet score in accordance with the classification of the individual components in this score). Stata version 14 was used for all analyses (StataCorp LP, College Station, Texas).
RESULTS
Baseline Characteristics
Baseline characteristics of study participants included in this analysis of the ARIC study were similar to the total ARIC study population (Table S4). The subset of excluded study participants (n=910 [5.8% of total ARIC study population]) was more likely to be African-American and overweight or obese, to have diabetes and hypertension, and less likely to have a high school education. By definition, excluded participants had worse kidney function at baseline.
Study participants with a DASH diet score in the lowest tertile were younger, more likely to be male and African-American, and less likely to have completed high school than participants (Table 1). They also had lower levels of physical activity, were more likely to smoke, and had a higher prevalence of overweight/obesity status. Higher DASH diet score was also associated with lower systolic blood pressure and higher prevalence of diabetes. Baseline eGFR was statistically but not clinically different across tertiles of the DASH diet score.
Table 1.
Baseline Demographics, Clinical Characteristics, and Dietary Factors According to Tertile of DASH Diet Score
| Tertile 1: 8-22 (Low Score) | Tertile 2: 23-26 (Moderate Score) | Tertile 3: 27-40 (High Score) | Pa | |
|---|---|---|---|---|
| Age, y | 53.5 (5.7) | 54.1 (5.7) | 54.9 (5.7) | <0.001 |
| Female sex | 43.7 (2,517) | 55.8 (2,383) | 68.3 (3,306) | <0.001 |
| African-American | 35.5 (2,044) | 22.4 (958) | 17.9 (867) | <0.001 |
| Diabetes | 9.2 (530) | 12.4 (528) | 13.0 (629) | <0.001 |
| Hypertension | 35.9 (2,070) | 33.5 (1,430) | 32.7 (1,586) | 0.002 |
| SBP, mmHg | 122.3 (19.1) | 120.9 (18.2) | 119.6 (18.3) | <0.001 |
| ACEi or ARB use | 2.9 (165) | 3.7 (159) | 3.4 (164) | 0.05 |
| Current smoker | 35.7 (2,059) | 23.3 (997) | 17.2 (832) | <0.001 |
| At least HS graduate | 67.7 (3,903) | 80.4 (3,435) | 84.3 (4,081) | <0.001 |
| Physical activity index | 2.27 (0.74) | 2.44 (0.79) | 2.63 (0.82) | <0.001 |
| Serum creatinine, mg/dL | 0.75 (0.18) | 0.72 (0.18) | 0.68 (0.17) | <0.001 |
| eGFR, mL/min/1.73 m2 | 104.4 (15.1) | 102.5 (14.2) | 102.4 (13.4) | <0.001 |
| BM ≥25 kg/m2 | 67.7 (3,903) | 69.5 (2,969) | 62.8 (3,042) | <0.001 |
| Caloric intake, kcal/d | 1,687 (582) | 1,588 (565) | 1,570 (489) | <0.001 |
| Caloric intake, kcal/kg | 21.9 (8.8) | 20.8 (8.1) | 21.4 (7.5) | <0.001 |
| Protein intake, g/d | 69.1 (27.1) | 71.0 (28.2) | 74.1 (27.5) | <0.001 |
| Protein intake, g/kg | 0.89 (0.39) | 0.93 (0.39) | 1.01 (0.40) | <0.001 |
| Red and processed meat, servings/day | 1.4 (0.8) | 1.0 (0.7) | 0.7 (0.6) | <0.001 |
Note: Values for categorical variables are given as number (percent); for continuous variables, as mean ± standard deviation. Conversion factor for creatinine in mg/dL to μmol/L, ×88.4.
p-value from linear regression for continuous variables and from χ2 test for categorical variables.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; DASH, Dietary Approaches to Stop Hypertension; eGFR, estimated glomerular filtration rate; HS, high school; SBP, systolic blood pressure
DASH Diet Score and Subsequent Kidney Disease
There were 3,720 cases of kidney disease during a median follow-up of 23 years. After adjusting for age, sex, race-center, education level, smoking status, physical activity, and total caloric intake, baseline eGFR, overweight/obese status, diabetes, hypertension, systolic blood pressure, and use of ACE inhibitors or ARBs, participants with a DASH diet score in the lowest tertile were 1.16-times more likely to develop kidney disease than those with the highest tertile of the DASH score (Model 3: HR for tertile 3 versus 1. 1.16; 95% CI, 1.07-1.26; p for trend across tertiles <0.001; Table 2).
Table 2.
Risk of Kidney Disease by Tertile of the DASH Diet Score
| Effect Estimate | Tertile 1: Score of 8-22 (Low) | Tertile 2: Score of 23-26 (Moderate) | Tertile 3: Score of 27-40 (High) | P for trend | |
|---|---|---|---|---|---|
| Unadjusted | IR (95% CI) | 13.3 (12.7 to 14.0) | 12.8 (12.1 to 13.6) | 11.8 (11.1 to 12.5) | 0.002 |
| IRD (95% CI) | −1.6 (−0.6 to −2.5) | −1.0 (−0.0 to −2.1) | 1.00 (reference) | 0.002 | |
| Model 1 | HR (95% CI) | 1.11 (1.03 to 1.21) | 1.10 (1.02 to 1.20) | 1.00 (reference) | 0.01 |
| Model 2 | HR (95% CI) | 1.10 (1.01 to 1.20) | 1.09 (1.00 to 1.18) | 1.00 (reference) | 0.03 |
| Model 3 | HR (95% CI) | 1.16 (1.07 to 1.27) | 1.09 (1.00 to 1.18) | 1.00 (reference) | <0.001 |
Note: Model 1: Adjusted for age, sex, race-center, education level, smoking status, physical activity, total caloric intake; Model 2: Model 1 + baseline eGFR (linear spline terms with one knot at 90 mL/min/1.73 m2); Model 3: Model 2 + overweight/obese status, diabetes, hypertension, systolic blood pressure, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers
Abbreviations and definitions: CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; HR, hazard ratio; IR, incidence rate per 1,000 person-years; IRD, incidence rate difference per 1,000 person-years
The association between DASH diet score and risk of kidney disease was likewise evident using the secondary DASH diet index that incorporates nutrients rather than food items (Model 3: HR for tertile 3 versus 1, 1.11; 95% CI, 1.02-1.22; p for trend=0.007; Table S5). Similar patterns were observed using indices modified to exclude dietary intake of sodium from the score (Table S6). In a sensitivity analysis using eGFR exclusively for the outcome definition, there were 2,030 cases of kidney disease (55% out of a total of 3,720 cases) and effect estimates were stronger than those for the primary method for ascertaining cases of kidney disease (Model 3: HR for tertile 3 versus 1, 1.22; 95% CI, 1.08-1.36; p for trend=0.001; Table S7).
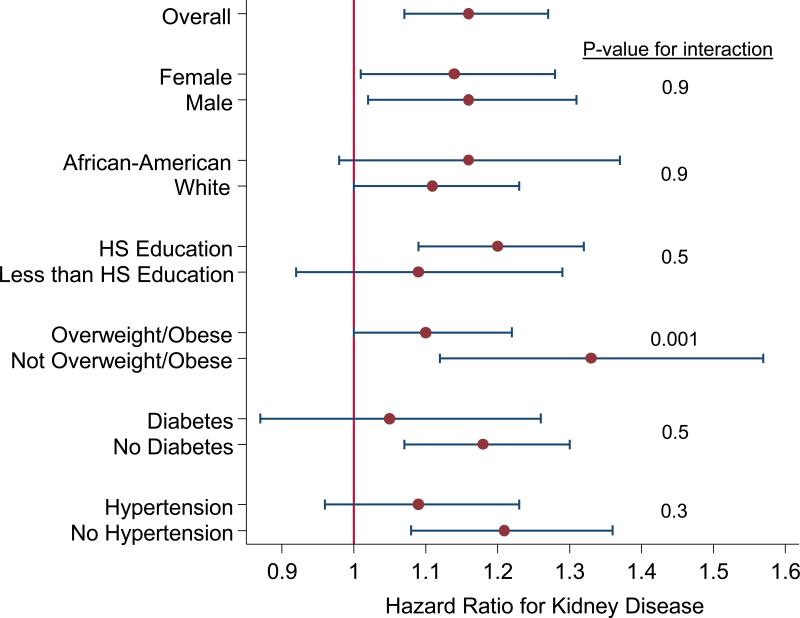
In stratified analysis, the association between DASH diet and kidney disease was similar by sex, race, and education level (Figure 1). The relationship between DASH diet and kidney disease appeared to be stronger among those without diabetes and without hypertension, but the test for interaction was not statistically significant. DASH diet score was more strongly associated with kidney disease among those who were not overweight/obese.
Figure 1.
Riska of Kidney Disease for Low (Tertile 1) vs. High (Tertile 3) DASH Diet Score According to Demographic, Socioeconomic, and Clinical Characteristics
a Hazard ratios for kidney disease are presented for the low (tertile 1) vs. high (tertile 3) DASH diet score, adjusted for age, sex, race-center, education level, smoking status, physical activity, total caloric intake, baseline eGFR (linear spline terms with one knot at 90 mL/min/1.73 m2), overweight/obese status, diabetes, hypertension, systolic blood pressure, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers
Components of DASH Diet Score and Subsequent Kidney Disease
Of the individual components of the DASH diet score, higher intake of red and processed meat was significantly associated with higher risk of kidney disease, and higher intake of nuts and legumes as well as low-fat dairy products was associated with a lower risk of kidney disease (Table 3). For the secondary DASH diet score, higher intake of magnesium and calcium was statistically significantly associated with a reduced risk of kidney disease, and higher dietary protein intake was associated with a higher risk of kidney disease (Table S8).
Table 3.
Dietary Intake of Individual Components of DASH Diet Score and Risk of Kidney Disease
| Component | Quintile 1 (Low Intake) | Quintile 2 | Quintile 3 (Moderate Intake) | Quintile 4 | Quintile 5 (High Intake) | P for trend | |
|---|---|---|---|---|---|---|---|
| Sodium | mg/d* | 251-1,021 | 1,022-1,287 | 1,288-1,553 | 1,554-1,906 | 1,907-5,030 | -- |
| Model 1 | 1 [Reference] | 0.98 (0.88, 1.09) | 0.94 (0.83, 1.07) | 0.97 (0.84, 1.12) | 0.97 (0.81, 1.17) | 0.5 | |
| Model 2 | 1 [Reference] | 0.97 (0.86, 1.08) | 0.92 (0.81, 1.04) | 0.94 (0.81, 1.08) | 0.92 (0.77, 1.11) | 0.2 | |
| Model 3 | 1 [Reference] | 0.95 (0.86, 1.07) | 0.93 (0.82, 1.06) | 0.92 (0.80, 1.06) | 0.91 (0.75, 1.09) | 0.2 | |
| Red and processed meat | servings/day* | 0.0-0.4 | 0.5-0.7 | 0.8-1.1 | 1.2-1.5 | 1.6-13.7 | -- |
| Model 1 | 1 [Reference] | 1.13 (1.01, 1.25) | 1.19 (1.06, 1.32) | 1.26 (1.12, 1.42) | 1.49 (1.31, 1.70) | <0.001 | |
| Model 2 | 1 [Reference] | 1.12 (1.00, 1.24) | 1.17 (1.05, 1.31) | 1.23 (1.09, 1.38) | 1.47 (1.29, 1.68) | <0.001 | |
| Model 3 | 1 [Reference] | 1.04 (0.93, 1.16) | 1.04 (0.93, 1.16) | 1.06 (0.94, 1.19) | 1.22 (1.07, 1.40) | 0.02 | |
| Sweetened beverages | glasses/day* | 0.0-0.0 | 0.0-0.1 | 0.2-0.4 | 0.5-0.9 | 1.0-10.0 | -- |
| Model 1 | 1 [Reference] | 0.83 (0.75, 0.92) | 0.85 (0.76, 0.94) | 0.84 (0.75, 0.93) | 0.86 (0.77, 0.97) | 0.01 | |
| Model 2 | 1 [Reference] | 0.82 (0.74, 0.91) | 0.85 (0.77, 0.94) | 0.86 (0.77, 0.95) | 0.86 (0.76, 0.97) | 0.02 | |
| Model 3 | 1 [Reference] | 0.91 (0.82, 1.01) | 0.94 (0.85, 1.04) | 0.93 (0.84, 1.04) | 0.94 (0.83, 1.06) | 0.3 | |
| Fruits | servings/day* | 0.0-0.9 | 1.0-1.5 | 1.6-2.2 | 2.3-3.0 | 3.1-23.6 | -- |
| Model 1 | 1 [Reference] | 1.03 (0.92, 1.14) | 1.03 (0.93, 1.15) | 1.09 (0.97, 1.22) | 1.22 (1.08, 1.37) | 0.002 | |
| Model 2 | 1 [Reference] | 1.02 (0.92, 1.14) | 1.04 (0.93, 1.16) | 1.08 (0.97, 1.21) | 1.24 (1.10, 1.40) | 0.001 | |
| Model 3 | 1 [Reference] | 0.99 (0.90, 1.11) | 0.97 (0.87, 1.08) | 0.99 (0.88, 1.10) | 1.06 (0.94, 1.20) | 0.5 | |
| Vegetables | servings/day* | 0.0-0.5 | 0.6-0.9 | 1.0-1.2 | 1.3-1.7 | 1.8-18.1 | -- |
| Model 1 | 1 [Reference] | 1.05 (0.95, 1.17) | 0.97 (0.87, 1.08) | 1.04 (0.93, 1.16) | 0.99 (0.88, 1.12) | 0.8 | |
| Model 2 | 1 [Reference] | 1.05 (0.94, 1.16) | 0.97 (0.87, 1.08) | 1.05 (0.94, 1.18) | 1.01 (0.89, 1.13) | 0.9 | |
| Model 3 | 1 [Reference] | 1.02 (0.92, 1.13) | 0.95 (0.85, 1.06) | 0.99 (0.89, 1.11) | 0.94 (0.83, 1.06) | 0.2 | |
| Nuts and legumes | servings/day* | 0.0-0.4 | 0.5-0.6 | 0.7-0.9 | 1.0-1.3 | 1.4-10.6 | -- |
| Model 1 | 1 [Reference] | 0.91 (0.82, 1.01) | 0.93 (0.83, 1.03) | 0.87 (0.78, 0.98) | 0.89 (0.79, 1.01) | 0.03 | |
| Model 2 | 1 [Reference] | 0.93 (0.84, 1.03) | 0.93 (0.83, 1.03) | 0.87 (0.78, 0.97) | 0.89 (0.79, 1.01) | 0.02 | |
| Model 3 | 1 [Reference] | 0.96 (0.87, 1.07) | 0.94 (0.85, 1.05) | 0.89 (0.79, 0.99) | 0.91 (0.81, 1.03) | 0.04 | |
| Whole grains | servings/day* | 0.0-0.2 | 0.3-0.5 | 0.6-0.9 | 1.0-1.5 | 1.6-8.6 | -- |
| Model 1 | 1 [Reference] | 0.97 (0.88, 1.07) | 0.95 (0.85, 1.05) | 1.03 (0.93, 1.14) | 0.89 (0.80, 1.00) | 0.2 | |
| Model 2 | 1 [Reference] | 0.98 (0.89, 1.08) | 0.96 (0.86, 1.07) | 1.05 (0.94, 1.16) | 0.93 (0.83, 1.04) | 0.5 | |
| Model 3 | 1 [Reference] | 0.94 (0.85, 1.04) | 0.94 (0.85, 1.05) | 1.01 (0.91, 1.12) | 0.91 (0.81, 1.02) | 0.3 | |
| Low-fat | servings/day* | 0.0-0.1 | 0.2-0.4 | 0.5-0.8 | 0.9-1.3 | 1.4-10.8 | -- |
| Model 1 | 1 [Reference] | 0.92 (0.83, 1.02) | 0.85 (0.76, 0.94) | 0.84 (0.75, 0.94) | 0.86 (0.77, 0.97) | 0.001 | |
| Model 2 | 1 [Reference] | 0.90 (0.81, 0.99) | 0.81 (0.73, 0.90) | 0.81 (0.73, 0.91) | 0.82 (0.73, 0.92) | <0.001 | |
| Model 3 | 1 [Reference] | 0.93 (0.84, 1.03) | 0.83 (0.75, 0.92) | 0.85 (0.76, 0.94) | 0.84 (0.75, 0.95) | <0.001 | |
Note: Unless otherwise indicated, values given as hazard ratio (95% confidence interval). Model 1: Adjusted for age, sex, race-center, education level, smoking status, physical activity, total caloric intake, and all other factors in the DASH diet score (all eight individual components of the DASH diet score were included in the same model, i.e., 1. sodium, 2. red and processed meat, 3. sweetened beverages, 4. fruits, 5. vegetables, 6. nuts and legumes, 7. whole grains, 8. low-fat dairy products); Model 2: Model 1 + baseline estimated glomerular filtration rate (linear spline terms with one knot at 90 mL/min/1.73 m2); Model 3: Model 2 + overweight/obese status, diabetes, hypertension, systolic blood pressure, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers
DASH, Dietary Approach to Stop Hypertension
range
DISCUSSION
Our study of 14,882 middle-aged African-American and Caucasian men and women suggests that following a low-sodium DASH-style diet is associated with lower risk of kidney disease. Specifically, individuals with the lowest DASH diet score were 16% more likely to develop kidney disease than those with the highest DASH diet score. Higher intake of red and processed meat was associated with elevated risk of kidney disease, whereas consumption of other sources of protein including nuts, legumes, and low-fat dairy products, was associated with lower risk of kidney disease.
The present study is to our knowledge the first to report a prospective association between a DASH-style dietary pattern and subsequent kidney disease in a diverse study population. A cross-sectional analysis of an 869-person subset of the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study showed that those in the lowest vs. highest tertile of the DASH diet score had a three-fold higher odds of reduced eGFR (<60 mL/min/1.73 m2) after adjusting for age, sex, race, education, health care access, diabetes, hypertension, smoking, and caloric intake among participants who were living in poverty (42% of the study population; OR, 3.15; 95% CI, 1.51-6.56), but there was no association among participants not living in poverty.16 In a prospective analysis of 3,121 older Caucasian women in the Nurses’ Health Study, the highest vs. lowest quartile of the DASH diet score was associated with lower risk of eGFR decline ≥30% after adjusting for age, hypertension, BMI, physical activity, caloric intake, smoking, diabetes, diabetes duration, cardiovascular disease, and use of ACE inhibitors or ARBs (OR, 0.55; 95% CI, 0.38-0.80), with no variation by diabetes status.15 Our study extends this research by reporting associations in the general population setting for a large (N=14,882) and broadly generalizable study population. We observed similar effect estimates for men and women, Caucasians and African-Americans, and according to education level as a proxy for socioeconomic status.
An interesting aspect of our study is that the DASH diet was more strongly associated with risk of kidney disease among individuals who were not overweight or obese at baseline. It is plausible that those who were overweight or obese at baseline were previously advised to modify their diet due to their weight. As such, characterizing their dietary pattern at baseline may overestimate the quality of their diet over the lifetime. Another possibility is that risk estimates were attenuated among those who were overweight or obese due to reporting bias of dietary intake.30 Among study participants without diabetes and those without hypertension, the DASH diet-kidney disease association appeared to be stronger, although the interaction was not statistically significant. Further research is necessary to replicate these findings among individuals with co-morbidities.
There are several possible mechanisms by which the DASH diet may affect risk of kidney disease. It may reduce blood pressure, as was the original intention of the diet. It also has a lower dietary acid load (−25.5 mEq/d) than a typical diet (50-75 mEq/day).31,32 We have previously demonstrated that higher dietary acid load was associated with incident kidney disease in the ARIC study.33 The association between dietary acid load and kidney disease, which has also been reported by other investigators, may be due to activation of the renin-angiotensin system or increase in endothelin 1 levels.34-45 Alternatively, as has been reported with other dietary patterns, not following a DASH-style diet may stimulate an inflammatory response and endothelial dysfunction, which is a shared pathophysiologic mechanism for the development of both cardiovascular and kidney disease.46-50
Individual components of the DASH diet score may also drive the association with risk of kidney disease. In the present study, after adjusting for age, sex, race-center, education, smoking, physical activity, caloric intake, baseline eGFR, overweight/obese status, diabetes, hypertension, systolic blood pressure, and use of ACE inhibitors or ARBs, higher intake of red and processed meat was associated with higher risk of kidney disease; and higher intake of nuts, legumes, and low-fat dairy was associated with lower risk of kidney disease. The significant associations from the secondary DASH diet score (protein, magnesium, calcium) were consistent with the main analysis: red and processed meat is a source of protein, nuts and legumes are rich sources of magnesium, and dairy products are a rich source of calcium.51 Increased dietary intake of protein is recommended for cardio-protection, whereas it is potentially harmful to the kidney.52 However, plant protein may protect against kidney disease through increases in serum bicarbonate and decreases in fibroblast growth factor 23.53 Lower serum levels of magnesium are associated with higher production of inflammatory and pro-atherogenic cytokines in endothelial cells, which is a pathway that might contribute to decreased kidney function.54,55 Milk protein contains peptides (casokinins and lactokinins) that have vasoactive properties, such as inhibiting the ACE and reducing blood pressure, an established kidney disease risk factor.56,57 Taken together, our results suggest that protein from meat confers higher risk of adverse kidney outcomes whereas vegetable and dairy sources of protein confer kidney protective effects. Future research and recommendations on dietary intake and kidney disease risk should differentiate between sources of protein.
There are certain strengths and limitations of our study. As with any observational study design, residual confounding may be present. However, participants were extensively characterized with respect to demographic, socioeconomic, clinical, and behavioral factors at ARIC study visits, allowing adjustment for many important confounders. The ascertainment of cases using a composite of criteria (eGFR, hospitalizations, deaths, USRDS registry) is clinically relevant, appropriate for research studies, and allows for the detection of a large number of cases.58 In a validation study, compared to medical chart review, this outcome demonstrated high specificity (96%) and low sensitivity (36%).58 Several ARIC study publications have used this composite outcome.59-61 In a sensitivity analysis of kidney disease based only on eGFR, the association between DASH diet and kidney disease was slightly stronger than that with the composite outcome. The lack of data on albuminuria, which is strongly associated with kidney function decline, is a limitation. The eGFR may have been affected by non-GFR determinants of serum creatinine level including protein intake and muscle mass.62
The strengths and limitations of dietary assessment deserve mention. Assessment of dietary intake by self-report is prone to reporting bias and other sources of measurement error.63 We reduced measurement error and reporting bias specifically by using data from questionnaires administered by trained interviewers following a standard protocol, using visual aids to represent portion sizes, and incorporating repeated measurements of dietary intake.22 In addition, administration of the food frequency questionnaire was repeated in a subset of 419 ARIC study participants to quantify reproducibility of dietary assessment.19 The 66-item food frequency questionnaire allows for ranking of dietary intake of the food items assessed. Absolute amounts of consumed food items and nutrients (especially sodium) were likely to be underestimated due to the limited number of items on the questionnaire and lack of information on food brands and snack foods.64 However, in a sensitivity analysis excluding sodium from the DASH diet score, the effect estimates were essentially unchanged. Further, our finding that high intake of red and processed meat was associated with higher risk of kidney disease may in part be due to the fact that meat is a leading source of sodium according to NHANES (National Health and Nutrition Examination Survey)—specifically, cold cuts/cured meat, pasta with meat sauce, and mixed meat dishes.65 Nonetheless, results of analyses that present individual food and nutrient relationships should be interpreted cautiously.
The evidence on dietary patterns such as the DASH diet should be evaluated for potential inclusion in clinical recommendations for kidney disease prevention. Our results provide support for promotion of a DASH-style diet in an even broader segment of the US population for reduced risk of kidney disease in addition to blood pressure reduction and cardiovascular disease prevention.
In conclusion, consumption of a DASH-style diet was associated with lower risk of kidney disease, independent of demographic characteristics, caloric intake, socioeconomic status, lifestyle factors, comorbid conditions, anti-hypertensive medication use, and baseline kidney function in this general population sample of African-American and Caucasian men and women. The DASH diet, designed for blood pressure reduction and now widely recommended for reducing the risk of cardiovascular disease and other chronic diseases, may also protect against kidney disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. Some of the data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Drs Crews and Grams are supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK097184 and K08 DK092287, respectively). The funders did not have a role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N Section: Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Steven M. Brunelli, MD, MSCE) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Contributions: Research idea and study design: CMR; data acquisition: JC; data interpretation: CMR, DCC, MEG, LMS, ASL, ERM, LJA, JC; statistical analysis: CMR; supervision and mentorship: LJA, JC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CMR takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Peer Review: Evaluated by 3 external peer reviewers and the acting Editor-in-Chief.
Supplementary Material
Table S1: Classification of components of DASH diet score based on food items.
Table S2: Classification of components of DASH diet score based on nutrients.
Table S3: Description of dietary intake for overall study population and according to case status.
Table S4: Baseline demographic and clinical characteristics for included and excluded participants and total ARIC population.
Table S5: Risk of kidney disease by tertile of alternative DASH diet score based on nutrients.
Table S6: Risk of kidney disease by tertile of DASH diet scores modified to exclude sodium.
Table S7: Risk of kidney disease based on eGFR by tertile of DASH diet score.
Table S8: Risk of kidney disease associated with individual components of alternative DASH diet score based on nutrients.
Figure S1: Flowchart of study participant selection.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Classification of components of DASH diet score based on food items.
Supplementary Table S2 (PDF). Classification of components of DASH diet score based on nutrients.
Supplementary Table S3 (PDF). Description of dietary intake for overall study population and according to case status.
Supplementary Table S4 (PDF). Baseline demographic and clinical characteristics for included and excluded participants and total ARIC population.
Supplementary Table S5 (PDF). Risk of kidney disease by tertile of alternative DASH diet score based on nutrients.
Supplementary Table S6 (PDF). Risk of kidney disease by tertile of DASH diet scores modified to exclude sodium.
Supplementary Table S7 (PDF). Risk of kidney disease based on eGFR by tertile of DASH diet score.
Supplementary Table S8 (PDF). Risk of kidney disease associated with individual components of alternative DASH diet score based on nutrients.
Supplementary Figure S1 (PDF). Flowchart of study participant selection.
REFERENCES
- 1.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 3.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 5.Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;172(20):1566–1572. doi: 10.1001/archinternmed.2012.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2015;115(5):780–800. e785. doi: 10.1016/j.jand.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47(2):296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Agriculture and US Department of Health and Human Services . Dietary Guidelines for Americans, 2010. U.S. Government Printing Office; Washington, DC: 2010. [Google Scholar]
- 9.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 10.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120–143. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 11.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013;3(1):1–150. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs-Smith SM, Subar AF, Reedy J. Examining Dietary Patterns in Relation to Chronic Disease: Matching Measures and Methods to Questions of Interest. Circulation. 2015;132(9):790–793. doi: 10.1161/CIRCULATIONAHA.115.018010. [DOI] [PubMed] [Google Scholar]
- 14.Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr. 2015;101(5):899–900. doi: 10.3945/ajcn.115.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses' Health Study. Am J Kidney Dis. 2011;57(2):245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crews DC, Kuczmarski MF, Miller ER, 3rd, Zonderman AB, Evans MK, Powe NR. Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr. 2015;25(2):103–110. doi: 10.1053/j.jrn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18.Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181(4):225–233. doi: 10.1093/aje/kwu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutrition Research. 1996;16(5):735–745. [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Shimakawa T, Sorlie P, Carpenter MA, et al. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev Med. 1994;23(6):769–780. doi: 10.1006/pmed.1994.1133. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 23.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 24.Powell-Wiley TM, Miller PE, Agyemang P, Agurs-Collins T, Reedy J. Perceived and objective diet quality in US adults: a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES). Public Health Nutr. 2014;17(12):2641–2649. doi: 10.1017/S1368980014000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18(11):1419–1422. [PubMed] [Google Scholar]
- 26.Parrinello CM, Grams ME, Couper D, et al. Recalibration of Blood Analytes over 25 Years in the Atherosclerosis Risk in Communities Study: Impact of Recalibration on Chronic Kidney Disease Prevalence and Incidence. Clin Chem. 2015;61(7):938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 29.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 30.Lissner L, Troiano RP, Midthune D, et al. OPEN about obesity: recovery biomarkers, dietary reporting errors and BMI. Int J Obes (Lond) 2007;31(6):956–961. doi: 10.1038/sj.ijo.0803527. [DOI] [PubMed] [Google Scholar]
- 31.Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium Dietary Approaches to Stop Hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29(1):8–18. doi: 10.1016/j.nutres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Scialla JJ, Anderson CA. Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis. 2013;20(2):141–149. doi: 10.1053/j.ackd.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rebholz CM, Coresh J, Grams ME, et al. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am J Nephrol. 2015;42(6):427–435. doi: 10.1159/000443746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee T, Crews DC, Wesson DE, et al. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15:137. doi: 10.1186/1471-2369-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee T, Crews DC, Wesson DE, et al. High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol. 2015;26(7):1693–1700. doi: 10.1681/ASN.2014040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna A, Simoni J, Hacker C, Duran MJ, Wesson DE. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol. 2004;15(9):2266–2275. doi: 10.1097/01.ASN.0000138233.78329.4E. [DOI] [PubMed] [Google Scholar]
- 38.Khanna A, Simoni J, Wesson DE. Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol. 2005;16(7):1929–1935. doi: 10.1681/ASN.2004121054. [DOI] [PubMed] [Google Scholar]
- 39.Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77(7):617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 40.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010;78(11):1128–1135. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 41.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300(4):F830–837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 42.Ng HY, Chen HC, Tsai YC, Yang YK, Lee CT. Activation of intrarenal reninangiotensin system during metabolic acidosis. Am J Nephrol. 2011;34(1):55–63. doi: 10.1159/000328742. [DOI] [PubMed] [Google Scholar]
- 43.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensinaldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;(99):S57–65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 44.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 45.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8(3):371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 47.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68(1):237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Garcia E, Hu FB. Nutrition and the endothelium. Curr Diab Rep. 2004;4(4):253–259. doi: 10.1007/s11892-004-0076-7. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 50.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory . USDA National Nutrient Database for Standard Reference. 2015. [Google Scholar]
- 52.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1996;27(5):652–663. doi: 10.1016/s0272-6386(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 53.Scialla JJ, Appel LJ, Wolf M, et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Ren Nutr. 2012;22(4):379–388. e371. doi: 10.1053/j.jrn.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferre S, Baldoli E, Leidi M, Maier JA. Magnesium deficiency promotes a proatherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta. 2010;1802(11):952–958. doi: 10.1016/j.bbadis.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87(4):820–827. doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kris-Etherton PM, Grieger JA, Hilpert KF, West SG. Milk products, dietary patterns and blood pressure management. J Am Coll Nutr. 2009;28(Suppl 1):103S–119S. doi: 10.1080/07315724.2009.10719804. [DOI] [PubMed] [Google Scholar]
- 57.FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134(4):980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- 58.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kummer AE, Grams M, Lutsey P, et al. Nephrolithiasis as a Risk Factor for CKD: The Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015;10(11):2023–2029. doi: 10.2215/CJN.10111014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rebholz CM, Grams ME, Crews DC, Appel LJ, Coresh J. Dietary acid load and incident chronic kidney disease: the Atherosclerosis Risk in Communities study. J Am Soc Nephrol. 2014;25:682A. [Google Scholar]
- 61.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–288. doi: 10.1016/S2213-8587(13)70199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 63.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103(14):1086–1092. doi: 10.1093/jnci/djr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015;181(7):473–487. doi: 10.1093/aje/kwu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease C, Prevention Vital signs: food categories contributing the most to sodium consumption - United States, 2007-2008. MMWR Morb Mortal Wkly Rep. 2012;61(5):92–98. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.