Abstract
Objective
To evaluate if injection of intravenous particles may provoke migraines in subjects with right‐to‐left shunts due to pulmonary arteriovenous malformations (AVMs).
Background
Migraine headaches commonly affect people with hereditary hemorrhagic telangiectasia (HHT), especially those with pulmonary AVMs that provide right‐to‐left shunts. In our clinical practice, patients occasionally reported acute precipitation of migraine headaches following injection of technetium‐labeled albumin macroaggregates for nuclear medicine scans.
Methods
Self‐reported migraine features and exacerbations were examined in HHT subjects with and without pulmonary AVMs, for a series of noninvasive and invasive investigations, using an unbiased online survey.
Results
One hundred and sixty‐six subjects were classified as having both HHT and migraines. HHT subjects with migraines were more likely to have pulmonary AVMs (P < .0001). HHT subjects with pulmonary AVMs were more likely to report photophobia (P = .010), “flashes of light” (P = .011), or transient visual loss (P = .040). Pulse oximetry, x‐rays, ultrasound, and computerized tomography (CT) scans without intravenous contrast medium rarely, if ever, provoked migraines, but unenhanced magnetic resonance imaging (MRI) was reported to exacerbate migraines by 14/124 (11.2%) subjects. One hundred and fourteen subjects had both enhanced and unenhanced CT examinations: studies with contrast media were more commonly reported to start (9/114 [7.8%]), and/or worsen migraines (18/114 [15.7%]), compared to those undertaken without contrast medium (P < .01), or after simple blood tests (P < .05). Additionally, migraine exacerbation was reported by 9/90 (10%) after contrast echocardiography, 2/44 (4.5%) after nuclear medicine scans, and 10/154 (6.5%) after blood tests.
Conclusions
HHT subjects frequently report migraine exacerbation following blood tests, contrast echocardiograms, MRI imaging, and CT studies performed with intravenous contrast medium. Since air emboli are recognized to complicate intravenous injections, particularly those given by a pressurized pump during contrast enhanced CT, future studies should re‐evaluate whether particulate emboli provoke migraines.
Keywords: hereditary hemorrhagic telangiectasia (HHT), pulmonary arteriovenous malformations (PAVMs), magnetic resonance imaging (MRI), computerized tomography (CT), right‐to‐left shunt, contrast injection
Abbreviations
- AVMs
arteriovenous malformations
- CT
computerized tomography
- HHT
hereditary hemorrhorrhagic telangiectasia
- MRI
magnetic resonance imaging
- R‐L shunt
right‐to‐left shunt
INTRODUCTION
Observations in a group of patients who have continuous right‐to‐left (R‐L) shunts led us to hypothesize that particulate paradoxical emboli may provoke migraines: Pulmonary arteriovenous malformations (AVMs) are abnormal vessels that allow blood to bypass the pulmonary capillary bed.1 Patients are at high risk of paradoxical embolic strokes2 and migraines,3 with risks reduced by pulmonary AVM treatment.1, 2, 3 Macroscopic pulmonary AVMs affect approximately 50% of people with the inherited vascular disorder, hereditary hemorrhagic telangiectasia (HHT).1 HHT patients also exhibit intrapulmonary R‐L shunting in the absence of overt pulmonary AVMs visible by computerized tomography (CT) scans or angiography.1, 4 Intrapulmonary R‐L shunts are considered part of the reason why HHT patients have a higher prevalence of migraines than the general population.3, 5, 6
In our clinical practice, patients occasionally reported acute precipitation of migraine headaches following R‐L shunt measurements by nuclear medicine scans involving the injection of 99mtechnetium‐labeled albumin macroaggregates (10–80 μm diameter).2 This led us to hypothesize that injection of intravenous particles in diagnostic studies may provoke migraines in HHT patients with pulmonary AVMs.
METHODS
Relevant questions were designed for inclusion in a wider HHT survey that did not specify specific study aims. The NRES Committee East Midlands‐Derby 1 Research Ethics Committee approved the study, which recruited between August 2013 and April 2015. The survey invitation was distributed by post using the Imperial College London HHTIC London Clinical Service databases (2012 to 2014); during attendance at the HHT clinics; and advertised by the HHT Foundation International, and HHT UK. The basic study design has been reported previously: each participant provided a single response.5 Briefly, online informed consent was obtained from all participants who were then asked to provide demographics, information on their HHT phenotype, and whether they had any of a number of common medical conditions, including migraine.
Participants stating that they had migraines were directed to a set of questions to distinguish between migrainous and non‐migrainous headaches. Relevant discerning questions were derived from standardized questions,7 and shrouded with dummy questions to reduce bias (see Supporting Information Data Supplement). Participants were also asked if something else happened before, during, or after the headache, and if they ticked “yes,” were directed to a series of options with tick boxes to indicate if occurrence was never; occasional; with some migraines; often; with most, or with all migraines. Participants reporting that they had received particular investigations were directed to “We would like to know if having […investigation….] seemed to make a difference to your migraines” with tick box options for “I have not had one”; “no different really”; “seemed a bit better”; “seemed a bit worse” “seemed to bring on a migraine” “seemed to stop a migraine” (see Supporting Information Data Supplement).
DATA ANALYSIS
Following preliminary analyses, data were downloaded from SurveyMonkey in December 2015 and transferred to numeric format using Microsoft Excel “replace” commands. New variables for HHT and migraine diagnoses were generated, and populated automatically according to the numeric data within the specific questions: Blinded to migraine reports, an HHT phenotype was assigned if at least four features were reported from nosebleeds at least once per year; telangiectasia (“red spots”) on the lips or finger pads; a stated family history of HHT; and any reported visceral AVMs, or if three features included visceral AVMs. Self‐reported migraines were classified as migraines if there was a formal medical diagnosis receiving migraine‐specified treatments, or, if not formally diagnosed, if described as a headache of moderate to severe intensity plus at least three associated features considered common in migraine headaches. Additionally, in order to provide a further numeric parameter with which to compare populations, the frequencies of associated features were assigned to a scale of 0 (never) to 5 (always).
Data were analyzed using STATA IC v12 (StataCorp, College Station, TX, USA) and GraphPad Prism (GraphPad Inc, La Jolla, CA, USA). Two group comparisons were performed using Mann Whitney or Fisher's exact test (for proportions). Multiple groups were compared using Kruskal Wallis with Dunn's multiple comparison test.
RESULTS
At study closure, 706 individuals had completed the survey. Five hundred and four survey respondents met the criteria for diagnosis of HHT and provided complete datasets for analysis. One hundred and sixty‐six of 504 (32.9%) reported migraines that met medical diagnosis or severity/association criteria (Table 1). In the migraine subgroup, ages ranged from 22 to 102 (median 49) years, 139/166 (83.7%) were female, and 41/166 (24.7%) were current or former smokers. HHT features were generally similar in the groups with and without migraines, but there was an excess of pulmonary AVMs (P < .0001) and cerebral AVMs (P = .0036) in those with migraines (Table 1).
Table 1.
Features of HHT in Subjects With and Without Migraines
| No migraine (N = 338) | Migraine (N = 166) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P value | |
| Nosebleeds | 301 | 89.1 | 152 | 91.6 | .38 |
| Daily | 182 | 53.9 | 72 | 43.4 | .23 |
| Weekly | 124 | 36.7 | 53 | 31.9 | .38 |
| Monthly | 50 | 14.8 | 30 | 18.1 | .34 |
| Telangiectasia | 309 | 91.4 | 144 | 86.8 | .69 |
| Lips | 263 | 77.8 | 132 | 79.5 | .66 |
| Finger pads | 224 | 66.3 | 107 | 64.5 | .74 |
| Pulmonary AVMs | 142 | 42.0 | 108 | 65.1 | .0001 |
| Cerebral AVMs | 22 | 6.5 | 24 | 14.5 | .0036 |
| Hepatic AVMs | 46 | 13.6 | 28 | 16.9 | .33 |
N, number with feature. P values calculated by Mann Whitney.
Migraines were typical in type, but not stereotypical in occurrence (Fig. 1). For example, 105/166 (63.3%) subjects reported nausea/vomiting with at least some migraines, but only 19/166 (11.5%) reported nausea/vomiting with all migraines. Subjects with pulmonary AVMs reported migraines of similar intensity and age progression to the rest of the HHT cohort, but compared to the other HHT subjects, were more likely to report a neurological association (P = .0005); photophobia (P = .010); “flashes of light” (P = .011); or transient visual loss (P = .040).
Figure 1.
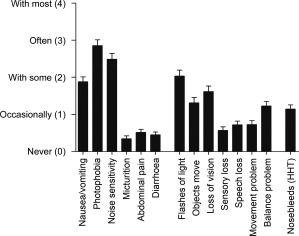
Reported associations with migraine headaches. Data from the 166 survey respondents meeting diagnostic criteria for HHT and migraines. The mean score, and standard error of the mean, are illustrated, noting individual responses ranged from never (0) to always (5). As reported elsewhere,5 almost half (70, 43.3%) described sometimes having an HHT nosebleed at the time of a migraine, and in 14 cases (8.4%) this occurred with most if not all migraines.
Pulse oximetry, x‐rays, ultrasound, and CT scans without intravenous contrast medium rarely if ever provoked migraines (Fig. 2).
Figure 2.
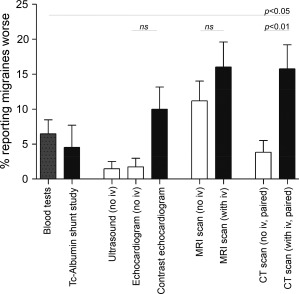
Reported exacerbation of migraines by investigations. Proportions of subjects reporting investigations exacerbated migraines, either starting or worsening a migraine. White bars: tests without injections, black bars, tests with injections, and stippled bar, venipuncture for blood tests. Mean and standard error of the mean displayed. Tests that never provoked migraines (pulse oximetry, x‐rays) are not displayed. The P values were calculated by Dunn's multiple comparison test after performing the Kruskal Wallis test across all 9 groups (overall P value < .0001).
MRI scans without any injection were reported to start a migraine by 6/124 (4.8%) subjects, and to worsen migraines by a further 8/124 (6.5%). MRI scans “with an injection,” were reported to start a migraine by 9/110 (8.1%) subjects, and worsen migraines by a further 12/110 (10.9%). Figure 2 illustrates the subjects reporting any exacerbation by MRI scans, either without (14/124 [11.2%]), or with injections (21/110 [19.1%]).
Two of 44 (4.5%) subjects having an albumin macroaggregate nuclear medicine “shunt” study reported that the scans started migraines. Similarly, of 90 migraineurs who had a microbubble contrast‐enhanced echocardiography study, nine (10%) reported migraines were started or made worse compared to only 2/115 (1.7%) after unenhanced echocardiograms (P = .0091). Similar rates of migraine provocation (3/154 [2.0%]) and/or exacerbation (10/154 [6.4%]) were reported after simple blood tests (Fig. 2).
Differences between contrast enhanced and non‐contrast scans were most pronounced for CT imaging: For the 114 subjects who described both types of CT scans, migraine exacerbations were reported by 18/114 (15.7%) receiving injected iodinated contrast medium, including 9/114 (7.8%) who stated the scans started a migraine. Lower proportions reported CT scans without contrast medium started (2/114 [1.7%]) or started/worsened (4/114 [3.5%]) a migraine (P values .059 and P < .01, respectively).
DISCUSSION
We have shown in a migraine‐prone population that MRI studies, blood tests, contrast echocardiograms, and intravenous injection of iodinated contrast medium associated with CT examinations are reported to provoke or exacerbate migraines. Migraine headaches were commonly associated with neurological features, particularly in subjects with R‐L shunts due to pulmonary AVMs.
The main study weaknesses are that it was survey based (hence bias from study participation, or participant recall cannot be excluded), and only modest numbers were recruitable representing the migraine‐affected subgroup of people affected by a rare medical condition. Direct causality cannot determined by the study methodology. Additionally, the precise type of intravenous injections that participants had received was not recorded.
Nevertheless, the actual numbers of subjects for which data are available is one of the paper's strengths, and the findings are provocative. Headaches as a result of MRI scans are usually attributed to noise, but there are emerging concerns regarding air emboli following intravenous access: It is accepted that air emboli can complicate intravenous injections (particularly with “pump” injectors for CT‐based contrast), or simple insertion of venous cannulae.8, 9 Patients with R‐L shunts are at a theoretical risk of paradoxical embolism of larger microbubbles before the gas is absorbed, and migraine headaches do not appear to have been included previously as a possible clinical outcome.
We cautiously suggest the findings may be relevant to wider groups of migraineurs. Migraine features and precipitants are similar in HHT patients to the general population.5, 7 More than 30% of the general population have patent foramen ovale that cause intermittent R‐L shunting (usually only after Valsalva maneuver reverse pressure differentials between left and right atria), while ∼5–8% are considered to have intrapulmonary R‐L shunts at rest, rising on exercise.10
Evaluation of migraines as a potential read‐out for paradoxical emboli is recommended. In the meantime, for people with HHT and migraines, pre‐test counseling may helpfully include advice to bring migraine preventers or treatments to help alleviate any symptoms promptly.
Acknowledgments: This study received support from donations from families and friends of HHT patients; Imperial College London BSc Student project funds (to Dr. Shovlin for T. Patel), and A. Elphick received an Imperial College Undergraduate Research Opportunities Programme (UROP) bursary. Dr. Shovlin also acknowledges support from the NIHR Biomedical Research Centre Funding Scheme (Imperial BRC).
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Amy Elphick, Claire L. Shovlin
(b) Acquisition of Data
Trishan Patel, Amy Elphick, Claire L. Shovlin
(c) Analysis and Interpretation of Data
Trishan Patel, James E. Jackson, Claire L. Shovlin
Category 2
(a) Drafting the Manuscript
Claire L. Shovlin
(b) Revising It for Intellectual Content
Trishan Patel, Amy Elphick, James E. Jackson, Claire L. Shovlin
Category 3
(a) Final Approval of the Completed Manuscript
Trishan Patel, Amy Elphick, James E. Jackson, Claire L. Shovlin
Supporting information
Supporting Information
Conflict of Interest: The authors report no conflict of interest.
REFERENCES
- 1. Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2014;190:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shovlin CL, Chamali B, Santhirapala V, et al. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: Associations with iron deficiency and platelets. PLoS One. 2014;9:e88812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Post MC, Thijs V, Schonewille WJ, et al. Embolization of pulmonary arteriovenous malformations and decrease in prevalence of migraine. Neurology. 2006;66:202–205. [DOI] [PubMed] [Google Scholar]
- 4. van Gent MW, Post MC, Snijder RJ, Westermann CJ, Plokker HW, Mager JJ. Real prevalence of pulmonary right‐to‐left shunt according to genotype in patients with hereditary hemorrhagic telangiectasia: A transthoracic contrast echocardiography study. Chest. 2010;138:833–839. [DOI] [PubMed] [Google Scholar]
- 5. Elphick A, Shovlin CL. Relationships between epistaxis, migraines, and triggers in hereditary hemorrhagic telangiectasia. Laryngoscope. 2014;124:1521–1528. [DOI] [PubMed] [Google Scholar]
- 6. Steele JG, Nath PU, Burn J, Porteous ME. An association between migrainous aura and hereditary haemorrhagic telangiectasia. Headache. 1993; 33:145–148. [DOI] [PubMed] [Google Scholar]
- 7. Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 8. Emby DJ, Ho K. Air embolus revisited – A diagnostic and interventional radiological perspective (bubble trouble and the dynamic Mercedes Benz sign). SA Journal of Radiology. 2006;10:3–7. [Google Scholar]
- 9. Groell R, Schaffler GJ, Rienueller R. The peripheral intravenous cannula: A cause of venous air embolism. Am J Med Sci. 1997;314:300–302. [DOI] [PubMed] [Google Scholar]
- 10. Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine‐induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113:1213–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


