Abstract
Purpose
Fulvestrant is an estrogen receptor (ER) antagonist and an approved treatment for metastatic estrogen receptor positive (ER+) breast cancer. With the exception of ER levels, there are no established predictive biomarkers of response to single agent fulvestrant. We attempted to identify a gene signature of response to fulvestrant in advanced breast cancer.
Experimental Design
Primary tumor samples from 134 patients enrolled in the phase III CONFIRM study of patients with metastatic ER+ breast cancer comparing treatment with either 250mg or 500mg fulvestrant were collected for genome-wide transcriptomic analysis. Gene expression profiling was performed using Affymetrix microarrays. An exploratory analysis was performed to identify biological pathways and new signatures associated with response to fulvestrant.
Results
Pathway analysis demonstrated that increased EGF pathway and FOXA1 transcriptional signaling is associated with decreased response to fulvestrant. Using a multivariate Cox model we identified a novel set of 37 genes whose expression is independently associated with progression free survival (PFS). TFAP2C, a known regulator of ER activity was ranked second in this gene set and high expression was associated with a decreased response to fulvestrant. The negative predictive value of TFAP2C expression at the protein level was confirmed by immunohistochemistry.
Conclusions
We identified biological pathways and a novel gene signature in primary ER+ breast cancers that predicts for response to treatment in the CONFIRM study. These results suggest potential new therapeutic targets and warrant further validation as predictive biomarkers of fulvestrant treatment in metastatic breast cancer.
Keywords: breast cancer, estrogen receptor, fulvestrant, EGF, FOXA1, TFAP2C
Introduction
Fulvestrant is a highly selective estrogen receptor alpha (ER) ligand devoid of agonist activity. When bound to ER, fulvestrant leads to ER degradation resulting in decreased cellular ER levels and to the inactivation of both transactivation domains, AF1 and AF2. This ability to induce ER degradation has led to its classification as a selective ER degrader (SERD). The early clinical trials of fulvestrant showed comparable activity to aromatase inhibitors in advanced breast cancer after progression on first line endocrine treatment1. In addition, fulvestrant was shown to have activity in metastatic disease after progression on an AI2. In a phase II prospective study in which post-menopausal patients received fulvestrant after progression on an AI, 30% of the patients derived clinical benefit at 24 weeks and previous response to an AI did not predict response to fulvestrant3. More recently, the phase II FIRST study indicated that first line therapy with fulvestrant at 500mg has superior activity compared to an AI4. Currently, there is an ongoing phase III study (FALCON) comparing fulvestrant (500mg) to an AI for first line treatment of metastatic disease (www.clinicaltrials.gov identifier NCT TO1602380), which could lead to the approval of fulvestrant as first line treatment. These studies and other studies, demonstrate that not all patients benefit from single agent fulvestrant, but there is a subgroup of patients with metastatic ER+ breast cancer that gain substantial and durable clinical benefit from single agent fulvestrant even after progression on previous endocrine treatment. However, there are no well-defined clinicopathological features or molecular markers other than ER expression to predict response to single agent endocrine treatment as first line or second line therapy in the metastatic setting.
A number of multi-gene expression based assays have been developed to assess prognosis and predict response to endocrine treatments and chemotherapy in early stage estrogen receptor positive (ER+) breast cancer. The OncotypeDx recurrence score (Genomic Health Inc, Redwood City, CA) is clinically available and widely employed in the primary setting to inform decisions concerning the benefit of the addition of adjuvant chemotherapy to adjuvant endocrine therapy5, 6. PAM50, which measures the expression of 50 genes that classify breast cancers into intrinsic subtypes and is applied for the calculation of a risk of recurrence score (RORS), has also been shown to be prognostic in the early disease context. An analysis of untreated breast cancer patients and patients that received neoadjuvant chemotherapy showed that the intrinsic subtype classification and RORs provide prognostic and predictive information in ER+ and ER-negative early stage breast cancer7. A molecular marker or gene set to predict response or resistance to endocrine treatment in the setting of ER+ metastatic disease would be helpful to stratify patients that are less likely to derive significant benefit from a single agent endocrine treatment and are candidates for treatments that combine endocrine treatment with newer targeted treatments or chemotherapy. The need for these tools is becoming more apparent as new treatment combinations are emerging as effective treatments for ER+ metastatic disease. The combination of letrozole and palbociclib was approved for first line treatment in ER+ metastatic disease8, but it is not clear that all patients need to be treated with this expensive regimen that is more toxic than letrozole alone. Such advances in the treatment of metastatic ER+ breast cancer as well as new endocrine treatments currently in clinical development underscore the importance of developing assays to predict response to endocrine treatment in metastatic disease.
The phase III Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) trial demonstrated that the 500 mg dose of fulvestrant compared to the 250 mg dose in post-menopausal women with locally advanced or metastatic ER+ breast cancer after recurrence or progression on prior endocrine treatment was associated with an improvement in PFS and overall survival (OS)9. In this analysis of the primary tumors from a subset of patients from the CONFIRM trial termed transCONFIRM, we evaluated the expression of the genes that make up the PAM50 intrinsic subtype–classifier and the oncotypeDx scoring system as predictors of response to fulvestrant. In addition, we performed pathway analyses and used a logistical regression model to identify potential new gene signatures to predict response to fulvestrant in the CONFIRM study.
Methods
CONFIRM patient population
The CONFIRM study population included post-menopausal patients who had locally advanced or metastatic ER+ breast cancer. Patients either experienced relapse on adjuvant endocrine therapy, within 1 year from completion of adjuvant therapy, had de novo advanced disease or recurrence more than 1 year after completion of adjuvant endocrine therapy. For patients with de novo metastatic disease or recurrences after more than 1 year from completion of adjuvant treatment, eligibility criteria required prior treatment with an anti-estrogen or an aromatase inhibitor as first line treatment. Central confirmation of ER status was not performed. The trial had a double blind, placebo-controlled design and patients were randomized to fulvestrant 500 mg or 250 mg given on days 0, 14, 28 and every 28 days thereafter. Treatment was continued until disease progression unless any criteria for early discontinuation were met first. The primary study end point was PFS defined as the time elapsing between the date of random assignment and the date of the earliest evidence of objective disease progression as evaluated by RECIST criteria.
TransCONFIRM tumor samples
From the 736 patients enrolled in the CONFIRM study, 134 formalin-fixed, paraffin-embedded (FFPE) primary tumor samples were collected with patient consent and local Institutional Review Board approval. Samples were centrally reviewed by a breast pathologist and tissue microarrays were created in quadruplicate. The distribution of the main clinicopathological variables was similar between the entire CONFIRM population and the transCONFIRM subpopulation (supplemental table 1).
Gene Expression microarrays
RNA was extracted from the FFPE tumor samples using RNeasy FFPE kits (Qiagen) and amplified with the WT-Ovation FFPE System V2 (NuGen). cDNA was labeled using the FL-Ovation cDNA Biotin Module V2 (Nugen). Samples were run on the Affymetrix Human Transcriptome Array (HTA) 2.0 following manufacturer’s instructions. For this workflow an Affymetrix Fluidics station 450 and Affymetrix GeneChip Scanner 3000 7G were used. Quality control and normalization was conducted using Affymetrix Expression Console. Of the 134 FFPE samples, 112 samples had sufficient RNA and met quality control criteria (Supplemental figure 1). The microarray data has been deposited in the Gene Expression Omnibus under the accession number GSE76040.
Surrogate Pam50 Intrinsic subtypes and surrogate OncotypeDX scoring
The PAM50 subtype predictor was applied using the publicly available algorithm7. The Affymetrix probesets with the highest inter-quartile range were selected and genes were median-centered using bootstrap samples of ER status by IHC. Each tumor sample was classified as one of the five different intrinsic breast cancer subtypes (luminal A, luminal B, HER2-enriched, basal-like and normal-like), and risk of relapse scores were calculated based on intrinsic subtype alone (RORS) and based on a combination of intrinsic subtype and a proliferation score (RORP). We next classified the tumors using a published algorithm to predict OncotypeDx recurrence score (RS) based on expression data for the 21 genes and standard thresholds to define scores as low, intermediate and high risk of recurrence10. The PAM50 intrinsic subtype predictions and RS classifications predictions in this study are considered surrogate classifiers since they are based on Affymetrix microarray gene expression analysis and not the original platforms that were used to establish these classifiers (Microarray data from Agilent human 1Av2 microarrays for the PAM50 and RT-PCR for OncotypeDx RS).
Immunohistochemistry Studies
Samples were centrally stained for ER, PR (ER/PR pharmDx™ Kit, Dako), HER2 (HercepTest™ Kit, Dako) and Ki67 (clone MIB-1, dilution 1:100, DAKO). ER and PR were scored according to the Allred scoring system and considered positive when the Allred score was 3 or above. HER2 positivity was defined as 3+ if more than 10% of cells displayed a strong complete membrane staining, according to the ASCO-CAP guidelines 2013.
All samples were stained for AP2γ (TFAP2C) by immunohistochemistry. Briefly, antigen retrieval was performed by heating in Target Retrieval Solution Citrate pH 6.0 (DAKO) using a pressure cooker for 10 min. Slides were incubated with the AP2γ primary antibody (clone 6E4/4, SantaCruz) at a dilution of 1:300 for 30 min at room temperature. The DAKO REAL™ Detection System Peroxidase/DAB+ was used with an automated protocol. Tumors were scored according to the Allred score (0–8). Tumors with scores 0–2 were defined as AP-2γ negative, while tumors with scores 3–8 were defined as AP-2γ positive. Whenever present, myo-epithelial cells served as positive internal control.
Statistical Analysis
Descriptive statistics were used to summarize patient and tumor characteristics, clinical outcomes, and expression-based classifiers. Contrasts of demographics and tumor characteristics between patient subgroups were evaluated using Pearson’s chi-squared test with continuity correction for categorical factors, and Wilcoxon rank sum tests for continuous factors. Correlation in IHC and expression-based phenotypes were evaluated using Spearman coefficients. Pathway-level analysis was performed in R/Bioconductor using the Significance Analysis of Function and Expression (SAFE) package with extensions for survival models described previously11. The following sources of annotation; accounting for multiple-comparisons within each group were used: 8527 Gene Ontology terms (by BioConductor), 670 Reactome pathways (by MSigDB v4), 196 PID genesets (by MSigDB v4), 186 KEGG pathways (by MSigDB v4), 217 Biocarta pathways, 2148 genesets from MSigBD C2: curated genesets, 93 ‘twoway’ genesets from MSigBD C6: Oncogenic signatures, 924 genesets from MSigBD C7: Immunologic pathways. Because SAFE allows for bi-directional testing of differential expression, pairs of genesets in MSigDB annotated as up- and down-regulated in each curated set were combined as a complete representation of the discovered pathway.
Clinical outcomes were summarized using the Kaplan-Meier product limit estimator. Hazard ratios (HR) and confidence intervals (CI) were estimated using univariate and multivariate Cox proportion hazards models. To identify a gene set associated with PFS, the adjusted hazard ratio (adj HR) was calculated for a 1-unit change in standardized expression, and the false discovery rate (FDR) was estimated using the Benjamini-Hochberg step-up method12. Hierarchical clustering of samples was performed using Euclidean distance and complete linkage. All statistical computations were carried out in R v.3.0.1 (http://cran.r-project.org) and SAS statistical software, v. 9.3 (SAS Institute Inc, Cary, NC).
Results
Clinicopathologic characteristics
The main clinicopathologic characteristics of the transCONFIRM cohort are shown in Table 1. With an average follow-up of 38.5 months at the time of analysis, median PFS was 8.3 months (95% CI 5.5–10.9 months). An equal number of patients received the 500mg and 250mg dose of fulvestrant, each dose group consisting of 56 patients. On central review the majority of the patients had ER+/HER2− or PR+/HER2− disease (92/112). The discordance rate for hormone receptor positivity between local and central review was 13% (ER negative at central review and positive at the local institution) and for HER2 positivity 9% (HER2 positive by central review and negative at local institution). Discordance between central and local review of ER and HER2 status has been described in the literature previously with rates of discordance similar to those detected in our study13. This discordance is likely due to differences in the staining methods, the scoring systems and possibly tumor heterogeneity. A strong correlation between the ER, PR and Ki67 protein levels by immunohistochemistry and microarray gene transcript levels was detected (ER, Spearman rho=0.72, p<0.0001, PR Rho=0.78 P=<0.0001, ki67, rho=0.72,p=<0.0001), Supplemental figure 2. Similar to the entire population in the CONFIRM study, the majority of the patients had either relapsed during adjuvant endocrine treatment or had de novo metastatic disease (84%) and only 6% of patients developed metastatic disease more than 1 year after completion of adjuvant endocrine treatment.
Table 1.
Patient characteristics.
| Characteristics | Variable | Value | % |
|---|---|---|---|
| Median age | years | 62 | |
| Median PFS | months | 8.3 | |
| Fulvestrant dose | 500mg | 56 | 50% |
| 250mg | 56 | 50% | |
|
Central review of receptors |
ER+/HER2− | 92 | 82% |
| ER−/PR+/HER2− | 2 | 1.7% | |
| ER+/HER2+ | 6 | 5% | |
| ER−/PR−/HER2+ | 2 | 1.7% | |
| ER−/PR−/HER2− | 10 | 8.9% | |
|
Time of relapse/progression |
During adjuvant endocrine therapy | 55 | 49% |
| 0–12 months after completion of adjuvant HT |
11 | 9.8% | |
| > 12 months after completion of adjuvant HT and after progression on first line HT |
7 | 6.2% | |
| Patients presenting with de novo advanced disease and experiencing progression on first-line HT |
39 | 35% |
HT=hormonal therapy.
PAM50 Intrinsic subtype distribution, comparison to clinical marker status and prediction of response to treatment
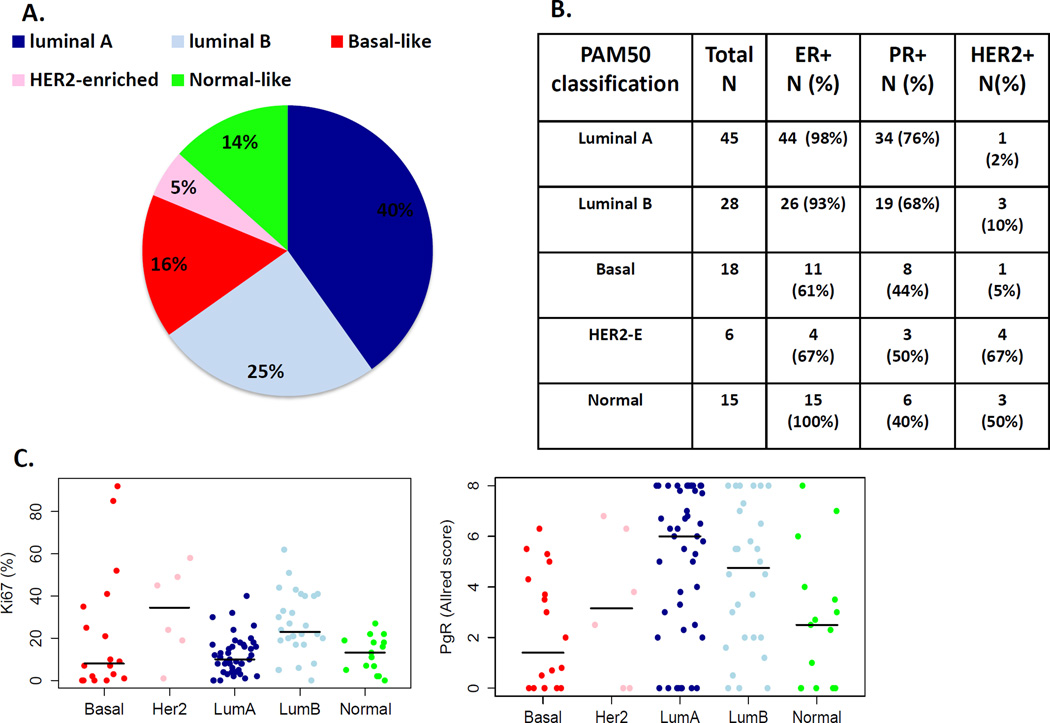
The distribution of the PAM50 intrinsic subtype and comparisons to clinical markers are detailed in figure 1. Of the 112 patients in the transCONFIRM cohort, 65% of patients had luminal subtype breast cancers (40% luminal A and 25% luminal B), 16% were basal–like, 14% were normal-like and 5% were HER2-enriched. This distribution of ER+ tumors is comparable to other studies, which also show that ER+ tumors are comprised of all the intrinsic subtypes with the majority consisting of luminal subtypes7. Focusing on the luminal A and luminal B subtype which included the majority of the tumor samples; 96% of the luminal A and luminal B subtypes were ER+. Luminal A subtype tumors compared to luminal B tumors had higher PR levels and lower Ki67 levels, which is consistent with the clinical luminal subtype classification14.
Figure 1.
PAM50 intrinsic subtype classification and comparison to clinicopathological features: A. Distribution of the intrinsic subtypes in the transCONFIRM cohort. B. ER, PR and HER2 status per each intrinsic subtype. C. Scatter plots showing ki67 and PR levels by IHC per intrinsic subtype.
We evaluated the association between the PAM50 intrinsic subtypes and PFS using a Cox model and found no significant relationship (4 d.f., p=0.91) (Supplemental figure 3A). Similarly, we assessed the ROR and ROR-P scores and did not detect an association with PFS or OS (ROR, 1 d.f., p=0.29, ROR-P, 1 d.f, p=0.35), Supplemental figure 4. In these analyses, no significant differences are seen between luminal A and luminal B subtypes. Surprisingly even tumors classified as having a basal PAM50 intrinsic subtype were not significantly different though the small number of such tumors may limit our ability to detect a difference. We next classified the transCONFIRM cohort by OncotypeDX recurrence score risk categories derived from the Affymetrix expression data. The majority of the tumors were within the high-risk group (high risk 63%, intermediate risk 15% and low risk 22%), but we did not identify an association between the risk categories and PFS (p=0.31), or OS (p=0.58) (Supplemental figure 3B).
Discovery of a gene set predictive of response to fulvestrant
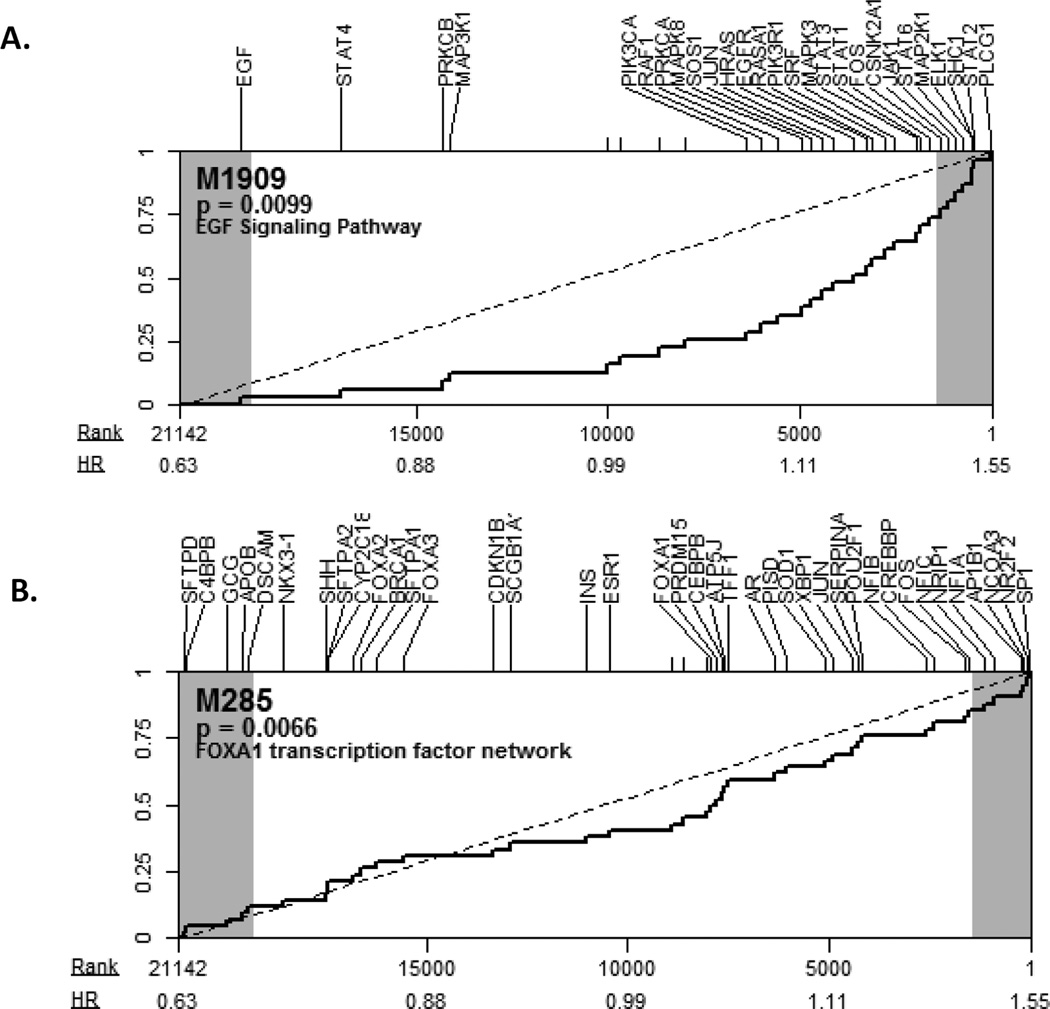
Because the PAM50 and OncotypeDx RS classifiers were not predictive of response to treatment in our analysis, we performed an exploratory analysis using SAFE to identify biological gene sets that correlate with response to fulvestrant. Using multiple sources of annotation and accounting for multiple-comparisons within each group we found several pathways that significantly correlated with PFS with fulvestrant treatment. The top ranked pathways included EGF signaling pathways (MSigDB, gene set ID M1909, number of genes: 31, p-value=0.0099, FDR=0.14 and GO:0007173, number of genes: 224, p-value=0.0044, FDR=0.3) and the FOXA1 transcription factor network (MSigDB, gene set ID M285, number of genes: 42, p-value=0.0066, FDR=0.13). As shown in figure 2, genes in these pathways strongly correlated with PFS, with the majority in each showing high expression associated with decreased PFS. This is consistent with previous reports in which these pathways have been implicated in endocrine resistance15–17.
Figure 2.
Pathway analysis of response to fulvestrant: A. SAFE plot for M1909 - EGFR signaling pathway; B. SAFE plot for M285 - FOXA1 transcriptional signaling. Transcript-level association to progress-free survival is ranked from high expression association with (left-most) prolonged PFS to (right-most) decreased PFS. Labels indicate the hazard ratio with a 1-unit change in standardized expression, and the shaded regions indicate which genes reached nominal significance (|Z| > 1.96) with the survival analysis used in SAFE. For each pathway shown, the distribution of ranks for gene-set members shows a shift toward decreased PFS with high expression.
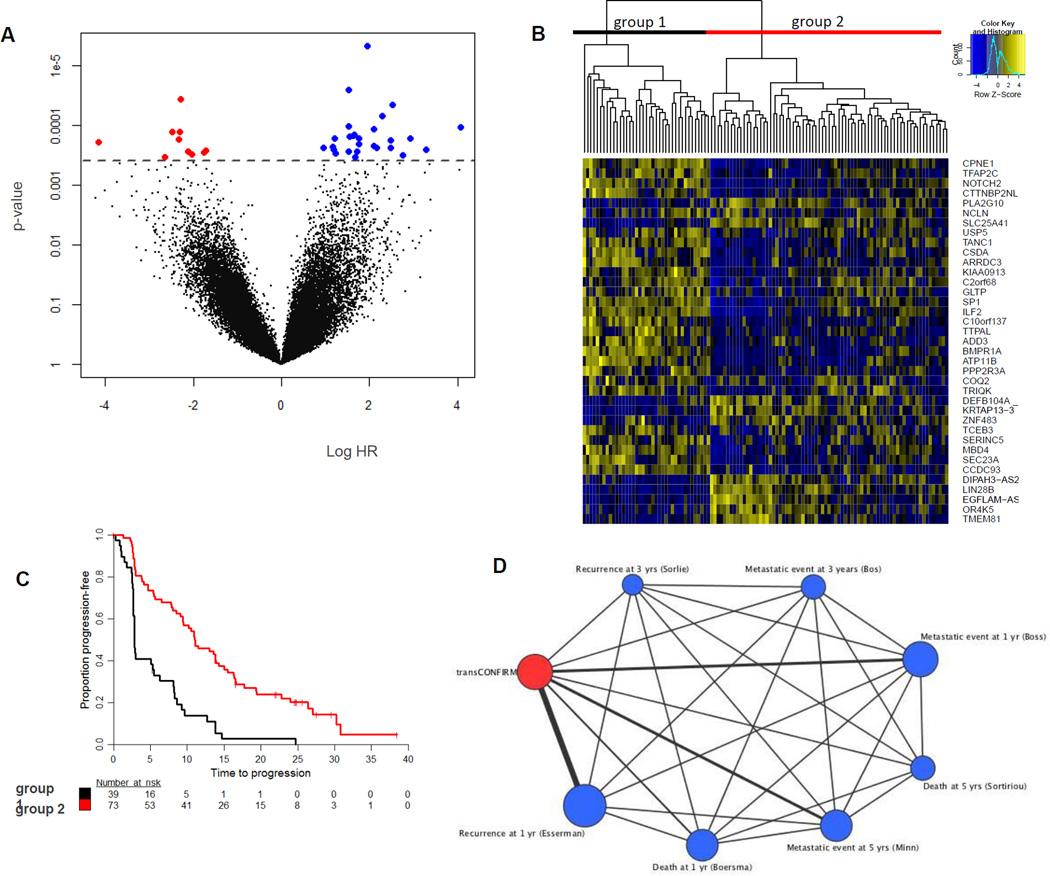
We next performed an exploratory analysis to identify a novel gene set associated with response to fulvestrant. We first applied a univariate proportional hazards model to find clinicopathological features of the transCONFIRM cohort associated with response to treatment. As shown in table 2, patient age and presentation with de novo metastatic disease versus development of metastatic disease after initial diagnosis of primary disease did not predict for PFS on fulvestrant. In addition, within this subpopulation of the CONFIRM study, the 500mg dose of fulvestrant compared to the 250mg dose was not significantly associated with PFS, although there was a trend for improved PFS with the higher dose. We evaluated ER and PR as continuous variables and for ER we did not detect a significant predictive cut point, whereas for PR, an Allred score of 6 or above was associated with better PFS with a HR of 0.59 (p=0.016, CI 0.38–0.91). Positive HER2 status conferred reduced PFS with a HR of 1.91 (p=0.037, CI 1.04–3.52), although the number of patients in this category was small. Second, we performed a multivariate Cox model adjusting for the significant clinicopathological factors and found that the expression of a set of 37 genes was independently associated with PFS (FDR<20%)(figure 3). Unsupervised clustering using these 37 genes differentiated samples into two groups; one group consisting of approximately 1/3 of the patients with worse clinical outcomes and the second group consisting of 2/3 of the patients with improved outcomes. In this gene set, upregulation of 27 genes was associated with poor PFS. These genes include genes that have known functions in the regulation of the ER transcriptional network (TFAP2C18, 19 and SP120, 21), genes that have been shown to be related to breast cancer biology and clinical outcomes (TFAP2C, BMPR1A22, ARRDC323, PPP2R3A24) and genes not yet reported to be related to breast cancer but with functional roles in tumorigenesis, metastases and response to treatment in other cancer types and may also have molecular functions in breast cancer (ILF225, ATP11B26, CSDA27, USP528,TANC129, NOTCH230, ADD331 and SEC23A32). In addition, there are a number of genes in the gene set that do not have known roles in cancer biology (CCDC93, CPNE1, GLTP and TCEB3). These genes are responsible for intra- and inter-cellular trafficking and transcription and studies to investigate their functional roles in breast cancer biology are needed. We used Oncomine Concepts Map analysis (Compendia Biosciences) to compare the 27-gene signature of poor PFS to published gene signatures of primary breast cancers and detected significant correlations with independent gene signatures of poor outcome33. Because the CONFIRM study showed a statistically significant improvement in PFS when the 500mg dose was compared with the 250mg, we performed a separate univariate Cox proportional hazards analysis for the 500mg dose and 250mg sub-groups and looked at the 37 gene set. As shown in supplemental table 2, in both subgroups the trends were similar to the multivariate analysis of the entire transCONFIRM, albeit not as significant statistically.
Table 2.
Univariate analysis of clinicopathological features.
| Variable | Unit Reference | HR (95% CI) | p-value* |
|---|---|---|---|
| Age | 10 year | 0.93 (0.63,1.39) | 0.74 |
| FULV. dose | 500 vs. 250mg | 0.95 (0.79,1.15) | 0.63 |
| ER levels | 1-unit increase in Allred score** |
1.00 (0.92,1.08) | 0.92 |
| PR levels | ≥6 / <6 Allred score |
0.59 (0.38,0.91) | 0.016 |
| HER2 status | 3 / 0–2 IHC | 1.91 (1.04,3.52) | 0.037 |
| Ki67 | 10% increase | 1.13 (0.99,1.29) | 0.064 |
Hazard ratio (HR) and 95% confidence interval (CI) are from a univariate Cox model, p-values are from a Wald-type hypothesis test.
ER was not significant (p > 0.5) for all possible cutpoints in Allred score.
Figure 3.
Discovery of a gene set predictive of response to fulvestrant: A. Volcano plot of the gene-level tests of association with PFS. The log fold-change in hazard ratio for a 1-unit increase in expression levels is plotted against the nominal p-value from a multivariate Cox regression model. B. Heatmap of unsupervised clustering of tumors using the 37 genes in the predictive gene set. C. Kaplan – Meier curve for PFS comparing the two clusters. D. Oncomine Concepts Map analysis (Compendia Biosciences) was used to compare the 27 upregulated gene set of poor PFS to published gene signatures from primary breast tumors, and reveals statistically significant correlations between this gene set and gene expression signatures of breast tumors with poor outcomes. The association between molecular concepts of different gene signatures or gene sets is represented as a graph using Cytoscape (http://cytoscape.org), in which a node represents a gene set and significantly associated sets (q ≤ 0.23) were connected by an edge.
AP2 gamma (TFAP2C) is a putative marker for resistance to fulvestrant treatment
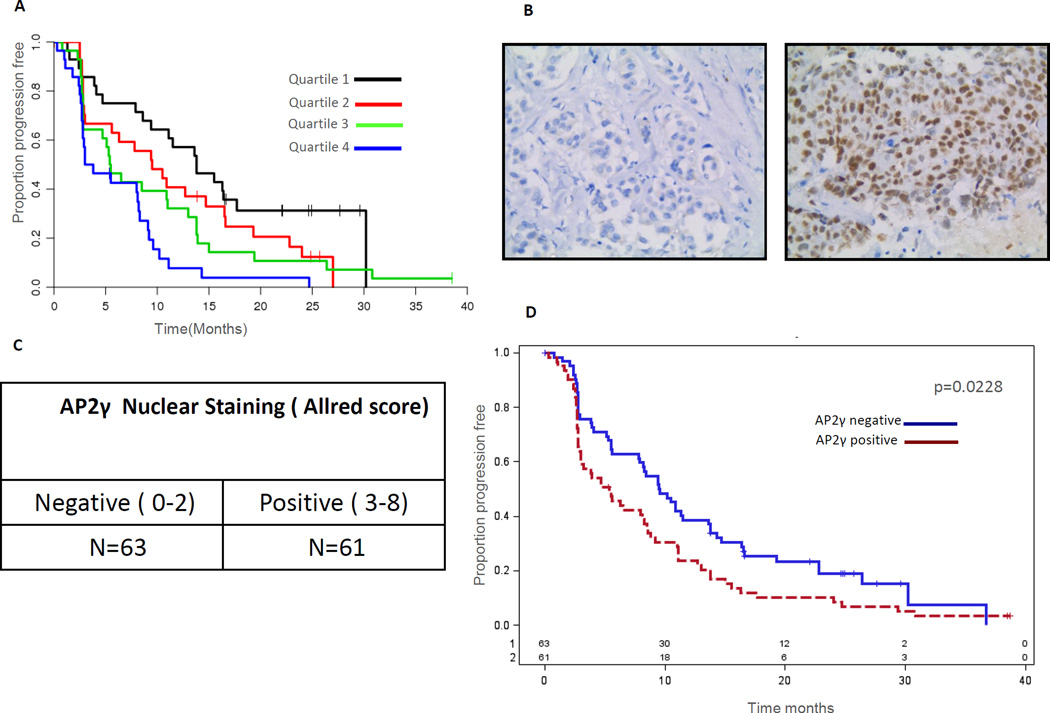
As mentioned above, the TFAP2C gene is a regulator of ER transcriptional activity. TFAP2C has also been previously implicated to play an adverse role in ER+ breast cancer34, 35. In addition, in The Cancer Genome Atlas (TGCA) dataset36, TFAP2C is amplified in 4% of luminal type breast cancers and gene amplification correlated with increased mRNA levels, suggesting a functional significance. In our study the expression of the TFAP2C gene at the mRNA level was strongly independently associated with poor PFS in the gene set discovery (rank = 2nd, adj HR = 2.86). To test whether the finding that TFAP2C expression at the mRNA level predicted for poor outcome could be corroborated at the protein level we performed IHC studies for its protein product the AP2-γ transcription factor. AP2-γ level was scored by the Allred scoring system and a score of 3 or above was considered positive. This analysis consisted of 124 of the transCONFIRM tumor samples. Consistent with the mRNA levels, AP2-γ protein status correlated with outcome with AP2-γ positive tumors exhibiting significantly reduced PFS (HR = 1.54, CI 1.05–2.25, p = 0.02) (figure 4) implying that AP2- γ levels could easily be analyzed for clinical use by IHC testing.
Figure 4.
High TFAP2C expression correlates with poor outcomes: A. Kaplan-Meier plot of PFS for quartiles of TFAP2C mRNA level. B Figures depicting examples of negative and positive IHC staining for AP2- γ (TFAP2C). C. Results of AP2- γ scoring in the transCONFIRM cohort. D. Kaplan-Meier plot of PFS for AP2- γ positive and negative tumors.
Discussion
In this study we comprehensively evaluated clinicopathological features, novel genetic markers and gene sets, and the established gene signatures of the PAM50 intrinsic subtype predictor and the OncotypeDX recurrence score risk classifier in a cohort of patients from the phase III CONFIRM study to test their ability to predict response to fulvestrant in metastatic disease. We found that high EGF pathway and FOXA1 transcriptional signaling are associated with decreased response to fulvestarnt. In addition, we identified a novel 37 gene set classifier of response to fulvestrant treatment that includes genes known to be associated with endocrine resistance and important for cell fate and proliferation, such as TFAP2C, BMPR1A and Notch237. Furthermore, TFAP2C could be evaluated by IHC and is thus a potential clinically assayed biomarker of resistance to fulvestrant.
We investigated gene expression profiles developed in primary tumors to determine if they would be determinants of response to endocrine treatment in metastatic disease. We chose to study archived primary tumors because of the difficulty obtaining sufficient tumor from metastatic biopsies to perform detailed molecular analyses. Because of the availability of primary tumor specimens, this design enables easy translation of results to clinical practice. However, this design is also a limitation of our study, since the prevailing model of metastases, based mainly on pre-clinical studies, suggests that the bulk of cells in a primary tumor do not metastasize and only a subpopulation of the cells in a primary tumor have metastatic capacity through the acquisition somatic mutations and epigenetic alterations38. These changes enable migration from the primary tumor, survival in the blood and lymphatic system, invasion of distant sites, growth of distant nodules with resistance to treatment. In addition, we and other groups have recently demonstrated in clinical samples the clonal selection of ESR1 mutations under the selective pressure of endocrine treatment as a mechanism of acquired resistance in metastatic ER+ breast cancer39. Thus, primary tumors cannot fully depict the molecular complexity of endocrine resistance in metastatic disease. Nonetheless, several studies have shown that in clinical samples of primary breast cancers and other cancer types there are molecular signatures of metastatic disease demonstrating the existence of gene expression events found early in primary tumors that reliably mark cellular phenotypes of metastatic behavior40–42. In addition, ER and HER2 expression, the most robust breast cancer molecular markers in clinical practice that dictate treatment in primary and metastatic disease, are highly concordant between primary and matching metastatic samples43. Collectively, it is conceivable that the clinical behavior of metastatic disease is governed by intrinsic biological characteristics inherent in primary tumor cells, as well as genetic and epigenetic changes that occur during the metastatic process under the pressure of treatments.
In our pathway analysis we show that increased EGF signaling correlated with poor response to fulvestrant in breast cancer patient samples. Both in vitro and xenograft studies have shown that activation of EGF and the downstream signaling including PI3K/AKT and MAPK pathways may provide an alternative survival pathway with reduced estrogen dependency leading to endocrine resistance16, 44. In pre-clinical studies, Gefitinib, a small molecule inhibitor of EGFR, was able to inhibit cellular proliferation of endocrine resistant breast cancer cell lines and delay the development of tamoxifen resistance in xenograft studies16, 45. Furthermore, high EGFR levels correlated with decreased response to endocrine treatment in elderly post-menopausal patients46. These findings have led to multiple clinical trials investigating the benefit of combining selective EGFR inhibitors to endocrine treatment as well as targeting downstream pathways such as the PI3K-AKT-MTOR pathway. Notable are two clinical trials including the BOLERO-2 phase III study and the TAMRAD phase II study that showed improved PFS with the addition of everolimus to endocrine treatment in patients with metastatic disease that progressed on prior treatment with an aromatase inhibitor47, 48. The addition of everolimus in early stage ER+ breast cancer was also shown to improve the efficacy of endocrine treatment in a neoadjuvant trial, suggesting that this pathway is important in primary disease and in-line with our findings that include evidence of increased EGF pathway signaling in primary tumors49.
Increased activation of FOXA1 transcriptional signaling was found to be associated with decreased response to fulvestrant in this study. FOXA1 is a pioneer factor that binds to highly compacted chromatin and dictates ER-DNA binding. Loss of FOXA1 in ER+ breast cancer cell lines leads to decreased ER chromatin binding and decreased cell proliferation. In primary breast cancer samples, FOXA1 protein expression levels correlated with ER and luminal A subtype breast cancers and in the luminal A sub-type, FOXA1 expression correlated with improved outcomes50, 51. However, FOXA1 has also been shown to have a role in endocrine resistance and metastatic disease. FOXA1 is co-expressed with ER in metastatic breast cancer samples and mediates ER reprogramming in poor outcome ER+ breast cancers17. Thus, FOXA1 likely has different functions in endocrine responsive and endocrine resistant breast cancers and our results support the notion that in poor outcome primary breast cancers, increased FOXA1 signaling is associated with decreased response to fulvetrant treatment. Additional studies are needed to better understand the changes in FOXA1 signaling in poor outcome ER+ breast cancers and how they contribute to endocrine resistance.
We found that mRNA levels of TFAP2C, a transcription factor expressed in breast cancers, correlated with the protein expression levels and high transcript and protein levels correlated with decreased response to fulvestrant treatment. Previous studies have shown that high levels of TFAP2C are associated with unfavorable clinical outcomes and decreased response to tamoxifen34, 35. However, the prognostic significance of TFAP2C expression is controversial, with a number of studies failing to show a prognostic role for this factor in primary tumors19, 52. A recent study showed that TFAP2C expression measured by AP2-γ IHC in primary breast cancer samples in a retrospective series of 451 breast cancer patients did not impact disease free survival or overall survival during the first 10 years after diagnosis of early stage disease, but was found to be associated with decreased overall survival after year 1035. Cell line studies demonstrated that TFAP2C is a pioneer factor and together with FOXA1 promotes ER recruitment followed by formation of long-range chromatin interactions required for ER transcriptional regulation53. Additionally, TFAP2C was shown to be essential for the maintenance of the luminal gene expression pattern of luminal type breast cancers and loss of TFAP2C led to basal differentiation18. Thus, as is the case for FOXA1 the association between TFAP2C expression and reduced response to endocrine treatment is complex. One possible mechanism by which TFAP2C may mediate endocrine resistance is through its down regulation of the cell cycle inhibitor p21 (CDKN1A) leading to cell cycle progression and treatment resistance54. However, this hypothesis and other potential mechanisms warrant further investigation.
Our study has several limitations that should be noted. The PAM50 and OncotypeDx RS classifications that we performed were surrogate assays since we used a microarray platform that is different from the microarray platform that was used for the development of the PAM50 assay and differs from the RT-PCR assay that is used for OncotypeDx testing. In addition the number of patients in our study was relatively small and this may limit the sensitivity of PAM50 intrinsic classification and OncotypeDx risk stratification to predict response to fulvestrant. Although our multivariate analysis was adjusted for several clinicopathological features that could influence response to fulvestrant, there may be other factors that we did not consider. In addition, the discovery studies herein are from a single clinical trial and thus require further validation.
In conclusion, central IHC and transcriptomic analysis of the transCONFIRM patient cohort showed that similar to earlier studies, high clinical PR levels is linked with increased response to fulvestrant and HER2 positivity is associated with decreased response. In addition, high EGF and FOXA1 signaling is associated with decreased response to fulvestrant and supports therapeutic strategies that combine inhibitors of the EGFR pathway with improved ER inhibition or new epigenetic therapeutic modalities. Lastly, we identified a novel gene set, consisting of genes with functions in ER regulation, cell fate and proliferation, that has the potential to predict resistance to fulvestrant in metastatic breast cancer.
Supplementary Material
Translational Relevance.
Endocrine therapy is the mainstay of treatment for metastatic ER+ breast cancer. However, not all patients have a durable response with single endocrine agents and therefore new therapies for ER+ disease are emerging. These new agents can add toxicity and cost underscoring the need to identify the patients who will respond well to endocrine therapy alone and could be spared the toxicities of the new agents and on the other hand, select patients resistant to existing endocrine therapy who are candidates for novel combinations. Herein, we report biological pathways and a putative gene signature that predict response to fulvestrant in a completed randomized phase III trial.
Acknowledgments
Financial Support:
This study was supported in part by the Susan F. Smith Center for Women’s Cancers and by grants from Susan G. Komen for the Cure (to MB) and Breast Cancer SPORE Career Development Award (to RJ).
We thank Dr Francesca Galardi and Dr Maria Caterina Truglia at Hospital of Prato, for gathering the tissue blocks and constructing the tissue microarrays
Footnotes
Disclaimers:
Angelo Di Leo and Luca Malorni have received honoraria from AstraZeneca for speaking at sponsored symposia and attending advisory boards.
Naomi Laing is an employee and shareholder of AstraZeneca
Contributor Information
Rinath Jeselsohn, Email: rinath_jeselsohn@dfci.harvard.edu.
William T. Barry, Email: bbarry@jimmy.harvard.edu.
Ilenia Migliaccio, Email: imigliaccio@uslcentro.toscana.it.
Chiara Biagioni, Email: cbiagioni@uslcentro.toscana.it.
Jin Zhao, Email: jin_zhao@dfci.harvard.edu.
Jonas De Tribolet-Hardy, Email: jonasdetribolet@gmail.com.
Cristina Guarducci, Email: cguarducci@uslcentro.toscana.it.
Martina Bonechi, Email: mbonechi@uslcentro.toscana.it.
Naomi Laing, Email: Naomi.Laing@astrazeneca.com.
Eric P. Winer, Email: eric_winer@dfci.harvard.edu.
Myles Brown, Email: myles_brown@dfci.harvard.edu.
Angelo Di Leo, Email: adleo@uslcentro.toscana.it.
Luca Malorni, Email: lmalorni@uslcentro.toscana.it.
References
- 1.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(16):3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(16):3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 3.Perey L, Paridaens R, Hawle H, Zaman K, Nole F, Wildiers H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18(1):64–69. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 4.Robertson JFRL-CA, Feltl D, Dewar J, Jasiówka M, Hewson N, Rukazenkov Y, Ellis MJ. University of Nottingham, Derby, Nottingham. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II ‘first’ study. 2014 doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England journal of medicine. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. The lancet oncology. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 9.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 10.Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, Nobel AB, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355(6):560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 11.Zhou YH, Barry WT, Wright FA. Empirical pathway analysis, without permutation. Biostatistics. 2013;14(3):573–585. doi: 10.1093/biostatistics/kxt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- 13.Badve SS, Baehner FL, Gray RP, Childs BH, Maddala T, Liu ML, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(15):2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 14.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. Journal of the National Cancer Institute. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilquin P, Villedieu M, Grisard E, Ben Larbi S, Ghayad SE, Heudel PE, et al. Molecular characterization of anastrozole resistance in breast cancer: pivotal role of the Akt/mTOR pathway in the emergence of de novo or acquired resistance and importance of combining the allosteric Akt inhibitor MK-2206 with an aromatase inhibitor. International journal of cancer Journal international du cancer. 2013;133(7):1589–1602. doi: 10.1002/ijc.28182. [DOI] [PubMed] [Google Scholar]
- 16.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer research. 2008;68(3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 17.Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481(7381):389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cyr AR, Kulak MV, Park JM, Bogachek MV, Spanheimer PM, Woodfield GW, et al. TFAP2C governs the luminal epithelial phenotype in mammary development and carcinogenesis. Oncogene. 2015;34(4):436–444. doi: 10.1038/onc.2013.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, et al. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer research. 1998;58(23):5466–5472. [PubMed] [Google Scholar]
- 20.Krishnan V, Wang X, Safe S. Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. The Journal of biological chemistry. 1994;269(22):15912–15917. [PubMed] [Google Scholar]
- 21.Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y, et al. MiR-200b expression in breast cancer: a prognostic marker and act on cell proliferation and apoptosis by targeting Sp1. Journal of cellular and molecular medicine. 2015;19(4):760–769. doi: 10.1111/jcmm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickup MW, Hover LD, Guo Y, Gorska AE, Chytil A, Novitskiy SV, et al. Deletion of the BMP receptor BMPR1a impairs mammary tumor formation and metastasis. Oncotarget. 2015;6(26):22890–22904. doi: 10.18632/oncotarget.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draheim KM, Chen HB, Tao Q, Moore N, Roche M, Lyle S. ARRDC3 suppresses breast cancer progression by negatively regulating integrin beta4. Oncogene. 2010;29(36):5032–5047. doi: 10.1038/onc.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puustinen P, Rytter A, Mortensen M, Kohonen P, Moreira JM, Jaattela M. CIP2A oncoprotein controls cell growth and autophagy through mTORC1 activation. The Journal of cell biology. 2014;204(5):713–727. doi: 10.1083/jcb.201304012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni T, Mao G, Xue Q, Liu Y, Chen B, Cui X, et al. Upregulated expression of ILF2 in non-small cell lung cancer is associated with tumor cell proliferation and poor prognosis. Journal of molecular histology. 2015;46(4–5):325–335. doi: 10.1007/s10735-015-9624-5. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Smith M, Halder JB, Meltzer PS, Gonda TA, Mangala LS, Rupaimoole R, et al. ATP11B mediates platinum resistance in ovarian cancer. The Journal of clinical investigation. 2013;123(5):2119–2130. doi: 10.1172/JCI65425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayagopal A, Yang JL, Haselton FR, Chang MS. Tight junction-associated signaling pathways modulate cell proliferation in uveal melanoma. Investigative ophthalmology & visual science. 2011;52(1):588–593. doi: 10.1167/iovs.10-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potu H, Peterson LF, Pal A, Verhaegen M, Cao J, Talpaz M, et al. Usp5 links suppression of p53 and FAS levels in melanoma to the BRAF pathway. Oncotarget. 2014;5(14):5559–5569. doi: 10.18632/oncotarget.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avirneni-Vadlamudi U, Galindo KA, Endicott TR, Paulson V, Cameron S, Galindo RL. Drosophila and mammalian models uncover a role for the myoblast fusion gene TANC1 in rhabdomyosarcoma. The Journal of clinical investigation. 2012;122(1):403–407. doi: 10.1172/JCI59877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu J, Song M, Xie J, Huang XY, Hu XM, Gan RH, et al. Notch2 signaling contributes to cell growth, invasion, and migration in salivary adenoid cystic carcinoma. Molecular and cellular biochemistry. 2015 doi: 10.1007/s11010-015-2575-z. [DOI] [PubMed] [Google Scholar]
- 31.Poon MW, Zhuang JT, Wong ST, Sun S, Zhang XQ, Leung GK. Co-expression of Cytoskeletal Protein Adducin 3 and CD133 in Neurospheres and a Temozolomide-resistant Subclone of Glioblastoma. Anticancer research. 2015;35(12):6487–6495. [PubMed] [Google Scholar]
- 32.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature medicine. 2011;17(9):1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gee JM, Eloranta JJ, Ibbitt JC, Robertson JF, Ellis IO, Williams T, et al. Overexpression of TFAP2C in invasive breast cancer correlates with a poorer response to anti-hormone therapy and reduced patient survival. The Journal of pathology. 2009;217(1):32–41. doi: 10.1002/path.2430. [DOI] [PubMed] [Google Scholar]
- 35.Perkins SM, Bales C, Vladislav T, Althouse S, Miller KD, Sandusky G, et al. TFAP2C expression in breast cancer: correlation with overall survival beyond 10 years of initial diagnosis. Breast cancer research and treatment. 2015;152(3):519–531. doi: 10.1007/s10549-015-3492-2. [DOI] [PubMed] [Google Scholar]
- 36.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nature reviews Genetics. 2012;13(9):654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature reviews Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 39.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nature reviews Clinical oncology. 2015 doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. The Journal of clinical investigation. 2005;115(1):44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature genetics. 2003;33(1):49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 42.Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van't Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, et al. Hormone Receptor Status and HER2 Expression in Primary Breast Cancer Compared With Synchronous Axillary Metastases or Recurrent Metastatic Disease. Clinical breast cancer. 2015 doi: 10.1016/j.clbc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Miller DL, el-Ashry D, Cheville AL, Liu Y, McLeskey SW, Kern FG. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for a role of EGFR in breast cancer growth and progression. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1994;5(12):1263–1274. [PubMed] [Google Scholar]
- 45.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson S, Sainsbury JR, Needham GK, Chambers P, Farndon JR, Harris AL. Quantitative assays of epidermal growth factor receptor in human breast cancer: cut-off points of clinical relevance. International journal of cancer Journal international du cancer. 1988;42(1):36–41. doi: 10.1002/ijc.2910420108. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England journal of medicine. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 49.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(16):2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 50.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132(6):958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, et al. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98(1):18–23. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- 53.Tan SK, Lin ZH, Chang CW, Varang V, Chng KR, Pan YF, et al. AP-2gamma regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. The EMBO journal. 2011;30(13):2569–2581. doi: 10.1038/emboj.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong PP, Miranda F, Chan KV, Berlato C, Hurst HC, Scibetta AG. Histone demethylase KDM5B collaborates with TFAP2C and Myc to repress the cell cycle inhibitor p21(cip) (CDKN1A) Molecular and cellular biology. 2012;32(9):1633–1644. doi: 10.1128/MCB.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.