Abstract
Leukemia is the most common pediatric cancer, affecting 3,800 children per year in the United States. Its annual incidence has increased over the last decades, especially among Latinos. Although most children diagnosed with leukemia are now cured, many suffer long-term complications, and primary prevention efforts are urgently needed. The early onset of leukemia – usually before age five – and the presence at birth of “pre-leukemic” genetic signatures indicate that pre- and postnatal events are critical to the development of the disease. In contrast to most pediatric cancers, there is a growing body of literature – in the United States and internationally – that has implicated several environmental, infectious, and dietary risk factors in the etiology of childhood leukemia, mainly for acute lymphoblastic leukemia, the most common subtype. For example, exposures to pesticides, tobacco smoke, solvents, and traffic emissions have consistently demonstrated positive associations with the risk of developing childhood leukemia. In contrast, intake of vitamins and folate supplementation during the pre-conception period or pregnancy, breastfeeding, and exposure to routine childhood infections have been shown to reduce the risk of childhood leukemia. Some children may be especially vulnerable to these risk factors, as demonstrated by a disproportionate burden of childhood leukemia in the Latino population of California. The evidence supporting the associations between childhood leukemia and its risk factors – including pooled analyses from around the world and systematic reviews – is strong; however, the dissemination of this knowledge to clinicians has been limited. To protect children’s health, it is prudent to initiate programs designed to alter exposure to well-established leukemia risk factors rather than to suspend judgement until no uncertainty remains. Primary prevention programs for childhood leukemia would also result in the significant co-benefits of reductions in other adverse health outcomes that are common in children, such as detriments to neurocognitive development.
INTRODUCTION
Cancer is the second most common cause of death in children 0–14 years of age, after accidents. Leukemia is the most common cancer in children, representing approximately one third of pediatric cancers. Approximately 3,800 children are diagnosed annually with acute lymphoblastic leukemia (ALL) or acute myeloblastic leukemia (AML) in the United States (U.S.).1 A small but steady annual increase from 1975 and 2012 in the age- adjusted incidence rate of childhood leukemia in the U.S. has resulted in an overall rise of 55% in the annual number of cases during the past three and a half decades. Modern treatment protocols cure 80 to 90% of children with leukemia with fewer sequelae than previous regimens. Still, even with improved treatments, the immediate and long-term consequences of childhood leukemia continue to exact a heavy toll.2,3 The impacts and costs of childhood leukemia extend beyond the care of the sick child; affecting family, friends, and the community. Long-term and late-appearing secondary effects include detriments to neurocognitive development, mental health, endocrine system function, and general health.4 To avoid these risks completely, it would be beneficial to prevent the disease altogether.
Though new genetic risk factors are likely still to be discovered, to date only a small fraction (less than 10%) of childhood leukemia cases can be attributed to the influence of genetics, including to genetic syndromes such as Down’s.5,6 Moreover, the aforementioned increase in childhood leukemia incidence – which is not fully explained by diagnostic trends -- indicates that causal factors for childhood leukemia have become more prevalent in the last few decades. Since genetic factors do not change on this time scale, it is probable that environmental factors play a significant role in the etiology of childhood leukemias and their recent upward trends.6 These facts underscore the importance of developing an approach to primary prevention of childhood leukemia focused on reducing exposure to environmental risk factors for the disease.
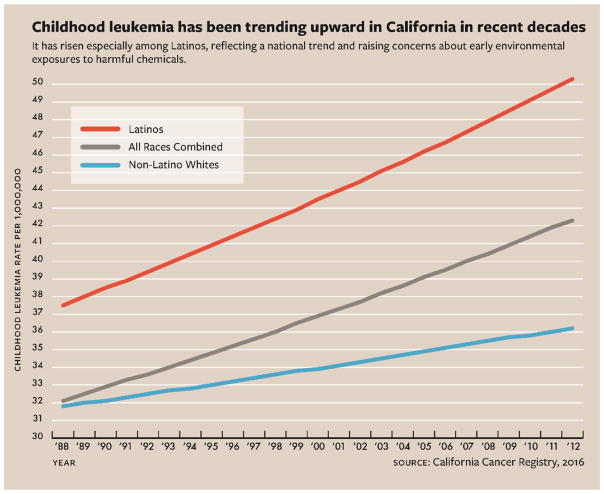
Children of Latino (also referred to as Hispanic) descent have a higher incidence of leukemia than whites, African-Americans or Asian-Americans nationally and in California, a highly-populated and ethnically-diverse State. Moreover, over the past 25 years, childhood leukemia incidence has been rising in California at a faster pace in Latino children, compared to white children (see Figure 1),7 suggesting that Latino children (or parents) are even more vulnerable to and/or more exposed to harmful environmental factors than others.
Figure 1.
Incidence of childhood leukemia in California by race-ethnicity, 1988–2012.
Figure adapted from Giddings B, Whitehead TP, Metayer C, Miller MD. Childhood Leukemia Incidence in California: High and Rising in the Hispanic Population. Cancer. (In Press).7
Exposure to carcinogens has generally been considered a non-threshold event and modeled in a linear fashion. However, there are chemicals for which a supra-linear dose response curve has been observed, indicating that the risk from low dose exposure may actually be proportionally greater than would be expected with a linear dose-response curve.8 One such example is the known leukemogen, benzene, which is metabolized to active intermediary compounds. This finding is consistent with other exposures of concern to pediatric environmental health, such as lead, where a non-linear dose-response relationship has resulted in worse-than-anticipated health effects from low-dose environmental exposures that were presumed to be safe.9 As such, findings from observational studies -- where participants are exposed to chemicals at environmental levels as opposed to higher dose occupational levels – are critical to avoid underestimating risk.
Research into environmental, infectious, and dietary causes of childhood leukemia has been limited, amounting to only a small fraction of the total Federal funding for childhood leukemia research (e.g., 3–7% of National Institutes of Health and 1% of National Cancer Institute total funding in 2010–11).10 Funding for treatment/outcome-related studies has been far greater than for those studies examining etiology. The dramatic increases in children cured clearly justifies the expenditures made to improve treatment, but research on etiologic factors that may lead to prevention is also important. Despite limited funding, evidence demonstrating associations between environmental, infectious, and dietary exposures and the risk of developing childhood leukemia is accumulating.
This issue provides an overview of key biological concepts in childhood leukemia research and summarizes the environmental, infectious, and dietary risk factors for childhood leukemia that have been identified in epidemiologic studies from around the world. Our purpose is not to conduct a systematic review of the field of childhood leukemia epidemiology – an endeavor which could fill an entire book – but rather to provide a concise perspective of the subject for the benefit of clinicians and policy makers. The literature cited in this issue spans a wide gamut. Whenever possible, the authors emphasize relevant findings from well-designed meta-analyses of published data and from large international collaborations that have pooled and analyzed individual-level data from multiple studies. In some instances, both pooled analyses and meta-analyses (including unpublished data) were conducted in parallel. Because most of these comprehensive studies fulfill criteria for systematic reviews, they provide the strongest evidence of associations between childhood leukemia and its risk factors. However, when warranted by the novelty of the research, the authors also discuss “one-off” findings of particular interest. The authors are associated with the California Childhood Leukemia Study (CCLS) and the Center for Integrative Research on Childhood Leukemia and the Environment (CIRCLE) at the University of California, Berkeley. This research group, begun by Patricia Buffler, PhD, has studied environmental causes of childhood leukemia for 25 years. In this paper, we identify many of the major epidemiologic investigations of childhood leukemia worldwide and we rely on our collective expertise to highlight the literature that we believe will be most instructive to clinicians and policy makers. However, we acknowledge that particular emphasis has been paid to the work of our research group and its collaborators in the Childhood Leukemia International Consortium (CLIC).
NATURAL HISTORY OF LEUKEMIA AS A DISEASE
Development of Blood Cells and the Formation of Leukemia
Hematopoiesis is a process involving extreme cellular blast growth, which produces 1011 blood cells per day throughout life. The activity of certain cell differentiation stages (defined by cell surface markers and the potential of the progeny) varies throughout life due to different needs of the organism. These changes in the activity of blood cell types over the course of development are reflected in the distinct types of blood cells with the propensity for cancer in childhood and adulthood.
Although the maternal immune system protects the child before and shortly after birth (with maternal antibodies), the child’s immune system must begin its education immediately after birth. This education of the adaptive immune system requires exposures to immune stimuli (e.g., infections) leading to the formation of billions of naïve pre-B and pre-T cells. Leukemias in children arise from these naïve, pre-antigen stages, typically leading to primitive precursor blast cell leukemic populations. That is, the majority of childhood leukemias are of the pre-B cell or, to a lesser extent, pre-T cell phenotype (exhibiting cell surface markers of normal pre-B or pre-T cells) and appear as clonal outgrowths of normal pre-B or pre-T cells “frozen” at a particular stage of differentiation.
Through the adaptive formation of antibodies and T-cell receptors that respond to specific antigens, a stable long-lived repertoire of cells is developed by the time a child reaches young adulthood. The pre-B and pre-T stages decrease in frequency after adolescence, exemplified by the involution of the thymus and the reduction in size of the pre-B cell population in the bone marrow. As a result, adults typically contract hematopoietic cancers (lymphomas and leukemias) in post-antigen stimulated B and T-cells and the myeloid lineage predominates in adult tumors. Distinct from adult leukemias, the unique properties of childhood leukemia cells are likely to be important for understanding the environmental sensitivities for leukemogenesis in this age group.
Childhood leukemia, like all cancers, is a product of two or more molecular changes in a stem-like cell that has the ability to divide while maintaining an immature state. Because they are formed from blood cells, leukemias have an inherent capacity for mobilization in the bloodstream and extravasation. Precursor blood cells also have an enormous capacity for “blast-like” growth, and an ability to travel throughout the body without restriction. These attributes are among the six “hallmarks of cancer”,11 and the fact that hematopoietic precursors harbor these “cancer-like” attributes may explain why leukemias seem to need far fewer genetic aberrations for tumor progression compared to solid tumors, which need to evolve additional metastatic capacities via genetic mutations. This simplicity may also help explain the short latency of childhood cancers.
The genetic simplicity of leukemia combined with the young age of onset has allowed researchers to delineate -- within the lifetime of the child -- the timing of the formation of characteristic genetic aberrations.12 These changes appear to occur within two time frames – prenatal initiating events that induce some cellular changes, and postnatal genetic and epigenetic events that allow the emergence of acute disease. This research was made possible by the availability of archived newborn blood spots that are routinely collected at birth by heel prick from neonates (e.g., by the State of California Newborn Screening Program), including from children with leukemia. Several translocations commonly found in leukemias that were assessed using archived newborn blood spots, including t(12;21) ETV6-RUNX1, t(8;21)RUNX1-MTG8, inv(16)CBFB-MYH11, have indicated a clear presence of the mutations in neonatal blood at birth in children who contract leukemia later in life.13–16 Several other mutations, including t(1;19)TCF3-PBX1, FLT3, and RAS mutations clearly occur postnatally.17–19 The mutations associated with leukemia are generally insufficient to cause disease by themselves. This is the case for ETV6-RUNX1and RUNX1-MTG8, the most common translocations for ALL and AML, respectively. Studies using cord blood from healthy children without cancer indicate that these translocations may occur at a rate of 1% or more in the general population,20,21 while the disease is much more rare.
Mutational Mechanisms in Leukemia
Recent investigations into the precise molecular attributes of leukemia point largely to three pathways: (1) aberrations in a small number of lineage-specific transcription factors such as ETV6, RUNX1, IKZF1, and PAX5 (2) defects in receptor protein tyrosine kinases and their downstream pathways (i.e., RAS/MEK/ERK) and (3) epigenetic modifiers. Mutations may involve translocations that comprise fusion genes, copy number alterations (most common are deletions), single nucleotide mutations, and broad changes in epigenetic features such as DNA methylation aberrations. The molecular formation of the initiating translocations may provide some clues as to their causes. In the past several years, many translocation breakpoints were shown to be induced by enzymatic mutagenic processes -- normally utilized by the immune system to diversify antibody and T-cell repertoire -- which were aberrantly targeted to oncogenes and genomic enhancers.22 These types of translocations are common in hematopoietic cancers that form after birth in response to antigenic stimulation. Interestingly, leukemia translocations including the most common in children such as ETV6-RUNX1 do not show signs of such activity and their origin remains a mystery. The lack of a clear endogenous path towards translocation formation points to exogenous causes such as in utero environmental exposures, and points again to the oncologic immaturity of childhood leukemia blasts. Environmental causes may also interact with endogenous mutagenic mechanisms in children after birth. Many of these postnatal secondary rearrangements show the clear involvement of the activity of the enzymes that create antibody diversity, i.e.., recombinase activating gene (RAG) and adenosine deaminase (AID) in the formation of secondary deletions and mutations.23 Illegitimate targeting of these endogenous enzymes has long been known to be stimulated by exposures as diverse as pesticides and smoking. As such, there is evidence that postnatal environmental exposures may initiate childhood leukemia via the stimulation of endogenous mutagenic mechanisms including recombinase activating gene activity.24,25
Apart from the chromosome breakage events mentioned above, additional mutations include point mutations and epigenetic modifications. Point mutations are caused by electrophilic chemicals that are either exposed to bone marrow cells directly or else they are metabolically activated, and their metabolites are exposed to bone marrow cells. Many oxidized reactive metabolites are produced from chemicals circulating in the bone marrow due to the presence of highly metabolically active cells such as neutrophils (containing myeloperoxidase, for instance) and the high levels of heme iron. Metabolites that adduct to DNA can directly cause miscoding mutations if unrepaired, or can result in repair-induced strand breaks. There is little current evidence that point mutations such as those found in RAS are caused by environmental chemicals26; however, the most common translocation in ALL, ETV6-RUNX1, appears to be strongly and specifically associated with parental smoking27 and home paint use28,29, which provides some credence to viability of this pathway.
Epigenetic modifications are a normal part of a blood cell’s development from a stem cell to a mature cell. Changes to the usual epigenetic programming can occur, however, as cells adapt to new environments. This type of adaptation is the essence of the “developmental origins of health and disease” initiatives that seek to understand when such adaptations early in life can lead to later disease risk, such as the demonstration that starvation in utero can lead to obesity and cardiac disease risk later in life.30 Adaptations can occur as the result of exposures to specific environmental factors – for instance the specific demethylation of CpG loci in the gene AHRR has been exquisitely related to cigarette smoke.31 Likewise, methylation changes have also been observed in response to folic acid exposures.32 Our understanding of the specific effects of environmental exposures on epigenetic features is just in its infancy, and it is unknown whether these perturbations could impact leukemogenesis.
The Role of Immune Factors in a Cancer of the Immune System
Infection is a direct cause of viral-induced cancers, such as human papillomavirus-induced cervical cancer and Merkel cell virus-induced skin cancer. Inflammation from infection is also a risk factor for many cancers, resulting from the collateral damage of tissue disorganization, tissue remodeling, and chronic exposure to reactive oxygen species following inflammatory processes. Childhood ALL is also a disease of the immune system, which begs the question can infection directly stimulate leukemia or stimulate leukemia via inflammatory processes?
Viruses that directly integrate into the genome have not been reported in childhood ALL. Epidemiological studies have, however, demonstrated clear effects of immune factors on leukemogenesis, most overtly in the form of a consistently-observed reduced risk of childhood ALL associated with more childhood contacts in daycare (odds ratio, OR=0.77, 95% Confidence Interval, 95% CI: 0.71–0.84; N=7399 cases) and other markers of early exposure to immune stimulus such as breastfeeding for at least six months (OR=0.86, 95% CI: 0.79–0.94), having an older sibling (OR=0.94, 95% CI: 0.88–1.00), or a history of four or more common infections in the first year of life (OR=0.88, 95% CI: 0.79–0.98).33 Just as regular immune stimulation appears to reduce risk for allergies and asthma, the same immune exercise can reduce the risk of leukemia. In the absence of these priming exposures, children may respond too strongly to the myriad of infections subsequently encountered in school, resulting in a cytokine “storm” and excess cell stimulation, secondary mutations, and, in some cases, leukemia. This putative pathology is referred to as the “Greaves hypotheses” after its first proponent.34 Other ancillary data on immune education seem to fit this pattern as well: normal childhood vaccines, the presence of older siblings, and breastfeeding were all associated with reduced risks of childhood leukemia. Population mixing, in which large numbers of a “new” population are mixed into a standing community, seems to transiently increase the risk of leukemia and also fit with the notion of an infectious stimulation for childhood ALL.35 A corollary hypothesis to Greaves’ was suggested by Kinlen who noticed that leukemia space-time clusters often followed recent population mixing events, such as the creation of new towns or population movements during warfare. He proposed that such mixing facilitated the transmission of a specific leukemia-initiating virus which, while plausible, was not followed up with biological validation as noted above. More likely, population mixing permits the transmission of common viruses to populations without herd immunity rather than spreading specific leukemia-initiating viruses.
Recent studies have noted that the child’s medical records confuse the infection issue somewhat: ALL patients visit their general practitioner for infections in the first year of life much more commonly than children who do not grow up to get leukemia.36,37 This suggests that the damage wrought by fulminant infections may occur much earlier than previously thought, and also that children who get leukemia may respond to infection differently, that is, more strongly to typical childhood infections. Part of this sensitivity may be a lack of immunomodulation from suppressive cytokines like IL-10 that tend to be present at lower levels in newborns who go on to develop leukemia.38
Chemicals as Leukemogens
The ability of a chemical to act as carcinogen was originally thought to hinge on its capacity as mutagen, but it is likely that many other more biologically-relevant activities are important in leukemogenesis as well. Some chemicals exhibit properties that allow them to target the bone marrow specifically, due to its metabolic activity (benzene for an example). Other chemicals may impact the immune system indirectly, setting up the individual for aberrant responses to infection. The role of exogenous factors such as chemicals, many of which are immunosuppressive, in this process is unknown and likely to be a major research field in the future. Other activities of chemicals are summarized in a recent review of chemicals as carcinogens.39 Below, we discuss chemical exposures that may cause leukemia and weigh the evidence for causal relationships.
ENVIRONMENTAL RISK FACTORS FOR CHILDHOOD LEUKEMIA
Exposure Science in Studies of Childhood Leukemia Risk
As mentioned earlier, childhood leukemia is the most common form of pediatric cancer; but, for the purposes of epidemiological study, it is a rare disease. As such, the vast majority of epidemiological investigations into the causes of childhood leukemia are forced to employ a case-control study design. In many instances, case-control studies of childhood leukemia are designed to assess children’s exposure to disease risk factors retrospectively. That is, a child is first diagnosed with leukemia, then s/he is enrolled in the case-control study, and only afterwards can investigators begin to assess the agents to which s/he has been exposed. This design imposes limitations on the epidemiologist, as etiologically relevant specimens – biological and environmental – are not necessarily available for collection by the time the child is under study. As discussed above, childhood leukemia can be initiated during the prenatal period, but most cases are not diagnosed until the child is between two to four years old. This leaves a long time interval between the first windows of susceptibility to leukemogenic agents and the time period when investigators can start measuring a child’s exposure to those agents. To overcome these challenges, childhood leukemia investigators have employed a variety of strategies to assess children’s exposures to potentially carcinogenic agents.
Using Parent Interviews To Assess Children’s Exposures to Chemicals
One simple way to circumvent the need for the collection of biological or environmental samples during etiologically relevant time periods is to interview participating parents to obtain information about their child’s history of exposure to specific agents. Such interviews can be used to pinpoint historical exposures during critical windows of a child’s development (e.g., the second trimester of pregnancy), they can be wide-ranging in scope, and they can be especially effective in assessing parents’ conscious behaviors (e.g., smoking habits, residential pesticide use, occupational histories). On the other hand, interview-based exposure assessments have inherent limitations; they are imprecise measures of chemical exposure, they provide little information about any of a child’s exposures that go unrecognized by the parents, and they are potentially subject to recall and reporting biases if parents of case and control children remember or report their children’s exposures in different ways. In practice, though, reproducibility studies have suggested that, for some of the environmental exposures that are of interest in childhood leukemia research -- ionizing radiation,40–42 pesticides,43 and smoking27 – there is minimal evidence of differential recall between cases and controls.
Measuring Chemicals in Settled Dust
Another strategy to obtain information about a child’s exposure to chemicals during etiologically relevant time periods is to measure levels of chemicals in stable environmental matrices. For example, persistent chemicals accumulate on settled dust particles, which can become trapped deep within a carpet, and this settled dust acts as a long-term reservoir for these chemicals.44 With limited exposure to direct sunlight and microbial action, persistent chemicals, like polychlorinated biphenyls (PCBs), are extremely slow to degrade on dust particles that settle indoors. As a result, collecting samples of settled dust from carpets or other household surfaces and measuring levels of persistent chemicals in these samples provides an integrated measure of chemical contamination over a long period of time.
As part of the California Childhood Leukemia Study, we have collected multiple dust samples from a large group of homes at time intervals of several years between sampling rounds. We found that while there was substantial variability in chemical levels between sampling rounds, there was also moderate correlation in the relative ranking of exposures (i.e., rankings from highest to lowest exposures) among homes over time.45–47 These findings support the hypothesis that chemical levels in dust samples collected after diagnosis may be informative surrogates for chemical contamination that was present in the home during important developmental periods of a child’s life.
Accidental ingestion of settled dust is an important route of human exposure to chemicals along with the consumption of contaminated food and the inhalation of contaminated air. For example, it has been suggested that dust ingestion is the major route of exposure to the flame retardant chemicals, polybrominated diphenyl ethers (PBDEs) in North America48 and positive relationships have been observed between PBDE levels in matched samples of dust and serum among U.S. adults.49,50 Due to their tendency to make hand-to-mouth contact and their proximity to the floor, young children are expected to receive a relatively large proportion of their total chemical intake via settled dust compared to adults,51 and a positive relationship has been observed between PBDE levels in matched samples of dust and serum in one investigation of toddlers from North Carolina.52 Likewise, research from the Center for Integrative Research on Childhood Leukemia and the Environment also indicates a relationship between levels of persistent chemicals in matched samples of settled dust and biospecimens.53,54 Taken together, the observation that chemical levels can be correlated in matched samples of settled dust and human serum as well as the observation that chemical levels in settled dust are relatively stable over time support the use of chemical levels measured in dust collected after diagnosis as surrogates for chemical exposures that children received during etiologically-relevant time periods (before diagnosis).
Estimating Ambient Environmental Exposures Using Geographic Information Systems
An alternative strategy for assessing a child’s history of environmental exposures is to estimate ambient pollution using Geographic Information Systems (GIS) and geospatial modeling and to use these estimates of ambient conditions as surrogates for the child’s total exposure to chemicals. Many governing bodies record a child’s home address on the birth certificate and this information can be obtained for research purposes contingent on appropriate ethical review and approval. Exposure models based on GIS data and geocoded birth addresses could provide useful information about children’s exposure to chemicals at the time of birth and, potentially, throughout the prenatal period as well (if participating mothers did not change residence during pregnancy). Moreover, complete residential histories can be obtained from parents via interview, which allows for a more comprehensive model of a child’s historic exposures to ambient chemicals. Agricultural pesticide application,55,56 traffic-related air pollution,57 and electromagnetic fields58 have been estimated using GIS in the context of epidemiological studies of childhood leukemia. One limitation of using estimates of ambient pollution as surrogates for total exposures is the inability to account for chemical exposures that occur indoors. This is a substantial drawback, because children tend to spend the vast majority of their time indoors, there are distinct chemical sources indoors, and chemical exposures tend to be higher indoors than outdoors.59,60
Measuring Chemicals in Archived Pre-Diagnostic Biospecimens
Perhaps the most straightforward way to obtain pre-diagnostic biospecimens would be to establish a prospective birth cohort and follow leukemia incidence in the participating children into adulthood. However, as discussed above, this study design is generally not feasible for childhood leukemia and will generally be limited by a small number of available cases. The International Childhood Cancer Cohort Consortium seeks to combine a number of large infant/child prospective studies (on the order of 100,000 participants per study) – which were originally designed to examine environmental and genetic determinants of common childhood diseases – for pooled analyses of childhood leukemia and other childhood cancers.61
Alternatively, it may be possible to utilize archived biospecimens – collected before diagnosis as part of routine medical testing – to measure prenatal chemical exposures.62 For example, in many countries, blood spots are collected from each newborn infant by heel-stick for the purposes of genetic screening. In the State of California, blood spots left over from genetic screening are archived in a State laboratory and made available for research contingent upon appropriate ethical review and approval. These archived neonatal blood spots provide a useful resource for childhood leukemia research, offering insight into the chemicals to which the developing fetus was exposed during the prenatal period, including folate,63 cotinine,64 and PBDEs65. Using current technology for exposure biology, investigators can characterize prenatal exposure to thousands of different chemicals with as little as a few drops of archived neonatal blood. Some important technical considerations include the potential instability of chemical analytes during long-term storage, the possibility of chemical contamination during storage, and the complex dynamics of newborn metabolite levels immediately after birth.66 Despite these limitations, the use of archived pre-diagnostic biospecimens for exposure assessment in epidemiological studies of childhood leukemia is very promising.
Using these strategies for assessing children’s exposure to environmental agents, epidemiologists have identified several suspected environmental risk factors for childhood leukemia, including pesticides, parental smoking, paint, petroleum solvents, traffic emissions, persistent organic pollutants, and radiation, as summarized in Table 1.
Table 1.
Environmental risk factors for childhood leukemia
| Childhood Leukemia Risk Factor | Exposure Measure | Level of Consensus | Subtype Specificity | Exposure Details | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Home pesticide use | Parental interview | Pooled analysis of 12 studies from CLIC29 | ALL | Before conception | 1.39 (1.25, 1.55) |
| ALL | During pregnancy | 1.43 (1.32, 1.54) | |||
| ALL | After birth | 1.36 (1.23, 1.51) | |||
| AML | Before conception | 1.49 (1.02, 2.16) | |||
| AML | During pregnancy | 1.55 (1.21, 1.99) | |||
| AML | After birth | 1.08 (0.76, 1.53) | |||
|
| |||||
| Parental occupational exposure to pesticides | Parental interview | Pooled analysis of 13 studies from CLIC & meta-analysis of CLIC + non-CLIC studies74 | ALL | Maternal during pregnancy | 1.01 (0.78, 1.30) |
| ALL | Paternal at conception | 1.20 (1.06, 1.38) | |||
| T-cell ALL | Paternal at conception | 1.42 (1.04, 1.94) | |||
| ALL after 5y | Paternal at conception | 1.38 (1.13, 1.67) | |||
| AML | Maternal during pregnancy | 1.94 (1.19, 3.18) | |||
| AML | Paternal at conception | 0.91 (0.66, 1.24) | |||
|
| |||||
| Proximity to agricultural pesticide application | GIS Model | Novel finding55 | ALL | Insecticides, moderate, lifetime | 1.5 (0.9, 2.4) |
| ALL | Fumigants, moderate, lifetime | 1.7 (1.0, 3.1) | |||
| ALL | Organophosphates, moderate, lifetime | 1.6 (1.0, 2.7) | |||
| ALL | Chlorinated phenols, moderate, lifetime | 2.0 (1.0, 3.8) | |||
| ALL | Triazines, moderate, lifetime | 1.9 (1.0, 3.7) | |||
|
| |||||
| Chlorthal, an herbicide | Settled dust levels | Novel finding80 | ALL | Detected, 1st Tertile vs. Not Detected | 1.49 (0.82, 2.72) |
| ALL | Detected, 2nd Tertile vs. Not Detected | 1.49 (0.83, 2.67) | |||
| ALL | Detected, 3rd Tertile vs. Not Detected | 1.57 (0.90, 2.73) | |||
|
| |||||
| Parental smoking | Parental interview | Pooled analysis of 14 studies from CLIC & meta-analysis of CLIC + non-CLIC studies83 | AML | Paternal at any time | 1.34 (1.11, 1.62) |
| AML | Maternal during pregnancy, Latinas | 2.08 (1.20, 3.61) | |||
| AMMoL | Paternal at any time | 1.87 (1.08, 3.25) | |||
|
| |||||
| Home paint use | Parental interview | Pooled analysis of 8 studies from CLIC86 | ALL | 1–3 months before conception | 1.54 (1.28, 1.85) |
| ALL | During pregnancy | 1.14 (1.04, 1.25) | |||
| ALL | After birth | 1.22 (1.07, 1.39) | |||
| ALL | Professional painter, during pregnancy | 1.66 (1.21, 2.28) | |||
| ALL - t(12;21) | During pregnancy | 1.51 (1.08, 2.11) | |||
|
| |||||
| Parental occupational exposure to paint | Parental interview | Pooled analysis of 13 studies from CLIC89 | ALL | Paternal at conception | 0.93 (0.76, 1.14) |
| ALL | Maternal during pregnancy | 0.81 (0.39, 1.68) | |||
| AML | Paternal at conception | 0.96 (0.65, 1.41) | |||
| AML | Maternal during pregnancy | 1.31 (0.38, 4.47) | |||
|
| |||||
| Meta- analysis88 | ALL | Maternal during pregnancy | 1.23 (1.02, 1.47) | ||
|
| |||||
| Parental occupational exposure to solvents | Parental interview | Meta- analysis88,90 | ALL | All solvents, maternal during pregnancy | 1.25 (1.09, 1.45) |
| ALL | Petroleum, maternal during pregnancy | 1.42 (1.10, 1.84) | |||
| ALL | Benzene, maternal during pregnancy | 1.71 (0.91, 3.24)* | |||
|
| |||||
| Replicated91 | ALL | Org. solvents, paternal at conception | 1.48 (1.01, 2.16) | ||
| ALL | Cl-hydrocarbon, paternal at conception | 2.28 (0.97, 5.37) | |||
|
| |||||
| Traffic- related air pollution | GIS model | Two independent meta- analyses92,93 | ALL | Traffic density | 1.25 (0.92, 1.69) |
| Any | Traffic density | 1.53 (1.12, 2.10) | |||
| ALL | Nitrogen dioxide estimates | 1.21 (1.04, 1.41) | |||
| AML | Benzene estimates | 2.28 (1.09, 4.75) | |||
|
| |||||
| PAHs | Settled dust levels, vacuum cleaners | Novel finding94 | ALL | Benzo[a]pyrene | 1.42 (0.95, 2.12) |
| ALL | Dibenzo[a,h]anthracene | 1.98 (1.11, 3.55) | |||
| ALL | Benzo[k]fluoranthene | 1.71 (0.91, 3.22) | |||
| ALL | Indeno[1,2,3-c,d]pyrene | 1.81 (1.04, 3.16) | |||
| ALL | PAH toxic equivalence | 2.35 (1.18, 4.69) | |||
|
| |||||
| PCBs | Settled dust levels | Novel finding81 | ALL | Any PCB detected vs. none detected | 1.97 (1.22, 3.17) |
| ALL | Top quartile Σ6PCB vs. bottom quartile | 2.78 (1.41, 5.48) | |||
|
| |||||
| PBDEs | Settled dust levels | Novel finding95 | ALL | Top quartile ΣPenta-BDEs vs. bottom | 0.7 (0.4, 1.3) |
| ALL | Top quartile ΣOcta-BDEs vs. bottom | 1.3 (0.7, 2.3) | |||
| ALL | Top quartile ΣDeca-BDEs vs. bottom | 1.0 (0.6, 1.8) | |||
| ALL | BDE-196 concentrations | 2.1 (1.1, 3.8) | |||
| ALL | BDE-203 concentrations | 2.0 (1.1, 3.6) | |||
| ALL | BDE-206 concentrations | 2.1 (1.1, 3.9) | |||
| ALL | BDE-207 concentrations | 2.0 (1.03, 3.8) | |||
ALL=acute lymphoblastic leukemia; AML=acute myeloblastic leukemia; AMMoL=acute myelomonocytic leukemia; CI=Confidence Interval; CLIC=Childhood Leukemia International Consortium; GIS=Geographic Information Systems
ALL=acute lymphoblastic leukemia; AML=acute myeloblastic leukemia; CI=Confidence Interval; CLIC=Childhood Leukemia International Consortium; GIS=Geographic Information Systems; PAHs=polycyclic aromatic hydrocarbons; PCBs=polychlorinated biphenyls; PBDEs=polybrominated diphenyl ethers
Indicates relative risk reported
Pesticides
Several studies have suggested that home pesticide exposure before birth and during a child's early years may increase the risk of childhood leukemia. Indeed, exposure to pesticides is one of the most frequently investigated chemical risk factors for childhood leukemia. A causal link between exposure to pesticides and childhood leukemia is supported by many studies, including the California Childhood Leukemia Study, which demonstrated a relationship between exposure to insecticides – as a general class – and childhood leukemia.67 Existing studies have generally used interviews with parents to assess children’s exposure to pesticides; as such, no specific pesticide, or class of pesticides, has been implicated as the causal agent underlying these observations.
Pooled Analyses of Home Pesticide Use and Childhood Leukemia
Investigators from the Childhood Leukemia International Consortium pooled individual parents’ responses to interview questions about children’s exposure to pesticides from 12 case-control studies.29 Each contributing study used a unique set of questions, often written in different languages, so exposure data were harmonized into compatible formats before pooled analyses could be conducted using multivariable logistic regression. ALL was associated with any pesticide exposure shortly before conception (OR=1.39, 95% CI: 1.25–1.55; N = 2,785 cases and 3,635 controls), during pregnancy (OR=1.43, 95% CI: 1.32–1.54; N = 5,055 cases and 7,370 controls), and after birth (OR=1.36, 95% CI: 1.23–1.51; N = 4,162 cases and 5,179 controls). Corresponding odds ratios for risk of AML were 1.49 (95% CI: 1.02–2.16; N=173 cases and 1,789 controls), 1.55 (95% CI: 1.21–1.99; N=344 cases and 4,666 controls) and 1.08 (95% CI: 0.76–1.53; N=198 cases and 2,655 controls), respectively.
The Childhood Leukemia International Consortium investigators29 confirmed the observed association between home pesticide exposure during pregnancy and childhood leukemia using meta-analyses as well. Other investigators have reported similar findings in independent meta-analyses,68–70 generally observing the strongest associations for indoor insecticide use.
Pooled Analyses of Parental Occupational Exposure to Pesticides and Childhood Leukemia
Given the consistently observed association between home pesticide use during early childhood and leukemia risk, a logical extension of this line of research has been to examine the effect of parental occupational exposure to pesticides on childhood leukemia risk. There is evidence that adults exposed to pesticides at work can track these chemicals back to their homes on their shoes, clothing, and skin, potentially exposing their families.71,72 Moreover, paternal exposure to pesticides before conception could result in germ cell damage, whereas maternal exposure to pesticides during pregnancy could also expose the fetus to these chemicals.73 The Childhood Leukemia International Consortium investigators pooled individual parents’ responses to interview questions about job histories and the data were harmonized to a compatible format that characterized parents’ pesticide exposures at work.74 ALL was associated with paternal exposure to pesticides at work around the time of conception (OR=1.20, 95% CI: 1.06–1.38; N=8,169 fathers of cases and 14,201 fathers of controls), but was not associated with maternal exposure during pregnancy (OR=1.01, 95% CI: 0.78–1.30; N=8,236 case, and 14,850 control mothers). In contrast, AML was associated with maternal exposure to pesticides at work during pregnancy (OR=1.94, 95% CI: 1.19–3.18, N=1,329 case and 12,141 control mothers), but was not associated with paternal exposure around the time of conception (OR=0.91, 95% CI: 0.66–1.24, N=1,231 case and 11,383 control fathers). The modest association between paternal exposure to pesticides around the time of conception and ALL risk in the offspring was more evident in children diagnosed at an older age (5+ years-old) and in children with the T-cell ALL subtype. The Childhood Leukemia International Consortium’s findings of a significant association between maternal exposure to pesticides during pregnancy and AML risk in the offspring is consistent with previous reports.75–77
In a meta-analysis that accompanied the above-referenced pooled analysis, the Childhood Leukemia International Consortium investigators74 found a positive association between maternal occupational exposures to pesticides during pregnancy and AML as well as between paternal occupational exposures around conception and T-cell ALL. This meta-analysis followed a systematic review and it included studies participating in the Childhood Leukemia International Consortium as well as non-participating, independent studies. Other investigators78,79 have reported similar findings in independent meta-analyses, observing an association between prenatal maternal exposure to pesticides at work and childhood leukemia..
GIS-Estimated Ambient Pesticide Levels and Childhood Leukemia
Investigators from the California Childhood Leukemia Study have also evaluated the association between residential proximity to agricultural pesticide applications and childhood ALL.55 For the families of 213 ALL cases and 268 matched controls, the authors linked residential histories together with agricultural pesticide-use reports from the California Department of Pesticide Regulation, to assess whether living within a half-mile (0.8 km) of pesticide applications was associated with childhood leukemia risk. Elevated ALL risk was associated with lifetime moderate exposure, but not high exposure, to certain physicochemical categories of pesticides, including organophosphates, chlorinated phenols, and triazines, and with pesticides classified as insecticides or fumigants.
Exposure to Herbicides and Childhood Leukemia
Epidemiological studies of childhood leukemia that use environmental or biological samples are relatively scarce. In one such study, investigators from the California Childhood Leukemia Study evaluated the relationship between childhood ALL and herbicide concentrations in settled dust as surrogates of herbicide exposures.80 The herbicide analysis included 269 ALL cases 0–7 years of age and 333 healthy controls matched on date of birth, sex, and race/ethnicity. Dust samples were collected from carpets using a high-volume small-surface sampler or from participant vacuum cleaners. Concentrations of agricultural or professional herbicides (alachlor, metolachlor, bromoxynil, bromoxynil octanoate, pebulate, butylate, prometryn, simazine, ethalfluralin, and pendimethalin) and residential herbicides (cyanazine, trifluralin, 2-methyl-4-chlorophenoxyacetic acid, mecoprop, 2,4-dichlorophenoxyacetic acid, chlorthal, and dicamba) were used in logistic regression adjusting for age, sex, race/ethnicity, household income, year and season of dust sampling, neighborhood type, and residence type. The risk of childhood ALL was associated with dust levels of chlorthal; compared to homes with a measurement below the analytical limit of detection, odds ratios for the first, second, and third tertiles were 1.49 (95% CI: 0.82–2.72), 1.49 (95% CI: 0.83–2.67), and 1.57 (95% CI: 0.90–2.73), respectively (p-value for linear trend=0.05). No other herbicides were identified as risk factors of childhood ALL. Metayer et al. postulated that 2,3,7,8-tetrachlorodibenzo-p-dioxin – a potent carcinogen and an impurity found in chlorthal – might be the causal agent underlying the observed association.80
Limitations of Existing Research on Pesticides and Childhood Leukemia
Previous studies of the relationship between pesticide exposure and childhood leukemia, including pooled analyses conducted by the Childhood Leukemia International Consortium have utilized interviews to assess pesticide exposures to children and their parents. Unfortunately, this study design has precluded investigators from identifying specific chemicals that may be causal agents underlying the observed associations. Findings from the California Childhood Leukemia Study seem to rule out organochlorine pesticides, such as DDT and chlordane, as the culpable pesticides underlying the observed association with childhood leukemia.81
Summary of Existing Research on Pesticides and Childhood Leukemia
Pooled analyses of data from studies around the world show a relationship between home pesticide use – especially of insecticides indoors – and the risk of childhood leukemia, which is confirmed by independent systematic reviews and meta-analyses. Pooled analyses of data from the Childhood Leukemia International Consortium demonstrate a relationship between prenatal maternal exposure to pesticides at work and the risk of childhood AML. Likewise, these findings were supported by independent systematic reviews and meta-analyses. Future studies will continue to exam relationships between pesticide exposures and the risk of specific leukemia subtypes and will also identify the specific pesticides which act as causal agents.
Parental Smoking
Parental tobacco use is another suspected risk factor for childhood leukemia that has received a lot of attention from researchers. Cigarettes contain numerous harmful constituents and tobacco use is well known to cause a variety of cancers in adults, including leukemia, via both direct and secondhand means of exposure. Likewise, there is evidence that parental cigarette smoking may also be associated with childhood cancer risk.
Investigators from the California Childhood Leukemia Study, for example, examined the association between parental smoking and childhood leukemia among 281 ALL cases, 46 AML cases, and 416 controls matched on age, sex, maternal race, and Latino ethnicity.82 Maternal smoking was not associated with an increased risk of either ALL or AML. Paternal preconception smoking was significantly associated with an increased risk of AML (OR=3.84, 95% CI: 1.04–14.17) and marginally associated with an increased risk of ALL (OR=1.32, 95% CI: 0.86–2.04).
Pooled Analyses of Parental Cigarette Smoking and Childhood AML
The Childhood Leukemia International Consortium pooled individual parents’ responses to interview questions about tobacco use from 14 case-control studies, representing 1,300 AML and 15,000 controls.83 Individual studies ascertained information about parental cigarette smoking at a number of stages of the child’s development with varying degrees of specificity, including maternal smoking during pregnancy and paternal smoking during the three months before conception. The findings from the pooled analyses strengthened the existing evidence of modest associations between paternal cigarette smoking at any time and childhood AML, with dose-response relationships (p<0.05). Maternal smoking during pregnancy was associated with an increased risk of AML for Latino children only.
Meta-analyses of Parental Cigarette Smoking and Childhood ALL
In 2009, a review of studies which evaluated the association between parental smoking and childhood leukemia revealed that 6 of 13 studies which had examined the relationship between paternal smoking and childhood leukemia reported significant positive associations.84 Subsequently, Liu et al.85 conducted a meta-analysis, which suggested that childhood ALL was associated with paternal smoking during preconception (OR=1.25, 95% CI: 1.08–1.46) during pregnancy (OR=1.24, 95% CI: 1.07–1.43), and after birth (OR=1.24, 95% CI: 0.96–1.60), with a dose-response relationships observed between childhood ALL and paternal smoking before conception or after birth.
Parental Cigarette Smoking and Childhood Leukemia Subtypes
There is some evidence that the strength of the association between parental cigarette smoking and childhood leukemia varies by the cytogenetic subtype of the tumor. For example, Metayer and colleagues27 reported that children with a history of paternal prenatal smoking combined with postnatal passive smoking had a 1.5-fold increased risk of ALL (95% CI: 1.01–2.23), compared to those without smoking history; but this joint effect was seen for B-cell precursor ALL with t(12;21) only (OR=2.08, 95% CI: 1.04–4.16), not for high hyperdiploid B-cell ALL. Similarly, the aforementioned pooled AML analysis conducted by the Childhood Leukemia International Consortium found that the highest smoking-related risk was seen for the myelomonocytic leukemia, a subtype common in treatment-related AML. Childhood leukemia comprises many subtypes and these findings demonstrate that each subtype may have a distinct set of characteristic risk factors corresponding to its unique etiology. As such, studies that evaluate subtype-specific chemical risk factors are the most likely to identify true relationships. Tellingly, when risk factors for each leukemia subtype are considered separately, higher odds ratios tend to be revealed.
Limitations of Existing Research on Tobacco Use and Childhood Leukemia
As was the case for pesticides above, previous studies of the relationship between parental tobacco use and childhood leukemia, including pooled analyses conducted by the Childhood Leukemia International Consortium, have utilized interviews to characterize parental smoking histories. This method of exposure assessment is useful, because it allows investigators to examine the effect of parental smoking at critical windows of a child’s development and because it enables investigators to untangle the separate effects of cigarette smoking done by the mother, father, or other family members. Moreover, in contrast to other environmental exposures that are of interest to leukemia researchers, parents are conscious of the number of cigarettes they tend to smoke each day and they can help quantify their own exposures to tobacco. However, recall and reporting biases are still concerns, as parents (especially parents of children with leukemia) may not accurately remember or may not feel comfortable discussing their past tobacco use history during the interview. The lack of an observed association between maternal smoking during pregnancy and childhood leukemia may be related to this potential for bias when using interview data to assess exposure. Alternatively, the lack of an observed association may also be the result of smoking-induced adverse birth outcomes (e.g., fetal loss, still birth) that preclude the subsequent development of childhood leukemia, thereby biasing epidemiological findings.
Summary of Existing Research on Tobacco Use and Childhood Leukemia
Pooled analyses of data from studies around the world show a relationship between paternal smoking before conception and AML risk. Likewise, these findings were supported by an independent systematic review and meta-analysis. Future studies will provide an increased focus on the role of prenatal maternal smoking on leukemia risk in Latino children and will pool data from the Childhood Leukemia International Consortium for an analysis of smoking-related ALL risk.
Chemicals Found in Paints, Petroleum Solvents, and Traffic Emissions
A collection of studies have investigated the associations between childhood leukemia and a loosely-related group of environmental exposures including paint, petroleum solvents, and vehicle traffic. These general exposure categories share characteristic chemical signatures, including the leukemogenic agent, benzene.
Investigators from the California Childhood Leukemia Study examined the association between childhood leukemia and the use of paint or petroleum solvents in the home before birth and in early childhood. The analysis included 550 ALL cases, 100 AML cases, and one or two controls per case individually matched for sex, age, Latino ethnicity, and race. Conditional logistic regression techniques were used to adjust for income. Home paint exposure was associated with ALL risk (OR=1.65; 95% CI: 1.26–2.15). The association was restricted to ALL with t(12;21) (OR=4.16, 95% CI: 1.66–10.4). Home use of petroleum solvents was associated with an increased risk for AML (OR=2.54, 95% CI: 1.19–5.42) but not ALL.
Pooled Analyses of Home Exposure to Paint and Childhood Leukemia
The Childhood Leukemia International Consortium pooled individual responses to questions about home paint exposures from eight case-control studies.86 Data were harmonized to account for inter-study differences in reported paint types, time periods of exposure, and leukemia subtypes and a compatible format was used in logistic regression. ALL risk was associated with home paint exposure in the 1–3 months before conception (OR=1.54, 95% CI: 1.28–1.85; N=3,002 cases and 3,836 controls), during pregnancy (OR=1.14, 95% CI: 1.04–1.25; N=4,382 cases and 5,747 controls), and after birth (OR=1.22, 95% CI: 1.07–1.39; N=1,962 cases and 2,973 controls). The risk was greater if someone other than the parents did the painting, e.g., a professional painter, which is indirect evidence of a dose-response relationship. The paint-leukemia association was stronger for ALL with t(12;21) than for other cytogenetic subtypes of leukemia.
Meta-Analyses of Parental Occupational Exposures To Paint, Solvents, Vehicle Exhaust, and Childhood Leukemia
As an extension of the research that has identified an association between exposure to paint in the home and childhood leukemia, meta-analyses have demonstrated associations between childhood leukemia risk and parental exposures in related occupational settings.
For example, a meta-analysis summarizing the existing literature on parental occupational exposures and childhood cancer found that relationships between childhood leukemia and paternal exposure to paints, solvents (e.g., benzene and trichloroethylene), and employment in motor vehicle-related occupations were among the strongest of the more than 1,000 occupation-childhood cancer combinations that were evaluated.87 The studies that comprised this meta-analysis had several limitations related to the quality of the exposure assessment including the small numbers of exposed cases studied, the likelihood of false positives due to multiple comparisons, and possibility of publication bias. Despite these limitations, this meta-analysis provides evidence that when parents are exposed to certain chemicals at work, the effects are harmful to their offspring.
Another meta-analysis summarized findings from 28 case-control studies and one cohort study that investigated the relationship between maternal occupational exposures and childhood leukemia in the offspring using 16,695 participating cases and 1,472,786 controls.88 ALL risk was associated with maternal paint exposures at work during pregnancy (OR=1.23, 95% CI: 1.02–1.47), with maternal solvent exposures at work during pregnancy (OR=1.25, 95% CI: 1.09–1.45), and with maternal petroleum exposure at work during pregnancy (OR=1.42, 95% CI: 1.10–1.84). No publication bias was found in this meta-analysis and consistent results were observed for subgroup and sensitivity analyses. However, an analysis of parental occupational paint exposures pooled from 13 case-controls studies revealed no increased risk for childhood ALL or AML.89
Carlos-Wallace et al.90 conducted meta-analyses to evaluate the risk of childhood leukemia associated with parental occupational exposure to benzene and solvents, as well as household use of products containing benzene and solvents. Maternal occupational exposure to benzene was associated with increased risk of childhood leukemia, yielding a summary relative risk of 1.71 (95% CI: 0.91–3.24). Use of household products containing benzene, aromatic hydrocarbons, solvents, or petroleum, was also associated with childhood leukemia risk, with a summary relative risk of 1.67 (95% CI: 1.01–2.78). The above associations were stronger for AML than for ALL and strongest for women who were exposed during pregnancy.
The California Childhood Leukemia Study examined the relationship between occupational exposure to organic solvents and the risk of childhood leukemia.91 Occupational histories were obtained via interview using 19 task-based job modules from parents of children with ALL (N=670), children with AML (N =104), and healthy control children (N =1021). Logistic regressions were used to estimate odds ratios adjusted for socio-demographic factors. Among children with non-Latino fathers, none of the exposures evaluated were associated with risks of ALL and AML. In contrast, exposure to any organic solvents in Latino fathers was associated with an increased risk of childhood ALL (OR=1.48, 95% CI: 1.01–2.16); in multivariable analyses and the odds ratio for exposure to chlorinated hydrocarbons, in particular, was elevated (OR=2.28, 95% CI: 0.97–5.37), whereas risk estimates for other exposures -- aromatic hydrocarbons, glycol ethers, and other hydrocarbon mixtures – were close to one. One common industrial chlorinated hydrocarbon, which might be the causal agent underlying the observed association, is trichloroethylene (TCE). As with other analyses that rely on interview-derived exposure data, the specific chlorinated hydrocarbon or mixture of chlorinated hydrocarbons responsible for the associations observed in these studies is not known. A large majority of mothers were not exposed to chemicals at work, and no associations were reported for risk of childhood ALL and AML
Traffic-related Air Pollution and Childhood Leukemia
Three recent meta-analyses independently demonstrated an association between childhood leukemia and postnatal traffic exposure.90,92,93 Boothe et al.92 combined findings from seven studies to estimate that childhood leukemia was positively associated with postnatal traffic density near the residence (OR=1.53, 95% CI: 1.12–2.10). There was no association between childhood leukemia and prenatal traffic exposures.
Filippini et al.93 combined 6 ecologic and 20 case-control studies that had assessed home exposure to traffic-related pollution by estimating traffic density in the neighboring roads, by estimating the vicinity to gas stations, or by modeling ambient nitrogen dioxide and benzene concentrations with GIS. Among high-quality studies that used traffic density to assign exposure, no significant increase in childhood leukemia risk was observed, even in the highest exposure category (OR=1.07, 95% CI: 0.93–1.24). Among studies that used NO2 estimates as the measure of exposure, there was a marginally significant association with childhood leukemia (OR=1.21, 95% CI: 0.97–1.52), which was stronger for ALL (OR=1.21, 95% CI: 1.04–1.41) than for AML (OR=1.06; 95% CI: 0.51–2.21). Among studies that used benzene estimates as the measure of exposure, there was a stronger association with AML (OR=2.28, 95% CI: 1.09–4.75) than ALL (OR=1.09, 95% CI: 0.67–1.77). Observed associations between childhood leukemia and exposure to traffic pollution were generally stronger for exposures in the postnatal period compared to the prenatal period.
Carlos-Wallace et al.90 conducted meta-analyses to evaluate the risk of childhood leukemia associated with traffic density and traffic-related air pollution. Both measures of traffic were associated with childhood leukemia; the summary relative risk was 1.48 (95% CI: 1.10–1.99). The relationship was stronger for AML than for ALL and stronger in studies that involved detailed models of traffic pollution than in those that estimated traffic density.
The findings from these three meta-analyses support a link between ambient exposure to traffic pollution and childhood leukemia risk, particularly due to benzene.
Benzene as the Potential Causal Agent
Once again, most of the studies that have evaluated the risk of childhood leukemia associated with paint, petroleum solvents, or traffic density are based on parent interviews. As such, it is challenging to identify the specific causal agent underlying the observed associations in these existing studies. Benzene is one potential culprit as it is a well-known leukemogen that is present in oil-based paints, petroleum solvents used in occupational and residential settings (e.g., in paint thinner), and vehicle exhaust. However, given that childhood leukemia subtype analyses have yielded disparate results depending on the specific exposure measure that was used in the meta/pooled analysis, it is also possible that this loose grouping of chemical exposures actually comprises several distinct chemical risk factors for leukemia. For example, in addition to benzene, other chemicals, such as 1,3-butadiane, styrene, xylene, and polycyclic aromatic hydrocarbons (PAHs) might also play a role in some of these observed relationships. The recent development of a mouse model for childhood leukemia will enable investigators to evaluate the role of specific chemical risk factors in the etiology of childhood leukemia.
Summary of Existing Research on Paint, Solvents, Traffic and Childhood Leukemia
Pooled analyses of data from studies around the world show a relationship between home exposure to paint and ALL risk. These findings were indirectly supported by a variety of systematic reviews and meta-analyses, which showed evidence of relationships between childhood leukemia and exposure to petroleum solvents (at home and at the mother’s work) and traffic (as measured by surrounding traffic density and modeled concentrations of traffic-related air pollutants). Several possible chemical risk factors may explain the observed association, including the well-known leukemogen, benzene.
Persistent Organic Pollutants
Epidemiological studies of childhood leukemia that use environmental or biological samples are relatively scarce. A series of analyses conducted as part of the California Childhood Leukemia Study have evaluated the relationship between childhood ALL and chemical concentrations in settled dust collected from participating homes as surrogates for chemical exposures.
Polycyclic Aromatic Hydrocarbons (PAHs) and Childhood Leukemia
The California Childhood Leukemia Study evaluated the relationship between childhood ALL and PAH concentrations in settled dust.94 As part of this population-based case-control study, dust samples were collected from 251 ALL cases and 306 birth-certificate controls using a high-volume small-surface sampler (N=185 cases, 212 controls) or directly from participants' household vacuum cleaner bags (N=66 cases, 94 controls). Logistic regression was used to evaluate the relationship between ALL risk and log-transformed concentrations of 9 individual PAHs, the summed PAHs, and the summed PAHs weighted by their carcinogenic potency (the toxic equivalence) while adjusting for demographic characteristics and duration between diagnosis/reference date and dust collection. Among participants with dust samples collected by high-volume small-surface sampler, risk of ALL was not associated with increasing concentration of any PAHs. However, among participants with dust samples collected by participants’ vacuum cleaners, a positive association was observed between ALL risk and increasing concentrations of benzo[a]pyrene (OR=1.42, 95% CI: 0.95–2.12), dibenzo[a,h]anthracene (OR=1.98, 95% CI: 1.11–3.55), benzo[k]fluoranthene (OR=1.71, 95% CI: 0.91–3.22), indeno[1,2,3-cd]pyrene (OR=1.81, 95% CI: 1.04–3.16), and the toxic equivalents (OR=2.35, 95% CI: 1.18–4.69). The observed association between ALL risk and PAH concentrations among participants with dust collected by vacuum suggests that PAH exposure may increase the risk of childhood ALL; however, understanding the reasons for the different results by sample type requires further scrutiny.
PAHs are byproducts of incomplete combustion that are found at high concentrations in cigarette smoke and vehicle exhaust. PAHs, especially dibenzo[a,h]anthracene, are potent human carcinogens. As such, it is possible that one PAH or a combination of PAHs may be the causal agent(s) responsible for the observed associations between parental smoking and childhood leukemia or between traffic density and childhood leukemia.
Polychlorinated Biphenyls (PCBs) and Childhood Leukemia
The California Childhood Leukemia Study has also evaluated the relationship between childhood ALL and levels of six PCBs – industrial chemicals that are probable human carcinogens and immune system disruptors – in settled dust.81 The PCB analysis included 184 ALL cases 0–7 years of age and 212 birth certificate controls matched to cases by birth date, sex, race, and Latino ethnicity. Dust samples were collected from the room where the child spent the most time using the high-volume small-surface sampler. In homes where any PCB was detected in the dust, there was a 2-fold increased risk of ALL (OR=1.97, 95% CI: 1.22–3.17). When considering the sum of the six PCBs analytes, compared to those in the lowest quartile of Σ6PCBs, the highest quartile was associated with about a 3-fold risk of ALL (OR=2.78, 95% CI: 1.41–5.48). The risk of ALL was positively associated with increasing concentrations of PCB congeners 118, 138, and 153 in dust. The associations with PCBs were stronger among non-Latino whites than among Latinos despite the presence of a similar distribution of PCB levels among controls in each racial/ethnic groups.
Polybrominated Diphenyl Ethers (PBDEs) and Childhood Leukemia
Along the same lines, the California Childhood Leukemia Study evaluated the relationship between childhood ALL and levels of PBDEs – chemical flame retardants – in settled dust.95 PBDEs are structural analogs to PCBs that also cause immune system perturbations. The PBDE analysis included 167 ALL cases 0–7 years of age and 214 birth certificate controls matched on date of birth, sex, and race/ethnicity. Dust samples were collected from carpets in the room where the child spent the most time while awake using a high-volume small-surface sampler or by sampling from participants' household vacuum cleaners. Concentrations of 14 PBDE congeners were measured including constituents of the Penta- (28, 47, 99, 100, 153, 154), Octa- (183, 196, 197, 203), and Deca-BDEs commercial mixtures (206–209). Odds ratios were calculated using logistic regression, adjusting for demographics, income, year of dust collection, and sampling method. Comparing the highest to lowest quartile showed no association with ALL for summed Penta- (OR=0.7, 95% CI: 0.4–1.3), Octa- (OR=1.3, 95% CI: 0.7–2.3), or Deca-BDEs (OR=1.0, 95% CI: 0.6–1.8). Comparing homes in the highest tertile to those below the analytical limit of detection, revealed a significant positive association with ALL risk for BDE-196 (OR=2.1, 95% CI: 1.1–3.8), BDE-203 (OR=2.0, 95% CI: 1.1–3.6), BDE-206 (OR=2.1; 95% CI: 1.1–3.9), and BDE-207 (OR=2.0, 95% CI: 1.03–3.8).
Interestingly, the significant associations with ALL risk observed in this analysis were for minor PBDE congeners that are found in dust at relatively low concentrations; whereas the most abundant PBDE congeners (e.g., BDEs 47, 99, and 209) were not associated with ALL risk. These low concentration PBDE congeners were measured with less analytical precision than their more common analogs, because the measured values were relatively close to the analytical limit of detection. In other words, the low-level PBDE congeners that were associated with ALL risk in this analysis were the ones measured with the least precision and, therefore, the ones with the greatest potential for a spurious finding. Still, there may be a plausible biological mechanism to explain the inconsistency of the risk estimates between PBDE congeners, as toxic and carcinogenic effects are expected to differ by congener.48,96 The fact that PCBs and PBDEs have a similar chemical structure lends credence to the hypothesis that these chemicals may be acting via the same mechanism of action, e.g., immune dysregulation.
Strengths of Existing Research on Persistent Organic Pollutants and Childhood Leukemia
Unlike much of the research described in this section, one strength of the existing research on persistent organic pollutants and childhood leukemia is the use of objective environmental measurements to assess chemical exposures, rather than interviews. Not only does this reduce the likelihood of recall bias, but it also allows investigators to identify specific chemicals as causal agents in the etiology of leukemia. Recognizing causal agents can be the first step in planning a successful intervention that will reduce future incidence of leukemia.
Limitations of Existing Research on Persistent Organic Pollutants and Childhood Leukemia
The relative stability of persistent organic pollutants in settled dust allows for exposure measurements that have limited temporal variability. This stability is a benefit of the sampling technique, because the resulting measurements represent long-term average levels of chemical contamination, which can be useful when trying to estimate past chemical exposures. However, this stability also obscures short-term fluctuations in chemical levels that might be important to investigators who want to identify critical windows of a child’s development when chemical exposures are especially harmful. That is, measuring chemical levels in settled dust will not enable a researcher to distinguish the leukemogenic effect of prenatal vs. postnatal chemical exposures, for example.
Moreover, children are exposed to chemicals through several other pathways in addition to the ingestion of contaminated settled dust. In particular, the ingestion of settled dust plays a relatively minor role in children’s exposure to PCBs compared to the ingestion of PCB-contaminated food. This is owing to the bioaccumulative nature of PCBs and the fact that they have been banned from production in the U.S. for several decades. Indeed, it is a testament to the persistence of these hazardous chemicals that they can still be so readily measured inside homes. In these analyses, the design of the California Childhood Leukemia Study did not account for chemical exposures received via the inhalation of contaminated air, the ingestion of contaminated food, or any other pathways. As such, the dust measurements are limited surrogates for total chemical exposure.
To date, the California Childhood Leukemia Study has only utilized dust samples to identify risk factors for ALL, the most common leukemia subtype. There is an insufficient number participants with dust samples available to analyze the risk of AML or to stratify by cytogenetic subtype.
Also of some concern is the fact that the subset of California Childhood Leukemia Study participants who were eligible for and consented to dust sampling had higher socioeconomic status than the full California Childhood Leukemia Study population and its underlying source population, the State of California. As such, the findings from these studies may not be representative of the general population. Fortunately, data from the California Childhood Leukemia Study indicates that both the case families and the control families participating in dust sampling had elevated socioeconomic status, which limits the risk of differential selection bias.
Perhaps the most important limitation of these studies is that they have not been substantiated by independent investigators. Whereas many members of Childhood Leukemia International Consortium and other studies have collected interview data about pesticides, smoking, and paint; very few have measured chemicals in environmental samples. As such, there are no meta-or pooled analyses published showing the risk of ALL associated with exposure to persistent organic pollutants, as there have been previously for the other, more well-studied, ALL risk factors that were discussed above.
Summary of Existing Research on Persistent Organic Pollutants and Childhood Leukemia
Three novel findings from the California Childhood Leukemia Study suggest that home exposures to persistent organic pollutants, such as PAHs, PCBs, and PBDEs, are associated with an increased risk of ALL. Future studies should attempt to interrogate these associations to replicate or refute their veracity.
Radiation
In utero exposure to low-dose radiation delivered from medical x-rays is one of the few widely-recognized risk factors for childhood leukemia.97 While the prevalence of fetal exposure to x-rays in utero has decreased markedly following radiation protection standards, the use of medical imaging procedures, including computerized tomography (CT) scans,98 has increased drastically during the past 30 years. In fact, CT scans are now the largest medical sources of radiation in economically developed countries.99 In addition, CT scans deliver an effective dose of radiation that is up to several hundred times stronger than conventional x-rays (depending on the target organ), which has led to major increases in the per capita radiation dose from medical sources (up 600% in the US since 1980).99 The carcinogenic effects of CT scans have not been established, but exposures to children are especially concerning because they are more sensitive to radiation-induced cell damage.97
Findings on children’s postnatal exposure to low-dose medical radiation and the risk of childhood leukemia are inconsistent, with modest positive associations reported in some studies, but not all.97,99 Risk prediction models have anticipated increased risks of childhood leukemia following CT scans, but these models were criticized for extrapolating from the effects of much higher levels of radiation that were observed in the Life Span Study of atomic-bomb survivors.100–102 Only one case-control study to date has published results on self-reported history of CT scans showing no increased risk of childhood ALL, based on small numbers of exposed children.103 Recent cohort studies with access to medical data in Europe 101,104–106, Australia107, and the US102, have reported small to moderate increases in the risk of leukemia in children exposed to CT scans; findings, however, were based on small number of excess cases ranging from 6 to 74. Pearce et al. 106 conducted a retrospective cohort study of 178,604 UK children and young adults with CT scans from 1985 to 2002. Patients were followed through 2008 and linked with 74 leukemia diagnoses and 135 brain cancer diagnoses. A cumulative dose of 50 milligray was estimated to triple the risk of childhood leukemia within 10 years of the first CT scan; likewise, a cumulative dose of 60 milligray tripled the risk of brain cancer.106 The main criticism of the study was the lack of an unexposed control group. Other cohort studies are underway108 including a pooled European study (EPI-CT)104 of about one million subjects (age 0–21 years). Although the number of excess childhood leukemia cases is still likely to be low (estimated to be approximately 60 to 100), this study will obtain more precise exposure information than previously possible, including individual doses of radiation for each organ.
Confounding by indication (reverse causation) is also a concern in studies of the relationship between medical radiation and childhood leukemia. In a French cohort study, the analyses accounting for child’s cancer-predisposing factors (mostly rare genetic conditions in less than 2% of children) showed a modest impact on the risk of childhood leukemia associated with CT scans.109 This was consistent with a case-control study of prenatal x-rays showing an overall 13% reduction in relative risk of childhood cancers, after adjusting for maternal illnesses during pregnancy.110 Others have argued that cancer-predisposing conditions may instead act as effect modifiers.111–113 Overall, despite methodological challenges, epidemiologic studies so far mostly support an association between post-natal exposure to CT scans and childhood leukemia, while results for lower dose x-rays are less consistent.
Pooled analyses reported that exposure to high levels of extremely low-frequency-electromagnetic fields over 0.3 or 0.4 μT is associated with an increased risk of childhood leukemia,114,115 which was the basis to classify extremely low-frequency-electromagnetic fields as possibly carcinogenic to humans (Group 2B).116 Methodological issues including possible confounding, selection bias, and measurement errors have been put forward as an alternate explanation for the observed association, and animal studies are ongoing to identify possible biological mechanisms.117,118 If the association between extremely low-frequency-electromagnetic fields and childhood leukemia is causal, the overall population attributable risk has been estimated to be 1.9% (1% to 4% depending on the countries).117,118
Future Steps in Identifying Environmental Causes of Childhood Leukemia
A multitude of studies on the environmental causes of childhood leukemia, including those conducted by our research team and others that are described above, have led to progress in identifying the etiological roles of environmental exposures in childhood leukemia. However, much work remains to be done. As discussed, several of the observed associations between chemical exposures and childhood leukemia are derived from interview data, an approach which can limit an investigator’s ability to identify specific chemical risk factors for childhood leukemia. As such one goal of future research will be to understand which specific causal agents underlie previously observed associations. For example, in collaboration with the National Cancer Institute, the California Childhood Leukemia Study is conducting a study to measure glyophosate in participating homes. This study will follow-up on a previously observed association between reported residential herbicide use and childhood ALL. The California Childhood Leukemia Study has observed associations between childhood leukemia risk and levels of certain PCBs, PBDEs, PAHs, and herbicides in settled dust. These unique findings need to be confirmed in independent studies, preferably in ones that use a distinct method for assessing children’s exposure to chemicals.
As an alternative approach for identifying the causal agents in childhood leukemia, the Center for Integrative Research on Childhood Leukemia and the Environment will employ a mouse model of ALL with t(12;21) to test the carcinogenic potential of a variety of chemicals, including, for example, compounds that comprise the broadly-defined groups of pesticides, solvents, or traffic pollutants. The mouse model will also be helpful in identifying mechanisms of action for the chemical risk factors of childhood leukemia. In fact, this is a central goal of the Center, as immunological factors and epigenetics will both be interrogated as possible cancer mechanisms. Moreover, state-of-the-science untargeted analytical approaches will allow investigators to identify novel chemical risk factors for childhood leukemia, by examining relationships between exposure and disease that have not been considered, to date.
DIETARY RISK FACTORS FOR CHILDHOOD LEUKEMIA
Introduction
Diet has been linked to several cancers in adults and children.119 These observations may be explained by various biological mechanisms such as exposure to dietary mutagens, mutagenesis due to nutrient deficiencies, and intake of micronutrients and other dietary components that may protect against the development of cancer by supporting cellular integrity, reducing inflammation and improving immune response.120–122 A growing body of research also suggests that the influence of particular nutrients on epigenetic processes may contribute to carcinogenesis.123
The role of the intrauterine environment is crucial in determining risk of disease later in life, and the “developmental origins of health and disease” hypothesis posits that nutritional and environmental exposures in utero permanently alter gene expression and the physical development of the fetus through a process called “programming”. 124,125 This hypothesis is likely central to the development of childhood leukemia, as well. Indeed, maternal nutrition during pregnancy may be related to both the occurrence of primary and secondary oncogenic events that lead to leukemia in the offspring. Subsequently, a child’s diet during the early years of life is likely to also play an important role in leukemogenesis. Breastfeeding has been consistently found to reduce leukemia risk in children, via intake of essential nutrients not readily available in newborns and due to the resulting beneficial effects of immune system priming. In contrast, the impact of diet – both the mother’s and the child’s – on childhood leukemia has been less studied.
Here we provide an overview of the current knowledge about the associations between diet and childhood leukemia.
Maternal Diet
Epidemiologic studies examining the relationship between maternal diet during pregnancy and the risk of childhood leukemia, have been mostly conducted in developed countries. Exposures of interest varied to include food groups (e.g. fruits, vegetables, proteins), micronutrients from dietary and supplement sources (e.g., folate and vitamins), food sources of topoisomerase II inhibitors (known risk factors for treatment-related leukemia), consumption of coffee, tea, and alcohol, and holistic measures of a healthy diet.
Food Groups
Fruits and vegetables contain a variety of vitamins and minerals that have anti-cancer, anti-proliferative, and anti-inflammatory effects,123 and the consumption of fruits and vegetables has been associated with a reduced risk of various types of cancer.119 Some food groups, in particular fruits and vegetables, have been associated with childhood leukemia risk in several studies. Research has found statistically significant negative associations between maternal consumption of fruits and vegetables and risk of childhood ALL,126–128 and one study found significant or near-significant inverse linear trends between the risk of infant leukemia and maternal consumption of fresh fruits and vegetables, especially for specific ALL subtypes.129 Negative associations have also been observed for childhood leukemia and maternal consumption of other food groups, specifically protein sources such as fish and seafood128 as well as beans and beef.126,127 One study demonstrated an increased risk of ALL with increased maternal consumption of meat or meat products and sugars or syrups.128
Folate and Other One-carbon Metabolism Nutrients
The one-carbon metabolism cycle is critical for the synthesis of DNA and RNA, the conversion of homocysteine to methionine, and the formation of s-adenosylmethionine (SAM), the primary methyl donor for DNA, RNA, proteins, and lipids.130 Folate and other B vitamins are important cofactors in the one-carbon metabolism cycle,130 and maternal folic acid supplementation during pregnancy has been demonstrated to influence DNA methylation in children.131 Maternal folic acid supplementation also protects against some childhood diseases, such as neural tube defects.132 High folate intake has been associated with reduced risk of breast133 and colorectal134 cancer and increased risk of prostate cancer135 among adults, whereas meta-analyses have indicated no effect of folic acid supplementation on adult cancer incidence.135–137 Maternal intake of folate and other nutrients involved in one-carbon metabolism may influence childhood leukemia risk due to the importance of these nutrients for DNA synthesis and repair, chromosomal integrity, and epigenetic processes that determine gene expression and influence cancer risk, including histone modification, levels of non-coding RNAs, and DNA methylation.123,130
Practices of prenatal folic acid supplementation have varied substantially across countries and over time, and individual epidemiologic studies that have evaluated the relationship between maternal folate intake through supplements and the risk of childhood ALL have yielded mixed findings. However, a recent Childhood Leukemia International Consortium analysis, the largest to date, pooled original interview data from 12 studies (representing 6,963 ALL, 585 AML, and 11,635 healthy controls) to observe that folic acid supplementation was protective against childhood leukemia.138 Folic acid taken before conception or during pregnancy-- with or without intake of other vitamins --was associated with a reduced risk of childhood ALL (OR=0.80, 95% CI: 0.71–0.89) and AML (OR=0.68, 95% CI: 0.48–0.96), after adjustment for study center.138 Interestingly, the reduced risks were seen only among women with low and medium education levels – a surrogate marker for low socio-demographic status and possibly for a low-quality (low-folate) diets -- suggesting that women who enter the peri-conception period with inadequate folate/vitamin levels would benefit the most from prenatal folate and vitamin supplementation.
Only a small number of studies, however, have examined the role of folate intake from food or the role of any other nutrients involved in the one-carbon metabolism cycle in the development of childhood leukemia. In addition, there has been only limited consideration of the role of maternal diet on risk of AML. A case-control study in Australia found some evidence that higher dietary intakes of folate and B12 from food in the last six months of pregnancy were associated with a decreased risk of ALL, whereas higher dietary intakes of vitamin B6 were unexpectedly associated with an increased risk of ALL.139 Previous analyses from a subset of the California Childhood Leukemia Study population examined the total intake of folate, vitamin B6 or vitamin B12 from both diet and supplements, and found no associations with ALL.126,127,140 However, in a recent and expanded California Childhood Leukemia Study analysis (681 ALL cases, 103 AML cases, and 1,076 controls) that employed principal components analysis to account for the high correlations between nutrients, higher maternal intake of one-carbon metabolism nutrients from food and supplements was associated with a reduced risk of ALL (OR=0.91, 95% CI: 0.84–0.99) and possibly AML (OR=0.83, 95% CI: 0.66–1.04).141 The association of ALL with nutrient intake exclusively from food (excluding supplements) was similar to the association of total nutrient intake from food and supplements, both in the study population overall and within racial/ethnic groups. However, high intake of B vitamins from supplements (versus none) was associated with a statistically significant reduced risk of ALL in children of Latinas (OR=0.36 95% CI: 0.17–0.74), but not in children of non-Latina white women (OR=0.76, 95% CI: 0.50–1.16) or Asian women (OR=1.51, 95% CI: 0.47–4.89).141 Racial/ethnic differences in nutrient intake and in genetic polymorphisms in the one-carbon (folate) pathway may partly explain the observed difference in leukemia risk.
In a biomarker analysis conducted as part of the California Childhood Leukemia Study, folate concentration measured in neonatal blood samples were similar between children with leukemia (313 ALL cases and 44 AML cases) and 405 controls, suggesting that folate levels at the end of pregnancy did not affect leukemia risk.63 The study was conducted in California where most pregnant women used prenatal vitamin supplementation, therefore limiting the ability to detect an association. Moreover, late pregnancy may not be the period of a child’s development during which a beneficial effect of folic acid and multivitamins, is critical to leukemia risk. However, this explanation would not be consistent with observations from the large pooled analyses of the Childhood Leukemia International Consortium that showed reduced risks of ALL and AML associated with self-reported supplementation during each trimester of the pregnancy.138
Newborn and child serum-nutrient levels are influenced by many factors, including maternal and child genetic polymorphisms.142 Many studies have found significant associations between single nucleotide polymorphisms (SNPs) in folate-related genes, such as MTHFR variants, and childhood leukemia; but, there are inconsistencies in the specific SNPs that have been identified across studies.140,143–147 A study examining the largest number of genes and SNPs in the folate pathway found statistically significant associations between SNPs in genes CBS, MTRR, and TYMS/ENOFS (but not MTHFR) and childhood ALL.140 Levels of maternal folate intake during pregnancy, and child’s Latino ethnicity were found to modify some of these associations.140 Other studies, however, did not report such interactions.144,148,149 Key findings regarding folate intake and other vitamins are summarized in Table 2.
Table 2.
Association between folate and other vitamins and risk of childhood leukemia: selected case-control studies.
| Population | No. of cases | Source of vitamins | Period of interest | OR( 95% CI) |
|---|---|---|---|---|
|
| ||||
| Childhood Leukemia International Consortium138 | 6,963 ALL 585 AML |
Maternal supplement | Prenatal | Folate only |
| ALL: OR = 0.80 (0.78, 0.92) | ||||
| AML: OR = 0.68 (0.48–0.96) | ||||
| Any vitamins | ||||
| ALL: OR = 0.85 (0.80, 0.94) | ||||
| AML: OR = 0.92 (0.75–1.14) | ||||
|
| ||||
| Australia | 333 ALL | Maternal diet | Pregnancy | Folate |
| ORQ2 = 0.68 (0.44, 1.06) | ||||
| ORQ3 = 0.58 (0.37, 0.91) | ||||
| ORQ4 = 0.44 (0.27, 0.71) | ||||
| ORQ5 = 0.70 (0.44, 1.12) | ||||
| p-trend=0.05 | ||||
| B12 vitamin | ||||
| ORQ2 = 0.72 (0.47, 1.10) | ||||
| ORQ3 = 0.79 (0.71, 1.21) | ||||
| ORQ4 = 0.85 (0.71, 1.31) | ||||
| ORQ5 = 0.49 (0.71, 0.77) | ||||
| p-trend=0.02 | ||||
| B6 vitamin | ||||
| ORQ2 = 1.04 (0.67, 1.62) | ||||
| ORQ3 = 1.15 (0.74, 1.81) | ||||
| ORQ4 = 1.28 (0.82, 2.00) | ||||
| ORQ5 = 1.60 (1.02, 2.51) | ||||
| p-trend=0.03 | ||||
|
| ||||
| California141 | 681 ALL 103 AML |
Maternal diet & supplements | Peri- conception | One-carbon metabolism nutrients (i.e., folate, B vitamins) |
| ALL: = 0.91 (0.84, ORPC 0.99) | ||||
| AML: ORPC = 0.91 (0.66, 1.04) | ||||
|
| ||||
| California63 | 317ALL 44 AML |
Blood levels | Neonatal | Hemoglobin concentration of folate at birth |
| No difference in between ALL, AML, and controls | ||||
ALL=acute lymphoblastic leukemia; AML=acute myeloblastic leukemia; Q=quintile; PC=principal component for one-carbon metabolism nutrients.
Topoisomerase II Inhibitors
The use of topoisomerase II inhibitors (a nuclear enzyme involved in DNA replication) in cancer chemotherapy has long been known to be associated with common MLL gene translocations that are characteristic of therapy-related AML.150 Consequently, it was hypothesized that topoisomerase inhibitors may be involved in the etiology of infant leukemia, because this subtype commonly involves the same MLL gene translocation that has been identified in these therapy-related leukemias.151 Aside from chemotherapeutic agents, topoisomerase inhibitors are found in diverse sources, including herbal medicines, quinolone antibiotics, certain types of laxatives, and pesticides.152 Certain dietary sources also contain topoisomerase inhibitors including, but not limited to, tea, coffee, wine, and certain fruits and vegetables.152 Recent laboratory studies, however, suggest that tea, wine and cocoa do not inhibit topoisomerase activity in vitro and thus are unlikely to increase the risk of MLL translocations.153
A small exploratory study examining maternal exposure to topoisomerase inhibitors during pregnancy and the risk of childhood leukemia found an increased risk of AML with increasing topoisomerase II inhibitor exposure (OR=10.2, 95% CI: 1.1–96.4; N=29 cases) for high exposure], but no increased risk for ALL (OR=1.1, 95% CI: 0.5–2.3; N=82).151 Subsequent studies confirmed a positive relationship between maternal dietary intake of topoisomerase II inhibitors and risk of infant AML with a MLL gene translocation, but have found no association between dietary intake of topoisomerase II inhibitors and risk of other subtypes of infant AML or any type of ALL.126,129
Coffee, Cola, and Tea
An early case-control study of 280 cases and 288 hospitalized controls found an increased risk of ALL among children of mothers reporting coffee consumption greater than four cups a day during pregnancy (OR=2.4, 95% CI: 1.3–4.7 for 4–8 cups, and OR=3.1, 95% CI: 1.0–9.5 for >8 cups), with similar ORs observed for acute non-lymphoblastic leukemia that did not reach statistical significance.154 Subsequent case-control studies have found maternal consumption of coffee during pregnancy to be associated with an increased risk of ALL, AML, and possibly infant leukemia, while others have failed to find an association, as summarized in a recent meta-analysis.155 There is some evidence from these studies that the increased risk of leukemia with maternal coffee consumption may be more pronounced among children born to non-smoking mothers.156,157 Similarly, cola-based drinks have been associated with increased risk of childhood ALL (summary OR=1.31, 95% CI: 1.09–2.47), while reduced risks have been reported with maternal tea consumption during pregnancy (summary OR=0.85, 95% CI: 0.75–0.97).155 A general limitation of those studies is the lack of information on the type of drinks (e.g., caffeinated or not, green or black tea), which contain different nutrients and other compounds with either anti-or pro carcinogenetic properties.
Alcohol
Maternal alcohol intake before or during pregnancy has been hypothesized to influence childhood leukemia risk by altering immune function or by teratogenic effects on cell differentiation.158 Alcohol is also an antagonist to folate metabolism and methionine synthase and may modify DNA methylation status in interaction with folate levels.159 A systematic review and meta-analysis of 21 case-control studies found that alcohol intake during pregnancy was associated with AML (summary OR=1.56, 95% CI: 1.13–2.15 produced from 9 studies comprising 731 cases) but not with ALL (summary OR=1.10, 95% CI: 0.93–1.29 produced from 11 studies comprising 5,108 cases).159 Heterogeneity between studies was explained in part by some studies 160,161 that demonstrated a negative association of childhood leukemia with maternal alcohol consumption during pregnancy. Repeating the meta-analysis by subgroup of alcohol indicated an increased risk of AML associated with reported consumption of wine but not beer or spirits, providing some additional support for the topoisomerase II hypothesis.159 For ALL, there was an association between maternal consumption of spirits during pregnancy, but not beer or wine.159 One subsequent study supported the finding of an increased risk of AML with maternal alcohol consumption during pregnancy162 while another did not find an association156; neither found a relationship between maternal alcohol consumption and ALL. In contrast, two other recent studies found that maternal consumption of alcohol during pregnancy was associated with a decreased risk of ALL163 and of infant leukemia.164
Healthy Diet Index
In contrast to studies that have evaluated the role of a limited number of specific nutrients or food components, measures of overall diet quality may better represent nutritional status and the complex biological interaction of multiple nutrients.165 Diet quality indices are often positively correlated with biological markers of micronutrient intake and have been associated with reduced risk of all-cause mortality, including cancer risk.166,167 Maternal dietary patterns and quality have also been associated with birth outcomes, such as neural tube and congenital heart defects.168
In a recent study169 overall maternal diet quality, as summarized by a diet quality index using a modified version of the 2010 Healthy Eating Index, was associated with a reduced risk of childhood ALL (OR for each five point increase on the index = 0.88, 95% CI: 0.78–0.98). A more pronounced reduction in risk was observed among younger children and children of women who did not use vitamin supplements before pregnancy. There was a similar reduced risk of AML with increasing maternal diet quality score, although this assosciation was not statistically significant (OR=0.76, 95% CI: 0.52–1.11). No single diet quality index component (i.e. food group or nutrient) appeared to account for the results, suggesting that the quality of the whole diet and the cumulative effects of many dietary components may be important in influencing childhood leukemia risk.169
Paternal Diet
In contrast to maternal diet, very few studies have examined the relationship between paternal diet before conception and childhood leukemia. One study suggested that the risk of childhood leukemia increased with increasing paternal consumption of hot dogs (sources of carcinogenic compounds from N-nitroso precursors).170 Also, there is no strong indication that paternal intake of folate and other vitamins from diet and supplements before the child’s conception reduced the risk of leukemia in the offspring.171,172
Child’s Diet
As mentioned earlier, breastfeeding and duration of breastfeeding (six months of more) have been associated with the risk of childhood ALL, as summarized in recent pooled173 and meta-analyses174 conducted by the Childhood Leukemia International Consortium. Besides breastfeeding, little is known about the influence of child’s early diet. Feeding with formula as early as 14 days after birth,175 alone or in combination with breastmilk, was associated with an increased risk of childhood ALL and dose-response relationships were reported for the duration of formula feeding.175,176 These studies contrasted previous null findings.177 Infants and children fed with milk formula have been found to have higher serum levels of IGF-1 than those breastfed, and fetal growth pathway has been hypothesized to play a role in leukemogenesis.176 Associations between a child’s consumption of various food groups and the risk for childhood ALL or for all leukemias combined are inconsistent, especially regarding the consumption of fruits and fruit juice, as well as the consumption of meat.170,175,177–179 Older age at introduction to solid food in general,176 and possibly older age at introduction to vegetables in particular,175 was associated with an increased risk of childhood ALL, while a reduced risk was reported for late introduction to eggs.175 Child’s consumption of cola-based drinks does not appear to be associated with leukemia, in contrast to maternal consumption during pregnancy.155
Conclusions
Most of the evidence to date supports the role of prenatal folate supplementation, alone or with other vitamins, in reducing childhood leukemia risk. Apart from the possible beneficial intake of fruits and vegetables both during pregnancy and in the early years of life, studies have led to mixed or isolated findings regarding the potential leukemogenic effect of other food groups, likely due to small sample sizes and challenges of collecting accurate descriptions of participants’ diets. In addition, there has been relatively limited consideration of the role of maternal diet on risk of AML, a less common subtype than ALL. Finally, measures of overall diet quality may represent nutritional status and the complex biological interaction of multiple nutrients better than single-nutrient assessment, and should be used in future studies.
A CLINICAL PERSPECTIVE ON ENVIRONMENTAL HEALTH LITERACY
Nurses, doctors, and other health care providers are highly respected as sources for health information; as such they play an important role in translating new scientific findings to the public.180 It has long been recognized that health care providers should act as resources for information on environmental health for their patients; unfortunately, U.S. medical education is largely void of training in Environmental Medicine.181 Pediatricians and other providers report low self-efficacy in basic skills of Environmental Medicine such as taking a history of environmental exposures.10,182,183 A survey of members of the American College of Obstetrics and Gynecology reported that although three quarters of those surveyed agreed that counseling patients could reduce exposure to environmental hazards, fewer than 20% routinely discussed environmental exposures – even known developmental toxicants – with their patients. Despite a lack of training in environmental health, clinicians report a high interest in learning more about current environmental health research as it may apply to their practice.10,182
Environmental Health Literacy is an evolving discipline that “combines key principles and procedural elements from the fields of risk communication, health literacy, environmental health sciences, communications’ research and safety culture.”184 A basic level of environmental health literacy will help clinicians to have a sense of self efficacy about environmental health and to fulfill roles as alert clinicians, educators, and advocates for children’s health.
The key studies on environmental risk factors for childhood leukemia are rarely published in clinical journals and presentations on new environmental epidemiologic research are rare at clinical meetings. These circumstances require clinicians to make a special effort to stay informed about the impact of the environment on health. Fortunately, many online resources are becoming available to make this easier (see Text Box 1).
Text Box 1. Online resources on environmental health literacy for clinicians.
Pediatric Environmental Health Toolkit online training. An introduction to the basics of children’s environmental health and approaches to anticipatory guidance. Free continuing medical education credits. (http://www.atsdr.cdc.gov/emes/health_professionals/pediatrics.html)
A Story of Health. Multimedia e-book explores how environments interact with genes to influence health across the lifespan; includes a chapter on childhood leukemia. Free continuing medical education credits available from the CDC (http://coeh.berkeley.edu/ucpehsu/soh.html)
Little Things Matter. Video illustrates key concepts in children’s environmental health (in multiple languages) (https://www.youtube.com/channel/UCblp9EePwfR8doGm9JbJOjA/videos).
Webinars, fact sheets, and other resources are available from the Pediatric Environmental Health Specialty Units, a network of experts in reproductive and children’s environmental health. (http://www.pehsu.net/health_professionals.html )
Childhood Leukemia: Is it Time for Primary Prevention?
As presented in the preceding sections of this paper, there is a large and growing body of literature that demonstrates the role of environmental agents in determining the risk for childhood leukemias. This is in contrast to other pediatric cancers that are more rare and for which there have been far fewer environmental epidemiologic studies conducted. The evidence implicating environmental causes of childhood leukemia comes from a variety of individual studies worldwide and includes meta- and pooled analyses from the Childhood Leukemia International Consortium, as discussed in the Environmental Risk Factors for Childhood Leukemia section. Exposures to agents such as pesticides, tobacco smoke, solvents, and traffic-related pollution have been consistently linked to an increased risk of developing childhood leukemia. On the other hand, intake of vitamins and folate supplementation during the preconception period or pregnancy has been associated with a reduced risk of childhood leukemia as have breastfeeding and early exposure to infection, as characterized by attendance at large daycare and pre-school settings. Despite the fact that many studies have identified modifiable risk factors (increased or decreased risk) for childhood leukemia we are aware of no current prevention program that specifically addresses childhood leukemia, anywhere in the world. The American Cancer Society does support programs that discourage tobacco use and promote healthy nutrition and exercise for children, with the understanding that addressing these factors early in life will reduce future cancer burden.1 Why is it that activities to reduce childhood leukemia have not been incorporated into cancer prevention programs when potentially modifiable risk factors have been identified? Recently, our research group has suggested that the time is right to develop activities focused on primary prevention of childhood leukemia.185,186
What Evidence is Needed Before We Take Action?
Historically, there have been many chemicals for which early warning signs of serious health impacts have been ignored due to a lack of scientific consensus. In some cases, protective efforts and corrective actions were not undertaken until many years or even decades after the first trouble was spotted.187 For example, long after initial concerns were raised about DDT, PCBs, and lead, these chemicals continued to be used in large quantities. While these chemicals were eventually banned or restricted in use, in the interim they continued accumulating in the environment, and left a long-term legacy of detrimental exposures that were unnecessary and avoidable.
The European Community recognizes the precautionary principle, which provides justification for public policy actions in situations of scientific uncertainly in order to reduce health threats. In contrast, in the U.S., the regulatory framework generally requires scientific consensus of proof of harm before policy actions are carried out. The lack of public health campaigns specifically focused on primary prevention of childhood leukemia may, in part, result from this tendency to avoid taking precautionary actions. In clinical medicine, our mandate to “do no harm” often dictates that we are wary of false positives, for example, when interpreting results from a clinical trial. However, in the context of environmental epidemiology, it is also important to avoid making interpretations that will yield false negatives, because a failure to identify a hazard and take preventive actions poses a threat to the public health.
At this time, despite steadily accumulating evidence that environmental exposures increase the risk of childhood leukemia, authoritative bodies, including the International Agency for Research on Cancer (IARC), consider only radiation and parents’ active smoking as “causative” factors in the development of childhood leukemia. The Agency reviews individual chemicals only periodically and many of the suspected environmental risk factors for childhood leukemia have not been reviewed in the last decade; a period of time during which the environmental epidemiology of childhood leukemia has developed substantially.
Clinical medicine has embraced an evidence-based approach and uses systematic reviews (e.g. Cochrane, GRADE) as the gold standard for determining the quality of evidence. The highest quality of evidence (within the evidence-based paradigm) is obtained from double blinded, randomized trials, but this study design would be impossible and unethical in the context of childhood leukemia research on environmental exposures. Prospective cohort studies, which generally are thought to provide high quality of evidence, are prohibitively expensive for a disease like childhood leukemia with an incidence of less than 100 per million population. Even an international effort to combine existing mother-child cohorts leads to small numbers of children diagnosed with leukemia and other cancers. Studies assessing the role of environmental exposures in childhood leukemia would almost exclusively be given a lower quality of evidence rating, given that they are usually, by necessity, observational studies with case-control-designs. Adaptations of the systematic review methodology have been developed to respond to the needs of environmental health and may allow for more precautionary assessments in the future.188,189
Primary prevention of cancer includes reducing exposures to risk factors or changing the underlying conditions which result in disease. While the CDC has been exploring opportunities for early life prevention of child and adult cancers, there are diverse opinions about whether there is an adequate evidence base for primary prevention of cancer.185 In a summary of expert opinion on what evidence should be necessary to support taking action, suggestions range from animal studies and toxicologic profiles to high quality systematic reviews (see Text Box 2).185
Text Box 2. The range of evidence suggested* as necessary to validate public health action.
Animal studies and toxicologic profiles
Human studies
Systematic structured reviews and meta-analyses
Prevalence of exposure to risk factor
Severe/dreaded outcomes
Risk benefit or cost benefit analysis
Likelihood of unintended consequences of potential actions
Difficulty of sustaining intervention
Co-morbidities also associated with exposure
Mechanistic basis for health impact
* Adapted from expert opinion comments in Holman DM and Buchanan N.185
Addressing Specific Evidence Needs for Action
Many of the requirements suggested as a rationale for undertaking primary prevention programs listed in Text Box 2 have already been satisfied in the context of childhood leukemia research. For example, many of the risk factors identified in recent research are common, resulting in widespread exposure to the general population. Moreover, childhood leukemia could certainly be considered “a severe and dreaded outcome”. Though childhood leukemia treatment results in an 80–90% cure rate, the treatment has long-term health implications for those treated, as well as profound impacts on the families and the communities who endure it. While most items on the checklist for taking preventive action (Text Box 2) have been completed, one notable exception is that, to date, animal studies of leukemia have been limited, since it is only recently that animal models of human childhood leukemia have been developed.190
Structured systematic reviews and meta-analyses are viewed as key elements that support the validity of findings from individual studies. Multiple meta-analyses have been conducted on the most highly-studied risk factors for childhood leukemia: pesticide use, tobacco smoke, and traffic-related air pollution. As noted in the Environmental Risk Factors for Childhood Leukemia section, the Childhood Leukemia International Consortium has investigated many of these key risk factors for childhood leukemia using meta-analyses and pooled analysis of original data from case-control studies. These studies have been carefully conducted and can be considered systematic reviews of the epidemiologic literature.
Each factor in this issue that has been associated with altered risk of childhood leukemia is also associated with altering the risk of other health outcomes in children as well as adults. In fact, these same exposures that are implicated in the risk for childhood leukemia have substantial documentation of their non-cancer health impacts, including neurobehavioral deficits and respiratory disorders. Thus, acting to prevent childhood leukemia would also reduce the incidence of other diseases, and vice versa. These potential co-benefits should lessen any concerns that an error in attribution would result in unwarranted actions with potential negative impacts on health or increased financial burdens without benefit to society. Moreover, some high-risk behaviors – such as parental smoking – already have clinical and/or public health recommendations that attempt to alter exposure patterns. Although these efforts are to be applauded, including an additional focus on the potential of reducing childhood leukemia in these existing public health campaigns may improve effectiveness. With the exception of tobacco control and lead poisoning prevention, efforts at wide-scale promotion of children’s environmental health activities have received limited funding and attention. When evaluating the costs and benefits of reducing exposure to an environmental hazard, one must consider the total social and economic impacts for risk reduction associated with all relevant health outcomes (such as cancer, neurobehavioral deficits, and respiratory disorders). Examples of risk factors and protective factors associated with childhood leukemia, other co-morbidities associated with these exposures, and current clinical or public health recommendations relevant to these exposures are presented in Table 3.
Table 3.
Examples of exposures associated with altered risk (increased or decreased) for developing childhood leukemia and co-benefits of improved health outcomes by clinical and public health actions.
| Exposure | Health Impacts other than Childhood Leukemia | Clinical Recommendations | Public Health Activities |
|---|---|---|---|
| Pesticides | Neurobehavioral disorders, asthma, adverse birth outcomes, adult cancer, reproductive toxicity | American Academy of Pediatrics recommends Integrated Pest Management, exposure reductions. Advocates clinicians become familiar with acute and chronic/subclinical effects and provide anticipatory guidance | Integrated Pest Management recommended by U.S. EPA and cooperative extension services |
| Tobacco | Respiratory disease and asthma, adverse birth outcomes, cardiovascular disease, adult cancers, neurocognitive disorders, sudden infant death syndrome | Smoking cessation, avoidance of secondhand smoke universally recommended | National and local tobacco control programs, cessation hotlines |
| Air Pollution (including traffic related) | Includes: Preterm birth, decreased birth wt., asthma and respiratory development, cardiovascular dis., neurobehavioral disorders | Less amenable to individual action. Recommendations to restrict outdoor activities during high air pollution days (AirNow.gov). Avoid wood fires. | Many programs for air pollution reduction. Local programs to encourage walking and biking. No idle zones, replacement of old diesel vehicles. School siting regulations. |
| Folate (risk reduction) supplementation/ healthy diet | Inadequate folate early in pregnancy associated with neural tube defects, increase in autism risk, other birth defects | Preconception or prenatal folate supplementation recommended by American College of Obstetrics and Gynecology and American Academy of Family Physicians and others | Fortification of supplementation recommended by U.S. Preventive Services Task Force |
| Breastfeeding (risk reduction) | Sudden infant death syndrome, diarrhea, bacteremia, otitis media, childhood obesity, respiratory infection204 | Promote breastfeeding as the norm, develop skills to assess and collaborate with obstetrical community and certified counselors, serve as advocates for breastfeeding | 2011 Surgeon General's Call to Action to Support Breastfeeding: Actions for non-governmental organizations, government, employers, etc. All states have breastfeeding coalitions, programs to promote and support breastfeeding in minority communities205 |
Moving Towards Prevention
There have been rare instances when the conventional American wisdom -- that a determination of causation is required before any action can be taken to protect the public health-- has been bypassed to great benefit. For example, in 1964, the U.S. Surgeon General supported action to reduce tobacco use stating;
“Although the causative role of cigarette smoking in deaths from coronary disease is not proven, the Committee considers it more prudent from the public health viewpoint to assume that the established association has causative meaning than to suspend judgment until no uncertainty remains”.191
Similarly, the “Back to Sleep” campaign sponsored by the U.S. National Institute of Child Health and Human Development to reduce sudden infant death syndrome (SIDS) is another example of a highly successful public health measure that has saved many lives despite being adopted with less-than-uniform agreement on causation.192 In 1992, the American Academy of Pediatrics issued its policy statement suggesting that infants be placed to sleep on their backs or sides rather than prone.193 At that time there were several case-control studies, but no prospective randomized clinical trials, to support the American Academy of Pediatrics recommendation. Indeed, the American Academy of Pediatrics statement acknowledged many limitations in the epidemiological literature that was available for SIDS at that time. As highlighted in the policy statement abstract,
“this recommendation is made with the full recognition that the existing studies have methodologic limitations and were conducted in countries with infant care practices and other SIDS risk factors that differ from those in the United States (eg, maternal smoking, types of bedding, central heating, etc)”.
In fact, the recommendation was in part informed by ecologic studies, which is the study design that generally provides the least convincing support for causality. These ecologic studies reported on SIDS incidence following large-scale changes in regional sleep positioning practice; observing that when parents started putting children to sleep in a prone position the incidence of SIDS increased.194 Subsequently, in 1994, the “Back to Sleep” campaign was launched to discourage prone sleeping for infants. By 2000, the U.S. mortality rate for SIDS had dropped to one half of the rate in 1990. A set of additional recommendations aimed at decreasing SIDS was included in the 2011 American Academy of Pediatrics policy statement based on findings of varying scientific rigor. 192
In making these policy statements, American Academy of Pediatrics adapted a strength of evidence scheme from the U.S. Preventive Services Task Force with a highest level of recommendation that requires:
“…good and consistent scientific evidence (i.e., there are consistent findings from at least 2 well-designed, well-conducted case-control studies, a systematic review, or a meta-analysis). There is high certainty that the net benefit is substantial, and the conclusion is unlikely to be strongly affected by the results of future studies”.
Many of the childhood leukemia risk factors listed in Table 1 have multiple studies demonstrating their effect and at least one meta-analysis if not a systematic review. Moreover, at least for the environmental agents that have been assessed via the comprehensive, pooled analyses conducted by the Childhood Leukemia International Consortium, it is unlikely that future studies will contradict the current findings. Finally, when one takes into account the multiple other diseases that are also associated with these exposures, the benefit of taking public health action to reduce human exposure should be considered substantial. Under the American Academy of Pediatrics rubric noted above many of the risk factors in Table 3 should receive the highest rating for evidence quality.
Examples of Primary Prevention in Clinical and Public Health Practice
Folate
As noted in the Dietary Risk Factors for Childhood Leukemia section, adequate folate during the preconception and very early pregnancy periods is not only associated with reductions in the incidence of childhood leukemia but it also protects against neural tube and other birth defects and possibly autism.195,196 In 1992, the U.S. Public Health Service recommended that all women capable of becoming pregnant take 400 μg of folic acid daily. In 1998, the United States introduced fortification of enriched cereal grain products with folate. It has been estimated that folate fortification has resulted in approximately 1,300 fewer children being born with neural tube defects, annually.197 Despite these dramatic results, nearly one quarter of women of childbearing age in the National Health and Nutrition Examination Survey (2007 to 2012) were found to have suboptimal folate-blood levels.198 In addition, Latinas were noted to have significantly lower blood folate levels than white women. Given that Latino children – those living in Central or South American as well as those living in California -- are recognized to be at greater risk of childhood leukemia than white children, a public health campaign targeting folate supplementation in Latinas of childbearing age could be a particularly important opportunity for childhood leukemia prevention.
A caution would be a concern about the possibility of some women exceeding the tolerable upper intake level for folic acid. The National Health and Nutrition Examination Survey estimated that 2.7% of participants (primarily women) exceed these recommended folate values.199 Nonetheless, a careful emphasis on improving nutritional status by ensuring fresh foods and folic acid supplementation for women of pregnancy age seems warranted.
Tobacco
Although tobacco control programs and policies have significantly reduced smoking, there are still between 15 and 23% of young adult men and women who smoke in the U.S.200 It is likely that most are not aware of the link between paternal smoking around the time of conception and a child’s subsequent risk of developing leukemia. To prevent soon-to-be-dads from smoking before conception, fuller implementation of proven tobacco control strategies aimed at preventing smoking initiation and reducing smoking in teenagers and young adults is needed.
Pesticides
Pesticide exposure, both prenatal and postnatal, has been associated with leukemia risk. This supports introduction of programs focused on the use of safe pesticide practices during the preconception period. An example of materials that target an audience of people who are considering becoming, or currently are, pregnant is available from the Program for Reproductive Health and the Environment at the University of California, San Francisco. These include Pesticides Matter: Steps to Reduce Exposure and Protect Your Health (http://prhe.ucsf.edu/prhe/pesticidesmatter.html).
Preconception Care
Recently, reproductive health professionals involved in the care and support of women of childbearing age have begun to emphasize preconception care. These professionals are interested in including environmental factors as part of their message to their patients about health promotion before conception and during pregnancy.201 Including both men and women in health promotion provides an ideal opportunity to address environmental exposures prior to early critical windows of development, even before conception. Initial studies on the effectiveness of strategies to promote behavior change to reduce chemical hazard exposures preconception and during pregnancy indicate that perceived normative pressure (perception of what is common among peers and important to family, friends and doctors) is a key element.202 The U.S. Centers for Disease Control and Prevention and the American College of Obstetrics and Gynecology have begun programs to address preconception health (http://www.cdc.gov/preconception/index.html) see Preconception Health and Health Care). An effort should be made to ensure that environmental health literacy and childhood cancer prevention activities are addressed within the context of these evolving programs and included in a new standard of care.
The cancer prevention activities suggested by our research on childhood leukemia and others203 largely reinforce programs already in existence by different public health entities (e.g., tobacco cessation, healthy diet during pregnancy and folate supplementation, avoidance of exposure to volatile organic compounds, pesticides, and paint). Public health agencies and health care systems can partner to extend current programs with similar goals. Innovative cross-agency, multidisciplinary program opportunities include:
Enhance the use of news media and add risk reduction for childhood leukemia to environmental health literacy education activities for the general public, as appropriate.
Include environmental health messaging in pre-conception, prenatal, and child preventive health care and as part of programs such as “Text 4 Baby” and “Bright Futures”.
Assure that preconception health programs include environmental health components and additional emphasis on healthy diet.
Use emerging systematic review methodologies to evaluate environmental health risks with the goal of having environmental health programs recognized as evidence-based medicine.
Improve health care provider counseling for women and couples to integrate concepts of environmental health – specifically the risk factors associated with childhood leukemia – into medical education at all levels (during schooling and post-graduate education) for nurses, physicians, and allied health professionals.
Develop policy initiatives to reduce exposures to chemicals associated with childhood leukemia (and other negative health outcomes) during pre-pregnancy, pregnancy, and early childhood.
The currently available toxicologic and observational epidemiologic studies (including meta and pooled analyses) provide a strong evidentiary basis for the presence of casual associations (of small to moderate sizes) between several environmental exposures and childhood leukemia. Awaiting a more complete evidentiary basis for decision-making, though ideal, will result in significant delays in safeguarding children’s health. Education of clinicians and the public on primary prevention -- actions that an individual can take to reduce their own family’s exposures to chemical risk factors for childhood leukemia as well as other disorders --- is something that can occur now. Ultimately, regulatory actions based on the evolving science are needed to shift the burden from the individual to producers.
Acknowledgments
Funding Sources: This publication was supported by the cooperative agreement award number 1 U61TS000237-02 from the Agency for Toxic Substances and Disease Registry (ATSDR). The U.S. Environmental Protection Agency (EPA) supports the Pediatric Environmental Health Specialty Units (PEHSU) by providing partial funding to ATSDR under Inter-Agency Agreement number DW-75-95877701. This work also was supported in part by the US National Institute of Environmental Health Sciences (NIEHS) (grants P01 ES018172 and P50ES018172) and the USEPA (grants RD83451101 and RD83615901), as part of the Center for Integrative Research on Childhood Leukemia and the Environment (CIRCLE). The California Childhood Leukemia Study was also supported in part by NIEHS (grant R01ES009137).
CALL OUTS
Natural History
-
#1
The development of childhood leukemia is a multi-step process; initiation occurs in utero for most leukemias, whereas progression into acute disease usually takes place after birth.
Environmental risk factors
-
#1
Chemicals measured in samples of settled dust collected from a child’s home after diagnosis are useful surrogates for the chemical exposures s/he received during etiologically-relevant periods before and after birth.
-
#2
Epidemiological studies have identified several environmental risk factors for childhood leukemia and those findings have been confirmed with large meta-analyses and pooled analyses that combined data from thousands of children with leukemia and healthy controls.
Diet
-
#1
Diet quality is a comprehensive measure of nutritional status, and healthy maternal diet at the time of conception and during pregnancy is linked to a reduced risk of leukemia in the offspring.
-
#2
Breastfeeding for six months or longer, is associated with a reduced risk of childhood leukemia, whereas early introduction to milk formula may increase leukemia risk.
Prevention
-
#1
Increased risks of childhood leukemia have been consistently associated with exposures to pesticides, tobacco smoke, solvents, and traffic-related pollution.
-
#2
Reduced risks of childhood leukemia are associated with adequate folate before conception and early in pregnancy, breastfeeding, and early exposure to routine childhood infections.
-
#3
Risk factors for childhood leukemia are also associated with the risk of developing other cancers, neurobehavioral deficits, and respiratory disorders.
Footnotes
Disclaimer: The ideas and opinions expressed herein are those of the authors and do not necessarily represent the official views of the ATSDR, EPA, or NIEHS. Endorsement of any product or service mentioned by ATSDR, EPA, or NIEHS is not intended nor should it be inferred.
Conflict of Interest Disclosure: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Society AC. [Accessed 6-17-16, 2016];Cancer Facts & Figures 2016. 2016 http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf.
- 2.Madhusoodhan PP, Carroll WL, Bhatla T. Progress and Prospects in Pediatric Leukemia. Current problems in pediatric and adolescent health care. 2016 Jul;46(7):229–241. doi: 10.1016/j.cppeds.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Nathan PC, Wasilewski-Masker K, Janzen LA. Long-term outcomes in survivors of childhood acute lymphoblastic leukemia. Hematology/oncology clinics of North America. 2009 Oct;23(5):1065–1082. vi–vii. doi: 10.1016/j.hoc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Iyer NS, Balsamo LM, Bracken MB, Kadan-Lottick NS. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015 Jul 16;126(3):346–353. doi: 10.1182/blood-2015-02-627414. [DOI] [PubMed] [Google Scholar]
- 5.Curtin K, Smith KR, Fraser A, Pimentel R, Kohlmann W, Schiffman JD. Familial risk of childhood cancer and tumors in the Li-Fraumeni spectrum in the Utah Population Database: implications for genetic evaluation in pediatric practice. International journal of cancer. Journal international du cancer. 2013 Nov 15;133(10):2444–2453. doi: 10.1002/ijc.28266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiemels J. Perspectives on the causes of childhood leukemia. Chemico-biological interactions. 2012 Apr 5;196(3):59–67. doi: 10.1016/j.cbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giddings B, Whitehead TP, Metayer C, Miller MD. Childhood Leukemia Incidence in California: High and Rising in the Hispanic Population. Cancer. 2016 doi: 10.1002/cncr.30129. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlaanderen J, Portengen L, Rothman N, Lan Q, Kromhout H, Vermeulen R. Flexible meta-regression to assess the shape of the benzene-leukemia exposure-response curve. Environmental health perspectives. 2010 Apr;118(4):526–532. doi: 10.1289/ehp.0901127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environmental health perspectives. 2005 Jul;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachek CM, Miller MD, Hsu C, et al. Children's Cancer and Environmental Exposures: Professional Attitudes and Practices. Journal of pediatric hematology/oncology. 2015 Oct;37(7):491–497. doi: 10.1097/MPH.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Wiemels JL. Chromosomal Translocations in Childhood Leukemia: Natural History, Mechanisms, and Epidemiology. JNCI Monographs. 2008 doi: 10.1093/jncimonographs/lgn006. in press. [DOI] [PubMed] [Google Scholar]
- 13.McHale CM, Wiemels JL, Zhang L, et al. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003 Jun 1;101(11):4640–4641. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 14.McHale CM, Wiemels JL, Zhang L, et al. Prenatal origin of TEL-AML1-positive acute lymphoblastic leukemia in children born in California. Genes Chromosomes Cancer. 2003 May;37(1):36–43. doi: 10.1002/gcc.10199. [DOI] [PubMed] [Google Scholar]
- 15.Wiemels JL, Cazzaniga G, Daniotti M, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 16.Wiemels JL, Xiao Z, Buffler PA, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99(10):3801–3805. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 17.Chang P, Kang M, Xiao A, et al. FLT3 mutation incidence and timing of origin in a population case series of pediatric leukemia. BMC cancer. 2010;10:513. doi: 10.1186/1471-2407-10-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiemels JL, Kang M, Chang JS, et al. Backtracking RAS mutations in high hyperdiploid childhood acute lymphoblastic leukemia. Blood cells, molecules & diseases. 2010 Oct 15;45(3):186–191. doi: 10.1016/j.bcmd.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiemels JL, Leonard BC, Wang Y, et al. Site-specific translocation and evidence of postnatal origin of the t(1;19) E2A-PBX1 fusion in childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2002 Nov 12;99(23):15101–15106. doi: 10.1073/pnas.222481199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori H, Colman SM, Xiao Z, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A. 2002;99(12):8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuna J, Ford AM, Peham M, et al. TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2004 Aug 15;10(16):5355–5360. doi: 10.1158/1078-0432.CCR-04-0584. [DOI] [PubMed] [Google Scholar]
- 22.Tsai AG, Lieber MR. Mechanisms of chromosomal rearrangement in the human genome. BMC genomics. 2010;11( Suppl 1):S1. doi: 10.1186/1471-2164-11-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009 Sep;41(9):1006–1010. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pluth JM, Nicklas JA, O'Neill JP, Albertini RJ. Increased frequency of specific genomic deletions resulting from in vitro malathion exposure. Cancer research. 1996 May 15;56(10):2393–2399. [PubMed] [Google Scholar]
- 25.Smith MT, Skibola CF, Allan JM, Morgan GJ. Causal models of leukaemia and lymphoma. IARC scientific publications. 2004;(157):373–392. [PubMed] [Google Scholar]
- 26.Kaur M, de Smith AJ, Selvin S, et al. Tobacco smoke and Ras mutations among Latino and non-Latino children with acute lymphoblastic leukemia. Archives of Medical Research. doi: 10.1016/j.arcmed.2016.11.016. Invited submission under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metayer C, Zhang L, Wiemels JL, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Sep;22(9):1600–1611. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scelo G, Metayer C, Zhang L, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environmental health perspectives. 2009 Jan;117(1):133–139. doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey HD, Infante-Rivard C, Metayer C, et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. International journal of cancer. Journal international du cancer. 2015 Dec 1;137(11):2644–2663. doi: 10.1002/ijc.29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker DJ. The origins of the developmental origins theory. Journal of internal medicine. 2007 May;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 31.Joubert BR, Felix JF, Yousefi P, et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. American journal of human genetics. 2016 Apr 7;98(4):680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonseth S, Roy R, Houseman EA, et al. Periconceptional folate consumption is associated with neonatal DNA methylation modifications in neural crest regulatory and cancer development genes. Epigenetics. 2015 Dec 2;10(12):1166–1176. doi: 10.1080/15592294.2015.1117889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudant J, Lightfoot T, Urayama K, et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: a Childhood Leukemia International Consortium (CLIC) Study. American Journal of Epidemiology. 2014 Sep; doi: 10.1093/aje/kwu298. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves MF, Alexander FE. An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia. 1993;7(3):349–360. [PubMed] [Google Scholar]
- 35.Kinlen LJ. Epidemiological evidence for an infective basis in childhood leukaemia [editorial] Br J Cancer. 1995;71(1):1–5. doi: 10.1038/bjc.1995.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crouch S, Lightfoot T, Simpson J, Smith A, Ansell P, Roman E. Infectious illness in children subsequently diagnosed with acute lymphoblastic leukemia: modeling the trends from birth to diagnosis. Am J Epidemiol. 2012 Sep 1;176(5):402–408. doi: 10.1093/aje/kws180. [DOI] [PubMed] [Google Scholar]
- 37.Chang JS, Tsai CR, Tsai YW, Wiemels JL. Medically diagnosed infections and risk of childhood leukaemia: a population-based case-control study. International journal of epidemiology. 2012 Aug;41(4):1050–1059. doi: 10.1093/ije/dys113. [DOI] [PubMed] [Google Scholar]
- 38.Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(8):1736–1740. doi: 10.1158/1055-9965.EPI-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MT, Guyton KZ, Gibbons CF, et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environmental health perspectives. 2016 Jun;124(6):713–721. doi: 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Infante-Rivard C, Jacques L. Empirical study of parental recall bias. Am J Epidemiol. 2000 Sep 1;152(5):480–486. doi: 10.1093/aje/152.5.480. [DOI] [PubMed] [Google Scholar]
- 41.Preston-Martin S, Bernstein L, Maldonado AA, Henderson BE, White SC. A dental x-ray validation study. Comparison of information from patient interviews and dental charts. Am J Epidemiol. 1985 Mar;121(3):430–439. doi: 10.1093/oxfordjournals.aje.a114015. [DOI] [PubMed] [Google Scholar]
- 42.Berrington de Gonzalez A, Ekbom A, Glass AG, et al. Comparison of documented and recalled histories of exposure to diagnostic x-rays in case-control studies of thyroid cancer. Am J Epidemiol. 2003 Apr 1;157(7):652–663. doi: 10.1093/aje/kwg026. [DOI] [PubMed] [Google Scholar]
- 43.Slusky DA, Metayer C, Aldrich MC, et al. Reliability of maternal-reports regarding the use of household pesticides: experience from a case-control study of childhood leukemia. Cancer epidemiology. 2012 Aug;36(4):375–380. doi: 10.1016/j.canep.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts JW, Wallace LA, Camann DE, et al. Monitoring and reducing exposure of infants to pollutants in house dust. Reviews of environmental contamination and toxicology. 2009;201:1–39. doi: 10.1007/978-1-4419-0032-6_1. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead TP, Brown FR, Metayer C, et al. Polychlorinated biphenyls in residential dust: sources of variability. Environmental science & technology. 2014 Jan 7;48(1):157–164. doi: 10.1021/es403863m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead TP, Brown FR, Metayer C, et al. Polybrominated diphenyl ethers in residential dust: sources of variability. Environment international. 2013 Jul;57–58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitehead TP, Metayer C, Petreas M, Does M, Buffler PA, Rappaport SM. Polycyclic aromatic hydrocarbons in residential dust: sources of variability. Environmental health perspectives. 2013 May;121(5):543–550. doi: 10.1289/ehp.1205821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. Journal of exposure science & environmental epidemiology. 2008 Jan;18(1):2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 49.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environmental science & technology. 2010 Jul 15;44(14):5627–5632. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins DJ, McClean MD, Fraser AJ, et al. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environmental science & technology. 2012 Jan 17;46(2):1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76(4):542–548. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 52.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environmental health perspectives. 2012 Jul;120(7):1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitehead TP, Crispo-Smith SM, Park JS, Petreas M, Rappaport SM, Metayer C. Concentrations of persistent organic pollutants in California women’s serum and vacuum dust. Environmental Research. 2014;136(Journal Article):57–66. doi: 10.1016/j.envres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitehead T, Crispo Smith SM, Park JS, Petreas M, Rappaport S, Metayer C. Concentrations of persistent organic pollutants in California children’s whole blood and residential dust. Chemosphere. 2014 doi: 10.1021/acs.est.5b02078. Submitted. [DOI] [PubMed] [Google Scholar]
- 55.Rull RP, Gunier R, Von Behren J, et al. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ Res. 2009 Oct;109(7):891–899. doi: 10.1016/j.envres.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunier RB, Ward MH, Airola M, et al. Determinants of agricultural pesticide concentrations in carpet dust. Environmental health perspectives. 2011 Jul;119(7):970–976. doi: 10.1289/ehp.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Von Behren J, Reynolds P, Gunier RB, et al. Residential traffic density and childhood leukemia risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008 Sep;17(9):2298–2301. doi: 10.1158/1055-9965.EPI-08-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slusky DA, Does M, Metayer C, Mezei G, Selvin S, Buffler PA. Potential role of selection bias in the association between childhood leukemia and residential magnetic fields exposure: a population-based assessment. Cancer epidemiology. 2014 Jun;38(3):307–313. doi: 10.1016/j.canep.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace LA, Pellizzari ED, Hartwell TD, et al. The TEAM (Total Exposure Assessment Methodology) Study: personal exposures to toxic substances in air, drinking water, and breath of 400 residents of New Jersey, North Carolina, and North Dakota. Environ Res. 1987 Aug;43(2):290–307. doi: 10.1016/s0013-9351(87)80030-0. [DOI] [PubMed] [Google Scholar]
- 60.Adgate JL, Eberly LE, Stroebel C, Pellizzari ED, Sexton K. Personal, indoor, and outdoor VOC exposures in a probability sample of children. Journal of exposure analysis and environmental epidemiology. 2004;14( Suppl 1):S4–S13. doi: 10.1038/sj.jea.7500353. [DOI] [PubMed] [Google Scholar]
- 61.Brown RC, Dwyer T, Kasten C, et al. Cohort profile: the International Childhood Cancer Cohort Consortium (I4C) International journal of epidemiology. 2007 Aug;36(4):724–730. doi: 10.1093/ije/dyl299. [DOI] [PubMed] [Google Scholar]
- 62.Funk WE, Waidyanatha S, Chaing SH, Rappaport SM. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008 Aug;17(8):1896–1901. doi: 10.1158/1055-9965.EPI-08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chokkalingam AP, Chun DS, Noonan EJ, et al. Blood levels of folate at birth and risk of childhood leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Jun;22(6):1088–1094. doi: 10.1158/1055-9965.EPI-12-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spector LG, Murphy SE, Wickham KM, Lindgren B, Joseph AM. Prenatal tobacco exposure and cotinine in newborn dried blood spots. Pediatrics. 2014 Jun;133(6):e1632–1638. doi: 10.1542/peds.2013-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma WL, Yun S, Bell EM, et al. Temporal trends of polybrominated diphenyl ethers (PBDEs) in the blood of newborns from New York State during 1997 through 2011: analysis of dried blood spots from the newborn screening program. Environmental science & technology. 2013 Jul 16;47(14):8015–8021. doi: 10.1021/es401857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass spectrometry reviews. 2016 May;35(3):361–438. doi: 10.1002/mas.21441. [DOI] [PubMed] [Google Scholar]
- 67.Ma X, Buffler PA, Gunier RB, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environmental health perspectives. 2002 Sep;110(9):955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Environmental health perspectives. 2010 Jan;118(1):33–41. doi: 10.1289/ehp.0900966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Residential exposure to pesticides and childhood leukaemia: a systematic review and meta–analysis. Environment international. 2011 Jan;37(1):280–291. doi: 10.1016/j.envint.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Chen M, Chang CH, Tao L, Lu C. Residential Exposure to Pesticide During Childhood and Childhood Cancers: A Meta-Analysis. Pediatrics. 2015 Oct;136(4):719–729. doi: 10.1542/peds.2015-0006. [DOI] [PubMed] [Google Scholar]
- 71.Curl CL, Fenske RA, Kissel JC, et al. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environmental health perspectives. 2002 Dec;110(12):A787–792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coronado GD, Vigoren EM, Thompson B, Griffith WC, Faustman EM. Organophosphate pesticide exposure and work in pome fruit: evidence for the take-home pesticide pathway. Environmental health perspectives. 2006 Jul;114(7):999–1006. doi: 10.1289/ehp.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrea EM, Jr, Bielawski DM, Posecion NC, Jr, et al. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ Res. 2009 Jan;109(1):116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailey HD, Fritschi L, Infante-Rivard C, et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: findings from the childhood leukemia international consortium. International journal of cancer. Journal international du cancer. 2014 Nov 1;135(9):2157–2172. doi: 10.1002/ijc.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexander FE, Patheal SL, Biondi A, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer research. 2001 Mar 15;61(6):2542–2546. [PubMed] [Google Scholar]
- 76.Buckley JD, Robison LL, Swotinsky R, et al. Occupational exposures of parents of children with acute nonlymphocytic leukemia: a report from the Childrens Cancer Study Group. Cancer research. 1989 Jul 15;49(14):4030–4037. [PubMed] [Google Scholar]
- 77.Ferreira JD, Couto AC, Pombo-de-Oliveira MS, Koifman S Brazilian Collaborative Study Group of Infant Acute L. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environmental health perspectives. 2013 Feb;121(2):269–275. doi: 10.1289/ehp.1103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wigle DT, Turner MC, Krewski D. A systematic review and meta–analysis of childhood leukemia and parental occupational pesticide exposure. Environmental health perspectives. 2009 Oct;117(10):1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer causes & control : CCC. 2010 Jun;21(6):787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- 80.Metayer C, Colt JS, Buffler PA, et al. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. Journal of exposure science & environmental epidemiology. 2013 Jul;23(4):363–370. doi: 10.1038/jes.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ward MH, Colt JS, Metayer C, et al. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environmental health perspectives. 2009 Jun;117(6):1007–1013. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006 Jun 15;163(12):1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 83.Metayer C, Petridou E, Mejia Arangure JM, et al. Parental Tobacco Smoking and Acute Myeloid Leukemia in Children: Pooled and Meta-analyses from the Childhood Leukemia International Consortium. American Journal of Epidemiology. doi: 10.1093/aje/kww018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang JS. Parental smoking and childhood leukemia. Methods in molecular biology (Clifton, NJ) 2009;472:103–137. doi: 10.1007/978-1-60327-492-0_5. [DOI] [PubMed] [Google Scholar]
- 85.Liu R, Zhang L, McHale CM, Hammond SK. Paternal smoking and risk of childhood acute lymphoblastic leukemia: systematic review and meta-analysis. Journal of oncology. 2011;2011:854584. doi: 10.1155/2011/854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey H, Metayer C, Milne E, et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: Findings from the Childhood Leukemia International Consortium. Cancer Causes and Control. 2015:26. doi: 10.1007/s10552-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colt JS, Blair A. Parental occupational exposures and risk of childhood cancer. Environmental health perspectives. 1998 Jun;106( Suppl 3):909–925. doi: 10.1289/ehp.98106909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Y, Zhang S, Li Z, et al. Maternal Benzene Exposure during Pregnancy and Risk of Childhood Acute Lymphoblastic Leukemia: A Meta-Analysis of Epidemiologic Studies. PloS one. 2014;9(10):e110466. doi: 10.1371/journal.pone.0110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bailey HD, Fritschi L, Metayer C, et al. Parental occupational paint exposure and risk of childhood leukemia in the offspring: findings from the Childhood Leukemia International Consortium. Cancer causes & control : CCC. 2014;25(10):1351–1367. doi: 10.1007/s10552-014-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am J Epidemiol. 2016 Jan 1;183(1):1–14. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metayer C, Scelo G, Kang A, et al. A Task-based Assessment of Parental Occupational Exposure to Organic Solvents and Other Compounds and the Risk of Childhood Leukemias in California. Environmental Research. doi: 10.1016/j.envres.2016.06.047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boothe VL, Boehmer TK, Wendel AM, Yip FY. Residential traffic exposure and childhood leukemia: a systematic review and meta-analysis. American journal of preventive medicine. 2014 Apr;46(4):413–422. doi: 10.1016/j.amepre.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. Journal of environmental science and health. Part C, Environmental carcinogenesis & ecotoxicology reviews. 2015;33(1):36–66. doi: 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deziel NC, Rull RP, Colt JS, et al. Polycyclic aromatic hydrocarbons in residential dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2014 Aug;133:388–395. doi: 10.1016/j.envres.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ward MH, Colt JS, Deziel NC, et al. Residential levels of polybrominated diphenyl ethers and risk of childhood acute lymphoblastic leukemia in california. Environmental health perspectives. 2014 Oct;122(10):1110–1116. doi: 10.1289/ehp.1307602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environmental health perspectives. 2004 Jan;112(1):9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linet MS, Kim KP, Rajaraman P. Children's exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatric radiology. 2009 Feb;39( Suppl 1):S4–26. doi: 10.1007/s00247-008-1026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA : the journal of the American Medical Association. 2012 Jun 13;307(22):2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA: a cancer journal for clinicians. 2012 Feb 3; doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 101.Journy N, Ancelet S, Rehel JL, et al. Predicted cancer risks induced by computed tomography examinations during childhood, by a quantitative risk assessment approach. Radiation and environmental biophysics. 2014 Mar;53(1):39–54. doi: 10.1007/s00411-013-0491-8. [DOI] [PubMed] [Google Scholar]
- 102.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA pediatrics. 2013 Aug 1;167(8):700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bailey HD, Armstrong BK, de Klerk NH, et al. Exposure to diagnostic radiological procedures and the risk of childhood acute lymphoblastic leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 Nov;19(11):2897–2909. doi: 10.1158/1055-9965.EPI-10-0542. [DOI] [PubMed] [Google Scholar]
- 104.Krille L, Jahnen A, Mildenberger P, et al. Computed tomography in children: multicenter cohort study design for the evaluation of cancer risk. European journal of epidemiology. 2011 Mar;26(3):249–250. doi: 10.1007/s10654-011-9549-6. [DOI] [PubMed] [Google Scholar]
- 105.Meulepas JM, Ronckers CM, Smets AM, et al. Leukemia and brain tumors among children after radiation exposure from CT scans: design and methodological opportunities of the Dutch Pediatric CT Study. European journal of epidemiology. 2014 Apr;29(4):293–301. doi: 10.1007/s10654-014-9900-9. [DOI] [PubMed] [Google Scholar]
- 106.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012 Aug 4;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. Bmj. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Einstein AJ. Beyond the bombs: cancer risks of low-dose medical radiation. Lancet. 2012 Aug 4;380(9840):455–457. doi: 10.1016/S0140-6736(12)60897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Journy N, Rehel JL, Ducou Le Pointe H, et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer. 2015 Jan 6;112(1):185–193. doi: 10.1038/bjc.2014.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knox EG, Stewart AM, Kneale GW, Gilman EA. Prenatal Irradiation and Childhood Cancer. J Soc Radiol. 1987;7(4):177–187. [Google Scholar]
- 111.Cardis E, de Basea MB. Comment on 'Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France'-Evidence of confounding by predisposing factors unclear. Br J Cancer. 2015 May 26;112(11):1842–1843. doi: 10.1038/bjc.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Journy N, Laurier D, Bernier MO. Comment on: Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer. 2015 May 26;112(11):1843–1844. doi: 10.1038/bjc.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muirhead CR. Response to 'Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France'. Br J Cancer. 2015 May 26;112(11):1841–1842. doi: 10.1038/bjc.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahlbom A, Day N, Feychting M, et al. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer. 2000 Sep;83(5):692–698. doi: 10.1054/bjoc.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greenland S, Sheppard AR, Kaune WT, Poole C, Kelsh MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood Leukemia-EMF Study Group. Epidemiology (Cambridge, Mass) 2000 Nov;11(6):624–634. doi: 10.1097/00001648-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 116.IARC. Non-ionizing Radiation: I, Static and Extremely Low-Frequency (ELF) Elctric and and Magnetic Fields. Lyon, France: International Agency for Research on Cancer; 2002. [Google Scholar]
- 117.Schuz J, Lagorio S, Bersani F. Electromagnetic fields and epidemiology: an overview inspired by the fourth course at the International School of Bioelectromagnetics. Bioelectromagnetics. 2009 Oct;30(7):511–524. doi: 10.1002/bem.20510. [DOI] [PubMed] [Google Scholar]
- 118.Teepen JC, van Dijck JA. Impact of high electromagnetic field levels on childhood leukemia incidence. International Journal of Cancer. 2012 Aug 15;131(4):769–778. doi: 10.1002/ijc.27542. [DOI] [PubMed] [Google Scholar]
- 119.Mosby TT, Cosgrove M, Sarkardei S, Platt KL, Kaina B. Nutrition in adult and childhood cancer: role of carcinogens and anti-carcinogens. Anticancer research. 2012 Oct;32(10):4171–4192. [PubMed] [Google Scholar]
- 120.Ferguson LR, Philpott M. Nutrition and mutagenesis. Annual review of nutrition. 2008;28:313–329. doi: 10.1146/annurev.nutr.28.061807.155449. [DOI] [PubMed] [Google Scholar]
- 121.Forster SE, Powers HJ, Foulds GA, et al. Improvement in nutritional status reduces the clinical impact of infections in older adults. Journal of the American Geriatrics Society. 2012 Sep;60(9):1645–1654. doi: 10.1111/j.1532-5415.2012.04118.x. [DOI] [PubMed] [Google Scholar]
- 122.Gibson A, Edgar JD, Neville CE, et al. Effect of fruit and vegetable consumption on immune function in older people: a randomized controlled trial. The American journal of clinical nutrition. 2012 Dec;96(6):1429–1436. doi: 10.3945/ajcn.112.039057. [DOI] [PubMed] [Google Scholar]
- 123.Stefanska B, Karlic H, Varga F, Fabianowska-Majewska K, Haslberger A. Epigenetic mechanisms in anti-cancer actions of bioactive food components--the implications in cancer prevention. British journal of pharmacology. 2012 Sep;167(2):279–297. doi: 10.1111/j.1476-5381.2012.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barker DJ. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public health. 2012 Mar;126(3):185–189. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annual review of nutrition. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 126.Jensen CD, Block G, Buffler P, Ma X, Selvin S, Month S. Maternal dietary risk factors in childhood acute lymphoblastic leukemia (United States) Cancer causes & control : CCC. 2004 Aug;15(6):559–570. doi: 10.1023/B:CACO.0000036161.98734.17. [DOI] [PubMed] [Google Scholar]
- 127.Kwan ML, Jensen CD, Block G, Hudes ML, Chu LW, Buffler PA. Maternal diet and risk of childhood acute lymphoblastic leukemia. Public health reports. 2009 Jul-Aug;124(4):503–514. doi: 10.1177/003335490912400407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petridou E, Ntouvelis E, Dessypris N, Terzidis A, Trichopoulos D Childhood Hematology-Oncology G. Maternal diet and acute lymphoblastic leukemia in young children. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005 Aug;14(8):1935–1939. doi: 10.1158/1055-9965.EPI-05-0090. [DOI] [PubMed] [Google Scholar]
- 129.Spector LG, Xie Y, Robison LL, et al. Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children's oncology group. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005 Mar;14(3):651–655. doi: 10.1158/1055-9965.EPI-04-0602. [DOI] [PubMed] [Google Scholar]
- 130.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature reviews. Cancer. 2013 Aug;13(8):572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim U, Song MA. Dietary and lifestyle factors of DNA methylation. Methods in molecular biology (Clifton, NJ) 2012;863:359–376. doi: 10.1007/978-1-61779-612-8_23. [DOI] [PubMed] [Google Scholar]
- 132.Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nature reviews. Neuroscience. 2006 Sep;7(9):724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen P, Li C, Li X, Li J, Chu R, Wang H. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer. 2014 Mar 25; doi: 10.1038/bjc.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kennedy DA, Stern SJ, Moretti M, et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer epidemiology. 2011 Feb;35(1):2–10. doi: 10.1016/j.canep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 135.Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Klemp M. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ open. 2012;2(1):e000653. doi: 10.1136/bmjopen-2011-000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Archives of internal medicine. 2010 Oct 11;170(18):1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 137.Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013 Mar 23;381(9871):1029–1036. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Metayer C, Milne E, Dockerty JD, et al. Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: a childhood leukemia international consortium study. Epidemiology (Cambridge, Mass) 2014 Nov;25(6):811–822. doi: 10.1097/EDE.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bailey HD, Miller M, Langridge A, et al. Maternal dietary intake of folate and vitamins b6 and B12 during pregnancy and the risk of childhood acute lymphoblastic leukemia. Nutrition and cancer. 2012 Oct;64(7):1122–1130. doi: 10.1080/01635581.2012.707278. [DOI] [PubMed] [Google Scholar]
- 140.Metayer C, Scelo G, Chokkalingam AP, et al. Genetic variants in the folate pathway and risk of childhood acute lymphoblastic leukemia. Cancer causes & control : CCC. 2011 Sep;22(9):1243–1258. doi: 10.1007/s10552-011-9795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singer AW, Selvin S, Block G, Golden C, Carmichael SL, Metayer C. Maternal prenatal intake of one-carbon metabolism nutrients and risk of childhood leukemia. Cancer causes & control : CCC. 2016 Jun 13; doi: 10.1007/s10552-016-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hay G, Clausen T, Whitelaw A, et al. Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. The Journal of nutrition. 2010 Mar;140(3):557–564. doi: 10.3945/jn.109.117424. [DOI] [PubMed] [Google Scholar]
- 143.Ajrouche R, Rudant J, Orsi L, et al. Childhood acute lymphoblastic leukaemia and indicators of early immune stimulation: the Estelle study (SFCE) Br J Cancer. 2015 Mar 17;112(6):1017–1026. doi: 10.1038/bjc.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amigou A, Rudant J, Orsi L, et al. Folic acid supplementation, MTHFR and MTRR polymorphisms, and the risk of childhood leukemia: the ESCALE study (SFCE) Cancer causes & control : CCC. 2012 Aug;23(8):1265–1277. doi: 10.1007/s10552-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 145.Koppen IJ, Hermans FJ, Kaspers GJ. Folate related gene polymorphisms and susceptibility to develop childhood acute lymphoblastic leukaemia. British journal of haematology. 2010 Jan;148(1):3–14. doi: 10.1111/j.1365-2141.2009.07898.x. [DOI] [PubMed] [Google Scholar]
- 146.Lightfoot TJ, Johnston WT, Painter D, et al. Genetic variation in the folate metabolic pathway and risk of childhood leukemia. Blood. 2010 May 13;115(19):3923–3929. doi: 10.1182/blood-2009-10-249722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wong NC, Ashley D, Chatterton Z, et al. A distinct DNA methylation signature defines pediatric pre-B cell acute lymphoblastic leukemia. Epigenetics. 2012 Jun 1;7(6):535–541. doi: 10.4161/epi.20193. [DOI] [PubMed] [Google Scholar]
- 148.Lupo PJ, Dietz DJ, Kamdar KY, Scheurer ME. Gene-environment interactions and the risk of childhood acute lymphoblastic leukemia: exploring the role of maternal folate genes and folic Acid fortification. Pediatr Hematol Oncol. 2014 Mar;31(2):160–168. doi: 10.3109/08880018.2013.825684. [DOI] [PubMed] [Google Scholar]
- 149.Milne E, Greenop KR, Scott RJ, et al. Folate pathway gene polymorphisms, maternal folic acid use, and risk of childhood acute lymphoblastic leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015 Jan;24(1):48–56. doi: 10.1158/1055-9965.EPI-14-0680. [DOI] [PubMed] [Google Scholar]
- 150.Mosad E, Abdou M, Zaky AH. Rearrangement of the myeloid/lymphoid leukemia gene in therapy-related myelodysplastic syndrome in patients previously treated with agents targeting DNA topoisomerase II. Oncology. 2012;83(3):128–134. doi: 10.1159/000338769. [DOI] [PubMed] [Google Scholar]
- 151.Ross JA, Potter JD, Reaman GH, Pendergrass TW, Robison LL. Maternal exposure to potential inhibitors of DNA topoisomerase II and infant leukemia (United States): a report from the Children's Cancer Group. Cancer causes & control : CCC. 1996 Nov;7(6):581–590. doi: 10.1007/BF00051700. [DOI] [PubMed] [Google Scholar]
- 152.Lightfoot TJ, Roman E. Causes of childhood leukaemia and lymphoma. Toxicology and applied pharmacology. 2004 Sep 1;199(2):104–117. doi: 10.1016/j.taap.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 153.Lanoue L, Green KK, Kwik-Uribe C, Keen CL. Dietary factors and the risk for acute infant leukemia: evaluating the effects of cocoa-derived flavanols on DNA topoisomerase activity. Experimental biology and medicine. 2010 Jan;235(1):77–89. doi: 10.1258/ebm.2009.009184. [DOI] [PubMed] [Google Scholar]
- 154.Menegaux F, Steffen C, Bellec S, et al. Maternal coffee and alcohol consumption during pregnancy, parental smoking and risk of childhood acute leukaemia. Cancer detection and prevention. 2005;29(6):487–493. doi: 10.1016/j.cdp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 155.Thomopoulos TP, Ntouvelis E, Diamantaras AA, et al. Maternal and childhood consumption of coffee, tea and cola beverages in association with childhood leukemia: a meta-analysis. Cancer epidemiology. 2015 Dec;39(6):1047–1059. doi: 10.1016/j.canep.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 156.Bonaventure A, Rudant J, Goujon-Bellec S, et al. Childhood acute leukemia, maternal beverage intake during pregnancy, and metabolic polymorphisms. Cancer causes & control : CCC. 2013 Apr;24(4):783–793. doi: 10.1007/s10552-013-0161-9. [DOI] [PubMed] [Google Scholar]
- 157.Milne E, Royle JA, Bennett LC, et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: results from an Australian case-control study. Cancer causes & control : CCC. 2011 Feb;22(2):207–218. doi: 10.1007/s10552-010-9688-1. [DOI] [PubMed] [Google Scholar]
- 158.Shu XO, Ross JA, Pendergrass TW, Reaman GH, Lampkin B, Robison LL. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a Childrens Cancer Group study. Journal of the National Cancer Institute. 1996 Jan 3;88(1):24–31. doi: 10.1093/jnci/88.1.24. [DOI] [PubMed] [Google Scholar]
- 159.Latino-Martel P, Chan DS, Druesne-Pecollo N, Barrandon E, Hercberg S, Norat T. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 May;19(5):1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- 160.Infante-Rivard C, Krajinovic M, Labuda D, Sinnett D. Childhood acute lymphoblastic leukemia associated with parental alcohol consumption and polymorphisms of carcinogen-metabolizing genes. Epidemiology (Cambridge, Mass) 2002 May;13(3):277–281. doi: 10.1097/00001648-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 161.Petridou E, Trichopoulos D, Kalapothaki V, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76(9):1241–1247. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Orsi L, Rudant J, Ajrouche R, et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: the ESTELLE study. Cancer causes & control : CCC. 2015 May 9; doi: 10.1007/s10552-015-0593-5. [DOI] [PubMed] [Google Scholar]
- 163.Milne E, Greenop KR, Scott RJ, et al. Parental alcohol consumption and risk of childhood acute lymphoblastic leukemia and brain tumors. Cancer causes & control : CCC. 2013 Feb;24(2):391–402. doi: 10.1007/s10552-012-0125-5. [DOI] [PubMed] [Google Scholar]
- 164.Slater ME, Linabery AM, Blair CK, et al. Maternal prenatal cigarette, alcohol and illicit drug use and risk of infant leukaemia: a report from the Children's Oncology Group. Paediatric and perinatal epidemiology. 2011 Nov;25(6):559–565. doi: 10.1111/j.1365-3016.2011.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002 Feb;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 166.Harnack L, Nicodemus K, Jacobs DR, Jr, Folsom AR. An evaluation of the Dietary Guidelines for Americans in relation to cancer occurrence. The American journal of clinical nutrition. 2002 Oct;76(4):889–896. doi: 10.1093/ajcn/76.4.889. [DOI] [PubMed] [Google Scholar]
- 167.Kant AK. Dietary patterns and health outcomes. Journal of the American Dietetic Association. 2004 Apr;104(4):615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 168.Carmichael SL, Yang W, Feldkamp ML, et al. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Archives of pediatrics & adolescent medicine. 2012 Feb;166(2):121–126. doi: 10.1001/archpediatrics.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singer AW, Carmichael SL, Selvin S, Fu C, Block G, Metayer C. Maternal diet quality before pregnancy and risk of childhood leukemia. British Journal of Nutrition. doi: 10.1017/S0007114516003469. Conditionally Accepted. [DOI] [PubMed] [Google Scholar]
- 170.Peters JM, Preston-Martin S, London SJ, Bowman JD, Buckley JD, Thomas DC. Processed meats and risk of childhood leukemia (California, USA) Cancer causes & control : CCC. 1994 Mar;5(2):195–202. doi: 10.1007/BF01830266. [DOI] [PubMed] [Google Scholar]
- 171.Bailey HD, Miller M, Greenop KR, et al. Paternal intake of folate and vitamins B6 and B12 before conception and risk of childhood acute lymphoblastic leukemia. Cancer causes & control : CCC. 2014 Dec;25(12):1615–1625. doi: 10.1007/s10552-014-0466-3. [DOI] [PubMed] [Google Scholar]
- 172.Wen W, Shu XO, Potter JD, et al. Parental medication use and risk of childhood acute lymphoblastic leukemia. Cancer. 2002 Oct 15;95(8):1786–1794. doi: 10.1002/cncr.10859. [DOI] [PubMed] [Google Scholar]
- 173.Rudant J, Amigou A, Orsi L, et al. Fertility treatments, congenital malformations, fetal loss, and childhood acute leukemia: The ESCALE study (SFCE) Pediatric blood & cancer. 2012 May 18; doi: 10.1002/pbc.24192. [DOI] [PubMed] [Google Scholar]
- 174.Amitay EL, Keinan-Boker L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA pediatrics. 2015 Jun;169(6):e151025. doi: 10.1001/jamapediatrics.2015.1025. [DOI] [PubMed] [Google Scholar]
- 175.Greenop KR, Bailey HD, Miller M, et al. Breastfeeding and nutrition to 2 years of age and risk of childhood acute lymphoblastic leukemia and brain tumors. Nutrition and cancer. 2015;67(3):431–441. doi: 10.1080/01635581.2015.998839. [DOI] [PubMed] [Google Scholar]
- 176.Schraw JM, Dong YQ, Okcu MF, Scheurer ME, Forman MR. Do longer formula feeding and later introduction of solids increase risk for pediatric acute lymphoblastic leukemia? Cancer causes & control : CCC. 2014 Jan;25(1):73–80. doi: 10.1007/s10552-013-0309-7. [DOI] [PubMed] [Google Scholar]
- 177.Kwan ML, Block G, Selvin S, Month S, Buffler PA. Food consumption by children and the risk of childhood acute leukemia. Am J Epidemiol. 2004 Dec 1;160(11):1098–1107. doi: 10.1093/aje/kwh317. [DOI] [PubMed] [Google Scholar]
- 178.Diamantaras AA, Dessypris N, Sergentanis TN, et al. Nutrition in early life and risk of childhood leukemia: a case-control study in Greece. Cancer causes & control : CCC. 2013 Jan;24(1):117–124. doi: 10.1007/s10552-012-0097-5. [DOI] [PubMed] [Google Scholar]
- 179.Sarasua S, Savitz DA. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer causes & control : CCC. 1994 Mar;5(2):141–148. doi: 10.1007/BF01830260. [DOI] [PubMed] [Google Scholar]
- 180.Rifkin R. American rate nurses highest on honesty, ethical standards. [Accessed 6-9-16, 2016];Gallup Poll, Social Issues. 2014 http://www.gallup.com/poll/180260/americansrate-nurses-highest-honesty-ethical-standards.aspx.
- 181.Medicine Io. Role of the primary care physicians in occupational and environmental medicine. Washington, DC: 1988. [Google Scholar]
- 182.Kilpatrick N, Frumkin H, Trowbridge J, et al. The environmental history in pediatric practice: a study of pediatricians' attitudes, beliefs, and practices. Environmental health perspectives. 2002 Aug;110(8):823–827. doi: 10.1289/ehp.02110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trasande L, Boscarino J, Graber N, et al. The environment in pediatric practice: a study of New York pediatricians' attitudes, beliefs, and practices towards children's environmental health. Journal of urban health : bulletin of the New York Academy of Medicine. 2006 Jul;83(4):760–772. doi: 10.1007/s11524-006-9071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Finn S, O'Fallon L. The Emergence of Environmental Health Literacy-From Its Roots to Its Future Potential. Environmental health perspectives. 2015 Jun 30; doi: 10.1289/ehp.1409337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Holman DM, Buchanan N. Cancer Prevention During Early Life Expert Group. Opportunities during Early Life for Cancer Prevention: 2016. Highlights from a Series of Virtual Meetings with Experts. Pediatrics. doi: 10.1542/peds.2015-4268C. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Metayer C, Dahl G, Wiemels J, Miller M. Childhood Leukemia: A Preventable Disease. Pediatrics. doi: 10.1542/peds.2015-4268H. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gee D. Late lessons from early warnings: Toward realism and precaution with endocrine-disrupting substances. Environmental health perspectives. 2006 Apr;114( Suppl 1):152–160. doi: 10.1289/ehp.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environmental health perspectives. 2014 Jul;122(7):711–718. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Woodruff TJ, Sutton P. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environmental health perspectives. 2014 Oct;122(10):1007–1014. doi: 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bergerson RJ, Collier LS, Sarver AL, et al. An insertional mutagenesis screen identifies genes that cooperate with Mll-AF9 in a murine leukemogenesis model. Blood. 2012 May 10;119(19):4512–4523. doi: 10.1182/blood-2010-04-281428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.US Department of Health E, and Welfare. Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: US Department of Health, Education, and Welfare, Public Health Service; 1964. [Google Scholar]
- 192.Task Force on Sudden Infant Death S. Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011 Nov;128(5):e1341–1367. doi: 10.1542/peds.2011-2285. [DOI] [PubMed] [Google Scholar]
- 193.American Academy of Pediatrics AAP Task Force on Infant Positioning and SIDS: Positioning and SIDS. Pediatrics. 1992 Jun;89(6 Pt 1):1120–1126. [PubMed] [Google Scholar]
- 194.Engelberts AC, de Jonge GA. Choice of sleeping position for infants: possible association with cot death. Archives of disease in childhood. 1990 Apr;65(4):462–467. doi: 10.1136/adc.65.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. The American journal of clinical nutrition. 2012 Jul;96(1):80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA : the journal of the American Medical Association. 2013 Feb 13;309(6):570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Williams J, Mai CT, Mulinare J, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995–2011. MMWR. Morbidity and mortality weekly report. 2015 Jan 16;64(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 198.Tinker SC, Hamner HC, Qi YP, Crider KS. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth defects research. Part A, Clinical and molecular teratology. 2015 Jun;103(6):517–526. doi: 10.1002/bdra.23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Orozco AM, Yeung LF, Guo J, Carriquiry A, Berry RJ. Characteristics of U.S. Adults with Usual Daily Folic Acid Intake above the Tolerable Upper Intake Level: National Health and Nutrition Examination Survey, 2003–2010. Nutrients. 2016;8(4) doi: 10.3390/nu8040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jamal A, Homa DM, O'Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR. Morbidity and mortality weekly report. 2015 Nov 13;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 201.Sutton P, Woodruff TJ, Perron J, et al. Toxic environmental chemicals: the role of reproductive health professionals in preventing harmful exposures. American journal of obstetrics and gynecology. 2012 Sep;207(3):164–173. doi: 10.1016/j.ajog.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mello S, Hovick SR. Predicting Behaviors to Reduce Toxic Chemical Exposures Among New and Expectant Mothers: The Role of Distal Variables Within the Integrative Model of Behavioral Prediction. Health education & behavior : the official publication of the Society for Public Health Education. 2016 May 13; doi: 10.1177/1090198116637600. [DOI] [PubMed] [Google Scholar]
- 203.Grason HA, Misra DP. Reducing exposure to environmental toxicants before birth: moving from risk perception to risk reduction. Public health reports. 2009 Sep-Oct;124(5):629–641. doi: 10.1177/003335490912400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 205.(US). OotSGUCfDCaPUOoWsH. The Surgeon General's Call to Action to Support Breastfeeding. Rockville, MD: 2011. [Google Scholar]