Abstract
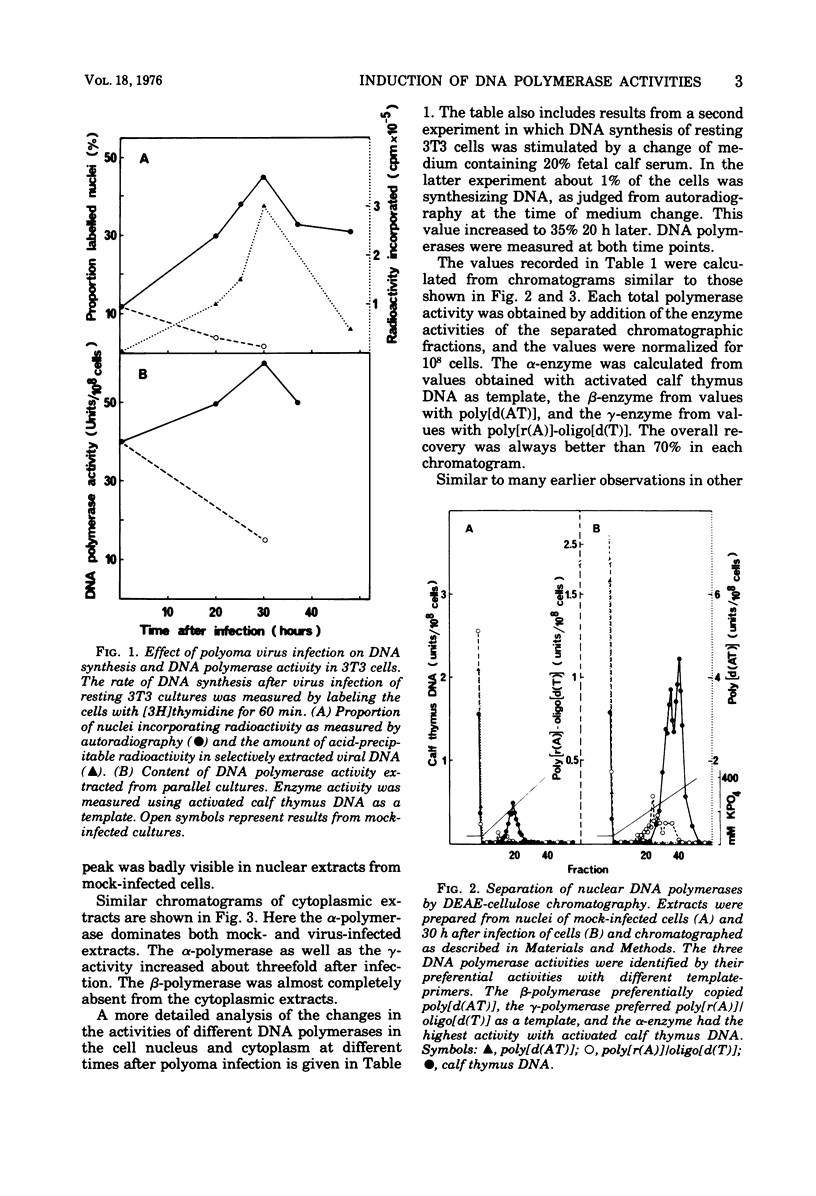
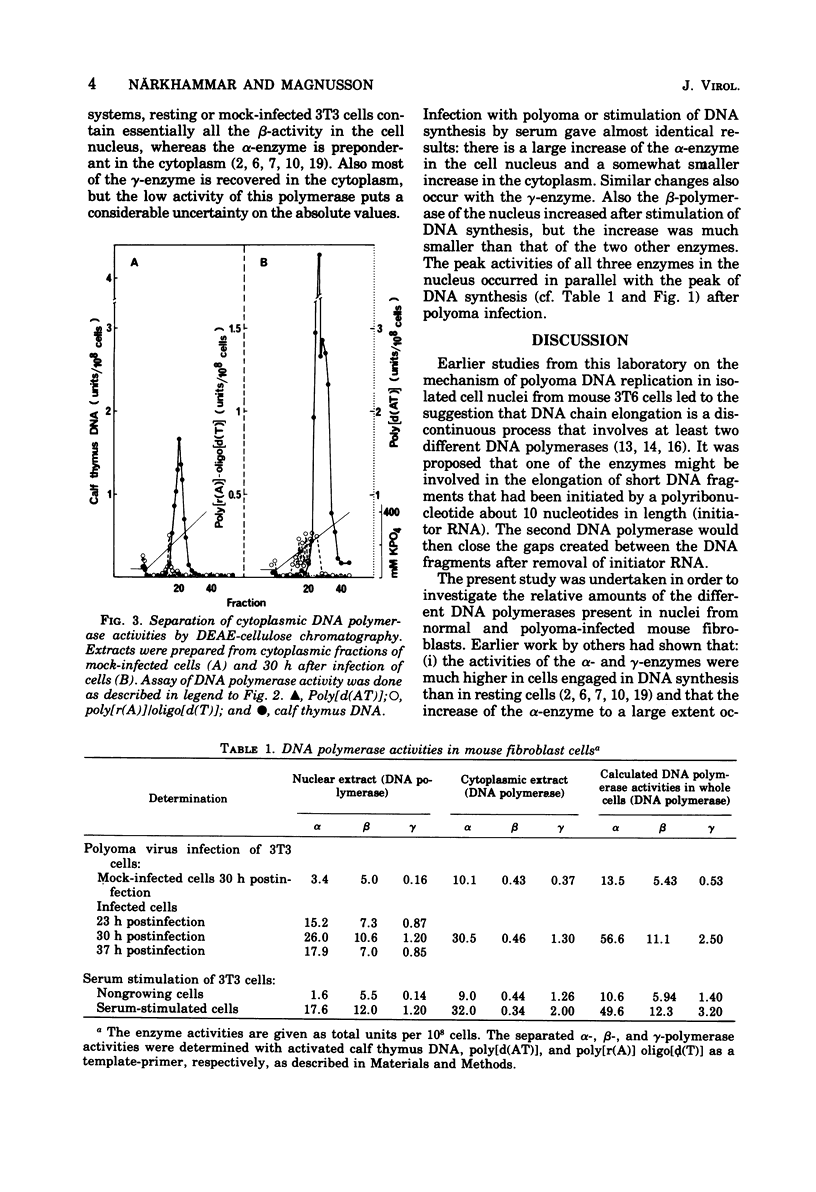
Resting mouse 3T3 fibroblasts were stimulated to synthesize DNA either by infection with polyoma virus or by injection of fresh serum. Changes in the levels of DNA polymerase (alpha-, beta-, and gamma-enzymes) were measured in the cytoplasm and the cell nucleus. Both types of stimulation gave very similar increases for all three enzyme activities. In the cell nucleus, both alpha- and gamma-polymerases increased almost tenfold, whereas the beta-enzyme only was stimulated twofold. In the cytoplasm alpha- and gamma-polymerases increased two- to four-fold. Only insignificant amounts of the beta-enzyme were found in the cytoplasm.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril E. F., Jenkins M. D., Brown O. E., Laszlo J., Morris H. P. DNA polymerases I and II in regenerating rat liver and Morris hepatomas. Cancer Res. 1973 Jun;33(6):1187–1193. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Variation of deoxyribonucleic acid polymerase activities during rat liver regeneration. J Biol Chem. 1972 Dec 25;247(24):7948–7950. [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Chang L. M. Replication of initiated polyriboadenylic acid by mammalian low molecular weight deoxyribonucleic acid polymerase. J Biol Chem. 1974 Dec 10;249(23):7441–7446. [PubMed] [Google Scholar]
- Chiu R. W., Baril E. F. Nuclear DNA polymerases and the HeLa cell cycle. J Biol Chem. 1975 Oct 10;250(19):7951–7957. [PubMed] [Google Scholar]
- Fried M., Pitts J. D. Replication of polyoma virus DNA. I. A resting cell system for biochemical studies on polyoma virus. Virology. 1968 Apr;34(4):761–770. doi: 10.1016/0042-6822(68)90097-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. DNA polymerase from HeLa cell nuclei: levels of activity during a synchronized cell cycle. Biochem Biophys Res Commun. 1970 Apr 8;39(1):100–109. doi: 10.1016/0006-291x(70)90763-1. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. I. Characterization of fragments. J Virol. 1973 Sep;12(3):600–608. doi: 10.1128/jvi.12.3.600-608.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Winnacker E. L., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. II. Evidence for semi-conservative replication. J Mol Biol. 1972 Dec 30;72(3):539–552. doi: 10.1016/0022-2836(72)90173-8. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. 3. The nucleotide sequence at the RNA-DNA junction of nascent strands. J Mol Biol. 1974 Mar 25;84(1):197–216. doi: 10.1016/0022-2836(74)90222-8. [DOI] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. The interrelation between DNA synthesis and various DNA polymerase activities in synchronized HeLa cells. J Mol Biol. 1974 Jun 15;86(1):11–20. doi: 10.1016/s0022-2836(74)80003-3. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. DNA polymerases in polyoma virus-infected mouse kidney cells. J Virol. 1975 Nov;16(5):1095–1100. doi: 10.1128/jvi.16.5.1095-1100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


