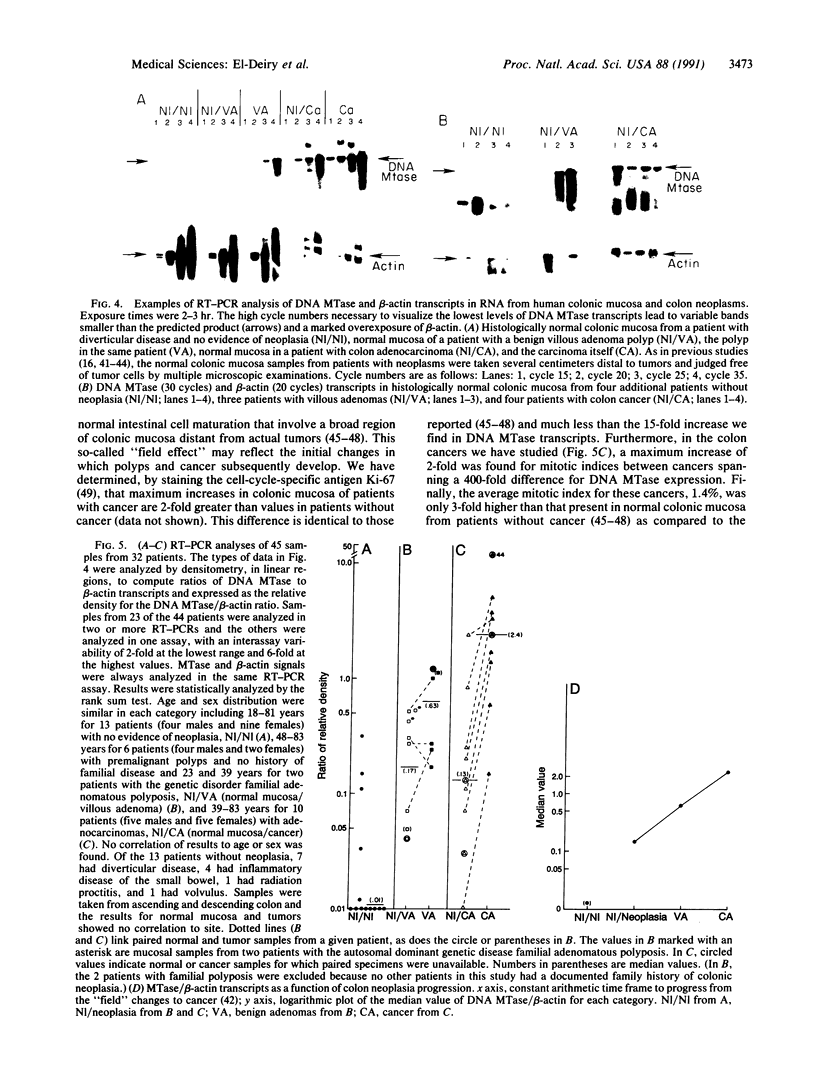
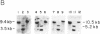Abstract
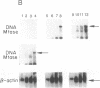
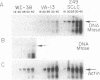
DNA methylation abnormalities occur consistently in human neoplasia including widespread hypomethylation and more recently recognized local increases in DNA methylation that hold potential for gene inactivation events. To study this imbalance further, we have cloned and localized to chromosome 19 a portion of the human DNA methyltransferase gene that codes for the enzyme catalyzing DNA methylation. Expression of this gene is low in normal human cells, significantly increased (30- to 50-fold by PCR analysis) in virally transformed cells, and strikingly elevated in human cancer cells (several hundredfold). In comparison to colon mucosa from patients without neoplasia, median levels of DNA methyltransferase transcripts are 15-fold increased in histologically normal mucosa from patients with cancers or the benign polyps that can precede cancers, 60-fold increased in the premalignant polyps, and greater than 200-fold increased in the cancers. Thus, increases in DNA methyltransferase gene expression precede development of colonic neoplasia and continue during progression of colonic neoplasms. These increases may play a role in the genetic instability of cancer and mark early events in cell transformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Antequera F., Macleod D., Bird A. P. Specific protection of methylated CpGs in mammalian nuclei. Cell. 1989 Aug 11;58(3):509–517. doi: 10.1016/0092-8674(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Fearon E. R., Vogelstein B., de Bustros A., Sharkis S. J., Burke P. J., Staal S. P., Nelkin B. D. Hypermethylation of the 5' region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood. 1987 Aug;70(2):412–417. [PubMed] [Google Scholar]
- Baylin S. B., Höppener J. W., de Bustros A., Steenbergh P. H., Lips C. J., Nelkin B. D. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res. 1986 Jun;46(6):2917–2922. [PubMed] [Google Scholar]
- Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988 Oct 20;203(4):971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Burt R. W., Bishop D. T., Cannon L. A., Dowdle M. A., Lee R. G., Skolnick M. H. Dominant inheritance of adenomatous colonic polyps and colorectal cancer. N Engl J Med. 1985 Jun 13;312(24):1540–1544. doi: 10.1056/NEJM198506133122403. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Skolnick M. H., Bishop D. T., Lee R. G., Burt R. W. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Chandler L. A., DeClerck Y. A., Bogenmann E., Jones P. A. Patterns of DNA methylation and gene expression in human tumor cell lines. Cancer Res. 1986 Jun;46(6):2944–2949. [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. J., Weidema W. F., Sandle G. I., Palmer L., Deschner E. E., DeCosse J. J. Sodium transport in a mouse model of colonic carcinogenesis. Cancer Res. 1987 Sep 1;47(17):4646–4650. [PubMed] [Google Scholar]
- Di Fiore P. P., Falco J., Borrello I., Weissman B., Aaronson S. A. The calcium signal for BALB/MK keratinocyte terminal differentiation counteracts epidermal growth factor (EGF) very early in the EGF-induced proliferative pathway. Mol Cell Biol. 1988 Feb;8(2):557–563. doi: 10.1128/mcb.8.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983 Feb 28;111(1):47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A. Review: assessment of cell proliferation in histological material. J Clin Pathol. 1990 Mar;43(3):184–192. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Genetics of cancer predisposition. Cancer Res. 1987 Nov 1;47(21):5518–5527. [PubMed] [Google Scholar]
- Hausheer F. H., Rao S. N., Gamcsik M. P., Kollman P. A., Colvin O. M., Saxe J. D., Nelkin B. D., McLennan I. J., Barnett G., Baylin S. B. Computational analysis of structural and energetic consequences of DNA methylation. Carcinogenesis. 1989 Jun;10(6):1131–1137. doi: 10.1093/carcin/10.6.1131. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Buckley J. D. The role of DNA methylation in cancer. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Jones P. A. DNA methylation and cancer. Cancer Res. 1986 Feb;46(2):461–466. [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautiainen T. L., Jones P. A. DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J Biol Chem. 1986 Feb 5;261(4):1594–1598. [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer. Their development and application to studies of cancer prevention. Gastroenterology. 1987 Apr;92(4):1083–1086. doi: 10.1016/0016-5085(87)90987-5. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988 Jan 15;48(2):235–245. [PubMed] [Google Scholar]
- Lipkin M., Sherlock P., Decosse J. J. Risk factors and preventive measures in the control of cancer of the large intestine. Curr Probl Cancer. 1980 Apr;4(10):1–57. doi: 10.1016/s0147-0272(80)80011-0. [DOI] [PubMed] [Google Scholar]
- Mabry M., Nakagawa T., Baylin S., Pettengill O., Sorenson G., Nelkin B. Insertion of the v-Ha-ras oncogene induces differentiation of calcitonin-producing human small cell lung cancer. J Clin Invest. 1989 Jul;84(1):194–199. doi: 10.1172/JCI114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Silverman A. L., Park J. G., Hamilton S. R., Gazdar A. F., Luk G. D., Baylin S. B. Abnormal methylation of the calcitonin gene in human colonic neoplasms. Cancer Res. 1989 Jul 1;49(13):3468–3473. [PubMed] [Google Scholar]
- Szyf M., Kaplan F., Mann V., Giloh H., Kedar E., Razin A. Cell cycle-dependent regulation of eukaryotic DNA methylase level. J Biol Chem. 1985 Jul 25;260(15):8653–8656. [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]