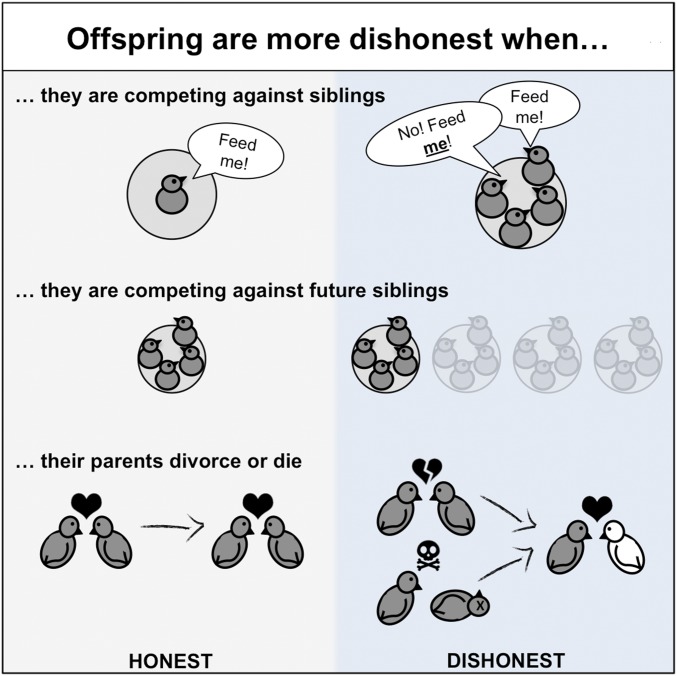
Significance
Should a chick beg for food even if it isn’t struggling to grow? Does it have anything to lose? The answer could be “yes” if it risks losing indirect fitness through the starvation of siblings. Evolutionary theory suggests that offspring may be more likely to exaggerate signals of need when they compete with less-related nestmates or a greater number of nestmates. We found clear support for this hypothesis in an analysis across 60 bird species. Offspring begging was less reliable in species where parents produce larger broods and more broods and where parental divorce or death reduces between-brood relatedness. This result helps explain why chicks of some species are more honest than others and tests general predictions of signaling theory.
Keywords: parent–offspring communication, signaling, begging, parent–offspring conflict, meta-analysis
Abstract
Offspring survival can often depend on successful communication with parents about their state of need. Theory suggests that offspring will be less likely to honestly signal their need when they experience greater competition from either a greater number of nestmates or less-related nestmates. We found support for this hypothesis with a comparative analysis, examining data from across 60 species of birds. We found that offspring are less honest about their level of need when (i) they face competition from current siblings; (ii) their parents are likely to breed again, and so they are in competition with future siblings; and (iii) parental divorce or death means that they are likely to be less related to future siblings. More generally, these patterns highlight the sensitivity of communication systems to conflict between signaler and receiver while also suggesting that when there is little conflict, natural selection favors the honest.
In almost every species where offspring live with their parents in family groups, they beg or signal to their parents for food (1, 2). Evolutionary theory suggests that signaling between offspring and their parents will evolve in response to the environment. At one extreme, if parents have enough resources to rear all their offspring, then offspring can be selected to honestly signal their need for food, so the offspring in the worst condition is fed (1–8). At the other extreme, if parents only have enough resources to rear one offspring, then offspring can be selected to competitively signal their quality or scramble for food, so the offspring in the best condition is fed (7–14). Empirical data support these predictions: offspring appear more likely to signal need when resources are relatively plentiful and quality when resources are scarce (15).
Theory also provides an understanding of when signals of need between offspring and parents are expected to break down or become distorted. If the self-interest of an offspring conflicts sufficiently with the interests of its parents, then it can be selected to exaggerate its need (4–7, 11, 13, 16–20). Increased competition between siblings can disrupt the alignment of interests between parents and their offspring. Competition between siblings can reduce the benefit of allowing needier siblings to be fed, selecting for each offspring to try to maximize its own share of parental resources, such that honest signaling of need is not stable. Both increased numbers of siblings and lower relatedness between those siblings could lead to greater sibling competition (4–6, 11, 13, 14, 16–20). Competition between siblings could also lead to exaggerated and dishonest signals of need, rather than the complete collapse of honest signaling (16). However, there is a lack of consistent empirical evidence demonstrating that sibling competition leads to either the breakdown of honest signaling or less honest signals (7, 21–33). One problem is that these hypotheses can be hard to test within a single species, where there may not be sufficient variation in offspring number and relatedness.
We exploited the variation in breeding behavior across birds to test whether offspring beg less honestly in species where there is greater competition between siblings. We estimated the honesty of offspring signals by calculating the strength of the relationship (correlation coefficient) between signals and long-term condition (Fig. S1) (34–37). “Condition,” “need,” and “quality” refer to the ultimate fitness effects of receiving additional food; our measures of long-term condition (body condition, health, rank within the brood and long-term food intake) therefore reflect the total requirement for food to improve overall quality before fledging and are likely to reflect both cryptic and public aspects of condition (SI Materials and Methods) (3, 4, 7, 9, 24, 38, 39). An advantage of focusing on the correlation between signaling and condition is that correlation coefficients are less likely to be influenced by nonsocial differences between species, such as body size, which can affect absolute measures of begging intensity, such as call volume (34). Our hypotheses were based on signal-of-need models (4–6, 11, 13, 14, 17–20), and so we first analyzed behavioral signals, such as begging calls and postures, which are more likely to be signals of need (15). We then analyzed structural signals, such as mouth color, which are more likely to be signals of quality (15).
Fig. S1.
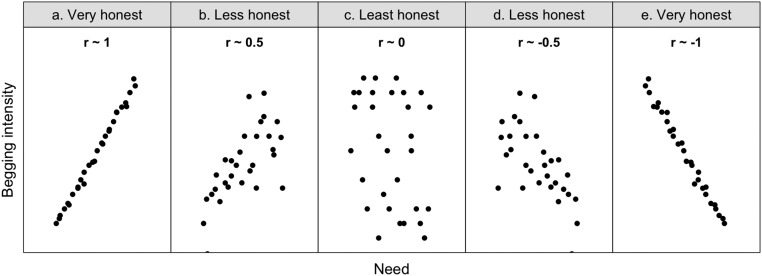
What is honesty? Species were considered honest if there was a strong relationship between offspring need and begging intensity, as measured by the correlation coefficient (37). (A) When the correlation coefficient approaches 1, begging is considered honest because it is almost perfectly informative. (B) When the correlation is weaker (e.g., r = 0.5), begging may provide some information about need, but it is less reliable. (C) When the correlation coefficient approaches 0, begging is considered dishonest because it provides no information about need. (D and E) Begging could also function as an honest signal of quality, rather than need, in which case the correlation coefficient would be negative rather than positive. Our analyses looked at whether differences in the relative strength of the correlation between need and begging across species can be explained by increases in within-family conflict as predicted by Hamilton’s rule.
SI Materials and Methods
Data Extraction and Effect Sizes.
All relevant statistical tests of the effect of offspring long-term need on begging intensity were included in the meta-analysis. We converted these test statistics to Pearson’s correlation coefficients, a standardized effect size that allows comparisons across studies (34, 35). Almost any test statistic (such as an F or χ2 value) or effect size (such as a risk ratio) can be transformed to a correlation coefficient. Sixteen papers also contained information such as raw means and SDs that could be used to calculate effect sizes. Correlation coefficients range from ±1 and describe the relationship between two variables. They account for different scales in their original measurements, are well suited to the ordered nature of the data and are more straightforward to interpret than standardized difference in means (34). We transformed the data extracted from the literature to correlation coefficients following Borenstein et al. (34), Grissom and Kim (36), Koricheva et al. (35), and Nakagawa and Schielzeth (62). Conversion formulas available on request.
Correlation coefficients were transformed to Fisher’s Z before analysis: Zr = 1/2 ln[(1 + r)/(1 − r)], so that the data could be weighted by the inverse variance 1/(n − 3), which approximates the variance on Fisher’s Z and is not dependent on the strength of the correlation (34). We used the number of broods used to generate the original test statistic for sample size, because this is a standard measure across studies and avoids the issue of pseudoreplication of having multiple nonindependent offspring from the same nest as the sample size. All analyses were conducted on the transformed values, and results were converted back into correlation coefficients for figures and discussion.
Tests for Publication Bias and Study Methodology Bias.
Although we did not expect to find one true effect size across all studies and species (34), we tested our meta-analysis for publication bias using the regression test for funnel plot asymmetry (Egger’s test) in the “metafor” package in R (60, 63). We calculated the mean effect size per study and compared it to its variance to determine whether studies with smaller sample sizes were more likely to show biased effects. We found no evidence of publication bias in begging analyses (z = 0.90, P = 0.37).
We also tested whether study methodology biased the strength or direction of the correlation coefficient. We recorded additional information on study methodology for each coefficient, including: whether the data were experimental or observational (two-level factor); whether the correlation coefficient was estimated or derived from a test statistic provided by the original study (two-level factor); the type of begging variable (two-level factor: continuous intensity measure, likelihood of signaling); the measure of long-term need (five-level factor: health, rank, weight, condition, brood-level effects); and whether the offspring contrast was dichotomous (bigger vs. smaller) or continuous (all offspring included). Analyses were run on the full dataset [Null (a)]. Presence/absence of siblings was included as a control factor, because some methodological factors, such as size rank within the brood and offspring contrast, were only available for species with siblings and the presence/absence of siblings influences the effect size (Table 1).
Table 1.
Results for all models: fixed effects
| Model no. | Fixed effects | Results | N species | N study | N observations |
| 1 | Siblings present y/n | F1,71.8 = 6.69, P = 0.0097 | 60 | 108 | 336 |
| 2 | Brood size | F1,31.0 = 4.57, P = 0.033 | |||
| 3 | Siblings present y/n | F1,67.0 = 8.45, P = 0.0050 | 51 | 98 | 317 |
| No. of future broods possible | F1,87.9 = 3.13, P = 0.080 | ||||
| 4 | Brood size | F1,82.2 = 11.87, P = 0.0009 | |||
| No. of future broods possible | F1,83.5 = 7.22, P = 0.0087 | ||||
| 5 | Siblings present y/n | F1,84.2 = 0.42, P = 0.42 | 49 | 96 | 314 |
| No. of future broods possible | F1,95.4 = 9.39, P = 0.0028 | ||||
| Full vs. half siblings likelihood | F1,112.5 = 6.94, P = 0.0096 | ||||
| 6 | Brood size | F1,87.1 = 3.07, P = 0.083 | |||
| No. of future broods possible | F1,86.4 = 13.09, P = 0.0005 | ||||
| Full vs. half siblings likelihood | F1,89.6 = 5.98, P = 0.016 | ||||
| 7 | Siblings present y/n | F1,60.5 = 0, P = 1 | 31 | 68 | 230 |
| No. of future broods possible | F1,58.4 = 3.10, P = 0.083 | ||||
| Full vs. half siblings likelihood | F1,76.0 = 3.77, P = 0.056 | ||||
| Extrapair paternity | F1,58.5 = 0.04, P = 0.84 | ||||
| 8 | Brood size | F1,66.2 = 3.87, P = 0.053 | |||
| No. of future broods possible | F1,58.3 = 4.94, P = 0.038 | ||||
| Full vs. half siblings likelihood | F1,67.1 = 1.00, P = 0.32 | ||||
| Extrapair paternity | F1,58.5 = 0.27, P = 0.61 |
Mean results (conditional Wald statistics) of 500 ASReml linear mixed models. Models controlled for phylogeny, repeated measures on studies, and species, and were weighted by study sample size (the number of broods used to calculate the original test statistic). Fixed effects in bold are significant at the P < 0.05 level and in italics at the P < 0.10 level. Models are grouped by the dataset used for analysis, as sample size decreased in later analyses due to unavailable life history data.
For begging analyses, we found no evidence that study methodology influences the correlation coefficient (P > 0.20 for all factors: experimental/observational Wald = 0.30, P = 0.58; estimated correlation coefficient Wald = 0.09, P = 0.77; begging variable type Wald = 0.00, P = 0.95; long-term need measure Wald = 1.53, P = 0.20, offspring contrast type Wald = 1.09, P = 0.36).
For structural signals, we previously tested the same dataset for publication bias and effects of study methodology and found no effects (15).
Detailed Explanation of Offspring Long-Term Condition.
Many aspects of offspring condition were reported in the literature, such as hunger, body mass to skeletal size ratio, dominance rank, experimentally reduced or enlarged broods, and experimental immune challenges. Following the common terminology of the field, low condition is equivalent to high need, and good condition is equivalent to high quality. We excluded correlation coefficients that examined only the effect of short-term food deprivation, i.e., hunger. Although hunger and condition may be intertwined, they represent very different selection pressures (5, 7, 39, 64). Each piece of food eaten increases the likelihood that an offspring will fledge, but the fitness benefit of food to fatally diseased offspring is zero, because they will not live to breed (38, 45). Furthermore, the influence of hunger on begging is already well established (1). Consequently, we focused on the influence of long-term condition, and so data on the relationship between hunger and signal intensity were not included in analyses of offspring strategies.
According to theory, costly signals of condition must contain information about a cryptic, rather than public, aspect of offspring condition (3, 9). Our estimate of honesty uses proxies of long-term need that may be obvious to parents without a signal, such as body condition. It is possible that all species are equally honest, but that public and cryptic condition are more tightly linked in species with less sibling competition and lower relatedness. Although we know of no reason this should be the case, the connection between cryptic and public condition is opaque. Given the strength of the effect of sibling competition in the predicted direction, it seems reasonable to assume that researchers in the field have captured a relevant aspect of offspring condition.
We assumed all measures of long-term condition were driving toward the same biological phenomenon, and so included all reported statistics in our analyses. Because test statistics were converted to a standardized scale, differences between the various measures of long-term should not influence the overall trends seen. The kind of measure of long-term need used to generate the original test statistic had no impact on effect size (P = 0.20).
• Rank: Relative condition within brood (e.g., body mass rank, hatching rank, dominance rank)
• Weight: Absolute measures of offspring size (e.g., body mass or skeletal size)
• Health: Offspring health indicators (e.g., experimental immune challenge, parasite load, carotenoid supplementation)
• Body condition: Measures such as body mass to skeletal size ratio and blood glucose levels
• Long-term changes to food intake: Changes in condition that are expected to affect all offspring within the brood over a period of at least 2 d (e.g., experimentally reduced or enlarged broods, with the assumption that offspring in larger broods receive less food per capita; or neck-taping which restricts food intake over multiple days)
Measures of Signaling Intensity Included in Analyses.
Many aspects of behavioral begging and structural signaling were reported in the literature, such as begging amplitude, duration, latency, likelihood, call structure and posture, mouth color, gape size, and UV reflectance of flanges. We assumed all measures were driving toward the same biological phenomenon and therefore included all reported statistics in our analyses. No measures including offspring height or proximity to parents or nest entrance were used as measures of begging intensity, because these combine both motivation and size effects (larger offspring can reach higher and may be better able to compete for access to feeding sites). Because test statistics were converted to a standardized scale, differences between the various measures of signal intensity should not influence the overall trends seen. The type of begging variable used to generate the original test statistic had no impact on effect size (P = 0.95).
• Probability: Whether or not signaling occurred
• Continuous intensity: Any continuous measure of signaling (such as vocalization duration, amplitude, rate, frequency, latency, playback; posture intensity; or color saturation or UV reflectance)
Measures of Sibling Competition.
Current siblings.
• Siblings present or absent: Two-level factor indicating whether or not species obligately lay only one egg per brood.
• Brood size: Continuous variable with the mean brood size for the study population or the species if population-level data were unavailable.
Future siblings.
• Number of future broods possible: Continuous variable calculated by multiplying adult life expectancy by the maximum number of broods parents can successfully rear each breeding season (species level).
Relatedness to nestmates or future broods.
• Full vs. half siblings likelihood: Two-level factor indicating whether or not there is a better than 50% chance that parents will breed together again in the following year. We calculated this by multiplying annual adult mortality and divorce rate and subtracting that value from 1 to generate the remarriage rate. The majority of divorce rate data were collected from a recent meta-analysis, which generated species-level mean divorce rates (51). Some species did not have exact divorce rate data available but did have qualitative descriptions of mating system (e.g., lifetime vs. seasonal monogamy). The 50% likelihood cutoff allowed us to increase our sample size by nine species: Casmerodius albus, Larus michahellis, Larus ridibundus, Luscinia svecica, Phoenicurus phoenicurus, Riparia riparia, Strix aluco, Sula nebouxii, and Toxostoma rufum. Divorce rates for these species were conservatively estimated as 0.05 for lifetime monogamy, 0.25 for multiple-season pair bonds, and 0.9 for no indication of pair bond persistence for calculations.
• Extrapair paternity (EPP): the percentage of broods containing at least one offspring sired by a different male. The percentage of EPP broods, rather than the percentage of EPP offspring, was used as the measure of promiscuity so we could include species that only lay one egg per breeding attempt.
Data Analysis: Life History Traits Used in Various Analyses.
Not all species had data on all life history traits. We ran separate models for each additional life history trait to take advantage of the greater sample size possible with fewer parameters (see Table 1 for sample size reduction with each model). Subsequent models controlled for life history traits analyzed using the larger dataset. For later models reported in the text, we used brood size, rather than the presence or absence of siblings, as the measure of current sibling competition to allow our models to converge properly. The six species that never experience sibling competition directly are also long-lived and have the potential to reproduce many times over their lives. This strong overlap between predictor variables makes it less likely that models will be able to detect the effect of the presence or absence of siblings when other terms are added to the model. The results of models using presence/absence as the measure of current sibling competition were qualitatively similar and can be found in Table 1.
Data Analysis: Example R Code.

Results and Discussion
We compared the strength of species’ correlation coefficients (effect sizes) between begging intensity and long-term need using phylogeny-based, linear mixed models, which were weighted by study sample size (34, 40, 41). A positive effect size indicates that offspring in worse condition beg more and therefore are more honestly signaling their need (37). Larger positive effect sizes suggest a clearer, more honest signal of need. In contrast, an effect size of zero indicates no correlation with begging intensity, such that begging provides no honest information about long-term need. A negative correlation indicates that offspring with less need beg more intensely.
Honesty and Sibling Rivalry.
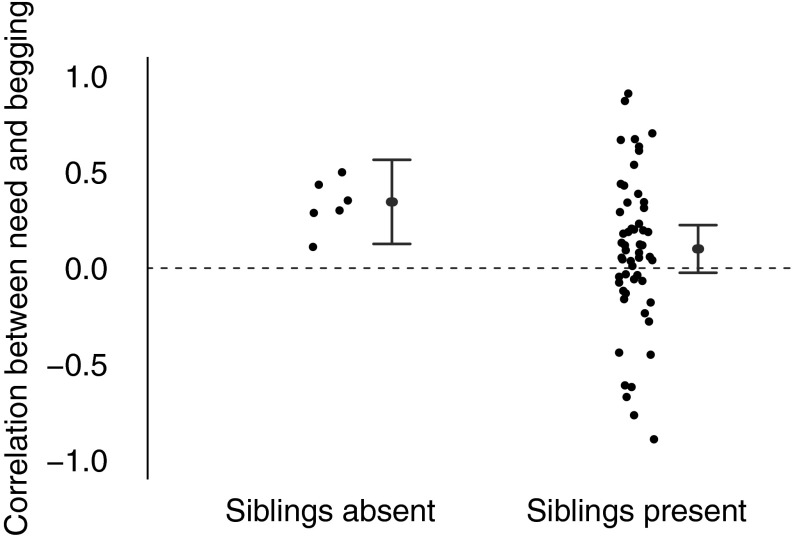
We first tested whether sibling conflict leads to less honest signaling. We estimated the effect of competition by dividing species into two categories: “siblings present” and “siblings always absent” (42). This categorization represents a true biological distinction, because some species of birds obligately lay only one egg per breeding attempt (42). Theory suggests that the difference between brood sizes of one and two will be substantially large, going from no to appreciable sibling competition (3, 4). Consistent with this, we found that the presence of siblings is correlated with less honest offspring: the relationship between need and begging is weaker in species with multiple offspring per brood (mean Wald statistic = 6.69, P = 0.0097, n = 60 species; Fig. 1 and Table 1, model 1). Offspring in worse condition beg more intensely in species where offspring never interact with siblings (95% CI of the correlation coefficient = 0.13–0.56, P = 0.0055) than in species where siblings compete for food (95% CI = −0.02 to 0.22, P = 0.075). Phylogeny did not influence how honest offspring are (Table S1).
Fig. 1.
The presence of siblings is associated with a reduction in offspring honesty. Data points represent species’ mean correlation coefficients of long-term need and begging intensity for species where parents rear only one (n = 6 species) or more than one offspring per brood (n = 54 species). Positive correlations indicate that offspring in worse condition beg more intensely, providing honest information about need. Gray lines represent the 95% CIs from the model, run on the full dataset, controlling for phylogeny and repeated measures. Species with siblings present have a weaker correlation between need and begging, suggesting less honest signaling of need (Wald = 6.69, P = 0.0097).
Table S1.
Results for all models: Random effects
| No. | Log likelihood | Total I2 | Random effects | Component | SE | N species | N study | N observations | Notes |
| Null (a) | 123.5 | 12.8% | Phylogeny | 0.0086 | 0.0182 | 60 | 108 | 336 | Full dataset |
| Species | 0.0005 | 0.0254 | |||||||
| Study | 0.1059 | 0.0265 | |||||||
| Residual | 0.0320 | 0.0077 | |||||||
| 1 | 124.3 | 12.0% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.1039 | 0.0224 | |||||||
| Residual | 0.0321 | 0.0077 | |||||||
| 2 | 121.7 | 12.0% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0056 | 0.0187 | |||||||
| Study | 0.0990 | 0.0277 | |||||||
| Residual | 0.0324 | 0.0078 | |||||||
| Null (b) | 117.5 | 12.7% | Phylogeny | 0.0060 | 0.0214 | 51 | 98 | 317 | Excluding 9 species missing data on adult mortality or the broods possible each breeding season |
| Species | 0.0048 | 0.0266 | |||||||
| Study | 0.1014 | 0.0294 | |||||||
| Residual | 0.0330 | 0.0080 | |||||||
| 3 | 115.0 | 11.6% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0986 | 0.0227 | |||||||
| Residual | 0.0330 | 0.0080 | |||||||
| 4 | 114.8 | 11.0% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0896 | 0.0213 | |||||||
| Residual | 0.0335 | 0.0080 | |||||||
| Null (c) | 119.0 | 12.4% | Phylogeny | 0.0104 | 0.0189 | 49 | 96 | 314 | Excluding 11 species missing data on adult mortality, the broods possible each breeding season, or mating system |
| Species | 0.0001 | 0.0245 | |||||||
| Study | 0.0984 | 0.0255 | |||||||
| Residual | 0.0328 | 0.0079 | |||||||
| 5 | 118.0 | 10.5% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0850 | 0.0205 | |||||||
| Residual | 0.0329 | 0.0079 | |||||||
| 6 | 117.6 | 10.1% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0787 | 0.0196 | |||||||
| Residual | 0.0333 | 0.0080 | |||||||
| Null (d) | 104.5 | 12.9% | Phylogeny | 0.0241 | 0.0274 | 31 | 68 | 230 | Excluding 29 species missing data on adult mortality, the broods possible each breeding season, mating system, or extra pair paternity |
| Species | 0.0000 | NA | |||||||
| Study | 0.0955 | 0.0283 | |||||||
| Residual | 0.0288 | 0.0079 | |||||||
| 7 | 95.8 | 11.3% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0985 | 0.0262 | |||||||
| Residual | 0.0285 | 0.0079 | |||||||
| 8 | 95.9 | 10.6% | Phylogeny | 0.0000 | NA | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0900 | 0.0244 | |||||||
| Residual | 0.0289 | 0.0079 |
Mean results of 500 ASReml linear mixed models. Models were weighted by study sample size (the number of broods used to calculate the original test statistic). Models are grouped by the dataset used for analysis, and the notes column describes the loss in sample size due to missing life history data. Sample error variance was constrained to 1. Total heterogeneity (I2) is a measure of the proportion of observed variance due to true differences in effect sizes in the null model (34). I2 values of 25%, 50%, and 75% indicate low, moderate, and high levels ratios of signal to noise (34). This measure does not take effect size dispersion into account (34). Model numbers correspond to Table 1. NA indicates that models could not estimate the standard error of this variance component.
We then examined whether increasing intensity of sibling competition led to a corresponding decrease in honesty using data on average brood size. The intensity of within-family conflict may increase as more offspring compete for food in bigger broods, and this conflict could favor exaggerated signals of need (13, 17, 18). For instance, offspring sharing a nest with ten siblings may experience more intense competition than those with only one sibling. It has also been suggested that parent-offspring communication can be affected in more complicated ways in larger broods, with only offspring in the most need selected to invest in costly signaling (18, 43, 44). We found that the strength of the correlation between need and begging was lower in species with larger brood sizes: offspring with more siblings are less honest with their parents about their level of need (mean Wald statistic = 4.57, P = 0.033; Table 1, model 2).
We might expect to see similar patterns within species that exhibit consistent variation in the intensity of sibling competition if individuals can assess the relative intensity of competition from siblings in their environment. Consistent with this, offspring of several species have been found to adjust their level of begging facultatively: American robins (21), great tits (30), tree swallows (27–29), and yellow-headed blackbirds (24, 45) all escalate their begging intensity when competing against more or needier nestmates. In contrast, barn swallows (33) and black-headed gulls (31) show the opposite pattern, reducing their begging intensity when faced with more or needier nestmates, and European starling offspring do not change how they beg at all based on the begging of their nestmates (25, 26). These discrepancies may be due to biological differences between species, particularly whether parents or offspring control offspring food distribution (18, 25). For instance, in black-headed gulls, parents regurgitate food to the nest floor, rather than distributing it, and so offspring completely determine food distribution (31). Because offspring control food distribution, they do not fit the assumptions of standard signal-of-need models; instead, theory predicts that offspring in better condition are selected to decrease, rather than increase, begging when their needier siblings beg more (18).
Honesty and Future Siblings.
Parental investment is not only shared within a brood, but also between broods produced throughout a parent’s lifetime. Therefore, unborn siblings, which may potentially exist in the future, could potentially impact the honesty of signaling between current offspring and their parents. If parents are saving resources for future breeding attempts, then this could make them less responsive to their current brood’s begging, and hence select for their offspring to exaggerate their signals (3, 6, 46, 47). We estimated conflict with future siblings from the relative number of potential future breeding attempts: adult life expectancy multiplied by the maximum number of successful broods parents can raise each year. These data were available for 51 of the 60 species in our dataset, and we controlled for current sibling competition (brood size) in these analyses.
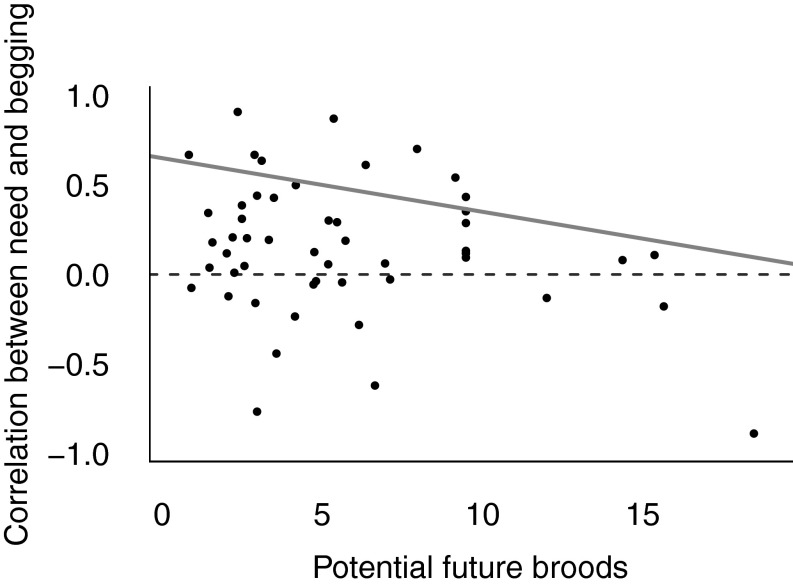
As we predicted, offspring were less honest with parents in species where offspring compete against more future siblings. The correlation between begging and need was significantly lower in species where parents can rear more broods over their lifetime (mean Wald statistic = 7.22, P = 0.0087; Fig. 2 and Table 1, model 4), and species with larger broods (mean Wald statistic = 11.87, P = 0.0009). Offspring compete with both their current and future siblings for resources by manipulating parental behavior through their begging. Overall, our analysis suggests that the honesty of offspring signaling varies in response to how parental investment is distributed over the parents’ lifetime.
Fig. 2.
Conflict with future siblings is associated with a reduction in offspring honesty. Data points show species’ mean correlation coefficient of long-term need and begging intensity. The number of potential future broods is the adult life expectancy multiplied by the number of successful broods that can be reared each breeding season (n = 51 species). The gray line is the regression coefficient from the model, run on the full dataset, controlling for phylogeny and repeated measures. Positive correlations indicate that offspring in worse condition beg more intensely, providing honest information about need. The dashed line at zero indicates no relationship between condition and begging. The correlation between need and begging is weaker in species where parents can potentially produce more broods over their lifetimes (Wald = 7.22, P = 0.0087), suggesting that future reproduction selects for less honest signaling of need.
We might also expect to see individuals adjusting their behavior conditionally in response to variation in the likelihood of competition with future siblings. For example, offspring born to younger parents, with greater reproductive potential, might make less reliable signals than offspring that represent a parents’ terminal investment (48). Previous studies within species that produce multiple broods in a season have found such patterns. For example, European starlings can lay two broods per year, and offspring only signal need honestly if they are in the second brood, when parents cannot lay another brood (49). Hihi bird parents’ response to offspring signals in their first brood decreases if their likelihood of breeding again is experimentally increased (50).
Honesty and Relatedness to Siblings.
When relatedness is lower between offspring sharing the same parent or parents, there will be stronger selection to monopolize parental investment and weaker selection on higher-quality offspring to show restraint in begging for food (3–7, 13, 18–20, 47). The survival of unrelated nestmates or brood fails to enhance the inclusive fitness of a focal offspring. Consequently, we predict that decreased relatedness between offspring will lead to less honest signaling of need. We examined the consequences of two factors that can reduce relatedness within families: (i) parents breeding with different partners, such that all future broods can only contain half (genetic relatedness, r = 0.25), rather than full (r = 0.50), siblings; and (ii) parents being promiscuous, such that some brood-mates are only half siblings.
We determined the likelihood of parents changing breeding partners by combining the rates of mortality and divorce. Divorce is the rate at which pairs mate with different partners when both original partners are still present in the population (51). In nine species where data on divorce rates were unavailable, we used mating system (lifetime vs. seasonal monogamy) to estimate the likelihood that parents will breed together again. Because the divorce rate was estimated in some cases, we binned species according to whether pairs had a higher or lower than 50% chance of breeding together again. Data on the likelihood of breeding together again were available for 49 of the 60 species in our original data set, and so we carried out analyses on this subset, controlling for the number of current and future siblings.
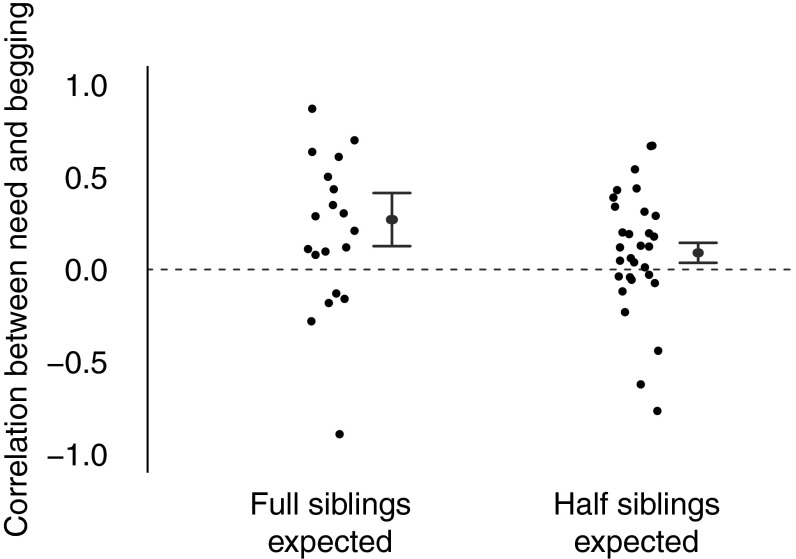
Offspring whose parents are unlikely to breed together again, through either death or divorce, are significantly less honest with their parents (mean Wald statistic = 5.98, P = 0.016; Fig. 3 and Table 1, model 6). The number of potential future siblings predicts species’ honesty in this model as well (Wald statistic = 13.09, P = 0.0005), but brood size was no longer significant, perhaps due to the reduction in sample size (Wald statistic = 3.07, P = 0.083). Overall, this suggests that an increased conflict of interest, due to lower relatedness between siblings, favors less honest signaling.
Fig. 3.
Parental divorce and death is associated with a reduction in offspring honesty. Data points represent species’ mean correlation coefficients of long-term need and begging intensity for species. We divided species by whether there is a higher or lower than 50% chance that parents will breed together again in the next year, based on survival and divorce rates (n = 19 species where full siblings are expected; n = 30 species where half siblings are expected). Positive correlations indicate that offspring in worse condition beg more intensely, providing honest information about need. Gray lines represent the 95% CIs from our analyses. The correlation between need and begging is weaker in species with higher rates of divorce and lower rates of survival, where half siblings are expected (Wald = 5.98, P = 0.016), suggesting less honest signaling of need.
We next looked at whether variation in within-brood relatedness due to promiscuity also impacts offspring honesty (3–6, 18–20). We used the percentage of broods with at least one extrapair offspring as our measure of promiscuity. These data were available for only 31 of the species in our dataset, and so we ran our analyses on this subset, controlling for the number of current and future siblings and the likelihood that parents will breed together again. We did not find an effect of extrapair paternity (mean Wald statistic = 0.27, P = 0.61; Table 1, model 8). Of the control variables, only the number of future broods remained significant in this model, again potentially because we are examining a much smaller data set (mean Wald statistic = 4.94, P = 0.038).
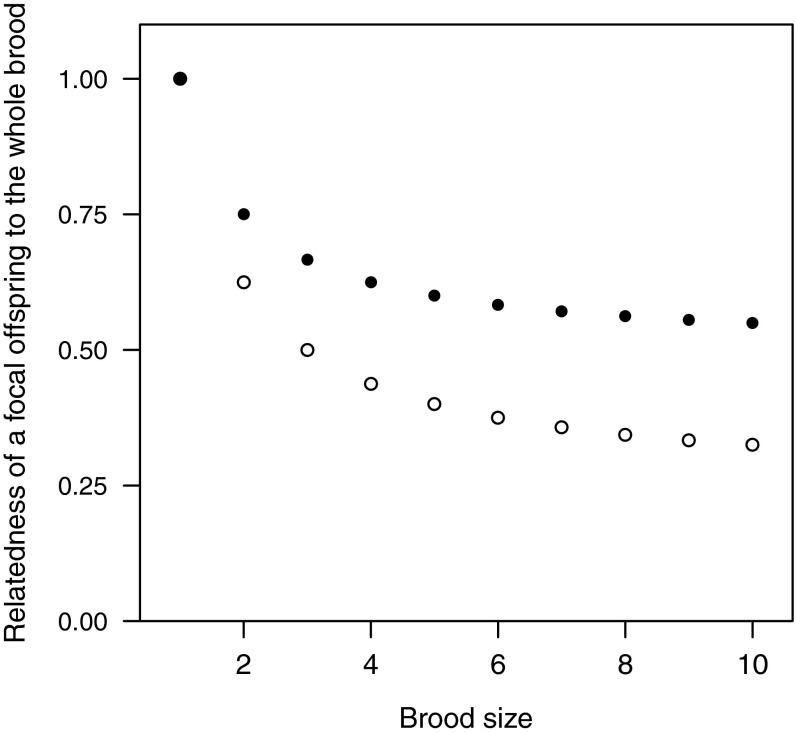
A possible explanation for a lack of a significant influence of promiscuity is that it selects relatively weakly on honesty. The likelihood that parents will breed together again could be a more consistent and reliable predictor of relatedness: although extrapair paternity may reduce relatedness to a proportion of nestmates, divorce or death means that all future offspring produced by a mother or a father must be half siblings (r ≤ 0.25). However, an experimental study on barn swallows found that offspring were less honest about their hunger when their nestmates were nonrelatives (52), and a previous comparative study on 11 species found that absolute begging intensity was significantly correlated with promiscuity, as would be expected if lower relatedness led to escalation in begging (22). This discrepancy may be because we assessed changes in honesty, rather than changes in absolute begging levels. Another possible reason we did not see an effect of promiscuity is that the influence of sibling number could obscure the influence of relatedness to those siblings. Specifically, species with larger broods may be less honest, irrespective of how many of those nestmates are half siblings (Fig. S2). This effect of brood size is a specific case of the more general point that local competition can reduce the importance of relatedness between interacting individuals (53).
Fig. S2.
Relatedness to the whole brood decreases as brood size increases. The average relatedness of a focal offspring to the whole brood decreases with each additional sibling, until reaching an asymptote r = 0.5 for full siblings (●) and r = 0.25 for half siblings (○). Whole brood relatedness is calculated by averaging a focal offspring’s relatedness to itself and each of its siblings. For example, r = 0.75 for two full siblings (1 + 0.5)/2, and r = 0.5 for three half siblings: (1 + 0.25 + 0.25)/3. Even without any extrapair paternity, the relatedness of a focal chick to the whole brood decreases rapidly as brood size increases: average relatedness to the whole brood is 75% with one full sibling, drops to 67% with two full siblings, and to 55% with ten full siblings.
Ecology and Life History.
Our above analyses explored the link between life history variables and signal honesty. Previous analyses have suggested that environmental quality and environmental predictability can also influence the extent to which offspring signal need (15). If these environmental variables covary with sibling number or relatedness, our results could have been driven purely by environmental variation. We tested whether environmental quality and predictability confounded our results in two ways: by assessing their relationship with life history traits (Table S2) and by comparing models with and without these variables (Table S3). We found no relationships between life history traits and environmental quality/predictability in our dataset, except for the presence/absence of siblings (Table S2). The obligate absence of siblings was nested within the “predictable” level of environmental predictability, and so this relationship was unavoidable. Models that included both environmental predictability and the presence/absence of siblings had somewhat variable results, as expected if these factors are partially collinear (models 1, 3, 5 and 7; Table S3). However, when we used brood size, rather than the presence/absence of siblings, the coefficients of fixed effects were equivalent across models, and both environmental factors and life history traits generally remained significant (models 2, 4, 6 and 8; Table S3). This result suggests that both life history and ecology influence parent-offspring communication.
Table S2.
Relationships between environmental and life history variables
| Life history variables | Environmental variables | N species | |
| Predictability | Quality | ||
| Siblings y/n | −33.07 to −2.56 | −4.24 to 23.28 | 60 |
| Brood size | −0.76 to 0.47 | −0.28 to 0.59 | |
| Siblings y/n | −19.37 to −9.42 | −2.18 to 9.03 | 51 |
| Brood size | −0.72 to 0.56 | −0.31 to 0.52 | |
| No. future broods possible | −0.02 to 0.02 | −0.01 to 0.01 | |
| Siblings y/n | −33.19 to −13.06 | −3.69 to 8.12 | 49 |
| Brood size | −0.89 to 0.41 | −0.30 to 0.53 | |
| No. future broods possible | −0.02 to 0.02 | −0.01 to 0.01 | |
| Full vs. half siblings likelihood | −5.50 to 2.81 | −3.54 to 2.22 | |
| Siblings y/n | −61.65 to −5.45 | −6.66 to 25.08 | 33 |
| Brood size | −0.91 to 0.74 | −0.64 to 0.43 | |
| No. future broods possible | −0.03 to 0.03 | −0.01 to 0.01 | |
| Full vs. half siblings likelihood | −9.01 to 37.59 | −80.76 to 7.21 | |
| Promiscuity | −0.03 to 0.03 | −0.01 to 0.01 | |
Table reports the 95% CI for the relationship of environmental variables on each of the life history variables used with each subset of the overall dataset (n = 60 species corresponds to models 1 and 2; n = 51 species corresponds to models 3 and 4; n = 49 species corresponds to models 5 and 6; n = 33 species corresponds to models 8 and 7). Results are from MCMCglmm models, controlling for phylogeny. MCMCglmm was used instead of ASReml because ASReml models did not converge properly when accounting for phylogenetic variance in some models with smaller sample sizes. Cells in bold indicate the relationship is significant (the 95% CI does not include 0).
Table S3.
Effect of adding environmental variables to models
| Model no. | Fixed effects | Variables included in model | N | ||
| Only life history | Only environmental | All variables | |||
| Model 1 | Intercept | Coef = 0.10 ± 0.04 | Coef = −0.09 ± 0.09 | Coef = −0.09 ± 0.09 | 60 species, 108 studies, 336 effect sizes |
| F1,89.9 = 13.57, P = 0.0005 | F1,91.0 = 13.23, P = 0.0002 | F1,88.9 = 13.57, P = 0.0005 | |||
| Environmental predictability | Coef = 0.20 ± 0.08 | Coef = 0.14 ± 0.09 | |||
| F1,91.7 = 6.73, P = 0.011* | F1,92.7 = 2.66, P = 0.11 | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,191.8 = 7.13, P = 0.0010** | F2,192.2 = 7.14, P = 0.0010** | ||||
| Siblings present y/n | Coef = −0.28 ± 0.11 | Coef = −0.20 ± 0.12 | |||
| F1,71.8 = 6.69, P = 0.0097** | F1,73.7 = 2.86, P = 0.095. | ||||
| Model 2 | Intercept | Coef = 0.31 ± 0.09 | Coef = −0.09 ± 0.09 | Coef = 0.05 ± 0.12 | |
| F1,34.1 = 12.61, P = 0.0002 | F1,91.0 = 13.23, P = 0.0002 | F1,89.6 = 13.45, P = 0.0004 | |||
| Environmental predictability | Coef = 0.20 ± 0.08 | Coef = 0.18 ± 0.08 | |||
| F1,91.7 = 6.73, P = 0.011* | F1,92.6 = 4.86, P = 0.03* | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,191.8 = 7.13, P = 0.0010** | F2,192.2 = 7.18, P = 0.0010** | ||||
| Brood size | Coef = −0.04 ± 0.02 | Coef = −0.04 ± 0.02 | |||
| F1,31.0 = 4.57, P = 0.033* | F1,96.1 = 3.07, P = 0.083. | ||||
| Model 3 | Intercept | Coef = 0.21 ± 0.07 | Coef = −0.06 ± 0.09 | Coef = 0.03 ± 0.11 | 51 species, 98 studies, 317 effect sizes |
| F1,79.1 = 9.14, P = 0.0034 | F1,81.7 = 15.70, P = 0.0002 | F1,78.6 = 9.04, P = 0.0036 | |||
| Environmental predictability | Coef = 0.19 ± 0.08 | Coef = 0.09 ± 0.09 | |||
| F1,81.9 = 5.26, P = 0.024* | F1,81.2 = 0.92, P = 0.34 | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,183.8 = 6.93, P = 0.0013** | F2,182.4 = 6.86, P = 0.0013** | ||||
| Siblings present y/n | Coef = −0.33 ± 0.11 | Coef = -0.27 ± 0.13 | |||
| F1,67.0 = 8.45, P = 0.0050** | F1,69.3 = 4.14, P = 0.046* | ||||
| No. future broods possible | Coef = -0.02 ± 0.01 | Coef = −0.02 ± 0.01 | |||
| F1,87.9 = 3.13, P = 0.080. | F1,89.9 = 2.03, P = 0.16 | ||||
| Model 4 | Intercept | Coef = 0.65 ± 0.14 | Coef = −0.06 ± 0.09 | Coef = 0.39 ± 0.18 | |
| F1,78.1 = 20.21, P = 0.0000 | F1,81.7 = 15.70, P = 0.0002 | F1,80.6 = 19.77, P = 0.0000 | |||
| Environmental predictability | Coef = 0.19 ± 0.08 | Coef = 0.12 ± 0.08 | |||
| F1,81.9 = 5.26, P = 0.024* | F1,80.7 = 2.02, P = 0.16 | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,183.8 = 6.93, P = 0.0013** | F2,184.3 = 6.86, P = 0.0013** | ||||
| Brood size | Coef = −0.08 ± 0.02 | Coef = −0.07 ± 0.03 | |||
| F1,82.2 = 11.87, P = 0.0009*** | F1,85.1 = 8.2, P = 0.005** | ||||
| No. future broods possible | Coef = −0.03 ± 0.01 | Coef = −0.03 ± 0.01 | |||
| F1,83.5 = 7.22, P = 0.0087** | F1,85.5 = 5.13, P = 0.026* | ||||
| Model 5 | Intercept | Coef = 0.64 ± 0.18 | Coef = −0.06 ± 0.09 | Coef = 0.46 ± 0.20 | 49 species, 96 studies, 314 effect sizes |
| F1,76.5 = 8.58, P = 0.0045 | F1,80.7 = 14.47, P = 0.0003 | F1,76.1 = 8.42, P = 0.0049 | |||
| Environmental predictability | Coef = 0.17 ± 0.08 | Coef = 0.07 ± 0.09 | |||
| F1,81.0 = 4.44, P = 0.038* | F1,79.1 = 0.63, P = 0.43 | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,184.5 = 6.94, P = 0.0012** | F2,185.3 = 6.79, P = 0.0014** | ||||
| Siblings present y/n | Coef = −0.11 ± 0.14 | Coef = −0.07 ± 0.15 | |||
| F1,84.2 = 0.42, P = 0.42 | F1,83.7 = 0.19, P = 0.67 | ||||
| No. future broods possible | Coef = −0.05 ± 0.01 | Coef = −0.04 ± 0.02 | |||
| F1,95.4 = 9.39, P = 0.0028** | F1,96.4 = 7.87, P = 0.0061** | ||||
| Full vs. half siblings likelihood | Coef = −0.39 ± 0.15 | Coef = −0.39 ± 0.15 | |||
| F1,112.5 = 6.94, P = 0.0096** | F1,111.5 = 6.76, P = 0.011* | ||||
| Model 6 | Intercept | Coef = 0.83 ± 0.16 | Coef = −0.06 ± 0.09 | Coef = 0.62 ± 0.20 | |
| F1,76.5 = 21.95, P = 0.0000 | F1,80.7 = 14.47, P = 0.0003 | F1,77.7 = 21.23, P = 0.0000 | |||
| Environmental predictability | Coef = 0.17 ± 0.08 | Coef = 0.06 ± 0.08 | |||
| F1,81.0 = 4.44, P = 0.038* | F1,78.9 = 0.62, P = 0.43 | ||||
| Environmental quality | Coef = 0.32 ± 0.09 | Coef = 0.32 ± 0.09 | |||
| F2,184.5 = 6.94, P = 0.0012** | F2,186.8 = 6.78, P = 0.0014** | ||||
| Brood size | Coef = −0.05 ± 0.03 | Coef = -0.04 ± 0.03 | |||
| F1,87.1 = 3.07, P = 0.083. | F1,87.1 = 2.51, P = 0.12 | ||||
| No. future broods possible | Coef = −0.05 ± 0.01 | Coef = −0.05 ± 0.01 | |||
| F1,86.4 = 13.09, P = 0.00050*** | F1,89.7 = 10.15, P = 0.0020** | ||||
| Full vs. half siblings likelihood | Coef = −0.33 ± 0.14 | Coef = −0.31 ± 0.14 | |||
| F1,89.6 = 5.98, P = 0.016* | F1,90.3 = 5.14, P = 0.026* | ||||
| Model 7 | Intercept | Coef = 0.65 ± 0.23 | Coef = 0.08 ± 0.11 | Coef = 0.56 ± 0.25 | 31 species, 68 studies, 230 effect sizes |
| F1,55.6 = 4.93, P = 0.030 | F1,2.7 = 9.24, P = 0.0000 | F1,54.8 = 4.32, P = 0.045 | |||
| Environmental predictability | Coef = 0.13 ± 0.10 | Coef = 0.07 ± 0.11 | |||
| F1,33.1 = 1.43, P = 0.24 | F1,58.1 = 4.73, P = 0.036* | ||||
| Environmental quality | Coef = 0.22 ± 0.09 | Coef = 0.22 ± 0.09 | |||
| F2,128.2 = 2.95, P = 0.056. | F2,127.4 = 3.09, P = 0.049* | ||||
| Siblings present y/n | Coef = 0.00 ± 0.22 | Coef = 0.06 ± 0.24 | |||
| F1,60.5 = 0, P = 1 | F1,60.2 = 1.11, P = 0.30 | ||||
| No. future broods possible | Coef = -0.03 ± 0.02 | Coef = −0.03 ± 0.02 | |||
| F1,58.4 = 3.10, P = 0.083. | F1,60.0 = 0.08, P = 0.79 | ||||
| Full vs. half siblings likelihood | Coef = -0.43 ± 0.22 | Coef = −0.44 ± 0.22 | |||
| F1,76.0 = 3.77, P = 0.056. | F1,74.9 = 1.07, P = 0.31 | ||||
| Promiscuity (% EPPbr) | Coef = 0.00 ± 0.01 | Coef = 0.00 ± 0.01 | |||
| F1,58.5 = 0.04, P = 0.84 | F1,57.9 = 1.22, P = 0.28 | ||||
| Model 8 | Intercept | Coef = 0.81 ± 0.19 | Coef = 0.08 ± 0.11 | Coef = 0.68 ± 0.23 | |
| F1,55.9 = 17.26, P = 0.0001 | F1,2.7 = 9.24, P = 0.0000 | F1,55.4 = 18.95, P = 0.0001 | |||
| Environmental predictability | Coef = 0.13 ± 0.10 | Coef = 0.05 ± 0.10 | |||
| F1,33.1 = 1.43, P = 0.24 | F1,57.5 = 4.73, P = 0.035* | ||||
| Environmental quality | Coef = 0.22 ± 0.09 | Coef = 0.22 ± 0.09 | |||
| F2,128.2 = 2.95, P = 0.056. | F2,128.0 = 2.94, P = 0.057. | ||||
| Brood size | Coef −0.07 ± 0.03 | Coef= −0.06 ± 0.03 | |||
| F1,66.2 = 3.87, P = 0.053. | F1,64.6 = 3.29, P = 0.076. | ||||
| No. future broods possible | Coef = −0.04 ± 0.02 | Coef = −0.04 ± 0.02 | |||
| F1,58.3 = 4.94, P = 0.038* | F1,60.5 = 0.03, P = 0.87 | ||||
| Full vs. half siblings likelihood | Coef = -0.19 ± 0.18 | Coef = −0.18 ± 0.19 | |||
| F1,67.1 = 1.00, P = 0.32 | F1,67.8 = 2.45, P = 0.12 | ||||
| Promiscuity (% EPPbr) | Coef = 0.00 ± 0.01 | Coef = 0.00 ± 0.01 | |||
| F1,58.5 = 0.27, P = 0.61 | F1,57.5 = 1.24, P = 0.27 | ||||
Coefficient, Wald statistic, and P value for fixed effects for three sets of models: (i) a model with life history variables only (models correspond to Table 1); (ii) a model with environmental variables only; and (iii) a model with all fixed effects included. Results are the mean from 500 ASReml linear mixed models. Models controlled for phylogeny, repeated measures, and were weighted by study sample size (the number of broods used to calculate the original test statistic). Fixed effects in bold are significant at the P < 0.05 level, and in italics at the P < 0.10 level.
Signaling Theory and Empirical Data.
The hypotheses tested here are based on existing theory that examines the conditions required for the evolutionary stability of honest signaling of need (3–6, 11, 13, 16–20). These previous models tend to predict that honest signaling is either stable or not, rather than a continuum of more gradual shifts between these extremes (43). In contrast, the empirical data show much more gradual variation, with a wide range of correlation coefficients between condition and signaling (Figs. 1–3). Our extrapolation is to take predictions for when stable signaling is or is not favored (extremes) and use them to make predictions about how honest signaling should be (variation across intermediates). This mismatch between the predictions of theoretical models (extremes), and the patterns observed empirically (intermediates) has previously been analyzed in the context of sex allocation. Sex allocation represents a much simpler game theoretic case, where it was found that by increasing biological complexity, models were able to move from the prediction of extremes (all or nothing) to the prediction of more gradual and realistic variation (54, 55). We suggest that increased biological complexity could similarly lead to the prediction of more gradual shifts in signaling models, and further theory is clearly required to examine the evolutionary stability of intermediate levels of honesty (16). Another complication is that many signaling models predict multiple possible equilibria (56) without distinguishing which of the possible equilibria natural selection will lead to (57). There is clearly also a need for signaling theory that can be better linked to the kind of variation in signaling systems that is observed in empirical studies.
Another complication with linking to data to theory is that empirical estimates of need could be unreliable or fail to capture character states in a way that is relevant to theory (7). These errors would, however, be most likely to obscure any underlying biological pattern, and it is unlikely that such error would have produced the patterns we observe. Indeed, by finding patterns in the predicted direction, our results suggest that empirical researchers have successfully managed to capture biologically relevant aspects of condition.
Signals of Quality.
Offspring can solicit food using either signals of need or quality, and the same life history traits may influence the evolution of both kinds of signals (1, 2). Our predictions were derived from signal-of-need models, where parents are assumed to have enough food to feed all their offspring and are selected to feed the offspring with the greatest need (1–8). In contrast, when parents do not have enough food to feed all their offspring, they are expected to pay attention to signals of quality and to feed the highest quality offspring (7–14). Consequently, we predict the opposite patterns with signals of quality as those we observed with signals of need. For example, when relatedness is higher, chicks in better condition should reduce their signaling to allow related nestmates in worse condition to be fed. This restraint could create a situation where chicks in worse condition give more intense signals—increasing the correlation between need and signals but decreasing the correlation between quality and signals.
To determine whether signals of quality respond to life history traits, we repeated our above analyses, but examining structural signals, such as mouth size or color, which are more likely to represent measures of quality (15). We could not find an effect of any of our life history traits on structural signals, whether or not environmental factors were included as covariates (Tables S4 and S5). Our null result could be because the evolution of structural signaling is influenced by other factors or because our sample size was too low to identify true effects: we only had data on 18 species and had no data on species without siblings present. More empirical studies on structural signals could help reveal the selection pressures on such signals. Furthermore, theory that explicitly models offspring signals of quality, without the assumption that all offspring will survive, is necessary to generate testable predictions about which life history traits could influence the evolution of signals of quality.
Table S4.
Results for all signal-of-quality models: fixed effects
| Model no. | Log Lik | Fixed effects | df | Wald statistic | P value | N species | N study | N observations | Notes |
| 9 | 107.5 | Intercept | 1, 16.8 | 4.23 | 0.056 | 18 | 33 | 140 | Full dataset |
| Brood size | 1, 18.8 | 1.06 | 0.32 | ||||||
| 10 | 107.0 | Intercept | 1, 13.9 | 2.67 | 0.12 | ||||
| Brood size | 1, 17.0 | 0.18 | 0.68 | ||||||
| Predictability | 1, 11.3 | 0.69 | 0.42 | ||||||
| Quality | 2, 133.0 | 7.61 | 0.00075 | ||||||
| Predictability * Quality | 2, 133.0 | 3.74 | 0.026 | ||||||
| 11 | 84.0 | Intercept | 1, 12.4 | 2.24 | 0.16 | 16 | 30 | 115 | Excluding 2 species missing data on adult mortality or the broods possible each breeding season |
| Brood size | 1, 14.7 | 0.88 | 0.36 | ||||||
| No. of future broods possible | 1, 10.1 | 0.11 | 0.75 | ||||||
| 12 | 86.3 | Intercept | 1, 10.5 | 1.83 | 0.20 | ||||
| Brood size | 1, 12.1 | 0.38 | 0.55 | ||||||
| No. of future broods possible | 1, 11.4 | 0.21 | 0.66 | ||||||
| Predictability | 1, 9.6 | 0.03 | 0.88 | ||||||
| Quality | 2, 107.0 | 10.18 | 0.00009 | ||||||
| 13 | 75.0 | Intercept | 1, 5.2 | 0.78 | 0.42 | 10 | 20 | 80 | Excluding 8 species missing data on adult mortality, the broods possible each breeding season, or mating system |
| Brood size | 1, 5.7 | 0.00 | 0.97 | ||||||
| No. of future broods possible | 1, 4.3 | 0.24 | 0.65 | ||||||
| Full vs. half siblings likelihood | 1, 4.3 | 0.46 | 0.53 | ||||||
| 14 | 73.6 | Intercept | 1, 4.0 | 0.99 | 0.38 | ||||
| Brood size | 1, 4.3 | 0.10 | 0.77 | ||||||
| No. of future broods possible | 1, 3.7 | 0.13 | 0.74 | ||||||
| Full vs. half siblings likelihood | 1, 3.4 | 0.06 | 0.82 | ||||||
| Predictability | 1, 3.6 | 0.16 | 0.72 | ||||||
| Quality | 1, 73.0 | 4.83 | 0.011 |
Mean results of 500 ASReml linear mixed models. Models controlled for phylogeny, repeated measures on studies, and species, and were weighted by study sample size (the number of broods used to calculate the original test statistic). Fixed effects in bold are significant at the P < 0.05 level and in italics at the P < 0.10 level. Models are grouped by the dataset used for analysis, and the notes column describes the loss in sample size due to missing life history data. Models did not converge when promiscuity was included as a fixed effect.
Table S5.
Results for all signal-of-quality models: random effects
| No. | Log likelihood | Total I2 | Random effects | Component | SE | N species | N study | N observations | Notes |
| Null (a) | 110.4 | 5.87% | Phylogeny | 0.0143 | 0.0329 | 18 | 33 | 140 | Full dataset |
| Species | 0.0197 | 0.0259 | |||||||
| Study | 0.0118 | 0.0105 | |||||||
| Residual | 0.0166 | 0.0068 | |||||||
| 9 | 107.5 | 5.79% | Phylogeny | 0.0149 | 0.0267 | ||||
| Species | 0.0147 | 0.0215 | |||||||
| Study | 0.0146 | 0.0116 | |||||||
| Residual | 0.0162 | 0.0067 | |||||||
| Null (b) | 90.2 | 13.1% | Phylogeny | 0.0980 | 0.0354 | 16 | 30 | 115 | Excluding 2 species missing data on adult mortality or the broods possible each breeding season |
| Species | 0.0222 | 0.0285 | |||||||
| Study | 0.0106 | 0.0108 | |||||||
| Residual | 0.0197 | 0.0078 | |||||||
| 11 | 84.0 | 6.04% | Phylogeny | 0.0110 | 0.0320 | ||||
| Species | 0.0213 | 0.0259 | |||||||
| Study | 0.0128 | 0.0118 | |||||||
| Residual | 0.0192 | 0.0077 | |||||||
| Null (c) | 82.2 | 4.74% | Phylogeny | 0.0046 | 0.1095 | 10 | 20 | 80 | Excluding 8 species missing data on adult mortality, the broods possible each breeding season, or mating system |
| Species | 0.0450 | 0.0460 | |||||||
| Study | 0.0002 | 0.0035 | |||||||
| Residual | 0.0000 | NA | |||||||
| 13 | 75.0 | 9.07% | Phylogeny | 0.0988 | 0.0769 | ||||
| Species | 0.0000 | NA | |||||||
| Study | 0.0009 | 0.0044 | |||||||
| Residual | 0.0000 | NA |
Mean results of 500 ASReml linear mixed models. Models were weighted by study sample size (the number of broods used to calculate the original test statistic). Models are grouped by the dataset used for analysis, and the notes column describes the loss in sample size due to missing life history data. Sample error variance was constrained to 1. Total heterogeneity (I2) is a measure of the proportion of observed variance due to true differences in effect sizes in the null model (34). I2 values of 25%, 50%, and 75% indicate low, moderate, and high levels ratios of signal to noise (34). This measure does not take effect size dispersion into account (34). Model numbers correspond to Table 1. NA indicates that models could not estimate the standard error of this variance component.
Conclusion
We found support for the prediction that increased conflict between siblings disfavors honest signaling of need from offspring to their parents (Fig. 4). Specifically, offspring are less likely to honestly signal their need when they have more siblings, when their parents are more likely to breed again, and when they are less related to their future siblings, due to parental divorce or death. These results suggest that siblings that are not even born yet, and, indeed, may never be born, cast a competitive shadow back in time, which selects for exaggerating need to parents. The logical next step would be to explore how parents’ response to begging is affected by the same life history factors (6, 49, 50, 58). Longevity and lifetime fecundity have already been shown to influence other aspects of parental care, such as how parents respond to nest predators, with species that have less potential for future reproduction engaging in riskier defense behavior (59). The results of this study suggest that parents within a stable pair-bond may be selected to be the most responsive parents, especially if they produce few offspring.
Fig. 4.
Family conflict is associated with a reduction in the honesty of offspring signals of need. Offspring in species with siblings present are less honest when signaling need (Wald statistic = 6.69, P = 0.0097). The probability that parents will breed again is also associated with more dishonest offspring, as the current brood competes against future broods for parental investment (Wald = 7.22, P = 0.0087). Finally, offspring are less honest in species where parents are likely to divorce or die before breeding again, and all future siblings will be half siblings (Wald = 5.98, P = 0.016).
Materials and Methods
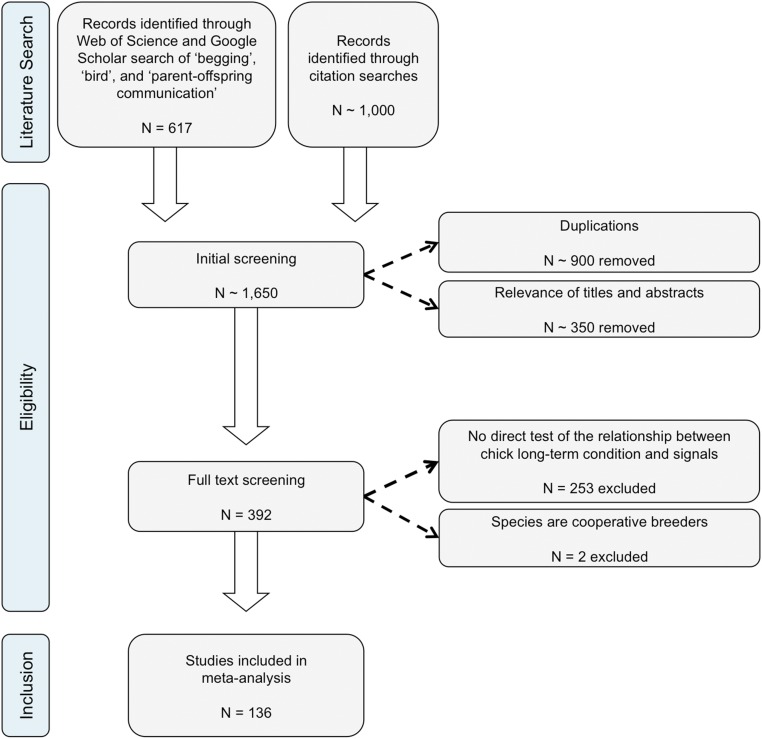
We searched the literature for data relating to the effect of offspring condition on begging intensity, leading to a dataset of 336 test statistics from 108 studies on 60 species of birds (see Fig. S3 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of study selection, Dataset S1 for the data included in the meta-analysis and life history references, and Dataset S2 for a list of excluded studies). We included any reported measures of behavioral begging, such as vocalizations and postures. We also collected data on the effect of condition on structural signals, leading to a dataset of 140 effect sizes from 33 studies on 18 species. We included any reported measures of structural signals, such as mouth color or UV reflectance. We calculated the correlation coefficient between condition and signals to generate a standardized effect size across studies and species (34–37). This coefficient varies between ±1, with positive values indicating that offspring in worse condition signal more, and negative values indicating that offspring in better condition signal more. We assumed offspring were dishonest if there was no correlation between condition and signal intensity. We used health, body condition, rank within the brood, and experimental manipulations that affected food intake over multiple days as proxies for long-term condition (SI Materials and Methods).
Fig. S3.
PRISMA flowchart of search results and the study selection process. See Dataset S2 for a list of papers excluded from the analysis. The final dataset for begging analyses contained 336 effect sizes from 108 studies on 60 species of birds. The final dataset for structural signal analyses contained 140 effect sizes from 33 studies on 18 species of birds. Overall, we found data in 136 studies on 72 species.
We analyzed differences in honesty across species using ASReml linear mixed models in R that were weighted by sample size and controlled for phylogeny and repeated measures on studies and species (40, 60). We averaged the results of 500 models using different trees to account for uncertainty in the phylogeny, which we obtained from www.Bird.tree.org, with both Ericson and Hackett backbones (61). Results relating to random effects can be found in Table S1. We compared honesty to a variety of relevant life history traits related to sibling completion and relatedness. We estimated current sibling competition in two ways: (i) presence or obligate absence of siblings and (ii) mean brood size of the study population, or species if population data were unavailable. We estimated species-level competition against potential future siblings by multiplying annual adult survival and the maximum number of broods parents can raise each year. We estimated species-level relatedness to siblings in two ways: (i) whether parents have a better than 50–50 chance of mating together again based on mortality and divorce rates, leading to half siblings; and (ii) the percentage of broods with extrapair paternity. We classified environmental predictability and quality following our previous comparative study (15).
Supplementary Material
Acknowledgments
We thank Charles Godfray, Camilla Hinde, Douglas Mock, Pat Barclay, and Katherine Coyte for useful discussion and/or comments on the manuscript and the Royal Society, The European Research Council, and the Calleva Foundation, Magdalen College for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13554.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606378113/-/DCSupplemental.
References
- 1.Wright J, Leonard ML. In: The Evolution of Begging: Competition, Cooperation and Communication. Wright J, Leonard ML, editors. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. [Google Scholar]
- 2.Bradbury JW, Vehrencamp SL. Principles of Animal Communication. 2nd Ed Sinauer Associates; Sunderland, MA.: 2011. [Google Scholar]
- 3.Godfray HCJ. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. [Google Scholar]
- 4.Godfray HCJ. Signaling of need between parents and young: Parent-offspring conflict and sibling rivalry. Am Nat. 1995;146(1):1–24. [Google Scholar]
- 5.Godfray HCJ, Johnstone RA. Begging and bleating: The evolution of parent-offspring signalling. Philos Trans R Soc Lond B Biol Sci. 2000;355(1403):1581–1591. doi: 10.1098/rstb.2000.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grodzinski U, Johnstone RA. Parents and offspring in an evolutionary game: The effect of supply on demand when costs of care vary. Proc Biol Sci. 2012;279(1726):109–115. doi: 10.1098/rspb.2011.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock DW, Dugas MB, Strickler SA. Honest begging: Expanding from signal of need. Behav Ecol. 2011;22(5):909–917. [Google Scholar]
- 8.Moreno-Rueda G, Soler M, Martín-Vivaldi M, Palomino JJ. Brood provisioning rate and food allocation rules according to nestling begging in a clutch-adjusting species, the Rufous-tailed Scrub-robin Cercotrichas galactotes. Acta Ornithol. 2009;44(2):167–175. [Google Scholar]
- 9.Grafen A. Biological signals as handicaps. J Theor Biol. 1990;144(4):517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- 10.Davis JN, Todd PM, Bullock S. Environment quality predicts parental provisioning decisions. Proc Biol Sci. 1999;266(1430):1791–1797. [Google Scholar]
- 11.Royle NJ, Hartley IR, Parker GA. Begging for control: When are offspring solicitation behaviours honest? Trends Ecol Evol. 2002;17(9):434–440. [Google Scholar]
- 12.Mock DW, Parker GA. The Evolution of Sibling Rivalry. Oxford Univ Press; Oxford, NY: 1997. [Google Scholar]
- 13.Parker GA, Royle NJ, Hartley IR. Begging scrambles with unequal chicks: Interactions between need and competitive ability. Ecol Lett. 2002;5(2):206–215. [Google Scholar]
- 14.Parker GA, Mock DW, Lamey TC. How selfish should stronger sibs be? Am Nat. 1989;133(6):846. [Google Scholar]
- 15.Caro SM, Griffin AS, Hinde CA, West SA. Unpredictable environments lead to the evolution of parental neglect in birds. Nat Commun. 2016;7:10985. doi: 10.1038/ncomms10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone RA, Grafen A. Dishonesty and the handicap principle. Anim Behav. 1993;46(4):759–764. [Google Scholar]
- 17.Rodríguez-Gironés MA, Cotton PA, Kacelnik A. The evolution of begging: Signaling and sibling competition. Proc Natl Acad Sci USA. 1996;93(25):14637–14641. doi: 10.1073/pnas.93.25.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone RA. Begging and sibling competition: How should offspring respond to their rivals? Am Nat. 2004;163(3):388–406. doi: 10.1086/375541. [DOI] [PubMed] [Google Scholar]
- 19.Akçay E. Incentives in the family II: Behavioral dynamics and the evolution of non-costly signaling. J Theor Biol. 2012;294:9–18. doi: 10.1016/j.jtbi.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Bossan B, Hammerstein P, Koehncke A. We were all young once: An intragenomic perspective on parent-offspring conflict. Proc Biol Sci. 2013;280(1754):20122637–20122637. doi: 10.1098/rspb.2012.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HG, Montgomerie R. Nestling American robins compete with siblings by begging. Behav Ecol Sociobiol. 1991;29(4):307–312. [Google Scholar]
- 22.Briskie JV, Naugler CT, Leech SM. Begging intensity of nestling birds varies with sibling relatedness. Proc Biol Sci. 1994;258(1351):73–78. [Google Scholar]
- 23.Price K, Ydenberg R. Begging and provisioning in broods of asynchronously-hatched yellow-headed blackbird nestlings. Behav Ecol Sociobiol. 1995;37(3):201–208. [Google Scholar]
- 24.Price K. Begging as competition for food in yellow-headed blackbirds. Auk. 1996;113(4):963–967. [Google Scholar]
- 25.Kacelnik A, Cotton PA, Stirling L, Wright J. Food allocation among nestling starlings: Sibling competition and the scope of parental choice. Proc Biol Sci. 1995;259(1356):259–263. [Google Scholar]
- 26.Cotton PA, Kacelnik A, Wright J. Chick begging as a signal: Are nestlings honest? Behav Ecol. 1996;7(2):178–182. [Google Scholar]
- 27.Leonard ML, Horn AG. Need and nestmates affect begging in tree swallows. Behav Ecol Sociobiol. 1998;42(6):431–436. [Google Scholar]
- 28.Leonard ML, Horn AG, Gozna A, Ramen S. Brood size and begging intensity in nestling birds. Behav Ecol. 2000;11(2):196–201. [Google Scholar]
- 29.Marques PAM, Leonard ML, Horn AG, Contasti A. How nestling tree swallows (Tachycineta bicolor) integrate their responses to hunger and signalling by nestmates. Ethology. 2011;117(2):163–170. [Google Scholar]
- 30.Neuenschwander S, Brinkhof MWG, Kölliker M, Richner H. Brood size, sibling competition, and the cost of begging in great tits (Parus major) Behav Ecol. 2003;14(4):457–462. [Google Scholar]
- 31.Mathevon N, Charrier I. Parent-offspring conflict and the coordination of siblings in gulls. Proc Biol Sci. 2004;271(Suppl_4):S145–S147. doi: 10.1098/rsbl.2003.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marques PAM, Marquez R, Vicente L. Brood size and nestling vocal begging behaviour in the Spanish Sparrow Passer hispaniolensis. Ardea. 2006;94(2):203–210. [Google Scholar]
- 33.Romano A, Caprioli M, Boncoraglio G, Saino N, Rubolini D. With a little help from my kin: Barn swallow nestlings modulate solicitation of parental care according to nestmates’ need. J Evol Biol. 2012;25(9):1703–1710. doi: 10.1111/j.1420-9101.2012.02571.x. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons; New York: 2011. [Google Scholar]
- 35.Koricheva J, Gurevitch J, Mengersen K. Handbook of Meta-analysis in Ecology and Evolution. Princeton Univ Press; Princeton: 2013. [Google Scholar]
- 36.Grissom RJ, Kim JJ. Effect Sizes for Research: Univariate and Multivariate Applications. 2nd Ed Taylor & Francis; Hoboken, NJ: 2011. [Google Scholar]
- 37.Carazo P, Font E. “Communication breakdown”: The evolution of signal unreliability and deception. Anim Behav. 2014;87(C):17–22. [Google Scholar]
- 38.Wells JC. Parent-offspring conflict theory, signaling of need, and weight gain in early life. Q Rev Biol. 2003;78(2):169–202. doi: 10.1086/374952. [DOI] [PubMed] [Google Scholar]
- 39.Hinde CA, Godfray HCJ. Quality, need, or hunger: Begging the question. Behav Ecol. 2011;22(6):1147–1148. [Google Scholar]
- 40.Butler DG, Cullis BR, Gilmour AR, Gogel BJ. ASReml-R Reference Manual: Mixed Models for S Language Environments. DPI&F Publications; Brisbane, Australia: 2009. [Google Scholar]
- 41.Nakagawa S, Santos ESA. Methodological issues and advances in biological meta-analysis. Evol Ecol. 2012;26(5):1253–1274. [Google Scholar]
- 42.Quillfeldt P. Begging in the absence of sibling competition in Wilson’s storm-petrels, Oceanites oceanicus. Anim Behav. 2002;64(4):579–587. [Google Scholar]
- 43.Johnstone RA. Signaling of need, sibling competition, and the cost of honesty. Proc Natl Acad Sci USA. 1999;96(22):12644–12649. doi: 10.1073/pnas.96.22.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnstone RA, Roulin A. Sibling negotiation. Behav Ecol. 2003;14(6):780–786. [Google Scholar]
- 45.Price K, Harvey H, Ydenberg R. Begging tactics of nestling yellow-headed blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim Behav. 1996;51(2):421–435. [Google Scholar]
- 46.Griffin AS, Alonzo SH, Cornwallis CK. Why do cuckolded males provide paternal care? PLoS Biol. 2013;11(3):e1001520. doi: 10.1371/journal.pbio.1001520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker GA, Royle NJ, Hartley IR. Intrafamilial conflict and parental investment: A synthesis. Philos Trans R Soc Lond B Biol Sci. 2002;357(1419):295–307. doi: 10.1098/rstb.2001.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stearns SC. The Evolution of Life Histories. Oxford Univ Press; Oxford, UK: 1992. [Google Scholar]
- 49.Bize P, Piault R, Moureau B, Heeb P. A UV signal of offspring condition mediates context-dependent parental favouritism. Proc Biol Sci. 2006;273(1597):2063–2068. doi: 10.1098/rspb.2006.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorogood R, Ewen JG, Kilner RM. Sense and sensitivity: Responsiveness to offspring signals varies with the parents' potential to breed again. Proc Biol Sci. 2011;278(1718):2638–2645. doi: 10.1098/rspb.2010.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Culina A, Radersma R, Sheldon BC. Trading up: The fitness consequences of divorce in monogamous birds. Biol Rev Camb Philos Soc. 2015;90(4):1015–1034. doi: 10.1111/brv.12143. [DOI] [PubMed] [Google Scholar]
- 52.Boncoraglio G, Saino N. Barn swallow chicks beg more loudly when broodmates are unrelated. J Evol Biol. 2008;21(1):256–262. doi: 10.1111/j.1420-9101.2007.01441.x. [DOI] [PubMed] [Google Scholar]
- 53.West SA, Pen I, Griffin AS. Cooperation and competition between relatives. Science. 2002;296(5565):72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 54.Wild G, West SA. A sex allocation theory for vertebrates: Combining local resource competition and condition-dependent allocation. Am Nat. 2007;170(5):E112–E128. doi: 10.1086/522057. [DOI] [PubMed] [Google Scholar]
- 55.West S. Sex Allocation. Princeton Univ Press; Princeton: 2009. [Google Scholar]
- 56.Lachmann M, Bergstrom CT. Signalling among relatives. II. Beyond the tower of Babel. Theor Popul Biol. 1998;54(2):146–160. doi: 10.1006/tpbi.1997.1372. [DOI] [PubMed] [Google Scholar]
- 57.Maynard Smith J, Harper D. Animal Signals. Oxford Univ Press; New York: 2003. [Google Scholar]
- 58.Stucki D, Kölliker M. Coevolutionary feedbacks between family interactions and life history. Evolution. 2013;67(11):3208–3220. doi: 10.1111/evo.12187. [DOI] [PubMed] [Google Scholar]
- 59.Ghalambor CK, Martin TE. Fecundity-survival trade-offs and parental risk-taking in birds. Science. 2001;292(5516):494–497. doi: 10.1126/science.1059379. [DOI] [PubMed] [Google Scholar]
- 60.R Core Team 2013 R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available at www.R-project.org. Accessed October 1, 2013.
- 61.Jetz W, et al. Global distribution and conservation of evolutionary distinctness in birds. Curr Biol. 2014;24(9):919–930. doi: 10.1016/j.cub.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–142. [Google Scholar]
- 63.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 64.Wright J, Hinde C, Fazey I, Both C. Begging signals more than just short-term need: Cryptic effects of brood size in the pied flycatcher (Ficedula hypoleuca) Behav Ecol Sociobiol. 2002;52(1):74–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.