Abstract
Interleukin-2 (IL2) was among the earliest reagents used for cancer immunotherapy due to its ability to support the survival and function of tumor-reactive T cells. However, treatment with IL2 is accompanied by off-target toxicity and low response rates in patients. In mouse models, these issues are largely overcome when IL2 is administered as a cytokine/antibody complex (IL2c). The complex has a longer serum half-life and can be designed for preferential cytokine delivery to specific cells of interest. Early studies showed IL2c could boost antitumor immunity in mice by activating tumor-reactive CD8+ T cells. But such functional T cells are often limited in the tumor microenvironment, where instead unresponsive tolerant T cells are eventually eliminated by apoptosis, representing a major obstacle to the success of cancer immunotherapy. We found that IL2c treatment rescued tumor-specific CD8+ T cells from a state of established tolerance, providing effective immunotherapy in tumor-bearing mice. Expression of the transcription factor T-bet was necessary to drive intratumoral IFNγ production and effector activity by T cells rescued with IL2c. Furthermore, IL2c promoted T-bet expression in human CD4+ and CD8+ T cells in humanized tumor-bearing mice, but also increased the frequency of Foxp3+ regulatory T cells. Our study reveals a novel role for IL2c as a powerful immunotherapeutic reagent capable of reversing tolerance in tumor-reactive T cells, and provides the first evidence that IL2c influences human T cells in vivo, highlighting the translational potential to modulate human antitumor immune responses.
Keywords: cancer immunotherapy, CD8+ T-cell tolerance, Interleukin-2, T-bet, cytokine complex
Introduction
Interleukin-2 (IL2) is a well-characterized T-cell growth factor known to enhance the activity of CD8+ T cells. For this reason IL2 was developed into one of the earliest reagents for cancer immunotherapy (1, 2). However, soluble IL2 therapy is impaired by a short serum half-life, necessitating large and often toxic doses of cytokine. The pleiotropic nature of IL2 is also problematic as it engages many different cell types and can cause a potentially lethal vascular leak syndrome by binding IL2 receptor alpha (IL2Rα) on vascular endothelium in the lungs (3, 4). Additionally, efforts to boost the activity of tumor-reactive effector T cells and NK cells with soluble IL2 are hindered by simultaneous expansion of IL2Rα+ regulatory T cells (Tregs) that dampen antitumor immunity (5, 6). Thus, harnessing the full potential of IL2 for cancer immunotherapy requires circumvention of these obstacles.
When IL2 is complexed with anti-IL2 (IL2c), its bioactivity in the serum of mice is extended from hours to days by preventing clearance in the kidneys (7–9). A completely separate benefit of IL2c is that the antibody component dictates receptor engagement and can be manipulated to focus IL2 toward specific cells of interest (8, 10, 11). The IL2 receptor (IL2R) is a heterotrimeric transmembrane complex consisting of CD25 (IL2Rα), CD122 (IL2Rβ) and the common gamma (γc) receptor. Depending on where the antibody binds to IL2, it can mask sites of natural receptor chain interactions (11). Because different immune cells like CD8+ T cells, NK cells, and Tregs have distinct CD25 and CD122 expression profiles, antibodies that alter binding to these receptor chains allow selective delivery of IL2 to these lymphocytes (10). This is particularly significant for cancer immunotherapy, as these cells have very different and potentially antagonistic roles.
A major barrier to successful cancer immunotherapy is T-cell tolerance, where T cells that are otherwise capable of recognizing tumor antigens are rendered dysfunctional or removed altogether by mechanisms of peripheral deletion. This has been observed for both endogenous T cells and those infused for adoptive immunotherapy. Restoring function of tolerant tumor-reactive T cells holds great therapeutic promise and is the basis of many immunotherapy strategies (12, 13). Whereas IL2c has been shown to enhance antitumor immunity in animal models (9, 14, 15), the ability of IL2c to influence tolerant tumor-reactive CD8+ T cells has not been explored. It is worth noting that IL2c induces proliferation of naive autoreactive T cells, which can subsequently mediate autoimmunity in mice (15, 16). However, this illustrates improved T-cell priming via IL2c stimulation and should not be confused with breaking established T-cell tolerance.
To model CD8+ T-cell tolerance, we studied T cells specific for the Gag glycoprotein expressed in a naturally transformed murine leukemia, FBL (17–19). When these T cells are transferred into FBL-bearing Alb:Gag recipients, which express the same Gag protein in healthy liver tissue, they become tolerant and die by apoptosis within 8–10 days (20–22). Genome-wide transcriptional analysis revealed that this tolerance stems from a failed effector cell differentiation program involving insufficient levels of Tbx21 (T-bet), and increased expression of inhibitory receptors (PD-1, CTLA-4, LAG-3) and apoptotic molecules (23, Gene Expression Omnibus (GEO) accession code GSE58722). This model provides a discrete window of time to evaluate tumor-reactive CD8+ T cells after tolerance has been established but before deletion is complete. Here we report that in vivo treatment with IL2c rescued tolerant tumor/self-reactive T cells despite having already initiated a tolerant gene expression profile. Administration of IL2c promoted tumor infiltration by rescued T cells and provided a long-term survival benefit to mice with established and disseminated leukemia. This IL2c-mediated immunotherapy was reliant on T-bet expression by rescued T cells, as transfer of T-bet deficient T cells failed to provide a therapeutic benefit. Using a humanized mouse model, these findings were extended to human T cells, where IL2c induced T-bet expression in CD4+ and CD8+ T cells, and expanded Foxp3+ regulatory T cells within human tumors. These results provide the first evidence that human T cells respond to human-specific IL2c in vivo, and underscore the promise of IL2c as a potent immunotherapeutic reagent that may be customized to achieve diverse outcomes during immunotherapy.
Materials and Methods
Mice
Alb:Gag, and TCRGag transgenic mice on wild type and Tbx21−/− backgrounds have been previously described (17, 22). Alb:Gag x TCR mice have been described elsewhere (24). NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(HLA-A2.1)1Enge/SzJ (NSG–HLA-A2) mice were acquired from The Jackson Laboratory. All mice were maintained under specific pathogen-free conditions and used in accordance with protocols established by the Institutional Animal Care and Use Committee of the Department of Comparative Medicine, SLU School of Medicine.
Cell lines, peptides and antibodies
The FBL cell line was a gift from Dr. Philip Greenberg (University of Washington) in 2008 and has been described previously (20, 21). FBL has not been authenticated. The FBL cell line is maintained in vivo and cells are harvested from ascites fluid on the day of experiment setup. The HLA-A2+ human melanoma line MeWo was purchased from ATCC in 2014. Peptides from FBL-Gag (CCLCLTVFL) and ovalbumin (SIINFEKL) were obtained from GenScript. Mouse blocking antibodies to CTLA-4 (9D9), PD-1 (RMP1–14) and LAG-3 (C9B7W) were purchased from BioXCell. Human antibodies against CTLA-4, PD-1, and LAG-3 were provided by Bristol-Myers Squibb. All blocking antibodies were administered intraperitoneally (i.p.) at a dose of 100 µg/mouse every 3 days. Fluorochome-conjugated antibodies to mouse CD90.1 (OX-7), CD90.2 (53–2.1), IFNγ (XMG1.2), TNF (MP6-XT22), and anti-CD16/CD32 Fc block (2.4G2) and antibodies to human CD45 (HI30), CD3 (UCHT1), CD4 (RPA-T4), CD8 (SK1), and Foxp3 (259D/C7) were purchased from BD Biosciences. Fluorochrome-conjugated antibody to CD8 (53–6.7) was purchased from BioLegend. Fluorochrome-conjugated antibodies to mouse CD4 (GK1.5), NK1.1 (PK136), Eomes (Dan11mag), and Foxp3 (FJK-16s) and antibody to human T-bet (ebio4b10) were purchased from eBioscience.
Quantitative RT-PCR
Transferred T cells were sorted to >95% purity and total RNA isolated using an RNeasy Plus Mini Kit (QIAGEN) and cDNA synthesized using SuperScript® III RT (Life Technologies). Quantitative real-time PCR was performed with SYBR® Select Master Mix (Life Technologies) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Beta-actin (Actb) was used as the endogenous amplification control. The following primer probe sets were used:
Bcl2l11 sense 5′- CCTCCCTACAGACAGAACCGC −3′, and antisense 5′- GTACCAGGCATCACCGTGG −3′;
Bbcl2l1 sense 5′-CACCTAGAGCCTTGGATCCAGG-3′, and antisense 5′-CACACCAGCCACAGTCATGC −3′;
Tbx21 sense 5′-CAACAACCCCTTTGCCAAAG-3′, and antisense 5′-TCCCCCAAGCAGTTGACAGT-3′;
Eomes sense 5′-GCCTACCAAAACACGGATA-3′, and antisense 5′-TCTGTTGGGGTGAGAGGAG-3′,
Ifng sense 5′-CACGGCACAGTCATTGAAAGC-3′, and antisense 5′-GAGATAATCTGGCTCTGCAGG-3′;
Fasl sense 5′-AACCCCAGTACACCCTCTG-3′, and antisense 5′-CGTTGATCACAAGGCCACC-3′;
Actb sense 5′-CCTTCGTTGCCGGTCCACAC-3′, and antisense 5′-ACCTCTCTTGCTCTGGGCCT-3′.
Adoptive cell transfer
Intravenous injections of no more than 2 × 106 T cells per 0.5 ml volume were performed via tail. T cells were labeled prior to transfer with eFluor-v450 cell proliferation dye (eBioscience) according to manufacturer’s protocol. In vivo killing assays were performed as described previously (21).
Cytokine treatments
All IL2 complexes were prepared at a 2:1 molar ratio of cytokine to antibody and allowed to form complexes at room temperature for 15 minutes before administration (8). Each dose contained 1.5 µg of cytokine and 15 µg of antibody. Murine IL2c (mIL2c) was prepared using recombinant carrier-free murine IL2 (rmIL2) from Gold Biotechnology and anti-mIL2 mAb clone JES6-5H4 from BioXCell. Human recombinant IL2 was obtained from BioLegend, and anti-hIL2 mAb MAB602 (clone 5355) and anti-hIL2 mAb MAB202 (clone 5334) were purchased from R&D Systems. Murine IL15c was made with recombinant carrier-free mouse IL15 from BioLegend and recombinant mouse IL15R alpha Fc Chimera Protein (R&D Systems) as previously described (25) Soluble IL2 treatment consisted of either 1.5 µg mrIL2 or 1 × 105 units of recombinant human IL2 as indicated.
Immunotherapy assays
Disseminated leukemia was established by intravenous injection of 1 × 105 viable FBL tumor cells. Four days later, mice received an adoptive transfer of 2 × 106 Gag-specific CD8+ T cells. Mice were treated with IL2c daily on days 3–9 relative to adoptive transfer and survival was followed for 100 days.
Humanized mouse engraftment
Human cord blood (CB) was obtained from the St. Louis Cord Blood Bank at SSM Health Cardinal Glennon. Mononuclear cells from HLA-A2+ CB samples were enriched for CD34+ hematopoietic stem cells (HSCs) using CD34 MicroBead Kit UltraPure on an AutoMACS (Miltenyi Biotec). Six to eight-week-old NSGHLA-A2 mice were non-lethally irradiated at 230 cGy and intravenously injected with 2 × 105 CD34+ cells within 24 hours of irradiation. Engrafted mice were rested 12–16 weeks to allow for immune cell development prior to use in experiments.
Statistical analysis
Results are expressed as mean ± standard deviation (SD) unless otherwise noted. Differences between groups were examined for statistical significance by using a Mann-Whitney U-test when comparing two groups. When comparing three or more groups, a one-way ANOVA with Bonferroni’s post-test or Dunn’s multiple comparison test were used as appropriate. Paired data was evaluated using the Wilcoxon signed rank test. Survival curves were analyzed by the log-rank test. Statistics were generated using GraphPad Prism 7 (GraphPad Software).
Results
IL2c prevents the induction of CD8+ T-cell tolerance
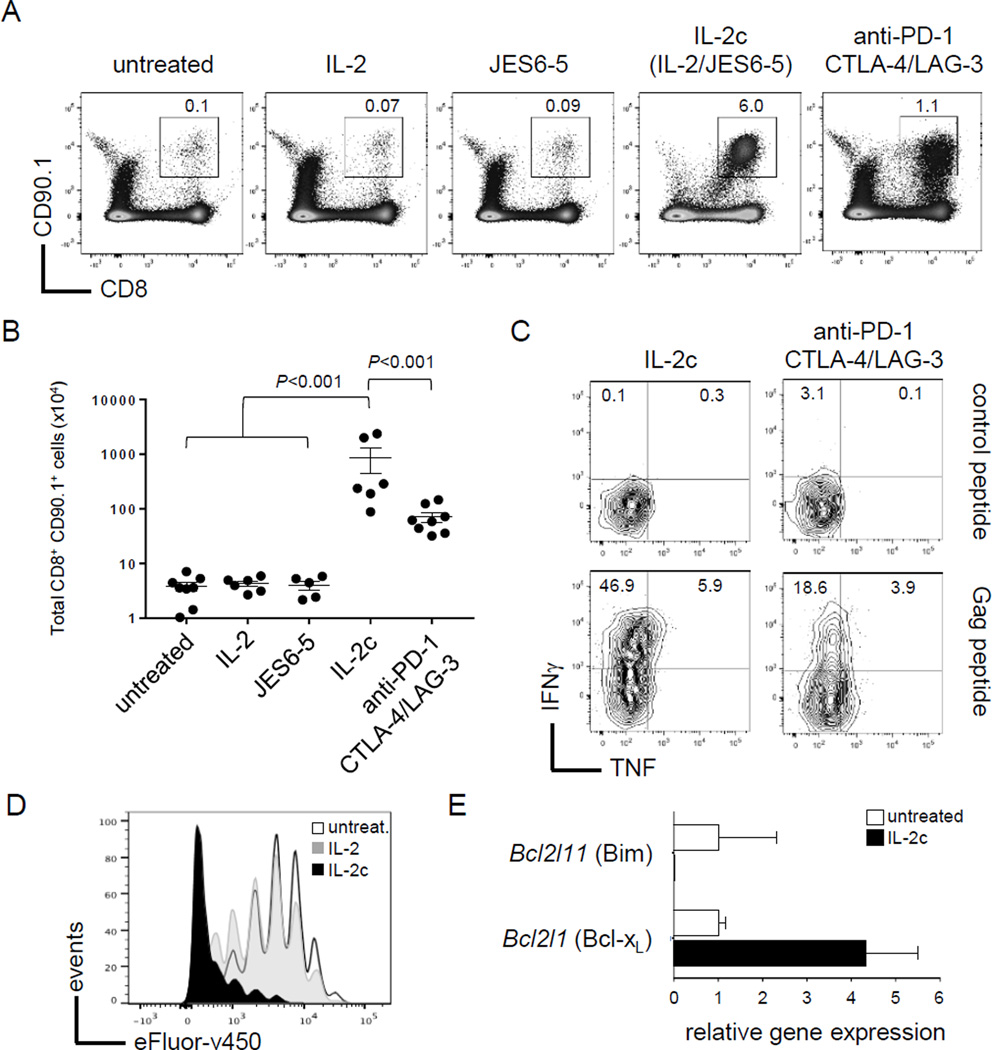
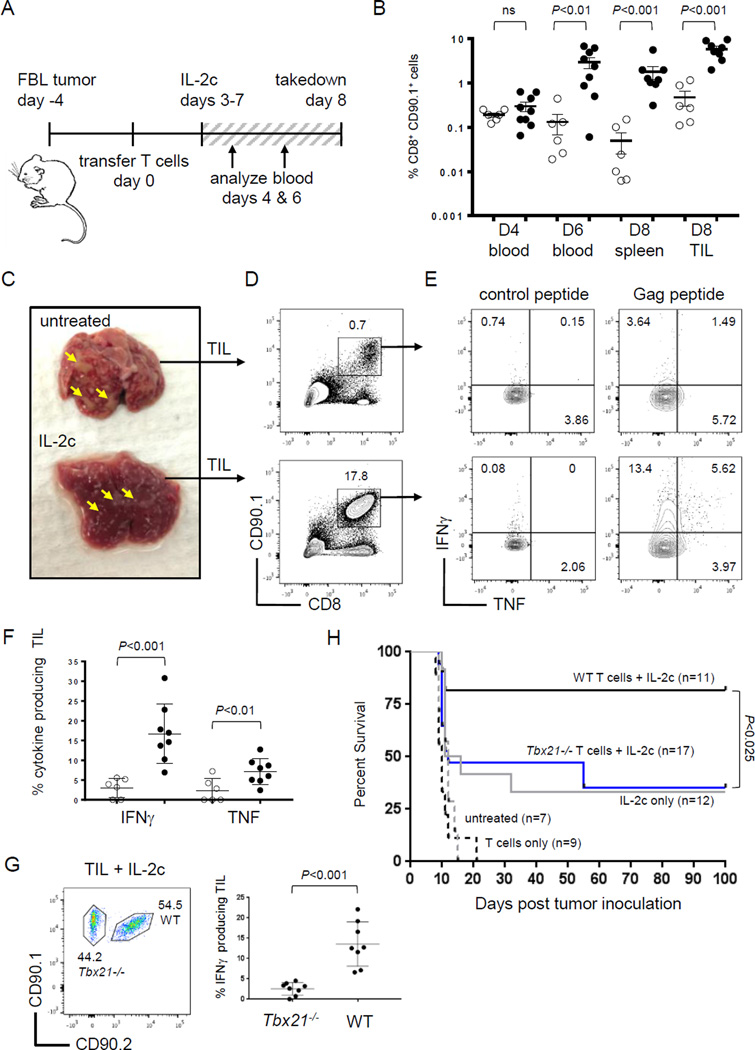
The complex of IL2 cytokine and anti-IL2 (IL2c) boosts CD8+ T-cell responses against cancer in mouse models (9, 14, 15). However, IL2c has not been tested as an immunotherapeutic agent under tolerizing conditions where tumor-reactive T cells are rendered dysfunctional and apoptotic. To address this, we used the well-characterized Alb:Gag model, where B6 mice express a tumor-associated antigen (Gag) in hepatocytes (17). Gag-specific CD8+ T cells transferred into Alb:Gag recipients become tolerant, characterized by the failure to acquire cytolytic capabilities followed by apoptotic deletion (20, 21). To determine if IL2c treatment prevents the initiation of CD8+ T-cell tolerance, IL2c was administered daily to Alb:Gag recipients at the time of T-cell transfer. Gag-specific CD8+ T cells (CD90.1+) were infused intravenously into Alb:Gag mice and the frequency of these T cells was assessed in spleens 8 days later. Without treatment, most transferred T cells were deleted (Fig. 1A). Administration of recombinant murine IL2 did not improve T-cell persistence, similar to our previous report using high-dose human IL2 (21). Likewise, anti-IL2 antibody (JES6-5) had no effect on T-cell survival. But when recipients were treated with IL2c, a high frequency of tumor/self-reactive T cells persisted (Fig 1A), suggesting stimulation with IL2c was able to override mechanisms of deletional tolerance.
Figure 1.
IL2c prevents CD8+ T-cell tolerance. (A) Gag-reactive T cells (CD90.1+ CD8+) were transferred into Alb:Gag mice and left untreated or treated as indicated. The frequency of transferred T cells was assessed in spleens 8 days later, and the percent of total splenocytes is indicated. (B) Total number of transferred T cells in spleens 8 days after transfer with error bars representing SEM. (C) Splenocytes were restimulated ex vivo with control or Gag peptide and assessed for intracellular IFNγ and TNF in transferred T cells. (D) T-cell division was assessed by fluorescent dye dilution 3 days after transfer. (E) Transferred T cells were sorted 3 days after infusion and relative gene expression assessed by qRT-PCR normalized to actin.
We previously reported that treatment with combination checkpoint blockade (anti-PD-1/CTLA-4/LAG-3) prevented T-cell deletion and promoted effector cytokine production during cancer immunotherapy (21, 23). Here, treatment with IL2c resulted in more than a 5-fold increase in transferred T-cell numbers at day 8 compared to checkpoint blockade treatment (Fig. 1A–B), and induced more interferon gamma (IFNγ) and tumor necrosis factor (TNF) production following ex vivo restimulation (Fig. 1C). These data highlight IL2c as a particularly potent immunomodulatory reagent able to boost CD8+ T-cell responses under conditions that are decidedly unfavorable for T-cell immunity.
IL2c works in part by enhancing the bioavailability of IL2 by stabilizing its serum half-life to promote durable signaling in T cells via the IL2 receptor (IL2R) (8, 9, 26). These signals culminate to induce T-cell proliferation and survival under many different in vivo conditions (27). Therefore, T-cell persistence here could reflect an increase in proliferation, prevention of apoptosis or both. To examine proliferation, T cells were labeled with proliferation dye (eFluor-v450) and transferred into Alb:Gag recipients left untreated, treated with soluble IL2 cytokine, or with IL2c daily for 3 days. Consistent with our previous study (21), encounter with tolerizing self-antigen in Alb:Gag mice induced several early rounds of division by Gag-reactive T cells prior to deletion, and this was essentially unchanged in recipients administered soluble IL2 (Fig. 1D). However, T-cell proliferation was markedly greater in mice treated with IL2c, suggesting rapid cell division contributed to the high frequency of transferred T cells persisting out to day 8.
To determine if IL2c also influenced T-cell apoptosis under tolerizing conditions, expression of apoptosis-related genes was assessed 3 days after T-cell transfer (just prior to deletion in untreated hosts). We previously identified the pro-apoptotic protein Bim (encoded by Bcl2l11) as necessary for T-cell deletion in Alb:Gag hosts (21, 23). Here, Bcl2l11 gene expression was observed in untreated T cells from the tolerizing environment, but was nearly undetectable following treatment with IL2c (Fig. 1E). Conversely, expression of the anti-apoptotic pro-survival molecule Bcl-xL (encoded by Bcl2l1) was increased 5-fold in T cells treated with IL2c relative to untreated. Together, these results suggest that IL2c prevents apoptosis and promotes robust proliferation to expand and sustain large numbers of CD8+ T cells under otherwise tolerizing conditions.
IL2c induces T-bet expression in responding CD8+ T cells
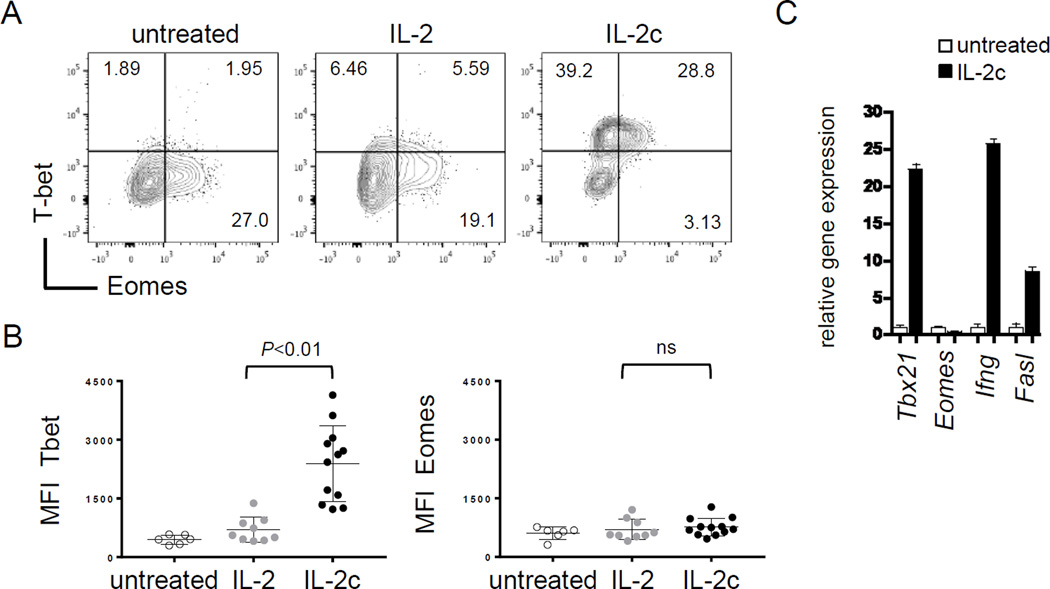
We have reported that CD8+ T-cell tolerance is regulated by a transcriptional program that involves repression of Tbx21 (T-bet) gene expression, whereas overcoming tolerance requires T-bet (22, 23, GEO accession code GSE58722). T-bet is a T-box transcription factor known to enforce CD4+ T helper-1 cell differentiation (28, 29) and CD8+ T-cell differentiation toward a cytolytic effector phenotype by promoting gene expression of effector molecules like IFNγ, GzmB and FasL (30, 31). To determine if IL2c induced T-bet in the prevention of tolerance, T-bet expression was assessed in Gag-specific T cells 3 days after transfer into Alb:Gag hosts. Here, IL2c induced high levels of intracellular T-bet protein, but not the related T-box transcription factor Eomesodermin (Eomes), which was maintained at basal levels under all conditions tested (Fig. 2A–B). This was mirrored at the gene expression level, as Eomes was unaffected by IL2c treatment whereas Tbx21 (T-bet) was markedly increased, as were the T-bet target genes Ifng and Fasl (Fig. 2C). Thus, IL2c is capable of converting a tolerizing signal into a robust T-cell activation and differentiation signal involving T-bet.
Figure 2.
IL2c treatment induces T-bet. (A) Intracellular T-bet and Eomes protein expression of was determined in transferred T cells from Alb:Gag recipients left untreated, treated with high-dose soluble human IL2 or mouse IL2c. Inset numbers are the percent of transferred CD8+ CD90.1+ T cells in each quadrant. (B) MFI of T-bet and Eomes 3 days after T-cell transfer pooled from 4 separate experiments. (C) Transferred T cells were sorted from recipient spleens 3 days after transfer and gene expression analyzed by qRT-PCR normalized to actin. Data are representative of 3 independent studies.
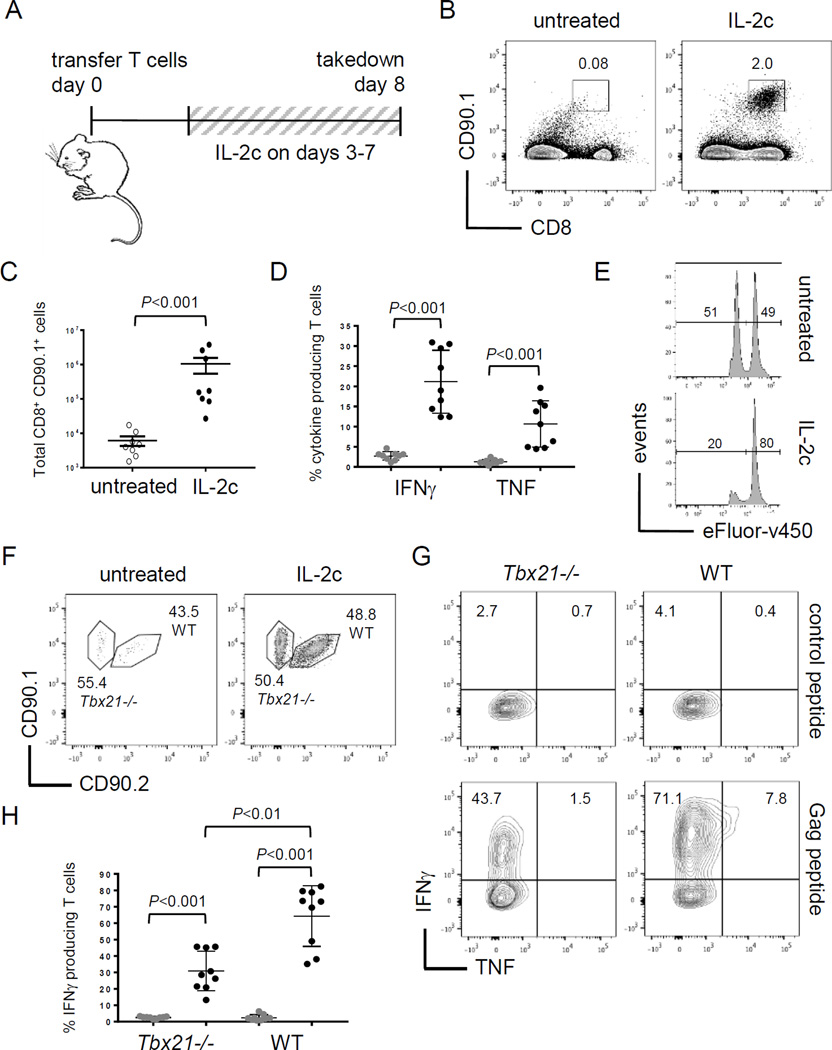
IL2c rescues tolerant CD8+ T cells
The ability of IL2c to prevent naive T cells from becoming tolerant demonstrates therapeutic promise, but this scenario does not reflect conditions in patients where immune tolerance is already established prior to diagnosis and treatment. To assess the ability of IL2c to rescue established T-cell tolerance, treatment of Alb:Gag recipients was delayed until 3 days after Gag-specific T-cell transfer to allow the induction of tolerance in vivo (Fig. 3A). At this time, T cells display a distinctly tolerant transcriptional profile (low Tbx21), are becoming apoptotic (high Bcl2l11), and do not make effector cytokines or mediate cytolytic activity (21, 23). Despite the onset of tolerance, intervention with IL2c rescued CD8+ T cells from this fate. Transferred CD8+ T cells persisted and were readily detected in the spleens of mice treated with IL2c after 8 days (Fig. 3B), which coincided with a significant increase in total numbers of transferred T cells in the spleens of IL2c treated mice compared to untreated (Fig. 3C). As with prevention of tolerance in Figure 1, intervention with checkpoint blockade did not efficiently rescue tolerant T cells compared to IL2c (Suppl. Fig S1), and was not pursued further here. In contrast, persisting T cells rescued with IL2c were functional, able to produce the effector cytokines IFNγ and TNF after ex vivo restimulation (Fig. 3D), and specifically kill antigen-positive target cells in vivo (Fig. 3E).
Figure 3.
IL2c rescues CD8+ T cells with established tolerance. (A) Diagram of experimental setup. (B) Transferred T-cell frequency in spleens on day 8. (C) Total number of transferred T cells in spleens at day 8 pooled from 3 separate experiments and error bars represent SEM. (D) Frequency of IFNγ or TNF positive cells from IL2c treated mice after restimulation with control (gray circles) or Gag peptide (black circles). (E) Relative frequency of Gag (efluorlow) and control (efluorhigh) peptide pulsed target cells. Inset numbers are the percent under each peak. (F) Tbx21−/− (CD90.1+) and WT (CD90.1+ CD90.2+) T cells were transferred into Alb:Gag recipients at a 1:1 ratio and the frequency of transferred cells in spleens assessed 8 days later. (G) Tbx21−/− and WT Gag-specific T cells were restimulated with control or Gag peptide and the frequency producing IFNγ or TNF was assessed. (H) Percent of transferred T cells producing IFNγ after restimulation with control peptide (gray circles) or Gag (black circles) pooled from 3 separate experiments.
Because T-bet was required to overcome T-cell tolerance with checkpoint blockade immunotherapy (23) and was induced by IL2c (Fig. 2), we tested whether T-bet was required for T-cell rescue with IL2c. Gag-specific CD8+ T cells from T-bet deficient mice (Tbx21−/−) and those with intact wild type Tbx21 (WT) were co-transferred into Alb:Gag recipients. After 8 days, both WT and Tbx21−/− T cells were similarly deleted without treatment and persisted equivalently when recipients were administered IL2c (Fig. 3F), suggesting T-bet is dispensable for survival of T cells rescued with IL2c. Persisting T cells, regardless of genotype, produced IFNγ after ex vivo restimulation, but T-bet was necessary for optimal cytokine production (Fig. 3G). The frequency of Tbx21−/− T cells expressing IFNγ was approximately half compared to WT T cells (Fig. 3H), and those that were positive produced less cytokine as indicated by lower median fluorescence intensity (MFI) (Suppl. Fig S2) perhaps the result of Ifng gene regulation by Eomes in the absence of T-bet (32), which is expressed at basal protein levels in tolerant T cells (Fig. 2A). These data demonstrate the potential of IL2c to re-engage dysfunctional and dying CD8+ T cells that had already been rendered tolerant. In light of this, it should be noted that IL2c treatment did not induce autoimmune liver toxicity in these Alb:Gag recipients, as measured by liver enzymes (ALT and AST) in the serum. This is consistent with similar analyses in this model, which appears highly resistant to T cell-mediated liver damage (18–21, 24).
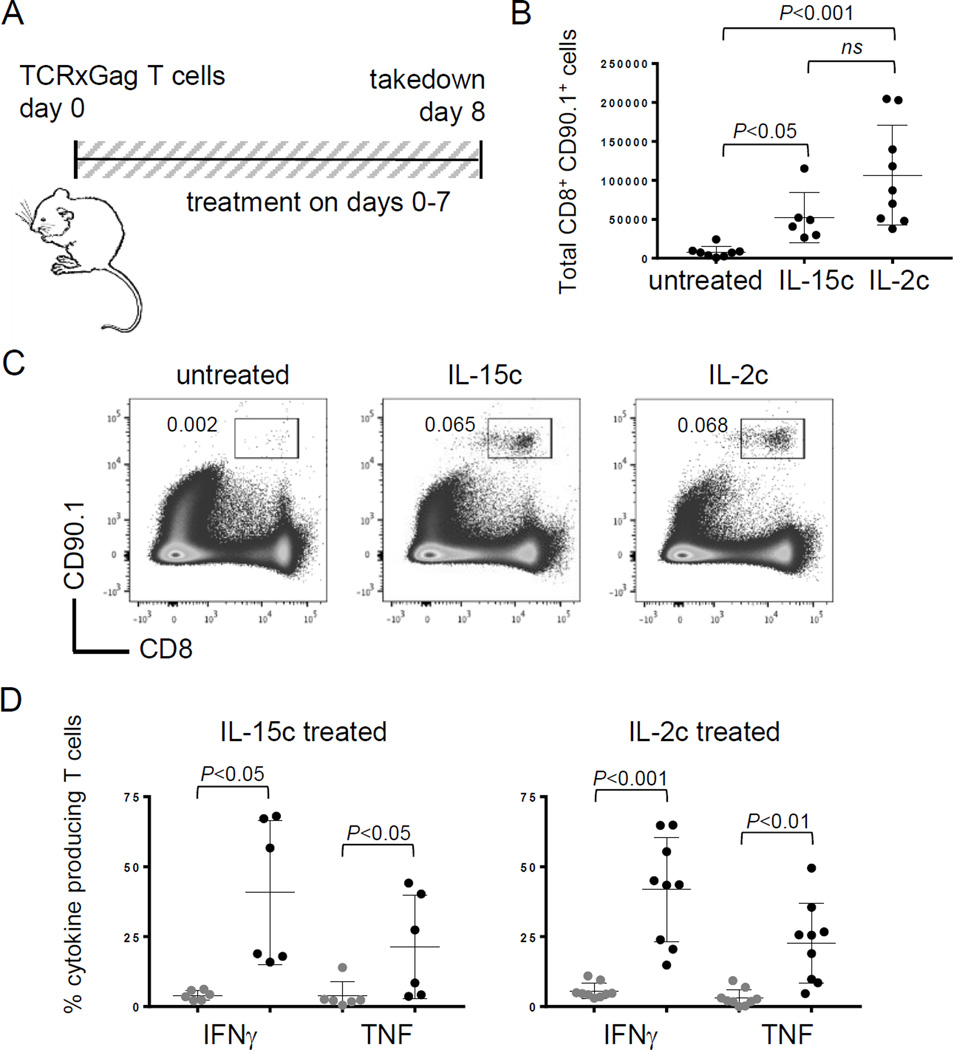
An alternative explanation for these results is that IL2c induced the expansion of a small subset of functional (non-tolerant) T cells that had somehow escaped or were resistant to tolerance. To address this, we tested the ability of IL2c to rescue tolerant T cells from TCRxGag mice (24). In this model, Alb:Gag mice are crossed with TCR transgenic mice expressing Gag-specific CD8+ T cells. These T cells undergo development and selection in the Alb:Gag thymus (19, 33). Some of these self-reactive T cells emigrate and persist in the periphery, but are tolerant and unable to proliferate or produce effector cytokines upon encounter with Gag antigen (18, 24, 33). These tolerant T cells can be rescued by inducing proliferation but regain a tolerant phenotype, which is regulated at the epigenetic level (19, 33). In vitro stimulation with IL15 (or high dose IL2) induces proliferation and temporary rescue of TCR x Gag T cells (18), but whether these tolerant CD8+ T cells can be rescued in vivo with cytokine complexes has not been reported. We transferred 2 × 105 purified TCR x Gag T cells into Alb:Gag recipients left untreated or treated daily with IL2 or IL15 complexes for 8 days (Fig. 4A). Both cytokine complexes induced similar increases in the number of transferred T cells in spleens 8 days after infusion (Fig. 4B and 4C). Production of effector cytokines (IFNγ and TNF) was also similar between IL2c and IL15c treatment groups, with both inducing similar frequencies of cytokine producing T cells as well as similar cytokine staining intensity (Fig. 4D and Suppl. Fig S3). These data reaffirm the capacity of IL2c (and IL15c) to rescue tolerant CD8+ T cells in vivo, with clear potential for therapeutic applications.
Figure 4.
CD8+ T-cell tolerance is rescued by IL2c or IL15c. (A) Diagram of experimental setup. (B) The total number of transferred T cells in spleens on day 8 pooled from 3 independent experiments. (C) Spleens were analyzed on day 8 for the frequency of persisting of CD8+ CD90.1+ cells. Inset numbers are the percent of transferred CD8+ CD90.1+ T cells. (D) Frequency of transferred cells producing IFNγ or TNF after restimulation with control (gray circles) or Gag peptide (black circles). Data are pooled from 2 and 3 separate experiments, respectively.
Rescue of T-cell tolerance with IL2c enhances cancer immunotherapy
To determine if IL2c–mediated rescue of tolerant T cells could provide a therapeutic benefit in mice with cancer, we returned to the transfer model, where Gag-specific CD8+ T cells become tolerant after 3 days in Alb:Gag recipients. T cells were infused into recipients bearing an established and disseminated FBL leukemia (Fig. 5A), and some received daily IL2c treatment starting 3 days later. The frequency of transferred T cells was tracked in peripheral blood on days 4 and 6 after infusion, and in spleen and tumor on day 8 (Fig. 5B). IL2c had no discernable impact on T-cell frequency in blood after 24 hours (day 4), but T-cell expansion was obvious in the blood 2 days later (day 6). Enhanced T-cell persistence in IL2c–treated mice was also observed in the spleen at day 8, and this was mirrored within solid tumor foci in the liver (Fig. 5B). Mice treated with IL2c had fewer and smaller foci compared to untreated mice (Fig. 5C), likely reflecting tumor regression or at least a stabilization of disease. Regardless of reduced tumor burden, sufficient numbers of tumor-infiltrating lymphocytes (TIL) were isolated for functional analysis (Fig. 5D). The CD90.1+ CD8+ TIL isolated from untreated mice displayed a tolerant phenotype and failed to produce effector cytokines after restimulation with Gag-peptide, whereas TIL from mice treated with IL2c made IFNγ and TNF (Fig. 5E and 5F). Production of IFNγ by rescued TIL was dependent on T-bet, as cytokine levels were near background inTbx21−/− TIL (Fig. 5G).
Figure 5.
T-cell rescue for immunotherapy requires T-bet. (A) Diagram of experimental setup. (B) T cells were transferred into Alb:Gag recipients left untreated (open circles) or treated with IL2c (closed circles) and frequencies in peripheral blood, spleen and tumor were analyzed. Data are pooled from 3 independent experiments, and error bars are SEM. (C) Representative livers from untreated and IL2c treated mice on day 8, denoting tumor foci with yellow arrows. (D) Frequency of TIL isolated from liver tumor foci. Inset numbers are the percent of TIL within the inscribed region (E) TIL were restimulated ex vivo and the frequency of cells producing IFNγ or TNF was determined. Inset numbers are the percent of CD8+ CD90.1+ cell in each quadrant (F) Frequency of IFNγ or TNF producing TIL from untreated (open circles) or IL2c treated (closed circles) mice pooled from 3 independent experiments. (G) The frequency of Tbx21−/−and WT T cells producing IFNγ after restimulation pooled from 3 separate experiments. (H) Percent survival (y-axis) over time in days (x-axis) of FBL-bearing Alb:Gag mice treated as indicated.
In similar studies, the survival of leukemia-bearing Alb:Gag recipients was followed for 100 days. Because Gag-specific T cells become tolerized shortly after infusion into Alb:Gag mice, adoptive T-cell transfer alone did not improve recipient survival over that of untreated mice (Fig. 5H). In contrast, rescue of tolerant transferred T cells with IL2c resulted in more than 80% of mice surviving long-term. Because T-bet was required for IFNγ production by TIL rescued with IL2c (Fig. 5G), we hypothesized that T-bet is necessary for IL2c–mediated immunotherapy. Indeed, transfer of Gag-specificTbx21−/− T cells and treatment with IL2c was no more effective than IL2c alone (Fig. 5H). These data support a model whereby IL2c requires induced T-bet expression to rescue tolerant tumor-reactive T cells for durable immunotherapy.
Our immunotherapy studies also revealed that IL2c alone (no T-cell transfer) provided a substantial benefit to Alb:Gag recipients (∼30% long-term survival) compared to untreated mice (Fig. 5H). This occurred despite the lack of endogenous Gag-reactive T cells in Alb:Gag mice due to negative selection in the thymus (17). Therefore, this therapeutic effect must rely on recognition of leukemia by other endogenous immune cells stimulated by IL2c. Indeed, tumor-bearing Alb:Gag mice treated with IL2c had expanded endogenous CD8+ T cells and natural killer (NK) cells in the spleen (Suppl. Fig. S4) in agreement with other reports (9, 34). In the same mice, IL2c had no apparent impact on the frequencies of peripheral CD4+ T cells or Foxp3+ Tregs. However, among endogenous TIL we observed a modest increase in CD4+ T cells and Treg frequencies along with enhanced CD8+ T cell and NK cell frequencies. It has yet to be determined whether higher frequencies of CD4+ T cells and Treg among TIL reflects a direct response to IL2c treatment not induced in spleen, or an indirect effect caused by increased inflammation, cellular influx or tumor cell apoptosis during therapy. Recent X-ray crystallography studies comparing different IL2 complexes composed of either the JES6-1A12 or S4B6 anti-IL2 clones demonstrated that specificity for distinct T-cell subsets stems from a conformational shift that favors high affinity interactions with cells expressing high IL2Rβ (CD8+ T cells and NK cells) or high IL2Rα (Tregs) (11). While such structural analysis has not been performed on IL2c using the JES6-5H4 clone, our studies suggest it functions similarly to S4B6 by selectively targeting CD8+ T cells and NK cells, which have particular significance for antitumor immunity. During adoptive immunotherapy, we expect these endogenous T cells and NK cells cooperate with transferred Gag-reactive T cells rescued by IL2c for optimal immunotherapy in recipients with leukemia (Fig. 5H).
Human IL2 complexes influence human T cells in vivo
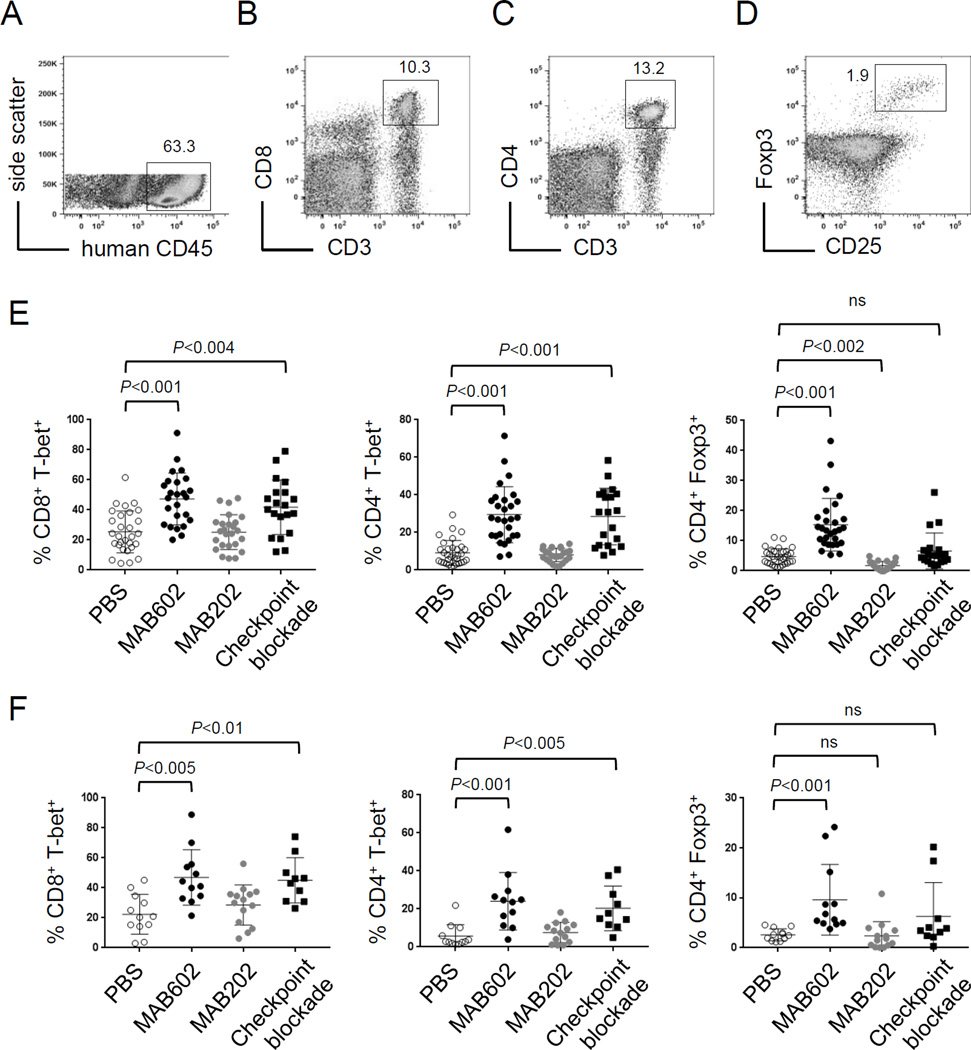
Our studies in mice demonstrate that rescue of CD8+ T-cell tolerance with IL2c relies on increased expression of T-bet to induce antitumor activity. However, the ability to elicit human immune responses in vivo with human IL2 complex (hIL2c) has not been reported. To examine this, human CD34+ hematopoietic stem cells (HSC) were purified and transferred into NSG mice expressing the human leukocyte antigen A2.01 (NSGHLA-A2) (35) to generate diverse human immune cells. After 12–14 weeks, mice had a large proportion (>60%) of human CD45+ lymphocytes in the spleen (Fig. 6A), including CD8+ T cells (Fig. 6B), CD4+ T cells (Fig. 6C), and CD4+ CD25+ Foxp3+ regulatory T cells (Fig. 6D).
Figure 6.
Human IL2c influences human immune cells. (A) Human lymphocytes were distinguished from murine cells by expression of human CD45. Flow plots show (B) CD8+ T cells and (C) CD4+ T cells, and inset numbers are the percent of total human CD45+ cells. (D) Tregs were distinguished by Foxp3 and CD25 expression, and inset number is the percent of total human CD45+ CD4+ cells. (E) Frequency of CD8+ and CD4+ T cells expressing T-bet or Foxp3 in (E) spleen and (F) tumor was assessed by intracellular flow cytometry. Data are pooled from 5 independent experiments for spleen and 3 for tumor.
Following immune cell reconstitution, NSGHLA-A2 mice were challenged with 1×106 HLA-A2+ human melanoma tumor cells by intravenous injection. Four weeks later, recipients were treated with one of two versions of hIL2c using the antibodies MAB602 or MAB202 daily for one week. For comparison, another group of mice received human checkpoint blockade antibodies against PD-1, CTLA-4 and LAG-3. None of these treatments significantly altered the frequency of CD4+ or CD8+ T cells (Suppl. Fig. S5), and none altered disease progression during this brief treatment window in humanized mice with advanced and disseminated melanoma. However, compared to PBS controls, mice treated with hIL2c (MAB602) had increased frequencies of CD8+ and CD4+ T cells expressing T-bet, and a marked increase in the percentage of Foxp3+ Tregs in the spleen (Fig. 6E). This increase in T-bet and Foxp3 expressing T cells was mirrored in tumors isolated from lungs (Fig. 6F). The other hIL2c (MAB202) had none of these effects, but rather showed a reduction in the frequency of Foxp3+ Tregs in spleen (Fig. 6E), but this did not reach statistical significance in lung tumors (Fig. 6F). Like IL2c (MAB6202), treatment with human checkpoint blockade antibodies resulted in a higher frequency of T-bet+ CD4+ and CD8+ T cells in spleen (Fig. 6E) and tumor (Fig. 6F). In contrast, checkpoint blockade did not significantly increase the frequency of Tregs in these tissues.
These data provide the first experimental evidence that human T cells can be engaged with hIL2c in vivo. As with mouse T cells, the use of different anti-human IL2 antibody clones in the complex produced divergent results (10). It is tempting to speculate that the ability to target different T-cell subsets comes from unique structural confirmations induced with different anti-IL2 antibodies (11). However, the crystal structures have not yet been solved for any versions of human IL2c. This has important implications for the potential use of IL2c in patients, as the cells being targeted here could dramatically alter the outcome of immunotherapy. Much more work is needed to identify and characterize the optimal hIL2c components necessary to achieve desired clinical outcomes.
Discussion
One of the formidable challenges to providing durable immunotherapy for cancer is the prevalence of immune tolerance, where T cells equipped to recognize and eliminate tumor cells are instead rendered dysfunctional in the tumor microenvironment. Successful immunotherapies like checkpoint blockade are designed to disrupt tolerizing signals, producing dramatic results for some patients. Still, many do not benefit from this approach, highlighting the need to better understand the mechanisms regulating immune tolerance and to develop new therapeutic strategies.
Since the earliest days of immunotherapy, the use of IL2 to elicit antitumor immunity has been a tantalizing prospect (2). Indeed, IL2 has some capacity to rescue tolerant T cells at least temporarily (18), but has limited clinical efficacy and can cause significant toxicity in patients (3). This led to the development of strategies to extend the in vivo half-life of IL2 and selectively target only cells of interest (8). One promising approach is the use of IL2/anti-IL2 complexes (IL2c) (10), which has yielded promising antitumor responses under non-tolerizing conditions in a mouse melanoma model (9, 14). We demonstrate here that immunotherapy with IL2c rescues tolerant tumor-reactive CD8+ T cells, and restoration of function is dependent on the transcription factor T-bet. Our results showcase a novel benefit of IL2c for cancer immunotherapy, and reveal new insight into the molecular mechanisms dictating T-cell tolerance versus immunity.
Induction of CD8+ T-cell tolerance is characterized by early proliferation upon encounter with tolerizing antigen, but failure to acquire effector function and eventual T-cell death by apoptosis. This phenotype is regulated by a transcriptional program involving low expression of effector genes such asTbx21 (T-bet), Ifng and Gzmb, and high expression of the pro-apoptotic gene Bcl2l11 (Bim) (21, 23, 33, 36). Despite the initiation of this tolerant program, intervention with IL2c was able to rescue T cells in vivo and provide immunotherapy for mice with disseminated and progressive leukemia. In fact, T cells rescued with IL2c displayed an altered profile, with decreased Bim and increased expression of the pro-survival gene Bcl2l1 (Bcl-xL). Rescued T cells also had high levels of T-bet, and the effector cytokine and T-bet target gene, Ifng. Restored T-bet expression was important for optimal IFNγ production by rescued T cells in the periphery, but absolutely vital for effector activity within tumors. The importance of T-bet was further illustrated during immunotherapy, as tumor-reactive T cells lacking T-bet provided no survival benefit for mice receiving IL2c treatment. This suggests that T-bet expression in responding tumor-reactive CD8+ T cells is essential for rescue of effector function and successful immunotherapy with IL2c. This finding supports other studies by our group that independently came to a similar unifying conclusion: T-bet is a key molecular determinant in the fate of tumor-reactive T cells, with important implications for immunotherapy (22, 23).
It is not difficult to imagine that induced T-bet expression in tumor-reactive T cells boosts antitumor immunity, as T-bet regulates several genes known to encode effector molecules like IFNγ and GzmB (23, 28). Certainly, such molecules are likely to aid immunotherapy, but our findings hint that T-bet may have other utilities as well. Because T-bet expression is induced under conditions that favor positive outcomes during immunotherapy, T-bet could serve as a biomarker to track therapeutic responses. Given the need for informative biomarkers in patients, we tested if T-bet expression could be tracked in human lymphocytes during immunotherapy. Humanized mice with disseminated melanoma were treated with hIL2c (MAB602) or checkpoint blockade antibodies and both immunotherapy regimens induced similar increases in the frequencies of human CD4+ and CD8+ T cells expressing T-bet in peripheral and tumor tissues. These results extend our findings in mouse to human immunology, providing translational evidence that relative T-bet expression may be used to gauge human T-cell responses to immunotherapy.
Human T-cell responses to these distinct immunotherapies were not identical. By inducing T-bet expression in conventional T cells without an increase in the frequency of Foxp3+ Tregs, checkpoint blockade appeared to drive a T-cell response geared toward effector function and antitumor immunity. Responses to hIL2c (MAB602) were more ambiguous, inducing robust T-bet expression in effector T cells, but also increasing the frequency of Tregs at the same time, potentially suppressing effector responses. While this outcome may not be advantageous in the context of cancer immunotherapy, we envision that human IL2c formulations could be alternatively designed for treatment of autoimmunity by preferentially boosting regulatory T cells and minimizing harm cause by self-reactive effector T cells. Whether different anti-human IL2 antibodies can be used to build hIL2 complexes that selectively target effector versus regulatory immune cell subsets (as in mice) to produce the desired therapeutic outcome requires further structural analysis and in vivo study. Our findings suggest such studies are warranted, and that human IL2c represents a powerful and customizable reagent for immunotherapy capable of eliciting distinct responses from diverse human T-cell populations.
Supplementary Material
Acknowledgments
The authors would like to thank Joy Eslick and Sherri Koehm of the Flow Cytometry Core Facility at Saint Louis University for their time and expertise in the acquisition of data by flow cytometry.
This work was supported by a grant from the NIH/NIAID (R01AI087764), a Cancer Research Institute CLIP award, and a grant from the Siteman Cancer Center and The Foundation for Barnes-Jewish Hospital to R.M. Teague. L.E. Klevorn was supported by a Saint Louis University Presidential Graduate Fellowship.
Footnotes
COI: The authors declare no potential conflicts of interest.
References
- 1.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 Consecutive Patients with Metastatic Melanoma or Renal-Cell Cancer Using High-Dose Bolus Interleukin-2. Jama-J Am Med Assoc. 1994;271:907–913. [PubMed] [Google Scholar]
- 2.Rosenberg SA. IL2: the first effective immunotherapy for human cancer. Journal of immunology. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. [PubMed] [Google Scholar]
- 4.Lentsch AB, Miller FN, Edwards MJ. Mechanisms of leukocyte-mediated tissue injury induced by interleukin-2. Cancer Immunol Immun. 1999;47:243–248. doi: 10.1007/s002620050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends in immunology. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue JH, Rosenberg SA. The fate of interleukin-2 after in vivo administration. Journal of immunology. 1983;130:2203–2208. [PubMed] [Google Scholar]
- 8.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, et al. IL2/anti-IL2 antibody complexes show strong biological activity by avoiding interaction with IL2 receptor alpha subunit CD25. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL2 and anti-IL2 monoclonal antibody as novel approach of cancer immunotherapy. Journal of immunology. 2009;183:4904–4912. doi: 10.4049/jimmunol.0900284. [DOI] [PubMed] [Google Scholar]
- 10.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 11.Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, et al. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity. 2015;42:815–825. doi: 10.1016/j.immuni.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson SR, Yuan J, Teague RM. Targeting CD8+ T-cell tolerance for cancer immunotherapy. Immunotherapy. 2014;6:833–852. doi: 10.2217/imt.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in immunology. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL2 immunotherapy by selective stimulation of IL2 receptors on lymphocytes and endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waithman J, Gebhardt T, Davey GM, Heath WR, Carbone FR. Cutting edge: Enhanced IL2 signaling can convert self-specific T cell response from tolerance to autoimmunity. Journal of immunology. 2008;180:5789–5793. doi: 10.4049/jimmunol.180.9.5789. [DOI] [PubMed] [Google Scholar]
- 17.Ohlen C, Kalos M, Hong DJ, Shur AC, Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. Journal of immunology. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- 18.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nature medicine. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 19.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, et al. Peripheral CD8(+) T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morimoto J, Tan X, Teague RM, Ohlen C, Greenberg PD. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. Journal of immunology. 2007;178:6849–6860. doi: 10.4049/jimmunol.178.11.6849. [DOI] [PubMed] [Google Scholar]
- 21.Berrien-Elliott MM, Jackson SR, Meyer JM, Rouskey CJ, Nguyen TL, Yagita H, et al. Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer research. 2013;73:605–616. doi: 10.1158/0008-5472.CAN-12-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson SR, Yuan J, Berrien-Elliott MM, Chen CL, Meyer JM, Donlin MJ, et al. Inflammation programs self-reactive CD8+ T cells to acquire T-box-mediated effector function but does not prevent deletional tolerance. Journal of leukocyte biology. 2014;96:397–410. doi: 10.1189/jlb.1A0913-500RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrien-Elliott MM, Yuan J, Swier LE, Jackson SR, Chen CL, Donlin MJ, et al. Checkpoint blockade immunotherapy relies on T-bet but not Eomes to induce effector function in tumor-infiltrating CD8+ T cells. Cancer immunology research. 2015;3:116–124. doi: 10.1158/2326-6066.CIR-14-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL15 to a superagonist by binding to soluble IL15R{alpha} Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Votavova P, Tomala J, Kovar M. Increasing the biological activity of IL2 and IL15 through complexing with anti-IL2 mAbs and IL15Ralpha-Fc chimera. Immunology letters. 2014;159:1–10. doi: 10.1016/j.imlet.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature Reviews Immunology. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 28.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 29.Zhu JF, Jankovic D, Oler AJ, Wei G, Sharma S, Hu GQ, et al. The Transcription Factor T-bet Is Induced by Multiple Pathways and Prevents an Endogenous Th2 Cell Program during Th1 Cell Responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarevic V, Glimcher LH. T-bet in disease. Nature immunology. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 33.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MT, Richer MJ, Gross BP, Norian LA, Badovinac VP, Harty JT. Enhancing Dendritic Cell-based Immunotherapy with IL2/Monoclonal Antibody Complexes for Control of Established Tumors. Journal of immunology. 2015;195:4537–4544. doi: 10.4049/jimmunol.1501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson T, Greiner DL, Shultz LD, et al. In: Creation of “humanized” mice to study human immunity. Current protocols in immunology. Coligan John E., editor. 2008. Chapter 15:Unit 15 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redmond WL, Wei CH, Kreuwel HT, Sherman LA. The apoptotic pathway contributing to the deletion of naive CD8 T cells during the induction of peripheral tolerance to a cross-presented self-antigen. Journal of immunology. 2008;180:5275–5282. doi: 10.4049/jimmunol.180.8.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.