Abstract
Inflammatory bowel disease is characterized by disturbed cytokine signalling in the mucosa. Inhibition of the proinflammatory interleukin (IL)-6 pathway is a promising new therapeutic strategy, but safety concerns arise as IL-6 signalling also contributes to epithelial repair of the intestinal mucosa. To which extent IL-6 classic or trans-signalling contributes to intestinal repair remains elusive. We tested the influence of IL-6 classic signalling on intestinal repair and proliferation. Whereas IL-6 induced STAT3 phosphorylation in the colonic cancer cell lines, primary non-malignant intestinal organoids did not respond to IL-6 classic signalling. Mice deficient in intestinal IL-6R (IL-6RΔIEC mice) did not display increased susceptibility to acute dextran sulfate sodium (DSS)-induced colitis. In the azoxymethane DSS model IL-6RΔIEC mice were not protected from inflammation-induced carcinogenesis but showed comparable tumor load to wild-type mice. These data indicate that classic signalling is not the major pathway to transduce IL-6 stimuli into the intestinal epithelium.
Introduction
Proficient epithelial regeneration is a prerequisite to maintain intestinal homeostasis.1 Within the intestinal epithelium, regeneration is elicited by pro-regenerative signals, which either derive from invading immune cells or are secreted in a paracrine and autocrine manner from intestinal epithelial cells (IECs).2 Many cytokines that elicit proliferative signals in the intestinal epithelium (for example, interleukin (IL)-17, IL-22, IL-11, IL-10 or IL-36) employ epithelial Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling, mostly by engaging STAT3 as the major signal transducer.3, 4, 5 Mice lacking epithelial STAT3 or JAK3 signalling display impaired epithelial regeneration6, 7 and increased T cell-driven intestinal inflammation.8 On the other hand, overactivation of the JAK/STAT pathway renders mice more susceptible to colon cancer formation, which highlights the dual role of JAK/STAT in tightly controlling intestinal regeneration.9
IL-6 is a key cytokine in intestinal inflammation with pleiotropic functions both as a pro-inflammatory and as a regeneration-promoting factor.10, 11 IL-6 also activates JAK/STAT signalling, mainly by STAT3. Upon binding of IL-6 to the membrane-bound IL-6 receptor (IL-6R), a dimer of the IL-6 family signal transducer glycoprotein gp130 is recruited, which leads to subsequent intracellular autophosphorylation of the gp130-associated tyrosine kinase Jak1 and finally to phosphorylation and activation of STAT3.12
While all body cells express the signal-transducing beta-receptor gp130, only few cell types (mainly hepatocytes and some leukocytes) express the non-signalling, specific IL-6R.13 Activation of gp130 via the membrane-bound IL-6R is called 'classic signalling'. Soluble IL-6R (sIL-6R) is mainly produced by protease shedding of the IL-6R ectodomain and, to a minor extent, by alternative splicing, and IL-6/sIL-6R complexes can activate also cells only expressing gp130, that is, practically all cells of the body. This process is called 'trans-signalling' and is thought to be a major sensitizing pathway for chronic inflammation.14 IL-6 trans-signalling contributes to intestinal proliferation, and overshooting IL-6 trans-signalling leads to murine colon cancer formation.15
IL-6 as a therapeutic target is currently under investigation in various chronic immune-mediated diseases, including rheumatic arthritis and inflammatory bowel disease (IBD). The interest in using IL-6 blockade as a therapeutic strategy in IBD is driven by the finding that the pathophysiology of IBD is strongly influenced by host genetics and many enriched genes are involved in pathways that influence host−environmental interactions and intestinal immune homeostasis.16, 17, 18 A small phase 2 trial showed favorable results, but no mucosal healing.19 Whereas IL-6 blockade in rheumatic arthritis has been shown to be safe and efficacious,20 concerns about the safety of IL-6 blockade in the context of intestinal inflammation were raised by various findings: (i) the incidence of intestinal perforations in rheumatic arthritis patients is increased under therapy with tocilizumab (Tcz), a human anti-IL-6R antibody, when compared to therapy with conventional disease-modifying anti-rheumatic drugs;21 (ii) IL-6R salvage therapy induces exacerbation in ulcerative colitis with increased ulcer formation;22 (iii) a clinical phase 2 study testing the anti-IL-6 agent BMS-945429 in Crohn's disease had to be prematurely terminated due to two cases of intestinal perforations (Clinicaltrials.gov Identifier: IM133-055). These observations raised questions about the role of IL-6 signalling in mediating intestinal regeneration. Whereas the role of common (classic and trans) IL-6 signalling in the context of intestinal regeneration has been studied,11 the contribution of specific IL-6 classic signalling in intestinal epithelial regeneration remains disputable. Although IL-6 classic signalling has been described for intestinal epithelial tumor cell lines,23, 24 the influence of IL-6 classic signalling on intestinal proliferation in vivo is rather circumstantial.25, 26 More precisely, very few studies have shown a biological effect of IL-6 classic signalling on epithelial proliferation.26
By employing in vitro methods of intestinal regeneration and in vivo mouse models of experimental colitis using a conditional deletion of the IL-6R in the intestinal epithelium (IL-6RΔIEC), we therefore investigated the influence of epithelial IL-6R on intestinal proliferation and repair.
Results and discussion
IECs receive IL-6 signals via classic and trans-signalling
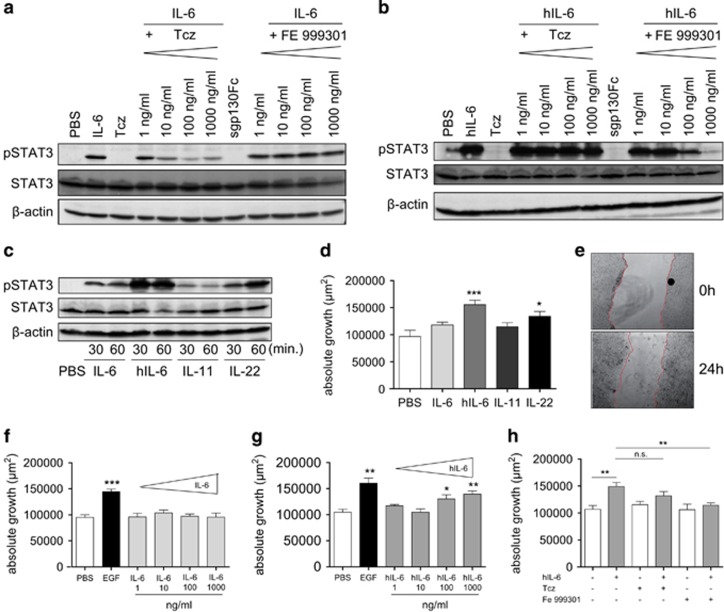
To test whether IECs receive IL-6 signals via classic and/or trans-signalling, human HT-29 colonic carcinoma cells were stimulated with different concentrations of IL-6 to activate the classic signalling pathway. Alternatively, trans-signalling was activated in these cells by using hyper-IL-6 (hIL-6), a fusion protein consisting of human IL-6 linked by a flexible peptide chain to human sIL-6R.27, 28 Cells were stimulated for 30 min, with or without pre-incubation with Tcz, a neutralizing human anti-IL6R antibody, or optimized sgp130Fc, a fusion protein of the extracellular domain of gp130 dimerized by the Fc domain of human IgG1 that selectively blocks trans-signalling.29, 30 IL-6 stimulation induced phosphorylation of STAT3, which was inhibited by pre-incubation with Tcz in a dose-dependent manner. As expected, pre-incubation with sgp130Fc did not interfere with IL-6-induced STAT3 phosphorylation (Figure 1a). Conversely, hIL-6-induced STAT3 phosphorylation was inhibited in a dose-dependent manner by pre-treatment with sgp130Fc, whereas Tcz had no inhibitory effect on hIL-6-induced STAT3 phosphorylation (Figure 1b), which is in line with previous observations and is due to the fact that Tcz, which is designed to block binding of IL-6 to (s)IL-6R, cannot significantly interfere with any formation of the already covalently formed IL-6/sIL-6R complex.31 To further investigate the role of classic and trans-signalling in epithelial wound healing in vitro, we assessed the effect of IL-6 and hIL-6 in an epithelial scratch assay in HT29 cells32 using IL-6 and hIL-6 concentrations, which were able to induce STAT3 in a robust manner. We also used IL-11 and IL-22 for comparison, as those cytokines have also been described to induce epithelial proliferation.33, 34 Interestingly, whereas all cytokines were able to induce STAT3 phosphorylation with different efficacies and kinetics (Figure 1c), only hIL-6 and IL-22 were able to induce a moderate, but statistically significant increase of intestinal wound healing (Figure 1d). Wound healing was assessed as the reduction of the area between the wound edges, as shown in representative micrographs of wound areas before (0 h) and 24 h after wound induction (Figure 1e). IL-11 or IL-6 did not show any effect on intestinal epithelial regeneration.
Figure 1.
Functional classic IL-6 signalling in IECs does not lead to increased cellular proliferation. (a) Tcz inhibits STAT3 phosphorylation (pSTAT3) after induction of classic signalling in a dose-dependent manner. Western blot analysis of lysates from HT-29 colon carcinoma cells, pre-treated with Tcz (1–1000 ng/ml) or sgp130Fc (1–1000 ng/ml) for 6 h and stimulated with human IL-6 (100 ng/ml) for 30 min. (b) sgp130Fc dose-dependently blocks trans-signalling: western blot analysis of lysates from HT-29 colon carcinoma cells, pre-treated with Tcz or sgp130Fc (1–1000 ng/ml) for 6 h and stimulated with hyper-IL-6 (hIL-6) (10 ng/ml) for 30 min. (c) HT-29 colon carcinoma cells were stimulated with IL-6 (100 ng/ml), hIL-6 (100 ng/ml), IL-11 (100 ng/ml) or IL-22 (100 ng/ml) for 30 or 60 min, and protein lysates were probed for (p)STAT3, STAT3 or β-actin. (d) IL-6 trans-signalling and IL-22, but not IL-6 classic signalling or IL-11 induce epithelial regeneration: confluent HT-29 cells (n=8 wells/stimulation) were scratched with a sterile 200 μl pipette and stimulated with IL-6 (100 ng/ml), hIL-6 (100 ng/ml), IL-11 (100 ng/ml) or IL-22 (100 ng/ml) for 24 h. Absolute and relative growth was assessed 24 h after scratching. (e) Representative photomicrographs were taken at 0 and 24 h after scratch induction in PBS-treated HT-29 cells. (f) IL-6 classic signalling does not alter intestinal epithelial proliferation and migration: absolute growth of HT-29 colon carcinoma cells after scratching with a sterile pipette and treatment with human EGF (10 ng/ml) or IL-6 (1–1000 ng/ml) for 24 h (n=8 wells per stimulation). (g) IL-6 trans-signalling increases intestinal epithelial proliferation and migration: HT-29 colon carcinoma cells were scratched and stimulated with human EGF (10 ng/ml) or hIL-6 (1–1000 ng/ml), and absolute growth was assessed after 24 h (f). (h) sgp130Fc, but not Tcz, inhibits hIL-6-induced epithelial proliferation and migration: HT-29 colon carcinoma cells were pre-incubated with Tcz (1000 ng/ml) or sgp130Fc (1000 ng/ml) for 4 h, scratched and stimulated with hIL-6 (100 ng/ml), and absolute growth was assessed after 24 h (n=8 wells per stimulation). Note the slight variations of absolute growth between g, h, which results from biological replicates of the experiment. Data are representative of n=2 individual experiments. Significance was determined using the two-tailed Student's t-test, and data are expressed as mean±s.d. *P<0.05; **P<0.01; ***P<0.001.
We further performed a detailed titration of IL-6 and hIL-6 in the scratch assay to determine whether higher doses of the cytokines would lead to increased proliferation and migration. However, the results again indicated that IL-6 classic signalling did not induce intestinal epithelial proliferation and migration (Figure 1f). In contrast, hIL-6 was able to induce wound healing, albeit at very high concentrations (100 or even 1000 ng/ml, corresponding to 1.67 or 16.7 nM, respectively) (Figure 1g). For comparison, a maximum cell proliferation of the very hIL-6-responsive cell line Ba/F3-gp130,35, 36 is achieved with 10 ng/ml hIL-6, similar to the 10 ng/ml EGF giving a maximum reference signal in the scratch assay used in the present study (Figures 1f and g). Lastly, we could show that only sgp130Fc, but not Tcz, could inhibit the hIL-6 induced wound healing (Figure 1h).
Intestinal organoids respond to IL-6 trans-signalling, but not to classic signalling
The results described above prompted us to test the impact of the presence of the epithelial membrane-bound IL-6R on IL-6-dependent intestinal regeneration in a clean genetic model of epithelial IL-6R deletion. For this purpose, we crossed IL-6Rflox mice37 with C57Bl/6VillinCre mice to generate conditional knockouts in the intestinal epithelium, which were termed IL-6RΔIEC (for ‘deleted in intestinal epithelial cells'). IL-6Rfl/fl (IL-6Rfl) littermates were used as controls.
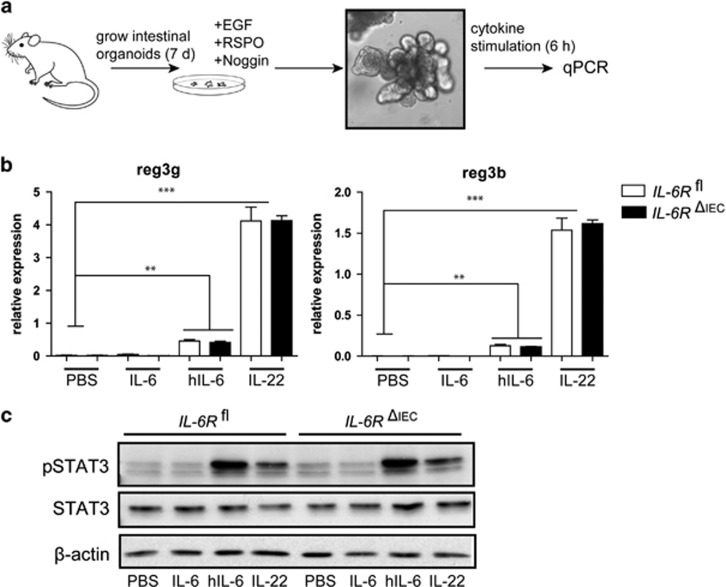
IL-6 has been shown to act as a canonical STAT3 inducer in multiple colon cancer epithelial cell lines.23 As epithelial regenerative responses occur independently from malignant transformations, we wanted to assess the role of IL-6 classic signalling in a epithelial non-tumor-derived model. For this purpose we cultured intestinal organoids from IL-6Rfl and IL-6RΔIEC mice as described previously38 (Figure 2a). Intestinal organoids were stimulated with IL-6, hIL-6 or IL-22, and the expression of the STAT3 target genes Reg3b and Reg3g was analyzed. Whereas IL-22 and hIL-6 induced a strong expression of STAT3 target genes, IL-6 did not induce any STAT3-driven gene expression (Figure 2b). Interestingly, no difference was seen between intestinal organoids from IL-6Rfl and IL-6RΔIEC mice, indicating that, if at all, the epithelial IL-6R does not interact with IL-6 trans-signalling. To further delineate whether impaired expression of Reg3b and Reg3g is indeed a surrogate of impaired phosphorylation of STAT3, IECs were stimulated with indicated cytokines for 30 min and probed for pSTAT3. Indeed, only hIL-6 and IL-22 induced phosphorylation of STAT3, whereas IL-6-stimulated cells showed no response (Figure 2c). This finding demonstrates that in non-malignant epithelial cells the epithelial IL-6R does not transduce STAT3-driven signals into the epithelium via classic IL-6R signalling.
Figure 2.
IL-6 trans-signalling, but not classic signalling, induces STAT3 target gene expression in intestinal organoids. (a) Mouse intestinal organoids from IL-6Rfland IL-6RΔIEC mice were cultivated as described previously.21 The medium was changed every other day and organoids were stimulated after 7 days of cultivation with hIL-6 (100 ng/ml), IL-6 (100 ng/ml) or IL-22 (100 ng/ml). (b) Expression of Reg3b and Reg3 was assessed by qPCR, and data are expressed as the expression relative to the housekeeping gene β-actin. (c) Western blot of isolated IECs, stimulated for 30 min with hIL-6 (100 ng/ml), IL-6 (100 ng/ml) or IL-22 (100 ng/ml) and probed for pSTAT3, STAT3 and β-actin. Data are representative of n=2 individual experiments. Significance was determined using the two-tailed Student's t-test, and data are expressed as mean ± s.d. **P<0.01; ***P<0.001.
Il6-R ΔIEC mice do not display increased susceptibility to dextran sulfate sodium (DSS)-induced colitis
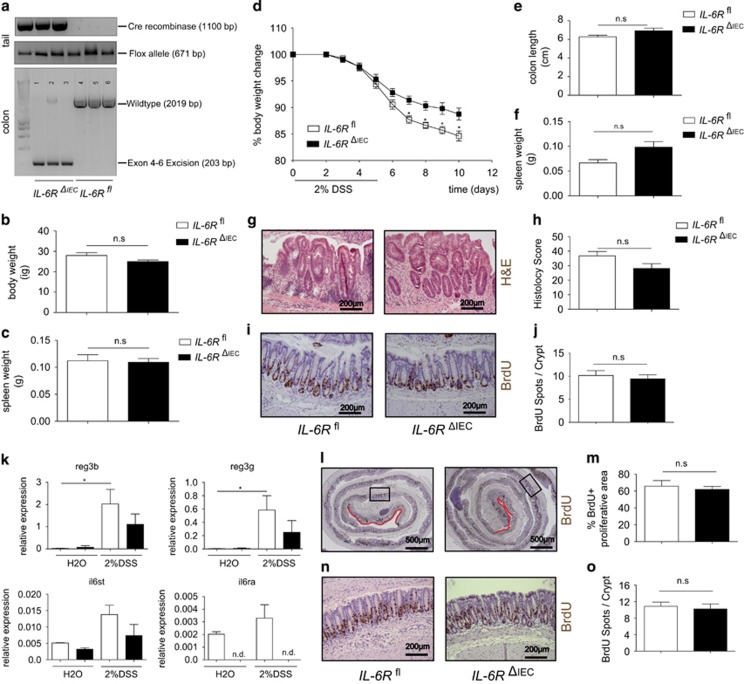
Having shown that classic IL-6 signalling is not active in non-malignant IECs in vitro, we wanted to test the role of the epithelial IL-6R in vivo. Deletion efficiency and specificity were tested in genotyping PCR from tail and colon IEC DNA (Figure 3a). Macromorphological analysis of the small and large intestine of IL-6RΔIEC mice did not reveal any spontaneous abnormalities when compared to wild-type littermates (data not shown), and baseline body weight (Figure 3b) and spleen weight (Figure 3c) were comparable between age- and sex-matched littermates. To determine the impact of IL-6R signalling on intestinal proliferation and repair under exogenous stress, we exposed IL-6Rfl and IL-6RΔIEC mice to DSS, a chemical irritant that disrupts the intestinal epithelial barrier and results in induction of colitis.39 Mice of both genotypes started to lose weight, but IL-6RΔIEC mice seemed to cope better with DSS-induced colitis than their wild-type littermates (Figure 3d) as they significantly lost less weight than IL-6Rfl mice. Remarkably, IL-6RΔIEC lost significantly less weight than the IL-6Rfl littermates, but showed no significant differences in other parameters of inflammation. Next, we aimed to rule out environmental factors that could influence the overall outcome of the DSS colitis. We performed 16srRNA sequencing of the luminal microflora after DSS colitis. IL-6Rfl and IL-6RΔIEC showed no difference in the overall composition of the colonic microflora and the relative abundance of major phylotypes (data not shown). In post-mortem analyses, no significant difference was seen in colon length (Figure 3e) or spleen weight (Figure 3f), although a non-significant trend points towards a diminished inflammatory response in IL-6RΔIEC mice.This was in line with histological evaluation of the diseased colon, which showed a non-significant trend towards less histological inflammation in IL-6RΔIEC in the overall histological score (Figures 3g and h) or in any subscore (mononuclear infiltration, crypt hyperplasia, epithelial erosion, polymorphonuclear infiltrates, transmural inflammation; details not shown). In order to investigate the impact of IL-6R signalling on intestinal regeneration, we also evaluated the overall bromodeoxyuridine-positive (BrdU+) areas of the entire colon. Interestingly, IL-6RΔIEC mice showed significantly increased mucosal areas with BrdU incorporation, thereby indicating more proficient epithelial regeneration than IL-6Rfl littermates. (Figures 3i and j). It must be noted that the acute DSS model is driven by an acute toxic destruction of the intestinal epithelium, in which partial IL-6-dependent regenerative responses can be completely overridden by the overt inflammation. In order to describe presumable partial regenerative effects of the epithelial IL-6R on the intestinal epithelium, a DSS colitis was induced only for 3 days. Colonic IECs were isolated and tested for STAT-dependent gene expression. DSS induction induced an upregulation of the STAT3 target genes Reg3g and Reg3b in IL-6RΔIEC and IL-6Rfl, indicating that epithelial IL-6R is not primarily involved in epithelial STAT3 signalling. As expected, Il6ra expression was absent in epithelial cells of IL-6RΔIEC compared to IL-6Rfl, whereas gp130(IL6st) expression levels in IECs were comparable (Figure 3k). BrdU staining revealed again comparable numbers of proliferative cells in the colon mucosa of IL-6RΔIEC and IL-6Rfl (Figures 3l–o). Although we cannot entirely rule out an incremental beneficial effect of IL-6 signalling on the intestinal epithelium, our data support the hypothesis that epithelial IL-6R does not orchestrate the regenerative response of the intestinal mucosa to inflammatory conditions.
Figure 3.
IL-6RΔIEC are not susceptible to DSS colitis and display regular epithelial regeneration. (a) Genotyping of IL-6Rfl and IL-6RΔIEC, showing expression of Cre recombinase and the mutant IL-6R allele in tail PCR. IEC-specific deletion of IL-6R was verified by isolating colon DNA and using primer pairs spanning the exon 4–6 region of the IL-6R gene (il6ra). PCR products show an amplicon of 2016 bp in IL-6Rfl and a truncated amplicon of 203 bp in IL-6RΔIECplus a faint 2016 bp signal, indicating DNA from non-epithelial origin. (b, c) Body weight and spleen weight of sex-matched untreated IL-6Rf(n=4) mice and IL-6RΔIEC (n=4) at the age of 8–12 weeks. (d) Sex-matched littermate IL-6Rfl (n=8) and IL-6RΔIEC (n=18) mice aged 8–12 weeks were subjected to experimental colitis induced by adding 2% of DSS to the drinking water. Body weight changes relative to the starting weight on day 0 showed that IL-6RΔIEC mice lost less weight than their IL-6Rfl littermates. (e, f) Postmortem analyses showed no significant difference between IL-6RΔIEC and IL-6Rfl in colonic shortening (e) or relative spleen weight (f). (g, h) H&E staining of colon Swiss rolls (g) and corresponding histological disease scores42 (h) showed no significant differences in disease severity between IL-6RΔIEC and IL-6Rflmice. (i, j) Representative pictures of BrdU staining of IL-6RΔIEC and IL-6Rfl are shown in (i). 10 mg/kg bodyweight of BrdU was injected i.p. 1.5 h before sacrifice. A minimum of 15 crypts/intestine was counted and genotype pooled data from IL-6Rfl (n=6) and IL-6RΔIEC (n=12) are depicted for statistical evaluation. No significant difference was seen in the number of BrdU+ cells/crypt between IL-6RΔIEC and IL-6Rfl mice (j). (k) IEC-specific expression of STAT3 target genes. Mice (n>3 per genotype) were challenged with 2% DSS or left untreated (water, H2O) for 3 days, and colonic IECs were isolated. Gene expression was assessed using qPCR. DSS induction led to significant upregulation of STAT3 target genes in IL-6Rfl animals, which was reduced or abrogated in IL-6RΔIEC mice. (l, m) Representative pictures of BrdU staining of IL-6RΔIEC and IL-6Rfl are shown in (i). 10 mg/kg bodyweight of BrdU was injected i.p. 1.5 h before sacrifice. The percentage of intact BrdU+ mucosa was expressed as 1−(length of BrdU-negative mucosa in μm/total mucosal length in μm)*100. (n, o) A minimum of 15 crypts/intestine were evaluated for BrdU-positive stained cells. Genotype pooled data from IL-6Rfl (n=4) and IL-6RΔIEC (n=4) are depicted for statistical evaluation. Significance was determined using the two-tailed Student's t-test, and data are expressed as mean±s.d. *P<0.05.
IL-6R ΔIEC mice are not protected from azoxymethane (AOM)-DSS-induced carcinogenesis
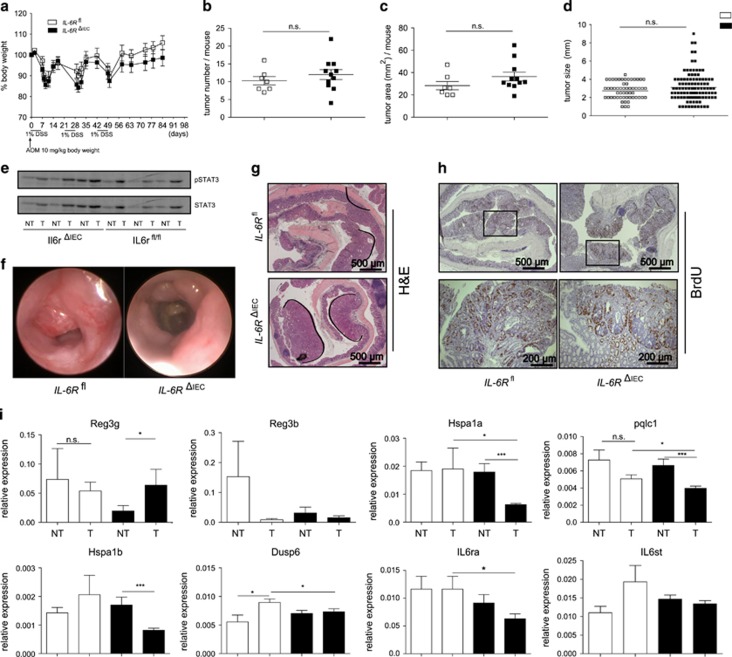
It has been recently postulated that classic IL-6R signalling contributes to intestinal carcinogenesis in a model of inflammasome-triggered carcinogenesis.40 We therefore tested the hypothesis that deletion of the IL-6R in IECs protects from inflammation-induced carcinogenesis using the AOM-DSS model. Mice received a single dose of 10 mg/kg AOM on day 0, and chronic DSS colitis was induced using three cycles of 1% DSS (Figure 4a). During the colitis cycles four IL-6RΔIEC and two IL-6Rfl mice died prematurely (data not shown). Postmortem analysis revealed no difference in total numbers of tumors/mouse (Figure 3b), total tumor area/mouse (Figure 3c) or in tumor size (Figure 3d) between IL-6RΔIEC and IL-6Rfl/fl mice. Western blot of mucosa from tumors or adjacent non-tumor mucosa showed increased tumor-dependent STAT3 phosphorylation levels. In line, no genotype-specific differences were seen, which indicates that classical IL-6R signalling does not contribute to malignant STAT3 activation in colon carcinogenesis (Figure 3e). Endoscopic assessment of tumors showed, again, no difference between IL-6RΔIEC and IL-6Rfl mice (Figure 3f). Histopathological assessment showed no difference in histological H&E staining (Figure 3g) or in BrdU staining in colonic tumors (Figure 3h). As these data suggested that epithelial-specific deletion of the IL-6R does not inhibit carcinogenesis, we investigated the impact of impaired classic IL-6 signalling in the context of tumor or non-tumor lesions. IL-6 has been described to induce a specific set of genes in the context of intestinal carcinogenesis, which is distinct from the STAT3-signalling cytokines like IL-11.34 Indeed, a set of genes were exclusively downregulated in tumors from IL-6RΔIEC but not IL-6Rfl mice, suggesting that IL-6 signalling is biologically active in the context of intestinal carcinogenesis but does not affect the overall tumor development (Figure 3i). Interestingly, gene expression of IL-6R itself (il6ra) was still detectable in tumor and non-tumor mucosal specimens from IL-6RΔIEC mice, which was most probably due to the invasion of leukocytes into the inflamed mucosa. Thus we conclude that functional classic IL-6R signalling in IECs is not involved in AOM-DSS-driven carcinogenesis. En masse we have shown that the IL-6 classic signalling pathway does not activate epithelial pSTAT3 and is not essentially involved in the orchestration of STAT3-driven regenerative responses in the context of intestinal inflammation. This finding contrasts observations made in epithelial cancer cells, where IL-6 signalling induces pSTAT3 activation and contributes to epithelial cancer proliferation.41 Indeed, we observed in our tested colon cancer cell line a great variety of IL-6 classic signalling responsiveness (data not shown). This finding leaves room for the speculation that classic IL-6 signalling is not an essential epithelial growth signal, but is rather acquired as a second proliferative signal during malignancy. With regard to regenerative response in acute or chronic inflammatory disorder we have shown that interception of the epithelial classic IL-6 signalling does not disrupt epithelial growth and mucosal healing.
Figure 4.
IL-6RΔIEC mice are not protected from colitis-associated cancer. (a) Sex-matched littermate IL-6Rfl (n=9) and IL-6RΔIEC (n=16) mice aged 8–12 weeks were subjected to an AOM-DSS colitis model. A single dose of 10 mg/kg of AOM was injected at day 0, followed by three cycles of 1% DSS added to the drinking water. Body weight changes relative to the starting weight on day 0 were not different between IL-6RΔIEC and IL-6Rfl mice. (b) Absolute number of tumors/mouse. (c) Average tumor area/mouse. (d) Overall distribution of tumor size between genotypes. (e) Protein lysates from colonic tumor and adjacent non-tumor tissue were probed on western blot against pSTAT3 and STAT3. (f) Representative pictures of mouse colonoscopy at the day of sacrifice showing no difference in endoscopic tumor growth between IL-6RΔIEC and IL-6Rflmice. (g, h) Representative histological pictures of colon Swiss rolls of H&E or BrdU staining revealed no difference in histological tumor growth or tumor proliferation between IL-6RΔIEC and IL-6Rflmice. (i) Gene expression patterns in tumor vs non-tumor colon tissues in IL-6RΔIEC and IL-6Rfl mice showed significant changes. Significance was determined using the two-tailed Student's t-test, and data are expressed as mean±s.d. *P<0.05; ***P<0.001.
Therefore our data do not support the clinical preoccupation about intestinal perforations as a complication in the therapeutic IL-6 interference, but rather come to the conclusion that the IL-6 classic signalling is indispensable for intestinal epithelial regeneration.
Acknowledgments
We gratefully appreciate the technical assistance of Melanie Nebendahl, Katharina Göbel, Maren Reffelmann, Tatjana Schmidtke, Karina Greve, Dorina Oelsner and Sabine Kock. This work was supported by DFG Excellence Cluster Inflammation at Interfaces, SFB877 (project A1, B9) and the Graduate School RTG1743 TP5.
Author contributions
KA and PR conceived the study concept and design, performed acquisition of data, analysis and interpretation of data, statistical analysis, and wrote the manuscript. AB and JS performed acquisition of data and analysis and interpretation of data, and JS contributed to writing the manuscript. SS, GHW and SR-J performed study concept and design, interpretation of data, and contributed to writing the manuscript.
Footnotes
GHW is employed by CONARIS Research Institute AG (a company commercially developing sgp130Fc proteins); SS and SR-J are shareholders of CONARIS, and both are inventors of patents owned by CONARIS. The other authors declare no conflict of interest.
References
- Shah SC, Colombel J-F, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 1245–1255.e8. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proc Natl Acad Sci USA 2015; 112: 10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Jacob S, Tato Cristina M, Joyce-Shaikh B, Gulen Muhammet F, Cayatte C, Chen Y et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015; 43: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut (e-pub ahead of print 18 January 2016; doi:10.1136/gutjnl-2015-310374). [DOI] [PubMed]
- Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature [Letter] 2015; 528: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Role of Janus Kinase 3 in mucosal differentiation and predisposition to colitis. J Biol Chem 2013; 288: 31795–31806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009; 206: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TA, Jurickova I, Collins M, Denson LA. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm Bowel Dis 2013; 19: 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreiter L, Fritz TMJ, Adolph TE, Krismer A-M, Offner FA, Tschurtschenthaler M et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med 2013; 210: 2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis 2007; 13: 1016–1023. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu G, Vallabhapurapu S. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009; 15: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 2015; 34: 75–82. [DOI] [PubMed] [Google Scholar]
- Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 2006; 63: 321–329. [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 2000; 6: 583–588. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble–IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol 2010; 184: 1543–1551. [DOI] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 2011; 43: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, McGovern DPB, Barrett JC, Wang K, Radford-Smith GL, Ahmad T et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 2010; 42: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology 2004; 126: 989–996. [DOI] [PubMed] [Google Scholar]
- Lindegaard HM, Johansen P, Gröndal G, Jensen EC, Juul L, Schlemmer AM et al. Doubling the single-dose infusion rate of tocilizumab in rheumatoid arthritis is safe and efficacious. Scand J Rheumatol 2016; 45: 1–5. [DOI] [PubMed] [Google Scholar]
- Gout T, Östör AJK, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol 2011; 30: 1471–1474. [DOI] [PubMed] [Google Scholar]
- Atreya RBU, Rath T, Mudter J, Vieth M, Neumann H, Neurath MF. First case report of exacerbated ulcerative colitis after anti-interleukin-6R salvage therapy. World J Gastroenterol 2015; 21: 12963–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivarthi H, Gordziel C, Themanns M, Kramer N, Eberl M, Rabe B et al The ratio of STAT1 to STAT3 expression is a determinant of colorectal cancer growth. Oncotarget 2016; 7: 51096–51106. [DOI] [PMC free article] [PubMed]
- Lee MJ, Lee J-K, Choi JW, Lee C-S, Sim JH, Cho C-H et al. Interleukin-6 induces S100A9 expression in colonic epithelial cells through STAT3 activation in experimental ulcerative colitis. PLoS ONE 2012; 7: e38801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest 2013; 123: 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KA, Manieri NA, Liu T-C, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014; 9: e114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer AI. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol 1997; 15: 142–145. [DOI] [PubMed] [Google Scholar]
- van Dam M, Müllberg J, Schooltink H, Stoyan T, Brakenhoff JP, Graeve L et al. Structure-function analysis of interleukin-6 utilizing human/murine chimeric molecules. Involvement of two separate domains in receptor binding. J Biol Chem 1993; 268: 15285–15290. [PubMed] [Google Scholar]
- Waetzig GH, Rose-John S. Hitting a complex target: an update on interleukin-6 trans-signalling. Expert Opin Ther Targets 2012; 16: 225–236. [DOI] [PubMed] [Google Scholar]
- Holmer R, Wätzig GH, Tiwari S, Rose-John S, Kalthoff H. Interleukin-6 trans-signaling increases the expression of carcinoembryonic antigen-related cell adhesion molecules 5 and 6 in colorectal cancer cells. BMC Cancer 2015; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F et al. Inhibition of classic signaling is a novel function of soluble Glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem 2011; 286: 42959–42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocols 2007; 2: 329–333. [DOI] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte J-M et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol 2006; 290: G827–G838. [DOI] [PubMed] [Google Scholar]
- Putoczki Tracy L, Thiem S, Loving A, Busuttil Rita A, Wilson Nicholas J, Ziegler Paul K et al. Interleukin-11 Is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013; 24: 257–271. [DOI] [PubMed] [Google Scholar]
- Fischer M, Goldschmitt J, Peschel C, Brakenhoff JPG, Kallen K-J, Wollmer A et al. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotech 1997; 15: 142–145. [DOI] [PubMed] [Google Scholar]
- Jostock T, Müllberg J, Özbek S, Atreya R, Blinn G, Voltz N et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 2001; 268: 160–167. [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol 2010; 184: 7219–7228. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protocols 2007; 2: 541–546. [DOI] [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci USA 2013; 110: 9862–9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D et al. Th17-type cytokines, IL-6 and TNF-[alpha] synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015; 34: 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph TE, Tomczak MF, Niederreiter L, Ko H-J, Bock J, Martinez-Naves E et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013; 503: 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]